1. Introduction
Cancer is the second leading cause of mortality
worldwide and results in an increasing number of deaths annually.
The World Health Organization postulates a 60% increase in cancer
cases over the next 20 years globally (1). The medical treatment of the majority
of cancers almost always involves several traditional approaches,
such as surgery, chemotherapy and radiotherapy. Surgical resection
is a suitable approach for tumor management in the early stages of
primary tumors. However, surgery is still limited by post-operative
recurrence and metastasis (2,3).
Of late, chemoradiotherapy, molecular targeted therapy and immune
checkpoint inhibitors have been considered the treatment approach
for advanced stages of cancers; however, severe adverse events
limit their use (4,5). Therefore, alternative therapeutic
methods are required to address these existing shortcomings.
Accordingly, traditional Chinese medicines (TCMs), such as ginseng,
Radix Astragali (RA), Scutellaria barbata, Curcumae
and turmeric, are used to enhance the efficacy and reduce the
side-effects of chemoradiotherapy. TCMs are effective in
suppressing tumor progression, relieving surgery-associated
discomfort, improving immune function and preventing complications
caused by the use of other treatment modalities (6).
RA is a dietary complement widely used in TCM and is
known to modulate the immune system and attenuate the adverse
effects of cytotoxic agents (7).
Saponins are the primary constituents that are responsible for the
suppression of tumor growth, which exert their effects via
intrinsic and extrinsic apoptotic pathways, modulating
intracellular signaling pathways, and inhibiting metastasis and
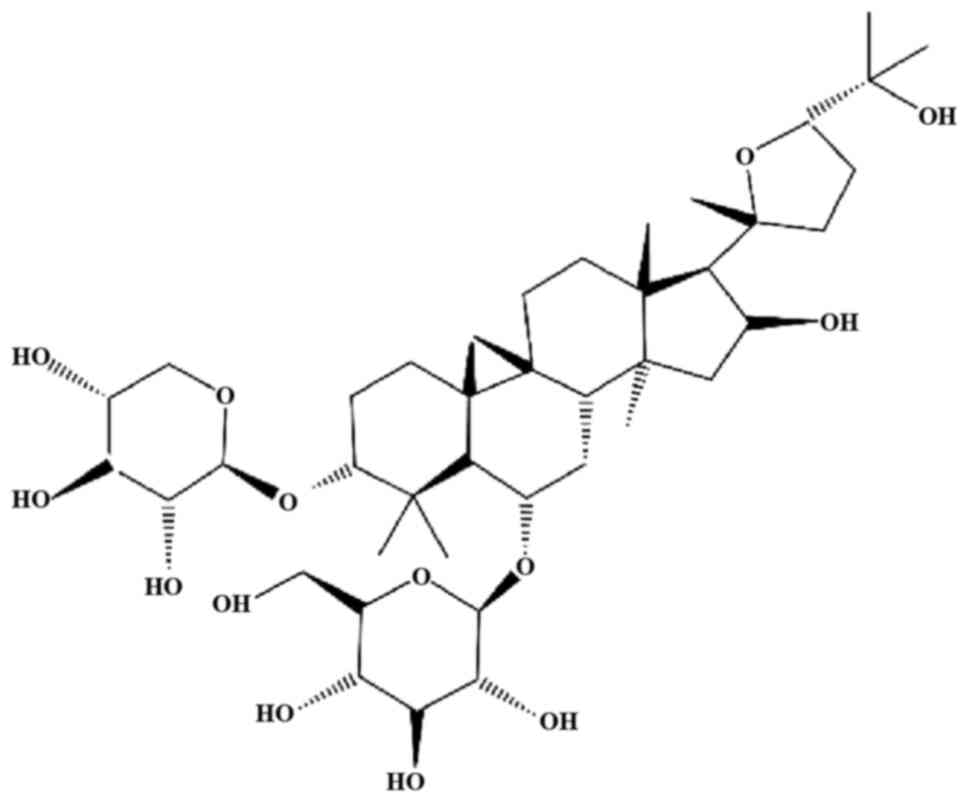
angiogenesis. Astragaloside IV (AS-IV; chemical structure presented
in Fig. 1) and astragaloside II
are the 2 main components of RA (8).
AS-IV, chemically known as
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol
(C14H68O14), is a lanolin-alcohol
type of tetracyclic triterpenoid saponin. It is included in the
Chinese and European Pharmacopoeia as a quality-control indicator
of RA. It has long been used since ancient times in China without
any evident hepatotoxic and nephrotoxic effects. Moreover, no
side-effects have been reported in rats following 14 weeks of the
continuous oral administration of AS-IV (10 mg/kg/day) (9,10).
However, there is currently no data available regarding the safety
of AS-IV in humans, at least to the best of our knowledge. The
methods used to extract AS-IV include ultrafiltration, high-speed
centrifugation, ultrasonic extraction and alcohol precipitation.
The present review article aimed to obtain and collate data from
studies conducted over the past 20 years on the effects of AS-IV on
tumors. In addition, the mechanisms of action of AS-IV on malignant
cells both in vivo and in vitro are summarized in
order to provide insight into the effects of AS-IV on cancer in
humans.
2. Literature search
Search strategy
Studies in English and Chinese, as well as trials
published before June 1, 2020, were searched on online databases.
The databases in the English language that were used were PubMed,
MEDLINE, Embase, ScienceDirect, Web of Science, BIOSIS Previews and
the Cochrane Library and Cochrane Central Register of Controlled
Trials (CENTRAL). The Chinese databases used for the searches
included the China National Knowledge Infrastructure (CNKI)
database and Wanfang Med Online.
In the present review, 'astragaloside IV', 'Cancer'
and 'mechanism' were used as the key search concepts. Additionally,
their synonyms were also included. Moreover, manual searches were
also carried out using the aforementioned terms. The search
methodology is described as follows as an example: i) astragaloside
IV; ii) astraloside; iii) ASIV; iv) i OR ii OR iii; v)
cancer[MeSH]; vi) tumor[MeSH]; vii) v OR vi; viii) pathway[MeSH];
ix) mechanism[MeSH]; x) viii OR ix; and xi) iv AND vii AND ix.
Inclusion criteria
The inclusion criteria were as follows: Studies
exploring the molecular mechanisms of AS-IV in cancer; studies with
comparable experimental and control groups, and those that
successfully established animal models of cancer; studies in which
animal experiments were approved by an ethics committee; and
studies that investigated related pathways involving upstream and
downstream molecular mechanisms and published experimental
findings, which could be retrieved.
Exclusion criteria
The exclusion criteria were as follows: Studies that
included only AS-IV or astragalus polysaccharide (APS) as the
experimental group; studies that had an obvious risk of bias,
including selection bias, performance bias, detection bias,
reporting bias and attrition bias; case studies, cross-over studies
and studies without a separate control group; studies combining
AS-IV with other TCM interventions, in which data specific to the
effect of AS-IV interventions on cancer could not be extracted
separately.
3. Effects of AS-IV in cancer models
AS-IV has been widely used in the management of
cardiovascular, digestive, endocrine, and nerve-related diseases
(11-13). Furthermore, it exerts significant
anticancer effects when used alone or as an adjuvant to other
treatment modalities, as it sensitizes the host to other drugs
(Table I).
 | Table IEffects of AS-IV on anti-cancer
properties depending on dose and various signaling pathways. |
Table I
Effects of AS-IV on anti-cancer
properties depending on dose and various signaling pathways.
| Cancer type | Observation | Cell type | Effects | Mechanism of
action | (Refs.) |
|---|
| Colorectal
cancer | In vitro
(10, 20, 40 µg/ml); in vivo BAlB/c mice (20
mg/kg) | HT29, SW480 | Inhibit
proliferation, induce cell cycle G1 arrest, induce apoptosis | p21↑, Bax/Bcl-2↑,
cleavage of PARP↑, caspase-3/9 ↑ | (16) |
| Breast cancer | In vitro
(10, 20, 40 µg/ml); in vivo BAlB/c nude mice (20
mg/kg) | MDA-MB-231 | Inhibit
proliferation | pERK1/2↓, pJNK↓,
MMP-2/-9↓, Vav3↓, Rac1/MAPK pathway↓ | (75) |
| Lung cancer | In vitro
(≥20 µg/ml) | A549 | Inhibit viability,
invasion and migration | MMP-2↓, MMP-9↓,
Integrin β1↓, E-cadherin↑, TGF-β1↓, TNF-α↓, IL-6↓,
PKC-α-ERK1/2-NF-κB↓ | (65) |
| Lung cancer | In vitro (40
µM-100 µM)l in vivo (40 mg/kg) male C57BL/6 J
mice | A549, H1299 | Inhibit invasion,
migration, angiogenesis | AMPKα↓, blocking
the M2 polarization of macrophages through AMPK signaling
pathway | (99) |
| Cervical
cancer | In vitro (5,
10, 25 µM); in vivo BAlB/c nude mice (25
mg/kg/day) | HeLa, SiHa | Inhibit tumor
growth, inhibit invasion, induce autophagy | LC3I/II↑, DCP1A↑,
TMSB4X↑, MGST3↓, AKR1C2↓, ERL1N1↓, Atg7↑, Atg12↑ | (105) |
| Gastric cancer | In vitro
(≥10 µmol/l) | BGC-823 | Inhibit
cancer-associated fibroblasts, regulate tumor microenvironment,
inhibit proliferation-, migration- and invasion-promoting
capacities of GCAFs | miR-214↑, miR-301a↓
SOX2↑, NANOG↑, M-CSF↓, TIMP2↑ | (108) |
| Non-small cell lung
cancer | In vitro
(12, 24 ng/ml) | HCC827, A549,
NCI-H1299 | Inhibit migration
and proliferation, induce apoptosis | Bax↑, Bcl-2↓,
caspase-3↑, Akt/GSK3β/b-catenin↓ | (18) |
| Gastric cancer | In vitro (10
ng/ml, 20 ng/ml) | BGC-823,
MKN-74 | Inhibit cell
viability, invasion and migration | Inhibit
TGF-β1-induced EMT through inhibition of PI3K/Akt/NF-κB
pathway | (113) |
| Lung cancer | In vitro
(10, 20, 50 ng/ml) | A549 | Inhibit cell
growth | VEGF↑, NF-κBp65↑,
MMP-2↓ | (66) |
| Liver cancer | In vitro
(0.1 mM) | 5-FU-resistant
Bel-7402/FU human hepatic cancer cells | Reverse drug
resistance of Bel-7402/FU cells | JNK/c-Jun/AP-1↓,
p-JNK↓, p-c-Jun↓ | (82) |
| Liver cancer | In vitro
(0.08 mg/ml) | 5-FU-resistant
Bel-7402/FU human hepatic cancer cells | Reverse drug
resistance of Bel-7402/FU cells to 5-FU, enhance intracellular
accumulation of 5-FU | P-gp↓, MDR1↓ | (83) |
| Liver cancer | In vitro
(100 µg/ml) | Huh7, MHCC97-H | Suppress migration
and invasion | Suppress EMT by
regulation of the Akt/GSK-3β/β-catenin pathway, E-cadherin↑,
N-cadherin↓, Vimentin↓, α-SMA↓, Slug↓ | (63) |
| Liver cancer | In vitro
(10, 20, 40, 80, 160 µg/ml) | SMMC-7721,
Huh-7 | Inhibit migration
and cell viability, induce apoptosis | LncRNA-ATB↓,
IL-11/STAT3 pathway↓ | (74) |
| Vulvar squamous
cell carcinoma | In vitro
(100, 200, 400, 600 and 800 µg/ml) | SW962 | Inhibit cell
proliferation, induce apoptosis and autophagy, induce cell-cycle
arresting in G0/G1 phase | P53↑, P21↑, Cyclin
D1↓, Bax↑, cleaved caspase-3↑, Bcl-2↓, Bcl-xl↓, Beclin-1↑, LC3-B↑,
P62↓, reverse dysregulation of TGF-β/Smad signaling by TGF-βRII↑
and Smad4↑ | (19) |
| Osteosarcoma | In vitro (20
mg/kg) In vivo (40 µM) BALB/c nude mice | MG-63, 143B | Inhibit cell
survival, increase chemosensitivity, enhance cisplatin-induced
apoptosis | Cleaved caspase-8↑,
cleaved caspase-3↑, cleaved PARP↑, GAPDH↑, regulate Fas/FasL
signaling | (23) |
| Glioma | In vitro
(100 µg/ml); in vivo (20 mg/kg) athymic BAlB/c
mice | U251 | Inhibit
proliferation in vitro and attenuate tumor growth in
vivo, suppress migration and invasion | PCNA↓, ki67↓,
MMP-2↓, MMP-9↓, VEGF↓, inactivation of MAPK/ERK signaling
pathway | (53) |
| Liver cancer | In vitro
(150 µg/ml) | HepG2, T47D,
MB-AMD-231, PC-3, 293T | Attenuate the
clonogenic survival and anchorage-independent growth of cancer
cells, inhibit the colony formation | Vav3.1↓, alter
level of proteins like BiP/GRP78, HSP70-2, HSPA1A, HSPA8 | (76) |
| Liver cancer | In vitro
(200, 400 µM) | SK-Hep1, Hep3B | Induced
cytotoxicity, inhibit proliferation, suppress invasion, trigger G1
arrest | Caspase-3/-8/-9↑,
XIAP↓, MCL1↓, CFLIP↓, Survivin↓ | (33) |
| Cervical
cancer | In vitro
(50, 200, 800 µg/ml); in vivo BABLc/nude mice (120
mg/kg) | SiHa | Inhibit invasion
and migration in vitro and in vivo, inhibit EMT | p-p38↓, p-MAPK↓,
p-PI3K↓, p-AKT↓, p-mTOR↓, TGF-β1↓, N-cadherin↓, Vimentin↓,
E-cadherin↑ | (64) |
| Colorectal
cancer | In vitro
(≥50 ng/ml) | SW620, HCT | Reduce cell
proliferation, arrest cell cycle in G0/G1 phase | B7-H3↓, miR-29c↑,
cyclin D1↓, CDK4↓ | (41) |
| Lung cancer | In vitro
(80, 160 µg/ml); in vivo C57Bl/6 mice with
3LL-luc-EGFP, 3LL-luc-IDO (40 mg/kg) | Lewis lung
carcinoma cell | Inhibit tumor
progression and prolong survival time | Enhance immune
response by inhibiting the Treg frequency and induce the activity
of CTLs, blocked IDO induction in vitro and in
vivo. | (98) |
| Glioma | In vitro
(20, 40, 80 µg/ml) | U251 | Inhibit migration
and invasion, promote apoptosis, inhibit proliferation | Interfered with the
TGF-β1-induced Wnt/β-catenin signaling pathway to inhibit EMT | (61) |
| Liver cancer | In vitro
(≥20 µg/ml) | SMMC-7721,
Huh7 | Increase
apoptosis | miR-150−5p↑,
β-catenin↓, Bax↓, Bcl-2↑ | (20) |
| Colorectal
cancer | In vitro (5,
10, 20 µg/ml) | SW-480 | Inhibit migration
and invasion, increase chemosensitivity | CREB1↓, miR-134↑,
EMT↓ | (70) |
| Macrophages and
Lewis lung carcinoma | In vitro
(100 µg/ml) | RAW264.7 | Enhance immune
function, induce G2/M phase arrest | NO↑, IL-4↓, IL-6↑,
CD40↑, CD86↑, IL-1β↑, TNF-α↑, iNOS↑, cyclin D1↑, CDK4↑, CDK6↑,
p50↑, p-p65↑, p50/β-actin↑, p-p65/p65↑, p-p38↑, pERK↑, pJNK↑, p38↓,
ERK↓, JNK↓, NF-κB/MAPK signaling pathway↑ | (102) |
| Breast cancer | In vivo (12
g/kg) 7, 12-dimethylbenzanthracene-induced SD rats | 7,
12-dimethylbenzanthracene-induced liver cancer | Inhibit tumor
progression | IL-2↑, IFN-γ↑,
CD3+↑, CD4+↑,
CD4+/CD8+↑ IL-1↓, IL-6↓, TNF-α↓,
CD8+↓ | (101) |
| Gastric cancer | In vitro
(20, 40, 60, 80 µmol/l) | MGC-803 | Induce apoptosis,
trigger G1 arrest | AKT/NF-κB↓, BAX↑,
BCL-2↓, BCL-2/BAX↓, caspase-3↑ | (21) |
| Liver cancer | In vitro (40
µg/ml) | HepG2/GCS | Increase
chemosensitivity, Reverse MDR | GCS↓,
caspase-9↑ | (87) |
| Glioma | In vivo
(AS-IV 12 g/kg, Cis 2 ml/kg) BABLc mice | C6 | Increase
chemosensitivity | Bcl-2↓, survivin↓,
caspase-3↑ | (34) |
| Gastric cancer | In vitro (5
µg/ml) | BGC823 | Inhibit tumor
growth and metastasis | MMP-2↓, MMP-7↓,
MMP-9↓, TIMP-1↑, nm23 mRNA↑, MDR1/P-gp↓, MRP-1↓, Bcl-2↓, Bax↑,
Bcl-2/Bax↓ | (59) |
| Gastric cancer | In vitro
(0.625 g/l) | SGC7901 | Inhibit tumor
growth | COX-2↓, VEGF↓,
PGE2↓ | (79) |
| Gastric cancer | In vitro
(100 µg/ml) | SGC7901 | Inhibit
invasion | MMP-2↓, MMP-9↓,
p-ERK↓ | (54) |
| Liver cancer | In vivo (100 g/ml,
50 g/ml) C57 mice | BN-75 | Inhibit tumor
growth | CD4+↑,
CD4+/CD8+↑, IFN-γ↑, IL-4↑ | (100) |
| Lung cancer | In vitro
(10-40 µmol) | SPC-A-1 | Inhibit
proliferation | SOD↑, GSH-Px↑,
Bcl2↓, Bax↑, Bcl2/Bax↓ | (37) |
| Liver cancer | In vitro
(2.5, 5, 10 µM/ml) | HepG2 | Promote apoptosis,
inhibit proliferation | ROS/NF-κB pathway↓,
Ki67↓, Bcl-2↓, NF-κB↓, IKK-α↓, IKK-β↓, ROS↑, Caspase-3↑, Bax↑ | (43) |
| Liver cancer | In vivo
(0.3, 1, 3 mg/kg) BALB/C mice | H22 | Inhibit ascites,
angiogenesis, metastasis | VEGF↓, MMP-2↓,
MMP-9↓, AQP-1↓, CD31↓ | (55) |
| Liver cancer | In vitro (40
µM) In vivo (50 mg/kg AS-IV) | HepG2 cell Balb/c
mice with H22 tumors | Increase
chemosensitivity, decrease cisplatin-induced kidney damage | Reverse MRP2
overexpression after Cis treatment | (114) |
| Non-small cell lung
cancer | In vitro
(10, 20, 40 ng/ml) | A549, HCC827,
NCI-H1299 | Increase
chemosensitivity, induce apoptosis | B7-H3↓ | (42) |
| Non-small cell lung
cancer | In vitro
(≥12 ng/ml) | NCI-H1299, HCC827,
A549 | Suppress cell
viability, increase chemosensitivity | SIRT6 ↓ | (85) |
| Breast cancer | In vitro (10
to 90 µM) In vivo Balb/c nude mice (50 mg/kg) | MCF-7,
MDA-MB-231 | Increase
chemosensitivity with high safety, induce G2/M cell cycle
arrest | Cleaved PARP↑,
Bcl-2↓, Bax↑, p-ERK/p-JNK↓, p-p38↑, activate eNOS/NO/3NT signaling
by inhibiting CAV-1 | (17) |
| Colorectal
cancer | In vitro
(10, 15 ng/ml) | HCT116, SW480 | Suppress tumor cell
growth, Elevate chemosensitivity | NOTCH3↓ | (86) |
| Precancerous
lesions of gastric carcinoma | In vivo (50,
100 mg/kg male Sprague-Dawley rats | MNNG-induced
PLGC | Reverse
MNNG-induced PLGC, ameliorate dysplasia of gastric mucosa | LDHA↓, p53↑,
TIGAR↑, MCT1↓, MCT4↓, HIF-1α↓, CD147↓, and miRNA-34a↑, reverse PLGC
via regulating p53/miRNA-34a/LDHA pathway | (107) |
| Doxorubicin
treatment | In vivo (40
mg/kg) C57Bl/6 mice; in vitro (20 µM) | Neonatal
cardiomyocytes of Sprague Dawley (SD) rats | Alleviate body
weight loss, myocardial injury, apoptosis of cardiomyocytes,
cardiac fibrosis and cardiac dysfunction in DOX-treated mice and
in vitro | NOX2↓, NOX4↓,
relieve oxidative stress | (111) |
To the best of our knowledge, there are no
systematic reviews available that discuss the role of AS-IV in
cancer; therefore, in the present review article, the efficacy and
mechanisms of action of AS-IV in cancer therapy are presented and
discussed.
Induction of apoptosis
Apoptosis, also known as programmed cell death,
includes the initiation stage, effect stage and degradation stage.
Apoptosis is characterized by surface blebbing, chromatin
condensation, fragmentation of chromosomal DNA and the appearance
of apoptotic bodies.
As shown in Table
I and Fig. 2, AS-IV leads to
apoptosis mainly by the mitochondrial-dependent intrinsic pathway
and the death receptor-dependent extrinsic pathway. The intrinsic
pathway leads to the release of cytochrome c (Cyt C) from
the mitochondria, which activates caspase-9, -3 and -7 (14). However, Bcl-2 can inhibit the
release of Cyt C and avoid the intrinsic apoptosis induced by Bax
(15). Research has indicated
that AS-IV can enhance the Bax/Bcl-2 ratio to induce intrinsic
apoptosis in a number of types of cancer, including colorectal
cancer (CRC), breast cancer, lung cancer, vulvar squamous cell
cancer (VSCC) and hepatocellular carcinoma (HCC) (16-21).
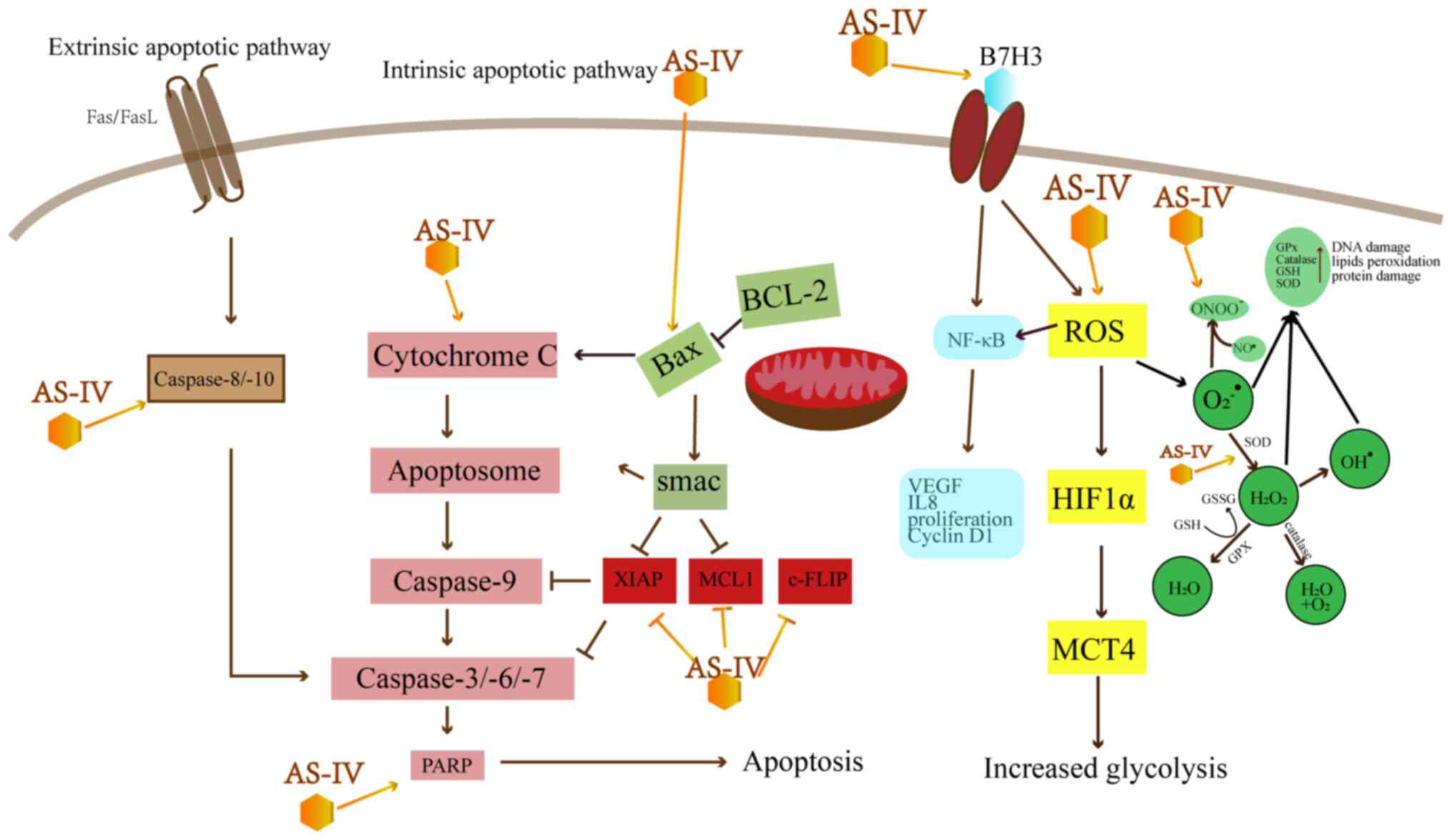 | Figure 2Effect of AS-IV on apoptosis-related
pathways. AS-IV, astragaloside IV; XIAP, x-linked mammalian
inhibitor of apoptosis; MCL1, myeloid cell leukemia 1; c-FLIP,
cellular FLICE-like inhibitory protein; ROS, reactive oxygen
species; VEGF, vascular endothelial growth factor; MCT,
monocarboxylic acid transporter; HIF, hypoxia-inducing factor; GPx,
glutathione peroxidase; GSH, glutathione; SOD, superoxide
dismutase. |
In terms of extrinsic apoptosis, certain receptors,
e.g., the Fas ligands and tumor necrosis factor (TNF)-α, can set
off the caspase-8-dependent extrinsic apoptotic pathways and become
activated following the caspase cascade, which finally triggers
apoptosis (22). It has been
reported that combined treatment with AS-IV and cisplatin (10
µM) markedly promotes the cleavage of caspase-8 and -3, and
poly(ADP-ribose) polymerase (PARP) in MG-63 and 143B cells via the
Fas/FasL signaling pathway, which considerably sensitizes the
osteosarcoma cells to the effects of cisplatin (23). In CRC, AS-IV alone can increase
the release of Cyt C into the cytoplasm and upregulate the
Bax/Bcl-2 ratio, as well as activate PARP and the caspase cascade
(16).
The IAP protein family may be the most important
apoptotic regulator involved in both intrinsic and extrinsic
apoptosis pathways, including the x-linked mammalian inhibitor of
apoptosis (XIAP), survivin and cellular inhibitor of apoptosis
protein 1 (cIAP1) (22,24).
HCC is associated with a high morbidity and
mortality rate globally, and presents with increased levels of
anti-apoptotic proteins, including myeloid cell leukemia 1 (MCL1),
cellular FLICE-like inhibitory protein (c-FLIP) and XIAP. c-FLIP
can suppress death receptor-mediated apoptosis, which inhibits
caspase-8 (25-28). Additionally, studies have
demonstrated that MCL1 can block apoptosis induced by various
apoptotic stimuli, including chemoradiotherapy (29-31). Its high protein expression levels
in cancer cells are associated with drug resistance (32). AS-IV has been shown to
significantly decrease XIAP, MCL1, c-FLIP and survivin expression
in HCC and C6 glioma cells (33,34).
Inhibition of proliferation
High levels of reactive oxygen species (ROS) are
considered to be a driver of a number of diseases, such as cancer
and neurodegeneration. ROS are capable of increasing the
carcinogenic potential of cancer cells and activating
hypoxia-inducing factor (HIF) in hypoxic tumor cells to maintain
cell viability (32,35). On the other hand, cells are
capable of eliminating surplus ROS via mechanisms involving
superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px)
(36). Yang proved that AS-IV
interrupted the proliferation of Spc-A-1 cells and suggested that
the mechanism was related to the activity of the antioxidant
enzymes, SOD and GSH-Px, which modulate ROS levels in cancer
(37).
In the B7/cluster of differentiation (CD)28
superfamily, the overexpression of B7-H3 is observed in various
types of cancer. It can downregulate the T-cell-mediated immune
responses, leading to immune escape (38-40). AS-IV can reduce B7-H3 by
upregulating miR-29c, which inhibits cell growth and reduces the
protein level of the cell-cycle regulators, cyclin D1 and CDK4, in
CRC cells. Thus, the anticancer effects of AS-IV may be mediated
via the B7-H3/nuclear factor (NF)-κB/cyclin D1 axis (41). It also increases the cytotoxicity
of cisplatin in non-small cell lung cancer (NSCLC) by suppressing
the expression of B7-H3 (42).
Moreover, another study reported that AS-IV inhibited the
proliferation of HCC HepG2 cells and promoted apoptosis by
regulating oxidative stress and the NF-κB signaling pathway
(43).
Inhibition of metastasis
Matrix metalloproteinases (MMPs) are a group of
proteolytic enzymes containing active Zn2+. Its
functions include, but are not limited to, degrading the
extra-cellular matrix (ECM). The interaction between MMPs and
cell-surface ECM receptors affects the function of integrins and
contributes to cell invasion (44). MMP-2 and MMP-9, in particular,
have been considered to play a vital role in tumor progression
(45).
The extracellular signal-regulated kinase pathway
(ERK), an important upstream switch, has been known to regulate the
secretion of MMPs in cells (46).
Mitogen-activated extracellular signal-regulated kinase (MAPK) is a
serine/threonine (Ser/Thr) kinase involved in cell proliferation,
differentiation, growth and apoptosis. In general, the MAPK/ERK
pathway, i.e., Ras-Raf-MEK-ERK pathway, is deregulated in various
types of cancers (47). Recently,
inhibitors against the MAPK/ERK pathway have been designed to
combat glioma and have been shown to be effective in the U251, as
well as the SGC7901 cell lines with the downregulation of the
expression of MMP-2 and MMP-9 (48-52). Li et al and Cao reported
that AS-IV inhibited the progression of glioma and gastric cancer
by interfering with the MAPK/ERK signaling pathway (53,54). Moreover, ascites in
H22-tumor-bearing mice have been shown to be decreased by AS-IV by
inhibiting the angiogenesis- and metastasis-associated genes, as
well as the expression of aquaporins (AQPs) (55).
Tissue inhibitors of metalloproteinases (TIMPs)
comprise TIMP-1, TIMP-2, TIMP-3 and TIMP-4, all of which can form
complexes with several MMPs via covalent bonds, thereby inhibiting
MMPs (56,57). The NM23 gene is a widely studied
metastasis suppressor gene. The protein encoded by NM23 has the
function of inhibiting tumor metastasis (58). AS-IV can downregulate the mRNA and
protein expression of MMP-2, -7 and -9, can mediate multidrug
resistance/P-glycoproteins (MDR1/P-gp) and multidrug
resistance-associated protein 1(MRP-1), and upregulate TIMP-1 and
NM23 to inhibit the proliferation of BGC823 (gastric cancer) cells
and reverse drug resistance (59).
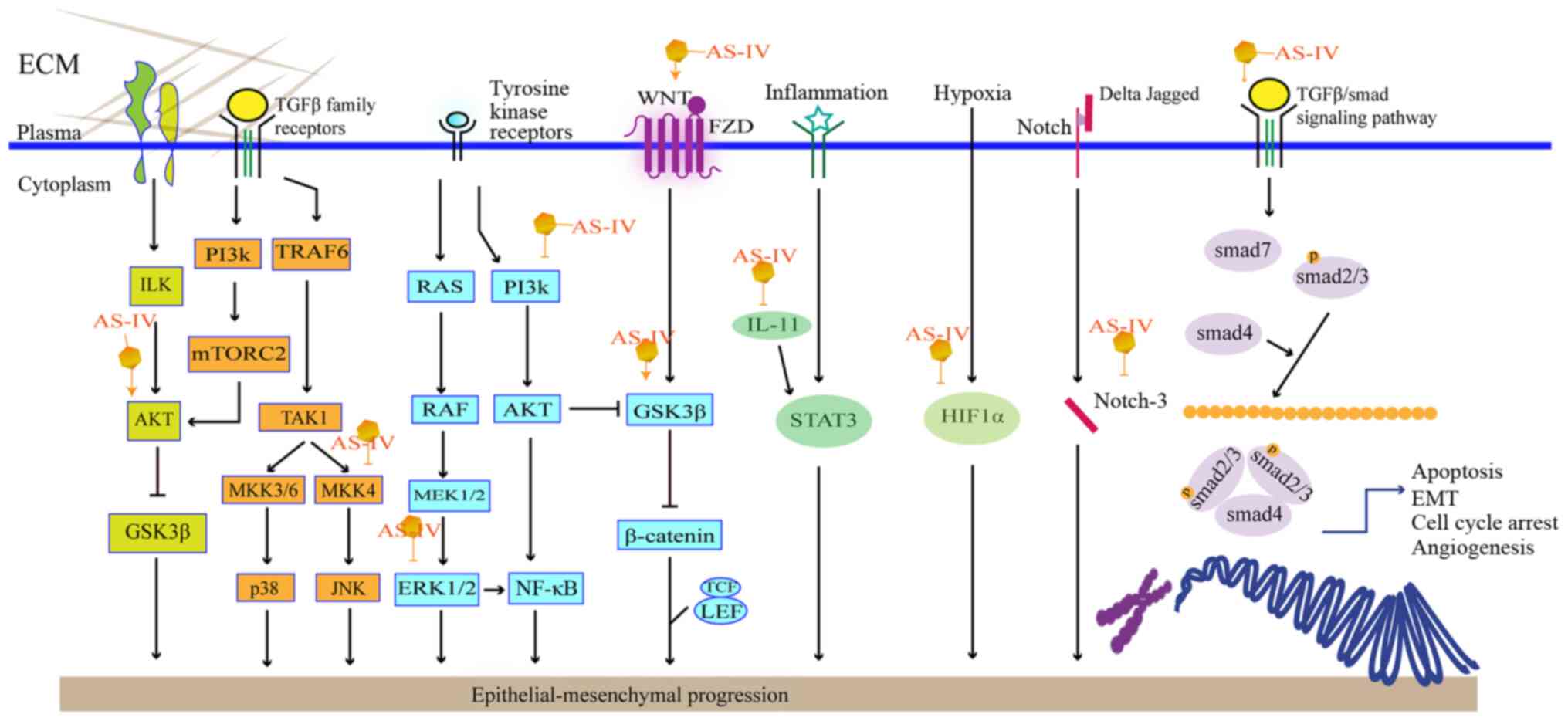
Epithelial-mesenchymal transformation (EMT) is the
conversion of a polarized epithelial cell, which interacts with the
basement membrane by means of its basal surface, to a mesenchymal
cell. As regards the metastatic process, EMT can be detected based
on specific molecular changes, such as diminished E-cadherin and
cytokeratin levels, and elevated levels of N-cadherin and vimentin
(60). As the transforming growth
factor β1 (TGF-β1) is a known factor in triggering the initiation
and execution of EMT, the downregulation of TGF-β1signaling can
prevent EMT in tumor cells. As shown in Fig. 3, AS-IV can affect EMT via several
pathways.
The Wnt/β-catenin signaling pathway regulates EMT.
Using U251 cells, Han et al found that AS-IV treatment
inhibited TGF-β1-guided EMT by interrupting the Wnt/β-catenin
pathway (61). β-catenin can also
modulate glycogen synthase kinase 3β (GSK3β). AKT is an upstream
molecule that activates GSK3β phosphorylation, eventually leading
to the accumulation of β-catenin in the cell nucleus (62). AS-IV has also been shown to
attenuate EMT in HCC and NSCLC via the modulation of the
Akt/GSK-3β/β-catenin pathway (18,63).
Phosphoinositide-3-kinase/protein kinase B/nuclear
factor κB (PI3K/Akt/NF-κB) is another common pathway suppressing
TGF-β1-induced EMT. It has been reported that AS-IV can inhibit
TGF-β1-induced EMT by interfering with the PI3K/Akt/NF-κB signaling
pathway in SiHa and MGC-803 cells (21,64). Moreover, it inhibits the
phosphorylation of MAPK and mTOR to varying degrees, which is
related to the proliferation of cancer cells.
Apart from the signaling pathways discussed, AS-IV
may also interrupt the migration and invasion of A549 cells. This
process is associated with the suppression of PKC-α-ERK1/2-NF-κB
and can be detected based on specific proteins, e.g., E-cadherin,
integrin β1 and MMPs (65). PKC-α
expression can be affected by ROS, which can induce the downstream
signaling of ERK1/2 and activate NF-κB to initiate the metastasis
of carcinoma cells (66).
In parallel, several miRNAs take part in the
inhibition of EMT signaling (67). For example, miR-134 from the miRNA
gene family has been proven to inhibit EMT (68,69). CREB1 is an important transcription
enhancer. A previous study reported that miR-134 activated by AS-IV
markedly inhibiting EMT signaling and increasing the
chemosensitivity of SW-480 cells to oxaliplatin by inhibiting CREB1
expression (70).
Similar to miR-134, miR-150-5p markedly
downregulates β-catenin in liver carcinoma, functioning as an
inhibitor to attenuate the proliferation of cancer cells. It has
been well-established that AS-IV can regulate the
miR150-5p/β-catenin axis to induce the apoptosis of HCC cells
(20).
Long non-coding RNAs (lncRNAs) are long nucleotide
chains without protein-coding capability (71,72). LncRNAs have been identified to
participate in several biological processes and are known to play a
crucial role in the emergence and progression of cancers. For
example, lncRNA-ATB promotes EMT by connecting to the miR-200
family, maintaining the viability of malignant cells via
IL-11/STAT3 signaling, which can be prevented by AS-IV (73,74).
Vav3, a member of the Vav protein family, functions
as an exchange factor for Rho family GTPases, such as Rac1. It
consists of 8 domains, and the complexity of the structure
contributes to its various functions. Vav3 modulates different
members in the Rho family to participate in the MAPK, PI3K/Akt and
NF-κB signaling pathways. Previous studies have demonstrated that
MMPs and Rho GTPases play a pivotal role in the migration of the
majority of malignant cells. AS-IV has been shown to have antitumor
and anti-metastasis functions both in vivo and in
vitro. These functions are accomplished by the downregulation
of Vav3 in liver and breast cancer by blocking the Rac1/MAPK
signaling pathway, as well as by decreasing MMP-2, MMP-9, and the
proteins related to cellular responses during stress and cell
signaling (75,76).
Inhibition of angiogenesis
Neovascularization relies on the secretion of
vascular endothelial growth factor (VEGF) by tumor cells and the
proliferation of endothelial cells (77). VEGF serves as a signal for
cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2). PGE2 is involved
in the major process of COX-2 acting on malignant cells (78). AS-IV inhibits the growth of
SGC7901 cells with the downregulation of COX-2, which leads to the
suppression of its downstream product, PGE2 expression, and the
downregulation of VEGF, thereby decreasing tumor growth (79). Apart from SGC7901, a reduction in
VEGF expression has also been reported in studies using A549 and
U251 cells.
MDR and increase in chemosensitivity
MDR is the leading cause of the failure of
chemotherapy and cancer renascence. The key to reversing tumor drug
resistance is to prevent MDR pathways to reduce drug efflux, which
can enhance the chemosensitivity of tumor cells (80). It has been found that MDR can be
attributed to several factors, including P-gps, lung
resistance-related proteins (LRPs), breast cancer resistance
protein (BCRP) and multidrug resistance-associated protein 2
(MRP2), all of which can pump drugs out from tumor cells and reduce
the anticancer efficacy of drugs (81). Several studies have reported that
AS-IV can reverse MDR and increase the chemosensitivity or
radiosensitivity of tumors (17,82-87) (Fig.
4)
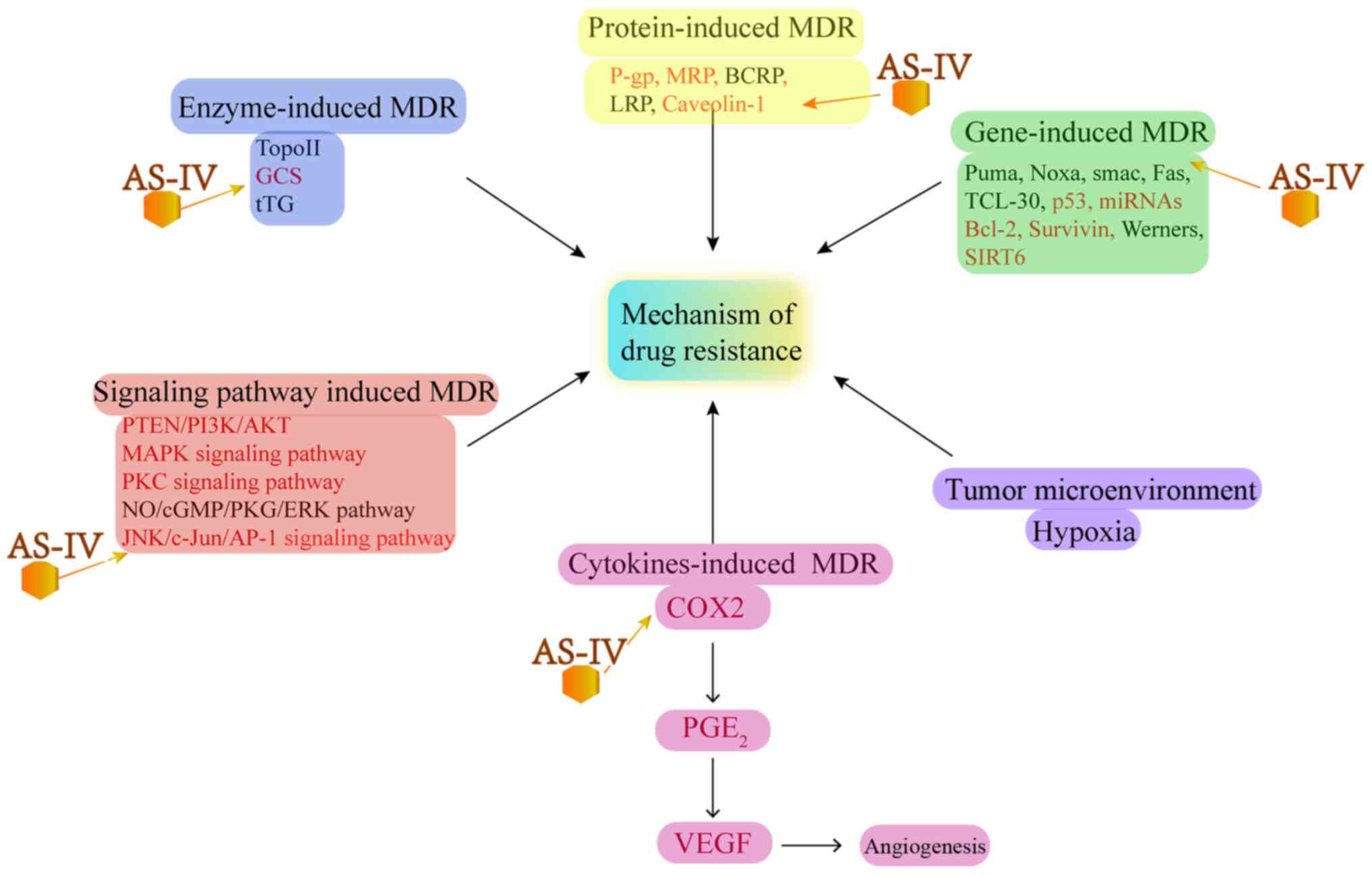 | Figure 4Effect of AS-IV on MDR-related
molecular mechanisms. MDR, multidrug resistance; GCS,
glucosylceramide synthase; P-gp, P-glycoprotein; LRP, lung
resistance-related proteins; BCRP, breast cancer resistance
protein; MRP, multidrug resistance-associated protein; COX2,
cyclooxygenase 2; PGE2, prostaglandin E2; VEGF, vascular
endothelial growth factor; SIRT6, sirtuin 6. |
Caveolin-1 (CAV-1) is a constituent protein playing
a role in signal transduction and other cellular activities. It has
been confirmed that the expression of CAV-1 is positively
associated with cancer metastasis and has, therefore, been
identified as a potential target to reverse MDR (88). Zheng et al reported that
AS-IV reduced CAV-1 expression and reversed the Taxol-induced
increase in CAV-1 expression; furthermore, AS-IV administration
resulted in initiating the endothelial nitric oxide synthase
(eNOS)/nitric oxide (NO)/peroxynitrite (ONOO−) pathway
and inhibiting CAV-1, which can induce severe oxidative stress and
apoptosis (17).
Moreover, the MAPK pathway, which comprises the ERK,
JNK and p38 pathways, controls several biological and cellular
processes in cancer. Therefore, its activation is vital to MDR
(89). Co-treatment with AS-IV
and Taxol lowers ERK and JNK in malignant cells, which are
associated with chemosensitizing effects (17).
Studies have found that inhibiting the JNK signaling
pathway suppresses the expression of c-Jun and drug-resistant
genes, e.g., MDR1 and P-gp, and increases drug-induced the
apoptosis of tumor cells. Wang et al demonstrated that AS-IV
may reverse MDR by inhibiting the JNK/c-Jun/AP-1 pathway in
Bel-7402/FU cells (82,83).
Silent information regulator 6 (SIRT6), an
NAD+-dependent deacetylase, plays a key regulatory role
in genomic stability, metabolism, chromatin regulation, telomere
integrity, gene transcription and glucose and lipid metabolism.
Further exploration of these molecular mechanisms has indicated
that the multiple roles of SIRT6 in tumorigenesis are realized by
regulating the ERK, SMAD and Raf pathways (84).
SIRT6 also triggers lethal autophagy in human cancer
cells (90). Recent studies have
reported that the upregulation of SIRT6 enhances the sensitivity of
NSCLC cells to other drugs and treatment modalities (91-93). Accordingly, the study by Dai et
al illustrated that AS-IV acted on SIRT6 to heighten the tumor
responses to gefitinib in the NCI-H1299, HCC827 and A549 lung
cancer cell lines (85).
Studies have indicated that NOTCH3 is highly
expressed in tumor cells. It also has been shown that the depletion
of NOTCH3 by sorafenib and adriamycin can increase the expression
of p53, promote GSK3β phosphorylation and downregulate p21, thereby
enhancing the efficacy of chemotherapy (94,95). In addition, NOTCH3 may be used as
a biomarker for RC. In a previous in vitro study, AS-IV was
reported to enhance the chemosensitivity of CRC towards cisplatin
by suppressing NOTCH3 (86).
The glucosylceramide synthase (GCS)-mediated
abolishment of ceramide-induced apoptosis is one of the underlying
mechanisms of acquired drug resistance in some resistant cells
(96). AS-IV can reverse drug
resistance to doxorubicin in HepG2/GCS cells, suggesting that MDR
can be prevented using AS-IV as it reduces the expression of GCS
(87).
Improvement of immunity
Owing to their high cytotoxicity and proliferation
ability, cytotoxic T lymphocytes (CTLs) are useful in the
monitoring and elimination of cancer cells. During tumor
progression, the tumor microenvironment (TME) results in the
suppression of immune function, which results in a loss of the
functions of CTL, leading to immune escape.
Tumor-associated macrophages (TAMs) constitute the
most important inflammatory cell group in the TME. Recent studies
have revealed that TAM may polarize to the M2-type in terms of
phenotypic characteristics. Macrophage colony-stimulating factor-1
(CSF-1), interleukin (IL)-4, IL-10, TGF-β and IL-13 benefit M2
subgroup differentiation. Moreover, M2 and Tregs can reduce the
levels of CTLs. Type 2 (M2) macrophages do not exert antitumor
effects, but rather participate in the occurrence, development,
invasion and metastasis of tumors; therefore, the phenotype M2 is a
novel potential target for tumor therapy (97).
There are multiple mechanisms by virtue of which
tumor cells escape recognition by CTLs. Indoleamine-2,3-dioxygenase
(IDO) is a tryptophan-degrading enzyme that participates in the
immune-escape program. In C57BL/6 mice bearing Lewis lung carcinoma
cells, AS-IV was shown to exert antineoplastic and
immunity-boosting effects to inhibit Tregs and augment CTL activity
by suppressing IDO expression (98). AS-IV has also been shown to
partially block M2 differentiation via the AMPK signaling pathway,
thereby inhibiting invasion, migration and angiogenesis (99).
In 7,12-dimethylbenzanthracene-induced liver and
breast cancer in tumor-bearing mice, the effect of co-treatment of
cisplatin and AS-IV against breast cancer in vivo was more
prominent than that of cisplatin alone. The mechanism of action may
be related to the effective upregulation of the levels of immune
factors IL-2, IFN-γ, CD3+, CD4+,
CD4+/CD8+, and the downregulation of IL-1,
IL-6, TNF-α and CD8+ in liver and breast cancer
(100,101).
Moreover, in vivo experiments have
demonstrated that AS-IV promotes host immunity by regulating the
levels of cytokines, NO and cycle-related mRNA and/or protein
expression, particularly IL-1β, IL-6 and TNF-α, under the influence
of the NF-κB/MAPK pathway. As an inhibitor of proliferation, AS-IV
also modulates the levels of cyclin D1, CDK4 and CDK6 in the host,
promotes the secretion of CDs, such as CD40 and CD86, and arrests
cells in the G2/M stage (102).
Promotion of autophagy
Autophagy is a process in which proteins or
organelles are engulfed into vesicles and fused with lysosomes to
form an autophagosome. Subsequently, the enclosed contents are
degraded, thus achieving the metabolic needs of cells and the
renewal of some organelles (103). Autophagy has a dual-directional
effect on the progression and survival of malignant tumors. This
progression could be measured based on the distribution of LC3-I
and LC3-II, which are biomarkers indicating autophagy vesicle
accumulation (104).
AS-IV elevates the level of autophagy-associated
proteins, such as LC3I/II, Atg7 and Atg12 in cervical cancer cells.
It also mediates differentially expressed proteins, including
MGST3, AKR1C2, and ERL1N1, which are related to cancer
proliferation and cytoskeleton composition. Two autophagy-related
proteins, namely, DCP1A and TMSB4X, have been found to be increased
in HeLa and SiHa cells following the administration of AS-IV
(105).
The TGF-β/SMAD signaling pathway plays a crucial
role in a number of types of cancer and the dysfunction of this
pathway is an important pathogenic mechanism in cancers. SMAD and
downstream TGF-β intracellular signaling transfer the
ligand-receptor interaction signal from the cytoplasm to the
nucleus. In a previous study, in VSCC cells, AS-IV was shown to
improve the dysfunctions of the TGF-β/SMAD pathway, determined
based on the elevated TGF-βRII and Smad4 levels; it was also found
that AS-IV induced autophagy in SW962 cells, and markedly increased
Beclin-1 and LC3-II levels, and decreased p62 protein levels
(19).
Prevention of cancer
Aerobic glycolysis and oxidative phosphorylation are
common energy sources in tumor cells. Owing to the rapid growth and
high energy demand of tumor cells, there is a tendency for an
increased glucose uptake and lactate production. Monocarboxylic
acid transporters (MCT)1 and MCT4 can transport large amounts of
lactic acid produced by tumor cells to the extracellular
environment and play a key role in maintaining the acidic
environment required for the glycolysis in tumor cells (106). CD147 is indispensable to the
activity of MCT1 and MCT4 in gastric cancer. The study by Zhang
et al suggested that AS-IV reduced the precancerous lesions
of gastric carcinoma (PLGC), inhibited glycolysis by regulating the
p53/miRNA-34a/LDHA and p53/TIGAR pathways, and restored the levels
of MCT1/4, CD147 and HIF-1α (107).
AS-IV inhibits the activity of gastric
cancer-associated fibroblasts (GCAFs) with an increased miR-214 and
decreased miR-301a expression. AS-IV also inhibits GCAFs from
increasing key factors, such as SRY-box2 (SOX2) and NANOG, in
inducing pluripotency in somatic cells, decreasing M-CSF expression
and increasing TIMP2 expression (108). All these studies demonstrate
that AS-IV hinders the development of gastric cancer. This topic is
worthy of further exploration in a clinical setting.
Remission of side-effects from
chemotherapy
NADPH oxidase (NOX) is a plasma membrane-related
enzyme protein family consisting of 7 members of DUOX1-2 and NOX1-5
families. Among the NOXs, NOX2 and NOX4 are expressed in the heart
and are responsible for increasing intracellular ROS levels.
Oxidative stress has been identified as a main cause of doxorubicin
(DOX)-induced cardiomyopathy (109,110). DOX administration has been shown
to increase the levels of NOX2 and NOX4 in animal hearts, thereby
increasing ROS-induced cardiomyopathy. By contrast, AS-IV
noticeably reduces the cardiomyopathy induced by DOX, decreases the
oxidative stress caused by NOX2 and NOX4, attenuates the
complications of doxorubicin, and, thus, appears suitable as an
adjuvant to chemotherapy (111).
4. Conclusions and future perspectives
TCMs are commonly used in clinical treatment in
several Asian countries. They significantly contribute towards
enhancing the effects of other therapies and reducing toxicity. The
in vitro and in vivo effects of AS-IV in inhibiting
tumor proliferation and invasion and in promoting tumor cell
apoptosis have been well-documented. Current findings highlight the
role of AS-IV in suppressing EMT, as EMT plays a role in the
majority of processes related to AS-IV in cancer. Furthermore,
AS-IV has also been proven to exert significant preventive effects
against MDR and in the regulation of immunity in antitumor therapy.
In addition, the low-cost and ready availability of AS-IV further
accentuates its potential in tumor therapy.
Despite these advantages, the use of AS-IV is still
limited by several means: i) Its mechanisms of action have not been
adequately elucidated. A previous study demonstrated that AS-IV
enhanced the efflux activity of P-gp and BCRP through the Nrf2-ARE
signaling pathway, exerting the opposite effect on P-gp protein in
liver cancer and gastric cancer cells, which may lead to herb-drug
interactions following treatment with AS-IV (112); ii) there are no clinical studies
(to the best of our knowledge) available that explore the role and
safety of AS-IV in human cancers. The human body is complex
compared to model organisms (in vivo or in vitro)
used in a laboratory setting; iii) finally, the dose of AS-IV used
in studies varies greatly; therefore, the safety window and
effective dose of AS-IV need to be accurately established. Thus,
further studies are warranted to determine the effects of AS-IV and
large cohort clinical studies are required to further validate its
efficacy in a clinical setting.
Funding
The present study was supported by Tianjin Science
& Technology Plan Projects (no. 17ZXMFSY00190), the Tianjin
Traditional Chinese Medicine Research Project, Tianjin health and
family planning commission (no. 2017003) and the National Natural
Science Foundation of China (no. 81403220).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (TC, PY and YJ) were involved in the
conception and design of the study. TC was invovled in the drafting
of the manuscript and in the processing of the figures. PY and YJ
were involved in the critical revision of the manuscript for
important intellectual content. YJ was responsible for obtaining
funding. TC and PY provided administrative, technical, or material
support. PY and YJ supervised and edited the study. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the First Teaching
Hospital of Tianjin University of Traditional Chinese Medicine for
providing the laboratory.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Ni Y, Sun G, Zhu S, Zhao J, Wang
Z, Zhang H, Zhu X, Zhang X, Dai J, et al: Survival outcomes of
radical prostatectomy + extended pelvic lymph node dissection and
radiotherapy in prostate cancer patients with a risk of lymph node
invasion over 5%: A population-based analysis. Front Oncol.
10:6075762020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Cintio F, Dal Bo M, Baboci L, De Mattia
E, Polano M and Toffoli G: The molecular and microenvironmental
landscape of glioblastomas: Implications for the novel treatment
choices. Front Neurosci. 14:6036472020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C,
Hui B, Liu R, Ma H and Ren J: A review of neoadjuvant
chemoradiotherapy for locally advanced rectal cancer. Int J Biol
Sci. 12:1022–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhoday J, Glimelius B, Tait D,
Glynne-Jones R, Adams R and Brown G: Session 4: What should we do
for poor responders after chemoradiotherapy: Bad biology or should
the fight go on? Colorectal Dis. 20(Suppl 1): 97–99. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung Y, Jerng U and Lee S: A systematic
review of anticancer effects of radix astragali. Chin J Integr Med.
22:225–236. 2016. View Article : Google Scholar
|
|
8
|
Li X, Qu L, Dong Y, Han L, Liu E, Fang S,
Zhang Y and Wang T: A review of recent research progress on the
astragalus genus. Molecules. 19:18850–18880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SY, Ouyang HT, Yang JY, Huang XL, Yang
T, Duan JP, Cheng JP, Chen YX, Yang YJ and Qiong P: Subchronic
toxicity studies of Radix Astragali extract in rats and dogs. J
Ethnopharmacol. 110:352–355. 2007. View Article : Google Scholar
|
|
10
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Yang P, Li F, Tao L, Ding H, Rui
Y, Cao Z and Zhang W: Therapeutic effects of astragaloside IV on
myocardial injuries: Multi-target identification and network
analysis. PLoS One. 7:e449382012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Curcumin activated both receptor-mediated and mitochondria-mediated
proteolytic pathways for apoptosis in human glioblastoma T98G
cells. Neurosci Lett. 407:53–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bagci EZ, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: Roles of bax,
bcl-2, and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar
|
|
16
|
Sun P, Liu Y, Wang Q and Zhang B:
Astragaloside IV inhibits human colorectal cancer cell growth.
Front Biosci (Landmark Ed). 24:597–606. 2019. View Article : Google Scholar
|
|
17
|
Zheng Y, Dai Y, Liu W, Wang N, Cai Y, Wang
S, Zhang F, Liu P, Chen Q and Wang Z: Astragaloside IV enhances
taxol chemosensitivity of breast cancer via caveolin-1-targeting
oxidant damage. J Cell Physiol. 234:4277–4290. 2019. View Article : Google Scholar
|
|
18
|
Jia L, Lv D, Zhang S, Wang Z and Zhou B:
Astragaloside IV Inhibits the progression of non-small cell lung
cancer through the Akt/GSK-3β/β-Catenin Pathway. Oncol Res.
27:503–508. 2019. View Article : Google Scholar
|
|
19
|
Zhao Y, Wang L, Wang Y, Dong S, Yang S,
Guan Y and Wu X: Astragaloside IV inhibits cell proliferation in
vulvar squamous cell carcinoma through the TGF-β/Smad signaling
pathway. Dermatol Ther. 32:e128022019.
|
|
20
|
Cui X, Jiang X, Wei C, Xing Y and Tong G:
Astragaloside IV suppresses development of hepatocellular carcinoma
by regulating miR-150-5p/β-catenin axis. Environ Toxicol Pharmacol.
78:1033972020. View Article : Google Scholar
|
|
21
|
Ying G: Astragaloside induces gastric
MGC-803 cells apoptosis by inhibiting AKT and NF-KB pathway. Int J
Lab Med. 19:2341–2344. 2018.
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI
|
|
23
|
Hu T, Fei Z and Wei N: Chemosensitive
effects of Astragaloside IV in osteosarcoma cells via induction of
apoptosis and regulation of caspase-dependent Fas/FasL signaling.
Pharmacol Rep. 69:1159–1164. 2017.PubMed/NCBI
|
|
24
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252.
1999.PubMed/NCBI
|
|
25
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006.PubMed/NCBI
|
|
26
|
Du X, Bao G, He X, Zhao H, Yu F, Qiao Q,
Lu J and Ma Q: Expression and biological significance of c-FLIP in
human hepatocellular carcinomas. J Exp Clin Cancer Res.
28:242009.PubMed/NCBI
|
|
27
|
Fleischer B, Schulze-Bergkamen H,
Schuchmann M, Weber A, Biesterfeld S, Müller M, Krammer PH and
Galle PR: Mcl-1 is an anti-apoptotic factor for human
hepatocellular carcinoma. Int J Oncol. 28:25–32. 2006.
|
|
28
|
Che Y, Ye F, Xu R, Qing H, Wang X, Yin F,
Cui M, Burstein D, Jiang B and Zhang DY: Co-expression of XIAP and
cyclin D1 complex correlates with a poor prognosis in patients with
hepatocellular carcinoma. Am J Pathol. 180:1798–1807.
2012.PubMed/NCBI
|
|
29
|
Cameron BD, Traver G, Roland JT, Brockman
AA, Dean D, Johnson L, Boyd K, Ihrie RA and Freeman ML:
Bcl2-Expressing Quiescent Type B neural stem cells in the
Ventricular-Subventricular zone are resistant to concurrent
Temozolomide/X-Irradiation. Stem Cells. 37:1629–1639.
2019.PubMed/NCBI
|
|
30
|
Tanaka N, Patel AA, Wang J, Frederick MJ,
Kalu NN, Zhao M, Fitzgerald AL, Xie TX, Silver NL, Caulin C, et al:
Wee-1 kinase inhibition sensitizes high-risk HPV+ HNSCC to
apoptosis accompanied by downregulation of MCl-1 and XIAP
antiapoptotic proteins. Clin Cancer Res. 21:4831–4844.
2015.PubMed/NCBI
|
|
31
|
Lin YC, Chen RY, Liang JA, Hung YC, Yeh
LS, Chang WC, Lin WC, Chang YY and Chen SW: Immunohistochemical
biomarkers of survival in patients with adenocarcinoma of the
uterine cervix receiving chemoradiotherapy. Anticancer Res.
39:3231–3240. 2019.PubMed/NCBI
|
|
32
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003.PubMed/NCBI
|
|
33
|
Su CM, Wang HC, Hsu FT, Lu CH, Lai CK,
Chung JG and Kuo YC: Astragaloside IV induces apoptosis,
G1-phase arrest and inhibits anti-apoptotic signaling in
hepatocellular carcinoma. In Vivo. 34:631–638. 2020.PubMed/NCBI
|
|
34
|
Chang XY: Effects of cisplatin combined
with astragaloside iv on apoptosis genes in C6 glioma mice. Chin J
Gerontol. 13:3282–3284. 2019.In Chinese.
|
|
35
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
|
|
36
|
Bułdak RJ, Bułdak Ł, Kukla M, Gabriel A
and Zwirska-Korczala K: Significance of selected antioxidant
enzymes in cancer cell progression. Pol J Pathol. 65:167–175.
2014.
|
|
37
|
Yang JY: Effect of astragaloside IV on the
proliferation of Spc-A-1 cells in human lung cancer and its
mechanism. Chin Traditional Patent Med. 8:1818–1820. 2016.In
Chinese.
|
|
38
|
Wang L, Kang FB and Shan BE:
B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer.
134:2764–2771. 2014.
|
|
39
|
Flem-Karlsen K, Fodstad Ø, Tan M and
Nunes-Xavier CE: B7-H3 in cancer - beyond immune regulation. Trends
Cancer. 4:401–404. 2018.PubMed/NCBI
|
|
40
|
Ling V, Wu PW, Spaulding V, Kieleczawa J,
Luxenberg D, Carreno BM and Collins M: Duplication of primate and
rodent B7-H3 immunoglobulin V- and C-like domains: Divergent
history of functional redundancy and exon loss. Genomics.
82:365–377. 2003.PubMed/NCBI
|
|
41
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He CS, Liu YC, Xu ZP, Dai PC, Chen XW and
Jin DH: Astragaloside IV enhances cisplatin chemosensitivity in
non-small cell lung cancer cells through inhibition of B7-H3. Cell
Physiol Biochem. 40:1221–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
An XC: Mechanism of astragaloside IV
promoting proliferation and apoptosis of hepatoma cells by
inhibiting ROS/NF-κB signaling pathway. Mod Dig Intervent.
12:1399–1403. 2019.In Chinese.
|
|
44
|
Brooks PC, Strömblad S, Sanders LC, von
Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP and
Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the
surface of invasive cells by interaction with integrin alpha v beta
3. Cell. 85:683–693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Trepat X, Chen Z and Jacobson K: Cell
migration. Compr Physiol. 2:2369–2392. 2012.
|
|
46
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rizzo D, Ruggiero A, Amato M, Maurizi P
and Riccardi R: BRAF and MEK inhibitors in pediatric glioma: New
therapeutic strategies, new toxicities. Expert Opin Drug Metab
Toxicol. 12:1397–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
50
|
Nickl-Jockschat T, Arslan F, Doerfelt A,
Bogdahn U, Bosserhoff A and Hau P: An imbalance between Smad and
MAPK pathways is responsible for TGF-beta tumor promoting effects
in high-grade gliomas. Int J Oncol. 30:499–507. 2007.PubMed/NCBI
|
|
51
|
Wu QN, Liao YF, Lu YX, Wang Y, Lu JH, Zeng
ZL, Huang QT, Sheng H, Yun JP, Xie D, et al: Pharmacological
inhibition of DUSP6 suppresses gastric cancer growth and metastasis
and overcomes cisplatin resistance. Cancer Lett. 412:243–255. 2018.
View Article : Google Scholar
|
|
52
|
Jiang X, Zhu X, Huang W, Xu H, Zhao Z, Li
S, Li S, Cai J and Cao J: Garlic-derived organosulfur compound
exerts antitumor efficacy via activation of MAPK pathway and
modulation of cytokines in SGC-7901 tumor-bearing mice. Int
Immunopharmacol. 48:135–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cao Y: The anti-invasion effects of
astragaloside IV on gastric cancer cell line SGC7901 and its
related mechanism. Shanxi Med J. 6:656–659. 2015.In Chinese.
|
|
55
|
Wu JY: Inhibition and mechanism of
astragaloside IV on H22 ascites in BALB/C mice. Chin J
Pharmacovigilance. 3:138–142. 2016.In Chinese.
|
|
56
|
Ogata Y, Miura K, Ohkita A, Nagase H and
Shirouzu K: Imbalance between matrix metalloproteinase 9 and tissue
inhibitor of metalloproteinases 1 expression by tumor cells
implicated in liver metastasis from colorectal carcinoma. Kurume
Med J. 48:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hajitou A, Sounni NE, Devy L,
Grignet-Debrus C, Lewalle JM, Li H, Deroanne CF, Lu H, Colige A,
Nusgens BV, et al: Down-regulation of vascular endothelial growth
factor by tissue inhibitor of metalloproteinase-2: Effect on in
vivo mammary tumor growth and angiogenesis. Cancer Res.
61:3450–3457. 2001.PubMed/NCBI
|
|
58
|
Keshavarz-Pakseresht B, Shandiz SA and
Baghbani-Arani F: Imatinib induces up-regulation of NM23, a
metastasis suppressor gene, in human Hepatocarcinoma (HepG2) cell
line. Gastroenterol Hepatol Bed Bench. 10:29–33. 2017.PubMed/NCBI
|
|
59
|
Zhou K: Effects of astragaloside IV on
gastric cancer cells and its related mechanisms. Hebei Medical
University. 2016.
|
|
60
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar
|
|
61
|
Han J, Shen X, Zhang Y, Wang S and Zhou L:
Astragaloside IV suppresses transforming growth factor-β1-induced
epithelial-mesenchymal transition through inhibition of
Wnt/β-catenin pathway in glioma U251 cells. Biosci Biotechnol
Biochem. 84:1345–1352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang L, Zhou J, Qin X, Huang H and Nie C:
Astragaloside IV inhibits the invasion and metastasis of SiHa
cervical cancer cells via the TGF-β1-mediated PI3K and MAPK
pathways. Oncol Rep. 41:2975–2986. 2019.PubMed/NCBI
|
|
65
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Su CC, Chiou TL, Chan MH and Lin JG:
Astragaloside IV increases MMP-2 mRNA and protein expression in
human lung cancer A549 cells. Mol Med Rep. 2:107–113.
2009.PubMed/NCBI
|
|
67
|
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG,
Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, et
al: MiR-1 and miR-200 inhibit EMT via Slug-dependent and
tumorigenesis via Slug-independent mechanisms. Oncogene.
32:296–306. 2013. View Article : Google Scholar
|
|
68
|
Kitamura K, Seike M, Okano T, Matsuda K,
Miyanaga A, Mizutani H, Noro R, Minegishi Y, Kubota K and Gemma A:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther.
13:444–453. 2014. View Article : Google Scholar
|
|
69
|
Liu Y, Zhang M, Qian J, Bao M, Meng X,
Zhang S, Zhang L, Zhao R, Li S, Cao Q, et al: miR-134 functions as
a tumor suppressor in cell proliferation and
epithelial-to-mesenchymal Transition by targeting KRAS in renal
cell carcinoma cells. DNA Cell Biol. 34:429–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ye Q, Su L, Chen D, Zheng W and Liu Y:
Astragaloside IV Induced miR-134 expression reduces EMT and
increases chemotherapeutic sensitivity by suppressing CREB1
signaling in colorectal cancer cell line SW-480. Cell Physiol
Biochem. 43:1617–1626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C,
Li J, Ye Y, Yao J, Liang K, et al: The LINK-A lncRNA interacts with
PtdIns(3,4,5) P3 to hyperactivate AKT and confer
resistance to AKT inhibitors. Nat Cell Biol. 19:238–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-beta promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Ye Y and Chen H: Astragaloside IV
inhibits cell migration and viability of hepatocellular carcinoma
cells via suppressing long noncoding RNA ATB. Biomed Pharmacother.
99:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang K, Lu Q, Li Q, Ji Y, Chen W and Xue
X: Astragaloside IV inhibits breast cancer cell invasion by
suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol.
42:195–202. 2017. View Article : Google Scholar
|
|
76
|
Qi H, Wei L, Han Y, Zhang Q, Lau AS and
Rong J: Proteomic characterization of the cellular response to
chemopreventive triterpenoid astragaloside IV in human
hepatocellular carcinoma cell line HepG2. Int J Oncol. 36:725–735.
2010.PubMed/NCBI
|
|
77
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar
|
|
79
|
Cao LP: Mod J Integr Tradit Chin West Med.
7:798–800. 2010.In Chinese.
|
|
80
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Choi YH and Yu AM: ABC transporters in
multidrug resistance and pharmacokinetics, and strategies for drug
development. Curr Pharm Des. 20:793–807. 2014. View Article : Google Scholar
|
|
82
|
Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ,
Yang CY, Zhu YH and Wang YQ: Astragaloside IV downregulates the
expression of MDR1 in Bel-7402/FU human hepatic cancer cells by
inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol Med Rep.
16:2761–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang PP, Xu DJ, Huang C, Wang WP and Xu
WK: Astragaloside IV reduces the expression level of P-glycoprotein
in multi-drug-resistant human hepatic cancer cell lines. Mol Med
Rep. 9:2131–2137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sebastián C, Zwaans BM, Silberman DM,
Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber
D, et al: The histone deacetylase SIRT6 is a tumor suppressor that
controls cancer metabolism. Cell. 151:1185–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xie T, Li Y, Li SL and Luo HF:
Astragaloside IV enhances cisplatin chemosensitivity in human
colorectal cancer via regulating NOTCH3. Oncol Res. 24:447–453.
2016. View Article : Google Scholar
|
|
87
|
Tian YZ: The function and mechanism of
astragaloside IV on the chemoresistance of HepG2/GCS cell lines.
Chin J Hepatobiliary Surg. 8:555–559. 2018.In Chinese.
|
|
88
|
Wang Z, Wang N, Liu P, Peng F, Tang H,
Chen Q, Xu R, Dai Y, Lin Y, Xie X, et al: Caveolin-1, a
stress-related oncotarget, in drug resistance. Oncotarget.
6:37135–37150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Morrison DK: MAP kinase pathways. Cold
Spring Harb Perspect Biol. 4:a0112542012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Iachettini S, Trisciuoglio D, Rotili D,
Lucidi A, Salvati E, Zizza P, Di Leo L, Del Bufalo D, Ciriolo MR,
Leonetti C, et al: Pharmacological activation of SIRT6 triggers
lethal autophagy in human cancer cells. Cell Death Dis. 9:9962018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shang JL, Ning SB, Chen YY, Chen TX and
Zhang J: MDL-800, an allosteric activator of SIRT6, suppresses
proliferation and enhances EGFR-TKIs therapy in non-small cell lung
cancer. Acta Pharmacol Sin. 42:120–131. 2021. View Article : Google Scholar
|
|
92
|
Krishnamoorthy V and Vilwanathan R:
Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in
non-small cell lung cancer cell lines. Genomics. 112:3703–3712.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang J, Cai Y and Sheng Z: Experimental
studies on the protective effects of the overexpression of
lentivirus-mediated sirtuin 6 on radiation-induced lung injury. Adv
Clin Exp Med. 29:873–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Giovannini C, Baglioni M, Baron Toaldo M,
Ventrucci C, D'Adamo S, Cipone M, Chieco P, Gramantieri L and
Bolondi L: Notch3 inhibition enhances sorafenib cytotoxic efficacy
by promoting GSK3b phosphorylation and p21 down-regulation in
hepatocellular carcinoma. Oncotarget. 4:1618–1631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Giovannini C, Gramantieri L, Chieco P,
Minguzzi M, Lago F, Pianetti S, Ramazzotti E, Marcu KB and Bolondi
L: Selective ablation of Notch3 in HCC enhances doxorubicin's death
promoting effect by a p53 dependent mechanism. J Hepatol.
50:969–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu YY, Hill RA and Li YT: Ceramide
glycosylation catalyzed by glucosylceramide synthase and cancer
drug resistance. Adv Cancer Res. 117:59–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Farhood B, Najafi M and Mortezaee K:
CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J
Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar
|
|
98
|
Zhang A, Zheng Y, Que Z, Zhang L, Lin S,
Le V, Liu J and Tian J: Astragaloside IV inhibits progression of
lung cancer by mediating immune function of Tregs and CTLs by
interfering with IDO. J Cancer Res Clin Oncol. 140:1883–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu TG: Effects of cisplatin combined with
astragaloside IV on inflammatory factors and immune function in
rats with breast cancer. Chin J Gerontol. 4:863–865. 2020.In
Chinese.
|
|
101
|
Lin L: The antitumor effect ASIV and
β-elemene to the immune system of mice with liver tumor. Nanjing
University of Chinese Medicine. 2011.
|
|
102
|
Li Y, Meng T, Hao N, Tao H, Zou S, Li M,
Ming P, Ding H, Dong J, Feng S, et al: Immune regulation mechanism
of Astragaloside IV on RAW264.7 cells through activating the
NF-κB/MAPK signaling pathway. Int Immunopharmacol. 49:38–49. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Singh SS, Vats S, Chia AY, Tan TZ, Deng S,
Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, et al: Dual role of
autophagy in hall-marks of cancer. Oncogene. 37:1142–1158. 2018.
View Article : Google Scholar
|
|
104
|
Tanida I, Ueno T and Kominami E: LC3 and
Autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xia C, He Z and Cai Y: Quantitative
proteomics analysis of differentially expressed proteins induced by
astragaloside IV in cervical cancer cell invasion. Cell Mol Biol
Lett. 25:252020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hong CS, Graham NA, Gu W, Espindola
Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PAL,
Gardner BK, et al: MCT1 modulates cancer cell pyruvate export and
growth of tumors that co-express MCT1 and MCT4. Cell Rep.
14:1590–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang C, Cai T, Zeng X, Cai D, Chen Y,
Huang X, Gan H, Zhuo J, Zhao Z, Pan H and Li S: Astragaloside IV
reverses MNNG-induced precancerous lesions of gastric carcinoma in
rats: Regulation on glycolysis through miRNA-34a/LDHA pathway.
Phytother Res. 32:1364–1372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY,
Feng L, Yang H, Liang JQ, Liu YY, Liu L and Lu HW: Astragaloside IV
inhibits pathological functions of gastric cancer-associated
fibroblasts. World J Gastroenterol. 23:8512–8525. 2017. View Article : Google Scholar
|
|
109
|
Lou H, Kaur K, Sharma AK and Singal PK:
Adriamycin-induced oxidative stress, activation of MAP kinases and
apoptosis in isolated cardiomyocytes. Pathophysiology. 13:103–109.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cave A: Selective targeting of NADPH
oxidase for cardiovascular protection. Curr Opin Pharmacol.
9:208–213. 2009. View Article : Google Scholar
|
|
111
|
Lin J, Fang L, Li H, Li Z, Lyu L, Wang H
and Xiao J: Astragaloside IV alleviates doxorubicin induced
cardiomyopathy by inhibiting NADPH oxidase derived oxidative
stress. Eur J Pharmacol. 859:1724902019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lou Y, Guo Z, Zhu Y, Zhang G, Wang Y, Qi
X, Lu L, Liu Z and Wu J: Astragali radix and its main bioactive
compounds activate the Nrf2-mediated signaling pathway to induce
P-glycoprotein and breast cancer resistance protein. J
Ethnopharmacol. 228:82–91. 2019. View Article : Google Scholar
|
|
113
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang G, Ou R, Li F, Wu J, Zheng L, Tong
Y, Liu Y, Liu Z and Lu L: Regulation of drug-metabolizing enzymes
and efflux transporters by Astragali radix decoction and its main
bioactive compounds: Implication for clinical drug-drug
interactions. J Ethnopharmacol. 180:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|