Introduction
Psoriasis is a recalcitrant disease characterized by
immune-mediated skin inflammation, manifesting clinically as scaly,
itchy, well-defined red patches on the skin (1). Psoriasis is known to affect ~2% of
the population worldwide (at least 200 million patients) and
considered to be the most common autoimmune skin disease in adults
(2). Several risk factors, such
as age, bacterial infection, family history of psoriasis, smoking,
alcohol consumption and physical inactivity, are known to trigger
psoriasis (3-8). Psoriasis may cause severe disruption
to the daily lives of the patients (9) and it is considered to be a serious
global health concern.
Abnormal keratinocyte proliferation and inflammatory
infiltration of the skin lesions are the major characteristics of
psoriasis (10). Several studies
have indicated that the imbalance between pro-inflammatory
mediators, such as tumour necrosis factor (TNF)-α, interferon
(IFN)-γ, interleukin (IL)-2 and IL-6, and anti-inflammatory
cytokines, such as transforming growth factor (TGF)-β and IL-4,
plays an important role in the underlying disease aetiology
(11). Furthermore, extensive
experimental and clinical evidence indicates that the accumulation
of immune cells, particularly a large number of activated T cells,
plays a key role at the initial stages of psoriasis (12,13). The initial T cells differentiate
into Th17 cells (CD4+ T cells), which in turn produce
several cytokines, forming a large network headed by Th1- and
Th17-type cytokines (14). The
formation pathway of Th17 cells is also known as the IL-23/IL-17
inflammatory reaction axis (10,15), the activation of which can quickly
involve the neutrophils and lead to the production of inflammatory
mediators.
Signal transducer and activator of transcription
(STAT) is a family of primary effectors that can generate numerous
pro-inflammatory cytokines that are involved in the development and
progression of psoriasis (16).
The STAT member STAT4 transports signals from IL-12, IL-23 and
IFN-α/β to the nucleus, resulting in the activation of dendritic
cells, differentiation of Th17 cells and production of IFN-γ
(17). Moreover, the vitamin D
receptor (VDR) is a ligand-activated transcription factor that
belongs to the steroid/retinoid/thyroid hormone receptor
superfamily (18,19). Vitamin D3 [1,25(OH)2D3], a
metabolite of vitamin D (VD), is a ligand that binds to VDR and
exerts biological activity (19).
VDR is expressed in activated T lymphocytes and is involved in
anti-proliferative and pro-differentiation pathways in
monocytes/macrophages (20). The
lack of VDR causes activation of the STAT3 signalling pathway and
inhibition of the VDR to regulate high-level glucose-induced
retinal ganglion cell damage through induction of the STAT3 pathway
(21,22). Therefore, the VDR/STAT4 signalling
pathway may be of value as a target for psoriasis treatment.
Although patients with psoriasis are prescribed
various chemical formulations for treatment, the prevalence of this
disease continues to increase. Although systemic biological drugs
that successfully target specific inflammatory cytokines have been
developed, they are not recommendable for long-term treatment due
to their high cost (23). As
compared with Western medicines, Chinese medicines and their active
extracts are more cost-effective and low-toxicity, and may they
therefore be more suitable and effective for psoriasis treatment
(24,25). Triptolide is one of the effective
components of Tripterygium wilfordii Hook. F., a traditional
Chinese herb that exerts anticancer and immunosuppressive effects
by suppressing the function of T cells, as well as the secretion of
IL-1, IL-6, IL-8, TNF-α and prostaglandin E2 by human peripheral
blood monocytes. 'Psoriasis 1' is composed of 13 Chinese herbs, and
it has been successfully used for treating patients with psoriasis
in China over the last few decades. Following treatment with
'Psoriasis 1,' several patients observe an improvement in skin
lesions, and our earlier studies have also demonstrated that
'Psoriasis 1' plays a protective role in patients with psoriasis by
inhibiting T-lymphocyte-mediated inflammation (26,27). However, the molecular mechanism of
action of 'Psoriasis 1' has yet to be fully elucidated.
Consequently, in the present study, T lymphocytes were collected
from the peripheral blood of patients with psoriasis and the
effects of 'Psoriasis 1' and underlying molecular mechanisms were
investigated.
Materials and methods
Reagents and materials
Ficoll-Hypaque solution was supplied by Becton,
Dickinson and Company. CD4 beads were purchased from Miltenyi
Biotec GmbH. PE anti-human CD3 (cat. no. 300307-1), APC anti-human
CD4 (cat. no. 317415-1), and FITC anti-human CD8 (cat. no.
344703-1) were obtained from BioLegend, Inc. RPMI-1640 medium and
FBS were obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Anti-human TNF-α (cat. no. STA00D), IFN-γ (cat. no. SIF50), IL-2
(cat. no. S2050), IL-6 (cat. no. S6050), TGF-β (cat. no. SB100B),
IL-4 (cat. no. S4050), IL-12 (cat. no. 10018-IL), IL-23 (cat. no.
S2300B) and VD (cat. no. DVDBP0B) ELISA kits were supplied by
R&D Systems, Inc. The bicinchoninic acid (BCA) protein assay
kit was purchased from Beyotime Institute of Biotechnology. RNAiso
Plus reagent, PrimeScript® RT reagent and
SYBR® PremixEx Taq™ II (TliRNaseH Plus) were purchased
from TaKaRa Biotechnology Co., Ltd. Protein extraction kit was
supplied by KeyGen Biotech. Co., Ltd. Rabbit anti-VDR (cat. no.
ab3508), anti-STAT4 (cat. no. ab235946), and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (cat. no. ab6721) were
supplied by Abcam. NC-siRNAs (cat. no. SIC001) and VDR-siRNAs (cat.
no. EHU010441) were purchased from RiboBio Co., Ltd. VD3 was
purchased from Sigma-Aldrich; Merck KGaA.
Components of the 'Psoriasis 1'
formulation
'Psoriasis 1' was supplied by the First Affiliated
Hospital of Guangzhou University (Guangzhou, China). Its components
include Rhizoma smilacis glabrae (30 g), Folium
isatidis (30 g), Radix isatidis (15 g), Angelica
sinensis (15 g), Hedyotis diffusa (15 g), Szechuan
lovage rhizome (12 g), plantain herb (12 g), Fructus kochiae
(12 g), Lobelia chinensis (15 g), Nidus vespae
(12 g), Rhizoma alismatis (12 g), Cortex dictamni (12
g) and Radix glycyrrhizae (6 g) (28).
Subjects and blood sampling schedule
A total of 40 patients with psoriasis (15 men and 25
women) and 40 healthy indi-viduals (18 men and 22 women) were
enrolled in the present study. The mean age of the patients was
43.2±11.6 years, and the mean disease duration was 5.8±1.3 years.
The mean age of the healthy individuals was 41.6±12.7 years. Serum
samples from the patients and healthy subjects were collected
between March 2018 and October 2019. The baseline characteristics
of the patients with psoriasis are summarized in Table I.
 | Table IBaseline characteristics of patients
with psoriasis vulgaris. |
Table I
Baseline characteristics of patients
with psoriasis vulgaris.
| No. | Sex | Age (years) | PASI | Familiarity for
psoriasis | Comorbidities |
|---|
| 1 | F | 59 | 8.5 | No | None |
| 2 | M | 42 | 13.5 | No |
Hypercholesterolaemia |
| 3 | F | 24 | 6.5 | No | None |
| 4 | M | 60 | 20.5 | No | None |
| 5 | F | 48 | 9 | No | None |
| 6 | M | 40 | 7.2 | No | None |
| 7 | F | 35 | 12.9 | Aunts | None |
| 8 | F | 42 | 4.2 | No | None |
| 9 | F | 32 | 14 | No | None |
| 10 | F | 49 | 10.5 | No | Enteritis |
| 11 | M | 22 | 18.4 | No | None |
| 12 | M | 23 | 15 | No | None |
| 13 | F | 25 | 7 | No | None |
| 14 | M | 58 | 5.5 | No | Arterial
hypertension |
| 15 | F | 30 | 19 | Grandmother | None |
| 16 | F | 19 | 8 | No | None |
| 17 | F | 28 | 8.2 | No | None |
| 18 | F | 21 | 12.5 | No | Arterial
hypertension |
| 19 | M | 52 | 11 | No | None |
| 20 | M | 30 | 10.5 | No | None |
| 21 | F | 26 | 10.3 | No | None |
| 22 | F | 30 | 12.7 | No | None |
| 23 | F | 35 | 18 | No | None |
| 24 | M | 37 | 8 | No | None |
| 25 | M | 45 | 9 | No | Nephritis |
| 26 | F | 28 | 15 | No | Anaemia |
| 27 | F | 49 | 18.2 | No | None |
| 28 | F | 41 | 12.4 | No | None |
| 29 | M | 52 | 18 | No | None |
| 30 | F | 53 | 20.6 | No | Coronary heart
disease |
| 31 | F | 42 | 16.3 | No | None |
| 32 | F | 33 | 13.4 | Both parents | None |
| 33 | M | 25 | 12.8 | No | None |
| 34 | M | 24 | 11 | No | None |
| 35 | M | 29 | 10.9 | No | None |
| 36 | M | 55 | 11.7 | No | Arterial
hypertension |
| 37 | F | 43 | 15.8 | No | None |
| 38 | F | 57 | 15 | No | None |
| 39 | F | 29 | 10.3 | Aunts | None |
| 40 | F | 37 | 10.8 | No | None |
The inclusion criteria were as follows: i)
Diagnostic criteria of psoriasis vulgaris and patients with typical
lesions; ii) men and women aged between 18 and 60 years; iii)
patients without serious heart, liver, kidney, and other chronic
systemic or autoimmune diseases; iv) patients who had not used
immunosuppressants, corticosteroids, vitamin A, or any other
anti-psoriasis drugs within the last 4 weeks; v) patients who
signed the informed consent form.
The exclusion criteria included the following: i)
Patients with erythrodermic psoriasis, psoriasis affecting the
joints, and pustular psoriasis; ii) patients with skin diseases
other than psoriasis vulgaris; and iii) pregnant and lactating
women.
All investigational procedures were approved by the
Institutional Review Board of the First Affiliated Hospital of
Guangzhou University [ZYYECK(2017)020 and ZYYECK(2019)030], and
written informed consent was obtained from all the
participants.
Preparation of peripheral blood
mononuclear cells (PBMCs)
Peripheral blood samples from each patient were
obtained by venipuncture and collected in heparin tubes BD
Vacutainer® CPT™ (BD Biosciences). Subsequently, PBMCs
were isolated from the whole blood by Ficoll-Hypaque density
gradient centrifugation as per the manufacturer's instructions. The
PBMCs were carefully collected from the thin interface layer
between the plasma and red blood cells, and then rinsed to remove
the platelets. Finally, they were suspended in 1ml of PBS solution
and the number of cells was counted. The viable and dead cells were
distinguished by staining with 0.1% trypan blue at room temperature
for 5 min.
T lymphocyte isolation, culture and
identification
Under sterile conditions, T lymphocytes were
segregated using MACS prep HLA T Cell Isolation kit (Milteny Biotec
GmbH, 130-110-128) following the manufacturer's instructions. A
total of 1×107 cells were centrifuged at 300 × g for 10
min at 4°C and suspended in 80 µl buffer. CD3 microbeads
were added to the cells and incubated at 4°C for 15 min. The
suspension was centrifuged (800 × g for 5 min at 4°C), cells were
added to an MS column, and collected for CD3+ T-cell
identification. The purity of T lymphocytes was identified using
PE-conjugated CD3 antibody (Thermo Fisher Scientific, Inc., cat.
no. 17-0032-82) for 30 min by flow cytometry (FCM). CD3+
T cells with a purity of >95% were cultured in RPMI-1640 medium
with 10% FBS at 37°C and 5% CO2.
Cell treatment
To investigate the effects of 'Psoriasis 1' on T
lymphocytes of patients with psoriasis, the T lymphocytes were
divided into the following five groups: Control; low dose of
'Psoriasis 1' (1 mg/ml); medium dose of 'Psoriasis 1' (2 mg/ml);
high dose of 'Psoriasis 1' (4 mg/ml), and positive control (10
µM triptolide; Cayman Chemical Company) groups. Various
concentrations of 'Psoriasis 1' were prepared by dissolving in
sterile saline and used to pretreat T lymphocytes for 24 h
(29-32); the control group was pretreated
with sterile saline for 24 h.
The role of the VDR/STAT4 signaling pathway in the
anti-psoriasis action of 'Psoriasis 1' was investigated. T
lymphocytes were therefore divided into six groups: Normal control
siRNA (NC-siRNA); VDR-siRNA; NC-siRNA plus 'Psoriasis 1' (4 mg/ml);
VDR-siRNA plus 'Psoriasis 1' (4 mg/ml); NC-siRNA plus triptolide
(10 µM); and VDR-siRNA plus triptolide (10 µM)
groups.
Furthermore, T cells were treated with VD3 (1 nM),
high dose of 'Psoriasis 1' (4 mg/ml), or VD3 (1 nM) plus 'Psoriasis
1' (4 mg/ml) for 48 h.
siRNA transfection
T lymphocytes from patients with psoriasis were
collected, and T lymphocytes (1.8×106 cells/well) were
seeded onto 6-well plates. After 24 h, 10 µM NC-siRNA
(Sigma-Aldrich; Merck KGaA, cat. no. SIC001) and 10 µM
VDR-siRNA (Sigma-Aldrich; Merck KGaA, cat. no. EHU010441) were
dissolved separately in Opti-MEM and then mixed gently with
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
for 20 min for siRNA liposome formation. The mixture was added to
the cells. After 48 h of incubation at 37°C, subsequent
experimentation was conducted.
FCM analyses
The changes in CD4+ and CD8+ T
cells were investigated using FCM assay to detect the effects of
'Psoriasis 1' on T lymphocyte subsets. The cells (1×105)
were incubated with fluorochrome-conjugated antibodies directed at
the CD markers: PE anti-human CD3 and APC anti-human CD4 (or FITC
anti-human CD8). Gated CD3-positive events were investigated for
CD4+ and CD8+ T cells. FCM analyses were
conducted using FACSCalibur (Becton, Dickinson and Company).
ELISA
The supernatant from T lymphocytes was collected,
and the TNF-α, IFN-γ, IL-2, IL-6, TGF-β, IL-4, IL-12, IL-23 and VD
levels were evaluated using ELISA kits as per the manufacturer's
instructions (R&D Systems, Inc.). Undiluted supernatant medium
was incubated for 2 h at room temperature in a 96-well plate. Then,
diluted biotin-labelled antibody mixture (100 µl) was added
into each well and incubated for 1 h. The diluted
streptavidin-HRP-conjugated secondary anti-bodies were added and
incubated for 60 min. Finally, 100 µl of substrate solution
was added into each well. A microplate reader (Thermo Fisher
Scientific, Inc.) was used to read the plate.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
To extract the total RNA from T lymphocytes
(1×106), RNAiso Plus reagent was used as per the
manufacturer's protocol (TaKaRa Biotechnology Co., Ltd.). To
synthesize cDNA, the PrimeScript® RT reagent kit was
used as per the manufacturer's instructions (TaKaRa Biotechnology
Co., Ltd.) in a TC-512 PCR system (Techne Ltd.).
TransScript® Top Green qPCR SuperMix in an ABI 7500
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) was used to perform qPCR, using GAPDH for normalization. The
thermocycling conditions were as follows: 94°C for 34 sec followed
by 40 cycles of 94°C for 5 sec, 60°C for 15 sec and 72°C for 10
sec. The pre-primers and post-primers of VDR and STAT4 are shown in
Table II. GAPDH was used as an
internal control, and the expression of mRNA was calculated using a
7900 Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and quantified using the 2−ΔΔCq method
(24).
 | Table IIPrimer sequences used for
quantitative PCR assay. |
Table II
Primer sequences used for
quantitative PCR assay.
| ID | Sequence
(5′-3′) | Product length
(bp) |
|---|
| GAPDH | Forward:
TGTTCGTCATGGGTGTGAAC | 154 |
| Reverse:
ATGGCATGGACTGTGGTCAT | |
| STAT4 | Forward:
TGTTGGCCCAATGGATTGAAA | 119 |
| Reverse:
GGAAACACGACCTAACTGTTCAT | |
| IL-17A | Forward:
TCACAATCCCACGAAATCCAG | 144 |
| Reverse:
GTGAGGTGGATCGGTTGTAG | |
| TNF-α | Forward:
CCTTCATCCACTCTCCCAC | 76 |
| Reverse:
CACATCTTTCACCCATCCCA | |
| IL-22 | Forward:
CTGTGAGCTCTTTCCTTATGGG | 149 |
| Reverse:
GGTGCGGTTGGTGATATAGG | |
| VDR | Forward:
GTGGACATCGGCATGATGAAG | 181 |
| Reverse:
GGTCGTAGGTCTTATGGTGGG | |
Western blot assay
Ice-cold RIPA buffer (Sigma-Aldrich; Merck KGaA,
cat. no. R0278) supplemented with 1 mM PMSF was used to extract
total protein (20 µg) from T lymphocytes. A BCA protein
assay kit was used for measuring the protein concentration. Samples
were subjected to 10% SDS-PAGE and transferred onto PVDF membranes.
The membranes were blocked with 10% (w/v) non-fat dried milk and
incubated over-night at 4°C using primary antibodies against VDR
(1:1,000) and STAT4 (1:1,000). After washing with PBS for 3 times,
the membranes were incubated with a secondary antibody (1:2,000,
cat. no. ab6721; Abcam) at room temperature for 1 h. The
expressions of VDR and STAT4 were visualized using ECL Plus
chemiluminescence reagent kit (Beyotime Institute of
Biotechnology). Finally, a ChemiDoc XRS bioimaging system (Bio-Rad
Laboratories, Inc.) was used for imaging the protein bands, and the
protein expressions were normalized using GAPDH.
Cell counting kit-8 (CCK-8) assay
The treated cells (1×104) were seeded
onto a 96-well plate. Then, 10 µl of CCK-8 was added to each
well according to the manufacturer's protocol. The absorbance was
measured at 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.) following incubation for 1 h.
High-performance liquid chromatography
(HPLC)
A C18 reverse-phase column (5 µm, Waters
Corporation) was used for the investigation. The mobile phase was
composed of acetonitrile and 0.1% trifluoroacetic solution, and
HPLC was conducted at room temperature at a flow rate of 1.0
ml/min. The detection was performed at 280 nm. The HPLC was
performed using RSLCnano system (Thermo Fisher Scientific,
Inc.).
Statistical analysis
SPSS 18.0 statistical software (SPSS, Inc.) was used
to perform statistical analysis. All values are presented as mean ±
standard deviation. An unpaired Student's t-test was used for
comparisons between two groups. One-way analysis of variance
followed by Tukey's post hoc test were used to evaluate the
differences between multiple independent groups, followed by the
least significance difference test. P<0.05 or P<0.01 was
considered to indicate statistically significant differences.
Results
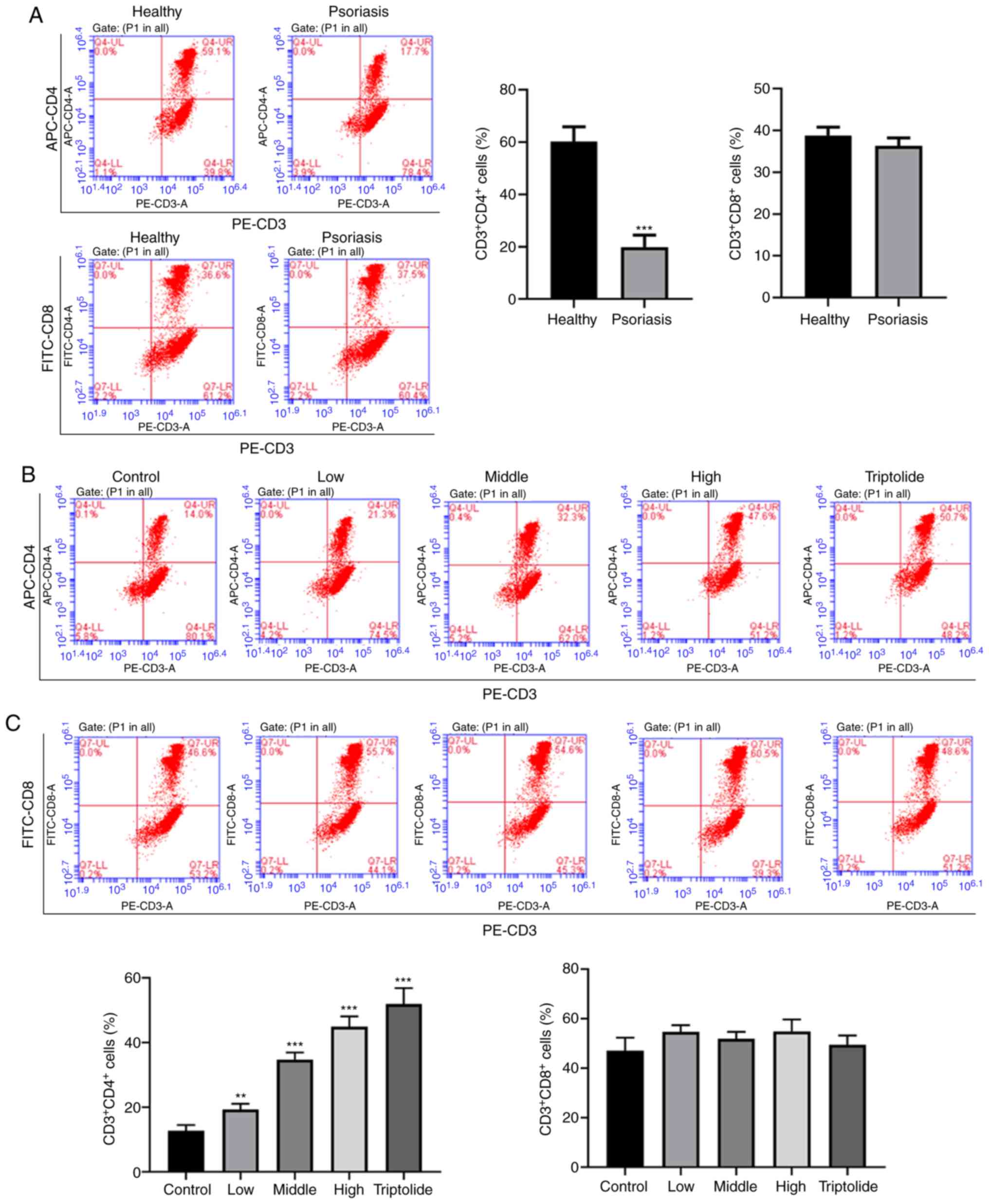
'Psoriasis 1' increases CD4+ T
lymphocyte subsets
First, FCM analysis was used to detect the
CD4+ T and CD8+ T cells in healthy
individuals and patients with psoriasis. The numbers of
CD4+ T cells were found to be significantly lower in the
peripheral blood of psoriatic patients compared with those in
healthy controls, whereas the numbers of CD8+ T cells
exhibited no obvious changes (Fig.
1A). Next, the psoriatic patients were treated with different
doses of 'Psoriasis 1' to investigate its effects on the
distribution of T lymphocyte subsets. As shown in Fig. 1B and C, 'Psoriasis 1' treatment
significantly increased the percentage of CD4+ T cells
compared with the control group, but no notable changes were
observed in CD8+ T cells. The numbers of Th1, Th2 and
regulatory T cells (Tregs) were also assessed, and the percentages
of Th2 and Tregs were also recorded corresponding to those of
CD4+ T cells (Fig.
S1). Moreover, a high dose of 'Psoriasis 1' exerted similar
effects as those of triptolide on the distribution of T lymphocyte
subsets (Fig. 1B and C). CCK-8
assay was also performed to examine the viability of cells on
treatment with 'Psoriasis 1' or triptolide, and it was observed
that a high concentration of 'Psoriasis 1' or triptolide reduced
cell proliferation (Fig. S2A).
These results indicate that 'Psoriasis 1' affected the distribution
of T lymphocyte subsets in patients with psoriasis.
'Psoriasis 1' inhibits the inflammatory
response of T lymphocytes
ELISA was used to examine the inflammation mediated
by T lymphocytes. A comparison between the control and 'Psoriasis
1' or triptolide groups is shown in Fig. 2A-F. The levels of inflammatory
factors, such as TNF-α, IFN-γ, IL-2 and IL-6, were found to be
significantly decreased following 'Psoriasis 1' treatment, and
those of anti-inflammatory media-tors, such as TGF-β and IL-4, were
recorded to be notably higher in the 'Psoriasis 1' and triptolide
groups. Moreover, both 'Psoriasis 1' and triptolide were found to
significantly reduce the expression of IL-23 and IL-17, indicating
that 'Psoriasis 1' and triptolide inhibit the activation of the
IL-23/IL-17 axis (Fig. 2G and H).
Moreover, 'Psoriasis 1' and triptolide reduced the expression of VD
(Fig. 2I).
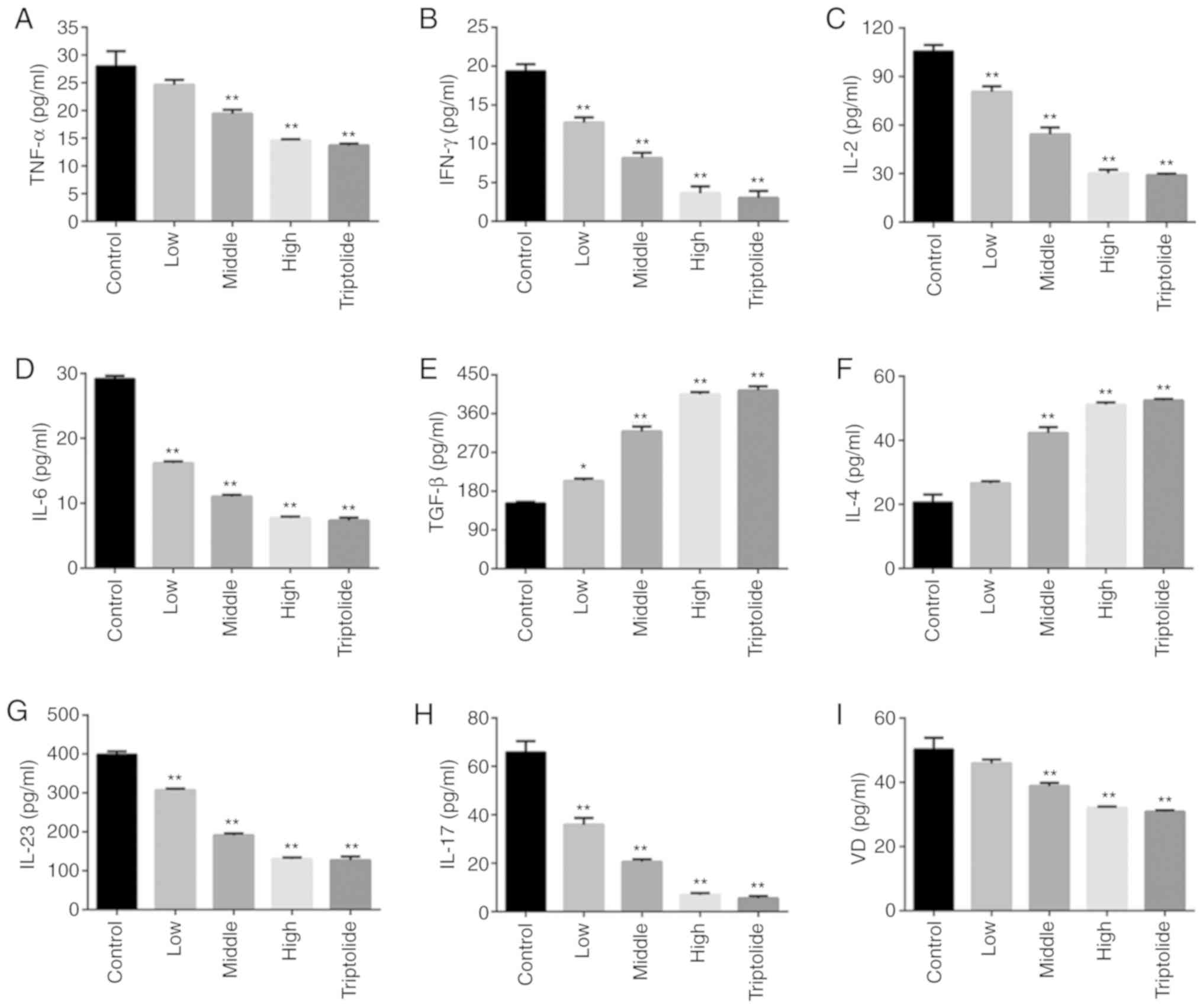 | Figure 2'Psoriasis 1' inhibits inflammatory
response in T lymphocytes. Effects of 'Psoriasis 1' at different
doses and triptolide on the levels of (A) TNF-α, (B) IFN-γ, (C)
IL-2, (D) IL-6, (E) TGF-β, (F) IL-4, (G) IL-23, (H) IL-17 and (I)
VD in T lymphocytes. Data are presented as mean ± standard
deviation. *P<0.05 and **P<0.01 vs.
control group. n=4. TNF, tumour necrosis factor; IFN, interferon;
IL, interleukin; TGF, transforming growth factor; VD, vitamin
D. |
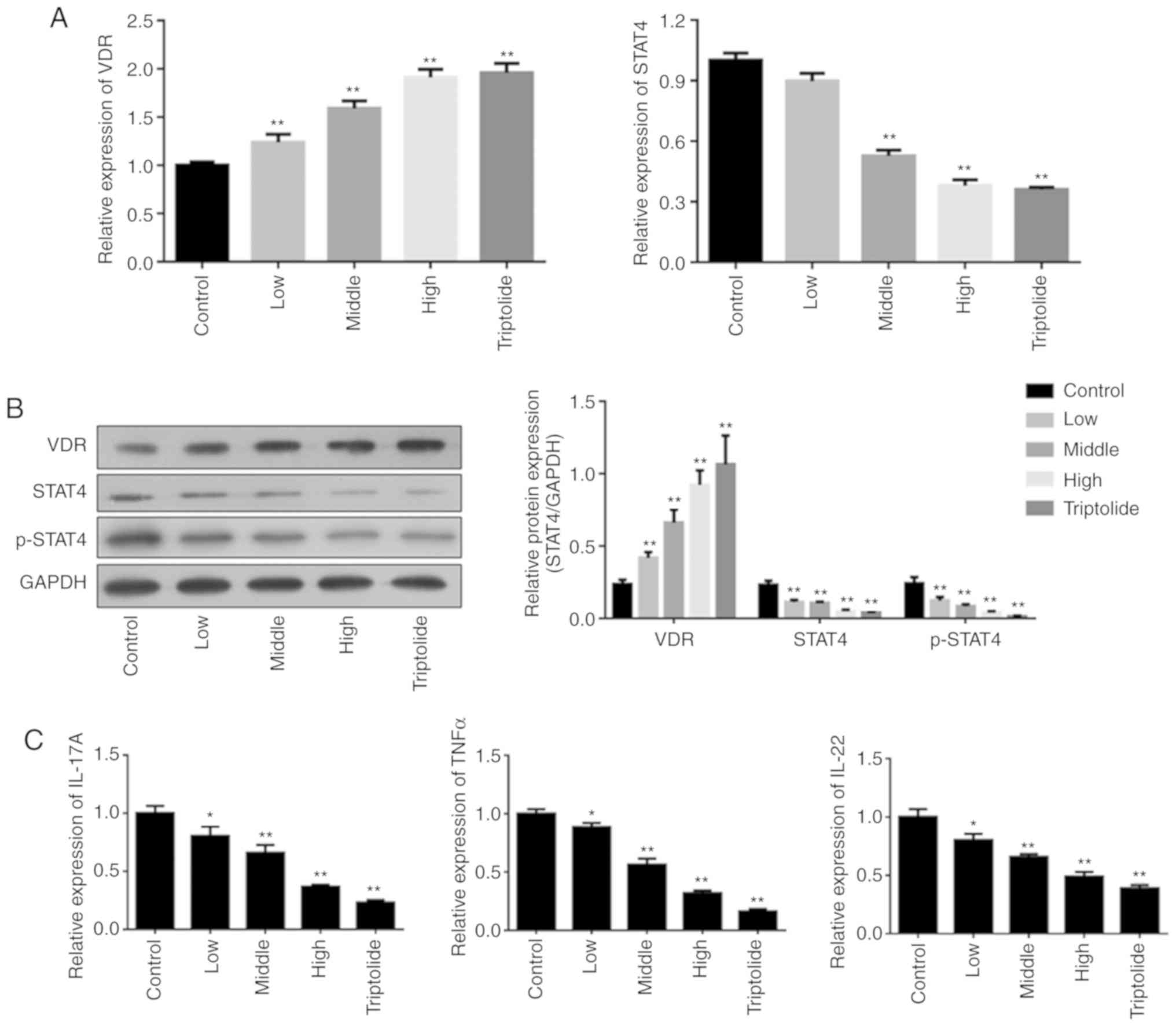
'Psoriasis 1' suppresses VDR-mediated
STAT4 signalling
As VD functions are primarily activated by its
nuclear receptor, VDR (33), the
effects of 'Psoriasis 1' on the mRNA and protein levels of VDR and
STAT4 were investigated. The findings indicated that 'Psoriasis 1'
and triptolide notably upregulated the VDR mRNA and protein
expression (Fig. 3A and B).
Moreover, 'Psoriasis 1' and triptolide markedly downregulated the
STAT4 mRNA and protein expression (Fig. 3A and B). Furthermore, a high dose
of 'Psoriasis 1' exerted a similar therapeutic effect as that of
triptolide. These findings suggest that 'Psoriasis 1' and
triptolide suppress VDR-mediated STAT4 signalling. The STAT3 level
was also measured, and no notable difference among groups was
observed (data not shown). Furthermore, ELISA results indicated
that the IL-17A, TNF-α and IL-22 levels decreased with 'Psoriasis
1' treatment in a dose-dependent manner (Fig. 3C).
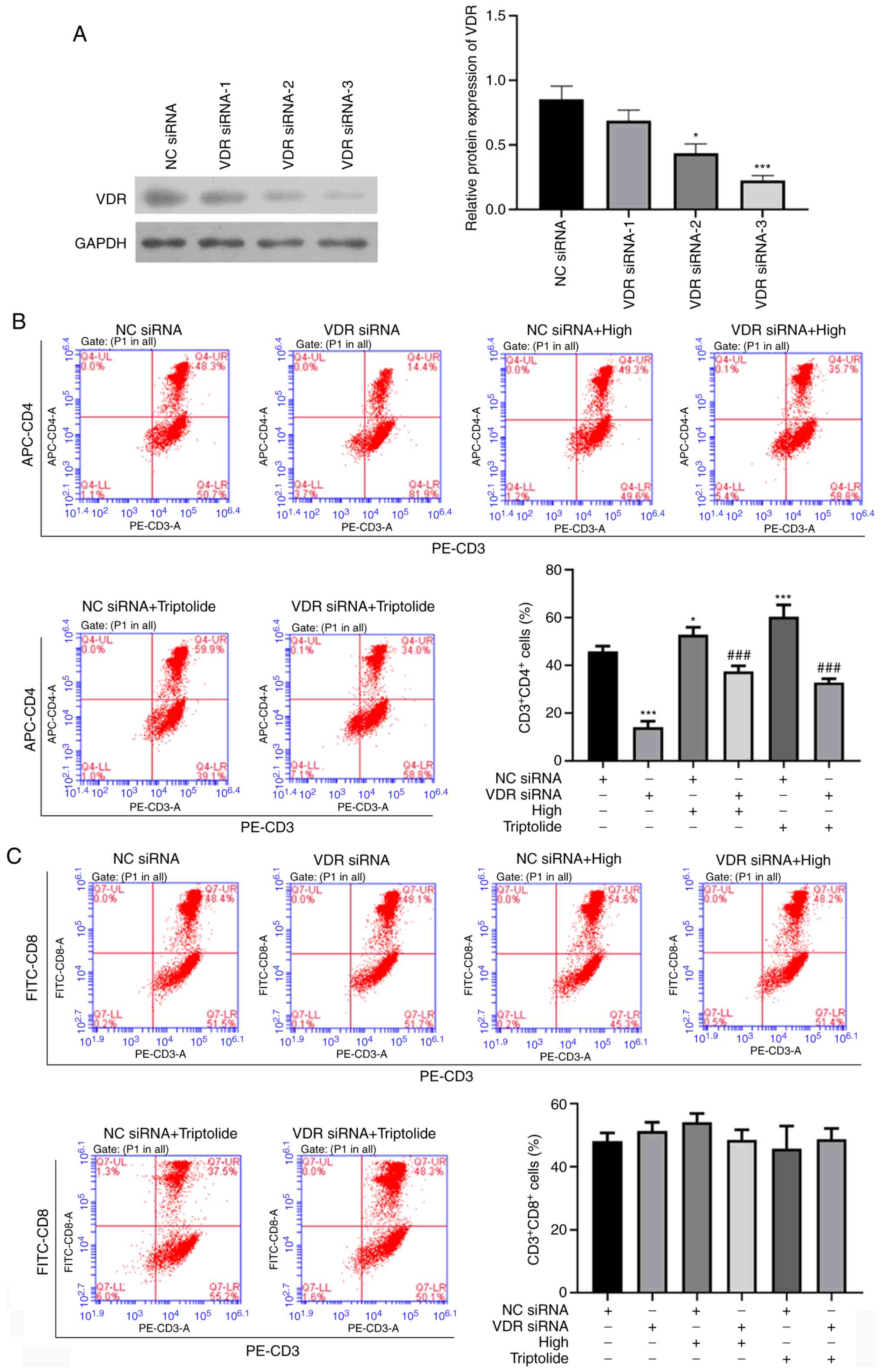
VDR mediates 'Psoriasis 1'-induced
regulation of CD4+ T lymphocyte subsets in patients with
psoriasis
To investigate the role of VDR on the effects of
'Psoriasis 1' against psoriasis, VDR-siRNA was used to reduce VDR
expression. Western blot assay was also employed to confirm the
transfection efficacy of VDR siRNAs, and we found that VDR
expression was significantly reduced in the VDR siRNA-2 and VDR
siRNA-3 groups compared to that in the NC-siRNA group, particularly
the VDR siRNA-3 group (Fig. 4A).
Therefore, VDR siRNA-3 was selected as it had the strongest
knock-down effect. Next, compared with NC-siRNA, the percentage of
CD4+ T cells was found to be significantly reduced by
VDR-siRNA. Both 'Psoriasis 1' and triptolide were observed to
notably upregulate the percentage of CD4+ T cells in the
VDR-siRNA group compared with the NC-siRNA group (Fig. 4B). However, the VDR suppression by
siRNA markedly reversed the effects of 'Psoriasis 1' or triptolide
on CD4+ T cells. Moreover, the percentage of
CD8+ T cells did not differ significantly among the
groups (Fig. 4C). Cell viability
was investigated after 'Psoriasis 1' or VDR siRNA treatment, and
the inhibition of VDR was found to reverse the decrease in cell
proliferation induced by high 'Psoriasis 1' concentration (Fig. S2B). Therefore, these outcomes
indicate that the VDR signalling pathway is involved in the
regulatory effect of 'Psoriasis 1' on T lymphocyte subsets.
VDR regulates 'Psoriasis 1'-induced
T-lymphocyte mediated inflammation
To assess the effects of VD and VDR on T lymphocyte
inflammation, VD3 alone or in combination with 'Psoriasis 1' was
added and cytokine expression was measured. The effects of VD3
alone were similar to those of 'Psoriasis 1' and are shown in
Fig. S3; a combination of the
two exerted no synergistic effect, indicating that 'Psoriasis 1'
affected VDR expression, resulting in modified cytokine expression.
Moreover, the inflammation mediated by T lymphocytes was examined
following inhibition of VDR; compared with NC-siRNA, VDR-siRNA was
found to significantly increase the levels of inflammatory factors,
including TNF-α, IFN-γ, IL-2 and IL-6, which were notably reduced
by a high dose of 'Psoriasis 1' or triptolide, as shown in Fig. 5A-D. VDR-siRNA markedly reduced the
levels of anti-inflammatory mediators, including TGF-β and IL-4,
which were augmented by a high dose of 'Psoriasis 1' or triptolide
(Fig. 5E and F). In addition, the
effects of 'Psoriasis 1' on the IL-23/IL-17 axis and VD level after
VDR-siRNA transfection were examined using ELISA, and both
'Psoriasis 1' and triptolide were found to reverse the promoting
effects of VDR siRNA on IL-23, IL-17 and VD levels (Fig. 5G-I). These findings indicated that
'Psoriasis 1' may inhibit the inflammatory response by way of the
VDR signalling pathway. Furthermore, to identify the ingredient of
'Psoriasis 1' that exerted the most prominent anti-inflammatory
effect, HPLC was performed and revealed that four compo-nents of
'Psoriasis 1,' namely caffeic acid, liquiritin, quercetin and
flavone, may be the active components that possess the most
prominent therapeutic properties (Fig. S4).
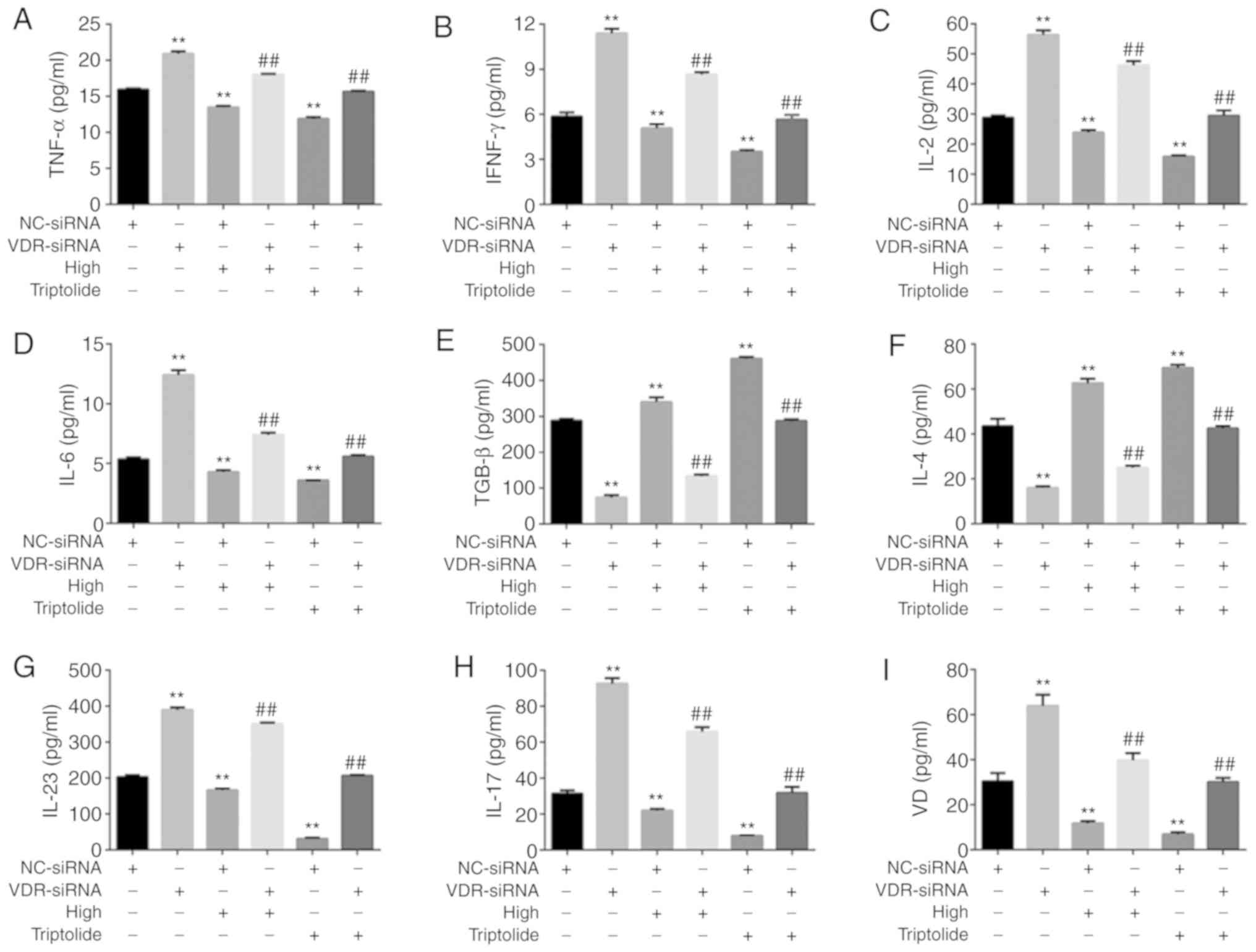 | Figure 5'Psoriasis 1' inhibited inflammatory
response following VDR-siRNA transfection. T lymphocytes were
isolated from patients with psoriasis and transfected with NC or
VDR siRNA. Effects of high-dose 'Psoriasis 1' and triptolide on the
levels of (A) TNF-α, (B) IFN-γ, (C) IL-2, (D) IL-6, (E) TGF-β, (F)
IL-4, (G) IL-23, (H) IL-17 and (I) VD in T lymphocytes after
VDR-siRNA transfection. Data are presented as mean ± standard
deviation. **P<0.01 vs. NC-siRNA group;
##P<0.01 vs. VDR-siRNA group. n=5. VD, vitamin D;
VDR, VD receptor; IL interleukin; TGF, transforming growth factor;
TNF, tumour necrosis factor. |
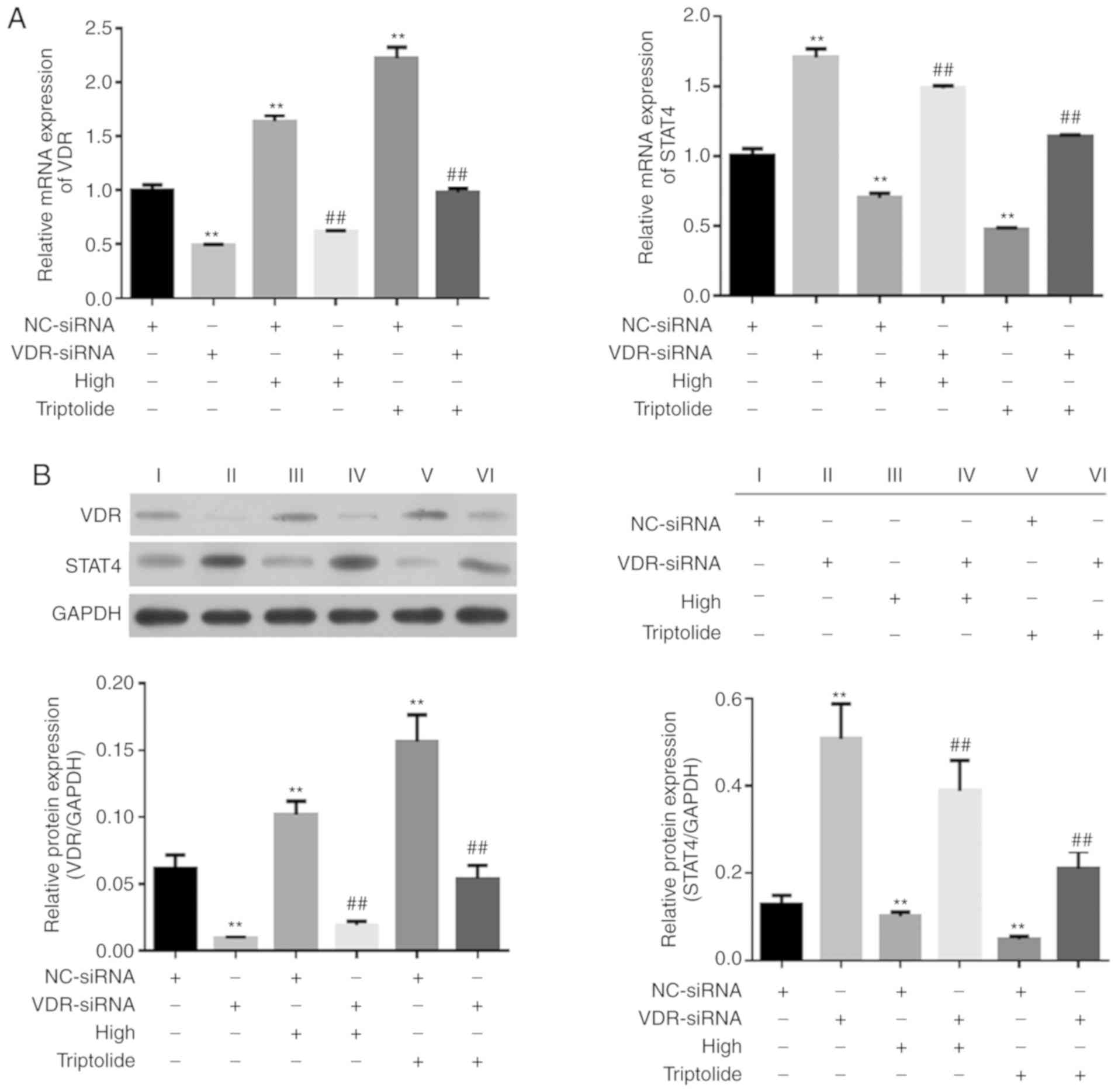
'Psoriasis 1' inhibits the VDR/STAT4
signalling pathway following VDR knockdown
As shown in Fig.
6A, compared with the NC-siRNA group, VDR-siRNA was observed to
significantly downregulate the mRNA and protein levels of VDR;
However, high doses of 'Psoriasis 1' and triptolide significantly
upregulated the mRNA and protein expressions of VDR. Furthermore,
compared with the NC-siRNA group, VDR-siRNA markedly upregulated
the mRNA and protein levels of STAT4, which were reversed by high
dose of 'Psoriasis 1' or triptolide (Fig. 6B).
Discussion
Psoriasis, an immune-mediated disease, is
characterized by T-lymphocyte-driven epidermal hyperplasia, which
is easily recurrent and refractory to treatment, severely affecting
the physical and mental health of the patients (10,34). Earlier epidemiological studies
have reported an increased risk of certain diseases among patients
with psoriasis, such as stroke, thromboembolism, coronary heart
disease and certain types of cancer. However, a significant number
of patients do not respond satisfactorily to the currently
available clinical treatments (24). 'Psoriasis 1' was recently
demonstrated to be successful in treating patients with psoriasis
in China (28). Therefore, the
effects of 'Psoriasis 1' on T lymphocytes in patients with
psoriasis and the possible underlying molecular mechanism were
investigated in the present study.
A negative correlation between the percentage of
CD4+ T cells and the severity of psoriasis has been
recorded in earlier studies (35,36). The percentage of CD4+ T
cells was observed to be significantly lower in psoriatic patients
compared with that in healthy controls and 'Psoriasis 1' was
reported to markedly increase the percentage of CD4+ T
cells, indicating that 'Psoriasis 1' may alleviate psoriasis. HPLC
demonstrated that four components of 'Psoriasis 1' may be the
active components possessing the most prominent therapeutic
properties.
The typical characteristics of psoriasis are
abnormal keratinocyte proliferation and inflammatory cell
infiltration (10,15). The effects of 'Psoriasis 1' on the
inflammatory response in T lymphocytes were examined, and
'Psoriasis 1' was observed to significantly decrease the levels of
pro-inflammatory mediators, such as TNF-α, IFN-γ, IL-2 and IL-6,
and increase the levels of anti-inflammatory cytokines, such as
TGF-β and IL-4. Moreover, IL-23 promotes the proliferation of Th17
cells and regulation of immune response (37), whereas IL-17, a pro-inflammatory
cytokine released by activated T cells, triggers the inflammation
cascade (38). The IL-23/IL-17
axis participates in autoimmune disorders, which indicates a new
direction for psoriasis treatment (12). In the present study, 'Psoriasis 1'
was shown to inhibit the activation of the IL-23/IL-17 axis by
downregulating the expression of IL-17A and IL-23. 'Psoriasis 1'
was also found to reduce the VD level. These findings indicate that
'Psoriasis 1' inhibits inflammatory response in T lymphocytes.
VDR has been reported to inhibit psoriasis-like skin
inflammation by suppressing the STAT signalling pathways (39). 'Psoriasis 1' has been reported to
reduce psoriasis-like skin inflammation by inhibiting VDR-mediated
nuclear NF-κB and STAT signalling pathways, including the
downregulation of STAT4 and pSTAT4 (40). Similar to previous reports, the
findings of the present study also revealed that the expression of
STAT4 and pSTAT4 decreased in a dose-dependent manner with
'Psoriasis 1' treatment. The effects of the STAT4/VDR signalling
pathway on the actions of 'Psoriasis 1' against
T-lymphocyte-mediated inflammation were further evaluated by
VDR-siRNA experiments. The inhibition of the VDR protein and
activation of the STAT4 protein achieved by VDR-siRNA markedly
reduced the percentage of CD4+ T cells and further
intensified the inflammatory response in T lymphocytes. In
addition, VDR silencing inhibited 'Psoriasis 1' that increased the
percentage of CD4+ T cells, induced T-lymphocyte
mediated inflammation and suppressed the activation of STAT4.
Therefore, these outcomes demon-strated that 'Psoriasis 1'
inhibited inflammatory responses in T lymphocytes via the VDR/STAT4
signalling pathway. The mRNA expression of VDR was highly dependent
on cell differentiation state, while VDR expression was found to be
regulated by Erk and PI3K signalling in a myeloid leukaemia cell
line wherein p38 activity appeared irrelevant (41,42), suggesting that, in addition to
various intracellular signalling pathways cooperating to regulate
the expression of VDR, the implicated signalling events differ
among various cell types and varying cell differentiation states.
Therefore, further investigation of the mechanism through which VDR
regulates alternative signalling pathways is required. Moreover, it
was recently discovered that the silencing of STAT4 led to a
reduction in the serum levels of IFN-γ and IL-2, with an elevation
in the serum levels of IL-6 and IL-10 (43). IFN-γ production was lowered in
STAT4 KO-derived splenocytes, but no significant differences in the
levels of IL-12 and TNF-α were found when compared to those in WT
mice (44). The effect of STAT4
reduction on VDR expression should be further elucidated in future
studies.
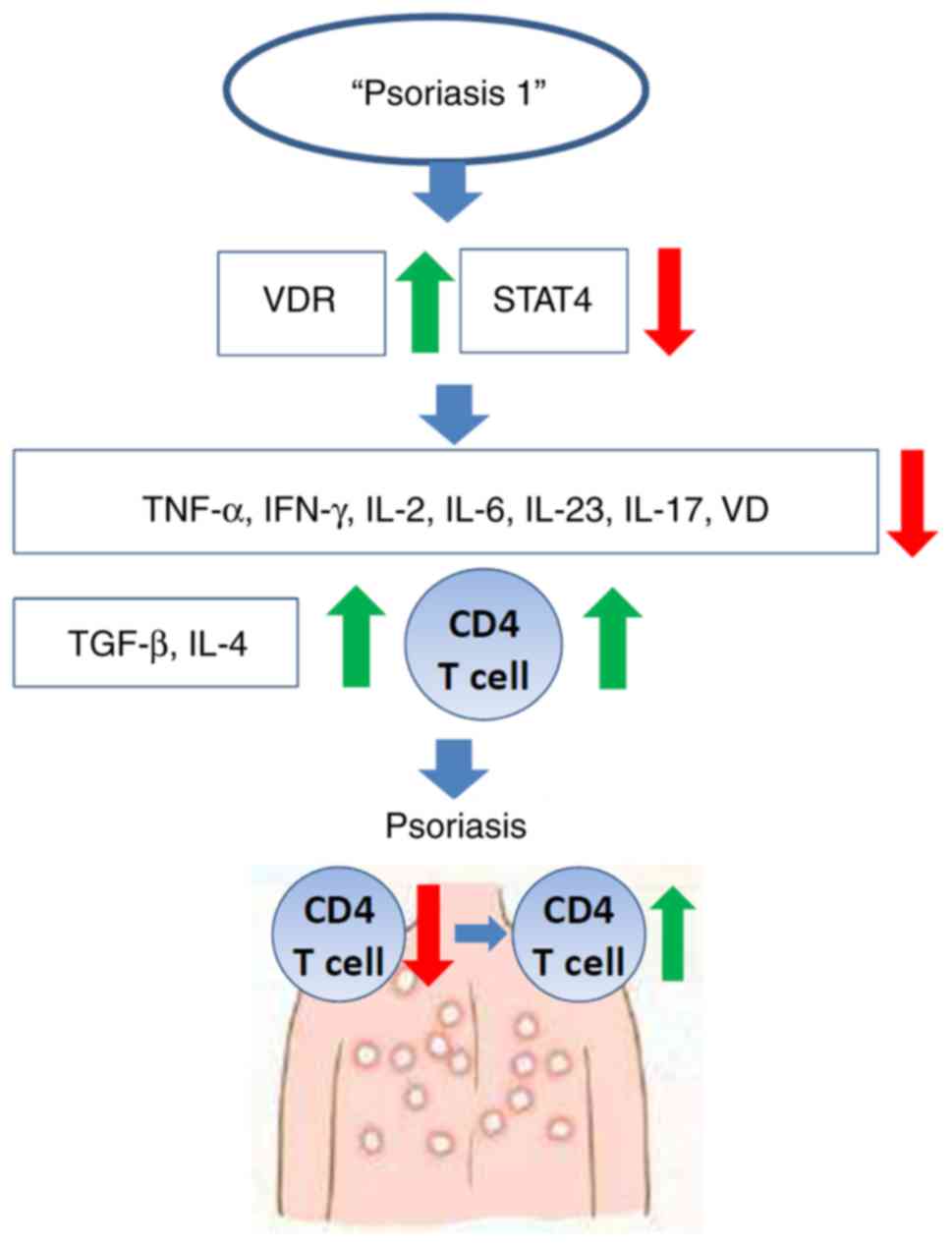
In conclusion, 'Psoriasis 1' was found to decrease
the inflammatory response in T lymphocytes and increase the
percentage of CD4+ T cells in patients with psoriasis
through inhibiting VDR-mediated STAT4 signalling (Fig. 7). The findings of the present
study highlight the clinical relevance of VDR and may enable
researchers to further investigate its therapeutic potential.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant. nos. 81573980
and 81673804), the Guangdong Science and Technology Department
Project (grant no. 2017A020215058), the Nature Science Foundation
of Hubei Province (grant no. 2018CFB289) and the Science Foundation
of Health Commission of Hubei Province (grant. no. WJ2019M074).
Availability of data and materials
All the datasets generated and analysed in the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YG and WS designed the study, performed the
experiments and conducted the statistical analysis. YG wrote the
manuscript. XC and HW revised the manuscript and procured the
funding. All the authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All investigational procedures were approved by the
Institutional Review Board of the First Affiliated Hospital of
Guangzhou University [ZYYECK(2017)020 and ZYYECK(2019)030]. Written
informed consent was obtained from all the participants.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Hung CH, Wang CN, Cheng HH, Liao JW, Chen
YT, Chao YW, Jiang JL and Lee CC: Baicalin ameliorates
imiquimod-induced psoriasis-like inflammation in mice. Planta Med.
84:1110–1117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parisi R, Symmons DP, Griffiths CE and
Ashcroft DM; Identification and Management of Psoriasis and
AssociatedComorbidiTy(IMPACT) project team: Global epidemiology of
psoriasis: A systematic review of incidence and prevalence. J
Invest Dermatol. 133:377–385. 2013. View Article : Google Scholar
|
|
3
|
Queiro R, Tejón P, Alonso S and Coto P:
Age at disease onset: A key factor for understanding psoriatic
disease. Rheumatology (Oxford). 53:1178–1185. 2014. View Article : Google Scholar
|
|
4
|
Jankovic S, Raznatovic M, Marinkovic J,
Jankovic J and Maksimovic N: Risk factors for psoriasis: A
case-control study. J Dermatol. 36:328–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huerta C, Rivero E and Rodriguez LA:
Incidence and risk factors for psoriasis in the general population.
Arch Dermatol. 143:1559–1565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armstrong AW, Harskamp CT, Dhillon JS and
Armstrong EJ: Psoriasis and smoking: A systematic review and
meta-analysis. Br J Dermatol. 170:304–314. 2014. View Article : Google Scholar
|
|
7
|
Brenaut E, Horreau C, Pouplard C,
Barnetche T, Paul C, Richard MA, Joly P, Le Maitre M, Aractingi S,
Aubin F, et al: Alcohol consumption and psoriasis: A systematic
literature review. J Eur Acad Dermatol Venereol. 27(Suppl 3):
S30–S35. 2013. View Article : Google Scholar
|
|
8
|
Frankel HC, Han J, Li T and Qureshi AA:
The association between physical activity and the risk of incident
psoriasis. Arch Dermatol. 148:918–924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Griffiths CEM, van der Walt JM, Ashcroft
DM, Flohr C, Naldi L, Nijsten T and Augustin M: The global state of
psoriasis disease epidemiology: A workshop report. Br J Dermatol.
177:e4–e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiricozzi A, Romanelli P, Volpe E,
Borsellino G and Romanelli M: Scanning the immunopathogenesis of
psoriasis. Int J Mol Sci. 19:1792018. View Article : Google Scholar :
|
|
12
|
Lovato P, Norsgaard H, Tokura Y and Røpke
MA: Calcipotriol and betamethasone dipropionate exert additive
inhibitory effects on the cytokine expression of inflammatory
dendritic cell-Th17 cell axis in psoriasis. J Dermatol Sci.
81:153–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jadali Z and Eslami MB: T cell immune
responses in psoriasis. Iran J Allergy Asthma Immunol. 13:220–230.
2014.PubMed/NCBI
|
|
14
|
Boonstra A, Barrat FJ, Crain C, Heath VL,
Savelkoul HF and O'Garra A: 1alpha,25-dihydroxyvitamin d3 has a
direct effect on naive CD4(+) T cells to enhance the development of
Th2 cells. J Immunol. 167:4974–4980. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Owczarczyk-Saczonek A, Czerwińska J and
Placek W: The role of regulatory T cells and anti-inflammatory
cytokines in psoriasis. Acta Dermatovenerol Alp Pannonica Adriat.
27:17–23. 2018.PubMed/NCBI
|
|
16
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang Y, Pan HF and Ye DQ: Therapeutic
potential of STAT4 in autoimmunity. Expert Opin Ther Targets.
18:945–960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mangelsdorf DJ, Thummel C, Beato M,
Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M,
Chambon P and Evans RM: The nuclear receptor superfamily: The
second decade. Cell. 83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HH, Mun MJ, Kim TH, Kim YS, Jang WC
and Hwang JY: Relationships between vitamin D receptor genetic
polymorphisms and endometriosis in Korean women. Clin Exp Obstet
Gynecol. 46:876–880. 2019.
|
|
20
|
O'Kelly J, Hisatake J, Hisatake Y, Bishop
J, Norman A and Koeffler HP: Normal myelopoiesis but abnormal T
lymphocyte responses in vitamin D receptor knockout mice. J Clin
Invest. 109:1091–1099. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gopinath SD: Inhibition of Stat3 signaling
ameliorates atrophy of the soleus muscles in mice lacking the
vitamin D receptor. Skelet Muscle. 7:22017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei XJ, Xu YL, Yang YQ, Bing YW and Zhao
YX: Vitamin D receptor regulates high-level glucose induced retinal
ganglion cell damage through STAT3 pathway. Eur Rev Med Pharmacol
Sci. 22:7509–7516. 2018.PubMed/NCBI
|
|
23
|
Rizvi S, Chaudhari K and Syed BA: The
psoriasis drugs market. Nat Rev Drug Discov. 14:745–746. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng S, Lin Z, Wang Y, Wang Z, Li P and
Zheng Y: Psoriasis therapy by Chinese medicine and modern agents.
Chin Med. 13:162018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu M, Deng Y, Li S, Chen Y, Guo D, Jin X,
Xu Q, Li B and Li F: The immunoregulatory effects of traditional
Chinese medicine on psoriasis via its action on interleukin:
Advances and considerations. Am J Chin Med. 46:739–750. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue M, Jiang ZZ, Liu JP, Zhang LY, Wang T,
Wang H, Liu L and Zhou ZX: Comparative study on the
anti-inflammatory and immune suppressive effect of Wilforlide A.
Fitoterapia. 81:1109–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menter A, Gottlieb A, Feldman SR, Van
Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA,
Korman NJ, et al: Guidelines of care for the management of
psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis
and guidelines of care for the treatment of psoriasis with
biologics. J Am Acad Dermatol. 58:826–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shan C, Yuan L, Xiuzhen B and Aiju Q:
Treatment of psoriasis vulgaris by oral administration of yin xie
ping granules - a clinical report of 60 cases. J Tradit Chin Med.
26:198–201. 2006.PubMed/NCBI
|
|
30
|
Chen M, Chen Z, Liu J, Tu S and Gao Y: The
effect of Chinese medicine 'Psoriasis 1' on TNF-α secretion of
psoriatic neutrophils in vitro. Lishizhen Med Mater Media Res.
29:565–567. 2018.In Chinese.
|
|
31
|
Sun W, Chen YJ, Deng WY, Huang SH, Li Y,
Liu J and Cha XS: Effects of Yinxieyihao on nuclear factor kappa B
for imiquimod induced psoriasis mice model. Chin J Derm Venereol.
94:79–81. 2017.
|
|
32
|
Gao Y, Sun W, Gao Y, Li Y, Liu J and Zha
X: Effects of Yinxieyihao on down-regulated inflammatory factors
and NF-κB in psoriasis model mices. Chin J Derm Venereol.
31:1131–1134. 2017.In Chinese.
|
|
33
|
Bouillon R, Carmeliet G, Verlinden L, van
Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C and Demay M:
Vitamin D and human health: Lessons from vitamin D receptor null
mice. Endocr Rev. 29:726–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hawkes JE, Chan TC and Krueger JG:
Psoriasis pathogenesis and the development of novel targeted immune
therapies. J Allergy Clin Immunol. 140:645–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim J, Lee J, Gonzalez J, Fuentes-Duculan
J, Garcet S and Krueger JG: Proportion of
CD4+CD49b+LAG-3+ type 1 regulatory
T cells in the blood of psoriasis patients inversely correlates
with psoriasis area and severity index. J Invest Dermatol.
138:2669–2672. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Diani M, Galasso M, Cozzi C, Sgambelluri
F, Altomare A, Cigni C, Frigerio E, Drago L, Volinia S, Granucci F,
et al: Blood to skin recirculation of CD4+ memory T
cells associates with cutaneous and systemic manifestations of
psoriatic disease. Clin Immunol. 180:84–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gooderham MJ, Papp KA and Lynde CW:
Shifting the focus-the primary role of IL-23 in psoriasis and other
inflammatory disorders. J Eur Acad Dermatol Venereol. 32:1111–1119.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ganti KP, Mukherji A, Surjit M, Li M and
Chambon P: Similarities and differences in the transcriptional
control of expression of the mouse TSLP gene in skin epidermis and
intes-tinal epithelium. Proc Natl Acad Sci USA. 114:E951–E960.
2017. View Article : Google Scholar
|
|
40
|
Sun W, Gao Y, Yu X, Yuan Y, Yi J, Zhang Z,
Cheng Y, Li Y, Peng X and Cha X: 'Psoriasis 1' reduces
psoriasis-like skin inflammation by inhibiting the VDR-mediated
nuclear NF-κB and STAT signaling pathways. Mol Med Rep.
18:2733–2743. 2018.PubMed/NCBI
|
|
41
|
Reinhardt TA and Horst RL: Phorbol
12-myristate 13-acetate and 1,25-dihydroxyvitamin D3 regulate
1,25-dihydroxyvitamin D3 receptors synergistically in rat
osteosarcoma cells. Mol Cell Endocrinol. 101:159–165. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gocek E, Kielbinski M and Marcinkowska E:
Activation of intracellular signaling pathways is necessary for an
increase in VDR expression and its nuclear translocation. FEBS
Lett. 581:1751–1757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue YL, Zhang SX, Zheng CF, Li YF, Zhang
LH, Hao YF, Wang S and Li XW: Silencing of STAT4 protects against
auto-immune myocarditis by regulating Th1/Th2 immune response via
inactivation of the NF-κB pathway in rats. Inflammation.
42:1179–1189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Varikuti S, Oghumu S, Natarajan G, Kimble
J, Sperling RH, Moretti E, Kaplan MH and Satoskar AR: STAT4 is
required for the generation of Th1 and Th2, but not Th17 immune
responses during monophosphoryl lipid A adjuvant activity. Int
Immunol. 28:565–570. 2016. View Article : Google Scholar : PubMed/NCBI
|