Introduction
Hepatocellular carcinoma (HCC) is one of the most
aggressive types of cancer worldwide and accounts for >80% of
primary hepatic cancers. The majority causes of HCC are liver
cirrhosis, genetic alterations and living habits (1). It is characterized by a delayed
diagnosis, complex pathogenesis, vascular invasion, metastasis and
multidrug resistance (2,3). Although interventional prevention
and therapeutic strategies, including vaccination, surgical
resection, transplantation and immunotherapy have been adopted
clinically, a high incidence and low survival rate are still
insurmountable challenges for HCC (4–6).
Therefore, the investigation of the biological mechanisms
responsible for the development of HCC and the identification of
novel biomarkers for HCC therapy are urgently required.
Long non-coding RNAs (lncRNAs) refer to a class of
RNAs with >200 endogenous nucleotides in length. Despite not
having a protein coding capacity, they participate in a series of
physiological and pathological processes, including
epithelial-mesenchymal transition (EMT), stem cell pluripotency
reprogramming, RNA splicing, transcriptional silencing/activation
and chromatin remodeling (7–9).
Homeobox A11 antisense RNA (HOXA11-AS) is a highly conserved gene
that has been shown to be involved in the regulation of
post-transcription during tumorigenesis and embryogenesis (10). The aberrant expression of
HOXA11-AS is associated with cell staging, and the metastasis and
invasion of various types of cancer, such as glioma, laryngeal
squamous cell carcinoma (LSCC) and HCC (11,12). For instance, HOXA11-AS expression
has been shown to be upregulated in glioma cells and the knockdown
of HOXA11-AS markedly suppresses glioma cell proliferation by
regulating the miR-214-3p/EZH2 axis (9). Similarly, it has been demonstrated
that the overexpression of HOXA11-AS significantly promotes
cisplatin resistance by modulating miR-454-3p/Stat3 in human lung
adenocarcinoma cells (13).
lncRNAs exert effects on tumor progression through
various mechanisms, including their microRNA (miRNA/miR) binding
function. miRNAs are non-coding RNAs comprised of 19–25 endogenous
nucleotides. Typically, they function as crucial regulators of
various types of cancer, including nasopharyngeal carcinoma (NPC),
pancreatic ductal adenocarcinoma (PDAC) and HCC by complementary
interacting with their target genes (14,15). As oncogenes or suppressors, miRNAs
play essential roles in cell proliferation, differentiation,
metabolism and apoptosis. For instance, miR-BART8-3p has been
proven to induce EMT and to promote NPC cell progression by
deactivating immune response (16,17). Inversely, the upregulation of
miR-200b has been shown to inhibit HCC cell adhesion and migration
by targeting high mobility protein HMGB3 (18). Currently, miR-506 is regarded as a
novel biomarker for cancer diagnosis. Moreover, starBase has
indicated that miR-506-3p is a target gene of HOXA11-AS. Thus, the
physiological role of miR-506-3p in HCC was investigated in the
present study.
In the present study, the expression of HOXA11-AS
and miR-506-3p was detected and the interaction between miR-506-3p
and HOXA11-AS or Slug was further investigated. It was found that
HOXA11-AS accelerates proliferation, invasion and EMT in HCC by
modulating the miR-506-3p/Slug axis, thus providing possible,
promising biomarkers for the treatment of HCC.
Materials and methods
Tissue samples
A total of 39 pairs HCC tumor and corresponding
non-tumor tissue samples were obtained from patients recruited from
the Third Hospital of Hebei Medical University. The characteristics
of the patients with HCC are presented in Table I. The patients had not received
any pre-operative therapy and their basic pathological
characteristics were recorded. The recruited patients signed
written informed consent forms. All the protocols were proved by
the Ethics Committee of the Third Hospital of Hebei Medical
University.
 | Table IAssociation between the clinical
characteristics of the patients with hepatocellular carcinoma
(n=78) and HOXA11-AS expression. |
Table I
Association between the clinical
characteristics of the patients with hepatocellular carcinoma
(n=78) and HOXA11-AS expression.
| Parameter | No. of
patients | HOXA11-AS
expression
| P-value |
|---|
| High (n=40) | Low (n=38) |
|---|
| Age (years) | | | | |
| ≤60 | 44 | 15 | 29 | 0.479 |
| ≥60 | 31 | 25 | 6 | |
| Sex | | | | |
| Male | 37 | 27 | 10 | 0.535 |
| Female | 41 | 13 | 28 | |
| Tumor size
(cm) | | | | |
| ≤7 | 46 | 12 | 34 | 0.032a |
| ≥7 | 32 | 28 | 4 | |
| Tumor staging
(HCC) | | | | |
| I+II | 36 | 10 | 26 | 0.019a |
| III | 42 | 30 | 12 | |
Cells and cell transfection
The HCC cell lines, Huh7, Hep3B and PLC/PRF/5, and
the human hepatic epithelial cell line, THLE-3, were purchased from
ATCC. In addition, the HCC cell line, MHCC97-H, was obtained from
the Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences. The Huh7, Hep3B and THLE-3 cells were maintained in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
and 0.05% penicillin/streptomycin and incubated in a 5%
CO2 incubator at 37°C. Small interfering RNA (siRNA)
targeting HOXA11-AS (si-HOXA11-AS, including si-HOXA11-AS#1:
5′-TCGTCACTCGGTGTTCTCACCGAAA-3′, si-HOXA11-AS#2:
5′-GCACGGTGACTTGATTACACTCTCT-3′, and si-HOXA11-AS#3:
5′-CGGAAACGGCTAACAAGGAGATTTG-3′) and siRNA negative control
(si-NC), pcDNA-HOXA11-AS overexpression vector (HOXA11-AS) and
negative control (Vector), pcDNA-Slug overexpression vector (Slug)
and its negative control (control) were synthesized by Genepharma.
The miRNA mimic (miR-506-3p) and negative control (miR-NC),
miR-506-3p inhibitor (anti-miR-506-3p) and negative control
inhibitor (anti-miR-NC) were purchased from Guangzhou RiboBio Co.,
Ltd. 0.2 μg of HOXA11-AS overexpression vector, Slug
overexpression vector or pcDNA vector was transfected in Huh7 and
Hep3B cells (4×104 cells) with 0.5 μl of
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). 0.5 μg of those oligonucleotides including
si-HOXA11-AS, si-NC, miR-506-3p, miR-NC, anti-miR-506-3p or
anti-miR-NC was transfected into cells using 0.6 μl of
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
After of 48 h transfection, the transfected cells were used in
subsequent experimentats.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The Huh7 and Hep3B cells were incubated with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) to obtain the
RNA. The All-in-One™ miRNA First-Strand cDNA Synthesis kit
(GeneCopoeia, Inc.) was applied to synthesize cDNA and the qPCR was
performed using TaqMan Gene Expression assay (Applied Biosystems).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6 were used
as internal controls. The amplification parameters were as follows:
Denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec and
extension at 72°C for 1 min. The primers used were as follows:
HOXA11-AS forward, 5′-GCCAAGTTGTACTTACTACGTC-3′ and reverse,
5′-GTTGGAGGAGTAGGAGTATGTA-3′; miR-506-3p forward,
5′-CGGGCTAAGGCACCCTTCTG-3′ and reverse, 5′-GTGCAGGGTCCGAGGTATTC-3′;
E-cadherin forward, 5′-GAGCCTGAGTCCTGCAGTCC-3′ and reverse,
5′-TGTATTGCTGCTTGGCCTCA-3′; N-cadherin forward,
5′-GTGCCATTAGCCAAGGGAATTCAGC-3′ and reverse,
5′-GCGTTCCTGTTCCACTCATAGGAGG-3′; Vimentin forward,
5′-TGCCCTTGAAGCTGCTAACTAC-3′ and reverse,
5′-CAACCAGAGGAAGTGCATCCAG-3′; GAPDH forward,
5′-CCCACTCCTCCACCTTTGAC-3′ and reverse,
5′-GGATCTCGCTCCTGGAAGATG-3′; and U6 forward,
5′-GCUUCGGCAGCACAUAUACUAAAAU-3′ and reverse,
5′-CGCUUCACGAAUUUGCGUGUCAU-3′. Relative expression was calculated
using the 2−ΔΔCq method (19).
Western blot analysis
Total protein was isolated from tissues and cell
lines using RIPA lysis buffer (EMD Millipore). The concentration of
total protein was measured using a BCA Protein assay kit (Nanjing
KeyGen Biotech Co., Ltd.). Protein (30 μg) was separated by
12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF)
membranes (EMD Millipore). The membranes were then blocked with 5%
skim milk at room temperature for 2 h. Subsequently, the membranes
were incubated with the following primary antibodies: E-cadherin
(1:1,000; cat. no. 14472), N-cadherin (1:1,000, cat. no. 13116),
Vimentin (1:1,000, cat. no. 5741), Slug (1:1,000, cat. no. 9585),
GAPDH (1:1,000, cat. no. 5174) (all from Cell Signaling Technology,
Inc.) overnight at 4°C. Following incubation with the primary
antibodies, the membranes were incubated with a the goat
anti-rabbit IgG H&L (HRP) (1:2,000, cat. no. ab205718, Abcam)
and goat anti-mouse IgG H&L (HRP) (1:2,000, cat. no. ab205719,
Abcam) secondary antibodies for 40 min at room temperature.
Finally, protein bands were visualized using the Enhanced
Chemiluminescence Plus kit (EMD Millipore) and quantified by
densitometric analysis of protein signals using ImageJ version 1.49
(National Institutes of Health).
Nuclear and cytoplasmic localization
analysis
RNA was isolated from the cell cytoplasm and nucleus
using the Cytoplasmic and Nuclear RNA Purification kit (Norgen
Biotek). Subsequently, total RNA in each fraction was measured by
RT-qRCR as described above. U6 and GAPDH functioned as internal
references for the nucleus and cytoplasm, respectively.
Cell proliferation and invasion
CCK-8 and Transwell assays were utilized for the
evaluation of cell proliferation and cell invasive ability. For
CCK-8 assay, the transfected Huh7 and Hep3B cells were seeded in
96-well plates (5,000 cells/well) and continuously incubated for a
further 24, 48, 72 and 96 h in a 5% CO2 incubator at
37°C. After rinsing with PBS 3 times, 10 μl CCK-8 (5 mg/ml;
Beyotime Institute of Biotechnology, Inc.) were added to each well
for 2 h. The absorption at 450 nm was measured using a microplate
reader (Bio-Rad Laboratories, Inc). For Transwell assay,
transfected Huh7 and Hep3B cells (2×105 cells/well) in
200 μl serum-free medium were seeded into the upper chamber
pre-treated with Matrigel (BD Biosciences). Complete medium with
10% FBS was seeded into the lower chamber. After incubation for 24
h at 37°C, the invasive cells in the lower chamber were stained
with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) at room
temperature for 10 min and counted using an inverted microscope
(Nikon Eclipse TS100; Nikon Corporation).
Luciferase reporter assay
StarBase (http://starbase.sysu.edu.cn/starbase2/) and TargetScan
(http://www.targetscan.org/vert_71/)
were used to screen the putative target. Wild-type and mutant type
sequences (HOXA11-AS-Wt, HOXA11-AS-Mut, Slug-Wt, Slug-Mut) were
constructed and cloned into the pGL3 basic vector (Invitrogen;
Thermo Fisher Scientific, Inc.). Huh7 and Hep3B cells were
co-transfected with the luciferase vectors and miR-506-3p mimics or
miR-NC for 24 h using Lipofectamine 2000 transfection reagent.
Luciferase activities were measured by a dual-luciferase assay
system (Promega). Firefly luciferase activities were normalized to
Renilla luciferase activities.
RNA pull-down assay
Biotinylated HOXA11-AS (Bio-HOXA11-AS), miR-506-3p
(Bio-miR-506-3p), HOXA11-AS Mut (Bio-HOXA11-AS-Mut), miR-506-3p Mut
(Bio-miR-506-3p-Mut), and negative control (Bio-miR-NC) (Sangon
Biotech Co., Ltd.) were transfected into the Huh7 and Hep3B cells.
Following incubation for 24 h, the transfected cells were lysed,
collected and incubated with Dynabeads M-280 Streptavidin
(Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min. The bound
RNAs were then subjected to RT-qPCR for quantification and analysis
as described above.
Statistical analysis
All the data were collected and analyzed using SPSS
software (SPSS, Inc.) and GraphPad Prism 7 (GraphPad Inc.). Data
are presented as the means ± SD. Comparisons between 2 groups were
evaluated using a Student's t-test. One-way ANOVA followed by
Tukey's test was used for differences among multiple groups. The
correlation between miR-506-3p and HOXA11-AS or Slug was analyzed
using Pearson's correlation coefficient. The association between
HOXA11-AS and the patient clinicopathological characteristics were
analyzed using the χ2 test or Fisher's exact test. A
P-value <0.05 (P<0.05) was considered to indicate a
statistically significant difference.
Results
HOXA11-AS is upregulated, whereas
miR-506-3p is downregulated in HCC tissues and cell lines
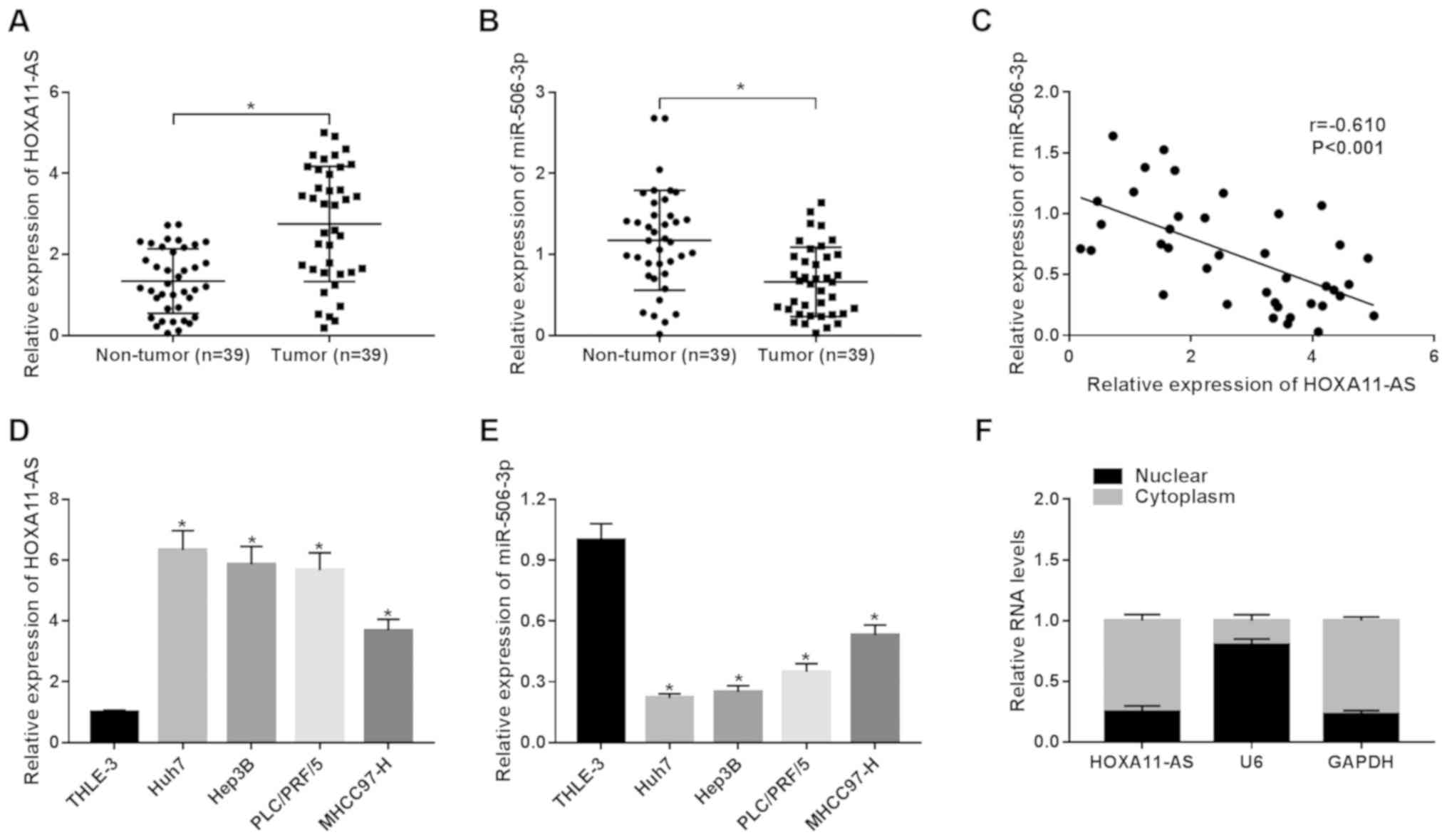
To explore the roles of HOXA11-AS and miR-506-3p in
HCC, the expression of HOXA11-AS and miR-506-3p in 39 pairs of HCC
tissues and the corresponding non-tumor tissues was detected by
RT-qPCR. HOXA11-AS was upregulated, whereas miR-506-3p was
downregulated in the HCC tumor samples in comparison to the
non-tumor samples (Fig. 1A and
B). Moreover, it was found that HOXA11-AS negatively correlated
with miR-506-3p (r=−0.610, P<0.001) (Fig. 1C). Furthermore, the expression of
HOXA11-AS was significantly enhanced in the HCC cell lines (Huh7,
Hep3B, PLC/PRF/5 and MHCC97-H) compared with the human hepatic
epithelial cells, THLE-3 (Fig.
1D). On the contrary, the expression of miR-506-3p was lower in
the HCC cells than in the THLE-3 cells (Fig. 1E). The data also suggested that
HOXA11-AS was mainly located in the cytoplasm (Fig. 1F). These findings demonstrate that
HOXA11-AS and miR-506-3p play vital roles in HCC.
HOXA11-AS directly interacts with
miR-506-3p
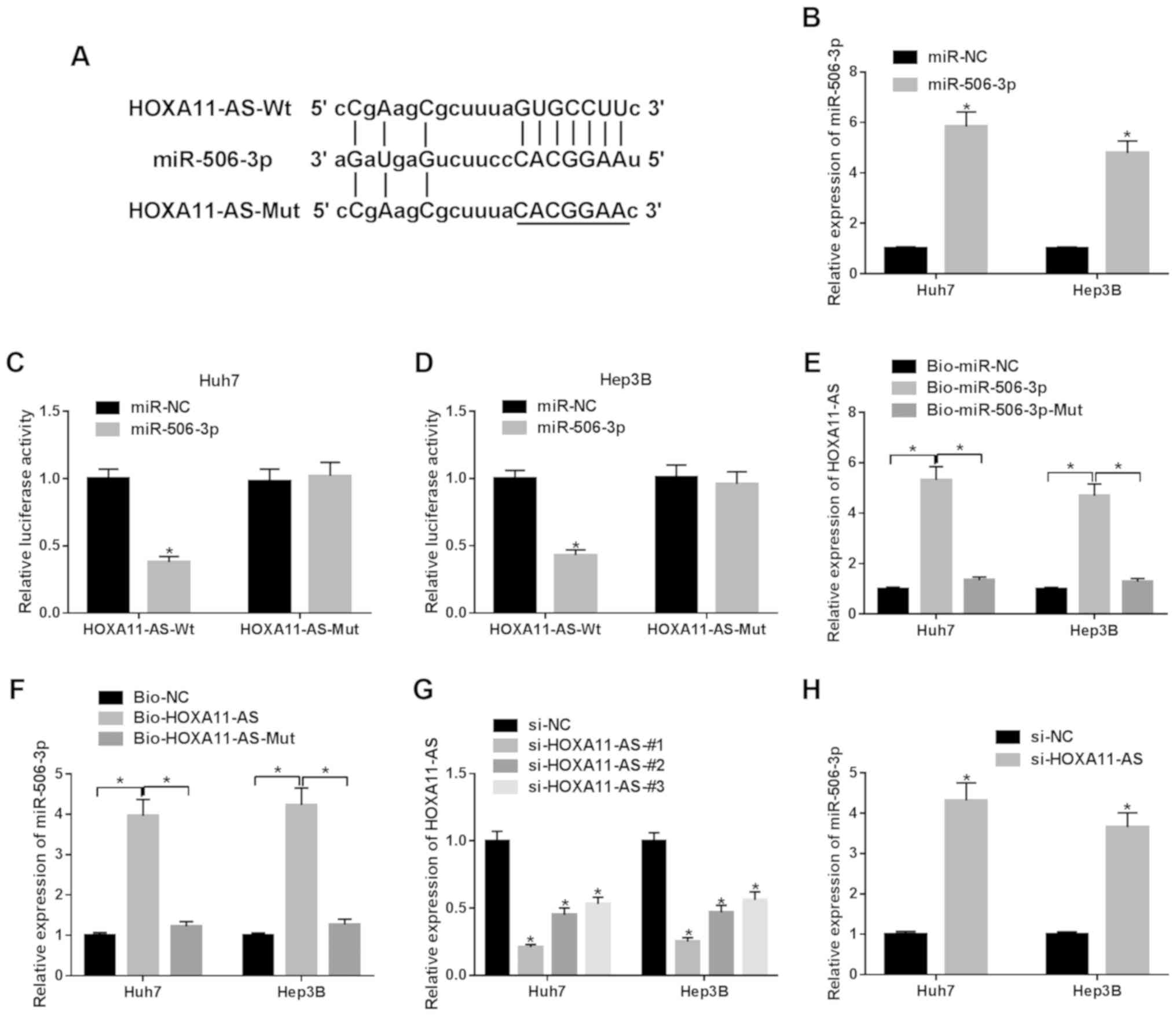
Bioinformatics prediction program StarBase was
employed to search the potential target miRNA for HOXA11-AS. It was
observed that miR-506-3p may bind to HOXA11-AS (Fig. 2A). As shown in Fig. 2B, miR-506-3p exhibited successful
efficiency in both the Huh7 and Hep3B cells. The luciferase
activity in the HCC cells co-transfected with miR-506-3p and
HOXA11-AS-Wt was markedly suppressed in comparison to the control
group (Fig. 2C and D). RNA
pull-down assay exhibited that the enrichment of HOXA11-AS was
observed in the HCC cells transfected with Bio-miR-506-3p compared
with Bio-miR-506-3p-Mut or Bio-miR-NC transfection (Fig. 2E). Similarly, the enrichment of
miR-506-3p was observed in the HCC cells transfected with
Bio-HOXA11-AS (Fig. 2F).
Furthermore, it was observed that the expression of HOXA11-AS was
decreased in the HCC cells transfected with si-HOXA11-AS#1,
si-HOXA11-AS#2, si-HOXA11-AS#3 compared with the si-NC group
(Fig. 2G). Due to the highest
knockdown efficiency, si-HOXA11-AS#1 was selected for use in
subsequent experiments. It was found that HOXA11-AS knockdown
increased the expression of miR-506-3p in HCC cells (Fig. 2H). Taken together, it was thus
concluded that miR-506-3p was negatively regulated by
HOXA11-AS.
HOXA11-AS promotes HCC progression by
targeting miR-506-3p
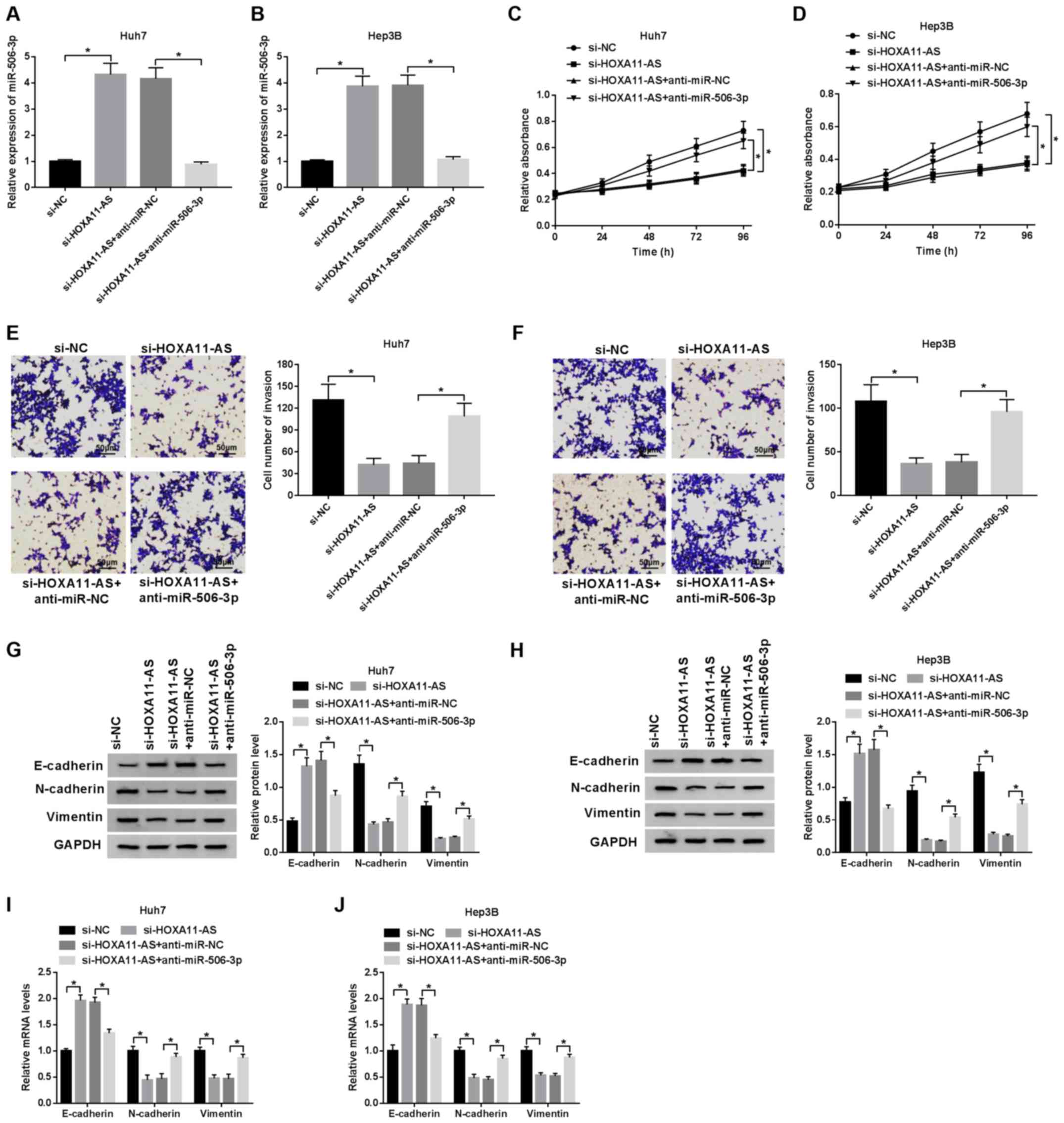
Subsequently, the regulatory mechanisms of the
HOXA11-AS/miR-506-3p axis were investigated in the HCC cells. The
successful transfection efficiency of anti-miR-506-3p and HOXA11-AS
in the HCC cells was observed (Fig.
S1A and B). The expression of miR-506-3p, elevated by HOXA11-AS
silencing, was reversed by transfection with miR-506-3p inhibitor
(Fig. 3A and B), while the
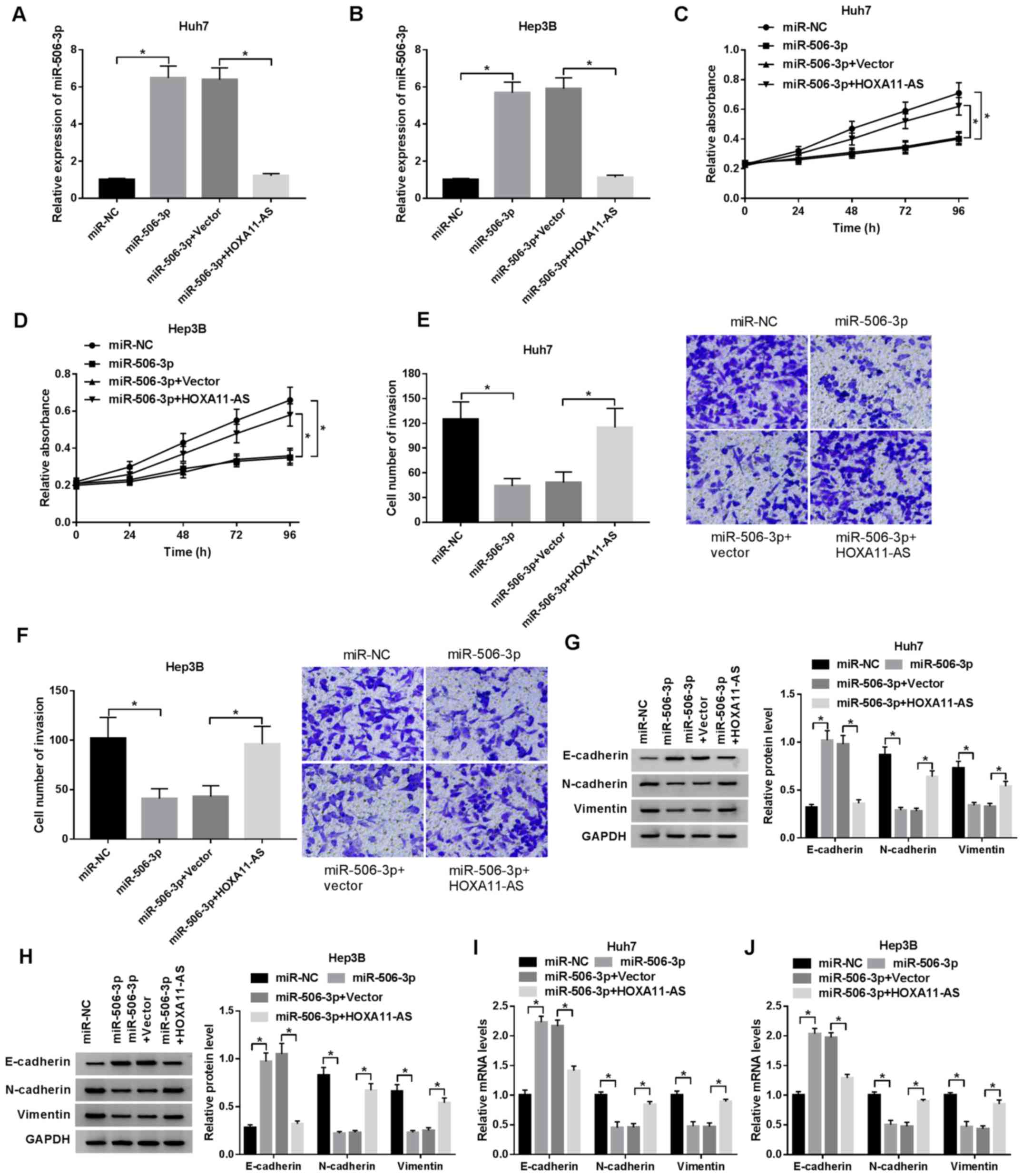
increase in miR-506-3p expression induced by transfection with
miR-506-3p mimic was suppressed by the overexpression of HOXA11-AS
in both the Huh7 and Hep3B cells (Fig. 4A and B). The results of CCK-8
assay revealed that the inhibitory effects of HOXA11-AS silencing
on cell proliferation were blocked by transfection with miR-506-3p
inhibitor (Fig. 3C and D), and
the overexpression of HOXA11-AS reversed the inhibitory effect of
miR-506-3p upregulation on cell proliferation (Fig. 4C and D). Moreover, cell invasion
was suppressed by HOXA11-AS silencing; however, this effect was
reversed by transfection with miR-506-3p inhibitor (Fig. 3E and F). In addition, the
inhibition effect of miR-506-3p overexpression on cell invasion was
abolished by the upregulation of HOXA11-AS (Fig. 4E and F). Furthermore, the protein
expression of EMT markers was examined by western blot analysis in
order to determine the effect of HOXA11-AS and miR-506-3p on EMT in
HCC cells. The mRNA and protein expression of E-cadherin enhanced
by HOXA11-AS silencing was suppressed by transfection with
miR-506-3p inhibitor, and the decreased mRNA and protein expression
of N-cadherin and vimentin induced by HOXA11-AS silencing was
promoted by miR-506-3p downregulation (Fig. 3G-J). Moreover, the promotion of
E-cadherin mRNA and protein expression, and the inhibition of the
mRNA and protein expression of N-cadherin and vimentin induced by
miR-506-3p overexpression were reversed by HOXA11-AS upregulation
in both the Huh7 and Hep3B cells (Fig. 4G-J). Collectively, these data
demonstrate that HOXA11-AS regulates cell proliferation, invasion
and EMT by targeting miR-506-3p.
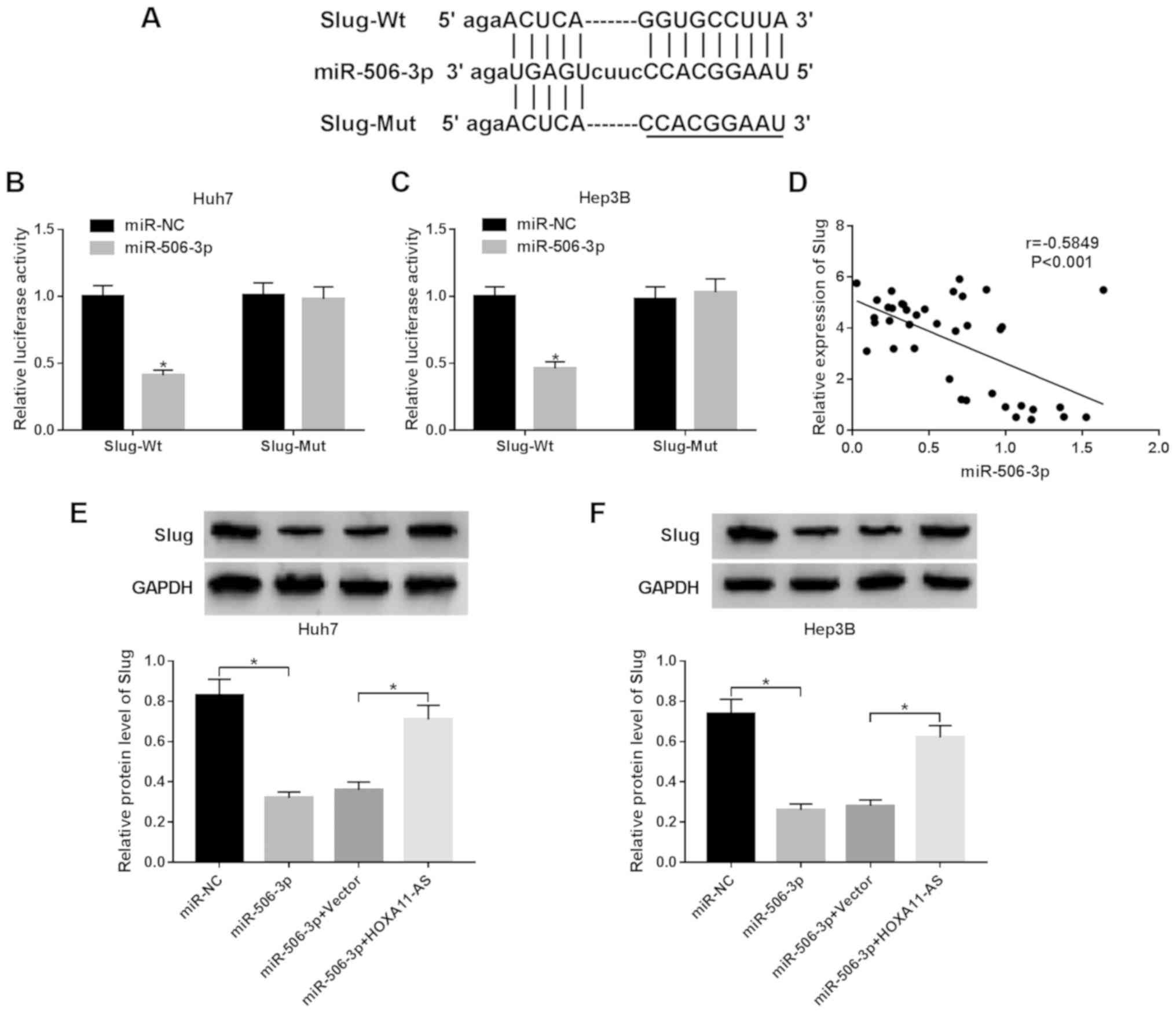
Slug is a target gene of miR-506-3p
TargetScan predicted that miR-506-3p contained a
complementary sequence of Slug (Fig.
5A). To confirm this prediction, wild-type Slug (Slug-Wt) or
mutant type Slug (Slug-Mut) was cloned into the luciferase gene and
co-transfected with miR-506-3p or miR-NC. The luciferase activity
was markeldy reduced in the Huh7 and Hep3B cells co-transfected
with Slug-Wt and miR-506-3p compared with miR-NC group, while the
luciferase activity was not altered in the HCC cell co-transfected
with Slug-Mut and miR-506-3p (Fig. 5B
and C). Moreover, we found a negative relationship between
miR-506-3p and Slug (r=−5849, P<0.001) (Fig. 5D). In addition, western blot
analysis revealed that the protein expression of Slug was decreased
by miR-506-3p overexpression, which was reversed by HOXA11-AS
overexpression in the Huh7 and Hep3B cells (Fig. 5E and F). These data indicate that
HOXA11-AS targets miT-506-3p to regulate Slug expression.
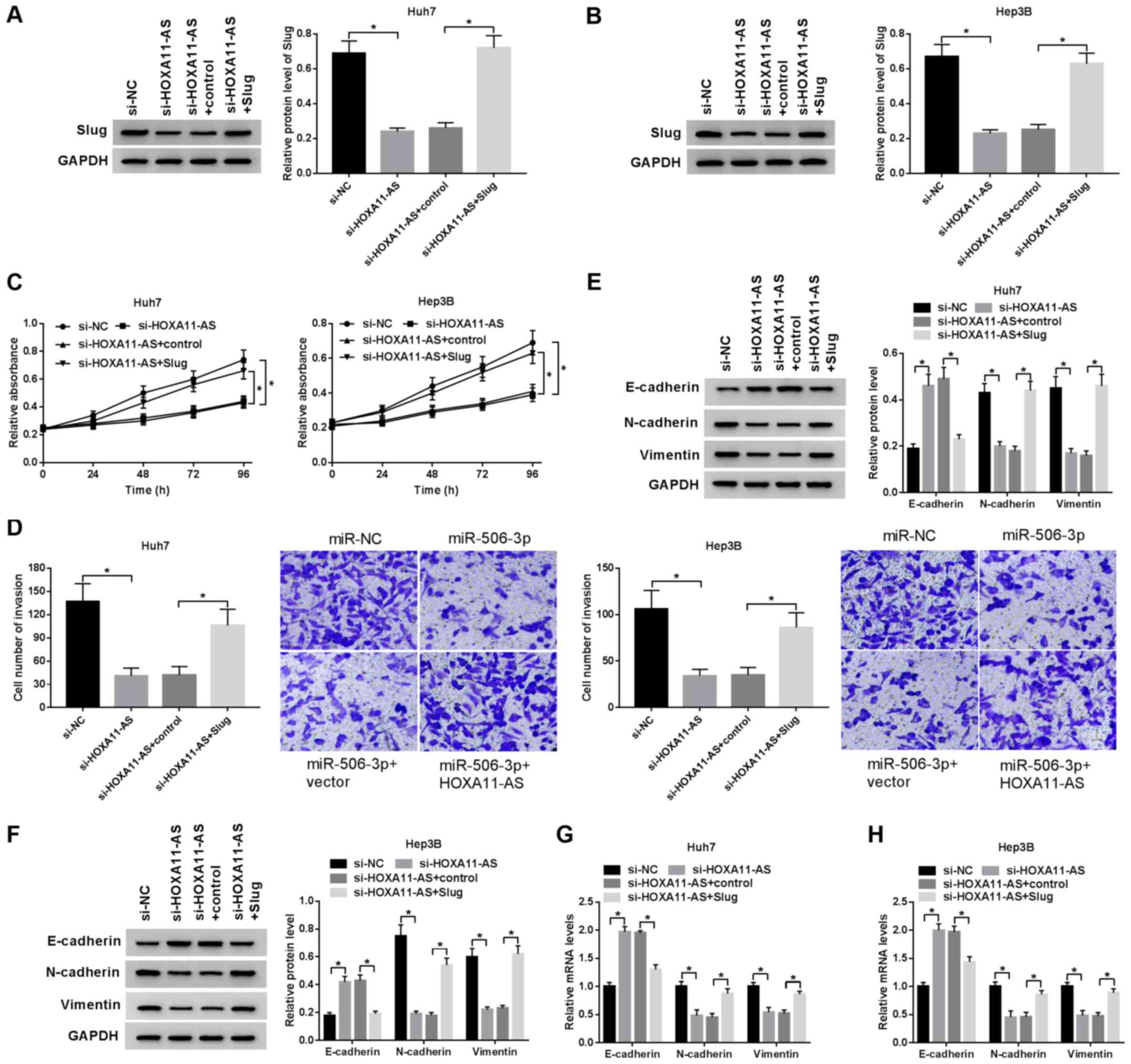
Slug attenuates the inhibitory effect on
HCC progression induced by HOXA11-AS silencing
To examine the regulatory effect of the
HOXA11-AS/Slug axis on HCC cell proliferation, invasion and EMT,
the Huh7 and Hep3B cells were transfected with si-HOXA11-AS+Slug,
si-HOXA11-AS, si-HOXA11-AS + control and si-NC. Western blot
analysis revealed that overexpression of Slug significantly
promoted Slug expression (Fig. S1C
and D). Slug protein expression inhibited by HOXA11-AS
silencing was recovered by Slug overexpression in the Huh7 and
Hep3B cells, demonstrating that Slug overexpression reversed the
inhibitory effects mediated by HOXA11-AS silencing (Fig. 6A and B). Moreover, the
upregulation of Slug reversed the inhibitory effects of HOXA11-AS
downregulation on the proliferation of HCC cells (Fig. 6C). Similarly, Slug overexpression
reversed the suppressive effects on cell invasion induced by
HOXA11-AS silencing (Fig. 6D).
Furthermore, the downregulation of HOXA11-AS promoted E-cadherin
mRNA and protein expression, but suppressed N-cadherin and vimentin
mRNA and protein expression in the HCC cells; these effects were
reversed by Slug overexpression (Fig.
6E-H). Overall, HOXA11-AS promoted HCC cell proliferation,
invasion and EMT by upregulating Slug expression.
Discussion
Previous studies have identified that lncRNAs play
fundamental roles as promoters or suppressors of the cell cycle,
infiltration, differentiation and metabolism in various types of
cancer, including HCC (20-22). For example, UCA1 has been shown to
function as a promoter to enhance HCC cell migration and G1/S
transition by improving CDK6 expression (23). Huang et al found that SNHG1
was highly expressed in HCC tissues and cells, which served as an
oncogene in HCC progression (24). miRNAs have also been confirmed to
be involved in various cellular biological behaviors, such as cell
proliferation, differentiation and death in HCC (25,26). For instance, miR-212 has been
shown to be expressed at low levels in HCC and to exert inhibitory
effects on tumor angiogenesis, migration and invasion by
inactivating Wnt/b-Catenin signaling (27). Wang et al suggested that
miR-383 functions as a suppressor of HCC cell growth (28).
In the present study, HOXA11-AS was found to be
significantly increased HCC in tissues and cells. Moreover,
HOXA11-AS has been confirmed to function as a ceRNA to participate
in tumor progression by interacting with specific miRNAs (29,30). For instance, HOXA11-AS
upregulation has been shown to regulate cell growth via targeting
miR-761 in papillary thyroid cancer (31). HOXA11-AS has been demonstrated to
play a promoting role in the progression of oral squamous cell
carcinoma by sponging miR-98-5p (32). Furthermore, HOXA11-AS has been
proven to function as a sponge for miR-125a-5p to upregulate PADI2
expression and further promote colorectal cancer cell metastasis
(33). In addition, Zhan et
al demonstrated that sufficiency of HOXA11-AS accelerated cell
growth and EMT by suppressing miR-214-3p in HCC (34).
StarBase predicted that miR-506-3p contained the
potential binding sites of HOXA11-AS. The results indicated that
miR-506-3p was a target of HOXA11-AS and negatively regulated by
HOXA11-AS. miR-506-3p has been identified to play tumor suppressive
role in several human cancers, such as ovarian cancer and
retinoblastoma (35,36). For example, miR-506-3p has been
demonstrated to suppress tumor progression in prostate cancer and
pancreatic cancer (37,38). Furthermore, miR-506-3p has been
shown to be downregulated in non-small lung cancer tissues and
cells, and the overexpression of miR-506-3p suppresses cell growth,
migration and invasion (39).
Consistent with these previous studies, in the present study,
miR-506-3p was found to be significantly decreased in HCC tissues
and cells. Moreover, HOXA11-AS exerted promoting effects on cell
growth, invasion and EMT via sponging miR-506-3p in HCC.
Slug (Snai2), a member of the Snail family
(transcription factor of C2H2-type zinc finger), is a transcription
factor involved in the process of EMT. Accumulating evidence has
indicated that Slug is related to cell invasion and metastasis in
various types of tumor (40). For
example, SNHG15 regulates Slug expression to enhance colon cancer
progression (41). Moreover, a
high expression of Slug has been observed in HCC, which plays a
promoting role in HCC progression (42). In the present research, the
results revealed that Slug was a target gene of miR-506-3p.
HOXA11-AS could sponge miR-506-3p to regulate the expression of
Slug in HCC cells. Furthermore, Slug overexpression blocked the
inhibitory effects of HOXA11-AS downregulation on cell growth,
invasion and EMT.
In conclusion, the present study demonstrated that
HOXA11-AS functioned as an oncogene to promote HCC progression and
EMT by elevating Slug expression via targeting miR-506-3p.
Furthermore, the regulatory mechanisms of the
HOXA11-AS/miR-506-3p/Slug axis in HCC development were elucidated,
providing promising biomarkers for the diagnosis and therapy of
HCC.
Supplementary Data
Funding
The present study was supported by the Task Force of
Hebei Education Department (grant no. 2008505) and Artificial liver
in patients with liver failure clinical study on the individualized
treatment (G201734).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YL was involved in the conceptualization of the
study and in data curation, and in the writing of the original
draft. YL and WY were involved in formal analysis and in the
investigative aspects of the study. WY was involved in the study
methodology. DZ was involved in project administration. GJ provided
resources. XC provided software. GJ and XC were also involved in
the writing, reviewing and editing of the manuscript. DZ, GJ and XC
were also involved in the conception and design of the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The recruited patients signed written informed
consent forms. All the protocols were proved by the Ethics
Committee of the Third Hospital of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Soliman B, Salem A, Ghazy M, Abu-Shahba N
and El Hefnawi M: Bioinformatics functional analysis of let-7a,
miR-34a, and miR-199a/b reveals novel insights into immune system
pathways and cancer hallmarks for hepatocellular carcinoma. Tumour
Biol. 40:10104283187736752018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Wang S, Xu J, Kou B, Chen D, Wang Y
and Zhu X: Extract of Stellerachamaejasme L(ESC) inhibits growth
and metastasis of human hepatocellular carcinoma via regulating
microRNA expression. BMC Complement Altern Med. 18:992018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hughes A and Dhoot GK: Dysregulated cancer
cell transdifferentiation into erythrocytes is an additional
metabolic stress in hepatocellular carcinoma. Tumour Biol.
40:10104283188114672018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Sun J, Zhang N, Yang R, Li H,
Zhang Y, Chen K and Kong D: PES1 enhances proliferation and
tumorigenesis in hepatocellular carcinoma via the PI3K/AKT pathway.
Life Sci. 219:182–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang S, Zhu P, Sun B, Guo J, Zhou H, Shu
Y and Li Q: Modulation of YrdC promotes hepatocellular carcinoma
progression via MEK/ERK signaling pathway. Biomed Pharmacother.
114:1088592019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheng N and Li Y, Qian R and Li Y: The
clinical significance and biological function of lncRNA RGMB-AS1 in
hepatocellular carcinoma. Biomed Pharmacother. 98:577–584. 2018.
View Article : Google Scholar
|
|
7
|
XU CH, Xiao LM, Liu Y, Chen LK, Zheng SY,
Zeng EM and Li DH: The lncRNA HOXA11-AS promotes glioma cell growth
and metastasis by targeting miR-130a-5p/HMGB2. Eur Rev Med
Pharmacol Sci. 2:241–252. 2019.
|
|
8
|
Jin QS, Huang LJ, Zhao TT, Yao XY, Lin LY,
Teng YQ, Kim SH, Nam MS, Zhang LY and Jin YJ: HOXA11-AS regulates
diabetic arteriosclerosis-related inflammation via PI3K/AKT
pathway. Eur Rev Med Pharmacol Sci. 22:6912–6921. 2018.PubMed/NCBI
|
|
9
|
Xu C, He T, Li Z, Liu H and Ding B:
Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth,
migration and invasion of glioma cells. Biomed Pharmacother.
95:1504–1513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu CW, Zhou DD, Xie T, Hao JL, Pant OP, Lu
CB and Liu XF: HOXA11 antisense long noncoding RNA (HOXA11-AS): A
promising lncRNA in human cancers. Cancer Med. 7:3792–3799. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu L, Jin M, Yang L, Sun C, Wang P, Li Y,
Tian L, Liu M and Sun Y: Expression of long non-coding RNA
HOXA11-AS is correlated with progression of laryngeal squamous cell
carcinoma. Am J Transl Res. 10:573–580. 2018.PubMed/NCBI
|
|
12
|
Yang FQ, Zhang JQ, Jin JJ, Yang CY, Zhang
WJ, Zhang HM, Zheng JH and Weng ZM: HOXA11-AS promotes the growth
and invasion of renal cancer by sponging miR-146b-5p to upregulate
MMP16 expression. J Cell Physiol. 233:9611–9619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Li X, Zhou L, Ni J, Yan W, Ma R,
Wu J, Feng J and Chen P: LncRNA HOXA11-AS drives cisplatin
resistance of human LUAD cells via modulating miR-454-3p/Stat3.
Cancer Sci. 109:3068–3079. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang TS, Zheng YJ, Wang J, Zhao JY, Yang
DK and Liu ZS: MicroRNA-506 inhibits tumor growth and metastasis in
nasopharyngeal carcinoma through the inactivation of the
Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin
Cancer Res. 38:972019. View Article : Google Scholar
|
|
15
|
He H, Liao X, Yang Q, Liu Y, Peng Y, Zhong
H, Yang J, Zhang H, Yu Z, Zuo Y, et al: MicroRNA-494-3p promotes
cell growth, migration, and invasion of nasopharyngeal carcinoma by
targeting sox7. Technol Cancer Res Treat. 17:15330338188099932018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin C, Zong J, Lin W, Wang M, Xu Y, Zhou
R, Lin S, Guo Q, Chen H, Ye Y, et al: EBV-miR-BART8-3p induces
epithelial-mesenchymal transition and promotes metastasis of
nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2
pathways. J Exp Clin Cancer Res. 37:2832018. View Article : Google Scholar
|
|
17
|
Yi F, Hao Y, Chong X and Zhong W:
Overexpression of microRNA-506-3p aggravates the injury of vascular
endothelial cells in patients with hypertension by downregulating
Beclin1 expression. Exp Ther Med. 15:2844–2850. 2018.PubMed/NCBI
|
|
18
|
Wang LK, Xie XN, Song XH, Su T, Chang XL,
Xu M, Liang B and Huang DY: Upregulation of miR-200b inhibits
hepatocellular carcinoma cell proliferation and migration by
targeting HMGB3 protein. Technol Cancer Res Treat.
17:15330338188064752018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Hu H, Yang L, Li L and Zeng C: Long
non-coding RNA KCNQ1OT1 modulates oxaliplatin resistance in
hepatocellular carcinoma through miR-7-5p/ABCC1 axis. Biochem
Biophys Res Commun. 503:2400–2406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu S, Jiang X, Su Z, Cui Z, Fu W and Tai
S: The role of the long non-coding RNA HOXA11-AS in promoting
proliferation and metastasis of malignant tumors. Cell Biol Int.
42:1596–1601. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Misawa A, Takayama K, Urano T and Inoue S:
Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes
cell growth and inhibits apoptosis in prostate cancer cells. J Biol
Chem. 291:17861–17880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YL, Liu JY, Yang JE, Yu XM, Chen ZL,
Chen YJ, Kuang M, Zhu Y and Zhuang SM: Lnc-UCID promotes G1/S
transition and hepatoma growth by preventing DHX9-mediated CDK6
down-regulation. Hepatology. 70:259–275. 2019.PubMed/NCBI
|
|
24
|
Huang D, Wei Y, Zhu J and Wang F: Long
non-coding RNA SNHG1 functions as a competitive endogenous RNA to
regulate PDCD4 expression by sponging miR-195-5p in hepatocellular
carcinoma. Gene. 714:1439942019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Q, Chen Y and Liu K: Erratum:
miR-185 inhibits cell migration and invasion of hepatocellular
carcinoma through CDC42. Oncol Lett. 19:10892020.PubMed/NCBI
|
|
26
|
Hu QL, Xu ZP, Lan YF and Li B: miR-636
represses cell survival by targeting CDK6/Bcl-2 in cervical cancer.
Kaohsiung J Med Sci. 36:328–335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia P, Wei G, Zhou C, Gao Q, Wu Y, Sun X
and Li X: Upregulation of MiR-212 inhibits migration and
tumorigenicity and inactivates Wnt/β-catenin signaling in human
hepatocellular carcinoma. Technol Cancer Res Treat.
17:15330346187652212018. View Article : Google Scholar
|
|
28
|
Wang J, Lu L, Luo Z, Li W, Lu Y, Tang Q
and Pu J: miR-383 inhibits cell growth and promotes cell apoptosis
in hepatocellular carcinoma by targeting IL-17 via STAT3 signaling
pathway. Biomed Pharmacother. 120:1095512019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cui Y, Yi L, Zhao JZ and Jiang YG: Long
noncoding RNA HOXA11-AS functions as miRNA sponge to promote the
glioma tumorigenesis through targeting miR-140-5p. DNA Cell Biol.
36:822–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu W, Peng W, Jiang H, Sha H and Li J:
LncRNA HOXA11-AS promotes proliferation and invasion by targeting
miR-124 in human non-small cell lung cancer cells. . Tumour Biol.
39:10104283177214402017. View Article : Google Scholar
|
|
31
|
Yin X, Zhang J, Li C, Zhang Z, Jin T, Song
L, Zhang R, Wang W, Tao Y and Wang X: LncRNA HOXA11-AS
accumulation-induced microRNA-761 downregulation regulates cell
growth by targeting TRIM29 in papillary thyroid cancer. Am J Transl
Res. 11:6826–6837. 2019.PubMed/NCBI
|
|
32
|
Niu X, Yang B, Liu F and Fang Q: LncRNA
HOXA11-AS promotes OSCC progression by sponging miR-98-5p to
upregulate YBX2 expression. Biomed Pharmacother. 121:1096232020.
View Article : Google Scholar
|
|
33
|
Chen D, Sun Q, Zhang L, Zhou X, Cheng X,
Zhou D, Ye F, Lin J and Wang W: The lncRNA HOXA11-AS functions as a
competing endogenous RNA to regulate PADI2 expression by sponging
miR-125a-5p in liver metastasis of colorectal cancer. Oncotarget.
8:70642–70652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhan M, He K, Xiao J, Liu F, Wang H, Xia
Z, Duan X, Huang R, Li Y, He X, et al: LncRNA HOXA11-AS promotes
hepatocellular carcinoma progression by repressing miR-214-3p. J
Cell Mol Med. 22:3758–3767. 2018. View Article : Google Scholar :
|
|
35
|
Wang Y, Lei X, Gao C, Xue Y, Li X, Wang H
and Feng Y: MiR-506-3p suppresses the proliferation of ovarian
cancer cells by negatively regulating the expression of MTMR6. J
Biosci. 44:1262019. View Article : Google Scholar
|
|
36
|
Wu L, Chen Z and Xing Y: MiR-506-3p
inhibits cell proliferation, induces cell cycle arrest and
apoptosis in retinoblastoma by directly targeting NEK6. Cell Biol
Int. Aug 6–2018.Epub ahead of print. View Article : Google Scholar
|
|
37
|
Hu CY, You P, Zhang J, Zhang H and Jiang
N: MiR-506-3p acts as a novel tumor suppressor in prostate cancer
through targeting GALNT4. Eur Rev Med Pharmacol Sci. 23:5133–5138.
2019.PubMed/NCBI
|
|
38
|
Huang B, Liu C, Wu Q, Zhang J, Min Q,
Sheng T, Wang X and Zou Y: Long non-coding RNA NEAT1 facilitates
pancreatic cancer progression through negative modulation of
miR-506-3p. Biochem Biophys Res Commun. 482:828–834. 2017.
View Article : Google Scholar
|
|
39
|
Guo S, Yang P, Jiang X, Li X, Wang Y,
Zhang X, Sun B, Zhang Y and Jia Y: Genetic and epigenetic silencing
of mircoRNA-506-3p enhances COTL1 oncogene expression to foster
non-small lung cancer progression. Oncotarget. 8:644–657. 2017.
View Article : Google Scholar :
|
|
40
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang H, Li T, Qu Y, Wang X, Li B, Song J,
Sun X, Tang Y, Wan J, Yu Y, et al: Long non-coding RNA SNHG15
interacts with and stabilizes transcription factor Slug and
promotes colon cancer progression. Cancer Lett. 425:78–87. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui RJ, Fan JL, Lin YC, Pan YJ, Liu C, Wan
JH, Wang W, Jiang ZY, Zheng XL, Tang JB and Yu XG: miR-124-3p
availability is antagonized by LncRNA-MALAT1 for Slug-induced tumor
metastasis in hepatocellular carcinoma. Cancer Med. 8:6358–6369.
2019. View Article : Google Scholar : PubMed/NCBI
|