Introduction
For several decades, phage display technology has
been widely used for the selection of fully human antibodies. At
present, the most advanced phage antibody library is the Cre-LoxP
recombinant antibody library, which was constructed based on the
single vector pDF (1). The
Cre-LoxP system mediates site-specific DNA recombination, and is
thereby the core to gene-targeting technology. Furthermore, the
phagemid vector pDF contains two non-homologous LoxP sites on both
ends of the antibody gene, which can be recognized by Cre
recombinant enzyme. With the application of Cre-LoxP recombination
in the construction of a phage display antibody library, different
heavy (VH) and light (VL) chain variable regions can be freely
recombined, allowing for the indefinite repetition of gene
recombination, so as to realize the construction of antibody
libraries with large repertoire size and diversity (2).
Proprotein convertase subtilisin/kexin 9 (PCSK9), a
member of the subtilisin-like proprotein convertase family, serves
a role in low-density lipoprotein receptor (LDLR)-lysosome
pathways, as well as cholesterol and fatty acid metabolism
(3,4). Cohen et al (5) reported that genetical alterations in
the PCSK9 gene, such as gain-of-function mutations, could
elevate serum LDL-C levels (>300 mg/dl), and by contrast, that
loss-of-function mutations may lower serum LDL-C (<100 mg/dl),
resulting in cardiovascular disease (6). Various approaches to inhibiting
PCSK9 have been reported for hypercholesterolemia treatment,
including the use of antisense oligonucleotides, lipidoid
nanoparticle formulated short interfering RNAs directed against
PCSK9 mRNA (7,8), antibodies and LDLR subfragments
(9).
Monoclonal antibodies are the primary type of
commer-cial PCSK9 inhibitors. As a first generation antibody, the
murine monoclonal antibody possesses desirable immunogenicity
(10), though fully-humanized
monoclonal antibodies exhibit superior properties, overcoming the
shortcomings of murine monoclonal and chimeric monoclonal
antibodies. As such, fully-humanized antibodies have become
integral to the development of antibody-based therapeutics
(11).
In the present study, three 5×107 primary
antibody libraries were constructed using 400 blood samples, 30
bone marrow samples and 10 cord blood samples, respectively, all
from healthy donors. By optimizing the recombination conditions, a
fully human recombinant single-chain fragment variable (scFv) phage
library (size, 1×1011) was obtained. Sequencing 60
random scFvs showed that the diversity of antibody genes in the
library was consistent with the antibody gene distribution of the
human embryo line. The PCSK9 antigen was used to screen the fully
human scFv library, and five different scFv sequences were obtained
that specifically targeted to PCSK9. After constructing the
expression vectors of five selected scFvs, transient expression
revealed that anti-body expression levels ranged from 50-200 mg/l;
3D2 was found to have a coordination effect when used with statins,
which promoted the absorption of LDLR and LDL in hepatic Hep-G2
cells. Animal experiments showed that 3D2 increased the expression
of LDLR and significantly reduced total serum cholesterol.
Therefore, the results of the present study indicate that the
constructed large fully human scFv phage display library can be
used to screen for therapeutic antibodies, and that 3D2 has great
potential for use as a candidate PCSK9 inhibitor.
Materials and methods
Construction of a fully human scFv phage
display antibody library RNA extraction
The blood of 400 healthy donors, the bone marrow of
30 healthy donors and cord blood samples from 10 healthy donors
were kindly provided by the Affiliated Hospital of Jilin Medical
University (Jilin, China) between January 2016 and June 2016. The
study was approved by the ethics committee of Jilin Medical
University (2016-LW012) prior to the start of the study. All donors
were healthy volunteers aged 18-60, and of both sexes. The
lymphocytes from these samples were isolated using a human blood
lymphocyte isolation kit (cat. no. P8610; Solarbio Science and
Technology Co., Ltd.) Total RNA was extracted from the lymphocytes
using TRIzol® reagent (SinoGene Scientific Co., Ltd.),
and the RNA samples were analysed by 1% agarose gel
electrophoresis.
cDNA synthesis
First chain cDNA was synthesized with a reverse
transcription kit (Shanghai Hifun Biotechnology Co., Ltd.)
according to the manufacturer's instructions, using total RNA as
the template.
PCR amplification of scFvs
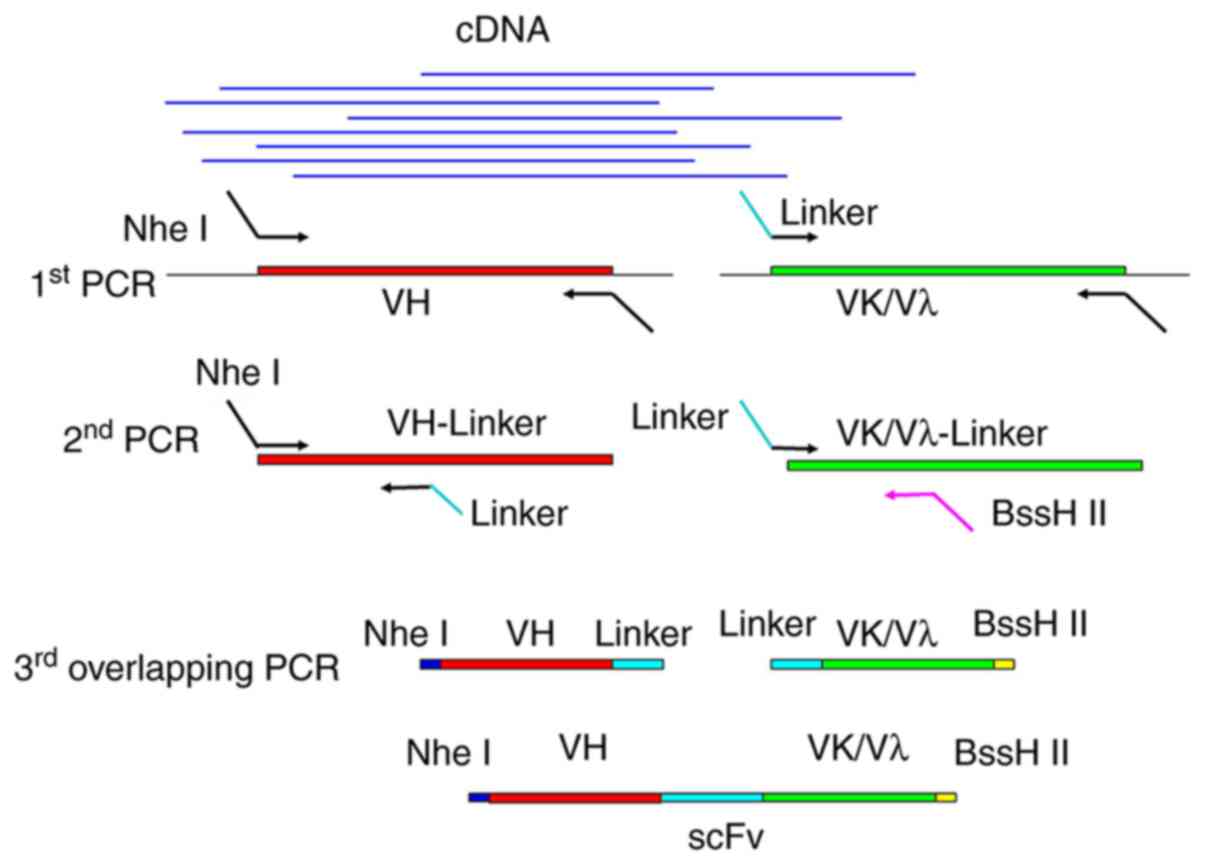
Stepwise PCR (Fig.
1) was used to synthesize the scFvs for library construction,
using a total of 22 different and degenerate primers as described
in Table I. Firstly, VH and VL
(Vκ/Vλ) chain variable regions were amplified using cDNA as the
template. The PCR reactions (50 µl each) contained 2
µl cDNA (40 ng/µl), 1 µl forward primer (10
µM), 1 µl reverse primer (10 µM), 0.5
µl Q5 DNA polymerase, 1 µl dNTP mixture (10
µM) and 34.5 µl ddH2O. The PCR
thermocycling conditions were as follows: 1 Cycle at 95°C for 3
min; 30 cycles at 95°C for 30 sec, 57°C for 30 sec and 72°C for 45
sec; and a final incubation at 72°C for 5 min. Then, the PCR
products (ranging around 400 bp) were recovered and further
amplified separately for VH- and VL-linkers with a
BssHII/NheI recognition site and LoxP511 sequence.
Finally, the VH- and VL-linkers were joined by overlap PCR. The PCR
program comprised two steps: 1 Cycle at 95°C for 3 min; and 2
cycles at 95°C for 1 min, 60°C for 30 sec, 55°C for 50 sec and 72°C
for 1 min. Then the HVKEXfor/PVHback primers were added according
to previous reports (1). The
subsequent PCR conditions were as follows: 5 Cycles of 95°C for 1
min, 60°C for 30 sec, 55°C for 50 sec and 72°C for 1 min; and 23
cycles at 95°C for 1 min, 60°C for 45 sec, and 72°C for 1 min. The
amplicons (ranging around 800 bp) were recovered and stored at
−80°C.
 | Table IPrimers used for gene
amplification. |
Table I
Primers used for gene
amplification.
| Forward primers for
VH | |
| PVHfor1 | TAT CCT CGA GCG GTA
CCS AGG TSC AGC TGG TRC AGT CTG G |
| PVHfor2 | TAT CCT CGA GCG GTA
CCS AGG TGC AGC TGK TGG AG |
| PVHfor3 | TAT CCT CGA GCG GTA
CCC AGR TCA CCT TGA AGG AGT CT |
| PVHfor4 | TAT CCT CGA GCG GTA
CCC AGS TGC AGCT RCA GSA GT |
|
| Reverse primer for
VH | |
|
| PVHback | GGC GGA TGC
GCT AGC TGA
RGA GAC RGT GAC C |
|
| Forward primers for
Vκ | |
|
| HVK1 | TAAT GCGCGC ATG CCG MCA TCC
RGW TGA CCC AGT CTC C |
| HVK2 | TAAT GCGCGC ATG CCG ATA TTG TGA TGA
CYC AGW CTC C |
| HVK3 | TAAT GCGCGC ATG CCG AAA TTG
TGW TGA CRC AGT CTC C |
| HVK4 | TAAT GCGCGC ATG CCG AAA CGA CAC TCA
CGC AGT CT |
|
| Reverse primers for
Vκ | |
|
| PVK1back | GGT CGA CCC TCC GGA
ACG TTT GAT HTC CAS CTT GGT |
| PVK2back | GGT CGA CCC TCC GGA
ACG TTT AAT CTC CAG TGC TGT |
|
| Forward primers for
Vλ | |
|
| HVL1 | TAAT GCGCGC ATG CCC AGT CTG
TGY TGA CKC AGC C |
| HVL2 | TAAT GCGCGC ATG CCC AGT CTG CCC TGA
CTC AGC C |
| HVL3 | TAAT GCGCGC ATG CCT CCT MTG
AGC TGA CWC AG |
| HVL4 | TAAT GCGCGC ATG CCC AGT YTG
TGC TGA CTC AAT C |
| HVL5 | TAAT GCGCGC ATG CCC AGR CTG
TGG TGA CYC AGG AG |
| HVL6 | TAAT GCGCGC ATG CCA ATT TTA TGC TGA
CTC AG |
| HVL7 | TAAT GCGCGC ATG CCC AGK MTG
RGC TGA YGC AGC CAC CCT C |
|
| Reverse primers for
Vλ | |
|
| PVLback | GGT CGA CCC TCC GGA
ACC TAG GAC GGT SAG CTT GGT CCC |
|
| Extended reverse
primer for VH, used for overlap PCR | |
|
| PVHL | CCA TAA CTT CGT
ATAATG TAT ACT ATA CGAAGT TAT_ CC_T CGA GCG GTA CC |
| Extended forward
primers for Vκ and Vλ | |
| HVKEXfor | GAG GAG GAG ATA ATG
CGC GCA TGC C |
| Extended reverse
primers for VL, used for overlap PCR | |
| PVLEX | GGA TAA CTT CGT ATA
GTA TAC ATT ATA CGAAGT TAT__ GGT CGA CCC TCC GGAAC |
Preparation of M13KO7-infected
electrocompetent E. Coli SS320 cells
According to previously described methods (12), 500 µl of E.
ColiSS320 (Tet+) at exponential phase
(OD600=0.8) was mixed with 6×108 pfu/ml of
M13K07 helper phage (Kana+) and 4 ml of 2YT top agar.
The mixture was poured onto LB agar plate (supplemented with 5
µg/ml tetracycline) and incubated for 20 h at 37°C. A single
plaque was selected and inoculated into 1 ml of 2YT/kana/tet medium
(10 g/l yeast extract, 16 g/l tryptone, 5 g/l NaCl, 15 g/l
granulated agar, 25 µg/ml kanamycin and 5 µg/ml
tetracycline; pH to 7.0) and incubated for 8 h at 37°C. The culture
was then transferred to 250 ml of 2YT/kana medium and incubated
with shaking at 200 × g at 37°C overnight. 5 ml of the overnight
culture was inoculated into 900 ml of superbroth/tet/kana medium
(12 g/l tryptone, 24 g/l yeast extract, 5 ml/l glycerol, 0.17 M
KH2PO4, 0.72 M K2HPO4,
5 µg/ml tetracycline and 25 µg/ml kanamycin) and
incubated with shaking at 200 × g at 37°C until the absorbance had
reached 0.8 (OD600). The culture was centrifuged at
5,000 × g for 15 min at 4°C. The supernatant was discarded and the
pellet was washed with cold 1 mM HEPES (pH 7.5) 3 times. By
centrifuging at 5,000 × g for 15 min at 4°C, the precipitate was
first resuspended with 20 ml of 1 mM HEPES containing 10% (v/v)
glycerol and finally resuspended with 1 ml of 10% (v/v) cold
glycerol. Then the cells were snap frozen with liquid nitrogen and
stored at −80°C.
Transformation and library
construction
According to previously described methods (12), the scFv fragments (1 µg)
were digested using BssHII and NheI (1 µg
each) and inserted into a pDF vector (also digested with
BssHII and NheI) (Fig.
4; Amp+, 1.78 µg) using T4 ligase at 16°C.
The constructs were purified using the QIAquick PCR purification
kit (cat. no. 28104; Qiagen, Dusseldorf) and electroporated into 25
µl M13KO7-infected SS320 electrocompetent cells at 2,500 V,
200 Ω, 25 µF and 4 msec. Then, 25 ml SOC media was added (5
g/l bactoyeast extract, 20 g/l bactotryptone, 0.5 g/l NaCl, 0.2 g/l
KCl, 0.01 M MgCl2 and 0.02 M glucose; pH 7.0) and the
cultures were incubated at 37°C for 40 min with agitation (200 ×
g). Subsequently, 10 µl of the culture was diluted
(1:108) and plated onto 2YT/Amp agar medium (2YT
supplemented with 50 µg/ml ampicillin) for overnight
incubation at 37°C, which was used to evaluate and calculate the
size of the scFv phage display library. The rest of the culture was
inoculated into 1 l 2YT/Amp/kan/IPTG media (supplemented with 50
µg/ml ampicillin, 25 µg/ml kanamycin and 25 mM IPTG)
and incubated overnight at 37°C with gentle shaking. The culture
was then centrifuged at 10,000 × g for 15 min at 4°C. The
supernatant was removed, mixed with 20% PEG 8000-2.5M NaCl
solution, and left to stand on ice for 20 min. After centrifuging
at 10,000 × g for 20 min at 4°C, the phage precipitate was
resuspended with 20 ml 1X PBS to generate the primary library. The
recombinant phage antibody library was constructed by infecting the
mid-log-phage-BS1365 strain (Cre+, OD600 0.8)
with 250 µl of the primary library (1×1012
pfu/ml) at a multiplicity of infection (MOI) of 200:1. After
standing at 37°C for 1 h, 400 ml 2YT/Amp media was added and the
culture was incubated at 37°C (200 × g) until the absorbance had
reached 0.5 (OD600). Then, 5 ml helper phage (M13KO7;
5×1010 pfu/ml) was added at a ratio of 3000:1, and the
culture was gently shaken. After standing at 37°C for 1 h, the
phage library was collected and used to infect 1 liter of the
mid-log-phase-OMNImax2 strain (Tet+, OD600
0.8) at an MOI ≤1; 10 ml of M13KO7 (5×1010 pfu/ml) was
then added and shaken at 30°C for 1 h. The resulting recombinant
human scFv phage library was stored in glycerol at −80°C. The
library size was calculated as follows: Library size
(cells/ml)=number of colonies ×103 × dilution ratio.
Human recombinant PCSK9 protein
preparation
Human PCSK9 (Genbank AX207686) was amplified from
Hep-G2 cells by reverse transcription PCR, and its sequence was
confirmed by sequencing. The Hep-G2 cells (Shanghai Biowing Applied
Biotechnology Co., Ltd.) were authenticated as a hepatocellular
carcinoma cell line using short tandem repeat profiling, which was
also conducted by Shanghai Biowing Applied Biotechnology Co., Ltd.
The cells were maintained in RPMI-1640 (Gibco) supplemented with
10% (v/v) fetal bovine serum (FBS, Gibco) and anti-biotics (100
IU/ml penicillin and 100 mg/ml streptomycin) at 37°C and 5%
CO2. Then, the gene was cloned into the pcDNA3.1-hIgG1FC
vector along with a 6X His tag, between the HindIII and
XhoI sites The recombinant plasmid (30 µg) was then
used to transfect 5×107 CHO-K1 cells using
Lipofectamine® 6000 transfection reagent (cat. no.
C0528; Beyotime Biotechnology Co., Ltd.). Recombinant PCSK9 protein
was purified from the cell supernatant using PrepEase®
His-tag protein purification resin (Biomart Co., Ltd.). The CHO-K1
cells were obtained from the American Type Culture Collection
(ATCC® CCL-61™) and maintained in RPMI-1640 (Gibco)
supplemented with 10% (v/v) FBS (Gibco) and antibiotics (100 IU/ml
penicillin and 100 mg/ml streptomycin) at 37°C and 5%
CO2.
Selection of antibodies against human
PCSK9
The recombinant human scFv phage library was
screened against PCSK9 as previously reported (13,14). Immunotubes were coated with
detection antigen (purified PCSK9) diluted to 100 µg/ml (2
ml/immunotube) and incubated at 4°C overnight. After washing 3
times with PBS, the immunotubes were blocked with 2% (w/v) BSA in
PBS. The diluted recombinant phage particles (1011
pfu/ml) were subsequently added into the coated immunotubes and
incubated at 37°C for 2 h. The immunotubes were then washed 10
times with PBST (PBS containing 0.1% Tween-20) and 10 times with
PBS only, to remove unbound phage. Bound phage was eluted with 100
mM triethylamine (Sigma-Aldrich; Merck KGaA) and neutralized with
500 µl of 1 M Tris-HCl (pH 7.5). Mid-log phase E.
coli TG1 cells were infected with the eluted phage, plated onto
several 2YT-agar plates and cultured until individual colonies had
formed. Each clone was used to transform mid-log phase E.
coli TG1 cells (using M13KO7 phages), which were screened using
a competitive phage-ELISA. The screening process was repeated for 4
rounds.
Expression and purification of antibodies
against PCSK9
The selected human PCSK9 scFvs in the phages were
amplified by PCR and inserted into a modified mammalian expression
vector (pFUSE2-CLIg-hk and pFUSE-CHIg-hG1; InvivoGen). The plasmids
were used to transfect 293 cells using the ExpiFectamine™ 293
Transfection Kit (Gibco; Thermo Fisher Scientific, Inc.), and the
culture supernatant was collected after 8 days. The antibodies were
separated and purified from the supernatant using a Protein A
Sepharose column (GE Healthcare Bio-Sciences). The integrity of the
antibodies was determined by ELISA and SDS-PAGE.
Biolayer interferometry (BLI)
analysis
The affinity measurements were analysed by BLI
technology using a Pall ForteBio's BLI-based Octet RED96e system
(ForteBio; Sartorius AG) combined with Octet version 13.1.0
software, according to the manufacturer's recommendation. The
purified anti-PCSK9 antibodies were biotinylated using the Biotin
Labelling Kit-NH2 (Dojindo Molecular Technologies,
Inc.). Briefly, 1 mg anti-PCSK9 antibody was mixed in a filtration
tube with 100 µl NH2-reactive biotin (10 mM), and incubated
at 37°C for 10 min. The tube was then centrifuged at 6,000 × g for
30 min at 4°C to remove uncombined biotin reagent. The biosensors
in the Pall ForteBio's BLI-based Octet® RED96e system
were hydrated as follows: 200 µl 1X Kinetics Buffer was
added to a each well of a round-bottom microplate (the hydration
plate), which was placed into the biosensor tray of the system. The
biosensors were dipped into the wells for 10 min; 200 µl 50,
25, 5, 2.5 or 0.5 µg/ml biotinylated anti-PCSK9 antibody was
added to the wells of a black 96-well polypropylene microplate (the
sample plate), followed by 200 µl PCSK9 antigen (200 nM).
Then, 200 µl biotinylated anti-PCSK9 antibody (50
µg/ml) was added to the wells, and biotinylated anti-PCSK9
antibody without PCSK9 antigen served as the negative control. The
plate temperature was set to 30°C in the Octet®
software, the sample plate was placed on the sample plate stage
inside the Octet® system, and the biosensor hydration
assembly was placed on the biosensor stage. The settings for the
affinity assay were as follows: Equilibration for 60 sec, loading
for 400 sec, baseline for 600 sec, association for 400 sec and
dissociation for 3,600 sec at a flow rate of 1,000 × g. After the
assay, the shapes of the individual binding curves were observed
and the binding association (Kon) and dissociation rate
constants (Koff) are calculated from a global fitting
analysis assuming a 1:1 binding model using the Octet Data Analysis
software. The affinity constant (KD) was determined from
the ratio of the rate constants:
KD=Koff/Kon.
Western blot analysis
Hep-G2 cells or liver tissues were lysed to
determine the expression levels of LDLR. Cells were treated with
radio-immunoprecipitation assay buffer and protease inhibitor
cocktail (both from Millipore Sigma) and incubated for 30 min to
stop proteolysis. The lysate was centrifuged at 12,000 × g for 30
min (4°C), and 60 µg total protein was electrophoresed on a
10% polyacrylamide gel; the proteins were transferred to a PVDF
membrane for 2 h at 300 mV, and then washed 5 times with TBST [50
mM Tris-Cl (pH 7.5), 100 mM NaCl and 0.5% Tween-20]. The membranes
were blocked with 5% BSA for 2 h at 37°C, and then incubated with
diluted primary antibodies (0.25 µg/ml goat anti-mouse LDLR,
rabbit anti-human LDLR or mouse anti-GAPDH antibodies) (Abcam,
Cambridge) at 4°C overnight, followed by HRP-labelled goat
anti-rabbit IgG (1:1,000) for 1 h at 37°C. The protein bands were
detected using an ECL kit.
Competitive phage ELISA screening and
indirect ELISA for selected clones
ELISA plates (96-well) were coated with 5
µg/ml PCSK9 protein (100 µl/well) at 4°C overnight.
After incubation with blocking buffer (2% BSA/PBS) for 1 h, 100
µl phage antibody (containing supernatants or purified
anti-PCSK9 antibodies) was added to each well, and the plates were
incubated at 37°C for 1 h. The plates were then washed three times
with PBST and incubated with a 1:5,000 dilution of HRP-labelled
mouse anti-M13 monoclonal antibody (cat. no. ab50370; Abcam) or
HRP-IgG at 37°C for 1 h. Then, 100 µl/well
tetramethylbenzidine peroxidase (TMB) substrate solution was added
to initiate the colour reaction, which was then terminated using 2
M sulfuric acid (100 µl/well). The absorbance at 450 nm was
measured using a Elx800 micro-plate reader (BioTeK Instruments,
Inc.), with 2% BSA as the negative control and PBS as the blank
control. All experiments were repeated three times.
PCSK9 inhibition in Hep-G2 cells using
purified anti-PCSK9 antibody
Hep-G2 cells (5×105 cells/ml) were
cultured in a 6-well plate overnight at 37°C, to generate
single-cell mono-layers. The supernatant was discarded and replaced
with DMEM containing 10% lipoprotein-deficient serum. After, 24 h,
the cells were treated with 1, 5 or 10 µg/ml purified
antibody or a combination of 0.5 or 1 µg/ml mevinolin, and
incubated for 24 h sequentially. After collection, the LDLR levels
of theHep-G2 cells were determined by western blotting.
Inhibitory effect of the anti-PCSK9
antibody on PCSK9-LDLR binding
A microtiter plate was coated with 5 µg/ml
rLDLR (recombinant human LDLR; Shanghai Xinfan Biological
Technology Co., Ltd.). The biotinylated PCSK9 protein was then
mixed with different concentrations of anti-PCSK9 anti-body, which
were then added to the plate. Bound biotinylated PCSK9 was detected
using avidin-HRP and TMB. The reactions were terminated using 50
µl H2SO4 (2 M) and absorbance was
measured at 450 nm.
Measurement for serum cholesterol liver
LDLR in mice
All animal experiments were approved by the Ethics
Committee of Jilin Medical University. Male C57BL/6J mice (weight,
18-22 g) were purchased from the experimental animal centre of
Jilin University (Changchun, China), and housed with food and water
ad libitum under a constant temperature (23±1°C) with a 12-h
light/dark cycle. The mice were randomly divided into three groups
and intravenously (i.v.) administered purified anti-PCSK9 antibody
(n=84), anti-keyhole limpet hemocyanin antibody (negative control,
n=21) or PBS (blank control, n=21).
A total of 21 mice were randomly selected from each
group and received a single i.v. injection of anti-PCSK9 antibody
(10 mg/kg). The serum was collected at different time points
post-injection (24, 48 and 72 h; 7 mice/group). The remaining 63
mice in the anti-PCSK9 antibody group were injected with different
concentrations of anti-PCSK9 antibody, and the serum was collected
at 3, 6 and 9 days after injection. The total cholesterol (TC)
levels were determined using a TC detection kit (cat. no. A111-1-1;
Nanjing Jiancheng Bioengineering Institute).
The mice were sacrificed by cervical dislocation,
and protein was extracted from the livers (24, 48 and 72 h
post-injection groups) using a Membrane and Cytosol Protein
Extraction Kit (cat. no. G4422-2; GBCBIO Technologies Inc.).
Western blotting was conducted using LDLR and GAPDH antibodies
(both Abcam) to determine the levels of LDLR following PCSK9
treatment.
Statistical analysis
Statistical analyses were conducted using Originpro
version 9.1 (OriginLab). All of the results conformed to a normal
distribution and are expressed as the mean ± SEM. The t-test was
used to compare two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction of fully human scFv phage
display library
Stepwise PCR was used to synthesize fully human
scFvs for library construction (Fig.
1). The concentration of total RNA was 2,164 ng/µl with
a A260/A280 ratio of 1.935 (data not shown). Following
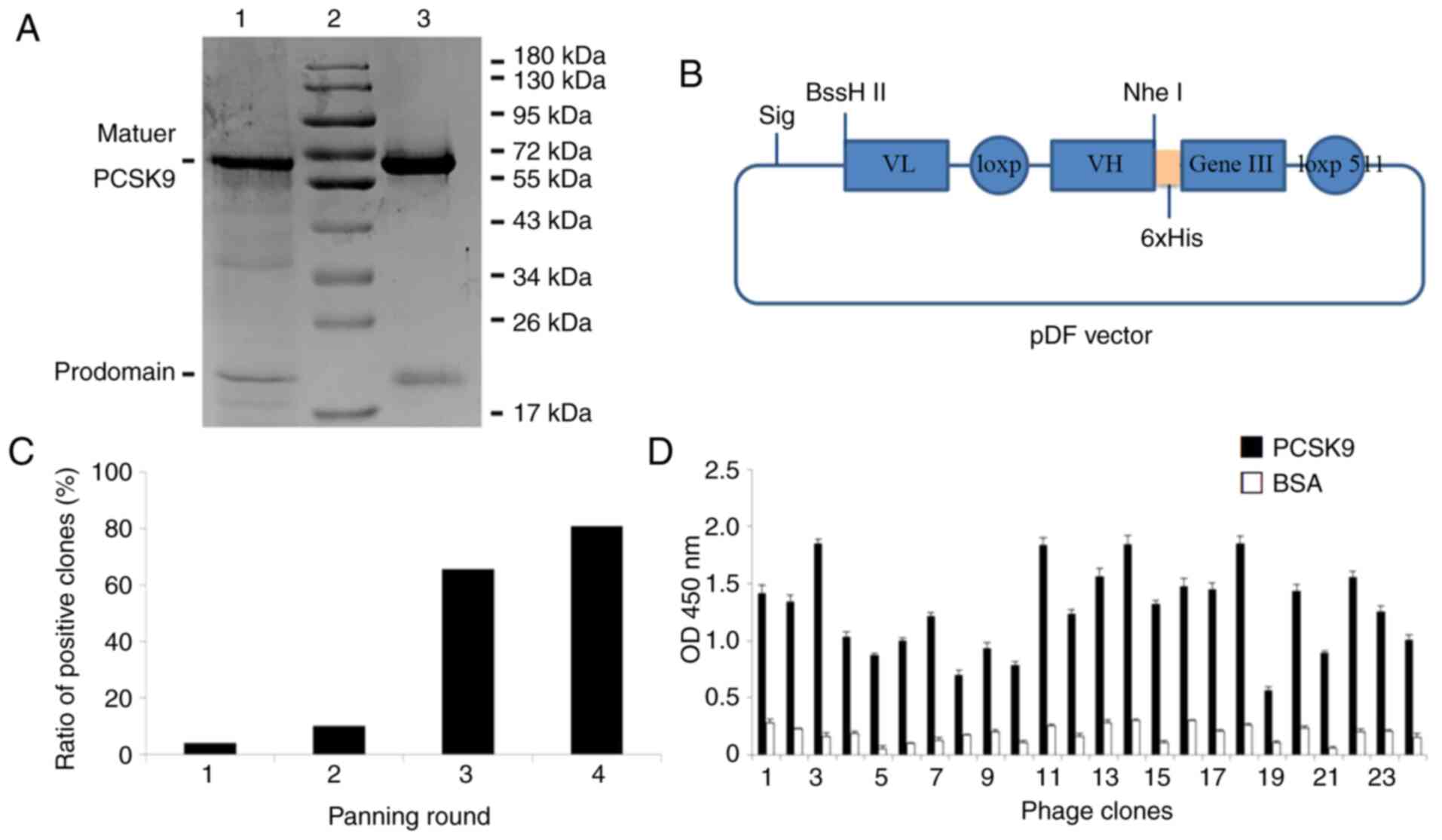
amplification of the VH, Vκ and Vλ genes (Fig. 2A-C), amplicons ranging around 400
bp were obtained and used as the templates for overlap PCR.
Ultimately, the amplicons ranging around 800 bp of the scFv
(Fig. 2D) were recovered and
cloned into pDF vectors. After recombination of the library in the
BS1365 strain, the library size was estimated to be
~1×1011 cells/ml (Fig.
3A). Tests for library accuracy and diversity, based on 60
colony PCR and scFv gene sequencing results, are shown in
Fig. 3B, which confirm >75%
sequence variation. Sequence blast results revealed that VL and VH
were consistent with the human antibody sequences (data not
shown).
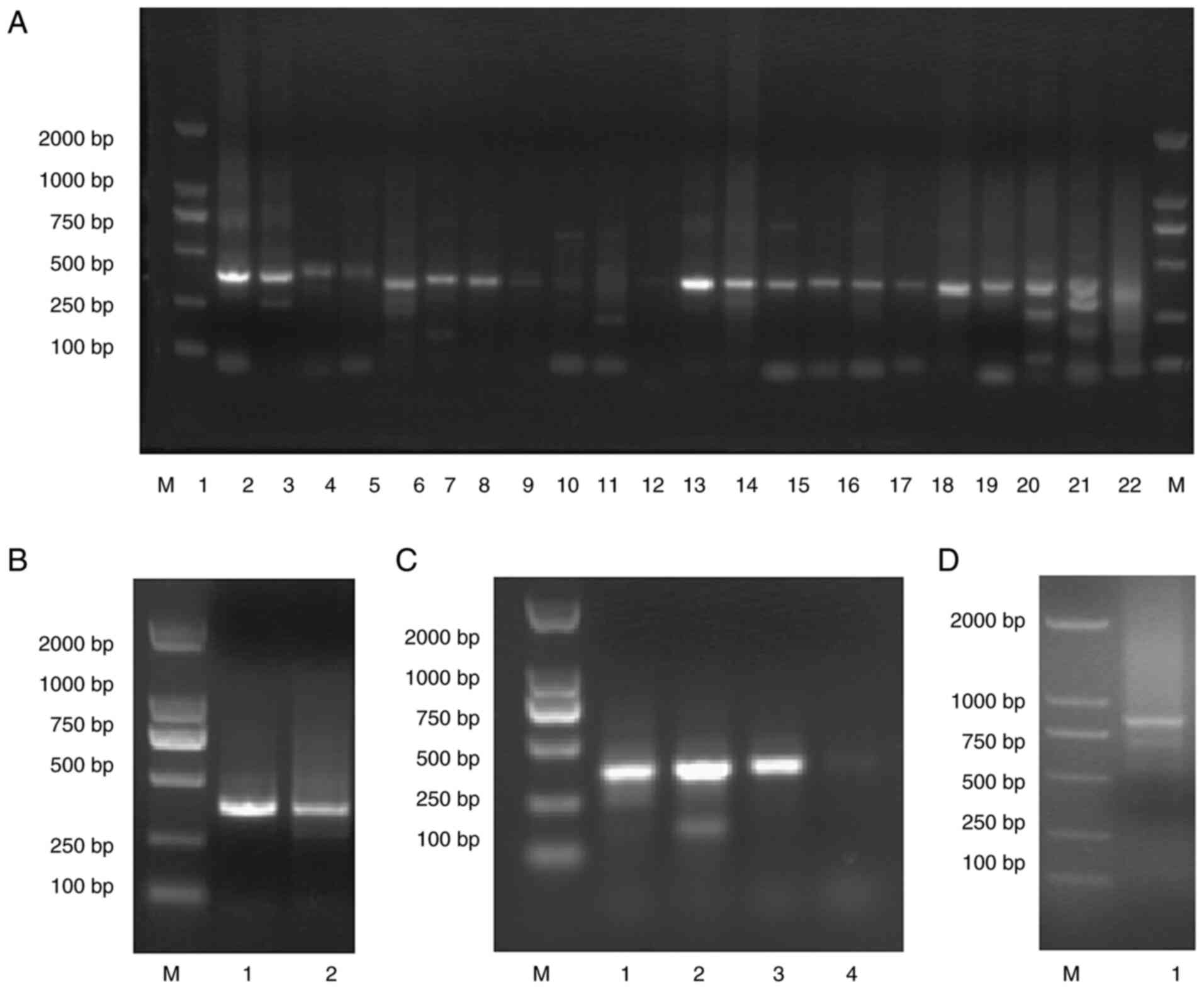 | Figure 2PCR amplification of scFvs by
stepwise PCR. (A) 1st Round PCR with amplicons ranging around 400
bp of the VH, Vκ and Vλ genes (lane M, DNA marker 2000; lane 1-11,
Vκ; lane 12-18, Vλ; lane 19-22, VH). (B-D) 1st Round products were
gel-purified and used as the templates for 2nd round PCR. 2nd Round
PCR with amplicons ranging around 400 bp of the Vκ-linker,
Vλ-linker (B, lane M, DNA marker 2000; lane 1, Vκ-linker; lane 2,
Vλ-linker) and VH-linker (C, lane M, DNA marker 2000; lane 1-4,
VH-linker) fragments were also gel-purified and used as the
templates for overlap PCR of the scFvs (D, Lane M, DNA marker 2000;
lane 1, scFv). Amplicons ranging around 800 bp were recovered and
stored at -80°C. scFv, single-chain fragment variable; VH, heavy
chain variable region; VL, light chain variable region. |
Generation of human PCSK9 recombinant
protein
The human PCSK9 gene is transcribed into a
3,637 bp linear mRNA, and is primarily expressed in the liver.
Thus, PCSK9 was amplified from hepatic Hep-G2 cells using reverse
transcription PCR (15). The
recombinant vector, pcDNA3.1-hIgG1FC-PCSK9, was successfully
constructed and expressed in Chinese hamster ovary (CHO)-K1 cells.
After purification, human PCSK9 in the cell supernatant was
analysed by SDS-PAGE (Fig. 4A).
Ultimately, two distinct bands were observed at 70 and 17 kDa,
which correspond to the catalytic C-terminal domains and the
prodomain, respectively (16).
Selection of human scFv antibodies
against PCSK9 using a phage display library
The human scFv phage library was based on
Cre-LoxP511 system in vivo recombination for VH and VL
generation, and was constructed using a pDF vector (Fig. 4B). The library comprised
1×1011 clones, which were screened against recombinant
PCSK9 protein. A total of 4 rounds of selection were performed on
immobilized PCSK9, detected by phage ELISA. As shown in Fig. 4C, a significant enrichment of 80%
was reached after the fourth selection. Ultimately, 24 colonies
recovered from the fourth round showed high binding affinity for
PCSK9 (Fig. 4D). All clones
exhibited the characteristics of the human antibody gene (Table II). A total of five different
scFv sequences were identified; the light chains of 2C7 and 3D2
were κ type, while 3B9, 7E4 and 12D6 were λ type. For comparison,
an NCBI online constraint-based multiple alignment tool showed that
there were differences in CDR region among the five clones, in
which both the VL κ types of CDR3 and the VL-CDRs of λ type, as
well as the CDRs of variable heavy chain regions, were
significantly different between the five clones.
 | Table IIFive sequences of scFvs obtained
after four rounds of screening, among which the
complementarity-determining regions (CDRs) were significantly
different. |
Table II
Five sequences of scFvs obtained
after four rounds of screening, among which the
complementarity-determining regions (CDRs) were significantly
different.
| Five sequences of
scFvs |
| CDR1
CDR2 |
| 12D6 |
DIVMTQTPS-VSVAPGKTARITCGGKDIGSKSVHWYQQKPGQAPVLVIYYDSDRPSGIPE |
| 3B9 |
DIVLTQSPS-VSVAPGKTARITCGGNDIGSKSVHWYQQKPGQAPVLVIYYDSDRPSGIPE |
| 7F4 |
DIVLTQAPS-VSVAPGKTARITCEPQEIGSKSVHWYQQKPGQAPVLVIYYDSDRPSGIPE |
| 3D2 |
EIVLTQSPVTLSVSPGERATLSCRASQSVIGNLAWYQQKPGQAPRLLIYGASARAAGIPD |
| 2C7 |
EIVLTQSPATLSVSPGERATLSCRASQSVLVNLAWYQQKPGQAPRLLIYGASRVESGIPD |
| CDR3 |
| 12D6 |
RFSGSNSGNTATLTISRVEAGDEADYYCAAWDDSLNGPVFGGGTKVEIKRSGGSTITSNN |
| 3B9 |
RFSGSNSGNTATLTISRVEAGDEADYYCHDWDESQRRPVFGGGTKGEIKRSGGSTITSNN |
| 7F4 |
RFSGSNSGNTATLTISRVEAGDEADYYCAEQDRRLHSQVFGGGTKVEIKRSGGSTITSNN |
| 3D2 |
RFSGSGSGTDFTLTISSLKSEDFAVYYCQQYGSS-PYTFGQGTKLEIKRSGGSTITSNN |
| 2C7 |
RFSGSGSGTEFTLTISSLKSEDFAVYYCQQYTLA-SGTFGQGTKLEIKRSGGSTITSNN |
| CDR1 |
| 12D6 |
VYLRKLSSSGTQVQLVQSGAEVKKPGASVKVSCKVSGYSFDTLGIHWVRRAPGKGLEWMG |
| 3B9 |
VYLRKLSSSGTQVQLVQSGAEVKKPGASVKVSCKSSGYGLEALGIQWVRWAPGTGLEWMG |
| 7F4 |
VYLRKLSSSGTQVQLVQSGAEVKKPGASVKVSCQASGVTDHGYDIHWVRQATGQGLEWMG |
| 3D2 |
VYLRKLSSSGTQVQLVQSGAEVKKPGSSVKVSCQASGGTFSGYDIHWVRQATGQGLEWMG |
| 2C7 |
VYLRKLSSSGTQVQLVQSGAEVKKPGASVKVSSKVSASRCVTLSIHWVRLAPGKGLEWMG |
| CDR2
CDR3 |
| 12D6 | WISPYNGNTDYAQNLQDRVSMTTDTSTSTAYMELRSLRSDDTAVYYCARVYSYSMDYWGQ |
| 3B9 | WISAYNGNTDYAQHFEDRVSMTTDTSTSTAYLELRSLRSDDTAVYYCVHGYSYRVGYWGQ |
| 7F4 | WMNPLVGADRYAQKFQGRVTMTRDTSTATAYMELTSLTSDDTAVYYCAR-YAHTSLEWGQ |
| 3D2 | WMNPHSGNAGYAQKFQGRVTMTRDTSTATAYMELTSLTSDDTAVYYCAR-YAQLSLEWGQ |
| 2C7 | WISRSTDSRVYAQNLHVTVFMTTDTSTSTAYMELRSLRSDDTAVYYCARVYRDRVDYWGQ |
| 241 |
| 12D6 | GTLVTVSS |
| 3B9 | GTLVTVSS |
| 7F4 | GTLVTVSS |
| 3D2 | GTLVTVSS |
| 2C7 | GTLVTVSS |
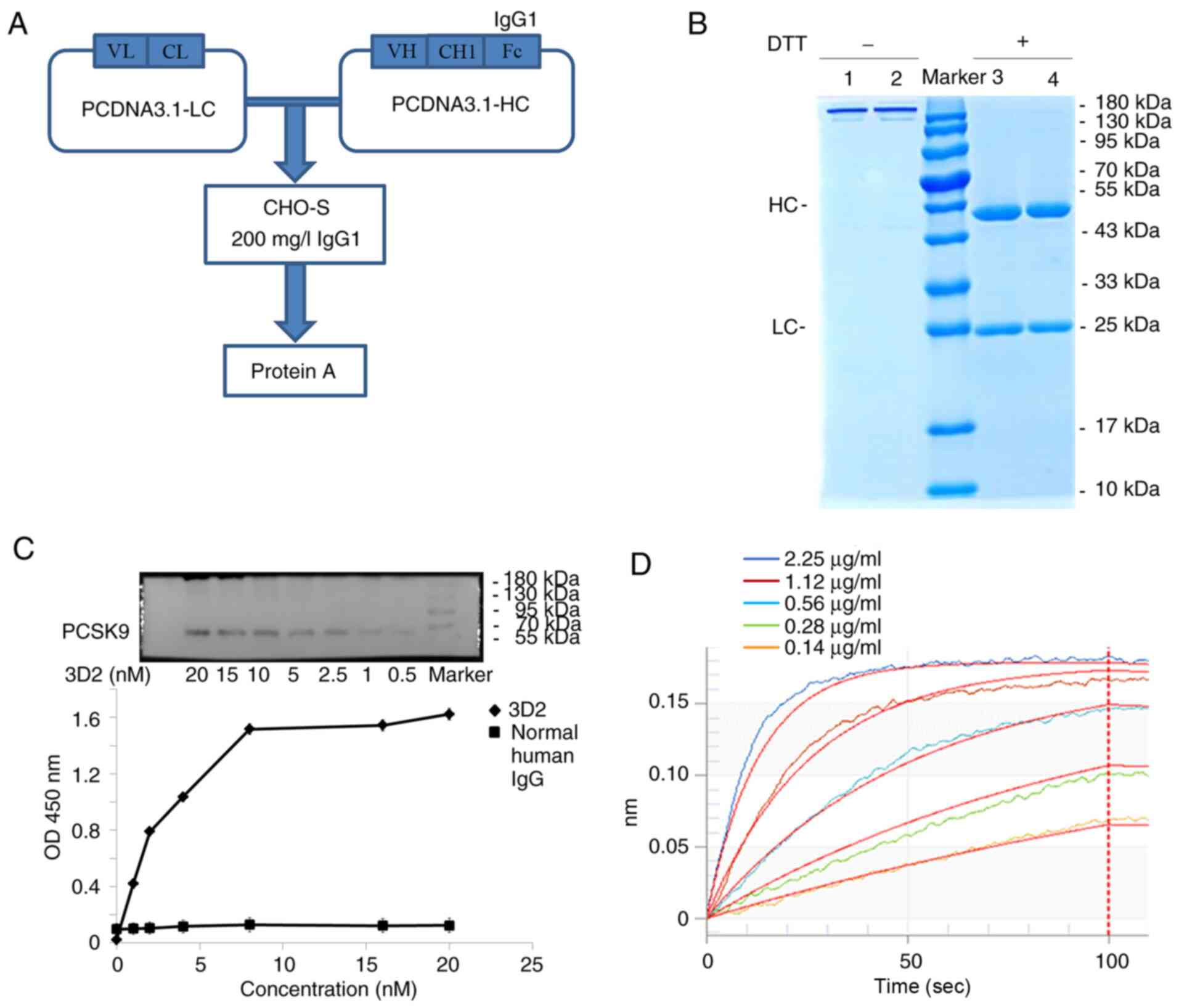
Construction and characterization of
fully human PCSK9 fragments
To generate fully human scFv antibodies, the VH and
VL regions of the five selected scFv clones were separately
subcloned into pcDNA3.1-CL and pcDNA3.1-CH expression vectors
(Fig. 5A). Antibodies were
expressed in 293 cells and purified using protein A affinity
chromatography. The yields of the five antibodies ranged between 50
and 200 mg/l per culture. SDS-PAGE analysis of the 3D2 antibody
revealed that it consisted of a 25 kDa VL and a 50 kDa VH region
under reducing conditions, and formed a 170 kDa homodimer under
non-reducing conditions, which are typical patterns of human IgG
(Fig. 5B). Western blotting and
indirect ELISA were used to assess the specificity of the five
selected antibodies against PCSK9. The data indicated that all of
the antibodies specifically bound the PCSK9 protein in a
dose-dependent manner (Fig. 5C).
Moreover, antibody affinity was determined using BLI technology,
revealing KD values ranging from 8.77ⅹ10−9 to
3.28ⅹ10−10 M (Fig. 5D
and T able III). These data suggest that the five fully human scFv
fragments were specific for PCSK9.
Human 3D2 antibody inhibits PCSK9
function in vitro
Functional properties of the five PCSK9 antibodies
were characterized using in vitro assays. Of these five
antibodies, only 3D2 was able to bind PCSK9, thereby blocking the
PCSK9-LDLR interaction with an IC50 of 2.25±1.23 nM
(n=3) (Fig. 6A). After 24-48 h of
incubation with 1, 3 or 10 µg/ml 3D2, the LDLR levels in the
Hep-G2 cells increased 1.3-2.5 fold compared with those in the
untreated cells (Fig. 6B). In
addition, previous reports have indicated that statins induce the
expression of both LDLR and PCSK9 (17); therefore, in the present study,
the effects of combining 3D2 antibody and statin administration on
Hep-G2 cell LDLR levels were investigated. Hep-G2 cells were
treated with the statin mevinolin and/or 3D2, and the levels of
LDLR protein in cell lysates were determined using western blotting
(Fig. 6C). The results indicate
that combined administration was more effective than that of
mevinolin or 3D2 alone; 1 µg/ml mevinolin combined with 10
µg/ml 3D2 increased the levels of LDLR protein up to 9.0
fold compared with untreated cells. These data suggest that the 3D2
antibody is a potential auxiliary treatment for decreasing cellular
lipid levels.
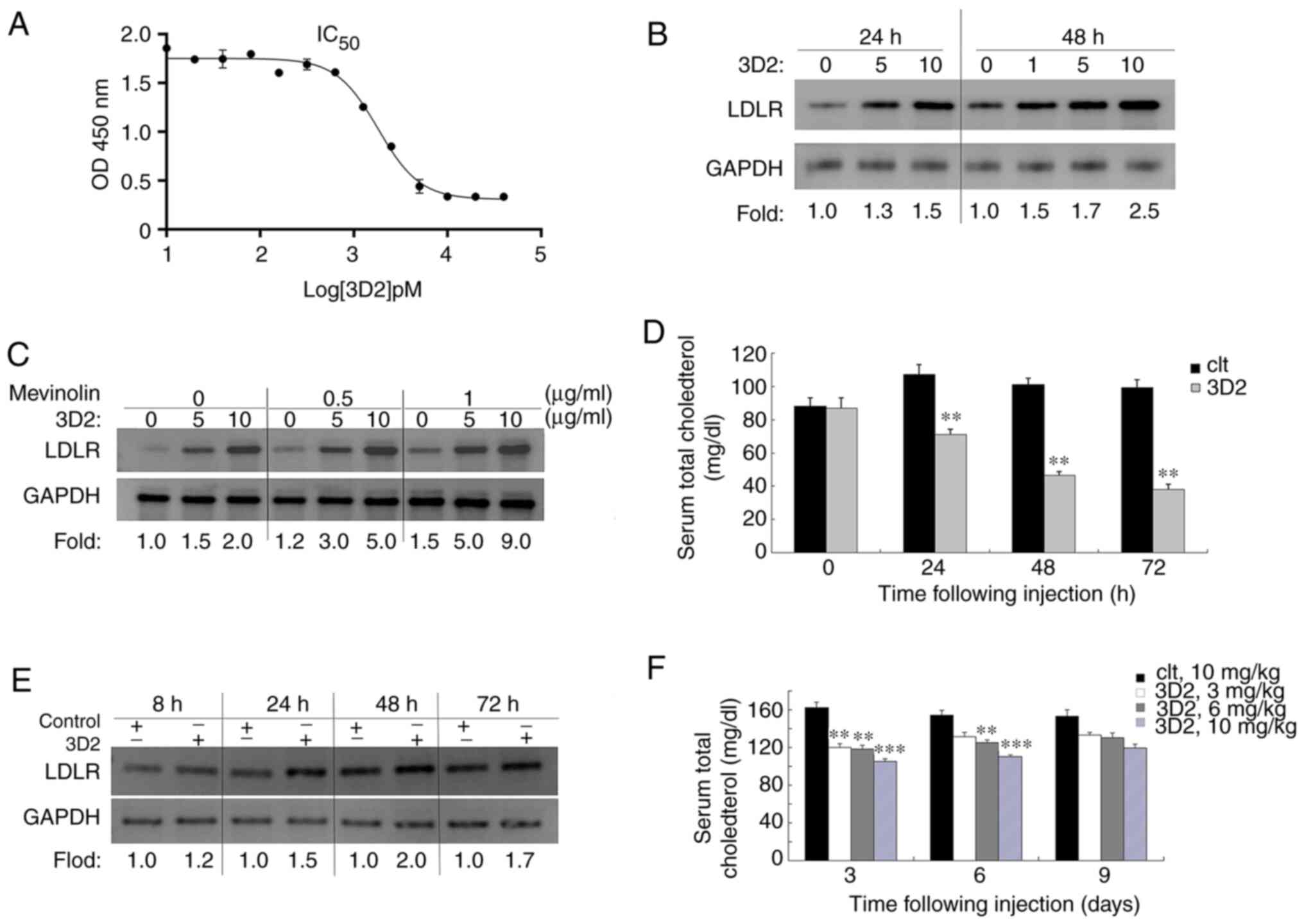 | Figure 6Human 3D2 antibody inhibits PCSK9
function. (A) 3D2 inhibits the binding of PCSK9 to LDLR in a
dose-dependent manner, with an IC50 of 2.25±1.23 nM. (B)
Western blotting indicated that after 24-48 h of incubation with 1,
3 or 10 µg/ml 3D2, Hep-G2 cell LDLR levels were increased
1.3- to 2.5-fold, compared with those in untreated cells. GAPDH
served as the loading control. (C) Hep-G2 cells were treated with
mevinolin (0.5 or 10 g/ml) and/or 3D2 (5 or 10 µg/ml) for 48
h, and the levels of LDLR protein in the cell lysates were assessed
by western blotting. (D) C57BL/6J mice (n=7 per group) were
administered a single i.v. injection of 3D2 antibody (10 mg/kg) and
serum TC levels were determined at 24, 48 and 72 h. (E) Hepatic
LDLR protein levels at 8, 24, 48 and 72 h post-injection were
analysed by western blotting; the fold change was calculated as the
ratio of LDLR in the presence or absence of 3D2 antibody for each
time point after normalization of the LDLR to GAPDH in each lane.
GAPDH served as the loading control. (F) Mice (n=7 per group) were
injected with a single dose of 3, 6 or 10 mg/kg 3D2 antibody, and
serum TC levels were determined at 3, 6 and 9 days, respectively.
Results are expressed as the mean ± SEM. **P<0.05 and
***P<0.01 vs. control. PCSK9, proprotein convertase
subtilisin/kexin 9; LDLR, low-density lipoprotein receptor; TC,
total cholesterol. |
Human 3D2 antibody reduces serum
cholesterol by increasing hepatic LDLR in mice
Experiments were performed to verify whether human
3D2 antibody could also increase hepatic LDLR levels in
vivo, and subsequently reduce serum TC. C57BL/6J mice (n=7 per
group) were treated with a single i.v. injection of 3D2 (10 mg/kg).
The results indicate that 3D2 significantly reduced serum TC levels
at 24, 48 and 72 h post-injection by 33.5±0.54% (P<0.05),
53.9±0.85% (P<0.05), and 61.5±1.2% (P<0.01), respectively
(Fig. 6D). However, the
phenomenon was reversible, and the effect was duration-dependent
(Fig. 6F). By day 9, the TC
levels in all treatment groups were not significantly different
from those in the control group. Furthermore, hepatic LDLR protein
levels were increased 2.0-fold (Fig.
6E), which indicates that the increase in LDLR level was
positively associated with a decrease in the lipid profile, and
that the 3D2 antibody reduces lipid levels in vivo.
Discussion
The fully human scFv phage display library holds
great value for the development of antibody-based treatments. The
construction of a human scFv library is theoretically simple, but a
high-quality library is difficult to obtain. There are three main
indexes used to evaluate the quality of a phage display library,
including gene diversity, library size and screening ability. In
the present study, these three key indexes were used to optimize
the construction of the human scFv library. A total of 400 blood,
30 bone marrow and 10 cord blood samples were collected from
healthy donors. Donors were recruited via different sources to
avoid the bias of individual antibody genes, and to ensure the
diversity of the scFv gene. The scFv gene was
amplified in strict accordance with the proportion of antibody
genes of different human germlines, and imitated the human antibody
gene repertoire to the maximal extent. The results of randomly
sequencing 60 scFvs revealed that the diversity of the constructed
phage display library was consistent with the antibody gene
distribution of the human germline. Table III
 | Table IIIBinding affinities of antibodies with
recombinant PCSK9 protein.a |
Table III
Binding affinities of antibodies with
recombinant PCSK9 protein.a
| Antibody | Kon
(106 M−1S−1) | Koff
(10−4S−1) | KD
(10−9 M) |
|---|
| 2C7 | 3.16±1.11 | 6.74±2.26 | 21.4±7.21 |
| 3B9 | 2.49±1.21 | 8.15±3.22 | 32.8±13.1 |
| 3D2 | 2.59±1.58 | 5.08±4.02 | 19.6±15.6 |
| 7F4 | 5.27±1.60 | 4.62±2.18 | 8.77±4.92 |
| 12D6 | 2.94±2.41 | 8.36±3.70 | 28.5±12.8 |
The simplest way to evaluate the quality of an
antibody library is by size. Theoretically, the larger the capacity
of the library, the higher the affinity of the screened antibodies.
The key factors that affect antibody library size are primarily the
efficiencies of gene splicing and electrotransformation. Splicing
of antibody light and heavy chain genes is generally conducted by
overlap PCR. After a long period of investigation, the optimal
protocol and conditions for overlap PCR amplification were
determined. A primary human scFv library (5×107 in size)
was constructed from peripheral and cord blood samples, and a
primary human scFv library (5×107 in size) was
constructed using bone marrow samples. By optimizing the
recombinant conditions, the primary library was used to infect the
BS1365 E. coli strain, which expresses the Cre enzyme. Using
Cre-LoxP enzyme-mediated heavy and light chain replacement
recombination, a recombinant human scFv phage display library with
a library size of 1×1011 was obtained. The most
important parameter for antibody library quality is the ability to
screen antibodies. The scFv library constructed in the present
study can be used to screen antibodies with an affinity of
3.28×10−10 M through the screening and verification of
those specific to PCSK9, which confirms the optimal quality of the
scFv phage display library.
Various studies have indicated that anti-PCSK9
antibodies are powerful inhibitors of PCSK9 (18,19), thus the generation of a novel and
less immunogenic human antibody with greater in vivo
efficacy is of great clinical significance. In the current study,
recombinant human PCSK9 protein was used to screen human scFv
antibodies. After 4 rounds of selection, an scFv antibody with high
affinity for PCSK9, 3D2, was obtained. Further experimentation
revealed that 3D2 increased hepatic LDLR levels in vivo and
vitro, which in turn reduced serum TC.
Due to low circulating LDL-C levels in their blood,
wild-type C57BL/6J mice are generally not suitable for the study of
cholesterol-lowering drugs (20).
However, some in vivo studies of PCSK9-targeted compounds
revealed enhanced levels of PCSK9 in the serum of mice. Rashid
et al (21) found that TC
levels were elevated in the serum, and the LDLR levels in
hepatocytes were reduced in mice receiving an i.v. injection of
PCSK9. To investigate its neutralizing activity, Schroeder et
al(22) treated mice with an
i.v. injection of an adeno-associated virus (AAV) vector carrying
the PCSK9 gene, followed by injection of the candidate antibody.
Furthermore, Barale et al (23) obtained a human anti-PCSK9 antibody
(mAb1) using the hybridoma technique, which had cross-reactivity
with murine PCSK9 and significantly decreased serum cholesterol in
WT C57BL/6J mice. In the present study, the 3D2 human anti-PCSK9
antibody, which also cross-reacted with murine PCSK9 (data not
shown), effectively reduced serum cholesterol by increasing hepatic
LDLR in mice.
In summary, the present study outlines the
construction of a human scFv phage antibody library, with large
library size and diversity, using Cre-LoxP in vitro
recombination. By screening with recombinant human PCSK9 antigen,
the diversity and screening ability of the constructed antibody
library was further confirmed, and it can be used for screening
other target antibodies. The fully human anti-PCSK9 antibody, 3D2,
was found to neutralize PCSK9 activity in vitro and in
vivo, demonstrating its potential for future clinical
development.
Funding
This study was supported by the following financial
programs: 'Research and Development of Industrial Technology'
Program of Jilin Province, PR China (grant nos. 20170204005YY and
20180623045TC), Program of Jilin Science and Technology bureau,
P.R. China (grant nos. 2019001179 and 20200104093), Health
Commission Program of Jilin Province (no. 2020Q029) and National
Training Program of Innovation and Entrepreneurship for
Undergraduates (nos. 201913706005, 201913706039 and
202013706008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of Jilin Medical University (approval no. 2016-LW012)
prior to the start of the study. Informed consents for the
collection of peripheral blood, bone marrow and cord were obtained
from all subjects.
Authors' contributions
YD and FM constructed the scFv phage display
library. ZW and SX prepared the antigen. TY was responsible for
cell culture. AC and JW screened the antibody. MY analyzed the data
regarding the characterization of antibody. LT and CH purified the
protein. HW determined the hypolipidemic effects of antibody. JC
analyzed all the data and wrote the manuscript. All authors read
and approved the final manuscript.
Patient consent for publication
Written informed consent for publication was
obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zhang Y, Wang W, Lv M, Lin Z, Geng J, Li
Y, Shen B, Ma Y, Li Y, Qiao C and Feng J: A single-chain antibody
using LoxP511 as the linker enables large-content phage library
construction via Cre/LoxP recombination. J Biomol Screen.
19:839–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang GM, Chen YP, Guan YZ, Wang Y and An
YQ: Modification and identification of a vector for making a large
phage antibody library. Chin Med J (Engl). 120:2011–2016. 2007.
View Article : Google Scholar
|
|
3
|
Baragetti A, Grejtakova D, Casula M,
Olmastroni E, Jotti GS, Norata GD, Catapano AL and Bellosta S:
Proprotein convertase subtilisin-Kexin type-9 (PCSK9) and
triglyceride-rich lipo-protein metabolism: Facts and gaps.
Pharmacol Res. 130:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abifadel M, Varret M, Rabès JP, Allard D,
Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich
D, et al: Mutations in PCSK9 cause autosomal dominant
hypercholesterolemia. Nat Genet. 34:154–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen J, Pertsemlidis A, Kotowski IK,
Graham R, Garcia CK and Hobbs HH: Low LDL cholesterol in
individuals of African descent resulting from frequent nonsense
mutations in PCSK9. Nat Genet. 37:161–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CC, Fornage M, Lloyd-Jones DM, Wei
GS, Boerwinkle E and Liu K: Longitudinal association of PCSK9
sequence variations with low-density lipoprotein cholesterol
levels: The Coronary Artery Risk Development in Young Adults Study.
Circ Cardiovasc Genet. 2:354–361. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank-Kamenetsky M, Grefhorst A, Anderson
NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R,
Fan Y, et al: Therapeutic RNAi targeting PCSK9 acutely lowers
plasma cholesterol in rodents and LDL cholesterol in nonhuman
primates. Proc Natl Acad Sci USA. 105:11915–11920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ray KK, Stoekenbroek RM, Kallend D, Leiter
LA, Landmesser U, Wright RS, Wijngaard P and Kastelein JJP: Effect
of an siRNA therapeutic targeting PCSK9 on atherogenic
lipoproteins: Prespecified secondary end points in ORION 1.
Circulation. 138:1304–1316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duff CJ, Scott MJ, Kirby IT, Hutchinson
SE, Martin SL and Hooper NM: Antibody-mediated disruption of the
interaction between PCSK9 and the low-density lipoprotein receptor.
Biochem J. 419:577–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dubuc G, Chamberland A, Wassef H, Davignon
J, Seidah NG, Bernier L and Prat A: Statins upregulate PCSK9, the
gene encoding the proprotein convertase neural apoptosis-regulated
convertase-1 implicated in familial hypercholesterolemia.
Arterioscler Thromb Vasc Biol. 24:1454–1459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Careskey HE, Davis RA, Alborn WE, Troutt
JS, Cao G and Konrad RJ: Atorvastatin increases human serum levels
of proprotein convertase subtilisin/kexin type 9. J Lipid Res.
49:394–398. 2008. View Article : Google Scholar
|
|
12
|
Tonikian R, Zhang Y, Boone C and Sidhu SS:
Identifying specificity profiles for peptide recognition modules
from phage-displayed peptide libraries. Nat Protoc. 2:1368–1386.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lakzaei M, Rasaee MJ, Fazaeli AA and
Aminian M: A comparison of three strategies for biopanning of
phage-scFv library against diphtheria toxin. J Cell Physiol.
234:9486–9494. 2019. View Article : Google Scholar
|
|
14
|
Tohidkia MR, Sepehri M, Khajeh S, Barar J
and Omidi Y: Improved soluble ScFv ELISA screening approach for
antibody discovery using phage display technology. SLAS Discov.
22:1026–1034. 2017.PubMed/NCBI
|
|
15
|
Grozdanov PN, Petkov PM, Karagyozov LK and
Dabeva MD: Expression and localization of PCSK9 in rat hepatic
cells. Biochem Cell Biol. 84:80–92. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lipari MT, Li W, Moran P, Kong-Beltran M,
Sai T, Lai J, Lin SJ, Kolumam G, Zavala-Solorio J, Izrael-Tomasevic
A, et al: Furin-cleaved proprotein convertase subtilisin/kexin type
9 (PCSK9) is active and modulates low density lipoprotein receptor
and serum cholesterol levels. J Biol Chem. 287:43482–43491. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez F and Harrington RA:
Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and
the future of LDL-C lowering. JAMA. 316:1967–1968. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harada LM, Carrilho AJ, Oliveira HC,
Nakandakare ER and Quintão EC: Regulation of hepatic cholesterol
metabolism in CETP/LDLr mice by cholesterol feeding and by drugs
(chole-styramine and lovastatin) that lower plasma cholesterol.
Clin Exp Pharmacol Physiol. 33:1209–1215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao Y, Yang H, Zhou X, Mao H, Gao T, Hu Z,
He L, Pan F and Guo Z: Selection and characterization of human
PCSK9 antibody from phage displayed antibody library. Biochem Bioph
Res Commun. 463:712–718. 2015. View Article : Google Scholar
|
|
20
|
Lie J, de Crom R, van Gent T, van Haperen
R, Scheek L, Sadeghi-Niaraki F and van Tol A: Elevation of plasma
phospholipid transfer protein increases the risk of atherosclerosis
despite lower apolipoprotein B-containing lipoproteins. J Lipid
Res. 45:805–811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rashid S, Curtis DE, Garuti R, Anderson
NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA and Horton JD: Decreased
plasma cholesterol and hypersensitivity to statins in mice lacking
Pcsk9. Proc Natl Acad Sci USA. 102:5374–5379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schroeder KM, Beyer TP, Hansen RJ, Han B,
Pickard RT, Wroblewski VJ, Kowala MC and Eacho PI: Proteolytic
cleavage of antigen extends the durability of an anti-PCSK9
monoclonal antibody. J Lipid Res. 56:2124–2132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barale C, Bonomo K, Frascaroli C, Morotti
A, Guerrasio A, Cavalot F and Russo I: Platelet function and
activation markers in primary hypercholesterolemia treated with
anti-PCSK9 mono-clonal antibody: A 12-month follow-up. Nutr Metab
Cardiovasc Dis. 30:282–291. 2020. View Article : Google Scholar
|