Circular RNAs (circRNAs), arising from the
non-canonical splicing of linear pre-mRNAs, are long non-coding
RNAs that lack 5' and 3' ends and a poly(A) tail, and exhibit a
circular form (1,2). They were initially identified in RNA
viruses as viroids extracted from plants in 1976 (3) and were considered to present a low
abundance and to represent errors in splicing (4). With the development of
high-throughput RNA sequencing and bioinformatics analysis,
>30,000 circRNAs have been discovered to date (5,6).
The discovery that the RNA sponge function of circRNAs is involved
in carcinogenesis and the malignant behavior of cancers has
revealed a novel molecular mechanism involved in cancer and a
number of other diseases, such as atherosclerosis and Alzheimer's
disease (7).
Gastrointestinal cancer represents a class of
cancers affecting the digestive system (8), such as colorectal cancer (CRC),
gastric cancer (GC), esophageal cancer, hepatocellular carcinoma
(HCC) and gallbladder cancer. These cancers constitute a serious
threat to human health worldwide (9). Due to a lack of effective markers
for early diagnosis, a number of patients are first diagnosed at an
advanced stage of gastrointestinal cancer with the characteristics
of regional or distant metastasis, which severely reduces the total
5-year survival rate (10). In
recent years, circRNAs have been found to possess clinical
potential for regulating the biological behavior and acting as
critical biomarkers and treatment targets of gastrointestinal
cancer. Therefore, in the present review, the potential
significance and latent function of circRNAs in cancer diagnosis,
prognosis and therapy in gastrointestinal cancer are briefly
discussed, with an aim to provide a better understanding of the
regulatory role of circRNAs in the pathogenesis of gastrointestinal
cancer and to assist in the identification of effective therapeutic
targets.
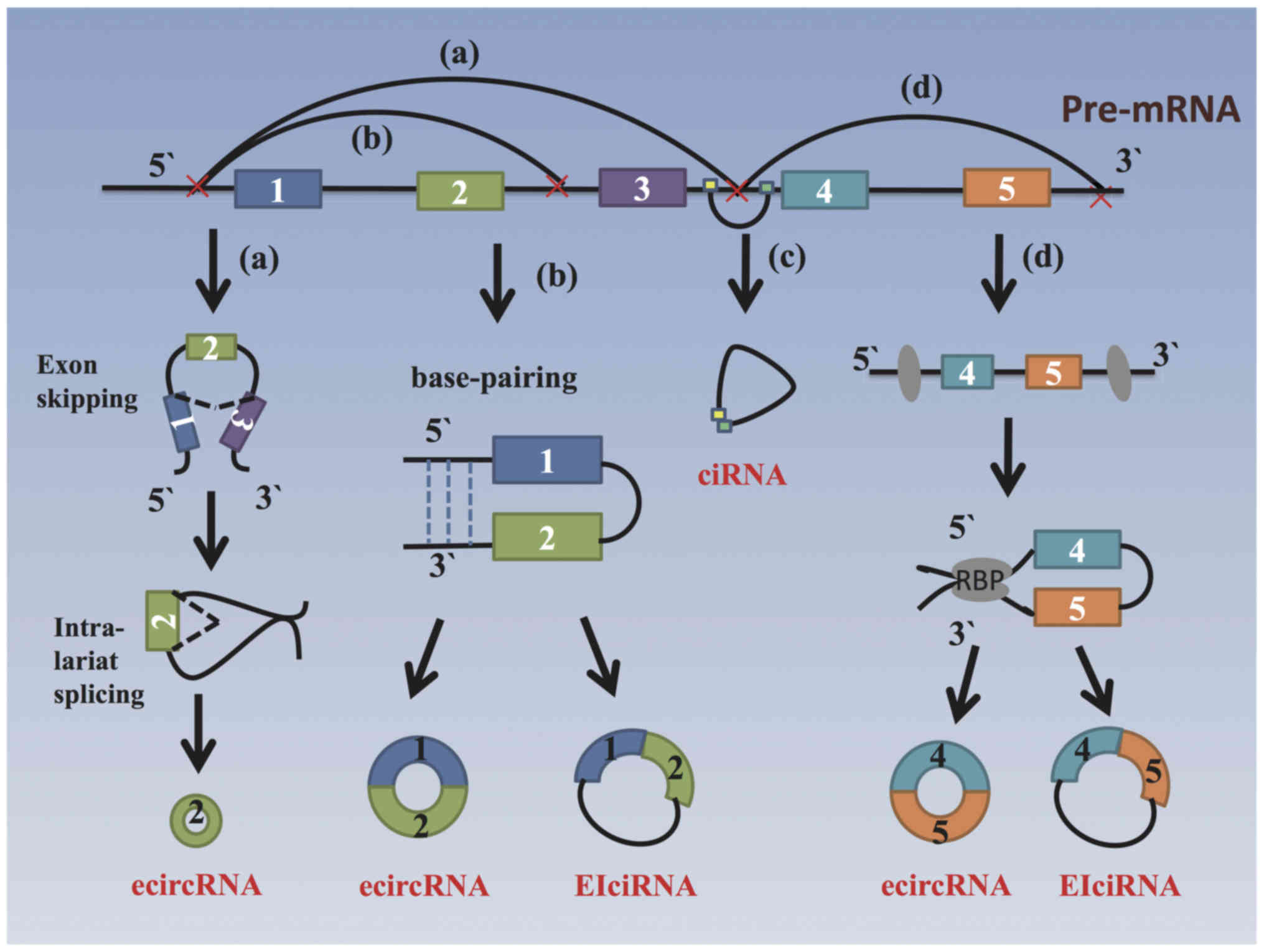
circRNAs are derived from the exons of coding
regions or from 5′ - or 3′ -untranslated regions, introns,
intergenic genomic regions as antisense RNAs (6). Among the identified circRNAs,
>80% originate from a single exon or several exons and can be
referred to as exonic circRNAs (ecircRNAs), which result from by
back-splicing, a process that differs from canonical linear RNA
splicing (11). The generation of
circRNAs can be facilitated by reverse complementary sequences,
specific protein factors or exon-skipping (12). Currently, there are 4 possible
models of circRNA biogenesis, as illustrated in Fig. 1: i) Exon-skipping mechanism or
lariat-driven circularization model: The pre-mRNA transcript brings
the original non-adjacent exons close to each other, followed by a
reverse covalent connection between the 3' donor of the downstream
exon and the 5' splice acceptor, which forms a lariat structure
containing exon 2 (13). ii)
Intron-pairing-driven circularization or back-splicing model: This
involves a circulation structure and a linear product can be formed
based on the pairing of the complementary motifs in the transcripts
(such as Alu elements) when introns are removed or retained
(11,14), forming an ecircRNA (circulatory
structure containing exons 1 and 2) or an exon-intron circRNA
(eiciRNA, a circulatory structure containing exons 1 and 2 and
introns). iii) Intron cyclization model: A circular intronic RNA
(ciRNA) is produced from intron lariats that escape debranching,
and the production of such ciRNAs is highly associated with
consensus motifs near splice sites and branchpoint sites (15). iv) RNA-binding protein
(RBP)-driven circularization model: In this case, RBPs such as
muscleblind-specific motifs within the flanking introns of the
pre-mRNA and non-sequential flanking introns can form a bridge
between the introns (16).
Due to the closed loop structures of circRNAs
without 5'-3' polarity or a polyadenylated tail, circRNAs are much
more stable than linear RNAs and impervious to deregulation by an
RNA exonuclease or RNase (6,17).
circRNAs with orthologous exons are highly structurally conserved
in the third codons compared with intergenic and ciRNAs (18). They are widely expressed in a
tissue-specific manner in the body. Although the abundance of
circRNAs accounts for approximately 2-4% of all mRNAs, some of them
are highly abundant in specific cell types, such as fibroblasts
(19). All the characteristics
mentioned above suggest that circRNAs are capable of indirectly or
directly targeting RNAs and proteins, thereby regulating gene
expression at multiple levels. Therefore, the development and
progression of non-cancerous and cancerous diseases are affected.
The various funcions of circRNAs are discussed below as
follows:
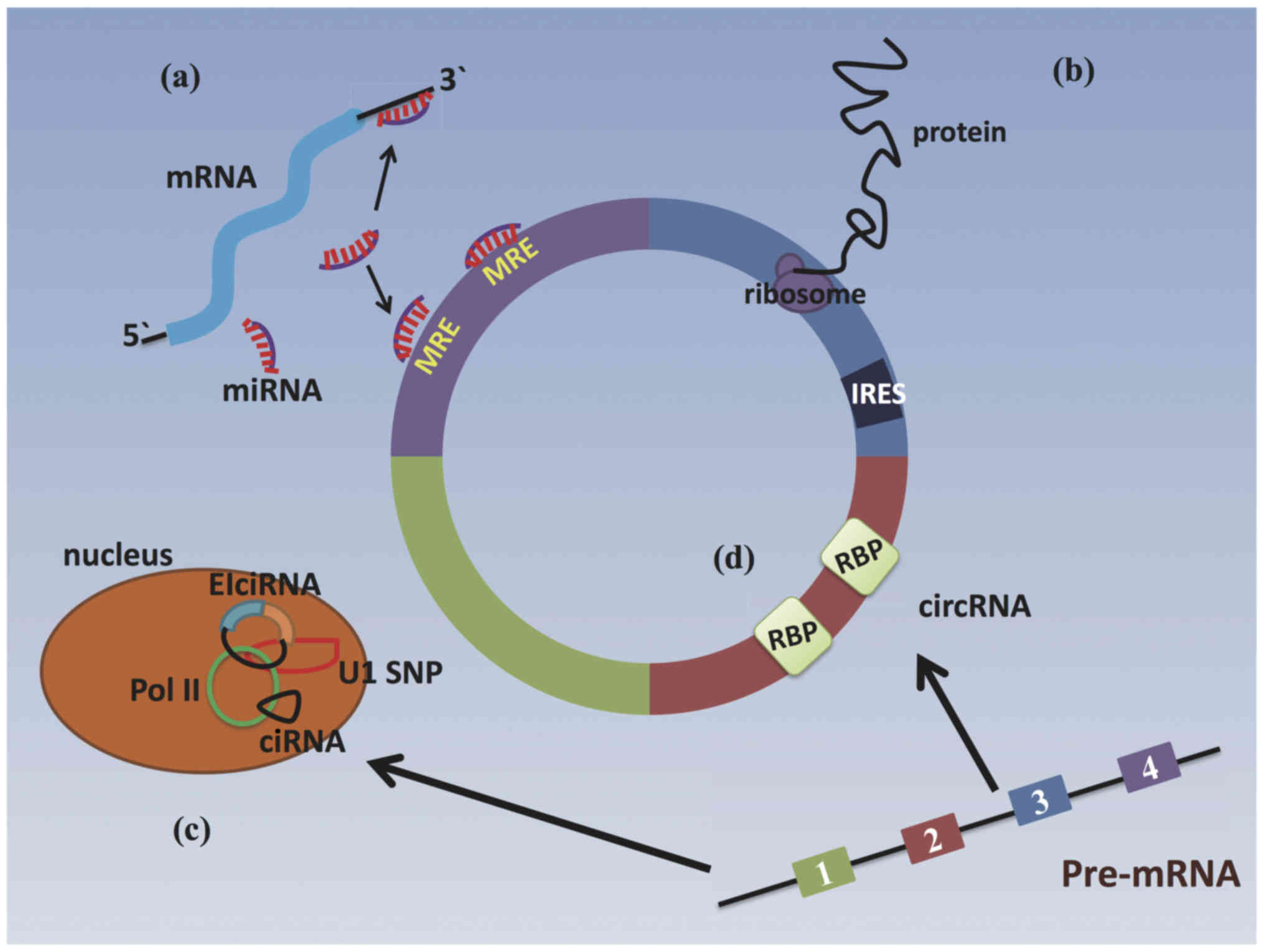
The most vital function of competing endogenous RNAs
(ceRNAs) is to function as inhibitors of microRNA (miRNA or miRs)
by sponging a specific miRNA at multiple binding sites in the
circular sequence. miRNAs are small, endogenous RNAs (22
nucleotides in length) that play critical regulatory roles in
animals and plants by targeting 3'UTRs of mRNAs for the cleavage or
translational repression of protein-coding genes (20). ceRNAs contain several different
miRNA response elements (MREs) within their sequences. ceRNAs act
as miRNA sponges to restrain miRNAs, suppressing the expression of
their target genes (Fig. 2). It
has previously been demonstrated that several abundant ceRNAs can
act as miRNA sponges in the cytoplasm, e.g., ciRS-7/CDR1 (21). CiRS-7, as an antisense transcript
of the human circRNA cerebellar degeneration-related protein 1
transcript (CDR1), contains over 70 conserved binding sites for
miRNA-7. The relatively high expression of CiRS-7 in a number of
human tissues increases the expression of miR-7 target genes by
suppressing miR-7 activity. The sponging of miR-7 by ciRS-7 affects
the proliferation, migration and invasion of cancer cells by
regulating oncogenes in cancer-related signaling pathways (16,22,23).
In addition to acting as miRNA sponges, circRNAs are
also able to interact with, sequester and transport RBPs and
subsequently influence target protein activities (24) (Fig.
2). Examples include circ-Foxo3, circ-MBL (muscleblind) and
circCcnb1, etc (25). circ-Foxo3
is highly expressed in non-cancer cells and can bind
cyclin-dependent kinase (CDK)2 and CDK inhibitor 1 (p21). The
circ-Foxo3-p21-CDK2 complex can suppress the formation of the
cyclin E/CDK2 complex, which results in the arrest of the cell
cycle from the G1 to the S phase (26). In p53 mutant cells, circ-Ccnb1 can
form a complex by interacting with H2AX and Bclaf1, which induces
the death of breast cancer cells, whereas circ-Ccnb1 can prevent
the cancer-suppressing effects by binding H2AX and wild-type p53
(25).
Some subclasses of circRNAs are enriched in the
nucleus. They can function as post-transcriptional regulators of
genes with various mechanisms. ciRNAs and eiciRNAs can participate
in parental gene transcription and splicing under different
regulatory models. ci-ankrd52 and ci-sirt7 positively promote
parental gene transcription in cis regulatory elements by
interacting with the RNA polymerase II (Pol II) complexes at the
transcription sites of their host genes (16). In HeLa and 293 cells, eiciRNAs,
such as circEIF3J and circPAIP can promote the transcription of
parental genes in a cis-acting manner by interacting with U1
small nuclear ribonucleoproteins (snRNPs) and further combining
with RNA polymerase II (Pol II) complexes (14) (Fig.
2).
circRNAs have long been considered to be a type of
endogenous non-coding RNA, which largely do not encode proteins
(27). However, some researchers
have demonstrated that a few circRNAs have the potential to be
translated into proteins in vitro and in vivo when
they possess internal ribosome entry sites (IRESs) upstream of
start codons (28) (Fig. 2). Circular zinc-finger protein 609
(Circ-ZNF609) contains an open reading frame (ORF) and can be
translated into a protein in murine myoblasts when driven by an
IRES (29). The ORFs of
circ-SHPRH and circ-FBXW7 are driven by IRESs and can be translated
into functional proteins in a similar pattern to circ-ZNF609
(30,31). In addition, the most abundant base
modification of RNAs, the N6-methy-ladenosine
(m6A) residues found in circRNAs, have been suggested to accelerate
the cap-independent translation of circRNAs (32). Furthermore, the translation of
certain linear mRNAs can be modulated by their cognate circRNAs. In
HeLa cells, circPABPN1 can bind HuR, an RNA-binding protein that
promotes translation, and the complex that is formed inhibits HuR
binding to PABPN1 mRNA. Finally, the translation of PABRN1 is
reduced (33).
Due to their conservation, specificity and
stability, circRNAs can be used as potential biomarkers for various
diseases, particularly cancers (34). Growing evidence has indicated that
a single circRNA possesses moderate diagnostic value, whereas
combined circRNAs improve the diagnostic efficacy (35). circLPAR1 exhibits a low expression
level in muscle-invasive bladder cancer (MIBC) and predicts a poor
prognosis. It may regulate the invasion and metastasis of MIBC by
targeting miR-762 and has the potential to serve as a stable
biomarker for the prognosis of MIBC (36). In HCC, circSMARCA5 can stimulate
apoptosis and suppress proliferation, invasion and metastasis. It
exhibits a high accuracy for the diagnosis of HCC and may function
as a potential biomarker for monitoring HCC (37). circ_0068871 can promote bladder
cancer progression by modulating the miR-181a-5p/FGFR3 axis and
activating STAT3 (38).
Therefore, it may function as a potential biomarker.
circRNAs are involved in the regulation of tumor
progression. The deregulation of circRNAs affects cell
proliferation, epithelial-mesenchymal transition, apoptosis,
angiogenesis and the cell cycle (39). Thus, circRNAs have the potential
to serve as tumor-targeted sites in tumor metastasis therapy. In
this section, the diagnostic and prognostic significance of
circRNAs in the development of tumor metastasis in gastrointestinal
cancer is emphasized.
Colorectal cancer is a common type of malignant
tumor with the third highest occurrence rate; however, it
represents the second leading cause of cancer-related mortality
worldwide. Over 1.8 million new cases and 881,000 deaths were
attributed to CRC in 2018 (40).
The majority of patients have already developed metastasis at the
initial diagnosis. The development of CRC is a multistep process
involving various factors, such as the BRAF gene, the APC gene and
the KRAS oncogene mutations, abnormal chromosome segregation,
abnormal hypermethy lation of gene promoter region, loss of
function of the p53 gene (41,42). The more in depth understanding of
the underlying molecular mechanisms of the development and
progression of CRC is of utmost importance in order to identify and
develop novel therapeutic markers and strategies. During CRC
progression, some circRNAs play a positive regulatory role in the
disease, while others do not. circRNAs play critical roles in the
biological behavior of tumors.
circ-1569, located on the plus strand of chromosome
16q13.1, is upregulated in CRC tissues and is associated with
aggressive characteristics in CRC. It directly inhibits miR-145
transcription by acting as a sponge. The miR-145 functional targets
(E2F5, BAG4 and FMNL2) are then upregulated, which carry out a
tumor-promoting function in CRC cells (43). This finding reveals a novel
mechanistic connection between miR-145 and circ-1569 in regulating
the progression of CRC, which may provide new insight into CRC
progression and may lead to the development of therapeutic
strategies for CRC prevention and treatment.
CDR1as (ciRS-7) harbors ~70 conserved binding sites.
It exhibits a higher expression in CRC tissues than in the adjacent
normal mucosa. CDR1as may influence tumor biological behavior as an
miRNA sponge. The tumor inhibitory effect of miR-7 can be reversed
by the overexpression of ciR-7 in HCT116 and HT29 cells. The target
gene EGFR is then activated, which results in the increased
proliferation, invasion and migration of CRC cells. This finding
reveals that aberrant CDR1as/miR-7/EGFR may serve as a promising
molecular target for the development of novel therapies to control
CRC progression (44).
Several studies have demonstrated that miR-21 and
miR-31 act as oncogenic molecules in various types of cancer.
miR-21 can increase the proliferation and migration of colon cancer
cells, such as SW480 and HCT116 cells (45). miR-31 can promote the resistance
of CRC cells to 5-fluorouracil (46). circ-0026344, which is
downregulated >5-fold in CRC tissues compared to normal tissues
(47), acts as an miRNA sponge
for miRNA-21 and miRNA-31. The low expression of circ-0026344
increases the expression of miR-21 and miR-31, which promotes the
growth and invasion, but suppresses the apoptosis of CRC cells. The
circ_0026344/miR-21/miR-31 regulatory axis plays an important role
in the progression of CRC, and circ-0026344 can be used as a
reliable prognostic biomarker in patients with CRC (41).
circRNA_103809 expression in colorectal cancer cell
lines is lower than that in normal colorectal epithelial cells and
can promote the proliferation and migration of CRC cells. As
circRNA-103809 has a binding site within miR-532-3p, the
deregulation of miR-532-3p can increase the expression of
circRNA-103809 and Foxo4 in SW620 cells. This indicates that
circRNA-103809 competitively binds to miR-532-3p as a ceRNA, which
in turn regulates FOXO4 expression and thereby restrains the
proliferation and migration of colorectal cancer cells (48).
Overall, almost 30 circRNAs participate in the
progression of the cell cycle, proliferation, invasion, metastasis
and apoptosis by functioning as oncogenes or antioncogenes in CRC.
Further information on this matter is presented in Table I.
circHIPK3 can sponge miR-124 and miR-29. The
upregulation of circHIPK3 decreases the expression of miR-124 and
miR-29b, which promotes GC cell proliferation. During this process,
the mRNA transcriptional levels of 3 target genes (COL1A1, COL4A1
and CDK6) of the circHIPK3-miRNA-124/miRNA-29b axes are upregulated
in GC cells. The survival status of GC can be predicted by
detecting the expression levels of COL1A1 and COL4A1. These
findings indicate that not only circHIPK3, but also COL1A1 and
COL4A1 can serve as prognostic biomarkers of the survival of
patients with GC, which expands our understanding of the
transcriptional mechanisms in GC (111).
miR-206 has been reported to function as an
inhibitory miRNA in GC, and its overexpression can inhibit the
expression of CXCR4. circ_0056618, which is upregulated in GC, can
sponge miR-206 in GC, and suppress cell proliferation and invasion
by upregulating CXCR4. Therefore, circ_0056618/miR-206/CXCR4 is
able to improve the survival rates of patients with GC by
inhibiting GC cell proliferation and metastasis (112).
circRNAs also function as anti-oncogenes. circLARP4,
derived from exons 9 and 10 of the LARP4 gene, is overex-pressed in
GC. It can inhibit DNA synthesis, cell proliferation and invasion
by sponging miR-424 and subsequently upregulates the expression of
the LATS1 and YAP genes in GC. Thus, circLARP4 may function as a
tumor-suppressive factor in GC by regulating the miR-424/LATS1/YAP
signaling pathway. circLARP4 can also serve as an independent
prognostic marker for the 5-year overall survival rate of patients
with GC and patients undergoing chemotherapy (113).
Phosphatase and tensin homolog (PTEN) is a tumor
suppressor that is mutated in various types of cancers at a high
frequency (114). It can be
targeted and regulated by miR-130a and miR-107, thereby affecting
the activity of cancer cells (115,116). circ-ZFR can regulate the
expression of miR-130a and miR-107 by sponging these miRNAs,
thereby regulating the expression of PTEN. This indicates that
circ-ZFR can suppress GC cell propagation and the cell cycle, and
can promote apoptosis by sponging miR-107/miR-130a and modulating
PTEN, which provides comprehensive insight into the regulatory role
of the circ-ZFR/miR-130a/miR-107/PTEN axis in GC and facilitates
the discovery of novel therapeutic targets for the treatment of GC
(117).
Although there are many circular RNAs that serve as
biomarkers for GC, further clinical and basic research is required
to improve the survival time of patients with GC. Over 15 circRNAs
participate in the cell cycle, proliferation, invasion, metastasis
and apoptosis by functioning as oncogenes or anti-oncogenes in GC.
For further information on circRNAs in GC, please refer to Table II.
The most well-known circRNA, ciRS-7, has been
reported to contain >70 binding sites for miR-7. It can suppress
miR-7 activity by acting as an miR-7 sponge (185,186). In addition, it also harbors 19
miR-876-5p-binding sites and functions as a sponge of miR-876-5p,
which directly targets the tumor antigen MAGE-A family and
suppresses the migration and invasion of esophageal squamous cell
carcinoma (ESCC) cells. The discovery of ciRS7 provides a novel
potential therapeutic target for the treatment of ESCC (187).
cir-ITCH is expressed in low levels in esophageal
carcinoma. As a potential tumor suppressor, it upregulates the
expression of the miRNA target gene, ITCH, by sponging miRNAs, such
as miR-7, miR-17 and miR-214, which results in the suppression of
the canonical Wnt pathway by inhibiting phosphorylated Dvl2,
preventing oncogenesis. This indicates that the low expression of
cir-ITCH increases cell viability and promotes proliferation via
the cir-ITCH/miR-7/miR-17/miR-214/ITCH/Dvl2/Wnt/β-catenin signaling
pathway in ESCC cells (188).
For further information regarding the regulation of signaling by
other circRNAs in esophageal cancer, please refer to Table III.
Hsa_circ_0005057 was first found to be expressed at
significantly higher levels in HCC tissues than in adjacent liver
tissues. Hsa_circ_0005057 inhibits the expression and function of
miR-23b-5p, and miR-23b-5p is subsequently downregulated. In
addition, a potential interaction between miR-23b-5p and the
Wnt/β-catenin signaling pathway has been identified. The ultimate
effect of hsa_circ_0005057 is to promote the growth of tumors
(120).
It has been demonstrated that Cdr1as expression is
upregulated in HCC tissues compared with adjacent non-tumor
tissues. The knockdown of Cdr1as inhibits HCC cell proliferation
and invasion by targeting miR-7 and CCNE1 and PIK3CD, and Cdr1as
functions as an oncogene partly by targeting miR-7 in HCC (202,205,206). Hsa_circ_0001649, which is
significantly downregulated in HCC tissues, exhibits miRNA-binding
sites for miR-1283, miR-4310 and miR-182-3p and is positively
associated with the metastasis and invasion of HCC (207). circRNA mitochondrial translation
optimization 1 homologue (circMTO1;
hsa_circRNA_0007874/hsa_circRNA_104135) is downregulated in HCC
tissues, and miR-9 is overexpressed in HCC, which results in the
HCC cell proliferation and invasion. The finding that circMTO1
co-localizes with miR-9 in the cytoplasm and that this
co-localization is decreased in tumors compared to adjacent
non-tumor tissues, has indicated that circMTO1 can inhibit the
progression of HCC by serving as a sponge of oncogenic miR-9 to
promote p21 expression (208).
For further information on circRNAs in HCC, please refer to
Table IV.
Gallbladder cancer is the most common malignant
neoplasm of the biliary tract, with a low 5-year overall survival
rate of 5-10% (275,276). Gallbladder cancers are detected
either incidentally or by clinical manifestation (277). However, patients with
gallbladder cancer who present symptoms are usually at the advanced
disease stage. Therefore, 90% of gallbladder cancers are diagnosed
at regional or metastatic stages. It is widely acknowledged that
surgical resection is the only curative method for gallbladder
cancer, and other treatment modalities, such as chemoradiotherapy,
are not effective in most cases without surgical resection
(278). Due to the high
recurrence, morbidity and mortality of gallbladder cancer, further
research is warranted to discover potential biomarkers for the
early detection and prognosis predication of this type of
cancer.
Recent studies have demonstrated that circHIPK3
plays a critical role, not only in CRC, but also in gallbladder
cancer. It is upregulated in human gallbladder cancer tissues and
can modulate miR-124 downregulation and ROCK1-CDK6 upregulation as
an miRNA sponge, thereby promoting gallbladder cancer cell growth
(279). circFOXP1
(hsa_circ_0008234), derived from the exon region of the FOXP1 gene,
is upregulated in GBC tissues and cells. It can promote PKLR
expression by sponging miR-370 in GBC cells, resulting in the
promotion of the Warburg effect in GBC progression. The effects
include the promotion of cell proliferation, migration and
invasion, and the inhibition of cell apoptosis in GBC (280). circERBB2, derived from the ERBB2
gene locus, differs from general circRNAs that are located in the
cytoplasm and function as miRNA sponges. circERBB2 is enriched in
nucleoli. It regulates the nucleolar localization of
proliferation-associated protein 2G4 (PA2G4), thereby forming a
circERBB2/PA2G4/TIFIA regulatory axis to upregulate Pol I activity
and rDNA transcription. Finally, it promotes GBC proliferation and
progression (281). Generally,
only 3 circRNAs to date have been found to participate in GBC
progression by functioning as oncogenes or anti-oncogenes.
An ideal biomarker should be non-invasive, accurate,
inexpensive, specific, sensitive and reliable, and reproducible
body fluids are ideal material for use in the diagnosis of human
cancer (282). circRNAs are
usually stable both inside cells and in extracellular plasma,
including blood and saliva (22).
In addition, they are involved in the pathogenesis of a variety of
diseases, such as diabetes, Alzheimer's disease and cancer.
Therefore, the stability of circRNAs in bodily fluids and the
specificity of circRNAs in diseases have made them promising
non-invasive alternatives for use as diagnostic biomarkers for
diseases, such as for the real-time monitoring of tumor progression
and therapeutic responses, cancer screening, and susceptibility
evaluation, particularly in gastrointestinal cancer (2). Currently, a few available clinical
biomarkers, such as CEA and CA19-9, have been reported to exhibit
low sensitivity and specificity for the early detection of CRC
(283,284). circ_0014717 exists stably in
human gastric juices and can potentially be used as a biomarker for
the screening of high-risk GC (285). It has been discovered that the
hsa_circ_0001017 and hsa_circ_0061276 levels are tightly associated
with the main clinicopathological features of patients with GC,
which indicates that they can serve as valuable blood-based
biomarkers (282).
Some circRNAs function as oncogenes, and several
therapeutic strategies have been proposed to target these. An
exogenously delivered siRNA can accurately target unique
back-splice junctions of circRNAs. The expression of circRNAs can
then be suppressed (119,286,287).
Another approach is the use of artificial miRNA sponges, such as
TuD, with the aim of deceasing the level of oncomiRs (288).
The early diagnosis of gastrointestinal cancer is a
main strategy to decrease the mortality rate associated with
gastrointestinal cancer, and the regulation of circRNA expression
will be the next therapeutic method tested for patients with
gastrointestinal cancer. It is considered that more accurate
disease detection will be achieved via the combined analysis of
body fluid circRNAs and specific biological biomarkers.
In summary, circRNAs have been discovered as novel
molecules in recent years and are no longer recognized as products
of transcription errors. Some circRNAs present unique advantages,
such as high abundance, stability and widespread expression. These
unique characteristics of circRNAs indicate that they are
potentially valuable biomarkers for assessing the prognosis and
diagnosis of gastrointestinal cancers. Some circRNAs function as
miRNA sponges and regulators of parental gene transcription and
possess the ability to bind with RBPs. The functions and regulatory
roles of circRNAs in tumors indicate that they are a potential
target for the treatment of cancer. These findings provide new
insight and potential therapeutic strategies for cancer prevention
and treatment in the future.
However, the underlying molecular mechanisms of
circRNAs are more complex than discussed in the present review.
They may exhibit multiple interactions in cancers, that are not yet
clearly understood. A number of studies have focused on the
'sponge' function of certain circRNAs. In fact, not all circRNAs
possess a sponge function, as only a small number of circRNAs
exhibit rich binding sites for certain target miRNAs (289). It is necessary to pay attention
to other roles and uncover additional functions of circRNAs, such
as the binding of RBPs and the mechanisms through which they affect
gene translation. Furthermore, as the current methods for the
detection and characterization of circRNAs are still limited and
challenging, circRNAs are far from being implicated into clinical
practice. These fundamental problems require further
investigation.
Therefore, due to the numerous amounts of unanswered
questions, circRNAs warrant further investigation. The regulatory
networks of circRNAs/miRNAs/mRNAs and circRNAs/RBPs and other roles
of circRNAs in gastrointestinal cancers need to be clarified in the
future. The full elucidation of all the ceRNA and RNA/protein
crosstalk that occurs under pathophysiological conditions in
gastrointestinal cancers will certainly have exciting applications
for the development of promising therapeutic approaches related to
circRNAs.
The current study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81760550
and 81460462), the Postgraduate Innovation Fund Project of Nanchang
University (grant no. CX2019168).
Not applicable.
XZ, YW, PY, QZ and XY collected the related
literature and drafted the manuscript. QY and DG participated in
the design of the review and drafted the manuscript. All authors
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Wang Y, Mo Y, Gong Z, Yang X, Yang M,
Zhang S, Xiong F, Xiang B, Zhou M, Liao Q, et al: Circular RNAs in
human cancer. Mol Cancer. 16:252017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Liang W, Zhang P, Chen J, Qian H,
Zhang X and Xu W: Circular RNAs: Emerging cancer biomarkers and
targets. J Exp Clin Cancer Res. 36:1522017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harder JM, Braine CE, Williams PA, Zhu X,
MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR
and John SWM: Early immune responses are independent of RGC
dysfunction in glaucoma with complement component C3 being
protective. Proc Natl Acad Sci USA. 114:E3839–E3848. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benitez-Herreros J, Lopez-Guajardo L,
Camara-Gonzalez C, Vazquez-Blanco M and Castro-Rebollo M:
Association between macular perfusion and photoreceptor layer
status in diabetic macular edema. Retina. 35:288–293. 2015.
View Article : Google Scholar
|
|
7
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar :
|
|
8
|
Ren X, Du Y, You L and Zhao Y: Potential
functions and implications of circular RNA in gastrointestinal
cancer. Oncol Lett. 14:7016–7020. 2017.
|
|
9
|
Ying X, Hanmin C, Wenqi Y, Zhichang L and
Zhengming Z: Research progress on the role of circRNA in
gastrointestinal tumor. China J Clin Oncol. 44:778–781. 2017.
|
|
10
|
Li B and Huang C: Regulation of EMT by
STAT3 in gastrointestinal cancer (Review). Int J Oncol. 50:753–767.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang XO, Wang HB, Zhang Y, Lu X, Chen LL
and Yang L: Complementary sequence-mediated exon circularization.
Cell. 159:134–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Z, Yang T and Xiao J: Circular RNAs:
Promising biomarkers for human diseases. EBioMedicine. 34:267–274.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Qu L, Chen X, Zhao YH and Luo Q:
Progress in understanding the relationship between circular RNAs
and neurological disorders. J Mol Neurosci. 65:546–556. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen LL: The biogenesis and emerging roles
of circular RNAs. Nat Rev Mol Cell Biol. 17:205–211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar :
|
|
20
|
Wei CC, Luo Z, Song YF, Pan YX, Wu K and
You WJ: Identification of autophagy related genes LC3 and ATG4 from
yellow catfish Pelteobagrus fulvidraco and their transcriptional
responses to waterborne and dietborne zinc exposure. Chemosphere.
175:228–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebbesen KK, Kjems J and Hansen TB:
Circular RNAs: Identification, biogenesis and function. Biochim
Biophys Acta. 1859:163–168. 2016. View Article : Google Scholar
|
|
22
|
Li X, Yang L and Chen LL: The biogenesis,
functions, and challenges of circular RNAs. Mol Cell. 71:428–442.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kulcheski FR, Christoff AP and Margis R:
Circular RNAs are miRNA sponges and can be used as a new class of
biomarker. J Biotechnol. 238:42–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qian L, Yu S, Chen Z, Meng Z, Huang S and
Wang P: The emerging role of circRNAs and their clinical
significance in human cancers. Biochim Biophys Acta Rev Cancer.
1870:247–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang L, Du WW, Lyu J, Dong J, Zhang C,
Yang W, He A, Kwok YSS, Ma J, Wu N, et al: Enhanced breast cancer
progression by mutant p53 is inhibited by the circular RNA
circ-Ccnb1. Cell Death Differ. 25:2195–2208. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilusz JE: A 360° view of circular RNAs:
From biogenesis to functions. Wiley Interdiscip Rev RNA.
9:e14782018. View Article : Google Scholar
|
|
28
|
Pamudurti NR, Bartok O, Jens M,
Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E,
Perez-Hernandez D, Ramberger E, et al: Translation of CircRNAs. Mol
Cell. 66:9–21.e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Legnini I, Di Timoteo G, Rossi F, Morlando
M, Briganti F, Sthandier O, Fatica A, Santini T, Andronache A, Wade
M, et al: Circ-ZNF609 Is a circular RNA that can be translated and
functions in myogenesis. Mol Cell. 66:22–37.e9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Huang N, Yang X, Luo J, Yan S,
Xiao F, Chen W, Gao X, Zhao K, Zhou H, et al: A novel protein
encoded by the circular form of the SHPRH gene suppresses glioma
tumorigen-esis. Oncogene. 37:1805–1814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar :
|
|
32
|
Yang Y, Fan X, Mao M, Song X, Wu P, Zhang
Y, Jin Y, Yang Y, Chen LL, Wang Y, et al: Extensive translation of
circular RNAs driven by N6-methyladenosine. Cell Res.
27:626–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdelmohsen K, Panda AC, Munk R,
Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM,
Martindale JL and Gorospe M: Identification of HuR target circular
RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA
Biol. 14:361–369. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li HM, Ma XL and Li HG: Intriguing
circles: Conflicts and controversies in circular RNA research.
Wiley Interdiscip Rev RNA. 10:e15382019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Li H, Lv X, Yang Z, Gao M, Bi Y,
Zhang Z, Wang S, Cui Z, Zhou B and Yin Z: Diagnostic performance of
circular RNAs in human cancers: A systematic review and
meta-analysis. Mol Genet Genomic Med. 7:e007492019.PubMed/NCBI
|
|
36
|
Lin G, Sheng H, Xie H, Zheng Q, Shen Y,
Shi G and Ye D: circLPAR1 is a novel biomarker of prognosis for
muscle-invasive bladder cancer with invasion and metastasis by
miR-762. Oncol Lett. 17:3537–3547. 2019.PubMed/NCBI
|
|
37
|
Li Z, Zhou Y, Yang G, He S, Qiu X, Zhang
L, Deng Q and Zheng F: Using circular RNA SMARCA5 as a potential
novel biomarker for hepatocellular carcinoma. Clin Chim Acta.
492:37–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao W, Huang X, Wang L, Zhang Z, Liu M, Li
Y, Luo M, Yao X, Fan J and Geng J: Circular RNA hsa_circ_0068871
regulates FGFR3 expression and activates STAT3 by targeting
miR-181a-5p to promote bladder cancer progression. J Exp Clin
Cancer Res. 38:1692019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuan Y, Liu W, Zhang Y, Zhang Y and Sun S:
CircRNA circ_0026344 as a prognostic biomarker suppresses
colorectal cancer progression via microRNA-21 and microRNA-31.
Biochem Biophys Res Commun. 503:870–875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang XL, Xu LL and Wang F:
Hsa_circ_0020397 regulates colorectal cancer cell viability,
apoptosis and invasion by promoting the expression of the miR-138
targets TERT and PD-L1. Cell Biol Int. 41:1056–1064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang W, Ji M, He G, Yang L, Niu Z, Jian M,
Wei Y, Ren L and Xu J: Silencing CDR1as inhibits colorectal cancer
progression through regulating microRNA-7. Onco Targets Ther.
10:2045–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Fu C, Xu Q and Wei X: Long
non-coding RNA CASC7 inhibits the proliferation and migration of
colon cancer cells via inhibiting microRNA-21. Biomed Pharmacother.
95:1644–1653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang CJ, Stratmann J, Zhou ZG and Sun XF:
Suppression of microRNA-31 increases sensitivity to 5-FU at an
early stage, and affects cell migration and invasion in HCT-116
colon cancer cells. BMC Cancer. 10:6162010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Zhang L, Su Y and Zhang X:
Screening potential biomarkers for colorectal cancer based on
circular RNA chips. Oncol Rep. 39:2499–2512. 2018.PubMed/NCBI
|
|
48
|
Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun
S, Li J, Sun Y and Qin J: Hsa_circRNA_103809 regulated the cell
proliferation and migration in colorectal cancer via
miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 505:346–352.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yong W, Zhuoqi X, Baocheng W, Dongsheng Z,
Chuan Z and Yueming S: Hsa_circ_0071589 promotes carcinogenesis via
the miR-600/EZH2 axis in colorectal cancer. Biomed Pharmacother.
102:1188–1194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weng W, Wei Q, Toden S, Yoshida K,
Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y and Goel A: Circular RNA
ciRS-7-A promising prognostic biomarker and a potential therapeutic
target in colorectal cancer. Clin Cancer Res. 23:3918–3928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peng L, Chen G, Zhu Z, Shen Z, Du C, Zang
R, Su Y, Xie H, Li H, Xu X, et al: Circular RNA ZNF609 functions as
a competitive endogenous RNA to regulate AKT3 expression by
sponging miR-150-5p in Hirschsprung's disease. Oncotarget.
8:808–818. 2017. View Article : Google Scholar :
|
|
52
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: CircHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu XW, Zheng BA, Hu ZM, Qian ZY, Huang CJ,
Liu XQ and Wu WD: Circular RNA hsa_circ_000984 promotes colon
cancer growth and metastasis by sponging miR-106b. Oncotarget.
8:91674–91683. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shen T, Cheng X, Liu X, Xia C, Zhang H,
Pan D, Zhang X and Li Y: Circ_0026344 restrains metastasis of human
colorectal cancer cells via miR-183. Artif Cells Nanomed
Biotechnol. 47:4038–4045. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fang G, Ye BL, Hu BR, Ruan XJ and Shi YX:
CircRNA_100290 promotes colorectal cancer progression th rough
miR-516b-induced downregulation of FZD4 expression and
Wnt/β-catenin signaling. Biochem Biophys Res Commun. 504:184–189.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He JH, Li YG, Han ZP, Zhou JB, Chen WM, Lv
YB, He ML, Zuo JD and Zheng L: The
CircRNA-ACAP2/Hsa-miR-21-5p/Tiam1 regulatory feedback circuit
affects the proliferation, migration, and invasion of colon cancer
SW480 cells. Cell Physiol Biochem. 49:1539–1550. 2018. View Article : Google Scholar
|
|
57
|
Li X, Wang J, Zhang C, Lin C, Zhang J,
Zhang W, Zhang W, Lu Y, Zheng L and Li X: Circular RNA circITGA7
inhibits colorectal cancer growth and metastasis by modulating the
Ras pathway and upregulating transcription of its host gene ITGA7.
J Pathol. 246:166–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang G, Zhu H, Shi Y, Wu W, Cai H and
Chen X: cir-ITCH plays an inhibitory role in colorectal cancer by
regulating the Wnt/β-catenin pathway. PLoS One. 10:e01312252015.
View Article : Google Scholar
|
|
59
|
Zhu M, Xu Y, Chen Y and Yan F: Circular
BANP, an upregu-lated circular RNA that modulates cell
proliferation in colorectal cancer. Biomed Pharmacother.
88:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xiong W, Ai YQ and Li YF, Ye Q, Chen ZT,
Qin JY, Liu QY, Wang H, Ju YH, Li WH and Li YF: Microarray analysis
of circular RNA expression profile associated with
5-fluorouracil-based chemo-radiation resistance in colorectal
cancer cells. Biomed Res Int. 2017:84216142017. View Article : Google Scholar
|
|
61
|
Zeng Y, Xu Y, Shu R, Sun L, Tian Y, Shi C,
Zheng Z, Wang K and Luo H: Altered expression profiles of circular
RNA in colorectal cancer tissues from patients with lung
metastasis. Int J Mol Med. 40:1818–1828. 2017.PubMed/NCBI
|
|
62
|
Hsiao KY, Lin YC, Gupta SK, Chang N, Yen
L, Sun HS and Tsai SJ: Noncoding effects of circular RNA CCDC66
promote colon cancer growth and metastasis. Cancer Res.
77:2339–2350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhong D, Li P and Gong PY:
Hsa_circ_0005075 promotes the proliferation and invasion of
colorectal cancer cells. Int J Biol Markers. 34:284–291. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jin YD, Ren YR, Gao YX, Zhang L and Ding
Z: Hsa_ circ_0005075 predicts a poor prognosis and acts as an
oncogene in colorectal cancer via activating Wnt/β-catenin pathway.
Eur Rev Med Pharmacol Sci. 23:3311–3319. 2019.PubMed/NCBI
|
|
65
|
Zhang Q, Zhang C, Ma JX, Ren H, Sun Y and
Xu JZ: Circular RNA PIP5K1A promotes colon cancer development
through inhibiting miR-1273a. World J Gastroenterol. 25:5300–5309.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pei FL, Cao MZ and Li YF: Circ_0000218
plays a carcinogenic role in colorectal cancer progression by
regulating miR-139-3p/RAB1A axis. J Biochem. 167:55–65. 2020.
View Article : Google Scholar
|
|
67
|
Lu X, Yu Y, Liao F and Tan S: Homo sapiens
circular RNA 0079993 (hsa_circ_0079993) of the POLR2J4 gene acts as
an oncogene in colorectal cancer through the microRNA-203a-3p1 and
CREB1 axis. Med Sci Monit. 25:6872–6883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lu C, Jiang W, Hui B, Rong D, Fu K, Dong
C, Tang W and Cao H: The circ_0021977/miR-10b-5p/P21 and P53
regulatory axis suppresses proliferation, migration, and invasion
in colorectal cancer. J Cell Physiol. 235:2273–2285. 2020.
View Article : Google Scholar
|
|
69
|
Zhang J, Liu H, Zhao P, Zhou H and Mao T:
Has_circ_0055625 from circRNA profile increases colon cancer cell
growth by sponging miR-106b-5p. J Cell Biochem. 120:3027–3037.
2019. View Article : Google Scholar
|
|
70
|
Li H, Jin X, Liu B, Zhang P, Chen W and Li
Q: CircRNA CBL.11 suppresses cell proliferation by sponging
miR-6778-5p in colorectal cancer. BMC Cancer. 19:8262019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ren TJ, Liu C, Hou JF and Shan FX:
CircDDX17 reduces 5-fluorouracil resistance and hinders
tumorigenesis in colorectal cancer by regulating miR-31-5p/KANK1
axis. Eur Rev Med Pharmacol Sci. 24:1743–1754. 2020.PubMed/NCBI
|
|
72
|
Zhang ZJ, Zhang YH, Qin XJ, Wang YX and Fu
J: Circular RNA circDENND4C facilitates proliferation, migration
and glycolysis of colorectal cancer cells through miR-760/GLUT1
axis. Eur Rev Med Pharmacol Sci. 24:2387–2400. 2020.PubMed/NCBI
|
|
73
|
Ding DY, Wang D and Shu ZB:
Hsa_circ_0007534 knockdown represses the development of colorectal
cancer cells through regulating miR-613/SLC25A22 axis. Eur Rev Med
Pharmacol Sci. 24:3004–3022. 2020.PubMed/NCBI
|
|
74
|
Zheng X, Ma YF, Zhang XR, Li Y, Zhao HH
and Han SG: Circ_0056618 promoted cell proliferation, migration and
angiogenesis through sponging with miR-206 and upregulating CXCR4
and VEGF-A in colorectal cancer. Eur Rev Med Pharmacol Sci.
24:4190–4202. 2020.PubMed/NCBI
|
|
75
|
Tu FL, Guo XQ, Wu HX, He ZY, Wang F, Sun
AJ and Dai XD: Circ-0001313/miRNA-510-5p/AKT2 axis promotes the
development and progression of colon cancer. Am J Transl Res.
12:281–291. 2020.PubMed/NCBI
|
|
76
|
Wu HB, Huang SS, Lu CG, Tian SD and Chen
M: CircAPLP2 regulates the proliferation and metastasis of
colorectal cancer by targeting miR-101-3p to activate the Notch
signalling pathway. Am J Transl Res. 12:2554–2569. 2020.PubMed/NCBI
|
|
77
|
Chen C, Huang Z, Mo X, Song Y, Li X, Li X
and Zhang M: The circular RNA 001971/miR-29c-3p axis modulates
colorectal cancer growth, metastasis, and angiogenesis through
VEGFA. J Exp Clin Cancer Res. 39:912020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen H, Pei L, Xie P and Guo G: Circ-PRKDC
contributes to 5-fluorouracil resistance of colorectal cancer cells
by regulating miR-375/FOXM1 axis and Wnt/β-catenin pathway. Onco
Targets Ther. 13:5939–5953. 2020. View Article : Google Scholar :
|
|
79
|
Chen HY, Li XN, Ye CX, Chen ZL and Wang
ZJ: Circular RNA circHUWE1 is upregulated and promotes cell
proliferation, migration and invasion in colorectal cancer by
sponging miR-486. Onco Targets Ther. 13:423–434. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen MS, Lin CH, Huang LY and Qiu XM:
CircRNA SMARCC1 sponges MiR-1403p to regulate cell progression in
colorectal cancer. Cancer Manag Res. 12:4899–4910. 2020. View Article : Google Scholar :
|
|
81
|
Chen P, Yao Y, Yang N, Gong L, Kong Y and
Wu A: Circular RNA circCTNNA1 promotes colorectal cancer
progression by sponging miR-149-5p and regulating FOXM1 expression.
Cell Death Dis. 11:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen ZL, Li XN, Ye CX, Chen HY and Wang
ZJ: Elevated levels of circRUNX1 in colorectal cancer promote cell
growth and metastasis via miR-145-5p/IGF1 signalling. Onco Targets
Ther. 13:4035–4048. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Deng Z, Li X, Wang H, Geng Y, Cai Y, Tang
Y, Wang Y, Yu X, Li L and Li R: Dysregulation of circRNA_0001946
contributes to the proliferation and metastasis of colorectal
cancer cells by targeting MicroRNA-135a-5p. Front Genet.
11:3572020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Du H, He Z, Feng F, Chen D, Zhang L, Bai
J, Wu H, Han E and Zhang J: Hsa_circ_0038646 promotes cell
proliferation and migration in colorectal cancer via
miR-331-3p/GRIK3. Oncol Lett. 20:266–274. 2020.PubMed/NCBI
|
|
85
|
Feng W, Gong H, Wang Y, Zhu G, Xue T, Wang
Y and Cui G: circIFT80 Functions as a ceRNA of miR-1236-3p to
promote colorectal cancer progression. Mol Ther Nucleic Acids.
18:375–387. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hu B, Xian Z, Zou Q, Zhang D, Su D, Yao J
and Ren D: CircFAT1 suppresses colorectal cancer development
through regulating miR-520b/UHRF1 axis or miR-302c-3p/UHRF1 axis.
Cancer Biother Radiopharm. May 5–2020.Epub ahead of print.
View Article : Google Scholar
|
|
87
|
Jian X, He H, Zhu J, Zhang Q, Zheng Z,
Liang X, Chen L, Yang M, Peng K, Zhang Z, et al: Hsa_circ_001680
affects the proliferation and migration of CRC and mediates its
chemore-sistance by regulating BMI1 through miR-340. Mol Cancer.
19:202020. View Article : Google Scholar
|
|
88
|
Li C and Zhou H: Circular RNA
hsa_circRNA_102209 promotes the growth and metastasis of colorectal
cancer through miR-761-mediated Ras and Rab interactor 1 signaling.
Cancer Med. Jul 24–2020.Epub ahead of print).
:101002/cam4.3332.
|
|
89
|
Li W, Xu Y, Wang X, Cao G, Bu W, Wang X,
Fang Z, Xu Y, Dong M and Tao Q: circCCT3 modulates vascular
endothelial growth factor A and Wnt signaling to enhance colorectal
cancer metastasis through sponging miR-613. DNA Cell Biol.
39:118–125. 2020. View Article : Google Scholar
|
|
90
|
Lu C, Fu L, Qian X, Dou L and Cang S:
Knockdown of circular RNA circ-FARSA restricts colorectal cancer
cell growth through regulation of miR-330-5p/LASP1 axis. Arch
Biochem Biophys. 689:1084342020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ma Z, Han C, Xia W, Wang S, Li X, Fang P,
Yin R, Xu L and Yang L: circ5615 functions as a ceRNA to promote
colorectal cancer progression by upregulating TNKS. Cell Death Dis.
11:3562020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ren C, Zhang Z, Wang S, Zhu W, Zheng P and
Wang W: Circular RNA hsa_circ_0001178 facilitates the invasion and
metastasis of colorectal cancer through upregulating ZEB1 via
sponging multiple miRNAs. Biol Chem. 401:487–496. 2020. View Article : Google Scholar
|
|
93
|
Shang A, Gu C, Wang W, Wang X, Sun J, Zeng
B, Chen C, Chang W, Ping Y, Ji P, et al: Exosomal circPACRGL
promotes progression of colorectal cancer via the
miR-142-3p/miR-506-3p-TGF-β1 axis. Mol Cancer. 19:1172020.
View Article : Google Scholar
|
|
94
|
Sun J, Liu J, Zhu Q, Xu F, Kang L and Shi
X: Hsa_circ_0001806 Acts as a ceRNA to facilitate the stemness of
colorectal cancer cells by increasing COL1A1. Onco Targets Ther.
13:6315–6327. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang J, Luo J, Liu G and Li X: Circular
RNA hsa_circ_0008285 inhibits colorectal cancer cell proliferation
and migration via the miR-382-5p/PTEN axis. Biochem Biophys Res
Commun. 527:503–510. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang X, Chen Y, Liu W, Liu T and Sun D:
Hsa_circ_0128846 promotes tumorigenesis of colorectal cancer by
sponging hsa-miR-1184 and releasing AJUBA and inactivating
Hippo/YAP signalling. J Cell Mol Med. Jul 18–2020.Epub ahead of
print. View Article : Google Scholar
|
|
97
|
Wang X, Ren Y, Ma S and Wang S: Circular
RNA 0060745, a novel circRNA, promotes colorectal cancer cell
proliferation and metastasis through miR-4736 sponging. Onco
Targets Ther. 13:1941–1951. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wang X, Zhang H, Yang H, Bai M, Ning T,
Deng T, Liu R, Fan Q, Zhu K, Li J, et al: Exosome-delivered circRNA
promotes glycol-ysis to induce chemoresistance through the
miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 14:539–555.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xiao H and Liu M: Circular RNA
hsa_circ_0053277 promotes the development of colorectal cancer by
upregulating matrix metallopeptidase 14 via miR-2467-3p
sequestration. J Cell Physiol. 235:2881–2890. 2020. View Article : Google Scholar
|
|
100
|
Yan Y, Su M and Qin B: CircHIPK3 promotes
colorectal cancer cells proliferation and metastasis via modulating
of miR-1207-5p/FMNL2 signal. Biochem Biophys Res Commun.
524:839–846. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang B, Du K, Yang C, Xiang L, Xu Y, Cao
C, Zhang J and Liu W: CircPRMT5 circular RNA promotes proliferation
of colorectal cancer through sponging miR-377 to induce E2F3
expression. J Cell Mol Med. 24:3431–3437. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang L, Sun H, Liu X, Chen J, Tian Z, Xu
J, Xiang B and Qin B: Circular RNA hsa_circ_0004277 contributes to
malignant phenotype of colorectal cancer by sponging miR-512-5p to
upregulate the expression of PTMA. J Cell Physiol. Jan 21–2020.Epub
ahead of print. View Article : Google Scholar
|
|
103
|
Yang Z, Zhang J, Lu D, Sun Y, Zhao X, Wang
X, Zhou W, He Q and Jiang Z: Hsa_circ_0137008 suppresses the
malignant phenotype in colorectal cancer by acting as a
microRNA-338-5p sponge. Cancer Cell Int. 20:672020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yin W, Xu J, Li C, Dai X, Wu T and Wen J:
Circular RNA circ_0007142 facilitates colorectal cancer progression
by modulating CDC25A expression via miR-122-5p. Onco Targets Ther.
13:3689–3701. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhang J, Wang H, Wu K, Zhan F and Zeng H:
Dysregulated circRNA_100876 contributes to proliferation and
metastasis of colorectal cancer by targeting microRNA-516b
(miR-516b). Cancer Biol Ther. 21:733–740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang L, Dong X, Yan B, Yu W and Shan L:
CircAGFG1 drives metastasis and stemness in colorectal cancer by
modulating YY1/CTNNB1. Cell Death Dis. 11:5422020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang X, Xu Y, Yamaguchi K, Hu J, Zhang L,
Wang J, Tian J and Chen W: Circular RNA circVAPA knockdown
suppresses colorectal cancer cell growth process by regulating
miR-125a/CREB5 axis. Cancer Cell Int. 20:1032020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Y, Zhang Z, Yi Y, Wang Y and Fu J:
CircNOL10 acts as a sponge of miR-135a/b-5p in suppressing
colorectal cancer progression via regulating KLF9. Onco Targets
Ther. 13:5165–5176. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhou C, Liu HS, Wang FW, Hu T, Liang ZX,
Lan N, He XW, Zheng XB, Wu XJ, Xie D, et al: circCAMSAP1 promotes
tumor growth in colorectal cancer via the miR-328-5p/E2F1 axis. Mol
Ther. 28:914–928. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sun HD, Xu ZP, Sun ZQ, Zhu B, Wang Q, Zhou
J, Jin H, Zhao A, Tang WW and Cao XF: Down-regulation of circPVRL3
promotes the proliferation and migration of gastric cancer cells.
Sci Rep. 8:101112018. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng
J, Hou J, Lin L and Cai J: Regulatory network of circRNA-miRNA-mRNA
contributes to the histological classification and disease
progression in gastric cancer. J Transl Med. 16:2162018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Li H, Yao G, Feng B, Lu X and Fan Y:
Circ_0056618 and CXCR4 act as competing endogenous in gastric
cancer by regulating miR-206. J Cell Biochem. 119:9543–9551. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang J, Liu H, Hou L, Wang G, Zhang R,
Huang Y, Chen X and Zhu J: Circular RNA_LARP4 inhibits cell
proliferation and invasion of gastric cancer by sponging miR-424-5p
and regulating LATS1 expression. Mol Cancer. 16:1512017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Namløs HM, Meza-Zepeda LA, Barøy T,
Østensen IH, Kresse SH, Kuijjer ML, Serra M, Bürger H,
Cleton-Jansen AM and Myklebost O: Modulation of the osteosarcoma
expression phenotype by microRNAs. PLoS One. 7:e480862012.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee KH, Lotterman C, Karikari C, Omura N,
Feldmann G, Habbe N, Goggins MG, Mendell JT and Maitra A:
Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent
kinase 6 expression in pancreatic cancer. Pancreatology. 9:293–301.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C,
Zeng Y and Luo H: Circular RNA-ZFR inhibited cell proliferation and
promoted apoptosis in gastric cancer by sponging miR-130a/miR-107
and modulating PTEN. Cancer Res Treat. 50:1396–1417. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Liu R, Lu Z, Gu J, Liu J, Huang E, Liu X,
Wang L, Yang J, Deng Y, Qian J, et al: MicroRNAs 15A and 161
activate signaling pathways that mediate chemotaxis of immune
regulatory B cells to colorectal tumors. Gastroenterology.
154:637–651.e7. 2018. View Article : Google Scholar
|
|
119
|
Hu ZY, Chen B, Zhang JP and Ma YY:
Up-regulation of autophagy-related gene 5 (ATG5) protects
dopaminergic neurons in a zebrafish model of Parkinson's disease. J
Biol Chem. 292:18062–18074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shang X, Li G, Liu H, Li T, Liu J, Zhao Q
and Wang C: Comprehensive circular RNA profiling reveals that hsa_
circ_0005075, a new circular RNA biomarker, is involved in
hepatocellular crcinoma development. Medicine (Baltimore).
95:e38112016. View Article : Google Scholar
|
|
121
|
Liu Z, Huang S, Cao Y, Yao Y, Li J, Chen
J, Jiang B, Yuan X, Xiang X, Xiong J and Deng J: YAP1 inhibits
circRNA-000425 expression and thus promotes oncogenic activities of
miR-17 and miR-106. Biochem Biophys Res Commun. 503:2370–2375.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Li G, Xue M, Yang F, Jin Y, Fan Y and Li
W: CircRBMS3 promotes gastric cancer tumorigenesis by regulating
miR-153-SNAI1 axis. J Cell Physiol. 234:3020–3028. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chang P, Wang F and Li Y: Hsa_circ_0000673
is down-regulated in gastric cancer and inhibits the proliferation
and invasion of tumor cells by targetting miR-532-5p. Biosci Rep.
38:BSR201805382018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhong S, Wang J, Hou J, Zhang Q, Xu H, Hu
J, Zhao J and Feng J: Circular RNA hsa_circ_0000993 inhibits
metastasis of gastric cancer cells. Epigenomics. 10:1301–1313.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li P, Chen H, Chen S, Mo X, Li T, Xiao B,
Yu R and Guo J: Circular RNA 0000096 affects cell growth and
migration in gastric cancer. Br J Cancer. 116:626–633. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Huang YS, Jie N, Zou KJ and Weng Y:
Expression profile of circular RNAs in human gastric cancer
tissues. Mol Med Rep. 16:2469–2476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Li J, Zhen L, Zhang Y, Zhao L, Liu H, Cai
D, Chen H, Yu J, Qi X and Li G: Circ-104916 is downregulated in
gastric cancer and suppresses migration and invasion of gastric
cancer cells. Onco Targets Ther. 10:3521–3529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Sun H, Tang W, Rong D, Jin H, Fu K, Zhang
W, Liu Z, Cao H and Cao X: Hsa_circ_0000520, a potential new
circular RNA biomarker, is involved in gastric carcinoma. Cancer
Biomark. 21:299–306. 2018. View Article : Google Scholar
|
|
129
|
Li X, He M, Guo J and Cao T: Upregulation
of circular RNA circ-ERBB2 predicts unfavorable prognosis and
facilitates the progression of gastric cancer via miR-503/CACUL1
and miR-637/MMP-19 signaling. Biochem Biophys Res Commun.
511:926–930. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lu J, Zhang PY, Li P, Xie JW, Wang JB, Lin
JX, Chen QY, Cao LL, Huang CM and Zheng CH: Circular RNA hsa_
circ_0001368 suppresses the progression of gastric cancer by
regulating miR-6506-5p/FOXO3 axis. Biochem Biophys Res Commun.
512:29–33. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kong S, Yang Q, Tang C, Wang T, Shen X and
Ju S: Identification of hsa_circ_0001821 as a novel diagnostic
biomarker in gastric cancer via comprehensive circular RNA
profiling. Front Genet. 10:8782019. View Article : Google Scholar :
|
|
132
|
Liu Z, Pan HM, Xin L, Zhang Y, Zhang WM,
Cao P and Xu HW: Circ-ZNF609 promotes carcinogenesis of gastric
cancer cells by inhibiting miRNA-145-5p expression. Eur Rev Med
Pharmacol Sci. 23:9411–9417. 2019.PubMed/NCBI
|
|
133
|
Dai X, Guo X, Liu J, Cheng A, Peng X, Zha
L and Wang Z: Circular RNA circGRAMD1B inhibits gastric cancer
progression by sponging miR-130a-3p and regulating PTEN and p21
expression. Aging (Albany NY). 11:9689–9708. 2019. View Article : Google Scholar
|
|
134
|
Guan E, Xu X and Xue F: circ-NOTCH1 acts
as a sponge of miR-637 and affects the expression of its target
gene Apelin to regulate gastric cancer cell growth. Biochem Cell
Biol. 98:164–170. 2020. View Article : Google Scholar
|
|
135
|
Cao C, Han S, Yuan Y, Wu Y, Lian W, Zhang
X, Pan L and Li M: Downregulated circular RNA hsa_circ_0000291
suppresses migration and proliferation of gastric cancer via
targeting the miR-183/ITGB1 axis. Cancer Manag Res. 11:9675–9683.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen LH, Wang LP and Ma XQ: Circ_SPECC1
enhances the inhibition of miR-526b on downstream KDM4A/YAP1
pathway to regulate the growth and invasion of gastric cancer
cells. Biochem Biophys Res Commun. 517:253–259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen Z, Ju H, Zhao T, Yu S, Li P, Jia J,
Li N, Jing X, Tan B and Li Y: hsa_circ_0092306 targeting miR-197-3p
promotes gastric cancer development by regulating PRKCB in MKN-45
cells. Mol Ther Nucleic Acids. 18:617–626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Du W, Li D, Guo X, Li P, Li X, Tong S,
Tong J, Kuang L and Liang D: Circ-PRMT5 promotes gastric cancer
progression by sponging miR-145 and miR-1304 to upregulate MYC.
Artif Cells Nanomed Biotechnol. 47:4120–4130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Liang M, Huang G, Liu Z, Wang Q, Yu Z, Liu
Z, Lin H, Li M, Zhou X and Zheng Y: Elevated levels of
hsa_circ_006100 in gastric cancer promote cell growth and
metastasis via miR-195/GPRC5A signalling. Cell Prolif.
52:e126612019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wang Q, Wang T, Hu Y, Jiang W, Lu C, Zheng
W, Zhang W, Chen Z and Cao H: Circ-EIF4G3 promotes the development
of gastric cancer by sponging miR-335. Pathol Res Pract.
215:1525072019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang S, Tang D, Wang W, Yang Y, Wu X, Wang
L and Wang D: circLMTK2 acts as a sponge of miR-150-5p and promotes
proliferation and metastasis in gastric cancer. Mol Cancer.
18:1622019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Wu L, Liu D and Yang Y: Enhanced
expression of circular RNA circ-DCAF6 predicts adverse prognosis
and promotes cell progression via sponging miR-1231 and miR-1256 in
gastric cancer. Exp Mol Pathol. 110:1042732019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhang H, Wang X, Huang H, Wang Y, Zhang F
and Wang S: Hsa_circ_0067997 promotes the progression of gastric
cancer by inhibition of miR-515-5p and activation of X
chromosome-linked inhibitor of apoptosis (XIAP). Artif Cells
Nanomed Biotechnol. 47:308–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Zhu Z, Rong Z, Luo Z, Yu Z, Zhang J, Qiu Z
and Huang C: Circular RNA circNHSL1 promotes gastric cancer
progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer.
18:1262019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Cai X, Nie J, Chen L and Yu F:
Circ_0000267 promotes gastric cancer progression via sponging
MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med.
8:e10932020. View Article : Google Scholar
|
|
146
|
Deng G, Mou T, He J, Chen D, Lv D, Liu H,
Yu J, Wang S and Li G: Circular RNA circRHOBTB3 acts as a sponge
for miR-654-3p inhibiting gastric cancer growth. J Exp Clin Cancer
Res. 39:12020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Guo X, Dai X, Liu J, Cheng A, Qin C and
Wang Z: Circular RNA circREPS2 acts as a sponge of miR-558 to
suppress gastric cancer progression by regulating RUNX3/β-catenin
signaling. Mol Ther Nucleic Acids. 21:577–591. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
He Y, Wang Y, Liu L, Liu S, Liang L, Chen
Y and Zhu Z: Circular RNA circ_0006282 contributes to the
progression of gastric cancer by sponging miR-155 to upregulate the
expression of FBXO22. Onco Targets Ther. 13:1001–1010. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Hu K, Qin X, Shao Y, Zhou Y, Ye G and Xu
S: Circular RNA MTO1 suppresses tumorigenesis of gastric carcinoma
by sponging miR-3200-5p and targeting PEBP1. Mol Cell Probes.
52:1015622020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Li B, Jin M, Cao F, Li J, Wu J, Xu L, Liu
X, Shi Y and Chen W: Hsa_circ_0017639 expression promotes gastric
cancer proliferation and metastasis by sponging miR-224-5p and
upregulating USP3. Gene. 750:1447532020. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Li C, Tian Y, Liang Y and Li Q:
Circ_0008035 contributes to cell proliferation and inhibits
apoptosis and ferroptosis in gastric cancer via miR-599/EIF4A1
axis. Cancer Cell Int. 20:842020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Li H, Shan C, Wang J and Hu C: CircRNA
hsa_circ_0001017 inhibited gastric cancer progression via acting as
a sponge of miR-197. Dig Dis Sci. Aug 1–2020.Epub ahead of print.
View Article : Google Scholar
|
|
153
|
Li Y, Gong Y, Ma J and Gong X:
Overexpressed circ-RPL15 predicts poor survival and promotes the
progression of gastric cancer via regulating miR-502-3p/OLFM4/STAT3
pathway. Biomed Pharmacother. 127:1102192020. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Liang L and Li L: Down-regulation of
circNRIP1 promotes the apoptosis and inhibits the migration and
invasion of gastric cancer cells by miR-182/ROCK1 axis. Onco
Targets Ther. 13:6279–6288. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lin J, Liao S, Li E, Liu Z, Zheng R, Wu X
and Zeng W: circ-CYFIP2 acts as a sponge of miR-1205 and affects
the expression of its target gene E2F1 to regulate gastric cancer
metastasis. Mol Ther Nucleic Acids. 21:121–132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lin S, Song S, Sun R, Zhang M, Du Y, Zhang
D, Xu W and Wang H: Oncogenic circular RNA Hsa-circ-000684
interacts with microRNA-186 to upregulate ZEB1 in gastric cancer.
FASEB J. 34:8187–8203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu J, Dai X, Guo X, Cheng A, Mac SM and
Wang Z: Circ-OXCT1 suppresses gastric cancer EMT and metastasis by
attenuating TGF-β pathway through the circ-OXCT1/miR-136/SMAD4
axis. Onco Targets Ther. 13:3987–3998. 2020. View Article : Google Scholar :
|
|
158
|
Liu J, Liu H, Zeng Q, Xu P, Liu M and Yang
N: Circular RNA circ-MAT2B facilitates glycolysis and growth of
gastric cancer through regulating the miR-515-5p/HIF-1α axis.
Cancer Cell Int. 20:1712020. View Article : Google Scholar
|
|
159
|
Liu P, Cai S and Li N: Circular
RNA-hsa-circ-0000670 promotes gastric cancer progression through
the microRNA-384/SIX4 axis. Exp Cell Res. 394:1121412020.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Lu J, Wang YH, Huang XY, Xie JW, Wang JB,
Lin JX, Chen QY, Cao LL, Huang CM, Zheng CH and Li P: circ-CEP85L
suppresses the proliferation and invasion of gastric cancer by
regulating NFKBIA expression via miR-942-5p. J Cell Physiol.
235:6287–6299. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Lu J, Wang YH, Yoon C, Huang XY, Xu Y, Xie
JW, Wang JB, Lin JX, Chen QY, Cao LL, et al: Circular RNA
circ-RanGAP1 regulates VEGFA expression by targeting miR-877-3p to
facilitate gastric cancer invasion and metastasis. Cancer Lett.
471:38–48. 2020. View Article : Google Scholar
|
|
162
|
Luo Z, Rong Z, Zhang J, Zhu Z, Yu Z, Li T,
Fu Z, Qiu Z and Huang C: Circular RNA circCCDC9 acts as a
miR-6792-3p sponge to suppress the progression of gastric cancer
through regulating CAV1 expression. Mol Cancer. 19:862020.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ma Y, Cong X, Zhang Y, Yin X, Zhu Z and
Xue Y: CircPIP5K1A facilitates gastric cancer progression via
miR-376c-3p/ZNF146 axis. Cancer Cell Int. 20:812020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Song H, Xu Y, Xu T, Fan R, Jiang T, Cao M,
Shi L and Song J: CircPIP5K1A activates KRT80 and PI3K/AKT pathway
to promote gastric cancer development through sponging miR-671-5p.
Biomed Pharmacother. 126:1099412020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Mo WL, Jiang JT, Zhang L, Lu QC, Li J, Gu
WD, Cheng Y and Wang HT: Circular RNA hsa_circ_0000467 promotes the
development of gastric cancer by competitively binding to MicroRNA
miR-326-3p. Biomed Res Int. 2020:40308262020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Quan J, Dong D, Lun Y, Sun B, Sun H, Wang
Q and Yuan G: Circular RNA circHIAT1 inhibits proliferation and
epithelial-mesenchymal transition of gastric cancer cell lines
through downregulation of miR-21. J Biochem Mol Toxicol.
34:e224582020. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Sun B, Sun H, Wang Q, Wang X, Quan J, Dong
D and Lun Y: Circular RNA circMAN2B2 promotes growth and migration
of gastric cancer cells by down-regulation of miR-145. J Clin Lab
Anal. 34:e232152020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Wang GJ, Yu TY, Li YR, Liu YJ and Deng BB:
Circ_0000190 suppresses gastric cancer progression potentially via
inhibiting miR-1252/PAK3 pathway. Cancer Cell Int. 20:3512020.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Wang J, Lv W, Lin Z, Wang X, Bu J and Su
Y: Hsa_ circ_0003159 inhibits gastric cancer progression by
regulating miR-223-3p/NDRG1 axis. Cancer Cell Int. 20:572020.
View Article : Google Scholar
|
|
170
|
Wang N, Lu K, Qu H, Wang H, Chen Y, Shan
T, Ge X, Wei Y, Zhou P and Xia J: CircRBM33 regulates IL-6 to
promote gastric cancer progression through targeting miR-149.
Biomed Pharmacother. 125:1098762020. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wang Y, Zhang J, Chen X and Gao L:
Circ_0001023 promotes proliferation and metastasis of gastric
cancer cells through miR-409-3p/PHF10 axis. Onco Targets Ther.
13:4533–4544. 2020. View Article : Google Scholar :
|
|
172
|
Wei W, Mo X, Yan L, Huang M, Yang Y, Jin
Q, Zhong H, Cao W, Wu K, Wu L, et al: Circular RNA profiling
reveals that circRNA_104433 regulates cell growth by targeting
miR-497-5p in gastric cancer. Cancer Manag Res. 12:15–30. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wu J, Chen Z, Song Y, Zhu Y, Dou G, Shen
X, Zhou Y, Jiang H, Li J and Peng Y: CircRNA_0005075 suppresses
carcinogenesis via regulating miR-431/p53/epithelial-mesenchymal
transition axis in gastric cancer. Cell Biochem Funct. Mar
4–2020.Epub ahead of print. View Article : Google Scholar
|
|
174
|
Wu Q, Wang H, Liu L, Zhu K, Yu W and Guo
J: Hsa_circ_0001546 acts as a miRNA-421 sponge to inhibit the
chemoresistance of gastric cancer cells via ATM/Chk2/p53-dependent
pathway. Biochem Biophys Res Commun. 521:303–309. 2020. View Article : Google Scholar
|
|
175
|
Xia T, Pan Z and Zhang J: CircSMC3
regulates gastric cancer tumorigenesis by targeting
miR-4720-3p/TJP1 axis. Cancer Med. 9:4299–4309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Xie M, Yu T, Jing X, Ma L, Fan Y, Yang F,
Ma P, Jiang H, Wu X, Shu Y and Xu T: Exosomal circSHKBP1 promotes
gastric cancer progression via regulating the miR-582-3p/HUR/VEGF
axis and suppressing HSP90 degradation. Mol Cancer. 19:1122020.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Xu W, Zhou B, Wu J, Jiang P, Chen H and
Yan F: Circular RNA hsa-circ-0007766 modulates the progression of
gastric carcinoma via miR-1233-3p/GDF15 axis. Int J Med Sci.
17:1569–1583. 2020. View Article : Google Scholar :
|
|
178
|
Yu X, Xiao W, Song H, Jin Y, Xu J and Liu
X: CircRNA_100876 sponges miR-136 to promote proliferation and
metastasis of gastric cancer by upregulating MIEN1 expression.
Gene. 748:1446782020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zhang L, Chang X, Zhai T, Yu J, Wang W, Du
A and Liu N: A novel circular RNA, circ-ATAD1, contributes to
gastric cancer cell progression by targeting miR-140-3p/YY1/PCIF1
signaling axis. Biochem Biophys Res Commun. 525:841–849. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Zhang Y, Xia L, Wu J, Xu X and Li G:
Hsa_circ_0023642 promotes proliferation, invasion, and migration of
gastric cancer by sponging microRNA-223. J Clin Lab Anal. Jun
19–2020.Epub ahead of print. View Article : Google Scholar
|
|
181
|
Zhang Z, Wang C, Zhang Y, Yu S, Zhao G and
Xu J: CircDUSP16 promotes the tumorigenesis and invasion of gastric
cancer by sponging miR-145-5p. Gastric Cancer. 23:437–448. 2020.
View Article : Google Scholar :
|
|
182
|
Zhang Z, Wu H, Chen Z, Li G and Liu B:
Circular RNA ATXN7 promotes the development of gastric cancer
through sponging miR-4319 and regulating ENTPD4. Cancer Cell Int.
20:252020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Li RC, Ke S, Meng FK, Lu J, Zou XJ, He ZG,
Wang WF and Fang MH: CiRS-7 promotes growth and metastasis of
esophageal squamous cell carcinoma via regulation of miR-7/HOXB13.
Cell Death Dis. 9:8382018. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Aliahmad P and Kaye J: Development of all
CD4 T lineages requires nuclear factor TOX. J Exp Med. 205:245–256.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Piwecka M, Glažar P, Hernandez-Miranda LR,
Memczak S, Wolf SA, Rybak-Wolf A, Filipchyk A, Klironomos F, Cerda
Jara CA, Fenske P, et al: Loss of a mammalian circular RNA locus
causes miRNA deregulation and affects brain function. Science.
357:eaam85262017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Geng HH, Li R, Su YM, Xiao J, Pan M, Cai
XX and Ji XP: The circular RNA Cdr1as promotes myocardial
infarction by mediating the regulation of miR-7a on its target
genes expression. PLoS One. 11:e01517532016. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Sang M, Meng L, Sang Y, Liu S, Ding P, Ju
Y, Liu F, Gu L, Lian Y, Li J, et al: Circular RNA ciRS-7
accelerates ESCC progression through acting as a miR-876-5p sponge
to enhance MAGE-A family expression. Cancer Lett. 426:37–46. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Li F, Zhang L, Li W, Deng J, Zheng J, An
M, Lu J and Zhou Y: Circular RNA ITCH has inhibitory effect on ESCC
by suppressing the Wnt/β-catenin pathway. Oncotarget. 6:6001–6013.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Su H, Lin F, Deng X, Shen L, Fang Y, Fei
Z, Zhao L, Zhang X, Pan H, Xie D, et al: Profiling and
bioinformatics analyses reveal differential circular RNA expression
in radioresistant esophageal cancer cells. J Transl Med.
14:2252016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zheng B, Wu Z, Xue S, Chen H, Zhang S,
Zeng T, Xu G, Wu W, Zheng W and Chen C: hsa_circRNA_100873
upregulation is associated with increased lymphatic metastasis of
esophageal squamous cell carcinoma. Oncol Lett. 18:6836–6844.
2018.
|
|
191
|
Pan Z, Lin J, Wu D, He X, Wang W, Hu X,
Zhang L and Wang M: Hsa_circ_0006948 enhances cancer progression
and epithelial-mesenchymal transition through the miR-490-3p/HMGA2
axis in esophageal squamous cell carcinoma. Aging (Albany NY).
11:11937–11954. 2019. View Article : Google Scholar
|
|
192
|
Liu J, Xue N, Guo Y, Niu K, Gao L, Zhang
S, Gu H, Wang X, Zhao D and Fan R: CircRNA_100367 regulated the
radiation sensitivity of esophageal squamous cell carcinomas
through miR-217/Wnt3 pathway. Aging (Albany NY). 11:12412–12427.
2019. View Article : Google Scholar
|
|
193
|
Huang E, Fu J, Yu Q, Xie P, Yang Z, Ji H,
Wang L, Luo G, Zhang Y and Li K: CircRNA hsa circ 0004771 promotes
esophageal squamous cell cancer progression via miR-339-5p/CDC 25A
axis. Epigenomics. 12:587–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Hou Y, Liu H and Pan W: Knockdown of
circ_0003340 induces cell apoptosis, inhibits invasion and
proliferation through miR-564/TPX2 in esophageal cancer cells. Exp
Cell Res. 394:1121422020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Lan X, Liu X, Sun J, Yuan Q and Li J:
CircRAD23B facilitates proliferation and invasion of esophageal
cancer cells by sponging miR-5095. Biochem Biophys Res Commun.
516:357–364. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Liu Z, Hu G, Zhao Y, Xiao Z, Yan M and Ren
M: Silence of cZNF292 suppresses the growth, migration, and
invasion of human esophageal cancer Eca-109 cells via upregulating
miR-206. J Cell Biochem. 121:2354–2362. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Ma Y, Zhang D, Wu H, Li P, Zhao W, Yang X,
Xing X, Li S and Li J: Circular RNA PRKCI silencing represses
esophageal cancer progression and elevates cell radiosensitivity
through regulating the miR-186-5p/PARP9 axis. Life Sci.
259:1181682020.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Shi Y, Guo Z, Fang N, Jiang W, Fan Y, He
Y, Ma Z and Chen Y: hsa_circ_0006168 sponges miR-100 and regulates
mTOR to promote the proliferation, migration and invasion of
esophageal squamous cell carcinoma. Biomed Pharmacother.
117:1091512019. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wu Y, Zhi L, Zhao Y, Yang L and Cai F:
Knockdown of circular RNA UBAP2 inhibits the malignant behaviours
of esophageal squamous cell carcinoma by microRNA-422a/Rab10 axis.
Clin Exp Pharmacol Physiol. 47:1283–1290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Xu Z, Tie X, Li N, Yi Z, Shen F and Zhang
Y: Circular RNA hsa_circ_0000654 promotes esophageal squamous cell
carcinoma progression by regulating the miR-149-5p/IL-6/STAT3
pathway. IUBMB Life. 72:426–439. 2020. View Article : Google Scholar
|
|
201
|
Chen Z, Yao N, Gu H, Song Y, Ye Z, Li L,
Lu P and Shao Q: Circular RNA_LARP4 sponges miR-1323 and hampers
progression of esophageal squamous cell carcinoma through
modulating PTEN/PI3K/AKT pathway. Dig Dis Sci. 65:2272–2283. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Xu L, Zhang M, Zheng X, Yi P, Lan C and Xu
M: The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of
hepatic microvascular invasion in hepatocellular carcinoma. J
Cancer Res Clin Oncol. 143:17–27. 2017. View Article : Google Scholar
|
|
203
|
Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma
JZ, Sun SH, Yang F and Zhou WP: Circular RNA cSMARCA5 inhibits
growth and metastasis in hepatocellular carcinoma. J Hepatol.
68:1214–1227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Lin X and Chen Y: Identification of
potentially functional circRNA-miRNA-mRNA regulatory network in
hepatocellular carcinoma by integrated microarray analysis. Med Sci
Monit Basic Res. 24:70–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Zhang X, Hu S, Zhang X, Wang L, Zhang X,
Yan B, Zhao J, Yang A and Zhang R: MicroRNA-7 arrests cell cycle in
G1 phase by directly targeting CCNE1 in human hepatocellular
carcinoma cells. Biochem Biophys Res Commun. 443:1078–1084. 2014.
View Article : Google Scholar
|
|
206
|
Yu L, Gong X, Sun L, Zhou Q, Lu B and Zhu
L: The circular RNA Cdr1as Act as an oncogene in hepatocellular
carcinoma through targeting miR-7 expression. PLoS One.
11:e01583472016. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomark. 16:161–169. 2016. View Article : Google Scholar
|
|
208
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Meng J, Chen S, Han JX, Qian B, Wang XR,
Zhong WL, Qin Y, Zhang H, Gao WF, Lei YY, et al: Twist1 regulates
vimentin through Cul2 circular RNA to promote EMT in hepatocellular
carcinoma. Cancer Res. 78:4150–4162. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Li X and Shen M: Circular RNA
hsa_circ_103809 suppresses hepatocellular carcinoma proliferation
and invasion by sponging miR-620. Eur Rev Med Pharmacol Sci.
23:555–566. 2019.PubMed/NCBI
|
|
211
|
Wei Y, Chen X, Liang C, Ling Y, Yang X, Ye
X, Zhang H, Yang P, Cui X, Ren Y, et al: A noncoding regulatory
RNAs network driven by Circ-CDYL acts specifically in the early
stages hepatocellular carcinoma. Hepatology. 71:130–147. 2020.
View Article : Google Scholar
|
|
212
|
Fu L, Chen Q, Yao T, Li T, Ying S, Hu Y
and Guo J: Hsa_ circ_0005986 inhibits carcinogenesis by acting as a
miR-129-5p sponge and is used as a novel biomarker for
hepatocellular carcinoma. Oncotarget. 8:43878–43888. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Fu L, Yao T, Chen Q, Mo X, Hu Y and Guo J:
Screening differential circular RNA expression profiles reveals
hsa_circ_0004018 is associated with hepatocellular carcinoma.
Oncotarget. 8:58405–58416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Su Y, Xu C, Liu Y, Hu Y and Wu H: Circular
RNA hsa_ circ_0001649 inhibits hepatocellular carcinoma progression
via multiple miRNAs sponge. Aging (Albany NY). 11:3362–3375. 2019.
View Article : Google Scholar
|
|
215
|
Cao S, Wang G, Wang J, Li C and Zhang L:
Hsa_circ_101280 promotes hepatocellular carcinoma by regulating
miR-375/JAK2. Immunol Cell Biol. 97:218–228. 2019. View Article : Google Scholar
|
|
216
|
Guan Z, Tan J, Gao W, Li X, Yang Y, Li X,
Li Y and Wang Q: Circular RNA hsa_circ_0016788 regulates
hepatocellular carcinoma tumorigenesis through miR-486/CDK4
pathway. J Cell Physiol. 234:500–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Guo J, Duan H, Li Y, Yang L and Yuan L: A
novel circular RNA circ-ZNF652 promotes hepatocellular carcinoma
metastasis through inducing snail-mediated epithelial-mesenchymal
transition by sponging miR-203/miR-502-5p. Biochem Biophys Res
Commun. 513:812–819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Jiang W, Wen D, Gong L, Wang Y, Liu Z and
Yin F: Circular RNA hsa_circ_0000673 promotes hepatocellular
carcinoma malignance by decreasing miR-767-3p targeting SET.
Biochem Biophys Res Commun. 500:211–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Li MF, Li YH, He YH, Wang Q, Zhang Y, Li
XF, Meng XM, Huang C and Li J: Emerging roles of hsa_circ_0005075
targeting miR-431 in the progress of HCC. Biomed Pharmacother.
99:848–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Liu H, Xue L, Song C, Liu F, Jiang T and
Yang X: Overexpression of circular RNA circ_001569 indicates poor
prognosis in hepa-tocellular carcinoma and promotes cell growth and
metastasis by sponging miR-411-5p and miR-432-5p. Biochem Biophys
Res Commun. 503:2659–2665. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Liu L, Qi X, Gui Y, Huo H, Yang X and Yang
L: Overexpression of circ_0021093 circular RNA forecasts an
unfavorable prognosis and facilitates cell progression by targeting
the miR-766-3p/MTA3 pathway in hepatocellular carcinoma. Gene.
714:1439922019. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Pan H, Tang L, Jiang H, Li X, Wang R, Gao
J and Li Q: Enhanced expression of circ_0000267 in hepatocellular
carcinoma indicates poor prognosis and facilitates cell progression
by sponging miR-646. J Cell Biochem. Feb 5–2019.Epub ahead of
print. View Article : Google Scholar
|
|
223
|
Qi SX, Sun H, Liu H, Yu J, Jiang ZY and
Yan P: Role and mechanism of circ-PRKCI in hepatocellular
carcinoma. World J Gastroenterol. 25:1964–1974. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Wang YG, Wang T, Ding M, Xiang SH, Shi M
and Zhai B: hsa_circ_0091570 acts as a ceRNA to suppress
hepatocellular cancer progression by sponging hsa-miR-1307. Cancer
Lett. 460:128–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Xu L, Feng X, Hao X, Wang P, Zhang Y,
Zheng X, Li L, Ren S, Zhang M and Xu M: CircSETD3
(Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting
hepatocellular carcinoma growth. J Exp Clin Cancer Res. 38:982019.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Zhang J, Chang Y, Xu L and Qin L: Elevated
expression of circular RNA circ_0008450 predicts dismal prognosis
in hepa-tocellular carcinoma and regulates cell proliferation,
apoptosis, and invasion via sponging miR-548p. J Cell Biochem.
120:9487–9494. 2019. View Article : Google Scholar
|
|
227
|
Zheng H, Chen T, Li C, Xu C, Ding C, Chen
J, Ju S, Zhang Z, Liang Z, Cui Z and Zhao J: A circular RNA
hsa_circ_0079929 inhibits tumor growth in hepatocellular carcinoma.
Cancer Manag Res. 11:443–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Song C, Li D, Liu H, Sun H, Liu Z, Zhang L
and Hu Y: The competing endogenous circular RNA ADAMTS14 suppressed
hepatocellular carcinoma progression through regulating
microRNA-572/regulator of calcineurin 1. J Cell Physiol.
234:2460–2470. 2019. View Article : Google Scholar
|
|
229
|
Zhang PF, Wei CY, Huang XY, Peng R, Yang
X, Lu JC, Zhang C, Gao C, Cai JB, Gao PT, et al: Circular RNA
circTRIM33-12 acts as the sponge of MicroRNA-191 to suppress
hepatocellular carcinoma progression. Mol Cancer. 18:1052019.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Song H, Bian ZX, Li HY, Zhang Y, Ma J,
Chen SH, Zhu JB, Zhang X, Wang J, Gu S, et al: Characterization of
hsa_circ_0000594 as a new biomarker and therapeutic target for
hepatoblastoma. Eur Rev Med Pharmacol Sci. 23:8274–8286.
2019.PubMed/NCBI
|
|
231
|
Ding Y, Fang A, Yan J, Duan J, Wang N, Yi
Y and Shen C: Selective downregulation of distinct circRNAs in the
tissues and plasma of patients with primary hepatic carcinoma.
Oncol Lett. 18:5255–5268. 2019.PubMed/NCBI
|
|
232
|
Zhu Q, Lu G, Luo Z, Gui F, Wu J, Zhang D
and Ni Y: CircRNA circ_0067934 promotes tumor growth and metastasis
in hepatocellular carcinoma through regulation of
miR-1324/FZD5/Wnt/β-catenin axis. Biochem Biophys Res Commun.
497:626–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Liu C, Zhong X, Li J and Xu F: Circular
RNA circVAPA promotes cell proliferation in hepatocellular
carcinoma. Hum Gene Ther Clin Dev. 30:152–159. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Tan A, Li Q and Chen L: CircZFR promotes
hepatocellular carcinoma progression through regulating
miR-3619-5p/CTNNB1 axis and activating Wnt/β-catenin pathway. Arch
Biochem Biophys. 661:196–202. 2019. View Article : Google Scholar
|
|
235
|
Zhang X, Luo P, Jing W, Zhou H, Liang C
and Tu J: circSMAD2 inhibits the epithelial-mesenchymal transition
by targeting miR-629 in hepatocellular carcinoma. Onco Targets
Ther. 11:2853–2863. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Yang W, Ju HY and Tian XF: Circular
RNA-ABCB10 suppresses hepatocellular carcinoma progression through
upregulating NRP1/ABL2 via sponging miR-340-5p/miR-452-5p. Eur Rev
Med Pharmacol Sci. 24:2347–2357. 2020.PubMed/NCBI
|
|
237
|
Li Z, Liu Y, Yan J, Zeng Q, Hu Y, Wang H,
Li H, Li J and Yu Z: Circular RNA hsa_circ_0056836 functions an
oncogenic gene in hepatocellular carcinoma through modulating
miR-766-3p/FOSL2 axis. Aging (Albany NY). 12:2485–2497. 2020.
View Article : Google Scholar
|
|
238
|
Wang Y, Gao R, Li J, Tang S, Li S, Tong Q
and Mao Y: Circular RNA hsa_circ_0003141 promotes tumorigenesis of
hepatocellular carcinoma via a miR-1827/UBAP2 axis. Aging (Albany
NY). 12:9793–9806. 2020. View Article : Google Scholar
|
|
239
|
Jin J, Liu H, Jin M, Li W, Xu H and Wei F:
Silencing of hsa_circ_0101145 reverses the epithelial-mesenchymal
transition in hepatocellular carcinoma via regulation of the
miR-548c-3p/LAMC2 axis. Aging (Albany NY). 12:11623–11635. 2020.
View Article : Google Scholar
|
|
240
|
Li Z, Hu Y, Zeng Q, Wang H, Yan J, Li H
and Yu Z: Circular RNA MYLK promotes hepatocellular carcinoma
progression by increasing Rab23 expression by sponging miR-362-3p.
Cancer Cell Int. 19:2112019. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Lin T, Dai Y, Guo X, Chen W, Zhao J, Cao L
and Wu Z: Silencing of hsa_circ_0008450 represses hepatocellular
carcinoma progression through regulation of microRNA-214-3p/EZH2
axis. Cancer Manag Res. 11:9133–9143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Liu L, Yang X, Li NF, Lin L and Luo H:
Circ_0015756 promotes proliferation, invasion and migration by
microRNA-7-dependent inhibition of FAK in hepatocellular carcinoma.
Cell Cycle. 18:2939–2953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Liu Z, Yu Y, Huang Z, Kong Y, Hu X, Xiao
W, Quan J and Fan X: CircRNA-5692 inhibits the progression of
hepatocellular carcinoma by sponging miR-328-5p to enhance DAB2IP
expression. Cell Death Dis. 10:9002019. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Tian F, Yu C, Wu M, Wu X, Wan L and Zhu X:
MicroRNA-191 promotes hepatocellular carcinoma cell proliferation
by has_ circ_0000204/miR-191/KLF6 axis. Cell Prolif. 52:e126352019.
View Article : Google Scholar
|
|
245
|
Yang X, Liu L, Zou H, Zheng YW and Wang
KP: circZFR promotes cell proliferation and migration by regulating
miR-511/AKT1 axis in hepatocellular carcinoma. Dig Liver Dis.
51:1446–1455. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Yang X, Song H, Zi Z, Kou J, Chen S, Dai
Y, Wang J, Yuan L and Gao K: Circ_0005075 promotes hepatocellular
carcinoma progression by suppression of microRNA-335. J Cell
Physiol. 234:21937–21946. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Yao Z, Xu R, Yuan L, Xu M, Zhuang H, Li Y,
Zhang Y and Lin N: Circ_0001955 facilitates hepatocellular
carcinoma (HCC) tumorigenesis by sponging miR-516a-5p to release
TRAF6 and MAPK11. Cell Death Dis. 10:9452019. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Zhu Y, Liu Y, Xiao B, Cai H, Liu M, Ma L,
Yin H and Wang F: The circular RNA PVT1/miR-203/HOXD3 pathway
promotes the progression of human hepatocellular carcinoma. Biol
Open. 8:bio0436872019. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Zou H, Xu X, Luo L, Zhang Y, Luo L, Yao Y,
Xiang G, Huang X and Wang G: Hsa_circ_0101432 promotes the
development of hepatocellular carcinoma (HCC) by adsorbing miR-1258
and miR-622. Cell Cycle. 18:2398–2413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Cao Y, Tao Q, Kao X and Zhu X:
Hsa-circRNA-103809 promotes hepatocellular carcinoma development
via MicroRNA-1270/PLAG1 like zinc finger 2 axis. Dig Dis Sci. Jul
18–2020.Epub ahead of print. View Article : Google Scholar
|
|
251
|
Ding Z, Guo L, Deng Z and Li P: Circ-PRMT5
enhances the proliferation, migration and glycolysis of hepatoma
cells by targeting miR-188-5p/HK2 axis. Ann Hepatol. 19:269–279.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Fu X, Zhang J, He X, Yan X, Wei J, Huang
M, Liu Y, Lin J, Hu H and Liu L: Circular RNA MAN2B2 promotes cell
proliferation of hepatocellular carcinoma cells via the
miRNA-217/MAPK1 axis. J Cancer. 11:3318–3326. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Gao J, Dai C, Yu X, Yin XB and Zhou F:
Circ-TCF4.85 silencing inhibits cancer progression through
microRNA-486-5p-targeted inhibition of ABCF2 in hepatocellular
carcinoma. Mol Oncol. 14:447–461. 2020. View Article : Google Scholar :
|
|
254
|
Gao S, Hu W, Huang X, Huang X, Chen W, Hao
L, Chen Z, Wang J and Wei H: Circ_0001178 regulates miR-382/VEGFA
axis to facilitate hepatocellular carcinoma progression. Cell
Signal. 72:1096212020. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
He Y, Huang H, Jin L, Zhang F, Zeng M, Wei
L, Tang S, Chen D and Wang W: CircZNF609 enhances hepatocellular
carcinoma cell proliferation, metastasis, and stemness by
activating the Hedgehog pathway through the regulation of
miR-15a-5p/15b-5p and GLI2 expressions. Cell Death Dis. 11:3582020.
View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Huang XY, Zhang PF, Wei CY, Peng R, Lu JC,
Gao C, Cai JB, Yang X, Fan J, Ke AW, et al: Circular RNA circMET
drives immunosuppression and anti-PD1 therapy resistance in
hepa-tocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol
Cancer. 19:922020. View Article : Google Scholar
|
|
257
|
Jia B, Yin X, Wang Y, Qian J, He Y, Yang
C, Yu G, Guo B and Meng X: CircRNA-PTN sponges miR-326 to promote
proliferation in hepatocellular carcinoma. Onco Targets Ther.
13:4893–4903. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Jia C, Yao Z, Lin Z, Zhao L, Cai X, Chen
S, Deng M and Zhang Q: circNFATC3 sponges miR-548I acts as a ceRNA
to protect NFATC3 itself and suppressed hepatocellular carcinoma
progression. J Cell Physiol. Jul 15–2020.Epub ahead of print.
View Article : Google Scholar
|
|
259
|
Li J, Qin X, Wu R, Wan L, Zhang L and Liu
R: Circular RNA circFBXO11 modulates hepatocellular carcinoma
progress and oxaliplatin resistance through miR-605/FOXO3/ABCB1
axis. J Cell Mol Med. 24:5152–5161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Li S, Weng J, Song F, Li L, Xiao C, Yang W
and Xu J: Circular RNA circZNF566 promotes hepatocellular carcinoma
progression by sponging miR-4738-3p and regulating TDO2 expression.
Cell Death Dis. 11:4522020. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Li Y, Shi H, Yuan J, Qiao L, Dong L and
Wang Y: Downregulation of circular RNA circPVT1 restricts cell
growth of hepatocel-lular carcinoma through downregulation of
Sirtuin 7 via microRNA-3666. Clin Exp Pharmacol Physiol.
47:1291–1300. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Lin Y, Huang G, Jin H and Jian Z: Circular
RNA Gprc5a promotes HCC progression by activating YAP1/TEAD1
signalling pathway by sponging miR-1283. Onco Targets Ther.
13:4509–4521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Liu W, Yin C and Liu Y: Circular RNA
circ_0091579 promotes hepatocellular carcinoma proliferation,
migration, invasion, and glycolysis through miR-490-5p/CASC3 axis.
Cancer Biother Radiopharm. Jul 14–2020.Epub ahead of print.
View Article : Google Scholar
|
|
264
|
Liu X, Yang L, Jiang D, Lu W and Zhang Y:
Circ-DENND4C up-regulates TCF4 expression to modulate
hepatocellular carcinoma cell proliferation and apoptosis via
activating Wnt/β-catenin signal pathway. Cancer Cell Int.
20:2952020. View Article : Google Scholar
|
|
265
|
Pu J, Wang J, Li W, Lu Y, Wu X, Long X,
Luo C and Wei H: hsa_circ_0000092 promotes hepatocellular carcinoma
progression through up-regulating HN1 expression by binding to
microRNA-338-3p. J Cell Mol Med. Feb 20–2020.Epub ahead of print.
View Article : Google Scholar
|
|
266
|
Song LN, Qiao GL, Yu J, Yang CM, Chen Y,
Deng ZF, Song LH, Ma LJ and Yan HL: Hsa_circ_0003998 promotes
epithelial to mesenchymal transition of hepatocellular carcinoma by
sponging miR-143-3p and PCBP1. J Exp Clin Cancer Res. 39:1142020.
View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Sun Q, Yu R, Wang C, Yao J and Zhang L:
Circular RNA circ-CSPP1 regulates CCNE2 to facilitate
hepatocellular carcinoma cell growth via sponging miR-577. Cancer
Cell Int. 20:2022020. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Wang W, Li Y, Li X, Liu B, Han S, Li X,
Zhang B, Li J and Sun S: Circular RNA circ-FOXP1 induced by SOX9
promotes hepatocellular carcinoma progression via sponging
miR-875-3p and miR-421. Biomed Pharmacother. 121:1095172020.
View Article : Google Scholar
|
|
269
|
Wei X, Zheng W, Tian P, He Y, Liu H, Peng
M, Li X and Liu X: Oncogenic hsa_circ_0091581 promotes the
malignancy of HCC cell through blocking miR-526b from degrading
c-MYC mRNA. Cell Cycle. 19:817–824. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Yang J, Li Y, Yu Z, Zhou Y, Tu J, Lou J
and Wang Y: Circular RNA Circ100084 functions as sponge of miR23a5p
to regulate IGF2 expression in hepatocellular carcinoma. Mol Med
Rep. 21:2395–2404. 2020.PubMed/NCBI
|
|
271
|
Yu X, Sheng P, Sun J, Zhao X, Zhang J, Li
Y, Zhang Y, Zhang W, Wang J, Liu K, et al: The circular RNA
circMAST1 promotes hepatocellular carcinoma cell proliferation and
migration by sponging miR-1299 and regulating CTNND1 expression.
Cell Death Dis. 11:3402020. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Zang H, Li Y, Zhang X and Huang G:
Circ_0000517 contributes to hepatocellular carcinoma progression by
upregulating TXNDC5 via sponging miR-1296-5p. Cancer Manag Res.
12:3457–3468. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Zhang W, Zhu L, Yang G, Zhou B, Wang J, Qu
X, Yan Z, Qian S and Liu R: Hsa_circ_0026134 expression promoted
TRIM25- and IGF2BP3-mediated hepatocellular carcinoma cell
proliferation and invasion via sponging miR-127-5p. Biosci Rep.
40:BSR201914182020. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Zhao M, Dong G, Meng Q, Lin S and Li X:
Circ-HOMER1 enhances the inhibition of miR-1322 on CXCL6 to
regulate the growth and aggressiveness of hepatocellular carcinoma
cells. J Cell Biochem. Feb 9–2020.Epub ahead of print.
|
|
275
|
Kim Y, Bang SS, Jee S, Park S, Shin SJ and
Jang K: Prevalence and clinicopathological significance of MET
overexpression and gene amplification in patients with gallbladder
carcinoma. Cancer Res Treat. 52:481–491. 2020. View Article : Google Scholar :
|
|
276
|
McNamara MG, Metran-Nascente C and Knox
JJ: State-of-the-art in the management of locally advanced and
metastatic gallbladder cancer. Curr Opin Oncol. 25:425–431. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Sung YN, Song M, Lee JH, Song KB, Hwang
DW, Ahn CS, Hwang S and Hong SM: Validation of the 8th edition of
the American joint committee on cancer staging system for
gallbladder cancer and implications for the follow-up of patients
without node dissection. Cancer Res Treat. 52:455–468. 2020.
View Article : Google Scholar :
|
|
278
|
Kakaei F, Beheshtirouy S, Nejatollahi SM,
Zarrintan S and Mafi MR: Surgical treatment of gallbladder
carcinoma: A critical review. Updates Surg. 67:339–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Kai D, Yannian L, Yitian C, Dinghao G, Xin
Z and Wu J: Circular RNA HIPK3 promotes gallbladder cancer cell
growth by sponging microRNA-124. Biochem Biophys Res Commun.
503:863–869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Wang S, Zhang Y, Cai Q, Ma M, Jin LY, Weng
M, Zhou D, Tang Z, Wang JD and Quan Z: Circular RNA FOXP1 promotes
tumor progression and Warburg effect in gallbladder cancer by
regulating PKLR expression. Mol Cancer. 18:1452019. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Huang X, He M, Huang S, Lin R, Zhan M,
Yang D, Shen H, Xu S, Cheng W, Yu J, et al: Circular RNA circERBB2
promotes gallbladder cancer progression by regulating
PA2G4-dependent rDNA transcription. Mol Cancer. 18:1662019.
View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Li T, Shao Y, Fu L, Xie Y, Zhu L, Sun W,
Yu R, Xiao B and Guo J: Plasma circular RNA profiling of patients
with gastric cancer and their droplet digital RT-PCR detection. J
Mol Med (Berl). 96:85–96. 2018. View Article : Google Scholar
|
|
283
|
Su BB, Shi H and Wan J: Role of serum
carcinoembryonic antigen in the detection of colorectal cancer
before and after surgical resection. World J Gastroenterol.
18:2121–2126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Lee WS, Baek JH, Kim KK and Park YH: The
prognostic significant of percentage drop in serum CEA post
curative resection for colon cancer. Surg Oncol. 21:45–51. 2012.
View Article : Google Scholar
|
|
285
|
Shao Y, Li J, Lu R, Li T, Yang Y, Xiao B
and Guo J: Global circular RNA expression profile of human gastric
cancer and its clinical significance. Cancer Med. 6:1173–1180.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Wang T, Shigdar S, Shamaileh HA, Gantier
MP, Yin W, Xiang D, Wang L, Zhou SF, Hou Y, Wang P, et al:
Challenges and opportunities for siRNA-based cancer treatment.
Cancer Lett. 387:77–83. 2017. View Article : Google Scholar
|
|
287
|
Zhang M and Xin Y: Circular RNAs: A new
frontier for cancer diagnosis and therapy. J Hematol Oncol.
11:212018. View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Ebert MS and Sharp PA: MicroRNA sponges:
Progress and possibilities. RNA. 16:2043–2050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Guo JU, Agarwal V, Guo H and Bartel DP:
Expanded identification and characterization of mammalian circular
RNAs. Genome Biol. 15:4092014. View Article : Google Scholar : PubMed/NCBI
|