Introduction
Pituitary neuroendocrine tumors (PitNETs) have five
main histological subtypes: somatotroph, lactotroph, thyrotroph,
corticotroph, and gonadotroph (1). Somatotroph adenomas (SOMA) are the
primary cause of acromegaly, leading to severe complications such
as hypertension, diabetes mellitus, cardiovascular disease,
osteoarthritis, and sleep apnea (2). Transsphenoidal surgery is the first
choice for patients with somatotrophic adenoma. For failed surgery,
poor surgical candidates, or residual tumors, drug therapy is used
as adjuvant treatment (3).
However, the normalization of serum growth hormone/insulin-like
growth factor 1 (GH/IGF-1) in patients treated with
octreotide and lanreotide for one year was 43% and 31-35%,
respectively (4,5). An international, non-interventional
multicenter study of 2,090 patients with pegvisomant proved that
the patient's ratio with normal IGF-1 increased from 53% at
1 year to 73% at 10 years. However, most patients (72.2%) had no
change in tumor size relative to the prior scan, and 22% of
patients had severe adverse events (6). Thus, it is imperative to find new
mechanisms and molecular for the medical treatment of SOMA.
Cell cycle dysregulation results in uncontrolled
proliferation in cancers, including lung cancer, hepatic carcinoma,
leukemia, and gynecologic oncology (7). Cyclin-dependent kinases (CDK) are a
family of protein kinases involved in regulating the cycle of
mammalian cell division, which in turn is limited by CDK inhibitors
(CKIs) (8,9). The cell cycle is negatively
regulated by CKIs (8,10), which includes Cip/Kip and INK4
family (11). Somatotroph cells
are usually characterized by aneuploidy, DNA damage, and cell cycle
disruption, including premature cell cycle arrest (12). Overexpression of cyclin
E/CDK2 and loss of p21CIP1/p27KIP1
appeared to be associated with poor prognosis in SOMA (13). The INK4 family shares similar
domains that competes with Cyclin D and relieves the activation of
CDK4/6 (14),
leading to cell cycle arrest in the G1/S phase (15). Loss of cyclin-dependent kinase
inhibitor 2A (CDKN2A) predicted sensitivity to the
CDK4/6 inhibitor, PD0332991, in melanoma cell lines
(16). CDKN2A specifically
prevented both nucleotide probe and palbociclib binding to
CDK4 in MCF7 cell line (14).
At present, the pathogenesis of SOMA has not been
well elucidated. However, disruption of the cell cycle is
considered to play an essential role in pituitary tumorigenesis.
The aim of the present study was to describe the characteristic
profile of the INK4 family in SOMA, which was different from that
of other subtype adenomas and further analyze the correlation of
INK4 family with angiogenesis, CDKs activity,
epithelial-mesenchymal transition (EMT) and the therapeutic target
of SOMA. Based on these results, we hope to provide the evidence of
combined the CDK4/6 inhibitor for the medical
treatment of SOMA.
Materials and methods
Patients and samples
Tumor specimens were collected at Beijing Tiantan
Hospital affiliated to Capital Medical University from May 2012
through December 2017 after transsphenoidal surgery. Isolated
specimens were stored in liquid nitrogen for 30-60 min. All
patients had sporadic adenomas and had no family history of
adenomas. The morpho-functional classification of pituitary
adenomas was diagnosed according to the 2017 World Health
Organization (WHO) classification of tumors of endocrine organs
(17). The patients comprised 112
(56.6%) males and 86 (43.4%) females with the average age of
46.3±13.5 years (range, 21-69). The study protocol was approved by
the Internal Review Board of Beijing Tiantan Hospital Affiliated to
Capital Medical University, and conformed to the ethical guidelines
laid down in the Declaration of Helsinki (no. KY-2013-015-02).
Knosp classification was based on coronal sections of unenhanced
and gadolinium diethylenetriamine-pentaacetic acid enhanced
magnetic resonance imaging scans, with the readily detectable
internal carotid artery serving as the radiological landmark.
Surgically proven invasion of the cavernous sinus space was present
in all Grade 4 and Grade 3 cases and in all but one of the Grade 2
cases; no invasion was present in Grade 0 and Grade 1 cases
(18). All the samples were
obtained after informed consent of patients, following
institutional review board-approved protocols. Three normal
pituitary samples were obtained from the Tianjin Red Cross Society
by autopsy through the human body donation program.
GH3 and GT1-1 cell lines were purchased from ATCC
(Manassas) and cultured in a humidified incubator at 37°C and 5%
CO2 in F-12K medium (ATCC), supplemented with 2.5% fetal
bovine serum and 10% horse serum (Gibco).
RNA extractions, sequencing, RNA-Seq data
processing and analysis
For RNA extractions, patient samples were assessed
with AllPrep DNA/RNA Mini kit (Qiagen) according to the
manufacturer's instructions. The quantity and quality of RNA was
evaluated by RNA Nano6000 assay kit (Aligent Technologies) (RIN
>6.8). Then, 3 µg RNA/sample was used for RNA
preparations, and the ribosomal RNA was removed by Epicentre
Ribo-zero™ rRNA Removal kit (Epicentre). Sequencing library was
generated by NEBNext® UltraTM Directional RNA Library
Prep kit (NEB). The library fragments (150-200 bp) were purified by
AMPure XP system (Beckman Coulter), and assessed by Agilent
Bioanalyzer 2100 system. The libraries were sequenced on an
Illumina Hiseq X platform, then 150 bp paired-end reads were
generated. Any reads containing adapters, containing ploy-N and
low-quality reads were removed. Clean reads were mapped to the
human reference genome (NCBI37/hg19) using hisat2 (v2.0.5) to get
reads counts/FPKM/TPM for each identified gene. RStudio was used to
analyze the correlation coefficient and significative degree. R
package ggplot2 (https://github.com/tidyverse/ggplot2) was used to
visualize the results.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA of 32 samples was extracted and purified
using the RNeasy®Mini kit (QiaGen), following the
manufacturer's instructions. Reverse transcriptase kit was used
(Applied biosystems, Thermo Fisher Scientific, Inc.), and
Quantitative real-time PCR (RT-qPCR) was performed on QuantStudio5
applied (Thermo Fisher Scientific, Inc.) using Power
SYBR®-Green PCR Master Mix (Life Technologies). The mRNA
level of crucial genes of angiogenesis, CDKs, EMT, and therapeutic
targets of SOMA were measured in this study. The fold-change in
differential expression for each gene was calculated using the
comparative CT method (2−∆∆Cq method) in R package with
'PCR' functions (https://github.com/MahShaaban/pcr) (19). GAPDH was used as the reference
gene (20).
Tissue microarray construction and
immunohistochemistry staining
A total of 198 formalin-fixed (4%, room temperature)
paraffin-embedded tissue blocks were sectioned. Three core biopsies
(2.0 mm in diameter) were selected from the paraffin-embedded
tissue. The cores were transferred to tissue microarrays using a
semi-automated system (Aphelys MiniCore). The microarrays were cut
into 4-µm sections and incubated with
anti-p16INK4A (rabbit monoclonal, 1:1,000, ab108349,
Abcam), anti-CDH2 (rabbit polyclonal, 1:300, ab18203),
anti-CDK4 (rabbit polyclonal, 1:300, ab137675, Abcam),
anti-EGFR (rabbit monoclonal, 1:200, ab52894), and
anti-SSTR2 (rabbit monoclonal, 1:400, ab134152) primary
antibodies. IHC staining was evaluated in normal pituitary and
PitNETs tissue concerning the pattern of expression either
cytoplasmic, membranous or nucleus. H-score was also calculated for
CDKN2A/CDH2/CDK4/EGFR/SSTR2
using the intensity and percentage of positive cells. The intensity
score (0-3) was multiplied by the percentage of cells that stained
with each level of intensity and the sum of these mathematical
products was expressed as a score of 0-300. H score formula was
calculated as: Strong intensity (3) x percentage + moderate intensity
(2) x percentage + mild intensity
(1) x percentage.
Cell viability and cell cycle
GH3 and GT1-1 cells were adjusted to a density of
1×105 cells/ml. A total of 100 µl of the cell
suspension was plated into each well of a 96-well plate and
cultured overnight with 37°C thermostatic. After palbociclib
treatment (1, 5 or 20 µM) for 24, 48 and 72 h, 20 µl
of
3-(4,5-diethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) solution was added to each well, and the cultures
were further incubated for 4 h, 37°C. Absorbance was measured at
490 nm using an ELISA plate reader (Thermo Fisher Scientific,
Inc.). After 72 h treatment, the cell cycle was determined with PI
Detection kit (Roche Diagnostics) by flow cytometry. Experiments
were performed in triplicate.
Enzyme-linked immunosorbent assay
The levels of GH and IGF-1 in cell culture
supernatant were measured by ELISA (DE3058-96T, DE2096-96T,
Applygen), according to the manufacturer's protocol. A total of 10
µl of supernatant was used per well. Absorbance was read at
450 nm using an ELISA plate reader (Thermo Fisher Scientific,
Inc.). Experiments were performed in triplicate.
SDS-PAGE and western blot analysis
Samples (up to 10 mg) were lysed in lysis buffer
containing 1% Nonidet P-40 (Calbiochem, Merk) and protease and
phosphatase inhibitor cocktails (Roche) overnight at 4°C. Total
extracts were centrifuged at 12,000 × g for 30 min at 4°C, and
protein concentration was determined with the BCA method (Pierce
Biotechnology). A total of 40 µg of protein per lane was
loaded onto 10% Bis-Tris SDS-PAGE gels, separated
electrophoretically, and blotted onto polyvinylidene fluoride
membranes (Merk). The blots were incubated with antibodies against
anti-p16INK4A antibody (1:1,000), anti-CDK4
antibody (1:2,000), anti-EGFR antibody (1:1,000) and
anti-CDH2 antibody (1:1,000) followed by a secondary
antibody (1:10,000) tagged with horseradish peroxidase (Santa Cruz
Biotechnology). Blots were visualized by enhanced
chemiluminescence, and densitometry was performed using a
fluorescence image analyzer (Amersham Imager 600, GE Healthcare).
GAPDH was used as a loading control.
Statistical analysis
Chi test and Fisher's exact test were used to
determine the significance of categorical variables. One-way ANOVA
(Tukey post-hoc test) and t-test (unpaired test) were applied in
patients or for in vitro experiments. Spearman correlation
coefficient was used for the relationship of INK4 family and
crucial genes of angiogenesis, CDKs, EMT, and therapeutic targets
of SOMA. P-values were two-sided, and 0.05 was applied as the
significance level.
Results
Clinical characteristics of patients
The 198 patients with PitNETs included 38 (19.2%)
corticotroph adenomas (CORT), 80 (40.4%) gonadotroph adenomas
(GONA), 40 (20.2%) lactotroph adenomas (LACT), 40 (20.2%) SOMA and
the clinical characteristics are shown in Table I. The patients comprised 112
(56.6%) males and 86 (43.4%) females with the average age of
46.3±13.5 years (range, 21-69). The distribution of tumor volume
classifications was 9 (4.54%) microadenomas (diameter ≤1 cm), 127
(64.14%) macroadenomas (1 cm< diameter ≤4 cm), and 62 (31.32%)
giant adenomas (diameter >4 cm). According to Knosp staging,
there were 94 (47.47%) invasive cases and 104 (52.53%) non-invasive
cases. The average follow-up time was 45.5±10.2 months (range,
23-78), and 5-year recurrence rate was 56/198 (28.28%).
 | Table IClinical characteristics of 198
pituitary adenoma patients. |
Table I
Clinical characteristics of 198
pituitary adenoma patients.
| Variable | Number | Percentage |
|---|
| Sex | | |
| Male | 112 | 56.57 |
| Female | 86 | 43.43 |
| Age (year) | | |
| Range | 21-69 | |
| Mean | 46.3±13.5 | |
| Tumor size | | |
| Micro | 9 | 4.54 |
| Macro | 127 | 64.14 |
| Giant | 62 | 31.32 |
| Invasive | | |
| Yes | 94 | 47.47 |
| No | 104 | 52.53 |
| Surgical
extent | | |
| Total
resection | 137 | 69.19 |
| Partial
resection | 61 | 30.81 |
| Recurrence | | |
| Yes | 56 | 28.28 |
| No | 142 | 71.72 |
| Subtypesa | | |
| CORT | 38 | 19.2 |
| GONA | 80 | 40.4 |
| LACT | 40 | 20.2 |
| SOMA | 40 | 20.2 |
Characteristic profile of the INK4 family
in SOMA
A total of 24 SOMA specimens were sequenced to
obtain the transcriptome reading data after matching, then
normalized them to transcripts per million (TPM) values. With these
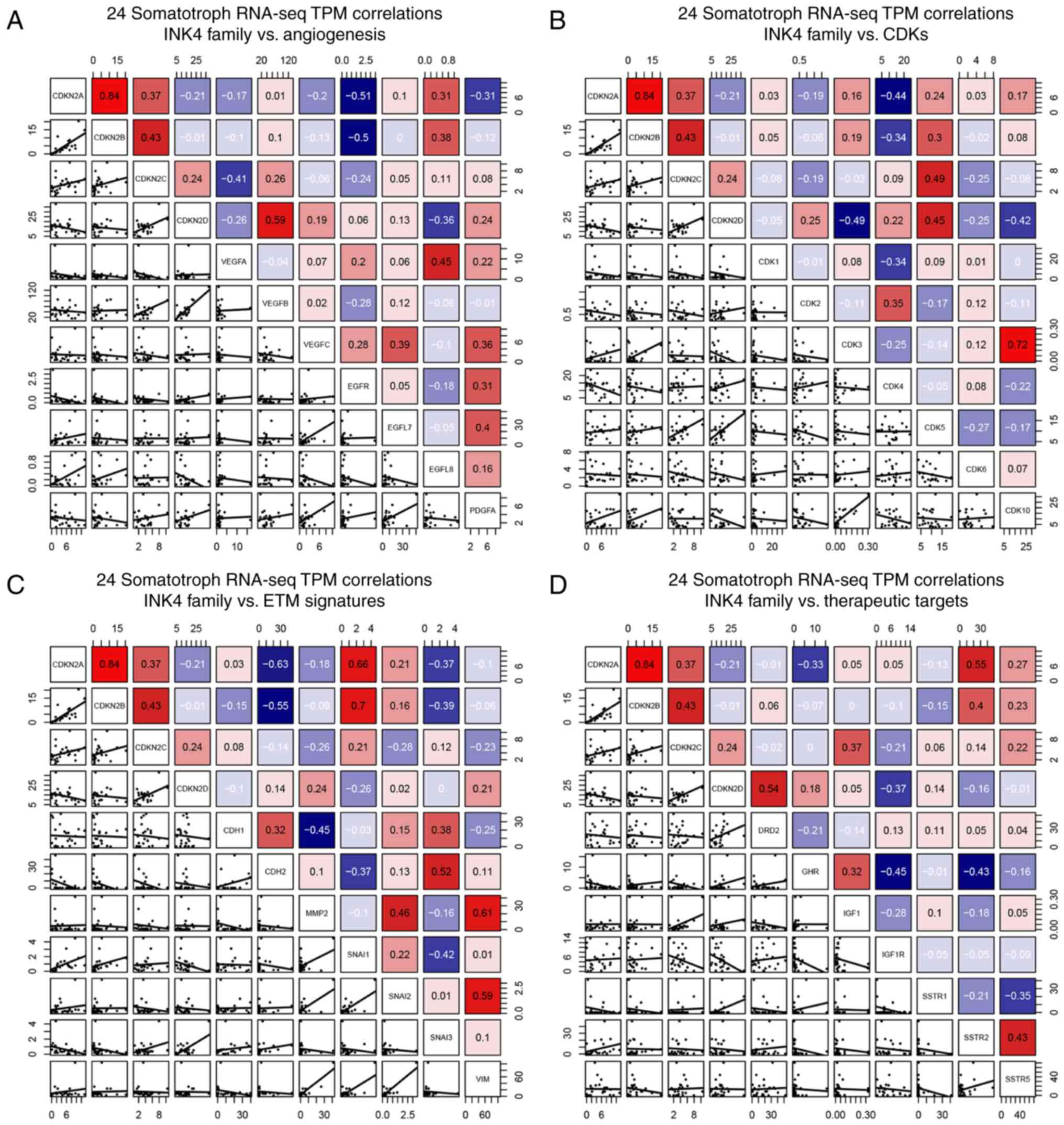
data, the mRNA levels correlation of INK4 family members with
crucial genes of angiogenesis, CDKs, EMT, and therapeutic targets
of SOMA were analyzed in Fig. 1.
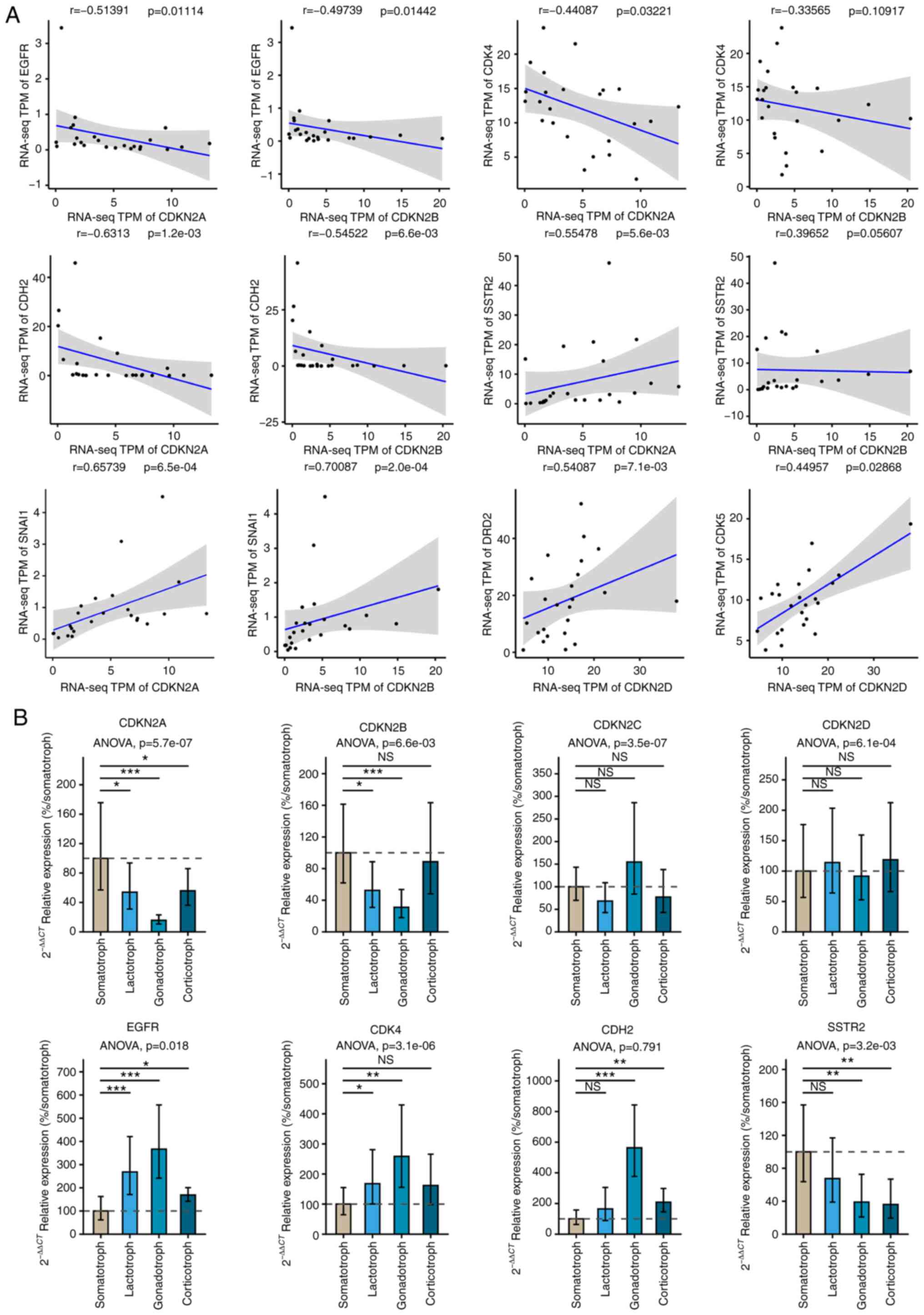
Among them, Spearman correlation coefficient of CDKN2A and
epidermal growth factor receptor (EGFR) (r=−0.534, P=0.032),
Cadherin 2 (CDH2) (r=−0.631, P=0.001), CDK4
(r=−0.441, P=0.032), Snail family transcriptional repressor 1
(SNAI1) (r=0.657, P=0.001), Somatostatin receptor 2
(SSTR2) (r=−0.555, P=0.006) as well as CDKN2B and
EGFR (r=−0.497, P=0.014), CDH2 (r=−0.545, P=0.007),
SNAI1 (r=0.70, P=0.001); CDKN2D and CDK5
(r=0.45, P=0.029), Dopamine receptor D2 (DRD2) (r=0.541,
P=0.007) had reached the significance level as shown in Fig. 2A. According to the results, EGFR,
CDK4, CDH2, and SSTR2 were filtered for the
next step.
Subsequently, we analyzed the mRNA levels of the
INK4 family and candidate molecules in SOMA, LACT, GONA, and CORT
in Fig. 2B via reverse
transcriptase-quantitative polymerase chain reaction (RT-qPCR). The
levels of CDKN2A and CDKN2B in SOMA were the highest
compared to other subtypes (P<0.01), and the levels of
EGFR and CDK4 in SOMA were the lowest (P<0.05).
There was no statistical difference of CDKN2C or
CDKN2D among these subtypes (P>0.05). Based on these
results, we chose CDKN2A for further research.
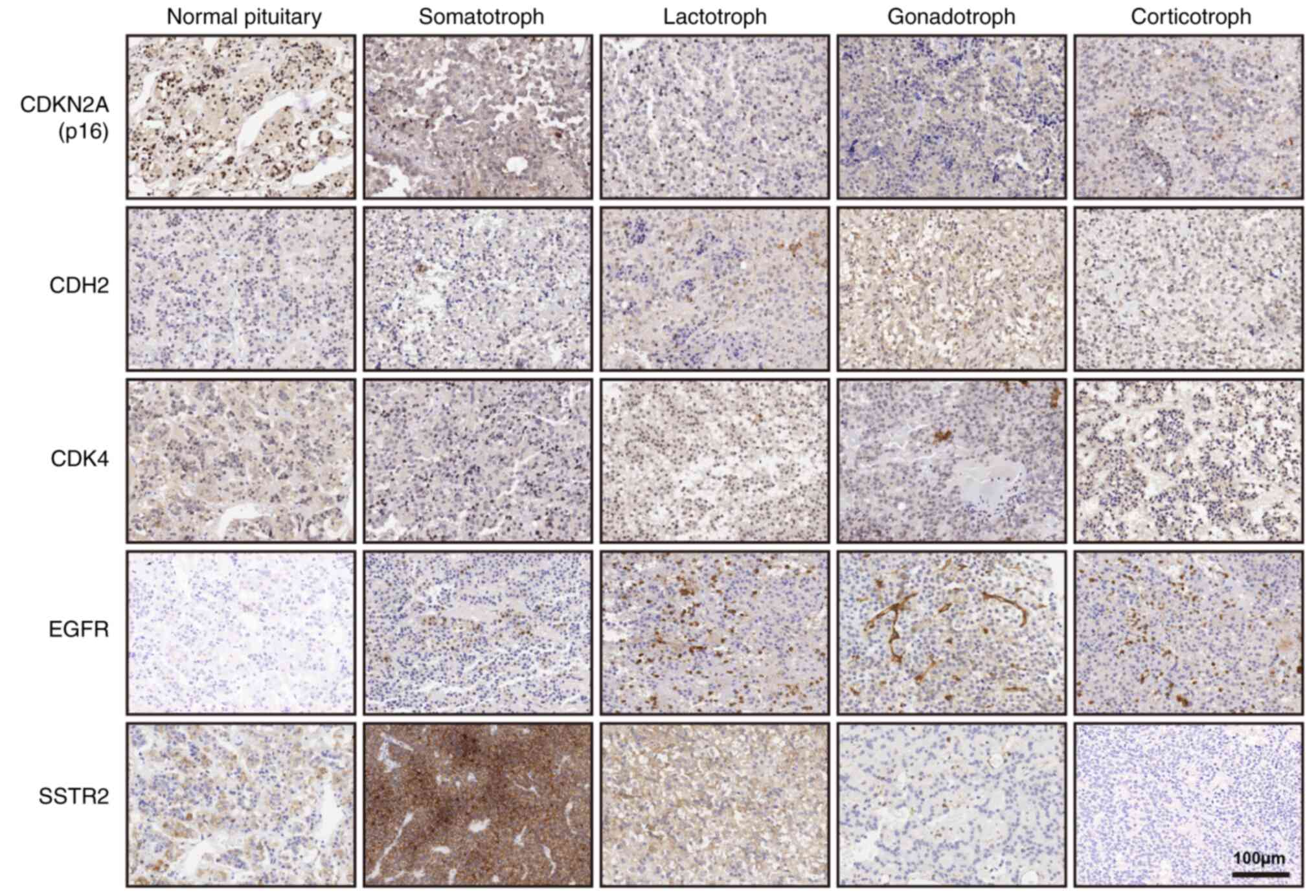
Expression profile of CDKN2A in 198
patients by tissue microarray analysis
Protein levels of CDKN2A, EGFR,
CDK4, CDH2, and SSTR2 were studied in 198
specimens by immunohistochemistry (IHC) (Fig. 3). Among the five groups, SOMA had
the lowest H-score compared to CDK4, CDH2, and
EGFR, but had a higher H-score compared to CDKN2A and
SSTR2 (P<0.01). Patients were divided into high and low
groups based on the median H-score. Patients with lower
CDKN2A had larger tumor size (58/99 vs. 41/99, P=0.016) and
higher invasive behavior (55/99 vs. 39/99, P=0.023) (Table II). There was also a negative
correlation between CDKN2A and invasive behavior (r=−0.207,
P=0.0004). Higher CDK4 might cause more invasive potential
(54/99 vs. 40/99, P=0.046). Positive staining of SSTR2 was
25/80 (31.3%) in GONA. The average H-scores of SSTR2 were
57.9±20.6 in SSTR2+GONA compared to 143.4±9.17 in SOMA. In
addition, we analyzed the correlation of DRD2 and
CDKN2A in 40 SOMA patients and 4 normal pituitary samples by
IHC experiment. H-scores of DRD2 in SOMA were 160.1±7.2
(range: 50-270), and 195±25.69 (160, 210, 210 and 270) in normal
pituitary. Correlation coefficient was 0.22 (P=0.151) in Fig. S1.
 | Table IIRelationship between CDKN2A, CDKN2C,
CDKN2D and various clinical parameters in 198 pituitary adenoma
patients. |
Table II
Relationship between CDKN2A, CDKN2C,
CDKN2D and various clinical parameters in 198 pituitary adenoma
patients.
| Variable | CDKN2A
| P-value | CDKN2C
| P-value | CDKN2D
| P-value |
|---|
| High | Low | High | Low | High | Low |
|---|
| Sex | | | P=0.086 | | | P=0.774 | | | P=0.251 |
| Male | 50 | 62 | | 57 | 55 | | 60 | 52 | |
| Female | 49 | 37 | | 42 | 44 | | 39 | 47 | |
| Age | | | P=0.065 | | | P=0.320 | | | P=0.118 |
| ≤46.3 | 43 | 56 | | 46 | 53 | | 55 | 44 | |
| >46.3 | 56 | 43 | | 53 | 46 | | 44 | 55 | |
| Tumor size
(cm3) | | | P=0.016 | | | P=0.201 | | | P=0.033 |
| ≤17.57 | 41 | 58 | | 45 | 54 | | 57 | 42 | |
| >17.57 | 58 | 41 | | 54 | 45 | | 42 | 57 | |
| Invasion | | | P=0.023 | | | P=0.090 | | | P=0.046 |
| Yes | 39 | 55 | | 42 | 52 | | 54 | 40 | |
| No | 60 | 44 | | 57 | 47 | | 45 | 59 | |
| Resection | | | P=0.045 | | | P=0.644 | | | P=0.090 |
| Total | 75 | 62 | | 67 | 70 | | 63 | 74 | |
| Partial | 24 | 37 | | 32 | 29 | | 26 | 25 | |
| Recurrence | | | P=0.115 | | | P=0.528 | | | P=0.058 |
| Yes | 23 | 33 | | 30 | 26 | | 34 | 22 | |
| No | 76 | 66 | | 69 | 73 | | 65 | 77 | |
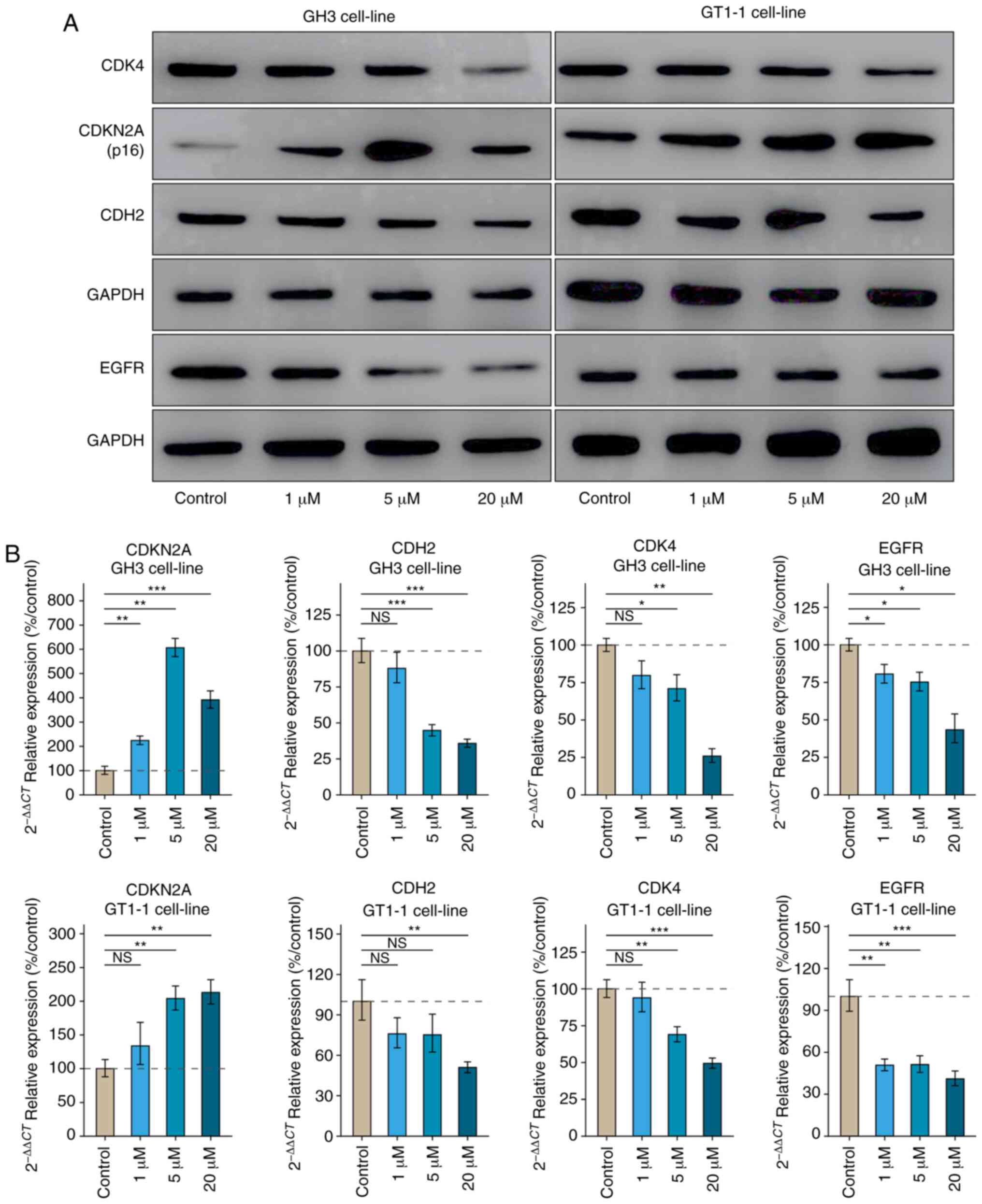
Inhibition induced by palbociclib in GH3
and GT1-1 cell line
The bioactivity effect of CDK4/6
inhibitor, palbociclib, was tested on the GH3 and GT1-1 cell lines.
The cell viability experiment showed that inhibition by palbociclib
occurred in a dose- and time-dependent manner (Fig. 4A and D). Levels of
GH/IGF-1 in cell culture supernatant declined in a
dose-dependent manner after 72 h treatment in Fig. 4B and E. Flow cytometry showed that
Palbociclib induced G1 phase arrest, whether in GH3 or GT1-1 cells
(Figs. 4C and F, S2). It was reported that the level of
CDKN2A was correlated with the sensitivity of CDK4
inhibitor, palbociclib. Statistical analysis of cell viability, and
secretion of GH/IGF-1 both showed the inhibition of
palbociclib in the GH3 cell line was stronger than that in the
GT1-1 cell line after 72 h treatment (Fig. 4G) (P<0.05).
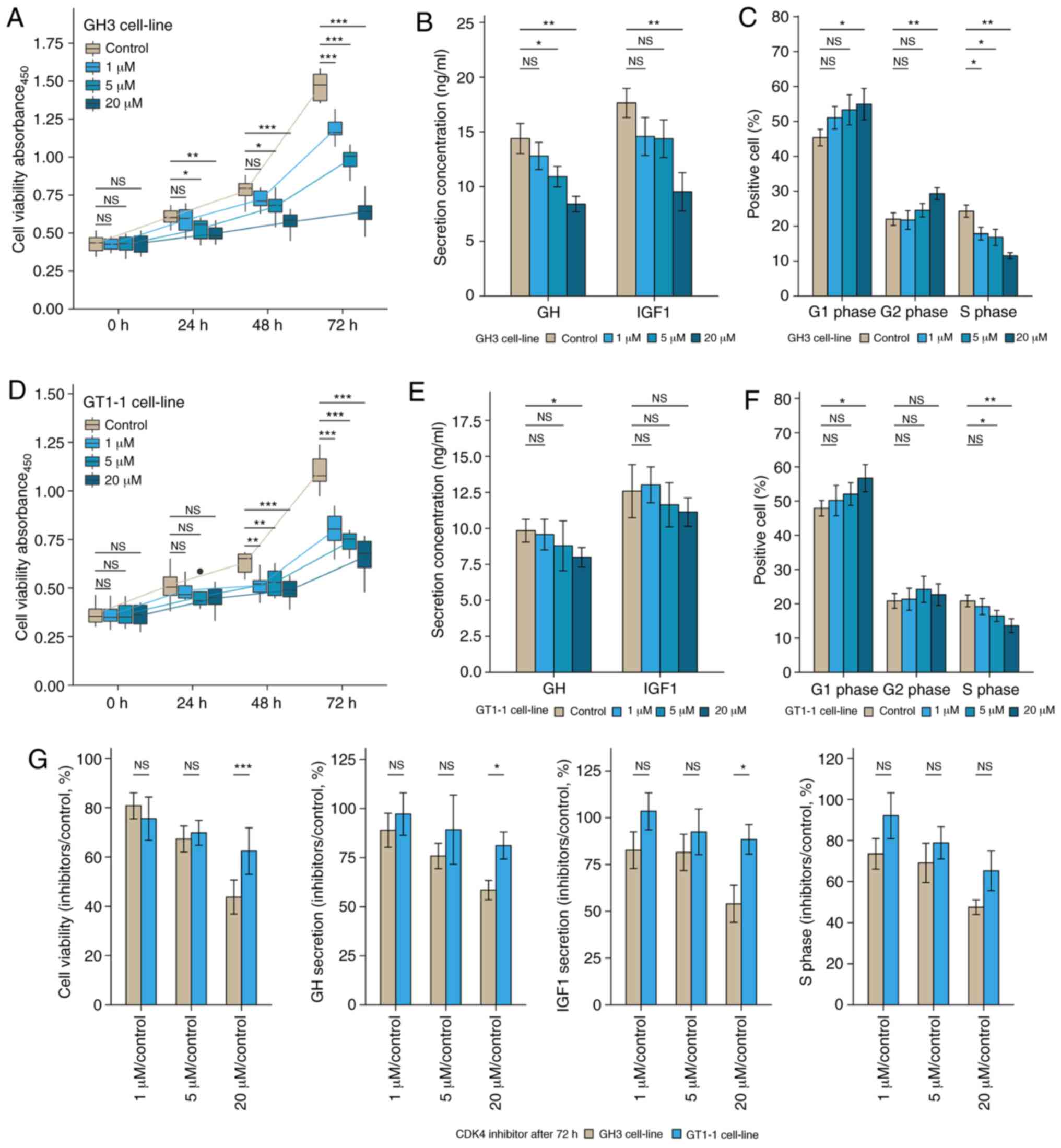 | Figure 4The bio-activity effect of
CDK4/6 inhibitor, Palbociclib, of GH3 and GT1-1 cell
line in various concentrations (1, 5, and 20µM). (A and D)
The inhibition induced by Palbociclib occurred in a time- and
dose-dependent manner, whether GH3 or GT1-1 cells.
*P<0.05, **P<0.01,
***P<0.001, n=8. (B and E) Palbociclib inhibited the
secretion of GH/IGF-1 in GH3 and GT1-1 cell lines. (C
and F) Palbociclib induced the G1 phase arrest in GH3 and GT1-1
cell lines. (G) Statistical analysis of the change of bio-activity
after 72 h treatment between GH3 and GT1-1 cell lines, compared to
the control group (%). *P<0.05,
**P<0.01, ***P<0.001, n=3. |
Results of the RT-qPCR and western blot assays
showed palbociclib undermined the level of CDK4,
CDH2, and EGFR (Fig.
5). Western blot analysis revealed that the level of
CDKN2A in GH3 cells was lower than that in GT1-1 cells.
Palbociclib has more potent inhibition on the activity of
CDK4 in GH3 cell line than in GT1-1 cell line.
Discussion
SOMA accounts for 13-15% of functional PitNETs and
are more common in males with a high standardized mortality ratio
compared with the general population (21). Factors associated with successful
treatment include tumor size, preoperative serum
GH/IGF-1 level, and parasellar exten-sion (22). Disruption of cell cycle played a
crucial role in pituitary tumorigenesis. Classifying the
relationship of cell cycle involving SOMA biology may provide new
therapeutic molecule against SOMA. Herein, we described the profile
of INK4 family in SOMA. The level of CDKN2A should be
evaluated for the strategy combined with CKIs in future medical
treatment.
Excess proliferation and gene dysregulation are the
hall-marks of cancer. Checkpoints block cells from passing into the
next phase, and CDKs control critical cell cycle checkpoints and
key transcriptional events. The first checkpoint occurs at the G1-S
phase, and the G1-S enzymes include CDK4, CDK6, and
the D-type cyclins (23). The
INK4 family regulates the G1-to-S phase transition by specifically
inhibiting the activity of CDK4/6.
CDKN2A/CDKN2B have unique structures and are
essential tumor suppressor genes; loss of CDKN2A function
was correlated with an increased risk of pancreatic cancer
(24-26). In addition, low level or loss of
CDKN2A was associated with shorter disease-free survival and
disease-specific survival times, independent of tumor size and WHO
grade of pancreatic neuroendocrine tumors (27). In this study, patients with low
CDKN2A had larger tumor volume and a higher likelihood of
invasive behavior than patients with high CDKN2A.
Correlation analysis of mRNA levels was measured in CDKN2A
and CDKs. CDK4 was filtered as the regulatory protein of
CDKN2A. The negative correlation between CDKN2A and
CDH2 was identified. CDH2 knockdown markedly
inhibited cell proliferation, colony formation, cell migration and
invasion, and induced cell cycle arrest and apoptosis (28).
The ankyrin-repeat protein CDKN2D functions
as a key regulator of G1/S transition, and phosphorylation of
CDKN2D dissociates the CDK6-CDKN2D inhibitory
complex, thereby, activating CDK6, which triggers entry into
S-phase (29). Considering the
dual role of CDKN2D in controlling differentiation and
proliferation (30), the positive
correlation between CDKN2D and CDK5 was not
significant in SOMA. CDKN2A and CDKN2D played a
converse role in the tumor proliferation and invasion of SOMA
heeding the relationship with genes related to EMT, at least,
albeit CDKN2D was not an inhibitor of CDK4 activity
in SOMA.
Recent evidence indicates epithelial-mesenchymal
transition (EMT) plays a critical role in stemness, metabolic
reprogramming, immune evasion and therapeutic resistance of cancer
cells. Transcriptional repressors including Snail (SNAI1),
Slug (SNAI2) and the ZEB family constitute key players for
EMT in cancer as well as in the developmental process (31). However, Tamura et al
reported that the expression of SNAIL1 was significantly associated
with suprasellar expansion, which was not related to tumor invasion
(32). Jia et al reported
that SNAI1 had a significant correlation of PitNET
proliferation, and SNAI2 had a significant correlation of
sella destruction (33). In
summary, the bio-activity of SNAI1 in PitNETs should be
investigated to clarify its role further in future research.
Therefore, we chose the most significant molecule, CDH2,
which is related to EMT. RT-qPCR and IHC experiments both indicated
the negative correlation between CDKN2A and CDH2.
CDK4/6 was known to promote continued
cell-cycle progression and growth in cancer. The imbalance of
CDK4/6 causes resistance to endocrine therapy in
hormone receptor-positive breast cancer (34). Palbociclib, combined with the
aromatase inhibitor, letrozole, significantly prolonged
progression-free survival as compared with letrozole alone in a
double-blind study of advanced breast cancer (35). An ovarian patient with loss of
CDKN2A derived significant, prolonged clinical benefit from
Palbociclib with letrozole (36).
High CDKN2Ashould be one of the exclusion criteria for
CDK4/6 inhibitor therapy for high CDK4 target
engagement by Palbociclib in cells without functional CDKN2A
and attenuated target engagement when CDKN2A is abundant
(14). GH3 cells mainly secrete
growth hormone and insulin-like growth factor, and GT1-1 cells
mainly secrete luteinizing hormone accompanied with a little growth
hormone. Theoretically, CDKN2A should be increased with the
increase of Palbociclib. However, the level of CDKN2A in 20
µM group did not support our hypothesis. We speculated that
the dose of Palbociclib (20 µM) was overdosed for the GH3
cell line. In summary, IC50 value of CDK4/6 inhibitor
is 23 nM to 10 µM in several cell lines including MU4-11,
MCF7, WM2664, BV173 and H69 (37).
In this study, we identified the lower level of
CDKN2A in GH3 cell line compared to that in the GT1-1 cell
line based on the western blot experiment. Palbociclib showed a
more potent inhibition of the level of CDK4, cell
proliferation, and cell cycle in the GH3 cell line compared to the
GT1-1 cell line. There was a negative correlation between
CDKN2A and CDK4 in SOMA specimens. Future study aims
to examine the RNAi-CDKN2A and plasmid overexpression in
in vitro/in vivo experiments, which can enhance the
results of anti-CDKN2A antibody blockade in the current
study. Palbociclib-combined SSAs should be a potent strategy in
SOMA patients with low CDKN2A level. Anderson et al
reported that metastatic breast cancer combined with non-functional
adenoma was treated with Palbociclib, and routine imaging
demonstrated significant regression of pituitary adenoma after
one-year treatment (38).
Furthermore, we found nearly one-third of
SSTR2-positive cases in GONA, which provided the evidence of
35±13% control ratio in non-functioning pituitary tumors by
SSTR2 agonists (1).
Combined with a low level of CDKN2A in GONA,
Palbociclib-combined SSAs should be evaluated in future clinical
trials. Lack of animal experiments and non-availability of
biomolecular details were some of the limitations of the current
study. For DNA hypomethylation and chromosomal instability in SOMA,
the epigenetic signature of the INK4 family should be explored in
future research.
In conclusion, correlations between CDKN2A
and tumor biological behaviors were established in SOMA.
Furthermore, nearly one-third of GONA had a positive SSTR2
expression. CDKN2A expression involved the inhibition of
cell proliferation and GH/IGF-1 secretion induced by
CDK4/6 inhibitor, and Palbociclib in in vitro
experiments. Therefore, we suggest the combination of CDK4
inhibitor and SSAs, can be used as potential therapeutic candidates
for targeting residual SOMA or GONA with high CDKN2A
expression.
Supplementary Data
Abbreviations:
|
inner salt MTS
|
3-(4,5-diethylthiazol-2-yl)-5-(3-
carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
|
|
CDH2
|
Cadherin 2
|
|
CKIs
|
CDK inhibitors
|
|
CORT
|
Corticotroph adenoma
|
|
CDK
|
cyclin-dependent kinases
|
|
CDKN2A
|
cyclin-dependent kinase inhibitor
2A
|
|
CDKN2B
|
cyclin-dependent kinase inhibitor
2B
|
|
CDKN2C
|
cyclin-dependent kinase inhibitor
2C
|
|
CDKN2D
|
cyclin-dependent kinase inhibitor
2D
|
|
DRD2
|
dopamine receptor D2
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
EGFR
|
epidermal growth factor receptor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
GONA
|
gonadotroph adenoma
|
|
GH
|
growth hormone
|
|
IHC
|
immunohistochemistry
|
|
1IGF-1
|
insulin-like growth factor
|
|
LACT
|
lactotroph adenoma
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SNAI1
|
Snail family transcriptional
repressor 1
|
|
SSA
|
somatostatin
|
|
SSTR2
|
somatostatin receptor 2
|
|
SOMA
|
somatotroph adenoma
|
|
TPM
|
transcripts per million
|
|
WHO
|
World Health Organization
|
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 81602182
and 81601205), Beijing Natural Science Foundation of China (grant
no. 7162035), and the Beijing High-level Talent Plan (grant no
2015-3-040).
Availability of data and materials
We agree that all datasets on which the conclusions
of the manuscript rely to be either deposited in publicly available
repositories (where available and appropriate) or presented in the
main paper or additional supporting files, in machine-readable
format (such as spreadsheets rather than PDFs) whenever
possible.
Authors' contributions
YC was involved in IHC experiments and writing the
manuscript. ZL was responsible for cell functional experiments. QF
conducted the PCR experiments. HW was responsible for performing
cell culture and functional experiments. CL carried out RNA-seq and
data analysis. HG was involved in data collection and statistical
analysis thereof. YZ designed the protocol and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all individuals
and ethical approval was obtained from the Institutional Review
Board of Beijing Tiantan Hospital Affiliated to Capital Medical
University (KY2013-015-02).
Patient consent for publication
All patients signed the informed consent.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors thank the laboratory technicians, data
collectors, and medical editors.
References
|
1
|
Neou M, Villa C, Armignacco R, Jouinot A,
Raffin-Sanson ML, Septier A, Letourneur F, Diry S, Diedisheim M,
Izac B, et al: Pangenomic classification of pituitary
neuroendocrine tumors. Cancer Cell. 13:S15356108193052272019.
|
|
2
|
Racine MS and Barkan AL: Medical
management of growth hormone-secreting pituitary adenomas.
Pituitary. 5:67–76. 2002. View Article : Google Scholar
|
|
3
|
Molitch ME: Diagnosis and treatment of
pituitary adenomas: A Review. JAMA. 317:516–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Melmed S, Popovic V, Bidlingmaier M,
Mercado M, van der Lely AJ, Biermasz N, Bolanowski M, Coculescu M,
Schopohl J, Racz K, et al: Safety and efficacy of oral octreotide
in acromegaly: Results of a multicenter phase III trial. J Clin
Endocrinol Metab. 100:1699–1708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alquraini H, Del Pilar Schneider M,
Mirakhur B and Barkan A: Biochemical efficacy of long-acting
lanreotide depot/autogel in patients with acromegaly naïve to
somatostatin-receptor ligands: Analysis of three multicenter
clinical trials. Pituitary. 21:283–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchfelder M, van der Lely AJ, Biller BM,
Webb SM, Brue T, Strasburger CJ, Ghigo E, Camacho-Hubner C, Pan K,
Lavenberg J, et al: Long-Term treatment with pegvisomant:
Observations from 2090 acromegaly patients in ACROSTUDY. Eur J
Endocrinol. 179:419–427. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rouse J and Jackson SP: Interfaces between
the detection, signaling, and repair of DNA damage. Science.
297:547–551. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sherr CJ and Roberts JM: CDK inhibitors:
Positive and negative regulators of G1-phase progression. Genes
Dev. 13:1501–1512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otto T and Sicinski P: Cell cycle proteins
as promising targets in cancer therapy. Nat Rev Cancer. 17:93–115.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roussel MF: The INK4 family of cell cycle
inhibitors in cancer. Oncogene. 18:5311–5317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melmed S: Pathogenesis of pituitary
tumors. Nat Rev Endocrinol. 7:257–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong W, Zhu H, Gao H, Shi W and Zhang Y,
Wang H, Li C, Song G and Zhang Y: Expression of cyclin
E/Cdk2/p27Kip1 in growth hormone adenomas. World Neurosurg.
121:e45–e53. 2019. View Article : Google Scholar
|
|
14
|
Green JL, Okerberg ES, Sejd J, Palafox M,
Monserrat L, Alemayehu S, Wu J, Sykes M, Aban A, Serra V and
Nomanbhoy T: Direct CDKN2 modulation of CDK4 alters target
engagement of CDK4 inhibitor drugs. Mol Cancer Ther. 18:771–779.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun F, Li N, Tong X, Zeng J, He S, Gai T,
Bai Y, Liu L, Lu K, Shen J, et al: Ara-C induces cell cycle G1/S
arrest by inducing upregulation of the INK4 family gene or directly
inhibiting the formation of the cell cycle-dependent complex
CDK4/cyclin D1. Cell Cycle. 18:2293–2306. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young RJ, Waldeck K, Martin C, Foo JH,
Cameron DP, Kirby L, Do H, Mitchell C, Cullinane C, Liu W, et al:
Loss of CDKN2A expression is a frequent event in primary invasive
melanoma and correlates with sensitivity to the CDK4/6 inhibitor
PD0332991 in melanoma cell lines. Pigment Cell Melanoma Res.
27:590–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lloyd R, Osamura R, Klöppel G and Rosai J:
2017, WHO classification of tumours of the endocrine organs. 4th
edn. International Agency for Research on Cancer; Lyon:
|
|
18
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–617. 1993.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Pabinger S, Rödiger S, Kriegner A,
Vierlinger K and Weinhäusel A: A survey of tools for the analysis
of quantitative PCR (qPCR) data. Biomol Detect Quantif. 1:23–33.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dekkers OM, Biermasz NR, Pereira M, Romijn
JA and Vandenbroucke JP: Mortality in acromegaly: A metaanalysis. J
Clin Endocrinol Metab. 93:61–67. 2008. View Article : Google Scholar
|
|
22
|
Starke RM, Raper DM, Payne SC, Vance ML,
Oldfield EH and Jane JA: Endoscopic vs microsurgical
transsphenoidal surgery for acromegaly: Outcomes in a concurrent
series of patients using modern criteria for remission. J Clin
Endocrinol Metab. 98:3190–3198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tigan AS, Bellutti F, Kollmann K, Tebb G
and Sexl V: CDK6-A review of the past and a glimpse into the
future: From cell-cycle control to transcriptional regulation.
Oncogene. 35:3083–3091. 2016. View Article : Google Scholar
|
|
24
|
Kim WY and Sharpless NE: The regulation of
INK4/ARF in cancer and aging. Cell. 127:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang B, Li Y, Qi G, Yuan S, Wang Z, Yu S,
Li B and He S: Clinicopathological significance of CDKN2A promoter
hyper-methylation frequency with pancreatic cancer. Sci Rep.
5:135632015. View Article : Google Scholar
|
|
26
|
Tian X, Azpurua J, Ke Z, Augereau A, Zhang
ZD, Vijg J, Gladyshev VN, Gorbunova V and Seluanov A: INK4 locus of
the tumor-resistant rodent, the naked mole rat, expresses a
functional p15/p16 hybrid isoform. Proc Natl Acad Sci USA.
112:1053–1058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roy S, LaFramboise WA, Liu TC, Cao D,
Luvison A, Miller C, Lyons MA, O'Sullivan RJ, Zureikat AH, Hogg ME,
et al: Loss of chromatin-remodeling proteins and/or CDKN2A
associates with metastasis of pancreatic neuroendocrine tumors and
reduced patient survival times. Gastroenterology. 154:2060–2063.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Da C, Wu K, Yue C, Bai P, Wang R, Wang G,
Zhao M, Lv Y and Hou P: N-Cadherin promotes thyroid tumorigenesis
through modulating major signaling pathways. Oncotarget.
8:8131–8142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar A, Gopalswamy M, Wolf A, Brockwell
DJ, Hatzfeld M and Balbach J: Phosphorylation-Induced unfolding
regulates p19INK4d during the human cell cycle. Proc Natl Acad Sci
USA. 115:3344–3349. 2018. View Article : Google Scholar
|
|
30
|
Wang Y, Jin W, Jia X, Luo R, Tan Y, Zhu X,
Yang X, Wang X and Wang K: Transcriptional repression of CDKN2D by
PML/RARα contributes to the altered proliferation and
differentiation block of acute promyelocytic leukemia cells. Cell
Death Dis. 5:e14312014. View Article : Google Scholar
|
|
31
|
Cho ES, Kang HE, Kim NH and Yook JI:
Therapeutic implications of cancer epithelial-mesenchymal
transition (EMT). Arch Pharm Res. 42:14–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tamura R, Ohara K, Morimoto Y, Kosugi K,
Oishi Y, Sato M, Yoshida K and Toda M: PITX2 expression in
non-functional pituitary neuroendocrine tumor with cavernous sinus
invasion. Endocr Pathol. 30:81–89. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia W, Zhu J, Martin TA, Jiang A, Sanders
AJ and Jiang WG: Epithelial-Mesenchymal transition (EMT) markers in
human pituitary adenomas indicate a clinical course. Anticancer
Res. 35:2635–2643. 2015.PubMed/NCBI
|
|
34
|
Shapiro GI: Cyclin-Dependent kinase
pathways as targets for cancer treatment. J Clin Oncol.
24:1770–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frisone D, Charrier M, Clement S,
Christinat Y, Thouvenin L, Homicsko K, Michielin O, Bodmer A,
Chappuis PO, McKee TA and Tsantoulis P: Durable response to
palbociclib and letrozole in ovarian cancer with CDKN2A loss.
Cancer Biol Ther. 21:197–202. 2020. View Article : Google Scholar :
|
|
37
|
Bisi JE, Sorrentino JA, Jordan JL, Darr
DD, Roberts PJ, Tavares FX and Strum JC: Preclinical development of
G1T38: A novel, potent and selective inhibitor of cyclin dependent
kinases 4/6 for use as an oral antineoplastic in patients with
CDK4/6 sensitive tumors. Oncotarget. 8:42343–42358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Anderson E, Heller RS, Lechan RM and
Heilman CB: Regression of a nonfunctioning pituitary macroadenoma
on the CDK4/6 inhibitor palbociclib: Case report. Neurosurg Focus.
44:E92018. View Article : Google Scholar : PubMed/NCBI
|