Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
disease that affects several organs, including the skin, joints,
central nervous system and kidneys. The occurrence of SLE is
related to hormonal, environmental and genetic factors; however,
the pathogenesis of SLE requires further study (1,2).
High titers of autoantibodies against double-stranded DNA (dsDNA)
and ribonucleoproteins (RNP) are often detectable in patients with
SLE several years before clinical manifestations arise. These
autoantibodies can bind to autoantigens and complement factors to
form circulating immune complexes (CICs), which are deposited in
target organs to induce inflammation and cause various diseases
(3).
As the main force of the immune response, B cells
play an important role in the humoral immune response. However,
studies have demonstrated that B lymphocytes acting as effector
cells can increase the occurrence and chronic persistence of SLE
(4,5). Signal transduction abnormity,
tolerance deficiency and immunoregulatory mechanism abnormity in B
cells, induced by environmental and genetic factors, play important
roles in the occurrence and development of SLE (6,7).
FcγRIIB, as an immunoregulatory receptor, is
expressed mainly on the surface of B cells. It contains the
extracellular region with the Ig-like domain, transmembrane region
and intracellular region with the immunoreceptor tyrosine
inhibitory motif (ITIM), which has an inhibitory function. The
extracellular domain is a low affinity binding IgG-Fc region.
Antigens and cytokines can stimulate the hydrolysis of the
extracellular domain of membrane-bound type FcγRIIB (mFcγRIIB) or
the selective splicing of the FcγRIIB gene to produce soluble
FcγRIIB (sFcγRIIB) (8). FcγRIIB
can regulate healthy B cells, induces immune tolerance by
inhibiting B cell expression of antibodies, and is a key inhibitory
receptor mediating B cells activation (9,10).
The inhibitory effect of FcγRIIB depends on the ITIM that is
phosphorylated upon FcγRIIB coaggregation with ITAM-bearing
receptors and recruits SH2 domain-containing phosphatases (11). The decrease in FcγRIIB expression
exerts adverse effects on the immune response, leading to the
occurrence of autoimmune diseases. The level of FcγRIIB on the
surfaces of immature B cells and memory B cells in patients with
autoimmune diseases has been found to be lower than that in healthy
subjects (12,13), causing B lymphocytes to produce a
large number of antibodies against autoantigens in a hyperimmune
response. However, it has been reported that increasing the
expression of FcγRIIB in B lymphocytes reduces the production of
antibodies and immune complexes in lupus mice, and improves the
symptoms of lupus (14). In
transgenic mice with FcγRIIB overexpression on the surface of B
lymphocytes, the production of T cell-dependent antibody IgG has
been shown to be significantly reduced, and the symptoms of SLE are
decreased (15). Therefore,
increasing the FcγRIIB levels on the surface of B cells may be a
promising approach for treating autoimmune diseases. Recombinant
human sFcγRIIB can be used as a potential target for the treatment
of autoimmune diseases mediated by antibodies and immune complexes.
However, to the best of our knowledge, there are no available
reports to date on the negative regulation of antibody secretion in
patients with SLE by recombinant human sFcγRIIB. More importantly,
the mechanisms of FcγRIIB in SLE remain unclear. In the present
study, the effects of mFcγRIIB and sFcγRIIB proteins on the IgG
antibody secretion of B cells form patients with SLE, and the
preventative and therapeutic effects of sFcγRIIB in mice with SLE
were examined. In addition, the underlying mechanisms were
investigated by measuring the phosphorylation levels of Bruton's
tyrosine kinase (BTK), DOK1, docking protein 1 (DOK1), Lyn
proto-oncogene (Lyn) and inositol polyphosphate-5-phosphatase D
(SHIP) in the downstream signaling pathways of B cell receptor
(BCR) and FcγRIIB in B cells.
Materials and methods
Construction of mFcγRIIB lentivirus
Total RNA from was isolated human B cells (isolated
from patients with SLE as described below) using TRIzol RNA
extraction reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, cDNA was synthesized using 1 µg of total RNA
and the reverse transcription system [Tiangen Biochemical
Technology (Beijing) Co., Ltd.]. The FcγRIIB gene was cloned by
nest PCR using Taq PCR MasterMix [Tiangen Biochemical Technology
(Beijing) Co., Ltd.] and outer primers (forward primer, 5′-ATC CGC
CAA GCT TTG AGA GAA GGC TGT GAC T-3′ and reverse primer, 5′-AGG GAG
CTT CAG GAC TCA GGT AGA TGA CT-3′) at 58°C annealing temperature
for the first PCR and inter primers (forward primer, 5′-GCC CCC GGG
ACG CGT ATG GGA ATC CTG TCA TTC TTA CC-3′ and reverse primer,
5′-CTA CCC GGT AGA ATT CCT AAA TAC GGT TCT GGT CAT CAG-3′) at 56°C
annealing temperature for the second PCR. The FcγRIIB gene was
inserted into the lentivirus-induced expression vector
pLVX-TRE3G-ZsGreen1 (Takara Bio, Inc.) with green fluorescent
protein (GFP) gene to construct the mFcγRIIB lentivirus.
Lentivirus packaging
The constructed human mFcγRIIB lentivirus
(106 TU/ml), mouse mFcγRIIB lentivirus (106
TU/ml) from our laboratory, and virus tetracycline (Tet)
(105 TU/ml) regulatory plasmid were transfected into
lenti-X 293T cells (Takara Bio, Inc.) to complete lentivirus
packaging using a Lenti-X Lentiviral packaging system (Takara Bio,
Inc.), according to the manufacturer's instructions.
HT-1080 cell culture
HT-1080 cells were purchased from the Shanghai
Institutes for Biological Sciences, and were cultured in Dulbecco's
modified Eagle's medium (HyClone; GE Healthcare Life Sciences)
supplemented with 10% (v/v) fetal bovine serum (HyClone; GE
Healthcare Life Sciences) and penicillin-streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.) in a 95% O2 and 5%
CO2 incubator at 37°C.
Establishment of HT-1080 cells stably
expressing mFcγRIIB
A total of 2×105 HT-1080 cells were
infected with lentivirus regulatory plasmid, followed by the
addition of 1 ml of poly-ethylene (10 mg/ml) virus diluent (diluted
6 times) to HT-1080 cells for infection, incubate at 37°C, 5%
CO2 for 12 h, and then add G418 (100
µg/µl) or Puro (0.3 µg/µl) working
solution, G418 (200 µg/ml) was used to screen single
G418-resistant HT-1080 cells. Subsequently, scale-up cultured
G418-resistant HT-1080 cells were infected with mFcγRIIB
lentivirus, and single G418- and Puro-resistant HT-1080 cells were
then screened using Puro (0.3 µg/ml, Takara Bio, Inc.),
cultured at 37°C, 5% CO2 for 7-14 days; the viral titer
was 4×107 TU/ml.
Immunofluorescence assay
G418- and Puro-resistant HT-1080 cells were
inoculated into 6-well plates, and inducer doxycycline (Dox)
(Takara Bio, Inc.) was added to induce the cells at a final
concentration of 1,000 ng/ml. The cells were then incubated for 48
h at 37°C with 5%CO2. After washing with PBS, PE-labeled
anti-FcγRIIB antibody (cat. no. 563019; 5 µl; BD
Biosciences) was incubated with the cells for 20 min on ice and in
the dark. After washing with PBS, cells were observed under
transmission light, green fluorescence and red fluorescence
microscopes (Olympus Corporation).
Western blot analysis
Double-resistant HT-1080 cells were induced by Dox
inducer at concentrations of 100, 500, 1,000 ng/ml and incubated
for 48 h at 37°C with 5% CO2. Total protein was
extracted from the cells using lysis buffer (KeyGEN BioTECH). After
determining the protein concentration using the BCA analysis kit
(KeyGEN BioTECH), an equal amount of proteins (100 ng) were
separated by 10% SDS-PAGE, and then transferred to polyvinylidene
difluoride membranes, which were blocked with 5% skim milk in TBS
with 1% Tween-20 for 90 min at 25°C. Subsequently, the membranes
were probed with primary antibodies at 4°C over-night, followed by
incubation with secondary antibodies for a further 2 h at room
temperature, each sample of total protein was assayed by western
blot analysis using mouse monoclonal anti-human FcγRIIB antibody
(cat. no. sc-166711; 1:2,000; Santa Cruz Biotechnology, Inc.) and
peroxidase-conjugated rabbit anti-mouse IgG antibody (cat. no.
sc-2357; 1:2,000; Santa Cruz Biotechnology, Inc.) (16). The immunoreactive bands were
visualized using an enhanced chemiluminescent (ECL) kit (KeyGEN
BioTECH). The blots were analyzed using ImageJ 1.48u software
(National Institutes of Health). β-actin (cat. no. sc-81178; 1:500;
Santa Cruz Biotechnology, Inc.) was used as the loading
control.
Preparation of recombinant sFcγRIIB
In order to prepare human recombinant sFcγRIIB,
PET-sFcγRIIB plasmid from our laboratory was transformed into
competent Escherichia coli BL21 cells (Takara Bio, Inc.). To
prepare mouse recombinant sFcγRIIB, mouse sFcγRIIB gene was cloned
using TRE-sFcγRIIB plasmid as a template and sFcγRIIB primers
(forward primer, 5′-GGA ATT CAT GGG AAT CCT GCC GTT CCT ACT GA-3′
and reverse primer, 5′-CCC AAG CTT TGT CAA TAC TGG TAA AGA CCT GCT
G-3′), and was inserted into pET-32a (+) (Beijing Solarbio Science
& Technology Co., Ltd.) vector to construct the PET-sFcγRIIB
plasmid. The PET-sFcγRIIB plasmid (2 µl) was subsequently
was transformed into competent Escherichia coli BL21 cells.
Both sets of transformed BL21 cells were induced by IPTG to produce
sFcγRIIB proteins, which were purified using a His Bind®
Purification kit (Merck & Co., Inc.) according to the
manufacturer's instructions. Purified sFcγRIIB proteins were
validated by western blot analysis.
ELISA for sFcγRIIB and IgG
A total of 30 female patients with SLE (35±10 years
old) from the Department of Rheumatism, General Hospital of Ningxia
Medical University were recruited into the present study based on
the American College of Rheumatology (ACR) criteria (17), and 30 healthy female subjects
(37±8 years old) from the Cardiovascular Hospital of Ningxia
Medical University were also recruited (October, 2018 to March,
2019). All subjects signed informed consent froms prior to the
start of the study. The present study was approved by the Ethics
Committee of Ningxia Medical University. Serum was collected from
all subjects. For serum sFcγRIIB detection, mouse anti-human
sFcγRIIB monoclonal antibody (cat. no. sc-166578; 1:2,000; Santa
Cruz Biotechnology, Inc.), serum (1:100; Biological Industries),
rabbit anti-human sFcγRIIB polyclonal antibody (cat. no.
bs-4991R;1:2,000; Beijing Bioss Biotechnology Co., Ltd.) and
HRP-labeled anti-IgG (cat. no. bs-0297G-HRP;1:8,000; Beijing Bioss
Biotechnology Co., Ltd.) were successively added into the plates
for incubation (37°C, 30 min). For serum sFcγRIIB-IgG
determination, mouse anti-human sFcγRIIB monoclonal antibody (cat.
no. bs-4991R; 1:2,000; Beijing Bioss Biotechnology Co., Ltd.),
serum (1:100; Biological Industries), and HRP-labeled anti-IgG
(cat. no. bs-0297G-HRP;1:8,000; Beijing Bioss Biotechnology Co.,
Ltd.) were successively incubated in plates (37°C, 30 min). For the
binding strength detection of recombinant human sFcγRIIB to IC in
serum, serum treated with 5 µg/ml calf thymus DNA (ctDNA;
Sigma-Aldrich; Merck KGaA) and HRP-labeled anti-IgG (cat. no.
bs-40295G-HRP; 1:8,000; Beijing Bioss Biotechnology Co., Ltd.) were
successively incubated (37°C, 30 min) in plates previously coated
with 1.25 µg/ml recombinant sFcγRIIB. Following these
incubations, TMB reagent was added to all plates for chromogenic
reaction. The optical density was determined using a microplate
reader (ELx800NB, BioTek Instruments, Inc.).
B cell preparation
Peripheral blood samples were collected from all
study participants and the lymphocytes were isolated with
lymphocyte separation liquid. A magnetic bead separation system
(Miltenyi Biotec GmbH) was used to sort CD19+ B cells
with anti-CD19+ antibody (cat. no. 130-113-733; 1:50;
Miltenyi Biotec GmbH). Sorted CD19+ B cells were
cultured for scale-up.
B cell treatment
For mFcγRIIB treatment, mFcγRIIB lentivirus,
lentivirus regulatory plasmid and Dox were added to the
CD19+ B cells. The expression of mFcγRIIB in the treated
B cells was then detected by immunofluorescence assay and western
blot analysis according to the procedures described above.
Subsequently, ctDNA and anti-ctDNA-IC (the mixture of SLE patient
serum and ctDNA at a final concentration of 5 µg/ml,
incubated at 37°C for 1 h) were incubated with treated
CD19+ B cells. After 24 h, the B cells were collected
for flow cytometry. Briefly, the collected cells were washed twice
with ice-cold PBS at pH 7.4, and were then resuspended in buffer
containing Annexin V (Elabscience); at a concentration of
1×106/ml cells/ml. Subsequently, 5 µl Alexa Fluor
555 (Thermo Fisher Scientific, Inc.) and 1 µl 100
µg/ml PI (Thermo Fisher Scientific, Inc.) were added to 100
µl cell suspension. After gentle vortexing, the cells were
incubated at 4°C in the dark for 15 min and analyzed by flow
cytometry (Merck KGaA). The percentages of positively stained cells
were determined. For sFcγRIIB treatment, recombinant sFcγRIIB and
ctDNA were used to treat CD19+ B cells for 72 h. Cell
medium from both virus and protein treatments was collected for IgG
detection using an ELISA kit (eBioscience), according to the
manufacturer's instructions. B cells were collected for the
determination of the BTK, Lyn, DOK1 and SHIP protein and
phosphorylation levels by western blot analysis using antibodies
against BTK (cat. no. ab208937; 1:500; Abcam), Lyn (cat. no.
ab1890; 1:1,000; Abcam), DOK1 (cat. no. ab8112; 1:1,000; Abcam),
SHIP (cat. no. ab45142; 1:1,000; Abcam), phosphorylated BTK (cat.
no. ab68217; 1:1,000; Abcam), phosphorylated DOK1 (cat. no.
ab75742; 1:1,000; Abcam), phosphorylated Lyn (cat. no. ab33914;
1:1,000; Abcam) and phosphorylated SHIP (cat. no. ab96402; 1:1,000;
Abcam), the membranes were probed with primary antibodies at 4°C
overnight, followed by incubation with secondary antibodies for a
further 2 h at room temperature.
Animal experiments
A total of 160 MRL/lpr SLE model mice (all female
mice) with a body weight of 16-20 g were purchased from the
Experimental Animal Center of Nanjing Military Region (no.
0032260). All animals were kept in cages with a light/dark cycle of
12 h at 25±1°C, with free access to food and water; the health and
behavior of the mice were monitored every 5 days. Among the 160
mice, mice (8 weeks old) that had no lupus symptoms were designated
as the prevention group (n=80/group) and 16-week-old mice that had
obvious lupus symptoms were designated as the treatment group
(n=80/group). The animal experiments were approved by the Animal
Ethics Committee of Ningxia Medical University and followed the
Guidelines for the Management and Use of Laboratory Animals
(National Academies Press). For mFcγRIIB treatment, the prevention
(pre) and treatment (tre) groups were treated with 100 µl or
200 µl suspensions of mFcγRIIB lentivirus, lentivirus
regulatory plasmid and Dox, 100 µl suspensions of lentivirus
empty vector, and healthy saline via the tail vein (n=10/group).
For sFcγRIIB treatment, the prevention group and treatment groups
were treated with intravenous 4.8 µg (60 µl), 9.6
µg (120 µl), and 14.4 µg (180 µl)
recombinant mouse FcγRIIB and normal saline, once per week for 4
consecutive weeks (n=10/group). Following observation for a week,
serum and urine were collected, and the mice were then anesthetized
by an intraperitoneal injection of chloral hydrate (4%, 400 mg/kg)
and were sacrificed by cervical dislocation. Mouse death was
confirmed by the inexistence of breath, heartbeat and corneal
reflex. No accidental deaths occurred during the experiment.
Kidney, liver and lymph tissues were obtained for
H&E staining. The mice were sacrificed and the kidney, liver
and lymph tissues of the mice were obtained and fixed in 4% (v/v)
paraformaldehyde, embedded in paraffin and sectioned at a 5-8
µm thickness. The fixed kidney, liver and lymph tissues were
stained with hematoxylin and eosin (H&E) for the evaluation of
the severity of kidney, liver and lymph injury. The samples were
dewaxed with xylene (cat. no. 10023428; Sinopharm Chemical Reagent
Co., Ltd.) and then dephenylated using a graded ethanol series
(100, 95, 80 and 70%) for 2 min. The tissues were then rehydrated
by rinsed in distilled water twice to, stained using 0.5%
hematoxylin (cat. no. G1120; Solarbio Life Science Co., Ltd.) for
20 min at room temperature and then washed under running water. The
slices were then rinsed in acidification solution comprised of
hydrochloric acid (cat. no. 10011018; Sinopharm Chemical Reagent
Co., Ltd.) and 75% ethanol for 30 sec and then washed with tap
water for 15 min. The tissues were then stained using 0.5% eosin
(cat. no. G1120; Solarbio Life Science Co., Ltd.) for 2 min at room
temperature, dehydrated in 100% ethanol for 1 min and treated with
xylene for 3 min. Finally, neutral gum was used to seal the
film.
The urine albumin (mouse albumin ELISA kit; cat. no.
AKRAL-121; Shibayagi), serum anti-dsDNA antibody [mouse anti-double
stranded DNA antibody (IgG) ELISA kit; cat. no. CSB-E11194m;
Cusabio] and serum anti-nuclear antibody [moue anti-nuclear
antibody (IgM) ELISA kit; cat. no. 88-50470-22; eBioscience] were
detected according to the manufacturer's instructions. Serum-free
FcγRIIB was measured using rabbit anti-mouse FcγRIIB monoclonal
anti-body (cat. no. sc-166711; 1:2,000, Santa Cruz Biotechnology,
Inc.) and goat anti-mouse FcγRIIB polyclonal antibody (cat. no.
sc-12815; 1:2,000; Santa Cruz Biotechnology, Inc.). Serum
FcγRIIB-IgG was assayed using rabbit anti-mouse FcγRIIB monoclonal
antibody (cat. no. sc-365864; 1:2,000; Santa Cruz Biotechnology,
Inc.) and HRP-labeled goat anti-mouse IgG antibody (cat. no.
bs-0296G-HRP; 1:5,000; Beijing Bioss Biotechnology Co., Ltd.).
Lymphocytes were isolated from spleens using lymphocyte separation
solution (Tianjin Hao Yang Hua Ke Co., Ltd.). B cells were sorted
using mouse anti-CD19 microbeads (Miltenyi Biotec GmbH). The
protein and phosphorylation levels of BTK, Lyn and SHIP in the B
cells were detected by western blot analysis using BTK polyclonal
antibody (cat. no. 21581-1-AP; 1:1,000; Proteintech), phospho-BTK
antibody (cat. no. 5082T; 1:1,000; Cell Signaling Technology,
Inc.), Lyn polyclonal antibody (cat. no. BS6657; 1:1,000;
Bioworld), phospho-Lyn (cat. no. BS64043; 1:500; Bioworld), SHIP
polyclonal antibody (cat. no. BS91238; 1:1,000; Bioworld),
phospho-SHIP (cat. no. BS94059; 1:1,000; Bioworld).
Statistical analysis
Statistical analyses were performed using SPSS 23.0
(IBM Corp.) and the results are presented as the means ± standard
error of mean (SEM). When the data exhibited a normal distribution,
one-way analysis of variance (ANOVA) with Tukey's post hoc test
were used for multiple comparisons, and the SNK-q test was used for
the comparison between 2 groups. When the data exhibited a
non-normal distribution, the Kruskal-Wallis test (Mann-Whitney U
with Bonferroni's correction applied) was used. Statistically
significant values were indicated by P<0.05. Graph construction
was performed using GraphPad Prism software version 5 (GraphPad
Software).
Results
Successful construction of mFcγRIIB
lentivirus and mFcγRIIB expression in HT-1080 cells
The human TRE-mFcγRIIB lentiviral vector was
successfully constructed with the sequencing results indicating
that the inserted mFcγRIIB gene was 100% homologous to that
provided by GenBank. The lentiviral vector titer was 106
TU/ml and the lentiviral regulatory plasmid titer was
105 TU/ml and exhibited good infectivity (data not
shown). After infecting the HT-1080 cells with the virus, mFcγRIIB
expression was induced with 1,000 ng/ml Dox. Immunofluorescence
staining revealed that mFcγRIIB lentivirus successfully infected
HT-1080 cells indicated by the green fluorescence emitted by
expressed GFP, and mFcγRIIB was expressed in the cytomembrane of
the HT-1080 cells indicated by red fluorescence (Fig. 1A). Western blot assays revealed
that the mFcγRIIB expression level was positively associated with
the inducer concentration, as shown in Fig. 1B.
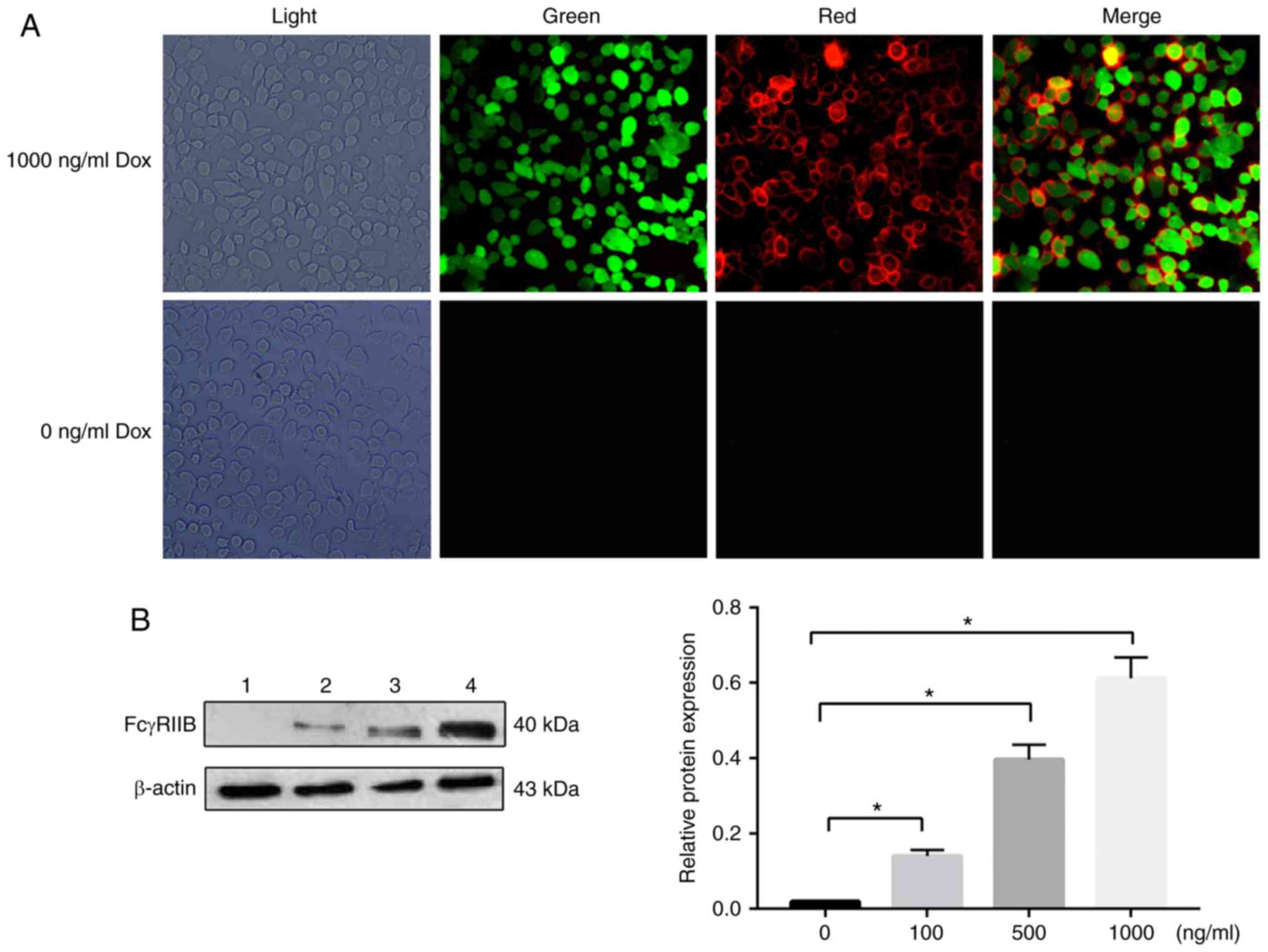 | Figure 1(A) Observation of double-resistant
HT-1080 cells by fluorescence microscopy (magnification ×400). The
expression of human mFcγRIIB in HT-1080 cells was detected by
immunofluorescence staining. Light, light field; green, green
fluorescent protein (GFP) expression in cells; red, mFcγRIIB
expression in cells; merge, GFP and mFcγRIIB expression in cells.
The experiments were repeated 3 times. (B) The protein expression
levels of sFcγRIIB in human were examined by western blot analysis,
with β-actin as a loading control. Lane 1, 0 ng/ml Dox inducer;
lane 2, 100 ng/ml Dox inducer; lane 3, 500 ng/ml Dox inducer; lane
4, 1,000 ng/ml Dox inducer. *P<0.05. Data are
presented as the means ± SD of 3 independent experiments. Dox,
doxycycline; mFcγRIIB, membrane-bound type FcγRIIB; sFcγRIIB,
soluble FcγRIIB. |
Expression of mFcγRIIB in human B
cells
Similarly, as shown in Fig. 2A, green fluorescence indicated
that the mFcγRIIB lentivirus was successfully infected into B
cells, and red fluorescence indicated that mFcγRIIB was expressed
in the cytomembrane of B cells. In addition, immunofluorescence
staining (Fig. 2A) and western
blot analysis (Fig. 2B) revealed
that the expression level of endogenous mFcγRIIB in the B cells of
patients with SLE was lower than that in the healthy controls.
mFcγRIIB expression in the virus-infected SLE group was
significantly higher than that in the control SLE group. Similarly,
the expression of mFcγRIIB in the virus-infected healthy group was
higher than that in the control healthy group.
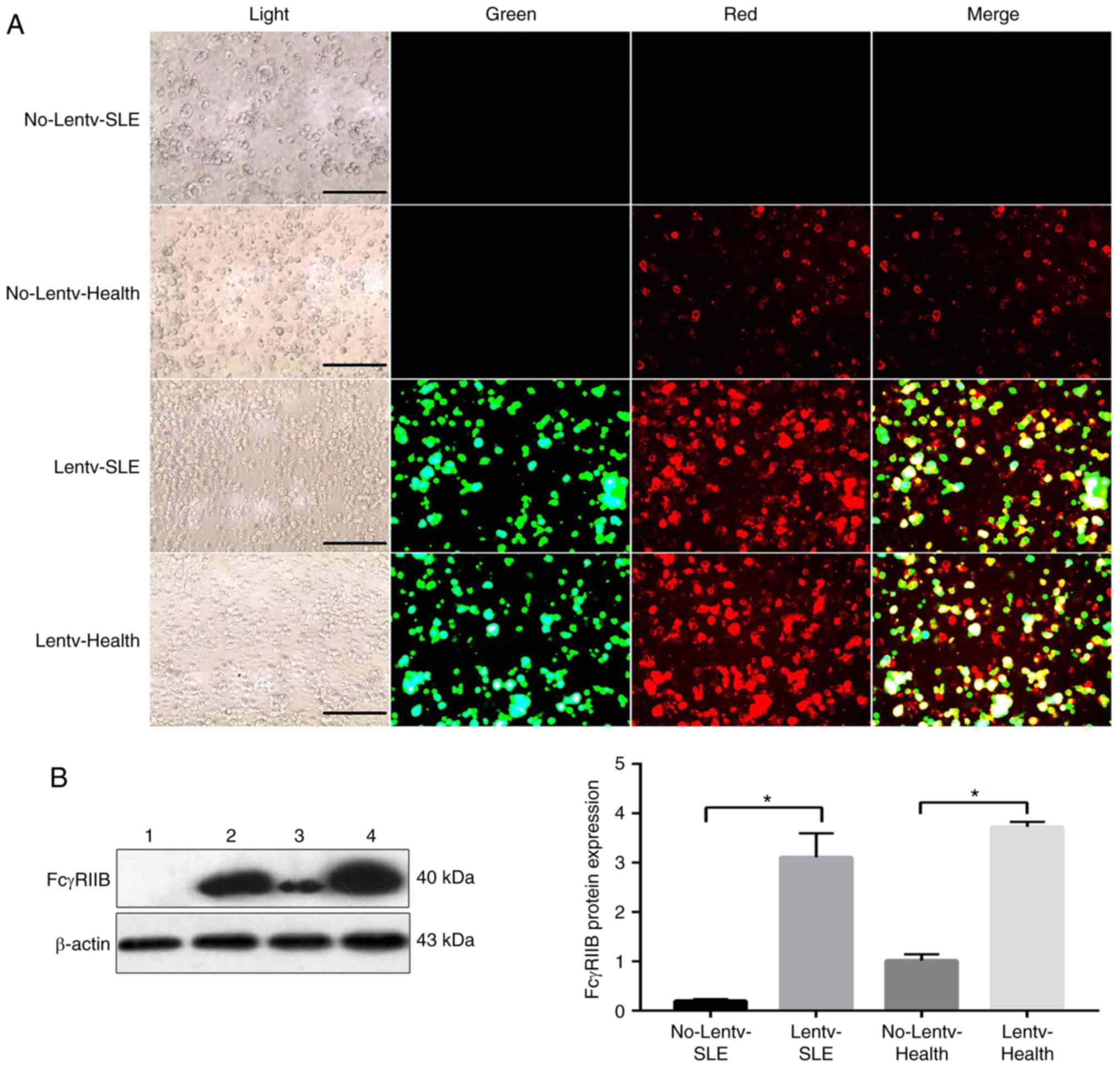 | Figure 2(A) Fluorescence microscopy to
observe the expression of mFcγRIIB in B lymphocytes (magnification
400x). B cells were transfected using mFcγRIIB-lentivirus for 72 h.
The infection efficiency of mFcγRIIB-lentivirus was observed by
fluorescence microscopy. No-Lentv-SLE, no transfection in the SLE
patients; Lentv-SLE, mFcγRIIB-lentivirus transfection in the SLE
patients; No-Lentv-health, no transfection in the healthy controls;
Lentiv-health, mFcγRIIB-lentivirus transfection in the healthy
controls. Light, light field; green, green fluorescent protein
(GFP) expression in cells; red, mFcγRIIB expres-sion in cells;
merge, GFP and mFcγRIIB expression in cells. The experiments were
repeated 3 times. (B) The protein expression levels of mFcγRIIB in
human were examined by western blot analysis, with β-actin as a
loading control. Lane 1, Control SLE group; lane 2, virus-infected
SLE group; lane 3, healthy control group; lane 4, virus-infected
healthy group. *P<0.05. Data are presented as the
means ± SD of 3 independent experiments. mFcγRIIB, membrane-bound
type FcγRIIB; sFcγRIIB, soluble FcγRIIB. |
Effects of overexpression of mFcγRIIB on
human B cells
ctDNA and anti-ctDNA-IC were used to stimulate
infected B cells. ELISA was performed to detect anti-IgG antibody
secreted by B cells, and the protein and phosphorylation levels of
BTK, Lyn, DOK1 and SHIP were detected by western blot analysis. The
results revealed that the IgG levels in the virus-infected SLE and
healthy groups were respectively significantly lower than those in
the control SLE and control healthy groups. Anti-ctDNA-IC treatment
significantly downregulated the B cell secretion of IgG antibody,
apart from the virus-infected healthy group (Fig. 3A). The relative expression levels
of p-DOK1/DOK1, p-SHIP/SHIP in the virus-infected SLE and
virus-infected healthy groups were respectively significantly
higher than those in the control SLE and control healthy groups;
the relative expression levels of p-Lyn/Lyn in the virus-infected
SLE, virus-infected healthy groups and control healthy groups were
respectively significantly higher than those in the control SLE
group, while the relative expression of p-BTK/BTK in the
virus-infected SLE and virus-infected healthy groups was
significantly lower than that in the control SLE and control
healthy groups following anti-ctDNA-IC stimulation (Fig. 3B and C). More importantly, the
apoptotic rate of the B cells in the virus-infected SLE and healthy
groups was respectively significantly higher than that in the
control SLE and control healthy groups, and the apoptotic rate of
the B cells in the anti-ctDNA-IC treatment group was higher than
that in the ctDNA treatment group (Fig. 4A).
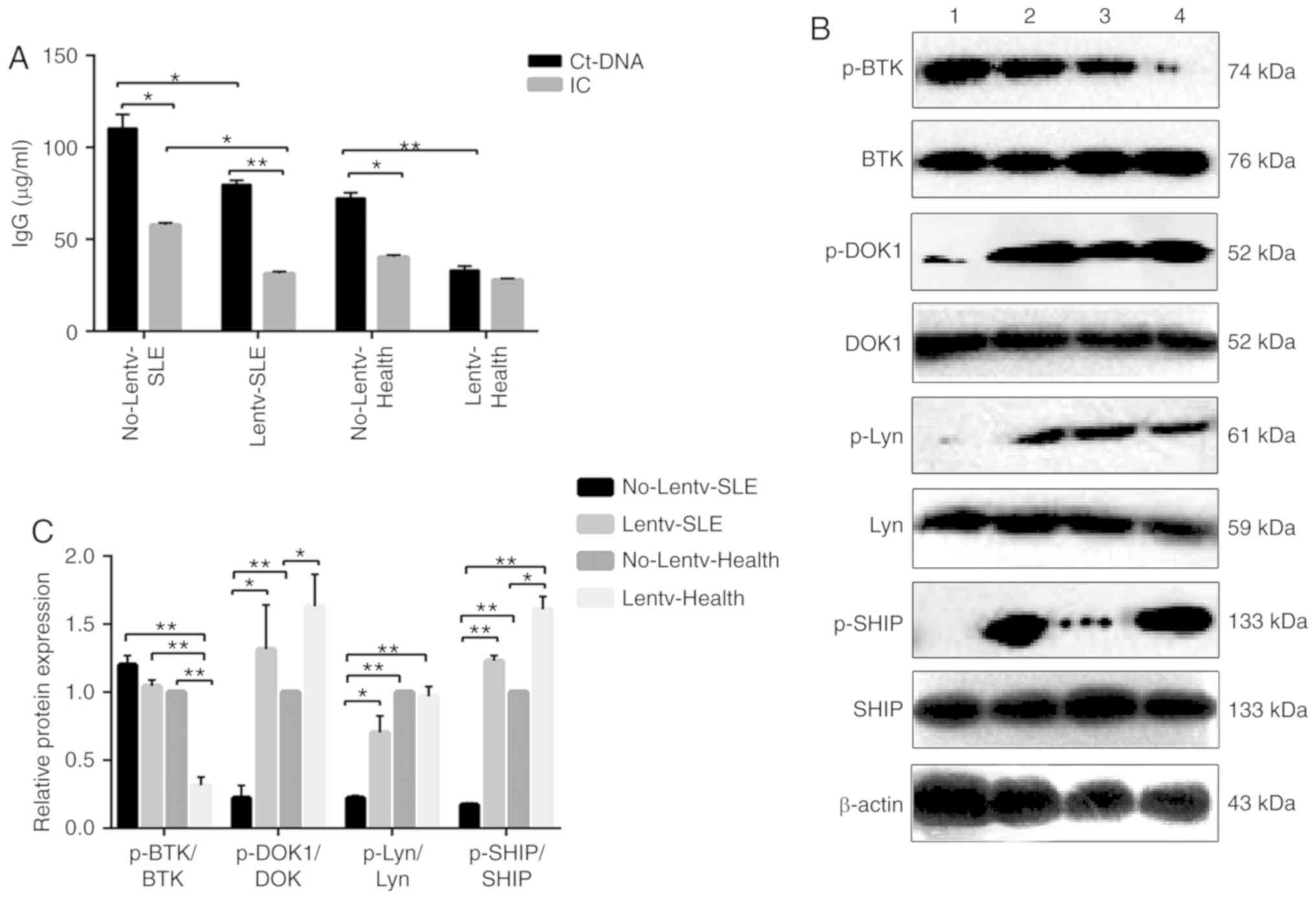 | Figure 3(A) ELISA detection of human IgG
antibody levels. No-Lentv-SLE, control SLE grou; Lentv-SLE,
virus-infected SLE group; No-Lentv-Health, healthy control group;
Lentv-Health, virus-infected healthy group; CtDNA, calf thymus DNA.
IC, anti-calf thymus DNA-immune complexes. *P<0.05;
**P<0.01. The experiments were repeated 3 times. (B
and C) The human protein expression levels of BTK, p-BTK, DOK1,
p-DOK1, Lyn, p-Lyn, SHIP and p-SHIP were examined by western blot
analysis, with β-actin as a loading control. The phosphorylation
and total protein levels of BTK, DOK1, Lyn and SHIP were detected
in B cells from patients with SLE and healthy subjects infected
with mFcγRIIB lentivirus following anti-ctDNA-IC stimulation, and
the relative level for each phosphorylation-protein/each respective
total protein was calculated. No-Lentv-SLE, control SLE group;
Lentv-SLE, virus-infected SLE group; No-Lentv-Health, healthy
control group; Lentv-Health, virus-infected healthy group.
*P<0.05; **P<0.01. Data are presented
as the means ± SD of 3 independent experiments. SLE, systemic lupus
erythematosus; mFcγRIIB, membrane-bound type FcγRIIB; sFcγRIIB,
soluble FcγRIIB; BTK, Bruton's tyrosine kinase; Lyn, Lyn
proto-oncogene, Src family tyrosine kinase; DOK-1, docking protein
1; SHIP, inositol polyphosphate-5-phosphatase D. |
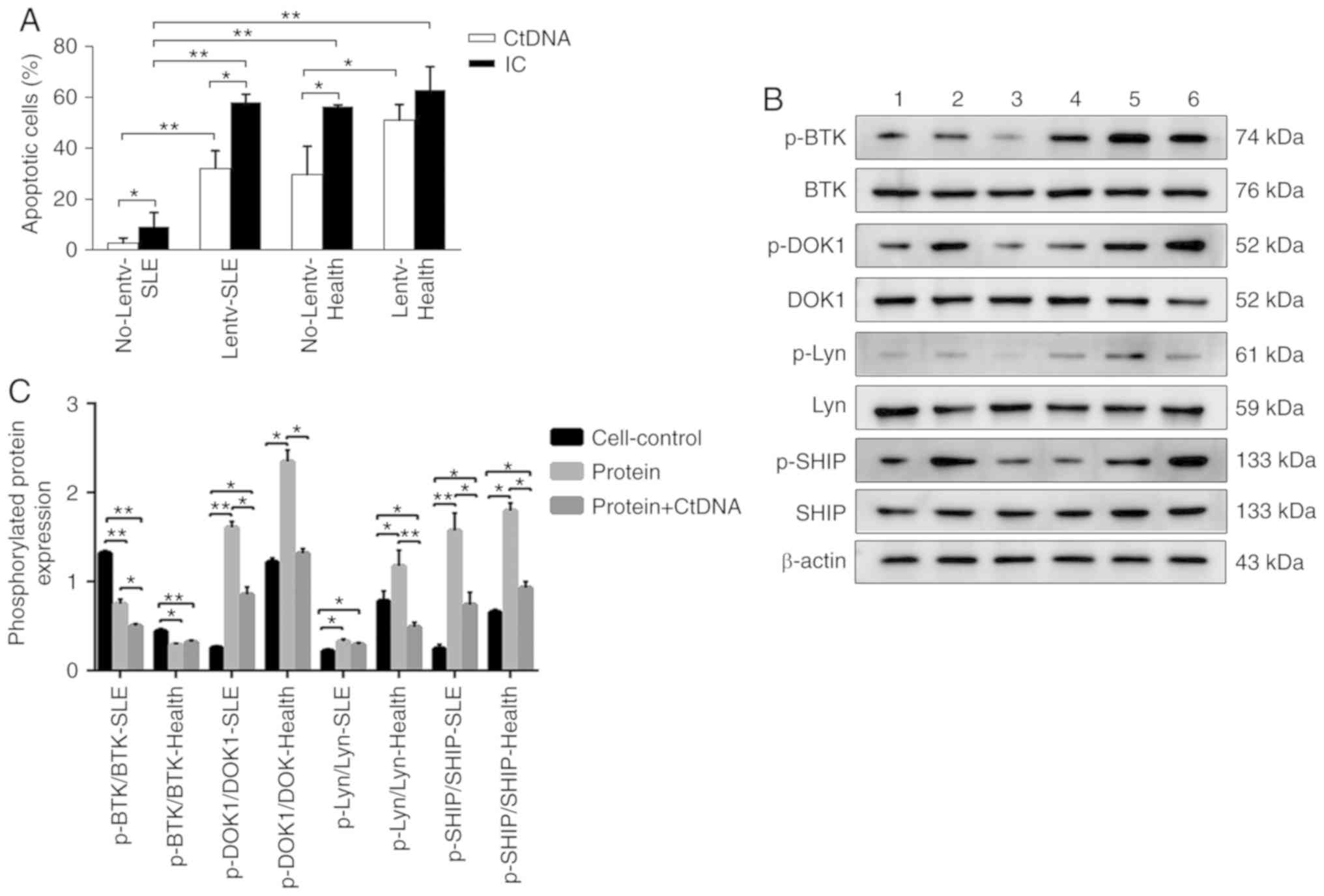 | Figure 4(A) Apoptotic rate of B cells
infected by human mFcγRIIB lentivirus. No-Lentv-SLE, control SLE
group; Lentv-SLE, virus-infected SLE group; No-Lentv-Health,
healthy control group; Lentv-Health, virus-infected healthy group;
CtDNA, calf thymus DNA; IC, anti-calf thymus DNA-immune complexes.
*P<0.05; **P<0.01. The experiments were
repeated 3 times. (B and C) The human protein expression levels of
BTK, p-BTK, DOK1, p-DOK-1, Lyn, p-Lyn, SHIP and p-SHIP were
examined by western blot analysis, with β-actin as a loading
control. The phosphorylation and total protein levels of BTK,
DOK-1, Lyn and SHIP were detected in B cells of patients with SLE
and healthy subjects following treatment with human sFcγRIIB or
sFcγRIIB plus ctDNA, and the relative level for each
phosphorylation-protein/each respective total protein was
calculated. Cell-Control-SLE, B cells of SLE patients; Protein-SLE,
B cells of SLE patients treated with sFcγRIIB; Protein + CtDNA-SLE,
B cells of SLE patients treated with sFcγRIIB and ctDNA;
Cell-Control-Health, B cells of healthy subjects; Protein-Health, B
cells of healthy persons treated with sFcγRIIB; Protein +
CtDNA-Health, B cells of healthy persons treated with sFcγRIIB and
ctDNA. *P<0.05; **P<0.01. Data are
presented as the means ± SD of 3 independent experiments. SLE,
systemic lupus erythematosus; mFcγRIIB, membrane-bound type
FcγRIIB; sFcγRIIB, soluble FcγRIIB; BTK, Bruton's tyrosine kinase;
Lyn, Lyn proto-oncogene, Src family tyrosine kinase; DOK-1, docking
protein 1; SHIP, inositol polyphosphate-5-phosphatase D. |
sFcγRIIB and sFcγRIIB-IgG levels in serum
of patients with SLE
The levels of sFcγRIIB and sFcγRIIB-IgG in the serum
of patients with SLE were lower than those in the serum of the
healthy subjects. The level of sFcγRIIB-IgG in the patients with
SLE was lower than that in the healthy subjects. No significant
difference was observed between sFcγRIIB-IgG and sFcγRIIB in the
serum of the healthy subjects (Table
I).
 | Table ITotal sFcyRIIB and sFcγRIIB-IgG
levels in human serum (n=30, ng/ml, means ± SD). |
Table I
Total sFcyRIIB and sFcγRIIB-IgG
levels in human serum (n=30, ng/ml, means ± SD).
| Group | Content |
|---|
| SLE total
sFcγRIIB | 125.11±4.63a |
| Health total
sFcγRIIB | 134.30±5.89 |
| SLE
sFcγRIIB-IgG | 89.23±13.07b |
| Health
sFcγRIIB-IgG | 132.64±6.20 |
Effect of sFcγRIIB on human B cells
Recombinant sFcγRIIB protein was successfully
expressed and identified by western blot analysis. The results
revealed that recombinant sFcγRIIB bound to immune complexes (ICs)
in serum and reduced the secretion of IgG antibodies in B cells,
suggesting that sFcγRIIB may inhibit the activation of B
lymphocytes by combining with IC (Tables II and III). The relative expression levels of
p-Lyn/Lyn p-DOK1/DOK1 and p-SHIP/SHIP in the SLE groups were lower,
while the relative expression of p-BTK/BTK was higher compared to
the corresponding healthy groups. Furthermore, among the SLE
groups, the relative expression levels of p-Lyn/Lyn, p-DOK1/DOK1
and p-SHIP/SHIP in the sFcγRIIB treatment subgroups were higher,
while the relative expression of p-BTK/BTK was lower than that in
the control subgroup (Fig. 4B and
C).
 | Table IISoluble FcγRIIB binding to immune
complexes, with OD values (n=30, means ± SD). |
Table II
Soluble FcγRIIB binding to immune
complexes, with OD values (n=30, means ± SD).
| Group DNA
(mg/ml) | OD value
|
|---|
| 0 | 0.05 | 0.1 | 0.2 | 0.4 | 0.8 | 1.0 |
|---|
| SLE | 1.239±0.061a | 1.439±0.065a | 1.507±0.065a | 1.569±0.068a | 1.653±0.075a | 1.763±0.068a | 1.776±0.065a |
| Healthy | 1.776±0.065 | 1.258±0.054 | 1.323±0.063 | 1.394±0.065 | 1.464±0.072 | 1.546±0.072 | 1.557±0.067 |
| PBS | 0.097 | 0.076 | 0.083 | 0.086 | 0.091 | 0.072 | 0.095 |
 | Table IIIIgG levels in B cells (n=30,
µg/ml, means ± SD). |
Table III
IgG levels in B cells (n=30,
µg/ml, means ± SD).
| Group | IgG
concentration |
|---|
| Control-Health | 19.608±4.838 |
|
sFcγRIIB-Health | 6.054±1.656a |
|
sFcγRIIB-ctDNA-Health |
12.400±1.803a,b |
| Control-SLE | 113.389±6.768 |
| sFcγRIIB-SLE |
33.392±1.521c |
|
sFcγRIIB-ctDNA-SLE |
70.679±6.595d,e |
Effect of mFcγRIIB lentivirus on MRL/lpr
SLE mice
The mouse mFcγRIIB gene was successfully inserted
into the TRE vector and packed into viruses. The titers of the
expressed and regulated viruses were measured to reach
106 TU/ml. mFcγRIIB lentivirus and lentivirus regulatory
plasmid were injected into the MRL/lpr SLE mice, and the effects
were examined. As a result, in the prevention group and treatment
group, mFcγRIIB lentivirus treatments significantly decreased urine
albumin, serum anti-dsDNA antibody and serum anti-nuclear antibody
levels, while increasing mFcγRIIB expression in spleen B cells
(Fig. 5). In the prevention and
treatment groups of B cells, the relative expression of p-BTK/BTK
was increased, while the relative expression of p-SHIP/SHIP was
decreased (Fig. 6). More
importantly, the results of H&E staining revealed that in the
MRL/lpr SLE mouse kidney tissue, a large number of lymphocytes
infiltrated around the glomerulus and the glomerular capillaries
became atrophied and hardened. In the liver tissue, mild
histological alterations were observed, such as ballooning in the
cytoplasm, dilatation in the central vein and hepatic sinusoids,
and lymphocytic infiltrate in the hepatic lobules. In the lymph
nodes, there were a large number of lymphocytes in the lymphoid
follicles, the lymphoid nodules were enlarged, and the lymphoid
follicles were proliferated. However, mFcγRIIB lentivirus
treatments slightly ameliorated these pathologies, including
decreased cytoplasmic swelling and lymphocyte infiltration
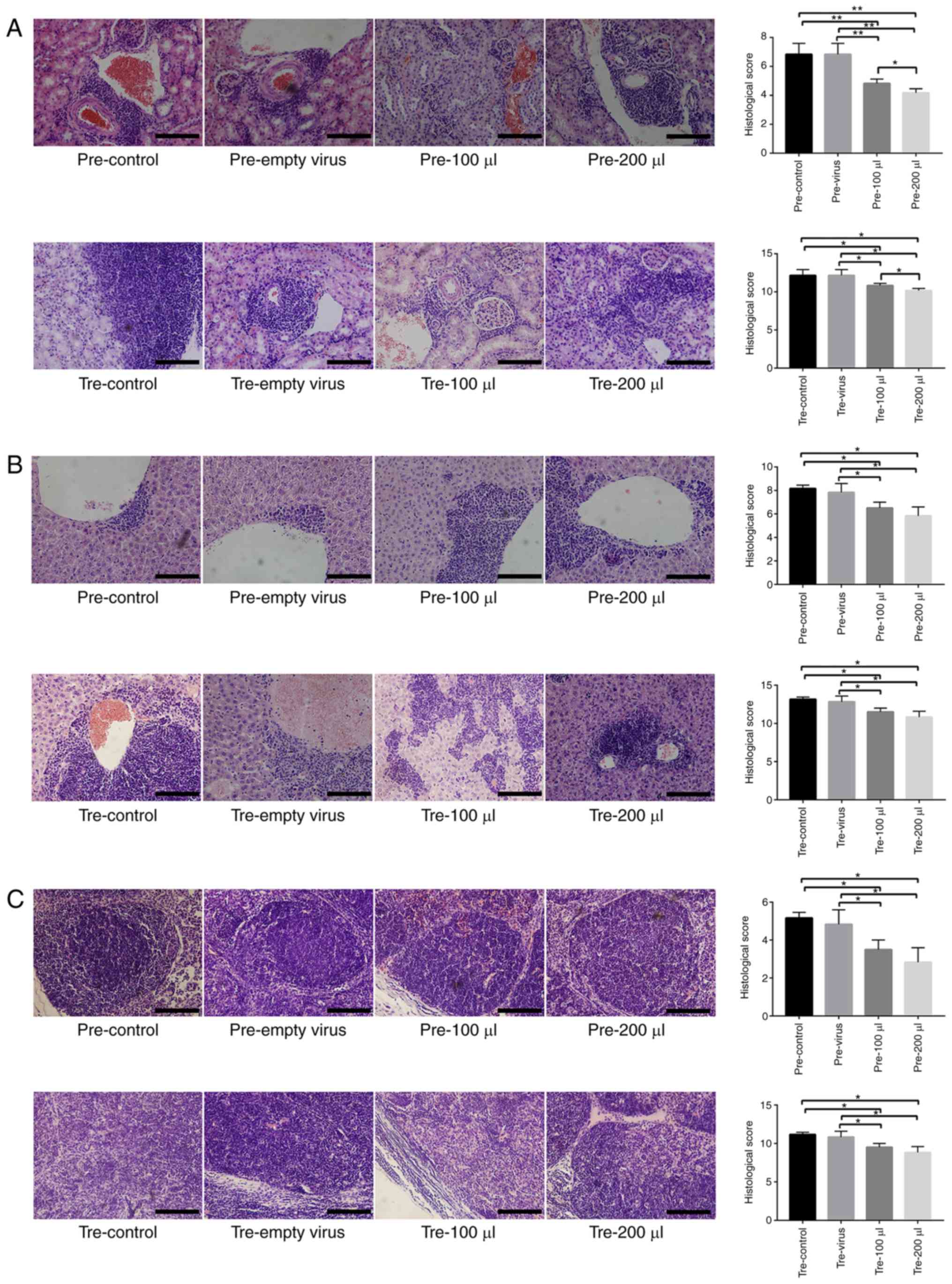
(Fig. 7).
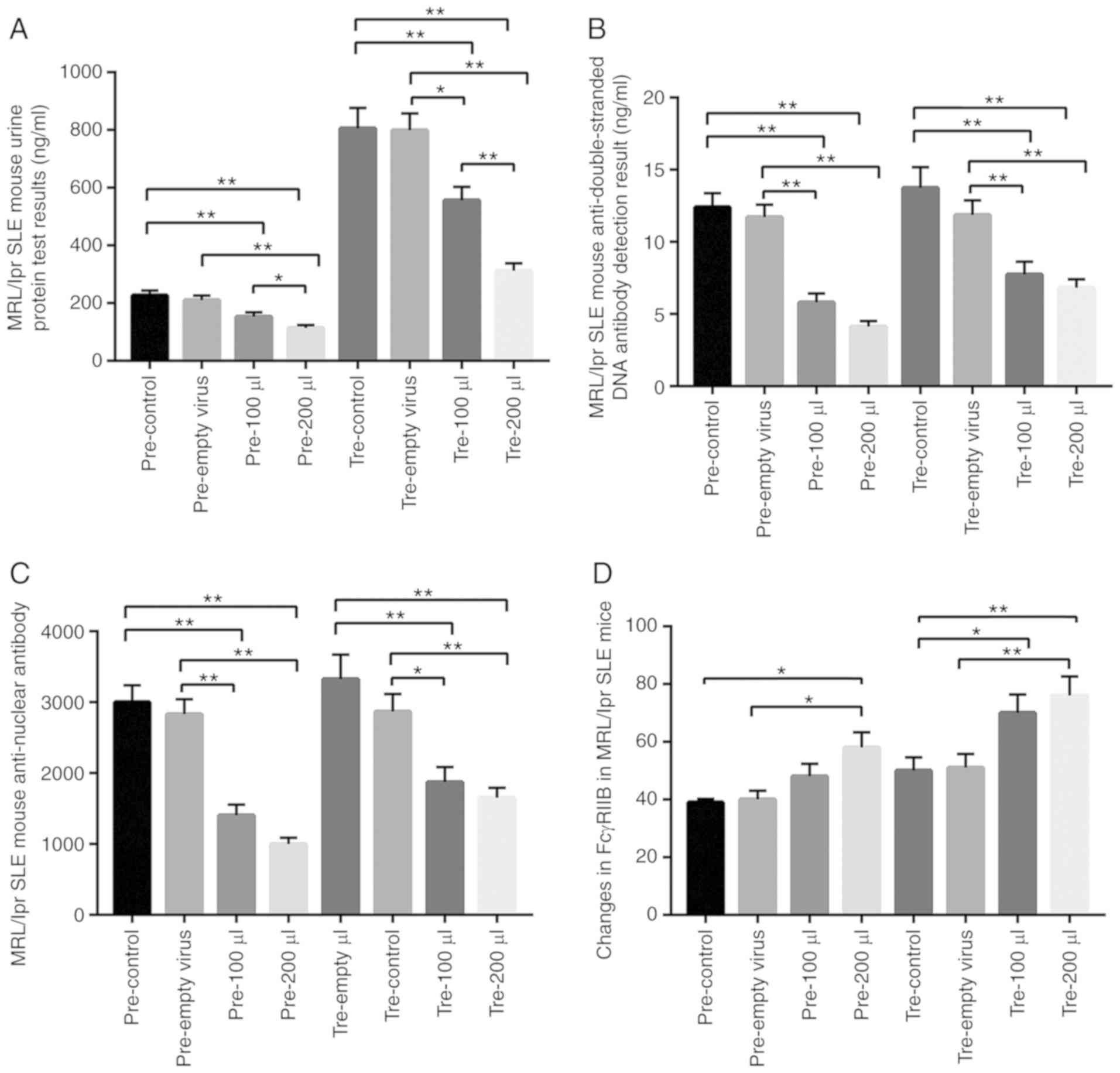 | Figure 5Target detection of MRL/lpr SLE mice
following mFcγRIIB treatment. (A) Changes in urine protein levels.
(B) Changes in serum anti-dsDNA antibody levels. (C) Changes in
serum anti-nuclear antibody levels. (D) Changes in mFcγRIIB levels
in B cells. pre-control, Control subgroup in the prevention group;
pre-empty virus, empty virus subgroup in the prevention group;
pre-100 µl, 100 µl mFcγRIIB lentivirus subgroup in
the prevention group; pre-200 µl, 200 µl mFcγRIIB
lentivirus subgroup in the prevention group; tre-control, control
subgroup in the treatment group; tre-empty virus, empty virus
subgroup in the treatment group; tre-100 µl, 100 µl
mFcγRIIB lentivirus subgroup in the treatment group; tre-200
µl, 200 µl mFcγRIIB lentivirus subgroup in the
treatment group. *P<0.05; **P<0.01;
n=10 in each group. SLE, systemic lupus erythematosus; mFcγRIIB,
membrane-bound type FcγRIIB; sFcγRIIB, soluble FcγRIIB. |
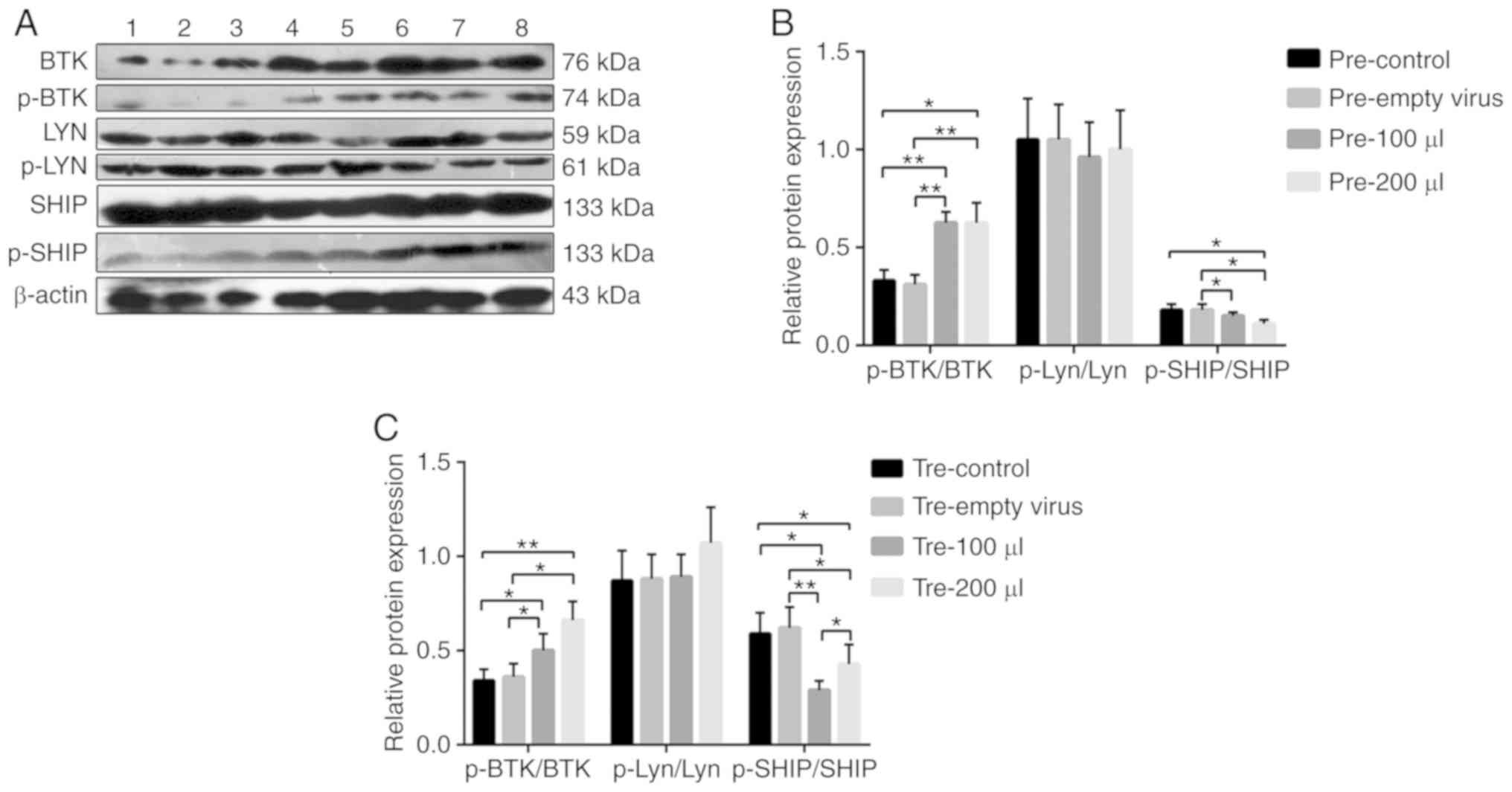 | Figure 6(A) The protein expression levels of
BTK, p-BTK, Lyn, p-Lyn, SHIP and p-SHIP in MRL/lpr SLE mouse B
cells were examined by western blot analysis, with β-actin as a
loading control. (B and C) The phosphorylation and total protein
levels of BTK, Lyn and SHIP were detected in B cells from MRL/lpr
SLE mice after the infection of mFcγRIIB lentivirus, and the
relative level for each phosphorylation-protein/each respective
total protein was calculated. Lane 1: pre-empty virus, empty virus
subgroup in the prevention group; lane 2: pre-control, control
subgroup in the prevention group; lane 3: pre-100 µl, 100
µl mFcγRIIB lentivirus subgroup in the prevention group;
lane 4: pre-200 µl, 200 µl mFcγRIIB lentivirus
subgroup in the prevention group; lane 5: tre-control, control
subgroup in the treatment group; lane 6: tre-empty virus, empty
virus subgroup in the treatment group; lane 7: tre-100 µl,
100 µl mFcγRIIB lentivirus subgroup in the treatment group;
lane 8: tre-200 µl, 200 µl mFcγRIIB lentivirus
subgroup in the treatment group. *P<0.05;
**P<0.01. Data are presented as means ± SD of 3
independent experiments. SLE, systemic lupus erythematosus;
mFcγRIIB, membrane-bound type FcγRIIB; sFcγRIIB, soluble FcγRIIB;
BTK, Bruton's tyrosine kinase; Lyn, Lyn proto-oncogene, Src family
tyrosine kinase; DOK-1, docking protein 1; SHIP, inositol
polyphosphate-5-phosphatase D. |
Preventive and therapeutic effects of
sFcγRIIB in MRL/lpr SLE mice
Mouse sFcγRIIB gene was successfully cloned into the
pET-sFcγRIIB plasmid, which was identified by DNA sequencing, and
mouse recombinant sFcγRIIB protein was obtained by induction and
was purified. Following treatment with mouse recombinant sFcγRIIB
protein, some indexes in the MRL/lpr SLE mice were detected. As a
result, in the prevention and treatment groups, in all sFcγRIIB
protein treatments, the levels of sFcγRIIB and sFcγRIIB-IgG were
significantly increased in a positive dose-dependent manner, while
serum anti-dsDNA antibody and urine albumin levels were markedly
decreased in a reverse dose-dependent manner (Fig. 8), as compared to the normal saline
treatment. Moreover, the relative expression levels of p-Lyn/Lyn
and p-SHIP/SHIP were increased, while the relative expression of
p-BTK/BTK in B cells was decreased following sFcγRIIB protein
treatment (Fig. 9).
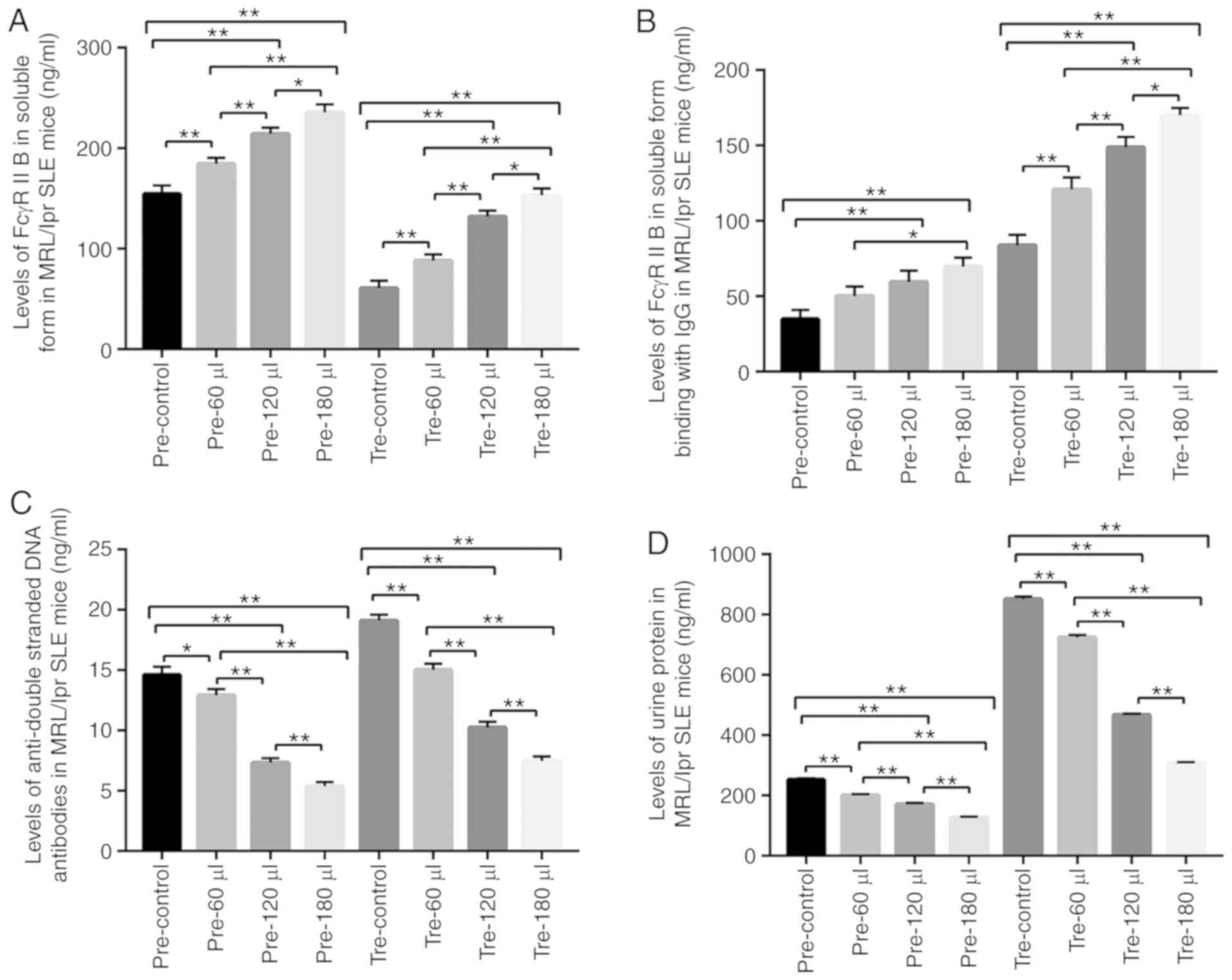 | Figure 8Target detection in MRL/lpr SLE mice.
(A) Detection of sFcγRIIB. (B) Detection of FcγRIIB binding with
IgG. (C) Detection of serum anti-double-stranded DNA antibody. (D)
Detection of urine protein. pre-control, control subgroup in the
prevention group; pre-60 µl, 4.8 µg sFcγRIIB subgroup
in the prevention group; pre-120 µl, 9.6 µg sFcγRIIB
subgroup in the prevention group; pre-180 µl, 14.4 µg
sFcγRIIB subgroup in prevention group; tre-control, control
subgroup in the treatment group; tre-60 µl, 4.8 µg
sFcγRIIB subgroup in the treatment group; tre-120 µl, 9.6
µg sFcγRIIB subgroup in the treatment group; tre-180
µl, 14.4 µg sFcγRIIB subgroup in the treatment group.
*P<0.05; **P<0.01; n=10 in each group.
SLE, systemic lupus erythematosus; mFcγRIIB, membrane-bound type
FcγRIIB; sFcγRIIB, soluble FcγRIIB. |
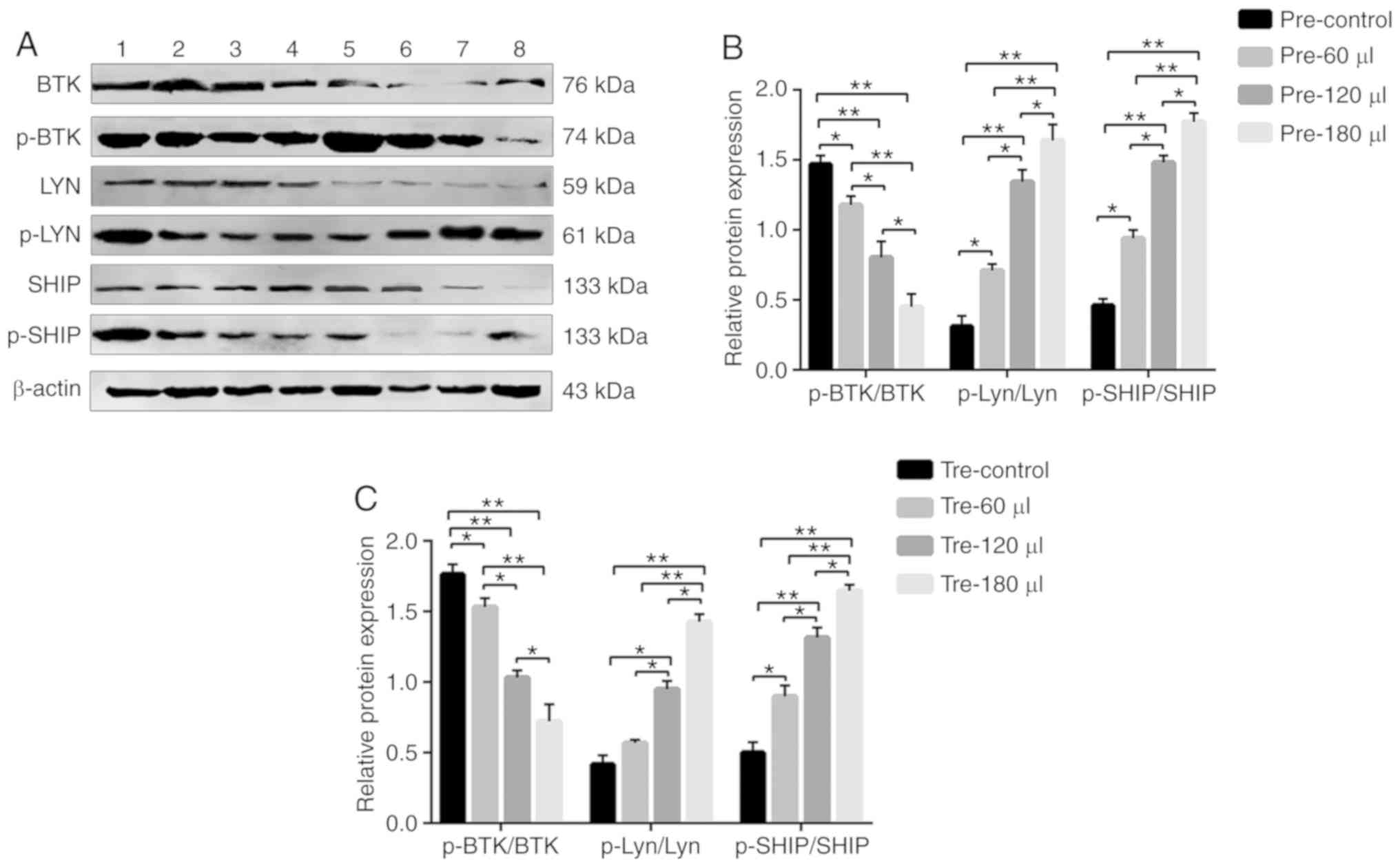 | Figure 9(A) The protein expression levels of
BTK, p-BTK, Lyn, p-Lyn, SHIP and p-SHIP in MRL/lpr SLE mouse B
cells were examined by western blot analysis, with β-actin as a
loading control. (B and C) The phosphorylation and total protein
levels of BTK, Lyn and SHIP were detected in B cells from MRL/lpr
SLE mice after the infection of sFcγRIIB lentivirus, and the
relative level for each phosphorylation-protein/each respective
total protein was calculated. Lane 1: pre-control, control subgroup
in the prevention group; lane 2: pre-60 µl, 4.8 µg
sFcγRIIB subgroup in the prevention group; lane 3: pre-120
µl, 9.6 µg sFcγRIIB subgroup in the prevention group;
lane 4: pre-180 µl, 14.4 µg sFcγRIIB subgroup in the
prevention group; lane 5: tre-control, control subgroup in the
treatment group; lane 6: tre-60 µl, 4.8 µg sFcγRIIB
subgroup in the treatment group; lane 7: tre-120 µl, 9.6
µg sFcγRIIB subgroup in the treatment group; lane 8: tre-180
µl, 14.4 µg sFcγRIIB subgroup in the treatment group.
*P<0.05; **P<0.01; n=10 in each group.
SLE, systemic lupus erythematosus; mFcγRIIB, membrane-bound type
FcγRIIB; sFcγRIIB, soluble FcγRIIB; BTK, Bruton's tyrosine kinase;
Lyn, Lyn proto-oncogene, Src family tyrosine kinase; DOK-1, docking
protein 1; SHIP, inositol polyphosphate-5-phosphatase D. |
Discussion
FcγRIIB, an inhibitory receptor in the FcγR family
(18), is composed of an
extracellular domain containing two Ig-like structures and an
intracellular region containing an ITIM motif (19,20). The extracellular region is an Fc
region with a low affinity for binding all IgG subclasses and
exists as a membrane-bound type and insoluble form. sFcγRIIB is
produced by the hydrolysis of the mFcγRIIB extracellular region
stimulated by antigens and cytokines or is produced by FcγRIIB gene
splicing (21,22). In the present study, in order to
examine the roles of mFcγRIIB and sFcγRIIB in SLE, patients with
SLE and MRL/lpr SLE mouse models were utilized.
The serum of patients with SLE contains a large
number of anti-nuclear antibodies and IgG type CICs (23), and research has indicated that
FcγRIIB can be crosslinked to BCR or activated FcR by IC to inhibit
the activation and antibody secretion of B cells (24,25). Therefore, in the present study,
ctDNA and anti-ctDNA-IC were used to stimulate B lymphocytes from
patients with SLE and healthy subjects following mFcγRIIB
lentivirus transfection. It was found that ctDNA stimulation
reduced B lymphocyte IgG antibody secretion following mFcγRIIB
lentivirus transfection, and exerted a more prominent effect on SLE
B cells than on normal B cells. Furthermore, when the added IC
reached a certain level, it directly inhibited the secretion of IgG
antibody by B cells. Therefore, B cell-derived FcγRIIB is involved
in preventing the in vivo production of autoreactive
antibodies, providing a peripheral checkpoint for maintaining
normal self-tolerance. The combination of FcγRIIB and IC provides
an inhibitory feedback loop for B lymphocytes, thus maintaining the
dynamic balance of antibody expression.
Following lentivirus vector transfection into
MRr/lpr mice, the expression level of mFcγRIIB was increased in a
dose-dependent manner with the lentiviral vector. In the presence
of the DOX inducer, the transcriptional expression of the target
gene was induced by the combining of expression product of
regulatory plasmid with rtTA of the response plasmid. Therefore,
the concentration of DOX inducer directly affects the transcription
and expression levels of target genes (26). The levels of anti-nuclear
antibodies, urinary albumin and anti-dsDNA in MRr/lpr mice were
downregulated after the injection of virus containing Dox, due to
the increase in mFcγRIIB which decreased the B cell secretion of
antibodies. These results could deactivate signal transduction
proteins by dephosphorylation to terminate BCR activation signal
transduction, inhibiting the activation, proliferation,
differentiation and antibody production of B cells (27).
The FcγRIIB signaling pathway is closely related to
IgG antibody secretion in B cells. It mainly inhibits cell
activation and proliferation through two signaling pathways
(28). Pauls and Marshall
(29) and Wang et al
(30) found that FcγRIIB
activated tyrosine kinase (Lyn) by crosslinking with BCR through
IC, causing the tyrosine phosphorylation of ITIM to recruit
molecules, such as SHIP (inositol phosphatase). SHIP acts on
3,4,5-phosphatidylinositol triphosphate to hydrolyze it to
3,4-phosphatidylinositol diphosphate. 3,4-Phosphatidylinositol
diphosphate prevents PIP3 from recruiting BTK and phospholipase cγ2
(PLCγ2) onto cell membranes following dephosphorylation, thereby
reducing the intracellular calcium levels, which finally inhibits
cell activation involving related kinases to reduce antibody
production. ITIM transduction can inhibit cell proliferation by the
activation of DOK1 and MAP kinase instead of SHIP (29,30). High concentrations of FcγRIIB lead
to the direct apoptosis of B cells by crosslinking with BCR to
increase the phosphorylation of Lyn and SHIP1, and the simultaneous
phosphorylation of BTK, independent of the ITIM pathway.
Corneth et al found that the cytoplasmic
regions of human and mouse FcγRIIB proteins contained ITIM motifs
(28). This highly conserved 13
amino acid sequence encoded by the C3 exon contains a common
inhibitory sequence, I/VxYxL, which can prevent the ITIM motif from
transmitting activation signaling by cross-linking BCR, FcγRI, and
FcγRIII. When FcγRIIB crosslinks other activation receptors
containing ITIM, ITIM can inhibit cell activation and proliferation
by activating Lyn to recruit SHIP1 or by activating DOK1 without
SHIP recruitment (28).
It has been reported that recombinant human sFcγRIIB
inhibited SLE membrane-type IC-mediated tissue damage by
interfering with the in vitro binding of IC to B cells
(31,32). Recombinant human sFcγRIIB plays a
role by competitively binding IgG sites with mFcγRIIB in
vivo; however, the mechanisms responsible for this blocking
effect have not been extensively investigated (33).
Recombinant human sFcγRIIB inhibits the in
vitro secretion of IgG-type antibodies by B cells. However, the
mechanisms involved remain unclear. In the present study, following
the stimulation of B cells from patients with SLE with recombinant
human sFcγRIIB to secrete IgG-type antibodies, BTK, Lyn, SHIP and
DOK1 were detected downstream of the sFcγRIIB and BCR signal
transduction pathways. As a result, the phosphorylation levels of
Lyn, SHIP and DOK1 were increased, while the phosphorylation level
of BTK was decreased, consistent with those mediated by mFcγRIIB
signal transduction, which proved that recombinant human sFcγRIIB
binds to mFcγRIIB by bridging IgG type anti-sFcγRIIB autoantibody,
and then initiated a series of signal transduction pathways.
Inhibitory signals produced by human sFcγRIIB antagonizes
activation signals produced by BCR, which eventually leads to B
cell inhibition.
Recombinant human sFcγRIIB has been shown to
alleviate the symptoms of proteinuria and weight loss in NZB/NZW F1
SLE model mice induced by IC, and to improve the survival rate of
model mice with prophylactic application (34). However, this previous study did
not explore the detailed mechanisms of sFcγRIIB alleviating SLE.
After injecting human sFcγRIIB into each group of model mice, it
was observed that the serum sFcγRIIB levels in the prevention and
treatment groups increased with the increased dose of injected
sFcγRIIB, and the sFcγRIIB level in the prevention group was higher
than that in the treatment group. Simultaneously, the titers of
anti-dsDNA antibodies and the urinary protein in the 2 groups
decreased. Recombinant human sFcγRIIB inhibited the in vivo
B cell secretion of IgG antibody. Of note, it was found in the
present study that the phosphorylation levels of Lyn and SHIP were
increased significantly with an increase in the sFcγRIIB dose
injected into SLE model mice, which may have inhibited the
downstream function of ITIM signal regulation and the release of
calcium ions through the BCR signal transduction pathway (35). The phosphorylation levels of BTK
in the prevention and treatment groups were significantly decreased
with the increase in exogenous sFcγRIIB, indicating that the
phosphorylation of BTK was not blocked when sFcγRIIB was deficient,
while the phosphorylation of BTK was inhibited when sFcγRIIB was
restored or enhanced. Therefore, it was surmised that sFcγRIIB
inhibited the activation of B cells by inhibiting the activation of
BCR pathway.
In conclusion, the present study demonstrated that
recombinant FcγRIIB inhibited B cell IgG antibody secretion by
altering the phosphorylation levels of downstream proteins involved
in the FcγRIIB signaling pathway. Simultaneously, the transduction
of mFcγRIIB lentivirus into MRr/lpr SLE mice further improved the
symptoms of SLE mice, suggesting that FcγRIIB may play a role in
improving the activation, proliferation and antibody secretion of B
cells, providing a new approach and method for the prevention and
treatment of SLE patients. However, further experiments are
required to address the conclusion on how FcγRIIB regulates the
phosphorylation of downstream proteins of FcγRIIB signaling
pathway, suppresses B cell activation to ameliorate systemic lupus
erythematosus, and promotes the apoptosis of B cells.
Funding
The present study was funded by the Ningxia High
School First-Class Disciplines (West China First-Class Disciplines
Basic Medical Sciences at Ningxia Medical University, no.
NXYLXK2017B07), the National Natural Sciencem Foundation of China
(no. 81160375) and the Ningxia Medical University Scientific
Research Project (XY201725).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LS and XC performed the experiments, analyzed the
data and wrote the manuscript. SC, JW, HX and HL performed the
experiments and collected the data. ZY conceived and designed the
study, obtained the funding and revised the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved the Ethics Committee
of Ningxia Medical University. All subjects signed informed consent
forms prior to their inclusion in the study. The animal experiments
were approved by the Animal Ethics Committee of Ningxia Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
SLE
|
systemic lupus erythematosus
|
|
dsDNA
|
double-stranded DNA
|
|
RNP
|
ribonucleoproteins
|
|
CICs
|
circulating immune complexes
|
|
ctDNA
|
calf thymus DNA
|
|
ICs
|
immune complexes
|
|
mFcγRIIB
|
membrane-bound type FcγRIIB
|
|
sFcγRIIB
|
soluble FcγRIIB
|
|
BTK
|
Bruton's tyrosine kinase
|
|
Lyn
|
Lyn proto-oncogene, Src family
tyrosine kinase
|
|
DOK1
|
docking protein 1
|
|
SHIP
|
inositol polyphosphate-5-phosphatase
D
|
|
BCR
|
B cell receptor
|
References
|
1
|
Das CM, Bezerra MC, Braga FN, da Justa
Feijão MR, Gois AC, Rebouças VC, de Carvalho TM, Carvalho LN and
Ribeiro ÁM: Clinical and immunological aspects and outcome of a
Brazilian cohort of 414 patients with systemic lupus erythematosus
(SLE): Comparison between childhood-onset, adult-onset, and
late-onset sle. Lupus. 25:355–363. 2016. View Article : Google Scholar
|
|
2
|
Pamuk ON, Balci MA, Donmez S and Tsokos
GC: The incidence and prevalence of systemic lupus erythematosus in
Thrace, 2003-2014: A 12-year epidemiological study. Lupus.
25:102–109. 2016. View Article : Google Scholar
|
|
3
|
Dema B and Charles N: Advances in
mechanisms of systemic lupus erythematosus. Discov Med. 17:247–255.
2014.PubMed/NCBI
|
|
4
|
Park MJ, Kwok SK, Lee SH, Kim EK, Park SH
and Cho ML: Adipose tissue-derived mesenchymal stem cells induce
expansion of interleukin-10-producing regulatory B cells and
ameliorate autoimmunity in a murine model of systemic lupus
erythematosus. Cell Transplant. 24:2367–2377. 2015. View Article : Google Scholar
|
|
5
|
Park MJ, Lee SH, Kim EK, Lee EJ, Park SH,
Kwok SK and Cho ML: Myeloid-Derived suppressor cells induce the
expan-sion of regulatory B cells and ameliorate autoimmunity in the
sanroque mouse model of systemic lupus erythematosus. Arthritis
Rheumatol. 68:2717–2727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGaha TL, Ma Z, Ravishankar B, Gabunia K,
McMenamin M and Madaio MP: Heterologous protein incites abnormal
plasma cell accumulation and autoimmunity in MRL-MpJ mice.
Autoimmunity. 45:279–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taher TE, Bystrom J, Ong VH, Isenberg DA,
Renaudineau Y, Abraham DJ and Mageed RA: Intracellular B lymphocyte
signal-ling and the regulation of humoral immunity and
autoimmunity. Clin Rev Allergy Immunol. 53:237–264. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sondermann P and Oosthuizen V: The
structure of Fc receptor/Ig complexes: Considerations on
stoichiometry and potential inhibitors. Immunol Lett. 82:51–56.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Li G, Wang J, Fan Y, Wan Z, Zhang S,
Shaheen S, Li J, Wang L, Yue C, et al: Through an ITIM-independent
mechanism the FcγRIIB blocks B cell activation by disrupting the
colocalized microclustering of the B cell receptor and CD19. J
Immunol. 192:5179–5191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pauls SD, Ray A, Hou S, Vaughan AT, Cragg
MS and Marshall AJ: FcγRIIB-Independent mechanisms controlling
membrane localization of the inhibitory phosphatase SHIP in human B
cells. J Immunol. 197:1587–1596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lesourne R, Bruhns P, Fridman WH and
Daëron M: Insufficient phosphorylation prevents fc gamma RIIB from
recruiting the SH2 domain-containing protein-tyrosine phosphatase
SHP-1. J Biol Chem. 276:6327–6336. 2001. View Article : Google Scholar
|
|
12
|
Tackenberg B, Nimmerjahn F and Lunemann
JD: Mechanisms of IVIG efficacy in chronic inflammatory
demyelinating poly-neuropathy. J Clin Immunol. 30:S65–S69. 2010.
View Article : Google Scholar
|
|
13
|
Chu SY, Yeter K, Kotha R, Pong E, Miranda
Y, Phung S, Chen H, Lee SH, Leung I, Bonzon C, et al: Suppression
of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody
that coengages B cell antigen receptor complex and Fcγ receptor IIb
inhibitory receptor. Arthritis Rheumatol. 66:1153–1164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gesheva V, Kerekov N, Nikolova K,
Mihaylova N, Todorov T, Nikolova M and Tchorbanov A: Suppression of
dsDNA-specific B lymphocytes reduces disease symptoms in SCID model
of mouse lupus. Autoimmunity. 47:162–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soni C, Domeier PP, Wong EB, Shwetank,
Khan TN, Elias MJ, Schell SL, Lukacher AE, Cooper TK and Rahman ZS:
Distinct and synergistic roles of FcγRIIB deficiency and 129
strain-derived SLAM family proteins in the development of
spontaneous germinal centers and autoimmunity. J Autoimmun.
63:31–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alegria-Schaffer A, Lodge A and Vattem K:
Performing and optimizing western blots with an emphasis on
chemiluminescent detection. Methods Enzymol. 463:573–599. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nossent HC and Rekvig OP: Is closer
linkage between systemic lupus erythematosus and
anti-double-stranded DNA antibodies a desirable and attainable
goal? Arthritis Res Ther. 7:85–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sondermann P: The FcγR/IgG interaction as
target for the treatment of autoimmune diseases. J Clin Immuno.
36:95–99. 2016. View Article : Google Scholar
|
|
19
|
Wang J, Li Z, Xu L, Yang H and Liu W:
Transmembrane domain dependent inhibitory function of FcγRIIB.
Protein Cell. 9:1004–1012. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roghanian A, Stopforth RJ, Dahal LN and
Cragg MS: New revelations from an old receptor: Immunoregulatory
functions of the inhibitory Fc gamma receptor, FcγRIIb (CD32B). J
Leukoc Biol. 6:10022018.
|
|
21
|
Zhang L, Cao XQ and Yang ZW: Preparation
and function of human soluble FcγRIIb. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 28:952–925. 2012.In Chinese. PubMed/NCBI
|
|
22
|
Magnusson SE, Andren M, Nilsson KE,
Sondermann P, Jacob U and Kleinau S: Amelioration of
collagen-induced arthritis by human recombinant soluble
FcgammaRIIb. Clin Immunol. 127:225–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Magro-Checa C, Schaarenburg RA, Beaart HJ,
Huizinga TW, Steup-Beekman GM and Trouw LA: Complement levels and
anti-C1q autoantibodies in patients with neuropsychiatric systemic
lupus erythematosus. Lupus. 25:878–888. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun JB, Xiang Z, Smith KG and Holmgren J:
Important role for FcγRIIB on B lymphocytes for mucosal
antigen-induced tolerance and Foxp3+ regulatory T cells. J Immunol.
191:4412–4422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daëron M and Lesourne R: Negative
signaling in Fc receptor complexes. Adv Immunol. 89:39–86. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong KE, Mora MC, Skinner M, Page SM,
Crisi GM, Arenas RB, Schneider SS and Emrick T: Evaluation of
PolyMPC-dox prodrugs in a human ovarian tumor model. Mol Pharm.
13:1679–1687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vásquez A, Baena A, González LA, Restrepo
M, Muñoz CH, Vanegas-García A, Ortiz-Reyes B, Abdoel N, Rojas M,
García LF and Vásquez G: Altered recruitment of lyn, syk and ZAP-70
into lipid rafts of activated B cells in systemic lupus
erythematosus. Cell Signal. 58:9–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corneth OB, Wolternil RG and Hendriks RW:
BTK signaling in B cell differentiation and autoimmunity. Curr Top
Microbiol Immunol. 393:67–105. 2016.
|
|
29
|
Pauls SD and Marshall AJ: Regulation of
immune cell signaling by SHIP1: A phosphatase, scaffold protein,
and potential therapeutic target. Eur J Immunol. 47:932–945. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Q, Vogan EM, Nocka LM, Rosen CE, Zorn
JA, Harrison SC and Kuriyan J: Autoinhibition of bruton's tyrosine
kinase (Btk) and activation by soluble inositol hexakisphosphate.
Elife. 4:e060742015. View Article : Google Scholar :
|
|
31
|
Akiyama M, Suzuki K, Yamaoka K, Yasuoka H,
Takeshita M, Kaneko Y, Kondo H, Kassai Y, Miyazaki T, Morita R, et
al: Number of circulating follicular helper 2 T cells correlates
with IgG4 and interleukin-4 levels and plasmablast numbers in
IgG4-related disease. Arthritis Rheumatol. 67:2476–2481. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Awe O, Hufford MM, Wu H, Pham D, Chang HC,
Jabeen R, Dent AL and Kaplan MH: Expression in T follicular helper
cells limits CD40L-dependent germinal center B cell development. J
Immunol. 195:3705–3715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwata S and Tanaka Y: The importance of B
cell-T cell inter-action in autoimmune diseases. Nihon Rinsho
Meneki Gakkai Kaishi. 38:398–402. 2015.In Japanese. View Article : Google Scholar
|
|
34
|
Zhang M, Yu G, Chan B, Pearson JT,
Rathanaswami P, Delaney J, Lim AC, Babcook J, Hsu H and Gavin MA:
Interleukin-21 receptor blockade inhibits secondary humoral
responses and halts the progression of preestablished disease in
the (NZB x NZW)F1 systemic lupus erythematosus model. Arthritis
Rheumatol. 67:2723–2731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seda V and Mraz M: B-Cell receptor
signalling and its cross-talk with other pathways in normal and
malignant cells. Eur J Haematol. 94:193–205. 2015. View Article : Google Scholar
|