Introduction
Atherosclerosis, a class of vascular diseases, is
the main cause of heart disease and stroke (1). Atherosclerosis induces thrombus
formation by disrupting the integrity of the arterial surface
(2). Vascular smooth muscle cells
(VSMCs) have been reported to be involved in the remodeling of the
arterial wall in atherosclerosis (3), and their viability and migration
serve a vital role in the progression of atherosclerosis (4,5).
Thus, it is imperative to determine the pathogenesis of
atherosclerosis and the roles of VSMCs in atherosclerosis.
Circular RNAs (circRNAs) are a type of
single-stranded RNAs with a covalently closed continuous loop that
are produced by backsplicing (6)
and are resistant to exonuclease-mediated degradation (7). Numerous studies have demonstrated
that circRNAs are associated with cardiac repair (8), silica-induced macrophage activation
(9), pulmonary arterial
hypertension (10) and
atherosclerosis (11). Microarray
analysis has revealed that circ_0010283 is upregulated in oxidized
low-density lipoprotein (ox-LDL)-induced VSMCs (12). However, the regulatory mechanism
of circ_0010283 in atherosclerosis needs to be further
investigated.
MicroRNAs (miRNAs) are short (~22 nucleotides)
conserved non-coding RNAs that mediate gene expression by binding
to the 3′-untranslated region (3′UTR) of target mRNAs at the
post-transcriptional level (13).
Emerging evidence has revealed a core position of miRNAs in the
regulation of atherosclerosis progression (14,15). miR-370-3p has been reported to be
associated with a number of human diseases including acute
pneumonia, cerebral aneurysm and tuberculosis (16-18), and a previous study has
demonstrated that miR-370-3p regulates coronary atherosclerosis
progression (19). However, the
precise mechanism of miR-370-3p in regulating atherosclerosis
progression remains to be studied.
High-mobility group box 1 (HMGB1) has been reported
to participate in the regulation of atherosclerosis progression. Wu
et al (20) demonstrated
that miR-328 mitigated ox-LDL-induced endothelial cell injury by
targeting HMGB1 in atherosclerosis. Moreno et al (21) reported that HMGB1 was highly
expressed in atherosclerotic plaques. Therefore, HMGB1 may be a
treatment target for atherosclerosis, and the regulatory mechanism
of HMGB1 needs to be explored.
The aim of the present study was to investigate the
potential diagnostic biomarkers for atherosclerosis and to further
understand the function and underlying regulatory mechanism of
circ_0010283 in ox-LDL-induced VSMCs.
Materials and methods
Cell culture and treatment
Human vascular smooth muscle cells (VSMCs) were
purchased from Otwo Biotech (Shenzhen), Inc. McCoy's 5A medium
(Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine
serum (FBS) (Sigma-Aldrich; Merck KGaA) was used to culture the
cells in an atmosphere containing 5% CO2 at 37°C.
Ox-LDL (Peking Union-Biology Co., Ltd.) was used to induce an
aberrant lipid environment. Transfected cells were cultured in the
medium with ox-LDL at concentrations ranging between 0 and 150
µg/ml for 24, 48 and 72 h (Fig. S1), and the normal control cells
were cultured in medium without ox-LDL.
Cell transfection
Small interfering RNA (siRNA) against circ_0010283
(si-circ_0010283, 5′-GCA GTC ATC TAC AGA TCA A-3′), miR-370-3p
mimic (referred to as miR-370-3p, 5′-GCC UGC UGG GGU GGA ACC UGG
U-3′) and miR-370-3p inhibitor (anti-miR-370-3p, 5′-ACC AGG UUC CAC
CCC AGC AGG C-3′), as well as the corresponding negative controls
(si-NC, 5′-CCA AAA CCA GGC UUU GAU UGA-3′; miR-NC, 5′-UUC UCC GAA
CGU GUC ACG UTT-3′; and anti-miR-NC, 5′-UCU ACU CUU UCU AGG AGG UUG
UGA-3′) were obtained from Shanghai GenePharma Co., Ltd. HMGB1
overexpression pcDNA3.1-plasmid (referred to as HMGB1) and its
negative control (referred to as vector) were acquired from
Guangzhou RiboBio Co., Ltd. VSMCs (1×105 cells/well)
were transfected with 100 nM plasmids using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After 6-h transfection at 37°C, the medium was replaced with fresh
medium with or without ox-LDL at 37°C. The transfected cells were
harvested after 48 h for use in subsequent experiments. The
efficiency of transfection was measured by reverse
transcription-quantitative (RT-q)PCR.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Briefly, transfected and 100 µg/ml
ox-LDL-treated VSMCs were seeded into 96-well plates
(1×105 cells/well) and incubated for 24, 48 or 72 h.
Subsequently, 20 µl MTT solution (5 mg/ml; Sigma-Aldrich;
Merck KGaA) was added to the well to incubate at 37°C for 4 h;
then, the medium was discarded, and 200 µl dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was added to the well. The
optical density values were determined at 490 nm using the
microplate reader (Bio-Rad Laboratories, Inc.).
RNA isolation, RT-qPCR and RNase R
treatment
Total RNA was isolated using TRIzol®
reagent (Sigma-Aldrich; Merck KGaA) and reverse-transcribed to
complementary DNA using a PrimeScript RT Master Mix kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's
instructions. The qPCR was performed using a SYBR® Green
PCR Master Mix (Vazyme Biotech Co., Ltd.), and the data were
analyzed using the 2−ΔΔCq method (22). U6 was used as the control of
miR-370-3p, and circ_0010283 and HMGB1 expression was normalized to
β-actin. The primers used in this study were as follows:
circ_0010283 forward, 5′-GAG GTG ATG AAC CAC CCT GG-3′ and reverse,
5′-CTG AGC TGG CTG TAA CCA CA-3′; linear mRNA ubiquitin protein
ligase E3 component n-recognin 4 (UBR4) forward, 5′-GGT GTT CCA GAG
GCT AGT GAT C-3′ and reverse, 5′-CCA ACT GCT TCT GCG GTT CCT T-3′;
miR-370-3p forward, 5′-TGT AAC CAG AGA GCG GGA TGT-3′ and reverse,
5′-TTT TGG CAT AAC TAA GGC CGA A-3′; HMGB1 forward, 5′-GCG GAC AAG
GCC CGT TA-3′ and reverse, 5′-AGA GGA AGA AGG CCG AAG GAA-3′;
β-actin forward, 5′-GCA CCA CAC CTT CTA CAA TG-3′ and reverse,
5′-TGC TTG CTG ATC CAC ATC TG-3′; U6 forward, 5′-TCC GGG TGA TGC
TTT TCC TAG-3′ and reverse, 5′-CGC TTC ACG AAT TTG CGT GTC AT-3′;
GAPDH forward, 5′-GAG TCA ACG GAT TTG GTC GT-3′ and reverse, 5′-TTG
ATT TTG GAG GGA TCT CG-3′.
Purified RNAs were treated with RNase R (Vazyme
Biotech Co., Ltd.) for subsequent experiments. circRNA stability
was assessed by RNase R experiment. The extracted RNAs from VSMCs
was separated into two parts; one part was treated with RNase R
digestion, whereas the other part (control) was digested with
digestion buffer. The expression level of circ_0010283 and linear
UBR4 mRNA were measured by RT-qPCR.
Nuclear-cytoplasmic fractionation
Cytoplasmic and nuclear RNAs were isolated using the
NE-PER™ Nuclear and Cytoplasmic Extraction reagents (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol as
previously described (23).
Briefly, following washing with PBS, 100 µg/ml
ox-LDL-treated VSMCs were centrifuged at 4°C to separate the
supernatant containing cytoplasm from the nuclear components.
Subsequently, the cytoplasmic and nuclear fractions were mixed with
the lysis/binding solution and ethanol, respectively. The RNAs of
these two fractions were obtained with an eluting solution. RT-qPCR
was used to examine circ_0010283 expression in the cell cytoplasm
and nucleus.
Western blotting
Proteins from VSMCs with or without ox-LDL treatment
were isolated using RIPA buffer (Vazyme Biotech Co., Ltd.), and the
protein concentration was determined by Detergent Compatible
Bradford Protein Quantification kit (Vazyme Biotech Co., Ltd.).
Equal amounts of protein (20 µg/lane) were separated by 10%
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and subsequently transferred to polyvinylidene
difluoride (PVDF) membranes (Vazyme Biotech Co., Ltd.), followed by
blocking with 5% skimmed milk (Vazyme Biotech Co., Ltd.) for 2 h at
37°C and washing with phosphate-buffered saline (PBS).
Subsequently, the membranes were incubated with the following
primary antibodies: Anti-cyclin D1 (1:1,000; cat. no. ab16663;
Abcam), anti-proliferating cell nuclear antigen (PCNA; 1:2,000;
cat. no. ab18197; Abcam), anti-matrix metalloproteinase 2 (MMP2;
1:3,000; cat. no. ab97779; Abcam), anti-MMP9 (1:1,000; cat. no.
ab38898; Abcam) and anti-HMGB1 (1:1,000; cat. no. ab18256; Abcam)
or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2,500;
cat. no. ab9485; Abcam) overnight at 4°C. Following washing with
PBS, the membranes were incubated with a horseradish
peroxidase-conjugated anti-rabbit IgG secondary antibody (1:3,000;
cat. no. ab205718; Abcam) for 3 h at 37°C. The membranes were
analyzed using the ChemiDoc™ MP Imaging System (Bio-Rad
Laboratories, Inc.) following treatment with an ECL kit (Vazyme
Biotech Co., Ltd.).
Transwell assay
Transwell chambers (Beijing Solarbio Science &
Technology Co., Ltd.) were used to determine the cell migratory
capacity. Transfected cells (1×105 cells/well) were
seeded into the upper chamber, and medium containing 10% FBS was
placed in the lower chamber. Following treatment with crystal
violet for 10 min at 37°C (Beijing Solarbio Science &
Technology Co., Ltd.), the cells were analyzed under an inverted
light microscope (MTX Lab Systems, Inc.) in five random fields of
view at ×100 magnification.
Dual-luciferase reporter assay
The potential complementary sequences of miR-370-3p
and circ_0010283 or HMGB1 were predicted using starBase (24). The wild-type (WT) sequences of
circ_0010283 or HMGB1 3′UTR harboring the target sites of
miR-370-3p were inserted into pGL3 vectors (Promega Corporation) to
generate the luciferase reporter vectors circ_0010283 WT or HMGB1
3′UTR WT. Analogously, circ_0010283 mutant (MUT) and HMGB1 3′UTR
MUT reporter vectors were established by mutating the binding sites
of miR-370-3p. The vectors were co-transfected with miR-370-3p or
miR-NC into VSMCs using Lipofectamine® 2000. The
Dual-Glo Luciferase Assay System kit (Promega Corporation) was used
to determine the relative luciferase activity. Firefly luciferase
activity was normalized to Renilla luciferase.
RNA immunoprecipitation (RIP) assay
RIP was performed using a Magna RIP RNA-Binding
Protein Immunoprecipitation kit (EMD Millipore) according to the
manufacturer's instructions. Briefly, ox-LDL treated VSMCs were
first washed with PBS. Then, the cells were centrifuged at 1,000 ×
g for 5 min at 4°C, lysed with RIP buffer (200 µl)
and incubated with magnetic beads conjugated with an anti-argonaute
2 (anti-Ago2) antibody (EMD Millipore), and an anti-immuno-globulin
G (anti-IgG) antibody (EMD Millipore) was used as the negative
control. The protein was removed by Proteinase K. The
immunoprecipitated RNA was purified and analyzed by RT-qPCR.
Statistical analysis
Experimental data were calculated using GraphPad
Prism 7 (GraphPad Software, Inc.) and presented as the mean ±
standard deviation. Student's t-test was used to analyze the
differences between two independent groups. For multiple groups,
one-way analysis of variance with Tukey's post hoc test was used to
evaluate the differences. All experiments were repeated
independently at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
circ_0010283 is induced by ox-LDL and
contributes to the viability and migration of ox-LDL-induced
VSMCs
Ox-LDL has been reported to regulate the viability
of VSMCs (25). In the present
study, VSMCs were first treated with ox-LDL at concentrations
ranging from 0 to 150 µg/ml for 24, 48 and 72 h. The results
demonstrated that the viability of VSMCs was gradually induced with
the increasing concentration of ox-LDL between 0 and 100
µg/ml and treatment time (Fig. S1). Therefore, VSMCs were treated
with 100 µg/ml ox-LDL for 48 or 72 h, or treated with 75 or
100 µg/ml ox-LDL for 72 h, and the level of circ_0010283 was
measured. As demonstrated in Fig. 1A
and B, the expression of circ_0010283 was significantly
increased by ox-LDL at different time points (48 and 72 h) and
concentrations (75 and 100 µg/ml) in VSMCs compared with
untreated cells. In addition, circ_0010283 was mainly distributed
in the cytoplasm (Fig. 1C).
Compared with linear UBR4 mRNA, circ_0010283 was more resistant to
RNase R (Fig. 1D). These results
suggested that circ_0010283 may function in atherosclerosis
progression. Thus, the role of circ_0010283 in ox-LDL-induced VSMCs
was explored. VSMCs were treated with ox-LDL, ox-LDL + si-NC or
ox-LDL + si-circ_0010283, and the knockdown efficiency of
si-circ_0010283 in VSMCs treated with ox-LDL or without treatment
was verified by RT-qPCR (Figs. 1E
and S2A). MTT assay results
demonstrated that downregulation of circ_0010283 notably suppressed
ox-LDL-induced viability in VSMCs (Fig. 1F). In addition, circ_0010283
silencing decreased the levels of the viability-associated proteins
cyclin D1 and PCNA in ox-LDL-induced VSMCs compared with those in
the ox-LDL + si-NC group (Fig.
1G). The migration of ox-LDL-induced VSMCs was also repressed
by circ_0010283 knockdown compared with that of VMSCs transfected
with si-NC (Fig. 1H).
Additionally, ox-LDL-induced MMP2 and MMP9 levels were
downregulated by si-circ_0010283 in VSMCs treated with ox-LDL
(Fig. 1I) Therefore, the results
of the present study suggested that circ_0010283 may serve a
promoting role in the viability and migration of ox-LDL-induced
VSMCs.
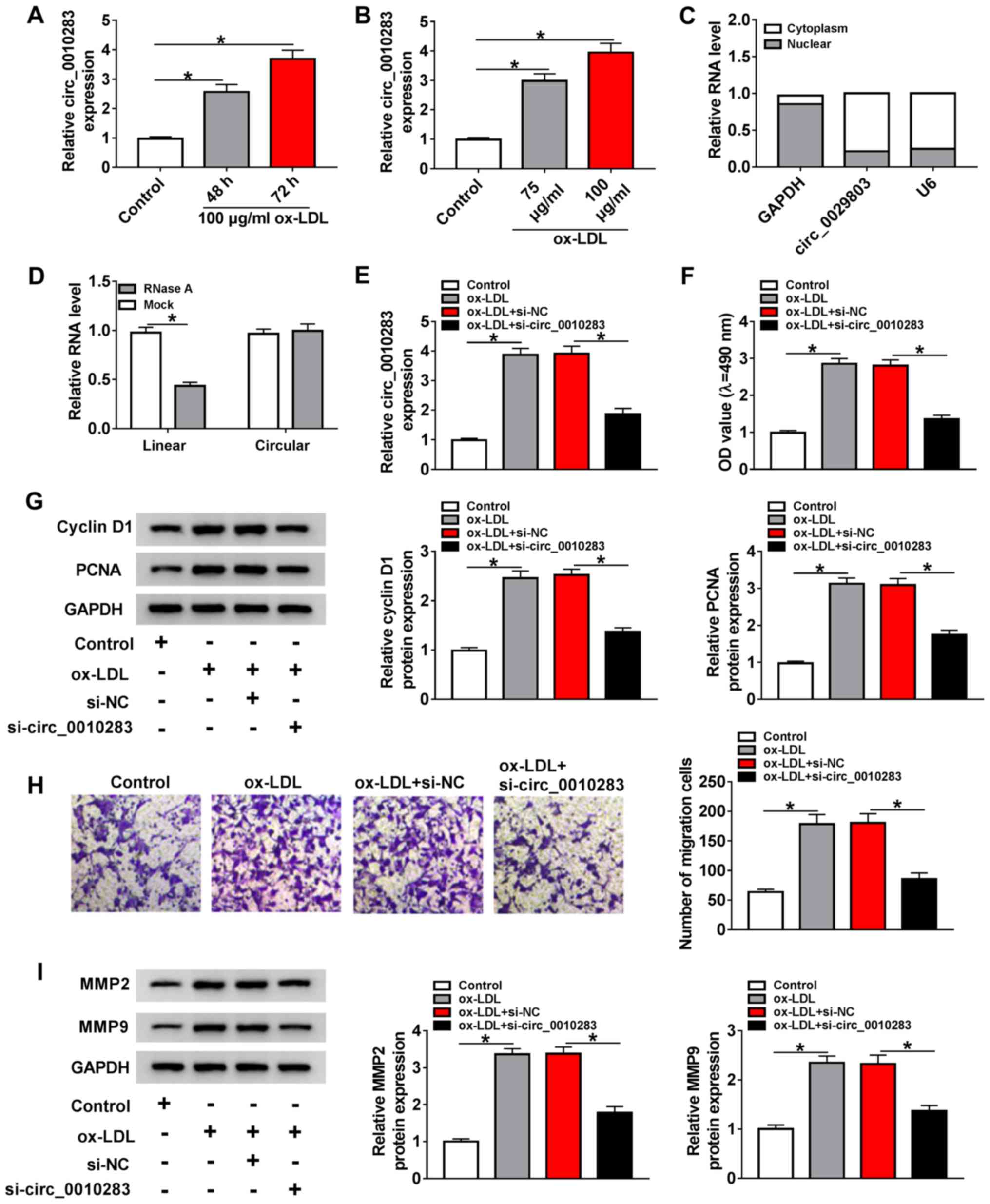 | Figure 1circ_0010283 expression is induced by
ox-LDL and facilitates the viability and migration of
ox-LDL-induced VSMCs. (A) The expression levels of circ_0010283 in
VSMCs treated with 100 µg/ml ox-LDL or for 48 or 72 h and
untreated cells were determined by RT-qPCR. (B) The expression
levels of circ_0010283 in VSMCs treated with 75 or 100 µg/ml
ox-LDL for 72 h was detected by RT-qPCR. (C) The expression levels
of circ_0010283 in the nucleus and the cytoplasm were detected by
RT-qPCR. GAPDH and U6 were used as positive controls in the nucleus
and cytoplasm, respectively. (D) The relative expression levels of
circ_0010283 in VSMCs treated with RNase R or not were measured by
RT-qPCR. (E) The levels of circ_0010283 in VSMCs treated with 100
µg/l ox-LDL or ox-LDL + si-circ_0010283 and the
corresponding controls was determined by RT-qPCR. (F) The viability
of VSMCs treated with 100 µg/ml ox-LDL or ox-LDL +
si-circ_0010283 and the corresponding controls was determined by
MTT assay. (G) The protein levels of cyclin D1 and PCNA were
detected by western blotting in VSMCs treated with 100 µg/ml
ox-LDL or ox-LDL + si-circ_0010283 and the corresponding controls.
(H) The migration of VSMCs treated with 100 µg/ml ox-LDL or
ox-LDL + si-circ_0010283 and the corresponding controls was
evaluated by Transwell assay. (I) The protein levels of MMP2 and
MMP9 were measured by western blotting in VSMCs treated with 100
µg/ml ox-LDL or ox-LDL + si-circ_0010283 and the
corresponding controls. *P<0.05. VMSCs, vascular
smooth muscle cells; ox-LDL, oxidized low-density lipoprotein;
RT-qPCR, reverse transcription-quantitative PCR; si, small
interfering; circ, circular RNA; NC, negative control; PCNA,
proliferating cell nuclear antigen; MMP, matrix metalloproteinase;
OD, optical density. |
circ_0010283 regulates cell viability and
migration by targeting miR-370-3p in ox-LDL-induced VSMCs
circRNAs have been reported to regulate a number of
human diseases by interacting with miRNAs (26,27). The potential miRNA targets for
circ_0010283 were screened in the present study using the starBase
database, and the results demonstrated that miR-370-3p harbored the
binding sites of circ_0010283 (Fig.
2A). To verify the interaction between circ_0010283 and
miR-370-3p, dual-luciferase and RIP assays were performed. The
dual-luciferase reporter assay results revealed that miR-370-3p
significantly diminished the luciferase activity of circ_0010283
WT, but not that of circ_0010283 MUT (Fig. 2B). The results of the RIP assay
demonstrated that the relative enrichment of circ_0010283 and
miR-370-3p was higher in the anti-Ago2 group compared with that in
anti-IgG group (Fig. 2C). In
addition, the expression levels of miR-370-3p were downregulated in
ox-LDL-induced VSMCs compared with those in the control group
(Fig. 2D and E). circ_0010283
silencing increased the expression level of miR-370-3p in
ox-LDL-induced VSMCs compared with that in the group transfected
with si-NC alone (Fig. 2F).
Subsequently, the overexpression or inhibition efficiency of
miR-370-3p mimic or anti-miR-370-3p in VSMCs was verified by
RT-qPCR (Fig. S2B and C). VSMCs
were treated with ox-LDL, ox-LDL + si-circ_0010283 or ox-LDL +
si-circ_0010283 + anti-miR-370-3p to investigate the interaction
between circ_0010283 and miR-370-3p. The RT-qPCR results
demonstrated that circ_0010283 silencing-induced miR-370-3p level
was reduced by anti-miR-370-3p in ox-LDL-induced VSMCs (Fig. 2G). In addition, anti-miR-370-3p
rescued the si-circ_0010283-suppressed viability of ox-LDL-induced
VSMCs (Fig. 2H and I). The
circ_0010283 knockdown-mediated inhibitory effect on cell migration
was also partly reversed by anti-miR-370-3p. As demonstrated by the
migratory rates and MMP2 and MMP9 levels, co-transfection with
si-circ_0010283 + anti-miR-370-3p partially restored the
suppressive effects of si-circ_0010283 on the migratory rate and
the expression of MMP2 and MMP9 in ox-LDL-induced VSMCs (Fig. 2J and K). These results suggested
that circ_0010283 affected the viability and migration of
ox-LDL-induced VSMCs by targeting miR-370-3p.
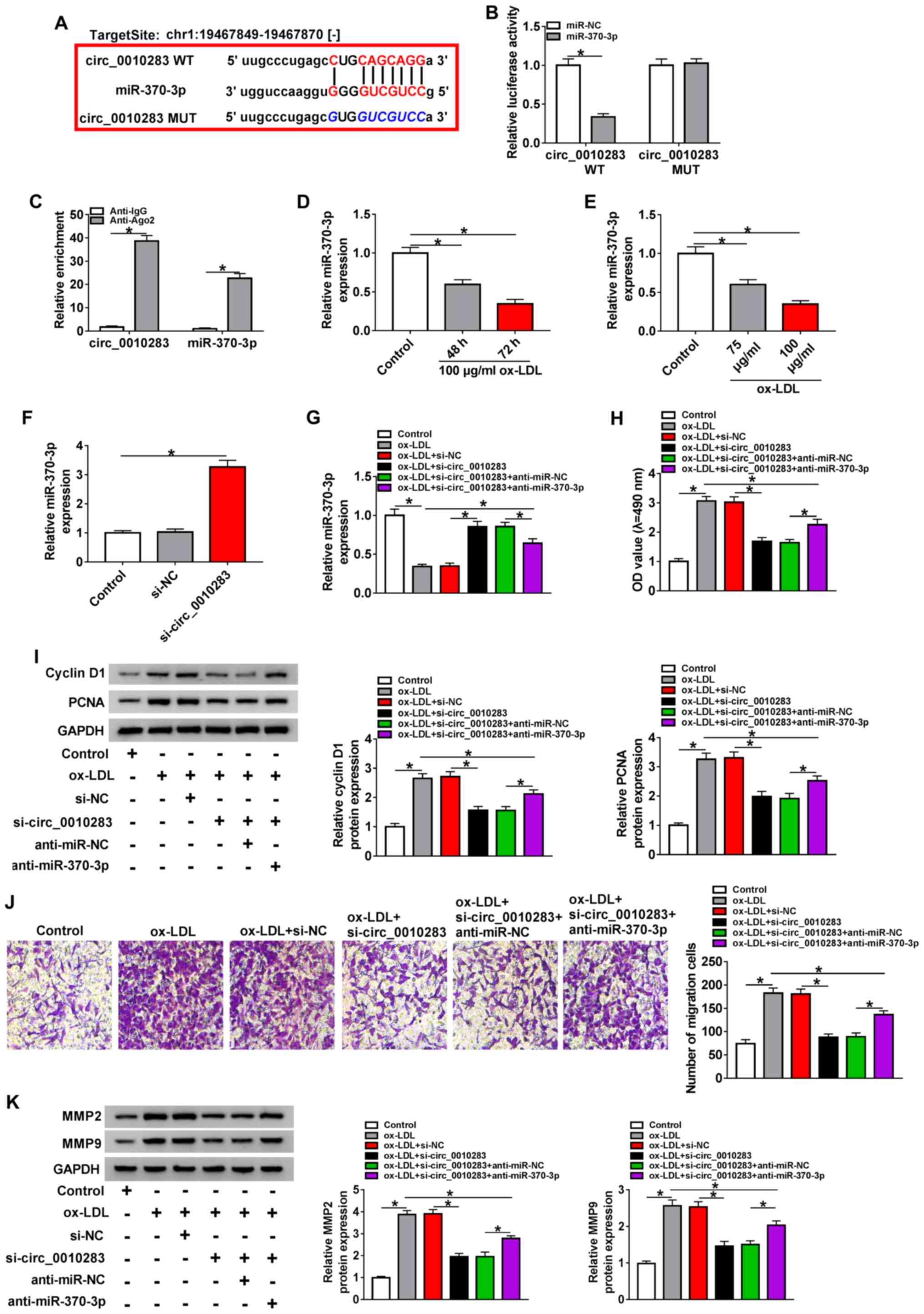 | Figure 2circ_0010283 regulates cell viability
and migration by targeting miR-370-3p in ox-LDL-induced VSMCs. (A)
The putative binding sites between circ_0010283 and miR-370-3p were
predicted by starBase. (B) The luciferase activity of circ_0010283
WT and circ_0010283 MUT was detected in VSMCs co-transfected with
miR-370-3p or miR-NC. (C) The RIP assay was performed to determine
the interaction between circ_0010283 and miR-370-3p, and anti-IgG
was used as the control. (D) The expression of miR-370-3p in VSMCs
treated with 0 or 100 µg/ml ox-LDL for 48 or 72 h was
assessed by RT-qPCR. (E) The level of miR-370-3p in VSMCs treated
with 75 or 100 µg/ml ox-LDL for 72 h was measured by
RT-qPCR. (F) The expression of miR-370-3p in VSMCs transfected with
si-circ_0010283 or si-NC was determined by RT-qPCR. (G) The levels
of miR-370-3p in VSMCs treated with 100 µg/ml ox-LDL, ox-LDL
+ si-circ_0010283 or ox-LDL + si-circ_0010283 + anti-miR-370-3p as
well as the corresponding controls were assessed by RT-qPCR. (H)
The viability of VSMCs treated with 100 µg/ml ox-LDL, ox-LDL
+ si-circ_0010283 or ox-LDL + si-circ_0010283 + anti-miR-370-3p as
well as the corresponding controls was determined by an MTT assay.
(I) The protein levels of cyclin D1 and PCNA in VSMCs treated with
100 µg/ml ox-LDL, ox-LDL + si-circ_0010283 or ox-LDL +
si-circ_0010283 + anti-miR-370-3p as well as the corresponding
controls were determined by western blotting. (J) The migratory
ability of VSMCs treated with 100 µg/ml ox-LDL, ox-LDL +
si-circ_0010283 or ox-LDL + si-circ_0010283 + anti-miR-370-3p as
well as the corresponding controls was assessed by Transwell assay.
(K) The protein levels of MMP2 and MMP9 in VSMCs treated with 100
µg/ml ox-LDL, ox-LDL + si-circ_0010283 or ox-LDL +
si-circ_0010283 + anti-miR-370-3p as well as the corresponding
controls were detected by western blotting. *P<0.05.
VMSCs, vascular smooth muscle cells; ox-LDL, oxidized low-density
lipoprotein; RT-qPCR, reverse transcription-quantitative PCR; si,
small interfering; circ, circular RNA; NC, negative control; miR,
microRNA; WT, wild-type; MUT, mutant; RIP, RNA immunoprecipitation;
Ago2, argonaute 2; IgG, immunoglobulin G; OD, optical density. |
circ_0010283 regulates HMGB1 expression
via miR-370-3p
To further determine the regulatory mechanism of
miR-370-3p, its possible target genes were predicted by starBase,
which identified that miR-370-3p could bind to the 3′UTR of HMGB1
(Fig. 3A). This interaction was
confirmed by the dual-luciferase reporter assay (Fig. 3B). The mRNA and protein levels of
HMGB1 were also measured, and the results demonstrated that HMGB1
was upregulated in VSMCs treated with 100 µg/ml ox-LDL for
48 or 72 h (Fig. 3C and D).
Analogously, the expression level of HMGB1 was significantly
increased in VSMCs treated with 75 or 100 µg/ml ox-LDL for
72 h (Fig. 3E and F). In
addition, miR-370-3p mimics decreased the mRNA and protein levels
of HMGB1 (Fig. 3G and H).
Knockdown of circ_0010283 downregulated HMGB1 expression, whereas
anti-miR-370-3p reversed this effect (Fig. 3I and J). These results suggested
that HMGB1 was a target of miR-370-3p, and circ_0010283 positively
regulated HMGB1 expression via miR-370-3p.
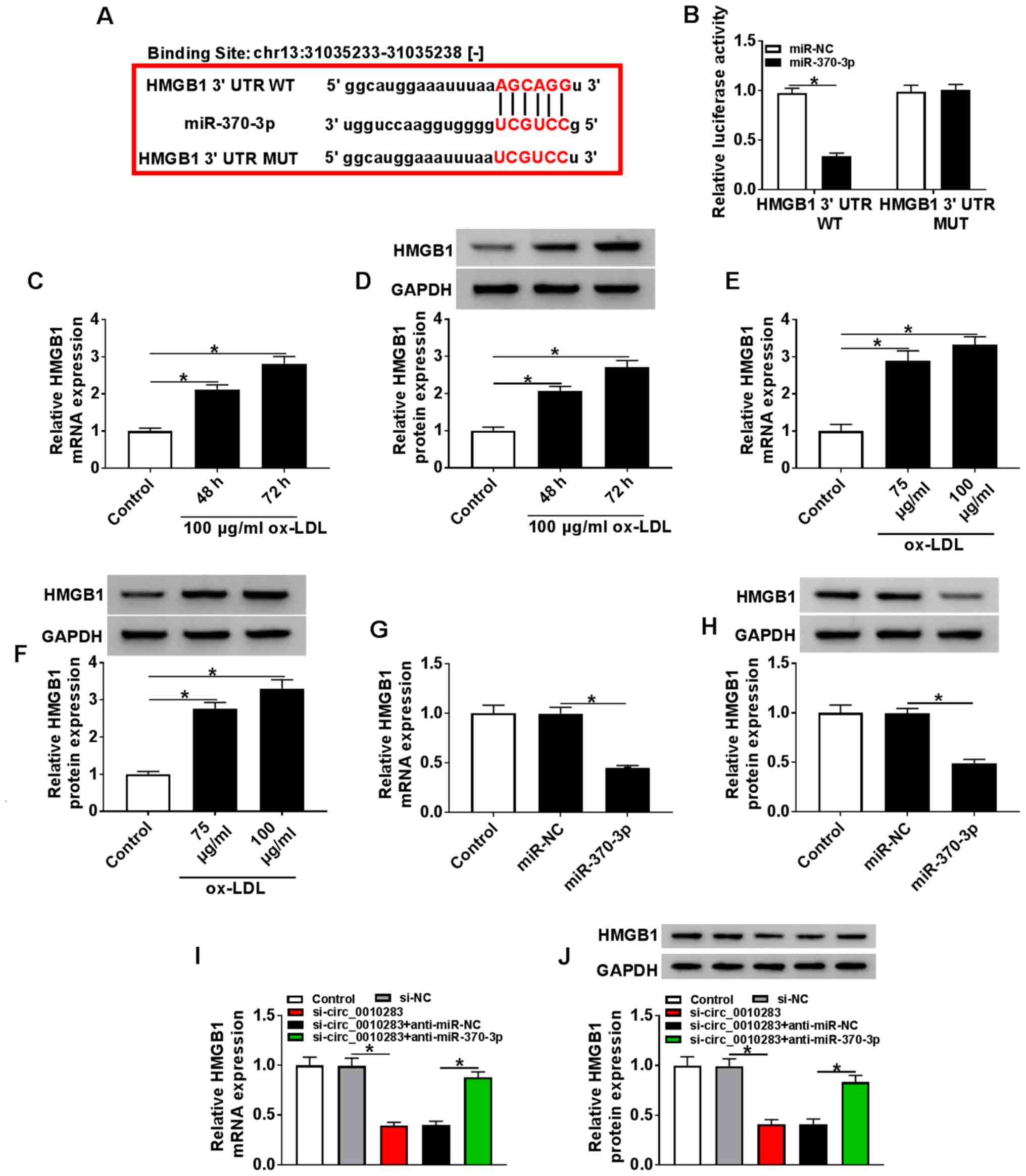 | Figure 3circ_0010283 regulates HMGB1
expression via miR-370-3p. (A) The potential target sites between
miR-370-3p and HMGB1 were predicted by starBase. (B) The luciferase
activity of HMGB1 3′UTR WT and HMGB1 3′UTR MUT was detected in
VSMCs co-transfected with miR-370-3p or miR-NC. (C and D) The mRNA
and protein levels of HMGB1 in VSMCs treated with 0 or 100
µg/ml ox-LDL for 48 or 72 h were determined by RT-qPCR and
western blotting, respectively. (E and F) The mRNA and protein
levels of HMGB1 in VSMCs treated with 75 or 100 µg/ml ox-LDL
for 72 h were assessed by RT-qPCR and western blotting,
respectively. (G and H) The mRNA and protein levels of HMGB1 in
VSMCs transfected with the miR-370-3p mimics or NC were detected by
RT-qPCR and western blotting, respectively. (I and J) The mRNA and
protein levels of HMGB1 in VSMCs treated with si-circ_0010283 or
si-circ_0010283 + anti-miR-370-3p as well as the corresponding
controls were determined by RT-qPCR and western blotting,
respectively. *P<0.05. VMSCs, vascular smooth muscle
cells; ox-LDL, oxidized low-density lipoprotein; RT-qPCR, reverse
transcription-quantitative PCR; si, small interfering; circ,
circular RNA; NC, negative control; miR, microRNA; UTR,
untranslated region; WT, wild-type; MUT, mutant; HMGB1, high
mobility group box 1. |
Overexpression of HMGB1 reverses the
miR-370-3p-mediated suppression on the viability and migration of
ox-LDL-induced VSMCs
To investigate the regulatory relationship between
miR-370-3p and HMGB1 in atherosclerosis progression, VSMCs were
transfected with an HMGB1 over-expression vector, and the
transfection efficiency was verified in ox-LDL-induced and
untreated VSMCs by RT-qPCR and western blotting (Figs. S3A, B, S2D and E). Subsequently,
the mRNA and protein levels of HMGB1 in VSMCs treated with ox-LDL,
ox-LDL + miR-370-3p or ox-LDL + miR-370-3p + HMGB1, as well as
matched controls were detected. The results demonstrated that HMGB1
expression was downregulated in ox-LDL-induced VSMCs treated with
the miR-370-3p mimic compared with those treated with ox-LDL and
miR-NC; however, the expression of HMGB1 was upregulated in the
ox-LDL + miR-370-3p group following transfection with the HMGB1
overexpression vector (Fig. 4A and
B). The results of the MTT assay revealed that overexpression
of HMGB1 significantly inhibited the miR-370-3p-mediated repression
of the viability of ox-LDL-induced VSMCs (Fig. 4C). In addition, the decreased
protein levels of cyclin D1 and PCNA in the ox-LDL + miR-370-3p
group were partially reversed following HMGB1 overexpression
(Fig. 4D). Analogously, the
miR-370-3p-mediated inhibitory effect on the migration of
ox-LDL-induced VSMCs was reversed by HMGB1 over-expression
(Fig. 4E). In addition, the low
protein levels of MMP2 and MMP9 in ox-LDL-induced VSMCs transfected
with the miR-370-3p mimics were rescued following HMGB1
overexpression (Fig. 4F). In
summary, these results suggested that miR-370-3p regulated the
viability and migration of ox-LDL-induced VSMCs by targeting
HMGB1.
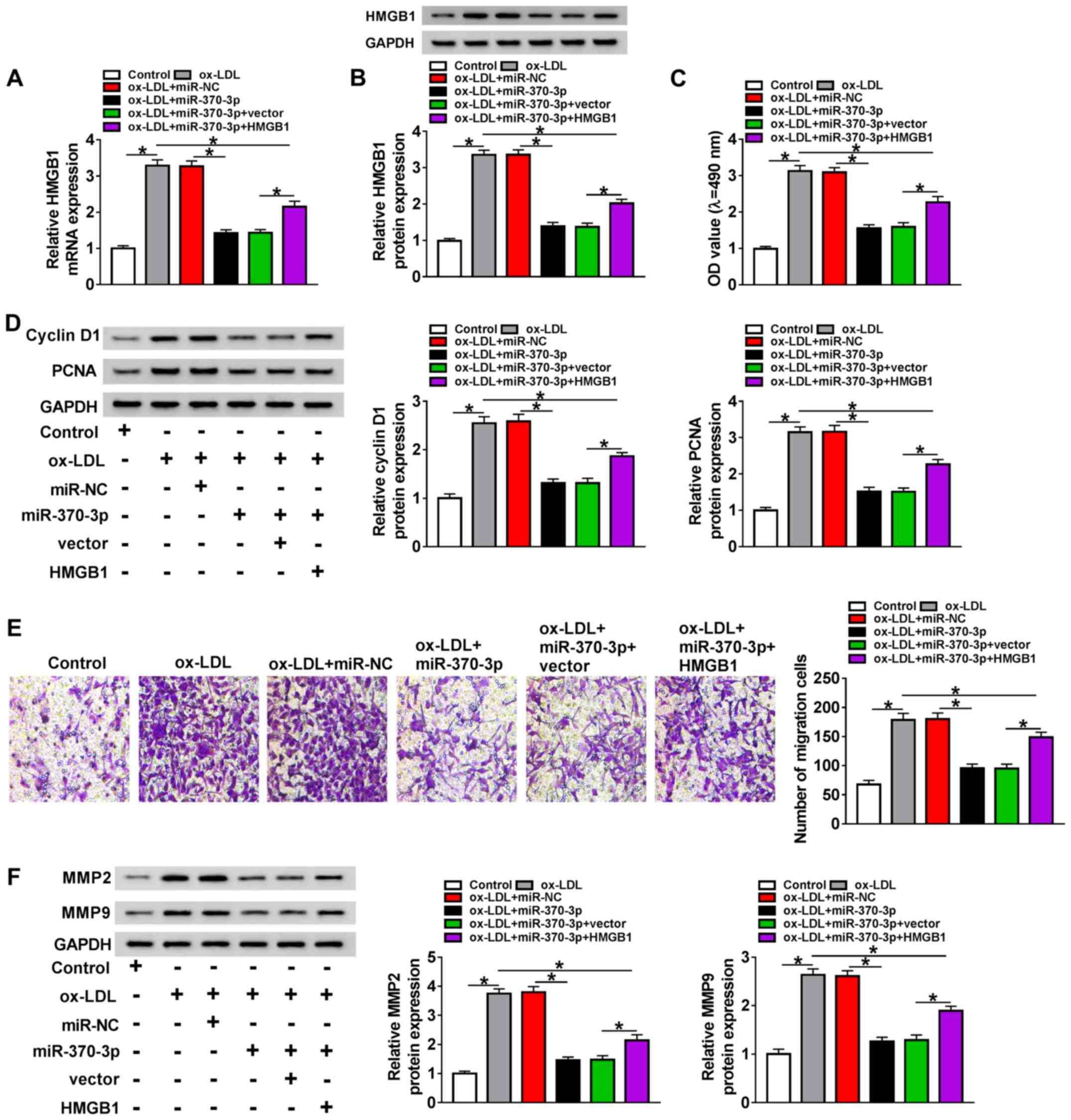 | Figure 4Overexpression of HMGB1 reverses the
miR-370-3p-mediated effects on VSMC viability and migration. VSMCs
were treated with 100 µg/ml ox-LDL, ox-LDL + miR-370-3p or
ox-LDL + miR-370-3p + HMGB1 as well as the corresponding controls.
(A and B) The mRNA and protein levels of HMGB1 were assessed by
reverse transcription-quantitative PCR and western blotting,
respectively. (C) Cell viability was determined by MTT assay. (D)
The protein levels of cyclin D1 and PCNA in all groups were
detected by western blot. (E) Cell migration was assessed by
Transwell assay. (F) The protein levels of MMP2 and MMP9 in the
samples were measured by western blotting. *P<0.05.
VMSCs, vascular smooth muscle cells; ox-LDL, oxidized low-density
lipoprotein; miR, microRNA; NC, negative control; HMGB1, high
mobility group box 1; PCNA, proliferating cell nuclear antigen;
MMP, matrix metalloproteinase; OD, optical density. |
Discussion
Atherosclerosis is a growing threat to human health,
and the dysfunction of VSMCs has been reported to be involved in
the formation of atherosclerosis (28,29). In the present study, VSMCs were
treated with ox-LDL to establish the cell model for atherosclerosis
based on previous studies (29,30). Recently, circRNAs have been
identified to serve a pivotal role in various human diseases.
Garikipati et al (8) have
reported that circular RNA circFndc3b regulate cardiac repair after
myocardial infarction. Zhou et al (9) have demonstrated that circRNA HECT
domain-containing E3 ubiquitin protein ligase 1 (HECTD1) mediates
silica-induced macrophage activation by regulating the
circHECTD1/HECTD1 axis and zinc finger CCCH-type containing
12A-dependent ubiquitination process. Zhou et al (10) have reported that circular RNA
hsa_circ_0016070 is associated with pulmonary arterial
hypertension. In addition, circRNA antisense non-coding RNA in the
INK4 locus and circRNA checkpoint with forkhead and ring finger
domains (circCHFR) have been confirmed to modulate the progression
of atherosclerosis (11,12). In the present study, the
expression of circ_0010283 was elevated in ox-LDL-induced VSMCs
compared with that in non-induced cells, which was also supported
by the microarray results of a previous study (12). In addition, the results of the
present study demonstrated that circ_0010283 was induced by ox-LDL
in a time- and concentration-dependent manner in VSMCs, suggesting
that circ_0010283 may be associated with the progression of
atherosclerosis. A previous study has demonstrated that circCHFR,
which is also induced by ox-LDL in VSMCs, promotes the development
of atherosclerosis (31).
Similarly, the present results indicated that knockdown of
circ_0010283 hindered the viability and migration of ox-LDL-induced
VSMCs, and reduced the expression levels of the
viability-associated proteins cyclin D1 and PCNA, and the
migration-associated proteins MMP2 and MMP9. Taken together, these
results suggested that circ_0010283 contributed to atherosclerosis
progression and may be a potential biomarker for
atherosclerosis.
Emerging evidence has revealed that circRNAs act as
miRNA sponges in various human diseases, including diabetic retinal
vascular dysfunction and myocardial fibrosis (26,27). The results of the present study
demonstrated that miR-370-3p was a target of circ_0010283.
miR-370-3p has been reported to partake in a number of human
diseases. For example, Zhang et al (16) have demonstrated that miR-370-3p is
associated with acute pneumonia, and Hou et al (17) have reported that miR-370-3p
suppresses VSMC viability in cerebral aneurysm. In addition,
circ_0003204 can directly target miR-370-3p to repress the
progression of atherosclerosis (32). In the present study, miR-370-3p
was downregulated in ox-LDL-induced VSMCs compared with non-induced
cells, and was negatively modulated by circ_0010283. In-depth
experiments demonstrated that inhibition of miR-370-3p reversed the
circ_0010283 silencing-mediated repressive effects on the viability
and migration of ox-LDL-induced VSMCs. These results suggested that
circ_0010283 may regulate the progression of atherosclerosis by
sponging miR-370-3p. In addition, the inhibitory role of miR-370-3p
in atherosclerosis progression was observed, which was consistent
with previous studies (19,32).
To deeply understand the regulatory mechanism of
miR-370-3p in atherosclerosis, its target genes were predicted, and
HMGB1 was identified as a target of miR-370-3p. HMGB1 has been
reported to accelerate foam cell formation and cholesterol
accumulation in VSMCs (33). In
addition, HMGB1 inhibition may be a potential therapeutic strategy
for atherosclerosis (34). In the
present study, the mRNA and protein levels of HMGB1 were measured,
and the results demonstrated that the expression levels of HMGB1
were upregulated in ox-LDL-induced VSMCs compared with those in
untreated cells, which was in line with a previous study (35). In addition, HMGB1 was demonstrated
to be negatively regulated by miR-370-3p in the present study, and
circ_0010283 upregulated HMGB1 expression via miR-370-3p. Further
results indicated that overexpression of HMGB1 reversed the
miR-370-3p-me-diated suppressive effects on the viability and
migration of ox-LDL-induced VSMCs. Additionally, the low mRNA and
protein levels of HMGB1 in the si-circ_0010283 group were reversed
following transfection with a miR-370-3p inhibitor. Taken together,
these results suggested that circ_0010283 may act as a sponge of
miR-370-3p to upregulate the expression of HMGB1, thus promoting
the viability and migration of ox-LDL-induced VSMCs (Fig. S4).
There were certain limitations in the current study.
Previous studies have elucidated that HMGB1 affects VSMC
development by regulating various signaling pathways, such as
PI3K/Akt and p38 mitogen-activated protein kinase/nuclear factor κB
path-ways (36,37). Whether the
circ_0010283/miR-370-3p/HMGB1 axis regulates atherosclerosis
progression via these pathways needs to be further explored.
Addtionally, the role of the circ_0010283/miR-370-3p/HMGB1 axis in
atherosclerosis in vivo also has not been demonstrated.
These issues will be the focus of our future study.
In conclusion, the results of the present study
demonstrated that circ_0010283 was significantly upregulated in
ox-LDL-induced VSMCs. In addition, circ_0010283 regulated the
viability and migration of ox-LDL-induced VSMCs via the
miR-370-3p/HMGB1 axis. This novel mechanism may provide new
effective therapeutic methods for athero-sclerosis.
Supplementary Data
Funding
No funding was received.
Availability of data and materials
The datasets analyzed and/or generated during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YD and YT conceived and designed the study. YT and
XL collected and analyzed the data. PD and YT performed and
validated the experiments. PD, YD and YT wrote, edited and reviewed
the manuscript. All authors read and approval the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansson GK, Libby P and Tabas I:
Inflammation and plaque vulnerability. J Intern Med. 278:483–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015. View Article : Google Scholar
|
|
4
|
Tapia-Vieyra JV, Delgado-Coello B and
Mas-Oliva J: Atherosclerosis and cancer; A resemblance with
far-reaching implications. Arch Med Res. 48:12–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennett MR, Sinha S and Owens GK: Vascular
smooth muscle cells in atherosclerosis. Circ Res. 118:692–702.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kristensen LS, Andersen MS, Stagsted LVW,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
8
|
Garikipati VNS, Verma SK, Cheng Z, Liang
D, Truongcao MM, Cimini M, Yue Y, Huang G, Wang C, Benedict C, et
al: Circular RNA CircFndc3b modulates cardiac repair after
myocardial infarction via FUS/VEGF-A axis. Nat Commun. 10:43172019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Z, Jiang R, Yang X, Guo H, Fang S,
Zhang Y, Cheng Y, Wang J, Yao H and Chao J: circRNA mediates
silica-induced macrophage activation via HECTD1/ZC3H12A-dependent
ubiquitination. Theranostics. 8:575–592. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou S, Jiang H, Li M, Wu P, Sun L, Liu Y,
Zhu K, Zhang B, Sun G, Cao C and Wang R: Circular RNA
hsa_circ_0016070 is associated with pulmonary arterial hypertension
by promoting PASMC viability. Mol Ther Nucleic Acids. 18:275–284.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holdt LM, Stahringer A, Sass K, Pichler G,
Kulak NA, Wilfert W, Kohlmaier A, Herbst A, Northoff BH, Nicolaou
A, et al: Circular non-coding RNA ANRIL modulates ribosomal RNA
maturation and atherosclerosis in humans. Nat Commun. 7:124292016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Yang F, Zhao H, Wang M and Zhang
Y: Circular RNA circCHFR facilitates the proliferation and
migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1
Pathway. Mol Ther Nucleic Acids. 16:434–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebert LFR and MacRae IJ: Regulation of
microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37.
2019. View Article : Google Scholar :
|
|
14
|
Schober A, Nazari-Jahantigh M, Wei Y,
Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H,
Hristov M, et al: MicroRNA-126-5p promotes endothelial
proliferation and limits atherosclerosis by suppressing Dlk1. Nat
Med. 20:368–376. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei Y, Nazari-Jahantigh M, Chan L, Zhu M,
Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C and
Schober A: The microRNA-342-5p fosters inflammatory macro-phage
activation through an Akt1- and microRNA-155-dependent pathway
during atherosclerosis. Circulation. 127:1609–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhu Y, Gao G and Zhou Z:
Knockdown XIST alleviates LPS-induced WI-38 cell apoptosis and
inflammation injury via targeting miR-370-3p/TLR4 in acute
pneumonia. Cell Biochem Funct. 37:348–358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou WZ, Chen XL, Wu W and Hang CH:
MicroRNA-370-3p inhibits human vascular smooth muscle cell
proliferation via targeting KDR/AKT signaling pathway in cerebral
aneurysm. Eur Rev Med Pharmacol Sci. 21:1080–1087. 2017.PubMed/NCBI
|
|
18
|
Lyu L, Zhang X, Li C, Yang T, Wang J, Pan
L, Jia H, Li Z, Sun Q, Yue L, et al: Small RNA profiles of serum
exosomes derived from individuals with latent and active
tuberculosis. Front Microbiol. 10:11742019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi X and Chen X: Effect of microRNA-370
on coronary atherosclerosis and its underlying mechanism. Exp Ther
Med. 17:115–122. 2019.PubMed/NCBI
|
|
20
|
Wu CY, Zhou ZF, Wang B, Ke ZP, Ge ZC and
Zhang XJ: MicroRNA-328 ameliorates oxidized low-density
lipoprotein-induced endothelial cells injury through targeting
HMGB1 in atherosclerosis. J Cell Biochem. Oct 15–2018.Epub ahead of
print.
|
|
21
|
Moreno JA, Sastre C, Madrigal-Matute J,
Muñoz-García B, Ortega L, Burkly LC, Egido J, Martín-Ventura JL and
Blanco-Colio LM: HMGB1 expression and secretion are increased via
TWEAK-Fn14 interaction in atherosclerotic plaques and cultured
monocytes. Arterioscler Thromb Vasc Biol. 33:612–620. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Tsai NP, Lin YL, Tsui YC and Wei LN: Dual
action of epidermal growth factor: Extracellular signal-stimulated
nuclear-cytoplasmic export and coordinated translation of selected
messenger RNA. J Cell Biol. 188:325–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42(Database Issue): D92–D97. 2014. View Article : Google Scholar
|
|
25
|
Hartley A, Haskard D and Khamis R:
Oxidized LDL and anti-oxidized LDL antibodies in
atherosclerosis-novel insights and future directions in diagnosis
and therapy <sup/>. Trends Cardiovasc Med. 29:22–26. 2019.
View Article : Google Scholar
|
|
26
|
Zhu K, Hu X, Chen H, Li F, Yin N, Liu AL,
Shan K, Qin YW, Huang X, Chang Q, et al: Downregulation of circRNA
DMNT3B contributes to diabetic retinal vascular dysfunction through
targeting miR-20b-5p and BAMBI. EBioMedicine. 49:341–353. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou B and Yu JW: A novel identified
circular RNA, circRNA_010567, promotes myocardial fibrosis via
suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res
Commun. 487:769–775. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kattoor AJ, Kanuri SH and Mehta JL: Role
of Ox-LDL and LOX-1 in atherogenesis. Curr Med Chem. 26:1693–1700.
2019. View Article : Google Scholar
|
|
29
|
Liu M, Song Y and Han Z: Study on the
effect of LncRNA AK094457 on OX-LDL induced vascular smooth muscle
cells. Am J Transl Res. 11:5623–5633. 2019.PubMed/NCBI
|
|
30
|
Xu K, Xiwen Liu, Ren G, Yin D, Guo S and
Zhao Y: Depletion of CPEB1 protects against oxidized LDL-induced
endothelial apoptosis and inflammation though SIRT1/LOX-1
signalling pathway. Life Sci. 239:1168742019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang JB, Li T, Hu XM, Ning M, Gao WQ,
Lang YH, Zheng WF and Wei J: Circ_CHFR expedites cell growth,
migration and inflammation in ox-LDL-treated human vascular smooth
muscle cells via the miR-214-3p/Wnt3/β-catenin pathway. Eur Rev Med
Pharmacol Sci. 24:3282–3292. 2020.PubMed/NCBI
|
|
32
|
Zhang S, Song G, Yuan J, Qiao S, Xu S, Si
Z, Yang Y, Xu X and Wang A: Circular RNA circ_0003204 inhibits
proliferation, migration and tube formation of endothelial cell in
atheroscle-rosis via miR-370-3p/TGFβR2/phosph-SMAD3 axis. J Biomed
Sci. 27:112020. View Article : Google Scholar
|
|
33
|
Wang R, Wu W, Li W, Huang S, Li Z, Liu R,
Shan Z, Zhang C, Li W and Wang S: Activation of NLRP3 inflammasome
promotes foam cell formation in vascular smooth muscle cells and
athero-genesis via HMGB1. J Am Heart Assoc. 7:e0085962018.
View Article : Google Scholar
|
|
34
|
Fan Z, Yang J, Yang J, Yang C and Guo X:
HMGB1: A promising therapeutic approach for atherosclerosis. Int J
Cardiol. 202:507–508. 2016. View Article : Google Scholar
|
|
35
|
Chen Z, Pan X, Sheng Z, Yan G, Chen L and
Ma G: Baicalin suppresses the proliferation and migration of
Ox-LDL-VSMCs in atherosclerosis through upregulating miR-126-5p.
Biol Pharm Bull. 42:1517–1523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang J, Chen L, Yang J, Ding J, Rong H,
Dong W and Li X: High mobility group box-1 induces migration of
vascular smooth muscle cells via TLR4-dependent PI3K/Akt pathway
activation. Mol Biol Rep. 39:3361–3367. 2012. View Article : Google Scholar
|
|
37
|
Chen J, Zhang J, Xu L, Xu C, Chen S, Yang
J and Jiang H: Inhibition of neointimal hyperplasia in the rat
carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis.
224:332–339. 2012. View Article : Google Scholar : PubMed/NCBI
|


















