Introduction
Osteoarthritis (OA) is a progressive disease,
characterized by cartilage erosion, osteophyte formation, chronic
low-grade inflammation of the synovium and abnormal bone
remodeling. Due to advances being made in analgesia and in
arthroplasty, the survival rate of patients with OA has improved,
and their quality of life has significantly improved. OA is not
traditionally regarded as an inflammatory disease, although a
number of studies have confirmed that oxidative stress affects the
pathogenesis of OA (1).
Oxidative stress is closely associated with vascular inflammation
as reactive oxygen species (ROS) overproduction creates a
pro-inflammatory microenvironment and elevates inflammatory
cytokine levels. The release of inflammatory cytokines into the
synovial fluid stimulates the production of pro-inflammatory
cytokines, which could contribute to the pathology of OA (2,3).
Chondrocytes (CHs), the only cell type present in
cartilage, are responsible for the development of cartilage
(4,5). CHs produce and maintain the
extracellular matrix, which normally has a low turnover rate. The
widespread use of non-steroidal anti-inflammatory drugs is
accompanied by an increase in gastrointestinal reactions, which
affects the survival of patients. To overcome bioavailability and
stability issues, intra-articular injections with small molecules
are rapidly cleared from the joint within hours via synovial
vasculature and larger macromolecules within days via synovial
lymphatics (5-7). However, drugs for OA have limited
success and display challenges with obtaining effective drug
loading and release profiles persist (8,9).
Therefore, attenuating the deleterious effects of oxidative stress
on CHs may serve as a strategy with which to repair cartilage
tissue, In particular, their application to oxidative stress
mechanisms requires further investigation.
Increased ROS generation and unfolded protein
responses (UPRs) in the CH microenvironment can activate
endoplasmic reticulum (ER) stress, and mitochondrial injury is
associated with rat CH apoptosis (10-12). ER stress in CHs plays an
important role in the pathogenesis of OA. Studies have revealed
that cells respond to ER stress by activating the UPR, a signaling
network consisting of three primary molecular sensors, including
eukaryotic translation initiation factor 2 α kinase 3, activating
transcription factor-6 (ATF6) and inositol-requiring enzyme 1α
(13-15). Under physiological conditions,
these proteins are associated with Bip or ER chaperone protein
glucose-regulated protein 78 (GRP78) and are engaged in
inactivation. Previous research has revealed that the
transcriptional activity of GRP78 is significantly elevated up to
10-25 fold during suffering from the stress response (16). There are 3 classical branches of
the signaling pathway: PERK, ATF6 and IRE do not operate
independently. They constitute an intricate signaling network and
crosstalk dynamics decide cell fate with respect to survival or
death following ER stress. All 3 pathways transduce information to
repair the ER status and restore protein-folding capacity in
ROS-exposed CHs. C EBP-homologous protein (CHOP) gadd153 (or simply
CHOP) is a transcription factor which is the most important in
regulating the ER stress-dependent apoptosis. In CHs in cartilage,
ER stress can increase the expression of CHOP (16); thus, inhibiting ER stress may
serve as a novel therapeutic approach for OA.
Hydrogen sulfide (H2S) has been proposed
as a potential regulator of inflammation and is a novel signaling
molecule with potent cytoprotective actions. Moreover,
H2S is an endogenously produced gasotransmitter that has
been reported to be involved in the inhibition of oxidative stress
(17). The physiological and
pathophysiological regulation of H2S in cellular
function has received increasing attention. Compared with patients
with rheumatoid arthritis, the concentrations of H2S in
synovial fluid aspirates and plasma samples have been shown to be
2-fold higher in paired patients with non-inflammatory arthritis
(18). GYY4137 has been
well-known as a novel H2S donor with its multiple
bioactivities (19-21). The present study examined the
effects of exogenous H2S on tert-Butyl hydroperoxide
(TBHP)-induced ER stress mitochondria-associated apoptotic
signaling pathways in CHs, as well as the underlying
mechanisms.
Materials and methods
Animals
A total of 40 male SD rats (weighing, 250-280 g;
age, 8 weeks) were obtained from the experimental animal center of
Harbin Medical University, and kept at the Key Laboratory Of
Molecular Imaging (Harbin, China). Rats were acclimated for 7 days
prior to the surgeries and were provided with unrestricted access
to food and water at room temperature (22-26°C) at 10-30% humidity.
Animals were performed according to guidelines approved by the
Institutional Experimental Animal Care and Ethics Committee of
Harbin Medical University (Harbin, China) and with approval from
the Harbin Medical University Ethics Review Board.
Cell isolation and culture
All animal care and experimental protocols complied
with the Guide for the Care and Use of Laboratory Animals of Harbin
Medical University (Harbin, China). Rats were anesthetized with
chloral hydrate (3.5%, 350 mg/kg, i.p.). The bilateral hip joints
of male rats were isolated immediately and washed thoroughly with
normal saline and then immediately processed for biochemical and
morphological analyses. Each bilateral hip joint was subjected to
the following analyses: Cell viability, western blot analysis for
ER stress pathway-associated protein indexes, ROS activity,
fluorescence staining and flow cytometric analyses. Primary CHs
were isolated from the bilateral hip joints of the male rats using
0.25% trypsin for digestion lasting approximately 1 h at 37°C and
incubated with collagenase II (1.5 mg/ml) in DMEM at 37°C and 5%
CO2 for 6 h. The CH suspensions were then centrifuged at
250 x g 4°C for 5 min and cultured at 37°C and 5% CO2 in
DMEM complete culture medium (10% FBS, 100 μg/ml
streptomycin and 100 U/ml penicillin). To avoid the loss of
phenotype, rat CHs were not used for >2 passages in the in
vitro experiments. At the end of the experimental procedure,
the animals were sacrificed by an i.p. injection of an overdose of
sodium pentobarbital (100 mg/kg).
Reagents
GYY4137 (SML0100; chemical structure shown in
Fig. 1A), tert-Butyl
hydroperoxide solution (416665), N-acetylcysteine (NAC,
A9165), chloroquine (CQ, C6628) were obtained from Sigma-Aldrich;
Merck KGaA. DMEM/F12 (SH30023.01), FBS and type II collagenases
were purchased from HyClone; Cytiva. 2,7-Dichlorofluorescein
diacetate (DCFH-DA) was purchased from Beyotime Institute of
Biotechnology. Lipofectamine 2000, calcium green-5N and ER-tracker
were both obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Primary antibodies [GRP78, #3177; ATF6, #65880; CHOP, #2895;
phospho-p70S6 kinase, #9208; p70S6 kinase, #2708; mammalian target
of rapamycin (mTOR), #2983; phospho-mTOR, #5536; SQSTM1/p62,
#23214; LC3A/B, #4108] were provided by Cell Signaling Technology,
Inc. and secondary antibodies (#SA00001-1 and #SA00001-2) were
purchased from ProteinTech Group, Inc., respectively.
Cell viability assay
Cell counting kit-8 (CCK-8) assay was performed for
the assessment of the cytotoxicity of GYY4137 (100 μM, 0-24
h) and TBHP (0-50 μM, 0-24 h) on CHs. A total of 10
μl CCK-8 solution was added to each 96-well plates
(5×103 cells per well) and co-incubated at 37°C for 4 h.
The absorbance was measured at 450 nm using a microplate reader
(InfiniteM200, Tecan Group, Ltd.).
Calculation of half maximal effective
concentration (EC50)
GraphPad Prism 7.0 software was used for the
calculation of the EC50 of GYY4137 in vitro. The
fitted dose-effect curve and EC50 value were generated
and with automatic output following the inputting rate data of
GYY4137 on chondrocytes and the setting of parameters on GraphPad
Prism 7.0.
MTT assay
CHs (5×103 cells/well) were cultured in
96-well plates and exposed to 0.4 mg/ml MTT diluted in complete
DMEM for 4 h at 37°C. Cells were washed with PBS and allowed to
air-dry overnight. Formazan crystals were resuspended in DMSO and
absorbance was measured at 560 nm with background at 670 nm on a
Cytation 5 plate reader (BioTek Instruments, Inc.).
Observation of intracellular ROS
content
The microscopic observation of intracellular ROS and
mitochondrial ROS was performed by the oxidative conversion of cell
permeable DCFH-DA to fluorescent 2′, 7′-dichlorofluorescein (DCF)
and MitoSOX (M36008, Invitrogen; Thermo Fisher Scientific, Inc.).
CHs were divided into the control, TBHP, TBHP + GYY4137 and TBHP +
NAC group, then stained with 10 μM DCFH-DA solution or 5
μM MitoSOX at 37°C for 20 min in the dark. The mean
fluorescence intensity (MFI) of DCF and MitoSOX from 4 random
fields was observed using an imaging system (BX50-FLA; Olympus
Corporation) and analyzed using ImagePro Plus software (version
6.0, Media Cybernetics).
Measurement of superoxide dismutase (SOD)
and catalase (CAT) activities
The Total Superoxide Dismutase Assay kit with NBT
(Beyotime Institute of Biotechnology) and a Catalase Assay kit
(Beyotime Institute of Biotechnology) were used to examine the
activities of SOD and CAT as previously described (14). The activity results are expressed
as U/mg protein.
Cell apoptosis analysis by flow
cytometry
At least 104-106 cells were
analyzed by flow cytometry with the Annexin V/PI kit (BD
Biosciences) using a Beckman Coulter MoFlo XDP. Briefly, the medium
was removed and cells were treated with serum-reduced medium (0.5%
FBS) with GYY4137 (100 μM) or NAC (5 μM) for 24 h.
Cells were suspended in 300 μl binding buffer, and then
stained with Annexin-FITC and propidium Iodide (PI). Positive
controls for cell apoptosis or necrosis were indicated by staining
with only Annexin-FITC or PI (Beckman Coulter, Inc.). Cell
apoptosis was counted using summit V5.2.0.7477 software.
Calcium retention assay
Calcium retention capacities of rat CH mitochondria
were measured as previously described (16) with some modifications. Briefly,
primary CHs were incubated with 0.25 M sucrose buffer for complex 1
without EGTA at 25°C (room temperature). Extra-mitochondrial
calcium was visualized using a 500 nm excitation wavelength and
emission wavelengths of 530 nm with 1 μM calcium green -5N.
Experiments were performed in the presence and absence of 1.2 mM
MgCl2 and 40 μM ADP, as well as 1 mM cyclosporine
A (CsA), an inhibitor of the mitochondrial permeability transition
pore (MPTP). The probe reversibly binds to calcium ions. The
inclusion of CsA allows for an increased Ca2+ retention
capacity prior to induction of the mPTP and the subsequent release
of Ca2+ from the mitochondria.
Caspase activity assays
Caspase-3 activity was assessed using a colorimetric
assay (ab39401, Abcam) according to the manufacturer's
instructions. The activities of caspase-3, caspase-8 and caspase-9
were determined using a Multiplex Activity Assay kit (ab219915,
Abcam) according to the manufacturer's instructions with some
modifications. The protocol in the following link was followed:
https://www.abcam.com/ps/products/219/ab219915/documents/Caspase-3-Caspase-8-and-Caspase-9-Multiplex-Activity-Assay-protocol-book-v1d-ab219915%20(website).pdf.
The caspase-3, -8 and -9 substrates were at room temperature prior
to the experiment. All controls and samples were assayed in
triplicate. The cells were plated overnight in DMEM at
2×104/90 μl per well. Blank wells (DMEM without
cells) contained 10 μl of compound buffer. To assay
caspase-3 activity in each well, assay loading solution was
prepared by the addition of 50 μl of caspase-3 substrate to
10 ml of Assay Buffer. To assay tri-caspase activity in the same
well, a mixed assay solution was prepared by the addition of 50
μl of tri-caspase substrate to 10 ml of assay buffer
together. This was followed by the addition of 100 μl/well
of solution directly into cell plates without removing DMEM at room
temperature for 45 min. A micro-plate reader (InfiniteM200, Tecan
Group Ltd.) was used to measure fluorescence at Ex/Em=535/620 nm
(caspase-3, red), Ex/Em=490/525 nm (caspase-8, green),
Ex/Em=370/450 nm (caspase-8, blue).
GRP78 short interfering RNA
transfection
GRP78 siRNA and scrambled siRNA (negative control)
were designed and synthesized by Invitrogen; Thermo Fisher
Scientific, Inc. (https://www.invivogen.com/sirnawizard/scrambled.php).
The siRNA sequences were as follow: GRP78 target siRNA,
5′-GAAAGTATACCTCCAGTTTTT-3′; and scrambled siRNA,
5′-GTTCTAACGTCTATGACATTA-3′. siRNA targeting rat GRP78 was
dissolved in siRNA suspension buffer and transfected into the CHs
at a concentration of 150 nM; scrambled siRNA served as a negative
control. All transient transfections were initially standardized to
improve knockdown efficiency and subsequently performed with 150 nM
GRP78 siRNAs using Lipofectamine 2000 as per the manufacturer's
protocol after 6 h; transfection mixtures were replaced with
regular medium. At 48 h following transfection, the cells were
incubated with DMEM with or without TBHP for processing assessment
of mitochondrial network and protein expression.
Western blot analysis
Total proteins from primary chondrocytes were
extracted with RIPA buffer containing protease and phosphatase
inhibitors (R0010, Beijing Solarbio Science & Technology Co.,
Ltd.) on ice. The concentration of proteins was measured by
bicinchoninic acid (BCA) protein assay (Beyotime Institute of
Biotechnology, Inc.). Equal amounts of protein (50 μg) were
separated by 10 or 12.5% SDS-PAGE (PG112/PG113, EpiZyme, inc.) and
transferred onto 0.22 μM NC membranes (P-N66485, Beijing
Solarbio Science & Technology Co., Ltd.). The membranes were
blocked with 5% dried skimmed milk in Tris-buffered saline with
0.05% Tween-20 (TBS-T) for 1 h at room temperature with sustained
shaking. The membrane was washed with TBS-T 3 times then probed
with primary antibodies (1:1,000) by incubation at 4°C overnight.
The following day, membranes were incubated with horseradish
peroxidase (HRP)-conjugated secondary anti-bodies (1:5,000) for 2 h
at room temperature. Immunoreactivity was visualized using ECL
reagent (Wanlei Biotechnology) using ChemiDoc™ MP Imaging System
(Tanon Science & Technology Co., Ltd.). The densities of the
bands were quantified using a Bio-Rad Chemi EQ densitometer and
Quantity One software (Tanon Science & Technology Co.,
Ltd.).
Staining procedures and microscopic
analyses
For ER staining experiments, 0.5 μl of
ER-tracker green DMSO stock solution (1 mM) was added into 2 ml of
pre-warmed DMEM incubated at 37°C, 5% CO2 at room
temperature for 30 min and washed with PBS solution 3 times. For
Hoechst 33342/PI (CA1120, Beijing Solarbio Science & Technology
Co., Ltd.) dual-staining, CHs were firstly stained with Hoechst
33342 (10 μM) for 30 min at 37°C and then stained with PI
(10 μM) for 5 min. Cells were observed under fluorescence
microscope (DMI4000B, Leica Microsystems, Inc.) after washing with
PBS.
Statistical analysis
Data are presented as the means ± the standard error
of the mean (SEM) from at least 3 independent experiment
replicates. One-way ANOVA followed by a Holm-Sidak test for
post-hoc analysis using Graph Pad Prism 7. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
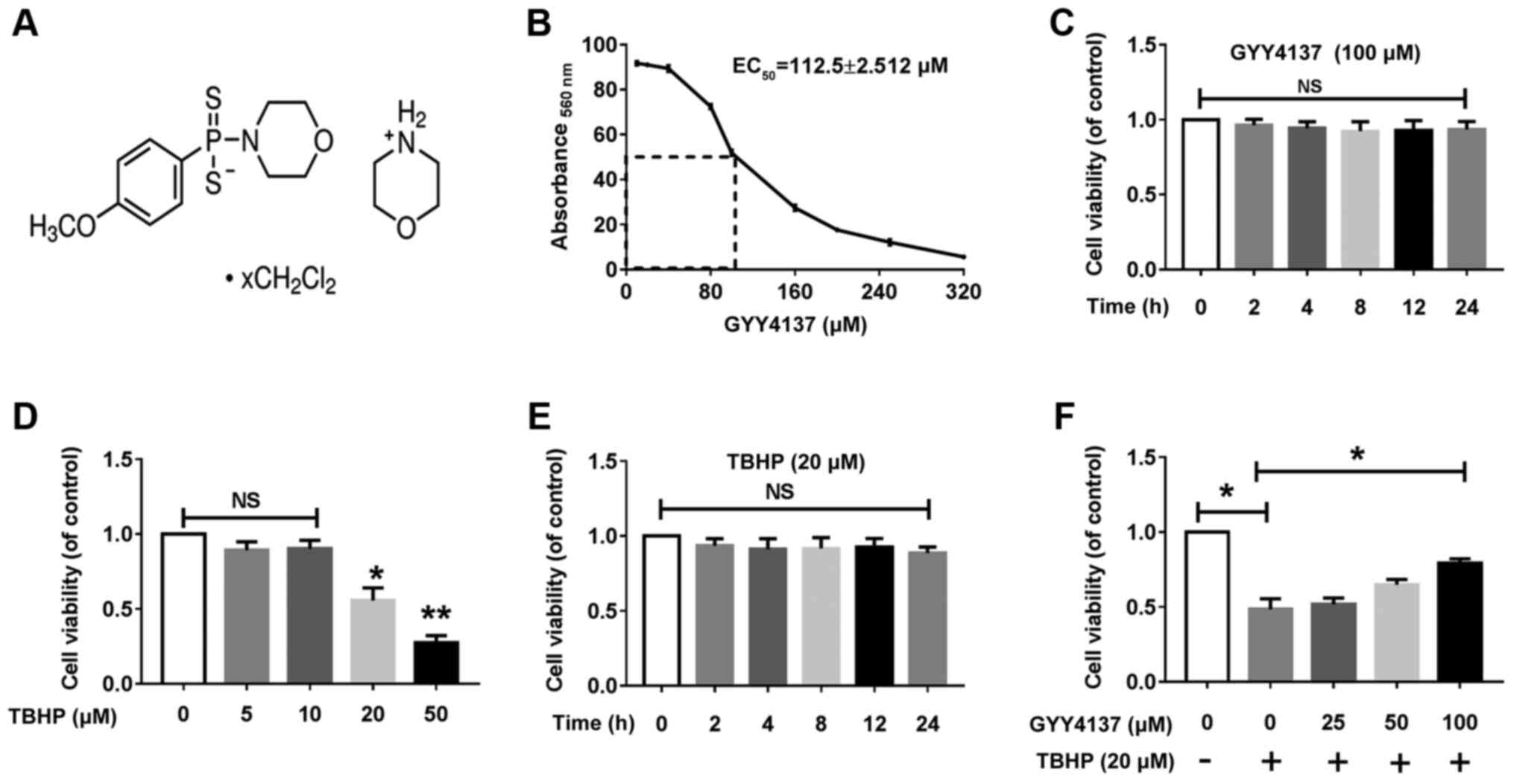
Effect of various concentrations of
H2S on CH viability with or without TBHP
The effect of GYY4137 on rat CHs viability was
analyzed by performing an MTT assay. The EC50
(calculated using GraphPad Prism7) of GYY4137 in the CHs at 24 h
was 112.5±2.5 μM (Fig.
1B). Treatment with 100 μM GYY4137 did not lead to any
significant cytotoxicity from 0 to 24 h, as shown by CCK-8 assay
(Fig. 1C). Stimulation with TBHP
at 20 μM was selected to mimic oxidative stress associated
with OA in the CHs, as TBHP has been reported to be more stable and
long-lasting as an oxidative stress inducer compared with
H2O2 (22). TBHP stimulation led to a
significant decrease in cell viability in a time-dependent manner
at the concentration of 20 μM (Fig. 1D and E), as shown by CCK-8 assay.
Moreover, GYY4137 reversed the decreased viability of CHs following
co-incubation with TBHP 20 μM (Fig. 1F). Collectively, these results
indicated that treatment with 100 μM GYY4137 reduced the
total number of CHs by inhibiting cell proliferation, and cell
apoptosis contributed more to the reduction in the total cell
number of CHs at toxic GYY4137 concentrations (>200 μM).
Therefore, GYY4137 at 100 μM and TBHP at 20 μM were
selected for use in subsequent experiments.
GYY4137 protects CHs from oxidative
stress-induced apoptosis
To determine whether treatment with an antioxidant
could attenuate the apoptotic effects of TBHP, chondrocytes were
treated with NAC. NAC is a derivative of L-cysteine that is well
known for its ability to protects cells from ROS-induced cellular
senescence. The results demonstrated that both cytoplasmic and
mitochondrial ROS production were significantly increased in the
TBHP group, an effect that was suppressed by GYY4137 or NAC
treatment (Fig. 2A and B).
Subsequently, SOD (Mn-SOD) and CAT (mito-CAT) activity in the
mitochondria of the CHs was detected to determine the mechanisms
through which GYY4137 suppresses ROS production. TBHP inhibited
Mn-SOD and mito-CAT activity, and this was attenuated by GYY4137 or
NAC treatment (Fig. 2C and D).
GYY4137 markedly increased mitochondrial Mn-SOD and mito-CAT
activity; therefore, it was hypothesized that exogenous
H2S can suppress mitochondrial ROS production. Flow
cytometry was conducted to detect CH apoptosis (Fig. 3A, C and D). The proportion of
early and late apoptotic cells (necrosis and dead cells) was
markedly decreased following treatment with GYY4137 for 24 h, which
suggested that GYY4137 reversed TBHP-induced cell apoptosis
(Fig. 3A, C and D).
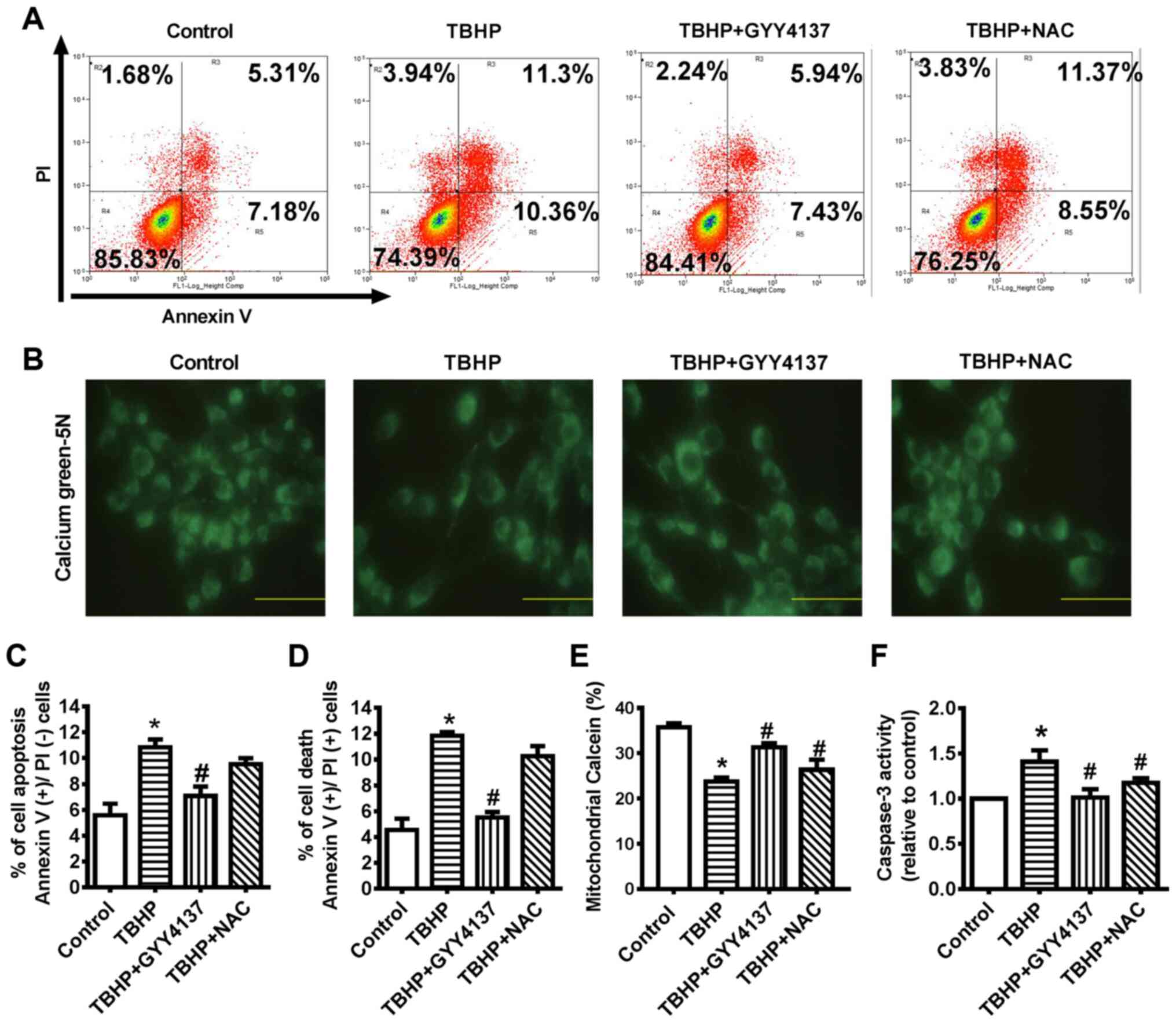 | Figure 3GYY4137 protects CHs from oxidative
stress-induced apoptosis. (A, C and D) Cells were treated with 0
μM (control), 20 μM TBHP, 100 μM GYY4137 and 5
μM NAC for 24 h. Subsequently, cells were analyzed using a
flow cytometer. Lower left quadrant, Annexin
V−/PI− (live cells); upper left quadrant,
Annexin V−/PI+ (mechanically damaged cells);
upper right quadrant, Annexin V+/PI+ (late
apoptotic or necrotic cells); lower right quadrant, Annexin
V+/PI− (early apoptotic cells). The
experiments were repeated at least 3 times. *P<0.05,
significant difference vs. the control (n=4). (B and E) CHs were
treated with 0 μM (control), 20 μM TBHP, 100
μM GYY4137 and NAC for 24 h. The mPTP opening was measured
by staining with calcein-AM and COCl2 (n=5). (F)
Caspase-3 activity in CHs. Data are presented as the means ± SEM.
*P<0.05 vs. control, #P<0.05 vs. TBHP
(n=3). TBHP, tert-Butyl hydroperoxide; NAC, N-acetyl cysteine; CH,
chondrocyte; mPTP, mitochondrial permeability transition pore. |
Calcein/Co2+ staining was conducted to
examine whether calcium-dependent apoptosis induced by ER stress
WAS mediated through the opening of mPTP. The results revealed that
mitochondrial calcein in the cytoplasm decreased gradually
following TBHP stimulation and that GYY4137 or NAC treatment
significantly inhibited mPTP opening at 24 h, while treatment with
NAC did not lead to any significant difference compared with
GYY4137 treatment (Fig. 3B and
E). The analysis of the activity of caspase-3 illustrated the
anti-apoptotic effect of GYY4137 in the TBHP-stimulated CHs.
Treatment with GYY4137 decreased caspase-3 activity compared with
the TBHP group (Fig. 3F).
GYY4137 attenuates ER stress via the GRP78/mTOR
signaling pathway
GRP78 is a key regulator of ER and previous research
has reported that GRP78 overexpression aggravates ER stress
(23). In the present study,
siRNA against GRP78 decreased the GRP78 expression levels by 80%
compared with the control group (Fig. 4A and B), which demonstrated the
transfection efficiency of si-GRP78. Compared with the control
group, DNA damage inducible transcript 3 (CHOP), GRP78 and ATF6
levels were significantly increased in the TBHP group, but
decreased in the GYY4137 group (Fig.
4C and D). Moreover, the results suggested that ER stress
occurs in CHs, indicating the possibility of induction of a
UPR-like event. ER-Tracker Green is highly selective for ER,
consisting of the green-fluorescent dye and glibenclamide (24). Following transfection with
si-GRP78, GYY4137 treatment protected the CHs against TBHP-induced
ER stress, as measured by ER-tracker (Fig. 5A). Furthermore, the results
demonstrated that the mean fluorescence intensity of ER-tracker was
increased in the CHs in the TBHP-cultured group but was decreased
following GYY4137 treatment for 24 h (Fig. 5B).
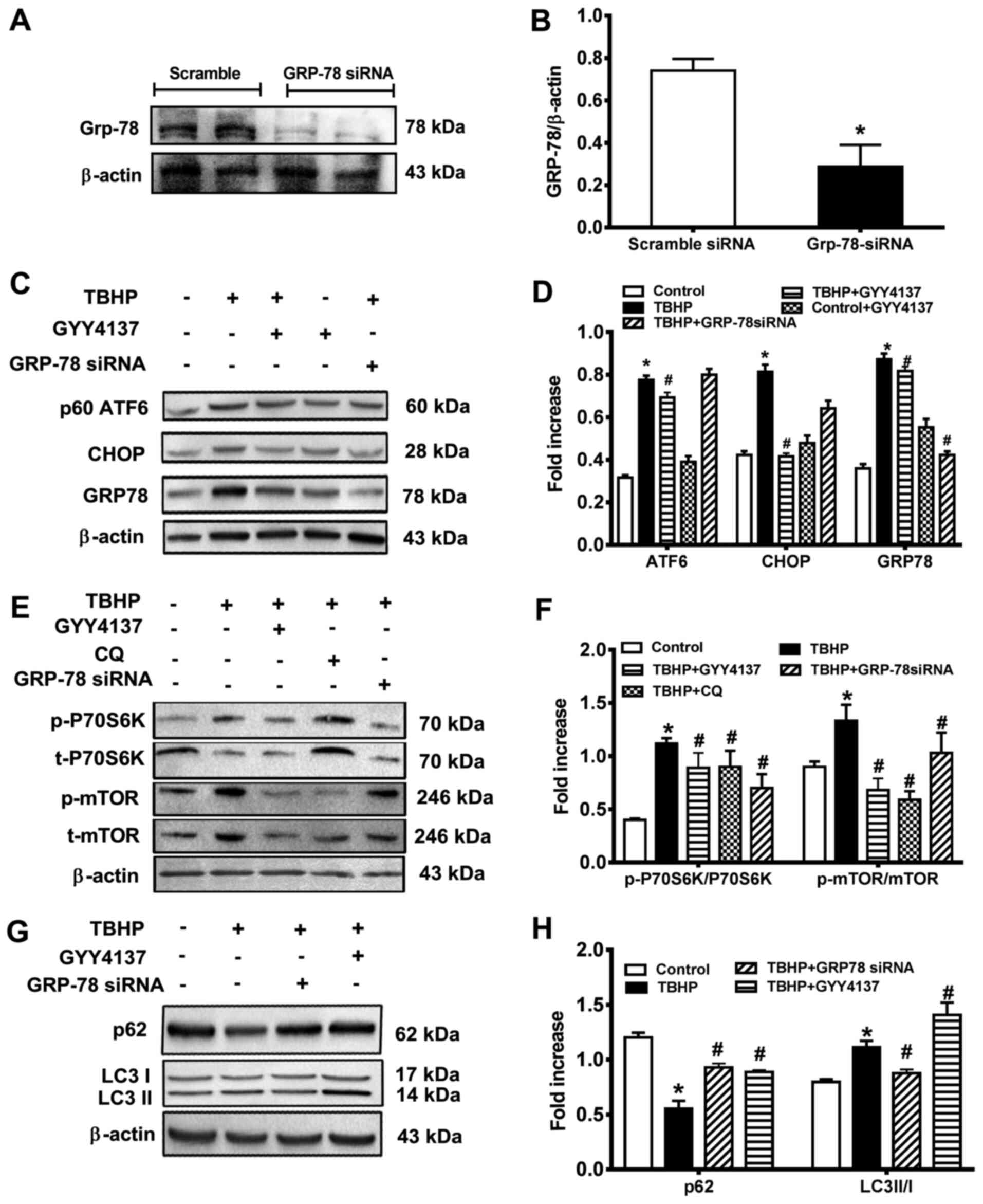 | Figure 4GRP78 knockdown leads to autophagy
inhibition and cell dysfunction. (A) Protein expression levels were
measured by western blot analysis. The following groups were
assessed: i) Scramble siRNA; and ii) Grp-78 siRNA. (B)
Densitometric results are expressed as a fold increase. (C) Protein
expression levels were measured by western blot analysis. The
following groups were assessed: i) Control, normal DMEM; ii) TBHP;
iii) TBHP + GYY4137, CHs pre-treated with 100 μM GYY4137;
and iv) TBHP + GRP-78 siRNA, CHs transfected with 150 nM GRP-78
siRNA for 48 h. The upper trace of each group displays
representative blots of the respective proteins. The trace of each
group displays representative blots of the respective proteins. (D)
Densitometric results are expressed as a fold increase. (E) Protein
expression levels were measured by western blot analysis. The
following groups were assessed: i) Control, normal DMEM; ii) TBHP;
iii) TBHP + GYY4137, CHs were pre-treated with 100 μM
GYY4137; iv) TBHP + GRP-78 siRNA, CHs were transfected with 150 nM
GRP-78 siRNA for 48 h; and v) TBHP + CQ, CHs were treated with
autophagic inhibitor CQ. The upper trace of each group displays
representative blots of the respective proteins. (F) Densitometric
results are expressed as a fold increase. (G) Protein expression
levels were measured via western blotting. The following groups
were assessed: i) Control, normal DMEM; ii) TBHP; iii) TBHP +
GYY4137, CHs were pre-treated with 100 μM GYY4137; and iv)
TBHP + GRP-78 siRNA, CHs were transfected with 150 nM GRP-78 siRNA
for 48 h. (H) Densitometric results are expressed as a fold
increase. *P<0.05 vs. control; #P<0.05
vs. TBHP. TBHP, tert-Butyl hydroperoxide; CH, chondrocyte; GRP78,
78-kDa glucose-regulated protein; siRNA, small interfering RNA; CQ,
chloroquine. |
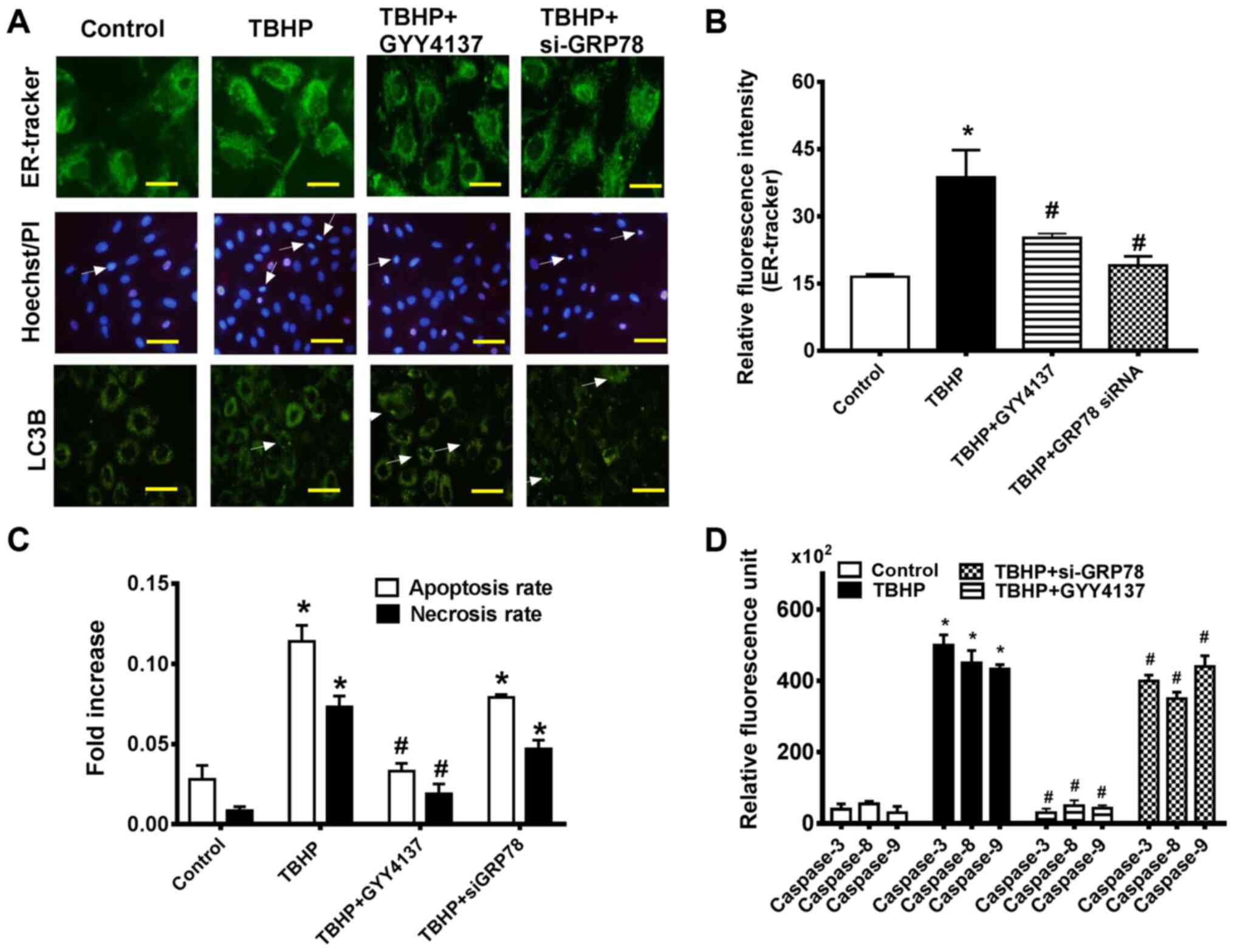 | Figure 5GYY4137 attenuates ER stress via the
GRP78/mTOR signaling pathway. (A) Upper panel images: Cells were
stained with ER-tracker (green). Fluorescence was observed using a
non-confocal fluorescence microscope (magnification, x400). Middle
panel images: The effect of GYY4137 and si-GRP78 on the number of
apoptotic CHs was assessed by performing Hoechst/PI staining
(magnification, x200). Lower panel images: The punctuate dots of a
green fluorescent-tagged LC3B protein in CHs following treatment
with or without GYY4137 (magnification, x200). (B) Analysis of
relative fluorescence intensity in CHs. (C) Quantification of
Hoechst-positive cells (as a percentage of total counted cells) and
PI-positive cells from 10 fields of view in 5 different areas. (D)
The activity levels of caspase-3, -8 and -9 in CHs by relative
activity assay. Data are presented as the means ± SEM.
*P<0.05 vs. control, #P<0.05 vs. TBHP
(n=3). ER, endoplasmic reticulum; si-, small interfering RNA;
GRP78, 78-kDa glucose-regulated protein; CH, chondrocytes; LC3B,
microtubule associated protein 1 light chain 3α. |
Chloroquine is an autophagy and Toll-like receptor
(TLR) inhibitor, which is used to lock the late stages of autophagy
in in vitro studies (chloroquine inhibits autophagic flux by
decreasing autophagosome-lysosome fusion). Compared with the
control group, the levels of p-P70S6K/P70S6K and p-mTOR/mTOR were
significantly increased in the TBHP group. Compared with the TBHP
group, the levels of p-P70S6K/P70S6K and p-mTOR/mTOR were
significantly decreased following treatment with GYY4137, GRP78
siRNA or chloroquine. This suggested that GYY4137 suppressed the
activation of the mTOR pathway induced by THBP through the
inhibition of autophagy (Fig. 4E and
F).
GRP78 knockdown leads to autophagy
inhibition and cell dysfunction
The present study then determined whether GRP78 is
necessary for increased ROS-induced cell apoptosis following TBHP
stimulation by using siRNA to silence of GRP78 in CHs. The CHs were
transfected with si-GRP78 to determine whether GRP78 was associated
with GYY4137-induced autophagy. The results suggested that
microtubule associated protein 1 light chain 3α (LC3) II/I
expression levels were increased in the GYY4137 group compared with
the TBHP group. Compared with the control group, the expression
levels of p62 were decreased in the TBHP group, which suggested the
suppression of autophagy (Fig. 4G
and H). The CHs displayed an increased LC3II expression in the
GYY4137 group compared with the TBHP group (Fig. 4G. There was no marked increase in
autophagy following treatment with GRP78 siRNA compared with the
control cells (Fig. 5A, LC3B).
These results indicated that H2S not only ameliorated
autophagy, but also inhibited ER stress in a GRP78-dependent
manner. Hoechst 33342/PI staining revealed that the ratio of
apoptosis (Hoechst) and necrosis (PI) in the CHs was reduced 24 h
following transfection with siRNA targeting GRP78 compared with the
TBHP group (Fig. 5C). As shown
in Fig. 5D, the CHs displayed a
marked decrease in caspase activity folowing treatment with
GYY4137. Moreover, the caspase activity of CHs decreased with GRP78
siRNA transfection, compared with the TBHP group. The results
suggested that GRP78 activation was essential for the protective
effects of H2S on the CHs under conditions of oxidative
stress.
Discussion
OA, which leads to joint disorders, is a common
disease that primarily affects elderly patients. For the past 20
years, intra-articular treatment options for the management of OA
have been limited to strategies that only provide symptomatic
relief and are associated with serious adverse effects (25,26). Therefore, improving the
therapeutic strategies is important to improve the efficacy of OA
therapy. CHs are the unique cell type in the cartilage, which are
implicated in multiple stresses, including oxidative stress,
inflammation and ER stress. ER stress induces CH apoptosis, which
is associated with the pathogenesis of OA (27). GRP78 has been reported to serve
as a critical mediator of ER stress, as demonstrated by decreased
mTOR induction (28-33). GRP78 was selected as a novel
biomarker, as its level associated with cell process, cell
integrity failure, and cell proliferation. In the present study,
the suppression of GRP78 was associated with increased mTOR levels
(Fig. 4E), which is consistent
with its role in cell autophagy (33).
In recent years, H2S has been recognized
as a target in cardiovascular diseases, including diabetic
cardiomyopathy, pulmonary arterial hypertension and
atherosclerosis, but it is relatively novel in CHs. GYY4137, a
H2S donor, releases H2S via hydrolysis both
in vitro and in vivo, and exhibits prolonged
biological activities, including anti-inflammatory, anti-autophagy,
anti-apoptotic effects (18-21). The present study aimed to
evaluate the protective effects of H2S against
OA-induced CH ER stress, as well as the possible effects of
H2S on oxidative stress. H2S is a useful
antioxidant due to its radical scavenging abilities. Previous in
vitro and in vivo studies have demonstrated that
exogenous H2S can alleviate cell apoptosis in humans and
animals. Both oxidative stress and ER stress have been associated
with CH apoptosis in OA. However, whether H2S can
protect CHs via suppressing ER stress-induced apoptosis is not yet
completely understood.
The present study investigated whether
H2S prevented apoptosis by inducing oxidative stress in
rat CHs, and also explored the possible underlying mechanisms. TBHP
was used as an oxidative stress inducer instead of
H2O2 due to its improved stability and longer
lasting effects. TBHP has been widely applied in the investigation
of the mechanisms underlying OA (31).
In the present study, it was hypothesized that
exogenous H2S could protect cartilage against
TBHP-induced oxidative stress. The present study relied on the
novel finding that GYY4137 displays physicochemical properties that
may be related to the inhibition of ER stress-induced apoptosis
(14,17,29) via the downregulation of the
expression levels of CHOP, GRP78 and ATF6.
Previous studies have suggested another important
role for the intrinsic or mitochondrial signaling pathway in
transducing a wide spectrum of death signals that originate both
inside and outside the cells (4,15). The stimulation of these pathways
is mediated by the translocation of death-promoting proteins from
the mitochondria to the cytoplasm, which initiates apoptosis via
the activation of a class of cysteine proteases (caspases)
(16,23). In the present study, GYY4137
treatment markedly decreased the activity of caspase-3 and
decreased the expression levels of p-mTOR (Figs. 3F and 4E) following TBHP stimulation. The
results suggested that treatment with exogenous H2S
protected against caspase family activation and decreased
TBHP-induced CH apoptosis. Moreover, the results indicated that
H2S inhibited TBHP-induced ER stress via the apoptotic
pathway (Fig. 5A and D).
Exogenous H2S treatment and si-GRP78 transfection
protected the CHs against TBHP-induced ER stress compared with the
TBHP-cultured group, as measured by ER-tracker and the activity
levels of caspase-3, -8 and -9 (Fig.
5A).
Autophagy plays as a prominent role in improving
cell survival. Previous studies have suggested that the interplay
between autophagy and apoptosis is complex (32). LC3 is initially synthesized in
its unprocessed form, as evidenced by conversion of LC3I to LC3II.
LC3II is localized in the autophagosome membrane and serves as a
reliable protein marker of autophagy (34). The results of the present study
suggested that the GYY4137-mediated cytoprotective effects against
TBHP-induced oxidative stress may be due to its activation of
autophagy via the GRP78/mTOR signaling pathway. The results
indicated a protective role of GRP78 in CHs. Moreover, si-GRP78
decreased mTOR phosphorylation, but successfully inhibited
autophagy, leading to increased apoptosis and cell injury. GRP78
knockdown augmented apoptosis compared with the control group, but
suppressed apoptosis compared with the TBHP group (Fig. 5C), suggesting the involvement of
GRP78/mTOR-dependent autophagy in cellular-protection. However, the
role of autophagy in OA is not yet completely understood, as it is
a repair mechanism that is activated by mild stress and with
increasing stress levels, apoptosis begins to occur.
A previous study demonstrated that miR-495 targeted
GRP78 and activated mTOR signaling to inhibit autophagy in
multidrug resistant gastric cancer (GC) cells (33), whereas the present study
demonstrated opposite results. First, the inconsistency between the
results of the present study and the aforementioned previous study
suggested that GC cells and CHs may be different cell types.
Moreover, the inconsistency indicated that there may be an upstream
microRNA modulating GRP78 at the post-transcriptional level. In
addition to activating mTOR and inhibiting ROS, H2S may
be implicated in preventing apoptosis or inducing autophagy via
modulating microRNAs, which should be investigated in future
studies. The past quarter-century has witnessed a tremendous
expansion in the knowledge of H2S that is associated
with anti-apoptotic and anti-oxidative mechanisms. It is worth
noting that the development of H2S-releasing drugs
rarely translates into preclinical success. Thus, it would be
important to not only identify the downstream signals of
H2S, but also production of H2S in various
types of cells under normal and disease conditions.
Funding
The present study was supported by grants from
National Natural Science Foundation of China (no. 81901853) to FY.
This study was also supported by the Key Project of Harbin
Municipal Science and Technology Bureau (no. 2016RAXYJ068) and dean
foundation of the Fourth Affiliated Hospital of Harbin Medical
University to DY. The present study was also supported by grants
from National Natural Science Foundation of China (no. 81900313) to
LY. The funders had no role in the study design, data collection
and analysis, decision to publish, or preparation of the
manuscript.
Availability of data and materials
The authors declare that the materials described in
the manuscript, including all relevant raw data, will be freely
available to any scientist wishing to use them for non-commercial
purposes, without breaching participant confidentiality.
Authors' contributions
DY made substantive contributions to the conception,
design of the study and critical revision. JW and FY performed
experiments and contributed to the design, data analysis, revision
of the original and final manuscript. XZ made contributions to the
initial draft of the article and performed experiments. GC, JZ and
LY made substantial contributions to the data analysis and
interpretation, and revision of the article. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from 4th Affiliated
Hospital of Harbin Medical University (Harbin, China). Animal
experiments were performed according to the Guide for the Care and
Use of Laboratory Animals of Harbin Medical University (Harbin,
China). All animal experiments were carried out according to animal
welfare standards and were approved by the Ethical Committee for
Animal Experiments of the Fourth Affiliated Hospital, Harbin,
China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Bo Yu from Key
Laboratory of Myocardial Ischemia, Ministry of Education, Harbin
Medical University (Harbin, China) who was more than generous with
his expertise and precious time. His vigorous academic observation
enlightens us not only in this thesis but also in our future
study.
Abbreviations:
|
OA
|
osteoarthritis
|
|
ROS
|
reactive oxygen species
|
|
NAC
|
N-acetyl-L-cysteine
|
|
ER
|
endoplasmic reticulum
|
|
H2S
|
hydrogen sulfide
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Xie J, Lin JT, Wei M, Teng Y, He Q, Yang G
and Yang X: Sustained Akt signaling in articular chondrocytes
causes osteoarthritis via oxidative stress-induced senescence in
mice. Bone Res. 7:232019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafara M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hui W, Young DA, Rowan AD, Xu X, Cawston
TE and Proctor CJ: Oxidative changes and signalling pathways are
pivotal in initiating age-related changes in articular cartilage.
Ann Rheum Dis. 75:449–458. 2016. View Article : Google Scholar :
|
|
4
|
Wu L and Liu Z: The molecular mechanisms
of preventing apoptosis of cartilage chondrocyte to target
osteoarthritis. Future Med Chem. 9:537–540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li XR, Li J, Ren Q and Sun S: The
molecular mechanism of treating osteoarthritis with dipsacus
saponins by inhibiting chondrocyte apoptosis. Exp Ther Med.
14:4527–4532. 2017.PubMed/NCBI
|
|
6
|
Mardones R, Jofre CM, Tobar L and Minguell
JJ: Mesenchymal stem cell therapy in the treatment of hip
osteoarthritis. J Hip Preserv Surg. 4:159–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasegawa A, Yonezawa T, Taniguchi N, Otabe
K, Akasaki Y, Matsukawa T, Saito Masahiko, Neo M, Marmorstein LY
and Lotz MK: Role of fibulin 3 in aging-related joint changes and
osteoarthritis pathogenesis in human and mouse knee cartilage.
Arthritis Rheumatol. 69:576–585. 2017. View Article : Google Scholar :
|
|
8
|
da Costa BR, Reichenbach S, Keller N,
Nartey L, Wandel S, Juni P and Trelle S: Effectiveness of
non-steroidal anti-inflammatory drugs for the treatment of pain in
knee and hip osteoarthritis: A network meta-analysis. Lancet.
390:e21–e33. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skrepnik N, Spitzer A, Altman R, Hoekstra
J, Stewart J and Toselli R: Assessing the impact of a novel
smartphone application compared with standard follow-up on mobility
of patients with knee osteoarthritis following treatment with hylan
G-F 20: A randomized controlled trial. JMIR MHealth UHealth.
5:e642017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walsh DA: Osteoarthritis: Nerve ablation-a
new treatment for OA pain? Nat Rev Rheumatol. 13:393–394. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Y, Chen J, Meng Z, Yao J, Ge W, Chen K,
Cheng S, Fu J, Peng L and Zhao YZ: Diazoxide prevents H2O2-induced
chondrocyte apoptosis and cartilage degeneration in a rat model of
osteoarthritis by reducing endoplasmic reticulum stress. Biomed
Pharmacother. 95:1886–1894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takada K, Hirose J, Yamabe S, Uehara Y and
Mizuta H: Endoplasmic reticulum stress mediates nitric
oxide-induced chondrocyte apoptosis. Biomed Rep. 1:315–319. 2013.
View Article : Google Scholar
|
|
13
|
Couasnay G, Bon N, Devignes CS, Sourice S,
Bianchi A, Veziers J, Weiss P, Elefteriou F, Provot S, Guicheux J,
et al: PiT1/Slc20a1 is required for endoplasmic reticulum
homeostasis, chondrocyte survival, and skeletal development. J Bone
Miner Res. 34:387–398. 2019. View Article : Google Scholar
|
|
14
|
Liu L, Zhang Y, Wang Y, Peng W, Zhang N
and Ye Y: Progesterone inhibited endoplasmic reticulum stress
associated apoptosis induced by interleukin-1β via the
GRP78/PERK/CHOP pathway in BeWo cells. J Obstet Gynaecol Res.
44:463–473. 2018. View Article : Google Scholar
|
|
15
|
Ye W, Zhu S, Liao C, Xiao J, Wu Q, Lin Z
and Chen J: Advanced oxidation protein products induce apoptosis of
human chondrocyte through reactive oxygen species-mediated
mitochondrial dysfunction and endoplasmic reticulum stress
pathways. Fundam Clin Pharmacol. 31:64–74. 2017. View Article : Google Scholar
|
|
16
|
González QM, Blondel A, Sagredo A, Hetz C,
Chevet E and Pedeux R: When endoplasmic reticulum proteostasis
meets the DNA damage response. Trends Cell Biol. 30:881–891. 2020.
View Article : Google Scholar
|
|
17
|
Yang F, Yu X, Li T, Wu J, Zhao Y, Liu J,
Sun A, Dong S, Wu J, Zhong X, et al: Exogenous H2S regulates
endoplasmic reticulum-mitochondria cross-talk to inhibit apoptotic
pathways in STZ-induced type I diabetes. Am J Physiol Endocrinol
Metab. 312:E190–E203. 2017. View Article : Google Scholar
|
|
18
|
Whiteman M, Haigh R, Tarr JM, Gooding KM,
Shore AC and Winyard PG: Detection of hydrogen sulfide in plasma
and knee-joint synovial fluid from rheumatoid arthritis patients:
Relation to clinical and laboratory measures of inflammation. Ann N
Y Acad Sci. 1203:146–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou X, Yuan Y, Sheng Y, Yuan B, Wang Y,
Zheng J, Liu CF, Zhang X and Hu LF: GYY4137, an H2S
slow-releasing donor, prevents nitrative stress and α-synuclein
nitration in an MPTP mouse model of Parkinson's disease. Front
Pharmacol. 8:7412017. View Article : Google Scholar
|
|
20
|
John A, Kundu S, Pushpakumar S, Fordham M,
Weber G, Mukhopadhyay M and Sen U: GYY4137, a hydrogen sulfide
donor modulates miR194-dependent collagen realignment in diabetic
kidney. Sci Rep. 7:109242017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drapala A, Koszelewski D, Tomasova L,
Ostaszewski R, Grman M, Ondrias K and Ufnal M: Parenteral Na2S, a
fast-releasing H2S donor, but not GYY4137, a slow-releasing H2S
donor, lowers blood pressure in rats. Acta Biochim Pol. 64:561–566.
2017. View Article : Google Scholar
|
|
22
|
Feng K, Ge Y, Chen Z, Li X, Liu Z, Li X,
Li H, Tang T, Yang F and Wang X: Curcumin Inhibits the
PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in
oxidative stress-induced rat chondrocytes and ameliorates
osteoarthritis progression in a rat model. Oxid Med Cell Longev.
2019:85743862019. View Article : Google Scholar
|
|
23
|
Zhang G, Wang X, Bi X, Li C, Deng Y,
Al-Hashimi AA, Luo X, Gillette TG, Austin RC, Wang Y and Wang ZV:
GRP78 (glucose-regulated protein of 78 kDa) promotes cardiomyocyte
growth through activation of GATA4 (GATA-binding protein 4).
Hypertension. 73:390–398. 2019. View Article : Google Scholar :
|
|
24
|
Kumari P, Verma SK and Mobin SM: A facile
two-photon fluorescent probe: An endoplasmic reticulum tracker
monitoring ER stress and vesicular transport to lysosomes. Chem
Commun (Camb). 55:294–297. 2019. View Article : Google Scholar
|
|
25
|
Allen KD, Golightly YM and White DK: Gaps
in appropriate use of treatment strategies in osteoarthritis. Best
Pract Res Clin Rheumatol. 31:746–759. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meng Z and Huang R: Topical treatment of
degenerative knee osteoarthritis. Am J Med Sci. 355:6–12. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao J, Zhang Y, Wang T and Li B:
Endoplasmic reticulum stress is involved in baicalin protection on
chondrocytes from patients with osteoarthritis. Dose Response.
16:15593258188106362018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ojha R, Leli NM, Onorati A, Piao S,
Verginadis II, Tameire F, Rebecca VW, Chude CI, Murugan S, Fennelly
C, et al: ER translocation of the MAPK pathway drives therapy
resistance in BRAF-mutant melanoma. Cancer Discov. 9:396–415. 2019.
View Article : Google Scholar
|
|
29
|
Liu Y, Wang X, Zhen Z, Yu Y, Qiu Y and
Xiang W: GRP78 regulates milk biosynthesis and the proliferation of
bovinemam-maryepithelial cells through the mTOR signaling pathway.
Cell Mol Biol Lett. 24:572019. View Article : Google Scholar
|
|
30
|
Thon M, Hosoi T, Yoshii M and Ozawa K:
Leptin induced GRP78 expression through the PI3K-mTOR pathway in
neuronal cells. Sci Rep. 4:70962014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng C, He K, Zhang C, Su S, Li B, Li Y,
Duan CY, Chen S, Chen R, Liu Y, et al: JNK contributes to the
tumorigenic potential of human cholangiocarcinoma cells through the
mTOR pathway regulated GRP78 induction. PLoS One. 9:e903882014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang Q, Zheng G, Feng Z, Chen Y, Lou Y,
Wang C, Zhang X, Zhang Y, Xu H, Shang P and Liu H: Trehalose
ameliorates oxidative stress-mediated mitochondrial dysfunction and
ER stress via selective autophagy stimulation and autophagic flux
restoration in osteoarthritis development. Cell Death Dis.
8:e30812017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D,
Kang J and Zhao G, Shi Y, Fan D and Zhao G: MicroRNA-495-3p
inhibits multidrug resistance by modulating autophagy through
GRP78/mTOR axis in gastric cancer. Cell Death Dis. 9:10702018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galluzzi L and Green DR:
Autophagy-independent functions of the autophagy machinery. Cell.
177:1682–1699. 2019. View Article : Google Scholar : PubMed/NCBI
|