Introduction
Osteosarcoma (OS) is a common primary malignant
tumour that severely affects the growth of bones and occurs mostly
in patients under the age of 25 years (1,2).
Although advanced therapeutic methods (particularly limb salvage
combined with neoadjuvant and adjuvant chemotherapy) have markedly
improved the limb salvage rates and long-term survival of patients
with OS, the risks of relapse and metastasis in patients with OS
remain high (3-5). Therefore, it is necessary to explore
novel prognostic molecular markers and the underlying mechanisms of
OS.
Long non-coding RNAs (lncRNAs) are endogenous RNA
molecules of >200 nucleotides in length and lack the ability to
encode proteins (6,7). It has been demonstrated that lncRNAs
are involved in the development of multiple tumours. For example,
lncRNA RP4 inhibits colorectal cancer cell proliferation and tumour
growth (8). LUCAT1 plays an
oncogenic role in ovarian cancer by promoting cell proliferation,
migration and invasion (9).
lncRNA-LOWEG inhibits the progression of gastric cancer by
suppressing cell invasion (10).
Moreover, increasing it has been demonstrated that lncRNAs exert
important functions in OS development. For instance, lncRNA
miR210HG has been shown to enhance cell invasion and metastasis by
sponging miR-503 in OS (11).
CAT104 has also been shown to promote OS development by sponging
miR-381 (12). TUG1 knockdown
also suppresses OS cell proliferation and invasion via miR-153
(13). Indeed, recent evidence
has revealed that TUSC8 serves as a tumour suppressor in the
development of cervical cancer by regulating the miR-641/PTEN
pathway (14). However, as a
novel lncRNA, the specific role of TUSC8 in OS has not yet been
clarified.
MicroRNAs (miRNAs or miRs) are a category of small
non-coding RNAs approximately 20 nucleotides in length (15). Previous studies have indicated
that miRNAs, such as miR-214, miR-153 and miR-146a-5p, play
important roles during the progression of various types of cancer
(16-18). As a type of miRNA, miR-197-3p has
been reported to facilitate the proliferation of breast cancer
cells (19). miR-197-3p functions
as a tumour promoter in bladder cancer by accelerating cell
proliferation, migration and invasion (20). It is well known that lncRNAs
function as competitive endogenous RNAs (ceRNAs) and regulate
protein expression by sponging miRNAs (21). For example, EPB41L4A-AS2
suppresses the progression of hepatocellular carcinoma by
regulating the miR-301a-5p/FOXL1 axis (22). MAGI2-AS3 inhibits bladder cancer
development by sponging miR-15b-5p and modulating CCDC1 (23). SNHG16 facilitates the
proliferation of OS cells via the miR-205/ZEB1 pathway (24). Nevertheless, little is known about
the exact regulatory mechanisms of TUSC8 in OS.
Therefore, the present study aimed to elucidate the
biological role and the underlying molecular mechanisms of TUSC8 in
OS. The results revealed that TUSC8 inhibits the development of OS
by modulating the miR-197-3p/EH-domain containing 2 (EHD2) pathway,
suggesting that TUSC8 may function as a novel target for the
treatment of OS.
Materials and methods
Tissue samples
A total of 52 OS tissues and normal bone tissues
were collected from patients at The Second Hospital of Jilin
University (Changchun, China) via surgery, and none of the patients
had been treated with chemotherapy before surgery. The cancerous
and normal tissues were identified according to the Tumor Node
Metastasis (TNM) Classification of Malignant Tumours (6th edition)
from the Union for International Cancer Control (UICC) by 2
experienced pathologists. All collected tissues were rapidly frozen
in liquid nitrogen at −80°C. The present study was approved by the
Human Research Ethics Committee of The Second Hospital of Jilin
University (Changchun, China). Written informed consent was signed
by each patient.
Cell culture and transfection
OS cell lines (MG-63, U2OS, Saos-2 and HOS) and the
osteoblastic cell line, OB3 (hFOB 1.19), were provided by the Cell
Bank of the Chinese Academy of Sciences (http://www.cellbank.org.cn/). These cells were
incubated in Roswell Park Memorial Institute (RPMI)-1640 medium
containing 10% foetal bovine serum (FBS; Gibco) and maintained in a
humid atmosphere with 5% CO2 at 37°C.
For TUSC8 overexpression experiments, the
full-length sequence of TUSC8 was subcloned into the pcDNA3.1
plasmid (Sangon Biotech Co., Ltd.) to generate the pcDNA3.1/TUSC8
construct. Short hairpin RNAs (shRNAs) targeting EHD2 were
purchased from Shanghai GenePharma Co., Ltd. to knock-down EHD2.
miR-197-3p mimics and controls (NC mimics) were purchased from
Shanghai GenePharma Co., Ltd. Lipofectamine 2,000 (Invitrogen;
Thermo Fisher Scientific, Inc.) was used for cell transfection
following the provided instructions. The mass of the miR-197-3p
mimics or miR-197-3p inhibitor was 20 µl. The
co-transfection of plasmid DNA and shRNA was performed using
Lipofectamine 2,000 reagent by the addition if 30 pmol of shRNA per
1 µg of DNA. Following 48 h of incubation at 37°C, cells
were harvested and used in the subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from tissues and cells (OB3,
MG-63, U2OS, Saos2 and HOS) using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and reverse transcribed into
complementary DNA (cDNA) using PrimeScript RT reagent kits (Takara
Biotechnology, Ltd.). A SYBR® Premix Ex Taq™ II reagent
kit (Takara Biotechnology, Ltd.) was utilized to perform qPCR. The
mirVana™ qRT-PCR microRNA Detection kit (Ambion Inc.) was used for
miRNA detection. U6 or GAPDH was regarded as the internal
reference. Relative quantification was evaluated by the
2−∆∆Cq method (25).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 3 min, followed by 40 cycles at 95°C for 5
sec and at 60°C for 30 sec. The sequences of the primers are
presented in Table SI.
Bioinformatics analysis
The starBase website (http://starbase.sysu.edu.cn/) was used to predict
potential miRNAs which interacted with TUSC8, and two candidate
miRNAs were predicted. Moreovoer, 6 candidate genes that containing
binding sites with miR-197-3p were screened out by overlap-ping the
bioinformatics prediction results of PITA and RNA22 under the
condition of Pan-Cancer (10 cancer types).
Luciferase reporter assay
The pmirGLO-TUSC8-Wt, pmirGLO-TUSC8-Mut,
pmirGLO-EHD2-Wt and pmirGLO-EHD2-Mut vectors were co-transfected
with miR-197-3p mimic or NC mimic into the MG-63 and U2OS cells
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). Following 48 h of incubation at room temperature, the
luciferase activity of the reporter plasmids was detected using the
Dual Luciferase Reporter Assay System (Promega Corp.). The relative
Firefly luciferase activity was normalized to Renilla
luciferase activity.
RNA immunoprecipitation (RIP) assay
RIP assay was performed with the EZ-Magna RIP kit
(EMD Millipore). Cells were cultivated with RIP buffer containing
magnetic beads conjugated with anti-Ago2 antibodies (1:20 dilution,
ab32381, Abcam). Anti-IgG (1:20 dilution, ab190475 Abcam) acted as
a negative control. The coprecipitated RNAs were then eluted from
the beads and measured by RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was analysed using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.).
Briefly, the cells were seeded into 96-well plates
(1×103 cells/well) and maintained in RPMI-1640 medium
supplemented with 10% FBS. CCK-8 solution (10 µl) was added
at 24, 48, 72 and 96 h. The absorption was recorded at 450 nm using
a microplate reader (EL340; BioTek Instruments, Inc.).
Colony formation assay
Transfected cells were seeded into 6-well plates at
a density of approximately 1×103 cells per well. The
cells were then cultured at 37°C for 2 weeks. Subsequently, the
cells were fixed with 5% paraformaldehyde for 15 min and stained
with 0.1% crystal violet (Beyotime Institute of Biotechnology,
Inc.) for 15 min at room temperature. Finally, the colonies were
visible and were counted under a light microscope (Olympus
Corp.).
Cell apoptotic analysis
Transfected cells were harvested and resuspended in
phosphate-buffered saline (PBS). The cells were then double-stained
with propidium iodide (PI) and Annexin V-fluorescein isothiocyanate
(Beyotime Institute of Biotechnolgoy, Inc.) according to the
manufacturer's instructions. Cell apoptosis was assessed using a
flow cytometer (BD Biosciences).
Transwell assay
Cells at a density of 1×105 cells per
well were added into the upper chamber, which had already been
coated with Matrigel and contained serum-free DMEM (Gibco; Thermo
Fisher Scientific, Inc.). DMEM containing 10% FBS was added to the
lower chamber. Following 24 h of incubation, the non-invaded cells
were removed, and the invaded cells were fixed with methanol and
stained with crystal violet (Beyotime Institute of Biotechnology,
Inc.) for 15 min at room temperature. The number of invaded cells
was counted using an inverted microscope (Olympus Corp.) to measure
the invasive ability. Cell migration was assessed in a similar
manner, with the exception that the upper chambers were not coated
with Matrigel.
Western blot analysis
Cells were lysed in RIPA buffer, and the protein
concentration was measured using the bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology, Inc.). The proteins
(30 µg) were then separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore). The membranes were cultured at 4°C overnight with
primary antibodies against E-cadherin (1:50 dilution, ab1416;
Abcam), N-cadherin (1:500 dilution, ab18203; Abcam), EHD2 (1:50,000
dilution, ab23935; Abcam) and GAPDH (1:10,000 dilution, ab181602;
Abcam). GAPDH antibody served as a control. The following day, the
bands were incubated with HRP-conjugated goat anti-rabbit secondary
antibodies (1:10,000 dilution, ab205718; Abcam) or HRP-conjugated
goat anti-mouse secondary antibodies (1:10,000 dilution, ab205719;
Abcam) for 1 h at room temperature and rinsed with TBST solution 3
times. Finally, proteins were visualized using an ECL
chemiluminescent detection system (Thermo Fisher Scientific,
Inc.).
Fluorescence in situ hybridization
(FISH)
FISH was conducted using the Ribo™ Fluorescent In
Situ Hybridization kit (Guangzhou RiboBio Co., Ltd.) as
previously described (26). The
TUSC8 probe was designed and synthesized by Guangzhou RiboBio Co.,
Ltd. DAPI (Guangzhou RiboBio Co., Ltd.) was used to stain the
nuclei. Images were obtained using a fluorescence microscope (Zeiss
AG).
Statistical analysis
Data are presented as the means ± standard deviation
(SD) and were analysed using SPSS v17.0 software (SPSS, Inc.). The
statistical significance of the data was determined by one-way
analysis of variance (ANOVA) or a Student's t-test (P<0.05). The
post hoc test used following one-way ANOVA was Tukey's test. Each
experiment was repeated in triplicate.
Results
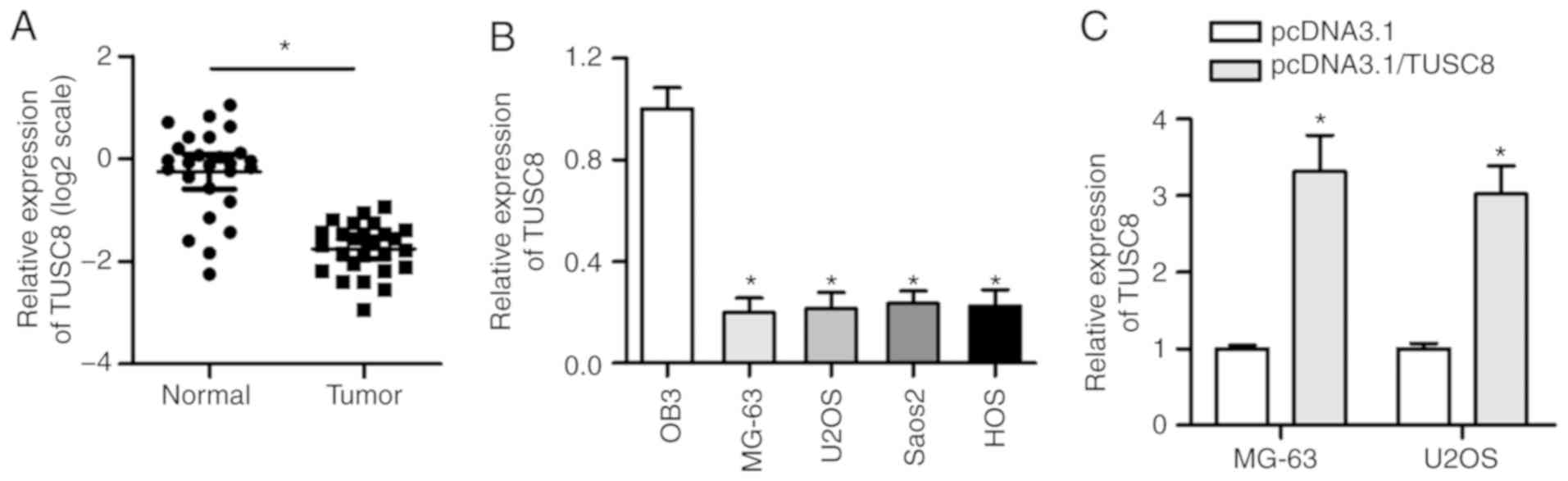
TUSC8 is prominently downregulated in OS
tissues and cells
To investigate the clinical significance of TUSC8 in
the development of OS, an RT-qPCR assay was conducted to detect the
expression status of TUSC8 in OS tissues and corresponding
non-cancerous tissues. As shown in Fig. 1A, the expression of TUSC8 was
significantly decreased in OS tissues compared with normal tissues.
Moreover, the TUSC8 level was consistently downregulated in the OS
cell lines (MG-63, U2OS, Saos-2 and HOS) compared with the
osteoblastic cell line OB3 (Fig.
1B). To verify the overexpression efficiency of pcDNA3.1/TUSC8
in the MG-63 and U2OS cells, RT-qPCR assays were carried out. As
shown in Fig. 1C, the
transfection of pcDNA3.1/TUSC8 triggered an obvious increase in
TUSC8 expression in the OS cells, suggesting that pcDNA3.1/TUSC8
could be used for follow-up experiments. Thus, these data indicate
that TUSC8 is prominently downregulated in OS tissues and
cells.
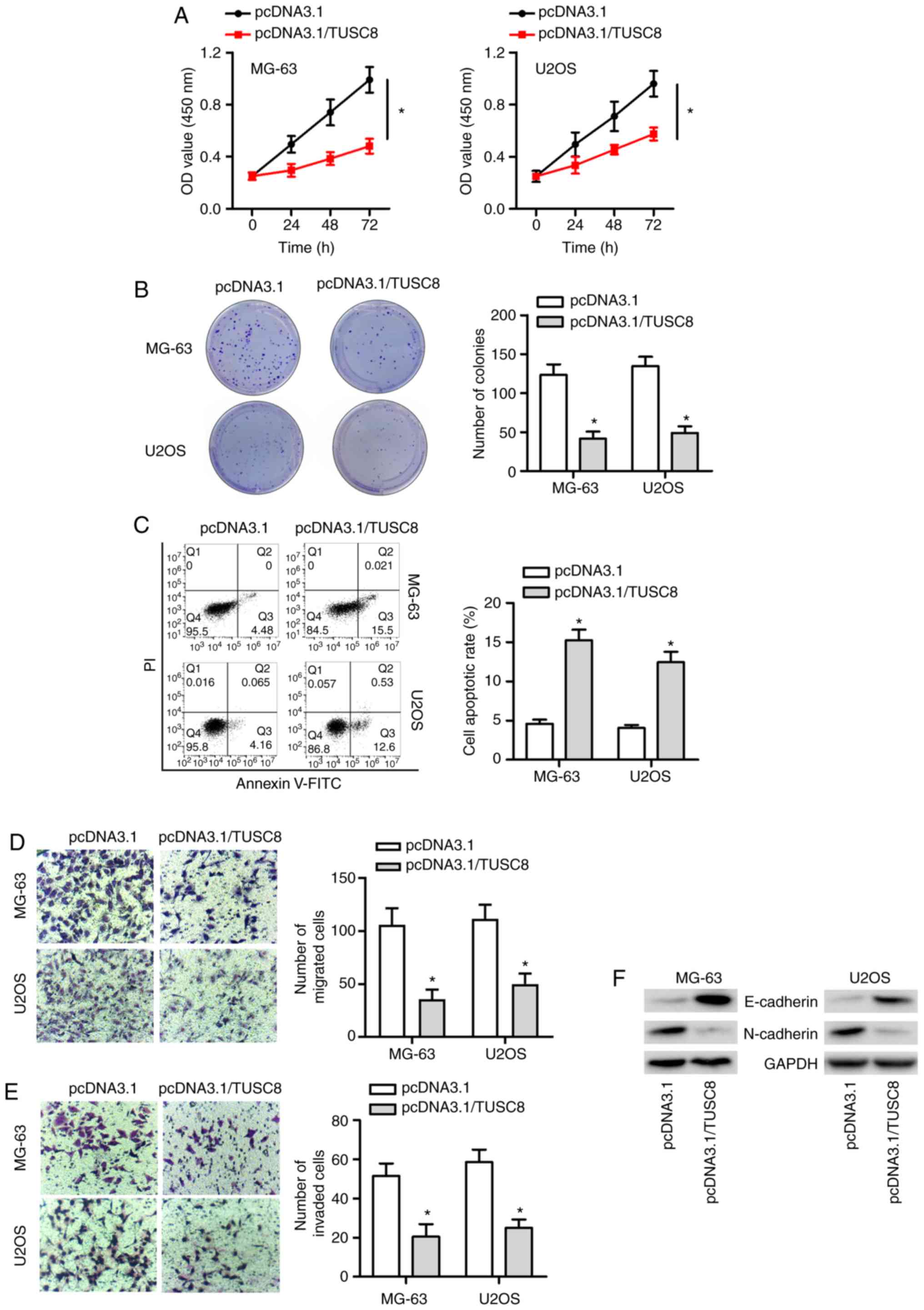
TUSC8 suppresses the progression of
OS
To confirm whether endogenous TUSC8 plays a role in
OS development, a series of functional assays were conducted. As
presented in Fig. 2A and B, CCK-8
and colony formation assays revealed that TUSC8 overexpression
markedly suppressed OS cell proliferation. Subsequently, flow
cytometric analysis demonstrated that the overexpression of TUSC8
increased the apoptosis of the MG-63 and U2OS cells (Fig. 2C). Furthermore, it was found that
the enhancement of TUSC8 expression markedly suppressed the
migratory and invasive capacity of the OS cells (Fig. 2D and E). It is well known that EMT
plays a crucial role in cancer development. Therefore, western blot
analysis was also conducted to validate whether TUSC8 affected the
EMT process in OS. As depicted in Fig. 2F, the protein expression of
E-cadherin (the epithelial marker) was markedly increased and the
protein level of N-cadherin (the mesenchymal marker) was notably
decreased by TUSC8 overexpression in the OS cells. Taken together,
TUSC8 suppressed the progression of OS by inhibiting cell
proliferation, migration, invasion and EMT, as well as by promoting
cell apoptosis in OS.
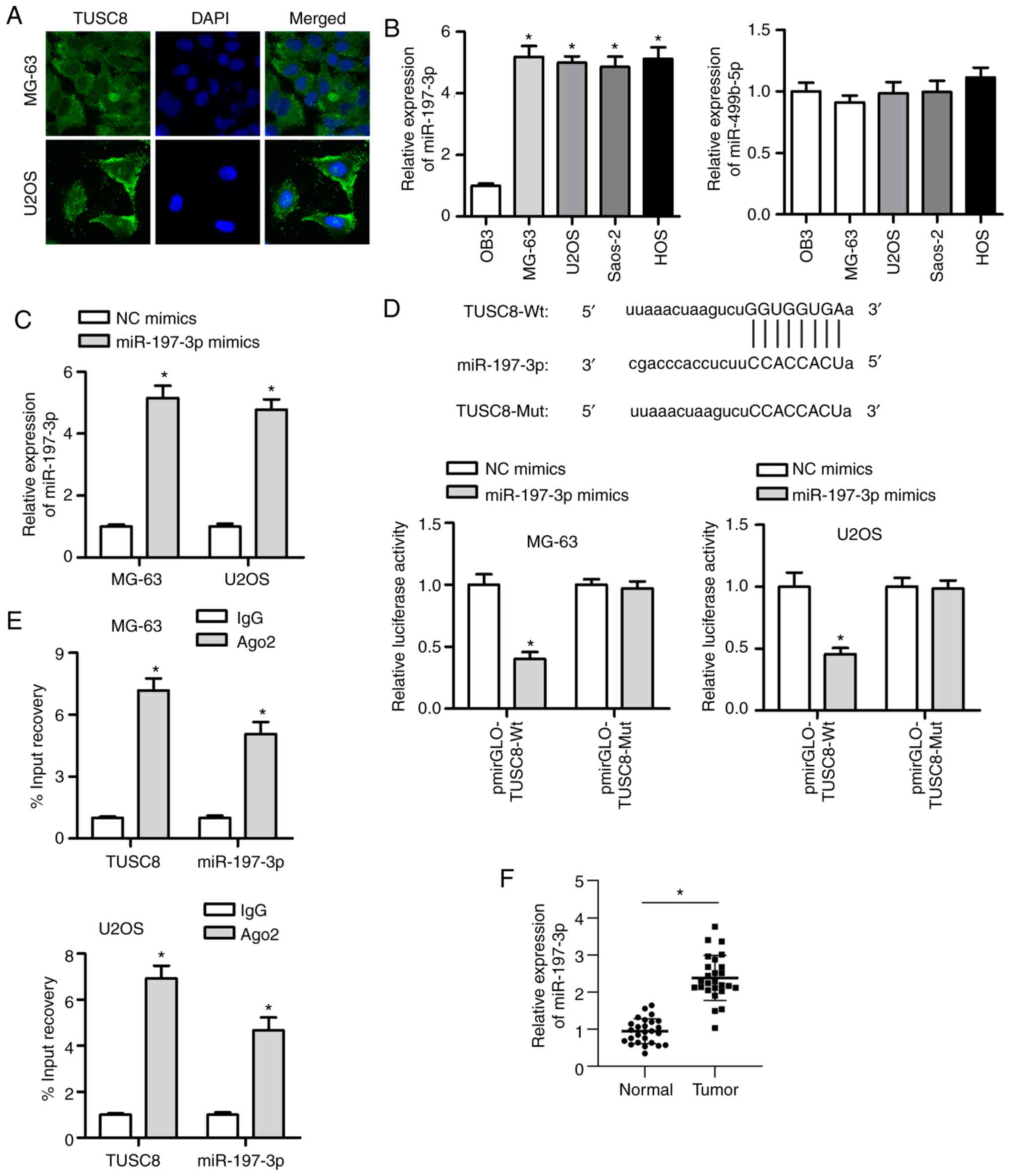
TUSC8 functions as a sponge of
miR-197-3p
Based on the above-mentoined functional experiments,
the antioncogenic role of TUSC8 was elucidated. To further explore
the under-lying mechanisms, the distribution of TUSC8 was measured
in the MG-63 and U2OS cells by FISH assay. The results indicated
that TUSC8 was predominantly distributed in the cytoplasm (Fig. 3A). Bioinformatics software
(http://starbase.sysu.edu.cn/) was used
to identify 2 candidate miRNAs (miR-197-3p and miR-499b-5p) that
exhibited binding sites for TUSC8. Subsequently, the expression of
these 2 miRNAs was analysed in OS cell lines and osteoblastic cell
lines. As shown by RT-qPCR, miR-197-3p was prominently upregulated
in OS cell lines compared with the osteoblastic cell line, while no
significant difference was found in the level of miR-499b-5p
(Fig. 3B). It has been reported
that miR-197-3p serves as a tumour promoter in various types of
cancer (19,27); thus, miR-197-3p was analysed in
subsequent explorations. It was observed that miR-197-3p expression
was substantially increased by transfection with miR-197-3p mimic
in the MG-63 and U2OS cells (Fig.
3C). A luciferase reporter assay was employed to verify the
interaction between TUSC8 and miR-197-3p. It was evident that the
luciferase activity of the pmirGLO-TUSC8-Wt vector was markedly
suppressed by transfection with miR-197-3p mimic (Fig. 3D). However, no significant changes
were observed in cells transfected with the pmirGLO-TUSC8-Mut
vector. Moreover, RIP assay revealed that both TUSC8 and miR-197-3p
were more enriched in Ago2-containing miRNA ribonucleoprotein
complexes than in IgG immunoprecipitates (Fig. 3E). In addition, it was found that
miR-197-3p expression was evidently higher in the OS tissues than
in matched normal tissues (Fig.
3F). Furthermore, the transfection efficiency of miR-197-3p was
confirmed by RT-qPCR (Fig. S1A).
The inhibition of miR-197-3p mark-edly suppressed cell
proliferation, migration and invasion in OS (Fig. S1B-D). Overall, the obtained
findings suggest that TUSC8 can directly bind with miR-197-3p.
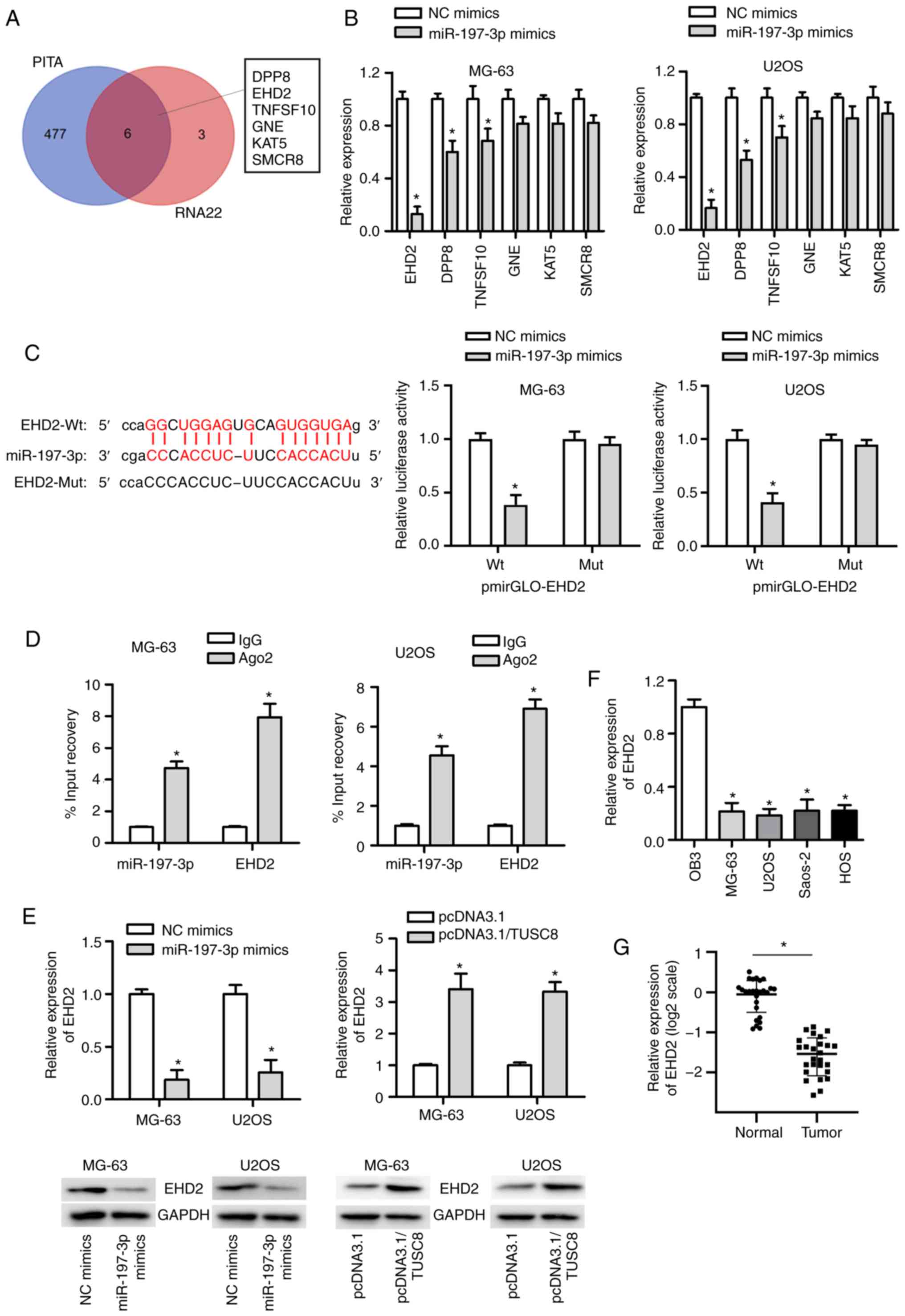
EHD2 is a downstream target of
miR-197-3p
It is well known that miRNAs exert their effects on
cancer development by targeting specific genes. To identify the
potential target genes of miR-197-3p, the starBase website was
used, and the most likely mRNAs (DPP8, EHD2, TNFSF10, GNE, KAT5 and
SMCR8) were identified (Fig. 4A).
Subsequently, the expression of these 6 mRNAs was analysed in OS
cells transfected with miR-197-3p mimic, and it was found that the
expression of EHD2 exhibited the most significant reduction
(Fig. 4B). As displayed in
Fig. 4C, miR-197-3p had a binding
site for EHD2, and the luciferase reporter assay suggested that the
luciferase activity of the pmirGLO-EHD2-Wt vector was markedly
decreased by co-transfection with miR-197-3p mimic, whereas no
significant change was observed in cells transfected with the
pmirGLO-EHD2-Mut vector. In addition, RIP assay further confirmed
the interactions between miR-197-3p and EHD2 (Fig. 4D). Furthermore, it was observed
that transfection with miR-197-3p mimics markedly diminished EHD2
mRNA expression, and the protein level of EHD2 was decreased in the
U2OS and MG63 cells. However, the opposite tendency was observed in
the pcDNA3.1/TUSC8-transfected U2OS and MG63 cells (Fig. 4E). In addition, the expression of
EHD2 in OS cell lines was much lower than that in the normal
osteoblastic cell line OB3 (Fig.
4F). Compared with the non-cancerous tissues, OS tissues
exhibited a lower expression of EHD2 (Fig. 4G). In summary, miR-197-3p targets
EHD2 in OS.
TUSC8 inhibits OS development by
regulating EHD2
To determine whether TUSC8 induces its suppressive
effects on OS by regulating EHD2, rescue assays were conducted.
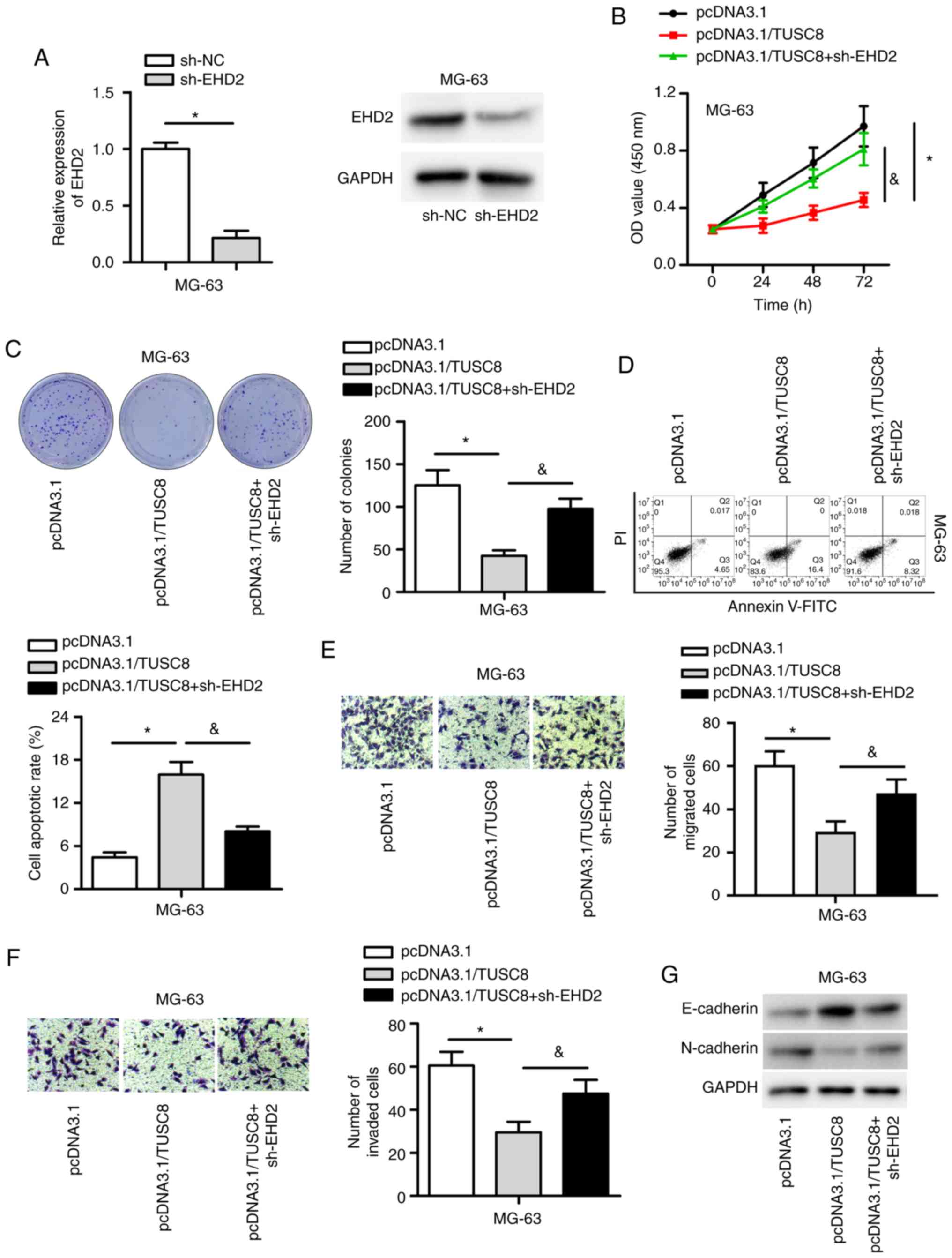
First, EHD2 was knocked down by transfection with sh-EHD2 into the
MG-63 cells, which resulted in a notable decrease in both the mRNA
and protein expression of EHD2 (Fig.
5A). As depicted in Fig. 5B and
C, EHD2 depletion markedly reversed the decreased proliferative
capability of MG-63 cells induced by TUSC8 overexpression. Flow
cytometric analysis also indicated that EHD2 knockdown
significantly reversed the elevated percentage of apoptotic cells
triggered by TUSC8 overexpression (Fig. 5D). In addition, the data
demonstrated that EHD2 silencing reversed the inhibitory effects of
TUSC8 overexpression on the migration and invasion of OS cells
(Fig. 5E and F). Moreover, the
pcDNA3.1/TUSC8-mediated decline in N-cadherin expression and the
increase in E-cadherin expression were partly reversed by EHD2
knock-down (Fig. 5G). Thus, TUSC8
inhibits OS development by regulating EHD2.
Discussion
Osteosarcoma (OS) is a common bone malignancy that
accounts for approximately 20% of all bone tumours and occurs
predominantly in the femur (28,29). The incidence of OS is increasing
rapidly at a rate of approximately 1.4% per year, and the prognosis
of patients with OS is poor due to the high risks of relapse and
distant metastasis (30-32). Hence, there is an urgent need for
the identification of novel therapeutic targets for OS
treatment.
Long non-coding RNAs (lncRNAs) are expressed in
specific differentiated tissues or cancers (33-35). TUSC8 is a novel lncRNA, and its
role in cancer progression is largely unknown. Increasing evidence
has revealed that the dysregulated expression of TUSC8 may be
considered as a potential biomarker in several types of cancer. A
recent study demon-strated that TUSC8 was downregulated in cervical
cancer and suppressed cell invasion and migration (14). The upregulation of TUSC8 has been
shown to significantly suppress tumour growth and the metastasis of
breast cancer (36). Likewise,
the results of the present study indicated that the expression of
TUSC8 was markedly decreased in OS tissues and cell lines.
Furthermore, it was discovered that the enhanced expression of
TUSC8 suppressed the proliferation, migration, invasion and EMT,
whereas it promoted the apoptosis of OS cells.
miRNAs are small non-coding RNA molecules of 20-24
nucleotides in length that play significant roles in the
progression of tumours, including OS (15). For example, miR-214 overexpression
suppresses cell migration and invasion in gastric cancer (37). miR-148a suppresses the metastasis
of non-small cell lung cancer via Wnt1 (38). miR-708 regulates cell
proliferation and apoptosis by targeting CUL4B in OS (39). It has been demonstrated that
lncRNAs regulate the development of multiple types of cancer by
sponging specific miRNAs. For example, HOXA11-AS contributes to the
tumorigenesis of glioma by sponging miR-140-5p (40). PVT1 functions as a sponge for
miR-152 in gastric cancer (41).
lncRNA RP4 suppresses the development of colorectal cancer by
acting as a sponge for miR-7-5p (8). It is worth noting that TUSC8 plays
an anti-oncogenic role in cervical cancer by sponging miR-641
(14). Moreover, a recent study
indicated that TUSC8 functions as a ceRNA of MYLIP by competitively
binding with miR-190b-5p to inhibit breast cancer growth (36). In the present study, TUSC8 was
found to be predominantly distributed in the cytoplasm, which
provided the possibility of ceRNA mechanism research. Subsequently,
a TUSC8 binding site was predicted in two candidate miRNAs
(miR-197-3p or miR-499b-5p) by the starBase website. Owing to the
differential expression of miR-197-3p in OS cell lines, the
underlying regulatory mechanisms were further analysed in OS. As
expected, the interaction between miR-197-3p and TUSC8 was
confirmed by luciferase reporter and RIP assays. According to
previous studies, miR-197-3p plays an oncogenic role in breast
cancer, bladder cancer and thyroid cancer (19,20,42,43). However, the anti-oncogenic role of
miR-197-3p has been found in prostate cancer and gastric cancer
(44,45). In the present study, the findings
were consisted with those of previous studies, which suggests the
promoting effects of miR-197-3p on the progression of OS.
EHD2 is a plasma membrane-associated member of the
EHD family and is related to the actin cytoskeleton (46). It has been reported that the
dysregulated expression of EHD2 is closely associated with the
metastasis of cancer. For example, EHD2 inhibits the metastasis of
hepatocellular carcinoma (47).
EHD2 knockdown promotes cell migration in oesophageal squamous cell
carcinoma (48). Extensive
studies have shown that lncRNAs containing miRNA binding sites can
function as ceRNAs to regulate mRNAs in cancers, including OS
(49-51). The ceRNA pattern mediated by TUSC8
also functions in other types of cancer. For example, TUSC8 sponges
miR-190b-5p and targets MTLIP to suppresses breast cancer growth
and metastasis (36). TUSC8
inhibits cell migration and invasion by regulating the miR-641/PTEN
axis in cervical cancer (14).
The present study, to the best of our knowledge, is the first to
certify that TUSC8 possesses the capacity to modulate EHD2
expression in OS. Through mechanistic analysis, EHD2 was identified
by starBase and further proved to bind with miR-197-3p.
Furthermore, the present study elucidated that the decreased EHD2
expression notably reversed the TUSC8 overexpression-mediated
effects on OS cellular processes.
Overall, the present study demonstrates that the
upregulation of TUSC8 is negatively associated with OS cell
proliferation, migration and invasion, whereas it is positively
associated with cell apoptosis. Mechanistically, TUSC8 serves as
competitive ceRNA to sponge miR-197-3p and target EHD2. These
findings indicate that TUSC8 may be a promising prognostic
biomarker and therapeutic target for OS therapy. However, further
experiments are required to investigate the mechanisms of TUSC8 in
OS in the future, including rescue assays of miR-197-3p on TUSC8
and in vivo experiments.
Supplementary Data
Funding
The present study was supported and funded by the
National Nature Science Foundation of China (grant no. 81601908)
and the Outstanding Youth Foundation from the Science and
Technology Department of Jilin Province (grant no.
20180520108JH).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
HF and SZ conceived and designed the experiments.
HF, TL and HT performed the experiments. HF and SZ analyzed the
data. HF, TL and SZ drafted the manuscript and reviewed the final
manuscript. All authors have read and approved the final manuscript
and agree to be accountable for all aspects of the work.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committee of The Second Hospital of Jilin University
(Changchun, China). Written informed consent was signed by each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Moore DD and Luu HH: Osteosarcoma. Cancer
Treat Res. 162:65–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biazzo A and De Paolis M:
Multidisciplinary approach to osteosarcoma. Acta Orthop Belg.
82:690–698. 2016.
|
|
3
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
Clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan XL, Cai GP, Zhu LL and Ding GM:
Efficacy and safety of ifosfamide-based chemotherapy for
osteosarcoma: A meta-analysis. Drug Des Devel Ther. 9:5925–5932.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang L, Froberg JE and Lee JT: Long
noncoding RNAs: Fresh perspectives into the RNA world. Trends
Biochem Sci. 39:35–43. 2014. View Article : Google Scholar :
|
|
7
|
Xiong XD, Ren X, Cai MY, Yang JW, Liu X
and Yang JM: Long non-coding RNAs: An emerging powerhouse in the
battle between life and death of tumor cells. Drug Resist Updat.
26:28–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu ML, Zhang Q, Yuan X, Jin L, Wang LL,
Fang TT and Wang WB: Long noncoding RNA RP4 functions as a
competing endogenous RNA through miR-7-5p sponge activity in
colorectal cancer. World J Gastroenterol. 24:1004–1012. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu H, Xu Y, Zhang D and Liu G: Long
noncoding RNA LUCAT1 promotes malignancy of ovarian cancer through
regulation of miR-612/HOXA13 pathway. Biochem Biophys Res Commun.
503:2095–2100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao JH, Sun JX, Song YX, Chen XW, Yang
YC, Ma B, Wang J, Gao P and Wang ZN: A novel long noncoding
RNA-LOWEG is low expressed in gastric cancer and acts as a tumor
suppressor by inhibiting cell invasion. J Cancer Res Clin Oncol.
142:601–609. 2016. View Article : Google Scholar
|
|
11
|
Li J, Wu QM, Wang XQ and Zhang CQ: Long
Noncoding RNA miR210HG Sponges miR-503 to facilitate osteosarcoma
cell invasion and metastasis. DNA Cell Biol. 36:1117–1125. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia B, Wang L, Feng L, Tian B, Tan Y and
Du B: Knockdown of long noncoding RNA CAT104 inhibits the
proliferation, migration, and invasion of human osteosarcoma cells
by regulating MicroRNA-381. Oncol Res. 27:89–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Yu Y, Fan S and Luo L: Knockdown
of long noncoding RNA TUG1 inhibits the proliferation and cellular
invasion of osteosarcoma cells by sponging miR-153. Oncol Res.
26:665–673. 2018. View Article : Google Scholar
|
|
14
|
Zhu Y, Liu B, Zhang P, Zhang J and Wang L:
LncRNA TUSC8 inhibits the invasion and migration of cervical cancer
cells via miR-641/PTEN axis. Cell Biol Int. 43:781–788. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer-a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar
|
|
16
|
Wang R, Sun Y, Yu W, Yan Y, Qiao M, Jiang
R, Guan W and Wang L: Downregulation of miRNA-214 in
cancer-associated fibroblasts contributes to migration and invasion
of gastric cancer cells through targeting FGF9 and inducing EMT. J
Exp Clin Cancer Res. 38:202019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang H, Ge F, Xu Y, Xiao J, Zhou Z, Liu R
and Chen C: miR-153 inhibits the migration and the tube formation
of endothelial cells by blocking the paracrine of angiopoietin 1 in
breast cancer cells. Angiogenesis. 21:849–860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iacona JR, Monteleone NJ, Lemenze AD,
Cornett AL and Lutz CS: Transcriptomic studies provide insights
into the tumor suppressive role of miR-146a-5p in non-small cell
lung cancer (NSCLC) cells. RNA Biol. 16:1721–1732. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu F, Li H and Hu C: LIFR-AS1 modulates
Sufu to inhibit cell proliferation and migration by miR-197-3p in
breast cancer. Biosci Rep. 39:BSR201805512019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Hong S and Liu Z: LncRNA LINC00641
predicts prognosis and inhibits bladder cancer progression through
miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem Biophys Res Commun.
503:1825–1829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan JJ and Tay Y: Noncoding RNA:RNA
regulatory networks in cancer. Int J Mol Sc. 19:13102018.
View Article : Google Scholar
|
|
22
|
Wang YG, Wang T, Shi M and Zhai B: Long
noncoding RNA EPB41L4A-AS2 inhibits hepatocellular carcinoma
development by sponging miR-301a-5p and targeting FOXL1. J Exp Clin
Cancer Res. 38:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie
H and Li G: Long noncoding RNA MAGI2-AS3 regulates CCDC19
expression by sponging miR-15b-5p and suppresses bladder cancer
progression. Biochem Biophys Res Commun. 507:231–235. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu C, Cheng D, Qiu X, Zhuang M and Liu Z:
Long Noncoding RNA SNHG16 promotes cell proliferation by sponging
MicroRNA-205 and upregulating ZEB1 expression in osteosarcoma. Cell
Physiol Biochem. 51:429–440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Liu H, Dai C, Wu Q, Liu H and Li F:
Expression profiling of long noncoding RNA identifies lnc-MMP31 as
a prognostic biomarker in external auditory canal squamous cell
carcinoma. Cancer Med. 6:2541–2551. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Wei T, Li W, Zhang R and Chen M:
Circular RNA hsa_circ_0002024 suppresses cell proliferation,
migration, and invasion in bladder cancer by sponging miR-197-3p.
Am J Transl Res. 11:1644–1652. 2019.PubMed/NCBI
|
|
28
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
29
|
Smith MA, Seibel NL, Altekruse SF, Ries
LA, Melbert DL, O'Leary M, Smith FO and Reaman GH: Outcomes for
children and adolescents with cancer: Challenges for the
twenty-first century. J Clin Onco. 28:2625–2634. 2010. View Article : Google Scholar
|
|
30
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: prognostic
factors and long-term outcome-the French pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiao X, Wang W and Wang Z: The role of
chemotherapy for metastatic, relapsed and refractory osteosarcoma.
Paediatr Drugs. 16:503–512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Zhang Y, Wan S, Li H, Li D, Xia J,
Yuan Z, Ren M, Yu S, Li S, et al: A comparative study between
limb-salvage and amputation for treating osteosarcoma. J Bone
Oncol. 5:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin PC, Huang HD, Chang CC, Chang YS, Yen
JC, Lee CC, Chang WH, Liu TC and Chang JG: Long noncoding RNA TUG1
is downregulated in non-small cell lung cancer and can regulate
CELF1 on binding to PRC2. BMC Cancer. 16:5832016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Zhang B, Jia Y, Shi H, Wang H, Guo Q
and Li H: LncRNA LOXL1-AS1 regulates the tumorigenesis and
development of lung adenocarcinoma through sponging miR-423-5p and
targeting MYBL2. Cancer Med. 9:689–699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jiang Y, Liu G, Ye W, Xie J, Shao C, Wang
X and Li X: ZEB2-AS1 Accelerates epithelial/mesenchymal transition
through miR-1205/CRKL pathway in colorectal cancer. Cancer Biother
Radiopharm. 35:153–162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao L, Zhou Y, Zhao Y, Li Q, Zhou J and
Mao Y: Long non-coding RNA TUSC8 inhibits breast cancer growth and
metastasis via miR-190b-5p/MYLIP axis. Aging (Albany NY).
12:2974–2991. 2020. View Article : Google Scholar
|
|
37
|
Wang Y, Wang X, Tang J, Su X and Miao Y:
The study of mechanism of miR-34c-5p targeting FLOT2 to regulate
proliferation, migration and invasion of osteosarcoma cells. Artif
Cells Nanomed Biotechno. 47:3559–3568. 2019. View Article : Google Scholar
|
|
38
|
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X,
Wang T, Wang F, Sun C and Zhang X: miRNA-148a serves as a
prognostic factor and suppresses migration and invasion through
Wnt1 in non-small cell lung cancer. PLoS One. 12:e01717512017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen G and Zhou H: MiRNA-708/CUL4B axis
contributes into cell proliferation and apoptosis of osteosarcoma.
Eur Rev Med Pharmacol Sci. 22:5452–5459. 2018.PubMed/NCBI
|
|
40
|
Cui Y, Yi L, Zhao JZ and Jiang YG: Long
noncoding RNA HOXA11-AS Functions as miRNA sponge to promote the
glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol.
36:822–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li T, Meng XL and Yang WQ: Long noncoding
RNA PVT1 acts as a 'sponge' to inhibit microRNA-152 in gastric
cancer cells. Dig Dis Sci. 62:3021–3028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye F, Gao G, Zou Y, Zheng S, Zhang L, Ou
X, Xie X and Tang H: circFBXW7 inhibits malignant progression by
sponging miR-197-3p and Encoding a 185-aa protein in
triple-negative breast cancer. Mol Ther Nucleic Acids. 18:88–98.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu K, Huang W, Yan DQ, Luo Q and Min X:
Overexpression of long intergenic noncoding RNA LINC00312 inhibits
the invasion and migration of thyroid cancer cells by
down-regulating microRNA-197-3p. Biosci Rep. 37:BSR201701092017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang Q, Ma B, Su Y, Chan K, Qu H, Huang
J, Wang D, Qiu J, Liu H, Yang X and Wang Z: miR-197-3 prepresses
the proliferation of prostate cancer by regulating the
VDAC1/AKT/β-catenin signaling axis. Int J Biol Sci. 16:1417–1426.
2020. View Article : Google Scholar :
|
|
45
|
Chen Z, Ju H, Zhao T, Yu S, Li P, Jia J,
Li N, Jing X, Tan B and Li Y: hsa_circ_0092306 Targeting miR-197-3p
promotes gastric cancer development by regulating PRKCB in MKN-45
cells. Mol Ther Nucleic Acids. 18:617–626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guilherme A, Soriano NA, Bose S, Holik J,
Bose A, Pomerleau DP, Furcinitti P, Leszyk J, Corvera S and Czech
MP: EHD2 and the novel EH domain binding protein EHBP1 couple
endocytosis to the actin cytoskeleton. J Biol Chem.
279:10593–10605. 2004. View Article : Google Scholar
|
|
47
|
Liu J, Ni W, Qu L, Cui X, Lin Z, Liu Q,
Zhou H and Ni R: Decreased expression of EHD2 promotes tumor
metastasis and indicates poor prognosis in hepatocellular
carcinoma. Dig Dis Sci. 61:2554–2567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li M, Yang X, Zhang J, Shi H, Hang Q,
Huang X, Liu G, Zhu J, He S and Wang H: Effects of EHD2
interference on migration of esophageal squamous cell carcinoma.
Med Oncol. 30:3962013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu C, Liu S, Wang L, Wang Y, Li Y and Cui
Y: Effect of EH domain containing protein 2 on the biological
behavior of clear cell renal cell carcinoma. Hum Exp Toxicol.
38:927–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Qu F and Cao P: Long noncoding RNA SOX2OT
contributes to gastric cancer progression by sponging miR-194-5p
from AKT2. Exp Cell Res. 369:187–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Y, Gu M, Liu C, Wan X, Shi Q, Chen Q
and Wang Z: Long noncoding RNA FOXC2-AS1 facilitates the
proliferation and progression of prostate cancer via targeting
miR-1253/EZH2. Gene. 686:37–42. 2019. View Article : Google Scholar
|