Introduction
Hashimoto's thyroiditis (HT) is a common
organ-specific autoimmune thyroid disease characterized by
autoantibodies against thyroid tissue and lymphocytic infiltration
in the thyroid parenchyma, resulting in the progressive destruction
and atrophy of thyrocytes and a diffusely enlarged thyroid gland,
which eventually evolves into hypothyroidism (1,2).
HT was initially described by Dr Hakaru Hashimoto in 1912 as a new
type of lymphomatous thyroid tumor in Japan (3), and was recognized as an autoimmune
disease of the thyroid several years later (4). HT is currently the most frequently
occurring autoimmune disease, with an incidence of ~1% per year
(5). According to National Health
and Nutrition Examination Survey of the United States, the
prevalence of HT is estimated to be 5% (6). HT is more common in women compared
with men, and in countries with iodine deficiency (7). The serological features of patients
with HT are characterized by elevated production of
anti-thyroglobulin-Ab (TgAb) and anti-thyroperoxidase-Ab (TPOAb)
(8,9). Additionally, some patients have high
concentrations of thyrotropin (10). HT is currently considered a
complex and continuously expanding disease caused by genetic
susceptibility, environmental factors, age, sex and immune
disorders (11). However, the
pathogenesis of HT remains unknown.
Long non-coding RNAs (lncRNAs) have been recently
recognized as a new class of non-coding RNAs >200 nucleotides in
size (12,13). lncRNAs are shorter than mRNA, have
low evolutionary sequence conservation and are highly prevalent in
the eukaryotic transcriptome (14). Based on various types of
transcriptional regulation and the position in the genome relative
to the protein-coding genes and enhancer regulatory elements,
lncRNAs have been classified as intronic lncRNAs, antisense
lncRNAs, long intergenic ncRNAs, enhancer RNAs and transcribed
pseudogene lncRNAs (15).
Multiple regulatory mechanisms of lncRNAs have been reported to
influence transcription and post-transcriptional events, as well as
the translation of diverse individual genes and the whole gene
networks (16). The regulatory
mechanisms of lncRNA vary depending on their location in the
nucleus or cytoplasm (17).
Numerous studies have reported that lncRNAs serve key roles in
pathogenesis of various diseases, including cancer, cardiovascular
diseases, nervous system disease and immune diseases (18-21). Certain lncRNAs are involved in
autoimmune diseases, such as multiple sclerosis, rheumatoid
arthritis (RA) and systemic lupus erythematosus (SLE) (22-24). However, the expression profiles
and function of lncRNAs in patients with HT are yet to be
elucidated.
The present study aimed to identify lncRNAs in the
peripheral blood mononuclear cells (PBMCs) from patients with HT
and to investigate the potential role of lncRNAs in the
pathogenesis of HT using next generation sequencing (NGS). Using
this approach, the present study aimed to elucidate the mechanism
of lncRNAs and to identify the potential diagnostic value of
lncRNAs in patients with HT.
Materials and methods
Subjects and samples
Patients with HT usually have no obvious clinical
symptoms at the early stage, and most only identify the elevated
serum levels of TgAb and TPOAb via physical examination. To obtain
a definitive diagnosis, strict inclusion criteria are required. The
samples were collected between December 2017 and May 2018 at The
Affiliated People's Hospital of Jiangsu University. In total, 33
patients with HT were enrolled in this study. Among them, five
female patients with HT were used for NGS. Moreover, 28 patients
with HT, including 21 women and seven men, were used to verify the
sequencing results. Their ages ranged from 27-75. The main clinical
characteristics of these patients are listed in Table I.
 | Table IClinical features of patients with HT
and healthy controls. |
Table I
Clinical features of patients with HT
and healthy controls.
| Variable | Patients with
HT | Healthy
controls | Range | P-values |
|---|
| Number | 28 | 27 | | |
| Sex (M/F) | | | | |
| Male | 7 | 14 | | |
| Female | 21 | 13 | | |
| Age, years | 41±13 | 35±9 | | 0.06 |
| FT3, pmol/l | 5.36±0.65 | 5.41±0.53 | 3.28-6.47 | 0.77 |
| FT4, pmol/l | 9.72±1.81 | 10.41±1.29 | 7.64-16.03 | 0.11 |
| TSH, uIU/ml | 4.95±4.54 | 1.73±0.54 | 0.56-5.91 | <0.01 |
| TgAb, IU/ml | 195.1±247.6 | 0.4±0.6 | 0-4 | <0.01 |
| TPOAb, IU/ml | 709.9±536.2.5 | 0.9±1.3 | 0-9 | <0.01 |
All patients were diagnosed based on clinical
manifestations and auxiliary examinations, including B-flow
ultrasonic imaging and laboratory criteria. The serum
concentrations of free triiodothyronine (3.28-6.47 pmol/l), free
thyroxine (FT4; 7.64-16.03 pmol/l), thyroid stimulating hormone
(TSH; 0.56-5.91 uIU/ml), TgAb (0-4 IU/ml) and TPOAb (0-9 IU/ml)
were measured using an LDX-800 system (Beckman Coulter, Inc.)
according to the manufacturer's instructions. All patients had
positive tests for TgAb or TPOAb. A total of nine patients with HT
and hypothyroidism had high levels of TSH with or without low
levels of FT4, and other patients with euthyroid had physiological
levels of TSH. In total, 32 age- and sex-matched healthy subjects
were included as controls, including five healthy female volunteers
for NGS and 27 healthy volunteers for validation of the sequencing
results. All healthy subjects were euthyroid and free of
thyroid-specific autoantibodies, as well as had no history of
thyroid disease, chronic inflammatory diseases, tumors and other
autoimmune diseases (25). All
subjects showed no obvious clinical symptoms of infection and were
not taking immunosuppressive drugs. The numbers of peripheral
leukocytes in all individuals were within the normal range
(3.50-9.50×109/l). After rigorous screening, five female
patients and five female volunteers were selected at random and
were involved in sequencing due to the significantly higher
incidence of HT in women (10).
The HT group criteria included: i) All patients with
HT were clinically diagnosed based on clinical manifestations and
auxiliary examinations; ii) there was no other autoimmune diseases
except HT in patients with HT; iii) all the individuals had not
recently taken immunosuppressive drugs for other diseases; iv) they
had not been infected recently; and v) the age ranged between
20-50. Healthy control group criteria were as follows: i) Thyroid
function or thyroid B-ultrasound were normal in healthy subjects;
ii) there was no occurrence of a recent infection; iii) all had no
autoimmune diseases and had not recently taken immunosuppressive
drugs; and iv) the age ranged between 20-50. Peripheral blood
samples (2 ml) were obtained from all patients and healthy
controls.
All protocols were approved by the Ethics Committee
of The Affiliated People's Hospital of Jiangsu University, and the
samples were collected at The Affiliated People's Hospital of
Jiangsu University after obtaining the written informed consent of
all subjects. All procedures described in this study were compliant
with the standard biosecurity and institutional safety procedures
(26).
Cell isolation and purification
Human PBMCs were isolated via density-gradient
centrifugation over Ficoll-Hypaque solution (Tianjin Haoyang
Biological Technology Co., Ltd.) according to the manufacturer's
instructions. The samples were centrifuged at 600 × g for 20 min
and the centrifugal temperature was maintained at 18-20°C. PBMCs
were stored at −80°C until use in lncRNA sequencing and reverse
transcription-quantitative (RT-q)PCR. Human PBMCs were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 5% CO2.
Library preparation for RNA and lncRNA
sequencing
The lncRNAs were derived from various genome
annotation projects, specialized lncRNA databases and
lncRNA-related literatures, including 'RefSeq' (https://www.ncbi.nlm.nih.gov/refseq/),
'UCSC_KnownGene' (http://genome.ucsc.edu/cgi-bin/hgGateway), 'Gencode'
(https://www.genco-degenes.org/),
'Ensembl' (http://asia.ensembl.org/index.html), 'lncRNAdb'
(https://lncipedia.org/), 'lncRNAdisease'
(http://www.cuilab.cn/lncrnadisease),
'Genbank' (https://www.uniprot.org/database/DB-0028), 'NRED'
(http://jsm-research.imb.uq.edu.au/nred/) and 'RNAdb'
(https://rnacentral.org/). Library preparation of
the RNA and high-throughput sequencing were performed by Cloud-Seq
Pte Ltd. A TruSeq stranded total RNA library prep kit (Illumina,
Inc.) was used for pretreatment of the RNA prior, extracted with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), to the construction of the sequencing library according to
the manufacturer's instructions. Quality control and quantitative
analysis of the libraries were performed using a Bio-Analyzer 2100
system (Agilent Technologies, Inc.). The 10 pM libraries were
desaturated into single-stranded DNA molecules, captured on
Illumina Flowcells (Illumina, Inc.) and amplified into clusters
in situ, which were sequenced for 150 cycles on an Illumina
Novaseq 6,000 sequencing instrument (Illumina, Inc.) in the
two-terminal mode (PE mode) according to the Illumina's
instructions.
lncRNA profiling analysis
Paired-end reads were obtained from an Illumina
Novaseq 6000 sequencing instrument, and quality control was
performed using Q30 (27). High
quality reads were obtained using Cut-adapt software (v1.9.3) to
remove low quality reads for the analysis of lncRNAs (28). High quality reads were
subsequently compared with the human reference genome (UCSC HG19)
using Hisat2 software (v2.0.4) (http://ccb.jhu.edu/software/hisat2/index.shtml). The
fragments per kilobase of exon per million fragments mapped (FPKM)
values of lncRNAs, as the lncRNA expression profiles in PBMCs of
patients with HT and healthy volunteers, were calculated using
Cuffdiff software (v2.2.1) (29),
as described in the GTF gene annotation file (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.13/).
Multiple changes and P-values of statistical indicators between the
experimental and control groups were calculated to screen for
lncRNAs with significantly different expression levels (fold change
≥2.0 and P-value <0.05).
Gene Ontology (GO) (http://www.geneontology.org) and Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway (https://www.genome.jp/kegg/) analyses of
differentially expressed lncRNA-associated genes were performed to
predict the potential function of lncRNAs (30,31).
RNA extraction and RT-qPCR
Total RNA was isolated from PBMCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) at 4°C for 15 min according to the manufacturer's
instructions. RNA concentrations were measured using a Nano-Drop
ND-1000 instrument (Thermo Fisher Scientific, Inc.). The quality of
RNA was determined via the optical density 260/280 ratio. RNA
integrity was measured via 1% agarose gel electrophoresis. RT was
performed to synthesize the cDNA with random primers in a
ReverTraAca® RT-qPCR kit (Toyobo Life Science), which
contained oligo-dT and random primers, according to the
manufacturer's instructions. RT-qPCR was performed in triplicate
using TB GreenTM Premix Ex Taq II (Takara Bio, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 3 min, followed by 40 cycles at 95°C for 12 sec, 62°C for
40 sec, and 72°C for 32 sec. The primer sequences are presented in
Table SI. The expression level
of each gene was normalized to the endogenous expression level of
the β-actin transcript and was calculated using 2−∆∆Cq
method (32). The data were
analyzed using Applied Biosystems 7500 Manager software v2.3
(Thermo Fisher Scientific, Inc.).
Small interfering (si)RNA
transfection
siRNA (Guangzhou RiboBio Co., Ltd.) was designed to
target the sequence of lncRNA-XLOC_I2_006631. A non-specific
scrambled siRNA was used as a negative control (NC). The siRNA
sequences were as follows: siRNA1, 5′-TTCTCCATAGAGATTTCGG-3′;
siRNA2, 5′-CTTGCATAGCTGAGCAACC-3′; and siRNA3,
5'-GCCTCTCAACAACCTCTTT-3′. The isolated human PBMCs were
transfected with the lncRNA-XLOC_ I2_006631 siRNA or NC at 100 nM
concentration using the Entranster-R reagent (Engreen Biosystem,
Co., Ltd.), according to the manufacturer's instructions in the
presence of 0.5 μg/ml functional anti-human CD3 Ab (cat. no.
300310; 20 μg/ml) and 2 μg/ml functional anti-human
CD28 Ab (cat. no. E-AB-F 11950; 0.5 mg/ml; Miltenyi Biotec GmbH).
After incubation at 37°C in 5% CO2 for 24 h, the PBMCs
were used for RT-qPCR.
Statistical analysis
GraphPad Prism version 5 software (GraphPad
Software, Inc.) was used to manage and analyze the data. A
Student's unpaired t-test was used for comparisons of two groups
when variables passed the normal distribution test. Mann-Whitney U
test was performed to analyze the differences between the two
groups of non-normally distributed data. One-way ANOVA test was
used for comparisons of multiple groups. Tukey's test was applied
for pair-to-pair comparisons of multiple groups after ANOVA.
Correlation between the variables was determined using the
Pearson's correlation coefficient. Receiver operating
characteristic (ROC) curve and the area under ROC curve (AUC) were
generated based on the logistic regression model. Sensitivity was
used as the Y-axis to represent true positive rate, while
100%-specificity% was used as the X-axis to represent false
positive rate. All experiments were performed three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
lncRNAs expression profiles in patients
with HT
NGS was performed to determine lncRNAs (GEO ID:
GSE156468) in the samples. Hierarchical clustering analysis
identified that lncRNA expression patterns in PBMCs were different
between the HT and control groups, with red and green representing
upregulated and downregulated lncRNAs, respectively (Fig. 1A). Volcano plots (Fig. 1B) and scatter plot (Fig. 1C) were used to identified
significantly dysregulated lncRNAs. A 2-fold expression difference
was used as a cutoff, it was found that the lncRNA expression
profiles in the HT group were distinctly different from those in
the control group. A total of 97,286 known lncRNAs were identified
in PBMCs of patients with HT and healthy controls, including 69,544
upregulated and 27,742 downregulated lncRNAs. A total of 218 novel
lncRNAs, including 94 upregulated and 124 downregulated, were
verified as having significantly aberrant expression (fold change
≥2; P<0.05; Fig. 1D).
Additionally, the 218 identified lncRNAs were divided into six
different categories according to the position of lncRNA in the
genome. Intergenic lncRNAs accounted for the largest proportion of
38.99% (85/218), exonic sense-overlapping lncRNAs accounted for
27.06% (59/218), intronic sense-overlapping lncRNAs accounted for
6.88% (15/218), natural antisense lncRNAs accounted for 7.8%
(17/218), intronic antisense lncRNAs accounted for 9.17% (20/218)
and bidirectional lncRNAs accounted for 10.09% (22/218) (Fig. 1E). These results were used in the
initial analysis of the sequencing data and were subsequently
validated by expanding the sample size.
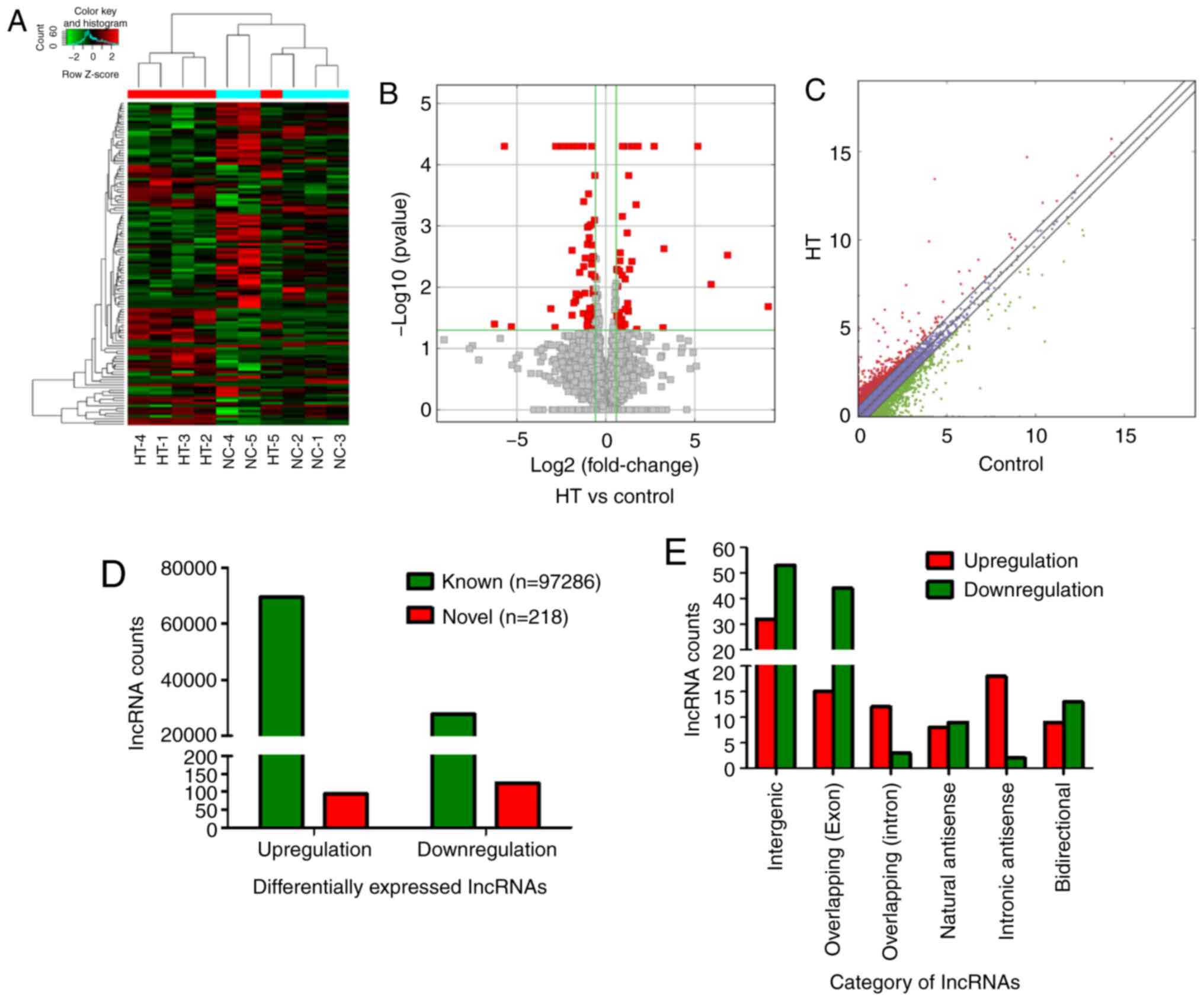 | Figure 1lncRNA expression profiles in
patients with HT. PBMCs of five patients with HT and five healthy
volunteers (controls) were enrolled into next-generation
high-throughput sequencing. (A) Hierarchical clustering analysis of
differentially expressed lncRNAs. Red and green represented
upregulated and downregulated lncRNAs, respectively. (B) Volcano
plots and (C) scatter plots were used to distinguish differentially
expressed lncRNAs. The red squares of volcano plots represented
statistically significant dysregulated lncRNAs. The red dots above
the diagonal line in the middle of the scatter plot indicated
significantly upregulated lncRNAs, and the green dots below
represented significantly downregulated lncRNAs. (D) A total of
97,286 known differentially expressed lncRNAs, including 69,544
upregulated and 27,742 downregulated lncRNAs, were identified.
Moreover, 218 novel lncRNAs were identified, 94 lncRNAs were
significantly upregulated and 124 lncRNAs were significantly
downregulated. (E) Number of upregulated (red) and downregulated
(green) lncRNAs according to the categories of formation mode. HT,
Hashimoto's thyroiditis; lncRNA, long non-coding RNA. |
GO and KEGG pathway analysis of
differentially expressed lncRNAs in patients with HT
GO and KEGG analyses were performed to determine the
potential biological functions of dysregulated lncRNAs. GO
enrichment analysis is composed of biological process (BP),
cellular component (CC) and molecular function (MF). A total of 257
GO enrichment items, including 128 upregulated and 129
downregulated lncRNAs, were counted (Fig. S1A). The top 10 GO enrichment
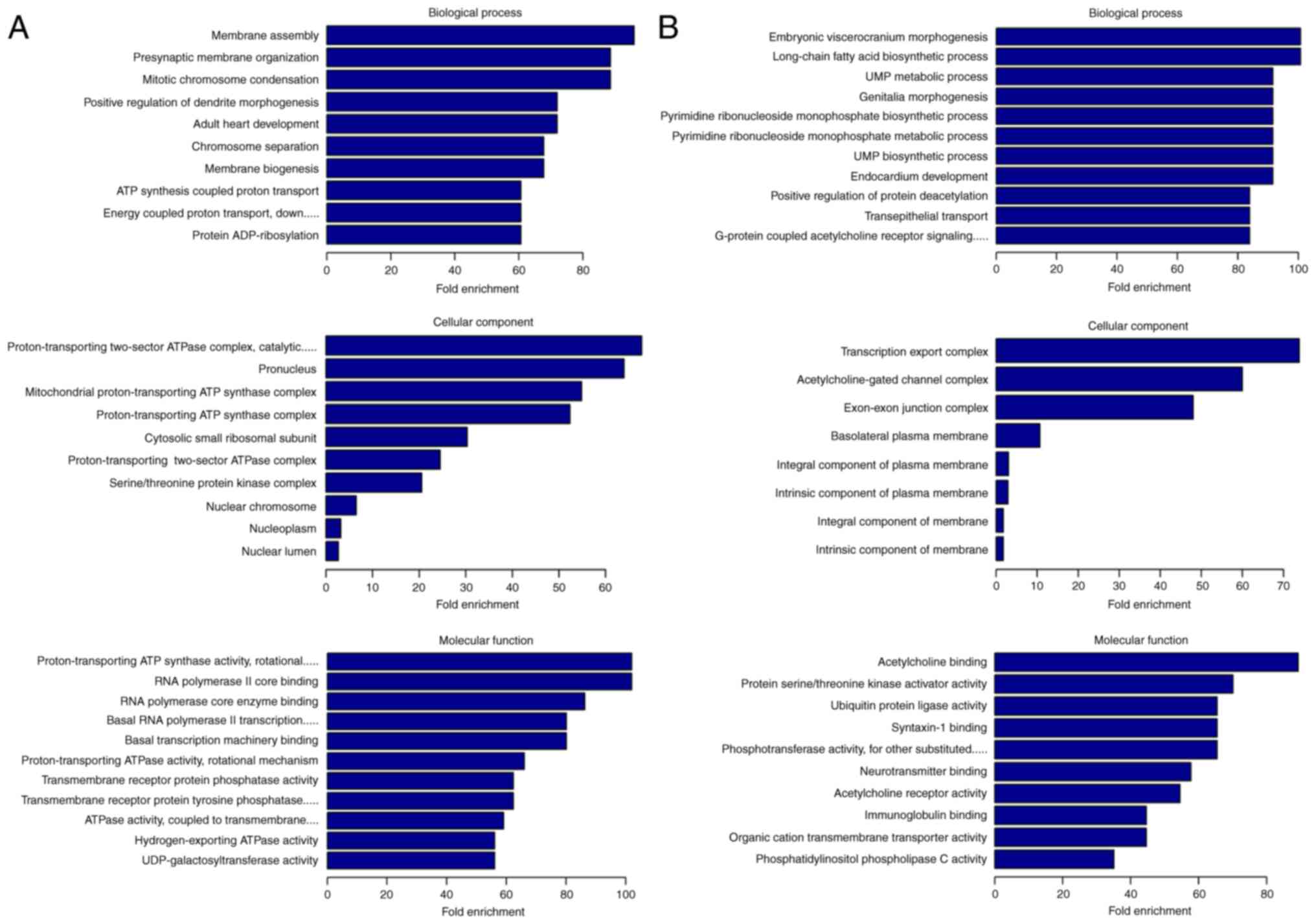
terms are presented in Fig. 2.
The most prominent GO enrichment terms of upregulated lncRNAs were
'membrane assembly' in BP, 'proton-transporting two-sector ATPase
complex, catalytic domain' in CC and 'proton-transporting ATP
synthase activity, rotational mechanism' in MF (Fig. 2A). Moreover, the most prominent GO
enrichment terms of downregulated lncRNAs were 'embryonic
viscerocranium morphogenesis' in BP, 'transcription export complex'
in CC (A total of eight terms) and 'acetylcholine binding' in MF
(Fig. 2B).
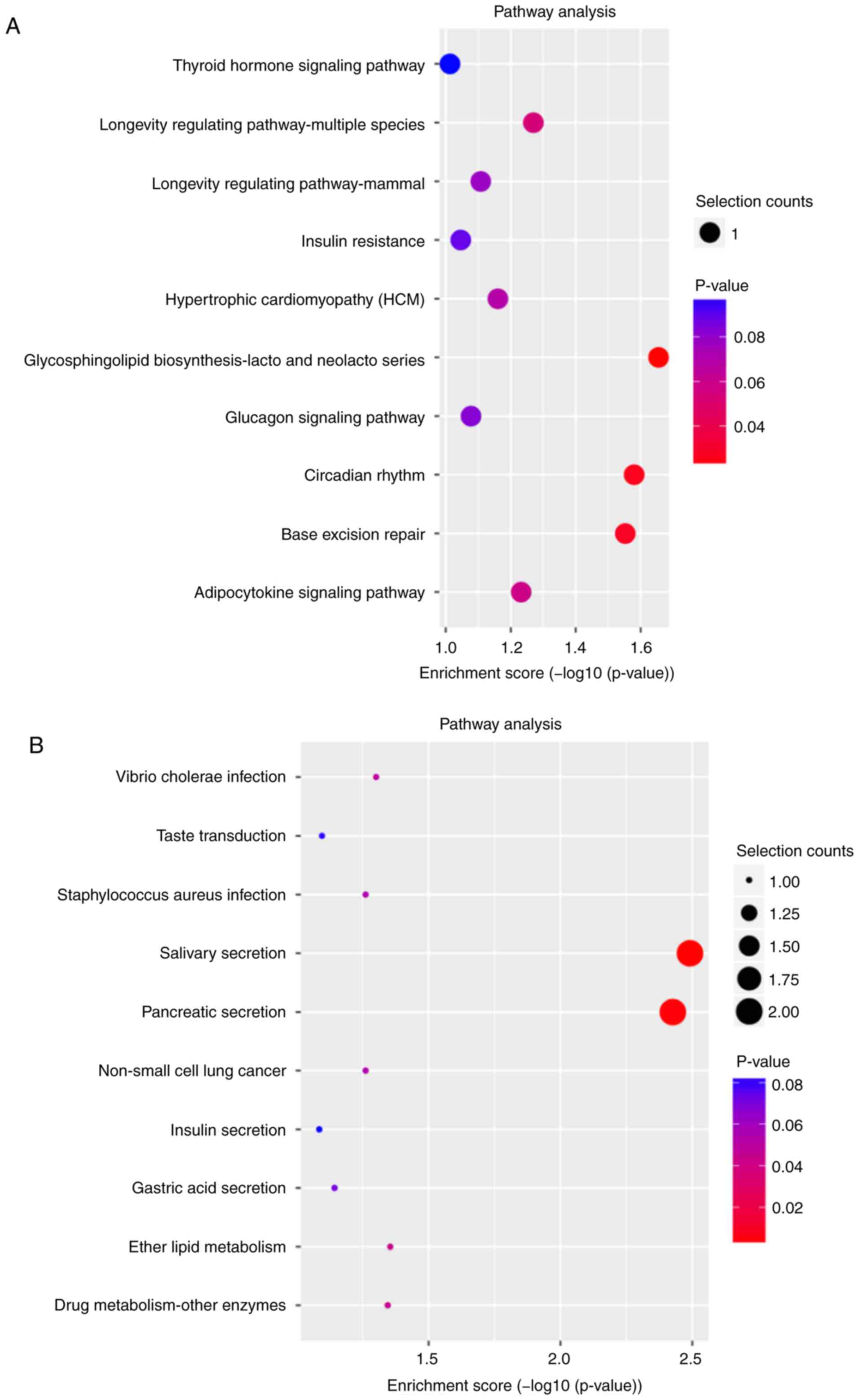
According to the KEGG classification, 17 signaling
pathways were associated with potential target genes of upregulated
lncRNAs (Fig. S1B), including
'thyroid hormone signaling pathway' (Fig. S2A), and 19 signaling pathways
were associated with downregulated lncRNAs (Fig. S1B), including 'calcium-regulated
signaling pathway' (Fig. S2B).
The top 10 KEGG pathways of dysregulated lncRNAs were shown in
Fig. 3A (upregulated lncRNAs) and
Fig. 3B (downregulated lncRNAs).
Among the 36 signaling pathways, 'longevity regulating
pathway-mammal', 'non-alcoholic fatty liver disease' and
'adipocytokine signaling pathway' (Fig. S3) were involved in the regulation
of the NF-κB, PI3K-Akt, TGF-β, MAPK and JAK-STAT signaling
pathways, which have been reported to participate in the
pathogenesis of HT (33-37).
Validation of selected lncRNAs via
RT-qPCR
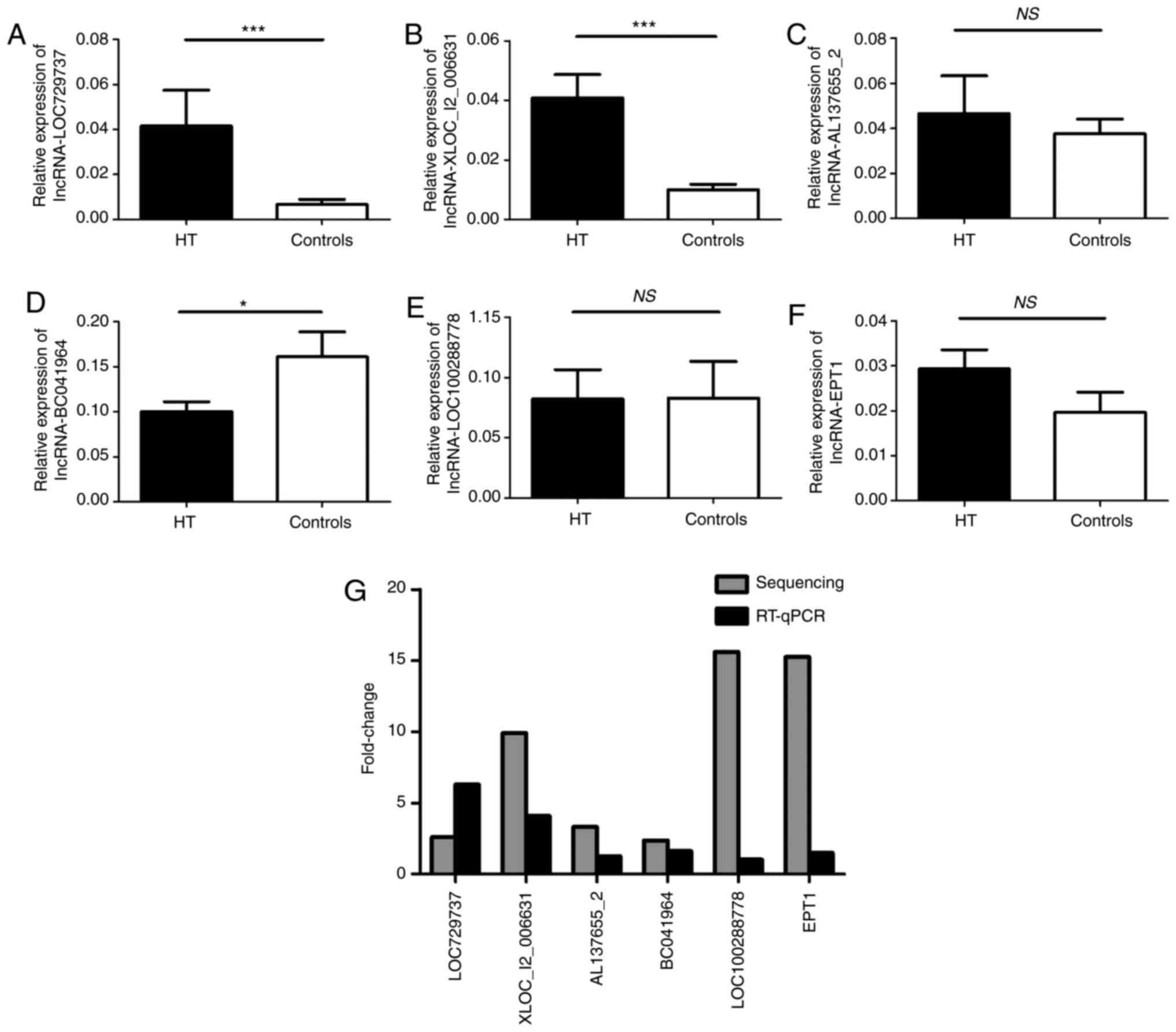
To confirm the NGS data, six differentially
expressed lncRNAs, including three upregulated [XLOC_I2_006631
(P=0.0051), AL137655_2 (P=0.0055) and LOC729737 (P=0.0053)] and
three down-regulated [LOC100288778 (P=0.0378), EPT1 (P=0.0181) and
BC041964 (P=0.0010)], were selected based on the differential
expression multiplier (fold change ≥2; P<0.05), the FPKM in ≥1
group (FPKM ≥0.5) and the abnormal uniform expression in each HT
sample.
These lncRNAs may be involved in the regulation of
potential target genes associated with autoimmune thyroid disease.
RT-qPCR was performed to verify the differences in selected lncRNAs
in PBMCs from 28 patients with HT and 27 healthy volunteers. The
results of the assays of five lncRNAs (LOC729737, XLOC_I2_006631,
AL137655_2, BC041964 and LOC100288778) were consistent with the NGS
data (Fig. 4A-E). However, only
lncRNA LOC729737, XLOC_I2_006631 and BC041964 expression levels had
statistically significant differences. The results of EPT1 were
inconsistent with the trend present in the sequencing data
(Fig. 4F). The fold changes of in
the expression of six selected lncRNAs in RT-qPCR and sequencing
are presented in Fig. 4G. The
results indicated that validation via RT-qPCR and sequencing
results were inconsistent with regard to the magnitude of changes
and significance.
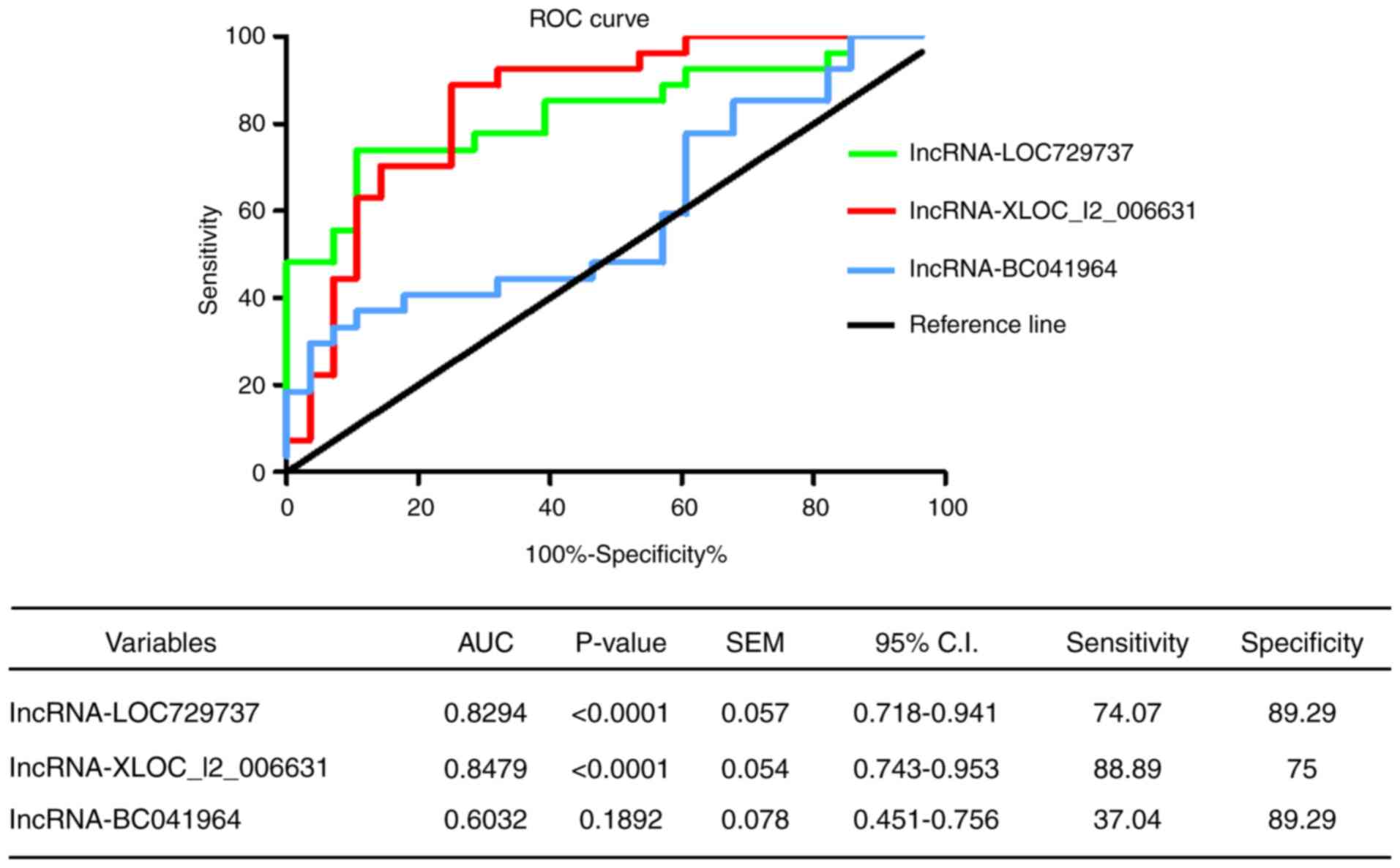
ROC curve analysis of confirmed
lncRNAs
The double-blind method was used in the experiments.
ROC curve analysis was used to evaluate the potential diagnostic
value of lncRNAs in the peripheral blood of patients with HT. The
AUC of lncRNA-XLOC_I2_006631 was 0.8479 (95% CI=0.743-0.953;
P<0.0001), and the sensitivity, specificity, likelihood ratio
and Jorden index were 88.89%, 75.00%, 3.56 and 0.64, respectively.
The AUC of lncRNA-LOC729737 was 0.8294 (95% CI=0.718-0.941;
P<0.0001), and the sensitivity, specificity, likelihood ratio
and Jorden index were 74.07%, 89.29%, 6.91 and 0.63, respectively.
The AUC of lncRNA-BC041964 was 0.6032 (95% CI=0.451-0.756;
P=0.1892), and the sensitivity, specificity, likelihood ratio and
Jorden index were 37.04%, 89.29%, 3.46 and 0.26, respectively
(Fig. 5). These data suggested
that lncRNA-XLOC_I2_006631 and lncRNA-LOC729737 could potentially
be used to differentiate patients with HT from healthy controls
and, thus, may be suitable biomarkers of HT.
Potential prediction of lncRNAs target
genes
Prediction of the target genes was performed to
evaluate the potential functions of validated lncRNAs in HT and to
determine whether they can participate in the regulation of gene
expression. lncRNA-LOC729737, lncRNA-XLOC_I2_006631 and
lncRNA-BC041964 were selected for these studies due to the
consistency of the results between the NGS and validation data.
Based on the list of the potential target genes associated with the
pathogenesis of HT, the potential target gene of lncRNA-LOC729737
was predicted to be STAT3; the potential target gene of
lncRNA-XLOC_I2_006631 was predicted to be MECP2 and the potential
target gene of lncRNA-BC041964 was predicted to be IL-21R. To
confirm these findings, the transcript expression levels of MECP2,
STAT3 and IL-21R were determined via RT-qPCR. The mRNA expression
of MECP2 was increased in PMBCs of patients with HT compared with
that in the healthy controls (Fig.
6A).
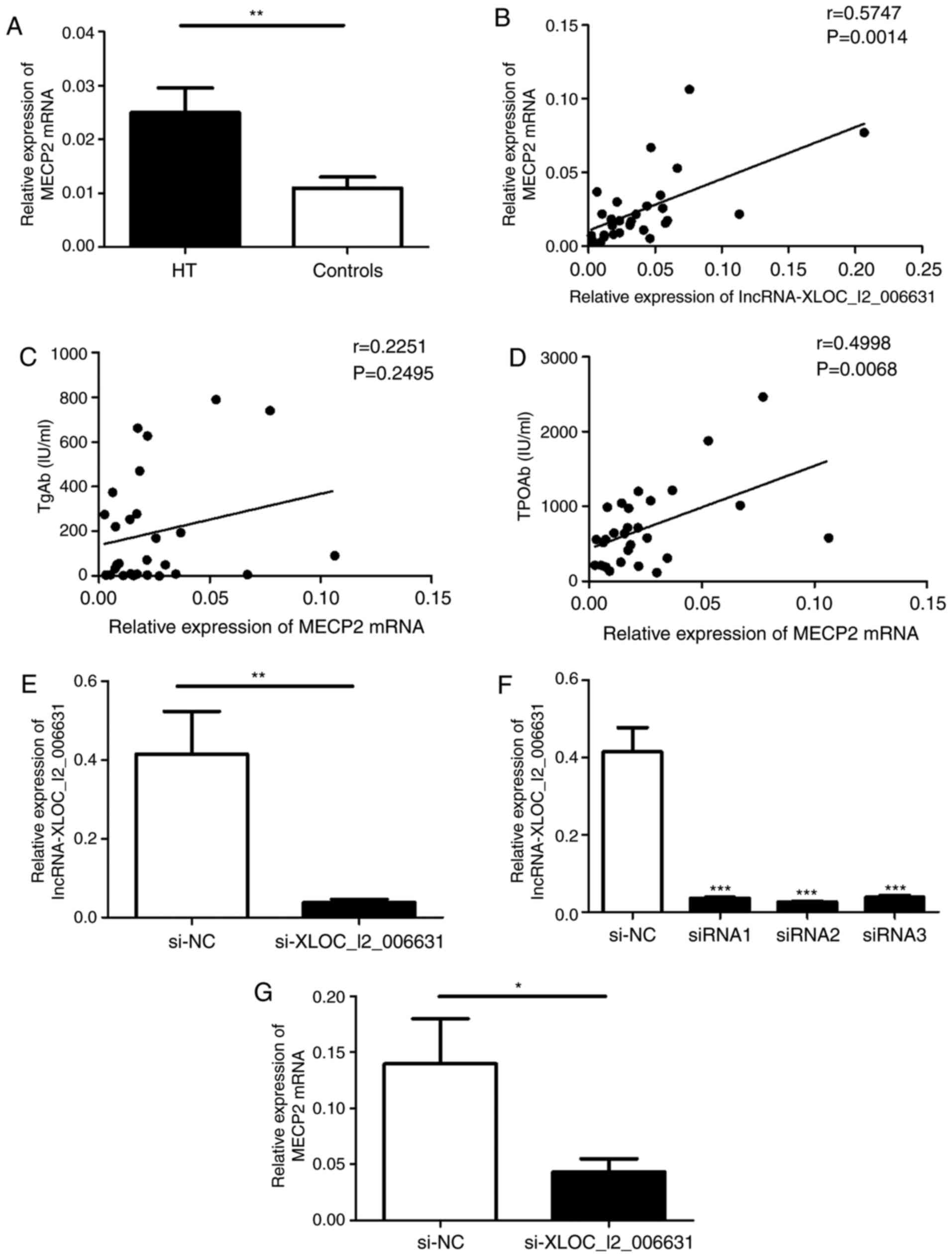 | Figure 6Potential prediction of lncRNAs
target genes and the effect of lncRNA-XLOC_I2_006631 on the
transcription of MECP2. (A) Relative expression of MECP2 mRNA in
PBMCs from patients with HT and healthy controls was detected via
RT-qPCR. (B) Correlation between the transcript levels of MECP2 and
lncRNA-XLOC_I2_006631 in patients with HT. The correlations between
the transcript levels of MECP2 and the serum concentrations of (C)
TgAb and (D) TPOAb. (E) Purified PBMCs was transfected with
lncRNA-XLOC_I2_006631-specific siRNAs and NC (100 nM) in the
presence of functional anti-human CD3 mAb and anti-human CD28 mAb.
(F) Transcript levels of lncRNA-XLOC_I2_006631 were detected via
RT-qPCR after transfection with lncRNA-XLOC_I2_006631-siRNA1-3 and
NC. (G) Transcript level of MECP2 was detected after transfection.
Each data point represents an individual subject, horizontal lines
represent the mean. *P<0.05, **P<0.01,
***P<0.001. lncRNA, long non-coding RNA; HT,
Hashimoto's thyroiditis; RT-qPCR, reverse
transcription-quantitative PCR; siRNA, small interfering RNA; NC,
negative control; MECP2, Methyl-CpG-binding protein 2; TgAb,
anti-thyroglobulin-Ab; TPOAb, anti-thyroperoxidase-Ab. |
A moderate positive correlation was observed between
the transcript levels of MECP2 and the transcript levels of
lncRNA-XLOC_I2_006631 (r=0.5747; P=0.0014; Fig. 6B). Additionally, the transcript
levels of STAT3 were decreased in patients with HT (Fig. S4A), and there was no correlation
between the transcript levels of STAT3 and the transcript levels of
lncRNA-LOC729737 (r=0.3318; P=0.0846; Fig. S4B). The transcript levels of
IL-21R were increased in patients with HT compared with controls
(Fig. S4C). An inconsistent
relationship was detected between downregulated lncRNA-BC041964
expression and upregulated IL-21R expression (Fig. S4D); the expression levels of
these two RNA species followed an opposing trend, but demonstrated
a positive correlation (r=0.5681; P=0.0016; Fig. S4D). These data suggested that
lncRNA-XLOC_I2_006631 was associated with MECP2 expression in
patients with HT.
Effect of lncRNA-XLOC_I2_006631 on the
transcription of MECP2
Utilizing lncRNA database and genome annotation
configuration, a gene, XLOC_I2_006631 (transcript ID:
TCONS_I2_00012362), was identified to transcribe a spliced,
non-coding RNA transcript of 2,768 in length and was located in
chromosome 19:18133183-18144152. According to the position compared
with the protein coding gene in the genome, lncRNA-XLOC_I2_006631
belonged to the intergenic lncRNA.
To determine whether lncRNA-XLOC_I2_006631
influences MECP2 transcription in patients with HT, the
relationship between MECP2 expression and the levels of
autoantibodies was initially analyzed. The results indicated that
the elevated levels of MECP2 were moderately positively correlated
with the serum levels of TPOAb (r=0.4998; P=0.0068; Fig. 6D). However, there was no
correlation between the levels of MECP2 and the levels of TgAb in
patients with HT (r=0.2251; P=0.2495; Fig. 6C).
Subsequently, purified human PBMCs were transfected
with lncRNA-XLOC_I2_006631-specific siRNA and NC. Manipulation of
lncRNA-XLOC_I2_006631-specific siRNA resulted in a decrease in the
transcript levels of lncRNA-XLOC_I2_006631 compared with that in NC
group (Fig. 6E and F). The
expression of MECP2 was significantly declined in PBMCs transfected
with the lncRNA-XLOC_ I2_006631-specific siRNA compared with that
in NC (Fig. 6G). These results
suggested that lncRNA-XLOC_I2_006631 regulated the expression of
MECP2 in vitro.
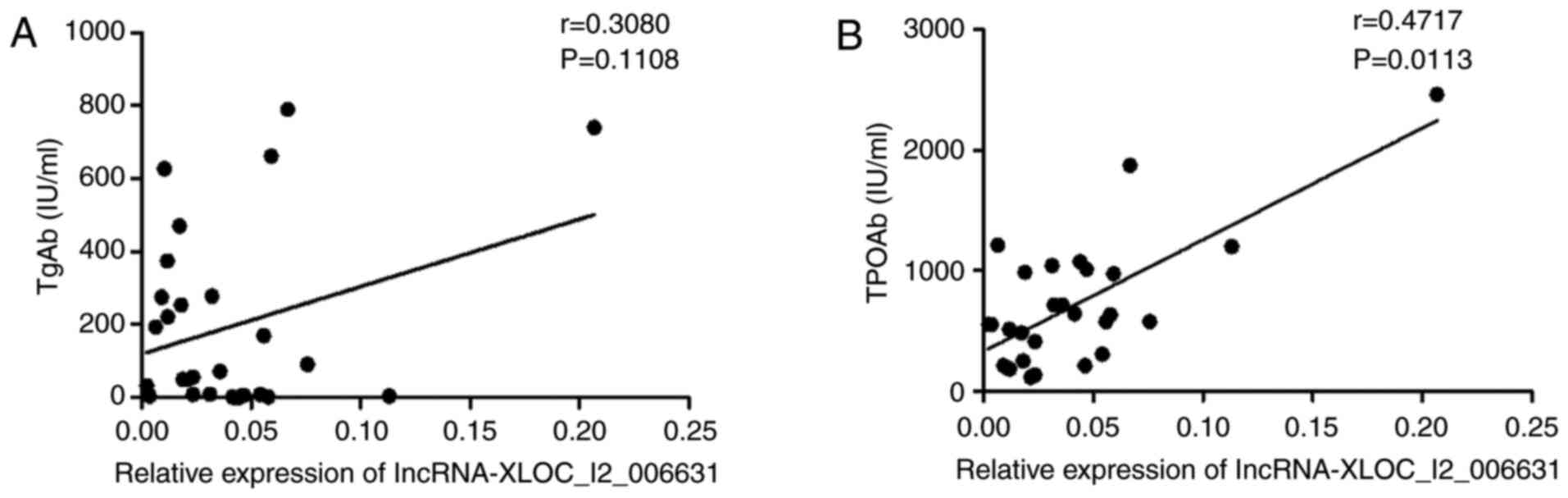
Increased expression of
lncRNA-XLOC_I2_006631 corre- lates with clinical severity of the
disease
TgAb and TPOAb are important antibodies for clinical
diagnosis of HT, and their levels can reflect the severity of the
disease (10). Correlations
between the expression of lncRNA-XLOC_I2_006631 and the serum
levels of TgAb or TPOAb were analyzed. A significant moderate
positive correlation between the expression of
lncRNA-XLOC_I2_006631 and the serum levels of TPOAb were detected
(r=0.4717; P=0.0113; Fig. 7B);
however, there was no correlation between the expression of
lncRNA-XLOC_I2_006631 and the serum levels of TgAb (r=0.3080;
P=0.1108; Fig. 7A). These data
indicated that dysregulated lncRNA-XLOC_I2_006631 expression was,
to a certain extent, associated with HT.
Discussion
Early symptoms of HT can be non-specific and are
easily confused with a number of other thyroid disorders, such as
simple goiter (38). Furthermore,
the diagnosis of HT requires a combination of clinical symptoms and
a variety of laboratory tests, including B-flow ultrasonic imaging
and indicators of thyroid function and autoantibodies, but there is
no single specific laboratory test that can be currently used to
diagnose HT (39). Hence,
understanding of the mechanisms of pathogenesis may lead to
identification of biomarkers that can predict the course of HT. The
present study focused on a class of intracellular regulatory
molecules, lncRNAs.
lncRNAs are increasingly recognized as important
versatile molecules involved in the regulation of DNA, proteins or
RNA (40). The mechanisms of
action of lncRNAs include modulation of adjacent protein-coding
genes in a cis or trans manner by binding to the promoter or
enhancer region of the target DNA based on recognition of specific
chromatin features, or by functioning as a bridge to connect the
DNA and regulatory elements (41). Additionally, lncRNAs can act as
precursor molecules of small RNAs or as 'sponges' for microRNAs
(miRs) (42). Numerous studies
have examined the roles of lncRNAs in the immune system. For
instance, HOTAIR acts as a scaffold to induce polycomb repressive
complex 2 to the target gene loci to suppress the transcription of
the HOXD gene by catalyzing histone H3 lysine 27 methylation
(43,44). Maternally expressed 3 has been
identified as a competing endogenous RNA of miR-17 that regulates
the level and function of Th17 cells in asthma (45). It has also been shown that
NONHSAT079547.2 could promote cell proliferation, as well as
control IL-17 expression and secretion via the miR-4716-5p/IL-17
axis in CD4+ T cells (25). lnc-DC regulates dendritic cells
differentiation by directly binging to STAT3 and promoting STAT3
phosphorylation (46). However,
the functional mechanism of lncRNAs in HT has not been fully
elucidated.
Previous studies have reported that the inflammatory
cells, including Th1 cells, Th17 cells and follicular helper T
cells, are significantly increased in the PBMCs from patients with
HT, and the proportion of regulatory T cells (Treg) is decreased in
the PBMCs from patients with HT (2,47,48). The present study aimed to
investigate the underlying causes of these unbalanced inflammatory
cells in the peripheral blood of patients with HT. Hence, human
PBMCs from patients with HT and healthy volunteers were selected
for lncRNAs sequencing. The present results demonstrated
differential expression profiles of lncRNAs in patients with HT and
healthy volunteers. A total of 218 significantly differentially
expressed lncRNAs, including 94 upregulated and 124 down-regulated
lncRNAs, were identified in PBMCs of patients with HT. To verify
the accuracy of the NGS data performed in a small set of samples,
six lncRNAs (XLOC_I2_006631, AL137655_2, LOC729737, LOC100288778,
EPT1 and BC041964) were selected for RT-qPCR. The data indicated
that five lncRNAs had a trend similar to the NGS results,
suggesting a certain accuracy of the NGS data. The differences of
lncRNA-XLOC_I2_006631, lncRNA-LOC729737 and lncRNA-BC041964 between
the HT and healthy groups were statistically significant. To
further investigate potential biological functions of these
lncRNAs, GO and KEGG analyses were subsequently performed to
examine the NGS data. The results indicated that 'activated T cell
proliferation', 'regulation of activated T cell proliferation',
'negative regulation of T cell proliferation' and 'immunoglobulin
binding' were the GO terms that may be involved in the occurrence
of HT. Additionally, the 'NF-kB', 'JAK-STAT', 'PI3K-Akt', 'TGF-β'
and 'MAPK signaling pathways' of the 36 relevant KEGG pathways are
involved in the mechanisms of HT (36,37,49-51). These data suggest the potential
biological functions of lncRNAs associated with HT.
Non-coding RNAs usually exert regulatory roles in
the organism; hence, the present study investigated the role of
validated lncRNAs in patients with HT. Prediction software was used
to identify the potential regulatory mRNAs of lncRNAs (LOC729737,
XLOC_I2_006631 and BC041964). The predicted mRNAs had to be
associated with HT. MECP2 was identified as the potential
regulatory gene of lncRNA-XLOC_I2_006631, and STAT3 was identified
as the potential target gene of lncRNA-LOC729737. It was also
demonstrated that lncRNA-BC041964 may regulate IL-21R.
MECP2 is a member of the MBD family and is a
pleio-tropic DNA binding protein that preferentially binds to
methylated cytosine-phosphate-guanine (CpG) and regulates the
expression of multiple methylation-sensitive genes, including T
lymphocytes overexpress CD70 (52), Foxp3 (53), secreted frizzled-related protein 4
(54) and patched 1 (55,56). MECP2 can selectively bind to
methylated DNA or interact with the histone deacetylase-containing
complexes, leading to two distinct epigenetic repression
mechanisms, including DNA methylation and histone deacetylation
(57). Moreover, MECP2 can
facilitate the transcription of genes by binding to unmethylated
CpG DNA or combining with cAMP-response element binding protein 1
in promoters (58). Previous
studies have revealed that MECP2 is involved in the pathogenesis of
various autoimmune diseases, such as RA, SLE and systemic sclerosis
(52,54,59). In RA, MECP2 exerts its role by
inhibiting secreted frizzled-related protein 4 expression, a
negative regulator of the canonical Wnt pathway (54). Research regarding SLE identified
the role of MECP2 in aberrant histone modifi-cations within the T
lymphocytes overexpress CD70 promoter in CD4+T cells
(52). Other studies have focused
on single nucleotide polymorphisms of the MECP2 gene in Sjögren's
syndrome and autoimmune thyroid diseases (AITD) (60,61). Therefore, it was hypothesized that
MECP2 may be associated with the pathogenesis of HT.
In the present study, MECP2 mRNA expression was
increased in PBMCs from patients with HT. Moreover, the transcript
levels of MECP2 were positively correlated with the serum levels of
TPOAb. There was also a significant positive correlation between
the elevated levels of lncRNA-XLOC_ I2_006631 and the transcript
levels of MECP2. Finally, transfection with
lncRNA-XLOC_I2_006631-specific siRNA resulted in a decline in MECP2
mRNA expression. Thus, the present results suggested that
lncRNA-XLOC_I2_006631 regulated the expression of MECP2 in patients
with HT. However, the underlying mechanism is yet to be elucidated,
and further investigations are required to expand on the current
findings.
Possible mechanisms of lncRNA-XLOC_I2_006631
involvement in HT were subsequently investigated in the present
study. The involvement of Treg in HT development may be one of the
possible mechanisms. Foxp3 is the master transcription factor of
Treg cells (62). MECP2 is a
central element of the upstream CpG-rich enhancer in the Foxp3 gene
that is methylated in naive CD4+T cells, activated
CD4+T cells and peripheral Treg cells (63). Importantly, induction of
demethylation of this CpG site by a DNA methyltransferase inhibitor
activates the upstream Foxp3 enhancer to induce Foxp3 expression
(64). Treg cells serve a key
role in autoimmune pathogenesis by maintaining immune homeostasis
and controlling activation of autoreactive CD4+T
effector cells (65,66). Our previous study reported that
the proportion of Treg cells and Foxp3 mRNA expression were
decreased in PBMCs from patients with HT (48). These data suggest that
lncRNA-XLOC_I2_006631 may be involved in the pathogenesis of HT by
regulating MECP2 expression. However, additional studies are
required.
STAT3 is the key transcription factor of Th17 cells,
which was identified as a new subset of CD4+T cells
involved in the pathogenesis of HT (64). A previous study revealed that
MECP2 was indispensable for the differentiation of Th17-mediated
pathologies by reinforcing STAT3 signaling in mice (67). The present study hypothesized that
the transcript levels of STAT3 should be consistent in Th17 cells,
whose proportion is increased in patients with HT. However, the
transcript levels of STAT3 were decreased in patients with HT. A
possible explanation for this phenomenon is that STAT3 functions
via phosphorylation regardless of its high or low levels in HT.
The IL-21/IL-21R signaling pathway is involved in
the development of AITD (68).
Interestingly, there was a positive correlation between the low
expression of lncRNA-BC041964 and high expression of IL-21R in
PBMCs from patients with HT in the current study. A positive
correlation suggests that two variables change in the same
direction, such as when the expression of IL-21R is downregulated
as the expression of lncRNA-BC041964 is downregulated in patients
with HT. However, the present data suggested that the expression of
IL-21R was upregulated in patients with HT. The correlation trend
and expression levels of lncRNA-BC041964 and IL-21R in patients
with HT were contradictory. Therefore, it was suggested that
lnc-BC041964 may participate in HT process via other target genes,
but this requires further investigation.
HT is an autoimmune disease characterized by
combined effects of multiple autoantibodies, and it is generally
accepted that elevated serum concentrations of TgAb and TPOAb are
the most impactful manifestations of HT (69). The present data demonstrated that
there was a positive correlation between lncRNA-XLOC_I2_006631
expression and serum concentrations of TPOAb. However,
lncRNA-XLOC_I2_006631 expression did not correlate with serum
concentrations of TgAb. Detection of TgAb and TPOAb is important in
HT; however, the levels of these antibodies are not specific in the
diagnosis of thyroid disease types (70). High concentrations of TgAb and
TPOAb can also be detected in other thyroid diseases, such as
Graves' disease and primary hypothyroidism (70,71). Moreover, thyroid autoantibodies
are present in a disease-free population (72). Hence, identification of novel
biomarkers for HT is important. Thus, the diagnostic value of
lncRNA-XLOC_I2_006631 in HT was investigated in the present study.
The findings indicated that the AUC of lncRNA-XLOC_I2_006631 in
PBMCs from patients with HT was 0.8479, and the sensitivity,
specificity, likelihood ratio, and Jorden index were 88.89%,
75.00%, 3.56, and 0.64, respectively. These data suggested that
lncRNA-XLOC_I2_006631 was in part associated with the disease
process of HT and may be as a potential biomarker, which in
combination with thyroid autoantibodies can improve the diagnosis
of HT.
The present study reports an intriguing phenomenon,
which provides a foundation for subsequent studies on the mechanism
and diagnostic value of lncRNAs in HT. However, there are certain
limitations to the current study. The validation cohort of
lncRNA-XLOC_I2_006631 expression was small. This study focused on
lncRNAs profiles in female patients with HT and did not examine
differences in lncRNAs profiles between the w and female
participants, potentially missing some lncRNAs signatures of male
vs. female participants. Additionally, the differences in lncRNAs
expression between various regions and ethnicities were not
examined. Larger cohorts of patients with HT are required in the
future studies to assess the possibility that lncRNA-XLOC_I2_006631
is a novel biomarker. In addition, the underlying mechanism and
specific functions of lncRNA-XLOC_I2_006631 in HT should be
examined using cellular and animal model experiments.
In conclusion, the present results demonstrate the
differential expression patterns of lncRNAs in patients with HT and
identified a significant increase in the expression of
lncRNA-XLOC_I2_006631. The relationship between
lncRNA-XLOC_I2_006631 and disease severity suggests that
lncRNA-XLOC_I2_006631 serves an important role in the development
of HT. Moreover, lncRNA-XLOC_I2_006631 may positively regulate
MECP2 expression. Overall, the present results indicated that
lncRNA-XLOC_I2_006631 may be involved in pathogenesis of HT via
regulation of MECP2. However, additional investigations are
required to confirm and expand these findings.
Supplementary Data
Acknowledgments
Not applicable.
Funding
This work was supported by National Natural Science
Foundation of China (grant nos. 81800698 and 81701616), Jiangsu
Provincial Key Medical Talents Project (grant nos. QNRC2016455 and
QNRC2016456), Jiangsu Province Fifth Phase '333' Project Training
Fund Support Project (grant no. BRA2018184) and the Zhenjiang
Science and Technology Planning Project (grant nos. SH2019045 and
SH2018051).
Availability of data and materials
The datasets generated for this study can be found
in the GEO/GSE156468, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE156468.
Authors' contributions
HP and SX performed the experiments, analyzed the
data and wrote the manuscript. XD, XW and LW helped with the
experiments and analyzed the data. XT participated in the design of
the experiments. YL conceived and designed the research, and
supervised all the work on this paper. All authors discussed the
results and commented on the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consents were obtained from all
subjects. All clinical sampling was performed with the approval of
the Ethics Committee of The Affiliated People's Hospital of Jiangsu
University (approval no. K-20180009-Y).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiong S, Peng H, Ding X, Wang X, Wang L,
Wu C, Wang S, Xu H and Liu Y: Circular RNA expression profiling and
the potential role of hsa_circ_0089172 in Hashimoto's thyroiditis
via sponging miR125a-3p. Mol Ther Nucleic Acids. 17:38–48. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu C, Ma J, Liu Y, Tong J, Tian J, Chen
J, Tang X, Xu H, Lu L and Wang S: Increased frequency of follicular
helper T cells in patients with autoimmune thyroid disease. J Clin
Endocrinol Metab. 97:943–950. 2012. View Article : Google Scholar
|
|
3
|
Hahsimoto H: Zur Kenntnis der
lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa).
Arch Klin Chir. 97:219–248. 1912.
|
|
4
|
Takami HE, Miyabe R and Kameyama K:
Hashimoto's thyroiditis. World J Surg. 32:688–692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanderpump MP, Tunbridge WM, French JM,
Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H,
Tunbridge F, et al: The incidence of thyroid disorders in the
community: A twenty-year follow-up of the whickham survey. Clin
Endocrinol (Oxf). 43:55–68. 1995. View Article : Google Scholar
|
|
6
|
Hollowell JG, Staehling NW, Flanders WD,
Hannon WH, Gunter EW, Spencer CA and Braverman LE: Serum TSH, T(4),
and thyroid antibodies in the United States population (1988 to
1994): National health and nutrition examination survey (NHANES
III). J Clin Endocrinol Metab. 87:489–499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penta L, Cofini M, Lanciotti L, Leonardi
A, Principi N and Esposito S: Hashimoto's disease and thyroid
cancer in children: Are they associated? Front Endocrinol
(Lausanne). 9:5652018. View Article : Google Scholar
|
|
8
|
Takasu N and Yoshimura Noh J: Hashimoto's
thyroiditis: TGAb, TPOAb, TRAb and recovery from hypothyroidism.
Expert Rev Clin Immunol. 4:221–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng H, Liu Y, Tian J, Ma J, Tang X, Yang
J, Rui K, Zhang Y, Mao C, Lu L, et al: Decreased expression of
microRNA-125a-3p upregulates interleukin-23 receptor in patients
with Hashimoto's thyroiditis. Immunol Res. 62:129–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caturegli P, De Remigis A and Rose NR:
Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun
Rev. 13:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu S and Rayman MP: Multiple nutritional
factors and the risk of Hashimoto's thyroiditis. Thyroid.
27:597–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu F, Jin L, Jin Y, Nie Z and Zheng H:
Long noncoding RNAs in autoimmune diseases. J Biomed Mater Res A.
107:468–475. 2019. View Article : Google Scholar
|
|
13
|
Tian X, Zheng Y, Yin K, Ma J, Tian J,
Zhang Y, Mao L, Xu H and Wang S: LncRNA AK036396 inhibits
maturation and accelerates immunosuppression of polymorphonuclear
myeloid-derived suppressor cells by enhancing the stability of
ficolin B. Cancer Immunol Res. 8:565–577. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Y, Tian X, Wang T, Xia X, Cao F,
Tian J, Xu P, Ma J, Xu H and Wang S: Long noncoding RNA Pvt1
regulates the immuno-suppression activity of granulocytic
myeloid-derived suppressor cells in tumor-bearing mice. Mol Cancer.
18:612019. View Article : Google Scholar
|
|
16
|
Zimmer-Bensch G: Emerging roles of long
non-coding rnas as drivers of brain evolution. Cells. 8:13992019.
View Article : Google Scholar
|
|
17
|
Zhang K, Shi ZM, Chang YN, Hu ZM, Qi HX
and Hong W: The ways of action of long non-coding RNAs in cytoplasm
and nucleus. Gene. 547:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Wu S, Zhu X, Zhang L, Deng J, Li
F, Guo B, Zhang S, Wu R, Zhang Z, et al: LncRNA-encoded polypeptide
ASRPS inhibits triple-negative breast cancer angiogenesis. J Exp
Med. 217:jem.201909502020. View Article : Google Scholar :
|
|
19
|
Lorenzen JM and Thum T: Long noncoding
RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol.
12:360–373. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Q, Han CL, Wang KL, Sui YP, Li ZB,
Chen N, Fan SY, Shimabukuro M, Wang F and Meng FG: Integrated
analysis of exosomal lncRNA and mRNA expression profiles reveals
the involvement of lnc-MKRN2-42:1 in the pathogenesis of
Parkinson's disease. CNS Neurosci Ther. 26:527–537. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Atianand MK, Caffrey DR and Fitzgerald KA:
Immunobiology of long noncoding RNAs. Annu Rev Immunol. 35:177–198.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moradi M, Gharesouran J, Ghafouri-Fard S,
Noroozi R, Talebian S, Taheri M and Rezazadeh M: Role of NR3C1 and
GAS5 genes polymorphisms in multiple sclerosis. Int J Neurosci.
130:407–412. 2020. View Article : Google Scholar
|
|
23
|
Moharamoghli M, Hassan-Zadeh V, Dolatshahi
E, Alizadeh Z and Farazmand A: The expression of GAS5, THRIL, and
RMRP lncRNAs is increased in T cells of patients with rheumatoid
arthritis. Clin Rheumatol. 38:3073–3080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang JB, Li J, Zhang TP, Lv TT, Li LJ, Wu
J, Leng RX, Fan YG, Pan HF and Ye DQ: Expression of several long
noncoding RNAs in peripheral blood mononuclear cells of patients
with systemic lupus erythematosus. Adv Med Sci. 64:430–436. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo J, Liu T and Teng W: LncRNA profile in
Hashimoto's thyroiditis and potential function of NONHSAT079547.2.
J Mol Endocrinol. 64:259–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilbert GL and Kerridge I: The politics
and ethics of hospital infection prevention and control: A
qualitative case study of senior clinicians' perceptions of
professional and cultural factors that influence doctors' attitudes
and practices in a large Australian hospital. BMC Health Serv Res.
19:2122019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Shen P, Zheng B, Yu W, Ji J and
Xiao Y: Comparative genomic analysis of 19 clinical isolates of
tigecycline-resistant acinetobacter baumannii. Front Microbiol.
11:13212020. View Article : Google Scholar :
|
|
28
|
Kechin A, Boyarskikh U, Kel A and
Filipenko M: CutPrimers: A new tool for accurate cutting of primers
from reads of targeted next generation sequencing. J Comput Biol.
24:1138–1143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar
|
|
31
|
Kanehisa M, Sato Y, Furumichi M, Morishima
K and Tanabe M: New approach for understanding genome variations in
KEGG. Nucleic Acids Res. 47(D1): D590–D595. 2019. View Article : Google Scholar :
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Liu J, Mao C, Dong L, Kang P, Ding C,
Zheng T, Wang X and Xiao Y: Excessive iodine promotes pyroptosis of
thyroid follicular epithelial cells in Hashimoto's thyroiditis
through the ROS-NF-KB-NLRP3 pathway. Front Endocrinol (Lausanne).
10:7782019. View Article : Google Scholar
|
|
34
|
Kotkowska A, Sewerynek E, Domanska D,
Pastuszak-Lewandoska D and Brzezianska E: Single nucleotide
polymorphisms in the STAT3 gene influence AITD susceptibility,
thyroid autoantibody levels, and IL6 and IL17 secretion. Cell Mol
Biol Lett. 20:88–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jankovic B, Le KT and Hershman JM:
Clinical review: Hashimoto's thyroiditis and papillary thyroid
carcinoma: Is there a correlation? J Clin Endocrinol Metab.
98:474–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stanilova SA, Gerenova JB, Miteva LD and
Manolova IM: The role of transforming growth factor-β1 gene
polymorphism and its serum levels in Hashimoto's thyroiditis. Curr
Pharm Biotechnol. 19:581–589. 2018. View Article : Google Scholar
|
|
37
|
Luo X, Zheng T, Mao C, Dong X, Mou X, Xu
C, Lu Q, Liu B, Wang S and Xiao Y: Aberrant MRP14 expression in
thyroid follicular cells mediates chemokine secretion through the
IL-1β/MAPK pathway in Hashimoto's thyroiditis. Endocr Connect.
7:850–858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thomas T, Sreedharan S, Khadilkar UN,
Deviprasad D, Kamath MP, Bhojwani KM and Alva A: Clinical,
biochemical & cytomorphologic study on Hashimoto's thyroiditis.
Indian J Med Res. 140:729–735. 2014.
|
|
39
|
Dias Lopes NM, Mendonca Lens HH, Armani A,
Marinello PC and Cecchini AL: Thyroid cancer and thyroid autoimmune
disease: A review of molecular aspects and clinical outcomes.
Pathol Res Pract. 216:1530982020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Satpathy AT and Chang HY: Long noncoding
RNA in hematopoiesis and immunity. Immunity. 42:792–804. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen YG, Satpathy AT and Chang HY: Gene
regulation in the immune system by long noncoding RNAs. Nat
Immunol. 18:962–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ballantyne MD, McDonald RA and Baker AH:
lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol
Ther. 99:494–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fang S, Shen Y, Chen B, Wu Y, Jia L, Li Y,
Zhu Y, Yan Y, Li M, Chen R, et al: H3K27me3 induces multidrug
resistance in small cell lung cancer by affecting HOXA1 DNA
methylation via regulation of the lncRNA HOTAIR. Ann Transl Med.
6:4402018. View Article : Google Scholar :
|
|
44
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiu YY, Wu Y, Lin MJ, Bian T, Xiao YL and
Qin C: LncRNA-MEG3 functions as a competing endogenous RNA to
regulate Treg/Th17 balance in patients with asthma by targeting
microRNA-17/RORүt. Biomed Pharmacother. 111:386–394. 2019.
View Article : Google Scholar
|
|
46
|
Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S,
Jiang Z, Xu J, Liu Q and Cao X: The STAT3-binding long noncoding
RNA lnc-DC controls human dendritic cell differentiation. Science.
344:310–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nanba T, Watanabe M, Inoue N and Iwatani
Y: Increases of the Th1/Th2 cell ratio in severe Hashimoto's
disease and in the proportion of Th17 cells in intractable Graves'
disease. Thyroid. 19:495–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu Y, Tang X, Tian J, Zhu C, Peng H, Rui
K, Wang Y, Mao C, Ma J, Lu L, et al: Th17/Treg cells imbalance and
GITRL profile in patients with Hashimoto's thyroiditis. Int J Mol
Sci. 15:21674–21686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng T, Xu C, Mao C, Mou X, Wu F, Wang X,
Bu L, Zhou Y, Luo X, Lu Q, et al: Increased interleukin-23 in
Hashimoto's thyroiditis disease induces autophagy suppression and
reactive oxygen species accumulation. Front Immunol. 9:962018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia N, Chen G, Liu M, Ye X, Pan Y, Ge J,
Mao Y, Wang H, Wang J and Xie S: Anti-inflammatory effects of
luteolin on experimental autoimmune thyroiditis in mice. Exp Ther
Med. 12:4049–4054. 2016. View Article : Google Scholar
|
|
51
|
Larson SD, Jackson LN, Riall TS, Uchida T,
Thomas RP, Qiu S and Evers BM: Increased incidence of
well-differentiated thyroid cancer associated with Hashimoto
thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg.
204:764–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou Y, Qiu X, Luo Y, Yuan J, Li Y, Zhong
Q, Zhao M and Lu Q: Histone modifications and methyl-CpG-binding
domain protein levels at the TNFSF7 (CD70) promoter in SLE CD4+ T
cells. Lupus. 20:1365–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vecellio M, Wu H, Lu Q and Selmi C: The
multifaceted functional role of DNA methylation in immune-mediated
rheumatic diseases. Clin Rheumatol. Jul 2–2020.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Miao CG, Huang C, Huang Y, Yang YY, He X,
Zhang L, Lv XW, Jin Y and Li J: MeCP2 modulates the canonical Wnt
pathway activation by targeting SFRP4 in rheumatoid arthritis
fibroblast-like synoviocytes in rats. Cell Signal. 25:598–608.
2013. View Article : Google Scholar
|
|
55
|
Sun ZH, Liu YH, Liu JD, Xu DD, Li XF, Meng
XM, Ma TT, Huang C and Li J: MeCP2 regulates PTCH1 expression
through DNA methylation in rheumatoid arthritis. Inflammation.
40:1497–1508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guy J, Cheval H, Selfridge J and Bird A:
The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 27:631–652.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Miao CG, Yang YY, He X and Li J: New
advances of DNA methylation and histone modifications in rheumatoid
arthritis, with special emphasis on MeCP2. Cell Signal. 25:875–882.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chahrour M, Jung SY, Shaw C, Zhou X, Wong
ST, Qin J and Zoghbi HY: MeCP2, a key contributor to neurological
disease, activates and represses transcription. Science.
320:1224–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Henderson J, Brown M, Horsburgh S, Duffy
L, Wilkinson S, Worrell J, Stratton R and O'Reilly S: Methyl cap
binding protein 2: A key epigenetic protein in systemic sclerosis.
Rheumatology (Oxford). 58:527–535. 2019. View Article : Google Scholar
|
|
60
|
Cobb BL, Fei Y, Jonsson R, Bolstad AI,
Brun JG, Rischmueller M, Lester SE, Witte T, Illei G, Brennan M, et
al: Genetic association between methyl-CpG binding protein 2
(MECP2) and primary Sjogren's syndrome. Ann Rheum Dis.
69:1731–1732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song RH, Qin Q, Yan N, Muhali FS, Meng S,
He ST and Zhang JA: Variants in IRAK1-MECP2 region confer
susceptibility to autoimmune thyroid diseases. Mol Cell Endocrinol.
399:244–249. 2015. View Article : Google Scholar
|
|
62
|
Nie J, Li YY, Zheng SG, Tsun A and Li B:
FOXP3(+) treg cells and gender bias in autoimmune diseases. Front
Immunol. 6:4932015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lal G, Zhang N, van der Touw W, Ding Y, Ju
W, Bottinger EP, Reid SP, Levy DE and Bromberg JS: Epigenetic
regulation of Foxp3 expression in regulatory T cells by DNA
methylation. J Immunol. 182:259–273. 2009. View Article : Google Scholar
|
|
64
|
Sopena F, Nerin JM, Prats E, Banzo J,
Ducons JA, López Zaborras J and Gomollón F: Gammagraphy with
labeled leukocytes as an activity and extension index of Crohn
disease. Rev Esp Enferm Dig. 79:387–392. 1991.In Spanish.
|
|
65
|
Li MO and Rudensky AY: T cell receptor
signalling in the control of regulatory T cell differentiation and
function. Nat Rev Immunol. 16:220–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dominguez-Villar M and Hafler DA:
Regulatory T cells in autoimmune disease. Nat Immunol. 19:665–673.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Jiang S, Li C, McRae G, Lykken E, Sevilla
J, Liu SQ, Wan Y and Li QJ: MeCP2 reinforces STAT3 signaling and
the generation of effector CD4+ T cells by promoting
miR-124-mediated suppression of SOCS5. Sci Signal. 7:ra252014.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guan LJ, Wang X, Meng S, Shi LF, Jiang WJ,
Xiao L, Shi XH, Xu J and Zhang JA: Increased IL-21/IL-21R
expression and its proinflammatory effects in autoimmune thyroid
disease. Cytokine. 72:160–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jia X, Zhai T and Zhang JA: Metformin
reduces autoimmune antibody levels in patients with Hashimoto's
thyroiditis: A systematic review and meta-analysis. Autoimmunity.
53:353–361. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Y, Teng D, Shan Z, Teng X, Guan H, Yu
X, Fan C, Chong W, Yang F, Dai H, et al: Antithyroperoxidase and
antithyroglobulin antibodies in a five-year follow-up survey of
populations with different iodine intakes. J Clin Endocrinol Metab.
93:1751–1757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Dong YH and Fu DG: Autoimmune thyroid
disease: Mechanism, genetics and current knowledge. Eur Rev Med
Pharmacol Sci. 18:3611–3618. 2014.PubMed/NCBI
|
|
72
|
Yan YR, Gao XL, Zeng J, Liu Y, Lv QG,
Jiang J, Huang H and Tong NW: The association between thyroid
autoantibodies in serum and abnormal function and structure of the
thyroid. J Int Med Res. 43:412–423. 2015. View Article : Google Scholar : PubMed/NCBI
|