Introduction
Acute kidney injury (AKI), a common complication
observed in critically ill patients admitted to the intensive care
unit, is characterized by a sharp and sustained decline in renal
function during a short period of time (1,2).
The occurrence of AKI was estimated to be 13 million cases
worldwide in 2016, and AKI results in ~1.7 million deaths per year
(3). Lipopolysaccharide (LPS), an
endotoxin from the outer membrane of gram-negative bacteria, has
been widely used to induce AKI-associated pathologies, including
inflammation and oxidative stress (OS) (4,5).
LPS has been reported to mediate both systemic and renal
inflammation through signaling by recognition of Toll-like receptor
4, which is present on immune and proximal tubule epithelial cells
(2). At present, there are no
targeted strategies for the treatment of AKI, and the underlying
mechanisms of development are incompletely understood. Therefore,
it is necessary to find effective drugs to treat AKI and understand
its molecular mechanisms.
Increasing evidence has demonstrated that
traditional Chinese medicine may prevent or ameliorate AKI
(6-8). Echinacea (Asteraceae) is
frequently utilized in North America and Europe to prevent and
treat the common cold (9). Root
preparations of Echinacea purpurea (E. purpurea) and
E. angustifolia have been applied to promote wound healing
and treat burns, and are widely used as an ingredient of cosmetic
or antimicrobial preservatives, or as a functional food ingredient
(10-12). In vitro studies have
suggested that Echinacea may act directly on monocytes,
natural killer cells, human keratinocytes and macrophages (11,13,14). Additionally, E. purpurea
extract has been employed as a prophylactic for treatment of a
range of viral infections, particularly for respiratory tract
infections in animals and humans (15). Pharmacological studies have
emphasized the immunomodulatory activities of cichoric acid,
alkamides and polysaccharides from E. purpurea (13,14,16). The immunostimulant effect of E.
purpurea appears to be due to polysaccharides surrounding
tissue cells that protect against pathogenic invasion (14), suggesting that polysaccharides may
serve as the primary active factor associated with the
anti-inflammatory effects of E. angustifolia. Aarland et
al (17) reported that E.
purpurea and E. angustifolia exhibited anti-oxidative,
anti-inflammatory and anti-proliferative effects in vitro.
Additionally, it was demonstrated that E. purpurea
polysaccharide exhibited anti-viral properties in herpes simplex
virus type-1 infections (14).
Accordingly, the present study hypothesized that E. purpurea
polysaccharide (EP) may be used to treat LPS-induced AKI.
Mitogen-activated protein kinases (MAPKs) are a
family of serine-threonine protein enzymes that control several
physiological processes including cell proliferation, apoptosis,
inflammation and OS (18,19). The MAPK signaling pathway
primarily encompasses three signaling cascades: Extracellular
signal-regulated kinase (ERK), c-Jun-N-terminal kinase (JNK) and
p38 (18). A previous study
demonstrated that a novel polysaccharide derived from algae
extracts inhibited proliferation and induced apoptosis, as well as
cell cycle arrest, of breast cancer cells (MCF-7 cells) via the JNK
signaling pathway (20).
Additionally, Sherif et al (21) revealed that the MAPK signaling
pathway was closely associated with the occurrence and development
of AKI. For example, the suppression of the MAPK signaling pathway
by baicalein (a bioflavonoid) prevented cisplatin-induced AKI
(22). Another study demonstrated
that polygonatum sibiricum polysaccharides improved
gentamicin-mediated AKI in rats via repressing p38 in the MAPK
signaling pathway (23). However,
whether EP can attenuate LPS-induced AKI via inhibition of the MAPK
signaling pathway has not been determined.
The aim of the present study was to investigate the
under-lying mechanism by which EP alleviated AKI using in
vivo and in vitro models. HBZY-1 cells have been
intensively utilized as a cell model in LPS-induced inflammation in
accordance with previous studies (24,25). Therefore, HBZY-1 cells were used
in the present study to establish an in vitro model to
evaluate the role of EP in inflammatory responses mediated by LPS.
The current results revealed that LPS-induced AKI was attenuated by
EP via inhibition of inflammation, OS and the activation of the
MAPK signaling pathway, suggesting that EP may be used as a
potential therapeutic for the treatment of AKI.
Materials and methods
Materials
LPS was provided by Shanghai Aladdin Biochemical
Technology Co., Ltd. EP was purchased from Nanjing Daosifu
Biological Technology Co., Ltd., extracted from Echinacea
purpurea Moench with a purity of 90.26%. EP was diluted using
DMSO for both the in vivo and in vitro experiments.
For the in vivo experiments, C57BL/6 mice were supplied by
Liaoning Changsheng Biotechnology Co., Ltd. HBZY-1 cells were
obtained from Procell Life Science & Technology Co., Ltd..
Mouse model of LPS-induced AKI
To establish a mouse model of AKI, 8-week-old male
C57BL/6 mice (30 mice; weight, 20 g) underwent an acclimatization
period for a week (living conditions: 12-h light/dark cycle,
25±1°C, 45-55% humidity and ad libitum access to food and
water). Mice were randomly divided into five groups (n=6 per
group), including a control group (without treatment), 10 mg/kg EP
group, LPS group, LPS+5 mg/kg EP group and LPS+10 mg/kg EP group.
Mice were given an intraperitoneal injection of LPS (10 mg/kg)
alone or followed by an intravenous injection of EP (5 or 10
mg/kg); the concentrations used were based on previous studies
(14,26). After 24 h from injection, mice
were anesthetized via intraperitoneal injection of sodium
pentobarbital (50 mg/kg). Peripheral blood samples (1-1.5 ml per
mouse) were collected from mice with the eyeball removed in the
animal experiment room, and mice were euthanized (weight, 22-24 g)
using 200 mg/kg sodium pentobarbital. Renal tissues were collected
and either fixed in 4% paraformaldehyde at 4°C for 15 min or frozen
at −70°C. All animal studies and proto-cols were approved by the
Animal Care Committee of Hebei Normal University of Science and
Technology (approval no. 201823; 5 September 2018) and performed in
accordance with the Committee's guidelines on animal care.
Histopathological evaluation
Fixed renal tissues were embedded in paraffin and
cut into 5-µm-thick sections. For hematoxylin and eosin
(H&E) staining, the sections were deparaffinized in xylene at
room temperature (RT) and stained with hematoxylin (Sangon Biotech
Co., Ltd.) for 5 min and eosin (Beijing Solarbio Science &
Technology Co., Ltd.) for 3 min, both at RT. For periodic
acid-Schiff (PAS) staining, tissues were cut into 5-µm-thick
sections. After deparaffinizing the sections as aforementioned, the
tissues were stained with periodic acid for 10 min and
counterstained with Schiff for 15 min, both at RT, using a PAS
staining solution obtained from BaSO Biotech Co., Ltd.. All the
sections were observed using a fluorescence microscope (Olympus
Corporation; magnification, ×200 for H&E and ×400 for PAS).
Inflammation was scored on a scale from 0 to 4: 0, None; 1, rare;
2, common; 3, frequent; and 4, severe (27). PAS-positive cells were quantified
using Image Pro-Plus v6.0 (Media Cybernetics, Inc.).
Serum analysis
After 24 h of EP and LPS treatment, blood samples
were collected, and the levels of blood urea nitrogen (BUN) and
creatinine (Cr) were determined using BUN and Cr detection kits
(Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocol. BUN levels were measured at a wavelength
of 640 nm, while Cr levels at a wavelength of 510 nm using a
UV-visible spectrophotometer (Shanghai Yoke Instrument Co.,
Ltd.).
Immunohistochemistry
Paraffin-embedded 5-µm-thick renal tissue
sections were deparaffinized with xylene for 30 min at RT and
rehydrated in a descending alcohol series (95, 85 and 75%). After
antigen retrieval, sections were incubated with 3%
H2O2 at room temperature for 15 min and
blocked with goat serum (Beijing Solarbio Science & Technology
Co., Ltd.) for 15 min at RT. Sections were incubated with primary
antibodies against inducible nitric oxide synthase (iNOS; 1:100;
cat. no. 18985-1-AP; ProteinTech Group, Inc.) or cyclo-oxygenase-2
(COX-2; 1:100; cat. no. ab133466; Abcam) overnight at 4°C, and
subsequently incubated with a biotin-labeled goat anti-rabbit IgG
secondary antibody (1:200; cat. no. A0277; Beyotime Institute of
Biotechnology) at 37°C for 30 min. The slides were labeled with
horseradish peroxidase enzyme (Beyotime Institute of
Biotechnology), stained with DAB (Beijing Solarbio Science &
Technology Co., Ltd.) and counterstained with hematoxylin for 3 min
at RT. All sections were observed under a fluorescence microscope
(Olympus Corporation) at a magnification of ×400 and quantified
using Image Pro-Plus v6.0.
LPS-induced HBZY-1 cell model
HBZY-1 cells were cultured in DMEM (Procell Life
Science & Technology Co., Ltd.) containing 10% FBS (Biological
Industries) at 37°C in a humidified incubator with 5%
CO2. Cells (3×103/well) were seeded in a
96-well plate and cultured for 24 h. Cells were pretreated with EP
(100 µg/ml), PD98095 (ERK inhibitor; 50 µmol/l;
Selleck Chemicals), SP600125 (JNK inhibitor; 10 µmol/l;
MedChemExpress) or SB203580 (p38 inhibitor; 10 µmol/l;
MedChemExpress) for 1 h and then stimulated with LPS (1
µg/ml) for 24 h, all at 37°C.
Cell viability assay
Cell viability was assessed after treatment with EP
and/or LPS using an MTT assay (Sigma-Aldrich; Merck KGaA). MTT (0.5
mg/ml; 100 µl) was added to each well, and cells were
cultured at 37°C with 5% CO2 for 4 h. DMSO (150
µl) was added to the cells to solubilize the formazan
crystals for 10 min at RT in the dark. The absorbance was measured
at 570 nm using a microplate reader (BioTek Instruments, Inc.).
Detection of inflammatory cytokines
The levels of tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6 were evaluated using commercial ELISA
kits (Wuhan USCN Business Co., Ltd.), including a TNF-α detection
kit (cat. no. SEA133Mu), IL-1β detection kit (cat. no. SEA563Mu)
and IL-6 detection kit (cat. no. SEA079Mu). Briefly, renal tissues
and cell supernatants by centrifugation (1,000 × g for 20 min at
RT) were collected after treatment with EP and/or LPS. The
concentrations of the proteins were determined using a
bicinchoninic acid (BCA) kit (Beyotime Institute of Biotechnology).
Subsequently, samples were analyzed using the corresponding kits
according to the manufacturer's protocols, and the absorbance was
measured at a wavelength of 450 nm using a microplate reader
(BioTek Instruments, Inc.).
Determination of nitric oxide (NO)
levels
NO content in renal tissues was detected using a
microwell plate method (5) and an
NO assay kit (cat. no. A013-2; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocol. The absorbance
was measured at a wavelength of 550 nm using a microplate reader
(BioTek Instruments, Inc.).
Determination of prostaglandin E2
(PGE2)
The levels of PGE2 in renal tissues were detected
using a PGE2 detection kit (cat. no. CEA538Ge; Wuhan USCN Business
Co., Ltd.) according to the manufacturer's protocol. The absorbance
was measured at a wavelength of 450 nm using a microplate reader
(BioTek Instruments, Inc.).
Measurement of reactive oxygen species
(ROS) production
The production of ROS was assessed using a ROS
detection kit (cat. no. E004; Nanjing Jiancheng Bioengineering
Institute). Briefly, kidney tissues or cells after treatment with
EP and/or LPS were washed with PBS twice and incubated with
dichloro-dihydro-fluorescein diacetate (DCFH-DA; 10 µmol/l)
at 37°C for 30 min in the dark. After washing with PBS, ROS
generation was observed using a fluorescence microplate reader
(Tecan Group, Ltd.).
Biochemical assays
The levels of malondialdehyde (MDA), superoxide
dismutase (SOD), catalase (CAT), reduced glutathione (GSH) and
oxidized glutathione (GSSG) were measured using specific kits
(Nanjing Jiancheng Bioengineering Institute), including MDA
detection kit (cat. no. A003-1), SOD detection kit (cat. no.
A001-1), CAT detection kit (cat. no. A007-1) and T-GSH/GSSG
detection kit (cat. no. A061-1). In addition, the activity of
glutathione reductase (GR) was assessed using a GR detection kit
(cat. no. BC1160; Beijing Solarbio Science & Technology Co.,
Ltd.). Briefly, renal tissues and cells after treatment with EP
and/or LPS were prepared by centrifugation (tissues at 420 × g for
10 min and cells at 1,500 g for 10 min, both at RT). The
concentrations of collected supernatants were determined using a
BCA kit. The absorbance of the samples was detected according to
the manufacturers' instructions of the aforementioned detection
kits using a microplate reader (BioTek Instruments, Inc.).
Western blotting
Kidney tissues and treated cells were lysed using
RIPA lysis buffer (Beyotime Institute of Biotechnology). The
protein concentrations were quantified using a BCA kit. A total of
20 µg protein/lane was separated via SDS-PAGE (5, 8 or 10%
gel) and transferred to a PVDF membrane (Thermo Fisher Scientific,
Inc.). After blocking non-specific binding sites with 5% BSA
(Biosharp Life Sciences) dissolved in TBS-Tween (0.15% Tween 20)
for 1 h at RT, the membranes were incubated with primary antibodies
against β-actin (1:2,000 in 5% BSA; cat. no. 60008-1-Ig), iNOS
(1:500; cat. no. 18985-1-AP) and COX-2 (1:300; cat. no. 12375-1-AP)
from ProteinTech Group, Inc.; phospho-(p-)ERK (Thr202/Tyr204;
1:1,000; cat. no. AF1015) and ERK (1:2,000; cat. no. AF0155) from
Affinity Biosciences; and p-JNK (Thr183/Tyr185; 1:1,000; cat. no.
4668), JNK (1:1,000; cat. no. 9252), p-p38 (Thr180/Tyr182; 1:1,000;
cat. no. 4511) and p38 (1:1,000; cat. no. 8690) from Cell Signaling
Technology, Inc., overnight at 4°C. β-actin was used as the
internal reference. Subsequently, membranes were incubated with
goat anti-rabbit IgG-HRP (1:5,000; cat. no. SA00001-2; ProteinTech
Group, Inc.) or goat anti-mouse IgG-HRP (1:5,000; cat. no.
SA00001-1; ProteinTech Group, Inc.), both diluted in 5% BSA, at
37°C for 40 min. The signals were visualized using an enhanced
chemiluminescent reagent (cat. no. E002-100; Shanghai Qihai Futai
Biological Technology Co., Ltd.) and quantified using Gel-Pro
Analyzer v4.0 (Media Cybernetics, Inc.).
Immunofluorescence analysis of HBZY-1
cells
Cells were grown on slides and treated with EP
and/or LPS. Subsequently, cells were fixed using 4%
paraformaldehyde for 15 min at 4°C, washed three times with PBS (5
min per wash) and incubated with 0.1% Triton X-100 (Beyotime
Institute of Biotechnology) for 30 min at RT. After washing three
times with PBS, the slides were blocked using goat serum (cat. no.
SL038; Beijing Solarbio Science & Technology Co., Ltd.) for 15
min at RT and incubated with primary antibodies against p-ERK
(1:200; cat. no. AF1015; Affinity Biosciences), p-JNK (1:200; cat.
no. bs-1640R; BIOSS) and p-p38 (1:200; cat. no. bs-0636R; BIOSS)
overnight at 4°C. Cells were incubated with a Cy3-labeled goat
anti-rabbit IgG secondary antibody (1:200; cat. no. A0516; Beyotime
Institute of Biotechnology) for 1 h at RT and counterstained with
DAPI (Beyotime Institute of Biotechnology) for 3 min at RT. Cells
were visualized using a fluorescence microscope (magnification,
×400).
Statistical analysis
The data are expressed as the mean ± standard
deviation. All data analyses were performed using GraphPad Prism
version 8.0 (GraphPad Software, Inc.). Comparisons between ≥3
groups were performed using a one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
EP attenuates LPS-induced AKI in
mice
The effects of EP on LPS-induced histological
changes in the kidney tissues were assessed using H&E and PAS
staining. Fig. 1A shows that
inflammation was successfully induced by LPS and that the
co-administration of EP significantly attenuated this process. As
shown in Fig. 1B, the glomerular
structure was intact, and no obvious pathological changes were
observed in the control and 10 mg/kg EP-treated mice. By contrast,
proliferation of mesangial cells, swelling of glomerular epithelial
cells, interstitial edema and glycogen deposition were observed in
the LPS-treated renal tissues. Pretreatment with EP markedly
alleviated LPS-induced glomerular and tubular damage, and
significantly decreased the number of PAS-positive cells.
Additionally, the levels of BUN in the blood were elevated
following LPS treatment for 24 h (Fig. 1C) compared with the control group.
Similarly, Cr levels were also increased in LPS-treated mice
(Fig. 1D). EP co-administration
markedly decreased BUN and Cr levels.
EP represses inflammation in LPS-treated
mice
The levels of indices of inflammation were measured
to evaluate the effect of EP on the inflammatory response. As shown
in Fig. 2A-E, TNF-α, IL-1β, IL-6,
NO and PGE2 levels were significantly increased in the LPS-treated
kidney tissues compared with in the control mice and were
significantly decreased following EP treatment compared with the
LPS group. Compared with the control group, the protein expression
levels of iNOS and COX-2 were significantly upregulated by LPS
treatment; however, pretreatment with EP decreased this
upregulation (Fig. 2F). In
addition, as shown in Fig. 2G and
H, immunohistochemistry results for iNOS and COX-2 revealed
that administration of EP decreased LPS-induced upregulation of
iNOS and COX-2 in kidney tissues. Additionally, there were few or
no iNOS and COX-2-positive cells in the control and EP-treated
control mice.
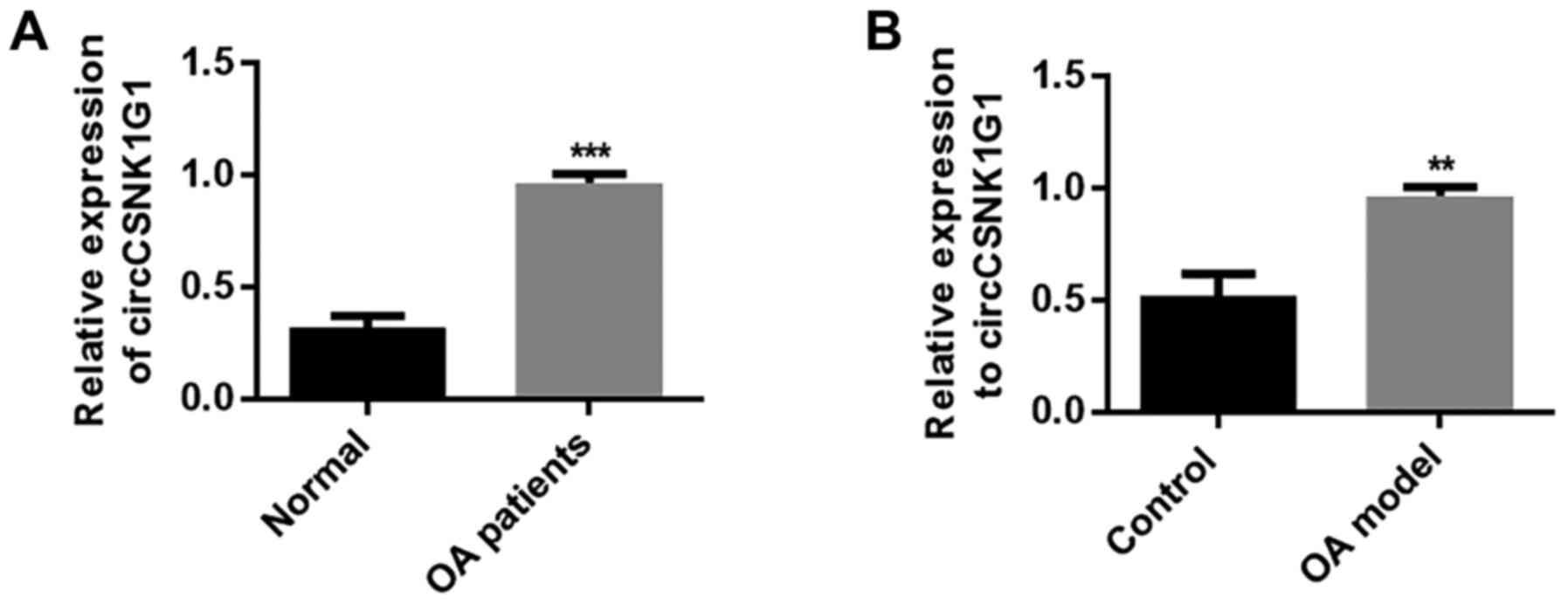
 | Figure 2Effect of EP on renal tissue
inflammation induced by LPS. Levels of (A) TNF-α, (B) IL-1β and (C)
IL-6 were evaluated in renal tissues using ELISA. (D) NO and (E)
PGE2 content. (F) Protein expression levels of iNOS and COX-2.
β-actin was used as the internal reference. Levels of (G) iNOS and
(H) COX-2 evaluated using immunohistochemistry. Arrows represent
iNOS/COX-2-positive cells. Results are presented as the mean ±
standard deviation (n=6). ###P<0.001 vs. control
group; *P<0.05, **P<0.01 and
***P<0.001 vs. LPS group. EP, Echinacea
polysaccharide; LPS, lipopolysaccharide; TNF-α, tumor necrosis
factor-α; IL, interleukin; NO, nitric oxide; PGE2, prostaglandin
E2; iNOS, inducible nitric oxide synthase; COX-2,
Cycol-oxygenase-2. |
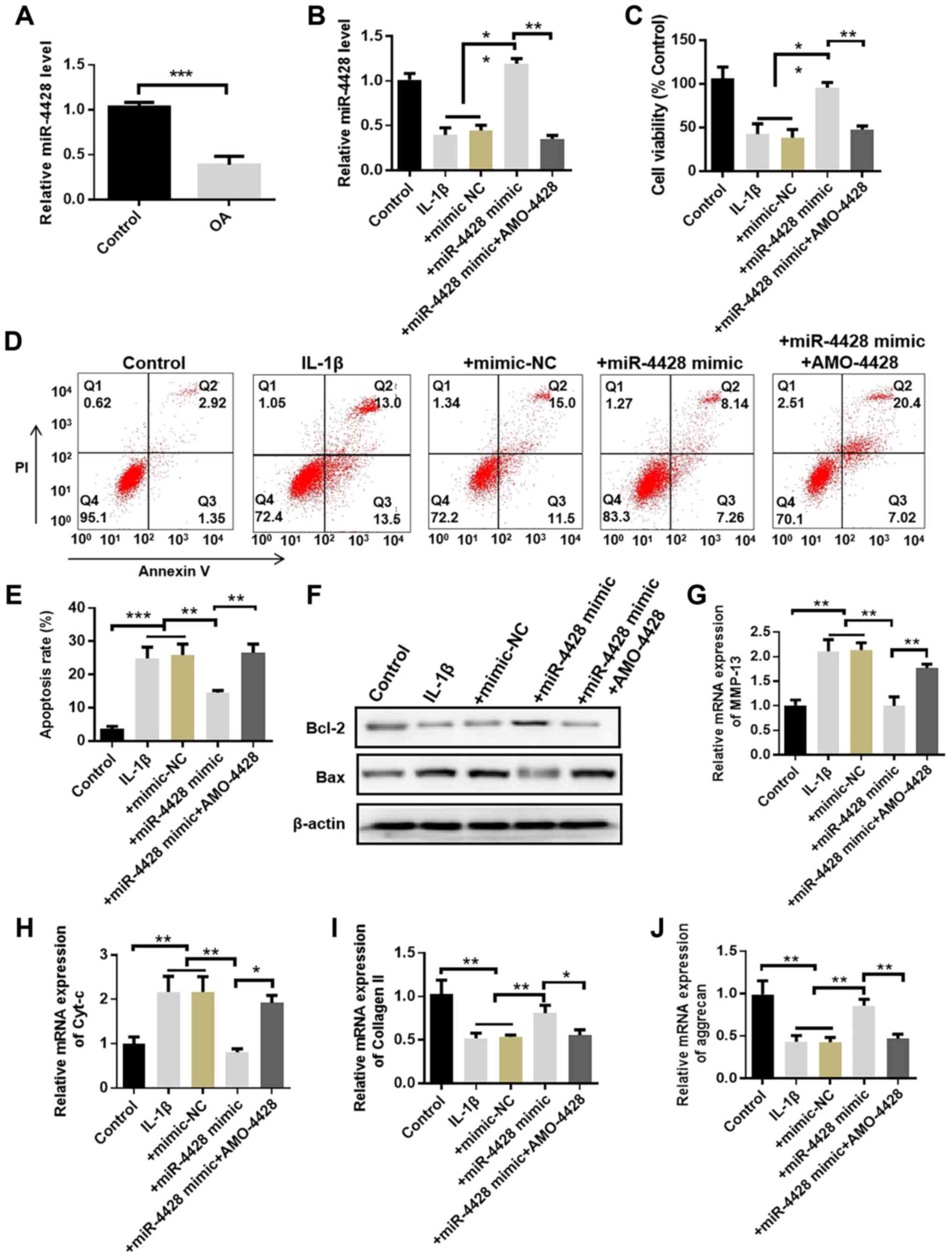
EP decreases OS induced by LPS in
mice
To investigate the role of EP in OS, the levels of
ROS and MDA, as well as SOD activity, were detected in kidney
tissues. DCFH-DA is a fluorescent probe of ROS. As illustrated in
Fig. 3A, the fluorescence
intensity of the LPS group was significantly higher than that of
the control group, indicating that the production of ROS in
LPS-treated mice was increased. EP administration significantly
decreased ROS generation compared with the LPS group. Furthermore,
the LPS-treated group exhibited a significant increase in MDA and
GSSG levels, and a significant decrease in SOD, CAT and GR levels,
as well as GSH activity, compared with the control mice (Fig. 3B-F). Additionally, compared with
in the control group, the ratio of GSH/GSSG was significantly
decreased in the LPS-treated group. Administration of EP markedly
attenuated these changes.
 | Figure 3Effect of EP on renal tissue
oxidative stress mediated by LPS. (A) Fluorescence intensity of
ROS. (B) MDA, (C) SOD, (D) CAT and (E) GR contents, and (F) GSH and
GSSG levels were detected using commercial kits. Results are
presented as the mean ± standard deviation (n=6).
##P<0.01 and ###P<0.001 vs. control
group; *P<0.05, **P<0.01 and
***P<0.001 vs. LPS group. EP, Echinacea
polysaccharide; LPS, lipopolysaccharide; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT,
catalase; GR, glutathione reductase; GSH, reduced glutathione;
GSSG, oxidized glutathione. |
EP inhibits the activation of the MAPK
signaling pathway in LPS-treated kidney tissues
To determine the effects of EP on the MAPK signaling
pathway in renal tissues following LPS treatment, the expression
levels of p-ERK, ERK, p-JNK, JNK, p-p38 and p38 were measured via
western blotting. As shown in Fig.
4, the levels of p-ERK, p-JNK and p-p38 were significantly
increased in LPS-treated kidneys compared with in the control
group. However, LPS-induced upregulation of p-ERK, p-JNK and p-p38
levels was attenuated by administration of EP (5 or 10 mg/kg).
Furthermore, there was no difference in ERK, JNK and p38 levels in
the kidney irrespective of treatment.
EP mitigates LPS-mediated HBZY-1 cell
damage
To further explore the role of EP in OS and
inflammation in vitro, an LPS-treated HBZY-1 cell model was
established. As shown in Fig. 5A,
compared with the control cells, LPS significantly decreased HBZY-1
cell viability, whereas pretreatment with EP restored the viability
of LPS-treated cells. Fig. 5B-G
shows that EP decreased OS in LPS-treated cells as demonstrated by
the lowered ROS, MDA and GSSG levels, and the elevated SOD, CAT and
GR levels, as well as GSH contents, compared with the LPS group.
Additionally, LPS treatment resulted in a significant increase in
TNF-α, IL-1β, IL-6, NO and PGE2 contents compared with the control
cells, while these levels were significantly decreased by
co-administration of EP compared with the LPS-treated cells
(Fig. 5H).
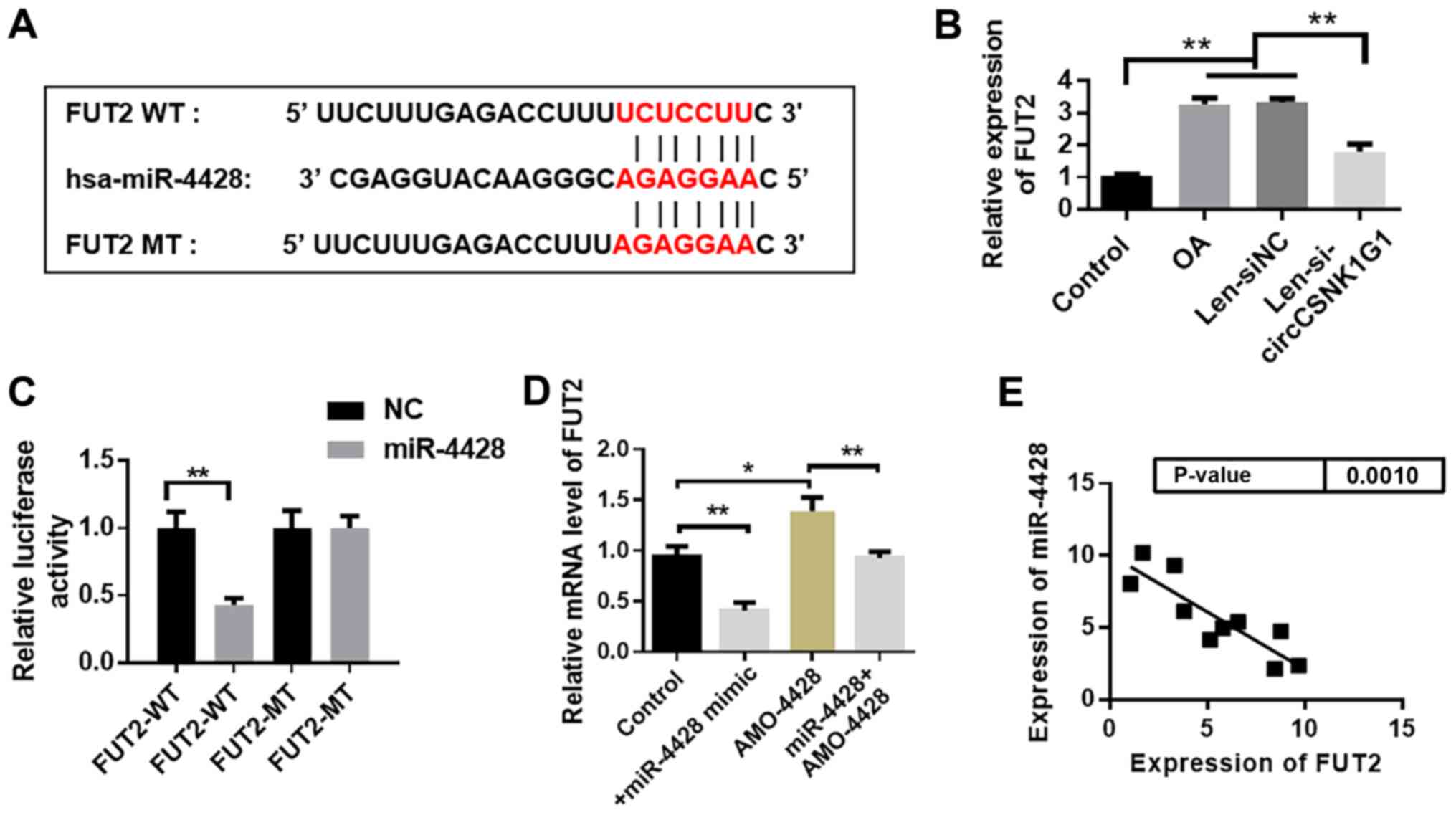 | Figure 5Effect of EP on LPS-treated HBZY-1
cells. (A) Cell viability was detected using an MTT assay. (B)
Fluorescence intensity of ROS. (C) MDA, (D) SOD, (E) CAT and (F) GR
levels, and (G) GSH and GSSG content were detected using commercial
kits. (H) TNF-α, IL-1β, IL-6, NO and PGE2 levels. Results are
presented as the mean ± standard deviation (n=3).
##P<0.01 and ###P<0.001 vs. control
group; *P<0.05, **P<0.01 and
***P<0.001 vs. LPS group. EP, Echinacea
polysaccharide; LPS, lipopolysaccharide; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT,
catalase; GR, glutathione reductase; GSH, reduced glutathione;
GSSG, oxidized glutathione; TNF-α, tumor necrosis factor-α; IL,
interleukin; NO, nitric oxide; PGE2, prostaglandin E2. |
EP suppresses the MAPK signaling pathway
in LPS-treated HBZY-1 cells
The effects of EP on proteins involved in the MAPK
signaling pathway in HBZY-1 cells were investigated using western
blotting. The expression levels of p-ERK, p-JNK and p-p38 were
significantly higher in LPS-treated cells compared with in the
control group, and these alterations were significantly attenuated
by pretreatment with EP (Fig.
6A). Notably, immunofluorescence analysis revealed that EP
markedly blocked nuclear translocation of p-ERK, p-JNK and p-p38 in
LPS-treated cells (Fig. 6B).
Additionally, cells were treated with EP, PD98095, SP600125 or
SB203580 for 1 h and then treated with LPS for 24 h. Cell viability
and the indicators associated with OS and inflammation were
evaluated. Cell viability was significantly decreased in the LPS
group compared with in control cells and was significantly reversed
with the administration of EP, PD98095, SP600125 or SB203580
(Fig. 6C). The results presented
in Fig. 6D-I confirmed that the
treatment with EP, PD98095, SP600125 or SB203580 attenuated OS
through the significantly decreased levels of ROS and MDA, and the
significantly increased SOD activity, as well as decreasing the
inflammatory response as demonstrated by the significantly lower
TNF-α, IL-1β and IL-6 levels compared with those in LPS-treated
cells. Therefore, EP may exhibit its therapeutic effect in AKI via
regulating the MAPK signaling pathway. However, the detailed
mechanism requires further study.
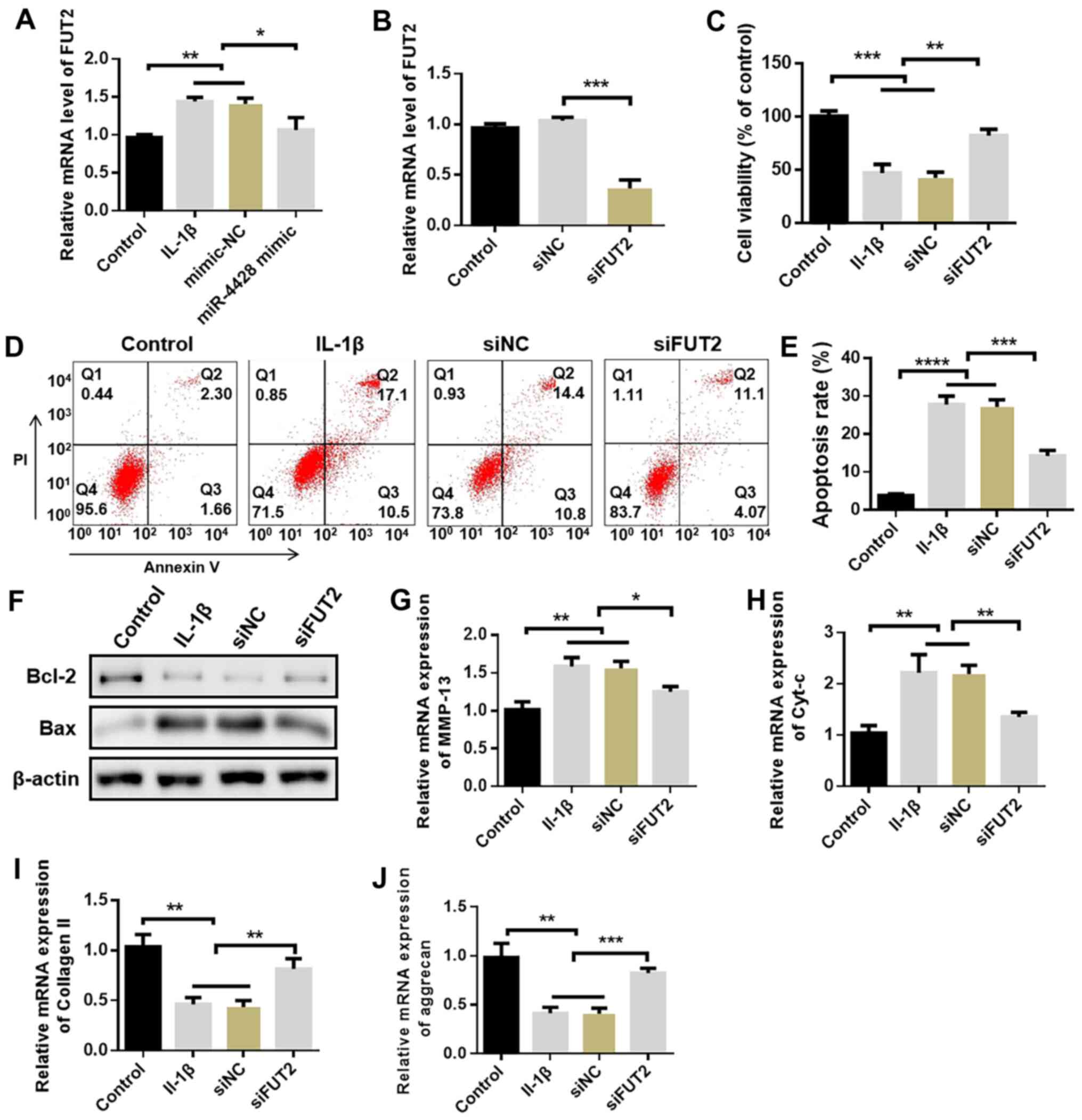 | Figure 6Effect of EP on the MAPK signaling
pathway in LPS-treated HBZY-1 cells. (A) Protein levels of p-ERK
(Thr202/Tyr204), ERK, p-JNK (Thr183/Tyr185), JNK, p-p38
(Thr180/Tyr182) and p38 were detected via western blotting. β-actin
was used as the internal reference. (B) p-ERK, p-JNK and p-p38
expression levels were evaluated using immunofluorescence analysis.
Scale bar, 50 µm. (C) An MTT assay was used to assess cell
viability. (D) Fluorescence intensity of ROS. (E) MDA content and
(F) SOD activity were detected using commercial kits. Levels of (G)
TNF-α, (H) IL-1β and (I) IL-6 were evaluated in cell supernatants
using the corresponding kits. Results are presented as the mean ±
standard deviation (n=3). ###P<0.001 vs. control
group; *P<0.05, **P<0.01 and
***P<0.001 vs. LPS group. EP, Echinacea
polysaccharide; LPS, lipopolysaccharide; MAPK, mitogen-activated
protein kinase; p-, phospho-; ERK, extracellular signal-regulated
protein kinase; JNK, c-Jun N-terminal kinase; ROS, reactive oxygen
species; MDA, malondialdehyde; SOD, superoxide dismutase; TNF-α,
tumor necrosis factor-α; IL, interleukin. |
Discussion
LPS treatment resulted in renal impairment in the
present mouse model of AKI, and EP effectively mitigated the
LPS-mediated AKI. BUN and Cr are established biomarkers used for
the diagnosis of AKI (28,29).
In the present study, there was a prominent decrease in the levels
of BUN and Cr following EP administration in LPS-treated kidney
tissues, in agreement with a previous study, which demonstrated
that AKI was attenuated by podocyte-specific soluble epoxide
hydrolase deficiency in mice and that BUN and Cr levels were
decreased (30). Furthermore, the
current histological analysis revealed that EP decreased
LPS-induced AKI.
A previous study highlighted the importance of
inflammation in burn-associated AKI (31). The inflammatory response is a
complex biological process, which includes the synthesis and
release of pro-inflammatory cytokines, such as TNF-α, IL-1β and
IL-6 (32). Additionally, LPS
induces the release of TNF-α and IL-1β via a Toll-like receptor 4
pathway, resulting in increased OS (33). TNF-α serves an essential role in
the development of AKI by activating kidney endothelial TNF
receptor 1 (34). A recent study
has indicated that IL-1β and IL-6 are important pro-inflammatory
indicators in renal diseases (35), which accelerate NO release.
Additionally, overproduction of NO generates peroxynitrite ions,
directly or indirectly resulting in the damage of target cells and
tissues (36). It has been
demonstrated that enhanced renal NO levels significantly increase
the expression levels of iNOS in glycerol-mediated AKI (37). Yu et al (36) reported that honokiol significantly
decreased the expression levels of iNOS in a rat model of renal
ischemia/reperfusion injury. COX-2, one of the downstream molecules
of NO, was upregulated in mononuclear cells, endothelial cells and
macrophages following elevated NO (38). Additionally, PGE2, the product of
COX-2, is an essential inflammatory mediator (38). As described by Hwang et al
(39), COX-2 and PGE2 serve
critical roles in the pathogenesis of kidney inflammation. A
previous study indicated that Aloe vera polysaccharides
suppressed TNF-α-induced secretion of inflammatory cytokines in
HaCaT cells (40). Another study
confirmed that EP exhibited anti-inflammatory properties in a rat
model of colitis induced by acetic acid (41). Consistent with previous studies
(3,42), the anti-inflammatory effect of EP
on LPS-induced AKI in the present study was achieved via decreasing
the levels of inflammatory indicators in vivo and in
vitro. Cellular injury and its associated molecular products
serve as vital triggers in inflammation following acute tissue
damage (6). Additionally, EP
pretreatment in the present study increased cell viability in
LPS-mediated HBZY-1 cells, which was similar to a previous study
demonstrating that Maerkang Lactarius Deliciosus Gray
polysaccharides promoted the proliferation of RAW264.7 cells
(43).
OS serves an important role in the occurrence and
development of AKI (44). It has
been demonstrated that OS is enhanced in LPS-induced models of AKI
(45) and that the MAPK signaling
pathway is an essential modulator of altered gene transcription in
response to increased OS (46).
OS can be caused by an imbalance between ROS generation and
anti-oxidant defense (47).
Excessive ROS production is detrimental to cellular functions; it
has been reported that ROS may damage biomolecules such as DNA,
RNA, proteins and lipids (3), and
may induce the release of inflammatory cytokines through activation
of the NF-κB signaling pathway (48). The production of endogenous ROS
activates ERK, while exogenous ROS generation results in p38
activation (46). Furthermore,
MDA, the end product of unsaturated lipid peroxidation, has been
widely utilized as a marker of oxidative damage (48). SOD, CAT, GR and GSH are vital
antioxidant indicators modulating oxidative injury (36,49). The anti-oxidative status is
closely associated with the ratio of GSH/GSSG (35). In the present study, ROS, MDA and
GSSG levels were elevated, while SOD, CAT, GR and GSH activities
were lowered in LPS-treated mice and cells. EP administration
reversed these levels, similarly to previous studies (4,50).
JNK may be activated by OS, and is considered vital
for the regulation of oxidative damage (47). Additionally, p38 is able to
participate in the progression and development of inflammation when
tissues or cells are impaired (51). Following injury, the majority of
inflammatory mediators, including TNF-α, IL-1β and IL-6, are
released (52). These molecules
can act on JNK and p38, phosphorylating and activating them;
subsequently, p-JNK and p-p38 are translocated to the nucleus
resulting in a series of responses, such as oxidative injury,
apoptosis and inflammatory responses (53-55). Additionally, the ERK signaling
pathway is an important signaling pathway, of which the abnormal or
dysregulated activation may lead to renal tissue impairment
(56). The MAPK signaling pathway
has been demonstrated to modulate pro-inflammatory cytokines, such
as IL-1β, in AKI (57). A
previous study revealed that JNK, ERK and p38 where inactivated by
hesperetin, suggesting that hesperetin may be used to inhibit
inflammation in cisplatin-induced AKI (58). In the present study, ERK, JNK and
p38 were notably activated by LPS in renal tissues and HBZY-1
cells. Notably, EP treatment decreased the expression levels of
p-ERK, p-JNK and p-p38. The current findings suggest that EP may
attenuate LPS-induced AKI in vivo and in vitro,
potentially via inhibiting the activation of the MAPK signaling
pathway, similarly to a previous study (5). However, another previous study
demonstrated that a novel polysaccharide derived from algae
extracts inhibited breast cancer progression via activation of the
JNK signaling pathway (20),
which is inconsistent with the results of the present study. This
may be due to the different sources of polysaccharides, cell types
and models. Additionally, the protein kinase B (Akt) signaling
pathway serves a critical role in inflammatory and autoimmune
diseases (59). A previous study
revealed that Terminalia bellirica (Gaertn.) Roxb. extract
decreased the inflammatory response and OS via activation of the
Akt signaling pathway in an LPS-shock mouse model (60). Zhao et al (61) reported that dexmedetomidine
protected against LPS-mediated AKI in rats via inhibiting the
activation of the Akt signaling pathway. Additionally, another
study demonstrated that water extracts from the roots of E.
purpurea containing a polysaccharide with anti-inflamma-tory
properties activated the Akt signaling pathway in human acute
monocytic leukemia (THP-1) cells (62). Therefore, future studies should
further investigate the effects of EP on the Akt signaling pathway
in LPS-induced AKI.
A previous study indicated that the absorption of
natural bioactive polysaccharides in the body was likely directly
via the gut, gut microbiota or Peyer's patches (63). As suggested by Yu et al
(64), pumpkin polysaccharides
may be metabolized by various enzymes in the liver. Dendrobium
aphyllum polysaccharides consist of mannose and glucose
(65), and glucose metabolism is
strongly involved in regulation of diabetes and immune responses.
Possible metabolic pathways involved with polysaccharides include
glucose dehydrogenase, glucose-6-phosphatase and glucose
transporters (GLUT1 and GLUT2). Notably, the aforementioned studies
provide some references for studying the in vivo metabolism
of EP; however, the full mechanism of how EP is metabolized in
vivo remains unknown. Therefore, the in vivo metabolism
of EP, the effect of EP on other possible signaling pathways and
the underlying mechanisms in LPS-induced AKI should be further
investigated in future studies. The present study illustrated the
inhibitory effect of EP on the MAPK signaling pathway, but how EP
exerts its function in AKI via this signaling pathway and detailed
mechanisms require further research.
In conclusion, the results of the present study
clearly indicated that EP attenuated AKI by decreasing inflammation
and OS. In addition, EP likely suppressed the activation of the
MAPK signaling pathway via decreasing the protein expression levels
of p-ERK, p-JNK and p-p38. Therefore, EP may be a potential
therapeutic agent for the treatment of AKI, given its
anti-inflammatory and anti-oxidative effects.
Abbreviations:
|
EP
|
Echinacea polysaccharide
|
|
LPS
|
lipopolysaccharide
|
|
AKI
|
acute kidney injury
|
|
H&E
|
hematoxylin and eosin
|
|
PAS
|
periodic acid-Schiff
|
|
BUN
|
blood urea nitrogen
|
|
Cr
|
creatinine
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL
|
interleukin
|
|
NO
|
nitric oxide
|
|
PGE2
|
prostaglandin E2
|
|
iNOS
|
inducible nitric oxide synthase
|
|
COX-2
|
cyclo-oxygenase-2
|
|
OS
|
oxidative stress
|
|
ROS
|
reactive oxygen species
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
GR
|
glutathione reductase
|
|
GSH
|
reduced glutathione
|
|
GSSG
|
oxidized glutathione
|
|
MAPK
|
mitogen-activated protein kinase
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
p-
|
phospho-
|
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 31472230),
the Third Batch of Giant Project of Hebei Province (grant no.
180416), the Top Talent Project for Youths of Hebei Province (grant
no. 180443), the Doctoral Startup Foundation of Hebei Normal
University of Science and Technology (grant no. 2018YB018), the
High School Hundred Excellent Innovation Talent Program of Hebei
Province, the Natural Science Foundation of Hebei Province (grant
no. C2019407111) and the Project of Department of Science and
Technology of Hebei Province (grant no. 18246629G).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ and QS contributed to the conception of the
study. QS, WL and SW designed the experiments. GL, XB and XY
performed the experiments. WL, SW and GL analyzed the data. QS
drafted the manuscript. HZ revised the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies and protocols were approved by
the Animal Care Committee of Hebei Normal University of Science and
Technology (approval no. 201823; 5 September 2018), and were
performed according to the Committee's guidelines on animal
care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gonsalez SR, Cortês AL, Silva RCD, Lowe J,
Prieto MC and Silva Lara LD: Acute kidney injury overview: From
basic findings to new prevention and therapy strategies. Pharmacol
Ther. 200:1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters E, Geraci S, Heemskerk S, Wilmer
MJ, Bilos A, Kraenzlin B, Gretz N, Pickkers P and Masereeuw R:
Alkaline phosphatase protects against renal inflammation through
dephosphorylation of lipopolysaccharide and adenosine triphosphate.
Br J Pharmacol. 172:4932–4945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang H, Chen MK, Li K, Hu C, Lu MH and
Situ J: Eupafolin nanoparticle improves acute renal injury induced
by LPS through inhibiting ROS and inflammation. Biomed
Pharmacother. 85:704–711. 2017. View Article : Google Scholar
|
|
4
|
Liu X, Lu J, Liao Y, Liu S, Chen Y, He R,
Men L, Lu C, Chen Z, Li S, et al: Dihydroartemisinin attenuates
lipopolysaccharide-induced acute kidney injury by inhibiting
inflammation and oxidative stress. Biomed Pharmacother.
117:1090702019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu
Z and Liu X: Chicoric acid supplementation prevents systemic
inflammation-induced memory impairment and amyloidogenesis via
inhibition of NF-κB. FASEB J. 31:1494–1507. 2017. View Article : Google Scholar
|
|
6
|
Rabb H, Griffin MD, McKay DB, Swaminathan
S, Pickkers P, Rosner MH and Kellum JA: Inflammation in AKI:
Current understanding, key questions, and knowledge gaps. J Am Soc
Nephrol. 27:371–379. 2016. View Article : Google Scholar :
|
|
7
|
Li HD, Meng XM, Huang C, Zhang L, Lv XW
and Li J: Application of herbal traditional Chinese medicine in the
treatment of acute kidney injury. Front Pharmacol. 10:3762019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Islam MS, Miao L, Yu H, Han Z and Sun H:
Ethanol extract of Illicium henryi attenuates LPS-induced acute
kidney injury in mice via regulating inflammation and oxidative
stress. Nutrients. 11:14122019. View Article : Google Scholar :
|
|
9
|
Karsch-Völk M, Barrett B and Linde K:
Echinacea for preventing and treating the common cold. JAMA.
313:618–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tubaro A, Tragni E, Del Negro P, Galli CL
and Della Loggia R: Anti-inflammation activity of a polysaccharidic
fraction of Echinacea angustifolia. J Pharm Pharmacol. 39:567–569.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sharifi-Rad M, Mnayer D, Morais-Braga MFB,
Carneiro JNP, Bezerra CF, Coutinho HDM, Salehi B, Martorell M, Del
Mar Contreras M, Soltani-Nejad A, et al: Echinacea plants as
anti-oxidant and antibacterial agents: From traditional medicine to
biotechnological applications. Phytother Res. 32:1653–1663. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hall C III: Echinacea as a functional food
ingredient. Adv Food Nutr Res. 47:113–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oláh A, Szabó-Papp J, Soeberdt M, Knie U,
Dähnhardt-Pfeiffer S, Abels C and Bíró T: Echinacea
purpurea-derived alkylamides exhibit potent anti-inflammatory
effects and alleviate clinical symptoms of atopic eczema. J
Dermatol Sci. 88:67–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghaemi A, Soleimanjahi H, Gill P, Arefian
E, Soudi S and Hassan Z: Echinacea purpurea polysaccharide reduces
the latency rate in herpes simplex virus type-1 infections.
Intervirology. 52:29–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hudson JB: Applications of the
phytomedicine Echinacea purpurea (Purple Coneflower) in infectious
diseases. J Biomed Biotechnol. 2012:7698962012. View Article : Google Scholar
|
|
16
|
Yang G, Li K, Liu C, Peng P, Bai M, Sun J,
Li Q, Yang Z, Yang Y and Wu H: A comparison of the
immunostimulatory effects of polysaccharides from tetraploid and
diploid Echinacea purpurea. Biomed Res Int. 2018:86285312018.
View Article : Google Scholar :
|
|
17
|
Aarland RC, Bañuelos-Hernández AE,
Fragoso-Serrano M, Sierra-Palacios ED, Díaz de León-Sánchez F,
Pérez-Flores LJ, Rivera-Cabrera F and Mendoza-Espinoza JA: Studies
on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and
antiproliferative activities of Echinacea purpurea and Echinacea
angustifolia extracts. Pharm Biol. 55:649–656. 2017. View Article : Google Scholar
|
|
18
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human disease: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santulli P, Marcellin L, Tosti C,
Chouzenoux S, Cerles O, Borghese B, Batteux F and Chapron C: MAP
kinases and the inflammatory signaling cascade as targets for the
treatment of endometriosis? Expert Opin Ther Targets. 19:1465–1483.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie P, Horio F, Fujii I, Zhao J, Shinohara
M and Matsukura M: A novel polysaccharide derived from algae
extract inhibits cancer progression via JNK, not via the p38 MAPK
signaling pathway. Int J Oncol. 52:1380–1390. 2018.PubMed/NCBI
|
|
21
|
Sherif IO, Al-Mutabagani LA, Alnakhli AM,
Sobh MA and Mohammed HE: Renoprotective effects of angiotensin
receptor blocker and stem cells in acute kidney injury: Involvement
of inflammatory and apoptotic markers. Exp Biol Med (Maywood).
240:1572–1579. 2015. View Article : Google Scholar
|
|
22
|
Sahu BD, Mahesh Kumar J and Sistla R:
Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney
injury by up-regulating antioxidant defenses and down-regulating
the MAPKs and NF-κB pathways. PLoS One. 10:e01341392015. View Article : Google Scholar
|
|
23
|
Han C, Sun T, Liu Y, Fan G, Zhang W and
Liu C: Protective effect of Polygonatum sibiricum polysaccharides
on gentamicin-induced acute kidney injury in rats via inhibiting
p38 MAPK/ATF2 pathway. Int J Biol Macromol. 151:595–601. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, Fu J, Zhao Y, Ji K, Luan T and Zang
B: Alpha-lipoic acid exerts anti-inflammatory effects on
lipopolysaccharide- stimulated rat mesangial cells via inhibition
of nuclear factor kappa B (NF-κB) signaling pathway. Inflammation.
38:510–519. 2015. View Article : Google Scholar
|
|
25
|
Yuan J, Hou K, Yao Y, Du Z, Lu C, Yuan Q
and Gao X: Gold clusters attenuate inflammation in rat mesangial
cells via inhibiting the activation of NF-κB pathway. Nanomaterials
(Basel). 10:–712. 2020. View Article : Google Scholar
|
|
26
|
Hou R, Xu T, Li Q, Yang F, Wang C, Huang T
and Hao Z: Polysaccharide from Echinacea purpurea reduces the
oxidant stress in vitro and in vivo. Int J Biol Macromol.
149:41–50. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beckert H, Meyer-Martin H, Buhl R, Taube C
and Reuter S: The canonical but not the noncanonical Wnt pathway
inhibits the development of allergic airway disease. J Immunol.
201:1855–1864. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burmeister DM, Gómez BI and Dubick MA:
Molecular mechanisms of trauma-induced acute kidney injury:
Inflammatory and metabolic insights from animal models. Biochim
Biophys Acta Mol Basis Dis. 1863:2661–2671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teo SH and Endre ZH: Biomarkers in acute
kidney injury (AKI). Best Pract Res Clin Anaesthesiol. 31:331–344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bettaieb A, Koike S, Chahed S, Zhao Y,
Bachaalany S, Hashoush N, Graham J, Fatima H, Havel PJ, Gruzdev A,
et al: Podocyte-specific soluble epoxide hydrolase deficiency in
mice attenuates acute kidney injury. FEBS J. 284:1970–1986. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark A, Neyra JA, Madni T, Imran J,
Phelan H, Arnoldo B and Wolf SE: Acute kidney injury after burn.
Burns. 43:898–908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gan Y, Tao S, Cao D, Xie H and Zeng Q:
Protection of resveratrol on acute kidney injury in septic rats.
Human Exp Toxicol. 36:1015–1022. 2017. View Article : Google Scholar
|
|
33
|
Chen Y, Jin S, Teng X, Hu Z, Zhang Z, Qiu
X, Tian D and Wu Y: Hydrogen sulfide attenuates LPS-induced acute
kidney injury by inhibiting inflammation and oxidative stress. Oxid
Med Cell Longev. 2018:67172122018.PubMed/NCBI
|
|
34
|
Xu C, Chang A, Hack BK, Eadon MT, Alper SL
and Cunningham PN: TNF-mediated damage to glomerular endothelium is
an important determinant of acute kidney injury in sepsis. Kidney
Int. 85:72–81. 2014. View Article : Google Scholar
|
|
35
|
Sharma M, Naura AS and Singla SK:
Modulatory effect of 4-phenyl butyric acid on hyperoxaluria-induced
renal injury and inflammation. Mol Cell Biochem. 451:185–196. 2019.
View Article : Google Scholar
|
|
36
|
Yu Y, Li M, Su N, Zhang Z, Zhao H, Yu H
and Xu Y: Honokiol protects against renal ischemia/reperfusion
injury via the suppression of oxidative stress, iNOS, inflammation
and STAT3 in rats. Mol Med Rep. 13:1353–1360. 2016. View Article : Google Scholar
|
|
37
|
Liu Y, Fu X, Gou L, Li S, Lan N, Zheng Y
and Yin X: L-citrulline protects against glycerol-induced acute
renal failure in rats. Ren Fail. 35:367–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hwang HS, Yang KJ, Park KC, Choi HS, Kim
SH, Hong SY, Jeon BH, Chang YK, Park CW, Kim SY, et al:
Pretreatment with paricalcitol attenuates inflammation in
ischemia-reperfusion injury via the up-regulation of
cyclooxygenase-2 and prostaglandin E2. Nephrol Dial Transplant.
28:1156–1166. 2013. View Article : Google Scholar
|
|
40
|
Leng H, Pu L, Xu L, Shi X, Ji J and Chen
K: Effects of aloe polysaccharide, a polysaccharide extracted from
Aloe vera, on TNF-α-induced HaCaT cell proliferation and the
under-lying mechanism in psoriasis. Mol Med Rep. 18:3537–3543.
2018.PubMed/NCBI
|
|
41
|
Dogan Z, Ergul B, Sarikaya M, Filik L,
Gonultaş MA, Hucumenoglu S and Can M: The protective effect of
Echinacea spp. (Echinacea angustifolia and Echinacea purpurea) in a
rat colitis model induced by acetic acid. Pak J Pharm Sci.
27:1827–1835. 2014.PubMed/NCBI
|
|
42
|
Mirzoyan K, Denis C, Casemayou A, Gilet M,
Marsal D, Goudounéche D, Faguer S, Bascands JL, Schanstra JP and
Saulnier-Blache JS: Lysophosphatidic acid protects against
endotoxin-induced acute kidney injury. Inflammation. 40:1707–1716.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su S, Ding X, Fu L and Hou Y: Structural
characterization and immune regulation of a novel polysaccharide
from maerkang Lactarius deliciosus gray. Int J Mol Med. 44:713–724.
2019.PubMed/NCBI
|
|
44
|
Tomsa AM, Alexa AL, Junie ML, Rachisan AL
and Ciumarnean L: Oxidative stress as a potential target in acute
kidney injury. PeerJ. 7:e80462019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rousta AM, Mirahmadi SM, Shahmohammadi A,
Nourabadi D, Khajevand-Khazaei MR, Baluchnejadmojarad T and Roghani
M: Protective effect of sesamin in lipopolysaccharide-induced mouse
model of acute kidney injury via attenuation of oxidative stress,
inflammation, and apoptosis. Immunopharmacol Immunotoxicol.
40:423–429. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chatterjee A and Chatterji U: All-trans
retinoic acid ameliorates arsenic-induced oxidative stress and
apoptosis in the rat uterus by modulating MAPK signaling proteins.
J Cell Biochem. 118:3796–3809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chenxu G, Minxuan X, Yuting Q, Tingting G,
Jinxiao L, Mingxing W, Sujun W, Yongjie M, Deshuai L, Qiang L, et
al: iRhom2 loss alleviates renal injury in long-term PM2.5-exposed
mice by suppression of inflammation and oxidative stress. Redox
Biol. 19:147–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun Q, Xin F, Wen X, Lu C, Chen R and Ruan
G: Protective effects of different kinds of filtered water on
hypertensive mouse by suppressing oxidative stress and
inflammation. Oxid Med Cell Longev. 2018:29173872018. View Article : Google Scholar
|
|
49
|
Sahu BD, Kuncha M, Sindhura GJ and Sistla
R: Hesperidin attenuates cisplatin-induced acute renal injury by
decreasing oxidative stress, inflammation and DNA damage.
Phytomedicine. 20:453–460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jia P, Wu X, Pan T, Xu S, Hu J and Ding X:
Uncoupling protein 1 inhibits mitochondrial reactive oxygen species
generation and alleviates acute kidney injury. EBioMedicine.
49:331–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Amos LA, Ma FY, Tesch GH, Liles JT,
Breckenridge D, Nikolic-Patersn DJ and Han Y: ASK1 inhibitor
treatment suppresses p38/JNK signalling with reduced kidney
inflammation and fibrosis in rat crescentic glomerulonephritis. J
Cell Mol Med. 22:4522–4533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma Y, Zhao Q, Shao Y, Cao MZ, Zhao M and
Wang D: Melatonin inhibits the inflammation and apoptosis in rats
with diabetic retinopathy via MAPK pathway. Eur Rev Med Pharmacol
Sci. 23(Suppl 3): S1–S8. 2019.
|
|
53
|
Zhu L, Yi X, Zhao J, Yuan Z, Wen L,
Pozniak B, Obminska-Mrukowicz B, Tian Y, Tan Z, Wu J and Yi J:
Betulinic acid attenuates dexamethasone-induced oxidative damage
through the JNK-P38 MAPK signaling pathway in mice. Biomed
Pharmacother. 103:499–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xing Y, Yang SD, Wang MM, Dong F, Feng YS
and Zhang F: Electroacupuncture alleviated neuronal apoptosis
following ischemic stroke in rats via midkine and ERK/JNK/p38
signaling pathway. J Mol Neurosci. 66:26–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen S, Zhao L, Sherchan P, Ding Y, Yu J,
Nowrangi D, Tang J, Xia Y and Zhang JH: Activation of melanocortin
receptor 4 with RO27-3225 attenuates neuroinflammation through
AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J
Neuroinflammation. 15:1062018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gupta KK, Donahue DL, Sandoval-Cooper MJ,
Castellino FJ and Ploplis VA: Abrogation of plasminogen activator
inhibitor-1-vitronectin interaction ameliorates acute kidney injury
in murine endotoxemia. PLoS One. 10:e01207282015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen WC, Liang CJ, Huang TM, Liu CW, Wang
SH, Young GH, Tsai JS, Tseng YC, Peng YS, Wu VC and Chen YL:
Indoxyl sulfate enhances IL-1β-induced E-selectin expression in
endothelial cells in acute kidney injury by the ROS/MAPKs/NFκB/AP-1
pathway. Arch Toxicol. 90:2779–2792. 2016. View Article : Google Scholar
|
|
58
|
Chen X, Wei W, Li Y, Huang J and Ci X:
Hesperetin relieves cisplatin-induced acute kidney injury by
mitigating oxidative stress, inflammation and apoptosis. Chem Biol
Interact. 308:269–278. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Gradinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
60
|
Tanaka M, Kishimoto Y, Sasaki M, Sato A,
Kamiya T, Kondo K and Iida K: Terminalia bellirica (Gaertn.) Roxb.
extract and gallic acid attenuate LPS-induced inflammation and
oxidative stress via MAPK/NF-κB and Akt/AMPK/Nrf2 pathways. Oxid
Med Cell Longev. 2018:93643642018. View Article : Google Scholar
|
|
61
|
Zhao Y, Feng X, Li B, Sha J, Wang C, Yang
T, Cui H and Fan H: Dexmedetomidine protects against
lipopolysaccharide-induced acute kidney injury by enhancing
autophagy through inhibition of the PI3K/AKT/mTOR pathway. Front
Pharmacol. 11:1282020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fast DJ, Balles JA, Scholten JD, Mulder T
and Rana J: Echinacea purpurea root extract inhibits TNF release in
response to Pam3Csk4 in a phosphatidylinositol-3-kinase dependent
manner. Cell Immunol. 297:94–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Han QB: Critical problems stalling
progress in natural bioactive polysaccharide research and
development. J Agric Food Chem. 66:4581–4583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yu M, Xiao B, Hao X, Tan J, Gu J, Wang G,
Wang W and Zhang Y: Pumpkin polysaccharide preparation, stimulated
gastrointestinal digestion, and in vivo biodistribution. Int J Biol
Macromol. 141:1293–1303. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu H, Ma L and Wang Q: Possible metabolic
pathway of a novel bioactive polysaccharide extracted from
Dendrobium aphyllum: An in vivo study. J Food Sci. 84:1216–1223.
2019. View Article : Google Scholar : PubMed/NCBI
|