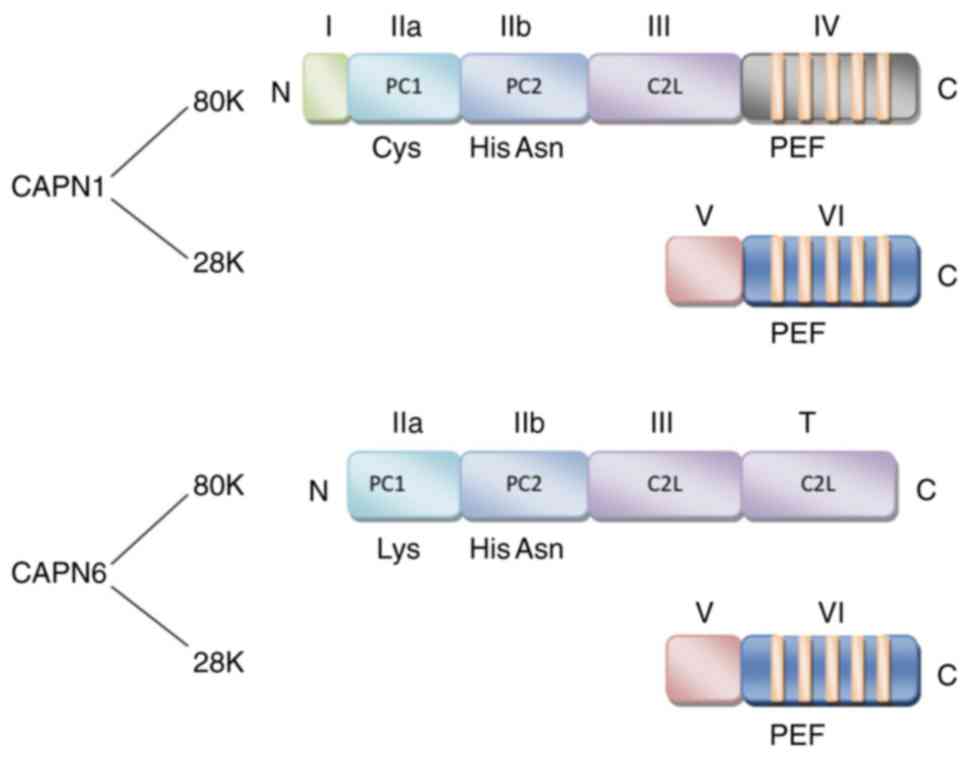
1. Overview of CAPN6
Discovery
Calpain is a Ca2+-dependent cysteine
protease (1). There have been 16
calpain genes identified to date (2). In 1997, Dear et al discovered
2 new calpain genes in vertebrates, one of these being calpain6
(CAPN6) (3), which is also
known as CANPX and calpamodulin (4). CAPN6 is located on chromosome
Xq23 (5). The mRNA expression of
CAPN6 has been identified to be high in the placental
chorionic plate, particularly during embryogenesis with a stronger
expression signaling the somite, the mandibular tissue of the first
branchial arch, developing skeletal muscle, the myocardium,
epithelial cells bordering the fourth ventricle, bronchial
epithelial cells, the tips of lung buds, the sacs of the lungs and
kidney (3,6,7).
However, CAPN6 expression is significantly downregulated after
birth (6).
Structure
Based on the differences in the C-terminal domains
of members within the calpain protein family, calpain is divided
into 2 groups, classic (typical) and non-classic (atypical)
(8).
Classic calpains, such as CAPN1, 2, 3, 8, 9, 11, 12,
13 and 14 comprise a large subunit (80 kDa) with catalytic activity
and a small subunit (28 kDa) with regulatory activity (2,4,8).
The large subunit contains 4 domains, namely I, II, III and IV.
Located on the N-terminus, domain I is the region where autolysis
occurs when the large subunit is activated by Ca2+, a
prerequisite for the hydrolysis of calpain. Domain II is the key
site for hydro-lytic activity with a conserved calpain-like
cysteine protease sequence motif defined in the conserved domain
database at the National Center for Biotechnology Information
(cd00044; CysPc) catalytic region, which consists of 2 protease
core (PC) domains (PC1 and PC2). Related to the conserved domain 2
originally defined for protein kinase C (C2), domain III is the
calpain-type β-sandwich (CBSW) domain that plays a regulatory role
by undergoing conformational changes during calcium-binding. Domain
IV at the C-terminus with a penta-EF-hand (PEF) is the
calcium-binding site (2,9,10).
Moreover, the small subunit contains 2 domains, namely V and VI.
The glycine-rich domain V is a hydrophobic region where calpain
binds to cell membranes or membrane proteins. Similar to domain IV,
the PEF-containing domain VI is the calmodulin binding region
(11).
Non-classic calpains, such as CAPN5, 6, 7, 10, 13,
15 and 16 have a different motif that replaces the IV domain
(4). Although most residues of
CAPN6 are highly conserved among members of the calpain family, it
lacks a C-terminal calmodulin-like domain, and it contains a CBSW
domain instead of a PEF domain at the catalytic subunit (7,12).
Due to the lack of a key active site that contains catalytic
cysteine residues for proteolytic activity, it is believed that the
CAPN6 protein may employ different sites for proteolysis. Since the
entire coding region exhibits a significant homology with TRA-3
that is involved in the sex determination of nematodes, the
C-terminal structure of CAPN6 is defined as a forked C2 domain
(also known as domain T) (3,5,13-15), rather than domain IV (summarized
in Fig. 1).
Physiological function
Classic calpain is a highly conserved protease
widely expressed in a number of tissues that participates in
various physiological and pathological processes of the cell,
including cell proliferation, apoptosis, cell migration and
invasion, cytoskeleton remodeling, signal transduction, etc.
(4,16-18).
To date, there are only a few studies available that
address the physiological functions of CAPN6 protein, even though
CAPN6 has a unique structure which may confer a different
functional and regulatory mechanism from that of members of the
classic calpain (7,12,19-21). During cytokinesis, CAPN6 is
co-localized to the microtubule structure, including the central
spindle and intermediates. As a microtubule-stabilizing protein,
the domain III of CAPN6 binds to microtubules to induce
stabilization through a non-proteolytic activity (7). Conversely, the inhibition of CAPN6
destroys the cytoskeletal tissues and the absorption capacity of
cells, which then lowers the microtubule stability, the levels of
acetylation and b3-integrin subunit proteins in osteoclasts,
further damages the factors that regulate osteoclast cytoskeleton
function, and ultimately causes cytoskeleton remodeling (19).
Rac Family Small GTPase1 (Rac1GTPase) and vimentin
are the key factors for cell movement (22). CAPN6 inhibits the activity of
Rac1GTPase through the interaction with Rho guanine nucleotide
exchange factor GEF-H1 (officially known as ARHGEF2) in order to
control lamellipodial formation and cell movement (20). The crosstalk between microtubules
and actin as the main cytoskeletal components is essential for cell
migration, diffusion and cytokinesis. Changes in microtubule
stability have an impact on microtubule-actin interactions and cell
movement (23). Therefore, the
inactivation of CAPN6 not only causes the instability of
microtubules, the loss of acetylated β-tubulin, and the
reorganization of actin, it also leads to the formation of
lamellipodium, laminar pseudofoot and laminar folds, which affect
the actin tissues, and ultimately promotes cell movement and
diffusion (7,20).
The activity of microtubule cytoskeleton and small
GTPases are the key regulators of muscle cell differentiation and
skeletal muscle development (24,25). CAPN6 regulates microtubule
dynamics and actin reorganization by affecting the activity of
Rac1GTPase. CAPN6 is highly expressed in embryonic muscle. The
knockout of CAPN6 gene in mice can promote the
differentiation of the muscle cells into myotubes. CAPN6
mRNA and CAPN6 protein can be detected in the regenerated skeletal
muscle of adult mice. Compared with that of
CAPN6lacZ/+ mice, the regenerated skeletal muscle
of CAPN6lacZ/lacZ mice has more nuclei per muscle
fiber and a larger cross-sectional area (21). Therefore, CAPN6 deficiency can
promote skeletal muscle differentiation during skeletal muscle
development and regeneration.
In addition, studies have demonstrated that in
uterine leiomyoma, cervical cancer, osteosarcoma and liver cancer
cells, an increased level of CAPN6 expression can promote cancer
cell proliferation and inhibit cell apoptosis (12,26-28). In white matter damage, a decreased
level of CAPN6 expression can promote oligodendrocyte precursor
cell apoptosis (29).
Overall, CAPN6 plays a role in maintaining cell
stability, in controlling cell movement and diffusion, and in
inhibiting skeletal muscle differentiation and growth. CAPN6 also
plays an anti-apoptotic role.
2. CAPN6 in disease: An introduction
Due to the abnormal protein expression levels,
members of the calpain protein family have been found to be
associated with the development of a number of diseases, such as
Alzheimer's disease, gastric cancer, muscular dystrophy, diabetes,
atherosclerosis, psoriasis, etc. (30-35). These are common diseases with a
high incidence rate and a significant impact on society. Based on
the different mechanisms involved in the pathogenesis of diseases,
calpain-related diseases can be classified into 3 categories as
follows: Diseases caused or aggravated by calpain activity,
diseases caused by pathogenic microorganisms which use the host
and/or their own calpain, and infection and survival caused by
calpain gene defects (36).
Calpain has been demonstrated as an effective therapeutic target
for a number of diseases (37).
Treatment strategies include the inhibition of calpain, such as
α-ketoamide inhibitor A-705253 (also known as BSF 419961 and CAL
9961) in treating Alzheimer's disease; and the activation of
calpain (direct or replacement), such as the restoration or
compensation of the loss of CAPN3 function in improving limb-girdle
muscular dystrophy type 2A (LGMD2A) (36,38,39). As a target of disease treatment,
the use of calpain is very challenging as classic calpains are
expressed in almost all cells. However, non-classic calpains are
mainly expressed in specific tissues and organs, exhibiting their
unique potential. In recent years, with an improved knowledge of
physiological functions of CAPN6, a number of diseases have been
identified to be specifically linked to CAPN6 (Table I), including tumors, neurological
diseases, vascular diseases, muscle diseases and skin diseases
(21,28,40-47). Among all the CAPN6-associated
diseases, tumors have gained the most research attention.
 | Table ICAPN6 expression and active pathways
in tissues in different diseases. |
Table I
CAPN6 expression and active pathways
in tissues in different diseases.
| Disease | Effect | Active
pathwaya | Refs. |
|---|
| Tumors | | | |
| Uterine
tumors | | | |
| Uterine
leiomyomas | Overexpression |
Rac1/PAK1/CAPN6 | (26,49) |
| Uterine
sarcoma | Overexpression | - | (40,50,52) |
| Cervical
cancer | Overexpression | PI3K/AKT/CAPN6 | (12,51) |
| Osteosarcoma | Overexpression | EDN-1/ERK1/2,
PI3K/AKT, NF-κB/CAPN6 | (27,41,60,61) |
| Liver cancer | Overexpression | PI3K/AKT/CAPN6 | (12,28,70) |
| Head and neck
squamous cell carcinoma | Low expression | - | (42) |
| Neurological
diseases | | | |
| White matter
injury | Low expression |
miR-142-3p/miR-466b-5p/CAPN6 | (29) |
| Prion
diseases | Overexpression | - | (43) |
| Vascular
diseases | | | |
|
Atherosclerosis | Overexpression | CWC22/EJC/Rac1 | (46,78-80) |
| Target organ
damage in hypertension | Low expression | - | (44) |
| Type 2 diabetic
nephropathy | Overexpression | - | (45) |
| Muscular
diseases | | | |
| Muscular
dystrophy | Overexpression | - | (21) |
| Muscular
atrophy | Overexpression | - | (21) |
| Skin diseases | | | |
| Atopic
dermatitis | - | Key factors related
to YWHAE | (47) |
3. CAPN6 in tumors
Uterine tumors
Uterine leiomyomas (UtLMs) are the most common
benign tumor of the female reproductive system. Overexpressed CAPN6
in UtLMs interacts with other abnormally expressed factors, such as
neuron navigator 2 (NAV2), kinesin family member 5C (KIF5C),
doublecortin (DCX), etc., which may lead to the pathological
transformation of the normal myometrium through related biochemical
path-ways (26,48). It has recently been found that,
whilst CAPN6 may regulate proliferation and apoptosis in UtLM
through the Rac1/p21-activated kinase 1 (PAK1) signaling pathway,
the downregulation of CAPN6 inhibits cell proliferation and
promotes cell apoptosis in UtLM (49). Therefore, the overexpression of
CAPN6 promotes the occurrence and development of UtLM.
CAPN6 has been found to be overexpressed in the
tissues of other malignant tumors of the uterus, such as
leiomyosarcoma, endometrial stromal sarcoma and cervical cancer
(12,40,50-52). As previously demonstrated, there
is a modest association between the intensity of CAPN6 expression
and tumor subtype, but not tumor stage or survival rate (50).
The expression of CAPN6 in cervical cancer is
regulated by the phosphatidylinositol 3 kinase (PI3K)/protein
kinase B (Akt) pathway (12),
which plays an important role in a number of cellular processes,
and promotes tumor growth and survival (53). The PI3K/Akt pathway inhibits the
proteasome degradation of CAPN6 protein through glycogen synthase
kinase (GSK)-3β, increases the stability of CAPN6 protein,
upregulates CAPN6mRNA expression, and uses transcription
factors, such as activator protein 1 (AP1), forkhead box D3 (FoxD3)
and organic cation transporter 1 (Oct-1) to stimulate the activity
of the CAPN6 promoter (12). A main prerequisite for tumor
formation and progression is to prevent apoptosis, and promote cell
proliferation and angiogenesis. Cisplatin is used to induce
cervical cancer cell apoptosis by activating the caspase-3 pathway
(54). A previous study using
cervical cancer cells (HeLa cells) transfected with CAPN6 cDNA
(Calpain 6) or CAPN6 siRNA (siCalpain 6) demonstrated that CAPN6
antagonized cisplatin-mediated apoptosis by inhibiting caspase-3
activity (51). Consistently,
another CAPN6 knockdown study using HeLa cells demonstrated an
upregulation of caspase-3 and a downregulation of Bcl-2 (12), indicating that CAPN6 induced
resistance to apoptosis. HeLa cells with a downregulated CAPN6
expression grow at a more rapid rate than their parental cells,
with a reduced S phase and cell-specific cyclin (cyclin D1)
expression, suggesting that CAPN6 can promote the proliferation of
cancer cells (12). Vascular
endothelial growth factor (VEGF) and basic fibro-blast growth
factor (bFGF) promote endothelial cell migration, proliferation and
angiogenesis, and activate calcium signal regulation in endothelial
cells (55-57). Since the secretion of VEGF and
bFGF is related to cervical cancer (58), a study using human umbilical vein
endothelial cells (HUVECs) demonstrated that CAPN6 overexpression
enhanced the VEGF-induced invasiveness of endothelial cells, and
the tissue formation of endothelial cell network, indicating that
calpain 6 promotes HUVEC invasion, migration and angiogenesis.
Since calpain 6 is a Ca2+-dependent cysteine protease,
it is considered that the intracellular calcium signals induced by
VEGF or bFGF can possibly activate CAPN6 (51). A recent study found that the
binding between the domain III of CAPN6 and the C-terminus of
VEGFA, produced a unique interaction that leads to the secretion of
VEGF, which in turn increases and promotes angiogenesis (59). Although it is generally accepted
that CAPN6 plays a role in promoting the occurrence and development
of cervical cancer, CAPN6 is not associated with cancer cell
migration and epithelial-mesenchymal transition (12).
Osteosarcoma
Osteosarcoma is one of the most common primary
malignant bone tumors. Metastasis and recurrence are important
factors leading to a poor prognosis. Chemoresistance is the main
cause of the poor treatment efficacy and disease recurrence in
patients with osteosarcoma.
It has been demonstrated that CAPN6 is expressed in
human osteosarcoma tissue, metastatic bone and lung, and the
majority of recurrent tumors at high levels (41). The CAPN6 level in the primary
tumor is found to be negatively associated with the
chemotherapeutic response. Compared with original U2OS sensitive
cells, cells with a resistant U2OS osteosarcoma origin have been
shown to express higher CAPN6 levels (27).
The inhibition of CAPN6 expression in osteosarcoma
cells can reduce the incorporation of 5-bromodeoxyuridine (BrdU)
into DNA, reduce cell proliferation, increase spontaneous
apoptosis, rescue the apoptotic response of resistant cells, and
increase the sensitivity to cytotoxic drugs. Therefore, CAPN6
promotes osteosarcoma cell proliferation, survival and growth, and
provides protection by increasing the chemical resistance of cells
(27). CAPN6 is a downstream
molecule of endo-thelin-1 (EDN-1) signaling during embryonic
development (7). EDN-1 can induce
the continuous activation of the nuclear factor-κ-gene binding
(NF-κB), extracellular regulated protein kinase ½ (ERK1/2) and AKT
pathways, and can promote the expression of CAPN6 in osteosarcoma
(60,61). Tumor cyto-chemical resistance is
associated with the activation of the NF-κB, Ras/MAPK and PI3K/AKT
pathways (62,63), further demonstrating the
association between CAPN6 expression and osteosarcoma cell chemical
resistance. In addition, in liposarcoma, it has been found that the
higher the tumor grade, the higher the CAPN6 expression (64), indicating that the expression of
CAPN6 is associated with the malignancy of sarcoma.
Syndecan-2 is a key regulator of cell death, and has
the function of promoting apoptosis. Since there is a decreased
level of expressed syndecan-2 in osteosarcoma tissue, it is
inferred that the abnormal expression or induction of syndecan-2 in
osteosarcoma is associated with the imbalanced apoptosis of tumor
cells (65). The overexpression
of syndecan-2 in osteosarcoma cells can enhance the apoptotic
response to cytotoxic drugs, restore the sensitivity of
drug-resistant osteosarcoma cells to chemotherapeutic drugs,
decrease the levels of CAPN6 in the tumor cells and ultimately
inhibit tumor growth; thus, a decrease in CAPN6 expression
contributes to syndecan-2-induced apoptosis (41,66). EDN-1 is overexpressed in
osteosarcoma to promote the invasion, metastasis and survival of
bone cancer cells, and to resist cisplatin-induced apoptosis
through EDNA receptor (67). Syndecan-2 can alter EDN-1
signaling. The PI3K/AKT and NF-κB pathways are controlled during
the pro-apoptotic process. In cells with syndecan-2 overexpression,
the steady-state activation of ERK1/2 and AKT is low (60,61). Therefore, syndecan-2 can inhibit
EDN-1 signal transduction, and can downregulate CAPN6 expression
through the ERK1/2, PI3K/AKT and NF-κB pathways to exert its
function to promote apoptosis (summarized in Fig. 2).
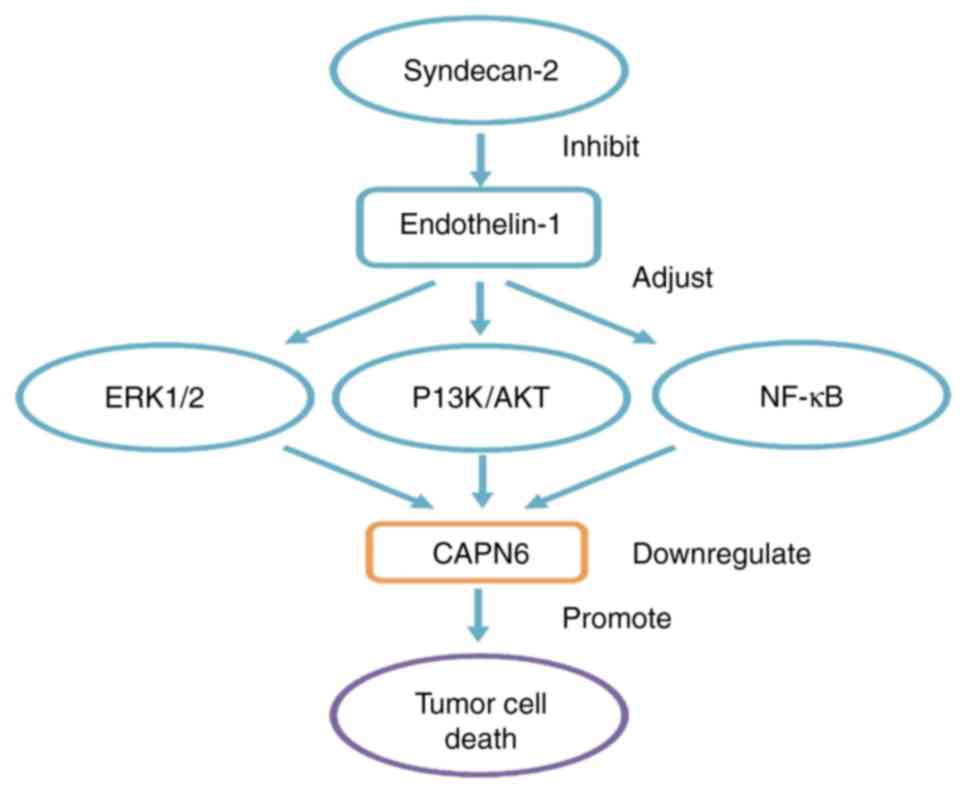 | Figure 2In osteosarcoma, CAPN6 involves
related signal pathways. CAPN6 is a downstream molecule of EDN-1
signaling. EDN-1 can induce the continuous activation of NF-κB,
ERK1/2 and AKT pathways, and promote the expression of CAPN6 in
osteosarcoma. Syndecan-2 is a key regulator of cell death.
Overexpression of syndecan-2 in osteosarcoma cells can decrease the
levels of CAPN6 in the tumor cells, and can alter EDN-1 signaling.
PI3K/AKT and NF-κB are new pathways controlled during their
pro-apoptotic process. In cells with syndecan-2 overexpression,
steady-state activation of ERK1/2 and AKT is low. Therefore,
syndecan-2 can inhibit EDN-1 signal transduction, and down-regulate
CAPN6 expression through ERK1/2, PI3K/AKT and NFκB pathways to
exert its function to promote apoptosis. CAPN6, calpain6; EDN-1,
endothelin-1; NF-κB, nuclear factor-κ-gene binding; ERK1/2,
extracellular regulated protein kinase 1/2; AKT, protein kinase
B. |
Cancer stem cells (CSC) have been shown to promote
the development of malignancies. Oct4, Nanog and Sox2 are stem cell
transcription factors that can be upregulated by hypoxia in
osteosarcoma. Hypoxia can also activate NF-κB and increase the
expression of EDN-1 receptors, thereby promoting the EDN-1/NF-κB
pathway to induce increased mRNA and protein levels of CAPN6,
resulting in a marked decrease in the silencing of Oct4, Nanog or
Sox2 (68). In a previous study,
in an osteosarcoma mouse model, it was found that cells expressing
CAPN6 had unique tumor initiation and metastasis capabilities;
CAPN6 knockdown inhibited hypoxia, promoted autophagy, prevented
aging, induced cancer stem cell population depletion and blocked
mouse tumor development (64).
CAPN6 expression also recognizes CSCs. The metastatic potential of
bone tumors is dependent on CSCs that express CAPN6, and CSCs
communicate with other tumor cells through exosomes to regulate the
cell migratory capacity (69).
Liver cancer
Consistent with that in cervical cancer, the
enhanced expression of CAPN6 in liver cancer (70), which is regulated by the PI3K/AKT
signaling pathway, plays a role in promoting cancer cell
proliferation and inhibiting apoptosis, but not necessarily tumor
metastasis (12). MicroRNAs
(miRNAs or miRs) play a key role in post-transcriptional gene
regulation (71), and an miRNA
imbalance may lead to the inactivation of liver cancer suppressor
genes and the activation of oncogenes, mainly by inhibiting the
expression of its target genes (72). miR-449a expression is decreased in
liver cancer (27). In a previous
study, luciferase assay confirmed that CAPN6 and
POU2F1 are the target genes of miR-449a (29). The downregulation of CAPN6
or POU2F1 increases the levels of Bax, decreases the levels
of Bcl-2, promotes G1 phase arrest, and inhibits cancer cell
proliferation and induces apoptosis, thereby preventing the
occurrence and development of liver cancer (28).
Head and neck squamous cell carcinoma
(HNSCC)
Xiang et al examined CAPN6 expression in
HNSCC, including tongue cancer, laryngeal cancer and nasal cancer
(42). The results of their study
demonstrated that the expression of CAPN6 in HNSCC tissues was
significantly decreased, which was in complete contrast to the
observations of other tumor studies, as mentioned above. CAPN6
expression also varies between different tumor stages of HNSCC, and
it differs significantly between different T stages, although there
is no significant difference between different N stages and
different tumor grades. In addition, the expression of CAPN6 and
the survival rate of patients with HNSCC exhibit a positive
association (42). However, it is
not clear whether the expression of CAPN6 is directly linked to the
occurrence of HNSCC, or whether HNSCC tumor growth or metastasis
leads to CAPN6 downregulation.
These studies have provided new knowledge for the
further understanding of the pathogenesis of these tumors. CAPN6
may be used as a biomarker, and in the diagnosis, prevention and
management of these tumors. Most importantly, CAPN6 may be an ideal
specific therapeutic target. For uterine tumors, osteosarcoma and
liver cancer that can be caused or aggravated by CAPN6 activity,
the inhibition of CAPN6 may be an effective treatment strategy,
particularly for chemoresistant and recurring tumors. Therefore,
CAPN6 can be inhibited to produce targeted drugs (including
targeted cell drugs and targeted vascular drugs) or tumor stem
cells to block the initiation, proliferation or metastasis of tumor
cells, to promote the occurrence of apoptosis or autophagy, and to
achieve the purpose of treatment. The combination of CAPN6
targeting with anticancer drug cisplatin may also produce a
synergistic effect. However, it is worth noting that studies have
found the pros and cons of calpain inhibition, depending on the
stage of tumor progression (73),
and that CAPN6 inhibition has an opposite effect in HNSCC.
Therefore, further systematic studies are warranted to determine
whether CAPN6 exerts differential effects in different stages of
the same tumor, and different cell types of tumors, as well as to
determine whether CAPN6 targeting is beneficial for the treatment
of tumors.
4. CAPN6 in neurological diseases
White matter injury (WMI)
Studies have found that hypoxia-ischemia (HI) can
lead to reduced myelin sheath, the death of oligodendrocyte
precursor cells and the dysplasia of oligodendrocytes, and can
alter the expression of specific mature miRNAs in demyelinating and
oligodendrocyte precursor cells, ultimately resulting in WMI
(29,71,74). The co-expression network of
miRNAs/mRNAs indicates that miR-142-3p, miR-466b-5p and miR-146a-5p
have differentially expressed targets. CAPN6 is the target
gene of miR-142-3p and miR-466b-5p. The abundance of CAPN6 mRNAs in
HI is significantly decreased. Therefore, it is considered that
miR-142-3p and miR-466b-5p may promote the apoptosis of
oligodendrocyte precursor cells by inhibiting CAPN6, and that
miR-142-3p/miR-466b-5p/CAPN6 pathway may be involved in the
pathogenesis of WMI (29).
Prion diseases
Prion diseases are a group of lethal
neurodegenerative disorders; however, the molecular mechanisms
responsible for these diseases are poorly understood. Other members
of the calpain family are associated with prion diseases and
several other neurodegenerative diseases (75). Calpain-mediated proteolytic
cleavage by prion protein (PrPSc) may be an important
event in prion reproduction (76). CAPN6 is overexpressed in the
medulla oblongata, spinal cord, cerebellar cortex and several other
areas of prion disease-affected brain tissues. CAPN6 is involved in
the pathogenesis of prion diseases (43).
CAPN6 participates in different pathogenesis of WMI
and prion diseases; thus, the corresponding targeted treatment
strategies vary. The decreased activity of CAPN6 in WMI the
promotes the development of disease. Hence, treatment strategies
should consider activating or replacing CAPN6, as no calpain
activator has been developed to date, at least to the best of our
knowledge. The only treatment option available is calpain
replacement or gene therapy (36). However, to the best of our
knowledge, to date, there is no research available on the
overexpression of CAPN6 in the treatment of diseases in live
animals; thus, it is difficult to assess whether gene therapy will
bring other effects. In prion diseases, it is speculated that prion
proteins can reproduce and survive to promote disease by mediating
CAPN6. Therefore, inhibiting CAPN6 to ameliorate the invasion and
growth of the infecting virus may be a novel treatment strategy.
For the inhibition of calpain in the treatment of neurodegenerative
diseases, mature drugs are already available, such as the
aforementioned α-ketoamide inhibitor, A-705253. There are also
drugs that have been tested on animals, such as Gabadul for the
treatment of Parkinson's disease and Lewy body dementia (36,77). Hence, therapeutic strategies with
the inhibition of CAPN6 may be worthy of investigation.
5. CAPN6 in vascular diseases
Degenerative vascular diseases
In degenerative vascular diseases, the dysfunction
of the calpain system has a prominent effect, since the increased
activity of calpain can induce diseases (78). As a non-proteolytic calpain, CAPN6
disrupts the CWC22/exon junction complex (EJC) system in
macro-phages to affect CWC22/EJC/Rac1 signal transduction, and
enhances the cell phagocytosis of natural low-density lipoprotein
(LDL), causing the cytosolic DL cholesterol deposition in cells,
thereby attenuating the clearance of dead cell corpses, and
eventually promoting the progression of degenerative vascular
diseases, such as atherosclerosis (46,78-80).
Hypertensive heart disease
Damage to target organs is the most harmful effect
caused by hypertension. Experiments have established a model of
target organ damage in hypertension. It has been found that in the
heart tissue of Dahl salt-sensitive rats with high salt (4% NaCl),
CAPN6 and CAPN9 are down-regulated by >50%, and that endogenous
calpain inhibitor calpastatin is upregulated by 225%. However, the
specific role of CAPN6 in hypertension target organ damage is
unknown, as CAPN6 lacks key amino acids in the catalytic triad and
may not have proteolytic activity (44).
Diabetic nephropathy
Diabetic nephropathy is one of the comorbidities of
diabetic systemic microangiopathies. Due to the higher expression
levels, CAPN6 is one of the 50 functional genes that may be
responsible for the occurrence of type 2 diabetes (45).
In vascular diseases, the expression and site of
action of CAPN6 differ due to the nature of the disease and the
different organs involved. In degenerative vascular diseases, CAPN6
mainly affects lipid metabolism by participating in the
inter-ference of mRNA splicing in macrophages. It has been found
that the transgenic overexpression of calpastatin and calpain
inhibitors (such as MDL28170 and BDA 410), are effective in the
treatment of degenerative disorders (36). Although there is no drug that
inhibits calpain-6, the development of atypical calpain inhibitors
is promising. In acute cardiovascular diseases, calpain-mediated
myocardial proteolysis is involved in ischemia-reperfusion and
pressure overload mechanisms that lead to the pathogenesis. Studies
performing animal experiments have indicated that calpain
inhibitors can improve the symptoms of these diseases (81,82). However, it has also been reported
that the inhibition of calpain may lead to a decrease in endogenous
calpain activity, which is unfavorable (83). This is consistent with the
decrease in CAPN6 activity and the upregulation of endogenous
calpain inhibitors in the hypertensive heart disease model. The
inhibition of calpain can also cause cardiac dysfunction under
pressure overload (82).
Therefore, calpain may play multiple roles in cardiovascular
diseases, either in a therapeutically inhibited or activated
manner. As regards diabetic nephropathy, only the specific
biological pathways and sites of action of CAPN6 involved in the
pathogenesis are clarified, the treatment measures need to be
further addressed.
6. CAPN6 in muscle disorders
Muscular dystrophy and muscle
atrophy
With CAPN6 being overexpressed, the developing
skeletal muscle is in a continuous cycle of degeneration and
regeneration. CAPN1 is also related to muscle differentiation.
CAPN1 overexpression inhibits muscle cell differentiation (84). During muscle degeneration, the
expression of CAPN1 decreases, which then recovers during muscle
regeneration. However, CAPN6 deficiency delays the expression of
CAPN1 in regenerating skeletal muscles (21). The exact association between CAPN1
and CAPN6 needs to be clarified.
Muscular dystrophy is a muscle degeneration disease
caused by genetic mutations in slow progressive symmetrical muscle
weakness and atrophy; while muscle atrophy is muscle reduction and
rhabdomyolysis caused by the thinning or even disappearance of
muscle fibers. Studies have found that CAPN6 is one of the genes
associated with LGMD2A, and its expression is up-regulated in the
skeletal muscles of patients (85). As an inhibitor of skeletal muscle
differentiation, CAPN6 downregulation assists the growth of
skeletal muscles and the induction of pluripotent stem cells to
produce skeletal muscles in vitro. Therefore, the
modification of specific antibodies or siRNA to offset CAPN6 may
regulate the quality of skeletal muscles, and may improve the
conditions of muscular dystrophy or muscle atrophy. However, the
inhibition of calpain does not necessarily improve muscle function.
The inhibition of calpain through inhibitors or calpastatin
overexpression can lead to the compensatory upregulation of calpain
(86). Therefore, the results of
such treatments need to be analyzed dialectically. Limb-girdle
muscular dystrophy type 2 (LGMD2A) is a recessive genetic disease
caused by CAPN3 mutation and loss of function (85). The inhibition of CAPN6 alone may
not be able to treat LGMD2A completely. A combination of CAPN6
inhibition with other treatment methods may be more beneficial.
7. CAPN6 in skin diseases
Atopic dermatitis
Atopic dermatitis is a familial hereditary skin
disease. The tyrosine 3-monooxygenase/tryptophan 5-mono-oxygenase
activation protein, epsilon polypeptide (YWHAE) isoform located in
the human keratinocytes is involved in the pathogenesis of atopic
dermatitis (87). CAPN6 is one of
the key factors associated with YWHAE (47). Addressing the causal association
and signal transduction pathways between CAPN6 and YWHAE may
facilitate the discovery of novel clinical treatments for
YWHAE-related atopic dermatitis.
8. Conclusions and future perspectives
Calpain-related diseases are a threat to human
health. The development of therapeutic drugs is the focus of
current research. Although some studies have facilitated the
under-standing of the disease pathogenesis and the application of
calpain in disease treatment, there are several aspects that
require further investigations. For instance, knowledge about
calpains, particularly non-classical calpains with natural
advantages warrant further attention. CAPN6 has great potential as
an emerging therapeutic target, although there are a number of
research areas that require further clarifications, including the
characteristics of CAPN6 itself, the molecular pathways involved in
the associated diseases, the identification of targets, the
development of CAPN6 inhibitors or activators, and the effective
testing of these therapies. However, the future research direction
of CAPN6 holds promise.
Funding
The present study was supported by the National Key
R&D Programme of China (grant nos. 2017YFA 0104201 and 2017YFA
0104200), the National Science Foundation of China (grant nos.
81330016, 81630038 and 81771634), and the Science and Technology
Bureau of Chengdu City (grant no. 2015-HM01-00424-SF).
Availability of data and materials
Not applicable.
Authors' contributions
LC and DX were involved in the conceptualization of
the study. LC, DX, FT and XL were involved in software
applications. LC, DX, HG and XL provided the study resources. FT,
HG and XL also played a role in the conceptualization of the study.
LC and DX were involved in the writing and preparation of the
original draft, and in the writing, reviewing and editing of the
study. HG and XL were involved in the processing of the figures. XL
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Abbreviations:
|
CAPN6
|
calpain6
|
|
PI3K
|
phosphatidylinositol 3 kinase
|
|
AKT
|
protein kinase B
|
|
CysPc
|
calpain-like cysteine protease
sequence motif defined in the conserved domain database at the
National Center for Biotechnology Information (cd00044)
|
|
PC
|
protease core
|
|
PC1
|
protease core domain 1
|
|
PC2
|
protease core domain 2
|
|
C2
|
conserved domain 2 originally defined
for protein kinase C
|
|
CBSW
|
calpain-type β-sandwich
|
|
PEF
|
penta-EF-hand (E, E-helix, F,
F-helix)
|
|
GEF-H1
|
officially known as ARHGEF2
|
|
LGMD2A
|
limb girdle muscular dystrophy type
2A
|
|
UtLMs
|
uterine leiomyomas
|
|
Rac1
|
Rac Family Small GTPase1
|
|
PAK1
|
p21-activated kinase1
|
|
VEGF
|
vascular endothelial growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
BrdU
|
5-bromodeoxyuridine
|
|
EDN-1
|
endothelin-1
|
|
NF-κB
|
nuclear factor-κ-gene binding
|
|
ERK1/2
|
extracellular regulated protein kinase
1/2
|
|
CSC
|
cancer stem cells
|
|
miRNA/miRs
|
microRNAs
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
HI
|
hypoxia-ischemia
|
|
WMI
|
white matter injury
|
|
EJC
|
exon junction complex
|
|
LDL
|
low-density lipoprotein
|
|
YWHAE
|
tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein, epsilon polypeptide
|
References
|
1
|
Sorimachi H, Hata S and Ono Y: Calpain
chronicle-an enzyme family under multidisciplinary
characterization. Proc Jpn Acad Ser B Phys Biol Sci. 87:287–327.
2011. View Article : Google Scholar :
|
|
2
|
Moretti D, Del Bello B, Allavena G and
Maellaro E: Calpains and cancer: Friends or enemies? Arch Biochem
Biophys. 564:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dear N, Matena K, Vingron M and Boehm T: A
new subfamily of vertebrate calpains lacking a calmodulin-like
domain: Implications for calpain regulation and evolution.
Genomics. 45:175–184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorimachi H, Hata S and Ono Y: Impact of
genetic insights into calpain biology. J Biochem. 150:23–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matena K, Boehm T and Dear N: Genomic
organization of mouse Capn5 and Capn6 genes confirms that they are
a distinct calpain subfamily. Genomics. 48:117–120. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dear TN and Boehm T: Diverse mRNA
expression patterns of the mouse calpain genes Capn5, Capn6 and
Capn11 during development. Mech Dev. 89:201–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tonami K, Kurihara Y, Aburatani H,
Uchijima Y, Asano T and Kurihara H: Calpain 6 is involved in
microtubule stabilization and cytoskeletal organization. Mol Cell
Biol. 27:2548–2561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna RA, Campbell RL and Davies PL:
Calcium-bound structure of calpain and its mechanism of inhibition
by calpastatin. Nature. 456:409–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glading A, Lauffenburger DA and Wells A:
Cutting to the chase: Calpain proteases in cell motility. Trends
Cell Biol. 12:46–54. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki K and Sorimachi H: A novel aspect
of calpain activation. FEBS Lett. 433:1–4. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Campbell RL and Davies PL:
Structure-function relationships in calpains. Biochem J.
447:335–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Mei C, Sun L, Li X, Liu M, Wang L,
Li Z, Yin P, Zhao C, Shi Y, et al: The PI3K-Akt pathway regulates
calpain 6 expression, proliferation, and apoptosis. Cell Signal.
23:827–836. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barnes TM and Hodgkin J: The tra-3 sex
determination gene of Caenorhabditis elegans encodes a member of
the calpain regulatory protease family. EMBO J. 15:4477–4484. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mugita N, Kimura Y, Ogawa M, Saya H and
Nakao M: Identification of a novel, tissue-specific calpain htra-3;
a human homologue of the Caenorhabditis elegans sex determination
gene. Biochem Biophys Res Commun. 239:845–850. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosseini M, Najmabadi H and Kahrizi K:
Calpains: Diverse functions but enigmatic. Arch Iran Med.
21:170–179. 2018.PubMed/NCBI
|
|
17
|
Lebart MC and Benyamin Y: Calpain
involvement in the remodeling of cytoskeletal anchorage complexes.
FEBS J. 273:3415–3426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sato K and Kawashima S: Calpain function
in the modulation of signal transduction molecules. Biol Chem.
382:743–751. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong JM, Teitelbaum SL, Kim TH, Ross FP,
Kim SY and Kim HJ: Calpain-6, a target molecule of glucocorticoids,
regulates osteoclastic bone resorption via cytoskeletal
organization and microtubule acetylation. J Bone Miner Res.
26:657–665. 2011. View Article : Google Scholar
|
|
20
|
Tonami K, Kurihara Y, Arima S, Nishiyama
K, Uchijima Y, Asano T, Sorimachi H and Kurihara H: Calpain-6, a
microtubule-stabilizing protein, regulates Rac1 activity and cell
motility through interaction with GEF-H1. J Cell Sci.
124:1214–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tonami K, Hata S, Ojima K, Ono Y, Kurihara
Y, Amano T, Sato T, Kawamura Y, Kurihara H and Sorimachi H:
Calpain-6 deficiency promotes skeletal muscle development and
regeneration. PLoS Genet. 9:e10036682013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Margiotta A, Progida C, Bakke O and Bucci
C: Rab7a regulates cell migration through Rac1 and vimentin.
Biochim Biophys Acta Mol Cell Res. 1864:367–381. 2017. View Article : Google Scholar
|
|
23
|
Rodriguez OC, Schaefer AW, Mandato CA,
Forscher P, Bement WM and Waterman-Storer CM: Conserved
microtubule-actin interactions in cell movement and morphogenesis.
Nat Cell Biol. 5:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mogessie B, Roth D, Rahil Z and Straube A:
A novel isoform of MAP4 organises the paraxial microtubule array
required for muscle cell differentiation. Elife. 4:e056972015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bryan BA, Li D, Wu X and Liu M: The Rho
family of small GTPases: Crucial regulators of skeletal myogenesis.
Cell Mol Life Sci. 62:1547–1555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Skubitz KM and Skubitz AP: Differential
gene expression in uterine leiomyoma. J Lab Clin Med. 141:297–308.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marion A, Dieudonné FX, Patiño-Garcia A,
Lecanda F, Marie PJ and Modrowski D: Calpain-6 is an endothelin-1
signaling dependent protective factor in chemoresistant
osteosarcoma. Int J Cancer. 130:2514–2525. 2012. View Article : Google Scholar
|
|
28
|
Liu Y, Wang Y, Sun X, Mei C, Wang L, Li Z
and Zha X: miR-449a promotes liver cancer cell apoptosis by
downregulation of Calpain 6 and POU2F1. Oncotarget. 7:13491–13501.
2016. View Article : Google Scholar :
|
|
29
|
Su X, Xiao D, Huang L, Li S, Ying J, Tong
Y, Ye Q, Mu D and Qu Y: MicroRNA alteration in developing rat
oligodendrocyte precursor cells induced by hypoxia-ischemia. J
Neuropathol Exp Neurol. 78:900–909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mahaman YAR, Huang F, Kessete Afewerky H,
Maibouge TMS, Ghose B and Wang X: Involvement of calpain in the
neuropatho-genesis of Alzheimer's disease. Med Res Rev. 39:608–630.
2019. View Article : Google Scholar
|
|
31
|
Peng P, Wu W, Zhao J, Song S, Wang X, Jia
D, Shao M, Zhang M, Li L, Wang L, et al: Decreased expression of
Calpain-9 predicts unfavorable prognosis in patients with gastric
cancer. Sci Rep. 6:296042016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fichna JP, Macias A, Piechota M,
Korostyński M, Potulska- Chromik A, Redowicz MJ and Zekanowski C:
Whole-exome sequencing identifies novel pathogenic mutations and
putative phenotype-influencing variants in Polish limb-girdle
muscular dystrophy patients. Hum Genomics. 12:342018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stumvoll M, Goldstein BJ and van Haeften
TW: Type 2 diabetes: Principles of pathogenesis and therapy.
Lancet. 365:1333–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyazaki T, Taketomi Y, Takimoto M, Lei
XF, Arita S, Kim-Kaneyama JR, Arata S, Ohata H, Ota H, Murakami M
and Miyazaki A: m-Calpain induction in vascular endothelial cells
on human and mouse atheromas and its roles in VE-cadherin
disorganization and atherosclerosis. Circulation. 124:2522–2532.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsushita Y, Shimada Y, Kawara S,
Takehara K and Sato S: Autoantibodies directed against the protease
inhibitor calpastatin in psoriasis. Clin Exp Immunol. 139:355–362.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ono Y, Saido TC and Sorimachi H: Calpain
research for drug discovery: Challenges and potential. Nat Rev Drug
Discov. 15:854–876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Drag M and Salvesen GS: Emerging
principles in protease-based drug discovery. Nat Rev Drug Discov.
9:690–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nikkel AL, Martino B, Markosyan S,
Brederson JD, Medeiros R, Moeller A and Bitner RS: The novel
calpain inhibitor A-705253 prevents stress-induced tau
hyperphosphorylation in vitro and in vivo. Neuropharmacology.
63:606–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ono Y, Ojima K, Shinkai-Ouchi F, Hata S
and Sorimachi H: An eccentric calpain, CAPN3/p94/calpain-3.
Biochimie. 122:169–187. 2016. View Article : Google Scholar
|
|
40
|
Skubitz KM and Skubitz AP: Differential
gene expression in leiomyosarcoma. Cancer. 98:1029–1038. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marion A, Dieudonné F, Patiño-García A,
Lecanda F, Marie P and Modrowski D: Identification of calpain-6 as
a new target involved in cell death of bone cancer cells. Bone.
44:S2472009. View Article : Google Scholar
|
|
42
|
Xiang Y, Li F, Wang L, Zheng A, Zuo J, Li
M, Wang Y, Xu Y, Chen C, Chen S, et al: Decreased calpain 6
expression is associated with tumorigenesis and poor prognosis in
HNSCC. Oncol Lett. 13:2237–2243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Filali H, Vidal E, Bolea R, Márquez M,
Marco P, Vargas A, Pumarola M, Martin-Burriel I and Badiola JJ:
Gene and protein patterns of potential prion-related markers in the
central nervous system of clinical and preclinical infected sheep.
Vet Res. 44:142013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Markmann A, Schäfer S, Linz W, Löhn M,
Busch AE and Wohlfart P: Down-regulation of calpain 9 is linked to
hypertensive heart and kidney disease. Cell Physiol Biochem.
15:109–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guttula SV, Rao AA, Sridhar GR,
Chakravarthy MS, Nageshwararo K and Rao PV: Cluster analysis and
phylogenetic relationship in biomarker identification of type 2
diabetes and nephropathy. Int J Diabetes Dev Ctries. 30:52–56.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyazaki T, Tonami K, Hata S, Aiuchi T,
Ohnishi K, Lei XF, Kim-Kaneyama JR, Takeya M, Itabe H, Sorimachi H,
et al: Calpain-6 confers atherogenicity to macrophages by
dysregulating pre-mRNA splicing. J Clin Invest. 126:3417–3432.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yin SJ, Lee JR, Kwak H, Lee BN, Han JW,
Hahn MJ, Park YD and Yang JM: Functional study of 143-3 protein
epsilon (YWHAE) in keratinocytes: Microarray integrating
bioinformatics approaches. J Biomol Struct Dyn. 38:2633–2649. 2020.
View Article : Google Scholar
|
|
48
|
Xia L, Liu Y, Fu Y, Dongye S and Wang D:
Integrated analysis reveals candidate mRNA and their potential
roles in uterine leiomyomas. J Obstet Gynaecol Res. 43:149–156.
2017. View Article : Google Scholar
|
|
49
|
Zhu L, Sun Y, Wu Q, Zhang C and Ling J:
CAPN6 regulates uterine leiomyoma cell proliferation and apoptosis
through the Rac1-dependent signaling pathway. Ann Clin Lab Sci.
50:24–30. 2020.PubMed/NCBI
|
|
50
|
Lee SJ, Choi YL, Lee EJ, Kim BG, Bae DS,
Ahn GH and Lee JH: Increased expression of calpain 6 in uterine
sarcomas and carci-nosarcomas: An immunohistochemical analysis. Int
J Gynecol Cancer. 17:248–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rho SB, Byun HJ, Park SY and Chun T:
Calpain 6 supports tumorigenesis by inhibiting apoptosis and
facilitating angiogenesis. Cancer Lett. 271:306–313. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee SJ, Kim BG, Choi YL and Lee JW:
Increased expression of calpain 6 during the progression of uterine
cervical neoplasia: Immunohistochemical analysis. Oncol Rep.
19:859–863. 2008.PubMed/NCBI
|
|
53
|
LoRusso PM: Inhibition of the
PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 34:3803–3815.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu XX and Kakehi Y: Enhancement of
lexatumumab-induced apoptosis in human solid cancer cells by
Cisplatin in caspase-dependent manner. Clin Cancer Res.
15:2039–2047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grgic I, Eichler I, Heinau P, Si H,
Brakemeier S, Hoyer J and Köhler R: Selective blockade of the
intermediate-conductance Ca2+-activated K+
channel suppresses proliferation of micro-vascular and
macrovascular endothelial cells and angiogenesis in vivo.
Arterioscler Thromb Vasc Biol. 25:704–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tomatis C, Fiorio Pla A and Munaron L:
Cytosolic calcium microdomains by arachidonic acid and nitric oxide
in endothelial cells. Cell Calcium. 41:261–269. 2007. View Article : Google Scholar
|
|
57
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li J, Meng X, Hu J, Zhang Y, Dang Y, Wei L
and Shi M: Heparanase promotes radiation resistance of cervical
cancer by upregulating hypoxia inducible factor 1. Am J Cancer Res.
7:234–244. 2017.PubMed/NCBI
|
|
59
|
Oh M, Rho SB, Son C, Park K and Song SY:
Non-proteolytic calpain-6 interacts with VEGFA and promotes
angiogenesis by increasing VEGF secretion. Sci Rep. 9:157712019.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Marion A, Dieudonné FX, Marie PJ and
Modrowski D: Endothelin-1 up regulates the survival factor
calpain-6 in osteo-sarcoma cells through Mapk and PI3K pathways.
Bone. 47:S1122010. View Article : Google Scholar
|
|
61
|
Marion A, Dieudonné FX, Patiño-Garcia A,
Lecanda F, Marie PJ and Modrowski D: Abnormal expression of
calpain-6 due to endothelin-1/NFκB signalling contributes to cell
survival and chemoresistance in osteosarcoma cells. Bone.
48:S382011. View Article : Google Scholar
|
|
62
|
Jin W, Wu L, Liang K, Liu B, Lu Y and Fan
Z: Roles of the PI-3K and MEK pathways in Ras-mediated
chemoresistance in breast cancer cells. Br J Cancer. 89:185–191.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Geismann C, Schäfer H, Gundlach JP, Hauser
C, Egberts JH, Schneider G and Arlt A: NF-κB dependent chemokine
signaling in pancreatic cancer. Cancers (Basel). 11:14452019.
View Article : Google Scholar
|
|
64
|
Andrique C, Morardet L, Linares LK, Cissé
MY, Merle C, Chibon F, Provot S, Haÿ E, Ea HK, Cohen-Solal M and
Modrowski D: Calpain-6 controls the fate of sarcoma stem cells by
promoting autophagy and preventing senescence. JCI insight.
3:e1212252018. View Article : Google Scholar :
|
|
65
|
Orosco A, Fromigué O, Bazille C,
Entz-Werle N, Levillain P, Marie PJ and Modrowski D: Syndecan-2
affects the basal and chemotherapy-induced apoptosis in
osteosarcoma. Cancer Res. 67:3708–3715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dieudonné FX, Marion A, Marie PJ and
Modrowski D: Targeted inhibition of T-cell factor activity promotes
syndecan-2 expression and sensitization to doxorubicin in
osteosarcoma cells and bone tumors in mice. J Bone Miner Res.
27:2118–2129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhao Y, Liao Q, Zhu Y and Long H:
Endothelin-1 promotes osteosarcoma cell invasion and survival
against cisplatin-induced apoptosis. Clin Orthop Relat Res.
469:3190–3199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaci I, Mansouri R, Marie P and Modrowski
D: Hypoxia upregulates calpain-6expression in osteosarcoma cells:
Implication in cancer stem cells. J Bone Min Res. 28:2013.
|
|
69
|
Bouanga JT, Yoon J and Modrowski D:
Contribution of cancer stem cells to the metastatic capacities of
osteosarcoma. Calcif Tissue Int. 104:S852019.
|
|
70
|
Zha X: The PI3K-Akt pathway regulates
calpain 6 expression, proliferation, and apoptosis. FASEB J.
25:2011.
|
|
71
|
Singh NK: microRNAs databases:
Developmental methodologies, structural and functional annotations.
Interdiscip Sci. 9:357–377. 2017. View Article : Google Scholar
|
|
72
|
Wong CM, Tsang FH and Ng IO: Non-coding
RNAs in hepatocellular carcinoma: Molecular functions and
pathological implications. Nat Rev Gastroenterol Hepatol.
15:137–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Raimbourg Q, Perez J, Vandermeersch S,
Prignon A, Hanouna G, Haymann JP, Baud L and Letavernier E: The
calpain/calpastatin system has opposing roles in growth and
metastatic dissemination of melanoma. PLoS One. 8:e604692013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Birch D, Britt BC, Dukes SC, Kessler JA
and Dizon ML: MicroRNAs participate in the murine oligodendroglial
response to perinatal hypoxia-ischemia. Pediatr Res. 76:334–340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Guo Y, Gong HS, Zhang J, Xie WL, Tian C,
Chen C, Shi Q, Wang SB, Xu Y, Zhang BY and Dong XP: Remarkable
reduction of MAP2 in the brains of scrapie-infected rodents and
human prion disease possibly correlated with the increase of
calpain. PLoS One. 7:e301632012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yadavalli R, Guttmann RP, Seward T,
Centers AP, Williamson RA and Telling GC: Calpain-dependent
endoproteolytic cleavage of PrPSc modulates scrapie prion
propagation. J Biol Chem. 279:21948–21956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Donkor I: An update on the therapeutic
potential of calpain inhibitors: A patent review. Expert Opin Ther
Pat. 1–17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Miyazaki T and Miyazaki A: Dysregulation
of calpain proteolytic systems underlies degenerative vascular
disorders. J Atheroscler Thromb. 25:1–15. 2018. View Article : Google Scholar :
|
|
79
|
Miyazaki T and Miyazaki A: Emerging roles
of calpain proteolytic systems in macrophage cholesterol handling.
Cell Mol Life Sci. 74:3011–3021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Miyazaki T and Miyazaki A: Impact of
dysfunctional protein catabolism on macrophage cholesterol
handling. Curr Med Chem. 26:1631–1643. 2019. View Article : Google Scholar
|
|
81
|
Kang MY, Zhang Y, Matkovich SJ, Diwan A,
Chishti AH and Dorn GW II: Receptor-independent cardiac protein
kinase Calpha activation by calpain-mediated truncation of
regulatory domains. Circ Res. 107:903–912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Taneike M, Mizote I, Morita T, Watanabe T,
Hikoso S, Yamaguchi O, Takeda T, Oka T, Tamai T, Oyabu J, et al:
Calpain protects the heart from hemodynamic stress. J Biol Chem.
286:32170–32177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Galvez AS, Diwan A, Odley AM, Hahn HS,
Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y and Dorn GW
II: Cardiomyocyte degeneration with calpain deficiency reveals a
critical role in protein homeostasis. Circ Res. 100:1071–1078.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moyen C, Goudenege S, Poussard S, Sassi A,
Brustis J and Cottin P: Involvement of micro-calpain (CAPN 1) in
muscle cell differentiation. Int J Biochem Cell Biol. 36:728–743.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sáenz A, Azpitarte M, Armañanzas R,
Leturcq F, Alzualde A, Inza I, García-Bragado F, De la Herran G,
Corcuera J, Cabello A, et al: Gene expression profiling in
limb-girdle muscular dystrophy 2A. PLoS One. 3:e37502008.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hollinger K and Selsby J: The
physiological response of protease inhibition in dystrophic muscle.
Acta Physiol (Oxf). 208:234–244. 2013. View Article : Google Scholar
|
|
87
|
Raaby L, Otkjær K, Salvskov-Iversen ML,
Johansen C and Iversen L: Characterization of the expression of
143-3 isoforms in psoriasis, basal cell carcinoma, atopic
dermatitis and contact dermatitis. Dermatol Rep. 2:e142010.
View Article : Google Scholar
|