Introduction
Neuroblastoma is a common extracranial solid tumor
that occurs as an embryonal malignancy (1); it typically originates from
undifferentiated sympathetic nervous cells in the adrenal medulla
or paravertebral sympathetic ganglia (2,3).
Neuroblastoma accounts for ~7% of all childhood malignancies, but
13% of cancer-related mortalities in children (4). In the majority of cases, surgery
cannot remove the tumors entirely when they are diagnosed (5). Neoadjuvant chemotherapy therefore
becomes the first choice to create an opportunity for further
surgery; however, a number of patients with neuroblastoma exhibit a
minimal response to current chemotherapy treatments (6). The prognosis for high-risk
neuroblastoma is poor (5-year survival rate, <35%) and has not
markedly improved over the past few decades (7). In fact, the drugs currently used in
clinical settings for treating neuroblastoma, such as cisplatin and
cyclophosphamide, were developed in the middle of the previous
century, and none of them were specifically approved for pediatric
patients with neuroblastoma. Therefore, novel drugs with greater
efficacy are required.
Traditional Chinese medicine has been used as a
resource for developing antitumor drugs (8). Bufalin, one of the major components
in extracts obtained from the venom of the parotoid glands or skin
epidermal gland of the Chinese toad Bufo gargarizans, is a
Traditional Chinese medicine used to treat heart failure in Asian
Pacific countries (9). Previous
studies have reported that bufalin exerts anticancer effects in
various tumors, such as lung cancer (10), hepatic carcinoma (11) and prostate cancer (12). However, due to the unknown targets
of bufalin, the detailed mechanisms underlying its antitumor
activity remain unclear. Li et al (13) reported that bufalin induced the
death of breast cancer cells via reactive oxygen species
(ROS)-mediated receptor-inter-acting protein
(RIP)1/RIP3/poly(ADP-ribose) polymerase (PARP)-1 pathway
activation. Liu et al (14) observed that bufalin-induced
inhibition of tumorigenesis depended on cell cycle arrest via the
c-Myc/NF-κB pathway. In glioma, bufalin upregulated microRNA-203
expression to inhibit cellular proliferation and retain cancer stem
cell-like phenotypes (15). In
lung cancer, bufalin induced cell death by disrupting DNA damage
response pathways or the PI3K/Akt pathway (16,17). Degradation of the
Na+/K+-ATPase α1 subunit was reported to be
involved in bufalin-induced inhibition of glioblastoma (18). However, to the best of our
knowledge, no studies have examined the antitumor effects of
bufalin in neuroblastoma, and the direct targets of bufalin remain
unknown.
In the present study, the antitumor effects of
bufalin in neuroblastoma were investigated in vitro and
in vivo. Moreover, a bufalin probe was designed to explore
the potential targets of bufalin in neuroblastoma via chemical
proteomics analysis, with the results suggesting an important role
of electron transport chain (ETC) disruption in bufalin-induced
mitochondrial-dependent apoptosis.
Materials and methods
Cell lines and reagents
The neuroblastoma cell lines SK-N-BE(2) (MYCN amplification) and SH-SY5Y (MYCN
non-amplification), and lung cancer cell lines A549 were obtained
from Guangzhou Jennio Biotech Co., Ltd. Cell line authentication by
STR profiling was conducted by Genewiz, Inc. All cells were
cultured in a 5% CO2 humidified incubator at 37°C in
Dulbecco's modified Eagle's medium (DMEM; HyClone; Cytiva)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). Bufalin (MedChemExpress), N-acetyl-L-cysteine
(NAC; cat. no. S0077; Beyotime Institute of Biotechnology) and the
bufalin-derived probe CS-P1 were dissolved in dimethyl sulfoxide
(DMSO) at a stock concentration of 100 mM and diluted with medium
before experimentation.
Synthesis of CS-P1
N,N'-dicyclohexylcarbodiimide (12 mg, 0.056 mmol,
1.1 eq.) and 4-dimethylaminopyridine (1 mg, 0.008 mmol, 0.15 eq.)
were added to a solution of bufalin (20 mg, 0.052 mmol) and
pent-4-ynoic acid (6 mg, 0.056 mmol, 1.1 eq.) in 5 ml dry
dichloromethane; the reaction mixture was stirred at room
temperature overnight. The solution was diluted with 20 ml water
and extracted with dichloromethane (5 ml) for three times. The
combined organic layer was washed with water, dried with
MgSO4 and then evaporated. The residue was purified via
preparative thin-layer chromatography using petroleum ether:ethyl
acetate (4:1) to yield CS-P1 (13 mg, 54%). CS-P1 is a white solid
with electrospray ionization-mass spectrometry (MS)
m/z 467.3 [M+H]+. 1H NMR (500
MHz, CDCl3) δ 7.84 (1H, dd, J=2.6, 9.8 Hz,
H-22), 7.23 (1H, d, J=2.6 Hz, H-23), 6.27 (1H, d,
J=9.8 Hz, H-21), 5.13 (2H, br.s, H-3, CH≡), 0.70 (3H, s,
H-18), 0.95 (3H, s, H-19), 2.54 (1H, m, H-17), 2.00-0.85 (26H, m).
13CNMR 125 MHz, CDCl3) δ 171.2
(-CH2-COO-), 162.4
(C-24), 148.5 (C-21), 146.7 (C-22), 122.6 (C-20), 115.3 (C-23),
85.3 (C-14), 82.6 (≡C-), 77.2 (C-3), 70.8 (CH ≡), 51.2 (C-17), 48.4
(C-13), 42.4 (C-8), 40.8 (C-12), 36.8 (C-5), 35.9 (C-9), 35.1
(C-10), 33.8 (-CH2-COO), 32.8 (C-15), 30.4
(C-4), 28.7 (C-16), 26.6 (C-6), 25.6 (C-1), 24.7 (C-2), 23.7
(C-19), 21.4 (C-7), 21.3 (C-11), 16.5 (C-18), 14.3 (-CH2-C≡CH).
Cell proliferation and colony formation
assays
Cell Counting Kit-8 reagent (Dojindo Molecular
Technologies, Inc.) was used to assess cell proliferation ability.
SK-N-BE(2), SH-SY5Y and A549
cells were cultured in complete medium at 37°C in 96-well plates at
a density of 1x104 cells/well and were treated with
bufalin or CS-P1 in a dose-(1-1,000 nM) and time-dependent (24, 48
or 72 h) manner at 37°C. Then, CCK-8 reagent was added for 2 h
incubation. The absorbance value at 450 nm was measured with an
Automated Microplate Reader (Bio-Rad Laboratories, Inc.) to assess
cell proliferation ability over 24, 48 or 72 h. The half
maximal-inhibitory concentration (IC50) of each drug was
calculated based on the dose-effect curve.
In the colony formation assays, SK-N-BE(2) cells were treated as follows: 0.1%
DMSO for 48 h, 45 nM bufalin for 48 h, 90 nM bufalin for 48 h or 90
nM bufalin for 72 h. SH-SY5Y cells were treated as follows: 0.1%
DMSO for 48 h, 15 nM bufalin for 48 h, 30 nM bufalin for 48 h or 30
nM bufalin for 72 h. For CS-P1 experiments, SK-N-BE(2) and SH-SY5Y cells were treated with
CS-P1 (329 nM) for 72 h at 37°C. Then, SK-N-BE(2) and SH-SY5Y cells were seeded into
complete medium at a density of 1x104 cells/well in
six-well plates. After 1 week of culture, the colonies were fixed
in 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 15 min at
room temperature. The colonies were stained with 0.5% crystal
violet (Beyotime Institute of Biotechnology) for 15 min at room
temperature. Finally, a camera was used to acquire images, and the
number of colonies was counted.
Cell migration assays
Cell migration assays were performed in 24-well
Transwell chambers (Corning, Inc.). Briefly, SK-N-BE(2) cells were treated as follows: 0.1%
DMSO for 48 h, 45 nM bufalin for 48 h, 90 nM bufalin for 48 h or 90
nM bufalin for 72 h. SH-SY5Y cells were treated as follows: 0.1%
DMSO for 48 h, 15 nM bufalin for 48 h, 30 nM bufalin for 48 h and
30 nM bufalin for 72 h. For CS-P1 experiments, SK-N-BE(2) and SH-SY5Y cells were treated with
CS-P1 (329 nM) for 72 h at 37°C. Then, SK-N-BE(2) and SH-SY5Y cells were plated into the
upper chambers the Transwell inserts with 200 μl serum-free
DMEM at a density of 2x104 cells/well, and 500 μl
complete DMEM containing 10% FBS was added to the lower chambers.
After 48 h of culture at 37°C, 4% paraformaldehyde was used to fix
the cells at room temperature for 15 min, and 0.5% crystal violet
was used to stain the cells for 15 min at room temperature.
Finally, images (magnifications, x100 and x200) were acquired under
an optical microscope (Leica Microsystems GmbH). Five randomly
selected fields containing migrated cells in each lower chamber
were counted.
Analysis of intracellular ROS
SK-N-BE(2) cells
were treated as follows: 0.1% DMSO for 48 h, 45 nM bufalin for 48
h, 90 nM bufalin for 48 h or 90 nM bufalin for 72 h. SH-SY5Y cells
were treated as follows: 0.1% DMSO for 48 h, 15 nM bufalin for 48
h, 30 nM bufalin for 48 h or 30 nM bufalin for 72 h. For NAC
experiments, SK-N-BE(2) and
SH-SY5Y cells were pretreated with NAC (5 mM) for 2 h at 37°C, then
SK-N-BE(2) cells were treated
with bufalin (90 nM) for 48 h and SH-SY5Y were treated with bufalin
(30 nM) for 48 h. For CS-P1 experiments, SK-N-BE(2) and SH-SY5Y were treated with CS-P1
(329 nM) for 72 h at 37°C. SK-N-BE(2) and SH-SY5Y cells were incubated with
2',7'-dichloroflu-orescin-diacetate (DCHF-DA; cat. no. S0033;
Beyotime Institute of Biotechnology) in six-well plates at a
density of 2x105 cells/well for intracellular ROS
measurement. Briefly, after incubation with 10μM DCHF-DA for
30 min in the dark at 37°C, the cells were harvested, washed and
resuspended in serum-free DMEM. Images (magnification, x200) of
five randomly selected fields were captured under a fluorescence
microscope (Leica Microsystems GmbH). The relative optical density
(OD) was measured with ImageJ 1.4.3 software (National Institutes
of Health).
Mitochondrial membrane potential (ΔΨm)
assay
A ΔΨm assay kit with JC-1 (cat. no. C2006; Beyotime
Institute of Biotechnology) was used to determine the ΔΨm.
According to the manufacturer's protocols, cells treated with CS-P1
(329 nM, 72 h) at 37°C were incubated with 1X JC-1 for 30 min in
the dark at 37°C. Next, the cells were washed twice with
phosphate-buffered saline (PBS) and observed with a fluorescence
microscope (Leica Microsystems GmbH). The relative OD was measured
with ImageJ 1.4.3 software.
Apoptosis assay
SK-N-BE(2) cells
were treated as follows: 0.1% DMSO for 48 h, 45 nM bufalin for 48
h, 90 nM bufalin for 48 h or 90 nM bufalin for 72 h. SH-SY5Y cells
were treated as follows: 0.1% DMSO for 48 h, 15 nM bufalin for 48
h, 30 nM bufalin for 48 h or 30 nM bufalin for 72 h. For NAC
experiments, SK-N-BE(2) and
SH-SY5Y cells were pretreated with NAC (5 mM) for 2 h at 37°C, then
SK-N-BE(2) cells were treated
with bufalin (90 nM, 48 h) and SH-SY5Y were treated with bufalin
(30 nM, 48 h). The cell apoptosis assay was performed using an
Annexin V-FITC Apoptosis Detection kit (cat. no. C1062L; Beyotime
Institute of Biotechnology). According to the manufacturer's
protocol, 1x106 pretreated cells were washed with PBS
three times after being isolated via centrifugation at 4°C for 3
min at 1,000 x g. Next, 5 μl Annexin V-FITC in 195 μl
1X Annexin V-FITC binding buffer and 10 μl propidium iodide
(50 μg/ml) were added to the cell suspension. The mixture
was protected from light for 20 min for incubation at room
temperature. Apoptosis was analyzed using BD FACS Canto II (BD
Biosciences) and data were analyzed using BD FACSDiva software 7.0
(BD Biosciences). Early and late apoptotic cells were used to
calculate the apoptotic rate.
Seahorse XF assay
Briefly, cells were seeded in XF cell culture plates
(Agilent Technologies, Inc.) at a density of 3x104
cells/well and cultured at 37°C overnight. When cell monolayers
were ~90% confluent, the culture medium was replaced with
bicarbonate-free low-buffered assay medium (XF Base Medium; Agilent
Technologies, Inc.) supplemented with 10 mM glucose, 2 mM glutamine
and 1 mM pyruvate. Rotenone (cat. no. R105076; Shanghai Aladdin
Bio-Chem Technology Co., Ltd.) at a final concentration of 0.5
μM and bufalin at final concentrations of 10, 20 and 40
μM were loaded to reagent ports on the sensor cartridge and
added during oxygen consumption rate (OCR) measurement. Cells were
incubated in a non-CO2 incubator at 37°C for 1 h prior
to testing. The OCR was detected by a Seahorse XF96 Analyzer
(Agilent Technologies, Inc.) according to the manufacturer's
instructions.
Mitochondria isolation
Mitochondria isolation was performed using a Cell
Mitochondria Isolation kit (cat. no. C3601; Beyotime Institute of
Biotechnology). Briefly, the cells were incubated with Mitochondria
isolation reagent containing PMSF on ice for 15 min. After Dounce
homogenization, the suspension was centrifuged at 4°C and 1,000 x g
for 10 min. Next, the supernatant was centrifuged at 4°C and 12,000
x g for 10 min. The deposit (mitochondria) and supernatant
(cytoplasm) were separately stored at -80°C.
Western blotting
Total protein was extracted from cells, which had
been lysed with RIPA buffer (Cell Signaling Technology, Inc.).
After denaturation and reductive alkylation, proteins (10
μg/lane, determined by BCA assay) were separated via 10%
SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD
Millipore). All blots were blocked via incubation in 5% skimmed
milk for 2 h at room temperature. After washing with 1X TBS-0.1%
Tween-20 buffer, primary antibodies were added to polyvinylidene
fluoride membranes overnight at 4°C. Polyclonal antibodies against
voltage-depen-dent anion channel 1 (cat. no. 4866) and cleaved
caspase-3 (cat. no. 9661), and monoclonal antibodies against
cleaved PARP (cat. no. 5625), cytochrome c (cat. no. 4280),
Bax (cat. no. 5023), Bcl-2 (cat. no. 4223) and β-actin (cat. no.
4970) were acquired from Cell Signaling Technology, Inc. (all
rabbit; all 1:1,000). Finally, horseradish peroxidase-conjugated
secondary antibody (anti-rabbit; 1:1,000; cat. no. A0208; Beyotime
Institute of Biotechnology) was added for 2 h at room temperature.
All blots were visualized using BeyoECL Star reagent (cat. no.
P0018AM; Beyotime Institute of Biotechnology) using a Bio-Rad
ChemiDoc XRS+ (Bio-Rad Laboratories, Inc.).
Gel-based fluorescence and Coomassie blue
staining of SK-N-BE(2) cells in
vitro
Cell lysates of SK-N-BE(2) were lysed in 0.2% SDS containing 1X
protease inhibitor cocktail (Beyotime Institute of Biotechnology)
and then treated with 30, 60 or 120 μM CS-P1 for 4 h in the
dark at 37°C, whereas control cells were treated with 0.1% DMSO.
For competitive binding analysis, SK-N-BE(2) cell lysates were pretreated with 120
μM bufalin (37°C, 2 h) followed by 120 μM CS-P1.
After incubation on ice for 30 min and sonication at 4°C for 2 min
at 30%, the lysates were added to freshly prepared click reaction
cocktail [200 μM azide-fluor 488 (Sigma-Aldrich; Merck
KGaA), 1 mM cupric sulfate (CuSO4; Sangon Biotech Co.,
Ltd.), 2 mM Tris(2-carboxyethyl)phosphine (TCEP; Sangon Biotech
Co., Ltd.), 80 μM Tris[(1-benzyl-1
H-1,2,3-triazol-4-yl)methyl]amine (TBTA; TCI Shanghai Development
Co., Ltd.)], vortexed and incubated at room temperature for 1.5 h.
Finally, prechilled acetone was added to the mixture to terminate
the reaction and precipitate the proteins. After centrifugation at
21,130 x g at 4°C for 2 min and washing with prechilled acetone
twice, the pellet was re-dissolved in 1X SDS loading buffer, and 30
μg of each sample was separated via 10% SDS-PAGE. The gel
was completely covered by 0.25% Coomassie Blue R250 [cat. no.
20310ES25; Yeasen Biotechnology (Shanghai) Co., Ltd.] staining
solution (0.25% R250 dissolved in 50% methanol, 10% acetic acid and
40% water) for 4 h in the dark and then washed with 5% methanol,
7.5% acetic acid and 87.5% water for 24 h. Finally, the gel was
analyzed using the Bio-Rad ChemiDoc XRS+.
Cellular imaging
A549 cells were seeded in 6-well plates containing
sterile glass coverslips, and then treated with 20 μM CS-P1
or DMSO for 4 h after the confluence reached 80%. After removing
the medium, prewarmed (37°C) medium without FBS containing 500 nM
MitoTracker (cat. no. C1035; Beyotime Institute of Biotechnology)
were added to plates for 30 min in a humidified incubator with 5%
CO2 at 37°C. The cells were washed three times with PBS,
and then treated with 4% paraformaldehyde for 15 min at room
temperature to fix the cells. After further washing for three times
with PBS, the cells were permeabilized with 1% Triton X-100 for 15
min at room temperature, and then washed again with PBS. The cells
were further incubated with freshly prepared click reaction
cocktail for 1.5 h at room temperature with vigorous shaking, and
then washed three times with PBS. Images (magnification, x400) were
captured under a fluorescence microscope (Leica Microsystems
GmbH).
Enrichment by azide magnetic beads
The SK-N-BE(2)
cell lysates in 0.2% SDS with 1X protease inhibitor cocktail were
pretreated with 120 μM bufalin (37°C, 2 h) followed by 120
μM CS-P1 (37°C, 4 h) or DMSO (37°C, 4 h). After incubation
on ice for 30 min and sonication at 4°C for 2 min at 30%, the
lysates (400 μg total protein) were added to freshly
prepared click reaction cocktail [10 μl azide magnetic beads
(Click Chemistry Tools), 1 mM CuSO4, 2 mM TCEP and 80
μM TBTA] for 1.5 h at room temperature with rotation. After
reductive alkylation [100 mM ammonium bicarbonate
(NH4hco3; Sangon Biotech
Co., Ltd.) with 20 mM TCEP and 40 mM iodoacetamide (Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature in the dark], the beads
were washed twice with 8 M urea, followed by three washes with PBS.
Next, the beads were resuspended with 200 μl 100 mM
NH4hco3 containing 4 μg
trypsin (Promega Corporation) and incubated at 37°C overnight.
Finally, the supernatant was collected and desalted using a ZipTip
with 0.6 μl C18 column (5 μg; cat. no. ZTC18S960;
Millipore). After drying in a speed-vac, samples were stored at
-80°C prior to MS analysis. Each treated sample was prepared in
three biological replicates.
Nano-high-performance liquid
chromatography (HPLC)-MS/MS analysis
Q Exactive plus (Thermo Fisher Scientific, Inc.)
coupled to an U300 RSLCnano system (Thermo Fisher Scientific, Inc.)
was used to conduct Nano-HPLC-MS/MS analysis at room temperature.
Briefly, tryptic peptide fragments of proteins were dissolved in
solvent A (0.1% formic acid, 2% acetonitrile and 98%
H2O). Separation was performed over a linear gradient of
5-35% solvent B (0.1% formic acid, 90% acetonitrile and 10%
H2O) for 50 min and 35-80% for another 10 min at a
constant flow rate of 300 nl/min on a manually packed reversed
phase C18 column (170 mm x 79 μm, 3-μm particle size;
Dikma Technologies, Inc.) coupled to an U300 RSLCnano
chromatography system. The eluted peptides were ionized and
analyzed with a Q Exactive plus mass spectrometer using a nanospray
ion source. For full MS spectra, peptides ranging from 350-1,300
m/z were scanned in an Orbitrap at a resolution of 120,000 at m/z
200. Automatic gain control was set to 7,000 at rapid mode with 1
m/z isolation window. Higher energy collisional dissociation was
sequentially used to fragment ions with charge states 2+, 3+ and 4+
at a normalized collision energy of 32%. The dynamic exclusion
duration was set to 60 sec. Fragment ions were analyzed in a linear
ion trap.
MS data analysis
MaxQuant software (version 1.6.0.16) (19) was used for peak detection and
quantification for MS raw files. Raw data were searched against the
UniProt Human database (https://www.uniprot.org/uniprot/?query=organism:9606&sort=score)
using the Andromeda search engine (20), with cysteine alkylation by
iodoacetamide as a fixed modification, and methionine oxidation,
Lys6, and Arg10, protein N-terminal acetylation as variable
modifications. The proteolytic enzyme was trypsin and maximum
missing cleavages was 2. After searching using a mass tolerance of
20 ppm, the mass accuracy of the precursor ions in the main search
was 4.5 ppm and fragment ion mass tolerance was 0.02 Da for
Q-Exactive or 0.5 Da for Fusion. The maximum false discovery rate
was 0.01 for proteins and peptides, and a minimum peptide length of
seven amino acids was required. Proteins identified in three
replicates were considered as significant targets if they met the
following criteria: i) Only detected in the CS-P1 group but not in
the DMSO group; and ii) had at least one unique peptide.
Bioinformatics analysis
The Database for Annotation, Visualization and
Integrated Discovery (https://david.ncifcrf.gov/) v6.8 was used for
bioinformatics analysis. The candidates identified by chemical
proteomics were annotated by Gene Ontology (GO) analysis (21,22). The threshold conditions were set
to a count ≥5 and EASE score (a modified Fisher's exact P-value)
≤0.05.
Subcutaneous xenograft model in nude
mice
A total of 10 male nude mice (6 weeks, 18-22 g) were
purchased from the Shanghai Laboratory Animal Center of the Chinese
Academy of Sciences. Mice were housed under specific pathogen-free
conditions (humidity, 40-70%; temperature, 22±2°C; 12:12-h
light/dark cycle; free access to standard sterile food and water).
Ethical approval for animal experiments was obtained from the
Xinhua Hospital Ethics Committee (Shanghai, China; approval no.
XHEC-F-2020-011). The xenograft study was performed between
February 2020 and April 2020. SK-N-BE(2) cells (5x106 cells in 100
μl PBS) were injected subcutaneously into the left axilla of
each mouse. The mice were randomly divided into two groups (5
mice/group) and treated as follows: Group one, vehicle (90% of 10%
2-hydroxypro pyl-β-cyclodextrin mixed with 10% DMSO) was injected
intraperitoneally alone; and group two, bufalin (5 mg/kg) was
dissolved in vehicle and injected intraperitoneally three times
every week. Humane endpoints were set as the tumor volume exceeding
2,000 mm3 or the maximum diameter of a tumor exceeding 2
cm, at which point nude mice would be euthanized. However, no mice
were euthanized or found dead before the end of the 4-week
treatment period. The nude mice were checked when receiving
treatment. Tumor volumes were measured every week and calculated as
1/2 x width2 x length. The xenograft tumors were excised
(fixed in 4% paraformaldehyde at 4°C overnight) and weighed after 4
weeks of treatment. Nude mice were euthanized with carbon dioxide
(30% volume/min chamber displacement) until breathing stopped for 2
min, and then cervical dislocation was performed to ensure
death.
Immunohistochemistry staining
Immunohistochemistry staining for Ki67,
proliferating cell nuclear antigen (PCNA) and caspase-3 was
performed by Servicebio, lnc. Images (magnifications, x100, x200
and x400) were acquired under an optical microscope (Leica
Microsystems GmbH) and five randomly selected fields were analyzed.
A semiquantitative histological score (H-score) was applied for the
evaluation of the immunohistochemistry staining results. The
H-score considers both the degree of staining intensity and the
percentage of positive neoplastic cells. The intensity was scored
on a scale of 0 to 3 (0, negative; 1, weak; 2, medium; and 3,
strong). The formula for the H-score is as follows: H-score=Σ(Pi x
I)=(percentage of cells with weak intensity x1) + (percentage of
cells with moderate intensity x2) + (percentage of cells with
strong intensity x3) (23), where
Pi indicates the percentage of positive tumor cells and I indicates
staining intensity.
Statistical analysis
Statistical analysis was conducted using Prism 6
software (GraphPad Software, Inc.). Unpaired student's t-test was
used to analyze differences between two groups. One-way ANOVA was
used to analyze datasets containing ≥3 groups and Tukey's multiple
comparisons test was used following one-way ANOVA to determine
significant differences between specific groups. Two-way ANOVA was
used to analyze datasets where groups were divided by two factors,
and Sidak's multiple comparisons test was used following two-way
ANOVA. Experiments were conducted independently at least three
times and the results are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Bufalin inhibits the proliferation and
migration of neuroblastoma cells
The antitumor effects of bufalin in neuroblastoma
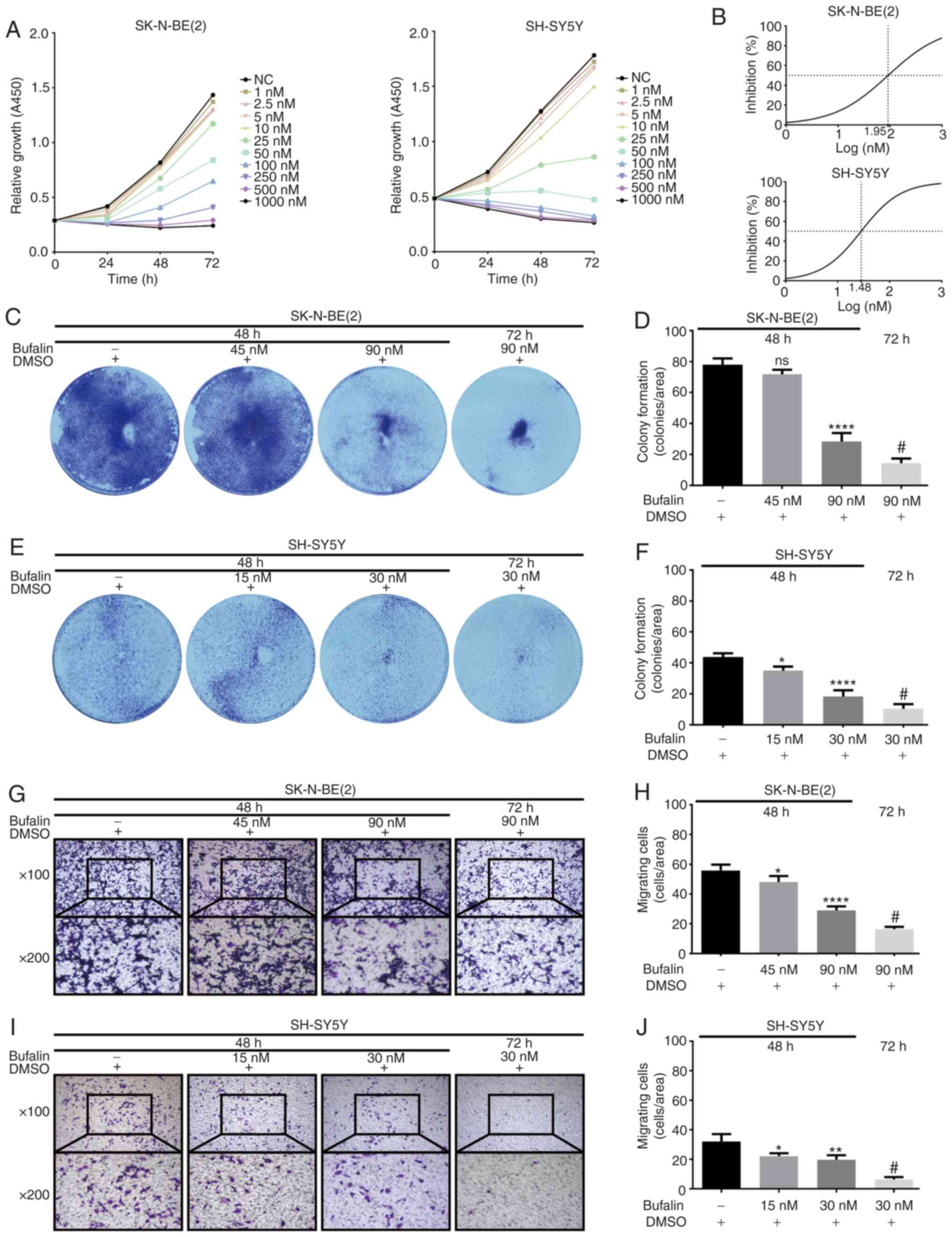
were evaluated. As shown in Fig.
1A, cell proliferation was markedly suppressed by bufalin in
dose-(1-1,000 nM) and time-dependent (24, 48 or 72 h) manners. The
IC50 values of bufalin in SK-N-BE(2) and SH-SY5Y cells were ~90 and 30 nM
at 72 h, respectively (Fig. 1B),
and the cells were selected for the subsequent experiments.
Single-cell proliferation and viability was investigated in a
colony formation assay. As shown in Fig. 1C-F, the number and size of
colonies formed by neuroblastoma cells treated with bufalin were
significantly reduced. Additionally, Transwell assays showed that
the ability to migrate to the lower chambers was significantly
suppressed in SK-N-BE(2) and
SH-SY5Y cells upon exposure to bufalin (Fig. 1G-J). Together, these results
indicated antitumor properties of bufalin in neuroblastoma by
inhibiting cell proliferation and migration.
Bufalin probe CS-P1 retains the antitumor
activity of bufalin in neuroblastoma
Identifying potential protein targets is the first
step towards understanding the molecular mechanism underlying the
antitumor activity of bufalin. Bufalin is a cardiotonic steroid
with an α,β-unsaturated carbonyl group (24). Through the electron-withdrawing
effect of the oxygen atom, α,β-unsaturated carbonyl is prone to
attack by nucleophiles for Michael additions, such as thiol and
amido (25). The results
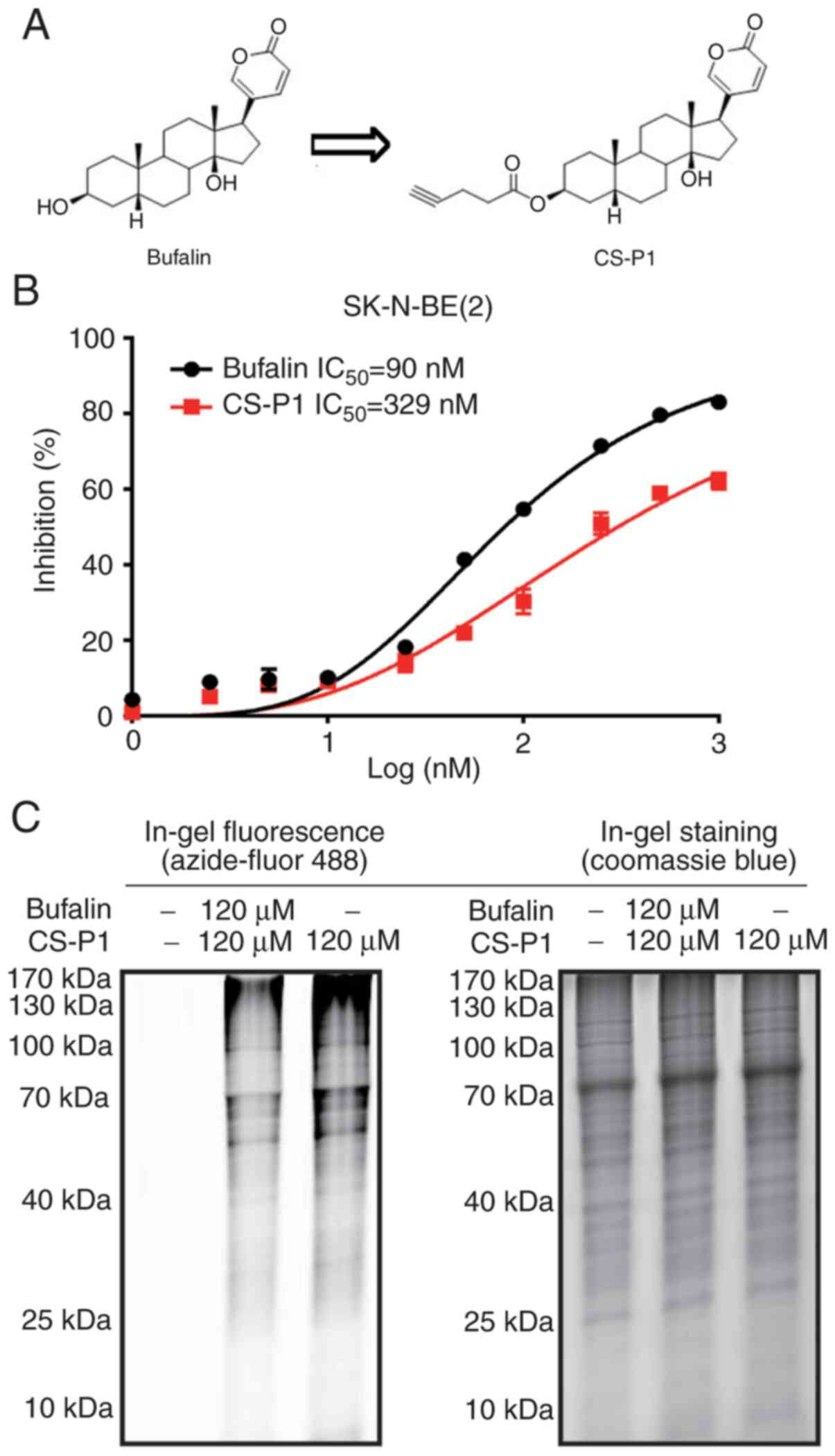
suggested that bufalin reacted with peptide molecules (Fig. S1A-E). To further investigate the
cellular target proteins of bufalin, the alkyne-containing chemical
probe CS-P1 was designed and synthesized (Fig. 2A). The biological activity of
CS-P1 was first assessed in the A549 cell line, a positive control
with an IC50 <10 nM for bufalin (26). Incubation of A549 cells with CS-P1
for 24 h significantly reduced the viability of A549 cells, showing
an IC50 of 104.7 nM, while bufalin exhibited an
IC50 of 9.8 nM (Fig.
S1F). Intracellular localization of CS-P1 was visualized by
Azide-fluor 488 and mitochondria were found to be potential targets
(Fig. S1G). Subsequently, the
antitumor activity of CS-P1 was evaluated in neuroblastoma cells.
As shown in Fig. 2B, the 72-h
IC50 of CS-P1 in SK-N-BE(2) cells was 329 nM, revealing that,
similar to bufalin, CS-P1 exhibited antitumor properties at
nanomolar concentrations. In addition, colony formation assays and
cell migration assays demonstrated that CS-P1 suppressed
neuroblastoma cell proliferation and migration (Fig. S2). Gel staining indicated
potential targets shared by CS-P1 and bufalin. In brief, total
proteins of SK-N-BE(2) cells
pretreated with bufalin and CS-P1 together or CS-P1 only were
subjected to a click reaction with Azide-fluor 488, and then
resolved by SDS-PAGE. Only CS-P1-targeted proteins were
fluorescently labeled, and the density of the sample co-treated
with bufalin and CS-P1 was markedly decreased (Fig. 2C). These results suggested that
CS-P1 retains antitumor properties by competitively binding to the
targets of bufalin.
Chemical proteomics highlights the ETC as
a potential target
Next, the potential antitumor targets shared by
bufalin and CS-P1 were investigated using a chemical proteomics
strategy as shown in Fig. 3A.
Briefly, after pretreatment with bufalin, proteins were then
treated with CS-P1 or DMSO. Proteins only detected in the CS-P1
group but not the DMSO group were considered to be targets. A total
of 99 proteins were identified in three replicates (Fig. 3B and Table SI). According to the fluorescence
imaging shown in Fig. S1G,
mitochondria-derived proteins were hypothesized to be potential
targets. GO term analysis was performed to annotate the cellular
localization of the identified proteins (Fig. 3C). 'Mitochondrial inner membrane'
(GO:0005743) was enriched according to the conditions of count ≥5
and a maximum EASE score of 0.05. There were 7 targets identified
that fell under the term 'mitochondrial inner membrane', including
CDS2, SDHD, COX7C, TMEM126A, MRPS31, MRPL34 and ATP5J, several of
which were localized to the ETC, suggesting that ETC disruption
occurred upon exposure to bufalin (Fig. 3D). Disruption of the ETC would
decrease the OCR of cells, which was validated by performing a
Seahorse assay after administration of bufalin in SK-N-BE(2) cells (Fig. 3E and F). It was previously
reported that ETC disruption led to excessive ROS levels (27). Therefore, ROS levels were
increased in SK-N-BE(2) cells
after treatment with CS-P1 (Fig. 3G
and H). Increased ROS levels reduce the ΔΨm (28). As shown in Fig. 3I and J, the ΔΨm of
SK-N-BE(2) cells was
significantly reduced following treatment with CS-P1. These results
suggested that bufalin induces its antitumor effects by targeting
the ETC.
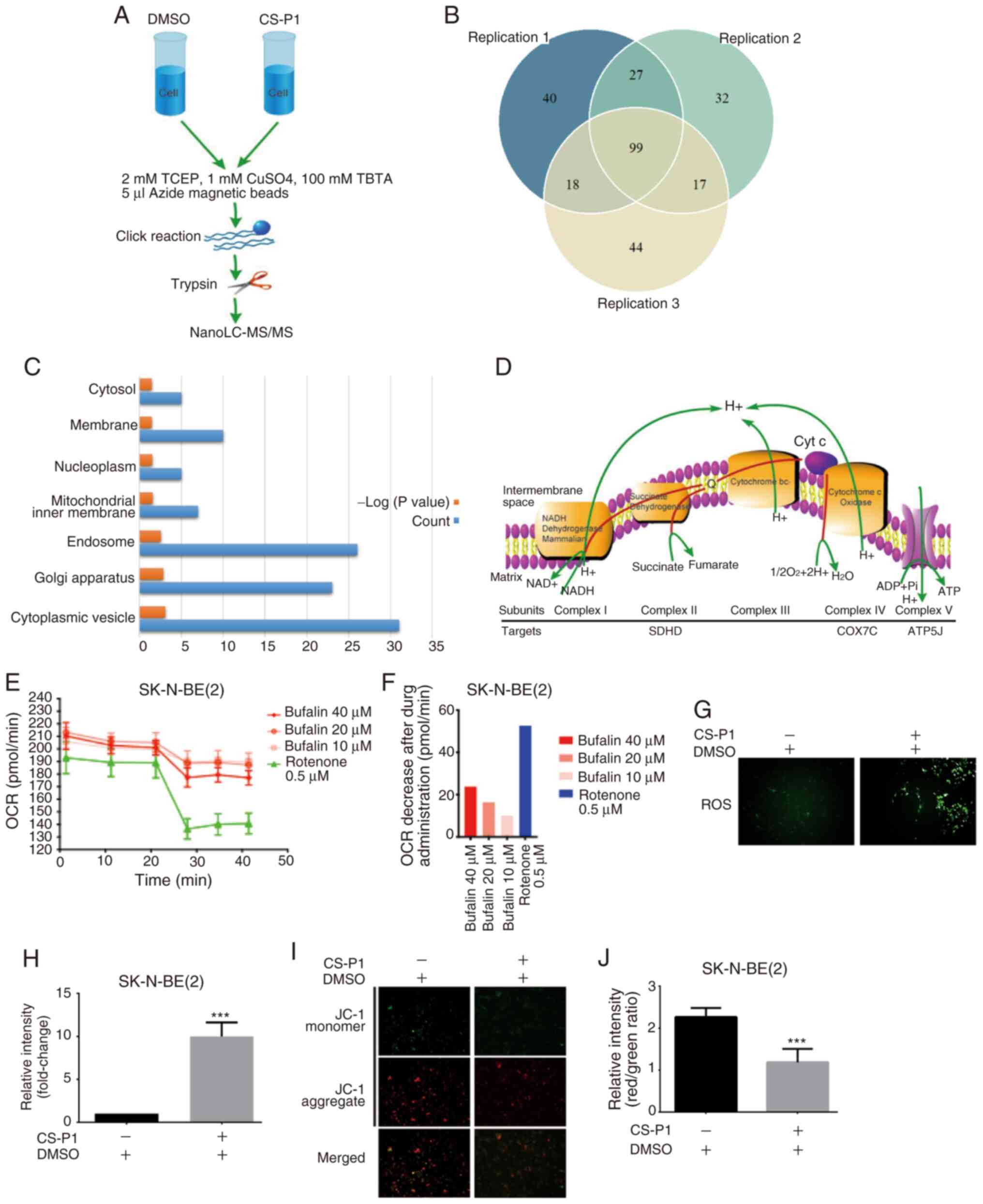 | Figure 3Chemical proteomics highlights the
ETC as a potential target. (A) Workflow diagram of nanoLC-MS/MS.
(B) Venn diagram of proteins identified in three experimental
repeats. (C) Gene Ontology analysis annotated cellular components
enriched by CS-P1. (D) Diagram of ETC and the possible targets of
CS-P1. (E) Seahorse assay of oxygen consumption and (F) OCR
decrease after administration of bufalin in SK-N-BE(2) cells. Rotenone was used as a positive
control. (G) Intracellular ROS detection and (H) increase in ROS
levels after CS-P1 treatment (329 nM, 72 h; magnification, x200).
(I) Mitochondrial membrane potential detection with JC-1 probes and
(J) reduction after CS-P1 treatment (329 nM, 72 h; magnification,
x200). Data are presented as the mean ± SD (n=3).
***P<0.001 vs. DMSO. ATP5J, ATP synthase-coupling
factor 6; COX7C, cytochrome c oxidase subunit 7C; DMSO,
dimethyl sulfoxide; ETC, electron transport chain; LC-MS/MS, liquid
chromatography-tandem mass spectrometry; OCR, oxygen consumption
rate; ROS, reactive oxygen species; SDHD, succinate dehydrogrenase
complex subunit D; TBTA,
Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine; TCEP,
Tris(2-carboxyethyl) phosphine. |
ETC disruption induces
mitochondria-dependent apoptosis of neuroblastoma cells
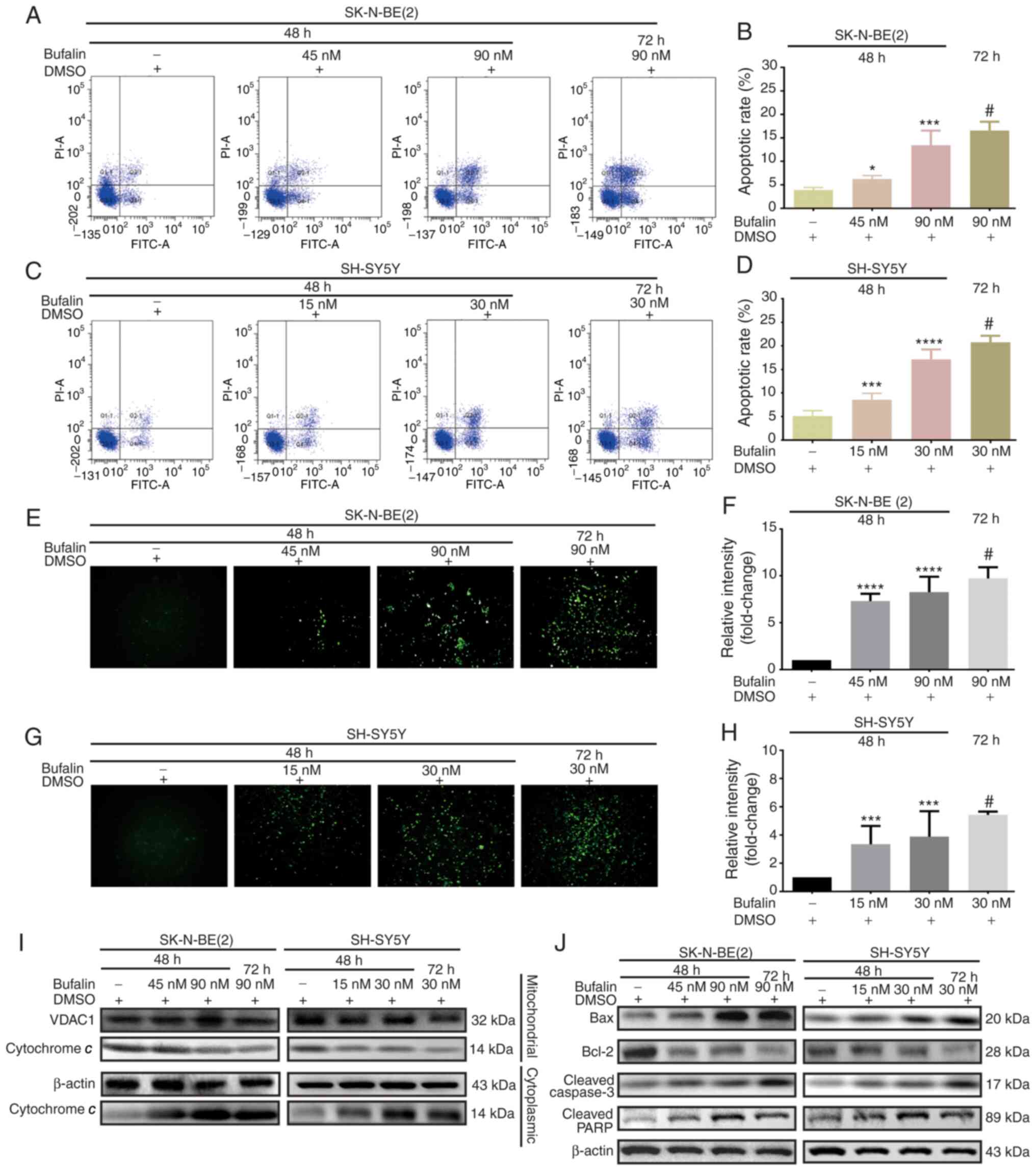
Reductions in ΔΨm result in cell apoptosis (29). To investigate the apoptotic
effects of bufalin in neuroblastoma cells, flow cytometric analysis
was performed for SK-N-BE(2) and
SH-SY5Y cells. Compared with that in negative control cells, the
apoptotic rate was significantly increased in bufalin-treated cells
in dose- and time-dependent manners (Fig. 4A-D). Additionally, intracellular
ROS levels were significantly increased after treatment with
bufalin, which was consistent with the results described above
(Fig. 4E-H). A decreased ΔΨm can
lead to increased permeability of the mitochondrial membrane,
causing cytochrome c to flow from the mitochondria into the
cytoplasm (30). Cytochrome
c may activate and cleave caspase-3 via induction of the
apoptotic cascade reaction by forming apoptosomes (31). Western blot analysis verified that
cytochrome c was decreased in the mitochondria but increased
in the cytoplasm after treatment with bufalin (Fig. 4I). Moreover, downregulation of
Bcl-2, as well as upregulation of Bax, cleaved caspase-3 and
cleaved PARP, was observed following bufalin treatment (Fig. 4J).
Bufalin-induced apoptosis of
neuroblastoma cells depends on ROS accumulation
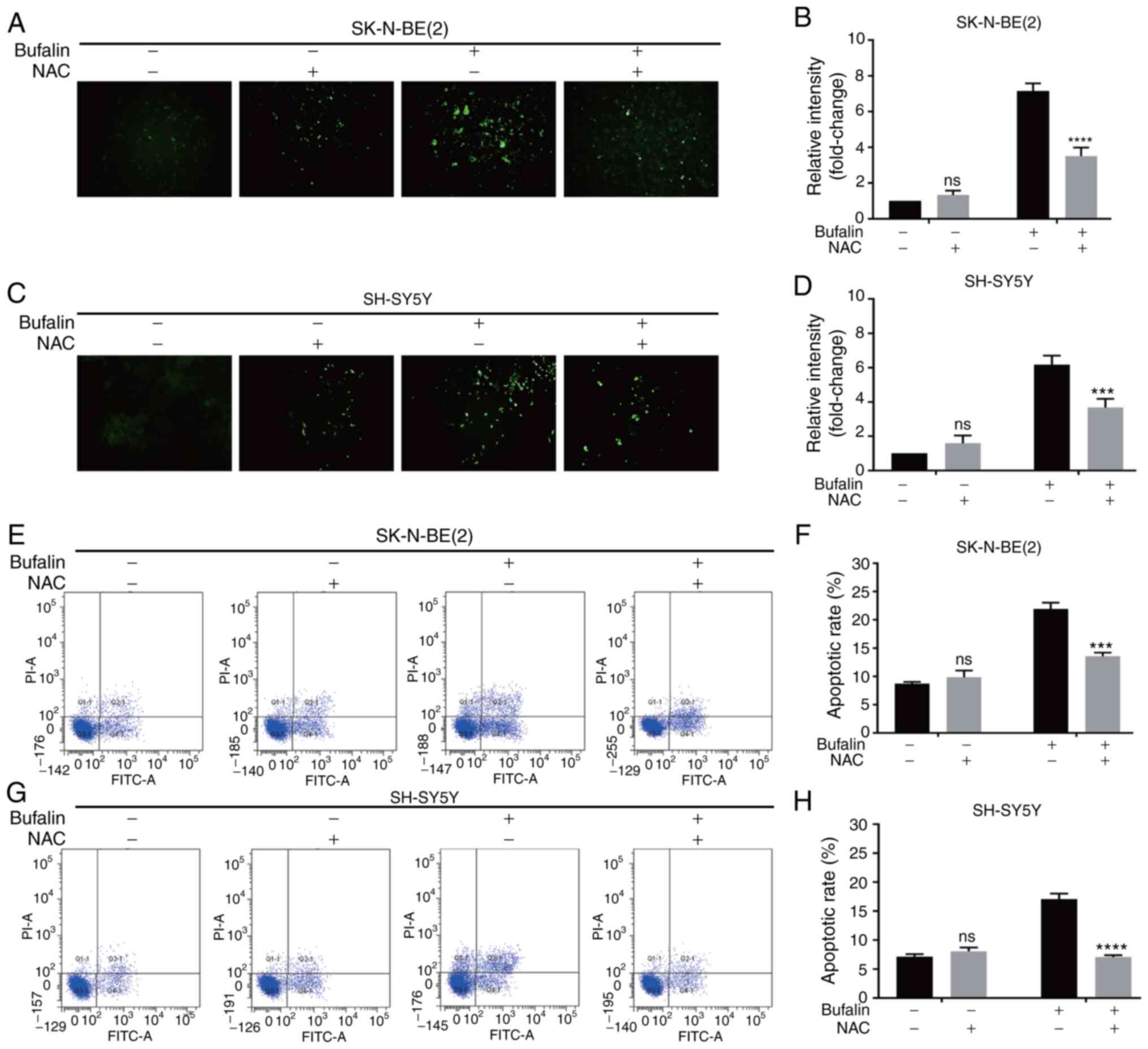
To investigate whether ROS play a central role in
the bufalin-induced apoptosis of neuroblastoma cells,
N-acetyl-L-cysteine (NAC), a well-known antioxidant, was used to
inhibit the effects of ROS. Neuroblastoma cells were treated with
NAC prior to exposure to bufalin. As shown in Fig. 5A-D, the increase in intracellular
ROS levels following treatment with bufalin was significantly
reversed by NAC in SK-N-BE(2) and
SH-SY5Y cells. Additionally, the apoptotic rate of cells pretreated
with NAC was significantly reduced compared with that of cells
treated with bufalin alone (Fig.
5E-H).
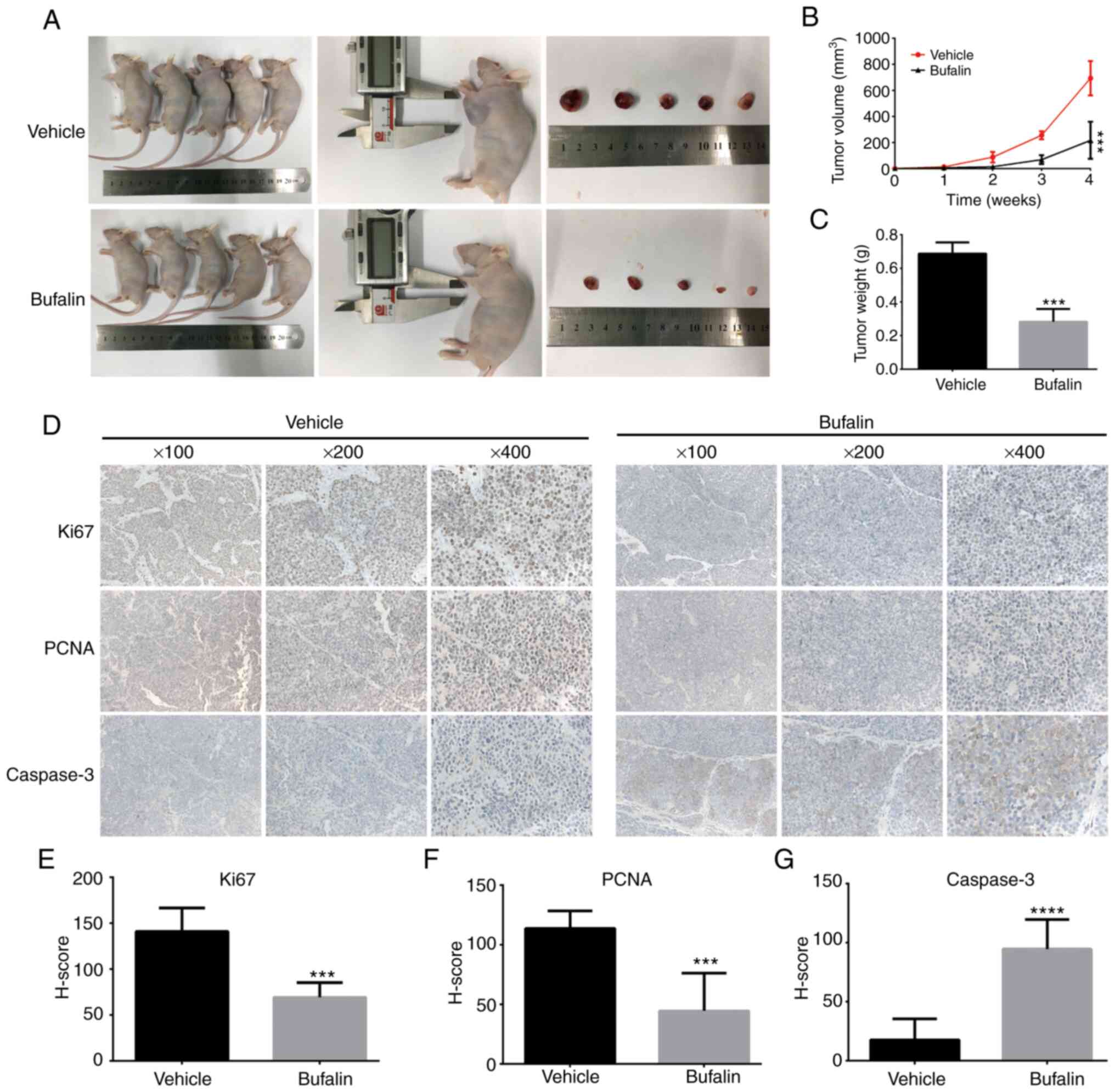
Bufalin inhibits tumor growth in
vivo
To establish animal models, SK-N-BE(2) cells were injected into nude mice
subcutaneously. Next, bufalin or vehicle was intraperitoneally
injected after cell transplantation. Tumor weight and volume in the
treatment group were significantly decreased compared with the
control group (Fig. 6A-C). To
further investigate the mechanisms underlying these effects,
immunohistochemical analysis was performed. As shown in Fig. 6D-G, the expression levels of the
proliferation indicators Ki67 and PCNA were significantly
decreased. Consistent with the in vitro results, caspase-3
expression was significantly increased.
Discussion
In various Asian Pacific countries, bufalin is used
as a cardiac glycoside for heart failure treatment, and low-dose
bufalin does not cause fatal damage to the human body (32-34). Following reports that bufalin
could potently induce the differentiation of human leukemia-derived
cell lines (35), bufalin has
subsequently been reported to induce antitumor effects in various
types of tumor (13,36,37). The octreotide-conjugated polymeric
prodrug of bufalin has shown promising effects on somatostatin
receptor 2-overexpressing breast cancer (38). Bufalin-loaded calcium
phosphate/DPPE-polyethylene glycol-epidermal growth factor
nanospheres were reported to have antitumor effects in colon cancer
via slow release from dialysis membranes (39). Bufalin inhibits cell proliferation
and induces apoptosis via DNA damage, chromatin condensation and
endoplasmic reticulum stress (37,40-42). However, there remains limited
knowledge regarding the potential mechanisms of bufalin, and the
substrates of bufalin remain unknown, which hinders our further
understanding of the molecular mechanism underlying its antitumor
effects, as well as its potential toxicity. Thus, identifying the
targets of bufalin is the first step towards its development as an
antitumor drug.
Chemical probes and chemical proteomics provide an
effective approach for identifying the potential cellular targets
of bioactive compounds, including approved drugs (43). Exploring novel targets may expand
our knowledge and avoid the potential side effects of these
compounds (44,45).
Therefore, a bufalin-derived probe was designed to
investigate its potential targets by modifying with an alkyne
group, enabling the probe to be immobilized with azide magnetic
beads through a click chemical reaction. Thus, the potential
targets of bufalin could be precipitated via pulldown. Cell
viability assays showed that the modified probe retained the potent
antitumor activity of bufalin. Chemical proteomics and
bioinformatics analyses revealed the ETC as a direct target of
bufalin. Downstream functional experiments suggested that ETC
function was impaired, leading to ROS burst and a reduction in ΔΨm,
as well as apoptosis. Collectively, the present findings identified
respiratory chain proteins as potential substrates of bufalin.
However, due to the limited specificity and accuracy of the probe,
other directly targeted proteins remain unclear. In addition, how
the compound binds to substrate proteins remains unclear, as well
as which domain of the substrate the compound binds to. Therefore,
further experiments are required to identify other targets.
To the best of the authors' knowledge, this is the
first report demonstrating the effective antitumor effects of
bufalin in neuroblastoma in vitro and in vivo.
Regardless of MYCN amplification status, bufalin exhibited
consistent antitumor activity, indicating that the antitumor
activity of bufalin was MYCN-independent. The Children's Oncology
Group stratifies the patients into low risk, medium risk and
high-risk group based on a number of variables, including age, MYCN
status, pathology, DNA polyploid and differentiation status; the
status of MYCN is an important factor for assessing clinical
malignant behavior, and is strongly associated with poor prognosis,
even with intensive chemotherapy regimens (5,7).
Indeed, MYCN amplification is an important in patients with
high-risk neuroblastoma (46).
SK-N-BE(2) is a MYCN
amplification human neuroblastoma cell line derived from children
with high-risk neuroblastoma (47,48). Thus, the present findings
suggested potential novel treatment strategies for patients with
high-risk neuroblastoma.
The in vivo experiments in the present study
also revealed that low-dose bufalin did not cause nude mouse death,
but effectively inhibited tumor growth. However, the physical
conditions of the mice, such as organ function, were not assessed
in detail. The mice were sacrificed following treatment and there
was no possibility of observing sequelae. Therefore, to evaluate
the clinical viability of bufalin, further experiments are required
to assess its safety. There have been a number of studies dedicated
to reducing the toxicity of bufalin and improving its efficacy
(49-51).
In conclusion, the present study demonstrated that
the antitumor effects of bufalin in neuroblastoma in vitro
occurred via targeting of the ETC. ETC disruption-induced ROS
accumulation reduced the ΔΨm and increased the permeability of the
mitochondrial membrane, causing accumulation of cytochrome c
in the cytoplasm. As a result, cytochrome c may activate the
downstream caspase-3 cascade, inducing apoptosis; however, further
studies are required to validate these proposed molecular
mechanisms, and elucidate the potential binding sites of
bufalin.
Supplementary Data
Acknowledgments
Immunohistochemistry staining was performed by
Servicebio, lnc.
Abbreviations:
|
GO
|
Gene Ontology
|
|
DMSO
|
dimethyl sulfoxide
|
|
IC50
|
half maximal-inhibitory
concentration
|
|
DCHF-DA
|
2',7'-dichlorofluorescin-diacetate
|
|
OCR
|
oxygen consumption rate
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
MS
|
mass spectrometry
|
|
ROS
|
reactive oxygen species
|
|
ETC
|
electron transport chain
|
|
NAC
|
N-acetyl-L-cysteine
|
|
PCNA
|
proliferating cell nuclear
antigen
|
Funding
The present study was supported by the Suzhou
Clinical Medicine Innovation Team Introduction Project (grant no.
SZYJTD201706 to YW) and the Natural Science Foundation of China
(grant no. 81874234 to ZW).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
LP, LN, SY and AB performed experiments. YY and YW
designed the study. ZT and ZW analyzed and interpreted data, and
managed the project. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval for animal experiments was
obtained from the Ethics Committee of Xinhua Hospital (approval no.
XHEC-F-2020-011).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ackermann S, Cartolano M, Hero B, Welte A,
Kahlert Y, Roderwieser A, Bartenhagen C, Walter E, Gecht J,
Kerschke L, et al: A mechanistic classification of clinical
phenotypes in neuroblastoma. Science. 362:1165–1170. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthay KK, Maris JM, Schleiermacher G,
Nakagawara A, Mackall CL, Diller L and Weiss WA: Neuroblastoma. Nat
Rev Dis Primers. 2:160782016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whittle SB, Smith V, Doherty E, Zhao S,
McCarty S and Zage PE: Overview and recent advances in the
treatment of neuroblastoma. Expert Rev Anticancer Ther. 17:369–386.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheung NK and Dyer MA: Neuroblastoma:
Developmental biology, cancer genomics and immunotherapy. Nat Rev
cancer. 13:397–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgenstern DA, Bagatell R, Cohn SL,
Hogarty MD, Maris JM, Moreno L, Park JR, Pearson AD, Schleiermacher
G, Valteau-Couanet D, et al: The challenge of defining
'ultra-high-risk' neuroblastoma. Pediatr Blood Cancer.
66:e275562019. View Article : Google Scholar
|
|
8
|
Ma Z, Fan Y, Wu Y, Kebebe D, Zhang B, Lu
P, Pi J and Liu Z: Traditional Chinese medicine-combination
therapies utilizing nanotechnology-based targeted delivery systems:
A new strategy for antitumor treatment. Int J Nanomedicine.
14:2029–2053. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Dong Y, Melkus MW, Yin S, Tang
SN, Jiang P, Pramanik K, Wu W, Kim S, Ye M, et al: Role of
P53-Senescence induction in suppression of LNCaP prostate cancer
growth by cardiotonic compound bufalin. Mol Cancer Ther.
17:2341–2352. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu SH, Hsiao YT, Chen JC, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin alters gene
expressions associated DNA damage, cell cycle, and apoptosis in
human lung cancer NCI-H460 cells in vitro. Molecules. 19:6047–6057.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu F, Han J, Zhai B, Ming X, Zhuang L, Liu
Y, Pan S and Liu T: Blocking autophagy enhances the apoptosis
effect of bufalin on human hepatocellular carcinoma cells through
endoplasmic reticulum stress and JNK activation. Apoptosis.
19:210–223. 2014. View Article : Google Scholar
|
|
12
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of androgen
dependent and independent prostate cancer cells. Prostate.
54:112–124. 2003. View Article : Google Scholar
|
|
13
|
Li Y, Tian X, Liu X and Gong P: Bufalin
inhibits human breast cancer tumorigenesis by inducing cell death
through the ROS-mediated RIP1/RIP3/parp-1 pathways. Carcinogenesis.
39:700–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Xiao XY, Shou QY, Yan JF, Chen L,
Fu HY and Wang JC: Bufalin inhibits pancreatic cancer by inducing
cell cycle arrest via the c-Myc/NF-κB pathway. J Ethnopharmacol.
193:538–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu T, Wu C, Weng G, Zhao Z, He X, Fu C,
Sui Z and Huang SX: Bufalin inhibits cellular proliferation and
cancer stem cell-like phenotypes via upregulation of MiR-203 in
glioma. Cell Physiol Biochem. 44:671–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu SH, Wu TY, Hsiao YT, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin induces cell death
in human lung cancer cells through disruption of DNA damage
response pathways. Am J Chin Med. 42:729–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lan YL, Wang X, Lou JC, Xing JS, Yu ZL,
Wang H, Zou S, Ma X and Zhang B: Bufalin inhibits glioblastoma
growth by promoting proteasomal degradation of the
Na+/K+-ATPase α1 subunit. Biomed
Pharmacother. 103:204–215. 2018. View Article : Google Scholar
|
|
19
|
Cox J and Mann M: MaxQuant enables high
peptide identification rates, individualized p.p.b.-range mass
accuracies and proteome-wide protein quantification. Nat
Biotechnol. 26:1367–1372. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cox J, Neuhauser N, Michalski A, Scheltema
RA, Olsen JV and Mann M: Andromeda: A peptide search engine
integrated into the MaxQuant environment. J Proteome Res.
10:1794–1805. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
The Gene Ontology Consortium: The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47:D330–D338. 2019. View Article : Google Scholar :
|
|
23
|
Yeo W, Chan SL, Mo FK, Chu CM, Hui JW,
Tong JH, Chan AW, Koh J, Hui EP, Loong H, et al: Phase I/II study
of temsirolimus for patients with unresectable hepatocellular
carcinoma (HCC)-a correlative study to explore potential biomarkers
for response. BMC Cancer. 15:3952015. View Article : Google Scholar
|
|
24
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar
|
|
25
|
Jackson PA, Widen JC, Harki DA and
Brummond KM: Covalent modifiers: A chemical perspective on the
reactivity of α,β-Unsaturated carbonyls with thiols via
hetero-michael addition reactions. J Med Chem. 60:839–885. 2017.
View Article : Google Scholar
|
|
26
|
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill
TG, Wang J, Qi R, Matzuk AJ, Song X, Madoux F, et al: Bufalin is a
potent small-molecule inhibitor of the steroid receptor
coactivators SRC-3 and SRC-1. Cancer Res. 74:1506–1517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Larosa V and Remacle C: Insights into the
respiratory chain and oxidative stress. Biosci Rep.
38:BSR201714922018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Battogtokh G, Choi YS, Kang DS, Park SJ,
Shim MS, Huh KM, Cho YY, Lee JY, Lee HS and Kang HC:
Mitochondria-targeting drug conjugates for cytotoxic,
anti-oxidizing and sensing purposes: Current strategies and future
perspectives. Acta Pharm Sin B. 8:862–880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou XS, Wang HS, Mugaka BP, Yang GJ and
Ding Y: Mitochondria: Promising organelle targets for cancer
diagnosis and treatment. Biomater Sci. 6:2786–2797. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai G, Zheng D, Guo W, Yang J and Cheng
AY: Cinobufagin induces apoptosis in osteosarcoma cells via the
mitochondria-mediated apoptotic pathway. Cell Physiol Biochem.
46:1134–1147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu Z, Feng H, Sun X, Zhuo Y, Li M, Zhou Z,
Huang L, Jiang Y, Zhu X, Zhang X, et al: Bufalin suppresses
hepatocarcinogenesis by targeting β-catenin/TCF signaling via cell
cycle-related kinase. Sci Rep. 8:38912018. View Article : Google Scholar
|
|
33
|
Liu J, Zhang Y, Sun S, Zhang G, Jiang K,
Sun P, Zhang Y, Yao B, Sui R, Chen Y, et al: Bufalin induces
apoptosis and improves the sensitivity of human glioma stem-like
cells to temozolamide. Oncol Res. 27:475–486. 2019. View Article : Google Scholar
|
|
34
|
Li H, Hu S, Pang Y, Li M, Chen L, Liu F,
Liu M, Wang Z and Cheng X: Bufalin inhibits glycolysis-induced cell
growth and proliferation through the suppression of Integrin β2/FAK
signaling pathway in ovarian cancer. Am J Cancer Res. 8:1288–1296.
2018.
|
|
35
|
Zhang L, Nakaya K, Yoshida T and Kuroiwa
Y: Induction by bufalin of differentiation of human leukemia cells
HL60, U937, and ML1 toward macrophage/monocyte-like cells and its
potent synergistic effect on the differentiation of human leukemia
cells in combination with other inducers. Cancer Res. 52:4634–4641.
1992.PubMed/NCBI
|
|
36
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su EY, Chu YL, Chueh FS, Ma YS, Peng SF,
Huang WW, Liao CL, Huang AC and Chung JG: Bufalin induces apoptotic
cell death in human nasopharyngeal carcinoma cells through
mitochondrial ROS and TRAIL pathways. Am J Chin Med. 47:237–257.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu T, Jia T, Yuan X, Liu C, Sun J, Ni Z,
Xu J, Wang X and Yuan Y: Development of octreotide-conjugated
polymeric prodrug of bufalin for targeted delivery to somatostatin
receptor 2 overexpressing breast cancer in vitro and in vivo. Int J
Nanomedicine. 11:2235–2250. 2016.PubMed/NCBI
|
|
39
|
Xu J, Sun Y, Yuan Z, Bao Y, Li R, Liu C,
Qiu Y, Xu K, Shi X, Yu H, et al: Bufalin-loaded CaP/DPPE-PEG-EGF
nanospheres: Preparation, cellular uptake, distribution, and
anti-tumor effects. J Biomed Nanotechnol. 15:329–339. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen S, Zhang Y, Wang Z, Liu R and Gong X:
Bufalin induces the interplay between apoptosis and autophagy in
glioma cells through endoplasmic reticulum stress. Int J Biol Sci.
10:212–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiang RF, Wang Y, Zhang N, Xu WB, Cao Y,
Tong J, Li JM, Wu YL and Yan H: MK2206 enhances the cytocidal
effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR
pathway. Cell Death Dis. 8:e27762017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Huang Q, Wang X, Xu Y, Xu R, Han
M, Huang B, Chen A, Qiu C, Sun T, et al: Bufalin enhances
radiosensitivity of glioblastoma by suppressing mitochondrial
function and DNA damage repair. Biomed Pharmacother. 94:627–635.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Drewes G and Knapp S: Chemoproteomics and
chemical probes for target discovery. Trends Biotechnol.
36:1275–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Klaeger S, Heinzlmeir S, Wilhelm M, Polzer
H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C,
et al: The target landscape of clinical kinase drugs. Science.
358:eaan43682017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bantscheff M, Eberhard D, Abraham Y,
Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau
C, et al: Quantitative chemical proteomics reveals mechanisms of
action of clinical ABL kinase inhibitors. Nat Biotechnol.
25:1035–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Seeger RC, Brodeur GM, Sather H, Dalton A,
Siegel SE, Wong KY and Hammond D: Association of multiple copies of
the N-myc oncogene with rapid progression of neuroblastomas. N Engl
J Med. 313:1111–1116. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Biedler JL and Spengler BA: Metaphase
chromosome anomaly: Association with drug resistance and
cell-specific products. Science. 191:185–187. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Biedler JL, Roffler-Tarlov S, Schachner M
and Freedman LS: Multiple neurotransmitter synthesis by human
neuroblastoma cell lines and clones. Cancer Res. 38:3751–3757.
1978.PubMed/NCBI
|
|
49
|
Liu T, Yuan X, Jia T, Liu C, Ni Z, Qin Z
and Yuan Y: Polymeric prodrug of bufalin for increasing solubility
and stability: Synthesis and anticancer study in vitro and in vivo.
Int J Pharm. 506:382–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Q and Liu J: Transferrin and folic
acid co-modified bufalin-loaded nanoliposomes: Preparation,
characterization, and application in anticancer activity. Int J
Nanomedicine. 13:6009–6018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shi XJ, Qiu YY, Yu H, Liu C, Yuan YX, Yin
PH and Liu T: Increasing the anticancer performance of bufalin
(BUF) by introducing an endosome-escaping polymer and
tumor-targeting peptide in the design of a polymeric prodrug.
Colloids Surf B Biointerfaces. 166:224–234. 2018. View Article : Google Scholar : PubMed/NCBI
|