Introduction
As determined in the mid-eighteenth century,
articular cartilage, once destroyed, cannot be repaired due to the
minimal regeneration capacity of articular cartilage. Small defects
or injuries may cause severe pain and joint disability owing to
secondary osteoarthritis (OA) (1-4).
Numerous surgical techniques have been developed over the past 2
centuries, which may help to partly alleviate symptoms; however,
these cannot regenerate tissue similar to native articular
cartilage (5-7). One strategy is to repair tissue with
properties identical to native cartilage, which integrates with the
tissue to encircle the lesion. An alternative approach would be to
improve the self-regeneration of articular cartilage using cells
and/or other biomaterials. For this strategy, autologous
mesenchymal stromal/stem cells (MSCs) have become an excellent
choice. MSCs are plastic-adherent fibroblast-like cells that can be
isolated from an array of mesenchymal tissues, including bone
marrow, adipose tissues, umbilical cord, placenta and dental pulp
(8-12). They can also differentiate into
adipocytes, osteoblasts, myocytes and chondrocytes both in
vivo and in vitro (13-17).
In recent years, accumulating evidence has suggested
that MSCs can migrate to injured sites and affect the local
microenvironment by secreting different bioactive factors,
including interleukin (IL)-6, IL-10, hepatocyte growth factor,
transforming growth factor-β, matrix metalloproteinases and tissue
inhibitors of metalloproteinases (18-24). Moreover, the paracrine effects of
MSCs are mediated by the secretion of extracellular vesicles (EVs)
(13,25,26). Exosomes are a subtype of EVs,
approximately 30-100 nm in diameter, containing a particular set of
protein families from intracellular compartments (27,28). MSC-derived exosomes have been
investigated in different disease models and have exhibited
therapeutic potential in managing stroke, Parkinson's disease and
OA (29-33). MicroRNAs (miRNAs or miRs),
including miR-222, miR-140 and miR-381, regulate chondrogenesis and
cartilage degeneration (34-36). Previous studies have demonstrated
that miR-140 plays essential roles in regulating cartilage
homeostasis and development by enhancing the expression of Sox9 and
aggrecan (ACAN) (37-39). Therefore, the role of miR-140 in
the repair of cartilage injury in a model of OA is worthy of
investigation. In the present study, miR-140-5p in was
overexpressed in dental pulp stem cells (DPSCs) and an improved
version of exosomes was produced from these cells, which promoted
the recovery of articular cartilage injuries (Fig. S1).
Materials and methods
Human DPSC isolation and culture
Human DPSCs were provided by S-Evans Biosciences.
The Ethics Committee of S-Evans Biosciences approved the collection
of the human samples (no. 2020-01). Briefly, regular human third
molars were extracted from adult donors (age, 18-28 years) during
orthodontic treatment or due to impaction after obtaining written
informed consent. Subsequently, the pulp tissue was lightly
harvested and then digested in a collagenase type I (2 mg/ml) and
dispase (4 mg/ml) solution for 1 h at 37°C. After passing through a
70-µm strainer (Falcon, BD Biosciences), the cell suspension
was seeded into tissue culture plates at a density of
1×105/well with the α modification of Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 15%
fetal bovine serum (FBS, Gibco; Thermo Fisher Scientific, Inc.), 2
mM l-glutamine, 100 units/ml penicillin and 100 µg/ml
streptomycin. The plates were then incubated at 37°C in 5%
CO2 using a humidified incubator and passaged by 0.25%
trypsin-ethylenediaminetetraacetic acid (EDTA, Gibco; Thermo Fisher
Scientific, Inc.) digestion at a 1:5 ratio.
Characterization of DPSCs and flow
cytometric analysis
Osteogenic, adipogenic and chondrogenic inductions
were conducted in vitro, as previously described, to
evaluate the multilineage differentiation capacity of the DPSCs
(40). To evaluate
mineralization, the cultures were stained with Alizarin Red S
according to the manufacturer' instructions (Cyagen Biosciences,
Inc.). Briefly, cells were fixed with 4% paraformaldehyde (Beyotime
Institute of Biotechnology) for 30 min and then stained with
Alizarin Red S for 5 min at room temperature. Lipid droplets formed
following adipogenesis induction were visualized using Oil Red O
staining according to the manufacturer' instructions (Cyagen
Biosciences, Inc.). Briefly, cells were fixed with 4%
paraformaldehyde for 30 min followed by Oil Red O staining for 30
min at room temperature. Successful chondrogenic differentiation
was tested with the use of Alcian blue staining according to the
manufacturer' instructions (Cyagen Biosciences, Inc.). Briefly,
cells were fixed with 4% paraformaldehyde for 30 min followed by
Alcian blue staining for 30 min at room temperature.
The phenotype of the DPSCs was examined at the third
passage by flow cytometry (BD FACSVerse) using antibodies,
including anti-CD14 (BD Biosciences, cat. no. 555397, at a dilution
of 1:5 and incubation at room temperature for 15 min), anti-CD34
(BD Biosciences, cat. no. 555822, at a dilution of 1:5 and
incubation at room temperature for 15 min), anti-CD73 (BioLegend,
cat. no. 344004, at a dilution of 1:20 and incubation at room
temperature for 15 min), anti-CD105 (BioGems, cat. no. 17111-60, at
a dilution of 1:20 and incubation at room temperature for 15 min),
anti-HLA-DR (BD Biosciences, Cat. no. 555811, at a dilution of 1:5
and incubation at room temperature for 15 min) and anti-CD90
(BioLegend, cat. no. 328110, at a dilution of 1:5 and incubation at
room temperature for 15 min) according to the manufacturer'
instructions. The suitable isotype-matched antibody (PE; BD
Biosciences, cat. no. 555749; FITC, BD Biosciences, cat. no.
555573, at dilution of 1:5 and incubation at room temperature for
15 min) was utilized as a negative control. The data were analyzed
using BD FACSuite software (1.0.5.3841).
Isolation and identification of
exosomes
Exosome isolation was performed using
ultracentrifugation as previously described (41). In brief, culture supernatants of
DPSCs were centrifuged at 3,000 × g for 30 min and then at 10,000 ×
g for a further 30 min at room temperature. Finally, exosomes were
pelleted following centrifugation at 64,000 × g for 110 min at 4°C
using an SW28 rotor (Beckman Coulter, Inc.) and washed once with
0.32 M sucrose. Nanosight 2000 analysis and transmission electron
microscopy (FEI) were utilized for the identification of exosomes
that were resuspended in phosphate-buffered saline.
Cell transfection
DPSCs at the third passage were transfected with
miR-140-5p mimic (20 µg/ml) or negative control (20
µg/ml) (Guangzhou RiboBio Co., Ltd.). The Amaxa
Nucleofection system was adopted according to the instructions of
the manufacturer of the Human Mesenchymal Stem Cell Nucleofector TM
kit (Lonza Group, Ltd.). Briefly, DNA and cells were mixed in the
Amaxa cuvette (Lonza Group, Ltd.) and placed in the nucleofector
device. Nucleofection was conducted using the U-23 program (Lonza
Group, Ltd.), which was optimized and performed as recommended by
the manufacturer. Subsequently, the cuvette was rinsed with culture
medium and the cells were transfer into the dish for further
culture.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using the Total Exosome RNA
and Protein Isolation kit (Invitrogen; Thermo Fisher Scientific,
Inc., cat. no. 4478545) according to the manufacturer's protocol,
and the amount of RNA isolated was quantified using a NanoDrop
spectrophotometer (NANO-100; Hangzhou Allsheng Instruments
Co.,Ltd.). RT reactions were performed using 100 ng total RNA with
the TaqMan miRNA RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc., cat. no. 4366596) according to the manufacturer's
instructions. qPCR reactions were conducted on a 7900 System
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using the
Taq-Man miR-140 probe and gene-specific primers (Thermo Fisher
Scientific, Inc., Assay ID 001187). Homo sapiens snRNA U6 qPCR
Primer (Thermo Fisher Scientific, Inc., Assay ID 001973) were used
as an internal control. qPCR was performed under the following
conditions: 10 min at 95°C, followed by 40 cycles of 95°C for 10
sec, 20 sec at 60°C and 10 sec at 72°C.
Analysis of relative gene expression in
cartilage cells
Cartilage cells subjected to IL-1β (10 ng/ml;
R&D Systems, Inc.) and DPSC-derived exosome (5×108
particles/ml) treatment for 48 h were collected and total RNA was
isolated using TRIzol reagent (Invitrogen, cat. no. 15596026) and
reversed transcribed into first-strand cDNA using reverse
transcriptase (Takara Bio, Inc.; cat. no. H2640A). RT-qPCR was
performed using a SYBR-Green Master Mix (Takara Bio, Inc.; cat. no.
RR820A) under the following conditions: 95°C for 4 min, 40 cycles
of 95°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec, followed by
melting curve analysis of 95°C for 30 sec, 72°C for 30 sec, and
95°C for 30 sec. Relative gene expression was calculated using the
2−ΔΔCq (42) method
and the data were normalized against GAPDH. The primers used
are listed in Table I. Each PCR
trial was performed in triplicate and repeated at least 3
times.
 | Table IPrimers used for
reverse-transcription polymerase chain reaction. |
Table I
Primers used for
reverse-transcription polymerase chain reaction.
| Genes | Forward | Reverse |
|---|
| Aggrecan |
TGAGCGGCAGCACTTTGAC |
TGAGTACAGGAGGCTTGAGG |
| Col2a1 |
TCAGGAATTTGGTGTGGACATA |
CCGGACTGTGAGGTTAGGATAG |
| Sox9 |
ATGAAGATGACCGACGAGCA |
CAGTCGTAGCCTTTGAGCAC |
| GAPDH |
GGCACAGTCAAGGCTGAGAATG |
ATGGTGGTGAAGACGCCAGTA |
Animal experiments
Specific pathogen-free (SPF) male Sprague-Dawley
(SD) rats (approximately 8 weeks old, weighing 200±20 g) were
procured from the Shanghai SLAC Laboratory Animal Co., Ltd.
[Certificate no. SCXK (Shanghai) 2017-0005] and maintained in the
SPF laboratory of a local animal facility. The experimental
protocol was approved by the local Medical Animal Experiment Ethics
Committee of Zhejiang Provincial People's Hospital (no.
2019-034).
A total of 24 SD rats were randomly divided into the
blank, OA model, exosome treatment and improved-exosome treatment
groups, with 6 animals per group. Subsequently, 50 µl of 25
mg/ml iodoacetic acid (Sigma-Aldrich; Merck KGaA) was administered
to the double knee joints to induce OA. The same amount of saline
was administered into the knee joint cavities of the rats in the
blank group. After 1 week, the exosome treatment group and
improved-exosome treatment group were injected with 50 µl of
exosomes (5×1010 particles/ml, isolated from the culture
supernatants of DPSCs as described above) and miR-140-enriched
exosomes (5×1010 particles/ml, isolated from the culture
supernatants of miR140-transfacted DPSCs as described above),
respectively, in the knee joint cavity of both hind limbs once a
week; a total of 4 such injections were administered. The rats in
the blank group and OA model group were administered the same
amount of saline. The rats were sacrificed by an overdose of
pentobarbital (150 mg/kg) intraperitoneally at 1 week after the end
of treatment in accordance with a previous study (43).
Gross morphological analysis of the knee
joints
At 1 week after the end of treatment, 3 rats were
randomly selected from each group and anesthetized with 3%
pentobarbital sodium (60 mg/kg) by intraperitoneal injection. An
X-ray examination of the knee joint structure was then performed,
and the grade of knee joint degradation of digital radiography
films (PerkinElmer, Inc., CLS136341/F) was scored using a modified
Kellgren-Lawrence scoring system for rats as previously described
(44,45).
Histopathological examination of the knee
joints
The rats were sacrificed, and their knee joints were
isolated and fixed in 10% neutral-buffered formalin (Sangon
Biotech) for 3 days and then decalcified for 2 weeks in buffered
12.5% EDTA (as previously described with some modifications)
(46,47) and formalin solution. The tissues
were then embedded in paraffin and were sliced along the
longitudinal axis of the lower extremity at a thickness of 5
µm. They were subsequently stained with Safranin O-Fast
Green (Sangon Biotech) and hematoxylin and eosin (H&E; Sangon
Biotech). For Safranin O-Fast Green staining, sections were
deparaffinized and stained with Safranin O for 5 min followed by
Fast Green staining for 5 min at room temperature. For H&E
staining, sections were deparaffinized and stained with hematoxylin
staining solution for 10 min and then counterstained in eosin
staining solution for 1 min at room temperature. The OARSI score
was used to evaluate histological changes as previously described
(48).
Primary isolation and culture of human
chondrocytes
Normal human cartilage tissues were obtained from
donation with written informed consent, and the protocol was
approved by the Ethics Committee of Zhejiang Provincial People's
Hospital in Hangzhou, China (2018 KY 012). Samples were obtained
aseptically and digested using a standard protocol. Briefly, the
tissues were cut into <1 mm3 sections; they were then
transferred to a culture flask, mixed with 5-fold volumes of 0.25%
trypsin, and placed in a 37°C, 5% CO2 incubator for 30
min. The trypsin digestion solution was then discarded and 5-fold
volumes of 0.05% type II collagenase (Sigma-Aldrich; Merck KGaA)
were added to the tissue fragments. Subsequently, cells were
collected every 4 h until the tissue block was digested. These
chondrocytes were then cultured in DMEM supplemented with 20% FBS
in a 37°C, 5% CO2 incubator.
Effects of DPSC-derived exosomes on
IL-1β-treated chondrocytes in vitro
Normal human chondrocytes were treated with IL-1β to
induce the inflammatory, in vitro model of OA as previously
described with some modifications (49). The cells were divided into 4
different groups as follows: i) Normal culture; ii) IL-1β (10
ng/ml, R&D Systems); iii) IL-1β (10 ng/ml) + DPSC-derived
exosomes (5×108 particles/ml); and iv) IL-1β (10 ng/ml)
+ miR-140 enriched exosomes (5×108 particles/ml). Cells
were collected for use in western blot analysis and apoptosis
analysis at different time points.
Western blot analysis
Western blot analysis was performed as previously
described (50). Briefly,
exosomes or chondrocytes were added to cold lysis buffer (Beyotime
Institute of Biotechnology, cat. no. P0013B) and incubated for 30
min on ice. The lysates were then centrifuged at 13,000 × g for 10
min at 4°C. Subsequently, the supernatants were collected and
quantified using the BCA protein assay kit (Beyotime Institute of
Biotechnology, cat. no. P0011), and samples (20 µg) were
separated on 10% polyacrylamide gels and transferred to Hybond-P
membranes. 5% BSA (Beyotime Institute of Biotechnology, cat. no.
ST025) was used for blocking at room temperature for 45 min prior
to incubation with antibodies. The following antibodies were used:
Rabbit anti-human CD63 (ProteinTech Group, Inc., cat. no.
25682-1-AP, at a dilution of 1:500), CD9 (ProteinTech Group, Inc.,
cat. no. 20597-1-AP at a dilution of 1:500) and Bcl-2 (ProteinTech
Group, Inc., cat. no. 12789-1-AP at a dilution of 1:1,000), as well
as rabbit anti-human Bax (Beyotime Institute of Biotechnology, cat.
no. AF0057, at a dilution of 1:500) and Bad (Beyotime Institute of
Biotechnology, cat. no. AF1009, at a dilution of 1:500). The
primary antibodies were incubated at room temperature for 45 min.
GAPDH (Beyotime Institute of Biotechnology, cat. no. AF1186, at a
dilution of 1:2,000) served as the loading control. The blots were
then incubated with HRP-conjugated goat anti-rabbit secondary
antibody (Beyotime Institute of Biotechnology, cat. no. A0208, at a
dilution of 1:1,000) for 45 min at room temperature and then
processed for analysis using an ECL chemiluminescence kit (Beyotime
Institute of Biotechnology). ImageJ (National Institutes of Health,
v1.8.0) was further employed to analyze the grayscale values
obtained by western blotting.
Apoptosis analysis
Apoptosis was determined using the Annexin
V-propidium iodide (PI) binding assay as previously reported
(51). The cells were harvested
and labeled with Annexin V-FITC and PI for 15 min using a FITC
Annexin V apoptosis detection kit I (BD Pharmingen). Apoptosis was
evaluated using flow cytometry (Navios, Beckman Coulter, Inc.)
within 30 min of staining, and the data were analyzed using FlowJo
software v11.0. The extent of apoptosis was quantified as the
percentage of Annexin V+ cells. All experiments were
performed in triplicate.
Statistical analysis
Data are presented as the means ± standard
deviations of the results of at least 3 independent studies.
Student's t-tests were utilized to identify differences among
groups, as suitable. One-way ANOVA with the Bonferroni test was
performed for multiple group comparisons. A P-value <0.05 was
considered to indicate a statistical significant difference.
Results
Isolation and characterization of
DPSCs
DPSCs were isolated from the pulp tissue of
extracted human third molar teeth without caries, periodontal
diseases, or infections. These cells are closely associated with
MSCs found in the stromal compartment of various tissues, including
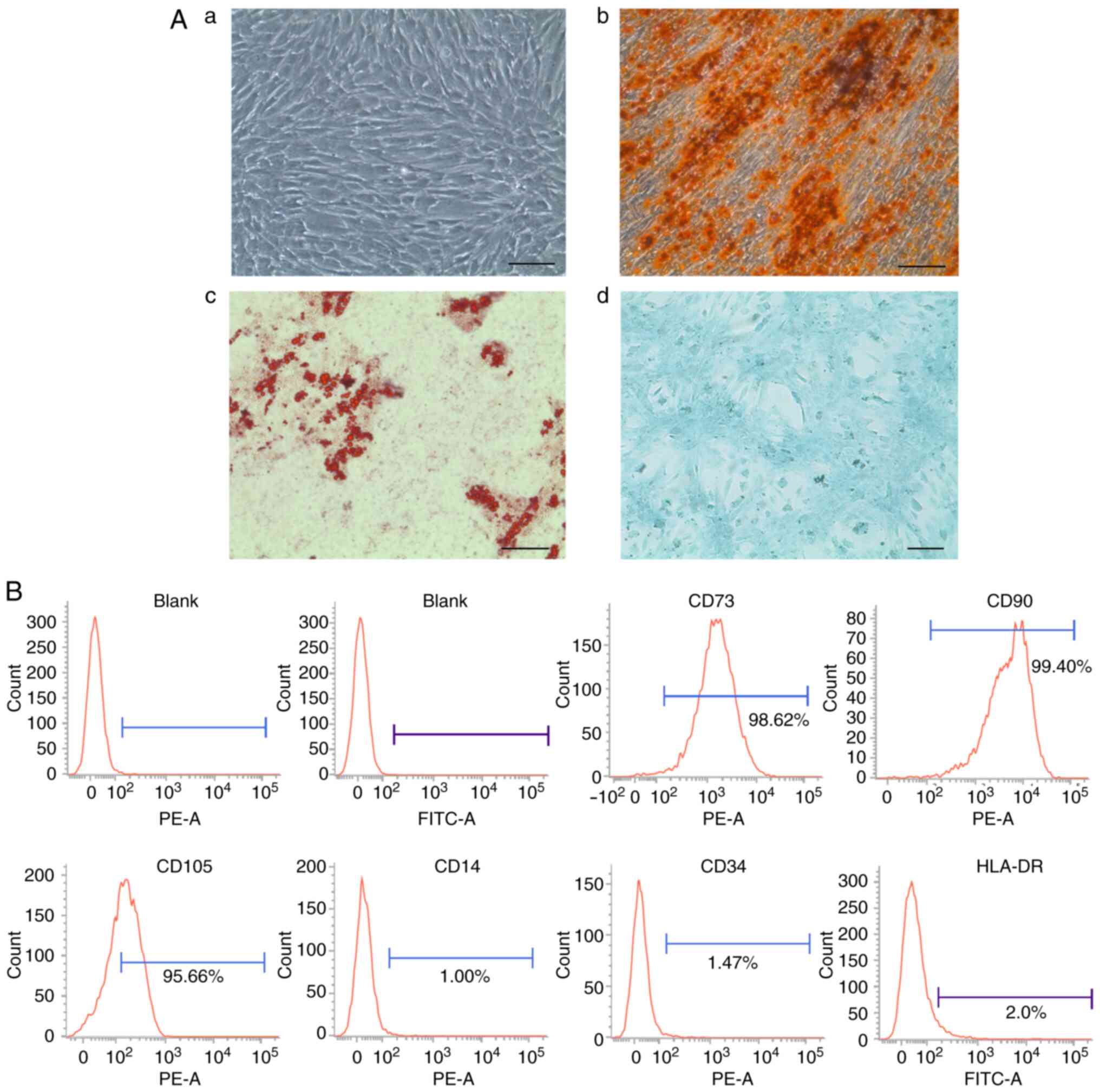
the bone marrow. As shown in Fig.
1A, the cells exhibited a typical spindle-shaped morphology.
DPSCs followed the criteria that defined multipotent MSCs, as
recommended by the International Society for Cellular Therapy
guidelines in 2006 (52).
Following culture in a specific differentiation medium for 2-3
weeks, the isolated DPSCs successfully differentiated into
mesenchymal derivatives, including osteoblasts (identified by
Alizarin Red labeling), adipocytes (identified by Oil Red O
labeling) and chondrocytes (identified by Alcian blue) (Fig. 1A). Furthermore, as revealed by
flow cytometric assay, these cells were positively stained for the
common MSC-associated markers, CD73, CD90 and CD105, and did not
exhibit an expression of the hematopoietic markers, CD14, CD34 and
HLA-DR (Fig. 1B).
Isolation and identification of exosomes
from dental pulp stem cell lines
To examine the effects of miR-140-5p-transfected
DPSCs on articular cartilage injuries, miR-140-5p mimic or negative
control oligonucleotide were transfected into third-passage DPSCs
via electroporation. The cells were collected 48 h later for total
RNA extraction. RT-qPCR revealed that the cells transfected with
the mimic exhibited a 10-fold increase in the miR-140-5p level
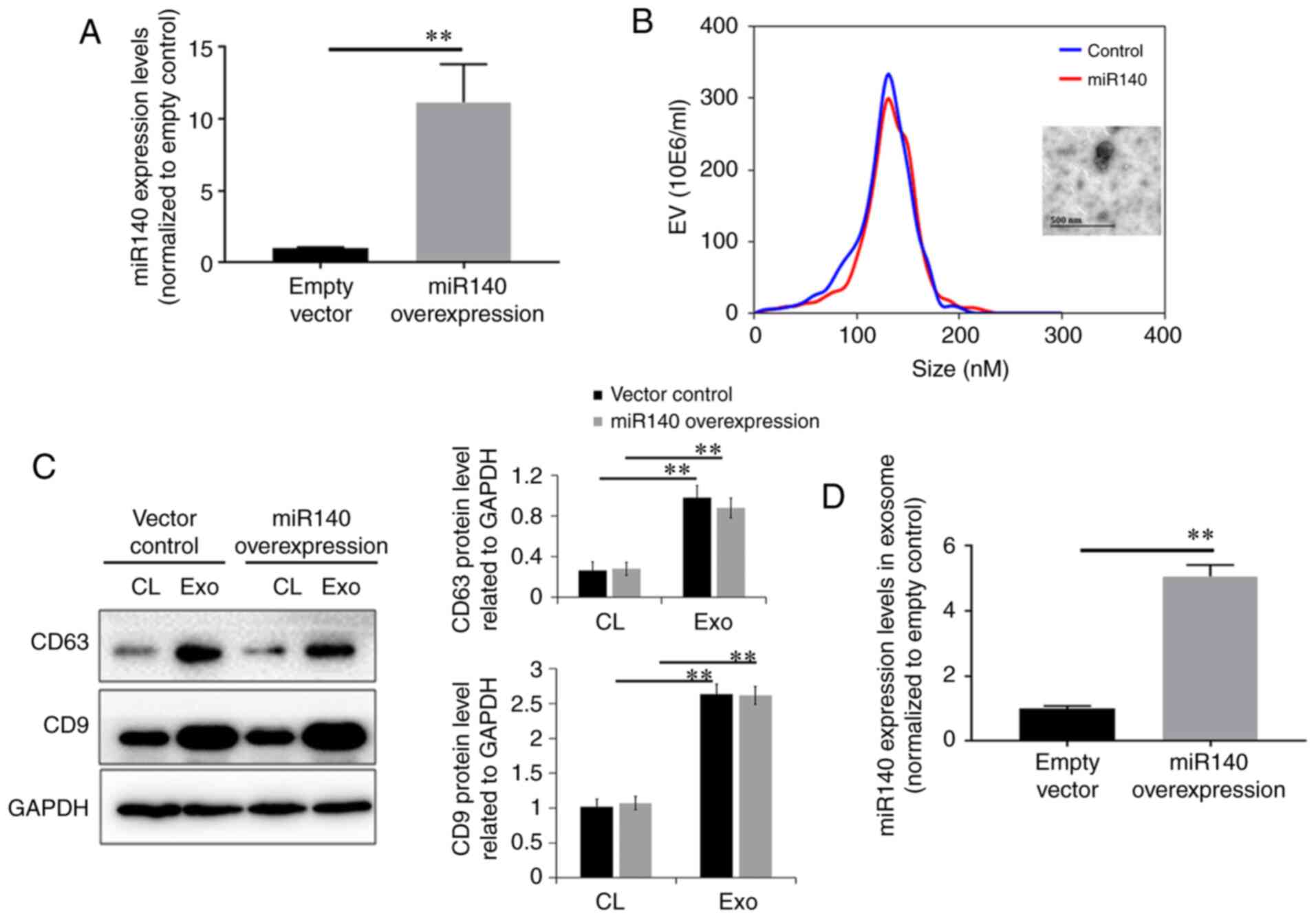
compared with the negative control cells (Fig. 2A). Exosomes were collected from
both types of DPSCs by centrifugation. Nanosight analysis indicated
that the diameter of the majority of the DPSC-derived exosomes was
approximately 134±29 nm (Fig.
2B). As shown in Fig. 2C,
western blot analysis revealed that the DPSC-derived exosomes
expressed exosomal markers, including CD9 and CD63. No significant
differences in the expression levels of CD9 and CD63 between
exosomes from the DPSCs with and without miR-140-5p transfection
were observed (P>0.05). Finally, the results of RT-qPCR revealed
a 4-fold increase in the miR-140-5p levels in exosomes derived from
the miR-140-5p-overexpressing DPSCs than those from the control
exosomes.
Effect of DPSC-derived exosomes on mRNA
levels in chondrocytes
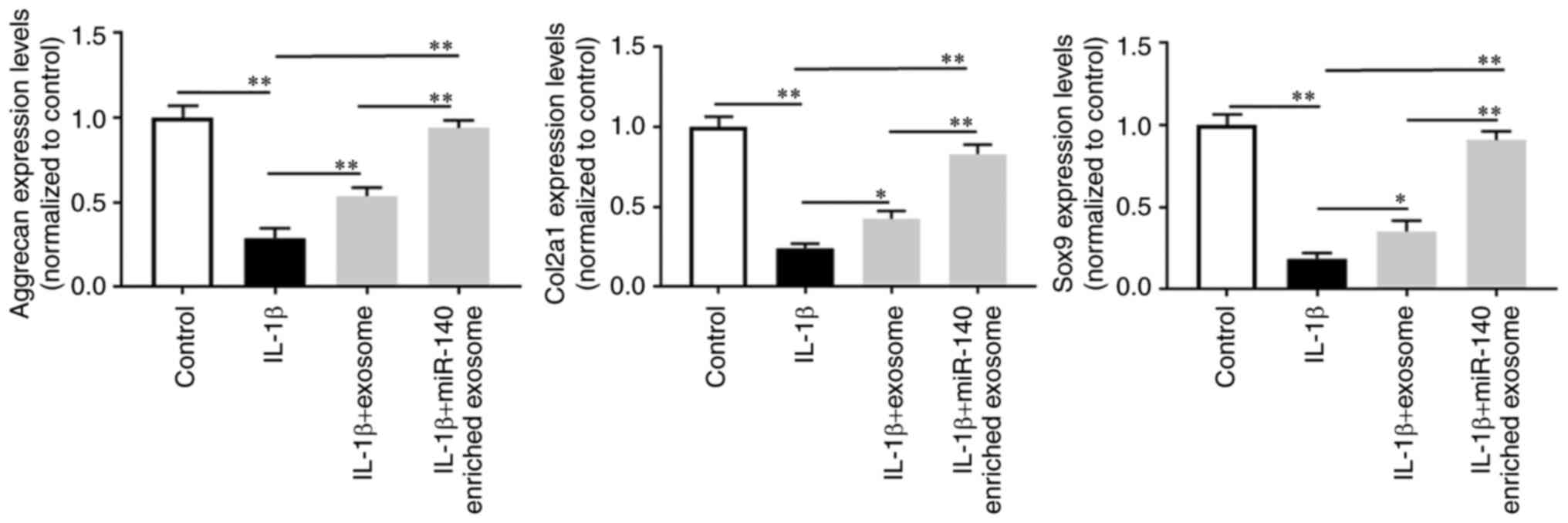
Subsequently, normal human chondrocytes were treated
with IL-1β (10 ng/ml) with or without DPSC-derived exosomes. As
shown in Fig. 3, IL-1β
stimulation substantially decreased the chondrocyte ACAN, Col2α1
and Sox9 mRNA levels. However, the use of DPSC-derived exosomes
elevated the corresponding mRNA levels, particularly the
miR-140-5p-enriched exosomes.
DPSC-derived exosomes inhibit the
IL-1β-induced apoptosis of chondrocytes
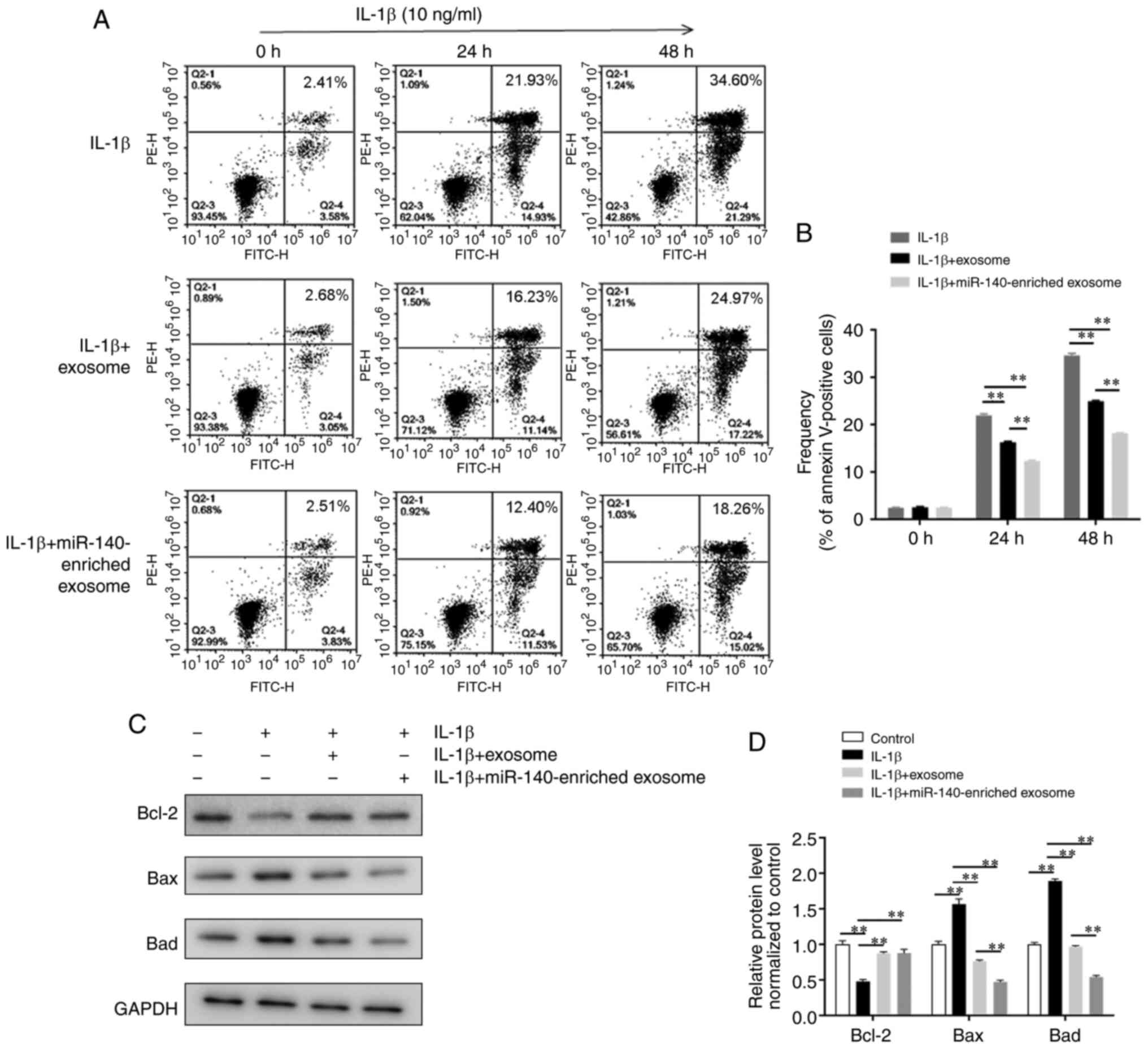
Chondrocytes from normal humans were treated with
IL-1β (10 ng/ml) with or without DPSC-derived exosomes. The cells
were trypsinized for apoptosis analysis at various time points. As
shown in Fig. 4A and B, when
exposed to IL-1β for 48 h, the DPSC-derived exosome groups
(DPSC-derived exosomes and miR-140-5p-enriched exosomes) exhibited
a decreased apoptosis compared with the control group (16.29±0.1%
and 12.32±0.2% vs. 21.91±0.3% Annexin V+ cells at 24 h;
24.91±0.1% and 18.14± 0.1% vs. 34.58±0.5% Annexin V+
cells at 48 h; all P<0.01). The miR-140-5p-enriched exosome
group exhibited a more significant reduction in cell apoptosis than
the DPSC-derived exosome group (P<0.01) (Fig. 4A and B). Likewise, western blot
analysis indicated a significant inhibition of the expression of
apoptosis-related proteins in chondrocytes treated with
miR-140-5p-enriched exosomes (Fig. 4C
and D).
Influence of exosome treatments on knee
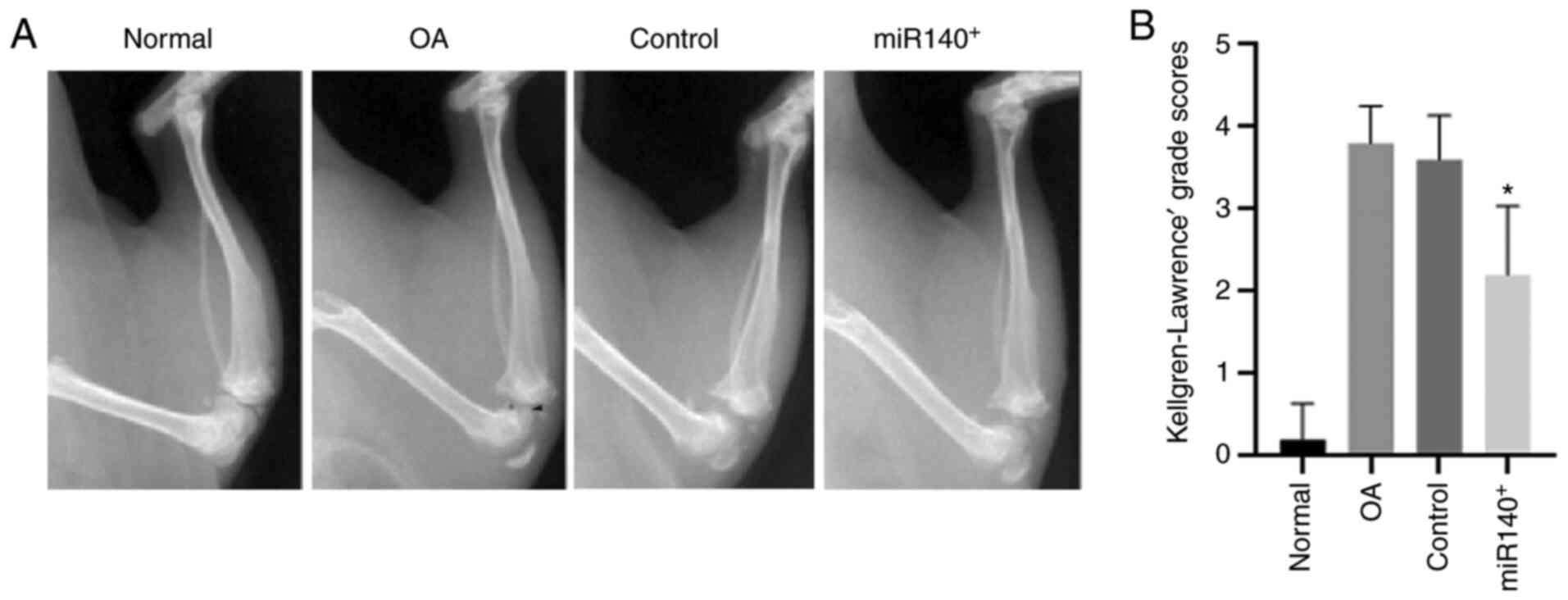
joint X-ray scores in a rat model of OA
Subsequently, the rat model of OA was treated with
the 2 above-mentioned types of DPSC-derived exosomes and then
assessed using X-rays to determine the effects of the treatments
(Fig. 5). In comparison with the
sham-operated group, the OA model group exhibited evident stenosis,
with visible osteophytes and osteosclerosis. This was in line with
previous findings (53,54), indicating the generation of the
rat model of OA was successfully established. Both DPSC-derived
exosomes reduced the grade and severity of knee joint damage in
rats with OA, as determined by X-ray score, improved the joint
cavity structure, and reduced the formation of osteophytes,
articular surface irregularity and osteosclerosis. Moreover, these
effects were substantially enhanced following miR-140-5p-enriched
exosome treatment.
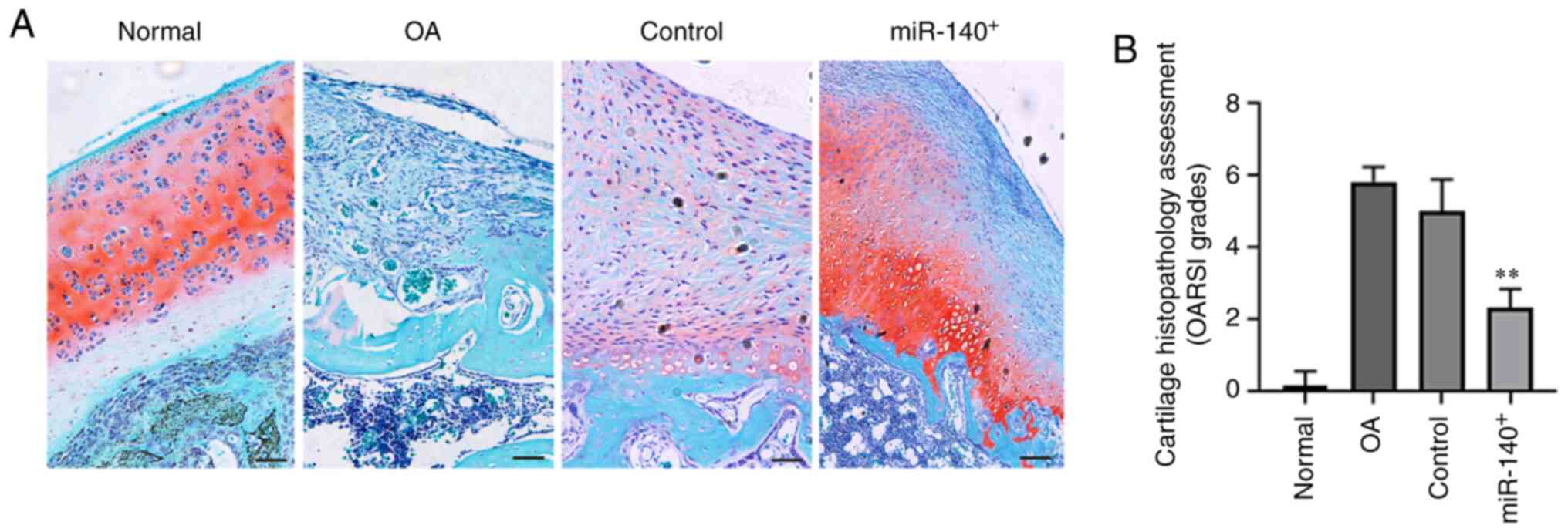
Effect of exosome treatments on the
pathology of the knee joint in rats with OA
To further assess the effects of exosome treatment
on the knee condition in rats with OA, the histological sections of
rat cartilage in all 4 groups were examined. As observed following
Safranin O-Fast Green and H&E staining (Figs. 6A and S2A), the knee joint cartilage was
uneven in the rat model OA group, and the thickness of the
cartilage was reduced with visible apoptotic bodies. Furthermore,
the lower edge of the cartilage was uneven, and pathological
changes, including inflammatory cell infiltration, could be
observed. The multiple administration of DPSC-derived exosomes
improved the pathology of the rat knee joints to varying degrees,
and the miR-140-5p-enriched exosomes substantially reduced the
total pathological OARSI grading score (Figs. 6B and S2B).
Discussion
OA is a chronic degenerative process that is a
common cause of disability in middle-aged and older adults. Its
common clinical phenotypes include progressive cartilage
deterioration, subchondral bone remodeling, loss of joint space,
marginal osteophytosis, and loss of joint function, and it affects
>80% of individuals aged of ≥55 years (55). The majority of patients with OA
are treated with a combination of non-pharmacological (such as
physiotherapy) and pharmacological treatments to reduce pain and
inflammation. Although some patients exhibit temporary relief, the
efficacy of these interventions is not consistent, and studies
assessing the efficacy of these interventions are still being
conducted. In recent years, it has been proposed that MSCs improve
chondrocyte regeneration and maintain articular cartilage in
patients with OA (56).
MSCs are multipotent stromal cells that can
differentiate into various cell types, including osteoblasts,
adipocytes and chondrocytes (13-17). Apart from their ability to
differentiate, the immune regulating function of MSC has received
more attention in the past decades. Indeed, MSCs are used to treat
various clinical conditions, particularly autoimmune diseases,
including Crohn's disease, multiple sclerosis, lupus, COPD,
Parkinson's and OA (32,57-60). However, these cells do not
directly cure these conditions; the efficacy of MSC-based
therapeutic strategies is ascribed more to paracrine secretion
(61). MSCs influence the local
microenvironment by secreting different bioactive factors. EVs are
critical mediators of cell-to-cell communication in these
situations. MSC-derived exosomes play a crucial role in regulating
cell migration, proliferation, differentiation, and matrix
synthesis. Moreover, MSC-derived exosomes have been shown to
shuttle many bioactive components (proteins, lipids, mRNAs, miRNAs,
lncRNAs, circRNAs and DNA) (31,62). Therefore, MSC exosomes may
represent a new therapeutic alternative for OA. DPSCs are type of
MSCs derived from dental pulp, which have become one of the best
cells for periodontal tissue engineering owing to their excellent
growth and differentiation capacity in ex vivo cultures
(63,64). Reportedly, cultured DPSCs have a
pale, round, or oval central nucleus with multiple nucleoli,
showing active DNA transcription, and RNA synthesis. Numerous
cytoplasmic vacuoles also show exuberant cellular secretory
vesicles (65).
Consistent with previously published data, the
current study proposed that miR-140 plays a role in chondrogenesis.
miR-140 has been reported to play essential roles in regulating
cartilage homeostasis and development by enhancing the expression
of SOX9 and ACAN (37-39). In addition, miR-140-5p can
regulate the proliferation and differentiation of DPSC and inhibit
the differentiation of DPSCs into odontoblastic cells (66,67). In the present study, miR-140-5p
was first overexpressed in DPSCs and the increased burden of
miR-140-5p in exosomes isolated from DPSC culture supernatant was
validated. The data indicated that DPSC-derived exosomes promoted
the expression of chondrocyte-related mRNAs, including AGAN, Col2α1
and Sox9, in cultured normal human chondrocytes. Sox9 is the master
regulator of a transcription factor for chondrogenesis. In
particular, it has been shown to activate Col11A1, Col2A1 and ACAN,
all of which play pivotal functions during chondrogenesis. Col11A1
has been proven to be essential in cartilage architecture during
chondrogenesis by modulating chondrocyte behavior (68). ACAN can improve chondrogenesis by
delaying hypertrophic chondrocyte maturation (69). Additionally, the effect of
DPSC-derived exosomes on the expression of chondrocyte-related
mRNAs was substantially enhanced by miR-140-5p enriched exosomes.
The findings from Annexin V-PI flow cytometry and western blot
analysis suggested that the miR-140-5p-enriched exosomes
significantly inhibited the IL-1β-induced apoptosis of primary
cultured human chondrocytes. Furthermore, the effects of
DPSC-derived exosomes were explored in a rat model of OA, and the
results were consistent with the in vitro findings.
In conclusion, the findings presented herein
demonstrate that the overexpression of exosomal-miR-140-5p
substantially attenuates OA both in vitro and in
vivo. Although previous stem cell-related studies (70,71) have also indicated a protective
effect of stem cell-derived exosomes in models of OA, the present
study determined that exosomes from miR140-overexpressing DPSCs
relieved symptoms of OA by inhibiting the apoptosis of chondrocytes
and promoting cartilage repair. Hence, the present study provides a
more efficient, safer and stem cell-independent strategy for the
treatment of OA. To the best of our knowledge, this is the first
study to adopt genetically modified DPSCs to produce
miRNA-140-enriched exosomes for the treatment of OA. Nonetheless,
further studies are required to clarify the specific function of
exosomal-miR-140-5p in models of OA prior to the clinical
application of exosome-based therapy in OA.
Supplementary Data
Funding
The present study was supported by the Social
Development Project of Public Welfare Technology Application in
Zhejiang Province (grant no. LGF18H060007), and the Key
Technologies R&D Program of Zhejiang Province (grant no.
2019C03041).
Availability of data and materials
All data generated or analyzed in the current study
are included in this published article or relevant supplementary
material, or are available from the corresponding author upon
reasonable request.
Authors' contributions
TL participated in the study design. NW performed
the experiments. LW and RZ analyzed the data. YFC contributed to
the study design and data acquisition, and provided valuable advice
and drafted the manuscript. RP supervised the study, performed the
experiments and revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of S-Evans Biosciences approved
the collection of human samples (no. 2020-01). The Ethics Committee
of Zhejiang Provincial People's Hospital approved the experiments
using human cartilage (2018 KY 012). All patients provided written
informed consent. The experimental protocol was approved by the
local Medical Animal Experiment Ethics Committee of Zhejiang
Provincial People's Hospital (no. 2019-034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests. The company 'S-Evans Biosciences', with which RP is
affiliated, had no role in the study design or outcomes.
Acknowledgments
Not applicable.
References
|
1
|
Bhosale AM and Richardson JB: Articular
cartilage: Structure, injuries and review of management. Br Med
Bull. 87:77–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA, Anderson DD, Brown TD,
Tochigi Y and Martin JA: The roles of mechanical stresses in the
pathogenesis of osteoarthritis: Implications for treatment of joint
injuries. Cartilage. 4:286–294. 2013. View Article : Google Scholar
|
|
3
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Osch GJ, Brittberg M, Dennis JE,
Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT and Luyten FP:
Cartilage repair: Past and future-lessons for regenerative
medicine. J Cell Mol Med. 13:792–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sgaglione NA, Miniaci A, Gillogly SD and
Carter TR: Update on advanced surgical techniques in the treatment
of traumatic focal articular cartilage lesions in the knee.
Arthroscopy. 18(Suppl 1): S9–S32. 2002. View Article : Google Scholar
|
|
6
|
Temenoff JS and Mikos AG: Review: Tissue
engineering for regeneration of articular cartilage. Biomaterials.
21:431–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simon TM and Jackson DW: Articular
cartilage: Injury pathways and treatment options. Sports Med
Arthrosc Rev. 26:31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park D, Spencer JA, Koh BI, Kobayashi T,
Fujisaki J, Clemens TL, Lin CP, Kronenberg HM and Scadden DT:
Endogenous bone marrow MSCs are dynamic, fate-restricted
participants in bone maintenance and regeneration. Cell Stem Cell.
10:259–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar
|
|
12
|
Valtieri M and Sorrentino A: The
mesenchymal stromal cell contribution to homeostasis. J Cell
Physiol. 217:296–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romanov YA, Darevskaya AN, Merzlikina NV
and Buravkova LB: Mesenchymal stem cells from human bone marrow and
adipose tissue: Isolation, characterization, and differentiation
potentialities. Bull Exp Biol Med. 140:138–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timper K, Seboek D, Eberhardt M, Linscheid
P, Christ-Crain M, Keller U, Müller B and Zulewski H: Human adipose
tissue-derived mesenchymal stem cells differentiate into insulin,
somatostatin, and glucagon expressing cells. Biochem Biophys Res
Commun. 341:1135–1140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sonomoto K, Yamaoka K, Oshita K, Fukuyo S,
Zhang X, Nakano K, Okada Y and Tanaka Y: Interleukin-1β induces
differentiation of human mesenchymal stem cells into osteoblasts
via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2
pathway. Arthritis Rheum. 64:3355–3363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZJ, Zhuge Y and Velazquez OC:
Trafficking and differentiation of mesenchymal stem cells. J Cell
Biochem. 106:984–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu DA, Han J and Kim BS: Stimulation of
chondrogenic differentiation of mesenchymal stem cells. Int J Stem
Cells. 5:16–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caplan AI: MSCs: The sentinel and
safe-guards of injury. J Cell Physiol. 231:1413–1416. 2016.
View Article : Google Scholar
|
|
19
|
Anton K, Banerjee D and Glod J:
Macrophage-associated mesenchymal stem cells assume an activated,
migratory, pro-inflammatory phenotype with increased IL-6 and
CXCL10 secretion. PLoS One. 7:e350362012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Gh, Liu Y, Lu Y, Liu J, Wu C and Duan
HF: IL-6 secreted from senescent mesenchymal stem cells promotes
proliferation and migration of breast cancer cells. PLoS One.
9:e1135722014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu X, Liu X, Cheng K, Yang R and Zhao RC:
Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10
secretion. Exp Hematol. 40:761–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noh MY, Lim SM, Oh KW, Cho KA, Park J, Kim
KS, Lee SJ, Kwon MS and Kim SH: Mesenchymal stem cells modulate the
functional properties of microglia via TGF-beta secretion. Stem
Cells Transl Med. 5:1538–1549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mias C, Lairez O, Trouche E, Roncalli J,
Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N,
Salvayre AN, et al: Mesenchymal stem cells promote matrix
metalloproteinase secretion by cardiac fibroblasts and reduce
cardiac ventricular fibrosis after myocardial infarction. Stem
Cells. 27:2734–2743. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lozito TP and Tuan RS: Mesenchymal stem
cells inhibit both endogenous and exogenous MMPs via secreted
TIMPs. J Cell Physiol. 226:385–396. 2011. View Article : Google Scholar
|
|
25
|
Rani S, Ryan AE, Griffin MD and Ritter T:
Mesenchymal stem cell-derived extracellular vesicles: Toward
cell-free therapeutic applications. Mol Ther. 23:812–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo Sicco C, Reverberi D, Balbi C, Ulivi V,
Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin
C, et al: Mesenchymal stem cell-derived extracellular vesicles as
mediators of anti-inflammatory effects: Endorsement of macrophage
polarization. Stem Cells Transl Med. 6:1018–1028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bang OY and Kim EH: Mesenchymal stem
cell-derived extracellular vesicle therapy for stroke: Challenges
and progress. Front Neurol. 10:2112019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZG, Buller B and Chopp M:
Exosomes-beyond stem cells for restorative therapy in stroke and
neurological injury. Nat Rev Neurol. 15:193–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu B, Zhang X and Li X: Exosomes derived
from mesenchymal stem cells. Int J Mol Sci. 15:4142–4157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noël D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7:162142017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Lin L, Zou R, Wen C, Wang Z and Lin
F: MSC-derived exosomes promote proliferation and inhibit apoptosis
of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in
osteoarthritis. Cell Cycle. 17:2411–2422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong S, Yang B, Guo H and Kang F:
MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys
Res Commun. 418:587–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang J, Liu H and Zhou Y: Roles of micro
RNA s in prenatal chondrogenesis, postnatal chondrogenesis and
cartilage-related diseases. J Cell Mol Med. 17:1515–1524. 2013.
View Article : Google Scholar
|
|
36
|
Liu H, Sun Q, Wan C, Li L, Zhang L and
Chen Z: MicroRNA-338-3p regulates osteogenic differentiation of
mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2.
J Cell Physiol. 229:1494–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buechli ME, Lamarre J and Koch TG:
MicroRNA-140 expression during chondrogenic differentiation of
equine cord blood-derived mesenchymal stromal cells. Stem Cells
Dev. 22:1288–1296. 2013. View Article : Google Scholar
|
|
39
|
Karlsen TA, Jakobsen RB, Mikkelsen TS and
Brinchmann JE: microRNA-140 targets RALA and regulates chondrogenic
differentiation of human mesenchymal stem cells by translational
enhancement of SOX9 and ACAN. Stem Cells Dev. 23:290–304. 2014.
View Article : Google Scholar
|
|
40
|
Hilkens P, Gervois P, Fanton Y,
Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I and
Bronckaers A: Effect of isolation methodology on stem cell
properties and multilineage differentiation potential of human
dental pulp stem cells. Cell Tissue Res. 353:65–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greening DW, Xu R, Ji H, Tauro BJ and
Simpson RJ: A protocol for exosome isolation and characterization:
Evaluation of ultracentrifugation, density-gradient separation, and
immunoaffinity capture methods. Methods Mol Biol. 1295:179–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Yan L, Zhou L, Xie D, Du W, Chen F, Yuan
Q, Tong P, Shan L and Efferth T: Chondroprotective effects of
platelet lysate towards monoiodoacetate-induced arthritis by
suppression of TNF-α-induced activation of NF-KB pathway in
chondrocytes. Aging (Albany NY). 11:2797–2811. 2019. View Article : Google Scholar
|
|
44
|
Jay GD, Elsaid KA, Kelly KA, Anderson SC,
Zhang L, Teeple E, Waller K and Fleming BC: Prevention of cartilage
degeneration and gait asymmetry by lubricin tribosupplementation in
the rat following anterior cruciate ligament transection. Arthritis
Rheum. 64:1162–1171. 2012. View Article : Google Scholar
|
|
45
|
Teeple E, Elsaid KA, Jay GD, Zhang L,
Badger GJ, Akelman M, Bliss TF and Fleming BC: Effects of
supplemental intra-articular lubricin and hyaluronic acid on the
progression of posttraumatic arthritis in the anterior cruciate
ligament-deficient rat knee. Am J Sports Med. 39:164–172. 2011.
View Article : Google Scholar
|
|
46
|
Gao X, Jiang S, Du Z, Ke A, Liang Q and Li
X: KLF2 protects against osteoarthritis by repressing oxidative
response through activation of Nrf2/ARE signaling in vitro and in
vivo. Oxid Med Cell Longev. 2019:85646812019. View Article : Google Scholar :
|
|
47
|
Inomata K, Tsuji K, Onuma H, Hoshino T,
Udo M, Akiyama M, Nakagawa Y, Katagiri H, Miyatake K, Sekiya I, et
al: Time course analyses of structural changes in the infrapatellar
fat pad and synovial membrane during inflammation-induced
persistent pain development in rat knee joint. BMC Musculoskelet
Disord. 20:82019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar
|
|
49
|
Castro Martins M, Peffers MJ, Lee K and
Rubio-Martinez LM: Effects of stanozolol on normal and
IL-1β-stimulated equine chondrocytes in vitro. BMC Vet Res.
14:1032018. View Article : Google Scholar
|
|
50
|
Fang QX, Zheng XC and Zhao HJ: L1CAM is
involved in lymph node metastasis via ERK1/2 signaling in
colorectal cancer. Am J Transl Res. 12:837–846. 2020.PubMed/NCBI
|
|
51
|
Crowley LC, Marfell BJ, Scott AP and
Waterhouse NJ: Quantitation of apoptosis and necrosis by Annexin V
binding, propidium iodide uptake, and flow cytometry. Cold Spring
Harb Protoc. 2016:2016.
|
|
52
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kohn MD, Sassoon AA and Fernando ND:
Classifications in brief: Kellgren-lawrence classification of
osteoarthritis. Clin Orthop Relat Res. 474:1886–1893. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qi Y, Feng G and Yan W: Mesenchymal stem
cell-based treatment for cartilage defects in osteoarthritis. Mol
Biol Rep. 39:5683–5689. 2012. View Article : Google Scholar
|
|
57
|
Voswinkel J, Francois S, Simon JM,
Benderitter M, Gorin NC, Mohty M, Fouillard L and Chapel A: Use of
mesenchymal stem cells (MSC) in chronic inflammatory fistulizing
and fibrotic diseases: A comprehensive review. Clin Rev Allergy
Immunol. 45:180–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bai L, Lennon DP, Caplan AI, DeChant A,
Hecker J, Kranso J, Zaremba A and Miller RH: Hepatocyte growth
factor mediates mesenchymal stem cell-induced recovery in multiple
sclerosis models. Nat Neurosci. 15:862–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu S, Liu D, Chen C, Hamamura K,
Moshaverinia A, Yang R, Liu Y, Jin Y and Shi S: MSC transplantation
improves osteopenia via epigenetic regulation of notch signaling in
lupus. Cell Metab. 22:606–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gu W, Song L, Li XM, Wang D, Guo XJ and Xu
WG: Mesenchymal stem cells alleviate airway inflammation and
emphysema in COPD through down-regulation of cyclooxygenase-2 via
p38 and ERK MAPK pathways. Sci Rep. 5:1–11. 2015.
|
|
61
|
Lee JW, Fang X, Krasnodembskaya A, Howard
JP and Matthay MA: Concise review: Mesenchymal stem cells for acute
lung injury: Role of paracrine soluble factors. Stem Cells.
29:913–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Deng H, Sun C, Sun Y, Li H, Yang L, Wu D,
Gao Q and Jiang X: Lipid, protein, and microRNA composition within
mesenchymal stem cell-derived exosomes. Cell Reprogram. 20:178–186.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ashri NY, Ajlan SA and Aldahmash AM:
Dental pulp stem cells: Biology and use for periodontal tissue
engineering. Saudi Med J. 36:1391–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Graziano A, d'Aquino R, Laino G and
Papaccio G: Dental pulp stem cells: A promising tool for bone
regeneration. Stem Cell Rev. 4:21–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Merckx G, Hosseinkhani B, Kuypers S,
Vanspringel L, Irobi J, Michiels L, Lambrichts I and Bronckaers A:
Extracellular vesicles from human dental pulp stem cells as
proangiogenic strategy in tooth regeneration. J Extracellular
Vesicles. 7:134. 2018.
|
|
66
|
Lu X, Chen X, Xing J, Lian M, Huang D, Lu
Y, Feng G and Feng X: miR-140-5p regulates the odontoblastic
differentiation of dental pulp stem cells via the Wnt1/β-catenin
signaling pathway. Stem Cell Res Ther. 10:2262019. View Article : Google Scholar
|
|
67
|
Sun DG, Xin BC, Wu D, Zhou L, Wu HB, Gong
W and Lv J: miR-140-5p-mediated regulation of the proliferation and
differentiation of human dental pulp stem cells occurs through the
lipopolysaccharide/toll-like receptor 4 signaling pathway. Eur J
Oral Sci. 125:419–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li A, Wei Y, Hung C and Vunjak-Novakovic
G: Chondrogenic properties of collagen type XI, a component of
cartilage extracellular matrix. Biomaterials. 173:47–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Caron MMJ, Janssen MPF, Peeters L,
Haudenschild DR, Cremers A, Surtel DAM, van Rhijn LW, Emans PJ and
Welting TJM: Aggrecan and COMP improve periosteal chondrogenesis by
delaying chondrocyte hypertrophic maturation. Front Bioeng
Biotechnol. 8:10362020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran
M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H and Hamblin
MR: Mesenchymal stem cell-derived exosomes: A new therapeutic
approach to osteoarthritis? Stem Cell Res Ther. 10:3402019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhu Y and Wang Y, Zhao B, Niu X, Hu B, Li
Q, Zhang J, Ding J, Chen Y and Wang Y: Comparison of exosomes
secreted by induced pluripotent stem cell-derived mesenchymal stem
cells and synovial membrane-derived mesenchymal stem cells for the
treatment of osteoarthritis. Stem Cell Res Ther. 8:642017.
View Article : Google Scholar : PubMed/NCBI
|