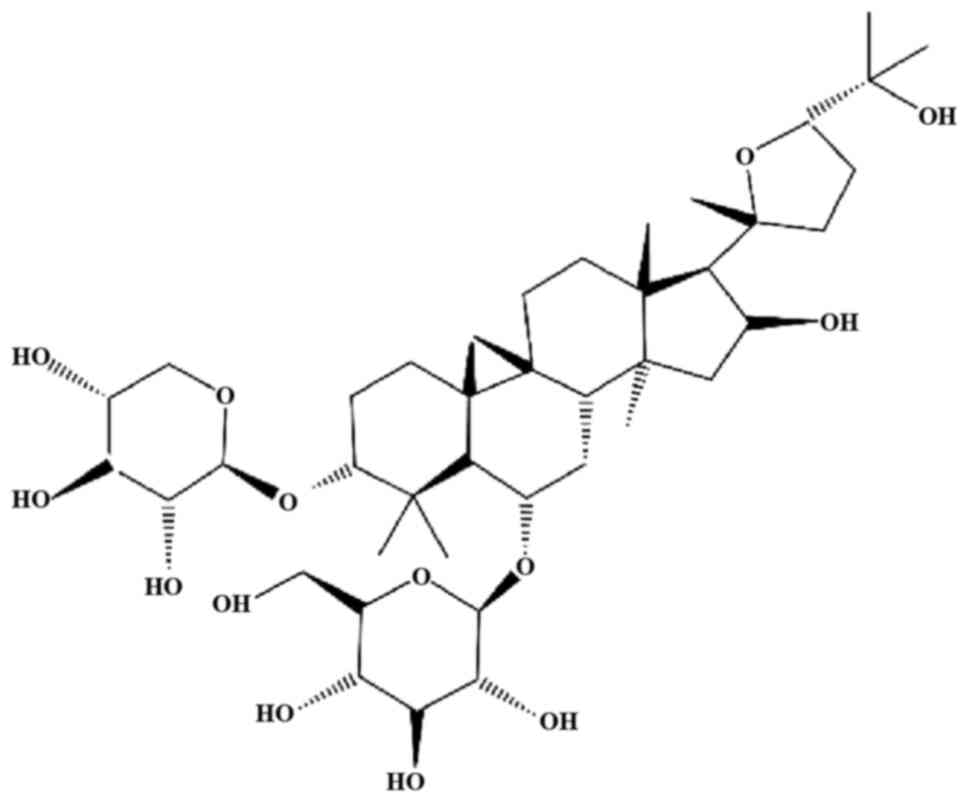
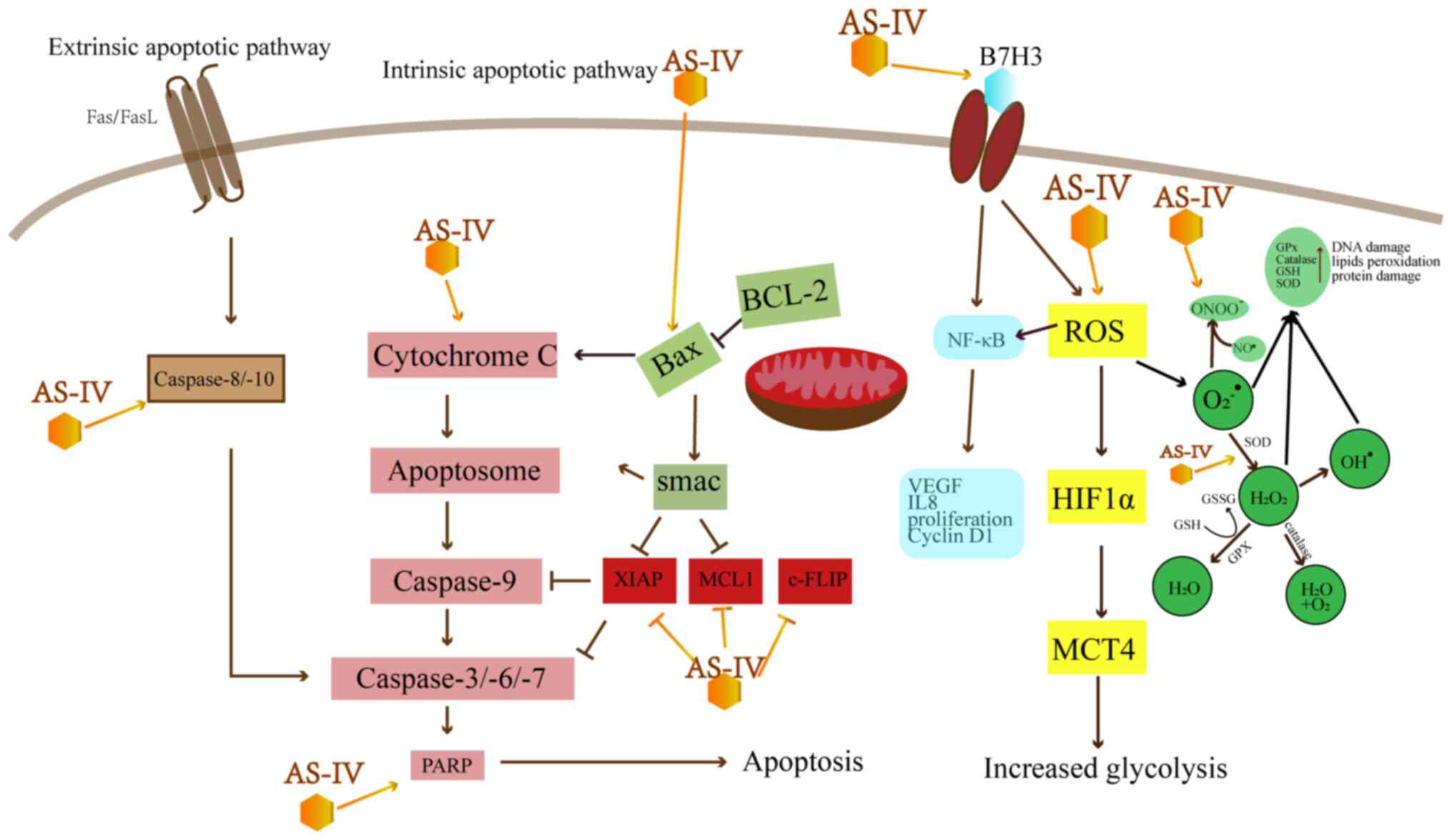
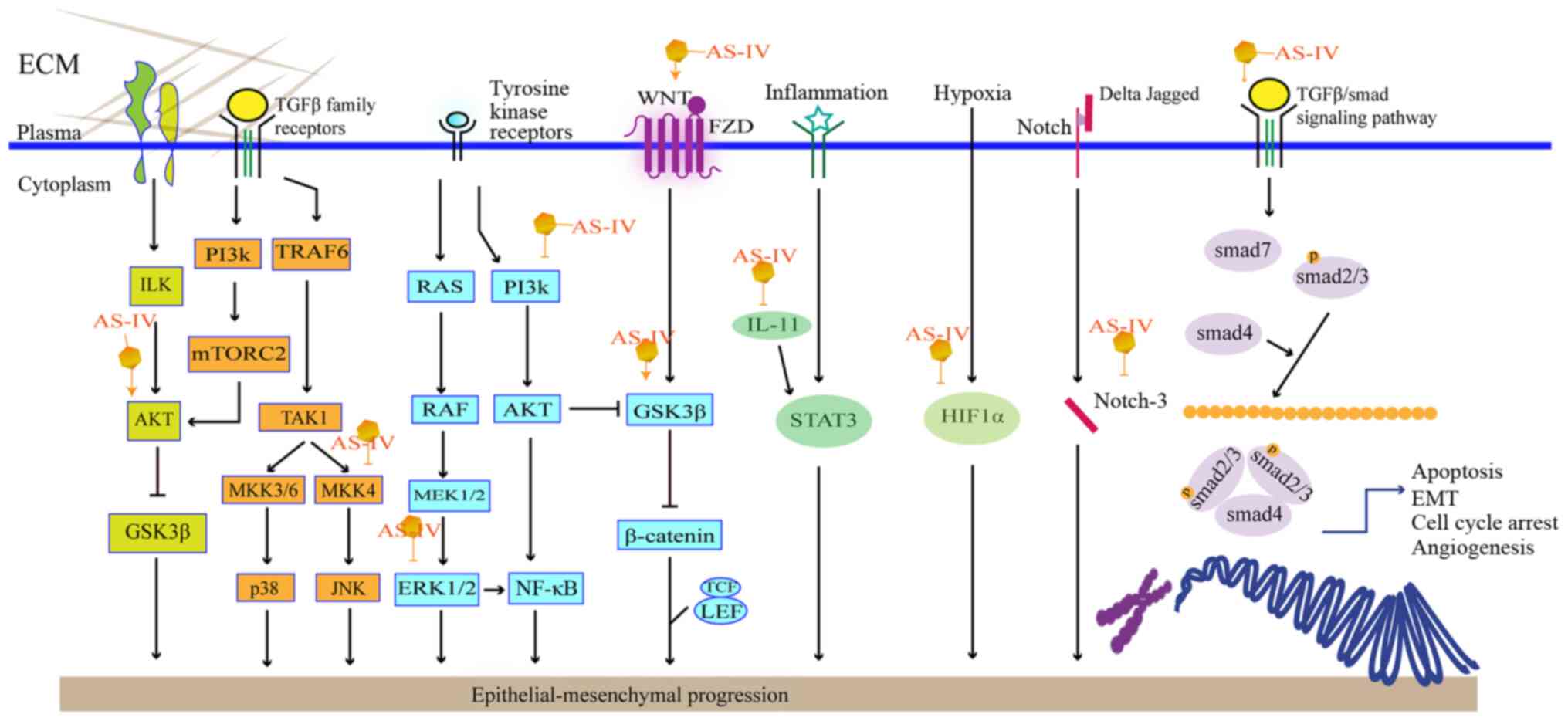
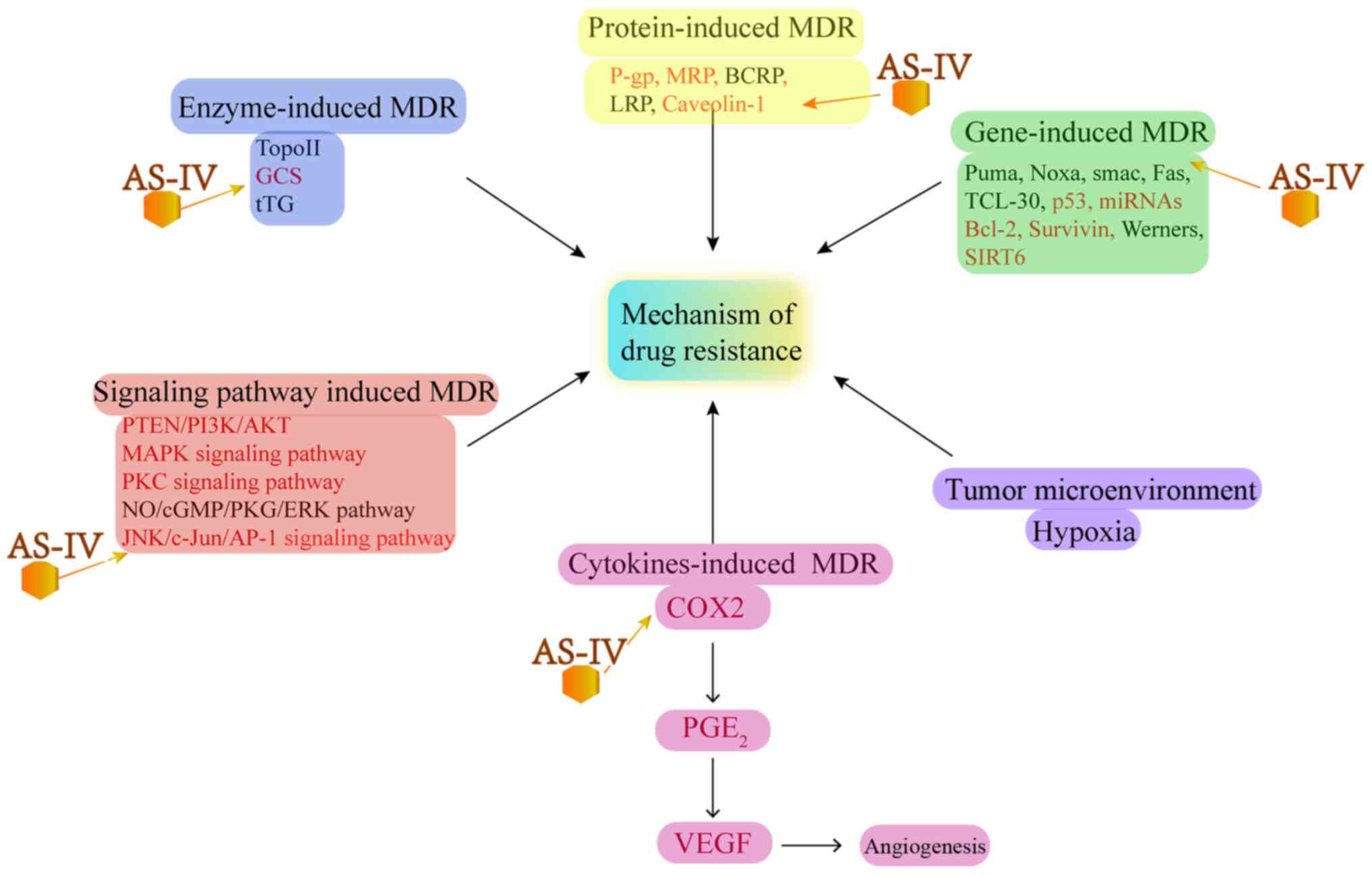
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Ni Y, Sun G, Zhu S, Zhao J, Wang
Z, Zhang H, Zhu X, Zhang X, Dai J, et al: Survival outcomes of
radical prostatectomy + extended pelvic lymph node dissection and
radiotherapy in prostate cancer patients with a risk of lymph node
invasion over 5%: A population-based analysis. Front Oncol.
10:6075762020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Cintio F, Dal Bo M, Baboci L, De Mattia
E, Polano M and Toffoli G: The molecular and microenvironmental
landscape of glioblastomas: Implications for the novel treatment
choices. Front Neurosci. 14:6036472020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C,
Hui B, Liu R, Ma H and Ren J: A review of neoadjuvant
chemoradiotherapy for locally advanced rectal cancer. Int J Biol
Sci. 12:1022–1031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhoday J, Glimelius B, Tait D,
Glynne-Jones R, Adams R and Brown G: Session 4: What should we do
for poor responders after chemoradiotherapy: Bad biology or should
the fight go on? Colorectal Dis. 20(Suppl 1): 97–99. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung Y, Jerng U and Lee S: A systematic
review of anticancer effects of radix astragali. Chin J Integr Med.
22:225–236. 2016. View Article : Google Scholar
|
|
8
|
Li X, Qu L, Dong Y, Han L, Liu E, Fang S,
Zhang Y and Wang T: A review of recent research progress on the
astragalus genus. Molecules. 19:18850–18880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SY, Ouyang HT, Yang JY, Huang XL, Yang
T, Duan JP, Cheng JP, Chen YX, Yang YJ and Qiong P: Subchronic
toxicity studies of Radix Astragali extract in rats and dogs. J
Ethnopharmacol. 110:352–355. 2007. View Article : Google Scholar
|
|
10
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of Astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Yang P, Li F, Tao L, Ding H, Rui
Y, Cao Z and Zhang W: Therapeutic effects of astragaloside IV on
myocardial injuries: Multi-target identification and network
analysis. PLoS One. 7:e449382012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karmakar S, Banik NL, Patel SJ and Ray SK:
Curcumin activated both receptor-mediated and mitochondria-mediated
proteolytic pathways for apoptosis in human glioblastoma T98G
cells. Neurosci Lett. 407:53–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bagci EZ, Vodovotz Y, Billiar TR,
Ermentrout GB and Bahar I: Bistability in apoptosis: Roles of bax,
bcl-2, and mitochondrial permeability transition pores. Biophys J.
90:1546–1559. 2006. View Article : Google Scholar
|
|
16
|
Sun P, Liu Y, Wang Q and Zhang B:
Astragaloside IV inhibits human colorectal cancer cell growth.
Front Biosci (Landmark Ed). 24:597–606. 2019. View Article : Google Scholar
|
|
17
|
Zheng Y, Dai Y, Liu W, Wang N, Cai Y, Wang
S, Zhang F, Liu P, Chen Q and Wang Z: Astragaloside IV enhances
taxol chemosensitivity of breast cancer via caveolin-1-targeting
oxidant damage. J Cell Physiol. 234:4277–4290. 2019. View Article : Google Scholar
|
|
18
|
Jia L, Lv D, Zhang S, Wang Z and Zhou B:
Astragaloside IV Inhibits the progression of non-small cell lung
cancer through the Akt/GSK-3β/β-Catenin Pathway. Oncol Res.
27:503–508. 2019. View Article : Google Scholar
|
|
19
|
Zhao Y, Wang L, Wang Y, Dong S, Yang S,
Guan Y and Wu X: Astragaloside IV inhibits cell proliferation in
vulvar squamous cell carcinoma through the TGF-β/Smad signaling
pathway. Dermatol Ther. 32:e128022019.
|
|
20
|
Cui X, Jiang X, Wei C, Xing Y and Tong G:
Astragaloside IV suppresses development of hepatocellular carcinoma
by regulating miR-150-5p/β-catenin axis. Environ Toxicol Pharmacol.
78:1033972020. View Article : Google Scholar
|
|
21
|
Ying G: Astragaloside induces gastric
MGC-803 cells apoptosis by inhibiting AKT and NF-KB pathway. Int J
Lab Med. 19:2341–2344. 2018.
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007.PubMed/NCBI
|
|
23
|
Hu T, Fei Z and Wei N: Chemosensitive
effects of Astragaloside IV in osteosarcoma cells via induction of
apoptosis and regulation of caspase-dependent Fas/FasL signaling.
Pharmacol Rep. 69:1159–1164. 2017.PubMed/NCBI
|
|
24
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252.
1999.PubMed/NCBI
|
|
25
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006.PubMed/NCBI
|
|
26
|
Du X, Bao G, He X, Zhao H, Yu F, Qiao Q,
Lu J and Ma Q: Expression and biological significance of c-FLIP in
human hepatocellular carcinomas. J Exp Clin Cancer Res.
28:242009.PubMed/NCBI
|
|
27
|
Fleischer B, Schulze-Bergkamen H,
Schuchmann M, Weber A, Biesterfeld S, Müller M, Krammer PH and
Galle PR: Mcl-1 is an anti-apoptotic factor for human
hepatocellular carcinoma. Int J Oncol. 28:25–32. 2006.
|
|
28
|
Che Y, Ye F, Xu R, Qing H, Wang X, Yin F,
Cui M, Burstein D, Jiang B and Zhang DY: Co-expression of XIAP and
cyclin D1 complex correlates with a poor prognosis in patients with
hepatocellular carcinoma. Am J Pathol. 180:1798–1807.
2012.PubMed/NCBI
|
|
29
|
Cameron BD, Traver G, Roland JT, Brockman
AA, Dean D, Johnson L, Boyd K, Ihrie RA and Freeman ML:
Bcl2-Expressing Quiescent Type B neural stem cells in the
Ventricular-Subventricular zone are resistant to concurrent
Temozolomide/X-Irradiation. Stem Cells. 37:1629–1639.
2019.PubMed/NCBI
|
|
30
|
Tanaka N, Patel AA, Wang J, Frederick MJ,
Kalu NN, Zhao M, Fitzgerald AL, Xie TX, Silver NL, Caulin C, et al:
Wee-1 kinase inhibition sensitizes high-risk HPV+ HNSCC to
apoptosis accompanied by downregulation of MCl-1 and XIAP
antiapoptotic proteins. Clin Cancer Res. 21:4831–4844.
2015.PubMed/NCBI
|
|
31
|
Lin YC, Chen RY, Liang JA, Hung YC, Yeh
LS, Chang WC, Lin WC, Chang YY and Chen SW: Immunohistochemical
biomarkers of survival in patients with adenocarcinoma of the
uterine cervix receiving chemoradiotherapy. Anticancer Res.
39:3231–3240. 2019.PubMed/NCBI
|
|
32
|
Finkel T: Oxidant signals and oxidative
stress. Curr Opin Cell Biol. 15:247–254. 2003.PubMed/NCBI
|
|
33
|
Su CM, Wang HC, Hsu FT, Lu CH, Lai CK,
Chung JG and Kuo YC: Astragaloside IV induces apoptosis,
G1-phase arrest and inhibits anti-apoptotic signaling in
hepatocellular carcinoma. In Vivo. 34:631–638. 2020.PubMed/NCBI
|
|
34
|
Chang XY: Effects of cisplatin combined
with astragaloside iv on apoptosis genes in C6 glioma mice. Chin J
Gerontol. 13:3282–3284. 2019.In Chinese.
|
|
35
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
|
|
36
|
Bułdak RJ, Bułdak Ł, Kukla M, Gabriel A
and Zwirska-Korczala K: Significance of selected antioxidant
enzymes in cancer cell progression. Pol J Pathol. 65:167–175.
2014.
|
|
37
|
Yang JY: Effect of astragaloside IV on the
proliferation of Spc-A-1 cells in human lung cancer and its
mechanism. Chin Traditional Patent Med. 8:1818–1820. 2016.In
Chinese.
|
|
38
|
Wang L, Kang FB and Shan BE:
B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer.
134:2764–2771. 2014.
|
|
39
|
Flem-Karlsen K, Fodstad Ø, Tan M and
Nunes-Xavier CE: B7-H3 in cancer - beyond immune regulation. Trends
Cancer. 4:401–404. 2018.PubMed/NCBI
|
|
40
|
Ling V, Wu PW, Spaulding V, Kieleczawa J,
Luxenberg D, Carreno BM and Collins M: Duplication of primate and
rodent B7-H3 immunoglobulin V- and C-like domains: Divergent
history of functional redundancy and exon loss. Genomics.
82:365–377. 2003.PubMed/NCBI
|
|
41
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He CS, Liu YC, Xu ZP, Dai PC, Chen XW and
Jin DH: Astragaloside IV enhances cisplatin chemosensitivity in
non-small cell lung cancer cells through inhibition of B7-H3. Cell
Physiol Biochem. 40:1221–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
An XC: Mechanism of astragaloside IV
promoting proliferation and apoptosis of hepatoma cells by
inhibiting ROS/NF-κB signaling pathway. Mod Dig Intervent.
12:1399–1403. 2019.In Chinese.
|
|
44
|
Brooks PC, Strömblad S, Sanders LC, von
Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP and
Cheresh DA: Localization of matrix metalloproteinase MMP-2 to the
surface of invasive cells by interaction with integrin alpha v beta
3. Cell. 85:683–693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Trepat X, Chen Z and Jacobson K: Cell
migration. Compr Physiol. 2:2369–2392. 2012.
|
|
46
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rizzo D, Ruggiero A, Amato M, Maurizi P
and Riccardi R: BRAF and MEK inhibitors in pediatric glioma: New
therapeutic strategies, new toxicities. Expert Opin Drug Metab
Toxicol. 12:1397–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zohrabian VM, Forzani B, Chau Z, Murali R
and Jhanwar-Uniyal M: Rho/ROCK and MAPK signaling pathways are
involved in glioblastoma cell migration and proliferation.
Anticancer Res. 29:119–123. 2009.PubMed/NCBI
|
|
50
|
Nickl-Jockschat T, Arslan F, Doerfelt A,
Bogdahn U, Bosserhoff A and Hau P: An imbalance between Smad and
MAPK pathways is responsible for TGF-beta tumor promoting effects
in high-grade gliomas. Int J Oncol. 30:499–507. 2007.PubMed/NCBI
|
|
51
|
Wu QN, Liao YF, Lu YX, Wang Y, Lu JH, Zeng
ZL, Huang QT, Sheng H, Yun JP, Xie D, et al: Pharmacological
inhibition of DUSP6 suppresses gastric cancer growth and metastasis
and overcomes cisplatin resistance. Cancer Lett. 412:243–255. 2018.
View Article : Google Scholar
|
|
52
|
Jiang X, Zhu X, Huang W, Xu H, Zhao Z, Li
S, Li S, Cai J and Cao J: Garlic-derived organosulfur compound
exerts antitumor efficacy via activation of MAPK pathway and
modulation of cytokines in SGC-7901 tumor-bearing mice. Int
Immunopharmacol. 48:135–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cao Y: The anti-invasion effects of
astragaloside IV on gastric cancer cell line SGC7901 and its
related mechanism. Shanxi Med J. 6:656–659. 2015.In Chinese.
|
|
55
|
Wu JY: Inhibition and mechanism of
astragaloside IV on H22 ascites in BALB/C mice. Chin J
Pharmacovigilance. 3:138–142. 2016.In Chinese.
|
|
56
|
Ogata Y, Miura K, Ohkita A, Nagase H and
Shirouzu K: Imbalance between matrix metalloproteinase 9 and tissue
inhibitor of metalloproteinases 1 expression by tumor cells
implicated in liver metastasis from colorectal carcinoma. Kurume
Med J. 48:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hajitou A, Sounni NE, Devy L,
Grignet-Debrus C, Lewalle JM, Li H, Deroanne CF, Lu H, Colige A,
Nusgens BV, et al: Down-regulation of vascular endothelial growth
factor by tissue inhibitor of metalloproteinase-2: Effect on in
vivo mammary tumor growth and angiogenesis. Cancer Res.
61:3450–3457. 2001.PubMed/NCBI
|
|
58
|
Keshavarz-Pakseresht B, Shandiz SA and
Baghbani-Arani F: Imatinib induces up-regulation of NM23, a
metastasis suppressor gene, in human Hepatocarcinoma (HepG2) cell
line. Gastroenterol Hepatol Bed Bench. 10:29–33. 2017.PubMed/NCBI
|
|
59
|
Zhou K: Effects of astragaloside IV on
gastric cancer cells and its related mechanisms. Hebei Medical
University. 2016.
|
|
60
|
Du B and Shim JS: Targeting
epithelial-mesenchymal transition (EMT) to overcome drug resistance
in cancer. Molecules. 21:9652016. View Article : Google Scholar
|
|
61
|
Han J, Shen X, Zhang Y, Wang S and Zhou L:
Astragaloside IV suppresses transforming growth factor-β1-induced
epithelial-mesenchymal transition through inhibition of
Wnt/β-catenin pathway in glioma U251 cells. Biosci Biotechnol
Biochem. 84:1345–1352. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH,
Wang YC, Ye BG, Cao MQ, Gao DM and Tang ZY: Astragaloside IV
inhibits metastasis in hepatoma cells through the suppression of
epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin
pathway. Oncol Rep. 37:1725–1735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang L, Zhou J, Qin X, Huang H and Nie C:
Astragaloside IV inhibits the invasion and metastasis of SiHa
cervical cancer cells via the TGF-β1-mediated PI3K and MAPK
pathways. Oncol Rep. 41:2975–2986. 2019.PubMed/NCBI
|
|
65
|
Cheng X, Gu J, Zhang M, Yuan J, Zhao B,
Jiang J and Jia X: Astragaloside IV inhibits migration and invasion
in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB
pathway. Int Immunopharmacol. 23:304–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Su CC, Chiou TL, Chan MH and Lin JG:
Astragaloside IV increases MMP-2 mRNA and protein expression in
human lung cancer A549 cells. Mol Med Rep. 2:107–113.
2009.PubMed/NCBI
|
|
67
|
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG,
Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, et
al: MiR-1 and miR-200 inhibit EMT via Slug-dependent and
tumorigenesis via Slug-independent mechanisms. Oncogene.
32:296–306. 2013. View Article : Google Scholar
|
|
68
|
Kitamura K, Seike M, Okano T, Matsuda K,
Miyanaga A, Mizutani H, Noro R, Minegishi Y, Kubota K and Gemma A:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther.
13:444–453. 2014. View Article : Google Scholar
|
|
69
|
Liu Y, Zhang M, Qian J, Bao M, Meng X,
Zhang S, Zhang L, Zhao R, Li S, Cao Q, et al: miR-134 functions as
a tumor suppressor in cell proliferation and
epithelial-to-mesenchymal Transition by targeting KRAS in renal
cell carcinoma cells. DNA Cell Biol. 34:429–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ye Q, Su L, Chen D, Zheng W and Liu Y:
Astragaloside IV Induced miR-134 expression reduces EMT and
increases chemotherapeutic sensitivity by suppressing CREB1
signaling in colorectal cancer cell line SW-480. Cell Physiol
Biochem. 43:1617–1626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C,
Li J, Ye Y, Yao J, Liang K, et al: The LINK-A lncRNA interacts with
PtdIns(3,4,5) P3 to hyperactivate AKT and confer
resistance to AKT inhibitors. Nat Cell Biol. 19:238–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yan X, Hu Z, Feng Y, Hu X, Yuan J, Zhao
SD, Zhang Y, Yang L, Shan W, He Q, et al: Comprehensive genomic
characterization of long non-coding RNAs across human cancers.
Cancer Cell. 28:529–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-beta promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li Y, Ye Y and Chen H: Astragaloside IV
inhibits cell migration and viability of hepatocellular carcinoma
cells via suppressing long noncoding RNA ATB. Biomed Pharmacother.
99:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jiang K, Lu Q, Li Q, Ji Y, Chen W and Xue
X: Astragaloside IV inhibits breast cancer cell invasion by
suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol.
42:195–202. 2017. View Article : Google Scholar
|
|
76
|
Qi H, Wei L, Han Y, Zhang Q, Lau AS and
Rong J: Proteomic characterization of the cellular response to
chemopreventive triterpenoid astragaloside IV in human
hepatocellular carcinoma cell line HepG2. Int J Oncol. 36:725–735.
2010.PubMed/NCBI
|
|
77
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hashemi Goradel N, Najafi M, Salehi E,
Farhood B and Mortezaee K: Cyclooxygenase-2 in cancer: A review. J
Cell Physiol. 234:5683–5699. 2019. View Article : Google Scholar
|
|
79
|
Cao LP: Mod J Integr Tradit Chin West Med.
7:798–800. 2010.In Chinese.
|
|
80
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Choi YH and Yu AM: ABC transporters in
multidrug resistance and pharmacokinetics, and strategies for drug
development. Curr Pharm Des. 20:793–807. 2014. View Article : Google Scholar
|
|
82
|
Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ,
Yang CY, Zhu YH and Wang YQ: Astragaloside IV downregulates the
expression of MDR1 in Bel-7402/FU human hepatic cancer cells by
inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol Med Rep.
16:2761–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang PP, Xu DJ, Huang C, Wang WP and Xu
WK: Astragaloside IV reduces the expression level of P-glycoprotein
in multi-drug-resistant human hepatic cancer cell lines. Mol Med
Rep. 9:2131–2137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sebastián C, Zwaans BM, Silberman DM,
Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber
D, et al: The histone deacetylase SIRT6 is a tumor suppressor that
controls cancer metabolism. Cell. 151:1185–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Dai PC, Liu DL, Zhang L, Ye J, Wang Q,
Zhang HW, Lin XH and Lai GX: Astragaloside IV sensitizes non-small
cell lung cancer cells to gefitinib potentially via regulation of
SIRT6. Tumour Biol. 39:10104283176975552017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xie T, Li Y, Li SL and Luo HF:
Astragaloside IV enhances cisplatin chemosensitivity in human
colorectal cancer via regulating NOTCH3. Oncol Res. 24:447–453.
2016. View Article : Google Scholar
|
|
87
|
Tian YZ: The function and mechanism of
astragaloside IV on the chemoresistance of HepG2/GCS cell lines.
Chin J Hepatobiliary Surg. 8:555–559. 2018.In Chinese.
|
|
88
|
Wang Z, Wang N, Liu P, Peng F, Tang H,
Chen Q, Xu R, Dai Y, Lin Y, Xie X, et al: Caveolin-1, a
stress-related oncotarget, in drug resistance. Oncotarget.
6:37135–37150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Morrison DK: MAP kinase pathways. Cold
Spring Harb Perspect Biol. 4:a0112542012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Iachettini S, Trisciuoglio D, Rotili D,
Lucidi A, Salvati E, Zizza P, Di Leo L, Del Bufalo D, Ciriolo MR,
Leonetti C, et al: Pharmacological activation of SIRT6 triggers
lethal autophagy in human cancer cells. Cell Death Dis. 9:9962018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shang JL, Ning SB, Chen YY, Chen TX and
Zhang J: MDL-800, an allosteric activator of SIRT6, suppresses
proliferation and enhances EGFR-TKIs therapy in non-small cell lung
cancer. Acta Pharmacol Sin. 42:120–131. 2021. View Article : Google Scholar
|
|
92
|
Krishnamoorthy V and Vilwanathan R:
Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in
non-small cell lung cancer cell lines. Genomics. 112:3703–3712.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang J, Cai Y and Sheng Z: Experimental
studies on the protective effects of the overexpression of
lentivirus-mediated sirtuin 6 on radiation-induced lung injury. Adv
Clin Exp Med. 29:873–877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Giovannini C, Baglioni M, Baron Toaldo M,
Ventrucci C, D'Adamo S, Cipone M, Chieco P, Gramantieri L and
Bolondi L: Notch3 inhibition enhances sorafenib cytotoxic efficacy
by promoting GSK3b phosphorylation and p21 down-regulation in
hepatocellular carcinoma. Oncotarget. 4:1618–1631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Giovannini C, Gramantieri L, Chieco P,
Minguzzi M, Lago F, Pianetti S, Ramazzotti E, Marcu KB and Bolondi
L: Selective ablation of Notch3 in HCC enhances doxorubicin's death
promoting effect by a p53 dependent mechanism. J Hepatol.
50:969–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu YY, Hill RA and Li YT: Ceramide
glycosylation catalyzed by glucosylceramide synthase and cancer
drug resistance. Adv Cancer Res. 117:59–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Farhood B, Najafi M and Mortezaee K:
CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J
Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar
|
|
98
|
Zhang A, Zheng Y, Que Z, Zhang L, Lin S,
Le V, Liu J and Tian J: Astragaloside IV inhibits progression of
lung cancer by mediating immune function of Tregs and CTLs by
interfering with IDO. J Cancer Res Clin Oncol. 140:1883–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Liu TG: Effects of cisplatin combined with
astragaloside IV on inflammatory factors and immune function in
rats with breast cancer. Chin J Gerontol. 4:863–865. 2020.In
Chinese.
|
|
101
|
Lin L: The antitumor effect ASIV and
β-elemene to the immune system of mice with liver tumor. Nanjing
University of Chinese Medicine. 2011.
|
|
102
|
Li Y, Meng T, Hao N, Tao H, Zou S, Li M,
Ming P, Ding H, Dong J, Feng S, et al: Immune regulation mechanism
of Astragaloside IV on RAW264.7 cells through activating the
NF-κB/MAPK signaling pathway. Int Immunopharmacol. 49:38–49. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Singh SS, Vats S, Chia AY, Tan TZ, Deng S,
Ong MS, Arfuso F, Yap CT, Goh BC, Sethi G, et al: Dual role of
autophagy in hall-marks of cancer. Oncogene. 37:1142–1158. 2018.
View Article : Google Scholar
|
|
104
|
Tanida I, Ueno T and Kominami E: LC3 and
Autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Xia C, He Z and Cai Y: Quantitative
proteomics analysis of differentially expressed proteins induced by
astragaloside IV in cervical cancer cell invasion. Cell Mol Biol
Lett. 25:252020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hong CS, Graham NA, Gu W, Espindola
Camacho C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PAL,
Gardner BK, et al: MCT1 modulates cancer cell pyruvate export and
growth of tumors that co-express MCT1 and MCT4. Cell Rep.
14:1590–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang C, Cai T, Zeng X, Cai D, Chen Y,
Huang X, Gan H, Zhuo J, Zhao Z, Pan H and Li S: Astragaloside IV
reverses MNNG-induced precancerous lesions of gastric carcinoma in
rats: Regulation on glycolysis through miRNA-34a/LDHA pathway.
Phytother Res. 32:1364–1372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY,
Feng L, Yang H, Liang JQ, Liu YY, Liu L and Lu HW: Astragaloside IV
inhibits pathological functions of gastric cancer-associated
fibroblasts. World J Gastroenterol. 23:8512–8525. 2017. View Article : Google Scholar
|
|
109
|
Lou H, Kaur K, Sharma AK and Singal PK:
Adriamycin-induced oxidative stress, activation of MAP kinases and
apoptosis in isolated cardiomyocytes. Pathophysiology. 13:103–109.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Cave A: Selective targeting of NADPH
oxidase for cardiovascular protection. Curr Opin Pharmacol.
9:208–213. 2009. View Article : Google Scholar
|
|
111
|
Lin J, Fang L, Li H, Li Z, Lyu L, Wang H
and Xiao J: Astragaloside IV alleviates doxorubicin induced
cardiomyopathy by inhibiting NADPH oxidase derived oxidative
stress. Eur J Pharmacol. 859:1724902019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lou Y, Guo Z, Zhu Y, Zhang G, Wang Y, Qi
X, Lu L, Liu Z and Wu J: Astragali radix and its main bioactive
compounds activate the Nrf2-mediated signaling pathway to induce
P-glycoprotein and breast cancer resistance protein. J
Ethnopharmacol. 228:82–91. 2019. View Article : Google Scholar
|
|
113
|
Zhu J and Wen K: Astragaloside IV inhibits
TGF-β1-induced epithelial-mesenchymal transition through inhibition
of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother
Res. 32:1289–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang G, Ou R, Li F, Wu J, Zheng L, Tong
Y, Liu Y, Liu Z and Lu L: Regulation of drug-metabolizing enzymes
and efflux transporters by Astragali radix decoction and its main
bioactive compounds: Implication for clinical drug-drug
interactions. J Ethnopharmacol. 180:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|