Introduction
As an integral part of the digestive system, the
gastrointestinal (GI) tract not only plays an essential role in the
digestion and absorption of food, but also in the absorption and
metabolism of medication (1).
However, the digestive system experiences a series of senescence
and degeneration events from structure to function during aging
(2). These changes have a certain
impact on the intake, digestion, absorption and utilization of
nutrients in the elderly, and directly or indirectly participate in
the occurrence and development of digestive diseases in these
patients (3). It has been
reported that digestive diseases have become the second largest
disease group, following cardiovascular diseases, in hospitalized
elderly patients aged >60 years (4). The identification of effective
strategies with which to alleviate the degradation of GI structure
and function plays a vital role in improving the quality of life of
the elderly.
Natural aging models are time-consuming, and as a
result of individual differences, the process of aging is difficult
to control. D-galactose (D-gal) is a normal nutrient in the body;
however, if consumed in excess, it will lead to metabolic disorder;
under the action of galactose oxidase, D-gal is converted into
aldose and hydrogen peroxide, thus producing a large number of
superoxide anion free radicals, causing oxidative damage, which
leads to aging (5). Using D-gal
to model aging can speed up the aging process, shorten the study
time, and the animals have a higher survival rate throughout the
study period (5). Thus, the model
of D-gal-induced aging is often used for studies of aging and
anti-aging (6-8). Codonopsis pilosula is a
traditional Chinese medicine and food supplement in China. Gansu
Province is one of the main areas of production of Codonopsis
pilosula in China (9-11). Of note, it has been reported that
Codonopsis pilosula, a traditional Chinese medicine, exerts
an anti-aging effect and can regulate the GI system (12). Previous studies have focused on
antioxidant stress and immune regulation (13-16). However, there is still a lack of
systematic research at the molecular level, considering that
traditional Chinese medicine has the properties of being able to
affect multiple targets and organs simultaneously (17-19). In particular, the changes in the
GI tract occurring during D-gal-induced aging and Codonopsis
pilosula treatment in mice from the perspective of the
transcriptome remain unclear. Transcriptomics is the study of
researching the expression and regulation of genes under specific
conditions at the RNA level, and it has been used in a number of
fields (20,21). lncRNAs play a complex and precise
regulatory role in gene expression (22); thus, it is necessary to integrate
lncRNAs and mRNAs in order to explore the mechanisms underlying the
anti-aging effects of Codonopsis pilosula at the
transcriptome level.
In the present study, in order to comprehensively
explore the underlying molecular mechanisms of the anti-aging
effects and GI regulation by Codonopsis pilosula, a mouse
model of aging was successfully established with the use of D-gal,
and water extract of Codonopsis pilosula was then used for
treatment intervention. The structural and functional changes of
the GI tract were then observed in aging mice and in mice treated
with Codonopsis pilosula. Finally, biochip technology and
bioinformatics were performed to explore the targets, pathways and
network involved in the anti-GI senescence effects of Codonopsis
pilosula. The findings of the present study may provide basic
evidence for the anti-aging and GI regulatory functions of
Codonopsis pilosula.
Materials and methods
Animals
A total of 100 specific pathogen-free grade Kunming
mice (50 males and 50 females; age, 2 months; body mass, 20±2 g)
were purchased from the Gansu University of Traditional Chinese
Medicine Scientific Research Laboratory Animal Center [Lanzhou,
China; animal production certificate no. SCXK (Gan) 2015-0002;
animal use certificate no. SKXK (Gan) 2015-0005]. All mice were
raised at the Scientific Experimental Animal Center of Gansu
University of Chinese Medicine. Males and females were raised in
separate cages at room temperature (20-25°C), were fed with general
mouse feed and natural light, and had free access to double
distilled water. All experimental procedures and protocols were in
accordance with the guidelines of the Institutional Animal Ethics
Committee of Gansu University of Chinese Medicine (approval no.
2017-106).
Drugs and reagents
Codonopsis pilosula (Franch.) Nannf. was
collected from Min County (Gansu, China), and was identified as
white Codonopsis pilosula by Professor Tao Du (Teaching and
Research Department of Traditional Chinese Medicine Resources,
Gansu University of Traditional Chinese Medicine, Lanzhou, China).
In order to control the quality of Codonopsis pilosula, the
polysaccharide of Codonopsis pilosula was determined by the
phenol-sulfuric acid method (23,24), and the average polysaccharide
content of Codonopsis pilosula in the present study was
43.2%. The identified Codonopsis pilosula weighed 100 g, and
was boiled twice in water for 1 h each time. A total of 10-fold the
amount of water was added the first time, and 6-fold the amount of
water the second time. The two extracting solutions were mixed,
filtered and concentrated into water decoction containing 0.5 g/ml
original medicinal material, and were then placed in the
refrigerator (4°C) for maintenance. The water decoction was
prepared every 5 days. D-gal was purchased from Merck KGaA (lot no.
24895207) and 12 g/l was prepared with normal saline prior to use.
Enzyme-linked immunosorbent assay (ELISA) kits for D-xylose,
motilin (MTL) and vasoactive intestinal peptide (VIP) were
purchased from Shanghai Jianglai Industrial Limited By Share Ltd.
(lot no. 20171105). Animal food was purchased from Beijing Ke'ao
Xie Li Feed Co., Ltd. (lot no. 09092402).
Animal model
A total of 100 mice were randomly divided into the
normal control (NC group), aging model (AM group), and
Codonopsis pilosula low-, medium- and high-dose groups (L-,
M- and H-CP groups, respectively) (n=20, 50% males and 50% females
per group). The mice in the AM, and L-, M- and H-CP groups received
a subcutaneous injection of D-gal solution into the back of the
neck at a concentration of 50 g/l D-gal per mouse per day
(injection volume, 0.025 ml/g). The mice in the L-, M- and H-CP
groups were intragastrically administered 5, 10 and 15 g/kg
Codonopsis pilosula solution, with the NC and the AM group
receiving an equal amount of normal saline. Continuous modelling
was performed for 42 days. For the preparation of the model of
aging, a decrease in body weight loss and thymus index indicated
the successful establishment of the model (25-27).
Determination of body weight and thymus
index
The body weights of the mice in each group were
measured and recorded every 2 days. After 2 h of the final
intragastric administration, the mice were anesthetized by
intraperitoneal injection with 5% chloral hydrate at a dose of 0.4
g/kg, and the abdominal cavity was then rapidly cut open, and the
thymus glands were completely removed. Excess fat was then removed
from the surface, washed with saline and drained using filter
paper. The thymus glands were weighed using an electronic balance.
The thymus index was then calculated for each mouse as follows:
Thymus index (%)=thymus weight (g)/body weight (g) ×100%.
Hematoxylin and eosin (H&E)
staining
In order to observe the histopathological changes,
complete stomachs and small intestines were separated following
anesthesia by an intraperitoneal injection with 5% chloral hydrate
at a dose of 0.4 g/kg. The removed stomachs and intestines were
then dehydrated in a series of ethanol, embedded in paraffin and
cut into 3-µm-thick slices. The slices were dewaxed, fixed
with xylene and ethanol, stained with H&E (room temperature, 15
min), and then washed and sealed. The slices were observed under an
Olympus CX31 microscope by an experienced pathologist (YH), who was
not aware of the experimental procedure and grouping, and the
degree of glandular number decrease was divided into no, mild,
moderate and severe grades according to the Chinese consensus on
chronic gastritis (28), with
corresponding scores ranging from 0 to 3. The specific methods of
classification are as follows: i) Mild: The number of innate glands
decreases by not >1/3 of the original glands, leaving most of
the glands intact; ii) moderate: The number of proper glands
decreases by >1/3, but not by >2/3. The remaining glands are
irregularly distributed; iii) Severe: The number of innate glands
reduced by >2/3, with only a few glands remaining, or even
completely disappeared. For each pathological slice, the observer
randomly selected 5 visual fields to observe the number of glands,
and scored each visual field according to the reduction of the
number of glands. The average score of the 5 visual fields was used
for statistical analysis. Mucosal thickness was measured under an
Olympus CX31 microscope with a 20X lens objective Finally, typical
images were captured computer monitor (L197WA; Lenovo).
Determination of D-xylose, MTL and
VIP
Following the last intragastric administration of
the Codonopsis pilosula, the mice in each group were fasted
without water for 24 h and gavaged with 3% D-xylose suspension at a
volume of 20 ml/kg. After 2 h, the animals were anesthetized by an
intraperitoneal injection with 5% chloral hydrate at a dose of 0.4
g/kg, 0.5 ml blood was collected once from the femoral artery from
each mouse, and serum was prepared to measure the D-xylose
concentration. The small intestinal tissues near the gastric antrum
were then rapidly cut up and cleaned to yield homogenates. The
blood and tissue homogenates were centrifuged at 1,500 × g for 10
min at 4°C, and the serum and supernatant were collected. ELISA was
performed to detect the levels of D-xylose, MTL and VIP. First, the
kits were taken out, and were maintained at room temperature for 30
min. The standard, blank and sample holes were labelled. The
samples were then added in turn, and incubated at 37°C for 30 min.
The plate was washed 3 times, and the excess liquid was drained.
Chromogenic agent was then added and kept away from the light at
37°C for 15 min. Finally, the termination solution was added to
terminate the reaction. The blank holes were set to zero, the
absorbance value of each hole was detected at 450 nm within 15 min,
and the concentration of D-xyose, MTL and VIP in the samples was
calculated.
Transmission electron microscope
To observe the ultrastructural changes of the
stomach and intestinal tissue cells, the mice were anesthetized by
an intraperitoneal injection with 5% chloral hydrate at a dose of
0.4 g/kg, and their completed stomachs and intestines were
extracted, rinsed with normal saline and dried using filter paper.
The stomachs and intestines from the mice in the NC, AM and H-CP
groups were cut into small sections of ~1 mm3, which
were placed into a 2.5% glutaraldehyde solution precooled at 4°C
for overnight fixation, rinsed with PBS 3 times, and fixed with 1%
osmic acid for 1 h, then dehydrated, permeated, embedded, dried, so
they could be cut into thin, 70-nm-thick slices; they were then
dyed with uranyl acetate for 30 min (room temperature), rinsed, and
then re-dyed with lead citrate for 10 min (room temperature).
Finally, the ultrastructural changes were observed in the GI tract
by transmission electron microscopy (JEM-1230; Jeol). Mice were
euthanized by cervical dislocation when the following humanized
endpoints were reached: Weakness (inability to eat, drink and
activities). The criteria for the confirmation of death following
euthanasia were the following: No breathing for 3 consecutive
minutes, no heartbeat and no blinking reflex.
RNA extraction, labelling and
hybridization
Total RNA containing small RNA was extracted from
the stomachs and intestinal tissues from the mice in the NC, AM and
H-CP groups (n=3) using TRIzol reagent (Thermo Fisher Scientific,
Inc.) and purified using the mirVana miRNA Isolation kit (Thermo
Fisher Scientific, Inc.). The purity and concentration of the RNA
were determined from OD260/280 readings using a spectrophotometer
(NanoDrop ND-1000). The RNA integrity was determined using 1%
formaldehyde denaturing gel electrophoresis. The CapitalBio
Technology Mouse LncRNA Array V1 (4×180 K format) was performed in
the present study. All microarray experiments, including labelling
and hybridization, were conducted according to the manufacturer's
instructions.
Microarray data analysis
The lncRNA and mRNA array data were analyzed for
data summarization, normalization and quality control using the
GeneSpring software V13.0 (Agilent Technologies, Inc.). The
threshold values of |fold change|≥1.5 and a Benjamini-Hochberg
corrected value of P≤0.05 were used to select the differentially
expressed genes. The data were Log2-transformed and median-centered
by genes using the Adjust Data function of CLUSTER 3.0 software
(http://bonsai.hgc.jp/~mdehoon/software/cluster/).
Finally, the Java Treeview (Stanford University School of Medicine,
Stanford, USA) was performed to tree visualization. Total RNA
microarray data were uploaded onto the GEO database (http://www.ncbi.nlm.nih.gov/geo/; accession no.
GSE140949). Please note that as the GSE140949 database is
confidential and will be release on December 31, 2020.
Kyoto Encyclopedia of Genes and Genomes
(KEGG) and Gene Ontology (GO) enrichment analysis
In order to examine the biological functions of the
mRNAs in the stomach and intestine related to the anti-aging
effects of Codonopsis pilosula, the common differentially
expressed mRNAs between the AM vs. NC group and the H-CP vs. AM
group, were selected in the stomach and intestine, respectively.
KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/) was used to perform KEGG
pathway enrichment analysis and David 6.8 (https://david.ncifcrf.gov/) was used to perform GO
enrichment analysis for the mRNAs related to the anti-aging effects
of Codonopsis pilosula in the stomach and intestine,
respectively. KEGG pathways and GO terms were considered
significantly enriched with at a value of P<0.05.
Construction of lncRNA-mRNA co-expression
network in the stomach and intestine
lncRNA has the ability to regulate the expression of
mRNAs. In the present study, lncRNA-mRNA co-expression networks
were constructed to investigate the functions of lncRNAs and to
reveal the regulatory associationb between lncRNAs and mRNAs in the
stomach and intestine. In order to determine the contribution of
the lncRNA-mRNA co-expression network, pairwise Pearson's
correlation coefficients between lncRNAs and mRNAs were calculated
using their expression levels. Only the pairs with |R|>0.85 and
P<0.05 were considered relevant and used to contribute to the
co-expression network in the stomach and intestine, respectively.
Finally, these networks were visualized using Cytoscape 3.8.1
(https://www.bytesin.com/software/Cytoscape/).
Real time-quantitative polymerase chain
reaction (RT-qPCR)
Stomach and intestinal tissue from the NC, AM and
H-CP groups were homogenized in 1 ml TRIzol reagent. Total RNA was
isolated according to the manufacturer's instructions. SYBR-Green
PCR Master Mix (Arraystar) was used for qPCR analysis, and all
reactions were run on a QuantStudio TM 7 Flex Real-time PCR System
(Thermo Fisher Scientific, Inc.) using the following steps:
Denaturation step (95°C for 10 min), and 45 cycles of three step
amplification (denaturation, 95°C for 10 sec; annealing, 58°C for 5
sec; and extension, 72°C for 10 sec). All RNA expression data were
normalized to GAPDH. The relative expression of the genes was
calculated using the 2−ΔΔCq method (29). The primers used in the present
study are summarized in Table
I.
 | Table ISequences of primers used for RT-qPCR
in the present study. |
Table I
Sequences of primers used for RT-qPCR
in the present study.
| Gene | Direction | Primer sequence
5′→3′ |
|---|
| GAPDH | Forward |
GTTGTCTCCTGCGACTTCA |
| GAPDH | Reverse |
TGGTCCAGGGTTTCTTACTC |
| Fam132a | Forward |
GGCCTTCTACTGCCGTTTGA |
| Fam132a | Reverse |
TCCTTGCAGTTCCACCAGAG |
| 1200016E24Rik | Forward |
AGGTCCATTTGGGTTGGTCA |
| 1200016E24Rik | Reverse |
TACAGGGCAAGTGCGATTTC |
| RORC | Forward |
TGCCTGTTTTCTGGGACCTA |
| RORC | Reverse |
TATCTCCACCCCACCCTCATT |
| LOC105243318 | Forward |
AGGTCCATTTGAGTTGGTCGT |
| LOC105243318 | Reverse |
CTGAGGATACGCAATTTCCAG |
Statistical analysis
Data were analyzed using SPSS 24.0 statistical
software, and expressed as the means ± standard deviation. Based on
the Shapiro-Wilk normality test, one-way ANOVA was used to analyze
the differences among groups, and Tukey's post hoc test was then
used to correct multiple comparisons. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Codonopsis pilosula intervention can
improve weight and thymus index in aging mice
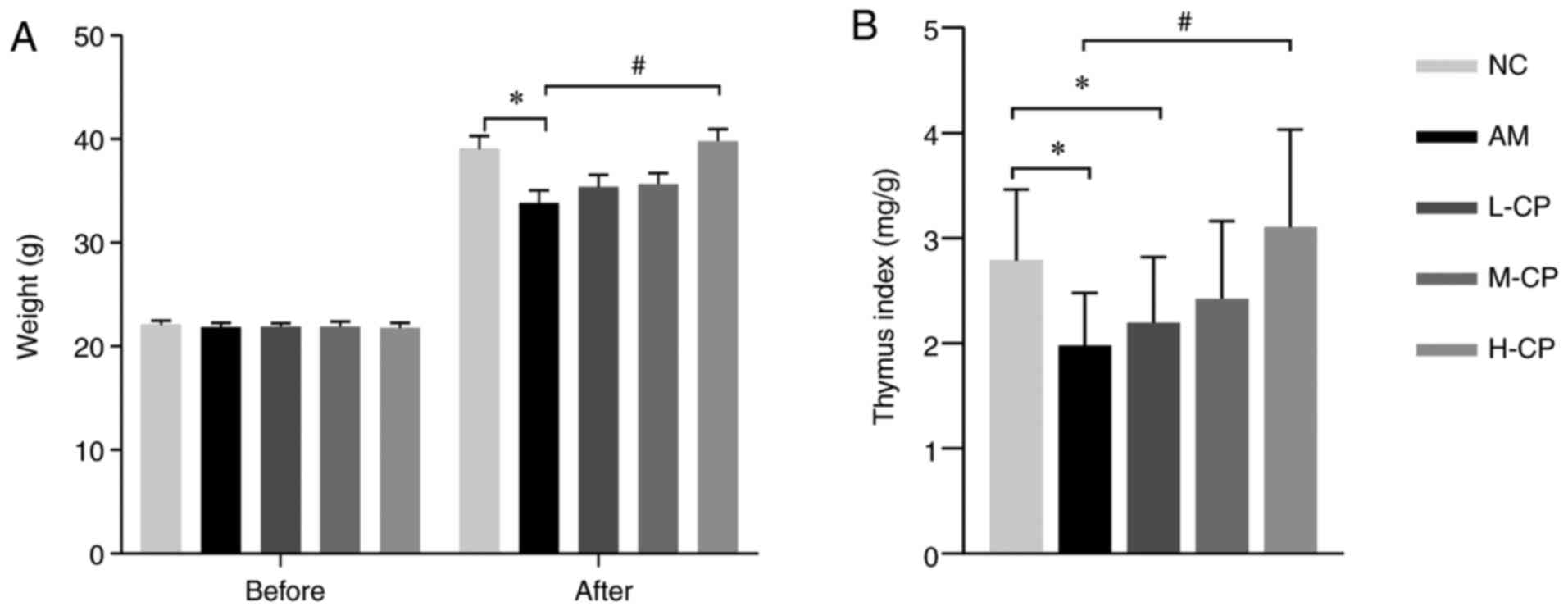
As shown in Fig.
1A, no significant differences in body weight were observed
among the groups prior to the experiment. However, following the
experiment, compared with the NC group, the body weights of the
mice in the AM group decreased significantly, with a maximum body
weight loss of 20% [maximum body weight loss=(maximum body weight
in NC group-minimum body weight in AM group)/the maximum body
weight in NC group: (40.8-32.6)/40.8 ×100] and an average weight
loss of 13%. Of note, a significant increase in body weight was
observed in the H-CP group, as compared with the AM group. A
significantly different diversity was observed in the thymus index
level in the AM and L-CP groups, as compared with the NC group, and
in the H-CP group, as compared with the AM group (Fig. 1B). These results revealed that
Codonopsis pilosula can ameliorate weight loss and thymus
index reduction induced by aging in the mouse model of aging.
Codonopsis pilosula can protect GI tissue
structure in the aging mice
In the stomach, the fundic glands were regular in
shape and abundant in quantity in the NC group (Fig. 2A). Compared with the NC group, the
deep glands and gastric mucosa of the AM group were atrophic to a
certain extent (Fig. 2B). The
results of the L-, M- and H-CP groups are shown in Fig. 2C-E, respectively. The fundus
glands and mucosa in the M group and the H-CP group were intact and
of regular shape (Fig. 2D and E).
The exact number of gastric mucosa thickness and inherent stomach
glands are presented in Table
SI. The results revealed that the medium and high dose of
Codonopsis pilosula reversed the changes of gastric
histomorphology in aged mice.
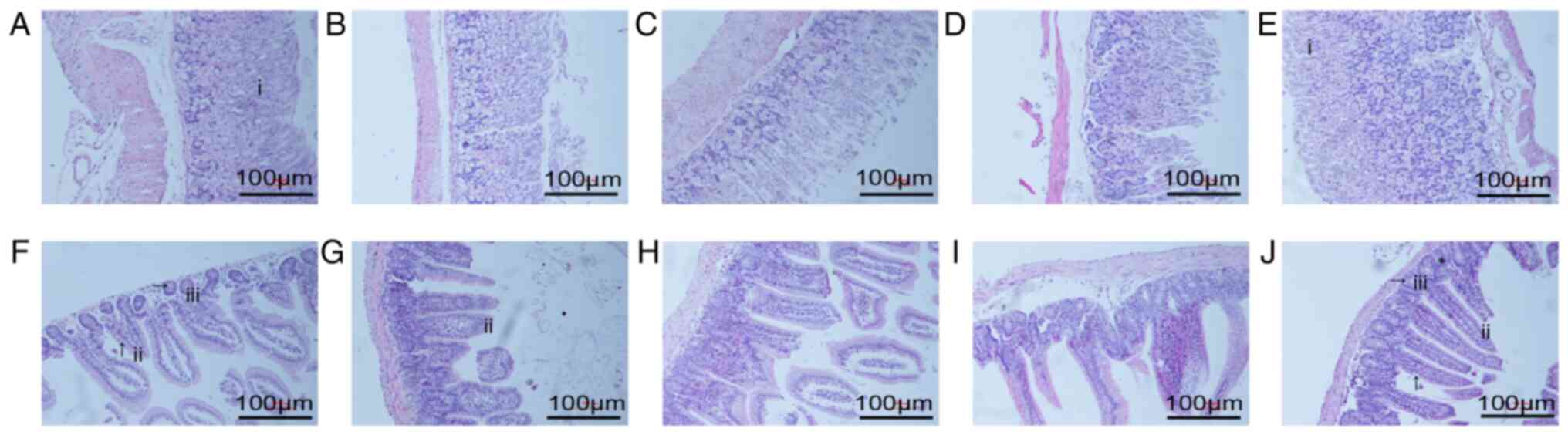 | Figure 2Effect of Codonopsis pilosula
on gastrointestinal structure (hematoxylin and eosin staining;
magnification, ×10). (A-E) Light microscopy of stomachs from the
NC, AM, and L-, M- and H-CP groups. (F-J) Light microscopy of
intestines from the NC, AM, and L-, M- and H-CP groups. i, fundic
gland; ii, intestinal villus; iii, small intestinal gland.
Rightward arrows (→) indicate Paneth cells. Upward arrows (↑)
indicate absorptive cells. MTL, motilin; VIP, vasoactive intestinal
peptide; NC group, normal control group; AM group, aging model
group; L-CP, low-dose Codonopsis pilosula group; M-CP,
medium-dose Codonopsis pilosula group; H-CP group, high-dose
Codonopsis pilosula group. |
In the intestine, the intestinal mucosa of the mice
was complete with regular arrangement of intestinal villi and
abundant glands in the NC group (Fig.
2F). The intestinal mucosal tissues and structures were
damaged, atrophic and sparsely arranged in the AM group (Fig. 2G). As compared with the AM group,
these damages were not improved in either the L- or M-CP groups
(Fig. 2H and I). However, this
was markedly ameliorated in the H-CP group (Fig. 2J); the intestinal mucosal tissue
and structural damage was reduced, and the arrangement was more
orderly. These results demonstrated that the high dose of
Codonopsis pilosula exerted protective effects on the
intestinal structure in aging mice.
Codonopsis pilosula can enhance D-xylose
absorption, as well as MTL and VIP secretion in the GI tract in
aging mice
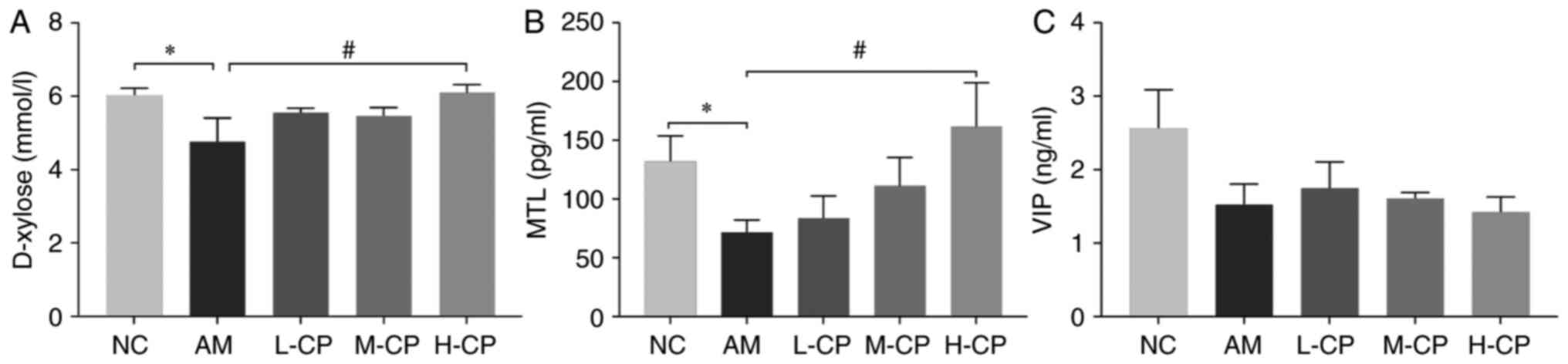
Three indexes were used to determine the digestive
function diversification in the present study, including D-xylose,
MTL and VIP (Fig. 3). As compared
with the NC group, the D-xylose level was markedly decreased in the
AM group. As compared with the AM group, the D-xylose level was
significantly increased in the H-CP group. The MTL level in the
mice in the AM group was considerably decreased, as compared with
the NC group, and it was significantly increased in the H-CP group
as compared with the AM group. These results demonstrated that
Codonopsis pilosula promoted D-xylose absorption and
increased MTL secretion in the GI tract; the higher the dose, the
more significant the effect. The VIP level in the AM group was
decreased as compared with the NC group. The L-CP group exhibited
the largest change in VIP levels as compared with the AM group,
followed by the M-CP group; the VIP level gradually decreased as
the concentration of the Codonopsis pilosula water extract
increased. This result indicated that the low of dose Codonopsis
pilosula stimulated VIP secretion; however, these changes were
not statistically significant (P>0.05).
Codonopsis pilosula protects the GI
tissue cell ultrastructure in aging mice
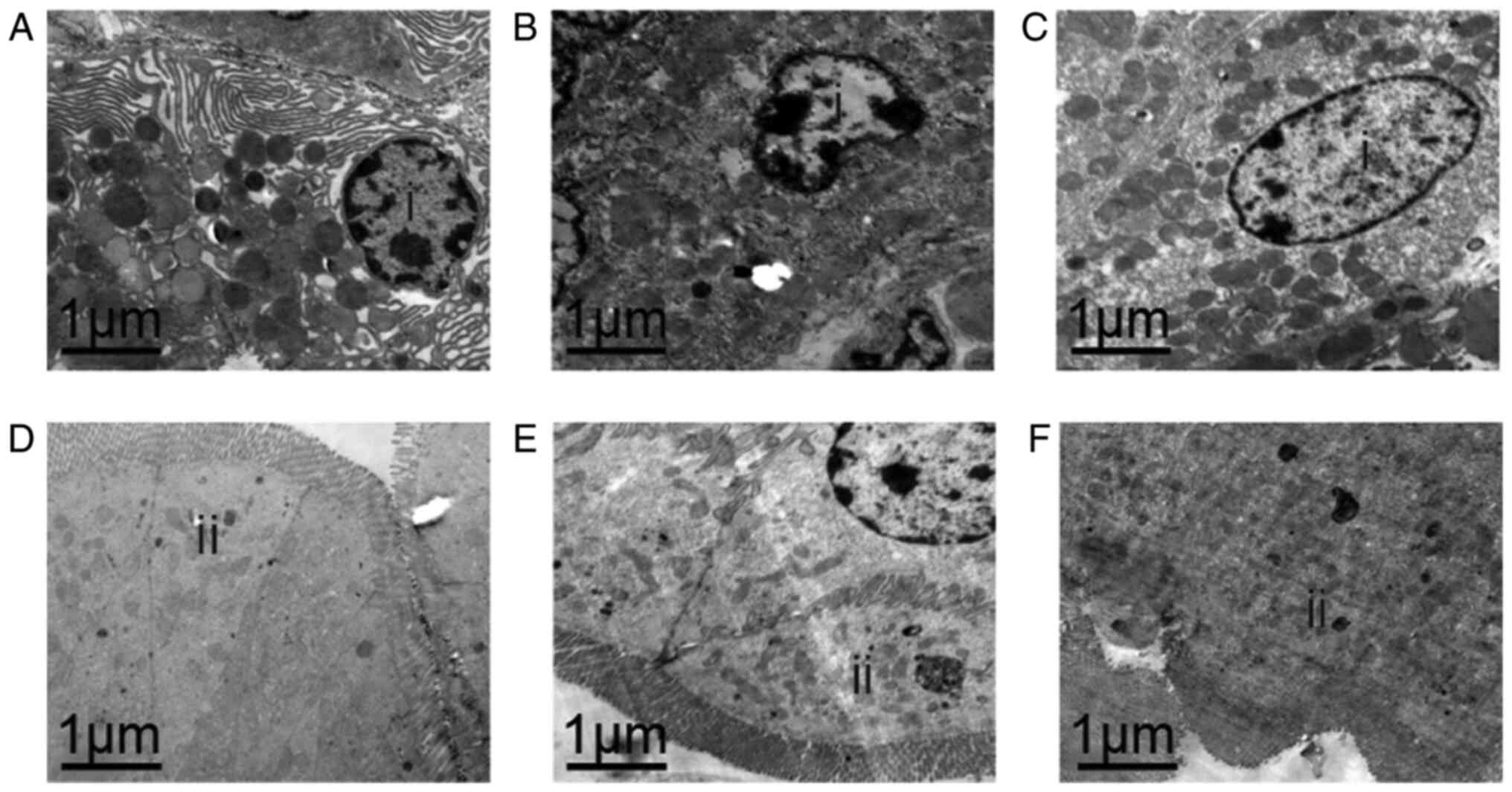
According to Figs.
1, 2 and 3A and B, it was found that compared with
the L-CP and M-CP groups, the H-CP group exhibited the most
significant anti-aging effect in the gastrointestinal tract of the
mice (in the H-CP group, body weight and thymus index increased
most evidently, and the improvement of pathological damage was the
most evident D-xylose absorption and MTL secretion were also were
the highest), therefore, we choose H-CP for our subsequent
transmission electron microscope. In the stomach, zymogenic cells
exhibited a normal structure in the NC group (Fig. 4A). However, organelle lysis and
structure destruction were observed in zymogenic cells in the AM
group (Fig. 4B). However, as
compared with the AM aging model group, the structure of the
zymogenic cells appeared to be improved (almost normal) in the H-CP
group (Fig. 4C).
In the intestine, normal intestinal epithelial cells
with a free surface were observed; the cytoplasm of the intestine
absorbing cells and the whole intestinal absorbing cells were also
observed in the NC group (Fig.
4D). As compared with the NC group, no marked changes were
observed in the AM group, apart from a certain degree of autophagy
(Fig. 4E). There was no damage to
the intestinal epithelial cells, and organelle changes were not
evident in the H-CP group (Fig.
4F). In combination, these results suggested that the high dose
of Codonopsis pilosula had the ability to protect the GI
tissue cell ultrastructure in aging mice to a certain extent.
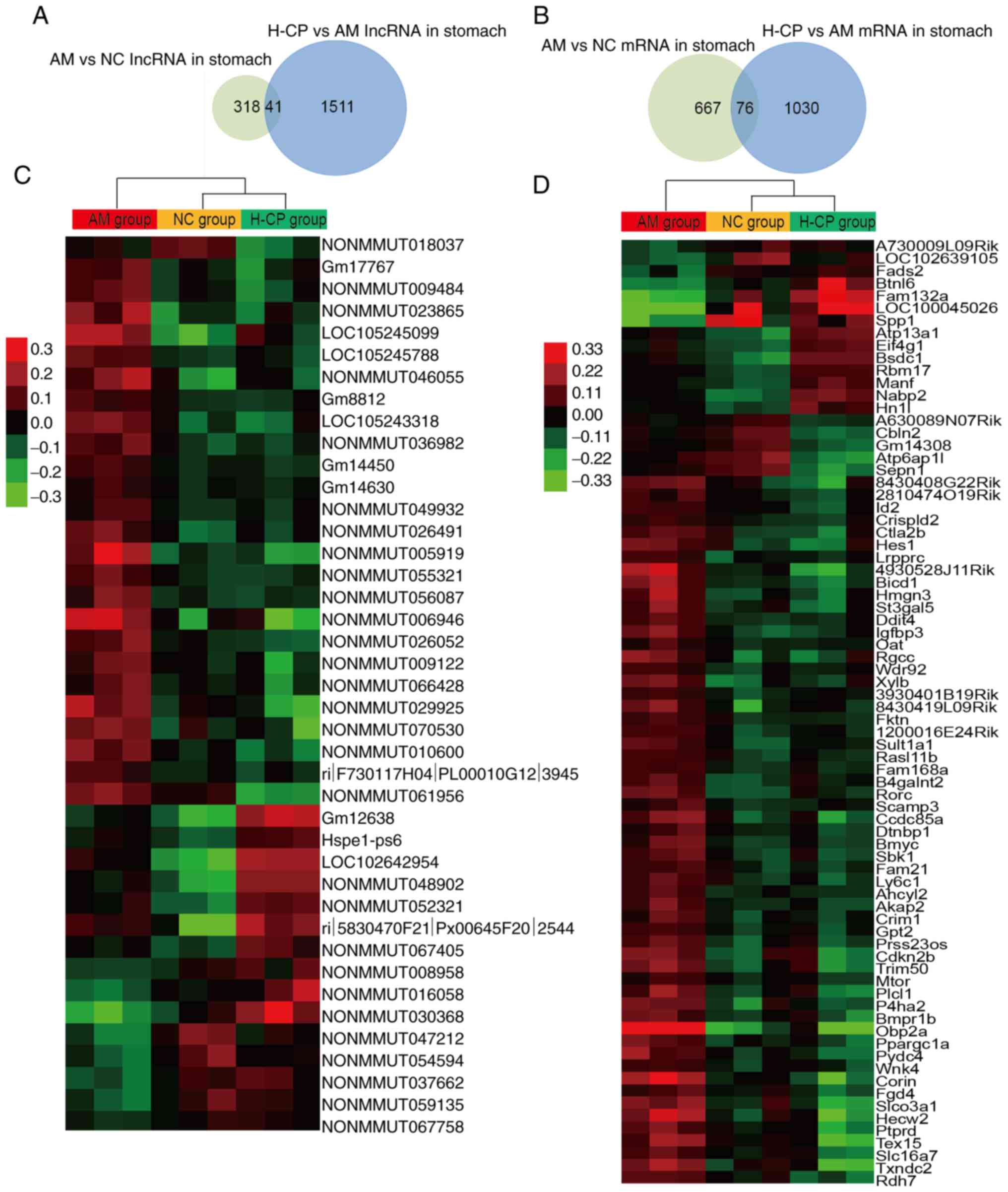
lncRNA and mRNA profiling in the stomach
of aging mice treated with Codonopsis pilosula
In the stomach, 359 and 1,552 differentially
expressed lncRNAs were identified between the AM and NC groups, and
between the H-CP and AM groups. A total of 743 and 1,106
significantly altered mRNAs were identified between the AM and NC
groups, and between the H-CP and AM groups (|Fold change|≥1.5;
P<0.05; Fig. 5A and B). Lists
of the top 50 up- and downregulated lncRNAs and mRNAs in the
stomachs from the mice in the AM vs. the NC groups, and H-CP vs.
the AM groups are presented in Table
SII.
In order to reveal which RNAs were involved in the
anti-aging effects of Codonopsis pilosula, the common
differentially expressed lncRNAs and mRNAs between the AM vs. NC
group and the H-CP vs. AM group, were selected as the RNAs involved
in the anti-aging effects of Codonopsis pilosula in the
stomach (Fig. 5C and D). A total
of 117 RNAs (41 lncRNAs and 76 mRNAs) associated with the
anti-aging effects of Codonopsis pilosula in the stomach
were identified, and the details are presented in Table SIII.
lncRNA and mRNA profiling in the
intestine of aging mice treated with Codonopsis pilosula
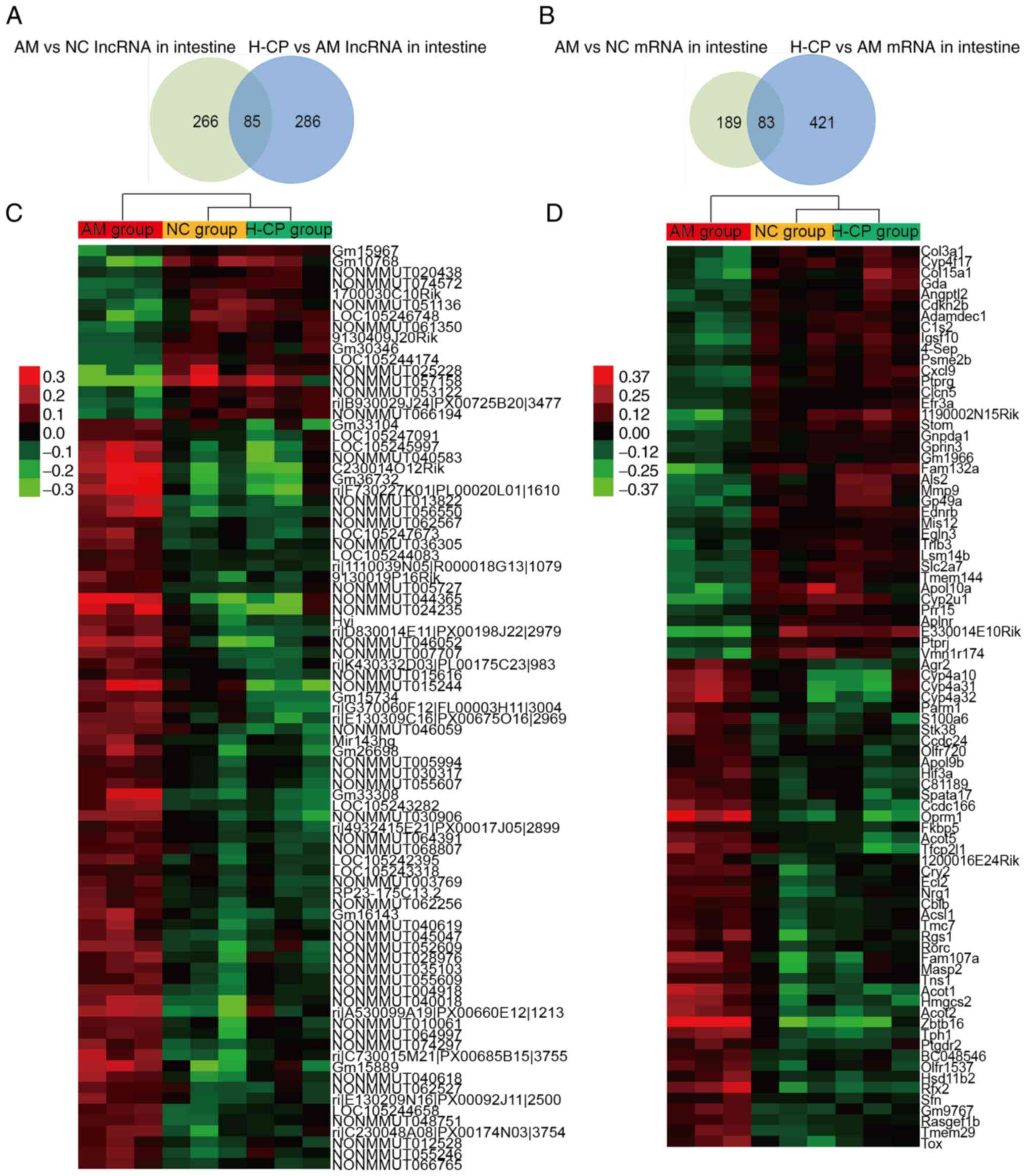
In the intestine, 351 and 371 differentially
expressed lncRNAs were identified between the AM and NC groups, and
between the H-CP and AM groups. A total of 272 and 504
significantly altered mRNAs were identified between the AM and NC
groups, and between the H-CP and AM groups (|Fold change|≥1.5;
P<0.05; Fig. 6A and B). Lists
of the top 50 up- and downregulated lncRNAs and mRNAs were
identified in intestines from the AM vs. NC group, and those from
the H-CP vs. AM groups are presented in Table SIV.
In order to discover which RNAs were involved in the
anti-aging effects of Codonopsis pilosula, the common
differentially expressed lncRNAs and mRNAs between the AM vs. NC
group and the H-CP vs. AM group, were found to be the RNAs
associated with the anti-aging effects of Codonopsis
pilosula in the intestine (Fig.
6C and D). There were 168 RNAs (85 lncRNAs and 83 mRNAs)
associated with the anti-aging effects of Codonopsis
pilosula in the intestine (Table
SV).
KEGG pathway and GO terms function
analysis for differentially expressed Codonopsis pilosula
anti-aging mRNAs in the stomach and intestine
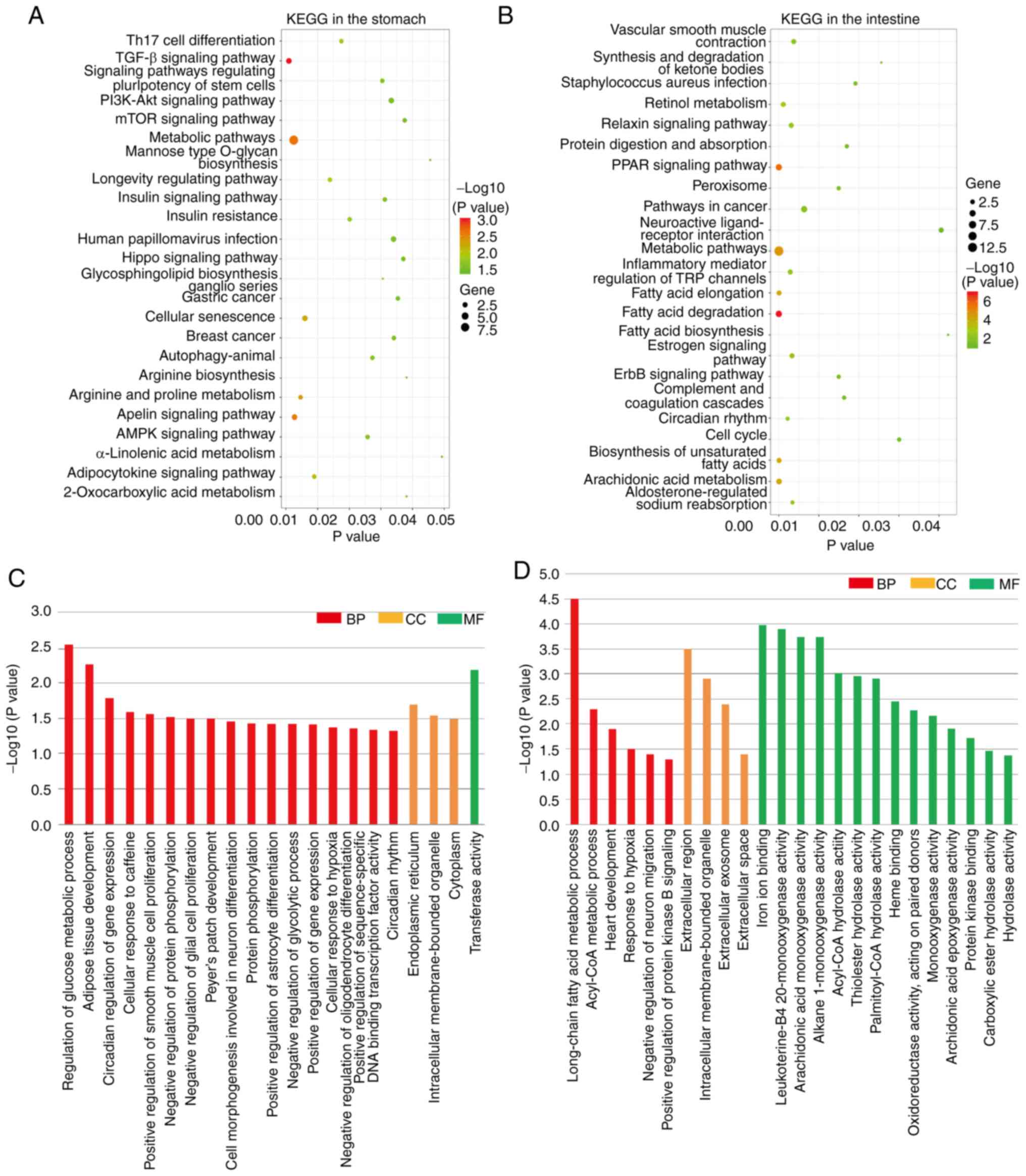
As shown in Fig. 7A
and B, 24 and 23 significant KEGG pathways were found in the
stomach and intestine, respectively, with the metabolic pathway
being the common pathway between the two sites. As shown in
Fig. 7C and D, the significant GO
terms included biological processes, cellular components and
molecular functions in the stomach and intestine. Intracellular
membrane-bound organelle was the common GO term between the stomach
and intestine.
lncRNA-mRNA co-expression network
construction of the anti-aging effects of Codonopsis pilosula in
the stomach, intestine and GI tract
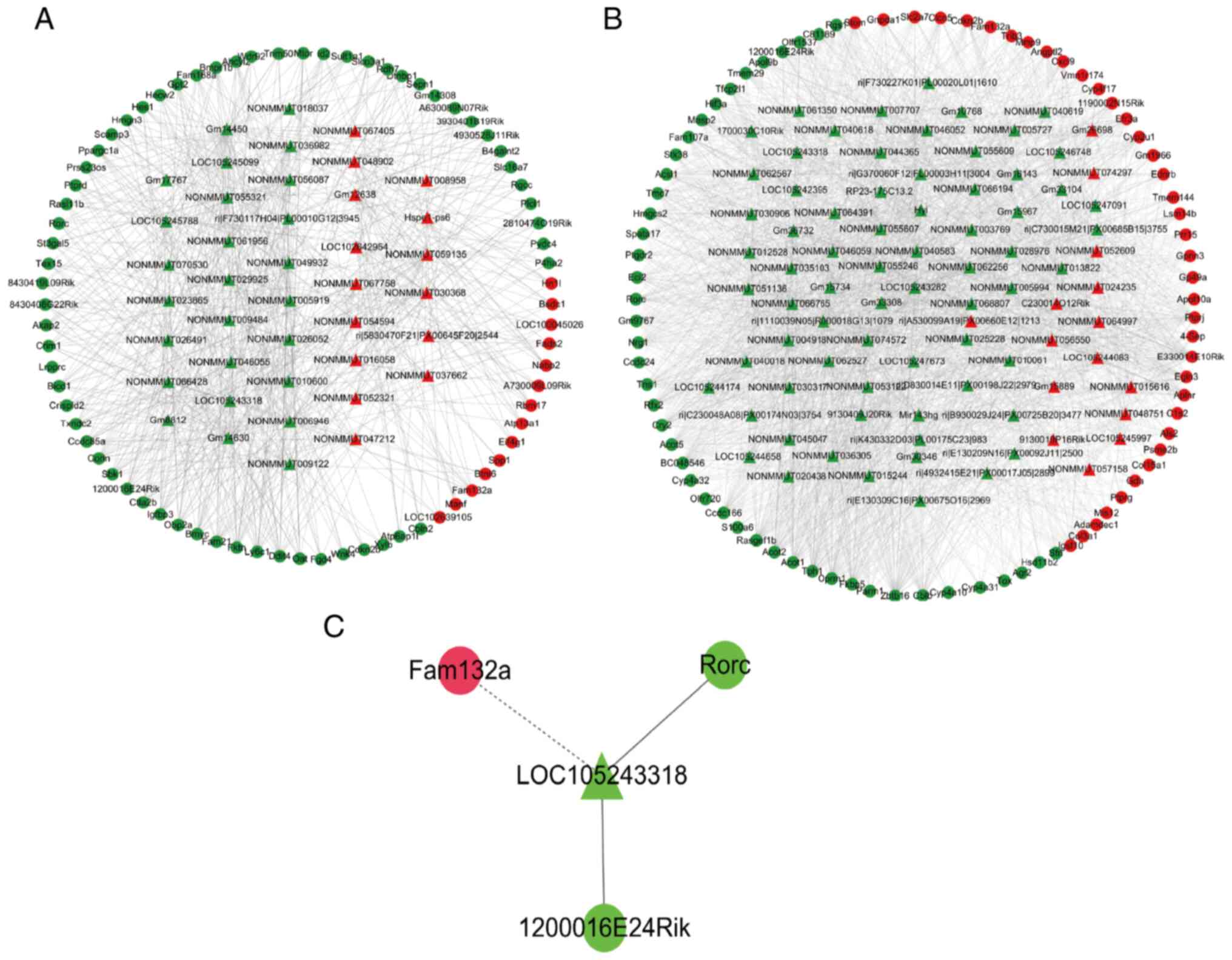
lncRNA-mRNA co-expression networks for the lncRNAs
and mRNAs related to the anti-aging effects of Codonopsis
pilosula were constructed by Pearson's correlation coefficient
in the stomach and intestine to explore the function of the
differently expressed lncRNAs (Fig.
8A and B). To reveal the precise molecular mechanisms of the
anti-aging effects of Codonopsis pilosula in the GI tract,
the co-expression networks in the stomach and intestine were
merged, and a sub-network, which participated in the anti-aging
effects of Codonopsis pilosula in the GI tract, was then
found (Fig. 8C), including 4
nodes (1 lncRNA and 3 mRNAs) and 3 edges.
RT-qPCR verification of gene expression
for the RNAs related to the anti-aging effects of Codonopsis
pilosula in the GI tract
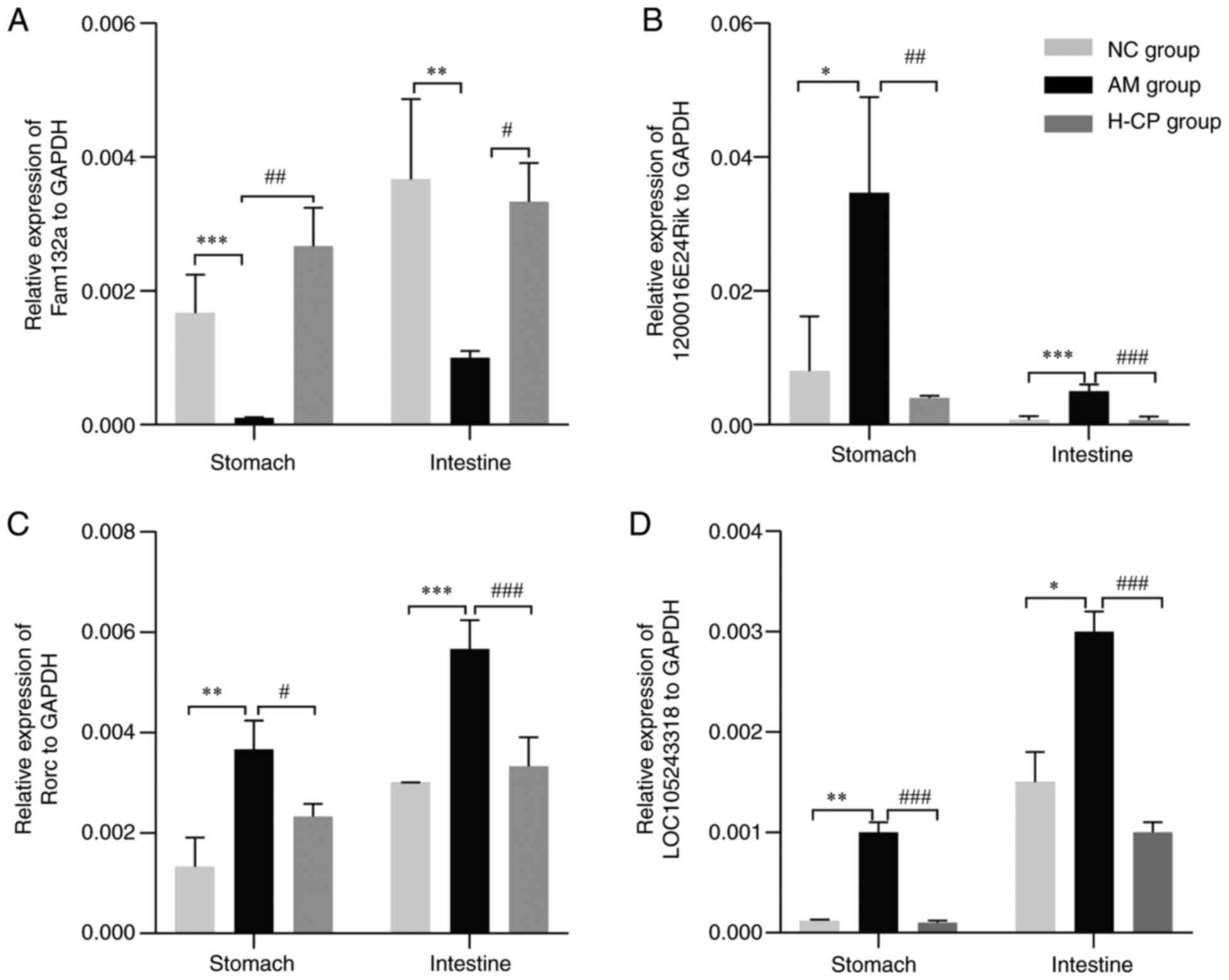
RT-qPCR was performed on 4 genes from the
Codonopsis pilosula anti-aging lncRNA-mRNA co-expression
network in the GI tract (Fam132a, 1200016E24Rik, RORC and
LOC105243318). The expression of Fam132a was increased in the H-CP
group, as compared with the AM group, and decreased in the AM
group, as compared with the NC group (Fig. 9A). 1200016E24Rik, RORC and
LOC105243318 were found to be decreased in the H-CP group, as
compared with the AM group, and increased in the AM, as compared
with the NC group (Fig. 9B-D).
These results were consistent with those of the microarray
expression profiling data.
Discussion
Biological aging is an intrinsic, complex and
irreversible process that occurs in living beings. However, aging
can be delayed, a fact that has attracted considerable medical
attention (6). In the present
study, it was confirmed that Codonopsis pilosula improved
the mouse body weight and thymus index, protected the GI structure,
improved GI function, and protected the ultrastructure of GI cells
in aging mice. Further analyses identified possible targets, action
networks and related pathways involved in the anti-aging effects of
Codonopsis pilosula in the GI tract of aging mice at the
molecular level.
It has been demonstrated that aging can lead to a
range of changes, including muscle mass loss, weight loss and organ
atrophy in varying degrees, which increase the risk of variously
age-related diseases in older individuals (30). The thymus index is one of the
reference indexes used to judge aging, since thymus atrophy occurs
during the aging process, which is associated with the immune aging
theory of the body (31,32). Pathological observation can
directly reflect the histomorphological changes, which can evaluate
the pathological changes of the body (33). During the aging process, tissues
and organs undergo a series of special pathological changes in the
GI tract, including atrophy and damage of the GI mucosa (34-36), which destroys the integrity of GI
mucosa structure and function, and is also closely associated with
the occurrence of several aging-related diseases (37,38). In the present study, the body
weights and thymus indexes were decreased in the aging mice, and
the mucosa of the GI tract of the aging mice shrank and was
destroyed; however, all these effects were alleviated following
intervention with Codonopsis pilosula, indicating that
Codonopsis pilosula attenuated aging and protected the
intact GI structure in aging mice.
Structural changes often lead to functional
alterations. In the present study, the blood concentration of
D-xylose was measured to evaluate the absorption function of the
small intestine (39), which is
associated with the integrity of the GI tract (40,41). The concentration of MTL and VIP
were measured to determine the motor and digestion functions of the
GI tract. It has been reported that the expression of MTL receptor
mRNA in different regions of the digestive tract decreases with age
(42), and the level of VIP has
been shown to be associated with several aging-related diseases
(43,44), particularly GI diseases. The
impaired digestive capacity of the GI tract is often caused by an
impaired cell secretion capacity (45). The gastric chief cell is a mature
type of cell that secretes digestive enzymes, and aging causes
degenerative changes in the gastric chief cells (46). Small intestinal absorption cells
are involved in the digestion and absorption of sugars and lipids;
the aging adult Drosophila intestine has obvious
characteristics, such as a multilayer of absorptive cells and a
wrong expression of cell type-specific genes (47). In the present study, the levels of
D-xylose, MTL and VIP were decreased, and the ultrastructure of the
gastric chief cells and small intestinal absorption cells was
destroyed in the GI tract of aging mice. These results demonstrated
that Codonopsis pilosula has the ability to regulate the GI
function and protect cell ultrastructure in the GI tract of aging
mice.
To comprehensively explore the underlying molecular
mechanisms of the anti-aging effects of Codonopsis pilosula
in the GI tract of aging mice, the stomach and intestine were
integrated, and 4 hub RNAs were identified, including 1 lncRNA
(LOC105243318) and 3 mRNAs (Fam132a, RORC and 1200016E24Rik).
LOC105243318 is a predicted non-coding RNA located in the negative
strand of chromosome 8 with a length of 443 bp, which begins with
3,594,757 and ends with 3,595,409. In the present study, it was
found to be able to negatively regulate Fam132a and positively
regulate RORC and 1200016E24Rik, which may play an important role
in the anti-GI senescence of Codonopsis pilosula. Fam132a is
located in chromosome 4, and encodes a newly identified
insulin-sensitizing adipokine, which has anti-inflammatory and
glucose-lowering properties (48). In the present study, Fam132a was
downregulated in aging mice and upregulated following Codonopsis
pilosula treatment by the regulation of LOC105243318. RORC is
the upstream regulatory gene of Th17 and Treg cells (49). Altering Th17 cytokine production
during the inflammatory response in the elderly is a novel method
for maintaining a healthy level of aging (50). Herein, RORC was upregulated
following aging, and downregulated following Codonopsis
pilosla intervention; therefore, the anti-aging effects of
Codonopsis pilosula in GI tract senescence may be associated
with the regulation of Th17 and Treg cells by RORC. Similar to
RORC, 1200016E24Rik was positively regulated by lncRNA
LOC105243318, upregulated in the GI tract of aging mice and
downregulated following Codonopsis pilosula
intervention.
In the present study, the metabolic pathway was
found to be an important pathway playing a vital role in the ageing
process of the GI tract of mice and the anti-aging effects of
Codonopsis pilosula. An overall metabolic stability decline
has been reported in elderly organisms (51), with an association observed
between these disorders and intestinal microbiota imbalance
(52). The regulation of the
intestinal microbiome has become an effective therapeutic tool
against age-related chronic diseases (53). Certain studies have indicated that
Codonopsis pilosula polysaccharide, one of the main
components of Codonopsis pilosula, can be used as a
prebiotic to regulate intestinal microbiota (54,55). The findings of the present study
support this point; the specific underlying mechanism warrant
further investigation in future studies.
In conclusion, the protective effects of
Codonopsis pilosula in senescence in the GI tract were
confirmed from the perspectives of tissue morphology, GI function
and cell ultrastructure. A total of 4 hub RNA molecules
(LOC105243318, Fam132a, RORC and 1200016E24Rik) and their
regulatory association network were found to be associated with the
anti-aging effects of Codonopsis pilosula in the GI tract of
aging mice. The metabolic pathway may play a vital role in the
process of aging and in the anti-aging effects of Codonopsis
pilosula. These results provide important insight and
directions for the future development of drugs and for the mining
of the mechanisms of aging intervention.
Supplementary Data
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81760835 and 82060829), and
the General Project of Scientific Research in Colleges and
Universities of Gansu Province (grant no. 2018A-052).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request. Please note that as the data on the GEO database are
confidential and will be released on December 31, 2020.
Authors' contributions
Each author has contributed significantly to the
present study. JM, JW, DL, JL and YD conceived and designed the
study. DL and JL performed the animal experiments. YH was
responsible for the histopathological and ultrastructural
observations. JM, DC and JK performed the statistical analyses. JM
drafted the manuscript. JW and JK revised the manuscript. YD edited
the original manuscript and guided the revision of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the experimental procedures and protocols were
accordance with the guidelines of and were performed following the
approval of the Institutional Animal Ethics Committee of Gansu
University of Chinese Medicine (approval no. 2017-106).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mr. Junjun Liu for
providing assistance with the animal experiments, and Dr Yong Wang
for assistance with the histopathological analysis.
Abbreviations:
|
NC
|
normal control
|
|
AM
|
aging model
|
|
L-CP
|
low-dose Codonopsis
pilosula
|
|
M-CP
|
medium-dose Codonopsis
pilosula
|
|
H-CP
|
high-dose Codonopsis
pilosula
|
|
H&E staining
|
hematoxylin and eosin staining
|
|
MTL
|
motilin
|
|
VIP
|
vasoactive intestinal peptide
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GO
|
Gene Ontology
|
|
DAVID
|
Database for Annotation, Visualization
and Integration Discovery
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
References
|
1
|
Dumic I, Nordin T, Jecmenica M, Stojkovic
Lalosevic M, Milosavljevic T and Milovanovic T: Gastrointestinal
tract disorders in older age. Can J Gastroenterol Hepatol.
2019:67575242019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Durazzo M, Campion D, Fagoonee S and
Pellicano R: Gastrointestinal tract disorders in the elderly.
Minerva Med. 108:575–591. 2017.PubMed/NCBI
|
|
3
|
Zhang XL and Zheng SB: Aging of digestive
system and clinic. Chin J Clin. 43:3–7. 2015.In Chinese.
|
|
4
|
Zhang HD: From 2014 to 2018, a
retrospective analysis was conducted on the hospitalization
situation of the elderly over 60 years old in a hospital. Jiangsu
Health System Management. 30:1561–1563. 2019.In Chinese.
|
|
5
|
Azman KF and Zakaria R:
D-Galactose-induced accelerated aging model: An overview.
Biogerontology. 20:763–782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai J, Ashraf MA, Luo L and Tang H:
Effects of Codonopsis pilosula water extract on MicroRNA expression
profile in D-galactose-induced senile mice. Pak J Pharm Sci.
30(Special): 1179–1183. 2017.PubMed/NCBI
|
|
7
|
Yang J, He Y, Zou J, Xu L, Fan F and Ge Z:
Effect of polygonum multiflorum thunb on liver fatty acid content
in aging mice induced by D-galactose. Lipids Health Dis.
18:1282019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen P, Chen F and Zhou BH: Leonurine
ameliorates D-galactose-induced aging in mice through activation of
the Nrf2 signalling pathway. Aging (Albany NY). 11:7339–7356. 2019.
View Article : Google Scholar
|
|
9
|
Wang HZ, Lian ZX, Lu GD, Huang YF, Cui ZJ,
Li JT and Du T: Relationship between seedling grade of Codonopsis
pilosula and yield and quality of medicinal materials. Zhongguo
Zhong Yao Za Zhi. 41:3950–3955. 2016.In Chinese.
|
|
10
|
Zhang JQ, Su X, Wu Q, Ding SS and Sun K:
Analysis of RAPD on medicinal plants of Codonopsis pilosula. Zhong
Yao Cai. 29:417–420. 2006.In Chinese. PubMed/NCBI
|
|
11
|
Xie Q, Sun Y, Cao L, Chen L, Chen J, Cheng
X and Wang C: Antifatigue and antihypoxia activities of
oligosaccharides and polysaccharides from Codonopsis pilosula in
mice. Food Funct. 11:6352–6362. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao SM, Liu JS, Wang M, Cao TT, Qi YD,
Zhang BG, Sun XB, Liu HT and Xiao PG: Traditional uses,
phytochemistry, pharmacology and toxicology of Codonopsis: A
review. J Ethnopharmacol. 219:50–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon IS and Cho SS: Effects of lobetyolin
on xanthine oxidase activity in vitro and in vivo: Weak and mixed
inhibition. Nat Prod Res. May 29–2019.Epub ahead of print.
View Article : Google Scholar
|
|
14
|
Deng X, Fu Y, Luo S, Luo X, Wang Q, Hu M,
Ma F, Ma CW and Zhou L: Polysaccharide from radix codonopsis has
beneficial effects on the maintenance of T-cell balance in mice.
Biomed Pharmacother. 112:1086822019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Zhang X, Cao L, Ji J and Gao J:
Three inulin-type fructans from Codonopsis pilosula (Franch.)
nannf. roots and their prebiotic activity on Bifidobacterium
longum. Molecules. 23:31232018. View Article : Google Scholar
|
|
16
|
Li J, Wang T, Zhu Z, Yang F, Cao L and Gao
J: Structure features and anti-gastric ulcer effects of inulin-type
fructan CP-A from the roots of Codonopsis pilosula (Franch.) nannf.
Molecules. 22:22582017. View Article : Google Scholar
|
|
17
|
Li L, Zhang L and Yang CC: Multi-target
strategy and experimental studies of traditional Chinese medicine
for Alzheimer's disease therapy. Curr Top Med Chem. 16:537–548.
2016. View Article : Google Scholar
|
|
18
|
Tian SS, Yang J, Zhao J and Zhang WD:
Application of network biology on study of traditional Chinese
medicine. Zhongguo Zhong Yao Za Zhi. 43:274–280. 2018.In Chinese.
PubMed/NCBI
|
|
19
|
Xu H, He L, Chen J, Hou X, Fan F, Wu H,
Zhu H and Guo Y: Different types of effective fractions from Radix
Isatidis revealed a multiple-target synergy effect against
respiratory syncytial virus through RIG-I and MDA5 signaling
pathways, a pilot study to testify the theory of superposition of
traditional Chinese Medicine efficacy. J Ethnopharmacol.
239:1119012019. View Article : Google Scholar
|
|
20
|
Grogan KE and Perry GH: Studying human and
nonhuman primate evolutionary biology with powerful in vitro and in
vivo functional genomics tools. Evol Anthropol. 29:143–158. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park HW and Weiss ST: Understanding the
molecular mechanisms of asthma through transcriptomics. Allergy
Asthma Immunol Res. 12:399–411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kazimierczyk M, Kasprowicz MK, Kasprzyk ME
and Wrzesinski J: Human long noncoding RNA interactome: Detection,
characterization and function. Int J Mol Sci. 21:10272020.
View Article : Google Scholar :
|
|
23
|
Tong H, Mao D, Zhai M, Zhang Z, Sun G and
Jiang G: Macrophage activation induced by the polysaccharides
isolated from the roots of Sanguisorba officinalis. Pharm Biol.
53:1511–1515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen F, Huang G, Yang Z and Hou Y:
Antioxidant activity of Momordica charantia polysaccharide and its
derivatives. Int J Biol Macromol. 138:673–680. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun K, Yang P, Zhao R, Bai Y and Guo Z:
Matrine attenuates D-Galactose-induced aging-related behavior in
mice via inhibition of cellular senescence and oxidative stress.
Oxid Med Cell Longev. 2018:71086042018. View Article : Google Scholar
|
|
26
|
Du HM, Wang YJ, Liu X, Wang SL, Wu SM,
Yuan Z and Zhu XK: Defective central immune tolerance induced by
high-dose D-Galactose resembles aging. Biochemistry (Mosc).
84:617–626. 2019. View Article : Google Scholar
|
|
27
|
Liu J, Chen D, Wang Z, Chen C, Ning D and
Zhao S: Protective effect of walnut on d-galactose-induced aging
mouse model. Food Sci Nutr. 3:969–976. 2019. View Article : Google Scholar
|
|
28
|
Fang JY, Du YQ, Liu WZ, Ren JL, Li YQ,
Chen XY, Lv NH, Chen YX and Lv B; Chinese Society of
Gastroenterology, Chinese Medical Association: Chinese consensus on
chronic gastritis (2017, Shanghai). J Dig Dis. 19:182–203. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Soenen S and Chapman IM: Body weight,
anorexia, and under-nutrition in older people. J Am Med Dir Assoc.
14:642–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fulop T, Witkowski JM, Pawelec G, Alan C
and Larbi A: On the immunological theory of aging. Interdiscip Top
Gerontol. 39:163–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martínez de Toda I, Vida C, Sanz San,
Miguel L and De la Fuente M: Function, oxidative, and inflammatory
stress parameters in immune cells as predictive markers of lifespan
throughout aging. Oxid Med Cell Longev. 2019:45742762019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao X, Yi R, Zhou X, Mu J, Long X, Pan Y,
Song JL and Park KY: Preventive effect of Lactobacillus plantarum
KSFY02 isolated from naturally fermented yogurt from Xinjiang,
China, on d-galactose-induced oxidative aging in mice. J Dairy Sci.
102:5899–5912. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tarnawski AS, Ahluwalia A and Jones MK:
Increased susceptibility of aging gastric mucosa to injury: The
mechanisms and clinical implications. World J Gastroenterol.
20:4467–4482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dinis-Ribeiro M and Kuipers EJ:
Identification of gastric atrophic changes: From histopathology to
endoscopy. Endoscopy. 47:533–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang
SC and Hu Y: Age-related changes in small intestinal mucosa
epithelium architecture and epithelial tight junction in rat
models. Aging Clin Exp Res. 26:183–191. 2014. View Article : Google Scholar
|
|
37
|
Hou P, Zhou X, Yu L, Yao Y, Zhang Y, Huang
Y, Chen M, Yi L and Mi M: Exhaustive exercise induces
gastrointestinal syndrome through reduced ILC3 and IL-22 in mouse
model. Med Sci Sports Exerc. 52:1710–1718. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Clark R and Johnson R: Malabsorption
syndromes. Nurs Clin North Am. 53:361–374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mansoori B, Rogiewicz A and Slominski BA:
The effect of canola meal tannins on the intestinal absorption
capacity of broilers using a D-xylose test. J Anim Physiol Anim
Nutr (Berl). 99:1084–1093. 2015. View Article : Google Scholar
|
|
40
|
Oteiza PI, Fraga CG, Mills DA and Taft DH:
Flavonoids and the gastrointestinal tract: Local and systemic
effects. Mol Aspects Med. 61:41–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng Y, Han X, Tang S, Li C, Xiao W and
Tan Z: Magnolol and Honokiol attenuate apoptosis of enterotoxigenic
escherichia coli-induced intestinal epithelium by maintaining
secretion and absorption homeostasis and protecting mucosal
integrity. Med Sci Monit. 24:3348–3356. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kitazawa T, Yoshida A, Tamano T, Teraoka H
and Kaiya H: Age-dependent reduction of ghrelin- and
motilin-induced contractile activity in the chicken
gastrointestinal tract. Peptides. 43:88–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ivic I, Solymar M, Fulop BD, Hashimoto H,
Toth G, Tamas A, Juhasz T, Koller A and Reglodi D: Aging-induced
modulation of pituitary adenylate cyclase-activating peptide- and
vasoactive intestinal peptide-induced vasomotor responses in the
arteries of mice. J Vasc Res. 54:359–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Korkmaz OT, Ay H, Aytan N, Carreras I,
Kowall NW, Dedeoglu A and Tuncel N: Vasoactive intestinal peptide
decreases β-amyloid accumulation and prevents brain atrophy in the
5xFAD mouse model of Alzheimer's disease. J Mol Neurosci.
68:389–396. 2019. View Article : Google Scholar
|
|
45
|
Planes-Muñoz D, López-Nicolás R,
González-Bermúdez CA, Ros-Berruezo G and Frontela-Saseta C: In
vitro effect of green tea and turmeric extracts on GLP-1 and CCK
secretion: The effect of gastrointestinal digestion. Food Funct.
9:5245–5250. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hollander D, Tarnawski A, Stachura J and
Gergely H: Morphologic changes in gastric mucosa of aging rats. Dig
Dis Sci. 34:1692–1700. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takeda K, Okumura T, Taniguchi K and
Adachi-Yamada T: Adult intestine aging model. Adv Exp Med Biol.
1076:11–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan BK, Chen J, Hu J, Amar O, Mattu HS,
Ramanjaneya M, Patel V, Lehnert H and Randeva HS: Circulatory
changes of the novel adipokine adipolin/CTRP12 in response to
metformin treatment and an oral glucose challenge in humans. Clin
Endocrinol (Oxf). 81:841–846. 2014. View Article : Google Scholar
|
|
49
|
Chen H, Ma X, Liu Y, Ma L, Chen Z, Lin X,
Si L, Ma X and Chen X: Gut microbiota interventions with
clostridium butyricum and norfloxacin modulate immune response in
experimental autoimmune encephalomyelitis mice. Front Immunol.
10:16622019. View Article : Google Scholar :
|
|
50
|
Castañeda-Delgado JE, Frausto-Lujan I,
González-Curiel I, Montoya-Rosales A, Serrano CJ, Torres-Juarez F,
Enciso-Moreno JA and Rivas-Santiago B: Differences in cytokine
production during aging and its relationship with anti-microbial
peptides production. Immunol Invest. 46:48–58. 2017. View Article : Google Scholar
|
|
51
|
Vemuri R, Shinde T, Gundamaraju R,
Gondalia SV, Karpe AV, Beale DJ, Martoni CJ and Eri R:
Lactobacillus acidophilus DDS-1 modulates the gut microbiota and
improves metabolic profiles in aging mice. Nutrients. 10:12552018.
View Article : Google Scholar :
|
|
52
|
Brasili E, Mengheri E, Tomassini A,
Capuani G, Roselli M, Finamore A, Sciubba F, Marini F and Miccheli
A: Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12
induce different age-related metabolic profiles revealed by 1H-NMR
spectroscopy in urine and feces of mice. J Nutr. 143:1549–1557.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Westfall S, Lomis N and Prakash S:
Longevity extension in Drosophila through gut-brain communication.
Sci Rep. 8:83622018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fu YP, Feng B, Zhu ZK, Feng X, Chen SF, Li
LX, Yin ZQ, Huang C, Chen XF, Zhang BZ, et al: The polysaccharides
from Codonopsis pilosula modulates the immunity and intestinal
microbiota of cyclophosphamide-treated immunosuppressed mice.
Molecules. 23:18012018. View Article : Google Scholar :
|
|
55
|
Jing Y, Li A, Liu Z, Yang P, Wei J, Chen
X, Zhao T, Bai Y, Zha L and Zhang C: Absorption Codonopsis pilosula
saponins by coexisting polysaccharides alleviates gut microbial
dysbiosis with dextran sulfate sodium-induced colitis in model
mice. Biomed Res Int. 2018:17810362018. View Article : Google Scholar
|