Introduction
Osteoporosis (OP) is a progressive bone disease
featured by a decrease in bone mass and density, regression of bone
condition contributing to increased incidence of bone fracture and
a series of serious bone-associated diseases (1,2).
Currently, OP has become a main public health concern, notably due
to the increased aging population worldwide (3). Menopausal OP is a bone metabolism
disorder featured by the reduction of bone mass, high fracture rate
and chronic alterations in the bone structure. This condition is
caused by an imbalance in intraosseous homeostasis (4-6).
Although complex factors induce postmenopausal OP,
estrogen-dependent bone resorption has become the major etiological
factor (7). The current
therapeutic methods for postmenopausal OP are mainly concentrated
on the inhibition of the absorption and identification of anabolic
agents, such as estrogen replacements. However, these treatments
also exhibit several side-effects, including high gynecological
oncology risk and hypocalcemia (8,9).
Therefore, it is of utmost significance to identify novel targets
and treatment methods for the clinical treatment of postmenopausal
OP.
MicroRNAs (miRNAs or miRs) are a type of non-coding
RNAs, with a nucleotide length ranging from 19-25. miRNAs regulate
gene expression by controlling post-transcriptional processes
(10,11). Multiple miRNAs exert different
biological functions in various organisms. More importantly, it has
been shown that miRNAs participate in bone-associated diseases
(12,13). In addition, previous studies have
concentrated on the function of miR-146a on rheumatoid arthritis
(RA) and on its involvement in the progression of certain tumors
(14,15). However, the underlying mechanisms
of miR-146a in the inhibition of osteoclast formation and the
potential therapeutic effects caused on OP remain unclear.
It has been shown that the Wnt/β-catenin signaling
pathway exerts a significant effect on OP and that the inhibition
of this pathway can improve bone density in an ovariectomized (OVX)
rat model (16,17). The induction of the receptor
activator of the nuclear factor kappa B ligand contributes to the
Wnt/β-catenin pathway-mediated regulation of osteoclastogenesis in
bone tissues. In the jawbones of OVX rats, the Wnt/β-catenin
pathway was activated by miR-141 (18). However, the interaction of
miR-146a with the Wnt/β-catenin pathway in the jawbones of OVX rats
remains unclear.
Therefore, the present study aimed to assess the
effects of miR-146a on the regulation of the Wnt/β-catenin
signaling pathway in the jawbone of OVX rats. In addition, the
results indicated that miR-146a was a negative factor of OP.
Although the OVX model does not strictly imitate postmenopausal OP,
the data reported in the present study provide evidence that
miR-146a may be a potential therapeutic target for postmenopausal
OP.
Materials and methods
Animals
A total of 54 female Sprague-Dawley rats (12 week
old, weighing 250±30 g) were purchased from the Beijing Weitong
Lihua Experimental Animal Technology Co., Ltd., [license no. SCXK
(Jing) 2016-0006]. The feeding environment was maintained under
standard conditions, including temperature range at 23±2°C, average
humidity of 55±5%, light and dark cycle of 12 h and free access to
food and water. The animal experiments followed the National
Institute of Health guidelines (NIH Pub. no. 85-23, revised 1996)
and were reviewed and approved by the Animal Protection and Use
Committee of the 960th Hospital of the PLA Joint Logistics Support
Force.
Construction of OP model
The experimental rats were anesthetized by 3% sodium
pentobarbital (50 mg/kg) by intraperitoneal injection. A bilateral
ovariectomy method was used to prepare the OP model, as previously
described (17). Basically, the
rats had both ovaries removed. The bone mineral density (BMD) of
the rat jaws was measured by dual-energy X-ray absorptiometry at 12
weeks following modeling in order to confirm whether the OP model
was successfully established.
The rats were randomly divided into 4 groups (n=6)
as follows: i) The sham-operated (sham) group, ii) OP group, iii)
miR-146a antagonist group (miR-146a-A) and iv) miR-146a antagonist
negative control group (miR-146-NC). In the sham group, part of the
adipose tissue near the ovaries was removed in the rats and saline
was injected into the tail vein once a week, whereas in the OP
group, both ovaries were removed in the rats and saline was
injected into the tail vein once a week. In the miR-146a-A group,
part of the adipose tissue near the ovaries was removed and
miR-146a antagonist (Guangzhou RiboBio Co., Ltd.) was injected at a
dose of 10 mg/kg into the tail vein once a week (18), whereas in the miR-146a-NC group,
part of the adipose tissue near the ovaries was removed and the
negative control (Guangzhou RiboBio Co., Ltd.) was injected into
the tail vein once a week (10 mg/kg). The treatment administration
was carried out each week for 12 weeks. At the end of the
treatment, the rats were sacrificed using 3% sodium pentobarbital
(120 mg/kg) by intraperitoneal injection. Blood samples from the
abdominal aorta and the bone of the jaws were collected. Certain
jawbones were fixed in 4% paraformaldehyde and 1 mm below the
center of the epiphyseal line was selected as the region of
interest for the analysis by dual-energy X-ray absorptiometry. The
analysis resulted in the determination of BMD. Certain jawbones
were stored at -80°C.
To further examine the effects of miR-146a on the
Wnt/β-catenin signaling pathway, another 30 rats were divided into
5 groups (n=6) as follows: i) The sham group, ii) OP group, iii)
miR-146a-A group, iv) Wnt activator group (DKK2-C2) and v) miR-146a
antagonist + Wnt inhibitor (endostatin) group (miR + E). The
DKK2-C2 group was established by an injection of recombinant DKK2
protein (20 µg/kg DKK2-C2; Prospec-Tany TechnoGene, Ltd.)
into the tail vein once a week (18). The miR + E group was treated with
the miR-146-A, the Wnt inhibitor and recombinant human endostatin
(Endostar, Shandong Xiansheng Mai Dejin Biopharmaceutical; 1.5
mg/kg) that were injected into the tail vein once a week (18).
Reverse transcription-quantitative PCR
(RT-qPCR)
The mandibular molars of the rats (100 mg) were
homogenized using a homogenizer (KZ-II, Servicebio) until there was
no visible tissue mass, then centrifuged at 4°C (800 × g, 15 min).
Total RNA was extracted using TRIzol reagent (Takara Bio, Inc.).
cDNA was synthesized using the reverse transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). A
Mastercycler® nexus X2 (Eppendorf) was used for qPCR.
SYBR-Green PCR kit (Qiagen, Inc.) was used as the fluorophore. The
following conditions were used: 95°C for 15 sec, 60°C for 60 sec
and 72°C for 40 sec (35 cycles). The data were processed using the
2−ΔΔCq method (19)
and the relative expression levels were calculated using U6 mRNA as
an internal reference. The sequences of the primers (Shanghai
Biotech Engineering Services Co., Ltd.) were the following:
miR-146a forward, 5′-CCT GAG AAG TGA ATT CCA TGG G-3′ and reverse:
5′-TGG TGT CGT GGA GTC G-3′; and U6 forward, 5′-ATT GGA ACG ATA CAG
AGA AGA TT-3′ and reverse, 5′-GGA ACG CTT CAC GAA TTT G-3′.
ELISA
The serum samples were prepared by centrifugation
(800 × g, 10 min, 4°C) of the collected blood samples at 4°C. The
osteocalcin concentration was measured according to the
instructions provided by the manufacturer (Rat ELISA kit;
E4764-100; BioVision, Inc.). Tartrate-resistant acid phosphatase
(TRAP) activity was determined using the TRAP ELISA kit (RA20761;
Bio-Swamp Life Science Lab).
Hematoxylin and eosin (H&E) and TRAP
staining
The molar area of the mandible of the rats was
obtained and stored in a refrigerator at -80°C. The other part was
fixed in a 4% paraformaldehyde solution for 2 days and was
subsequently transferred to 10% EDTA for 2 months for
decalcification. The mandible was dehydrated and paraffin-embedded
and fixed with 4% paraformaldehyde at 37°C for 48 h. The embedded
tissue was sectioned to 5-µm-thick slices. The slices were
routinely dewaxed and hydration, subsequently stained for 5 min in
hematoxylin at room temperature (Beijing Solarbio Science &
Technology Co., Ltd.). The sections were differentiated in
hydrochloric acid and ethanol for 30 sec, immersed in PBS for 15
min and finally placed in eosin staining solution (Beijing Solarbio
Science & Technology Co., Ltd.) for 2 min at room temperature.
They were routinely dehydrated, processed to form a transparent
structure and mounted. The histopathological changes of the jawbone
were photographed under a light microscope at ×200 magnification
(Olympus Corporation). The steps for the paraffin-embedded, dewaxed
and hydrated sections were the same as those for H&E staining.
The sections were stained with the TARP staining kit (Whatman plc;
GE Healthcare Life Sciences) for 1 min at 37°C. The sections were
dehydrated with gradient ethanol, cleared with dimethylbenzene and
sealed with neutral balsam.
Immunohistochemistry
The slices were baked at 60°C in a dryer (101-1A,
Nanjing Wohuan Science & Technology Industrial Co. Ltd.) for
120 min and dewaxed with xylene, and sequentially hydrated with a
gradient ethanol solution. A 3% H2O2 methanol
solution was used to inactivate processing for 20 min. The citrate
buffer (pH 6.0) was heated for 10 min and sealed with 5% BSA for 20
min. Rabbit anti-rat nuclear factor of activated T cells c1
(NFATc1); 1:100, sc-17834, Santa Cruz Biotechnology, Inc.), c-Fos
antibody (1:100, ab209794; Abcam) and cathepsin K (CTK) antibody
(1:100, ab19027; Abcam) were incubated with the samples at 4°C
overnight. Goat anti-rabbit IgG (1:1,000, ABIN101988;
antibodies-online Aachen) labeled with horseradish peroxidase was
used for secondary antibody incubation at room temperature for 60
min. The chromogenic reaction was detected using DAB. Following DAB
staining, the sections were re-dyed and dehydrated with gradient
ethanol solution. Subsequently, the sections were transparently
treated with xylene and sealed with neutral gum. The visual
inspection was made under an optical microscope at ×200
magnification (Olympus, Corporation). A total of 3 fields were
randomly selected from each section and image analysis was
performed using ImageJ 6.0 software, which was used to measure the
integral optical density.
Western blot analysis
The tissues were homogenized and the supernatant was
obtained following centrifugation (800 × g, 10 min) at room
temperature. The protein concentration was measured using the BCA
kit (Beijing Solarbio Science & Technology Co., Ltd.). A total
of 40 µg protein sample was mixed with 10% SDS gel buffer
and the protein was denatured by heating at 95°C for 5 min.
Subsequently, the proteins were subjected to 12% SDS-PAGE and
transferred to PVDF membranes, which were blocked with TBST
solution containing 5% skimmed milk powder for 1 h at 4°C. The
following antibodies were used: Rabbit anti-rat osteoprotegerin
(OPG; 1:300, ab203061; Abcam), TRAP (1:10,000, ab133288; Abcam),
dickkopf1 (DKK1; 1:2,000, ab61275; Abcam), Wnt2 (1:1,000, ab27794;
Abcam), β-catenin (1:8,000, ab32572, Abcam) and β-actin (1:2,000,
ab61275; Abcam). The polyclonal antibodies were diluted with TBST
solution containing 3% bovine serum protein and incubated overnight
at 4°C. Goat anti-rabbit IgG (1:1,000, ABIN 101988;
antibodies-online, Aachen) labeled with horseradish peroxidase was
incubated at room temperature for 1 h following pre-incubation. The
PVDF membrane was incubated with the ECL substrate for 3-5 min
following washing. The protein expression levels were normalized
with β-actin and the analysis was performed using ImageJ 6.0 (NIH)
software.
Statistical analysis
SPSS19.0 statistical software was used to analyze
the data. The results are expressed as the means ± SD. The analysis
between multiple groups was performed by the single factor analysis
of variance and by the Tukey's test. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of miR-146a on the expression of
osteocalcin and TRAP in the serum of OVX rats
The BMD in the jawbones of the rats in the OP group
was significantly decreased compared with that of the rats in the
sham group (Fig. 1A, P<0.05).
However, the BMD was increased in the miR-146a-A group compared
with that noted in the OP group (P<0.05). The expression levels
of miR-146a were measured by RT-qPCR (Fig. 1B). The data indicated that the
expression levels of miR-146a were significantly increased in the
OP group compared to those of the sham group (P<0.05). The
expression levels of miR-146a in the miR-146a-A group were notably
decreased compared to those of the OP group (P<0.05). The
contents of osteocalcin (Fig. 1C)
and TRAP (Fig. 1D) were also
analyzed in the serum samples derived from each group. The data
demonstrated that the levels of osteocalcin and TRAP were elevated
in the OP group compared to those of the sham group (P<0.05).
However, the contents of osteocalcin and TRAP in the serum of the
miR-146a-A group were significantly decreased compared to those of
the OP or the miR-146a-NC groups (P<0.05). Taken together, these
data indicated that inhibition of miR-146a attenuated OP.
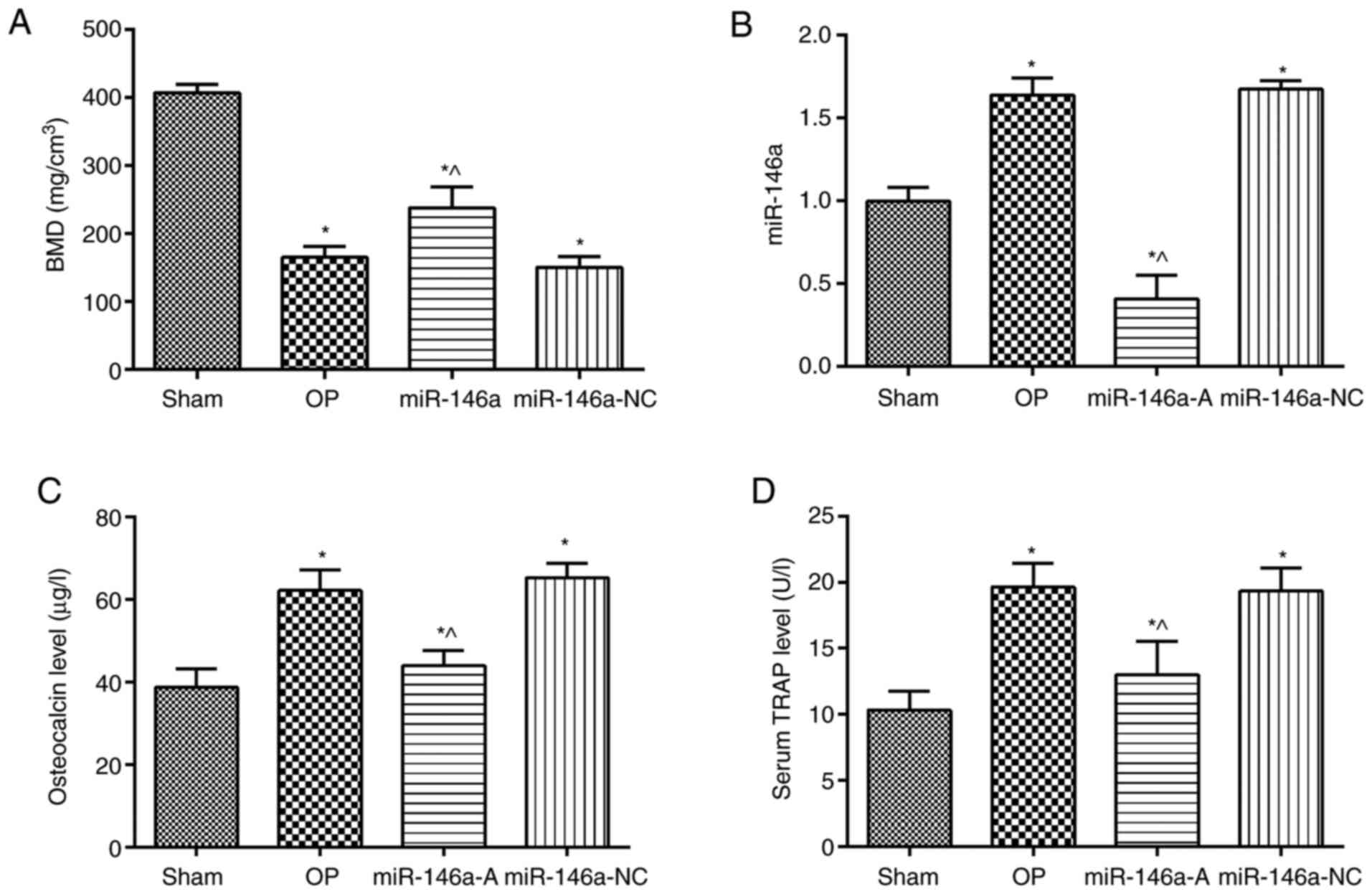 | Figure 1Evaluation of BMD, miR-146a,
osteocalcin and TRAP expression levels. BMD and miR-146a levels
were assessed in the jawbones of rats, whereas the expression
levels of osteocalcin and TRAP were investigated in serum. (A) BMD.
(B) miR-146a expression levels. (C) Osteocalcin levels. (D) Serum
TRAP levels. *P<0.05, compared with the sham group;
^P<0.05, compared with the OP group. BMD, bone
mineral density; miR-146a, microRNA-146a; TRAP, tartrate-resistant
acid phosphatase; sham, sham group; OP, osteoporosis group;
miR-146-A, miR-146a antagonist group; miR-146a-NC, miR-146a
antagonist negative control group. |
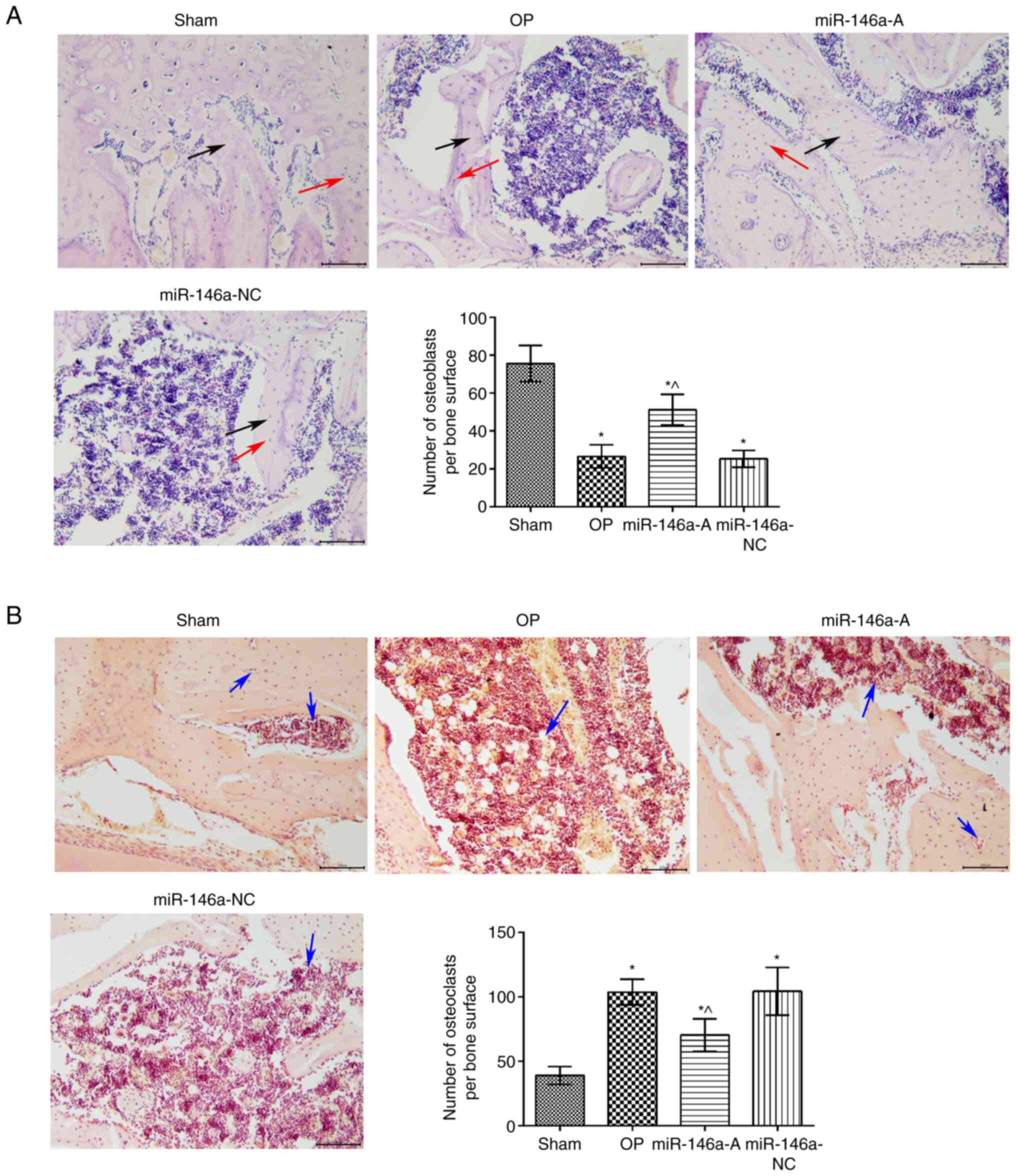
Effects of miR-146a on the pathological
changes of the jawbone
The results of H&E staining indicated that the
morphology and structure of the bone trabeculae in the sham group
were arranged regularly and the bone marrow cavity was relatively
small (Fig. 2A). However, the
structure of the bone trabeculae was sparsely arranged with poor
connectivity and a large number of blind ends in the trabecular
bone. The thickness of the bone trabecular wall was inconsistent
and the bone marrow cavity was increased in the OP and miR-146a-NC
groups. However, in the bone trabeculae were thick and the bone
marrow cavity was reduced in the miR-146a-A group compared with
that of the OP group. The number of osteoblasts in the OP and
miR-146a-NC groups was significantly lower than that noted in the
sham group (P<0.05). The inhibition of miR-146a expression
significantly increased the number of osteoblasts compared with
that noted in the OP group (P<0.05). TRAP staining (Fig. 2B) indicated that the number of
osteoclasts in the OP group was higher than that in the sham group
(P<0.05). The expression levels of TRAP in the OP and
miR-146a-NC groups were significantly higher than those noted in
the miR-146a antagonist group (P<0.05). Therefore, the results
indicated that the downregulation of miR-146a attenuated the
OP-associated pathological changes of the jawbone.
Effects of miR-146a on the expression of
NFATc1, c-Fos and CTK in the jawbone
The expression levels of NFATc1, c-Fos and CTK in
the jawbone were analyzed by immunohistochemistry (Fig. 3). The expression levels of NFATc1
(Fig. 3A), c-Fos (Fig. 3B) and CTK (Fig. 3C) in the jawbone tissues were
significantly increased in the OP group compared to those of the
sham group (P<0.05). However, the expression levels of NFATc1,
c-Fos and CTK were significantly decreased in the miR-146a-A group
(P<0.05). In addition, the levels of NFATc1, c-Fos and CTK were
significantly increased in the miR-146a-NC group compared to those
of the miR-146a-A group (P<0.05). Thus, the downregulation of
miR-146a decreased the expression levels of NFATc1, c-Fos and CTK
in the jawbone and relieved the effects of OP.
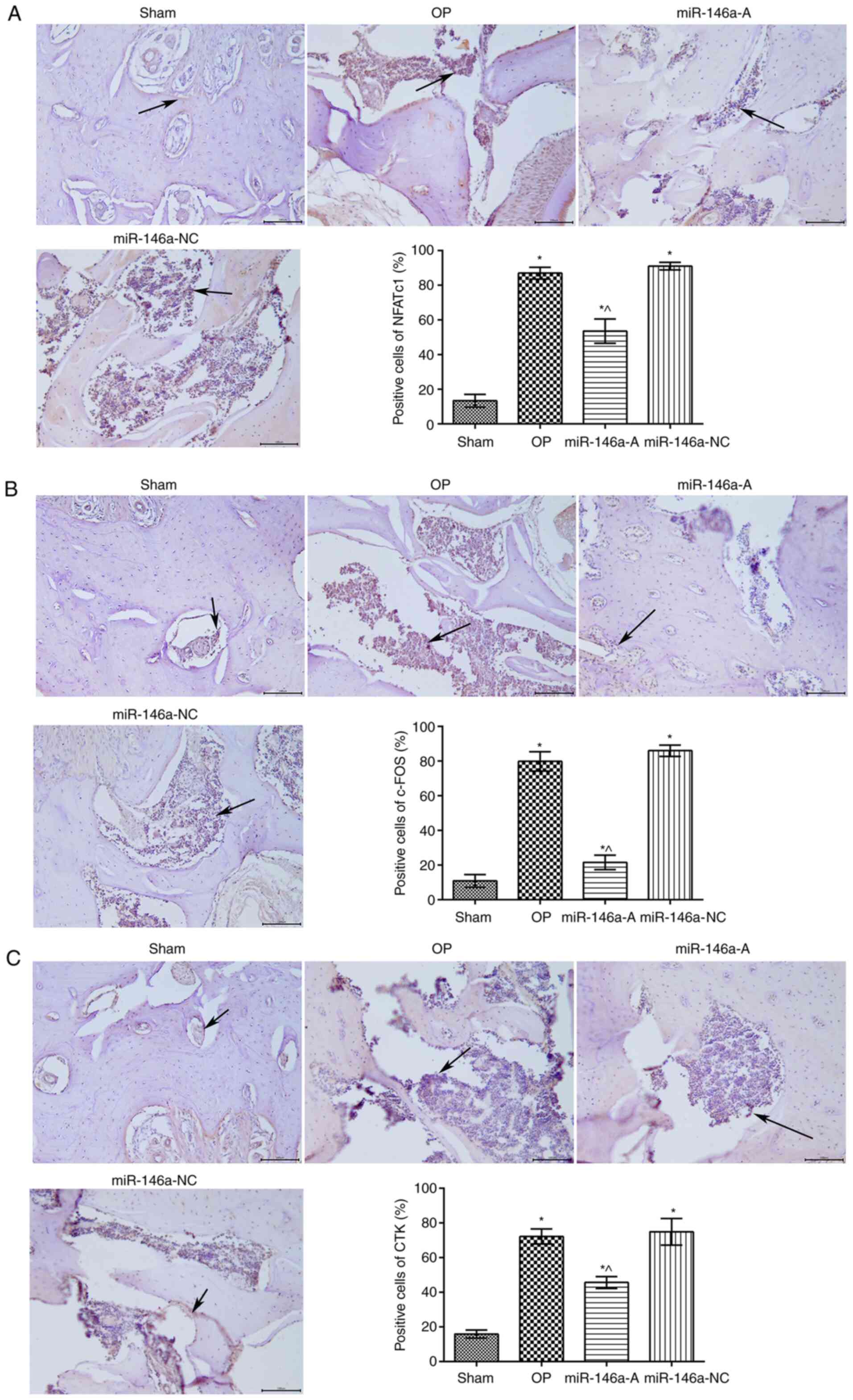 | Figure 3Effects of miR-146a were assessed on
the expression of (A) NFATc1, (B) c-Fos and (C) CTK in rat jawbone
tissues by immunohistochemical analysis (×200 magnification). Black
arrows represent the expression levels determined by
immunohistochemical analysis. *P<0.05, compared with
the sham group; ^P<0.05, compared with the OP group.
miR-146a, microRNA-146a; NFATc1, nuclear factor of activated T
cells c1; CTK, cathepsin K; sham, sham group; OP, osteoporosis
group; miR-146a-A, miR-146a antagonist group; miR-146a-NC, miR-146a
antagonist negative control group. |
Effects of miR-146a on the protein
expression of OPG, TRAP, DKK1, Wnt2 and β-catenin in the
jawbone
The expression levels of OPG, Wnt2 and β-catenin in
the jawbone were examined by western blot analysis (Fig. 4). The data indicated that the
expression levels of OPG, Wnt2 and β-catenin were significantly
decreased, while the expression levels of TRAP and DKK1 proteins
were significantly increased in the OP group compared to those
noted in the sham group (P<0.05). However, the expression levels
of OPG, Wnt2 and β-catenin proteins were significantly increased,
while the expression levels of TRAP and DKK1 proteins were
significantly decreased in the miR-146a-A group compared to those
noted in the OP group (P<0.05). The expression levels of OPG,
Wnt2 and β-catenin proteins were significantly decreased, while the
expression levels of TRAP and DKK1 proteins were significantly
increased in the miR-146a-NC group than those noted in the
miR-146a-A group (P<0.05). These results indicated that the
downregulation of miR-146a regulated the expression of
OP-associated proteins in the jawbone, which resulted in the
reduction of the progression of OP.
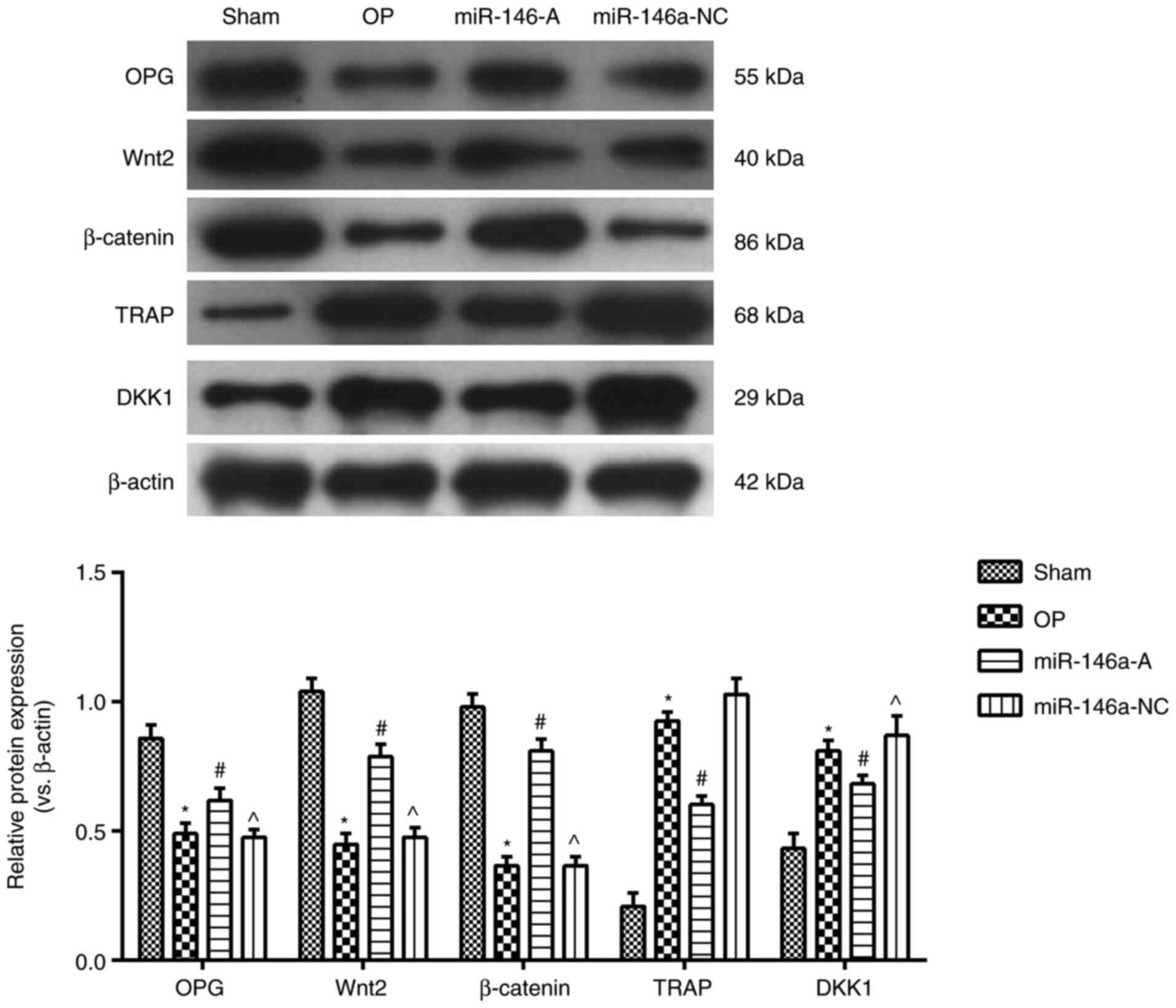 | Figure 4Effects of miR-146a on the expression
of OPG, TRAP, DKK1 and Wnt2, β-catenin proteins in jawbone tissues.
*P<0.05, compared with the sham group;
#P<0.05, compared with the OP group;
^P<0.05, compared with the miR-146a group. miR-146a,
microRNA-146a; OPG, osteoprotegerin; TRAP, tartrate-resistant acid
phosphatase; DKK1, dickkopf1; sham, sham group; OP, osteoporosis
group, miR-146a, miR-146a-A antagonist group; miR-146a-NC, miR-146a
antagonist negative control group. |
miR-146a affects OP by regulating the
Wnt/β-catenin signaling pathway
To further assess the effects of miR-146a on the
Wnt/β-catenin signaling pathway, the Wnt2 activator and inhibitor
were used. The BMD of each group was analyzed (Fig. 5A). The BMD of the latter groups
was significantly decreased compared with that of the sham group
(P<0.05), whereas the BMD in the miR-146a-A and DKK2-C2 groups
was significantly increased compared with that of the OP group
(P<0.05). In addition, the BMD was significantly decreased
following treatment of the cells with miR-146a-A and endostatin
(miR + E) compared with that of the miR-146a-A group
(P<0.05).
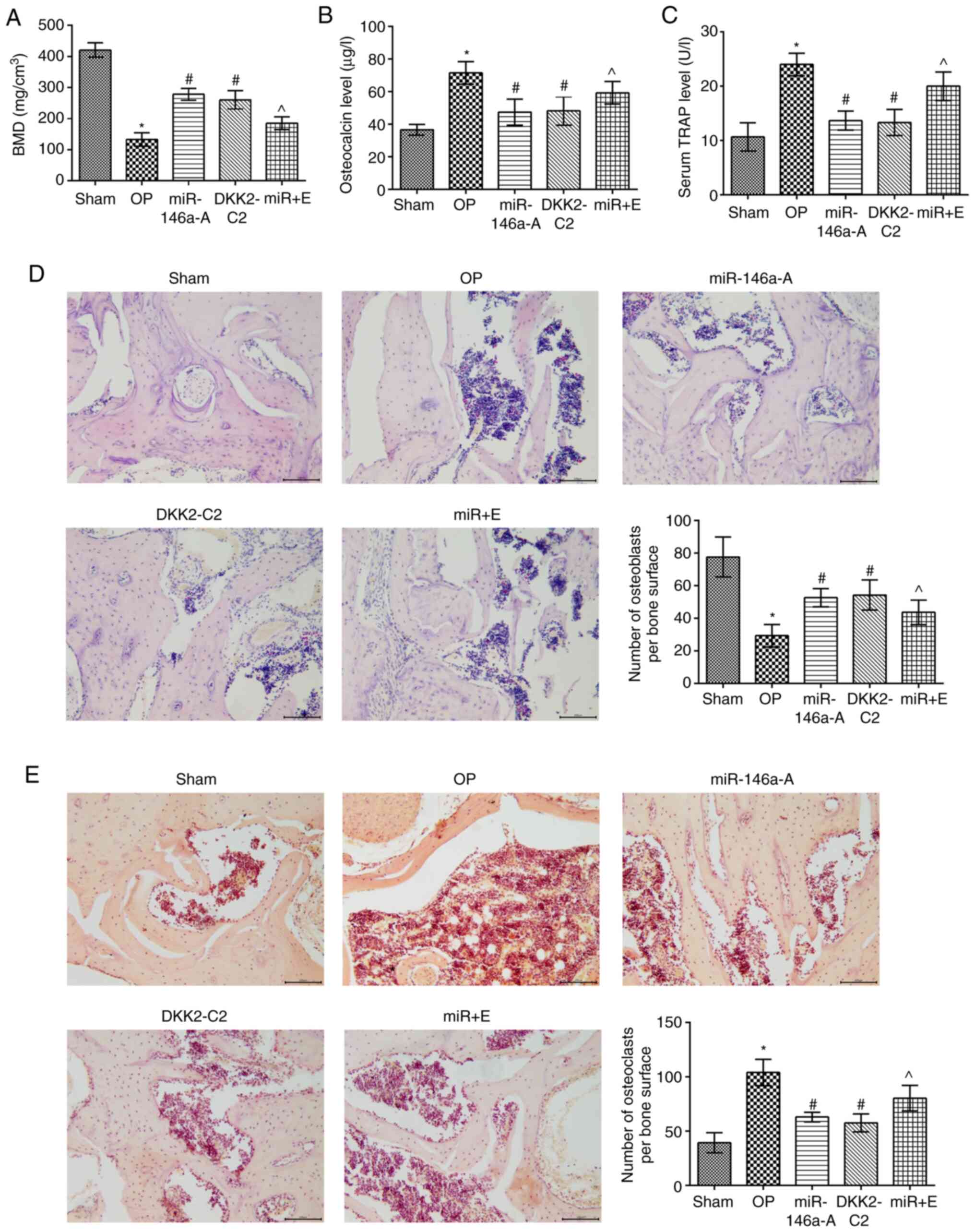 | Figure 5Activation of the Wnt/β-catenin
signaling pathway induces pathological alteration in the jaws and
reduces the serum levels of osteocalcin and TRAP. (A) BMD
determination; the expression levels of osteocalcin (B) and TRAP
(C) were measured in the serum by ELISA. (D) The pathological
alterations caused in the jaws were analyzed by H&E staining
(×200) (E). TRAP staining (×200 magnification).
*P<0.05, compared with the sham group;
#P<0.05, compared with the OP group;
^P<0.05, compared with the miR-146a group. TRAP,
tartrate-resistant acid phosphatase; BMD, bone mineral density;
H&E, hematoxylin-eosin; sham, sham group; OP, osteoporosis
group; miR-146a-A, miR-146a antagonist group; DKK2-C2, Wnt
activator group; miR + E: miR-146a antagonist + Wnt inhibitor
group. |
The contents of osteocalcin and TRAP in the serum of
the rats in the other groups were significantly increased compared
to those of the sham group (P<0.05; Fig. 5B and C). The levels of osteocalcin
and TRAP in the serum of the miR-146a-A and DKK2-C2 group rats were
significantly decreased compared to those of the OP group
(P<0.05). However, the serum levels of osteocalcin and TRAP in
the miR + E group were significantly increased compared to those of
the miR-146a-A group (P<0.05).
The trabecular structure was complete and arranged
tightly and regularly in the sham group, as determined by light
microscopy (Fig. 5D). Moreover,
the trabecular connections were meshed and the bone marrow cavity
was relatively small, while the trabecular structure of the OP and
miR + E groups was sparse and its total content was significantly
reduced. However, the trabeculae were thicker and the bone marrow
cavity was decreased in the miR-146a antagonist and DKK2-C2 groups
compared to those of the OP group.
TRAP staining (Fig.
5E) indicated that the osteoclast number of the miR-146a-A and
DKK2-C2 groups was significantly lower compared with that of the OP
group (P<0.05). However, the osteoclast number in the miR + E
group was significantly higher compared with that of the miR-146a-A
group (P<0.05), whereas no significant differences were noted
between the miR-146a-A and DKK2-C2 groups (P>0.05). These
results indicated that the downregulation of miR-146a expression
attenuated the pathological alterations of the jawbone via the
interaction with the Wnt/β-catenin signaling pathway.
miR-146a affects NFATc1, c-Fos and CTK
protein expression by regulating the Wnt/β-catenin signaling
pathway in the jawbone
The expression levels of NFATc1, c-Fos and CTK in
the jawbone were analyzed by immunohistochemistry (Fig. 6). The results indicated that the
expression of NFATc1 (Fig. 6A),
c-Fos (Fig. 6B) and CTK (Fig. 6C) in the other groups was
significantly increased compared with that noted in the sham group
(P<0.05). The expression levels of NFATc1, c-Fos and CTK were
significantly downregulated in the miR-146a-A and DKK2-C2 groups
compared with those of the OP group, (P<0.05). In addition, the
expression levels of NFATc1, c-Fos and CTK were notably increased
in the miR + E group compared to those in the miR-146a-A group
(P<0.05). No significant differences were noted between the
miR-146a-A and the DKK2-C2 groups with regard to the aforementioned
indices (P>0.05). Taken together, these results indicated that
the effects of miR-146-A were similar to those of DKK2-C2.
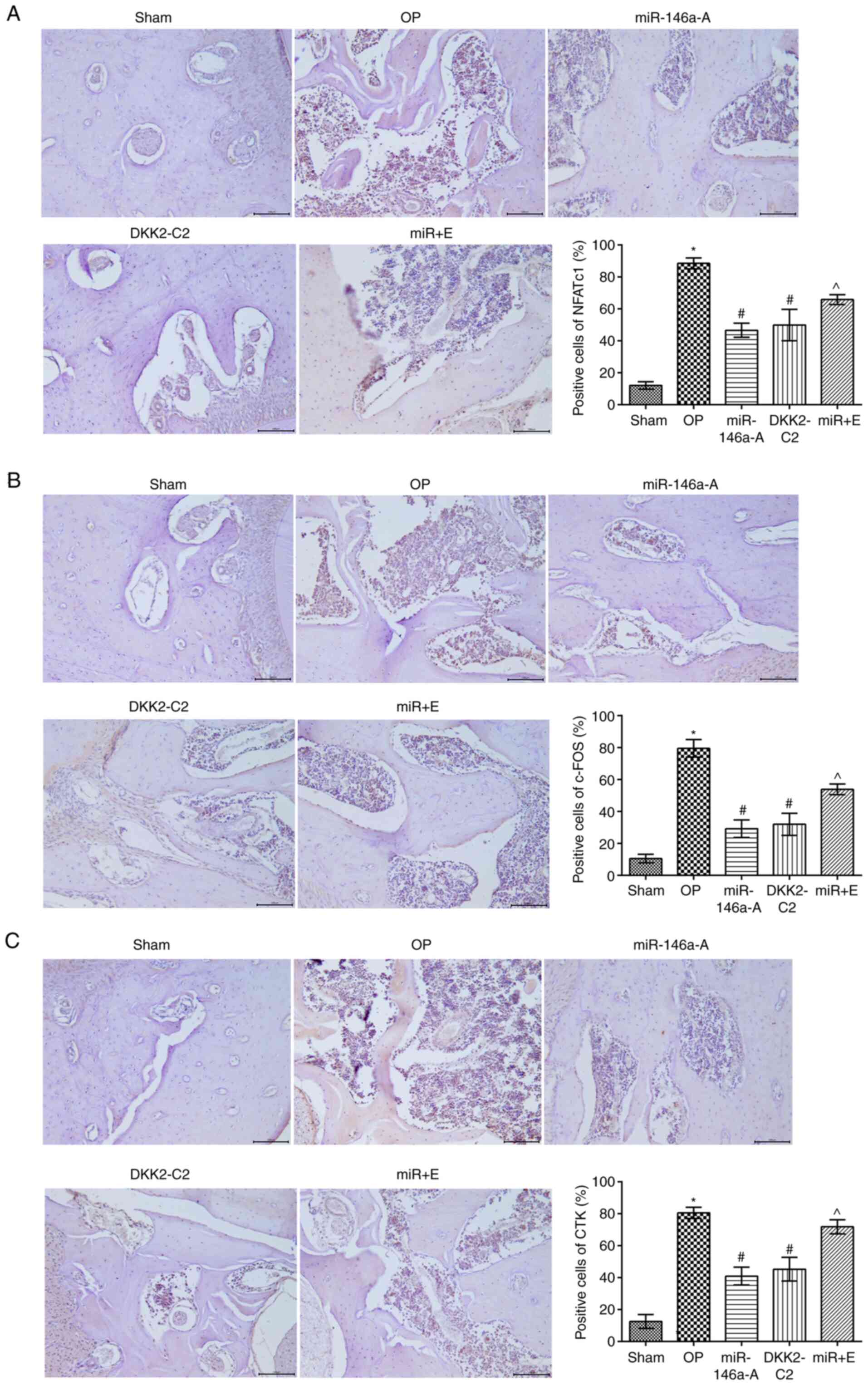 | Figure 6Activation of the Wnt/β-catenin
signaling pathway alters the expression levels of (A) NFATc1, (B)
c-Fos and (C) CTK proteins in the jaws of rats.
*P<0.05, compared with the sham group;
#P<0.05, compared with the OP group;
^P<0.05, compared with the miR-146a group. NFATc1,
nuclear factor of activated T cells c1; CTK, cathepsin K; sham,
sham group; OP, osteoporosis group; miR-146a-A, miR-146a antagonist
group; DKK2-C2, Wnt activator group; miR + E, miR-146a antagonist +
Wnt inhibitor group. |
miR-146a interacts with the Wnt/β-catenin
signaling pathway in the jawbone
The expression levels of OPG, Wnt2, β-catenin, TRAP
and DKK1 in the jawbone were further analyzed in each group by
western blot analysis (Fig. 7).
The expression levels of OPG, Wnt2 and β-catenin were significantly
decreased (P<0.05), while the expression levels of TRAP and DKK1
proteins were significantly increased in the other groups compared
to those of the sham group (P<0.05). The expression levels of
OPG, Wnt2 and β-catenin proteins were notably increased, while the
levels of TRAP and DKK1 proteins were significantly reduced in the
miR-146a-A and DKK2-C2 groups (P<0.05). Furthermore, the
expression levels of OPG, Wnt2 and β-catenin proteins in the miR +
E group were significantly decreased, while those of TRAP and DKK1
proteins were markedly increased in comparison with the miR-146a-A
group (P<0.05). The expression levels of these proteins were
similar between the miR-146a-A and DKK2-C2 groups (P>0.05).
Taken together, these data indicated that the downregulation of
miR-146a inhibited OP in the jaws of OVX rats by regulating the
Wnt/β-catenin signaling pathway.
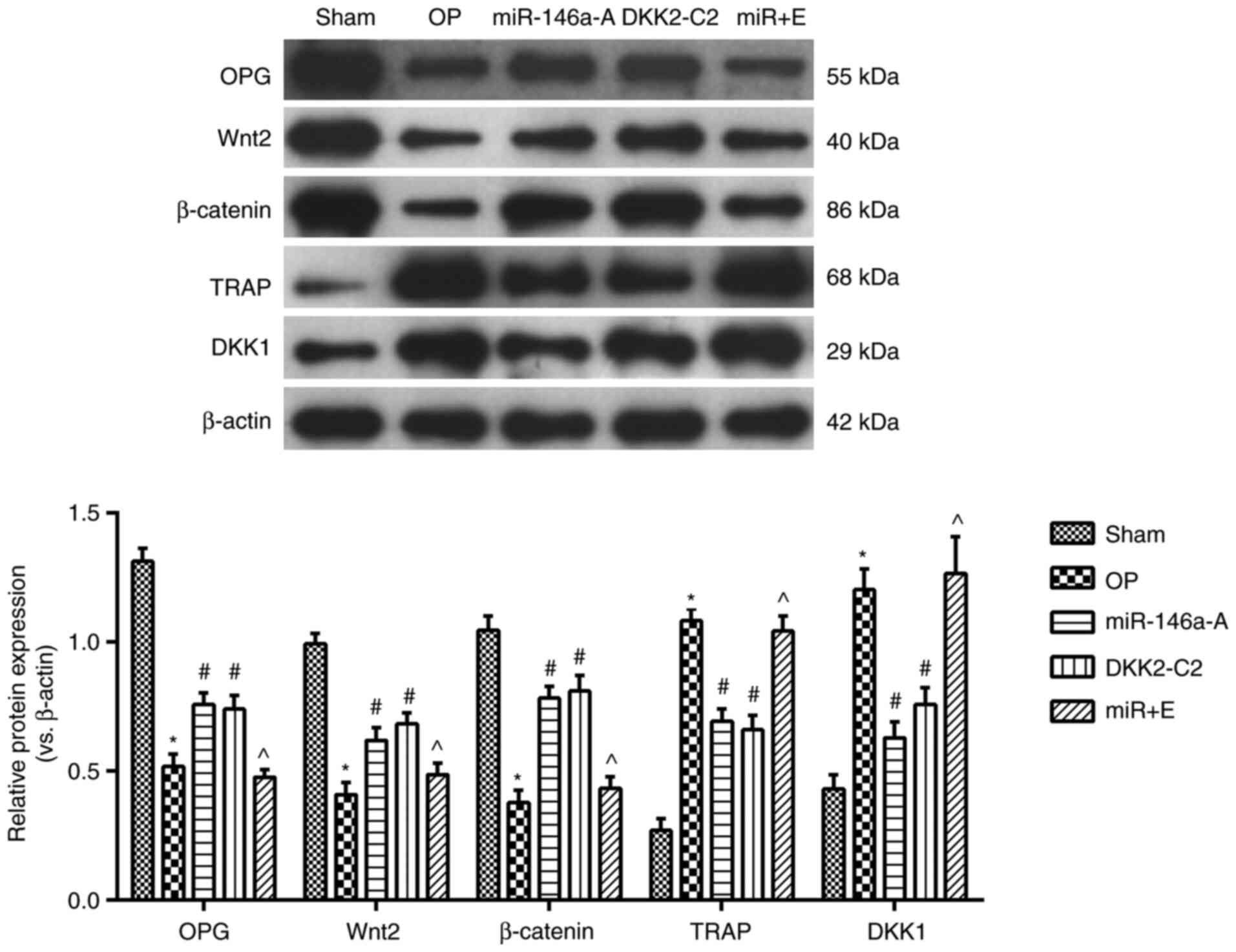 | Figure 7Activation of the Wnt/β-catenin
signaling pathway alters the expression levels of OPG, TRAP, DKK1,
Wnt2 and β-catenin proteins in the jaw. *P<0.05,
compared with the sham group; #P<0.05, compared with
the OP group; ^P<0.05, compared with the miR-146a
group. OPG, osteoprotegerin; TRAP, tartrate-resistant acid
phosphatase; DKK1, dickkopf1; sham, sham group; OP, osteoporosis
group; miR-146a-A, miR-146a antagonist group; DKK2-C2, Wnt
activator group; miR + E, miR-146a antagonist + Wnt inhibitor
group. |
Discussion
OP is a bone disease caused by metabolic
disturbance, which is characterized by a decrease in BMD and
microstructure abnormalities of the bone tissue (20). Several studies have demonstrated
that the impairment in the balance of bone marrow stem cell
differentiation into osteoblasts and osteoclasts is the main cause
of OP (21-23). In the present study, the results
indicated that the downregulation of miR-146a improved BMD and
increased bone formation markers in OVX rats, whereas the results
confirmed that downregulation of miR-146a inhibited OP in OVX rats
by activating the Wnt/β-catenin signaling pathway.
miR-146a is associated with the development of RA by
osteoclasts and synovium fibroblasts (24). Zhao et al (24) demonstrated that miR-146a played a
key role in OP induced by estrogen deficiency. miR-146a knockout
protected bone loss in OVX mice by increasing OPG and decreasing
TRAP levels (24). In the present
study, the results demonstrated that the downregulation of miR-146a
increased OPG and reduced TRAP expression in OVX rats. The data
further confirmed the negative role of miR-146a in OVX rats. In
addition, c-Fos is indispensable for the activation of NFATc1,
which translocates into the nucleus and adjusts the expression
levels of genes associated with osteoclast differentiation and
function, such as TRAP and CTK (25). The present study indicated that
downregulation of miR-146a reduced the expression levels of NFATc1,
c-Fos and CTK.
Furthermore, DKK1 can inhibit the classical Wnt
signaling pathway by binding to the Wnt-receptor LRP5 (26). In the present study, the
downregulation of miR-146a promoted osteogenic differentiation and
inhibited DDK1 expression. The Wnt/β-catenin signaling pathway
plays an important role in OP (16,18). β-catenin accumulates in the
nucleus and binds to enhancer-binding transcription factor-1 in
order to promote the expression of Runx2, which is one of the Wnt
target genes and elicits a series of reactions, such as the
proliferation and differentiation of osteoblasts (27,28). In the present study, the Wnt2
activator and inhibitor were used to confirm that miR-146a
regulates OP in OVX rats via the Wnt/β-catenin pathway. The results
indicated that the effects of the Wnt2 activator were similar to
those noted by the downregulation of miR-146a. This demonstrated
that miR-146a inhibited OP in OVX rats by activating the
Wnt/β-catenin signaling pathway.
In conclusion, the present study investigated the
role of miR-146a on OP in the jaws of OVX rats. The results
indicated that downregulation of miR-146a could inhibit OP in
castrated rats by activating the Wnt/β-catenin signaling pathway.
In addition, the results provided novel insight with regard to the
role of miR-146a in the induction of OP in the jawbone of OVX rats
and can be used further for the development of a potential
therapeutic strategy for OP.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
HL and XY carried out the experimental work and the
data collection and interpretation, participated in the design and
coordination of the experimental work, the acquisition of the data,
and in the preparation of the manuscript. GZ participated in the
data collection, analysis of the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments adhered to the NIH guidelines
(NIH Pub. no. 85-23, revised 1996) and were approved by the Animal
Protection and Use Committee of the 960th Hospital of the PLA Joint
Logistics Support Force.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Martel-Pelletier J, Barr AJ, Cicuttini FM,
Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl
AJ and Pelletier JP: Osteoarthritis. Nat Rev Dis Primers.
2:160722016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Spil WE, Kubassova O, Boesen M,
Bay-Jensen AC and Mobasheri A: Osteoarthritis phenotype novel
therapeutic targets. Biochem Pharmacol. 165:41–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Neill TW, McCabe PS and McBeth J: Update
on the epidemiology, risk factors and disease outcomes of
osteoarthritis. Best Pract Res Clin Rheumatol. 32:312–326. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalaitzoglou E, Griffin TM and Humphrey
MB: Innate immune responses and osteoarthritis. Curr Rheumatol Rep.
19:452017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JR, Jong Y and Hyun K: Therapeutics in
osteoarthritis based on an Understanding of its molecular
pathogenesis. Int J Mol Sci. 19:6742018. View Article : Google Scholar :
|
|
8
|
Herrero-Beaumont G, Pérez-Baos S,
Sánchez-Pernaute O, Roman-Blas JA, Lamuedra A and Largo R:
Targeting chronic innate inflammatory pathways, the main road to
prevention of osteoarthritis progression. Biochem Pharmacol.
165:24–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gómez R, Villalvilla A, Largo R, Gualillo
O and Herrero-Beaumont G: TLR4 signaling in osteoarthritis-finding
targets for candidate DMOADs. Nat Rev Rheumatol. 11:159–170. 2014.
View Article : Google Scholar
|
|
10
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Nowand the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Black DM and Rosen CJ: Clinical practice.
Postmenopausal osteoporosis. N Engl J Med. 374:254–262. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manolagas SC and Jilka RL: Bone marrow,
cytokines, and bone remodeling. Emerging insights into the
pathophysiology of osteoporosis. N Engl J Med. 332:305–311. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobacchi C, Schulz A, Coxon FP, Villa A
and Helfrich MH: Osteopetrosis: Genetics, treatment and new
insights into osteoclast function. Nat Rev Endocrinol. 9:522–536.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Almeida M, Laurent MR, Dubois V, Claessens
F, O'Brien CA, Bouillon R, Vanderschueren D and Manolagas SC:
Estrogens and androgens in skeletal physiology and pathophysiology.
Physiol Rev. 97:135–187. 2017. View Article : Google Scholar :
|
|
15
|
Khosla S, Oursler MJ and Monroe DG:
Estrogen and the skeleton. Trends Endocrinol Metab. 23:576–581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi HK, Kim GJ, Yoo HS, Song DH, Chung
KH, Lee KJ, Koo YT and An JH: Vitamin C activates
osteoblastogenesis and inhibits osteoclastogenesis via
Wnt/β-catenin/ATF4 signaling pathways. Nutrients. 11:5062019.
View Article : Google Scholar
|
|
17
|
Lee KY, Kim JH, Kim EY, Yeom M, Jung HS
and Sohn Y: Water extract of Cnidii Rhizoma suppresses
RANKL-induced osteoclastogenesis in RAW 264.7 cell by inhibiting
NFATc1/c-Fos signaling and prevents ovariectomized bone loss in
SD-rat. BMC Complement Altern Med. 19:2072019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu TJ and Guo JL: Overexpression of
microRNA-141 inhibits osteoporosis in the jawbones of
ovariectomized rats by regulating the Wnt/β-catenin pathway. Arch
Oral Biol. 113:1047132020. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Ji X, Chen X and Yu X: MicroRNAs in
osteoclastogenesis and function: Potential therapeutic targets for
osteoporosis. Int J Mol Sci. 17:3492016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46:D296–D302.
2018. View Article : Google Scholar :
|
|
22
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unifcation of biology. Te Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
David C, Mundo AF, Haw R, Milacic M,
Weiser J, Wu G, Caudy M, Garapati P, Gillespie M, Kamdar MR, et al:
The Reactome pathway knowledgebase. Nucleic Acids Res. 42(Database
issue): D472–D477. 2014. View Article : Google Scholar
|
|
24
|
Zhao J, Huang M and Zhang X, Xu J, Hu G,
Zhao X, Cui P and Zhang X: miR-146a deletion protects from bone
loss in OVX mice by suppressing RANKL/OPG and M-CSF in bone
microenvironment. J Bone Miner Res. 34:2149–2161. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JH, Kim EY, Lee B, Min JH, Song DU,
Lim JM, Eom JW, Yeom M, Jung HS and Sohn Y: The effects of Lycii
Radicis Cortex on RANKL-induced osteoclast differentiation and
activation in RAW 264.7 cells. Int J Mol Med. 37:649–658. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao GQ, Troiano N, Simpson CA and Insogna
KL: Selective deletion of the soluble colony-stimulating factor 1
isoform in vivo prevents estrogen-deficiency bone loss in mice.
Bone Res. 5:170222017. View Article : Google Scholar :
|
|
27
|
Hu L, Su P, Yin C, Zhang Y, Li R, Yan K,
Chen Z, Li D, Zhang G, Wang L, et al: Microtubule actin
crosslinking factor 1 promotes osteoblast differentiation by
promoting β-catenin/TCF1/Runx2 signaling axis. J Cell Physiol.
233:1574–1584. 2018. View Article : Google Scholar
|
|
28
|
Kahler RA and Westendorf JJ: Lymphoid
enhancer factor-1 and beta-catenin inhibit Runx2-dependent
transcriptional activation of the osteocalcin promoter. J Biol
Chem. 278:11937–11944. 2003. View Article : Google Scholar : PubMed/NCBI
|