Introduction
Head and neck squamous cell carcinoma (HNSCC),
including tumors arising in the nasopharynx, oropharynx and the
oral cavity, are frequent malignancies with a worldwide incidence
of >650,000 cases and 330,000 deaths per year (1,2).
HNSCC belongs to a family of tumors for which a strong association
with combined alcohol and tobacco use has been established
(1,3,4).
In contrast to the overall declining incidence rates, numerous
epidemiological studies recently highlighted a marked increase of
human papillomavirus (HPV)-associated HNSCC in various western
countries, emerging as a growing public health concern (5–7).
Interestingly, HPV-associated (HPV+) HNSCC appears to be
more sensitive to radiotherapy, which contributes in part to the
observed better prognosis (4,8).
Currently, the viral oncoproteins E6 and E7 are considered to be
responsible for impaired DNA damage response upon exposure to
ionizing radiation (IR) in HPV+ HNSCC due to degradation
of p53 and the inactivation of retinoblastoma family members, but
the precise mechanism underlying the enhanced radiosensitivity of
HPV-associated tumors remains elusive (9,10).
Numerous studies have highlighted the impact of
heterogeneous nuclear ribonucleoprotein (hnRNP) K as a key player
in tumorigenesis (11,12). This multifunctional protein acts
as a highly integrated docking platform for various signaling
pathways and regulates the expression of target genes, as well as
the translation of mRNA transcripts associated with cancer
initiation and progression (12,13). High hnRNP K expression was
repeatedly reported in various cancer tissues, including HNSCC, and
linked to advanced tumor stage as well as enhanced invasiveness,
increased propensity for metastasis and poor clinical outcome
(14–19). Accordingly, it was reported that
hnRNP K knockdown reduces the tumorigenic characteristics of
multiple cancer cells (18).
Furthermore, hnRNP K represents a crucial co-factor
for the p53-mediated DNA damage repair (DDR) upon exposure to IR.
DNA damage finally results in the upregulation of transcripts for
p53 target genes in a p53/hnRNP K-codependent manner (20). As a typical consequence,
IR-induced arrest of cell cycle progression at the G1 or G2 phase
is mediated by the p53/hnRNP K target proteins p21 or 14-3-3σ,
respectively (21).
Finally, hnRNP K has been shown to be indispensable
for an effective cellular IR-induced DDR in various cell models,
including HNSCC (22,23).
Interestingly, a recent proteomic study in HNSCC
cells revealed increased cellular hnRNP K levels in response to IR,
whereas hnRNP K knockdown impaired HNSCC cell migration, which is a
prerequisite for metastasis (24).
The aim of the present study was to analyze whether
hnRNP K expression contributes to the differences in
radioresistance between HPV+ and HPV- HNSCC.
It was hypothesized that the known responsiveness of HPV-dependent
HNSCC to radiotherapy may be associated with higher expression of
hnRNP K compared with HPV-independent HNSCC. Therefore, the
functional consequences of hnRNP K in the tumorigenic
characteristics of HNSCC cells were investigated applying
immunohistochemistry (IHC), knockdown analyses and a chick egg
chorioallantoic membrane (CAM) assay as a preclinical in
vivo tumor model. Furthermore, as the prognosis of patients
with HNSCC is associated with sex, potential differences in hnRNP K
expression between HNSCC samples from female and male patients were
investigated. The results of the present study may facilitate
further stratification of HNSCC patients beyond HPV status with
regard to dosage of radiotherapy and/or elucidate the role of hnRNP
K and associated pathways as possible therapeutic targets in
HNSCC.
Materials and methods
Tissue samples
Two slides each from two head and neck cancer tissue
microarrays (TMA) were obtained from a commercial provider (US
Biomax, Inc.; cat. nos. HN802 and HN803c). Each TMA contained 80
individual cases including head and neck tumor tissue derived from
oral, oropharyngeal and laryngeal SCC, as well as normal tissues.
The tissue specimens were formalin-fixed and paraffin-embedded
(FFPE). All TMA slides consisted of one single FFPE core per case
(HNSCC and normal tissue).
A total of 11 non-SCC cases and 8 cases representing
HNSCC metastases were excluded from the analysis. Additionally, 4
overlapping cases from both TMAs had to be considered. A total of 5
cases were excluded due to missing tissue cores. For 7 HNSCC cases,
no grading information was available. The detailed
clinicopathological sample characteristics are listed in Table I.
 | Table IAssociation of nuclear and
cytoplasmic hnRNP K expression levels (IHC score) in HNSCC tissues
with the clinicopathological characteristics of 117 patients with
HNSCC. |
Table I
Association of nuclear and
cytoplasmic hnRNP K expression levels (IHC score) in HNSCC tissues
with the clinicopathological characteristics of 117 patients with
HNSCC.
|
Characteristics | No. | IHC score hnRNP K
|
|---|
| Nucleara | P-valueb | cytoplasmica | P-valueb |
|---|
| Normal oral
tissue | 15 | 0.60±0.23 | 0.028 | 0.27±0.12 | 0.3 |
| HNScc | 117 | 1.16±0.08 | | 0.49±0.06 | |
| Sex | | | <0.001 | | 0.009 |
| Male | 59 | 1.46±0.10 | | 0.68±0.10 | |
| Female | 58 | 0.86±0.10 | | 0.29±0.07 | |
| HPV status | | | 0.298 | | 0.685 |
| p16-negative | 95 | 1.13±0.08 | | 0.47±0.07 | |
| p16-positive | 22 | 1.32±0.18 | | 0.55±0.16 | |
| Age, years | | | 0.791 | | 0.32 |
| <57 | 58 | 1.14±0.11 | | 0.43±0.09 | |
| ≥57 | 59 | 1.19±0.10 | | 0.54±0.09 | |
| UIcc stage | | | 0.052 | | 0.003 |
| I-III | 83 | 1.07±0.09 | | 0.34±0.06 | |
| IV | 34 | 1.38±0.14 | | 0.85±0.14 | |
| Gradec | | | 0.426 | | 0.545 |
| G1 | 37 | 1.27±0.13 | | 0.40±0.10 | |
| G2 | 49 | 1.04±0.12 | | 0.59±0.11 | |
| G3 | 24 | 1.21±0.17 | | 0.49±0.15 | |
IHC
IHC was performed as previously described (25). For IHC staining of TMAs, a rabbit
monoclonal antibody against hnRNP K [dilution 1:250, 60 min at room
temperature (RT), Biozol Diagnostica Vertrieb GmbH, cat. no.
LS-C138027], a rabbit polyclonal antibody against p16INK4A
(dilution 1:250, 60 min at RT, Cell Signaling Technology, Inc.,
cat. no. 4824) and the ZytoChem Plus AP Polymer Kit (Zytomed
Systems GmbH, cat. no. POLAP-100) were used according to the
manufacturer's instructions. An experienced pathologist determined
IHC scores separately for tumor cell nuclei and cytoplasm by
assessing the staining intensity for hnRNP K and p16, respectively,
using the following scoring method: Lack of immunoreactivity (0),
weak immunoreactivity (1) and
strong immunoreactivity (2).
Cell culture and transient
transfection
Cal-27 cells were obtained from ATCC (CRL-2095™, lot
no. 62278082) and UPCI-SCC-154 were obtained from DSMZ
(UPCI-SCC-154 ACC 669 lot no. 2) and were authenticated by STR
analysis. The HPV status was determined using a PCR-based assay by
the respective companies. Both cell lines were routinely tested to
ensure the absence of mycoplasma contamination. Cal-27 originated
from a 56-year old man suffering from SCC of the tongue and were
cultivated in DMEM GlutaMAX (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FCS (Roche Diagnostics GmbH) at 37°C and 5%
CO2. UPCI-SCC-154 cells were originally derived from an
oral SCC of a 54-year-old man and were incubated in MEM (with
Earle's salts) supplemented with 10% FCS, 2 mM L-glutamine plus
non-essential amino acids (Roche Diagnostics GmbH). According to
the provider, PCR analyses confirmed Cal-27 cells as derived from
an HPV-negative and UPCI-SCC-154 cells as derived from an
HPV-positive tumor. For the transfection experiments, we used
Lipofectamine™ 2000 as the transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), Silencer® Select negative
control siRNA #1 and Silencer® Select hnRNP K (sequence:
3′-AUAAUCAUAGGUUUCAUCGta; 5′-CGAUGAAACCUAUGAUUAUtt; both from
Thermo Fisher Scientific, Inc.). At 48 h after transfection, cells
were harvested and treated according to the respective experimental
procedures.
Radiation exposure
For radiation experiments, HNSCC cells were exposed
to 240 kV X-rays using the MaxiShot system (YXLON International
GmbH) including a 3-mm beryllium filter at a plateau dose rate of 1
Gy/min at 13 mA. Applied doses were monitored with a PTW Unidose
dosimeter (PTW Freiburg GmbH). During irradiation experiments,
control cells were stored under equivalent external environmental
conditions to ensure comparability.
Immunoblotting
For western blot analyses, the XCell Sure Lock™
Mini-Cell Electrophoresis System was used. Equalization of protein
concentrations was performed using the BCA Protein Assay Kit (both
from Thermo Fisher Scientific, Inc.). A rabbit monoclonal antibody
was used for hnRNP K detection (dilution 1:1,000; Biozol
Diagnostica Vertrieb GmbH, cat. no. LS-C138027) and HRP-conjugated
anti-GAPDH served as the loading control (dilution 1:10,000; Cell
Signaling Technology, Inc., cat. no. 3683S). Acquisition of digital
images was carried out with the myECL™ Imager system (Thermo Fisher
Scientific, Inc.). For densitometry greyscale intensity, the values
of hnRNP K and GAPDH were determined using ImageJ software, v. 1.51
(National Institutes of Health) before referencing to the
non-irradiated control values and calculating the hnRNP K/GAPDH
ratios.
Immunofluorescence (IF) microscopy
IF microscopy was performed as previously described
(26). For IF staining of HNSCC
cells, TexasRed-conjugated Phalloidin (dilution 1:40, Invitrogen;
Thermo Fisher Scientific, Inc.), mouse monoclonal anti-hnRNP K
(dilution 1:1,000, Cell Signaling Technology, Inc.) and a rabbit
polyclonal anti-p16INK4A (dilution 1:1,000, Cell Signaling
Technology, Inc.) were used following 60 min of incubation at RT.
The nuclei were stained using Fluoroshield Mounting Medium with
DAPI (Abcam). Fluorescence labeling was carried out with Alexa
Fluor® 488-conjugated goat polyclonal anti-mouse and
Alexa Fluor® 488-conjugated goat polyclonal anti-rabbit
antibodies (both from Thermo Fisher Scientific, Inc.; dilution
1:500). Image acquisition was performed with a Zeiss AxioImager 2i
fluorescence microscope (Carl Zeiss AG) and the ISIS fluorescence
imaging system (MetaSystems).
Apoptosis assay
Induction of apoptosis was examined 6 h post-IR
using the Bio-Plex® Multiplex Immunoassay System and the
Bio-Plex Pro™ RBM Apoptosis Panel 3 (both from Bio-Rad
Laboratories, Inc.) according to the manufacturers'
instructions.
Clonogenic survival assay
UPCI-SCC-154 and Cal-27 cells were seeded in 6-well
plates (seeding densities for 0 Gy: 200 cells/well; 2 Gy: 600
cells/well) and cultured at 37°C for 24 h. Subsequently, the cells
were transfected or irradiated (n=4). After 9 days, tumor cell
colonies were fixed with 70% ethanol at RT for 10 min before
staining with gentian violet at RT for 10 min. Colonies containing
>50 cells were manually counted using a Zeiss STEMI SV8
stereomicroscope (Carl Zeiss AG). Experiments were performed in
quadruplicate.
CAM assay
The CAM tumor xenograft assay was performed as
previously described (27,28).
Briefly, fertilized chicken eggs were incubated (37°C; 60% relative
air moisture), fenestrated and a silicone ring (diameter: 5 mm) was
placed on top of the vascularized CAM on day 7 of fertilization.
HNSCC cells (1.5×106 cells/egg) were dissolved in a 1:1
solution of medium and Matrigel (BD Diagnostics) and grafted on the
CAM surface within the ring. HNSCC xenografts were incubated for
another 4 days, harvested, imaged and fixed in phosphate-buffered
4% formaldehyde solution at RT for 24 h (Roti-Histofix®,
Carl Roth GmbH). After embedding in paraffin, the tumor blocks were
serially cut at 5 μm and the sections were mounted on slides
coated with poly-L-lysine. For subsequent IHC staining, the
following primary antibodies were used: Monoclonal rabbit
anti-hnRNP K (dilution 1:250; Biozol Diagnostica Vertrieb GmbH,
cat. no. LS-C138027), monoclonal mouse anti-Ki-67 and monoclonal
mouse anti-desmin (dilution 1:100; both from Dako; Agilent
Technologies, Inc., cat. nos. M7240 and M0760).
Tumor volume (TV; mm3) was calculated
according to the formula: TV=length (mm) × width2 (mm) ×
π/6 (29).
Statistical analysis
For calculation of differences between IHC
expression levels, clonogenic survival and cleaved caspase-3
expression, Kruskal-Wallis test was performed followed by the
appropriate post hoc test as indicated in the figure legends using
GraphPad Prism v.5.00 (GraphPad Software, Inc.) or SigmaPlot 14.0
(Systat Software, Inc.). The plating efficiency (PE) and surviving
fraction (SF) of colony formation assays were calculated as
follows: PE=(colonies counted)/(cells seeded per well) ×100;
SF=(colonies counted)/[(cells seeded per well) × (PE/100)].
According to the linear-quadratic model of cell survival
(S=e–α*D–β*D^2) we calculated the parameters α and β by
non-linear regression analysis using SigmaPlot 14.0 (Systat
Software, Inc.) and considered P<0.05 as statistically
significant.
Results
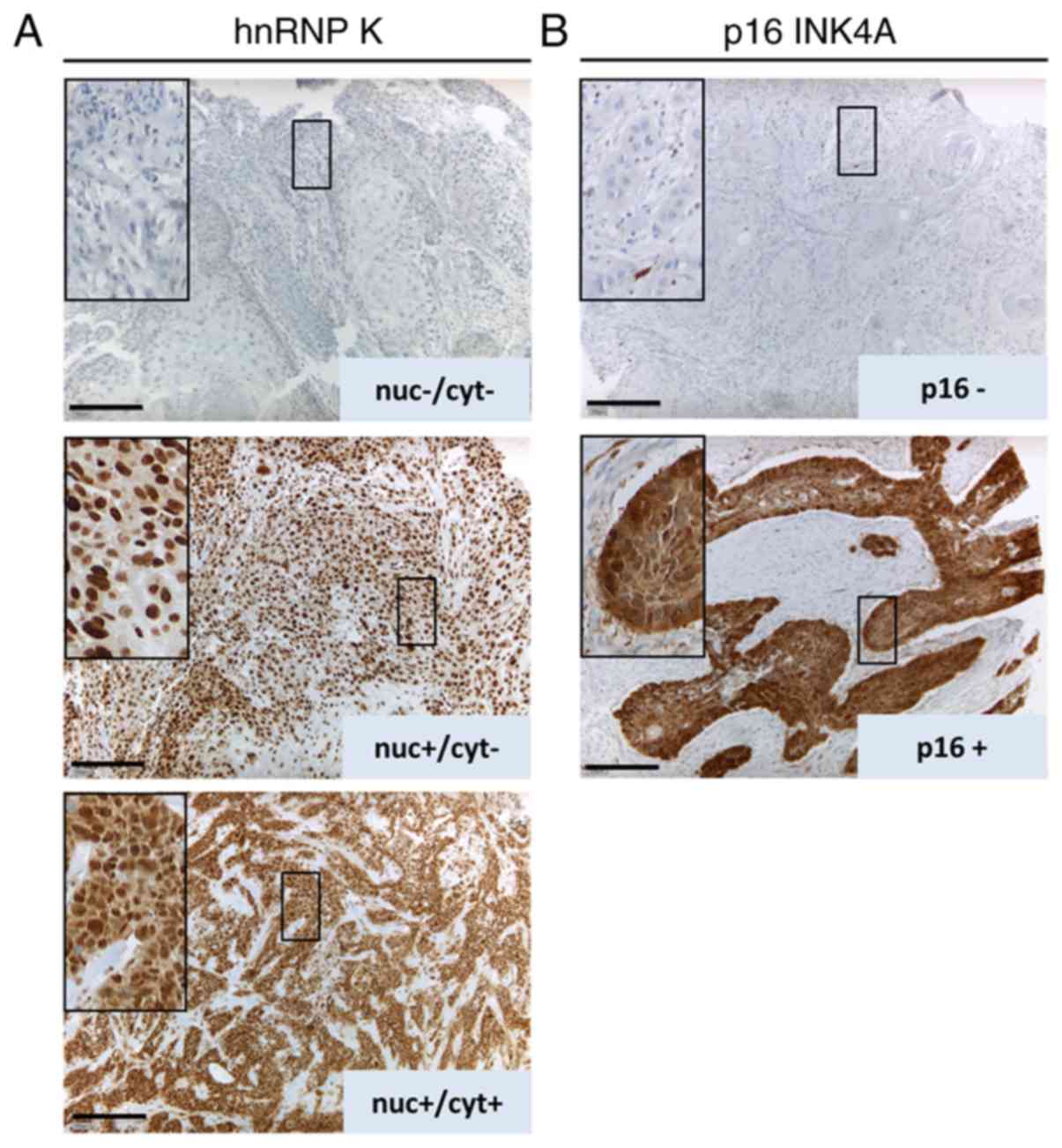
Strong hnRNP K expression in HNSCC is
associated with advanced tumor stage and male sex
Two sequential sections of tissue samples from 117
HNSCC patients and 15 samples of non-neoplastic oropharyngeal
epithelium were immunostained for hnRNP K and p16 (Fig. 1). Nuclear, but not cytoplasmic,
immunoreactivity with the antibody against hnRNP K was
significantly elevated in HNSCC tumor samples vs. normal epithelium
(Table I). Accordingly, the odds
ratio for nuclear hnRNP K IHC scores >0 was found to be
significantly increased in HNSCC tissue compared with normal oral
tissue by 5.5 [95% confidence interval (CI): 1.8–17.5; P=0.004;
Table II].
 | Table IIAssociation of hnRNP K IHC score with
the analyzed categories (tissue, sex and stage) among 117 HNSCC
cases and 15 normal oral tissue samples. |
Table II
Association of hnRNP K IHC score with
the analyzed categories (tissue, sex and stage) among 117 HNSCC
cases and 15 normal oral tissue samples.
| Model | OR | 95% cI | P-value |
|---|
| Nuclear hnRNP K IHc
score >0a | | | | 0.004 |
| Normal tissue | 1 | | | |
| HNScc | 5.5 | 1.8 | 17.5 | |
| Nuclear hnRNP K IHc
score 2a | | | | <0.001 |
| Female | 1 | | | |
| Male | 4.9 | 2.2 | 10.9 | |
| Cytoplasmic hnRNP K
IHc score >0a | | | | 0.005 |
| Female | 1 | | | |
| Male | 3.2 | 1.5 | 7.2 | |
| Cytoplasmic hnRNP K
IHc score 2a | | | | 0.002 |
| I-III
stageb | 1 | | | |
| IV stageb | 7.1 | 2.0 | 25.1 | |
Furthermore, elevated cytoplasmic hnRNP K levels
were observed in tissue samples from tumors with a high clinical
stage [Union for International Cancer Control (UICC) IV, Table I]. Advanced tumor progression was
shown to significantly increase the odds ratio by 7.1 (95% CI:
2.0–25.1; P=0.002) for the presence of strong cytoplasmic hnRNP K
immunoreactivity (IHC score 2) in the analyzed HNSCC samples
(Table II).
Both strong nuclear and cytoplasmic hnRNP K
immunoreactivity in HNSCC was shown to be statistically
significantly associated with male sex (Table I). In line with these findings,
the odds ratio for the presence of a higher hnRNP K expression was
found to be significantly increased in male compared with female
patients for both subcellular localizations, as shown in Table II.
By contrast, there was no significant difference
between the mean values of hnRNP K IHC scores according to the p16
status as a surrogate marker of HPV-induced tumorigenesis or any
other patient characteristics (Table
I).
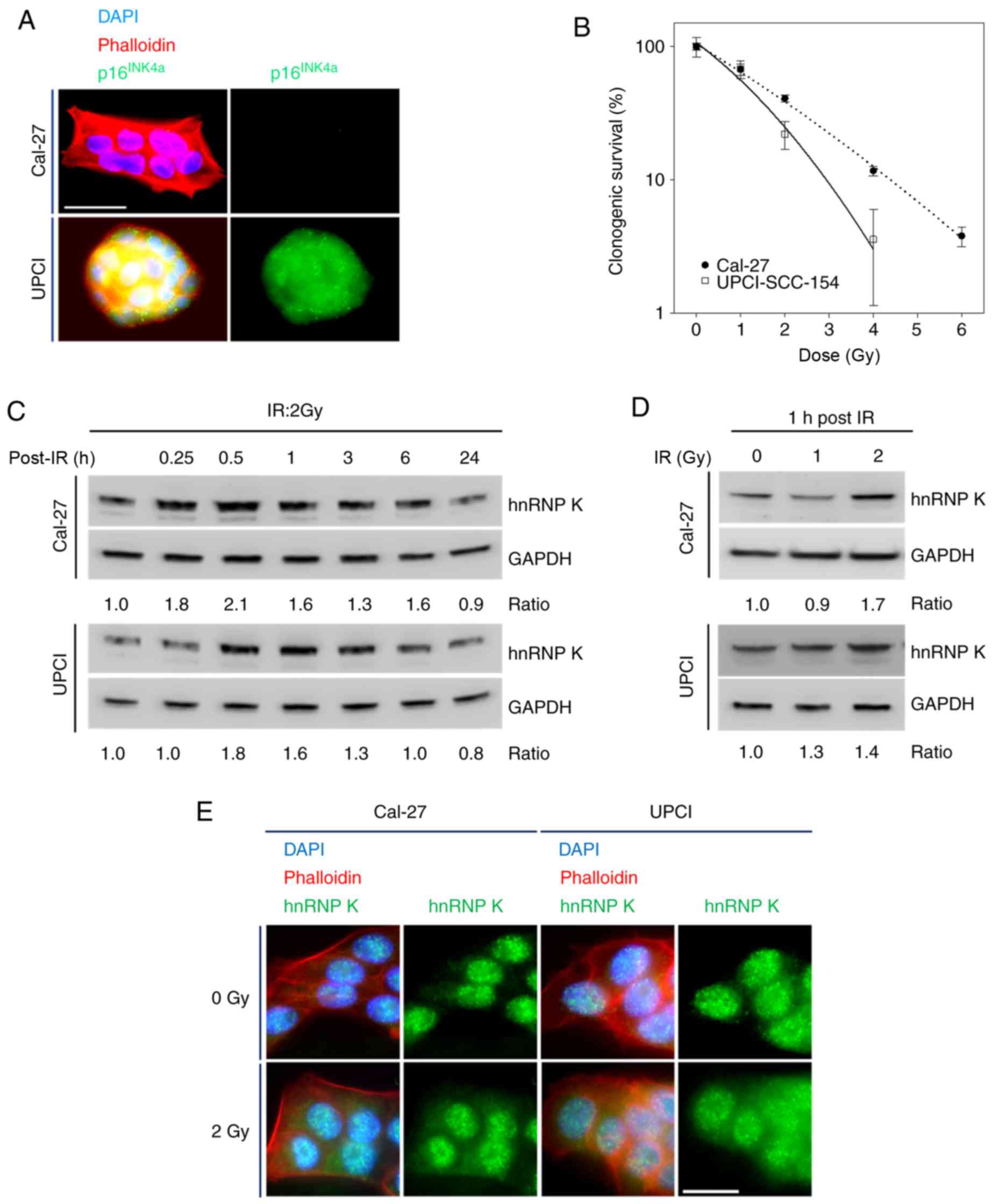
Accumulation of hnRNP K in HNSCC cells as
response to IR independent of HPV status
First, Cal-27 cells were verified as non-HPV- and
UPCI-SCC-154 cells as HPV-associated HNSCC cell lines by p16INK4A
immunostaining followed by fluorescence microscopy (Fig. 2A). Contrary to Cal-27, a dose of 6
Gy completely abrogated the clonogenicity of UPCI-SCC-154 cells
(Fig. 2B). Based on the
linear-quadratic model of cell survival, the linear (α) and
quadratic (β) constants of the fitted dose effect curves were
determined (Fig. 2B;
R2 of the fit 0.997 for Cal-27 and 0.989 for
UPCI-SCC-154) and calculated the α/β-ratio in Gy. For UPCI-SCC-154
cells, a lower α/β-ratio (6.39 Gy; 0.5164/0.0808) was found
compared to Cal-27 cells (23.63 Gy; 0.4395/0.0186). These results
revealed a higher radiosensitivity and a lower fractionation
sensitivity for HPV-associated UPCI-SCC-154 cells compared to
Cal-27 cells.
Acute radiation exposure using a clinically relevant
individual dose of 2 Gy increased cellular hnRNP K concentrations
in both cell lines, displaying a maximum plateau phase between 0.5
and 1 h post-exposure before reaching initial values after 24 h, as
shown by immunoblotting (Fig.
2C). This effect appeared to be dose-dependent when analyzing
cells 1 h after radiation exposure (Fig. 2D).
To analyze radiation-induced changes in hnRNP K
expression and subcellular localization, IF microscopy we
additionally performed. Upon applying IR at 2 Gy, both cell lines
responded with cytoplasmic hnRNP K accumulation within 1 h
(Fig. 2E).
Interestingly, all observed radiation-induced
changes in the expression levels and cellular localization of hnRNP
K were independent of the HPV status of the cell lines
investigated.
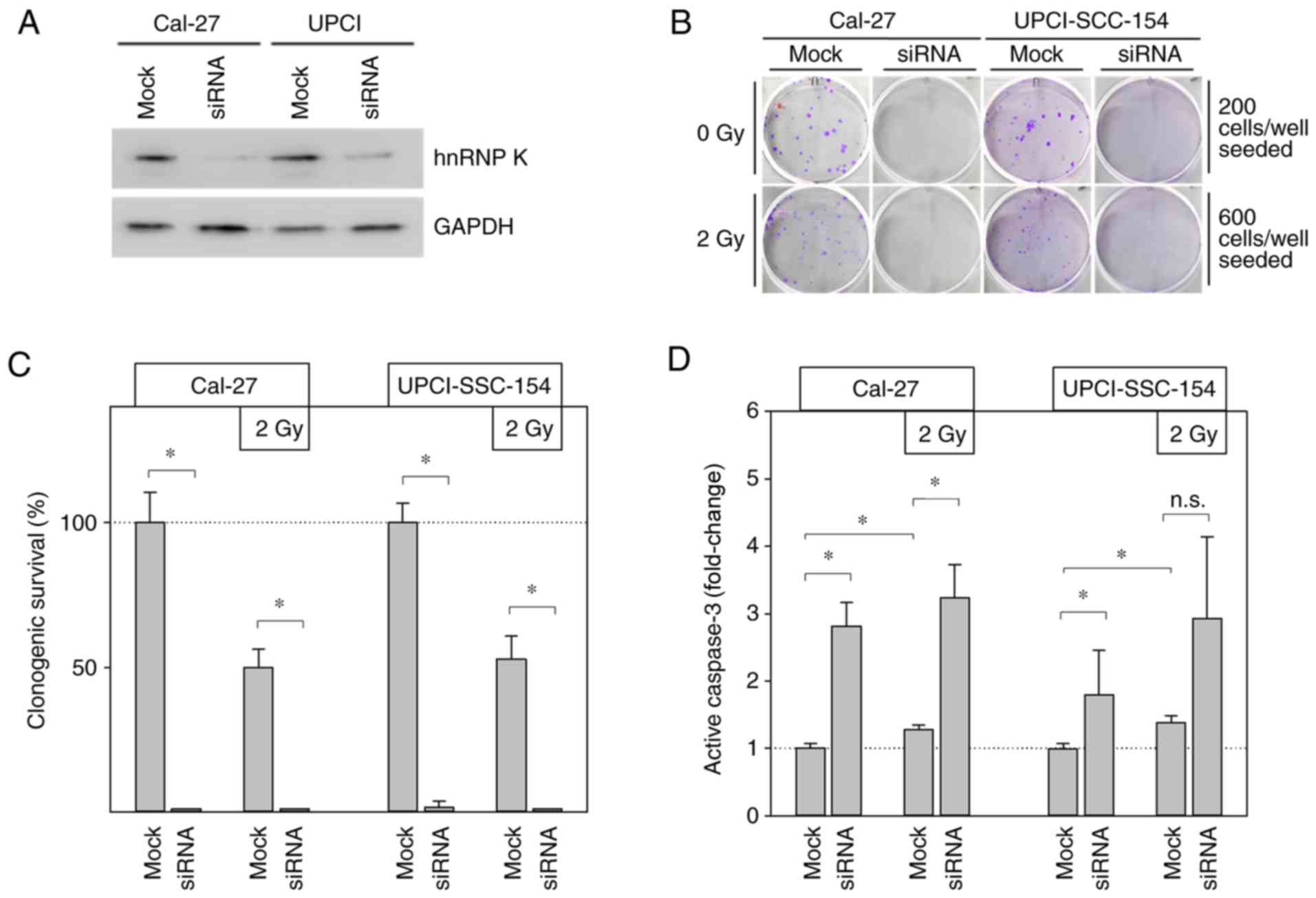
Transient hnRNP K knockdown induces
apoptosis in HNSCC cells
Knockdown experiments based on hnRNP K siRNA
transfection were performed to specify the functional effects of
cellular hnRNP K on the radiation response of HNSCC cells. The
efficiency of the transient hnRNP K knockdown was verified by
immunoblotting (Fig. 3A) and its
persistence after 4 days was demonstrated by CAM experiments
(Fig. 4A and B).
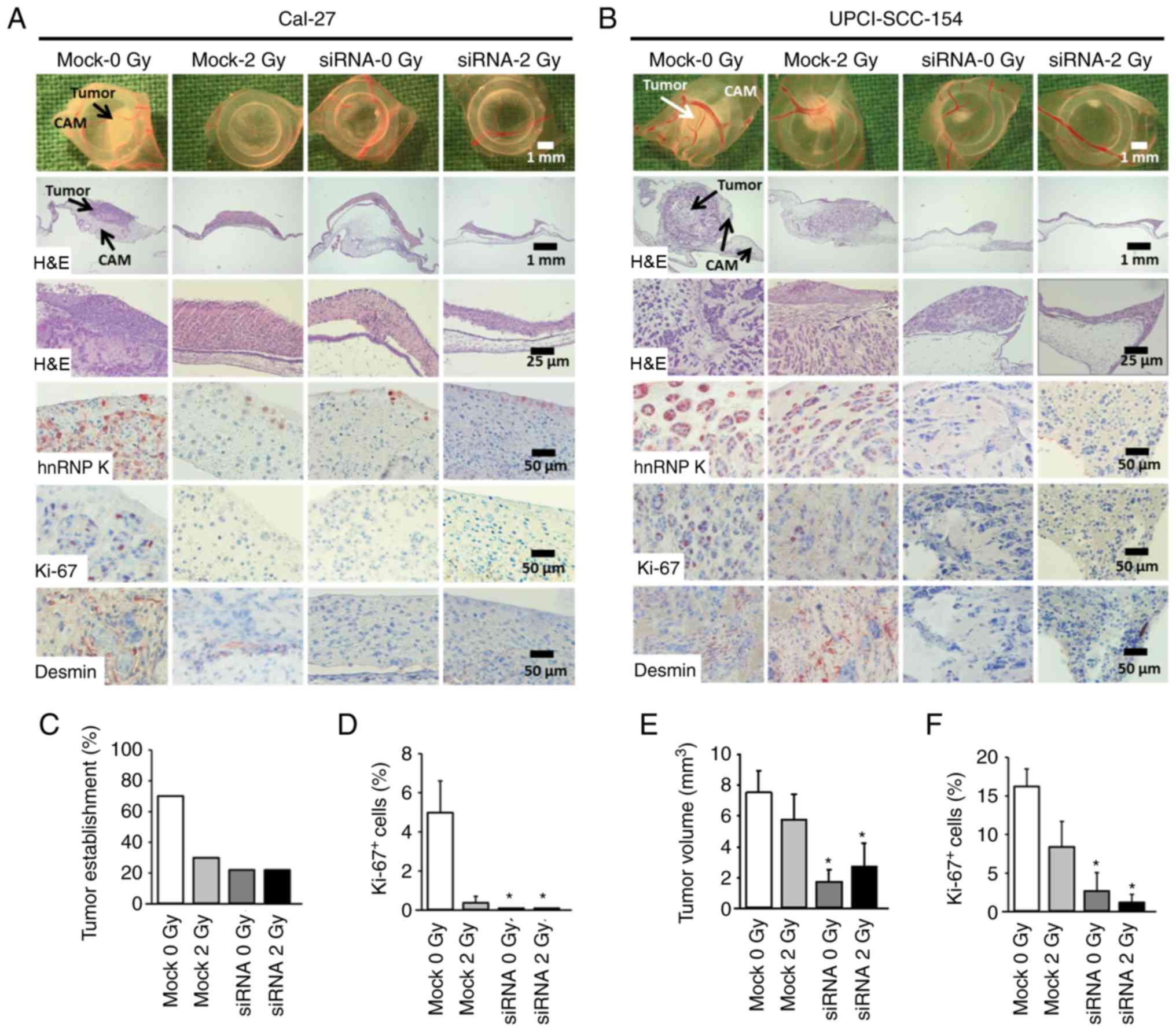 | Figure 4Knockdown of hnRNP K inhibits growth
of HNSSC xenografts on the chick egg CAM in vivo. Cells
(mock or siRNA, ± irradiation) were seeded on the CAM of fertilized
chick eggs 7 days after the start of incubation (1.5×106
cells/egg in medium/Matrigel 1:1). After an incubation period of 4
days at 37°C, the tumors were collected, imaged, fixed and embedded
in paraffin for immunohistochemical analysis. Sections (5
μm) were stained for hnRNP K, proliferation marker Ki-67 and
the angiogenesis marker desmin. Each group contained 9-10
tumor-bearing eggs. (A and B) Representative images of tumor
xenografts immediately after extraction (1st row), overview of
tumor and underlying CAM tissue (H&E staining, 2nd and 3rd
rows), immunohistochemical staining of hnRNP K-expressing cells
(4th row), Ki-67+ proliferative cells (5th row) and
desmin+ pericytes indicating angiogenesis (6th row). (C)
Percentage of solid tumor formation of Cal-27 cells 4 days after
xenotransplantation (9-10 tumors/group). (D) Percentage of
proliferating Ki-67+ cells in Cal-27 xenografts. A total
of 261-358 cells from each tumor were evaluated. Data are presented
as the mean ± SEM of 4 tumors/group. (E) Mean tumor volume of
UPCI-SCC-154 cancer xenografts 4 days after xenotransplantation as
assessed immediately after extraction. Tumor volume was calculated
according to the formula: π/6 × length × width2. Mean of
9-10 tumors/group. (F) Percentage of proliferating
Ki-67+ cells. A total of 298-632 cells from each tumor
were evaluated. Data are presented as the mean ± SEM of 5
tumors/group. *P<0.05 vs. control (mock 0 Gy)
(Kruskal Wallis test, Dunnett's post hoc test). hnRNP K,
heterogenous nuclear ribonucleoprotein K; HNSCC, head and neck
squamous cell carcinoma; CAM, chorioallantoic membrane; H&E,
hematoxylin and eosin. |
Clonogenic survival assays significantly impaired
the clonogenic ability in both HNSCC cell lines deficient of
endogenous hnRNP K (Fig. 3B and
C). Cal-27 cells, in particular, completely lost their ability
to form colonies under this condition. In UPCI-SCC-154 cells,
complete abrogation of colony formation was achieved by combined
treatment with hnRNP K knockdown and 2 Gy IR.
To highlight the potential role of apoptosis in this
observation, the cellular levels of the pro-apoptotic protein
active caspase-3 were analyzed (Fig.
3D). Radiation alone (2 Gy) significantly increased active
caspase-3 by ~1.3-fold under control conditions (mock) in both
investigated HNSCC cell lines. After additional hnRNP K knockdown,
cellular active caspase-3 levels were found to be elevated even up
to 3-fold. However, this finding reached statistical significance
level for Cal-27 cells only.
In summary, these findings indicate enhanced
apoptosis of HNSCC cells as the main cause for the deprivation of
clonogenicity following loss of cellular hnRNP K, and confirmed the
involvement of hnRNP K in the radioresistance of HNSCC cells.
hnRNP K knockdown inhibits HNSCC
xenograft formation on the CAM in vivo
The CAM model was used to investigate the impact of
cellular hnRNP K levels on the tumor-forming capacity of HNSCC
cells in vivo. HNSCC cells with hnRNP K knockdown and/or
irradiation were seeded on the CAM of fertilized chicken eggs. The
resulting tumors were imaged and harvested 4 days later. IHC of the
FFPE tumor sections confirmed effective knockdown of hnRNP K
(Fig. 4A and B). The tumor
forming capacity of Cal-27 cells was markedly decreased by both
hnRNP K knockdown and irradiation. In both conditions, macroscopic
analysis of the tumor volumes was not possible due to poor tumor
establishment in 30% (mock 2 Gy) and 22% (siRNA mock, siRNA 2 Gy)
of the grafted eggs compared to 70% in the control group (mock)
(Fig. 4C). This was confirmed by
an almost complete lack of staining for the proliferation marker
Ki-67 (Fig. 4D).
By contrast, UPCI-SCC-154 cells demonstrated a
strong tumor forming capacity. Knockdown of hnRNP K resulted in
significantly reduced tumor volume, impairment of proliferation, as
indicated by Ki-67 immunostaining, and loss of tumor
vascularization, as shown by desmin immunostaining, whereas
irradiation alone showed minor efficacy (Fig. 4B, E and F).
Discussion
The aim of the present study was to analyze the
expression levels of hnRNP K in HNSCC, with an additional focus on
its role in HNSCC radioresistance with respect to individual HPV
status and sex. These investigations were based on the established
role of hnRNP K in radioresistance of various malignant tumors
(20), the higher
radiosensitivity of HPV-associated HNSCC (4,8)
and sex-dependent prognosis of HNSCC patients (30,31).
Significant higher nuclear, but not cytoplasmic,
expression levels of hnRNP K were detected in HNSCC compared with
normal oral mucosa samples, which was in line with previous
findings (16,19,32). This observation further supports
the increasing evidence of high hnRNP K expression in
cancerous/malignant tissues.
Furthermore, hnRNP K expression was previously
suggested as a prognostic biomarker associated with worse outcome
(11). Consistently, the
capability of hnRNP K to promote migration, angiogenesis and the
expression of matrix metalloproteinases as prerequisites for
invasion have previously been discussed as key contributing factors
to a more malignant phenotype of hnRNP K-overexpressing tumors
(33,34). Furthermore, cytoplasmic hnRNP K
levels were herein demonstrated to be significantly associated with
advanced HNSCC tumor stages, which are characterized either by
extensive infiltration by the primary tumor or by the presence of
distant metastasis (UICC stage IV). In line with these
observations, particularly aberrant cytoplasmic hnRNP K
localization has been linked to unfavorable tumor characteristics
and patient outcome, including patients with HNSCC (16,19).
IHC demonstrated a significantly stronger hnRNP K
expression (both nuclear and cytoplasmic) in male compared to
female patients. To the best of our knowledge, this is the first
study to describe a sex-specific difference in hnRNP K expression
in HNSCC tumor samples. A previous study also reported higher hnRNP
K levels in HNSCC from male patients; however, the difference did
not reach statistical significance (16). The finding of higher hnRNP K
expression in male patients is of particular interest, since the
number of studies analyzing the impact of sex on HNSCC biology and
prognosis is limited and, in most studies, male patients are
significantly over-represented in the investigated cohort (16,30,35). Interestingly, two recent studies
highlighted a significantly increased hazard ratio for male
patients for HNSCC-related mortality (30,31).
The mechanisms underlying this phenomenon remain to
be fully elucidated; however, given the results presented herein,
differences in hnRNP K expression may, at least in part, contribute
to a worse prognosis in male HNSCC patients.
Despite our hypothesis, hnRNP K response to
irradiation was not found to be dependent on HPV status. The higher
baseline radiosensitivity of HPV-associated HNSCC cells was
confirmed, while upregulation and cytoplasmic redistribution of
hnRNP K upon exposure to IR were similar in both cell lines.
siRNA-based knockdown of hnRNP K significantly increased the levels
of active caspase-3 in HNSCC cells, indicating induction of
apoptosis. Comparable initiation of programmed cell death due to
hnRNP K knockdown has been previously demonstrated in various
cancer cells (17,18,36,37). However, loss of hnRNP K exerted no
synergistic effect on the radiosensitivity of HNSCC cell lines,
irrespective of their HPV status.
Further analyses using the CAM assay as an in
vivo tumor xenograft model confirmed that knockdown of hnRNP K
diminished the tumor-forming ability of HNSCC cells. Interestingly,
a concomitant decrease in neovascularization and proliferation
index within the tumor xenografts was observed upon hnRNP K
knockdown. In Cal-27 cells, hnRNP K knock-down prevented tumor
establishment. These observations may mainly be due to apoptosis
induction, as demonstrated in vitro, combined with the
abrogation of clonogenic survival upon hnRNP K knockdown in HNSCC
cells. Furthermore, an essential role of hnRNP K in tumor-related
angiogenesis has been described (33). At the molecular level, hnRNP
K-dependent translation of vascular endothelial growth factor mRNA
was demonstrated in renal tubular epithelial cells (38,39). Thus, an impairment of tumor
vascularization induced upon hnRNP K knockdown and a resulting lack
of nutrients may explain the reduced proliferation index observed
in HNSCC xenografts.
Taken together, the findings of the present study
demonstrated that hnRNP K is overexpressed in HNSCC compared with
normal squamous epithelium, and that high levels of hnRNP K are
statistically associated with male sex and advanced tumor stage.
There were no differences in hnRNP K expression levels between HPV-
and non-HPV-associated HNSCC. IR led to quick upregulation and
cytoplasmic redistribution of the hnRNP K protein, while hnRNP K
knockdown enhanced IR-induced apoptosis and slowed down tumor
growth in vitro and in vivo. This effect was
independent of the HPV status of the cellular tumor model.
Therefore, while hnRNP K appears to play a key role in
tumorigenesis and evasion of apoptosis upon exposure to IR, the
clinically observed radiosensitivity of HPV-associated HNSCC
appears to be independent of hnRNP K expression levels in these
tumors.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
Individual scientists contributed to the present
work as follows: JK and SE: Conceptualization and methodology. SH
and JK: CAM experiments and statistics. JK, SH, TP, CH, AR, MP and
SE: Data analysis, writing, reviewing and editing of the
manuscript. SK, CH and SE: IHC analysis and statistics. JK and SE:
In vitro experimental investigation. SE: Project
administration and supervision. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Acknowledgements
The authors would like to express their gratitude to
Roland Ridi, Claudia Schlosser and Andreas Huebsch for their
excellent technical assistance, to Barbara Couson and Jianqing Chen
for performing the colony formation assays, and to Eva Winkler for
supporting the grafting of the HNSCC cells.
Abbreviations:
|
CAM
|
chorioallantoic membrane
|
|
FFPE
|
formalin-fixed and
paraffin-embedded
|
|
IF
|
immunofluorescence
|
|
IHC
|
immunohistochemistry
|
|
IR
|
ionizing radiation
|
|
hnRNP K
|
heterogenous nuclear ribonucleoprotein
K
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HPV
|
human papillomavirus
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franceschi S, Talamini R, Barra S, Baron
AE, Negri E, Bidoli E, Serraino D and La Vecchia C: Smoking and
drinking in relation to cancers of the oral cavity, pharynx,
larynx, and esophagus in northern Italy. Cancer Res. 50:6502–6507.
1990.PubMed/NCBI
|
|
4
|
Ang KK and Sturgis EM: Human
papillomavirus as a marker of the natural history and response to
therapy of head and neck squamous cell carcinoma. Semin Radiat
Oncol. 22:128–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chaturvedi AK: Epidemiology and clinical
aspects of HPV in head and neck cancers. Head Neck Pathol. 6(Suppl
1): S16–S24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brouwer AF, Eisenberg MC and Meza R: Age
effects and temporal trends in HPV-Related and HPV-Unrelated oral
cancer in the United States: A multistage carcinogenesis modeling
analysis. PLoS One. 11:e01510982016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fakhry C, Westra WH, Li S, Cmelak A, Ridge
JA, Pinto H, Forastiere A and Gillison ML: Improved survival of
patients with human papillomavirus-positive head and neck squamous
cell carcinoma in a prospective clinical trial. J Natl Cancer Inst.
100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Mann D, Sinha UK and Kokot NC: The
molecular mechanisms of increased radiosensitivity of HPV-positive
oropharyngeal squamous cell carcinoma (OPSCC): An extensive review.
J Otolaryngol Head Neck Surg. 47:592018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moody CA and Laimins LA: Human
papillomavirus oncoproteins: Pathways to transformation. Nat Rev
Cancer. 10:550–560. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barboro P, Ferrari N and Balbi C: Emerging
roles of heterogeneous nuclear ribonucleoprotein K (hnRNP K) in
cancer progression. Cancer Lett. 352:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallardo M, Hornbaker MJ, Zhang X, Hu P,
Bueso-Ramos C and Post SM: Aberrant hnRNP K expression: All roads
lead to cancer. Cell Cycle. 15:1552–1557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bomsztyk K, Denisenko O and Ostrowski J:
hnRNP K: One protein multiple processes. Bioessays. 26:629–638.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carpenter B, McKay M, Dundas SR, Lawrie
LC, Telfer C and Murray GI: Heterogeneous nuclear ribonucleoprotein
K is over expressed, aberrantly localised and is associated with
poor prognosis in colorectal cancer. Br J Cancer. 95:921–927. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LC, Chung IC, Hsueh C, Tsang NM, Chi
LM, Liang Y, Chen CC, Wang LJ and Chang YS: The antiapoptotic
protein, FLIP, is regulated by heterogeneous nuclear
ribonucleoprotein K and correlates with poor overall survival of
nasopharyngeal carcinoma patients. Cell Death Differ. 17:1463–1473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CS, Chang KP, Chen LC, Chen CC, Liang
Y, Hseuh C and Chang YS: Heterogeneous ribonucleoprotein K and
thymidine phosphorylase are independent prognostic and therapeutic
markers for oral squamous cell carcinoma. Oral Oncol. 48:516–522.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Gu P, Xie R, Han J, Liu H, Wang B,
Xie W, Xie W, Zhong G, Chen C, et al: Heterogeneous nuclear
ribonucleoprotein K is associated with poor prognosis and regulates
proliferation and apoptosis in bladder cancer. J Cell Mol Med.
21:1266–1279. 2017. View Article : Google Scholar :
|
|
18
|
Eder S, Arndt A, Lamkowski A, Daskalaki W,
Rump A, Priller M, Genze F, Wardelmann E, Port M and Steinestel K:
Baseline MAPK signaling activity confers intrinsic radioresistance
to KRAS-mutant colorectal carcinoma cells by rapid upregulation of
heterogeneous nuclear ribonucleoprotein K (hnRNP K). Cancer Lett.
385:160–167. 2017. View Article : Google Scholar
|
|
19
|
Matta A, Tripathi SC, DeSouza LV, Grigull
J, Kaur J, Chauhan SS, Srivastava A, Thakar A, Shukla NK, Duggal R,
et al: Heterogeneous ribonucleoprotein K is a marker of oral
leukoplakia and correlates with poor prognosis of squamous cell
carcinoma. Int J Cancer. 125:1398–1406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moumen A, Masterson P, O'Connor MJ and
Jackson SP: hnRNP K: An HDM2 target and transcriptional coactivator
of p53 in response to DNA damage. Cell. 123:1065–1078. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haley B, Paunesku T, Protic M and
Woloschak GE: Response of heterogeneous ribonuclear proteins
(hnRNP) to ionising radiation and their involvement in DNA damage
repair. Int J Radiat Biol. 85:643–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wiesmann N, Strozynski J, Beck C,
Zimmermann N, Mendler S, Gieringer R, Schmidtmann I and Brieger J:
Knockdown of hnRNPK leads to increased DNA damage after irradiation
and reduces survival of tumor cells. Carcinogenesis. 38:321–328.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eder S, Lamkowski A, Priller M, Port M and
Steinestel K: Radiosensitization and downregulation of
heterogeneous nuclear ribonucleoprotein K (hnRNP K) upon inhibition
of mitogen/extracellular signal-regulated kinase (MEK) in malignant
melanoma cells. Oncotarget. 6:17178–17191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strozynski J, Heim J, Bunbanjerdsuk S,
Wiesmann N, Zografidou L, Becker SK, Meierl AM, Gouveris H, Lüddens
H, Grus F and Brieger J: Proteomic identification of the
heterogeneous nuclear ribonucleoprotein K as irradiation responsive
protein related to migration. J Proteomics. 113:154–161. 2015.
View Article : Google Scholar
|
|
25
|
Steinestel K, Lennerz JK, Eder S, Kraft K
and Arndt A: Invasion pattern and histologic features of tumor
aggressiveness correlate with MMR protein expression, but are
independent of activating KRAS and BRAF mutations in CRC. Virchows
Arch. 465:155–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liebau S, Steinestel J, Linta L, Kleger A,
Storch A, Schoen M, Steinestel K, Proepper C, Bockmann J,
Schmeisser MJ and Boeckers TM: An SK3 channel/nWASP/Abi-1 complex
is involved in early neurogenesis. PLoS One. 6:e181482011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuan SL, Fischer S, Hafner S, Wang T,
Syrovets T, Liu W, Tokura Y, Ng DYW, Riegger A, Förtsch C, et al:
Boosting antitumor drug efficacy with chemically engineered
multidomain proteins. Adv Sci (Weinh). 5:17010362018. View Article : Google Scholar
|
|
28
|
Zuo Z, Syrovets T, Wu Y, Hafner S,
Vernikouskaya I, Liu W, Ma G, Weil T, Simmet T and Rasche V: The
CAM cancer xenograft as a model for initial evaluation of MR
labelled compounds. Sci Rep. 7:466902017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fakhry C, Westra WH, Wang SJ, van Zante A,
Zhang Y, Rettig E, Yin LX, Ryan WR, Ha PK, Wentz A, et al: The
prognostic role of sex, race, and human papillomavirus in
oropharyngeal and nonoropharyngeal head and neck squamous cell
cancer. Cancer. 123:1566–1575. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Worsham MJ, Stephen JK, Chen KM, Mahan M,
Schweitzer V, Havard S and Divine G: Improved survival with HPV
among African Americans with oropharyngeal cancer. Clin Cancer Res.
19:2486–2492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roychoudhury P and Chaudhuri K: Evidence
for heterogeneous nuclear ribonucleoprotein K overexpression in
oral squamous cell carcinoma. Br J Cancer. 97:574–576. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao R, Yu Y, Inoue A, Widodo N, Kaul SC
and Wadhwa R: Heterogeneous nuclear ribonucleoprotein K (hnRNP-K)
promotes tumor metastasis by induction of genes involved in
extracellular matrix, cell movement, and angiogenesis. J Biol Chem.
288:15046–15056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chung IC, Chen LC, Chung AK, Chao M, Huang
HY, Hsueh C, Tsang NM, Chang KP, Liang Y, Li HP and Chang YS:
Matrix metalloproteinase 12 is induced by heterogeneous nuclear
ribonucleoprotein K and promotes migration and invasion in
nasopharyngeal carcinoma. BMC Cancer. 14:3482014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan
PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar
AK, et al: Randomized phase III trial of concurrent accelerated
radiation plus cisplatin with or without cetuximab for stage III to
IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 32:2940–2950.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao X, Feng J, He Y, Xu F, Fan X, Huang W,
Xiong H, Liu Q, Liu W, Liu X, et al: hnRNPK inhibits GSK3β Ser9
phosphorylation, thereby stabilizing c-FLIP and contributes to
TRAIL resistance in H1299 lung adenocarcinoma cells. Sci Rep.
6:229992016. View Article : Google Scholar
|
|
37
|
Yang JH, Chiou YY, Fu SL, Shih IY, Weng
TH, Lin WJ and Lin CH: Arginine methylation of hnRNPK negatively
modulates apoptosis upon DNA damage through local regulation of
phosphorylation. Nucleic Acids Res. 42:9908–9924. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feliers D, Lee MJ, Ghosh-Choudhury G,
Bomsztyk K and Kasinath BS: Heterogeneous nuclear ribonucleoprotein
K contributes to angiotensin II stimulation of vascular endothelial
growth factor mRNA translation. Am J Physiol Renal Physiol.
293:F607–F615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sataranatarajan K, Lee MJ, Mariappan MM
and Feliers D: PKCdelta regulates the stimulation of vascular
endothelial factor mRNA translation by angiotensin II through hnRNP
K. Cell Signal. 20:969–977. 2008. View Article : Google Scholar : PubMed/NCBI
|