Introduction
More than 350,000 individuals are diagnosed with
oral cancer annually, and oral cancer will ultimately prove fatal
in almost half of those diagnosed with the disease (1). Of the defined histological types of
oral cancer, >90% of patients are diag-nosed with oral squamous
cell carcinoma (OSCC), which typically arises on the lips or within
the oral cavity (2). The most
effective treatments currently available for OSCC depend on its
clinical stage at presentation. Although stage-I and -II OSCCs are
treated with surgery or radiotherapy, advanced stage-III and -IV
disease is treated with a combination of surgery, radiotherapy and
chemotherapy (3).
Chemotherapeutic regimens typically include cisplatin as a
first-line agent; it is often combined with docetaxel or
5-fluorouracil (4,5). Paclitaxel, methotrexate and
carboplatin can be also used in the treatment of OSCCs (6); however, there is only limited
information available on the efficacy of molecular targeting drugs
and/or antibody-based therapies for OSCC.
The epidermal growth factor receptor (EGFR) is a
member of the human epidermal growth factor receptor (HER) family
of receptor tyrosine kinases, and is involved in cell growth and
differentiation (7-9). EGFR forms homo- or heterodimers with
other HER family members, such as HER2 and HER3, and thereby
activate downstream signaling cascades. These pathways are
frequently dysregulated in malignant diseases, including OSCC,
often via the overexpression of EGFR (10). Nimotuzumab is a humanized
monoclonal antibody (mAb) directed against the extracellular domain
of the EGFR that has been shown to have clinical efficacy in
various types of cancer (11).
Although nimotuzumab has been approved in 29 countries for use in
the treatment of advanced head and neck carcinoma, esophageal
cancer, nasopharyngeal carcinoma and pancreatic cancer, only modest
success has been achieved with respect to the treatment of
recurrent and/or metastatic OSCC (12). Although a number of EGFR-targeted
therapies have been used in patients with OSCC, treatment failures
due to the low response rates and acquired resistance have been
reported (13).
In a previous study by the authors, mice were
immunized with purified recombinant EGFR, and successfully produced
monoclonal EMab-134 (mouse IgG1, kappa). This antibody
detected endogenous EGFR in oral cancers in applications including
flow cytometry, western blot analysis and immunohistochemistry
(14). For example, when used in
immunohistochemical analysis, EMab-134 reacted with its target
antigen in 36 of 38 (94.7%) oral cancer specimens. The minimum
epitope of EMab-134 was determined to be
377-RGDSFTHTPP-386 (15). Although EMab-134 has proven to be
very useful for the detection of EGFR, the mouse IgG1
subclass does not facilitate antibody-dependent cellular
cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC)
activities.
To address this issue, in the present study,
EMab-134 (IgG1 subclass) was converted into
134-mG2a of the mouse IgG2a subclass. It was
then determined whether 134-mG2a exhibits ADCC, CDC, and
in vivo antitumor activities against OSCCs.
Materials and methods
Antibodies
Anti-EGFR mAb EMab-134 (mouse IgG1,
kappa) was developed as previously described (14). To generate 134-mG2a,
VH cDNA of EMab-134 and CH mouse
IgG2a were subcloned into pCAG-Ble vector, and
VL and CL cDNAs of EMab-134 were subcloned
into pCAG-Neo vector (FUJIFILM Wako Pure Chemical Corporation),
respectively. Vectors were transfected into ExpiCHO-S cells using
the ExpiCHO Expression System (Thermo Fisher Scientific, Inc.). The
resulting mAb, 134-mG2a, was purified with Protein
G-Sepharose (GE Healthcare Bio-Sciences). Mouse IgG (cat. no.
I8765), IgG1 (cat. no. M7894), and IgG2a
(cat. no. M7769) were purchased from Sigma-Aldrich; Merck KGaA.
Cell lines
The CHO-K1 cell line was obtained from the American
Type Culture Collection (ATCC). Human EGFR-expressing CHO-K1 cells
(CHO/EGFR) were previously established by the transfection of
pCAG/PA-EGFR-RAP-MAP into CHO-K1 cells using Lipofectamine LTX
(Thermo Fisher Scientific, Inc.) (16). The amino acid sequences of each
tag were as follows: PA tag (17), 12 amino acids (GVAMPGAEDDVV); RAP
tag (18), 12 amino acids
(DMVNPGLEDRIE); and MAP tag (19), 12 amino acids (GDGMVPPGIEDK). OSCC
cell lines, including HSC-2 (oral cavity) and SAS (tongue) were
obtained from the Japanese Collection of Research Bioresources Cell
Bank (JCRB). CHO-K1 and CHO/EGFR were cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium (Nacalai Tesque, Inc.). The
HSC-2 and SAS cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Nacalai Tesque, Inc.). Cell culture medium was
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.), 100 units/ml of penicillin, 100
µg/ml streptomycin, and 0.25 µg/ml amphotericin B
(Nacalai Tesque, Inc.). Cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2.
Animals
All animal experiments were performed in accordance
with relevant guidelines and regulations to minimize animal
suffering and distress in the laboratory. Animal experiments for
ADCC and antitumor activity were approved by the Institutional
Committee for Experiments of the Institute of Microbial Chemistry
(Permit. no. 2019-049 for ADCC assays, 2019-046 for antitumor
experiments). Mice were maintained in a pathogen-free environment
(23±2°C, 55±5% humidity) on an 11-h light/13-h dark cycle with food
and water supplied ad libitum across the experimental
period. Mice were monitored for health and weight every 2 or 5 days
during the 3-week period of each experiment. The loss of original
body weight to a point >25% and/or a maximum tumor size
>3,000 mm3 were identified as humane endpoints for
euthanasia. Mice were euthanized by cervical dislocation; death was
verified by respiratory and cardiac arrest.
Flow cytometry
Cells were harvested by brief exposure to 0.25%
trypsin/1 mM ethylenediamine tetra acetic acid (EDTA, Nacalai
Tesque, Inc.). After washing with 0.1% bovine serum albumin in
phosphate-buffered saline (PBS), cells were treated with 1
µg/ml of anti-EGFR mAbs for 30 min at 4°C followed by Alexa
Fluor 488-conjugated anti-mouse IgG at a dilution of 1:1,000 (cat.
no. 4408S; Cell Signaling Technology, Inc.) for 30 min at 4°C.
Fluorescence data were collected using an SA3800 Cell Analyzer
(Sony Corp.).
Western blot analyses
Cell pellets were suspended using lysis buffer (1%
Triton X-100 and 50 µg/ml aprotinin in PBS) on ice for 15
min. Following centrifugation (20,630 × g, 15 min, 4°C), cell
lysates were boiled in sodium dodecyl sulfate sample buffer
(Nacalai Tesque, Inc.). The samples were electrophoresed on 5-20%
polyacrylamide gels (Nacalai Tesque, Inc.) and transferred onto
polyvinylidene difluoride (PVDF) membranes (Merck KGaA). After
blocking with 4% skim milk (Nacalai Tesque, Inc.) for 1 h, the
membranes were incubated with anti-EGFR mAbs or anti-β-actin (1
µg/ml) for 1 h, followed by incubation with HRP-conjugated
anti-mouse immunoglobulins at a 1:2,000 dilution (Agilent
Technologies, Inc.) for 1 h. The membranes were developed with the
ImmunoStar LD Chemiluminescence Reagent (FUJIFILM Wako Pure
Chemical Corporation) using the Sayaca-Imager (DRC Co., Ltd.). All
western blot analysis procedures were performed at room
temperature.
Immunohistochemical analyses
Histological sections (4-µm-thick) of an oral
cancer tissue array (cat. no. OR601c; US Biomax, Inc.) were
directly autoclaved in EnVision FLEX Target Retrieval Solution High
pH (Agilent Technologies, Inc.) for 20 min. The sections were then
incubated with 5 µg/ml anti-EGFR mAbs for 1 h at room
temperature and treated using an Envision+ kit (Agilent
Technologies, Inc.) for 30 min. Color was developed using
3,3′-diaminobenzidine tetrahydrochloride (DAB; Agilent
Technologies, Inc.) for 2 min, and the sections were then
counterstained with hematoxylin (FUJIFILM Wako Pure Chemical
Corporation). Hematoxylin and eosin (H&E) staining was
performed using consecutive tissue sections as follows: Hematoxylin
staining (FUJIFILM Wako Pure Chemical Corporation) for 5 min and
eosin staining (FUJIFILM Wako Pure Chemical Corporation) for 2 min
at room temperature. Leica DMD108 (Leica Microsystems GmbH) was
used to examine the sections and obtain images.
Determination of the binding
affinity
The cells were suspended in 100 µl of
serially diluted anti-EGFR mAbs (0.6-10 µg/ml) followed by
the addition of Alexa Fluor 488-conjugated anti-mouse IgG (1:1,000;
Cell Signaling Technology, Inc.). Fluorescence data were collected
using an EC800 Cell Analyzer (Sony Corp.). The dissociation
constant (KD) was calculated by fitting binding
isotherms to built-in one-site binding models in GraphPad Prism 7
(GraphPad Software, Inc.).
ADCC
A total of 6 female 6-week-old BALB/c nude mice
(weighing 15-18 g) were purchased from Charles River Laboratories,
Inc. Spleen cells from 6 mice were used as the source of natural
killer (NK) cells for the evaluation of ADCC, which has been
reported previously (20).
Following euthanasia by cervical dislocation, the spleens were
removed aseptically and single-cell suspensions were obtained by
forcing spleen tissues through a sterile cell strainer (352360, BD
Falcon, Corning, Inc.) using a syringe. Erythrocytes were lysed
with a 10-sec exposure to ice-cold distilled water. Splenocytes
were washed with DMEM and resuspended in DMEM with 10% FBS; this
preparation was used as effector cells. Target tumor cells were
labeled with 10 µg/ml Calcein AM (Thermo Fisher Scientific,
Inc.) and resuspended in the same medium. The target cells
(2×104 cells/well) were plated in 96-well plates and
mixed with effector cells (effector/target cell ratio, 50), 100
µg/ml of anti-EGFR antibodies or control IgGs. Following a
4-h incubation at 37°C, the release of Calcein AM into the
supernatant was measured in each well. The fluorescence intensity
was determined using a microplate reader (Power Scan HT; BioTek
Instruments, Inc.) with an excitation wave-length of 485 nm and an
emission wavelength of 538 nm. Cytolytic activity (% lysis) was
calculated using the equation % lysis=(E-S)/(M-S) ×100, where 'E'
is the fluorescence measured in combined cultures of target and
effector cells, 'S' is the spontaneous fluorescence of target cells
only, and 'M' is the maximum fluorescence measured following the
lysis of all cells with a buffer containing 0.5% Triton X-100, 10
mM Tris-HCl (pH 7.4) and 10 mM of EDTA.
CDC
The cells (2×104 cells/well) were plated
in 96-well plates and mixed with rabbit complement (final dilution
1:10; Low-Tox-M Rabbit Complement; Cedarlane Laboratories) together
with 100 µg/ml of anti-EGFR or control IgGs. Following 5 h
incubation at 37°C, MTS [3-(4,5-dimethylthi-azol-2-yl)-5-
(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; inner
salt] assay was performed using a CellTiter 96 AQueous assay kit
(Promega Corp.).
Antitumor activity of 134-mG2a
in xenografts of CHO/EGFR cells
A total of 24 female BALB/c nude mice (5 weeks old,
weighing 14-17 g) were purchased from Charles River Laboratories,
Inc. and used in experiments once they reached 7 weeks of age.
CHO/EGFR cells (0.3 ml of 1.33×108 cells/ml in DMEM)
were mixed with 0.5 ml BD Matrigel Matrix Growth Factor Reduced (BD
Biosciences); 100 µl of this suspension (5×106
cells) was injected subcutaneously into the left flanks of the
mice. On day 1 post-inoculation, 100 µg of EMab-134 (n=8),
134-mG2a (n=8), or control mouse IgG (n=8) in 100
µl PBS were injected intraperitoneally. Additional antibody
inoculations were performed on days 7 and 14. At 21 days following
cell implantation, all mice were euthanized by cervical
dislocation; tumor diameters and volumes were determined as
previously described (21).
Antitumor activity of 134-mG2a
in xenografts of oral cancers
A total of 48 female BALB/c nude mice (5 weeks old,
weighing 14-17 g) were purchased from Charles River Laboratories,
Inc. and used in experiments once they reached 7 weeks of age. The
HSC-2 and SAS cells (0.3 ml of 1.33×108 cells/ml in
DMEM) were mixed with 0.5 ml BD Matrigel Matrix Growth Factor
Reduced (BD Biosciences); 100 µl of this suspension
(5×106 cells) was injected subcutaneously into the left
flanks of the mice. On day 1 post-inoculation, 100 µg of
EMab-134 (n=8 in each group), 134-mG2a (n=8 in each
group), or control mouse IgG (n=8 in each group) in 100 µl
PBS were injected intraperitoneally. Additional antibody
inoculations were performed on days 7 and 14. At 18 days following
cell implantation, all mice were euthanized by cervical
dislocation, and tumor diameters and volumes were determined.
Statistical analyses
All data are expressed as the means ± standard error
of the mean (SEM). Statistical analysis was performed using one-way
ANOVA followed by Tukey-Kramer's test using R statistical (R
Foundation for Statistical Computing). A value of P<0.05 was
adopted as a level of statistical significance.
Results
Generation and characterization of
134-mG2a, a mouse IgG2a-type anti-EGFR
antibody
As mouse IgG2a subclass facilitates both
ADCC and CDC (22), in the
present study, a mouse IgG2a version of the
IgG1 EMab-134 (14)
was generated by subcloning VH cDNA of EMab-134 and
CH mouse IgG2a into pCAG-Ble vector, and
VL and CL cDNAs of EMab-134 into pCAG-Neo
vector. The IgG2a version of EMab-134 was named
134-mG2a. The sensitivity of 134-mG2a in
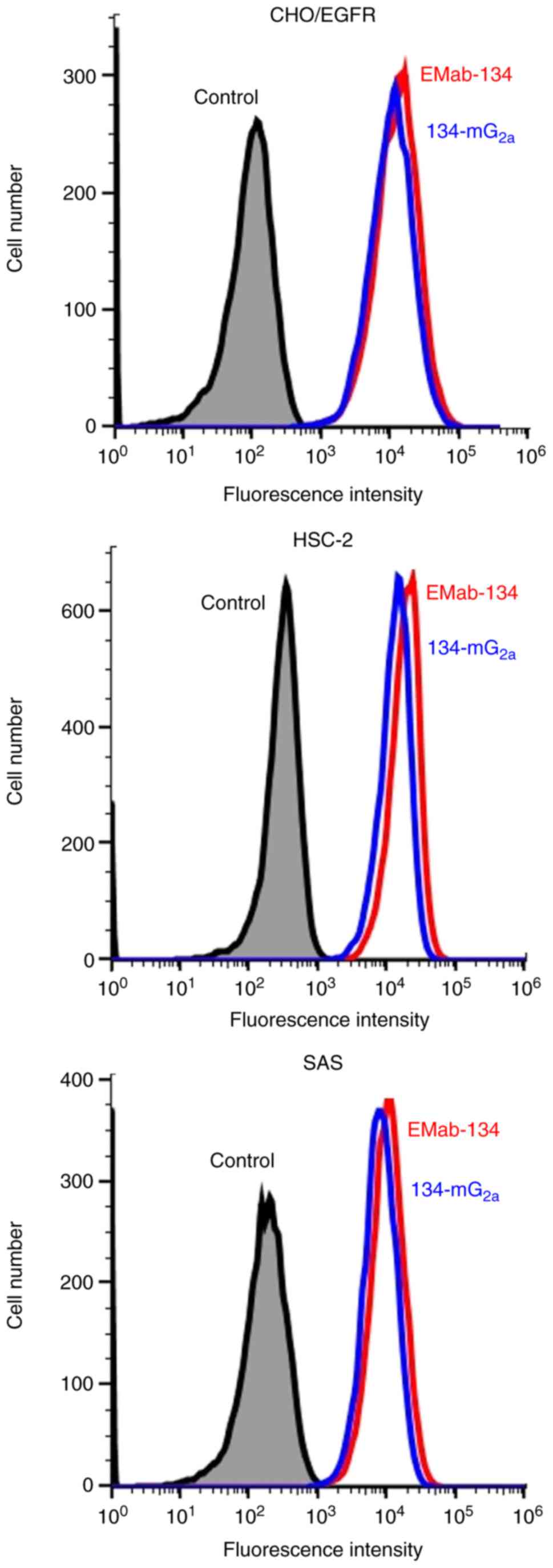
CHO/EGFR, HSC-2 and SAS cells was analyzed by flow cytometry. As
shown in Fig. 1, EMab-134 and
134-mG2a were equally effective at detecting CHO/EGFR,
HSC-2 and SAS cells using this method.
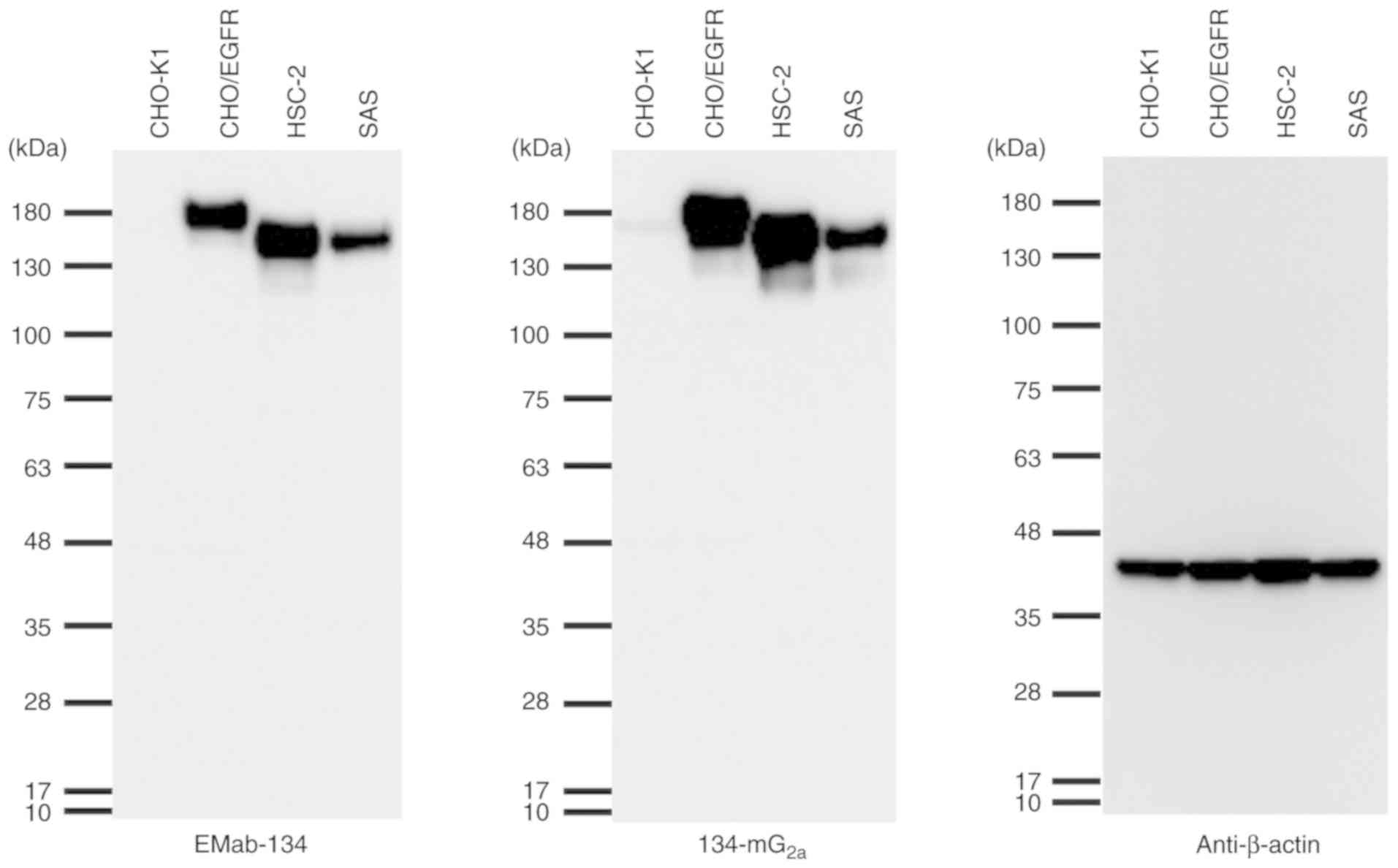
Subsequently, the sensitivities of EMab-134 and
134-mG2a were compared when used to probe lysates of
CHO/EGFR, HSC-2 and SAS cells by western blot analysis. Notably,
134-mG2a exhibited a relatively higher reactivity when
detecting their targets in cell lysates from CHO/EGFR and HSC-2
cells; both antibodies were faintly reactive against targets in SAS
cell lysates (Fig. 2). The
molecular weight of the EGFRs of HSC-2 and SAS cells was smaller
than that expressed in CHO-K1 cells, as PA-EGFR-RAP-MAP, in which 3
peptide tags, such as PA tag, RAP tag, and MAP tag were added, was
transfected into the CHO-K1 cells (16).
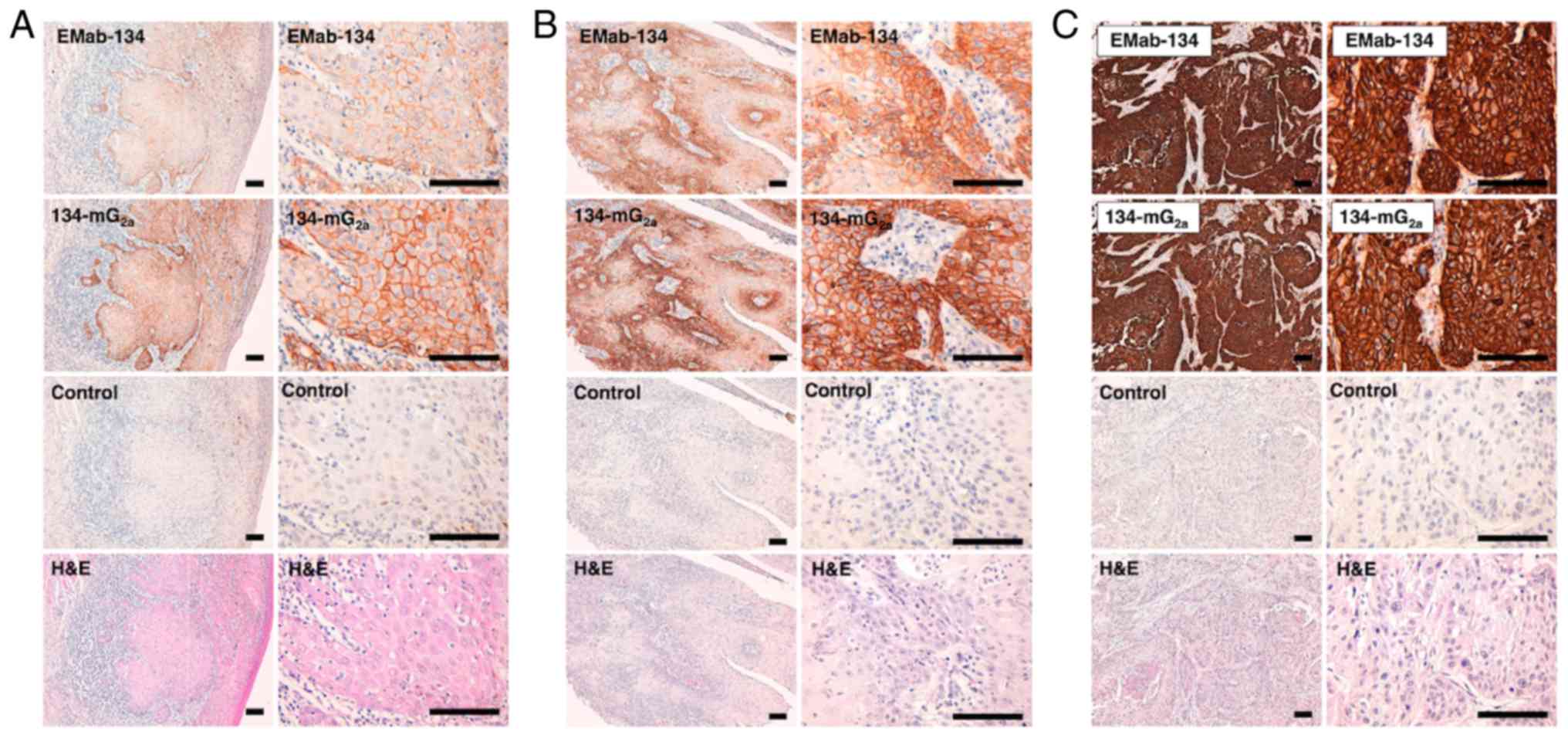
Immunohistochemical analysis revealed that both
EMab-134 and 134-mG2a detected membrane antigens in oral
cancer tissues (Fig. 3).
134-mG2a exhibited higher staining intensities when
compared to the results from EMab-134 in several oral cancer
tissues with various levels of EGFR expression; intensities
included 1+ (Fig. 3A), 2+
(Fig. 3B) and 3+ (Fig. 3C). No staining was observed in
tissues incubated with the buffer control.
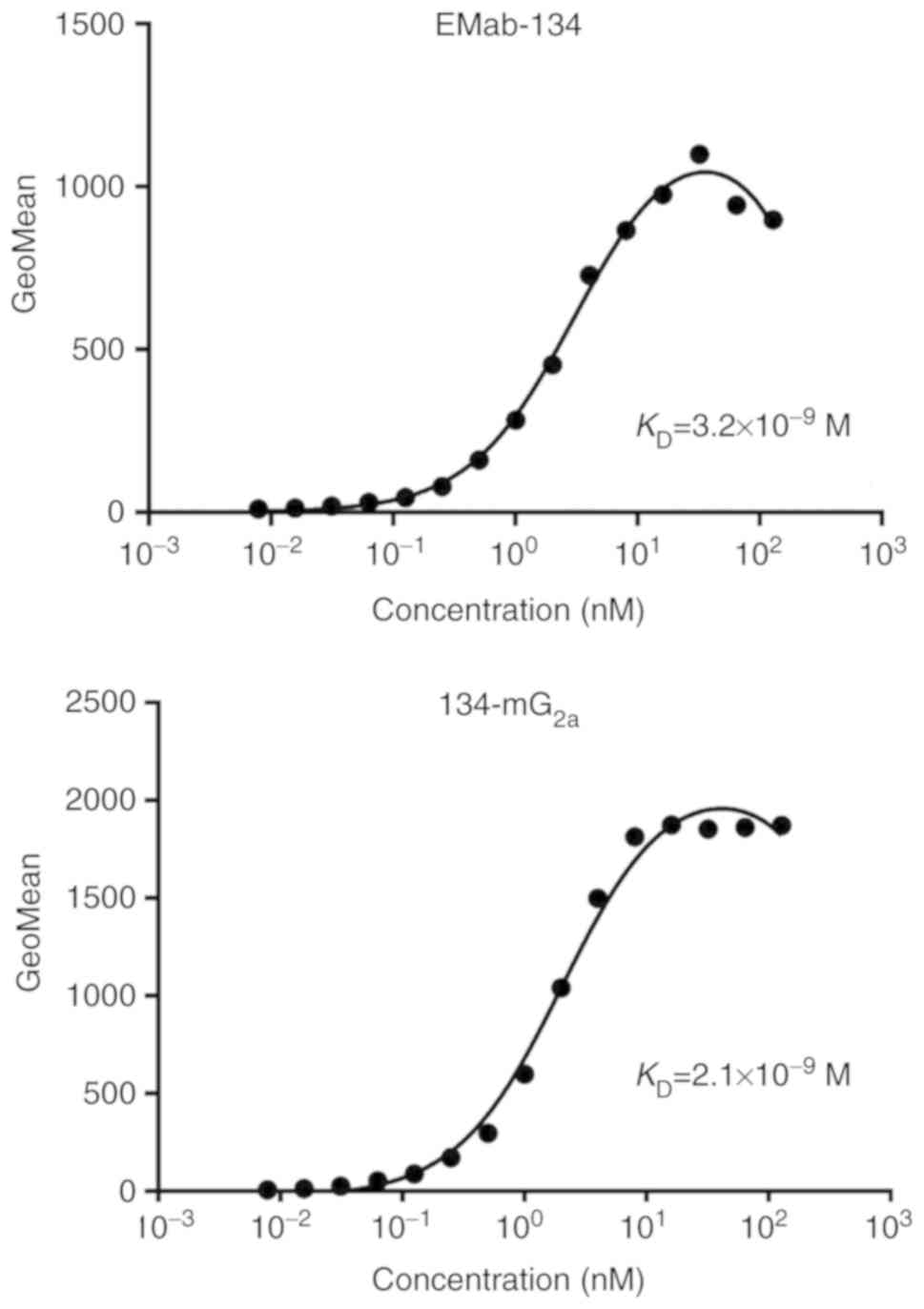
A kinetic analysis of the interactions of EMab-134
and 134-mG2a with CHO/EGFR cells was then performed
using flow cytometry. As shown in Fig. 4, the dissociation constant
(KD) for the interaction of EMab-134 with
CHO/EGFR cells was 3.2×10−9 M. By contrast, the
KD for the interaction of 134-mG2a
with CHO/EGFR cells was 2.1×10−9 M (Fig. 4). The binding affinity of
134-mG2a for CHO/EGFR cells was 1.5-fold higher than
that of EMab-134; taken together with the results from western blot
analysis, this result suggests that the higher binding affinity of
134-mG2a may result in the higher sensitivity observed
in western blot and immunohistochemical analyses.
134-mG2a-mediated ADCC in oral
cancer cell lines
Subsequently, whether the newly-developed
134-mG2a was capable of mediating ADCC against CHO/EGFR
cells or oral cancer cell lines, including HSC-2 and SAS cells was
examined. As shown in Fig. 5A,
134-mG2a elicited ADCC (66% cytotoxicity; P<0.01)
against CHO/EGFR cells more effectively than did control mouse
IgG2a (23% cytotoxicity). By contrast, EMab-134 promoted
no significant ADCC (23% cytotoxicity; n.s.) against CHO/EGFR cells
compared to that observed in response to control mouse
IgG1 (23% cytotoxicity). Similarly, 134-mG2a
elicited ADCC (53% cytotoxicity; P<0.01) against the HSC-2 cells
more effectively than did control mouse IgG2a (13%
cytotoxicity) (Fig. 5B). By
contrast, EMab-134 elicited no significant ADCC (14% cytotoxicity;
n.s.) against the HSC-2 cells compared to that observed in response
to mouse IgG1 control (13% cytotoxicity). Furthermore,
134-mG2a elicited higher ADCC (63% cytotoxicity;
P<0.01) against the SAS cells compared with that elicited by
control mouse IgG2a (32% cytotoxicity; Fig. 5C), while EMab-134 elicited no
significant ADCC (32% cytotoxicity; n.s.) against SAS cells
compared to that observed in response to control mouse
IgG1 (33% cytotoxicity). Taken together, the novel mAb
134-mG2a exhibited significantly higher ADCC for all 3
EGFR-expressing cell lines featured in the present study; by
contrast, no ADCC was observed in response to EMab-134.
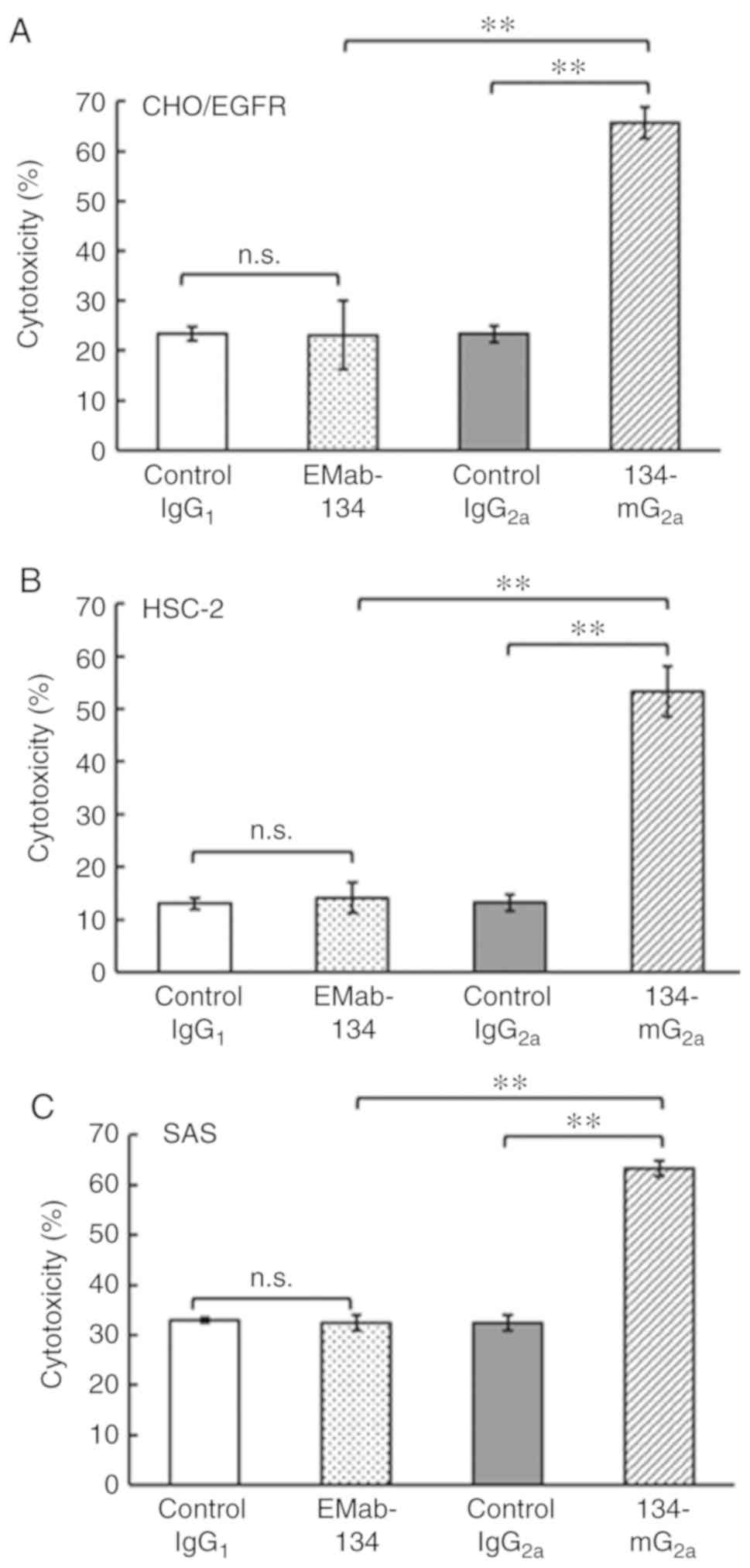 | Figure 5Evaluation of ADCC elicited by
anti-EGFR mAbs. (A) ADCC elicited by EMab-134, 134-mG2a,
control IgG1, or control IgG2a targeting
CHO/EGFR cells. (B) ADCC elicited by EMab-134, 134-mG2a, control
IgG1, or control IgG2a targeting HSC-2 cells. (C) ADCC
elicited by EMab-134, 134-mG2a, control IgG1,
or control mouse IgG2a targeting SAS cells. Values shown
are the means ± SEM. Asterisks indicate statistical significance
(**P<0.01; n.s., not significant; one-way ANOVA and
Tukey-Kramer's test). ADCC, antibody-dependent cellular
cytotoxicity; EGFR, epidermal growth factor receptor; mAbs,
monoclonal antibodies. |
134-mG2a-mediated CDC in oral
cancer cell lines
The present study then examined whether
134-mG2a induces CDC in CHO/EGFR cells or in oral cancer
cell lines, including HSC-2 and SAS cells. As shown in Fig. 6A, 134-mG2a elicited a
higher degree of CDC (46% cytotoxicity; P<0.01) in CHO/EGFR
cells compared with that elicited by control mouse IgG2a
(5.9% cytotoxicity). By contrast, EMab-134 elicited no significant
CDC (11% cytotoxicity; n.s.) against CHO/EGFR cells compared to
that observed in response to control mouse IgG1 (7.4%
cytotoxicity). Similarly, 134-mG2a elicited a higher
degree of CDC (79% cytotoxicity; P<0.01) against HSC-2 cells
compared with that elicited by control mouse IgG2a (19%
cytotoxicity; Fig. 6B). By
contrast, EMab-134 elicited no significant CDC (20% cytotoxicity;
n.s.) against the HSC-2 cells compared to that observed in response
to control mouse IgG1 (19% cytotoxicity). Furthermore,
134-mG2a elicited a higher degree of CDC (60%
cytotoxicity; P<0.01) against SAS cells compared with that
elicited by control mouse IgG2a (15% cytotoxicity;
Fig. 6C). By contrast, EMab-134
elicited no significant CDC (28% cytotoxicity; n.s.) against the
SAS cells compared to that observed in response to control mouse
IgG1 (20% cytotoxicity). Taken together, these results
demonstrated that 134-mG2a promoted significantly higher
levels of CDC against all EGFR-expressing cells evaluated in this
study; by contrast, EMab-134 was not effective in this role. As the
ADCC/CDC activities of 134-mG2a in oral cancer cells
were all potent and effective, this antibody may also exert
antitumor activity against oral cancer cells in vivo.
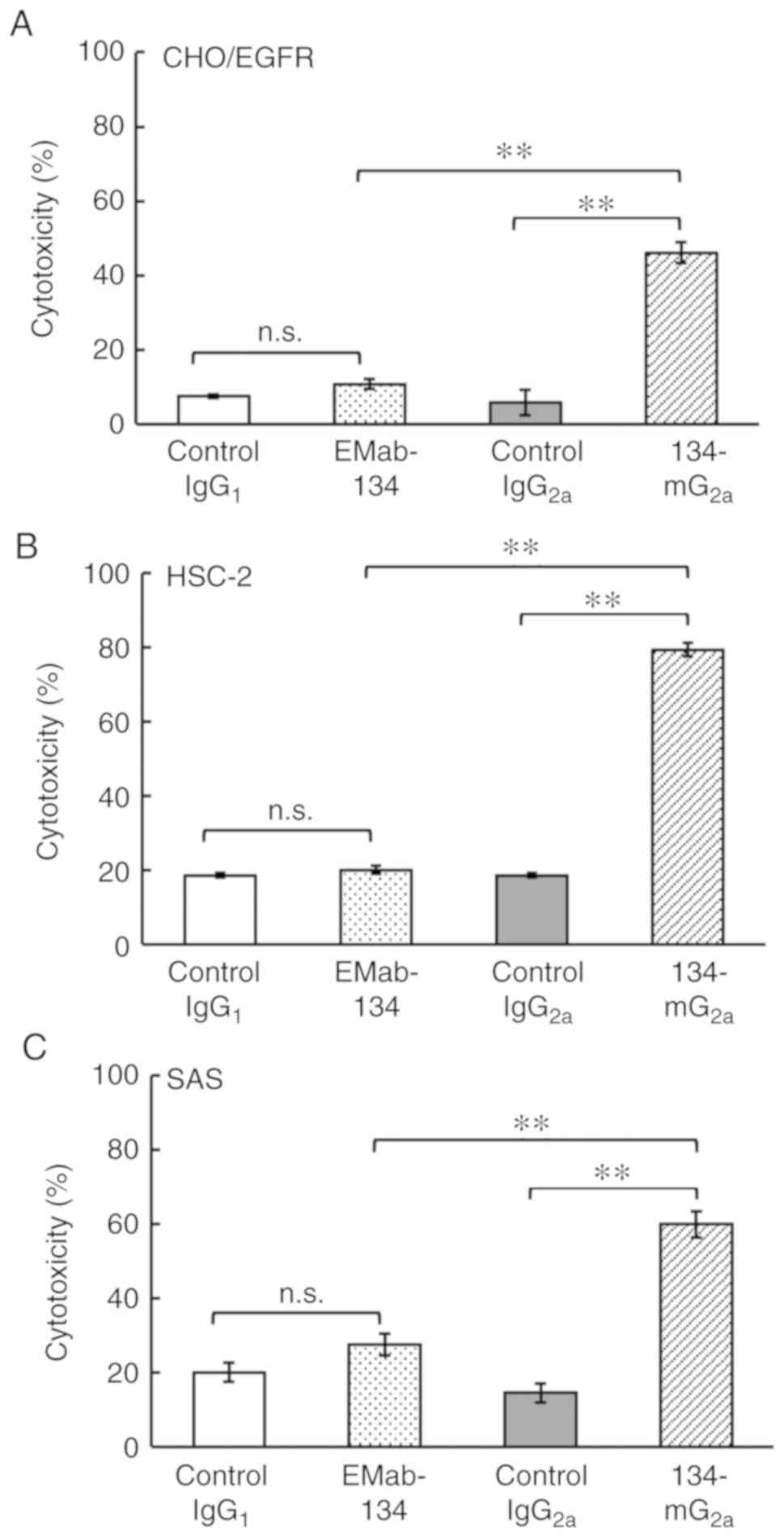 | Figure 6Evaluation of CDC elicited by
anti-EGFR mAbs. (A) CDC elicited by EMab-134, 134-mG2a,
control IgG1, or control IgG2a in target
CHO/EGFR cells. (B) CDC elicited by EMab-134, 134-mG2a,
control IgG1, or control IgG2a in target
HSC-2 cells. (C) CDC elicited by EMab-134, 134-mG2a,
control IgG1, or control IgG2a in target SAS
cells. Values shown are the means ± SEM. Asterisks indicate
statistical significance (**P<0.01; n.s. not
significant; one-way ANOVA and Tukey-Kramer's test). CDC,
complement-dependent cytotoxicity; EGFR, epidermal growth factor
receptor; mAbs, monoclonal antibodies. |
Antitumor activities of
134-mG2a in the mouse xenografts of CHO/EGFR cells
In the CHO/EGFR xenograft models,
134-mG2a (100 µg), EMab-134 (100 µg) and
control mouse IgG (100 µg) were injected intraperitoneally
into the mice on days 1, 7 and 14 following the injection of
CHO/EGFR cells. The tumor volume was measured on days 7, 9, 14, 18
and 21 after the injection. The administration of
134-mG2a resulted in a significant reduction in tumor
development on days 7 (P<0.01), 9 (P<0.01), 14 (P<0.05),
18 (P<0.01) and 21 (P<0.01) compared to the mice treated with
either EMab-134 or control mouse IgG (Fig. 7). No significant differences in
tumor volume were observed in a comparison between the EMab-134-
and control IgG-treated mice on days 7, 9, 14, 18, and 21. The
administration of 134-mG2a resulted in a 41% reduction
in tumor volume compared to the EMab-134-treated mice on day 21
post-injection. Furthermore, tumors from the
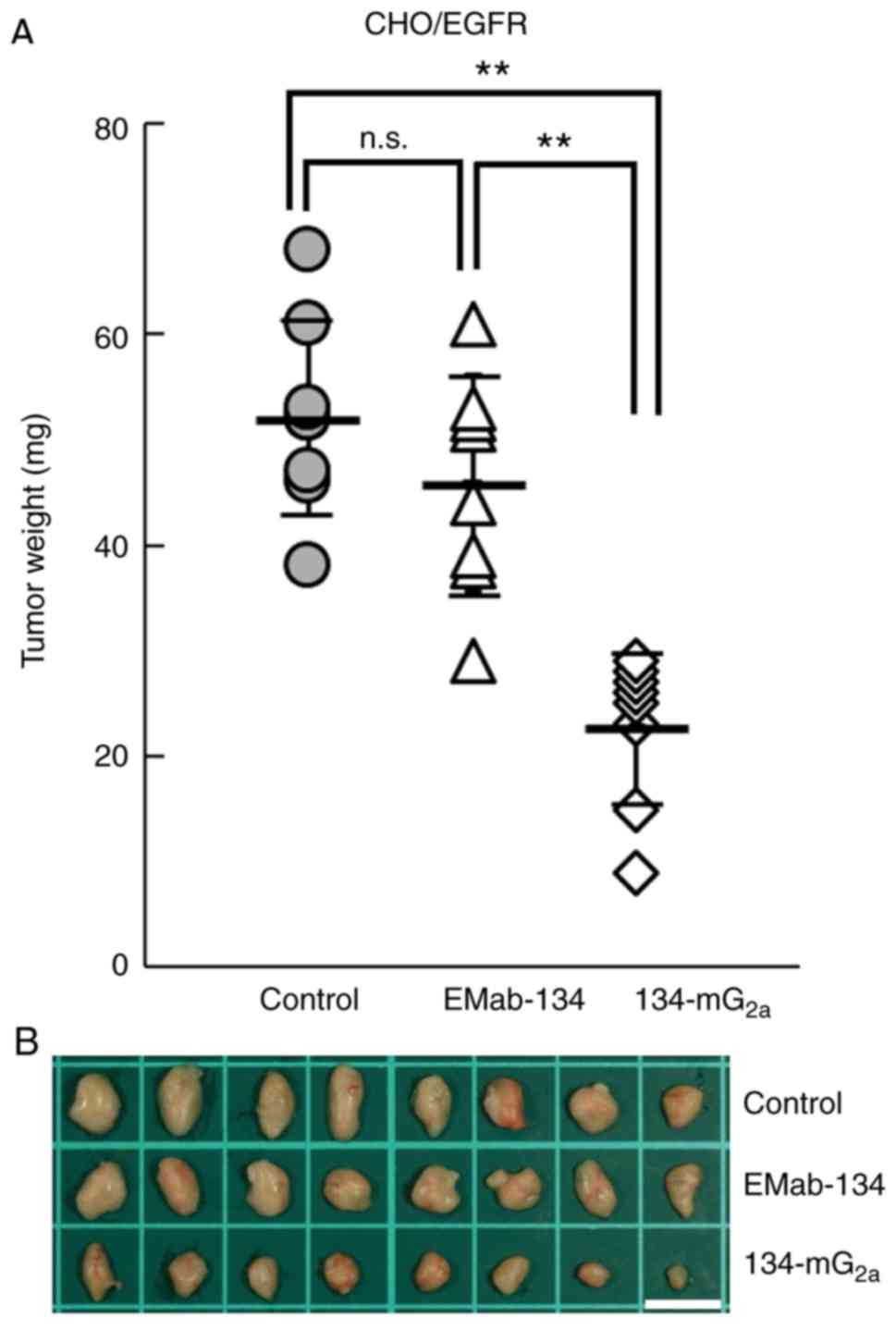
134-mG2a-treated mice weighed significantly less than
tumors from the EMab-134-treated mice (50% reduction; P<0.01,
Fig. 8A). No significant
differences in tumor weights were observed when comparing those
from the EMab-134- and control mouse IgG-treated mice (Fig. 8A). Tumors that were resected from
mice on day 21 are illustrated in Fig. 8B. Total body weights did not
differ significantly among the 3 groups (data not shown). Taken
together, these results indicated that the administration of
134-mG2a effectively reduced the growth of CHO/EGFR
xenografts.
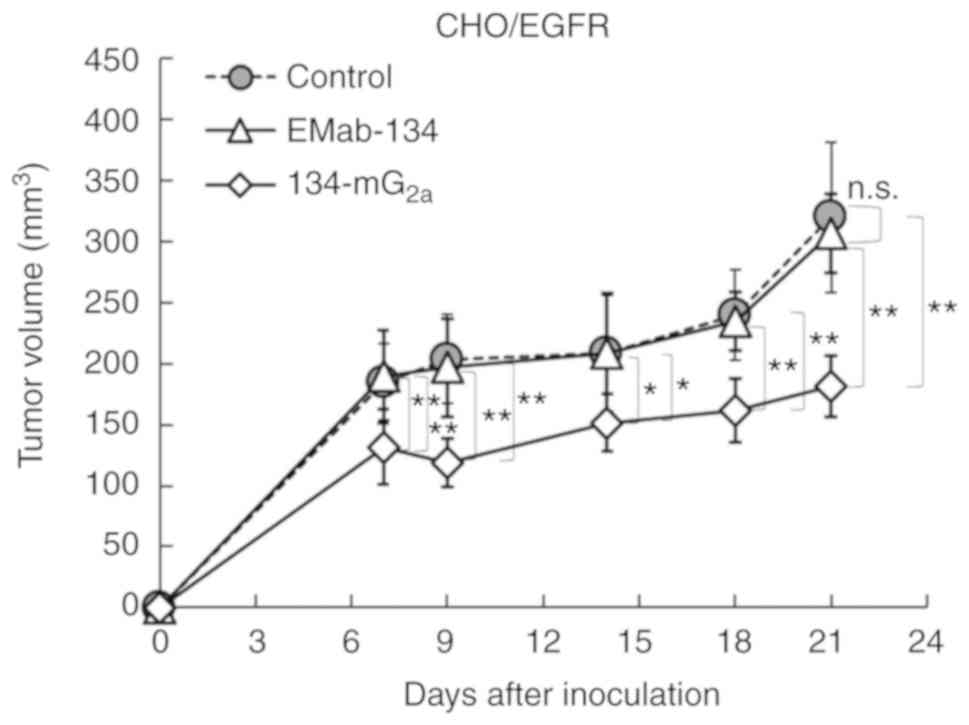 | Figure 7Evaluation of antitumor activity of
134-mG2a in CHO/EGFR xenografts (tumor size). CHO/EGFR
cells (5×106 cells) were injected subcu-taneously into
the left flank. After day 1, 100 µg of EMab-134,
134-mG2a, or control mouse IgG in 100 µl PBS were
injected intraperitoneally into mice; additional antibodies were
then injected on day 7 and day 14. Tumor volume was measured on
days 7, 9, 14, 18 and 21. Values shown are the means ± SEM.
Asterisks indicate statistical significance
(**P<0.01, *P<0.05; n.s., not
significant; one-way ANOVA and Tukey-Kramer's test). EGFR,
epidermal growth factor receptor; mAbs, monoclonal antibodies. |
Antitumor activities of
134-mG2a in mouse xenografts of HSC-2 oral cancer
cells
In the HSC-2 xenograft models, 134-mG2a
(100 µg), EMab-134 (100 µg), or control mouse IgG
(100 µg) were injected intraperitoneally into mice on days
1, 7 and 14 after the HSC-2 cell injections. Tumor volume was
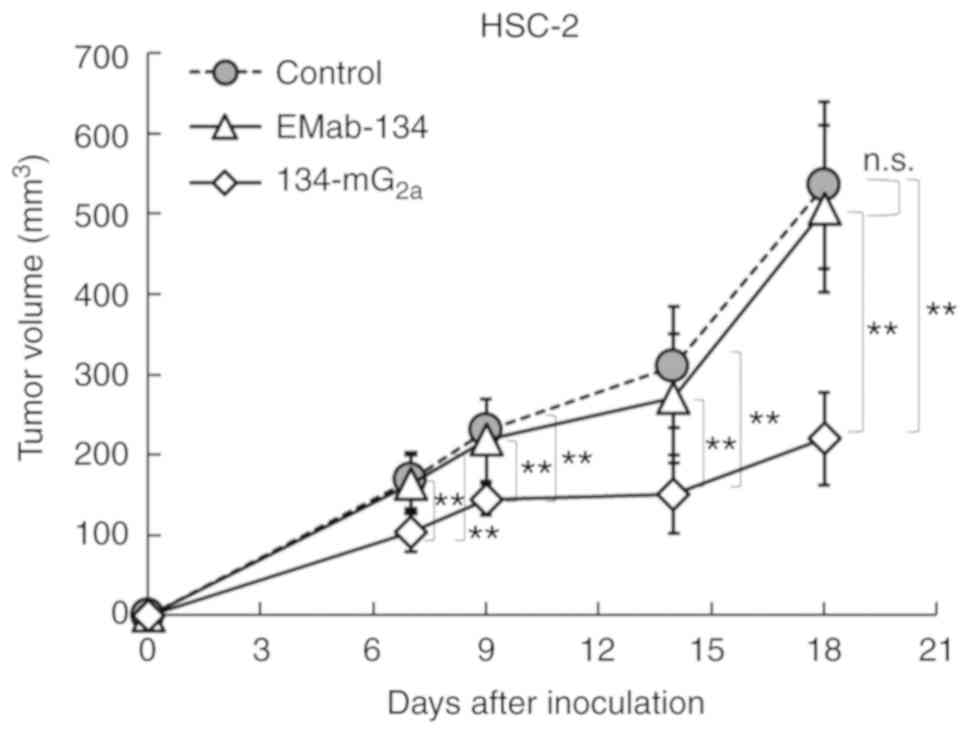
measured on days 7, 9, 14 and 18. The administration of
134-mG2a resulted in significantly decreased tumor
development on days 7 (P<0.01), 9 (P<0.01), 14 (P<0.01)
and 18 (P<0.01) in comparison to the EMab-134-treated mice
(Fig. 9). No significant
differences were observed between the EMab-134- and control
IgG-treated mice on days 7, 9, 14 and 18. The administration of
134-mG2a resulted in a 57% reduction in tumor volume
compared to the EMab-134-treated mice on day 18 post-injection.
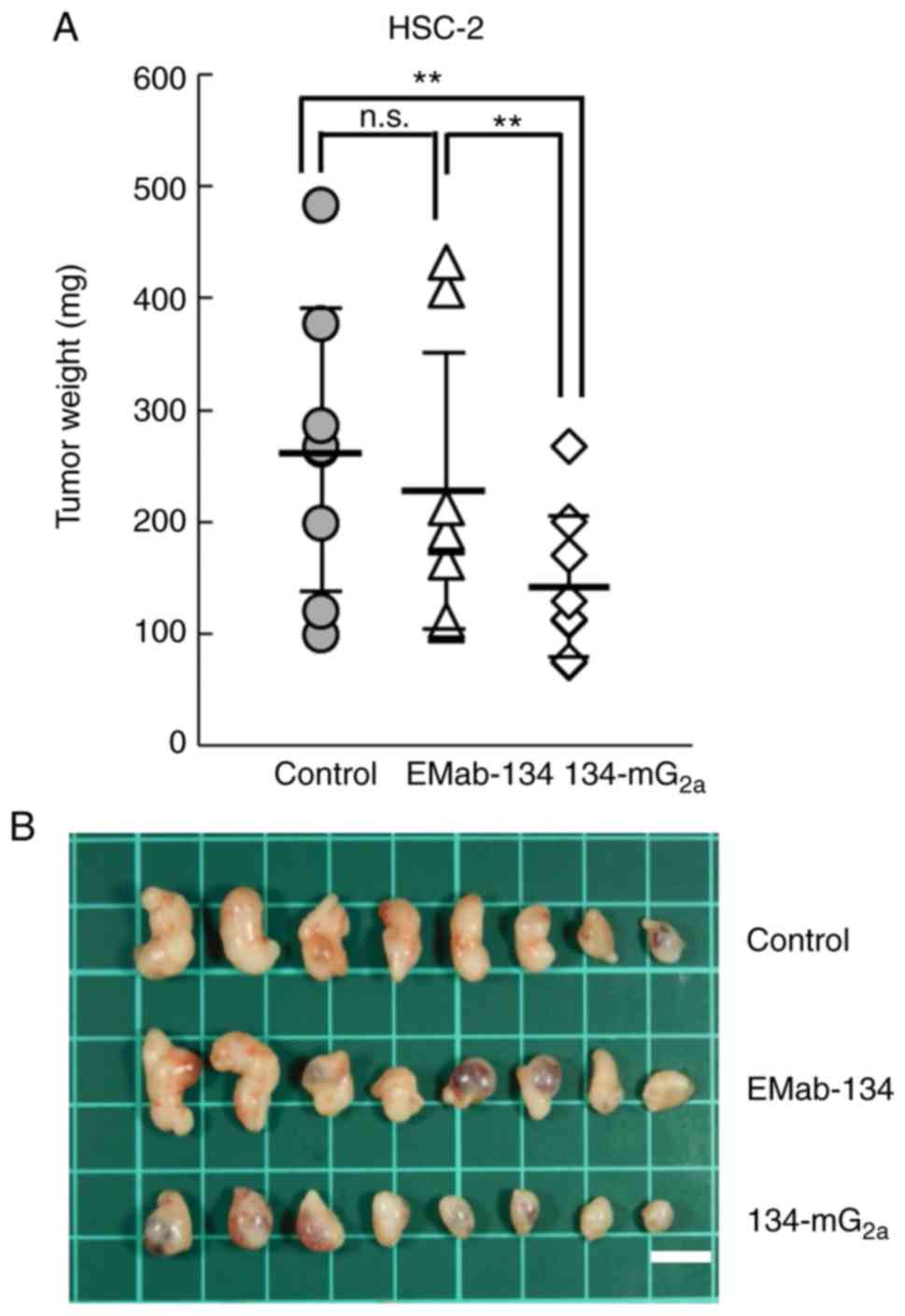
Tumors from the 134-mG2a-treated mice weighed
significantly less than the tumors from the EMab-134-treated mice
(37% reduction; P<0.01, Fig.
10A). No significant differences in tumor weight were observed
when comparing those from the EMab-134- and control mouse
IgG-treated mice. Tumors resected on day 18 are illustrated in
Fig. 10B. Total body weights did
not differ significantly among the 3 groups (data not shown). These
results indicated that the administration of 134-mG2a
effectively limited the growth of HSC-2 cell xenografts.
Antitumor activities of
134-mG2a in mouse xenografts of SAS oral cancer
cells
In the SAS xenograft models, 134-mG2a
(100 µg), EMab-134 (100 µg), and control mouse IgG
(100 µg) were injected intraperitoneally into the mice on
days 1, 7 and 14 after SAS cell injections. Tumor volumes were
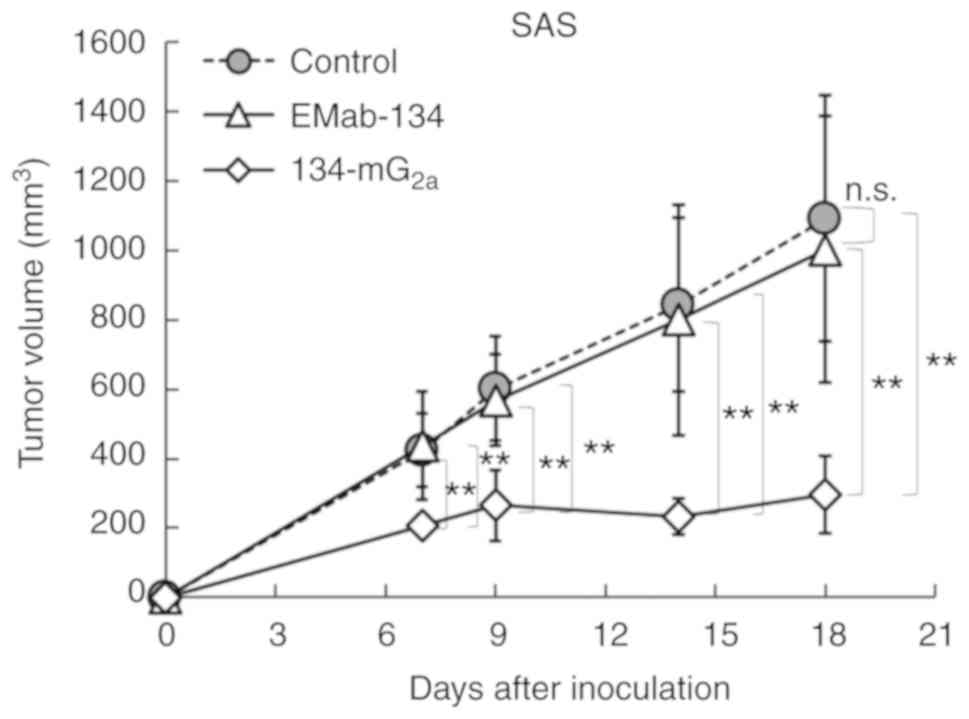
measured on days 7, 9, 14 and 18. The administration of
134-mG2a resulted in significantly reduced tumor
development as determined on days 7 (P<0.01), 9 (P<0.01), 14
(P<0.01) and 18 (P<0.01) when compared to tumors from the
EMab-134-treated mice (Fig. 11).
No significant differences between EMab-134 and control mouse
IgG-treated mice on days 7, 9, 14 and 18 were observed. The
administration of 134-mG2a resulted in a 70% reduction
in tumor volume on day 18 compared to the responses observed among
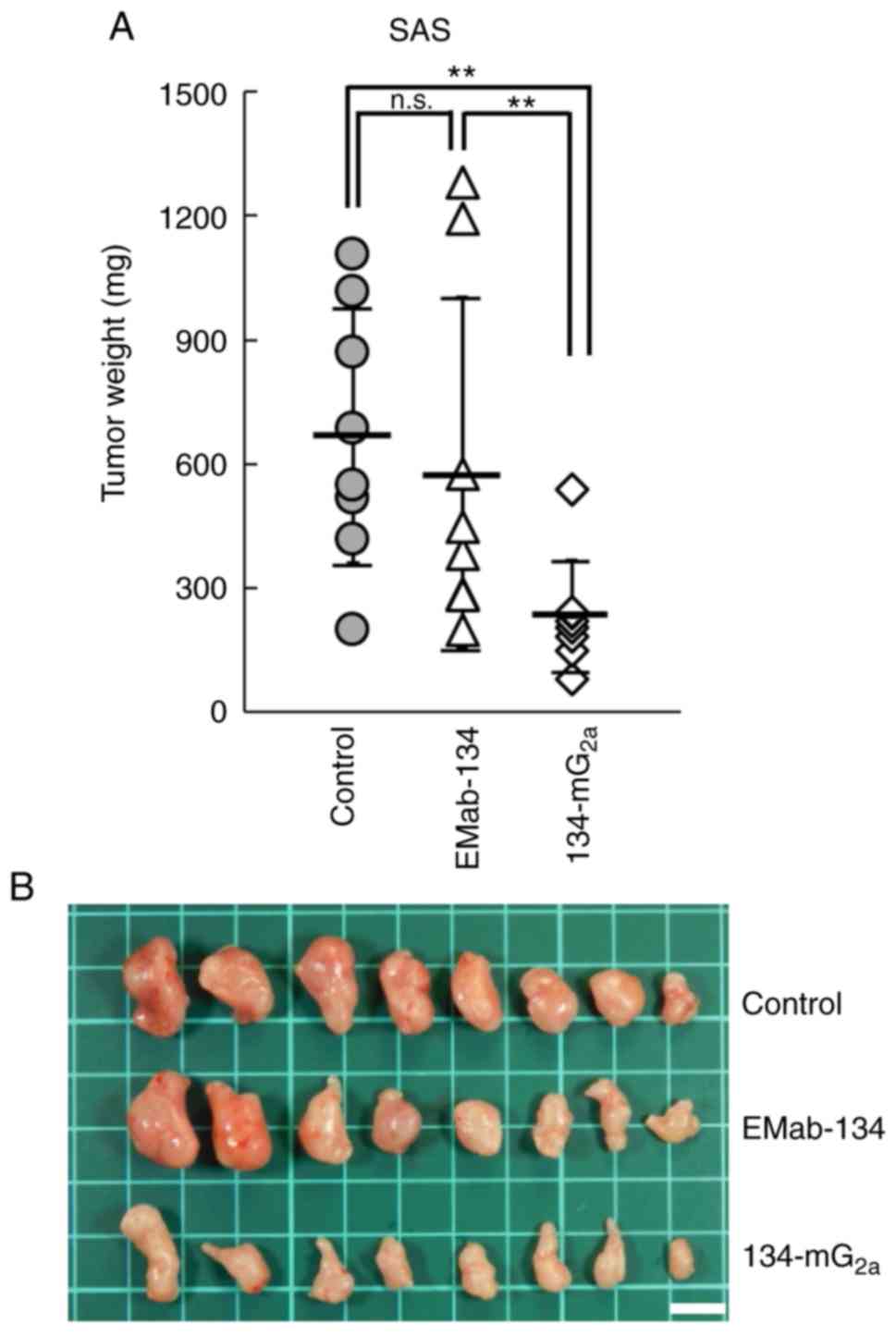
the EMab-134-treated mice. Tumors from the
134-mG2a-treated mice weighed significantly less than
the tumors from the EMab-134-treated mice (60% reduction;
P<0.01, Fig. 12A). No
significant differences in tumor weight were observed when
comparing the tumors from the EMab-134- and control mouse
IgG-treated mouse resected on day 18. The tumors resected from the
mice are shown in Fig. 12B.
Total body weights did not significantly differ among the three
groups (data not shown). These results indicated that the
administration of 134-mG2a effectively limited the
growth of SAS xenografts.
Discussion
The authors of the present study previously
established a sensitive and specific anti-EGFR mAb, EMab-134 (mouse
IgG1), which is very useful for several applications,
including flow cytometry, western blot analysis and
immunohistochemistry (14). This
antibody could not be used to investigate antitumor activity as the
IgG1 subclass does not exhibit ADCC/CDC activities.
Therefore, EMab-134 was converted into 134-mG2a
(IgG2a subclass). It was demonstrated that
134-mG2a elicits both ADCC and CDC in vitro
(Figs. 5 and 6), and antitumor activities against both
CHO/EGFR xenografts (Figs. 7 and
8) and OSCC xenografts (Figs. 9-12) in vivo. Importantly, the
administration of 134-mG2a efficiently reduced the
growth of OSCC xenografts at all time points examined when compared
to the responses to EMab-134. Nevertheless, only limited reductions
in HSC-2 and SAS tumor volume were observed in response to the
administration of 134-mG2a, to 57 and 70%, respectively.
These results suggest that targeting EGFR with this antibody may
not be sufficient to eliminate most OSCCs. The authors have
previously added EGF to HSC-2 and SAS cell lines; however, these
cell lines did not respond to EGF stimulation and did not grow well
compared to the control cells (21). The results indicated that
134-mG2a and EMab-134 antibodies could not neutralize
the EGF-EGFR axis. Taken together, antitumor activities by
134-mG2a were exerted by ADCC and CDC activities, not
neutralization.
In a previous study, it was found that HER2 was
expressed in oral cancers, and that the administration of an
anti-HER2 mAb (clone H2Mab-19, mouse IgG2b)
resulted in antitumor activity against HSC-2 and SAS xenografts
(23). By contrast, Mirza et
al (24) demonstrated that
only one case out of 140 OSCCs was HER2-positive; as such, the
feasibility of anti-HER2 therapy for OSCC remains uncertain. In
another study, the authors developed a sensitive and specific mAb
against EGFR that recognized a distinct epitope (clone EMab-17,
mouse IgG2a) and that elicited both ADCC and CDC, as
well as antitumor activity against HSC-2 and SAS xenografts
(21). The extent of ADCC, CDC or
antitumor activities of EMab-17 and 134-mG2a were not
yet compared, nor was the binding epitope of EMab-17 determined;
further investigations are warranted in order to select the optimal
anti-EGFR mAb for the treatment of OSCCs.
The authors previously converted an
anti-podocalyxin (PODXL) mAb of IgG1 subclass (PcMab-47)
into a mouse IgG2a-type mAb (47-mG2a) to
facilitate the evaluation of ADCC and CDC (25). The authors also developed
47-mG2a-f, a core fucose-deficient variant of
47-mG2a in order to increase its ADCC. In vivo
analysis revealed that 47-mG2a-f, but not
47-mG2a, exerted antitumor activity in HSC-2 and SAS
xenograft models at administered 3 times at doses of 100
µg/mouse/week; these results indicated that a core
fucose-deficient anti-PODXL mAb may also be useful for
antibody-based therapy against PODXL-expressing OSCCs. Moreover, a
cancer-specific mAb (CasMab) against podoplanin (PDPN) was
established, which is expressed in a number of types of cancer,
including oral cancers (26). In
xenograft models of HSC-2 cells, a mouse-human chimeric mAb,
chLpMab-23, exerted antitumor activity by engaging human NK cells;
these results suggest that chLpMab-23 may be advantageous for
antibody therapy against PDPN-expressing oral cancers (27). Antibody regimens that focus on
multiple targets, including EGFR, HER2, PODXL and PDPN, may
ultimately be effective with the goal of conquering oral cancers.
In the future, cancer-specific anti-EGFR mAbs may also be developed
that can reduce the adverse effects of traditional antibody
therapy.
Acknowledgments
The authors would like to thank Ms. Saori Handa,
Ms. Saki Okamoto and Mr. Yu Komatsu (Department of Antibody Drug
Development, Tohoku University Graduate School of Medicine) for
providing technical assistance with the in vitro
experiments, and Ms. Akiko Harakawa (Institute of Microbial
Chemistry (BIKAKEN), Numazu, Microbial Chemistry Research
Foundation) for providing technical assistance with the animal
experiments.
Abbreviations:
|
ADCC
|
antibody-dependent cellular
cytotoxicity
|
|
ATCC
|
American Type Culture Collection
|
|
CasMab
|
cancer-specific monoclonal
antibody
|
|
CDC
|
complement-dependent cytotoxicity
|
|
CHO
|
Chinese hamster ovary
|
|
DAB
|
3,3′-diaminobenzidine
tetrahydrochloride
|
|
DMEM
|
Dulbecco's modified Eagle's
medium
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
EGFR
|
epidermal growth factor receptor
|
|
FBS
|
fetal bovine serum
|
|
JCRB
|
Japanese Collection of Research
Bioresources Cell Bank
|
|
HER
|
human epidermal growth factor
receptor
|
|
mAb
|
monoclonal antibody
|
|
n.s.
|
not significant
|
|
OSCC
|
oral squamous cell carcinoma
|
|
PBS
|
phosphate-buffered saline
|
|
PDPN
|
podoplanin
|
|
PODXL
|
podocalyxin
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
PVDF
|
polyvinylidene difluoride
|
|
SEM
|
standard error of the mean
|
Funding
The present study was supported in part by the
Japan Agency for Medical Research and Development (AMED) under the
grant nos. JP20am0401013 (to YK), JP20am0101078 (to YK) and
JP20ae0101028 (to YK), and by the Japan Society for the Promotion
of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI)
grant no. 17K07299 (to MKK), grant no. 19K07705 (to YK), and grant
no. 20K16322 (to MS).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
HHo, TO, JT, TN, MS TA, YS and MY performed the
experiments. MKK analyzed the experimental data. MK, HHa and YK
conceived and designed the present study and wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments for ADCC and the antitumor
activity were approved by the institutional committee for
experiments of the Institute of Microbial Chemistry (Permit. no.
2019-049 for ADCC assays, 2019-046 for antitumor experiments). The
tissues used were from a purchased tissue microarray.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
3
|
Guneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vokes EE: Induction chemotherapy for head
and neck cancer: Recent data. Oncologist. 15:3–7. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcazzan S, Varoni EM, Blanco E, Lodi G
and Ferrari M: Nanomedicine, an emerging therapeutic strategy for
oral cancer therapy. Oral Oncol. 76:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Furness S, Glenny AM, Worthington HV,
Pavitt S, Oliver R, Clarkson JE, Macluskey M, Chan KK and Conway
DI: Interventions for the treatment of oral cavity and
oropharyngeal cancer: Chemotherapy. Cochrane Database Syst Rev.
13:CD0063862011.
|
|
7
|
Dokala A and Thakur SS: Extracellular
region of epidermal growth factor receptor: A potential target for
anti-EGFR drug discovery. Oncogene. 36:2337–2344. 2017. View Article : Google Scholar
|
|
8
|
Ogiso H, Ishitani R, Nureki O, Fukai S,
Yamanaka M, Kim JH, Saito K, Sakamoto A, Inoue M, Shirouzu M and
Yokoyama S: Crystal structure of the complex of human epidermal
growth factor and receptor extracellular domains. Cell.
110:775–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Downward J, Yarden Y, Mayes E, Scrace G,
Totty N, Stockwell P, Ullrich A, Schlessinger J and Waterfield MD:
Close similarity of epidermal growth factor receptor and v-erb-B
oncogene protein sequences. Nature. 307:521–527. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mendelsohn J: The epidermal growth factor
receptor as a target for cancer therapy. Endocr Relat Cancer.
8:3–9. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schultheis B, Reuter D, Ebert MP, Siveke
J, Kerkhoff A, Berdel WE, Hofheinz R, Behringer DM, Schmidt WE,
Goker E, et al: Gemcitabine combined with the monoclonal antibody
nimotuzumab is an active first-line regimen in KRAS wildtype
patients with locally advanced or metastatic pancreatic cancer: A
multicenter, randomized phase IIb study. Ann Oncol. 28:2429–2435.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen RB: Current challenges and clinical
investigations of epidermal growth factor receptor (EGFR)- and ErbB
family-targeted agents in the treatment of head and neck squamous
cell carcinoma (HNSCC). Cancer Treat Rev. 40:567–577. 2014.
View Article : Google Scholar
|
|
13
|
Ma H, Jin S, Yang W, Zhou G, Zhao M, Fang
S, Zhang Z and Hu J: Interferon-alpha enhances the antitumour
activity of EGFR-targeted therapies by upregulating RIG-I in head
and neck squamous cell carcinoma. Br J Cancer. 118:509–521. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itai S, Yamada S, Kaneko MK, Chang YW,
Harada H and Kato Y: Establishment of EMab-134, a sensitive and
specific anti-epidermal growth factor receptor monoclonal antibody
for detecting squamous cell carcinoma cells of the oral cavity.
Monoclon Antib Immunodiagn Immunother. 36:272–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaneko MK, Yamada S, Itai S, Chang YW,
Nakamura T, Yanaka M and Kato Y: Elucidation of the critical
epitope of an anti-EGFR monoclonal antibody EMab-134. Biochem
Biophys Rep. 14:54–57. 2018.PubMed/NCBI
|
|
16
|
Itai S, Kaneko MK, Fujii Y, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Handa S, Chang YW, Suzuki H, et al:
Development of EMab-51, a sensitive and specific anti-epidermal
growth factor receptor monoclonal antibody in flow cytometry,
western blot, and immunohistochemistry. Monoclon Antib Immunodiagn
Immunother. 36:214–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujii Y, Kaneko M, Neyazaki M, Nogi T,
Kato Y and Takagi J: PA tag: A versatile protein tagging system
using a super high affinity antibody against a dodecapeptide
derived from human podoplanin. Protein Expr Purif. 95:240–247.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujii Y, Kaneko MK, Ogasawara S, Yamada S,
Yanaka M, Nakamura T, Saidoh N, Yoshida K, Honma R and Kato Y:
Development of RAP tag, a novel tagging system for protein
detection and purification. Monoclon Antib Immunodiagn Immunother.
36:68–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujii Y, Kaneko MK and Kato Y: MAP Tag: A
novel tagging system for protein purification and detection.
Monoclon Antib Immunodiagn Immunother. 35:293–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawada M, Inoue H, Kajikawa M, Sugiura M,
Sakamoto S, Urano S, Karasawa C, Usami I, Futakuchi M and Masuda T:
A novel monoclonal antibody targeting coxsackie virus and
adenovirus receptor inhibits tumor growth in vivo. Sci Rep.
7:404002017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett. 19:2809–2816.
2020.PubMed/NCBI
|
|
22
|
Kato Y, Kunita A, Fukayama M, Abe S,
Nishioka Y, Uchida H, Tahara H, Yamada S, Yanaka M, Nakamura T, et
al: Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts
antitumor activity against human podoplanin through
anti-body-dependent cellular cytotoxicity and complement-dependent
cytotoxicity. Monoclon Antib Immunodiagn Immunother. 36:20–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: H2Mab-19, an anti-human epidermal growth
factor receptor 2 monoclonal antibody exerts antitumor activity in
mouse oral cancer xenografts. Exp Ther Med. 20:846–853. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirza S, Hadi N, Pervaiz S, Khan SZ,
Mokeem SA, Abduljabbar T, Al-Hamoudi N and Vohra F: Expression of
HER-2/neu in oral squamous cell carcinoma. Asian Pac J Cancer Prev.
21:1465–1470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Itai S, Ohishi T, Kaneko MK, Yamada S, Abe
S, Nakamura T, Yanaka M, Chang YW, Ohba SI and Nishioka Y:
Anti-podocalyxin antibody exerts antitumor effects via
antibody-dependent cellular cytotoxicity in mouse xenograft models
of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4:59242014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaneko MK, Nakamura T, Kunita A, Fukayama
M, Abe S, Nishioka Y, Yamada S, Yanaka M, Saidoh N, Yoshida K, et
al: ChLpMab-23: Cancer-specific human-mouse chimeric
anti-podoplanin antibody exhibits antitumor activity via
antibody-dependent cellular cytotoxicity. Monoclon Antib
Immunodiagn Immunother. 36:104–112. 2017. View Article : Google Scholar : PubMed/NCBI
|