Introduction
Cervical cancer is a common malignant tumor
affecting women worldwide. Its main risk factor is human
papilloma-virus (HPV) infection. The HPV genome can be integrated
into the host genome (1). The
detection of the HPV DNA content has been used to screen cervical
cancer (2). HPV types 16 and 18
were found in 22% of HPV-positive cases in Tampere, Finland
(3). Among HPV-positive cervical
cancers in Iranian women, 48.5% were HPV16 and 12.5% were HPV18
positive (4). HPV16 and HPV18 are
highly prevalent in cervical cancer, and belong to a subset of
high-risk HPVs.
HPV is a small non-enveloped virus containing a
single double-stranded DNA molecule approximately 8 kb in length.
The HPV genome contains 3 coding regions: The early region (E),
late region (L) and upstream regulatory region. The E region
comprising E1-E8 encodes proteins essential for replication,
transcription and transformation (5). E6 and E7 play a critical role in the
molecular pathogenesis of cervical cancer (6). The expression level of HPV E6/E7 is
closely related to the malignant degree of cervical cancer and the
poor prognosis of patients (7,8).
HPV E6 and E7 interact with regulatory proteins
essential to cell fate and the cell cycle. E6 binds to a short
leucine (L)-rich LXXLL consensus sequence within the cellular
ubiquitin ligase E6AP. Subsequently, the E6/E6AP heterodimer
recruits and degrades p53 (9-11)
and inhibits the transcriptional activity of p53 (12,13). E6 promotes retinoblastoma protein
(pRb) phosphorylation and cell cycle progression. The E6 protein
markedly increases the activity of cyclin A/cyclin-dependent kinase
2, which is involved in Rb1 phosphorylation (14). E7 also inhibits p53
transcriptional activity and impairs the control of cell cycle
checkpoints by p53 (15-17). The conserved N-terminal domain of
E7 can bind and inactivate the Rb protein, promote the activation
of the E2F transcription factor, and subsequently activates the
transcription of S-phase DNA replication-related genes. The E7
protein can inhibit the effects of the cyclin-dependent kinase
inhibitors, p27, p15, and p21, allowing cells to escape the G1/S
phase check point and then triggering abnormal chromosome
replication. Suppressing the action of E6 and E7 is a key approach
for cervical cancer therapy (6,18).
Resveratrol is a natural polyphenol compound with a
variety of biological activities, such as antioxidant,
anti-inflammatory, immunoregulatory, chemopreventive and antitumor
activities (19). Resveratrol
treatment has been shown to trigger the apoptosis of the T-acute
lymphoblastic leukemia (T-ALL) cell line, CCRF-CEM, via the
upregulation of the apoptotic BAX gene and the downregulation of
the anti-apoptotic BCL2 gene (20). Apoptosis has also been shown to be
induced in TRAIL-resistant lung cancer cells following co-treatment
with resveratrol and TRAIL via the suppression of Akt/NF-κB
signaling (21). Resveratrol is a
candidate for the treatment and prevention of various types of
cancer via the inhibition of reactive oxygen species (ROS)
(22). Resveratrol also plays a
role in killing cervical cancer cells through multiple molecular
mechanisms. Resveratrol has been shown to induce the autophagy and
apoptosis (23,24), and suppress the migration and
invasion of human cervical cancer cells (25). Resveratrol significantly inhibits
the occurrence and development of cervical cancer by regulating
phospholipid scramblase 1 (26).
Resveratrol has also exhibited antitumor activity on an HPV
E6-positive cervical cancer (27,28). The combination of resveratrol and
curcumin, epicatechin gallate and tricurin has been shown to exert
a unique synergistic suppressive effect on HPV E6, eliminating
HPV+ cancer cells, and inhibiting tumor progression
(29). However, whether or not
the effects of resveratrol on HPV E7 are the same as those on HPV
E6 remains unknown. The mechanisms through which resveratrol
affects cervical cancer cells remains to be further elucidated.
In the present study, the effects of resveratrol on
the expression of HPV E6 and E7, and the proliferation, apoptosis
and cycle of HPV16/18 cervical cancer cells were investigated by
using the HPV18-positive human cervical cancer cell line, HeLa
(30), and the HPV16- and
HPV18-positive Ca Ski cells (31,32). The present study demonstrates that
resveratrol inhibits cervical cancer development by suppressing HPV
E6 and E7 expression, and promoting p53, BAX and p16 expression,
thereby promoting cell apoptosis and cell cycle arrest at the G1/S
phase transition.
Materials and methods
Reagents and antibodies
High-glucose DMEM (11965-092) and 0.25% trypsin
(25300054) were from Gibco; Thermo Fisher Scientific, Inc. Fetal
Bovine Serum (FBS-HI-11A) was from Capricorn Scientific GmbH.
Resveratrol (1602105-100MG) was from Sigma-Aldrich; Merck KGaA. The
cell counting kit-8 (CCK-8; BS350B) was from Biosharp. The
apoptosis test kit (abs50001) was from Absin Bioscience Inc. and
the cell cycle detection kit (KGA512) was from KeyGEN BioTECH
Corp., Ltd. TRIzol reagent (93289-100ML) was from Sigma-Aldrich;
Merck KGaA. The reverse transcription kit (M1705) was from Promega
Corporation and SYBR-Green PCR Mix (1725204) and the Bio-Rad DC
Protein Assay kit (5000002) were from Bio-Rad Laboratories, Inc.
Goat anti-mouse IgG (115-035-003) and goat anti-rabbit IgG
(111-035-003) were from Jackson Immunoresearch Laboratories, Inc.
ECL substrate (46640) was from Thermo Fisher Scientific, Inc. Mouse
monoclonal anti-β-actin antibody (abs137975), mouse monoclonal
anti-GAPDH antibody (abs137959), rabbit anti-β-tubulin polyclonal
antibody (abs125209), mouse monoclonal anti-HPV18-E6 (abs128694),
rabbit polyclonal anti-p53 (abs130605), rabbit polyclonal
anti-phosphorylated pRb1 (p-pRb1; abs110536), rabbit anti-Rb1
(C-term) poly-clonal antibody (abs111662), mouse monoclonal
anti-BAX (abs100264), and rabbit polyclonal anti-BCL-2 (abs115024)
were from Absin Bioscience Co., Ltd. Mouse monoclonal anti-HPV18-E7
(ab100953) was from Abcam; rabbit polyclonal anti-p16-INK4A
(10883-1-AP), rabbit polyclonal anti-Cyclin E1 (11554-1-AP), rabbit
polyclonal anti-CDK4 (11026-1-AP) and rabbit polyclonal anti-E2F1
(12171-1-AP) were from Proteintech Group, Inc. DyLight 594 Orange
goat anti-mouse (A23310) and DyLight 488 green goat anti-rabbit
fluorescent antibody (A23240) were from Abbkine Scientific Co.,
Ltd.. DAPI dye (P0131) was obtained from the Beyotime Institute of
Biotechnology, Inc.
Cells and cell culture
HeLa and Ca Ski cells were purchased from Hunan Feng
Hui Biological Technology Co., Ltd. These cells were certified by
an STR test, consistent with published reports (33-35). These cells were cultured in
high-glucose DMEM containing 10% fetal bovine serum at 37°C in a 5%
CO2 incubator.
Determination of cell proliferation by
CCK-8 assay
HeLa and Ca Ski cells were seeded in 96-well plates
at a density 5×103 cells/100 µl/well. Following
culture in regular medium 37°C in a 5% CO2 incubator for
24 h, the cells were cultured in resveratrol-containing fresh
medium. Following further incubation 37°C in a 5% CO2
incubator for 24, 48 and 72 h, 10 µl of CCK-8 buffer were
added to each well and incubated at 37°C for 2 h. The plates were
measured for absorbance (OD) at 450 nm on a plate reader (Spectra
Max i3x, Molecular Devices, LLC). Cell viability and the
half-maximal inhibitory concentration (IC50) values were
calculated by using the analysis function of Graphpad Prism
(GraphPad Software, Inc.) software, transforming the resveratrol
concentrations to logarithms, then using non-linear regression to
fit curve and select dose-response-inhibition. CCK-8 reagent
contains WST-8, which is reduced to a highly water-soluble yellow
formazan dye by dehydrogenase in cell mitochondria under the action
of electron carrier (1-methoxy PMS). The amount of generated
formazan is in direct proportion to the number of living cells.
Determination of cell apoptosis by flow
cytometry
HeLa cells were treated with the vehicle (DMEM
complete medium containing 1/2,500 volume of ethanol) and
resveratrol (5, 10, 20 and 40 µM) for 24 h and then
collected. After washing with pre-cooled PBS, the cells were
collected by centrifugation at 300 × g for 5 min at 4°C,
resuspended with 1X Binding Buffer of an apoptosis test kit, and
mixed with 5 µl of Annexin V-FITC. The cells were incubated
at room temperature for 15 min and then stained with 5 µl of
PI for 5 min. Subsequently, 200 µl of 1X Binding Buffer were
added, and the cells were analyzed on a BD FACSAria II flow
cytometer (BD Biosciences) at 488 nm. The apoptotic ratio was
analyzed by FlowJo™ v10.7 (version X; TreeStar, Inc.).
Determination of cell cycle by flow
cytometry
HeLa and Ca Ski cells were treated with the vehicle
(DMEM complete medium containing 1/2,500 volume of ethanol) and
resveratrol (10, 20 and 40 µM) for 24 h and collected. After
washing with PBS, the cells were collected by centrifugation at 300
× g, room temperature, for 5 min and then resuspended at a density
1×106/ml. The cells (1 ml) were aliquoted and collected
by centrifugation at 300 × g, room temperature, for 5 min. After
the supernatant was removed, 500 µl of cold 70% ethanol were
added and stored at 4°C overnight. After washing with PBS, the
cells were resuspended with 500 µl of the PI/RNase A working
solution in the cell cycle detection kit in accordance with the
manufacturer's instructions. Following incubation in the dark at
room temperature for 45 min, the cells were analyzed on a BD
FACSAria II flow cytometer (BD Biosciences) at 488 nm. Cell
clusters meeting the analysis conditions were circled at the
interface of FSC and SSC, and then circled single cells at the
interface of PE-W and PE-A. Cell cycle curve fitting was completed
by using the cell cycle function of FlowJo™ v10.7 (version X;
TreeStar, Inc.).
Reverse transcription-quantitative
PCR
Total RNA was extracted from the Ca Ski cells or
tumor tissue grown from HeLa cells (as described below). A total of
2 µg total RNA was reverse-transcribed into cDNA in the
reaction system of 25 µl. cDNA (0.5 µl) was used as a
PCR template, and PCR analysis was performed using a SYBR-Green PCR
Mix by following the program: Pre-denaturation at 95°C for 5 min,
40 cycles of 95°C for 15 sec, 56°C for 30 sec, and 72°C for 30 sec.
The specificity of primers (Table
I) was examined using a melting curve. GAPDH was used as the
internal reference, and the relative expression of the target gene
was calculated using the 2−∆∆Cq method (36). The primers were synthesized by
Genecreate Biotechnology Co., Ltd.
 | Table IPrimer pair sequences used for
RT-qPCR. |
Table I
Primer pair sequences used for
RT-qPCR.
| Gene | Orientation | Sequence
(5′→3′) |
|---|
| HPV18-E6 | Sense |
GCCAGAAACCGTTGAATCC |
| Antisense |
AGTCTTTCCTGTCGTGCTCG |
| HPV18-E7 | Sense |
GCATGGACCTAAGGCAACA |
| Antisense C |
TCGTCGGGCTGGTAAAT |
| BCL-2 | Sense |
TTCTTTGAGTTCGGTGGGG |
| Antisense |
AGTCTTTCCTGTCGTGCTCG |
| BAX | Sense |
ACTGTGCGTGGAAAGCGTA |
| Antisense |
TGAGACACTCGCTCAGCTTC |
| BCL-xL | Sense |
TTCTGCCCTCAACCGCAAAGAT |
| Antisense |
ACCAGCGGTTGAAGCGTT |
| p16 | Sense |
TCCAGGTCATGATGATGGG |
| Antisense |
ATGCGGGCATGGTTACTG |
| p21 | Sense |
GCCAGAAACCGTTGAATCC |
| Antisense |
AGTCTTTCCTGTCGTGCTCG |
| p27 | Sense |
GCTAACTCTGAGGACACGCA |
| Antisense |
GGGGAACCGTCTGAAACAT |
| GAPDH | Sense |
AGAAGGCTGGGGCTCATTTG |
| Antisense |
AGGGGCCATCCACAGTTCTTC |
Western blot analysis
HeLa cells were treated with the vehicle (DMEM
complete medium containing 1/2,500 volume of ethanol) and
resveratrol (10, 20 and 40 µM) and Ca Ski cells were treated
with the vehicle (1/2,500 ethanol) and resveratrol (50 µM)
for 24 h, washed with cold PBS, collected, and then homogenized in
lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 50 mM
sodium fluoride, 1 mM EDTA and 1 µg/ml leupeptin]. Tissue
samples were homogenized in lysis buffer [10 mM Tris-HCl (pH 7.5),
150 mM NaCl, 1 mM EGTA, 50 mM NaF, 0.5 mM
phenylmethylsul-fonylfluoride, 1 mM sodium vanadate, 1% Triton
X-100, 0.5% Nonidet P-40 and and 1 µg/ml of aprotinin]. The
homogenized samples were centrifuged at 12,000 × g for 15 min at
4°C, and the supernatants were collected. An aliquot of the
super-natant was used to determine the protein concentration of
each supernatant by using a Protein Assay kit. All samples
containing 30 µg protein/sample were aliquoted, mixed with
5X loading buffer, and then loaded for electrophoresis on a 12%
SDS-polyacrylamide gel. After electrophoresis, the resolved
proteins in the gel were transferred onto PVDF (polyvinylidene
difluoride) membranes. The membranes were blocked in 5% non-fat
milk TBST buffer (20 mM Tris pH 7.4, 150 mM NaCl and 0.1% Tween-20)
for 1 h at room temperature and then probed with primary antibodies
over-night at 4°C. After washing with TBST buffer 3 times, the
membranes were incubated with corresponding horseradish
peroxidase-conjugated secondary antibody, goat anti-mouse IgG
(1:1,000 dilution) or goat anti-rabbit IgG (1:1,000 dilution) for 1
h at room temperature and developed using the ECL substrate. The
relative amount of protein on the blots was determined by
densitometry using Lab Works software (UVP, LLC). Mouse monoclonal
anti-β-actin (1:5,000 dilution) or mouse monoclonal anti-GAPDH
antibody (1:5,000 dilution) was used as loading controls. Proteins
were detected using the following primary antibodies: Mouse
mono-clonal anti-HPV18-E6 (1:500 dilution), mouse monoclonal
anti-HPV18-E7 (1:1,000 dilution), rabbit polyclonal anti-p53 (1:500
dilution), rabbit polyclonal anti-p-pRb1 (1:2,000 dilution), rabbit
polyclonal anti-Rb1 (1:2,000 dilution), mouse monoclonal anti-BAX
(1:500 dilution), rabbit polyclonal anti-BCL-2 (1:500 dilution),
rabbit polyclonal anti-p16-INK4A (1:500 dilution), rabbit
polyclonal anti-Cyclin E1 (1:1,000 dilution), rabbit polyclonal
anti-CDK4 (1:500 dilution), and rabbit polyclonal anti-E2F1 (1:500
dilution).
Immunocytochemical assay
HeLa cells were treated with resveratrol (40
µM) and the vehicle (DMEM complete medium containing 1/2,500
volume of ethanol) for 24 h and fixed in 4% paraformaldehyde for 15
min at room temperature. After washing with PBS 3 times, 0.5%
Triton X-100 was added followed by incubation at room temperature
for 15 min. After washing with PBS 3 times, the cells were blocked
with horse serum for 1 h at room temperature and then probed with
mouse monoclonal anti-HPV18-E6 (1:100 dilution), mouse monoclonal
anti-HPV18-E7 (1:100 dilution), rabbit polyclonal anti-p53 (1:100
dilution), rabbit polyclonal anti-p-pRb1 (1:100 dilution) overnight
at 4°C. After washing with PBS, the cells were probed with DyLight
594 Orange goat anti-mouse (1:2,000 dilution) and DyLight 488 green
goat anti-rabbit fluorescent antibody (1:2,000 dilution) in the
dark room at room temperature for 60 min. After washing with PBS 3
times, nuclei were labeled with DAPI at room temperature for 10 min
and then washed with PBS 3 times. The cells were examined under a
confocal laser microscope (Leica DMi8, Leica Microsystems GmbH)
within 1 h.
Animal models and treatment
A total of 12 Balb/C female nude mice (4-6 weeks
old, weighing 20.0±2.0 g) were purchased from Hunan SJA laboratory
animal Co. Ltd. The mice were housed at a temperature of 20-22°C
with 50-60% relative humidity and fed with standard laboratory chow
and tap water ad libitum, and were allowed to adapt for 1
week. These nude mice were subcutaneously injected with HeLa cells
(2×107/ml, 100 µl/mouse) into the right axillary
midline. The volume of the tumors was measured using a caliper as
follows: Tumor volume=(length) × (width)2 ×0.5 every 3
days. When length was ≥2 mm, the mice were randomly divided into 2
groups with 6 mice in each group: The vehicle group and the
resveratrol treatment group. The resveratrol treatment group was
administered intragastrically with 600 µl of 15 mg/kg
resveratrol, whereas the vehicle group was administered the same
volume of saline (containing 1/100 ethanol). Gastric administration
was performed 3 times per week and lasted for approximately 5
weeks. The key time nodes are explained respective figure (see
below). The maximum tumor volume in mice was not >1.5
cm3. During the administration, the mice in each group
were observed in terms of viability, activity, diet and water
intake. At the end of the treatment, all nude mice were euthanized
by cervical dislocation. Tumors were isolated, weighed and
aliquoted for RT-qPCR, western blot analysis, hematoxylin and eosin
(H&E) staining, and immunohistochemical (IHC) staining assay.
The present study was approved by the Ethics Committee for Animal
Experimentation of Xiangyang No.1 People's Hospital (no.
2017DW038).
Immunohistochemical staining of tissues
from HeLa-derived subcutaneous tumor xenografts in mice
Tumor tissues were fixed in 4% polyformaldehyde,
paraffin-embedded and then sectioned into 5-µm-thick slices.
The sections were then incubated with anti-HPV18-E6 (1:100
dilution) and mouse monoclonal anti-HPV18-E7 (1:100 dilution)
antibody at 4°C overnight, and a biotinylated goat anti-rabbit
antibody was used as a secondary antibody at room temperature for
50 min. The slides were then washed with PBS and incubated at room
temperature with diaminobenzidine chromogen for 3-5 min.
Brown-yellow staining was considered positive. The stained sections
were examined under a microscope (IX73P2F, Olympus Corporation),
and 4 fields were selected in areas with clear cell staining and
good background. The view was recorded in images and analyzed using
ImageJ (Fiji, National Institutes of Health) software. The relative
expression of HPV18-E6 and HPV18-E7 was calculated based on the
positively stained area of area and the total area in the
views.
Statistical analysis
Data were analyzed using GraphPad Prism 7.0
(GraphPad Software, Inc.) software. The results are expressed as
the means ± standard deviation for at least 3 independent
experiments. Statistical comparisons between groups were performed
using a Student's t-test or one-way ANOVA, followed by Dunnett's
post hoc test. Data with P<0.05 were considered to indicate
statistically significant differences.
Results
Resveratrol inhibits the proliferation of
cervical cancer cells
Firstly, it was confirmed that resveratrol
efficiently inhibited HeLa and Ca Ski cell growth. Under the
present experimental conditions, the resveratrol IC50
value at 24 h was 155.8 µM, at 48 h was 71.66 µM and
at 72 h it was 40.06 µM in the HeLa cells (Fig. S1A). Similar effects of
resveratrol on Ca Ski cells were observed, and the IC50
value at 24 h was 172.4 µM, at 48 h was 100.8 µM and
at 72 h it was 59.07 µM (Fig.
S1B). This suggested that resveratrol inhibited the
proliferation of cervical cancer cells in a dose- and
time-dependent manner.
Resveratrol promotes the apoptosis of
cervical cancer cells
Using resveratrol, the apoptosis of HeLa and Ca Ski
cells treated with resveratrol was examined. The apoptotic rate was
determined by flow cytometry, and the BCL-2, BCL-xL and BAX mRNA
and protein levels were measured by RT-qPCR and western blot
analysis. The results of flow cytometry revealed that resveratrol
increased the apoptotic rate of HeLa cells in a
concentration-dependent manner, particularly in the late stage of
apoptosis (Fig. 1A). The BCL-2
and BCL-xL mRNA levels (Fig. 1B)
and the BCL-2 protein level (Fig.
1C) significantly decreased, and the BAX mRNA (Fig. 1B) and protein level (Fig. 1C) significantly increased in the
HeLa cells treated with resveratrol compared with the cells treated
with the vehicle control. Similarly, the BCL-2 and BCL-xL mRNA
(Fig. 1D) and BCL-2 protein
(Fig. 1E) levels decreased, and
the BAX mRNA (Fig. 1D) and
protein (Fig. 1E) levels
increased in the Ca Ski cells treated with resveratrol compared
with the cells treated with the vehicle. These results indicated
that resveratrol promoted the apoptosis of cervical cancer
cells.
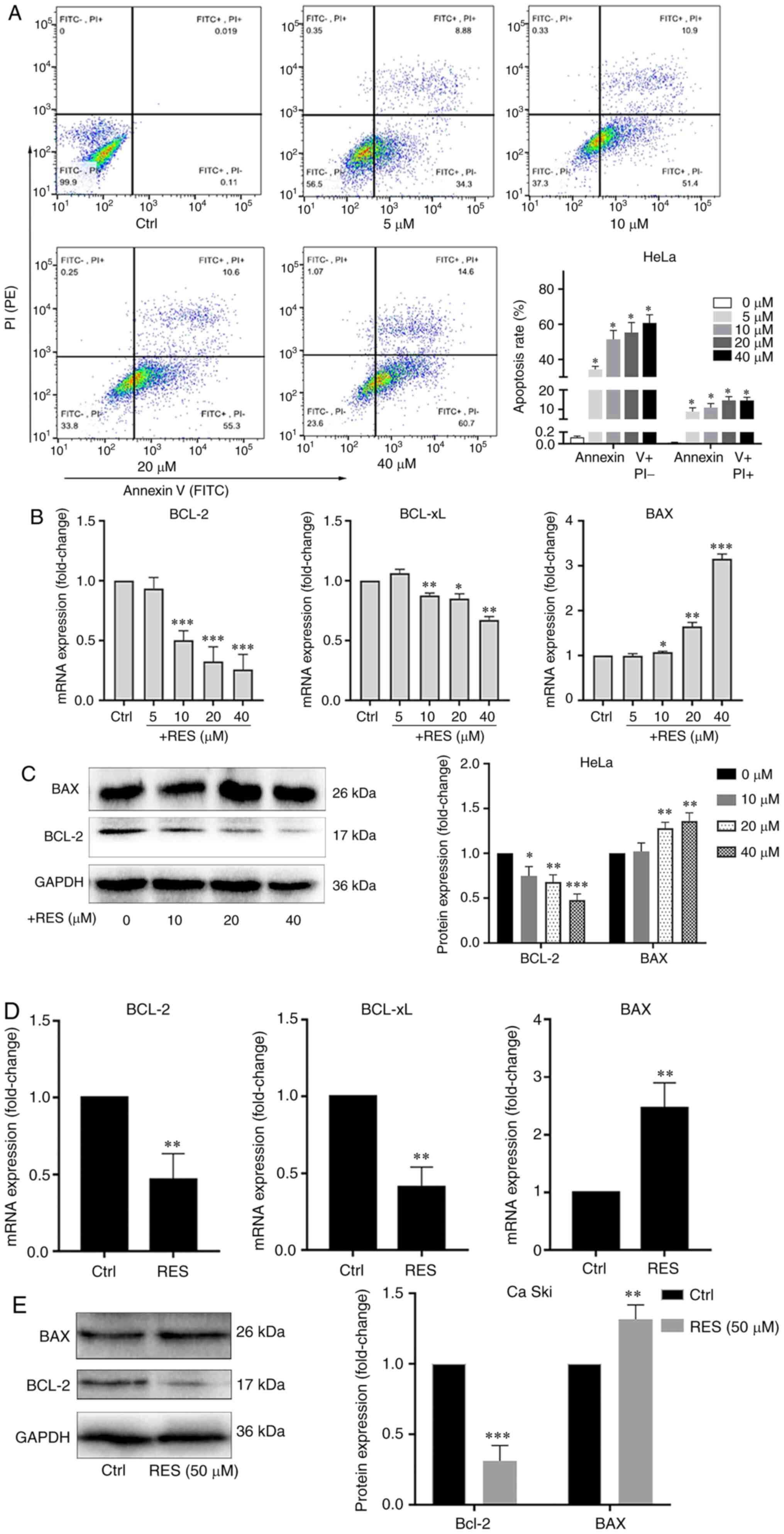 | Figure 1Resveratrol promotes the apoptosis of
cervical cancer cells. (A) HeLa cells were treated with the vehicle
(DMEM medium supplemented with 10% fetal bovine serum, and
containing 1/2,500 volume of ethanol) or resveratrol at various
concentrations (5, 10, 20 and 40 µM) for 24 h. Apoptotic
cells were detected by flow cytometry. (B) BCL-2, BCL-xL and BAX
mRNA levels in HeLa cells treated with resveratrol or the vehicle
were determined by RT-qPCR. (C) BCL-2 and BAX protein levels in
HeLa cells treated with resveratrol (10, 20 and 40 µM) or
the vehicle for 24 h were determined by western blot analysis. (D)
BCL-2, BCL-xL and BAX mRNA levels in Ca Ski cells treated with
resveratrol (50 µM) or the vehicle (DMEM medium supplemented
with 10% fetal bovine serum, and containing 1/2,500 volume of
ethanol) for 24 h were determined by RT-qPCR. (E) BCL-2 and BAX
protein levels in Ca Ski cells treated with resveratrol (50
µM) or the vehicle for 24 h were determined by western blot
analysis. Data are presented as the means ± SD.
*P<0.05, **P<0.01,
***P<0.001, compared with the vehicle control. |
Resveratrol promotes G1/S arrest in
cervical cancer cells
To examine the effects of resveratrol on the cell
cycle of cervical cancer, HeLa and Ca Ski cells were treated with
resveratrol or the vehicle. The results of flow cytometry revealed
that treatment of the HeLa cells with resveratrol significantly
increased the proportion of cells in the G1 phase and significantly
decreased the proportion of cells in the S phase in a
concentration-dependent manner (Fig.
2A). Similar effects of resveratrol on the cell cycle of Ca Ski
cells were observed (Fig. 2B). It
was also found that the p16, p21 and p27 mRNA levels significantly
increased in the HeLa cells treated with resveratrol in a
concentration-dependent manner (Fig.
2C). The CDK4, E2F1 and p-pRb1 protein levels significantly
decreased in the HeLa cells treated with resveratrol in a
concentration-dependent manner (Fig.
2D). Although the concentration of resveratrol used altered the
protein levels of Cyclin E and p16, the level of Cyclin E decreased
following treatment with resveratrol at up to 20 µM, but
then increased at 40 µM, and the level of p16 increased
following treatment with resveratrol at up to 20 µM, but
then decreased at 40 µM. Thus, the levels of Cyclin E and
p16 were not altered in a concentration-dependent manner (Fig. 2D). These results suggested that
resveratrol promoted G1/S phase arrest in cervical cancer cells.
Moreover, resveratrol reduced the expression of p-pRb1 and promoted
the activity of hypo-phosphorylated Rb1 binding to the
transcription factor, E2F1.
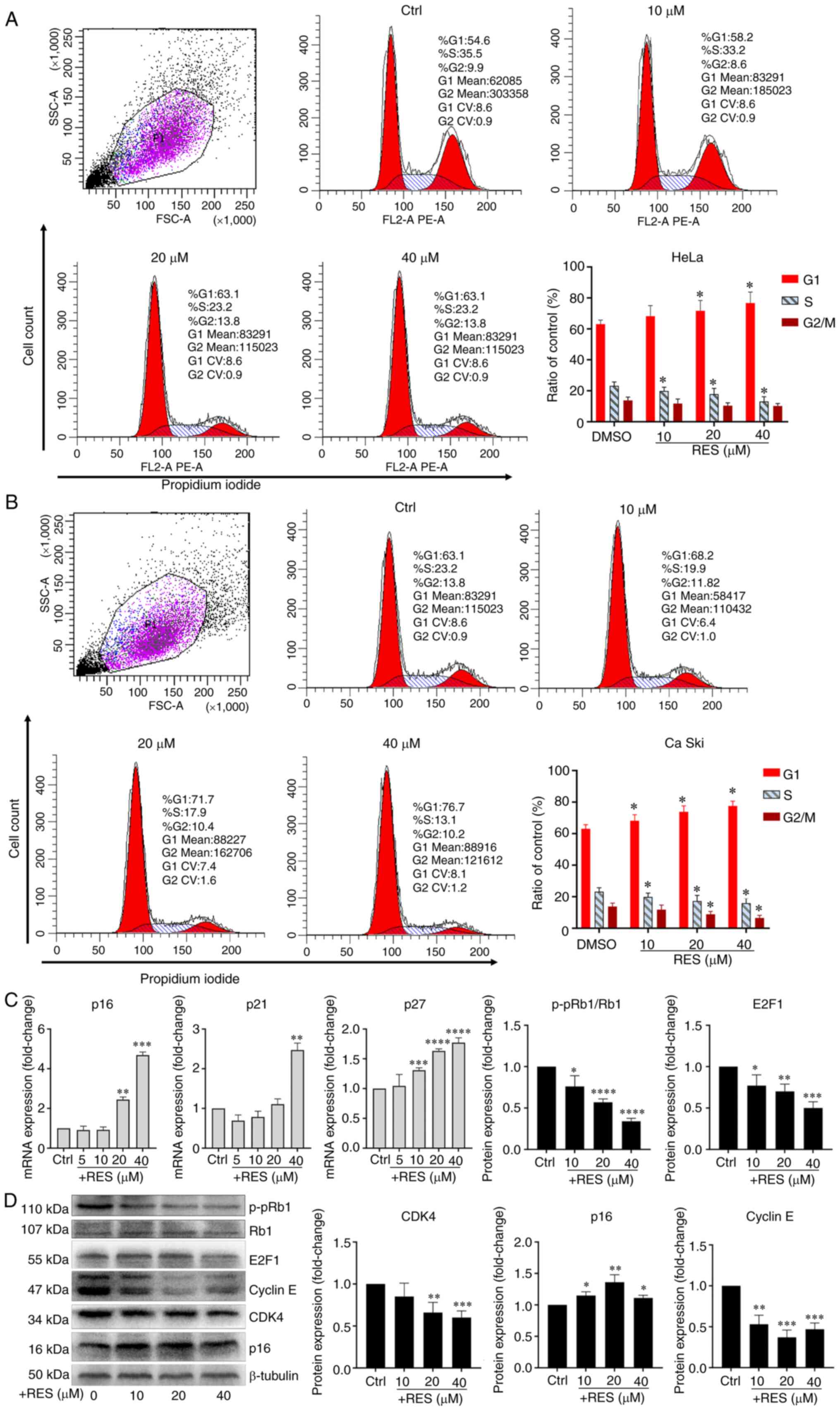 | Figure 2Resveratrol promotes G1/S phase
arrest of cervical cancer cells. (A) HeLa cells and (B) Ca Ski
cells were treated with resveratrol at various concentrations (0,
10, 20 and 40 µM) and the vehicle (DMEM medium supplemented
with 10% fetal bovine serum, and containing 1/2,500 volume of
ethanol) for 24 h. Cell cycle was determined by flow cytometry.
Cell cycle curve fitting was completed by using the cell cycle
function of FlowJo™ v10.7 (Version X). (C) p16, p21 and p27 mRNA
levels in HeLa cells treated with resveratrol or vehicle for 24 h
were determined by RT-qPCR. (D) p16, p21, p27, CDK4, Cyclin E, E2F1
and p-pRb1 protein levels in HeLa cells treated with resveratrol or
the vehicle for 24 h were determined by western blot analysis and
quantitative analysis of western blot analysis results. Data are
presented as the means ± SD. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001, compared with the vehicle control. |
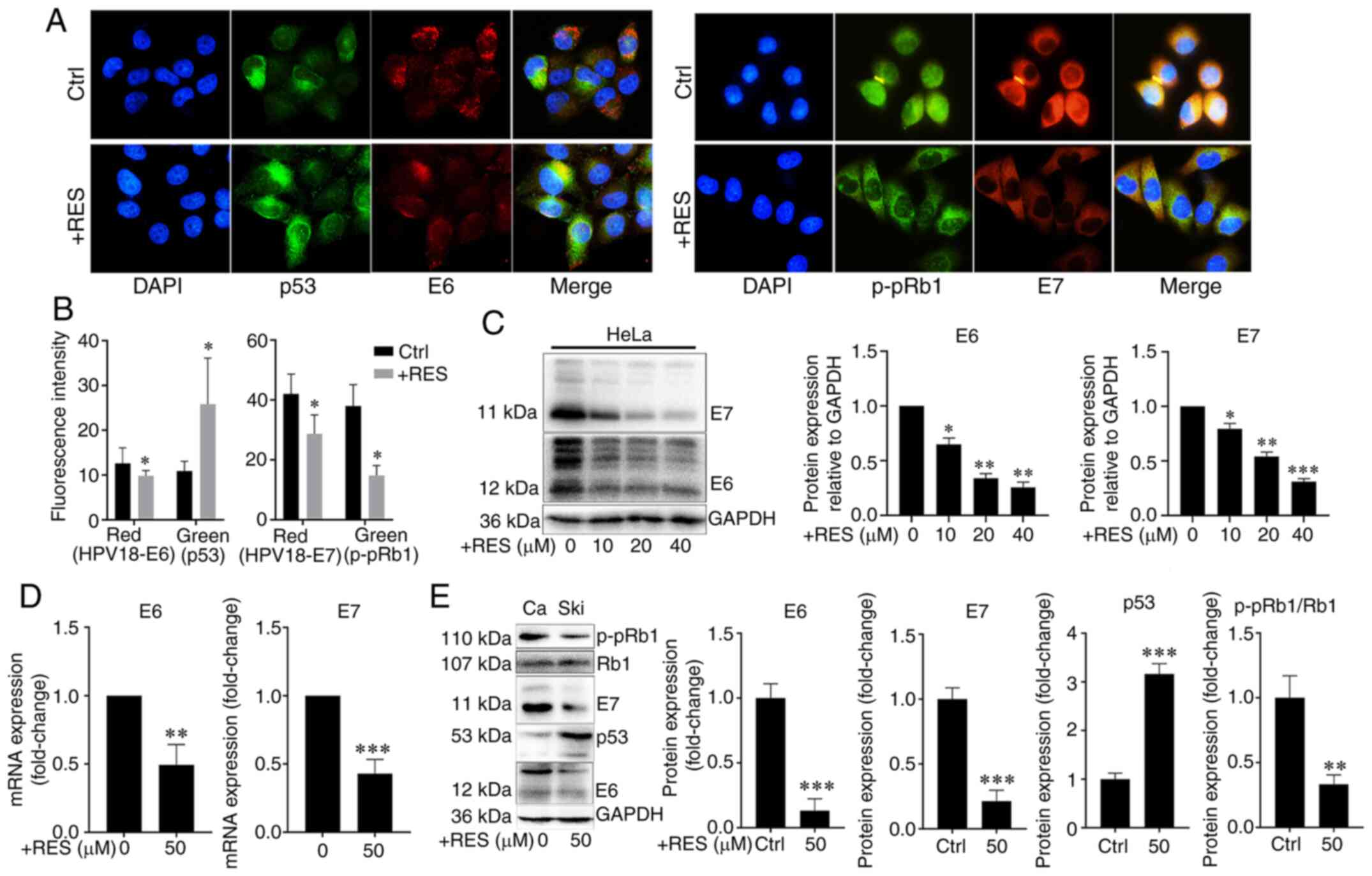
Resveratrol inhibits the expression of
HPV E6 and HPV E7 in cervical cancer cells
To examine the effects of resveratrol on the
expression of HPV E6 and HPV E7 in cervical cancer cells, HeLa and
Ca Ski cells were treated with resveratrol or the vehicle. The
expression and localization of HPV E6/E7, p53 and p-pRb1 in HeLa
cells were determined using immunocyto-chemistry and western blot
analysis, and the HPV E6/E7 mRNA levels were measured by RT-qPCR.
Immunofluorescence assay revealed that the amounts of HPV E7 and
p-pRb1 were reduced in the nuclei, whereas the nuclear localization
signal of HPV E6 and p53 exhibited no marked changes in the HeLa
cells treated with resveratrol (Fig.
3A). The HPV E6/E7 and p-pRb1 protein levels decreased and the
p53 protein levels increased in the HeLa cells treated with
resveratrol compared with those treated with the vehicle control
(Fig. 3A and B). Western blot
analysis revealed that the HPV E6 and E7 protein levels in the HeLa
cells were significantly inhibited by resveratrol in a
concentration-dependent manner (Fig.
3C). Similarly, the HPV E6/E7 mRNA levels significantly
decreased (Fig. 3D), the HPV E6
and E7 and p-pRb1 protein levels decreased, and the p53 protein
levels increased in the Ca Ski cells treated with resveratrol
compared with the cells treated with the vehicle control (Fig. 3E). These results suggested that
resveratrol significantly inhibited the expression of HPV E6 and
HPV E7 in cervical cancer cells. Therefore, it was hypothesized
that the effects of resveratrol on apoptosis and the cell cycle of
cervical cancer cells were due to its inhibition of HPVE6 and E7
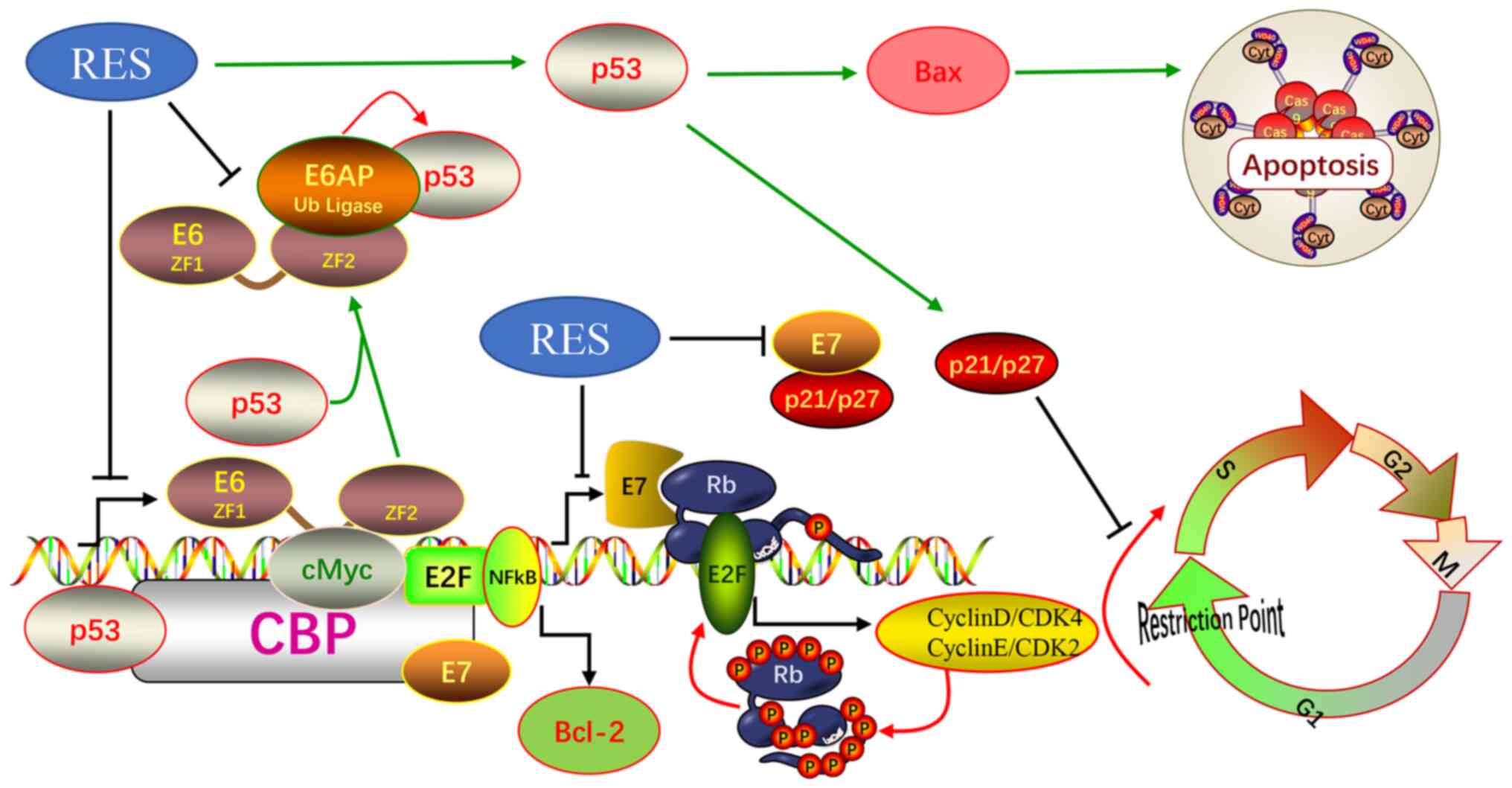
(Fig. 6).
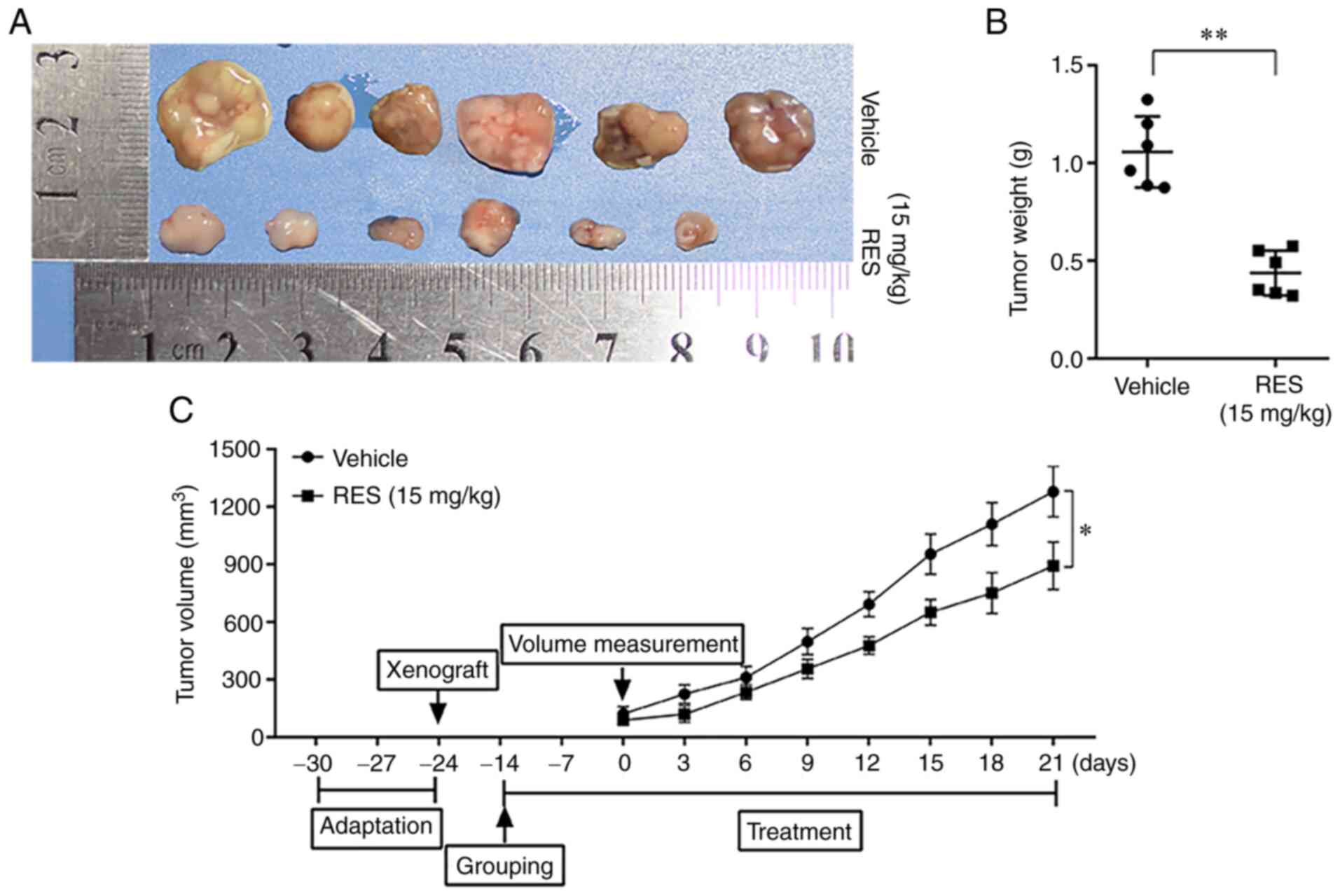
Resveratrol inhibits the growth of
HeLa-derived subcutaneous tumor xenografts in mice
To examine the effects of resveratrol on the growth
of cervical cancer cells in vivo, subcutaneous tumor
xenograft derived from HeLa cells were established in Balb/C female
nude mice and the mice were then treated with resveratrol or
saline. The tumor tissues were examined, and the weight of tumor
mass and the volumes of tumors were measured. The results revealed
that the weight and volumes of tumors in the resveratrol treatment
group were significantly lower than those in the saline treatment
group (Fig. 4). The results
suggested that resveratrol significantly inhibited the growth of
cervical cancer cells in nude mice.
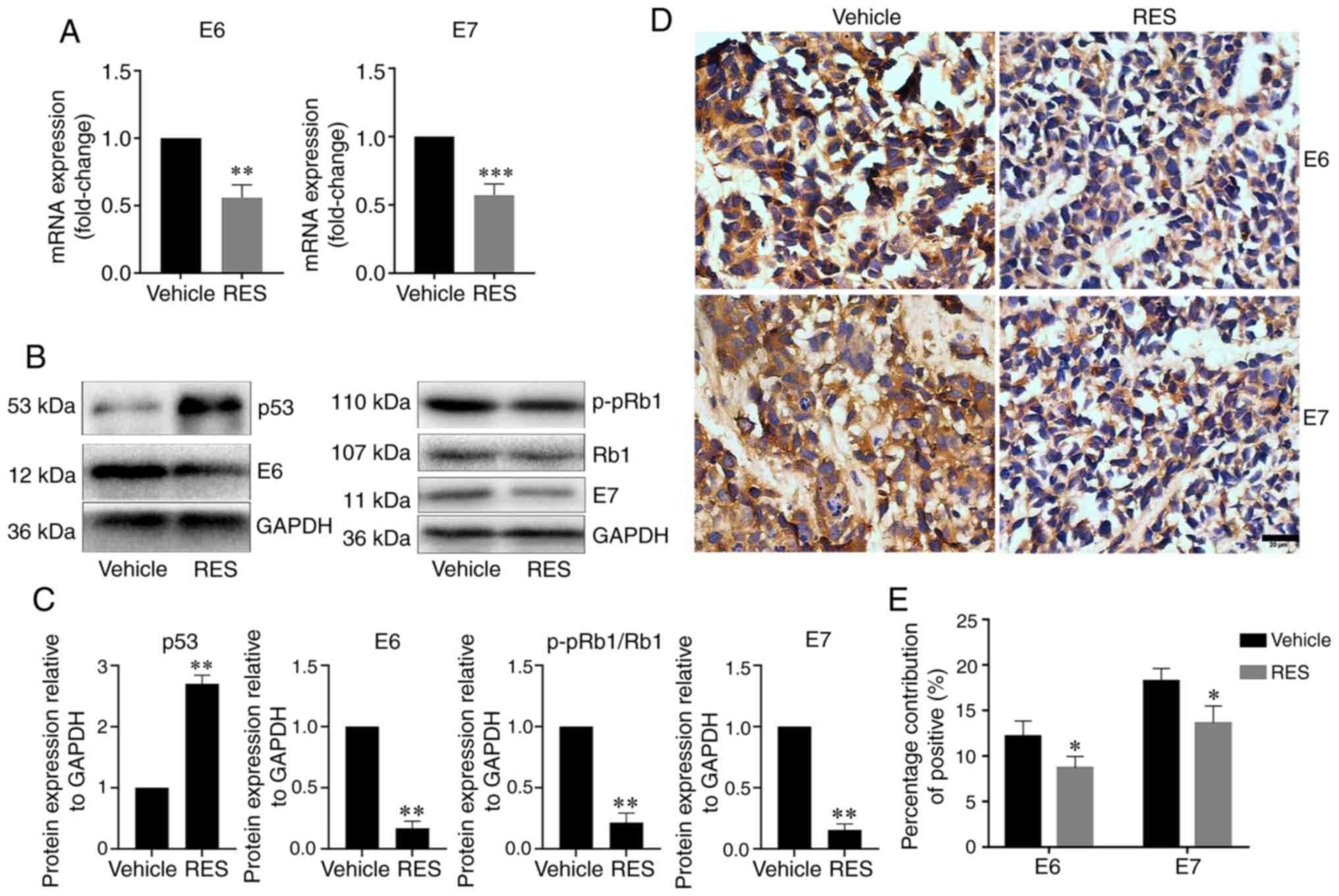
Resveratrol inhibits the expression of
HPV E6 and E7 in subcutaneous tumor xenograft tissues from nude
mice
To examine the effects of resveratrol on the
expression of HPV E6 and E7 in subcutaneous tumor xenograft tissues
in vivo, the HPV E6, E7, p53 and p-pRb1 mRNA and protein
levels in tumor tissues from subcutaneous xenograft were determined
by RT-qPCR, western blot analysis and immunohistochemistry as
appropriate. The results revealed that the tumor tissue HPV E6 and
E7 mRNA levels were lower in the resveratrol treatment group than
in the vehicle treatment group (Fig.
5A). The results of western blot analysis revealed that the
tumor tissue HPV E6, E7, and p-pRb1 protein levels significantly
decreased, and the p53 protein level significantly increased in the
resveratrol treatment group compared with the vehicle treatment
group (Fig. 5B and C).
Consistently, immunohistochemical assay revealed that HPV E6 and E7
expression in the cancer tissues significantly decreased in the
resveratrol treatment group compared with the vehicle treatment
group (Fig. 5D and E). These
results indicated that resveratrol significantly inhibited the
expression of HPV E6 and E7, promoted the expression of p53
protein, and inhibited the phosphorylation of Rb1 protein in
cervical cancer tissues in nude mice.
Discussion
In the present study, it was found that resveratrol
inhibited the proliferation of cervical cancer cells in a
concentration- and time-dependent manner. Resveratrol treatment
also decreased the weight and volumes of tumors derived from HeLa
cells in Balb/C female nude mice. These data were consistent with
those of previous studies (23,24,26-28). In addition, a previous study
demonstrated that resveratrol suppressed the migration and invasion
of human cervical cancer cells (25). Therefore, resveratrol inhibits the
development and progression of cervical cancer and is a candidate
for cervical cancer therapy.
E6 and E7 are highly expressed in HPV18-positive
HeLa cells (30) and HPV16- and
HPV18-positive Ca Ski cells (31,32). E6 and E7, both encoded by HPV,
play a critical role in the molecular pathogenesis of cervical
cancer (6). High-risk HPV E6 and
E7 are two viral oncoproteins that respectively, induce protein
degradation of tumor suppressors p53 and pRb1 (37). A previous study found that
resveratrol downregulated E6 and induces and upregulated p53
protein levels (28).
Consistently, the present study found that resveratrol inhibited
the HPV E6 mRNA and protein and phosphorylated pRb1 protein levels,
and also increased the p53 protein levels in HeLa and Ca Ski cells
and cervical cancer tissues grown from HeLa cells. In addition,
resveratrol inhibited the HPV18 E7 mRNA and protein levels in HeLa
and Ca Ski cells and cervical cancer tissues grown from HeLa cells.
These results suggest that resveratrol inhibits the development of
cervical cancer by suppressing the E6 and E7 mRNA and protein
levels in HPV16- and HPV18-positive cervical cancers.
In high-risk HPV, the splicing of the E6 intron from
a bicistronic E6E7 pre-mRNA is a crucial step to control expression
of E6 and E7 oncogenes (38,39). while the unspliced E6/E7 mRNA
encodes for the full length E6 protein, the E6*I and E6*II mRNAs
may favor the E7 expression by a termination-re-initiation process
or leaky scanning mechanisms and the production of shorter E6
peptides (38,40). The HPV16 E6*I isoform have
oncogenic activities, including disruption of mitochondrial
functions and promotion of ROS production (41). Resveratrol has mitochondrial
protection and ROS exert scavenging effects. It is hypothesized
that resveratrol may affect the splicing of E6 introns, thereby
inhibiting the transcription and translation of E6 and E7. Further
studies are required to understand the precise role of resveratrol
on splicing processes of E6/E7 mRNA.
p53 promotes apoptosis by boosting the expression of
the pro-apoptotic protein, BAX. A recent study associated the
chemopreventive activity of resveratrol with its ability to block
the NF-κB pathway through IκB kinase inhibition (28). In the present study, the results
of flow cytometry revealed that resveratrol increased the apoptotic
rate of HeLa cells in a concentration-dependent manner,
particularly in the late stage of apoptosis. In addition,
resveratrol decreased BCL-2 expression and increased BAX expression
in HeLa and Ca Ski cells. BCL-2 is an anti-apoptotic factor, and
BAX is a pro-apoptotic factor. The data of the present study are
consistent with those of previous studies (24,27,42). These results suggest that the
induction of apoptosis is a mechanism through which resveratrol
inhibits the development of cervical cancer. Of note, the published
literature indicate that resveratrol uses different mechanisms to
induce cell death in cervical cancer cell line with or without
expression of viral oncoproteins (24).
The expression of HPV E6 and E7 in human cervical
tissue destroys cell cycle G1/S checkpoints and promotes cells to
proliferate indefinitely (14-16). Resveratrol regulates the cancer
cell cycle. It impairs G1/S phase transition in MDA-MB-231 and
MCF-7 cells (43) and induces
G1/S arrest in human prostate cancer cell lines (44,45). The E2F1 complex binds specifically
hypophosphorylated Rb1. During the cell cycle, Rb1 becomes
phosphorylated in mid-to-late G1 phase, detaches from the DRTF1/E2F
complex, rendering E2F transcriptionally active. Viral oncoprotein
HPV E7 is capable of sequestering Rb1, thus releasing the active
complex (46). In the present
study, the treatment of HeLa and Ca Ski cells with resveratrol
significantly increased the proportion of G1 phase cells and
significantly decreased the proportion of S phase cells in a
dose-dependent manner. Furthermore, resveratrol significantly
increased the expression levels of p53, p16, p21 and p27, which
inhibited G1/S phase progression, and significantly decreased the
expression levels of CDK4, Cyclin E, E2F1 and p-pRb1, which promote
G1/S phase progression. These data suggest that resveratrol
promotes the G1/S arrest of HeLa and Ca Ski cells. The induction of
G1/S arrest is also a possible mechanism through which resveratrol
inhibits the development of cervical cancer. Therefore, resveratrol
can also reduce the inhibition of p53 transcriptional activity by
inhibiting the expression of HPV E6 and E7 and restore the role of
P53 at the G1/S detection point of cells, and the expression of
pro-apoptotic protein BAX, as shown in Fig. 6.
In conclusion, the data of the present study
indicate that resveratrol inhibits cervical cancer development by
suppressing E6 and E7 expression to promote apoptosis and cell
cycle arrest at the G1/S phase transition. The findings of the
present study may serve as a reference for the future development
of resveratrol as a candidate for cervical cancer therapy.
Supplementary Data
Funding
The present study was supported by grants from the
National Natural Science Foundation (no. 81903005) and the Natural
Science Foundation of Hubei Province of China (nos. 2020DFE025,
2019AHB068 and 2018CFB701). The present study was also supported by
the Open Project of Hubei Key Laboratory of Wudang Local Chinese
Medicine Research (grant no. WDCM2018009), the Innovative Team
Project (grant no. 2017YHKT02) from the Institute of Medicine and
Nursing at Hubei University of Medicine. The funding bodies were
not involved in the design of this study, in the collection,
analysis, and interpretation of the data, or in writing of the
manuscript
Availability of data and materials
All data generated or analyzed during this study are
included in this published article and its supplementary
information files. The original data are available upon request to
the corresponding author.
Authors' contributions
MS, XS and PF conceived and designed the
experiments. XS, PF, LX, QX and LZ performed the experiments. SC,
NJ and XW analyzed the data. XS and MS contributed to the reagents,
materials and analysis tools, MS, XS and PF wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee for Animal Experimentation of Xiangyang No.1 People's
Hospital (no. 2017DW038). All animal experiments in this study were
undertaken in accordance with the provisions of the Declaration of
Helsinki (as revised in Edinburgh 2000).
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing financial
interests.
Acknowledgments
The authors wish to thank Professor Xinsheng Gu of
the Department of Pharmacology (Hubei University of Medicine) for
his careful modification of the manuscript. The authors would also
like to thank the Director of the Key Laboratory of Wudang Local
Chinese Medicine Research (Hubei University of Medicine), Professor
Xuanbin Wang, for providing the facilities to carry out the
experiments.
References
|
1
|
Xu B, Chotewutmontri S, Wolf S, Klos U,
Schmitz M, Dürst M and Schwarz E: Multiplex identification of human
papillomavirus 16 DNA integration sites in cervical carcinomas.
PLoS One. 8:e666932013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duvlis S, Popovska-Jankovic K, Arsova ZS,
Memeti S, Popeska Z and Plaseska-Karanfilska D: HPV E6/E7 mRNA
versus HPV DNA biomarker in cervical cancer screening of a group of
Macedonian women. J Med Virol. 87:1578–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kares S, Veijalainen O, Kholova I,
Tirkkonen M, Vuento R, Huhtala H, Tuimala V, Mäenpää J and Kujala
P: HIGH-RISK HPV testing as the primary screening method in an
organized regional screening program for cervical cancer: The value
of HPV16 and HPV18 genotyping? APMIS. 127:710–716. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fani M, Mahmoodi P, Emadzadeh M, Avan A,
Karimi E, Ferns GA, Rezayi M and Amiri IS: Correlation of human
papil-lomavirus 16 and 18 with cervical cancer and their diagnosis
methods in Iranian women: A systematic review and meta-analysis.
Curr Probl Cancer. 44:1004932020. View Article : Google Scholar
|
|
5
|
DiGiuseppe S, Bienkowska-Haba M and Sapp
M: Human papillomavirus entry: Hiding in a bubble. J Virol.
90:8032–8035. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taghizadeh E, Jahangiri S, Rostami D,
Taheri F, Renani PG, Taghizadeh H and Gheibi Hayat SM: Roles of E6
and E7 human papillomavirus proteins in molecular pathogenesis of
cervical cancer. Curr Protein Pept Sci. 20:926–934. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang HY, Park S, Lee D, Kim S, Kim G, Park
KH and Lee H: Prevalence of type-specific oncogenic human
papillomavirus infection assessed by HPV E6/E7 mRNA among women
with high-grade cervical lesions. Int J Infect Dis. 37:135–142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang JJ, Cao XC, Zheng XY, Wang HY and Li
YW: Feasibility study of a human papillomavirus E6 and E7
oncoprotein test for the diagnosis of cervical precancer and
cancer. J Int Med Res. 46:1033–1042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huibregtse JM, Scheffner M and Howley PM:
Localization of the E6-AP regions that direct human papillomavirus
E6 binding, association with p53, and ubiquitination of associated
proteins. Mol Cell Biol. 13:4918–4927. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papilloma-virus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martinez-Zapien D, Ruiz FX, Poirson J,
Mitschler A, Ramirez J, Forster A, Cousido-Siah A, Masson M, Vande
Pol S, Podjarny A, et al: Structure of the E6/E6AP/p53 complex
required for HPV-mediated degradation of p53. Nature. 529:541–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crook T, Fisher C, Masterson PJ and
Vousden KH: Modulation of transcriptional regulatory properties of
p53 by HPV E6. Oncogene. 9:1225–1230. 1994.PubMed/NCBI
|
|
13
|
Mietz JA, Unger T, Huibregtse JM and
Howley PM: The transcriptional transactivation function of
wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6
oncoprotein. EMBO J. 11:5013–5020. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malanchi I, Accardi R, Diehl F, Smet A,
Androphy E, Hoheisel J and Tommasino M: Human papillomavirus type
16 E6 promotes retinoblastoma protein phosphorylation and cell
cycle progression. J Virol. 78:13769–13778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fischer M, Uxa S, Stanko C, Magin TM and
Engeland K: Human papilloma virus E7 oncoprotein abrogates the
p53-p21-DREAM pathway. Sci Rep. 7:26032017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slebos RJ, Lee MH, Plunkett BS, Kessis TD,
Williams BO, Jacks T, Hedrick L, Kastan MB and Cho KR:
P53-dependent G1 arrest involves pRB-related proteins and is
disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl
Acad Sci USA. 91:5320–5324. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Massimi P and Banks L: Repression of p53
transcriptional activity by the HPV E7 proteins. Virology.
227:255–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almeida AM, Queiroz JA, Sousa F and Sousa
A: Cervical cancer and HPV infection: Ongoing therapeutic research
to counteract the action of E6 and E7 oncoproteins. Drug Discov
Today. 24:2044–2057. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Z, Chen K, Cheng L, Yan B, Qian W,
Cao J, Li J, Wu E, Ma Q and Yang W: Resveratrol and cancer
treatment: Updates. Ann NY Acad Sci. 1403:59–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zadi Heydarabad M, Vatanmakanian M,
Abdolalizadeh J, Mohammadi H, Azimi A, Mousavi Ardehaie R,
Movasaghpour A and Farshdousti Hagh M: Apoptotic effect of
resveratrol on human T-ALL cell line CCRF-CEM is unlikely exerted
through alteration of BAX and BCL2 promoter methylation. J Cell
Biochem. 119:10033–10040. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rasheduzzaman M, Jeong JK and Park SY:
Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent
and suppression of Akt/NF-KB signaling. Life Sci. 208:208–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Casey SC, Amedei A, Aquilano K, Azmi AS,
Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR,
et al: Cancer prevention and therapy through the modulation of the
tumor microenvironment. Semin Cancer Biol. 35(Suppl 1): S199–S223.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu KF, Wu CL, Huang SC, Wu CM, Hsiao JR,
Yo YT, Chen YH, Shiau AL and Chou CY: Cathepsin L mediates
resveratrol-induced autophagy and apoptotic cell death in cervical
cancer cells. Autophagy. 5:451–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garcia-Zepeda SP, Garcia-Villa E,
Diaz-Chavez J, Hernandez-Pando R and Gariglio P: Resveratrol
induces cell death in cervical cancer cells through apoptosis and
autophagy. Eur J Cancer Prev. 22:577–584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YS, Sull JW and Sung HJ: Suppressing
effect of resveratrol on the migration and invasion of human
metastatic lung and cervical cancer cells. Mol Biol Rep.
39:8709–8716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Y, Yuan X, Li X and Zhang Y:
Resveratrol significantly inhibits the occurrence and development
of cervical cancer by regulating phospholipid scramblase 1. J Cell
Biochem. Oct 22–2018.Epub ahead of print. View Article : Google Scholar : 2018.
|
|
27
|
Chatterjee K, Mukherjee S, Vanmanen J,
Banerjee P and Fata JE: Dietary polyphenols, resveratrol and
pterostilbene exhibit anti-tumor activity on an HPV E6-Positive
cervical cancer model: An in vitro and in vivo Analysis. Front
Oncol. 9:3522019. View Article : Google Scholar
|
|
28
|
Chatterjee K, AlSharif D, Mazza C, Syar P,
Al Sharif M and Fata JE: Resveratrol and pterostilbene exhibit
anticancer properties involving the downregulation of HPV
Oncoprotein E6 in cervical cancer cells. Nutrients. 10:2432018.
View Article : Google Scholar :
|
|
29
|
Mukherjee S, Debata PR, Hussaini R,
Chatterjee K, Baidoo JNE, Sampat S, Szerszen A, Navarra JP, Fata J,
Severinova E, et al: Unique synergistic formulation of curcumin,
epicatechin gallate and resveratrol, tricurin, suppresses HPV E6,
eliminates HPV+ cancer cells, and inhibits tumor
progression. Oncotarget. 8:60904–60916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Popescu NC, DiPaolo JA and Amsbaugh SC:
Integration sites of human papillomavirus 18 DNA sequences on HeLa
cell chromosomes. Cytogenet Cell Genet. 44:58–62. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choo CK, Rorke EA and Eckert RL:
Differentiation-independent constitutive expression of the human
papillomavirus type 16 E6 and E7 oncogenes in the CaSki cervical
tumour cell line. J Gen Virol. 75:1139–1147. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pattillo RA, Hussa RO, Story MT, Ruckert
AC, Shalaby MR and Mattingly RF: Tumor antigen and human chorionic
gonadotropin in CaSki cells: A new epidermoid cervical cancer cell
line. Science. 196:1456–1458. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saha SK and Khuda-Bukhsh AR: Berberine
alters epigenetic modifications, disrupts microtubule network, and
modulates HPV-18 E6-E7 oncoproteins by targeting p53 in cervical
cancer cell HeLa: A mechanistic study including molecular docking.
Eur J Pharmacol. 744:132–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Souza RP, Bonfim-Mendonca PS, Gimenes F,
Ratti BA, Kaplum V, Bruschi ML, Nakamura CV, Silva SO, Maria-Engler
SS and Consolaro ME: Oxidative stress triggered by apigenin induces
apoptosis in a comprehensive panel of human cervical cancer-derived
cell lines. Oxid Med Cell Longev. 2017:15127452017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meissner JD: Nucleotide sequences and
further characterization of human papillomavirus DNA present in the
CaSki, SiHa and HeLa cervical carcinoma cell lines. J Gen Virol.
80:1725–1733. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Tornesello ML, Annunziata C, Tornesello
AL, Buonaguro L and Buonaguro FM: Human Oncoviruses and p53 tumor
suppressor pathway deregulation at the origin of human cancers.
Cancers (Basel). 10:2132018. View Article : Google Scholar
|
|
38
|
Filippova M, Evans W, Aragon R, Filippov
V, Williams VM, Hong L, Reeves ME and Duerksen-Hughes P: The small
splice variant of HPV16 E6, E6, reduces tumor formation in cervical
carcinoma xenografts. Virology. 450-451:153–164. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cerasuolo A, Buonaguro L, Buonaguro FM and
Tornesello ML: The role of RNA splicing factors in cancer:
Regulation of viral and human gene expression in human
papillomavirus-related cervical cancer. Front Cell Dev Biol.
8:4742020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ajiro M, Jia R, Zhang L, Liu X and Zheng
ZM: Intron definition and a branch site adenosine at nt 385 control
RNA splicing of HPV16 E6*I and E7 expression. PLoS One.
7:e464122012. View Article : Google Scholar
|
|
41
|
Evans W, Filippova M, Filippov V,
Bashkirova S, Zhang G, Reeves ME and Duerksen-Hughes P:
Overexpression of HPV16 E6* Alters β-Integrin and mitochondrial
dysfunction pathways in cervical cancer cells. Cancer Genomics
Proteomics. 13:259–273. 2016.PubMed/NCBI
|
|
42
|
Zhang P, Li H, Yang B, Yang F, Zhang LL,
Kong QY, Chen XY, Wu ML and Liu J: Biological significance and
therapeutic implication of resveratrol-inhibited Wnt, Notch and
STAT3 signaling in cervical cancer cells. Genes Cancer. 5:154–164.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Medina-Aguilar R, Marchat LA, Arechaga
Ocampo E, Gariglio P, García Mena J, Villegas Sepúlveda N, Martínez
Castillo M and López-Camarillo C: Resveratrol inhibits cell cycle
progression by targeting Aurora kinase A and Polo-like kinase 1 in
breast cancer cells. Oncol Rep. 35:3696–3704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang TT, Schoene NW, Kim YS, Mizuno CS and
Rimando AM: Differential effects of resveratrol and its naturally
occurring methylether analogs on cell cycle and apoptosis in human
androgen-responsive LNCaP cancer cells. Mol Nutr Food Res.
54:335–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hsieh TC and Wu JM: Differential effects
on growth, cell cycle arrest, and induction of apoptosis by
resveratrol in human prostate cancer cell lines. Exp Cell Res.
249:109–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fischer M and Muller GA: Cell cycle
transcription control: DREAM/MuvB and RB-E2F complexes. Crit Rev
Biochem Mol Biol. 52:638–662. 2017. View Article : Google Scholar : PubMed/NCBI
|