|
1
|
Bhosale AM and Richardson JB: Articular
cartilage: Structure, injuries and review of management. Br Med
Bull. 87:77–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buckwalter JA, Anderson DD, Brown TD,
Tochigi Y and Martin JA: The roles of mechanical stresses in the
pathogenesis of osteoarthritis: Implications for treatment of joint
injuries. Cartilage. 4:286–294. 2013. View Article : Google Scholar
|
|
3
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Osch GJ, Brittberg M, Dennis JE,
Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT and Luyten FP:
Cartilage repair: Past and future-lessons for regenerative
medicine. J Cell Mol Med. 13:792–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sgaglione NA, Miniaci A, Gillogly SD and
Carter TR: Update on advanced surgical techniques in the treatment
of traumatic focal articular cartilage lesions in the knee.
Arthroscopy. 18(Suppl 1): S9–S32. 2002. View Article : Google Scholar
|
|
6
|
Temenoff JS and Mikos AG: Review: Tissue
engineering for regeneration of articular cartilage. Biomaterials.
21:431–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
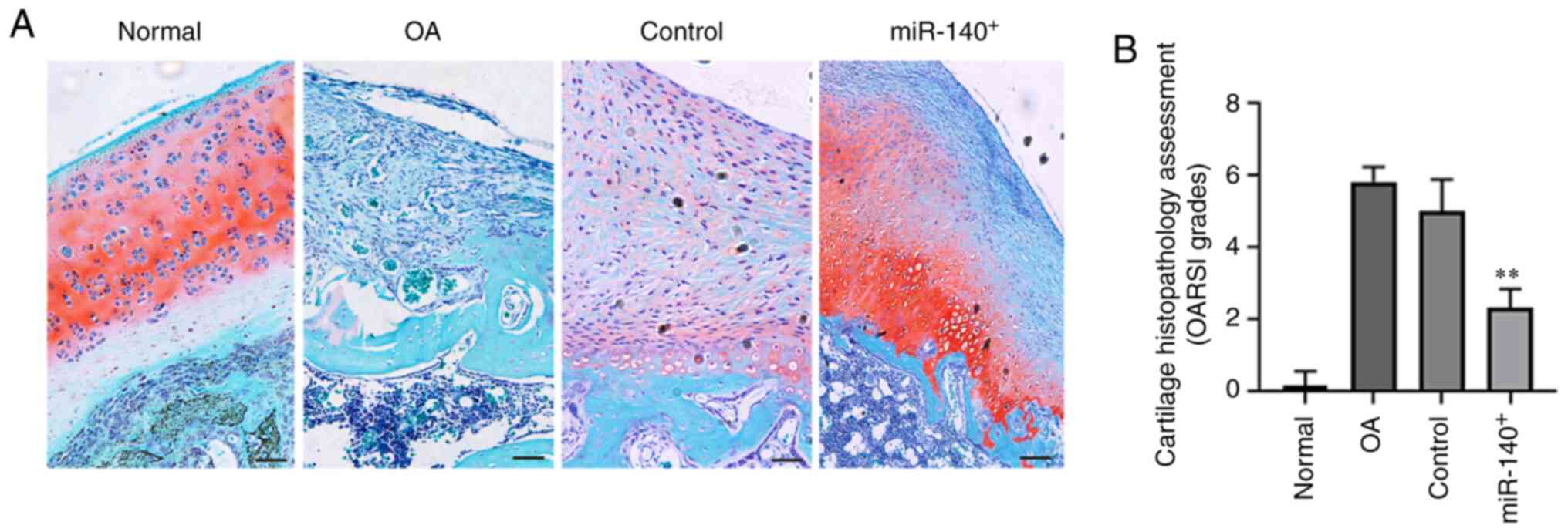
Simon TM and Jackson DW: Articular
cartilage: Injury pathways and treatment options. Sports Med
Arthrosc Rev. 26:31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
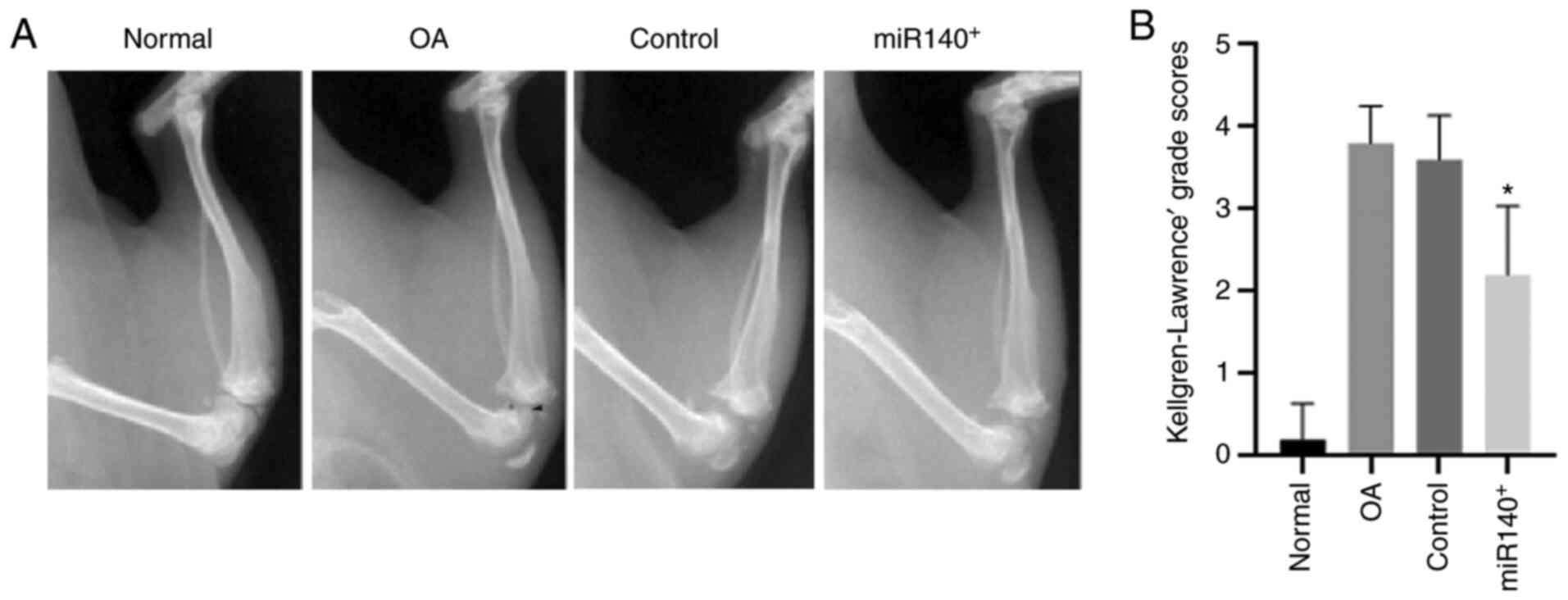
8
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park D, Spencer JA, Koh BI, Kobayashi T,
Fujisaki J, Clemens TL, Lin CP, Kronenberg HM and Scadden DT:
Endogenous bone marrow MSCs are dynamic, fate-restricted
participants in bone maintenance and regeneration. Cell Stem Cell.
10:259–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q,
Jiang R, Yan Y, Mao F, Yang H, et al: Human mesenchymal stem cells
isolated from the umbilical cord. Cell Biol Int. 32:8–15. 2008.
View Article : Google Scholar
|
|
12
|
Valtieri M and Sorrentino A: The
mesenchymal stromal cell contribution to homeostasis. J Cell
Physiol. 217:296–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romanov YA, Darevskaya AN, Merzlikina NV
and Buravkova LB: Mesenchymal stem cells from human bone marrow and
adipose tissue: Isolation, characterization, and differentiation
potentialities. Bull Exp Biol Med. 140:138–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Timper K, Seboek D, Eberhardt M, Linscheid
P, Christ-Crain M, Keller U, Müller B and Zulewski H: Human adipose
tissue-derived mesenchymal stem cells differentiate into insulin,
somatostatin, and glucagon expressing cells. Biochem Biophys Res
Commun. 341:1135–1140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sonomoto K, Yamaoka K, Oshita K, Fukuyo S,
Zhang X, Nakano K, Okada Y and Tanaka Y: Interleukin-1β induces
differentiation of human mesenchymal stem cells into osteoblasts
via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2
pathway. Arthritis Rheum. 64:3355–3363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu ZJ, Zhuge Y and Velazquez OC:
Trafficking and differentiation of mesenchymal stem cells. J Cell
Biochem. 106:984–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu DA, Han J and Kim BS: Stimulation of
chondrogenic differentiation of mesenchymal stem cells. Int J Stem
Cells. 5:16–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caplan AI: MSCs: The sentinel and
safe-guards of injury. J Cell Physiol. 231:1413–1416. 2016.
View Article : Google Scholar
|
|
19
|
Anton K, Banerjee D and Glod J:
Macrophage-associated mesenchymal stem cells assume an activated,
migratory, pro-inflammatory phenotype with increased IL-6 and
CXCL10 secretion. PLoS One. 7:e350362012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Gh, Liu Y, Lu Y, Liu J, Wu C and Duan
HF: IL-6 secreted from senescent mesenchymal stem cells promotes
proliferation and migration of breast cancer cells. PLoS One.
9:e1135722014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu X, Liu X, Cheng K, Yang R and Zhao RC:
Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10
secretion. Exp Hematol. 40:761–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noh MY, Lim SM, Oh KW, Cho KA, Park J, Kim
KS, Lee SJ, Kwon MS and Kim SH: Mesenchymal stem cells modulate the
functional properties of microglia via TGF-beta secretion. Stem
Cells Transl Med. 5:1538–1549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mias C, Lairez O, Trouche E, Roncalli J,
Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N,
Salvayre AN, et al: Mesenchymal stem cells promote matrix
metalloproteinase secretion by cardiac fibroblasts and reduce
cardiac ventricular fibrosis after myocardial infarction. Stem
Cells. 27:2734–2743. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lozito TP and Tuan RS: Mesenchymal stem
cells inhibit both endogenous and exogenous MMPs via secreted
TIMPs. J Cell Physiol. 226:385–396. 2011. View Article : Google Scholar
|
|
25
|
Rani S, Ryan AE, Griffin MD and Ritter T:
Mesenchymal stem cell-derived extracellular vesicles: Toward
cell-free therapeutic applications. Mol Ther. 23:812–823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lo Sicco C, Reverberi D, Balbi C, Ulivi V,
Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin
C, et al: Mesenchymal stem cell-derived extracellular vesicles as
mediators of anti-inflammatory effects: Endorsement of macrophage
polarization. Stem Cells Transl Med. 6:1018–1028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bang OY and Kim EH: Mesenchymal stem
cell-derived extracellular vesicle therapy for stroke: Challenges
and progress. Front Neurol. 10:2112019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZG, Buller B and Chopp M:
Exosomes-beyond stem cells for restorative therapy in stroke and
neurological injury. Nat Rev Neurol. 15:193–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu B, Zhang X and Li X: Exosomes derived
from mesenchymal stem cells. Int J Mol Sci. 15:4142–4157. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noël D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7:162142017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Lin L, Zou R, Wen C, Wang Z and Lin
F: MSC-derived exosomes promote proliferation and inhibit apoptosis
of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in
osteoarthritis. Cell Cycle. 17:2411–2422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong S, Yang B, Guo H and Kang F:
MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys
Res Commun. 418:587–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang J, Liu H and Zhou Y: Roles of micro
RNA s in prenatal chondrogenesis, postnatal chondrogenesis and
cartilage-related diseases. J Cell Mol Med. 17:1515–1524. 2013.
View Article : Google Scholar
|
|
36
|
Liu H, Sun Q, Wan C, Li L, Zhang L and
Chen Z: MicroRNA-338-3p regulates osteogenic differentiation of
mouse bone marrow stromal stem cells by targeting Runx2 and Fgfr2.
J Cell Physiol. 229:1494–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Buechli ME, Lamarre J and Koch TG:
MicroRNA-140 expression during chondrogenic differentiation of
equine cord blood-derived mesenchymal stromal cells. Stem Cells
Dev. 22:1288–1296. 2013. View Article : Google Scholar
|
|
39
|
Karlsen TA, Jakobsen RB, Mikkelsen TS and
Brinchmann JE: microRNA-140 targets RALA and regulates chondrogenic
differentiation of human mesenchymal stem cells by translational
enhancement of SOX9 and ACAN. Stem Cells Dev. 23:290–304. 2014.
View Article : Google Scholar
|
|
40
|
Hilkens P, Gervois P, Fanton Y,
Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I and
Bronckaers A: Effect of isolation methodology on stem cell
properties and multilineage differentiation potential of human
dental pulp stem cells. Cell Tissue Res. 353:65–78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greening DW, Xu R, Ji H, Tauro BJ and
Simpson RJ: A protocol for exosome isolation and characterization:
Evaluation of ultracentrifugation, density-gradient separation, and
immunoaffinity capture methods. Methods Mol Biol. 1295:179–209.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
43
|
Yan L, Zhou L, Xie D, Du W, Chen F, Yuan
Q, Tong P, Shan L and Efferth T: Chondroprotective effects of
platelet lysate towards monoiodoacetate-induced arthritis by
suppression of TNF-α-induced activation of NF-KB pathway in
chondrocytes. Aging (Albany NY). 11:2797–2811. 2019. View Article : Google Scholar
|
|
44
|
Jay GD, Elsaid KA, Kelly KA, Anderson SC,
Zhang L, Teeple E, Waller K and Fleming BC: Prevention of cartilage
degeneration and gait asymmetry by lubricin tribosupplementation in
the rat following anterior cruciate ligament transection. Arthritis
Rheum. 64:1162–1171. 2012. View Article : Google Scholar
|
|
45
|
Teeple E, Elsaid KA, Jay GD, Zhang L,
Badger GJ, Akelman M, Bliss TF and Fleming BC: Effects of
supplemental intra-articular lubricin and hyaluronic acid on the
progression of posttraumatic arthritis in the anterior cruciate
ligament-deficient rat knee. Am J Sports Med. 39:164–172. 2011.
View Article : Google Scholar
|
|
46
|
Gao X, Jiang S, Du Z, Ke A, Liang Q and Li
X: KLF2 protects against osteoarthritis by repressing oxidative
response through activation of Nrf2/ARE signaling in vitro and in
vivo. Oxid Med Cell Longev. 2019:85646812019. View Article : Google Scholar :
|
|
47
|
Inomata K, Tsuji K, Onuma H, Hoshino T,
Udo M, Akiyama M, Nakagawa Y, Katagiri H, Miyatake K, Sekiya I, et
al: Time course analyses of structural changes in the infrapatellar
fat pad and synovial membrane during inflammation-induced
persistent pain development in rat knee joint. BMC Musculoskelet
Disord. 20:82019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar
|
|
49
|
Castro Martins M, Peffers MJ, Lee K and
Rubio-Martinez LM: Effects of stanozolol on normal and
IL-1β-stimulated equine chondrocytes in vitro. BMC Vet Res.
14:1032018. View Article : Google Scholar
|
|
50
|
Fang QX, Zheng XC and Zhao HJ: L1CAM is
involved in lymph node metastasis via ERK1/2 signaling in
colorectal cancer. Am J Transl Res. 12:837–846. 2020.PubMed/NCBI
|
|
51
|
Crowley LC, Marfell BJ, Scott AP and
Waterhouse NJ: Quantitation of apoptosis and necrosis by Annexin V
binding, propidium iodide uptake, and flow cytometry. Cold Spring
Harb Protoc. 2016:2016.
|
|
52
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kohn MD, Sassoon AA and Fernando ND:
Classifications in brief: Kellgren-lawrence classification of
osteoarthritis. Clin Orthop Relat Res. 474:1886–1893. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Qi Y, Feng G and Yan W: Mesenchymal stem
cell-based treatment for cartilage defects in osteoarthritis. Mol
Biol Rep. 39:5683–5689. 2012. View Article : Google Scholar
|
|
57
|
Voswinkel J, Francois S, Simon JM,
Benderitter M, Gorin NC, Mohty M, Fouillard L and Chapel A: Use of
mesenchymal stem cells (MSC) in chronic inflammatory fistulizing
and fibrotic diseases: A comprehensive review. Clin Rev Allergy
Immunol. 45:180–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bai L, Lennon DP, Caplan AI, DeChant A,
Hecker J, Kranso J, Zaremba A and Miller RH: Hepatocyte growth
factor mediates mesenchymal stem cell-induced recovery in multiple
sclerosis models. Nat Neurosci. 15:862–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu S, Liu D, Chen C, Hamamura K,
Moshaverinia A, Yang R, Liu Y, Jin Y and Shi S: MSC transplantation
improves osteopenia via epigenetic regulation of notch signaling in
lupus. Cell Metab. 22:606–618. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gu W, Song L, Li XM, Wang D, Guo XJ and Xu
WG: Mesenchymal stem cells alleviate airway inflammation and
emphysema in COPD through down-regulation of cyclooxygenase-2 via
p38 and ERK MAPK pathways. Sci Rep. 5:1–11. 2015.
|
|
61
|
Lee JW, Fang X, Krasnodembskaya A, Howard
JP and Matthay MA: Concise review: Mesenchymal stem cells for acute
lung injury: Role of paracrine soluble factors. Stem Cells.
29:913–919. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Deng H, Sun C, Sun Y, Li H, Yang L, Wu D,
Gao Q and Jiang X: Lipid, protein, and microRNA composition within
mesenchymal stem cell-derived exosomes. Cell Reprogram. 20:178–186.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ashri NY, Ajlan SA and Aldahmash AM:
Dental pulp stem cells: Biology and use for periodontal tissue
engineering. Saudi Med J. 36:1391–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Graziano A, d'Aquino R, Laino G and
Papaccio G: Dental pulp stem cells: A promising tool for bone
regeneration. Stem Cell Rev. 4:21–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Merckx G, Hosseinkhani B, Kuypers S,
Vanspringel L, Irobi J, Michiels L, Lambrichts I and Bronckaers A:
Extracellular vesicles from human dental pulp stem cells as
proangiogenic strategy in tooth regeneration. J Extracellular
Vesicles. 7:134. 2018.
|
|
66
|
Lu X, Chen X, Xing J, Lian M, Huang D, Lu
Y, Feng G and Feng X: miR-140-5p regulates the odontoblastic
differentiation of dental pulp stem cells via the Wnt1/β-catenin
signaling pathway. Stem Cell Res Ther. 10:2262019. View Article : Google Scholar
|
|
67
|
Sun DG, Xin BC, Wu D, Zhou L, Wu HB, Gong
W and Lv J: miR-140-5p-mediated regulation of the proliferation and
differentiation of human dental pulp stem cells occurs through the
lipopolysaccharide/toll-like receptor 4 signaling pathway. Eur J
Oral Sci. 125:419–425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li A, Wei Y, Hung C and Vunjak-Novakovic
G: Chondrogenic properties of collagen type XI, a component of
cartilage extracellular matrix. Biomaterials. 173:47–57. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Caron MMJ, Janssen MPF, Peeters L,
Haudenschild DR, Cremers A, Surtel DAM, van Rhijn LW, Emans PJ and
Welting TJM: Aggrecan and COMP improve periosteal chondrogenesis by
delaying chondrocyte hypertrophic maturation. Front Bioeng
Biotechnol. 8:10362020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran
M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H and Hamblin
MR: Mesenchymal stem cell-derived exosomes: A new therapeutic
approach to osteoarthritis? Stem Cell Res Ther. 10:3402019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhu Y and Wang Y, Zhao B, Niu X, Hu B, Li
Q, Zhang J, Ding J, Chen Y and Wang Y: Comparison of exosomes
secreted by induced pluripotent stem cell-derived mesenchymal stem
cells and synovial membrane-derived mesenchymal stem cells for the
treatment of osteoarthritis. Stem Cell Res Ther. 8:642017.
View Article : Google Scholar : PubMed/NCBI
|