Introduction
Mesenchymal stem cells (MSCs) generally have
anti-inflammatory, tissue repairing and immunomodulatory properties
(1). The therapeutic effect of
MSCs is known to be mediated by their secretome (2-6).
Secretome refers to the secretary molecules, cytokines, chemokines
and growth factors that are released by MSCs and in broader terms,
includes extracellular vesicles (EVs), such as exosomes,
microvesicles and apoptotic bodies (3,5,7).
Despite decades of research on stem cells, the paucity of clinical
application is due to several limitations of stem cell research,
including limited in vivo cell survival, senescence-related
genetic instability, and potential of malignant transformation
(8,9). In this respect, utilizing the
secretome instead of stem cells themselves is expected to be one of
the prominent strategies which may be used to overcome the
limitations of stem cell therapy (8,10,11).
Adjusting the culture conditions of MSCs can lead to
a significant difference in the composition of the secretome
(7,12). This means that a greater
therapeutic potential could be obtained from the secretome with
adjusted culture conditions than from the naïve secretome. There
are mainly 2 methods use for adjusting the culture conditions of
MSCs: The adjustment of the physico-chemical conditions and genetic
engineering. As a method of improving the therapeutic potentials of
MSCs by adjusting the physico-chemical conditions, the authors have
previously attempted hypoxic and lipopolysaccharide
pre-conditioning (13-15). In addition, several genes have
been utilized to reinforce the therapeutic potentials of the
secretome, including Akt (16),
gene for heat shock protein 20 (17) and nuclear factor erythroid
2-related factor 2 (18).
Overall, these methods attempted thus far belong to the
non-specific stimulation of MSCs to generate the secretome.
Furthermore, the authors attempted to identify a method which may
be used to stimulate MSCs to generate a disease-specifical
secretome.
In the present study, it was hypothesized that
pre-sensitization of MSCs with disease-causing agents could harness
MSCs to release the secretome containing therapeutic materials
specialized for the disease. Herein, the induced secretome
(isecretome) was defined as the secretome released from MSCs that
had been stimulated by disease-causing materials for the treatment
of the specific disease. In a previous study, it was demonstrated
that the thioacetamide (TAA; a hepatotoxin)-induced secretome was
superior to the naïve secretome in restoring hepatic function,
while minimizing inflammatory processes in mice with
thioacetamide-induced hepatic failure (19). In the present study, the authors
aimed to construct the isecretome using hepatitis B virus (HBV) to
further generalize the hypothesis. If the HBx-induced secretome
(HBx-isecretrome) is shown to have a greater therapeutic efficacy
than the control secretome in the model of hepatitis B, it could
increase the possibility of generating the disease-specific
secretome.
Materials and methods
Chemicals, cells and cell culture
TAA was obtained from Sigma-Aldrich; Merck KGaA. The
AML12 mouse hepatocyte cell line was obtained from the American
Type Culture Collection (ATCC). AML12 cells were maintained in
Dulbecco's modified Eagle's medium/Ham's F-12; DMEM/F12 (Thermo
Fisher Scientific, Inc.). The medium was supplemented with 10% FBS
(fetal bovine serum; Gibco; Thermo Fisher Scientific, Inc.), 1%
antibiotics (Thermo Fisher Scientific, Inc.), 1X ITS supplement
(Insulin-Transferrin-Selenium-G supplement; Invitrogen; Thermo
Fisher Scientific, Inc.) and 40 ng/ml dexamethasone (Sigma-Aldrich;
Merck KGaA) at 37°C. Human adipose-derived stem cells (ASCs) were
kindly donated by Hurim BioCell Co. (Seoul). ASCs were cultured in
DMEM/lowGlucose (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with antibiotics (Penicillin-streptomycin; Gibco;
Thermo Fisher Scientific, Inc.) at 37°C.
Preparation of secretomes released from
ASCs
ASCs that reached 70-80% confluency were re-fed with
serum-free DMEM low-glucose medium (Thermo Fisher Scientific, Inc.)
at 37°C under 5% CO2. Thereafter, the ASCs were cultured
under 2 different conditions as follows: One subset was directly
transfected with 4 µg pcDNA-HBx for 24 h and the other
subset was indirectly treated with culture medium from AML12 cells
that had been transfected with 4 µg pcDNA-HBx for 24 h. In
the process of transfection, pcDNA3.1 (Thermo Fisher Scientific,
Inc.) we used as the plasmid backbone and Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used as a
transfection reagent. In more detail, 24 h following the
transfection of pcDNA-HBx into the ASCs, the transfected ASCs were
incubated in serum-free DMEM low-glucose medium at 37°C for 24 h.
The 25-fold-concentrated medium obtained from the culture medium of
the transfected ASCs was termed as the direct pcDNA-HBx secretome.
Subsequently, 24 h following the transfection of pcDNA-HBx into
AML12 cells, the transfected AML12 cells were incubated in
serum-free DMEM/F12 medium for 24 h. The 25-fold concentration of
the culture medium obtained from the transfected AML12 cells was
then obtained. Subsequently, the ASCs were treated with the 25-fold
concentrated medium in serum-free DMEM low-glucose medium at a
ratio of 1:100 for 24 h. The culture medium of these ASCs was
termed as the indirect pcDNA-HBx secretome. Finally, the control
secretome (CS) refers to a 25-fold concentration of the conditioned
medium that was obtained following incubation of the ASCs for 24 h.
The concentration of the culture medium was accomplished using
ultrafiltration units (Amicon Ultra-PL 3; EMD Millipore) with a
3-kDa cut-off.
Preparation of toxin-conditioned
medium
The AML12 cells was treated with 0.1 mM TAA for 24
h. Conditioned medium from the TAA-treated AML12 cells were
collected, and were concentrated approximately 25-fold using ultra
filtration units with a 3-kDa molecular weight cutoff. After
culturing the ASCs that reached 70-80% confluency, the cells were
re-fed with serum-free DMEM low-glucose medium. The ASCs was
treated with conditioned medium (1:1,000) for 24 h.
Toxin-conditioned medium from the CM-treated ASC cells was
collected, and was concentrated approximately 25-fold using ultra
filtration units with a 3-kDa molecular weight cut-off. The
toxin-conditioned medium was termed as the TAA-isecretome and
stored at -80°C until its use in the experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total cell RNA was extracted using FavorPrep TRI-RNA
reagent (Favorgen) according to the manufacturer's instructions.
Reverse transcription was performed with 1 µg RNA using
ReverTra Ace® qPCR RT Master mix (Toyobo Life Science)
according to the manufacturer's instructions. The primers used for
SYBR-Green quantitative PCR were as follows: Vascular endothelial
growth factor (VEGF) sense, 5′-TGT ACC TCC ACC ATG CCA AGT-3′ and
antisense, 5′-CGC TGG TAG ACA TCC ATG AA-3′; hepatocyte growth
factor (HGF) sense, 5′-TGC TGT CCT GGA TGA TTT TG-3′ and antisense,
5′-AGT GTA GCC CCA GCC ATA AA-3′; HBx sense, 5′-CCT CTA CCG TCC CCT
TCT TC-3′ and antisense, 5′-GTC GGT CGT TGA CAT TGT TG-3′; and
GAPDH sense (forward), 5′-GCA CCG TCA AGG CTG AGA AC-3′ and
antisense (reverse), 5′-TGG TGA AGA CGC CAG TGG A-3′. The PCR
parameters were as follows: An initial denaturation step (at 95°C
for 10 sec); amplification and quantification step (40 cycles: At
95°C for 15 sec and then 60°C for 60 sec); and final elongation
step (1 cycle: At 95°C for 15 sec, 60°C for 60 sec and then 95°C
for 15 sec). The reaction was performed using a Step one plus
Real-Time PCR system (Life Technologies; Thermo Fisher Scientific,
Inc.). Following normalization to the GAPDH gene, the expression
levels for each target gene were calculated using the
2−∆∆Cq method (20).
Western blot analysis
Mouse liver specimens were lysed using the EzRIPA
Lysis kit (ATTO Corporation), and quantified using Bradford reagent
(Bio-Rad Laboratories, Inc.). Cells and liver tissue lysates were
separated by SDS-polyacrylamide gel on 10% gels and
electrotransferred onto a nitrocellulose membrane (Bio-Rad
Laboratories, Inc.). The membrane was blocked with 1X blocking
solution (TransLab Biosciences) for 1 h at room temperature.
Proteins were visualized by western blot analysis by incubation
with the following primary antibodies (1:1,000 dilution) at 4°C
overnight and then with horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:2,000 dilution) for 1 h at 25°C. Primary
antibodies against Bcl-2-like protein 11 (Bim, cat no. 2933), HGF,
myeloid cell leukemia 1 (Mcl-1, cat no. 5453), poly(ADP-ribose)
polymerase (PARP, cat no. #9542), proliferating cell nuclear
antigen (PCNA, cat no. 2586), phosphorylated signal transducer and
activator of transcription 3 (p-STAT3, cat no. 9131) and cleaved
caspase 3 (c-caspase 3, cat no. 9664) were obtained from Cell
Signaling Technology, Inc. VEGF (cat no. ab46154) was obtained from
Abcam. β-actin (cat no. sc58673) was obtained from Santa Cruz
Biotechnology, Inc. HRP-conjugated secondary antibody (cat no.
ADI-SAB-100) was obtained from Enzo Life Sciences. Specific immune
complexes were detected using the Western Blotting Plus
Chemiluminescence Reagent (EMD Millipore). Relative densities of
individual markers were quantified using Image Lab software V3.0
(Bio-Rad Laboratories, Inc.).
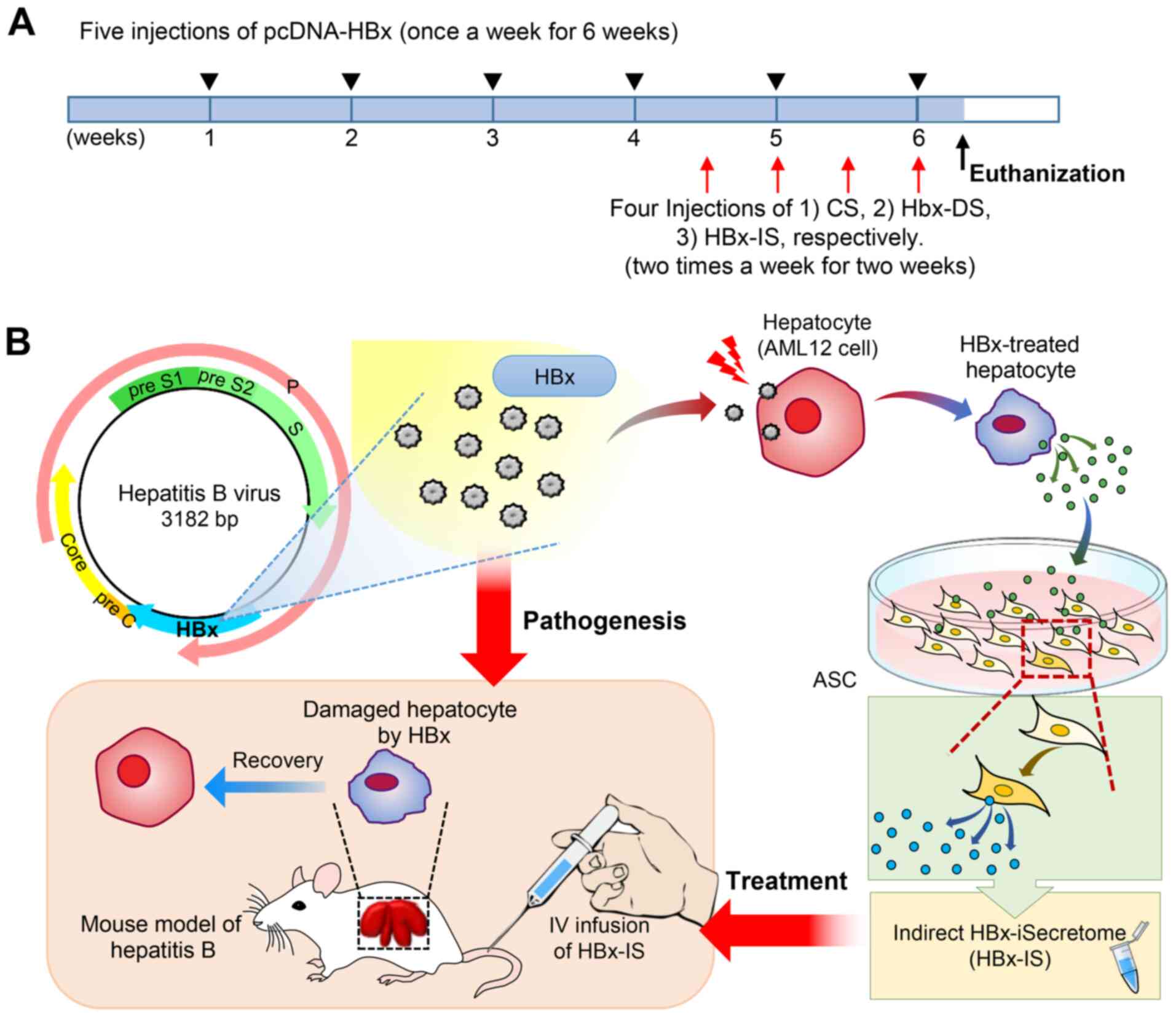
Animal experiments
BALB/c mice, weighing 25-30 g (n=52; 8 weeks old,
male; Damool Science) were used in the present study. The Ethics
Committee at Daejeon St. Mary's Hospital, the Catholic University
of Korea, approved the animal experiments for the research (IRB no.
CMCDJ-AP-2016-001). The in vivo model of chronic hepatitis B
was generated by an intravenous injection of pcDNA-HBx (4 µg
pcDNA-HBx and in vivo-jetPEI® Transfection mix,
once a week for 6 weeks) into the mice, as previously described
(21,22). Subsequently, the experimental mice
received 4 intravenous injections (twice a week for 2 weeks through
the tail vein) of secretome (100 µl for each injection).
Specifically, the control mice were injected with normal saline
(control group; n=5), the control secretome (CS; n=5), HBx-DS (n=5)
and HBx-IS (n=5), and the mice with hepatitis B were injected with
normal saline (control group; n=8), CS (n=8), HBx-DS (n=8) and
HBx-IS (n=8) (Fig. 1A). Herein,
CS refers to a 25-fold concentration of the conditioned medium that
was obtained following incubation of the ASCs at 37°C for 24 h.
Subsequently, the mouse model of TAA-induced hepatic failure was
generated using TAA (each amount, 300 mg/kg) for 2 consequent days
administered intraperitoneally. From the first day of the TAA
administration, the mice received 2 intravenous injections (100
µl for each injection) of CS (n=5), TAA-isecretome (n=5) and
HBx-IS (n=5) for 2 consequent days, via the tail vein. On the
following day of the final injection, the mice were euthanized and
the liver specimens were examined.
Serological test and ELISA
Blood samples (0.2 ml per one time) obtained from
each mouse from the tail vein on the day of euthanization, were
centrifuged at 4°C for 10 min at 10,000 × g and the sera were
collected. The concentrations of markers of liver injury, such as
aspartate transaminase (AST) and alanine transaminase (ALT) were
measured using an IDEXX VetTest Chemistry Analyzer (IDEXX
Laboratories, Inc.). In addition, the serum concentrations of mouse
interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) were
measured using a sandwich enzyme-linked immunosorbent (ELISA) assay
(Abcam, Inc.) according to the manufacturer's instructions.
Hematoxylin and eosin (H&E) staining,
immunohistochemistry and immunofluorescence
For analysis, formalin-fixed paraffin-embedded liver
tissue sections (<3 mm in thickness) were deparaffinized,
rehydrated in an ethanol series and subjected to epitope retrieval
using standard procedures. H&E staining was performed by
staining the specimens with Harris's hematoxylin (YD Dignostics)
for 7 min, rinsing with tap water for 1 min, and incubation with
Eosin Y solution (Duksan) for 150 sec at 25°C. The sections were
then dehydrated with 80% and then wtih 100% EtOH, dipped in Xylene
(Duksan) for 3 min and mounted in a permount mounting medium (cat.
no. #SP15-100; Thermo Fisher Scientific, Inc.). The specimens were
examined under a panoramic distal slide scanner system (3D
HISTECH). Immunohistochemical analysis were visualized by
incubation with the following primary anti-bodies at 4°C overnight
and then with peroxidase-conjugated secondary antibodies (cat nos.
PK-6101 and PK-6102; Vector Laboratories, Inc.) for 1 h at 25°C.
Antibodies against VEGF (1:200; Abcam, cat no. ab1316), B-cell
leukemia-extra large (Bcl-xL) (1:100; Abcam, ab98143) and
γ-glutamyl transpeptidase (γ-GTP; Santa Cruz Biotechnology, Inc.,
1:200; cat no. sc100746) were used for immunochemical staining. The
samples were then examined under a panoramic distal slide scanner
system (3D HISTECH). immunofluorescence analysis were visualized by
incubation with the following primary anti-bodies at 4°C overnight
and then with fluorescent-secondary antibodies (1:500; Invitrogen;
Thermo Fisher Scientific, Inc., cat no. A11005 and A11008) for 1 h
at 25°C. Antibodies against F4/80 (1:100; Santa Cruz Biotechnology,
Inc., cat no. sc37709) and CD68 (1:500; Abcam, cat no. #125212)
were used for immunofluorescence staining. The samples were then
examined under a fluorescence imaging system (EVOS M5000: Thermo
Fisher Scientific, Inc.) to analyze the expression of F4/80 and
CD68. Percentages of immunofluorescent areas were measured using
NIH Image J and expressed as relative values to those in control
mice.
Statistical analysis
All data were analyzed using SPSS 11.0 software
(SPSS Inc.), and are presented as the means ± standard deviation
(SD). Statistical comparison among groups was determined using the
Kruskal-Wallis test followed by the Mann-Whitney test with
Bonferroni correction as the post hoc analysis. A P-value <0.05
was considered to indicate a statistically significant
difference.
Results
Effects of HBx-isecretome on the
expression of HGF and VEGF mRNA in the liver
The present study aimed to determine whether the
secretome obtained by stimulating ASCs with specific
disease-causing agents (pathogens) was more effective in improving
the specific disease than the control secretome. In this
experiment, the pathogen and the specific disease were set to HBx
and hepatitis B, respectively. The present study used 2 methods to
obtain the HBx-isecretome. The first method involved the collection
of the secretome released from HBx-overexpressing ASCs that had
been produced by transfecting ASCs with pcDNA-HBx for 24 h. The
second method involved the collection of secretary materials
following the stimulation of ASCs with 100-fold diluted culture
medium of AML12 cells that had been transfected with pcDNA-HBx for
24 h (Fig. 1B). These were termed
as the direct HBx-isecretome (HBx-DS) and indirect HBx-isecretome
(HBx-IS), respectively. The successful transfection of HBx was
identified by confirming the upregulation of HBx through RT-qPCR.
Specifically, in vitro transfection was validated by
demonstrating the higher HBx-mRNA expression in the transfected
AML12 cells and ASCs, respectively (both P<0.05; Fig. 2A). Subsequently, in vivo
transfection was validated by demonstrating the higher HBx-mRNA
expression in the liver tissues of the mice that had been injected
with pcDNA-HBx via the tail vein (P<0.05; Fig. 2B). An animal model of hepatitis B
was then generated by injecting HBx into mice, and the mice were
subsequently intravenously administered CS, HBx-DS and HBx-IS. On
the following day of the final injection, the mice were euthanized
and the specimens were investigated. H&E staining of each
treatment revealed that HBx-IS infusion resulted in a higher
restoration of the hepatic tissue than HBx-DS infusion (Fig. 2C). RT-qPCR was performed to
compare the mRNA expression levels of HGF and VEGF in the mouse
liver specimens (Fig. 2D). The
HBx-DS injection did not increase the mRNA expression of HGF and
VEGF compared to the CS injection in the mice with hepatitis B.
However, the HBx-IS injection to the mice with hepatitis B
significantly increased the mRNA expression of HGF and VEGF
compared to the CS injection (P<0.05).
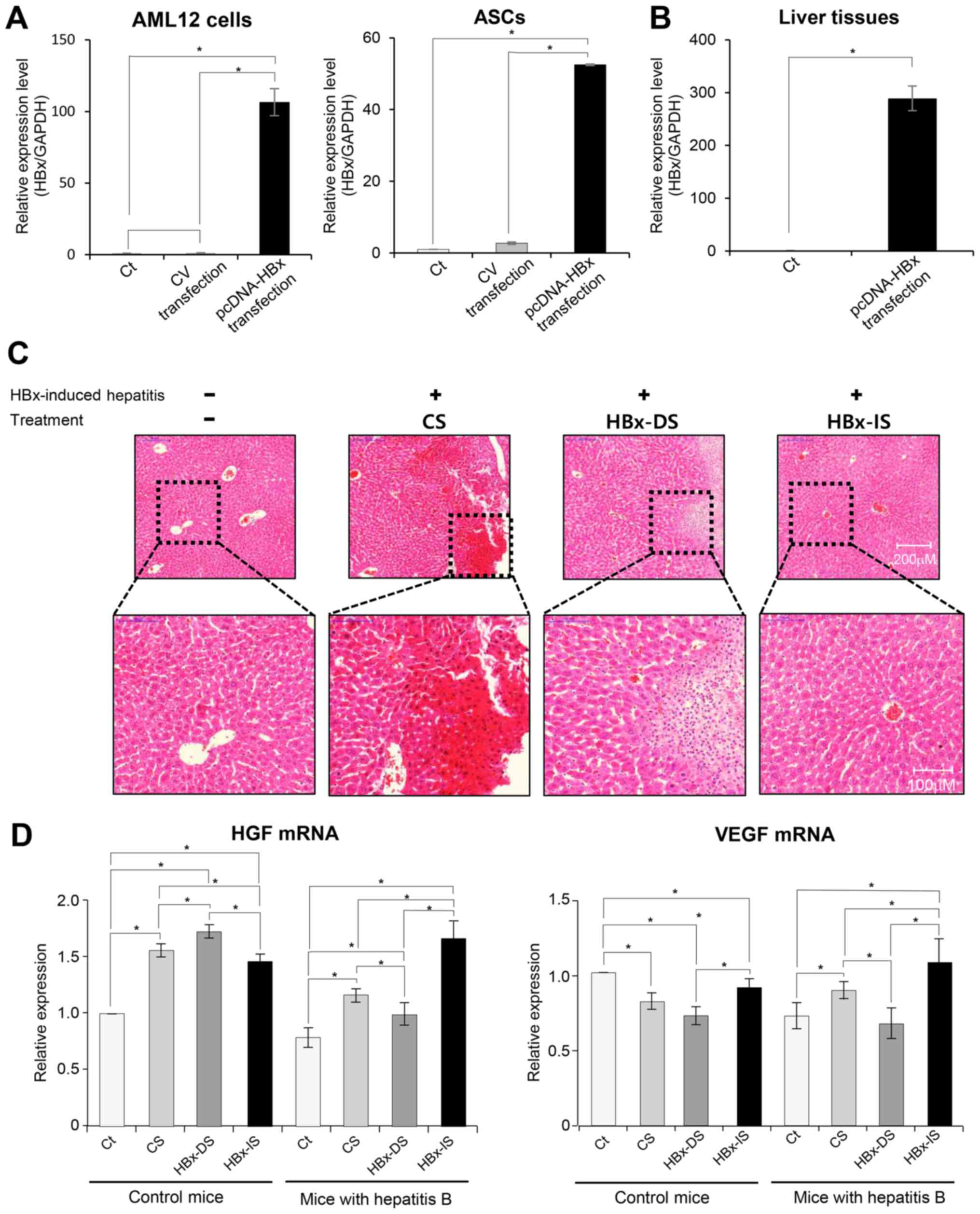 | Figure 2Validation of HBx transfection and the
effects of the HBx-isecretome on the expression of mRNAs reflecting
liver regeneration. (A) In vitro validation of HBx
transfection. Results of RT-qPCR showing the higher HBx-mRNA
expression in the transfected AML12 cells and ASCs, respectively
(both P<0.05). (B) In vivo validation of HBx
transfection. Results of RT-qPCR showing the higher HBx-mRNA
expression in the liver tissues of the mice that had been injected
with pcDNA-HBx via the tail vein (P<0.05). (C) Representative
histological comparison of each treatment. CS, HBx-DS and HBx-IS,
respectively, were intravenously administered to the mice with
HBx-induced hepatitis. H&E staining showing that the HBx-IS
infusion resulted in a greater restoration of the hepatic tissue
than the HBx-DS infusion. (D) Results of RT-qPCR showing the mRNA
expression of HGF and VEGF following individual treatments. HBx-DS
injection did not increase the expression of HGF and VEGF mRNA
compared to the CS injection in the mice with hepatitis B. However,
the HBx-IS injection to the mice with hepatitis B significantly
increased the expression of HGF and VEGF mRNA compared to the CS
injection. Values are presented as the means ± standard deviation
of 3 independent experiments. *P<0.05. ASCs,
adipose-derived stem cells; HBx, hepatitis B virus protein X; CS,
control secretome; CV, control vector; HBx-IS, indirect HBx-induced
secretome; HBx-DS, direct HBx-induced secretome; HGF, hepatocyte
growth factor; VEGF, vascular endothelial growth factor. |
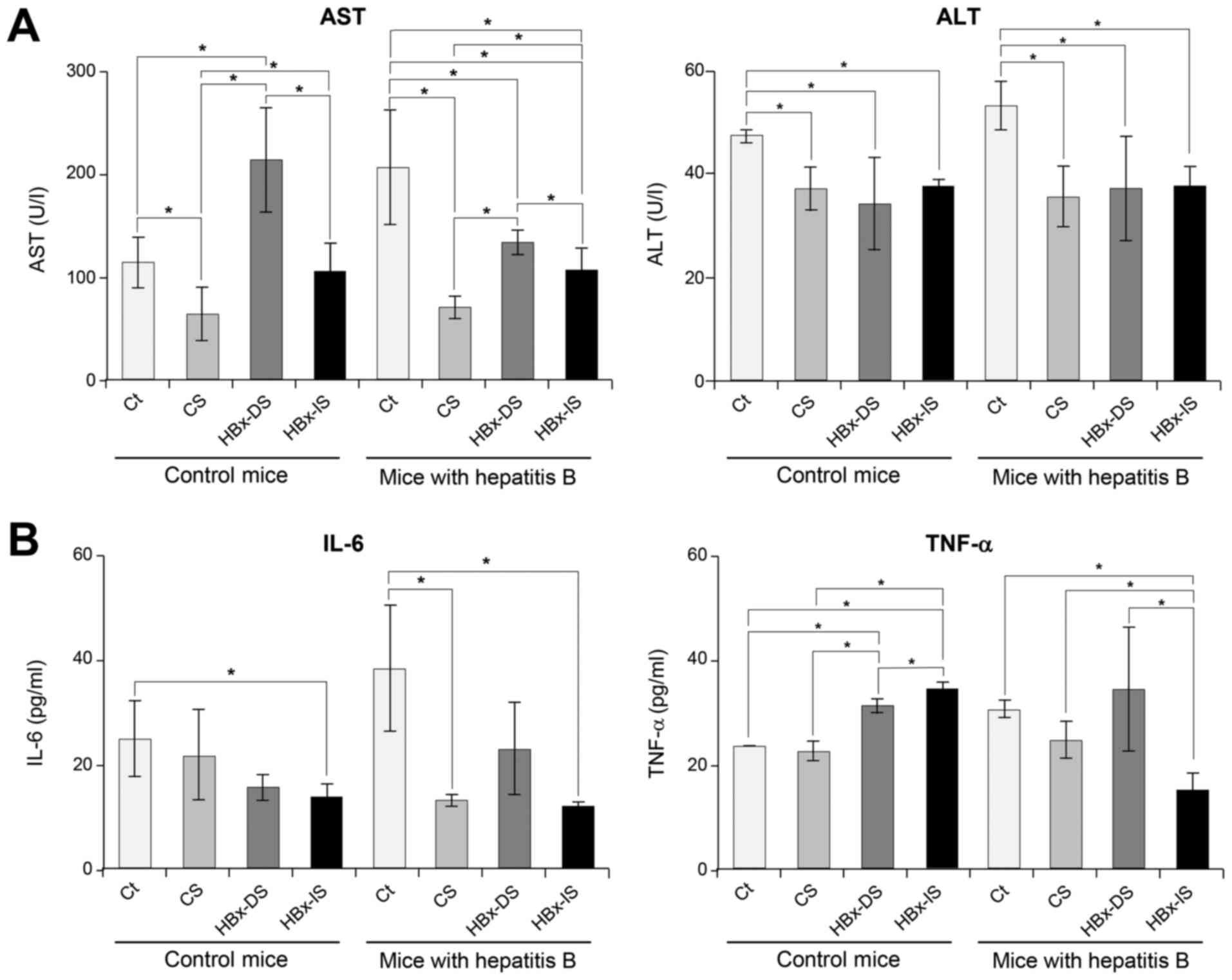
Effects of the HBx-isecretome on the
serum levels of liver enzymes and pro-inflammatory cytokines
Sera from mice were collected at the day of
euthanization and the serum levels of liver enzymes and
pro-inflammatory cytokines were compared. The mice with hepatitis B
exhibited elevated levels of AST, which were significantly
decreased following the individual secretome treatments (CS, HBx-DS
and HBx-IS) (P<0.05; Fig. 3A).
The serum levels of ALT were not significantly increased in the
mice with hepatitis B, and were significantly decreased following
the individual secretome treatments (P<0.05). Subsequently, the
serum levels of IL-6 and TNF-α were compared in each group
(Fig. 3B). In the mice with
hepatitis B, the HBx-DS injection did not significantly reduce the
serum levels of IL-6 and TNF-α compared to the CS injection.
However, the HBx-IS injection significantly decreased the serum
levels of IL-6 and TNF-α compared to the CS injection
(P<0.05).
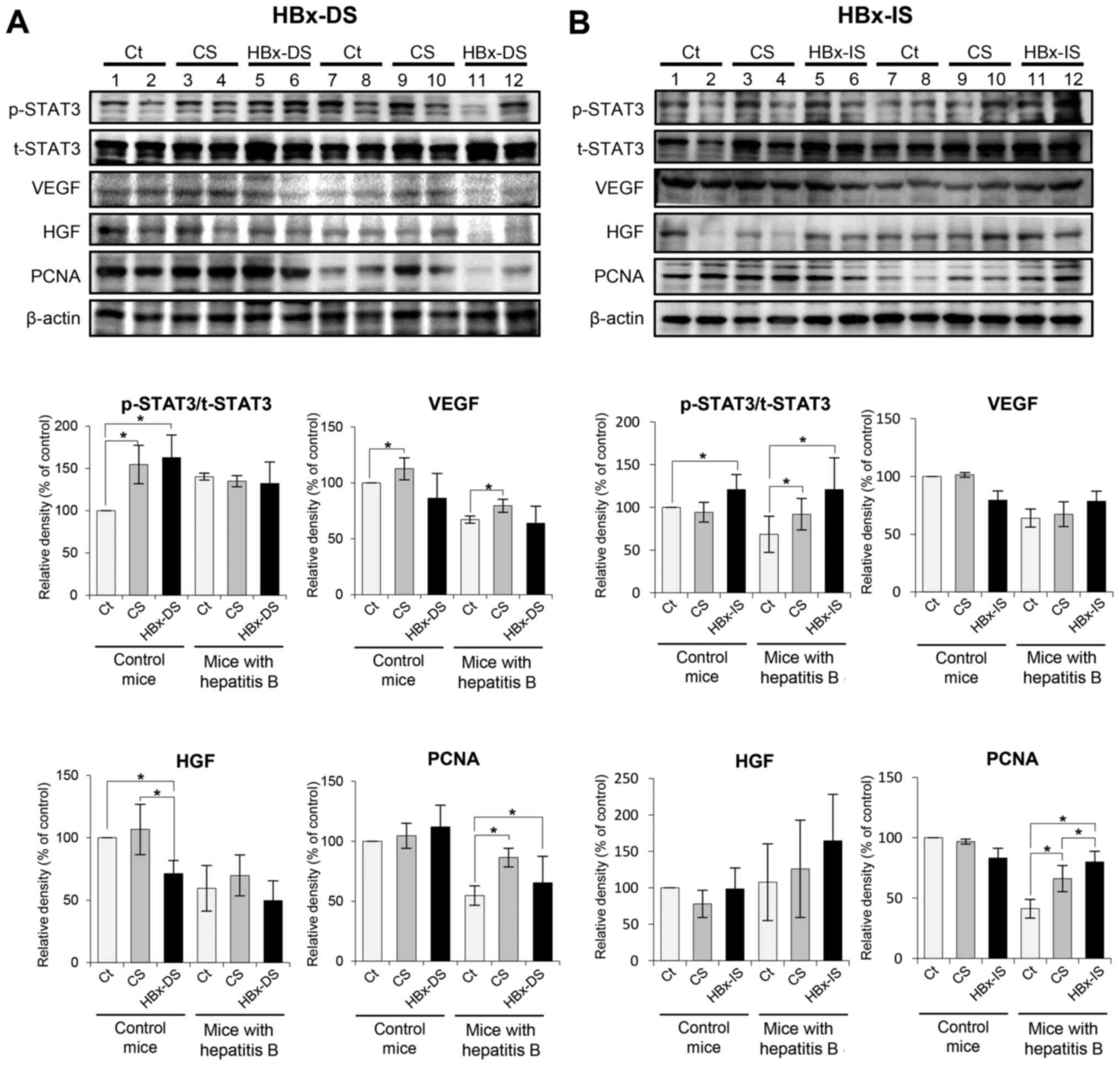
Effects of the HBx-isecretome on the
expression of proteins reflecting liver regeneration
Western blot analysis was performed to determine the
expression of liver regeneration-related proteins in the liver
specimens following each treatment. The liver regeneration-related
proteins, included p-STAT3/t-STAT3, VEGF, HGF and PCNA. In the mice
with hepatitis B, HBx-DS did not significantly increase the
expression levels of these liver regeneration-related proteins
compared with the CS (Fig. 4A).
By contrast, HBx-IS significantly increased the expression levels
of p-STAT3/t-STAT3 and PCNA compared with the CS (P<0.05;
Fig. 4B).
Effects of the HBx-isecretome on the
expression of apoptosis-related proteins
Western blot analysis was further performed to
determine the expression levels of apoptosis-related proteins in
the liver specimens following each treatment. Pro-apoptotic markers
included PARP, c-caspase 3 and BIM, and the examined anti-apoptotic
marker, was Mcl-1. In the mice with hepatitis B, HBx-DS
significantly increased the expression of certain pro-apoptotic
markers (c-caspase 3 and BIM) and significantly decreased the
expression of Mcl-1 compared with the CS (P<0.05; Fig. 5A). By contrast, HBx-IS
significantly decreased the expression of all the pro-apoptotic
markers tested (P<0.05), and insignificantly increased Mcl-1
compared with the CS (Fig.
5B).
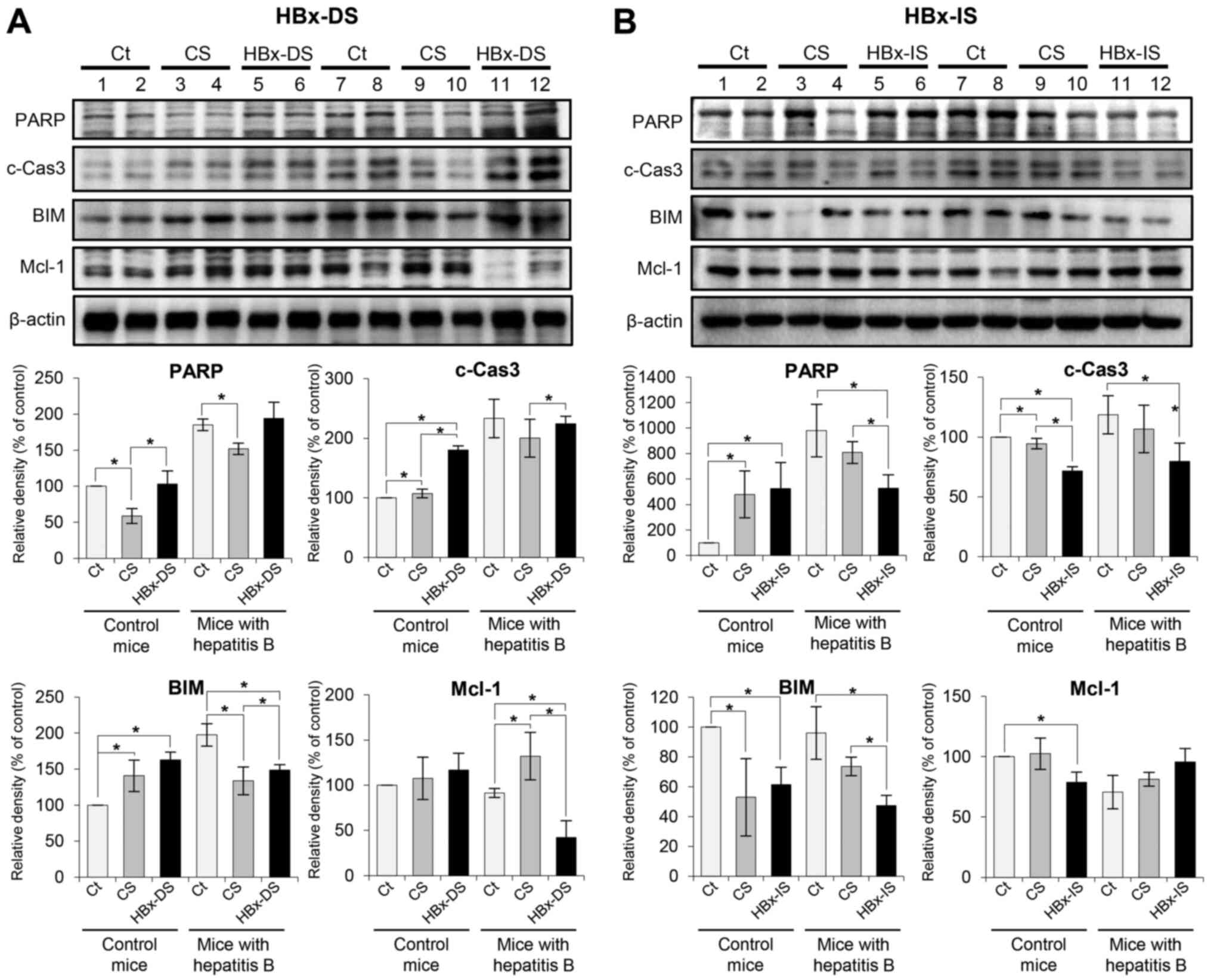 | Figure 5Effects of the HBx-isecretome on the
expression of apoptosis-related proteins. (A) Top panel illustrates
the effects of HBx-DS on the expression of apoptosis-related
proteins. In the mice with hepatitis B, HBx-DS significantly
increased the expression of certain pro-apoptotic markers
(c-caspase 3 and BIM) and significantly decreased the expression of
Mcl-1 compared with CS. Bottom panel illustrates relative densities
of apoptosis-related proteins in each group. (B) Top panel
illustrates the effects of HBx-IS on the expression of
apoptosis-related proteins. HBx-IS significantly decreased the
expression of all the pro-apoptotic markers tested (P<0.05), and
insignificantly increased Mcl-1 compared with CS. Bottom panel
illustrates relative densities of apoptosis-related proteins in
each group. Values are presented as the means ± standard deviation
of 3 independent experiments. Relative densities of individual
markers had been quantified using Image Lab software and were then
normalized to those of β-actin in each group.
*P<0.05. BIM, Bcl-2-like protein 11; HBx, hepatitis B
virus protein X; c-Cas3, cleaved caspase-3; CS, control secretome;
HBx-IS, indirect HBx-induced secretome; HBx-DS, direct HBx-induced
secretome; Mcl-1, myeloid cell leukemia 1; PARP, poly-ADP
(adenosine diphosphate)-ribose polymerase. |
Immunohistochemistry of the liver
following the administration of the HBx-isecretome
Immunostaining for VEGF (a liver
regeneration-related protein), Bcl-xL (an anti-apoptotic protein)
and γ-GTP (a marker for hepatitis) was performed using the liver
specimens. The mouse model of hepatitis B exhibited a decreased
expression of VEGF and Bcl-xL in the liver. However, the injection
of CS and HBx-IS increased the expression of VEGF and Bcl-xL in the
liver (Fig. 6A and B). When
comparing the CS and HBx-IS, the expression of VEGF and Bcl-xL was
significantly higher in the HBx-IS group than in the CS group
(P<0.05). By contrast, the mouse model of hepatitis B exhibited
an increased expression of γ-GTP in the liver. However, the
injection of CS and HBx-IS decreased the expression of γ-GTP in the
liver (Fig. 6C). When comparing
CS and HBx-IS, the expression of γ-GTP was significantly lower in
the HBx-IS group than in the CS group (P<0.05). Taken together,
these results suggest that HBx-IS has a higher liver regenerative
potential and a higher ability to inhibit cell apoptosis and
hepatitis than CS.
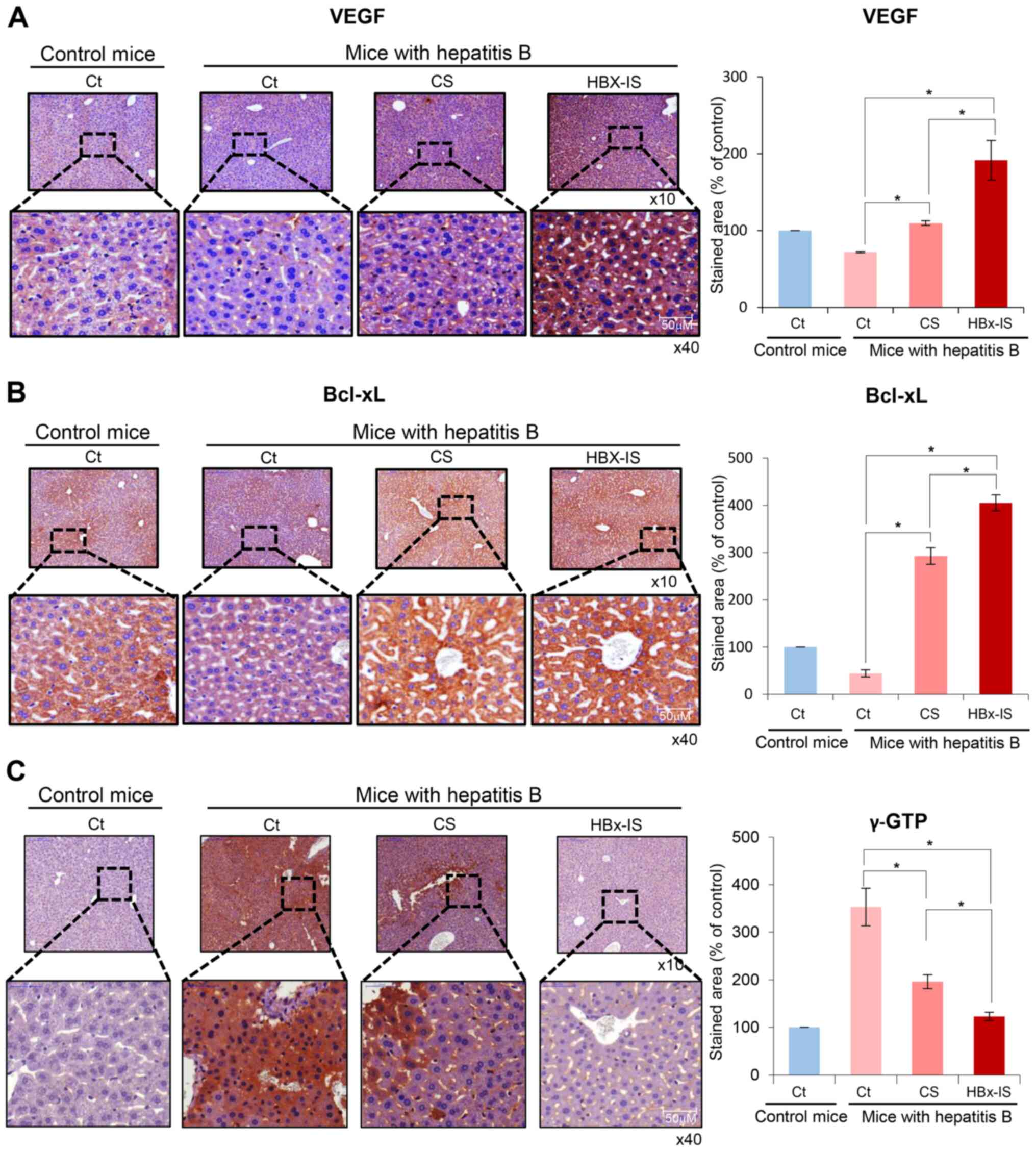 | Figure 6Immnohistochemical stains of the
liver following administration of the HBx-isecretome. (A) Left
panel illustrates VEGF immunohistochemistry of the liver following
the administration of each treatment. Injection of CS and HBx-IS
both significantly increased the expression of VEGF in the liver.
In addition, the expression of VEGF was significantly higher in the
HBx-IS group than in the CS group. Right panel presents the
percentages of VEGF immunoreactive areas. (B) Left panel
illustrates Bcl-xL immunohistochemistry of the liver following
administration of each treatment. Injection of CS and HBx-IS both
significantly increased the expression of Bcl-xL in the liver. In
addition, the expression of Bcl-xL was significantly higher in the
HBx-IS group than in the CS group. Right panel presents the
percentages of Bcl-xL immunoreactive areas. (C) Left panel
illustrates γ-GTP immunohistochemistry of the liver following
administration of each treatment. Injection of CS and HBx-IS both
significantly decreased the expression of γ-GTP in the liver. When
comparing CS and HBx-IS, HBx-IS, the expression of γ-GTP was
significantly lower in the HBx-IS group than in the CS group. Right
panel presents percentages of γ-GTP immunoreactive areas. Values
are presented as the means ± standard deviation of 3 independent
experiments. Percentages of immunoreactive areas were measured
using NIH image J and expressed as relative values to those in
normal livers. *P<0.05. Bcl-xL, B-cell leukemia-extra
large; HBx, hepatitis B virus protein X; CS, control secretome;
γ-GTP, γ-glutamyltranspeptidase; HBx-IS, indirect HBx-induced
secretome; HBx-DS, direct HBx-induced secretome; VEGF, vascular
endothelial growth factor. |
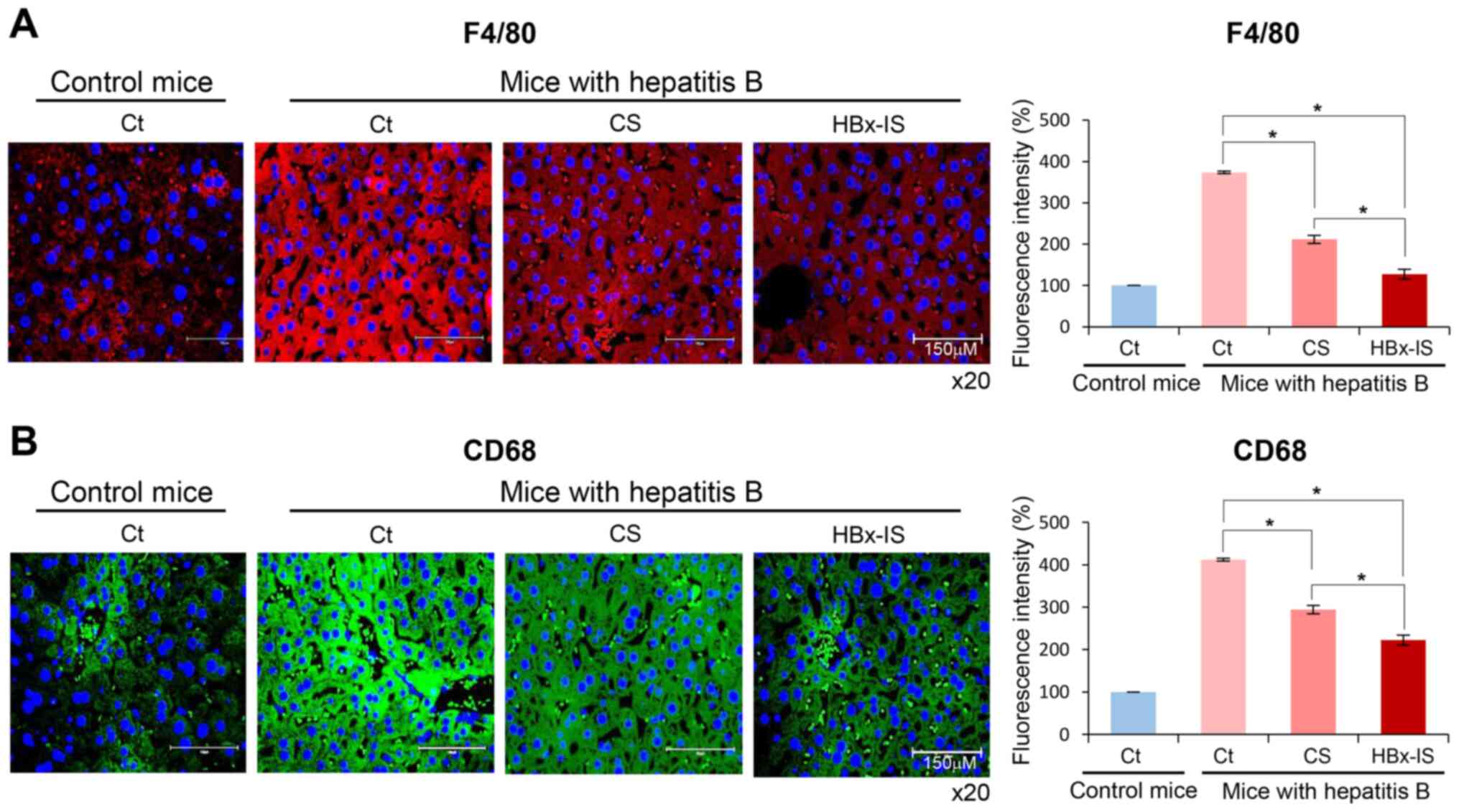
Immunofluorescence of the liver following
the administration of the HBx-isecretome
Immunostaining for pro-inflammatory markers, such as
F4/80 and CD68, in the liver specimens was finally performed
(Fig. 7A and B). The mouse model
of hepatitis B exhibited an increased expression of these markers
in the liver. However, the injection of CS and HBx-IS significantly
decreased the expression levels of these markers in the liver
(P<0.05). When comparing CS and HBx-IS, the expression of these
markers was significantly lower in the HBx-IS group compared to the
CS group (P<0.05), suggesting that the HBx-IS injection
inhibited the inflammatory reactions in the liver more effectively
than the CS injection.
Validation of the disease-specificity of
the HBx-isecretome
To validate the disease-specific effectiveness of
the HBx-isecretome, the control secretome, TAA-isecretome and
HBx-isecretome, we intravenously infused into the mice in the model
of TAA-induced hepatic failure. At 2 days after the first infusion,
the mice were euthanized and the specimens were examined. Western
blot analysis revealed that the TAA-isecretome infusion, rather
than the HBx-isecretome infusion, induced a higher expression of
proliferation markers (HGF, VEGF and PCNA) (all P<0.05; Fig. 8). Taken together, whereas the
HBx-isecretome exerted the optimal proliferative and
anti-inflammatory effects against HBx-induced hepatic failure, its
capacity for promoting the recovery of the liver appeared to be
much inferior to the TAA-isecretome in mice with TAA-induced
hepatic failure, suggestive of the potential for disease-specific
treatment.
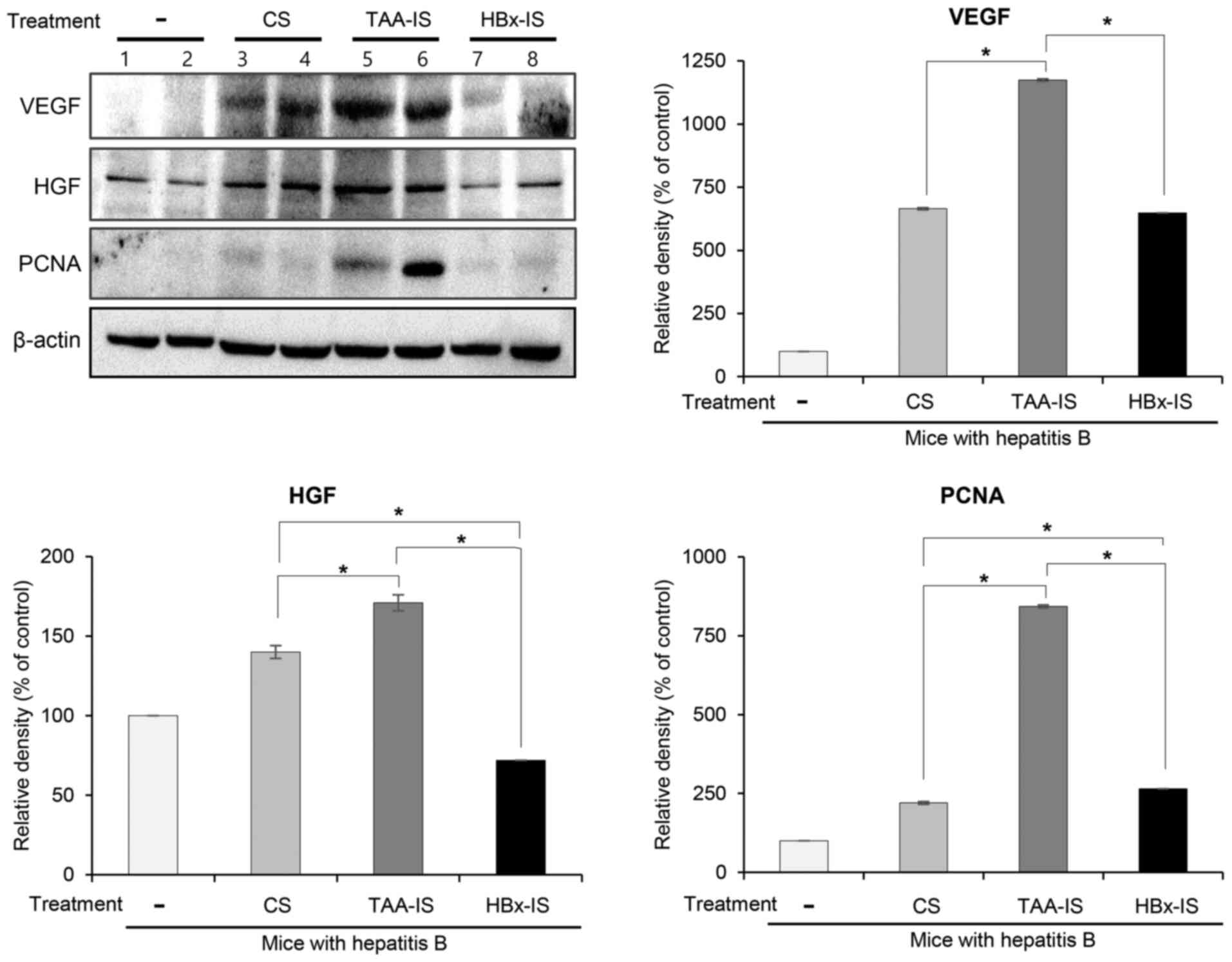 | Figure 8Validation of disease specificity of
HBx-isecretome. The mice with TAA-induced hepatic failure were
intravenously infused with the control secretome, TAA-isecretome
and HBx-isecretome, respectively. Western blot analysis of the
livers revealed that TAA-isecretome infusion, rather than
HBx-isecretome infusion, induced a higher expression of
proliferation markers (HGF, VEGF and PCNA) (all P<0.05). Values
are presented as the means ± standard deviation of 3 independent
experiments. *P<0.05. Relative densities of
individual markers were quantified using Image Lab software and
were then normalized to those of β-actin in each group. CS, control
secretome; Ct, control; HBx, hepatitis B virus protein X; HBx-IS,
indirect HBx-induced secretome; HGF, hepatocyte growth factor;
PCNA, proliferating cell nuclear antigen; TAA, thioacetamide;
TAA-IS, thioacetamide-induced secretome; VEGF, vascular endothelial
growth factor. |
Discussion
The secretome released by ASCs has the potential to
induce tissue regeneration and repair. The present study attempted
to identify a method which may be used to enhance the
disease-specific therapeutic effect of the secretome. Specifically,
the therapeutic potential of the HBx-isecretome in a mouse model of
hepatitis B was determined. The HBx-isecretome (HBx-IS) was
obtained by collecting the secretary materials following the
stimulation of ASCs with 100-fold diluted culture medium of AML12
cells that had been transfected with pcDNA-HBx for 24 h.
Subsequently, HBx-IS was intravenously administered to the mice
with hepatitis B. Compared with the CS injection, the HBx-IS
injection more significantly reduced tje serum levels of IL-6 and
TNF-α (pro-inflammatory cytokines). The HBx-IS injection led to the
higher expression of liver regeneration-related proteins and to the
lower expression of pro-inflammatory and pro-apoptotic proteins in
mouse livers than the CS injection. Taken together, these results
indicate that HBx-IS exhibits greater liver regenerative,
anti-inflammatory and anti-apoptotic properties, particularly in
the mouse model of hepatitis B than the CS. This suggests that
secretomes obtained by stimulating ASCs with disease-causing agents
could have a more promiment therapeutic effect in specific disease
than naïve secretomes.
In general, cells have the property of protecting
themselves when exposed to irritants or toxins. This property can
be expressed by the release of secretomes in response to external
stimuli (23-26). For example, when exposed to live
toxins, primary hepatocytes are known to release large amounts of
vital liver-specific proteins, including the enzymes carbamoyl
phosphate synthetase 1, S-adenosyl methionine synthetase 1 and
catechol-O-methyltransferase (27). However, there is a marked
difference in the amount and composition of the secretomes released
from mature cells and MSCs against the same stimuli. Since MSCs
have a stronger responsiveness and plasticity against external
stimuli than mature cells, they generally produce larger amounts of
secretome with greater therapeutic potential. Collectively, these
findings indicate that the stem cell secretome is more advantageous
than the mature cell secretome in therapeutic application.
Furthermore, obtaining a secretome with stimuli could enhance its
amount and potential.
Whereas non-specific stimulation has been utilized
to obtain the secretome from ASCs to date, the present study first
adopted the concept of disease-specific stimulation. First, the
term isecretome was created. Isecretome literally refers to
'induced secretome', which indicates a secretome induced by the
specific disease-causing agents. The concept of 'pre-sensitization
by disease-causing agents' has already been demonstrated. For
example, Prado et al proposed a method of treating a disease
using EVs obtained by pre-sensitizing mature cells using
disease-causing agents (28).
They pre-sensitized the mice by respiratory exposure to Ole e 1 (an
allergen), and then obtained EVs from bronchoalveolar lavage fluid.
Subsequently, the obtained EVs were administrated intranasally to
the new mice. It was demonstrated that the mice treated with the
EVs did not experience allergy to Ole e 1, reaching to a tolerant
status. This suggests that respiratory tract cells produced
protective materials in response to Ole e 1, which prevented
allergy by Ole e 1 in new mice. Unlike the research of Prado et
al in which mature respiratory cells were utilized, the present
study utilized ASCs with a higher responsiveness and plasticity,
thus, raising the possibility of their application to a variety of
therapeutics.
In the present study, the hypothesis put forth was
that the appropriate stimulation of MSCs with pathogenic agents
could lead to the production of a secretome specialized for
exerting protective effects against the pathogen. In a previous
study, the authors fist validated this hypothesis by demonstrating
the superiority of the TAA-isecretome in a mouse model of
TAA-induced hepatic failure (19). In that study, the authors
collected the secretory materials (named as inducers) released from
AML12 hepatocytes that had been pre-treated with TAA and generated
the TAA-induced secretome (TAA-isecretome) after stimulating the
ASCs with the inducers. The TAA-isecretome was intravenously
administered to mice with TAA-induced hepatic failure and those
with partial hepatectomy. TAA-isecretome infusion exhibited greater
therapeutic potential in terms of i) restoring disorganized hepatic
tissue to normal tissue; ii) inhibiting pro-inflammatory cytokines
(IL-6 and TNF-α); and iii) reducing abnormally elevated liver
enzymes (AST and ALT) compared to the naïve secretome infusion in
mice with TAA-induced hepatic failure. However, the TAA-isecretome
exhibited an inferior therapeutic potential for restoring hepatic
function in partially hepatectomized mice. Therefore, it was
concluded that the appropriate stimulation of MSCs with pathogenic
agents may lead to the production of a secretome specialized for
protecting against the pathogen.
In order to produce a disease-specific secretome, it
is essential to select appropriate pathogens that stimulate MSCs,
as well as the precise determination of the reaction condition. It
should be highlighted that pathogenic stimuli should not be too
weak or too strong as they could lead to a lack of a response or
destruction of MSCs, respectively. In order to determine the
appropriate isecretome for the mouse model of hepatitis B, the
present study deliberately examined a variety of conditions, and
compressed the isecretome candidates into HBx-DS and HBx-IS.
Subsequently, it was found that HBx-IS exerted more potent
anti-inflammatory, liver regenerative, and anti-apoptotic effects
in the mouse model of hepatitis B than HBx-DS in the present study.
Thus, it was concluded that HBs-IS could be a more acceptable
candidate of isecretome for hepatitis B than HBx-DS.
There are still many incurable diseases, most of
which are due to the inability of the mature cells of patients to
neutralize or inhibit pathogenic agents. However, it should be
emphasized that while the mature cells of patients are unable to
produce the protective materials against pathogenic agents, MSCs
could be able to produce these, as MSCs have superior
responsiveness and plasticity than mature cells (29-31). The isecretome produced by MSCs is
the collections of protective materials against pathogenic agents
most of which mature cells could not produce. It is expected that
the application of such an isecretome concept may pave the way for
the treatment of several incurable diseases.
HBx is the protein encoded by the HBx gene that is
one of four open reading frames comprising the HBV genome. HBx
plays a key role in HBV transcription and replication. This is
achieved by the regulation of viral promoters and enhancers by HBx
(32,33). Furthermore, HBx is involved in the
development of hepatocellular carcinoma, as well as in the
regulation of checkpoints in the cell cycle (34,35). In particular, HBx plays a dual
role in the regulation of the apoptotic process, which indicates
that HBx inhibits, as well as promotes cellular apoptosis (36). Anti-apoptosis by HBx is achieved
via activating NF-κB, an activator of anti-apoptotic signals
(37). On the other hand, HBx
exerts pro-apoptotic effects when NF-κB is inhibited (37). Since HBx exerts various effects on
cellular apoptosis, it is particularly difficult to generate
appropriate an HBx-isecretome against hepatitis B. The authors aim
to perform the component analysis of HBx-IS and HBx-DS, which is
expected to clarify the therapeutic mechanism of the
HBx-isecretome.
In conclusion, the present study demonstrated a
method which may be used to enhance the disease-specific
therapeutic effects of the secretome. Specifically, the therapeutic
potential of the HBx-isecretome (HBx-IS) in mice with hepatitis B
was determined. Compared with the CS injection, the HBx-IS
injection more significantly reduced the serum levels of
pro-inflammatory cytokines. In addition, the HBx-IS injection led
to a higher expression of liver regeneration-related markers, a
lower expression of pro-apoptotic markers in mouse livers, and a
lower expression of pro-inflammatory markers in the liver compared
to the CS injection. These results collectively indicate that the
HBx-IS exhibits greater liver regenerative, anti-inflammatory, and
anti-apoptotic properties, particularly in mice with hepatitis B,
than the CS. This suggests that the secretome obtained by
stimulating ASCs with the disease-causing agents may exert a more
potent therapeutic effect in the specific disease than naïve
secretomes. This approach is expected to pave the way to the
development of novel various specific therapeutics based on the
high plasticity and responsiveness of MSCs to disease-causing
agents.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to manuscript preparation.
SJK designed the research, analyzed the data and contributed to
manuscript preparation. HJK also analyzed the data and contributed
to manuscript preparation. OHK performed the in vitro
experiments and also contributed to manuscript preparation. HEH
performed various in vitro and in vivo experiments.
SCL was involved in designing the study and analyzing data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were carried out in compliance
with the guidelines of the Institute for Laboratory Animal
Research, Korea (IRB no. CMCDJ-AP-2016-001). The Ethics Committee
at Daejeon St. Mary's hospital, the Catholic University of Korea,
approved the animal experiments for the research (IRB no.
CMCDJ-AP-2016-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Meirelles Lda S, Fontes AM, Covas DT and
Caplan AI: Mechanisms involved in the therapeutic properties of
mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee
G, Park JY, Park SY, Kim JH, Do BR, et al: Milk fat globule-EGF
factor 8, secreted by mesenchymal stem cells, protects against
liver fibrosis in mice. Gastroenterology. 152:1174–1186. 2017.
View Article : Google Scholar
|
|
3
|
Makridakis M, Roubelakis MG and Vlahou A:
Stem cells: Insights into the secretome. Biochim Biophys Acta.
1834:2380–2384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parekkadan B, van Poll D, Suganuma K,
Carter EA, Berthiaume F, Tilles AW and Yarmush ML: Mesenchymal stem
cell-derived molecules reverse fulminant hepatic failure. PLoS One.
2:e9412007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul G and Anisimov SV: The secretome of
mesenchymal stem cells: Potential implications for
neuroregeneration. Biochimie. 95:2246–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salgado AJ, Oliveira JM, Martins A,
Teixeira FG, Silva NA, Neves NM, Sousa N and Reis RL: Tissue
engineering and regenerative medicine: Past, present, and future.
Int Rev Neurobiol. 108:1–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lavoie JR and Rosu-Myles M: Uncovering the
secretes of mesenchymal stem cells. Biochimie. 95:2212–2221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baglio SR, Pegtel DM and Baldini N:
Mesenchymal stem cell secreted vesicles provide novel opportunities
in (stem) cell-free therapy. Front Physiol. 3:3592012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubio D, Garcia S, Paz MF, De la Cueva T,
Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J and Bernad A:
Molecular characterization of spontaneous mesenchymal stem cell
transformation. PLoS One. 3:e13982008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konala VBR, Mamidi MK, Bhonde R, Das AK,
Pochampally R and Pal R: The current landscape of the mesenchymal
stromal cell secretome: A new paradigm for cell-free regeneration.
Cytotherapy. 18:13–24. 2016. View Article : Google Scholar :
|
|
11
|
Salgado AJ, Sousa JC, Costa BM, Pires AO,
Mateus-Pinheiro A, Teixeira FG, Pinto L and Sousa N: Mesenchymal
stem cells secretome as a modulator of the neurogenic niche: Basic
insights and therapeutic opportunities. Front Cell Neurosci.
9:2492015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waszak P, Alphonse R, Vadivel A, Ionescu
L, Eaton F and Thebaud B: Preconditioning enhances the paracrine
effect of mesenchymal stem cells in preventing oxygen-induced
neonatal lung injury in rats. Stem Cells Dev. 21:2789–2797. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SC, Jeong HJ, Lee SK and Kim SJ:
Lipopolysaccharide preconditioning of adipose-derived stem cells
improves liver-regenerating activity of the secretome. Stem Cell
Res Ther. 6:752015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SC, Jeong HJ, Lee SK and Kim SJ:
Hypoxic conditioned medium from human adipose-derived stem cells
promotes mouse liver regeneration through JAK/STAT3 signaling. Stem
Cells Transl Med. 5:816–825. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SC, Kim KH, Kim OH, Lee SK, Hong HE,
Won SS, Jeon SJ, Choi BJ, Jeong W and Kim SJ: Determination of
optimized oxygen partial pressure to maximize the liver
regenerative potential of the secretome obtained from
adipose-derived stem cells. Stem Cell Res Ther. 8:1812017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noiseux N, Gnecchi M, Lopez-Ilasaca M,
Zhang L, Solomon SD, Deb A, Dzau VJ and Pratt RE: Mesenchymal stem
cells overexpressing Akt dramatically repair infarcted myocardium
and improve cardiac function despite infrequent cellular fusion or
differentiation. Mol Ther. 14:840–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Zhao T, Huang W, Wang T, Qian J,
Xu M, Kranias EG, Wang Y and Fan GC: Hsp20-engineered mesenchymal
stem cells are resistant to oxidative stress via enhanced
activation of Akt and increased secretion of growth factors. Stem
Cells. 27:3021–3031. 2009.PubMed/NCBI
|
|
18
|
Mohammadzadeh M, Halabian R, Gharehbaghian
A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM and Roudkenar
MH: Nrf-2 overexpression in mesenchymal stem cells reduces
oxidative stress-induced apoptosis and cytotoxicity. Cell Stress
Chaperones. 17:553–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim OH, Hong HE, Seo H, Kwak BJ, Choi HJ,
Kim KH, Ahn J, Lee SC and Kim SJ: Generation of induced secretome
from adipose-derived stem cells specialized for disease-specific
treatment: An experimental mouse model. World J Stem Cells.
12:70–86. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Li X, Liu G, Chen M, Yang Y, Xie Y and
Kong X: A novel hydrodynamic injection mouse model of HBV genotype
C for the study of HBV biology and the anti-viral activity of
lamivudine. Hepat Mon. 16:e344202016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling LR, Zheng DH, Zhang ZY, Xie WH, Huang
YH, Chen ZX, Wang XZ and Li D: Effect of HBx on inflammation and
mitochondrial oxidative stress in mouse hepatocytes. Oncol Lett.
19:2861–2869. 2020.PubMed/NCBI
|
|
23
|
Chierchia A, Chirico N, Boeri L, Raimondi
I, Riva GA, Raimondi MT, Tunesi M, Giordano C, Forloni G and Albani
D: Secretome released from hydrogel-embedded adipose mesenchymal
stem cells protects against the Parkinson's disease related toxin
6-hydroxydopamine. Eur J Pharm Biopharm. 121:113–120. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Driscoll J and Patel T: The mesenchymal
stem cell secretome as an acellular regenerative therapy for liver
disease. J Gastroenterol. 54:763–773. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eleuteri S and Fierabracci A: Insights
into the secretome of mesenchymal stem cells and its potential
applications. Int J Mol Sci. 20:45972019. View Article : Google Scholar :
|
|
26
|
Li N, Sarojini H, An J and Wang E:
Prosaposin in the secretome of marrow stroma-derived neural
progenitor cells protects neural cells from apoptotic death. J
Neurochem. 112:1527–1538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodríguez-Suárez E, Gonzalez E, Hughes C,
Conde-Vancells J, Rudella A, Royo F, Palomo L, Elortza F, Lu SC,
Mato JM, et al: Quantitative proteomic analysis of
hepatocyte-secreted extracellular vesicles reveals candidate
markers for liver toxicity. J Proteomics. 103:227–240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prado N, Marazuela EG, Segura E,
Fernández-García H, Villalba M, Théry C, Rodriguez R and Batanero
E: Exosomes from bronchoalveolar fluid of tolerized mice prevent
allergic reaction. J Immunol. 181:1519–1525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bobis S, Jarocha D and Majka M:
Mesenchymal stem cells: Characteristics and clinical applications.
Folia Histochem Cytobiol. 44:215–230. 2006.
|
|
30
|
Filip S, Mokrý J, English D and Vojácek J:
Stem cell plasticity and issues of stem cell therapy. Folia Biol
(Praha). 51:180–187. 2005.
|
|
31
|
Frisén J: Stem cell plasticity? Neuron.
35:415–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen HS, Kaneko S, Girones R, Anderson RW,
Hornbuckle WE, Tennant BC, Cote PJ, Gerin JL, Purcell RH and Miller
RH: The woodchuck hepatitis virus X gene is important for
establishment of virus infection in woodchucks. J Virol.
67:1218–1226. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zoulim F, Saputelli J and Seeger C:
Woodchuck hepatitis virus X protein is required for viral infection
in vivo. J Virol. 68:2026–2030. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choe BH: Hepatitis B virus: Pathogenesis,
molecular diagnosis, and clinical significance of mutation. Korean
J Pediatr Gastroenterol Nutr. 10:51–65. 2007.
|
|
35
|
Neuveut C, Wei Y and Buendia MA:
Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol.
52:594–604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Slagle BL and Bouchard MJ: Role of HBx in
hepatitis B virus persistence and its therapeutic implications.
Curr Opin Virol. 30:32–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Clippinger AJ, Gearhart TL and Bouchard
MJ: Hepatitis B virus X protein modulates apoptosis in primary rat
hepatocytes by regulating both NF-kappaB and the mitochondrial
permeability transition pore. J Virol. 83:4718–4731. 2009.
View Article : Google Scholar : PubMed/NCBI
|