Introduction
Ischemic stroke is one of the most common causes of
death worldwide (1,2). Cerebral edema and elevated
intracranial pressure (ICP) are common complications following
ischemic stroke (3,4). Elevated ICP is a key factor
affecting the clinical and neurological outcomes of stroke
(5-7); however, the underlying mechanisms
remain unclear.
Inflammation in the central nervous system is an
important cause of secondary brain injury that occurs following
cerebral ischemia (8). The
microglial cells are the resident immunocytes in the brain and are
activated within the first few hours following cerebral infarction
and release a large number of inflammatory cytokines (9-12).
Recent studies have reported that intracellular NOD-like receptors,
including NOD-like receptor protein 3 (NLRP3), are widely expressed
in microglia (13-16). NLRP3 inflammasome serves a key
role in initiating and amplifying the inflammation in the central
nervous system (17-19). To exert its functions, the
activation of the NLRP3 inflammasome is first required. As several
studies have reported, the NLRP3 inflammasome can be triggered by
reactive oxygen species (ROS) (20-23). The expression of IL-1β and IL-18
can be upregulated following activation of the NLRP3 inflammasome,
which promotes inflammation in the central nervous system and
eventually leads to the aggravation of brain injuries following
ischemic stroke (24,25). Whether ICP can mediate NLRP3
inflammasome activation in ischemic microglia remains to be
clarified.
In the present study, it was hypothesized that
elevated ICP may aggravate nerve injury that occurs following
cerebral ischemia. The possible underlying mechanism was determined
to involve elevated ICP, which in-turn increases IL-1β and IL-18
secretion via activation of the NLRP3 inflammasome.
Materials and methods
Animals and treatment
Male adult Sprague-Dawley rats aged 3-4 months
weighing 220-250 g were provided by the Institute of Laboratory
Animal Science of Jinan University (Guangzhou, China). The rats
were fed standard chow and water, and housed under standard
experimental conditions (temperature, 20-25°C; humidity, 50-70%)
with a 12-h light/dark cycle for a week. As few animals as possible
were used in the experiments. A total of 160 rats were randomly
divided into four groups (n=40 rats per group): i) Sham-operated
group (sham group); ii) cerebral ischemia-reperfusion (IR) group
(IR group); iii) cerebral IR + normal saline group (NS group); and
iv) cerebral IR + 10% hypertonic saline group (HS group). Rats in
the IR, NS and HS groups were subjected to middle cerebral artery
occlusion (MCAO). Rats in the sham group were subjected to all the
procedures without occlusion. The tail vein was cannulated for
intravenous infusion of 10% HS or normal saline. After IR, the rats
in the NS group and HS group were continuously administered NS (0.3
ml/h) and 10% HS (0.3 ml/h) by intravenous injection, respectively.
All animals were observed closely for 24 h.
Rat model of cerebral ischemia
Before the surgical procedure, all rats were fasted
with access to water overnight. Cerebral ischemia was induced by
right-sided MCAO as described previously (26). The rats were anesthetized with
pentobarbital sodium (30 mg/kg intraperitoneal injection) followed
by a midline incision. The right common carotid artery, internal
carotid artery and external carotid artery were carefully exposed.
A head-end spherical nylon suture was inserted from the external
carotid artery into the middle cerebral artery until resistance was
felt. The suture remained in place for 2 h, after which it was
withdrawn to allow reperfusion. The health and behavior of the rats
were monitored every 2 h after surgery. The rats (n=23) who could
not walk spontaneously and had a depressed level of consciousness,
were excluded from the study. The rats were anesthetized with
sodium pentobarbital (30 mg/kg intraperitoneal injection) and
euthanized with 0.9% sodium chloride intravenous perfusion and
arterial exsan-guination. The Research Ethics Committee of
Guangdong Provincial People's Hospital and Guangdong Academy of
Medical Sciences approved all animal procedure protocols [approval
no. GDREC2012106A(R1); Guangzhou, China].
Measurement of ICP
The ICP was measured 0, 2, 4, 8, 12, 16, 20 and 24 h
after surgery (n=8 for each group). To evaluate the ICP, a midline
incision over the vertex was performed following anesthesia, and
then a hole caudal to the coronal suture was drilled, 4 mm from the
midline. The dura was punctured and a microsensor for ICP was
inserted intracranially (3). An
ICP monitor (Integra CAMO2; Integra LifeSciences) was used to
measure the ICP.
Measurement of ROS levels in brain
tissue
The ROS levels in brain tissues were evaluated using
a ROS ELISA kit (cat. no. DG21175D-96; DG Biotech) 24 h after IR.
Briefly, samples and standards (50 µl/well) were added to
the plate wells coated with HRP-conjugated antibodies, which were
used to capture ROS. The plates were incubated for 1 h at 37°C.
After washing completely, substrate A (50 µl/well) and
substrate B (50 µl/well) were added to incubate the plate in
the dark for 15 min at 37°C. Then, the stop buffer was added, and
the optical density was measured spectrophotometrically at a
wavelength of 450 nm. The concentrations of ROS in the samples were
then determined by comparing the optical density of the samples to
the standard curve.
BV-2 microglial cell cultures and
treatment
BV-2 microglial cells (cat. no. 7-1502) were
purchased from CHI Scientific, Inc., and were cultured and treated
as described in our previous study (27). Briefly, the cells were cultured on
six well plates at a density of 1.5×106 cells/well with
DMEM high glucose (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.).
The microglial cells were randomly divided into three groups: i)
Control group; ii) oxygen-glucose deprivation (OGD) + 20 mmHg
group; and iii) OGD group. The cells in the OGD group were cultured
for 2 h with glucose-free medium in an airtight hypoxia chamber
with 0.1% O2/5% CO2 at 37°C, and then the
cells were switched to DMEM high glucose in an incubator with 5%
CO2/95% air for 24 h. The cells in the OGD + 20 mmHg
group were exposed to a higher atmospheric pressure (20 mmHg) for
24 h with a high-pressure installation when the oxygen-glucose
supply was reinstated. The cells in the control group were cultured
in the DMEM high glucose containing 10% FBS.
ROS measurement in microglia
The ROS production in the BV-2 microglial cells was
evaluated using a ROS assay kit (cat. no. BB-4705-2; BestBio),
according to the manufacturer's protocol. Briefly, DCFH-DA was
diluted with DMEM high glucose without FBS (1:1,500). The
coverslips with adherent BV-2 microglial cells were cultured in
DMEM high glucose supplemented with 10% FBS. Following treatment,
the medium was changed to diluted DCFH-DA (2 ml/well). Then, the
plates were incubated for 20 min at 37°C and 5% CO2. The
coverslips were washed with DMEM high glucose without FBS. Finally,
the coverslips were mounted using a fluorescent mounting medium and
visualized under a fluorescence microscope (Olympus DP73
Microscope; Olympus Corporation).
Western blotting analysis
Total proteins from the peri-infarcted cerebral
cortex and BV-2 microglial cells (n=4 per group) were extracted
using a Total Protein Extraction kit (cat. no. BB-3101-100T;
BestBio) as described previously (26). Protein concentration was
determined using a Pierce™ BCA Protein assay kit (cat. no. 23227;
Pierce; Thermo Fisher Scientific, Inc.). Equal quantities of
protein from each sample (40 µg per lane) were separated via
10% SDS-PAGE, and then transferred to PVDF membranes, which were
blocked with 5% non-fat milk for 1 h at room temperature.
Subsequently, the following primary antibodies were added to
incubate the membranes overnight at 4°C: Caspase-1 (1:1,000; cat.
no. 24232S; Cell Signaling Technology, Inc.), IL-1β (1:1,000; cat.
no. 12703S; Cell Signaling Technology, Inc.), IL-18 (1:1,000; cat.
no. ab207323; Abcam) and gasdermin D-N domains (GSDMD-N; 1:1,000;
cat. no. 36425S; Cell Signaling Technology, Inc.). The membranes
were washed the following day, and the HRP-conjugated goat
anti-rabbit antibody (1:2,000; cat. no. 7074S; Cell Signaling
Technology, Inc.) was added and the membrane was incubated for 2 h
at 4°C. The immunoblots were visualized using a chemiluminescence
kit (Bioworld Technology, Inc.), and detected using an imaging
densitometer (ImageQuant™ LAS 500; Cytiva). The relative density
was semi-quantified using FluorChem 8900 software (version 4.0.1;
ProteinSimple). β-actin was used as the loading control.
Double immunofluorescence labeling
After 24 h of reperfusion, the rats were
anesthetized with sodium pentobarbital (30 mg/kg intraperitoneal
injection) and transcardially perfused with saline and 4%
paraformaldehyde sequentially. The brains were harvested and
post-fixed in 4% paraformaldehyde for 24 h at 4°C. These tissue
samples were then dehydrated in a graded series of sucrose
solutions, embedded in optimal cutting temperature compound and cut
into 10-µm thick sections. In vitro, the coverslips
with adherent BV-2 microglial cells were fixed with 4%
paraformaldehyde for 20 min at room temperature 24 h after
treatment.
The sections/coverslips were blocked in 5% normal
donkey serum (cat. no. ab7475; Abcam) for 0.5 h at room
tempera-ture. Subsequently, they were incubated with the following
primary antibodies overnight at 4°C: Caspase-1 (1:100; cat. no.
24232S; Cell Signaling Technology, Inc.), IL-1β (1:100; cat. no.
12703S; Cell Signaling Technology, Inc.), IL-18 (1:100; cat. no.
ab207323; Abcam), and Iba1 (1:100; cat. no. ab15690; Abcam). The
sections/coverslips were washed the following day, and the
secondary antibodies, Alexa Fluor® 549 goat anti-rabbit
IgG (H+L) (1:100; cat. no. ATRJN1301; Invitrogen; Thermo Fisher
Scientific, Inc.) and Alexa Fluor® 488 Goat anti-mouse
IgG (1:100; Invitrogen; cat. no. ATRMR2301; Thermo Fisher
Scientific, Inc.) were added to the sections and incubated for 1 h
at room temperature. Finally, the sections were mounted using a
fluorescent mounting medium with DAPI (Sigma-Aldrich; Merck KGaA)
and visualized using a fluorescence microscope.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 (SPSS, Inc.). All values are expressed as the mean ±
standard error of the mean. Repeated measures ANOVA was used to
analyze the repeated measurement data. A one-way ANOVA was used to
analyze the data of three or four-group univariate-factor
measurements. Following ANOVA, multiple comparisons were performed
using Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ICP levels following MCAO
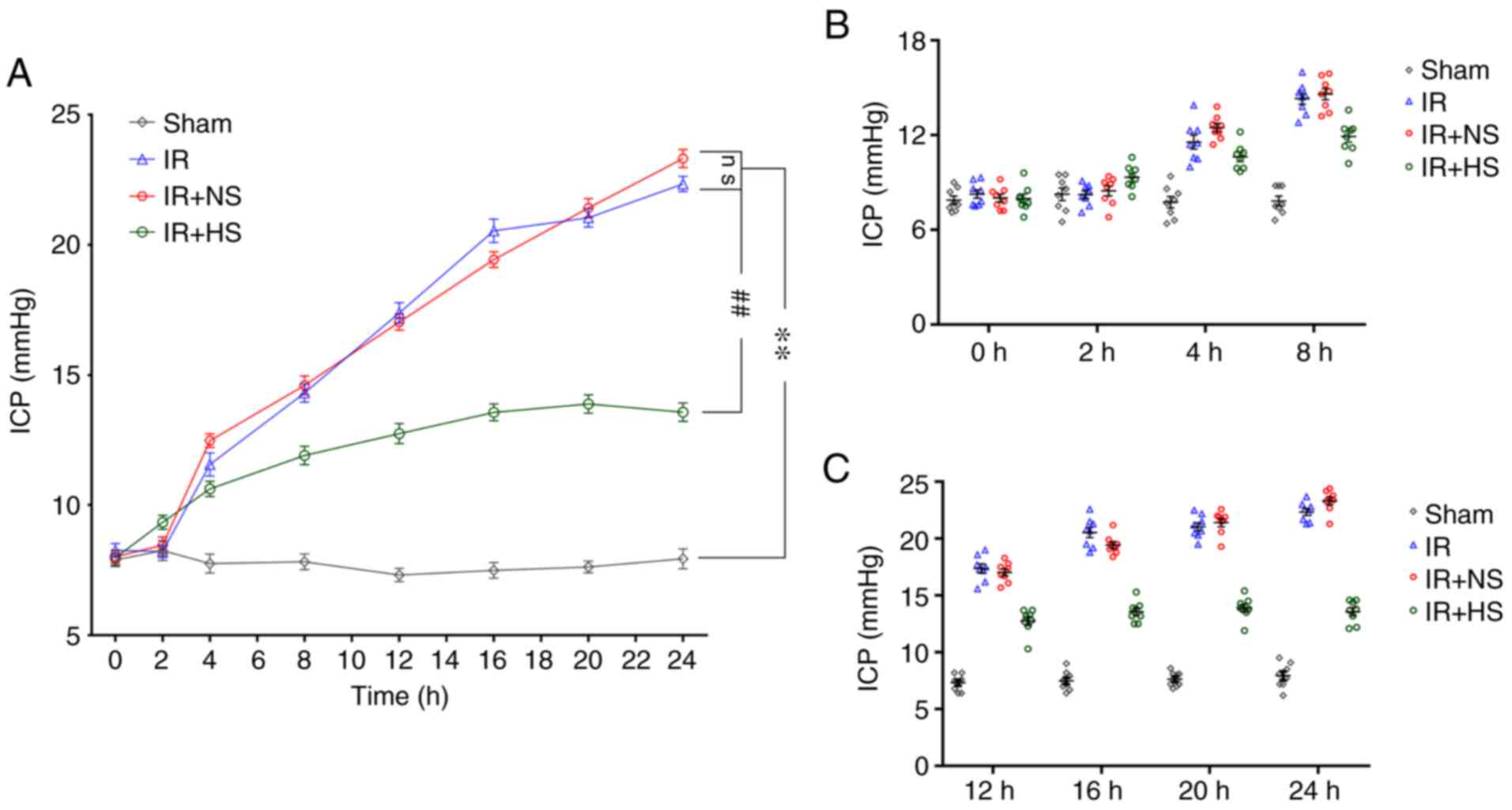
The ICP levels in the IR group and the IR + NS group
were significantly higher than the sham group (P<0.01). There
were no significant differences between the IR group and the IR +
NS group (P>0.05). The ICP levels in the IR + HS group were
significantly lower compared with the IR group (P<0.01; Fig. 1A-C; Table I).
 | Table IICP levels 0, 2, 4, 8, 12, 16, 20 and
24 h after ischemia-reperfusion in vivo. |
Table I
ICP levels 0, 2, 4, 8, 12, 16, 20 and
24 h after ischemia-reperfusion in vivo.
| Time Group, h | Sham, mmHg | IR, mmHg | IR + NS, mmHg | IR + HS, mmHg |
|---|
| 0 | 7.89±0.72 | 8.26±0.74a | 8.01±0.69a | 7.98±0.82b |
| 2 | 8.25±1.10 | 8.23±0.68a | 8.46±0.89a | 9.34±0.77b |
| 4 | 7.75±1.02 | 11.56±1.26a | 12.48±0.75a | 10.63±0.82b |
| 8 | 7.83±0.86 | 14.31±1.01a | 14.60±1.03a | 11.91±1.02b |
| 12 | 7.31±0.72 | 17.39±1.12a | 17.04±0.87a | 12.75±1.10b |
| 16 | 7.49±0.85 | 20.55±1.30a | 19.44±0.84a | 13.56±0.93b |
| 20 | 7.63±0.65 | 21.05±1.00a | 21.43±1.03a | 13.89±1.00b |
| 24 | 7.94±1.09 | 22.35±0.84a | 23.33±0.98a | 13.58±1.00b |
Elevated ICP promotes ROS
overproduction
The ROS levels in the IR group and the IR + NS group
were significantly higher compared with the sham group (P<0.01).
There was no significant difference between the IR group and the IR
+ NS group (P>0.05). The ROS levels in the IR + HS group were
significantly lower compared with the IR group when ICP levels were
reduced by HS (P<0.01; Fig.
2A). In vitro, increased ROS immunofluorescence was
observed in the OGD + 20 mmHg group compared with the control
group. Compared with the OGD + 20 mmHg group, ROS fluorescence was
notably reduced in the OGD group without high-pressure treatment
(Fig. 2B).
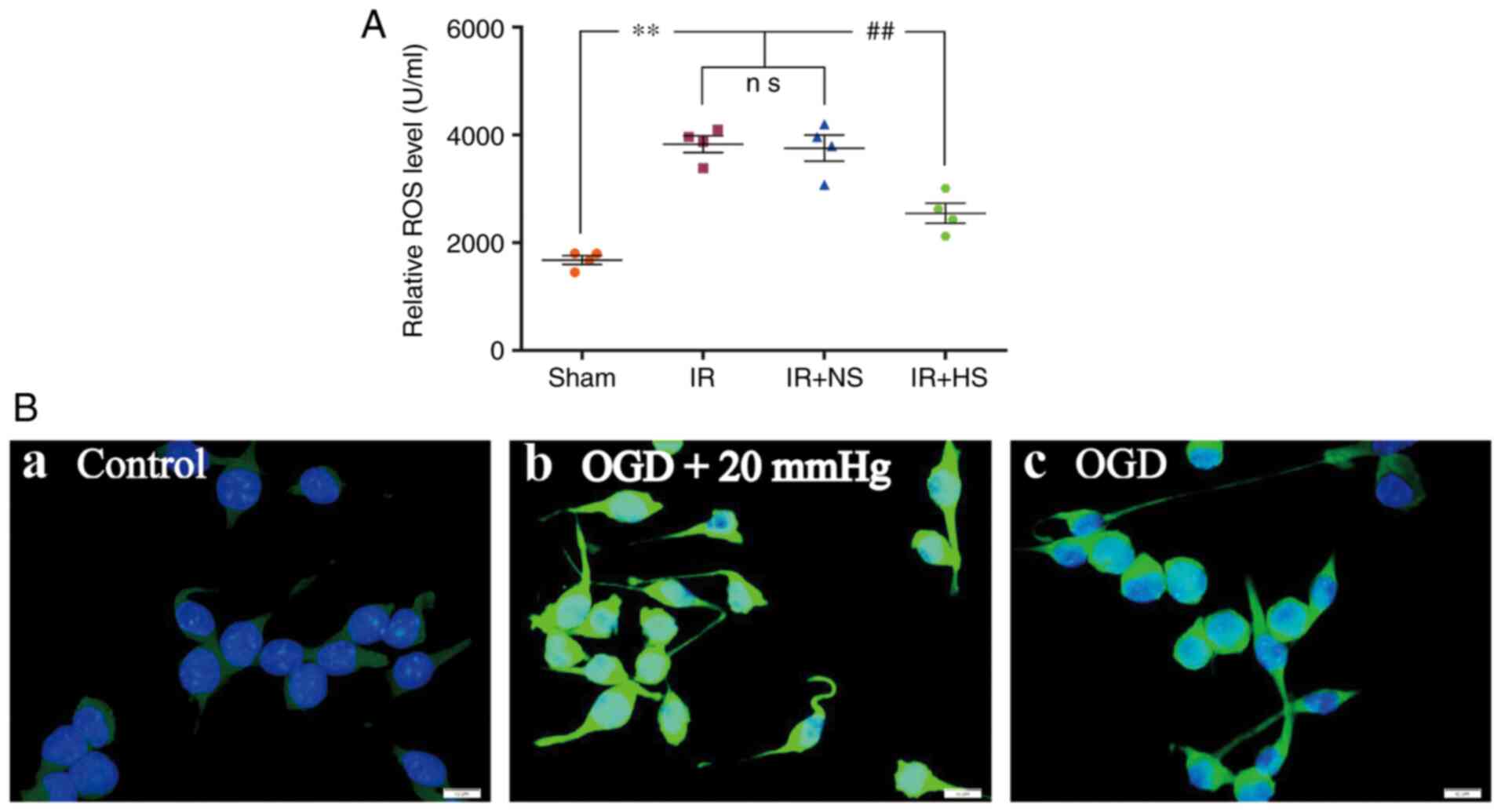 | Figure 2Elevated ICP promotes ROS
overproduction in ischemic microglia both in vivo and in
vitro. (A) ROS levels in the IR group and the IR + NS group
were significantly higher compared with the sham group. There was
no significant difference between the IR group and the IR + NS
group. ROS levels in the IR + HS group were significantly lower
compared with the IR group when ICP levels were reduced by HS. (B)
Immunofluorescence images show the production of ROS (green) in the
(B-a) control, (B-b) OGD + 20 mmHg and (B-c) OGD groups. Enhanced
ROS immunofluorescence was observed in the BV-2 microglial cells of
the OGD + 20 mmHg group compared with the control group. Compared
with the OGD + 20 mmHg group, ROS fluorescence was notably reduced
in the OGD group without high-pressure treatment. Scale bar, 10
µm. n=4 per group. **P<0.01 vs. sham group;
##P<0.01 vs. IR group. ICP, intracranial pressure;
sham, sham-operated; IR, ischemia-reperfusion; NS, normal saline;
HS, hypertonic saline group; OGD, oxygen-glucose deprivation; ns,
non-significant; ROS, reactive oxygen species. |
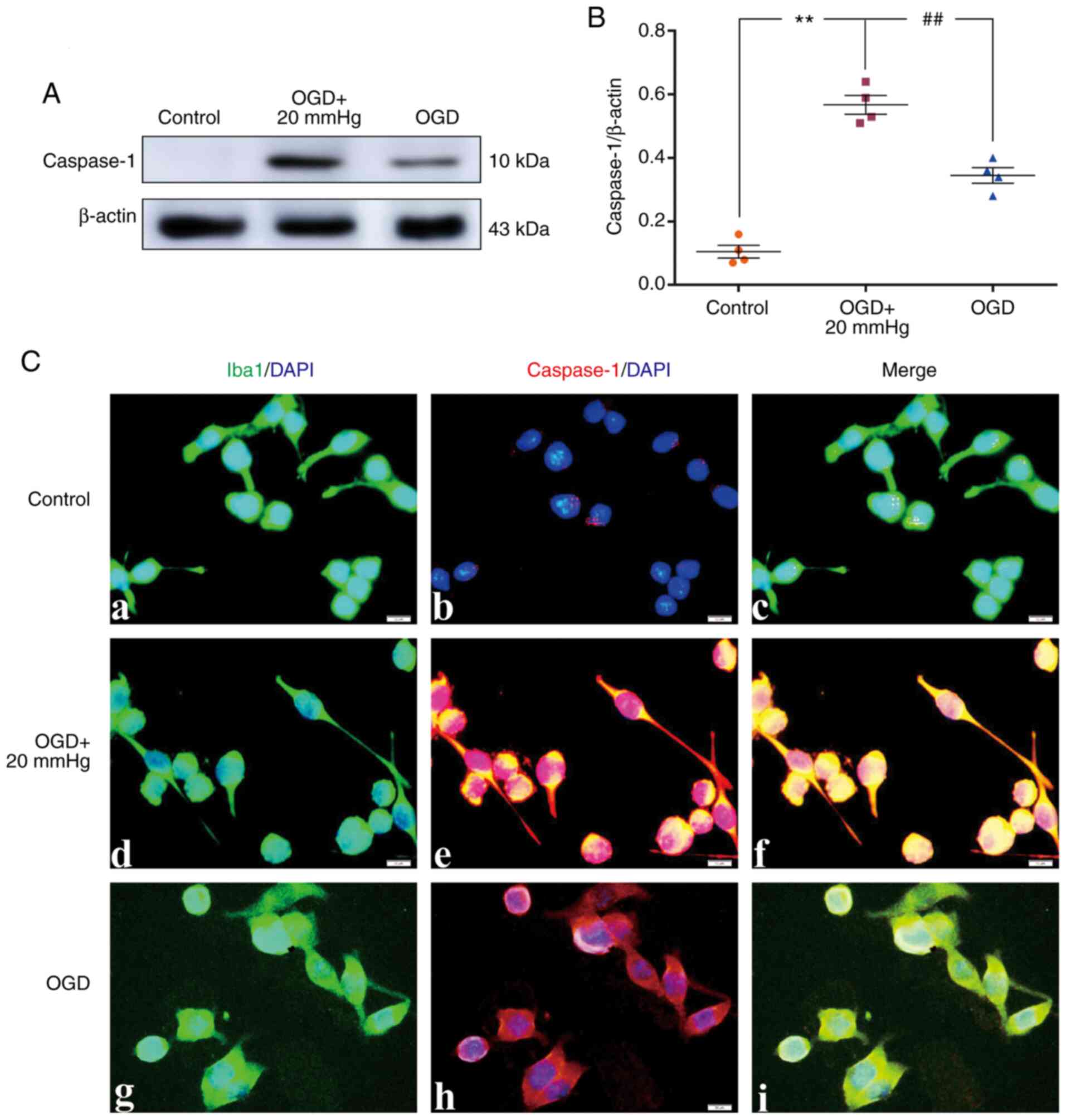
Elevated ICP promotes Caspase-1
activation
In vivo, the protein expression levels of
Caspase-1 were significantly increased in the IR group and the IR +
NS group compared with the sham group (P<0.01). There was no
significant difference between the IR group and the IR + NS group
(P>0.05). The levels in the IR + HS group were significantly
lower than the IR group when ICP levels were reduced by HS
(P<0.01; Fig. 3A and B).
Double immunofluorescence staining was used to examine Caspase-1
expression in the microglia of the peri-infarcted brain tissue.
Increased Caspase-1 immunofluorescence was observed in the IR group
and the IR + NS group compared with the sham group. When ICP levels
were reduced by HS, Caspase-1 fluorescence was noticeably
attenuated (Fig. 3C).
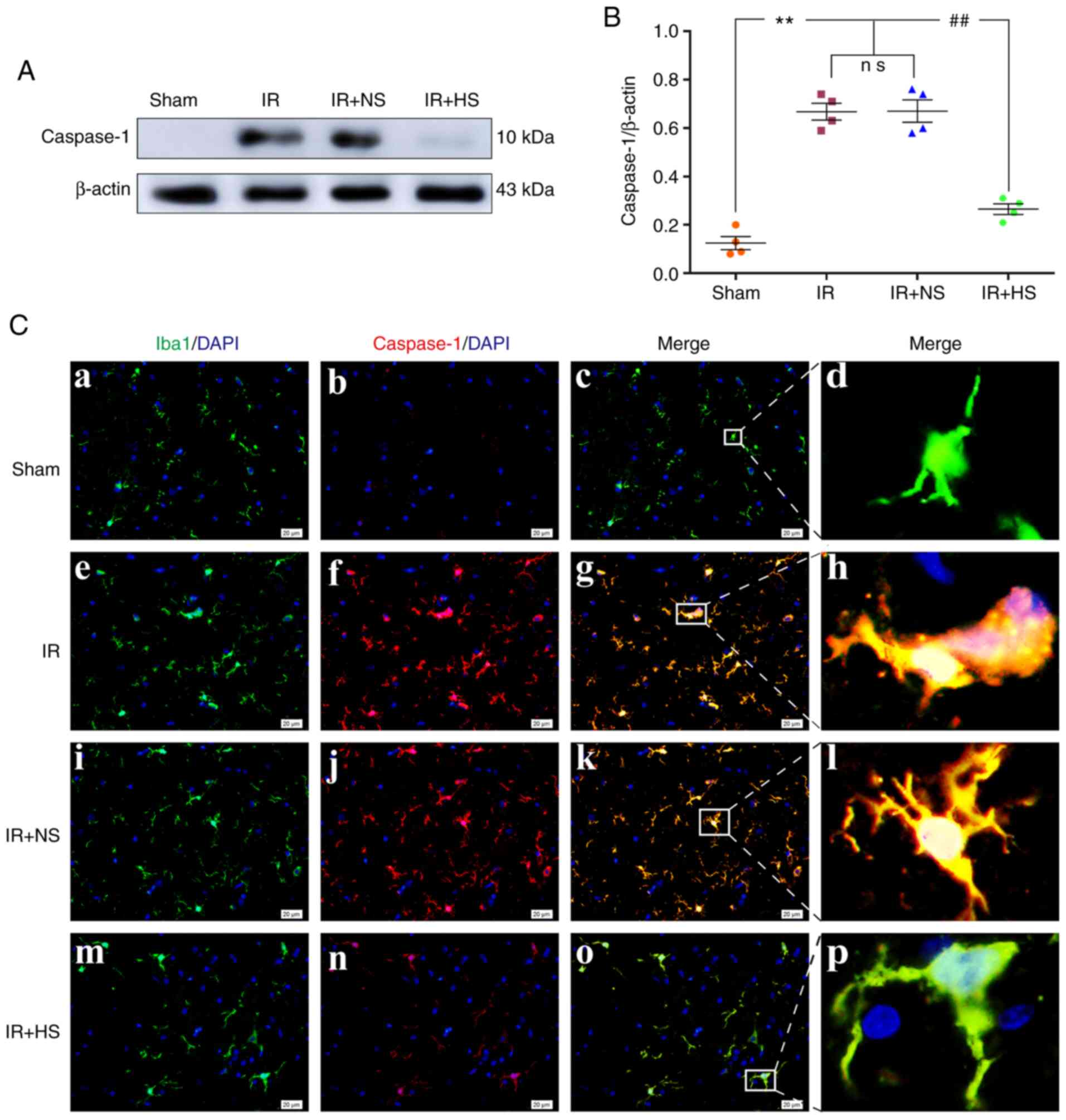 | Figure 3Elevated ICP promotes Caspase-1
expression in microglia following IR in vivo. (A)
Immunoreactive bands of Caspase-1 (10 kDa) and β-actin (43 kDa).
(B) Protein expression levels of Caspase-1 were significantly
increased in the IR group and the IR + NS group compared with the
sham group. There was no significant difference between the IR
group and the IR + NS group. The levels in the IR + HS group were
significantly lower than the IR group when ICP levels were reduced
by HS. (C) Immunofluorescence images showing the expression of
(C-a, C-e, C-i and C-m) Iba1+ microglia (green), (C-b, C-f, C-j and
C-n) Caspase-1 (red), (C-c, C-g, C-k and C-o) the co-localization
of Caspase-1 and microglia, and (C-d, C-h, C-l and C-p) high
amplification images of the microglial cells in peri-ischemic
cortex. Increased Caspase-1 immunofluorescence was observed in the
IR group and the IR + NS group compared with the sham group. When
ICP levels were reduced by HS, Caspase-1 fluorescence was notably
attenuated. Scale bar, 20 µm. n=4 per group.
**P<0.01 vs. sham group; ##P<0.01 vs.
IR group. ICP, intracranial pressure; sham, sham-operated; IR,
ischemia-reperfusion; NS, normal saline; HS, hypertonic saline
group; ns, non-significant. |
In vitro, the protein expression levels of
Caspase-1 were significantly increased in the OGD + 20 mmHg group
compared with the control group (P<0.01). Compared with the OGD
+ 20 mmHg group, the expression levels were significantly reduced
in the OGD group without high-pressure treatment (P<0.01;
Fig. 4A and B). Double
immunofluorescence staining was used to examine Caspase-1
expression in the BV-2 microglial cells. Increased Caspase-1
immunofluorescence was observed in the OGD + 20 mmHg group compared
with the control group. Compared with the OGD + 20 mmHg group, the
fluorescence was notably reduced in the OGD group without
high-pressure treatment (Fig.
4C).
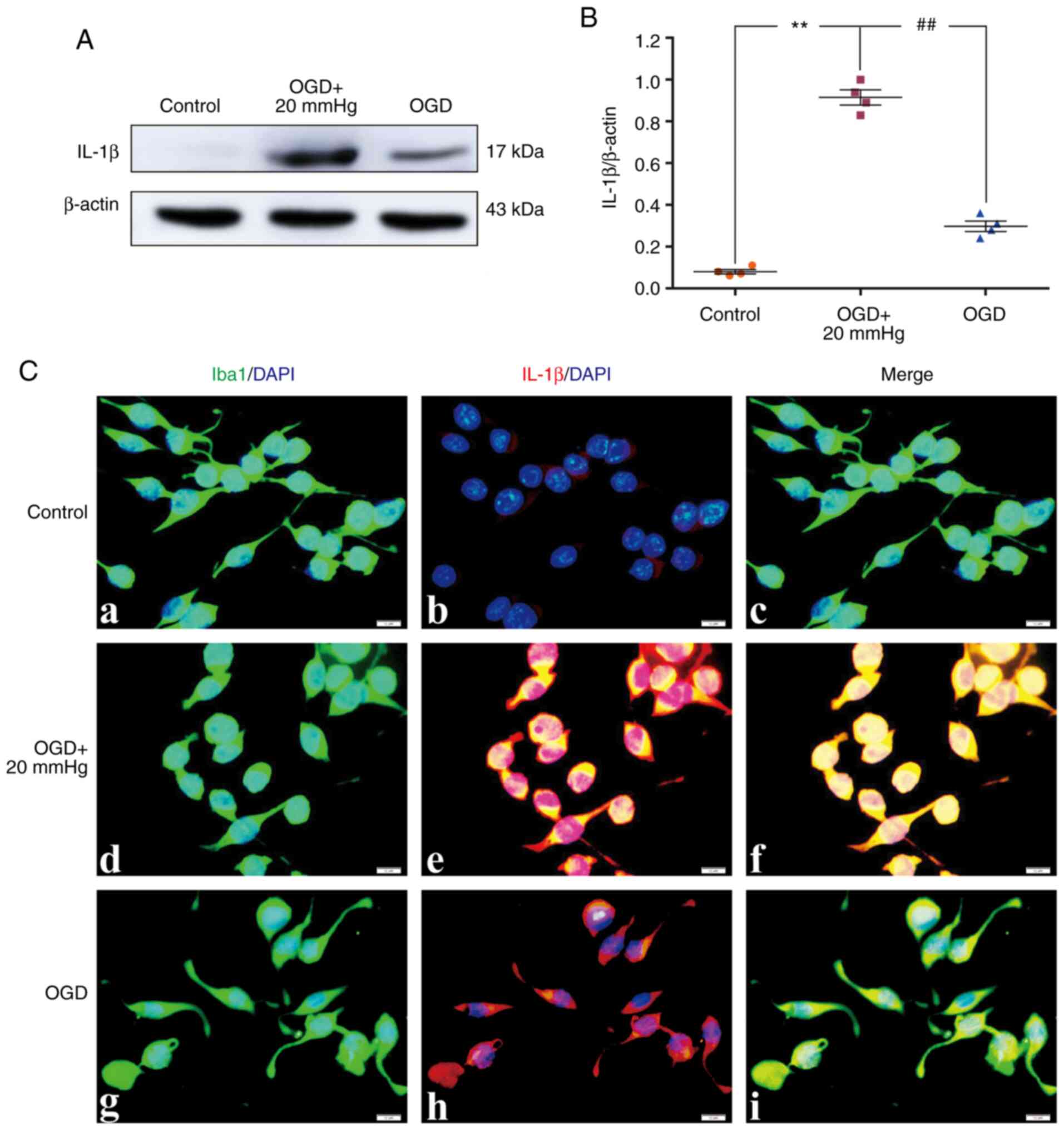
Elevated ICP increases IL-1β
expression
The protein expression levels of IL-1β in
vivo were significantly increased in the IR group and the IR +
NS group compared with the sham group (P<0.01). There were no
significant differences between the IR and the IR + NS group
(P>0.05). The IL-1β expression levels in the IR + HS group were
significantly lower than the IR group when ICP levels were reduced
by HS (P<0.01; Fig. 5A and B).
Double immunofluorescence staining was used to examine IL-1β
expression in the microglia of the peri-infarcted brain tissue.
Increased IL-1β immunofluorescence was observed in the IR group and
the IR + NS group compared with the sham group. When ICP levels
were reduced by HS, IL-1β fluorescence was notably attenuated
(Fig. 5C).
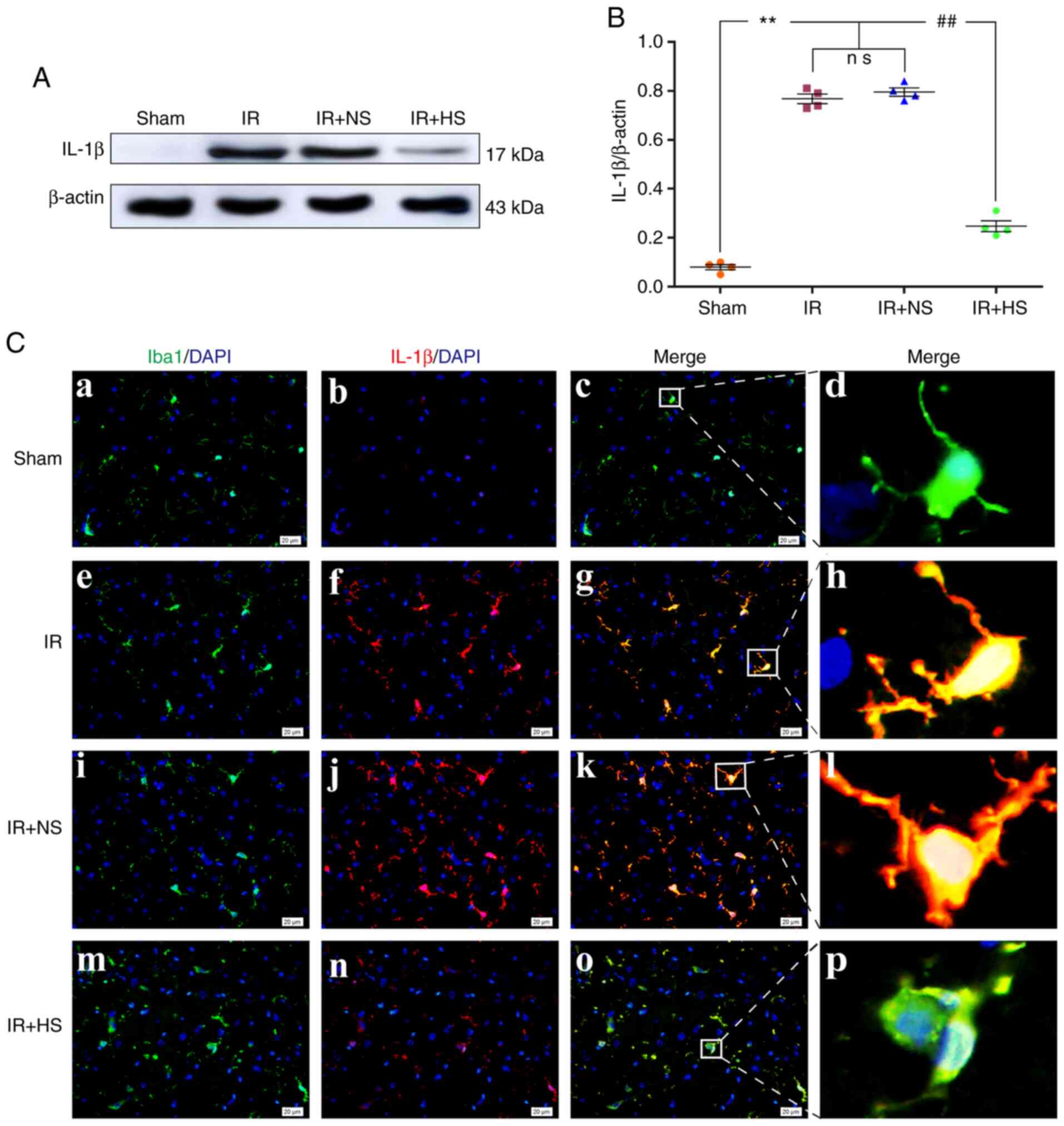 | Figure 5Elevated ICP increases IL-1β
expression in the microglia following IR in vivo. (A)
Immunoreactive bands of IL-1β (17 kDa) and β-actin (43 kDa). (B)
Protein expression levels of IL-1β were significantly increased in
the IR and the IR + NS groups compared with the sham group. There
were no significant differences between the IR and the IR + NS
group. The levels in the IR + HS group were significantly lower
than in the IR group when ICP levels were reduced by HS. (C)
Immunofluorescence images showing the expression of (C-a, C-e, C-i
and C-m) Iba1+ microglia (green), (C-b, C-f, C-j and C-n) IL-1β
(red), (C-c, C-g, C-k and C-o) the co-localization of IL-1β and
microglia, and (C-d, C-h, C-l and C-p) high amplification images of
the microglial cells in peri-ischemic cortex. Enhanced IL-1β
immunofluorescence was observed in the IR and the IR + NS groups
compared with the sham group. In the IR + HS group, IL-1β
fluorescence was notably decreased. Scale bar, 20 µm. n=4
per group. **P<0.01 vs. sham group;
##P<0.01 vs. IR group. ICP, intracranial pressure;
sham, sham-operated; IR, ischemia-reperfusion; NS, normal saline;
HS, hypertonic saline group; ns, non-significant. |
In vitro, the protein expression levels of
IL-1β were significantly increased in the OGD + 20 mmHg group
compared with the control group (P<0.01). Compared with the OGD
+ 20 mmHg group, the expression levels were significantly reduced
in the OGD group without high-pressure treatment (P<0.01;
Fig. 6A and B). Double
immunofluorescence staining was used to examine IL-1β expression in
the BV-2 microglial cells. Enhanced IL-1β immunofluorescence was
observed in the OGD + 20 mmHg group compared with the control
group. Compared with the OGD + 20 mmHg group, the fluorescence was
notably reduced in the OGD group without high-pressure treatment
(Fig. 6C).
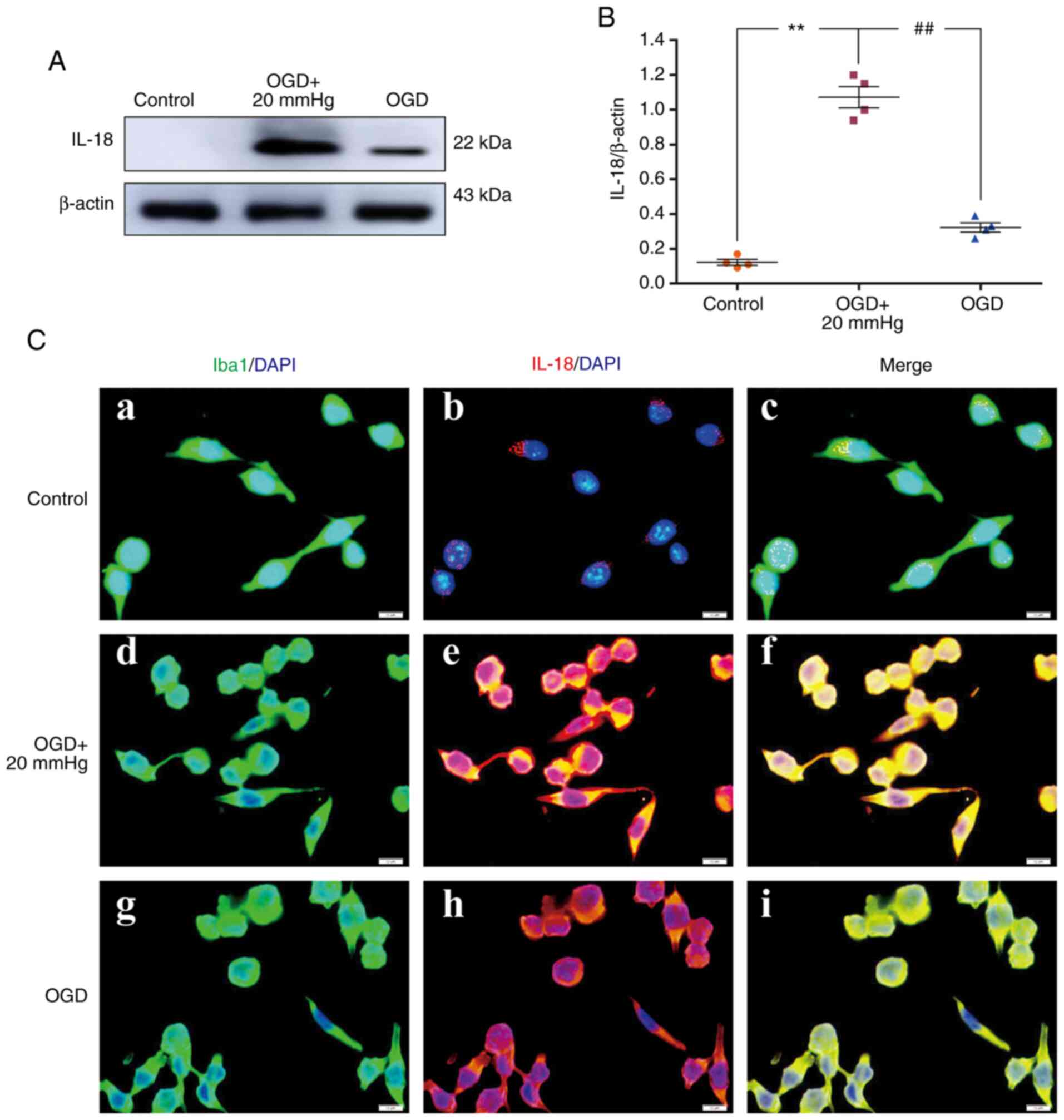
Elevated ICP increases IL-18
expression
The protein expression levels of IL-18 in
vivo were significantly increased in the IR group and the IR +
NS group compared with the sham group (P<0.01). There were no
significant differences between the IR group and the IR + NS group
(P>0.05). IL-18 expression levels in the IR + HS group were
significantly lower when ICP levels were reduced by HS compared
with the IR group (P<0.01; Fig. 7A
and B). Double immunofluorescence staining was used to examine
IL-18 expression in the microglia of the peri-infarcted brain
tissue. Enhanced IL-18 immunofluorescence was observed in the IR
group and the IR + NS group compared with the sham group. When ICP
levels were reduced by HS, IL-18 fluorescence was noticeably
attenuated (Fig. 7C).
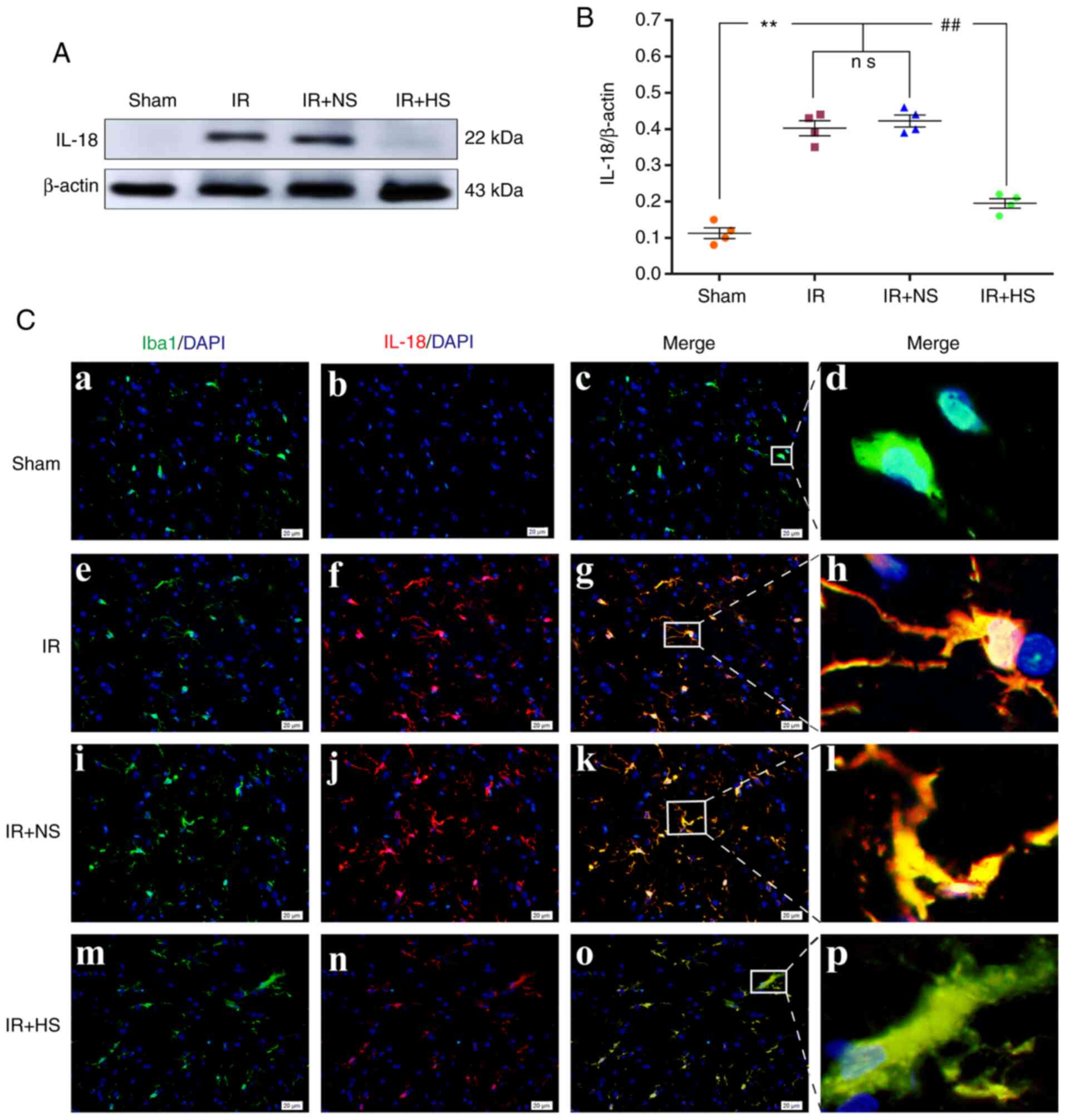 | Figure 7Elevated ICP increases IL-18
expression in microglia following IR in vivo. (A)
Immunoreactive bands of IL-18 (22 kDa) and β-actin (43 kDa). (B)
Protein expression levels of IL-18 were significantly increased in
the IR group and the IR + NS group compared with the sham group.
There was no significant difference between the IR group and the IR
+ NS group. The levels in the IR + HS group were significantly
lower than the IR group when ICP levels were reduced by HS. (C)
Immunofluorescence images showing the expression of (C-a, C-e, C-i
and C-m) Iba1+ microglia (green), (C-b, C-f, C-j and C-n) IL-18
(red), (C-c, C-g, C-k and C-o) the co-localization of IL-18 and
microglia, and (C-d, C-h, C-l and C-p) high amplification images of
the microglial cells in peri-ischemic cortex. Increased IL-18
immunofluorescence was observed in the IR group and the IR + NS
group compared with the sham group. In the IR + HS group, IL-18
fluorescence was notably reduced. Scale bar, 20 µm. n=4 per
group. **P<0.01 vs. sham group;
##P<0.01 vs. IR group. ICP, intracranial pressure;
sham, sham-operated; IR, ischemia-reperfusion; NS, normal saline;
HS, hypertonic saline group; ns, non-significant. |
In vitro, the protein expression levels of
IL-18 were significantly increased in the OGD + 20 mmHg group
compared with the control group (P<0.01). Compared with the OGD
+ 20 mmHg group, the expression levels were significantly reduced
in the OGD group without high-pressure treatment (P<0.01;
Fig. 8A and B). Double
immunofluorescence staining was used to examine IL-18 expression in
the BV-2 microglial cells. Enhanced IL-18 immunofluorescence was
observed in the OGD + 20 mmHg group compared with the control
group. Compared with the OGD + 20 mmHg group, fluorescence was
notably reduced in the OGD group without high-pressure treatment
(Fig. 8C).
Elevated ICP increases GSDMD-N
expression
The protein expression levels of GSDMD-N in
vivo were significantly increased in the IR group and the IR +
NS group compared with the sham group (P<0.01). There were no
significant differences between the IR and the IR + NS group
(P>0.05). The levels in the IR + HS group were significantly
lower than the IR group when ICP levels were reduced by HS
(P<0.01; Fig. 9A and B).
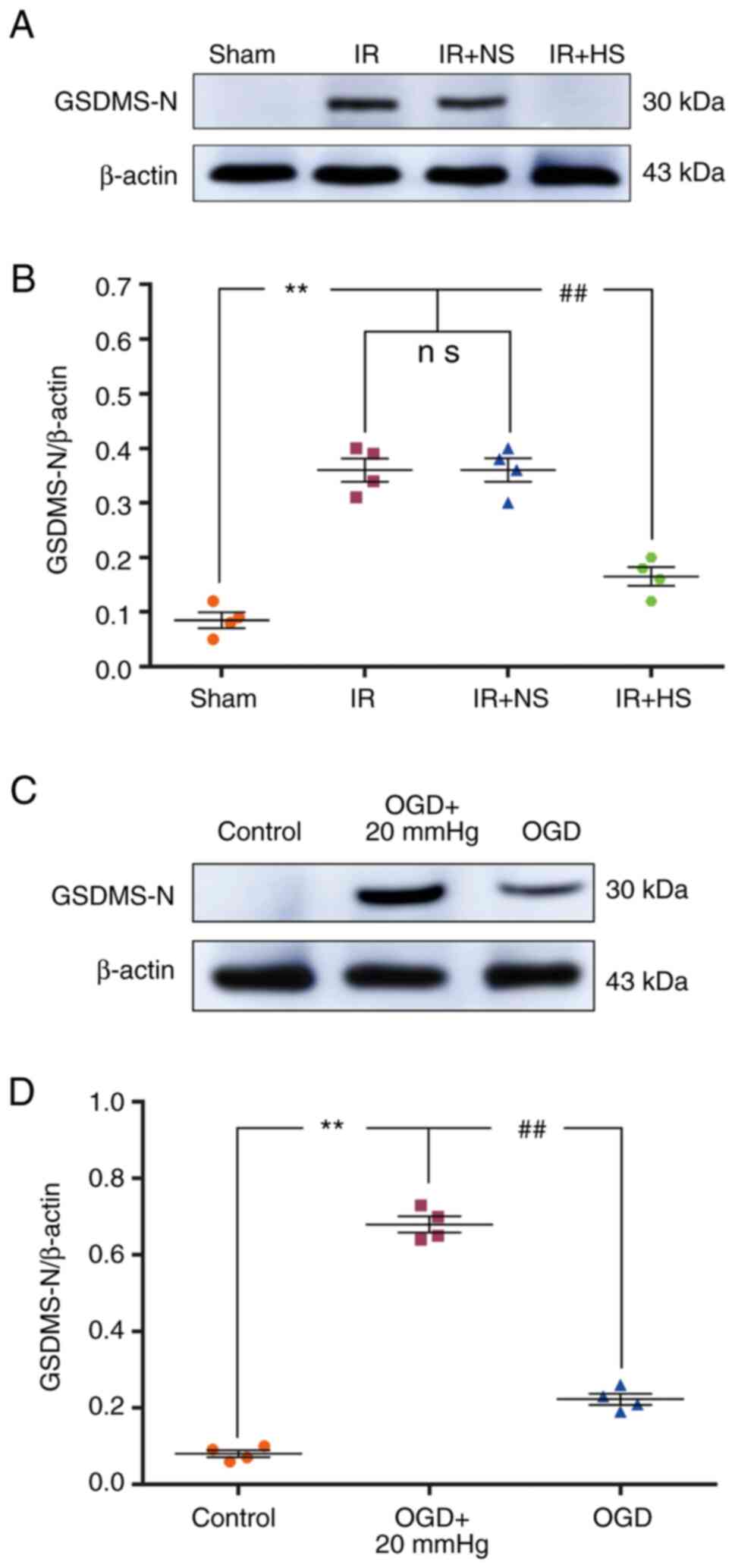 | Figure 9Elevated ICP increases GSDMD-N
expression in ischemic microglia both in vivo and in
vitro. (A and C) Immunoreactive bands of GSDMD-N (30 kDa) and
β-actin (43 kDa). (B) Protein expression levels of GSDMD-N were
significantly increased in the IR group and the IR + NS group
compared with the sham group. There was no significant difference
between the IR group and the IR + NS group. The levels in the IR +
HS group were significantly lower than the IR group when ICP levels
were reduced by HS. (D) In vitro, the protein expression
levels of GSDMD-N were significantly increased in the OGD + 20 mmHg
group compared with the control group. Compared with the OGD + 20
mmHg group, the expression levels were significantly reduced in the
OGD group without high-pressure treatment. n=4 per group.
**P<0.01 vs. sham group or control group;
##P<0.01 vs. IR group or OGD + 20 mmHg group. ICP,
intracranial pressure; sham, sham-operated; IR,
ischemia-reperfusion; NS, normal saline; HS, hyper-tonic saline
group; OGD, oxygen-glucose deprivation; ns, non-significant;
GSDMD-N, gasdermin D-N domains. |
In vitro, the protein expression levels of
GSDMD-N were significantly increased in the OGD + 20 mmHg group
compared with the control group (P<0.01). Compared with the OGD
+ 20 mmHg group, the expression levels were significantly reduced
in the OGD group without high-pressure treatment (P<0.01;
Fig. 9C and D).
Discussion
In the present study, it was shown that elevated ICP
promoted NLRP3 inflammasome activation in microglia of ischemic
adult rats. This was evident based on the increased expression
levels of Caspase-1, GSDMD-N, IL-18 and IL-1β in the
ischemia-activated microglial cells.
ICP management is an essential means of preventing
secondary injury in the central nervous system following ischemic
stroke (5-7). Osmotherapy has been used as a
foundation for managing elevated ICP levels induced by cerebral
edema (5,6). Commonly used osmotic agents include
HS and mannitol (28,29). In the present study, to determine
the effects of HS on ICP following ischemic stroke, the ICP of the
rats were examined 0, 2, 4, 8, 12, 16, 20 and 24 h after MCAO. The
ICP levels were significantly increased following cerebral IR.
Following 10% HS treatment by intravenous injection, the ICP of the
rats after MCAO was significantly reduced. This was consistent with
the effects of HS on traumatic brain injury (30) and subarachnoid hemorrhage
(31).
The NLRP3 inflammasome can be triggered by ROS
(20-23). It has been reported that ROS
levels are increased during high altitude exposure in lowlanders,
which induced passive hypobaric hypoxia; optic nerve sheath
diameter (ONSD), which is an indirect measurement of ICP, is
concurrently increased. However, regression analysis did not infer
a causal relationship between oxidative stress biomarkers and
changes in ONSD (32). In the
present study, the ROS levels of the brain tissue were increased
when ICP was increased following cerebral IR. Additionally, ROS
overproduction was inhibited by reduced ICP. Furthermore, high
pressure (20 mmHg) combined with OGD treatment increased ROS
production in the BV-2 microglial cells compared with those
subjected to OGD treatment alone in vitro. These results
suggested that elevated ICP can enhance oxidative stress. To
determine whether elevated ICP activated the NLRP3 inflammasome via
ROS overproduction in ischemia-activated microglia, expression of
Caspase-1, GSDMD-N, IL-18 and IL-1β in the microglia were
determined both in vivo and in vitro. It was shown
that elevated pressure upregulated the expression of Caspase-1,
GSDMD-N, IL-18 and IL-1β in IR- or OGD-treated microglia both in
vivo and in vitro. More importantly, Caspase-1, GSDMD-N,
IL-18 and IL-1β expression in microglia was significantly
downregulated when the elevated pressure was reduced or removed.
These results suggested that elevated ICP increased NLRP3
inflammasome activation in ischemia-activated microglial cells via
induction of ROS overproduction.
However, there are limitations to the present study.
Firstly, it has been reported that activated microglia secrete
pro-inflammatory cytokines, including TNF-α, IL-18 and IL-1β
(33-35). However, the lack of investigation
of other cytokines (such as TNF-α) in the present study, is a
limitation and an area for future research. Secondly, the present
study did not determine the upstream mechanism by which
elevated
ICP promotes ROS overproduction in ischemic stroke.
It has been found that ischemia damages mitochondria and induces
ROS production (36,37). Mitophagy can eliminate damaged
mitochondria, reduce ROS production and then alleviate NLRP3
inflammasome activation (38).
Elevated ICP may promote ROS overproduction by inhibiting
mitophagy. It has been reported that the NLRP3 inflammasome is
expressed in astrocytes (39).
However, the lack of determination of NLRP3 inflammasome expression
in astrocytes in the present study is another limitation of the
present study. Finally, all animals were observed closely for 24 h
after IR in the present study. However, the lack of determination
of factors after various time points is another limitation.
In summary, elevated ICP was found to upregulate the
expression of Caspase-1, GSDMD-N, IL-18 and IL-1β in ischemic
microglia, which was significantly downregulated when ICP was
reduced. Thus, elevated ICP-induced Caspase-1, GSDMD-N, IL-18 and
IL-1β overproduction in the microglia may be a potential target for
mitigating neuroinflammation following an ischemic stroke.
Abbreviations:
|
ICP
|
intracranial pressure
|
|
ROS
|
reactive oxygen species
|
|
MCAO
|
middle cerebral artery occlusion
|
|
GSDMD-N
|
gasdermin D-N domains
|
|
IR
|
ischemia-reperfusion
|
|
OGD
|
oxygen-glucose deprivation
|
|
NLRP3
|
NOD-like receptor protein 3
|
Acknowledgments
The authors would like to thank Mr Zhengkang Ding
and Miss Zixi Yang (Guangdong Provincial People's Hospital,
Guangzhou, China) for the technical support.
Funding
The present work was supported by the National
Natural Science Foundation for Young Scientists of China (grant no.
82002074), the Medical Scientific Research Foundation of Guangdong
Province (grant nos. A2019135 and A2017284), the Scientific
research project of Guangdong Traditional Chinese Medicine Bureau
(grant no. 20201045), and the Science and Technology Program of
Guangzhou (grant no. 202002030338).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
HZ conceived the project and designed the
experiments. HD carried out the assessment of ICP and ROS
evaluation. YL established the rat model of MCAO. MW carried out
BV-2 microglial cell cultures and treatment. XL and YH performed
western blotting and immunofluorescence staining. HD conducted the
statistical analysis. HD and YL wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The Research Ethics Committee of Guangdong
Provincial People's Hospital and Guangdong Academy of Medical
Sciences approved all animal procedure protocols [approval no.
GDREC2012106A(R1); Guangzhou, China].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Phipps MS and Cronin CA: Management of
acute ischemic stroke. BMJ. 368:169832020.
|
|
2
|
Khandelwal P, Yavagal DR and Sacco RL:
Acute ischemic stroke intervention. J Am Coll Cardiol.
67:2631–2644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blaha M, Schwab J, Vajnerova O, Bednar M,
Vajner L and Michal T: Intracranial pressure and experimental model
of diffuse brain injury in rats. J Korean Neurosurg Soc. 47:7–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caltagirone C, Cisari C, Schievano C, Di
Paola R, Cordaro M, Bruschetta G, Esposito E and Cuzzocrea S:
Coultramicronized palmitoylethanolamide/luteolin in the treatment
of cerebral ischemia: From rodent to man. Transl Stroke Res.
7:54–69. 2016. View Article : Google Scholar
|
|
5
|
Robinson JD: Management of refractory
intracranial pressure. Crit Care Nurs Clin North Am. 28:67–75.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah A, Almenawer S and Hawryluk G: Timing
of decompressive craniectomy for ischemic stroke and traumatic
brain injury: A review. Front Neurol. 10:112019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei CC, Zhang ST, Tan G, Zhang SH and Liu
M: Impact of anemia on in-hospital complications after ischemic
stroke. Eur J Neurol. 25:768–774. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ and
Liu F: Regulation of microglial activation in stroke. Acta
Pharmacol Sin. 38:445–458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang S, Zhang H and Xu Y: Crosstalk
between microglia and T cells contributes to brain damage and
recovery after ischemic stroke. Neurol Res. 38:495–503. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao TZ, Ding Q, Hu J, He SM, Shi F and Ma
LT: GPER expressed on microglia mediates the anti-inflammatory
effect of estradiol in ischemic stroke. Brain Behav. 6:e4492016.
View Article : Google Scholar
|
|
12
|
Ma Y, Wang J, Wang Y and Yang GY: The
biphasic function of microglia in ischemic stroke. Prog Neurobiol.
157:247–272. 2017. View Article : Google Scholar
|
|
13
|
Goldmann T, Tay TL and Prinz M: Love and
death: Microglia, NLRP3 and the Alzheimer's brain. Cell Res.
23:595–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS,
Kwon HJ, Kim HM, Kim DH and Yoon SY: Autophagy in microglia
degrades extracellular β-amyloid fibrils and regulates the NLRP3
inflammasome. Autophagy. 10:1761–1775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Houtman J, Freitag K, Gimber N,
Schmoranzer J, Heppner FL and Jendrach M: Beclin1-driven autophagy
modulates the inflammatory response of microglia via NLRP3. Embo J.
38:e994302019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panicker N, Sarkar S, Harischandra DS,
Neal M, Kam TI, Jin H, Saminathan H, Langley M, Charli A, Samidurai
M, et al: Fyn kinase regulates misfolded α-synuclein uptake and
NLRP3 inflammasome activation in microglia. J Exp Med.
216:1411–1430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD,
Shen W, Zhou ML, Zhu L and Hang CH: Expression of the NLRP3
inflammasome in cerebral cortex after traumatic brain injury in a
rat model. Neurochem Res. 38:2072–2083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang N, Zhang X, Liu X, Wang H, Xue J, Yu
J, Kang N and Wang X: Chrysophanol inhibits NALP3 inflammasome
activation and ameliorates cerebral ischemia/reperfusion in mice.
Mediators Inflamm. 2014:3705302014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gustin A, Kirchmeyer M, Koncina E, Felten
P, Losciuto S, Heurtaux T, Tardivel A, Heuschling P and Dostert C:
NLRP3 inflammasome is expressed and functional in mouse brain
microglia but not in astrocytes. PLoS One. 10:e1306242015.
View Article : Google Scholar
|
|
20
|
Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G
and Yu M: Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflamma-some
activation in BV2 cells after cerebral ischemia reperfusion.
Inflamm Res. 67:57–65. 2018. View Article : Google Scholar
|
|
21
|
Wang H, Zhong D, Chen H, Jin J, Liu Q and
Li G: NLRP3 inflammasome activates interleukin-23/interleukin-17
axis during ischaemia-reperfusion injury in cerebral ischaemia in
mice. Life Sci. 227:101–113. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20:33282019. View Article : Google Scholar :
|
|
23
|
Harijith A, Ebenezer DL and Natarajan V:
Reactive oxygen species at the crossroads of inflammasome and
inflammation. Front Physiol. 5:3522014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fann DY, Lee SY, Manzanero S, Chunduri P,
Sobey CG and Arumugam TV: Pathogenesis of acute stroke and the role
of inflammasomes. Ageing Res Rev. 12:941–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang F, Wang Z, Wei X, Han H, Meng X,
Zhang Y, Shi W, Li F, Xin T, Pang Q and Yi F: NLRP3 deficiency
ameliorates neurovascular damage in experimental ischemic stroke. J
Cereb Blood Flow Metab. 34:660–667. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang LQ, Zhu GF, Deng YY, Jiang WQ, Fang
M, Chen CB, Cao W, Wen MY, Han YL and Zeng HK: Hypertonic saline
alleviates cerebral edema by inhibiting microglia-derived TNF-α and
IL-1β-induced Na-K-Cl Cotransporter up-regulation. J
Neuroinflammation. 11:1022014. View Article : Google Scholar
|
|
27
|
Ding HG, Deng YY, Yang RQ, Wang QS, Jiang
WQ, Han YL, Huang LQ, Wen MY, Zhong WH, Li XS, et al: Hypercapnia
induces IL-1β overproduction via activation of NLRP3 inflammasome:
Implication in cognitive impairment in hypoxemic adult rats. J
Neuroinflammation. 15:42018. View Article : Google Scholar
|
|
28
|
Changa AR, Czeisler BM and Lord AS:
Management of elevated intracranial pressure: A review. Curr Neurol
Neurosci Rep. 19:992019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernando SM, Tran A, Cheng W, Rochwerg B,
Taljaard M, Kyeremanteng K, English SW, Sekhon MS, Griesdale D,
Dowlatshahi D, et al: Diagnosis of elevated intracranial pressure
in critically ill adults: Systematic review and meta-analysis. BMJ.
366:l42252019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu AG, Samadani U, Slusher TM, Zhang L and
Kiragu AW: 23.4% hypertonic saline and intracranial pressure in
severe traumatic brain injury among children: A 10-year
retrospective analysis. Pediatr Crit Care Med. 20:466–473. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pasarikovski CR, Alotaibi NM, Al-Mufti F
and Macdonald RL: Hypertonic saline for increased intracranial
pressure after aneurysmal subarachnoid hemorrhage: A systematic
review. World Neurosurg. 105:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Strapazzon G, Malacrida S, Vezzoli A, Dal
Cappello T, Falla M, Lochner P, Moretti S, Procter E, Brugger H and
Mrakic-Sposta S: Oxidative stress response to acute hypobaric
hypoxia and its association with indirect measurement of increased
intracranial pressure: A field study. Sci Rep. 6:324262016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdullah Z, Rakkar K, Bath PM and
Bayraktutan U: Inhibition of TNF-α protects in vitro brain barrier
from ischaemic damage. Mol Cell Neurosci. 69:65–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong R, Lénárt N, Hill L, Toms L, Coutts
G, Martinecz B, Császár E, Nyiri G, Papaemmanouil A, Waisman A, et
al: Interleukin-1 mediates ischaemic brain injury via distinct
actions on endothelial cells and cholinergic neurons. Brain Behav
Immun. 76:126–138. 2019. View Article : Google Scholar :
|
|
35
|
Kho DT, Johnson R, Robilliard L, du Mez E,
McIntosh J, O'Carroll SJ, Angel CE and Graham ES: ECIS technology
reveals that monocytes isolated by CD14+ve selection mediate
greater loss of BBB integrity than untouched monocytes, which
occurs to a greater extent with IL-1β activated endothelium in
comparison to TNFα. PLoS One. 12:e1802672017. View Article : Google Scholar
|
|
36
|
Liang JM, Xu HY, Zhang XJ, Li X, Zhang HB
and Ge PF: Role of mitochondrial function in the protective effects
of ischaemic postconditioning on ischaemia/reperfusion cerebral
damage. J Int Med Res. 41:618–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li H, Feng J, Zhang Y, Feng J, Wang Q,
Zhao S, Meng P and Li J: Mst1 deletion attenuates renal
ischaemia-reperfusion injury: The role of microtubule cytoskeleton
dynamics, mitochondrial fission and the GSK3β-p53 signalling
pathway. Redox Biol. 20:261–274. 2019. View Article : Google Scholar
|
|
38
|
Deretic V and Levine B: Autophagy balances
inflammation in innate immunity. Autophagy. 14:243–251. 2018.
View Article : Google Scholar :
|
|
39
|
Heneka MT, McManus RM and Latz E:
Inflammasome signal-ling in brain function and neurodegenerative
disease. Nat Rev Neurosci. 19:610–621. 2018. View Article : Google Scholar : PubMed/NCBI
|