Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common type of cancer worldwide and has a poor 5-year
survival rate (1). According to
statistics from the American Cancer Society, an estimated 53,000
new cases of HNSCC were diagnosed in the United States in 2019
(2) and globally, 600,000 new
cases of HNSCC are diagnosed annually (1). Although there has been significant
advancements in the diagnosis and treatment methods of HNSCC over
the past few years, the 5-year survival rate for HNSCC has not
significantly improved. Cervical lymph node metastasis (LNM) is an
important and independent prognostic factor affecting the treatment
of HNSCC, and is considered to be the main cause of the high
mortality of HNSCC (3).
Therefore, an improved understanding of the mechanisms of
metastasis and the identification of novel biomarkers are urgently
required to develop effective therapeutic strategies for HNSCC.
MicroRNAs (miRNAs/miRs) are a class of short
non-coding RNAs of 18-24 nucleotides in length, which regulate gene
expression by binding to the 3'-untranslated region (UTR) of target
mRNAs to inhibit protein translation and/or degrade the mRNA
(4,5). An increasing number of studies have
reported that miRNAs play important roles as oncogenes or tumor
suppressor by regulating the biological behaviors of cancer cells
(6-10). Moreover, accumulating studies
have reported that numerous miRNAs are abnormally expressed in
HNSCC tissues, which affects the cell migration and invasion of
HNSCC (11-13).
miR-411 belongs to the 14q32.31 miRNA cluster
(14). Previous studies have
reported that miR-411-5p inhibits the metastasis of breast cancer
cells (15) and the
overexpression of miR-411 suppresses renal cell cancer migration
(16). In non-small cell lung
cancer (NSCLC), miR-411-5p/3p can promote NSCLC cell proliferation,
tumor growth and metastasis both in vitro and in vivo
(17). However, these studies
mainly focused on the differences in miR-411-5p expression levels
between cancer tissues and paracancerous tissues. To further
understand the effect of miR-411-5p on metastasis in HNSCC, the
present study investigated the differences in miR-411-5p expression
levels between patients with and without LNM, and further analyzed
the mechanism of action of miR-411-5p in regulating HNSCC
metastasis.
Ring1 and YY1 binding protein (RYBP) is a member of
Polycomb group (PcG) proteins (18). It has been demonstrated that the
dysregulation of RYBP leads to a poorer prognosis in hepatocellular
carcinoma (19) and RYBP has
been found to act as a tumor suppressor in different types of
cancer (20-22). From previous research, it is
known that different miRNAs can regulate RYBP expression in
tumorigenesis in melanoma and gastric cancer (23,24).
Thus, it was hypothesized that miR-411-5p can affect
metastasis and epithelial-mesenchymal transition (EMT) by
regulating RYBP expression in HNSCC cells. The present study
investigated the effect of miR-411-5p on metastasis and EMT in
vitro and in vivo, and further clarify the potential
mechanisms of miR-411-5p in the development of HNSCC metastasis.
The results of the present study may provide a novel prognostic and
therapeutic strategy to treat HNSCC metastasis.
Materials and methods
The Cancer Genome Atlas (TCGA)
analysis
The miRNA expression profiles of TGCA-HNSC dataset
and the clinical information were obtained from TCGA database
(https://portal.gdc.cancer.gov/)
(25). A total of 528 patients
with HNSCC from TCGA database were selected for analysis in the
present study. The exclusion criteria were as follows: i)
Incomplete information on LNM; ii) incomplete patient clinical
data; and iii) the miR-411-5p expression data were incomplete.
Following the exclusion of patients, the miR-411-5p expression
levels and clinical information from 378 patients with HNSCC were
used in the present study.
Patient studies
A total of 52 tissues from patients with HNSCC were
collected from The Hospital of Stomatology, Sun Yat-Sen University
(Guangzhou, China) between October 2017 and June 2018. No patient
had received radiotherapy or chemotherapy before the biopsy, and
all patients were diagnosed with HNSCC. The tissue samples were
frozen at -80°C until required for use in further experiments. The
histology and pathology of all samples was examined by two
independent pathologists. Written informed consent was provided
from all patients prior to participation and the study was approved
by the Ethics Committee of Sun Yat-Sen University [approval no.
ERC-(2017)-32].
Cell lines and culture
The human HNSCC cell lines, HSC-3 and SCC-15, were
purchased from the American Type Culture Collection. HSC-3 cells
were cultured in DMEM supplemented with 10% FBS, 100 IU/ml
penicillin and 100 μg/ml streptomycin. SCC-15 cells were
cultured in DMEM/F12 (1:1) supplemented with 10% FBS, 100 IU/ml
penicillin and 100 μg/ml streptomycin. All culture reagents
were purchased from Gibco (Thermo Fisher Scientific, Inc.). All
cells were maintained at 37°C in a humidified atmosphere with 5%
CO2.
Cell transfection and lentiviral vector
construction
A total of 1x105 cells were transiently
transfected with 50 nM chemically synthesized miR-411-5p mimic, 100
nM miR-411-5p inhibitor, 50 nM small interfering RNA (si)-RYBP and
the corresponding negative controls (NCs; all Guangzhou RiboBio
Co., Ltd.) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. The
sequences were as follows: miR-411-5p mimic, 5'-UAG UAG ACC GUA UAG
CGU ACG-3'; miR-411-5p inhibitor, 5'-CGU ACG CUA UAC GGU CUA
CUA-3'; and si-RYBP, 5'-ACA GCA TAC AGT CTG CAA A-3'. Lentiviruses
encoding a reverse complementary sequence of miR-411-5p
(Anti-miR-411-5p) and an empty vector (Anti-ctrl) were constructed
by Shanghai GeneChem Co., Ltd. A total of 1x105 cells
HSC-3 and SCC-15 cells were infected with the lentiviral vectors
(1x108 TU/ml), and stable cell lines were selected
following treatment with 2 μg/ml puromycin for 2 weeks.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tissues or cultured
cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). To analyze the expression levels of miR-411-5p, Bulge-Loop™
miRNA qRT-PCR Primer sets were synthesized by Guangzhou RiboBio
Co., Ltd. Total RNA was reverse transcribed into cDNA using the
PrimeScript RT Master Mix (Takara Bio, Inc.), according to the
manufacturer's protocols. qPCR was subsequently performed using the
LightCycler® 480 SYBR Green I Master kit (Roche Diagnostics),
according to the manufacturer's protocols. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95°C for 5 min; followed by 40 cycles of denaturation for 10 sec
at 95°C, annealing for 20 sec at 60°C and extension for 20 sec at
72°C; and a final 10 min extension at 72°C. The relative expression
levels were calculated using the 2-ΔΔCq method (26). The following primers were used
for the qPCR: RYBP forward, 5'-GGG GTG GTG GGG TGG CAT ACT-3' and
reverse, 5'-CGC AGA CGA AGG GTT TTG GGA TT-3'; and GAPDH forward,
5'-GCA CCG TCA AGG CTG AGA AC-3' and reverse, 5'-TGG TGA AGA CGC
CAG TGG A-3'. GAPDH and U6 were used as the internal controls for
RYBP and miR-411-5p, respectively. GAPDH and RYBP were purchased
from Sangon Biotech Co., Ltd., while U6 and miR-411-5p were
purchased from Guangzhou RiboBio Co., Ltd.
Transwell assay
Cell migration and invasion assays were performed
using Transwell chambers (8-mm pore size; Corning, Inc.). Briefly,
for the migration assay, HSC-3 (3x104) or SCC-15
(8x104) cells were plated in serum-free medium into the
upper chambers of the Transwell plates. For the invasion assay,
HSC-3 (6x104) or SCC-15 (2x105) cells were
seeded into the upper chambers with Transwell plates precoated with
Matrigel for 6 h at 37°C. The lower chambers were filled with
medium supplemented with 10% FBS. Following incubation for 24 h at
37°C, the medium was removed and the cells were fixed with 4%
formaldehyde for 20 min at room temperature. Cells that had not
migrated or invaded through the membrane were gently removed using
a cotton swab. The fixed cells were subsequently stained with 0.4%
crystal violet for 15 min at room temperature and observed under a
light microscope (Zeiss GmbH). A total of five representative
fields at x100 magnification were randomly imaged. ImageJ v1.8.0
(National Institutes of Health) was used for quantification, and
the average counts of the five images represented the migrated
cells.
Cell Counting Kit-8 (CCK-8) assays
The proliferation of HNSCC cells transfected with
the miR-411-5p mimic or miR-411-5p inhibitor was determined using a
CCK-8 assay (Telenbiotech). Briefly, 2x103 cells were
seeded into a 96-well plate and cultured for 24 h, prior to
transfection with the miR-411-5p mimic or inhibitor. Following
incubation for 24, 48, 72, 96 or 120 h, CCK-8 reagent was added to
the culture medium for 2 h. The absorbance of each well was
measured at a wavelength of 450 nm. All experiments were performed
in triplicate.
Colony formation assay
For the colony formation assay, 5x102
cells were seeded into 6-well plates in triplicate 24 h
post-transfection. Following the culture of cells for 10 days, the
visible colonies were stained with 0.4% crystal violet for 10 min
at room temperature. Colonies with diameters >1 mm were defined
as colonies.
Dual-luciferase reporter assay
To further analyze the molecular mechanism of
miR-411-5p-mediated metastasis in HNSCC, miRDB (http://www.mirdb.org/miRDB), miRTar-Base (http://mirtarbase.mbc.nctu.edu.tw/php/)
and TargetScan 7.1 (http://www.targetscan.org/vert_71) databases were
used. The potential miR-411-5p binding site in RYBP 3'-UTR was
predicted using TargetScan (http://www.targetscan.org/vert_71). For luciferase
assays, wild-type (Wt) or Mutant (Mut) RYBP 3'-UTR sequences were
designed, synthesized and cloned into a pMIR-REPORT™ vector by OBiO
Technology (Shanghai) Co., Ltd. to generate RYBP-3'-UTR-Wt and
RYBP-3'-UTR-mut vectors, respectively. Then, HNSCC cells
(4x104) were co-transfected with 100 nM miR-411-5p
mimics or NC and RYBP-3'-UTR-Wt or RYBP-3'-UTR-mut vectors using
Lipofectamine 3000 reagent. Following 48 h of transfection, the
relative luciferase activity was measured using a Dual-Luciferase
Reporter Assay System (Promega Corporation), according to the
manufacturer's protocols, and was compared with the Renilla
luciferase activity of the samples. The assays were performed in
triplicate.
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with protease and phosphatase inhibitors. Total protein was
quantified using a BCA kit (Wuhan Boster Biological Technology Co.,
Ltd.), according to the manufacturer's protocols, and 20 μg
protein/lane was separated via SDS-PAGE on a 10% gel. The separated
proteins were subsequently transferred onto PVDF membranes (EMD
Millipore) and blocked with 5% non-fat milk for 1 h at room
temperature. The membranes were then incubated with the following
primary antibodies: Anti-E-cadherin (1:1,000; cat. no. 14472; Cell
Signaling Technology, Inc.), anti-N-cadherin (1:1,000; cat. no.
13116; Cell Signaling Technology, Inc.), anti-vimentin (1:1,000;
cat. no. 5741; Cell Signaling Technology, Inc.), anti-GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.) and
anti-RYBP (1:500; cat. no. sc-374235; Santa Cruz Biotechnology,
Inc.). Following the primary antibody incubation, the membrane was
washed three times with TBS with 2% Tween-20 and incubated with
HRP-conjugated anti-rabbit (cat. no. 7074) or anti-mouse (cat. no.
7076) secondary anti-bodies (1:5,000; Cell Signaling Technology,
Inc.) for 1 h at room temperature. Protein bands were visualized
using Immobilon Western Chemiluminescent HRP substrate (EMD
Millipore) and an ImageQuant™ LAS 4000 mini-imaging system
(Cytiva). Semi-quantification was performed using ImageJ
(v1.8.0).
Immunohistochemistry (IHC)
IHC staining was performed with paraffin-embedded
sections. Briefly, the lymph nodes were immediately fixed in 4%
paraformaldehyde for 24 h and soaked in ethanol (70 to 100%),
following which the sections (4 μm thick) were embedded in
paraffin. The tissue sections were incubated in 10% goat serum
blocking solution (Wuhan Boster Biological Technology, Ltd.) for 1
h at room temperature, followed by incubation with an
anti-pan-cytokeratin primary antibody (1:200; cat. no. ab9377;
Abcam) overnight at 4°C. Sections were then incubated with a
secondary antibody for 30 min at room temperature according to the
instructions of the GT Vision™ and Detection System/MoRb kit (Gene
Tech), which contained the secondary antibody and DAB, followed by
staining with DAB and counterstaining with hematoxylin for 1 min at
room temperature. Then, two senior pathologists blinded to the data
assessed and scored the IHC results. The extent of staining was
scored according to the percentage of positively stained cells: i)
1, <10% of cells; ii) 2, 10-35% of cells; iii) 3, 35-70% of
cells; and iv) 4, >70% of cells. The staining intensity was
graded as follows: i) 0, no staining; ii) 1, weak staining (light
yellow); iii) 2, moderate staining (yellow brown); and iv) 3,
strong staining (brown), as previously described (9). The staining index was calculated by
multiplying the percentage of positive cells and staining
intensity. Five random fields per sample were analyzed using a
light microscope (magnification, x100) to visualize sections.
In vivo xenograft experiments
All animal experiments were approved by the Ethical
Committee of Sun Yat-Sen University (approval no.
SYSU-IACUC-2020-000562) and were performed in accordance with the
guidelines for animal care and protection (27). A total of 14 BALB/c nude mice
(female; age, 4-6 weeks old; weight, 18-20 g) were obtained from
GemPharmatech Co., Ltd., and were housed in specific pathogen-free
conditions at 25°C and 50% humidity, with a 12-h light/dark cycle
and free access to food and water. The mice were randomly divided
into 2 groups (n=7). A total of 2x106 SCC-15 cells with
or without miR-411-5p expression knockdown were suspended in 100
μl PBS, and then injected into the tongue of the nude mice
following anesthesia by isoflurane [4% (0.5 l/min) for induction
and 2% for maintenance]. Following 8 weeks, the maximum tumor
diameter in the mice was recorded as 5.65 mm and the maximum tumor
volume was 62.40 mm3, all mice were sacrificed using
cervical dislocation and death was confirmed by the absence of
breathing, and the lack of a heartbeat and corneal reflex. Finally,
the cervical lymph nodes were collected and analyzed using IHC
analysis.
Statistical analysis
Statistical analysis was performed using SPSS
version 20.0 software (IBM Corp.) and GraphPad Prism 6.0 software
(GraphPad Software, Inc.). Statistical differences between two
groups were determined using an unpaired Student's t-test, while
multiple group comparisons were performed using a one-way ANOVA,
followed by a Tukey's post hoc test. χ2 tests were used
to determine the association between miR-411-5p expression levels
and clinicopathological features. Spearman's correlation analyses
were used to determine the correlation between miR-411-5p and RYBP
expression. The Kaplan-Meier method and a log-rank test were used
for overall survival analysis. To further determine the influence
of multiple independent prognostic factors, including miR-411-5p
expression levels, on the overall survival of patients with HNSCC,
multivariate Cox regression analysis was performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-411-5p expression levels are
upregulated in patients with HNSCC with LNM
In the present study, 378 patients with HNSCC from
TCGA were obtained, including 218 patients with LNM and 160
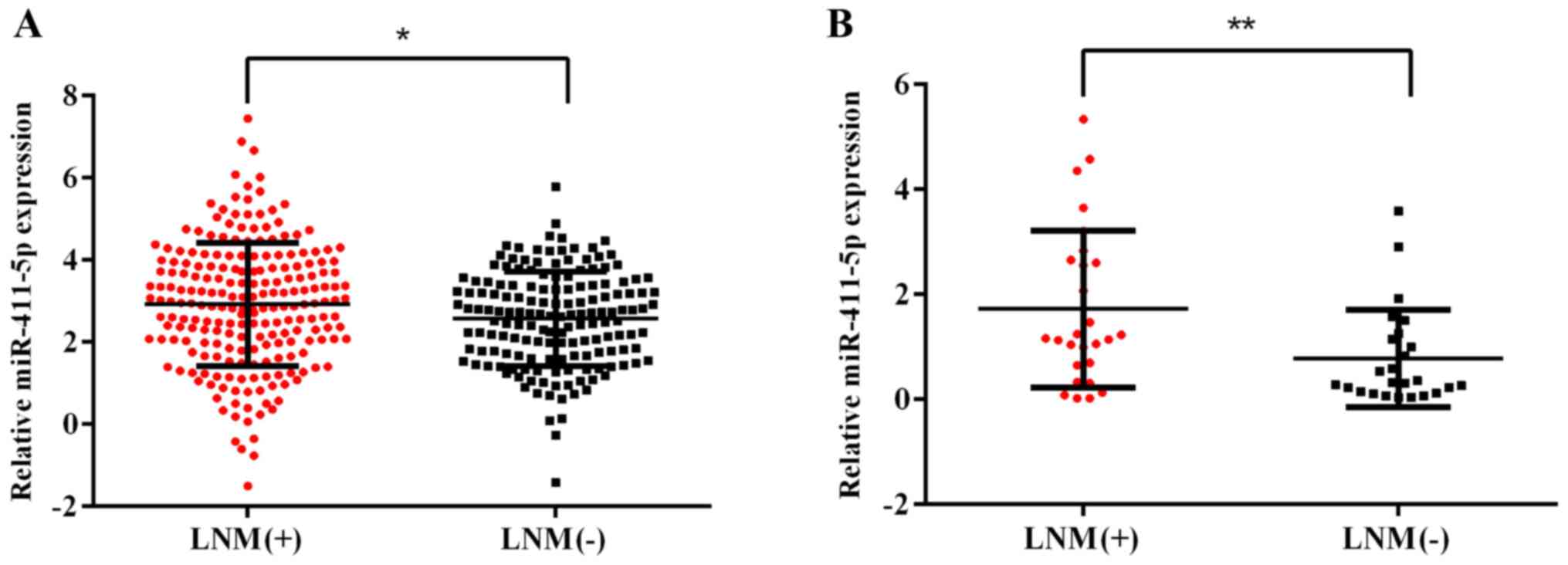
patients without LNM. As shown in Fig. 1A, the expression levels of
miR-411-5p were upregulated in patients with HNSCC with LNM
compared with patients without LNM. To validate the results
obtained via bioinformatics analysis, miR-411-5p expression levels
in 52 HNSCC clinical patient samples were analyzed by RT-qPCR, and
the results revealed the same trends in the expression levels as
the bioinformatics analysis (Fig.
1B). These findings suggested that the expression levels of
miR-411-5p may be associated with LNM in HNSCC.
Association between miR-411-5p expression
levels and clinicopathological characteristics in patients with
HNSCC
As shown in Table
I, χ2 analysis revealed that high miR-411-5p
expression levels were positively associated with LNM, pathological
differentiation and clinical stage in 52 clinical HNSCC patient
samples. Furthermore, the association between miRNA-411-5p
expression levels and the clinicopathological characteristics of
the 378 patients with HNSCC are shown in Table SI. The analysis identified that
miR-411-5p expression levels were associated with LNM; however, no
association was found between miR-411-5p expression levels and sex,
age, T stage, clinical stage and pathological differentiation. By
combining the analysis of TCGA databases and HNSCC clinical
samples, the data indicated that miR-411-5p expression levels may
be a useful biomarker for HNSCC.
 | Table IAssociation between the expression of
miR-411-5p and clinicopathological characteristics in HNSCC
clinical sample. |
Table I
Association between the expression of
miR-411-5p and clinicopathological characteristics in HNSCC
clinical sample.
| Clinicopathological
characteristics | Number of cases,
n | miR-411-5p
expression, n
| P-value |
|---|
| High | Low |
|---|
| Age, years | | | | |
| ≥55 | 26 | 11 | 15 | 0.406 |
| <55 | 26 | 15 | 11 | |
| Sex | | | | |
| Male | 37 | 17 | 20 | 0.541 |
| Female | 15 | 9 | 6 | |
|
Differentiation | | | | |
| Well | 23 | 7 | 16 | 0.025a |
| Moderate +
poor | 29 | 19 | 10 | |
| T stage | | | | |
| T1 + TII | 26 | 7 | 14 | 0.404 |
| TIII + TIV | 19 | 16 | 8 | |
| Clinical stage | | | | |
| I-II | 20 | 6 | 14 | 0.045a |
| III-IV | 32 | 20 | 12 | |
| LNM | | | | |
| LNM(−) | 25 | 7 | 18 | 0.005a |
| LNM(+) | 27 | 19 | 8 | |
Association between miR-411-5p expression
levels and prognosis in patients with HNSCC
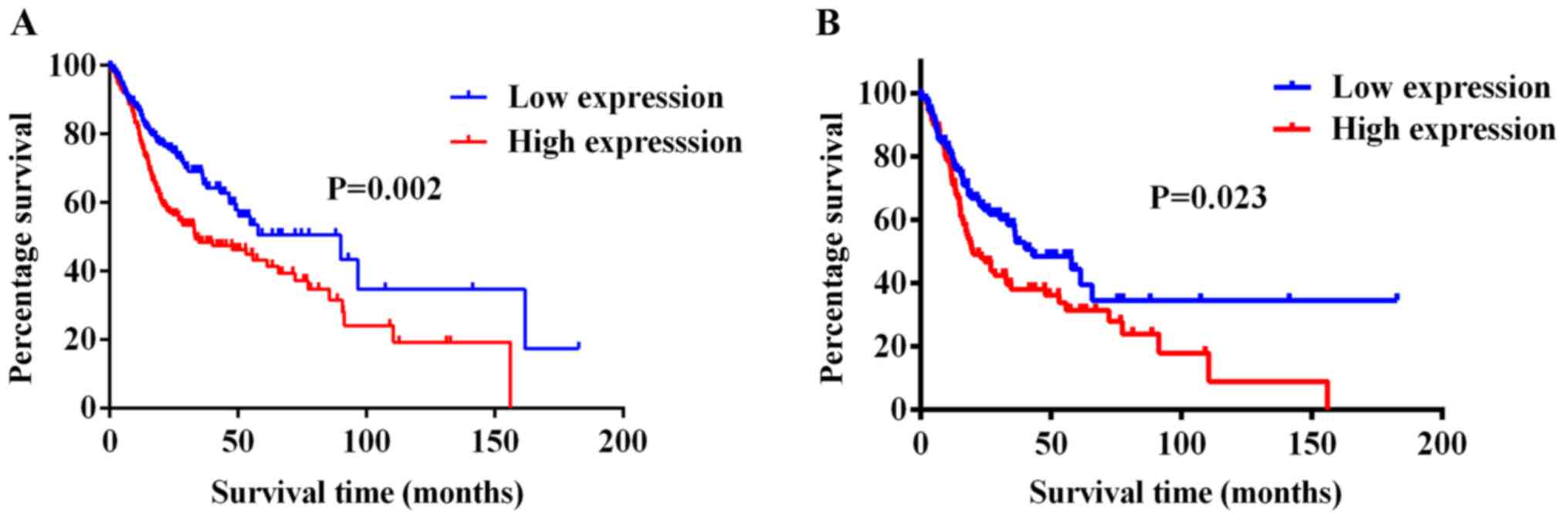
Kaplan-Meier survival analysis based on TCGA dataset
revealed that upregulated miR-411-5p expression levels were
associated with a poor prognosis in patients with HNSCC (Fig. 2A). In addition, the prognostic
value of miR-411-5p expression levels was also analyzed in patients
with LNM. The results revealed that upregulated miR-411-5p
expression levels were associated with a poor overall survival rate
in patients with HNSCC with LNM (Fig. 2B). Furthermore, univariate
analysis revealed that LNM and miR-411-5p expression levels were
prognostic factors for patients with HNSCC. Multivariate cox
analysis of the prognostic variables in 378 patients with HNSCC
from TCGA dataset identified that miR-411-5p expression levels
[hazard ratio (HR), 1.478; 95% CI, 1.066-2.048; P=0.019] and LNM
(HR, 1.911; 95% CI, 1.335-2.735; P<0.001) were significantly
associated with the prognosis of HNSCC (Table SII). Taken together, these
results suggested that the upregulated expression levels of
miR-411-5p may be positively associated with the metastatic
potential of HNSCC and may serve as a potential indicator for the
poor prognosis of patients with HNSCC.
miR-411-5p promotes HNSCC metastasis and
EMT
Clinicopathological analyses demonstrated that
miR-411-5p expression levels were positively associated with LNM in
HNSCC. Therefore, the effects of miR-411-5p on the migration and
invasion of HNSCC cells were analyzed using Transwell assays. HSC-3
and SCC-15 cells were transfected with miR-411-5p mimics or
inhibitors, compared with the negative control group, increased and
decreased miR-411-5p expression levels in HNSCC cells was confirmed
by RT-qPCR (Fig. 3A and B).
Meanwhile, HSC-3 and SCC15 cells were infected with lentiviral
vectors to establish stable cell lines that expressed short hairpin
RNA against miR-411-5p (anti-miR-411-5p), decreased miR-411-5p
expression was verified by RT-qPCR (Fig. S1A and B). In the miR-411-5p
inhibitor group, the knockdown of miR-411-5p expression
significantly reduced the migratory and invasive abilities of HSC-3
and SCC-15 cells (Fig. 3C).
Conversely, the overexpression of miR-411-5p significantly promoted
the migration and invasion of HSC-3 and SCC-15 cells (Fig. 3C). Furthermore, inguinal LNM
models were constructed by injecting SCC-15 cells with or without
knocked down miR-411-5p expression into the tongue of mice to
analyze the effect of miR-411-5p on metastasis in vivo.
Histological analysis revealed that the knockdown of miR-411-5p
decreased the number of pan-cytokeratin-positive metastatic tumor
cells and metastatic ratio of cervical lymph nodes (Fig. 3D and E). Taken together, these
findings indicated that the knockdown of miR-411-5p may suppress
LNM in vitro and in vivo.
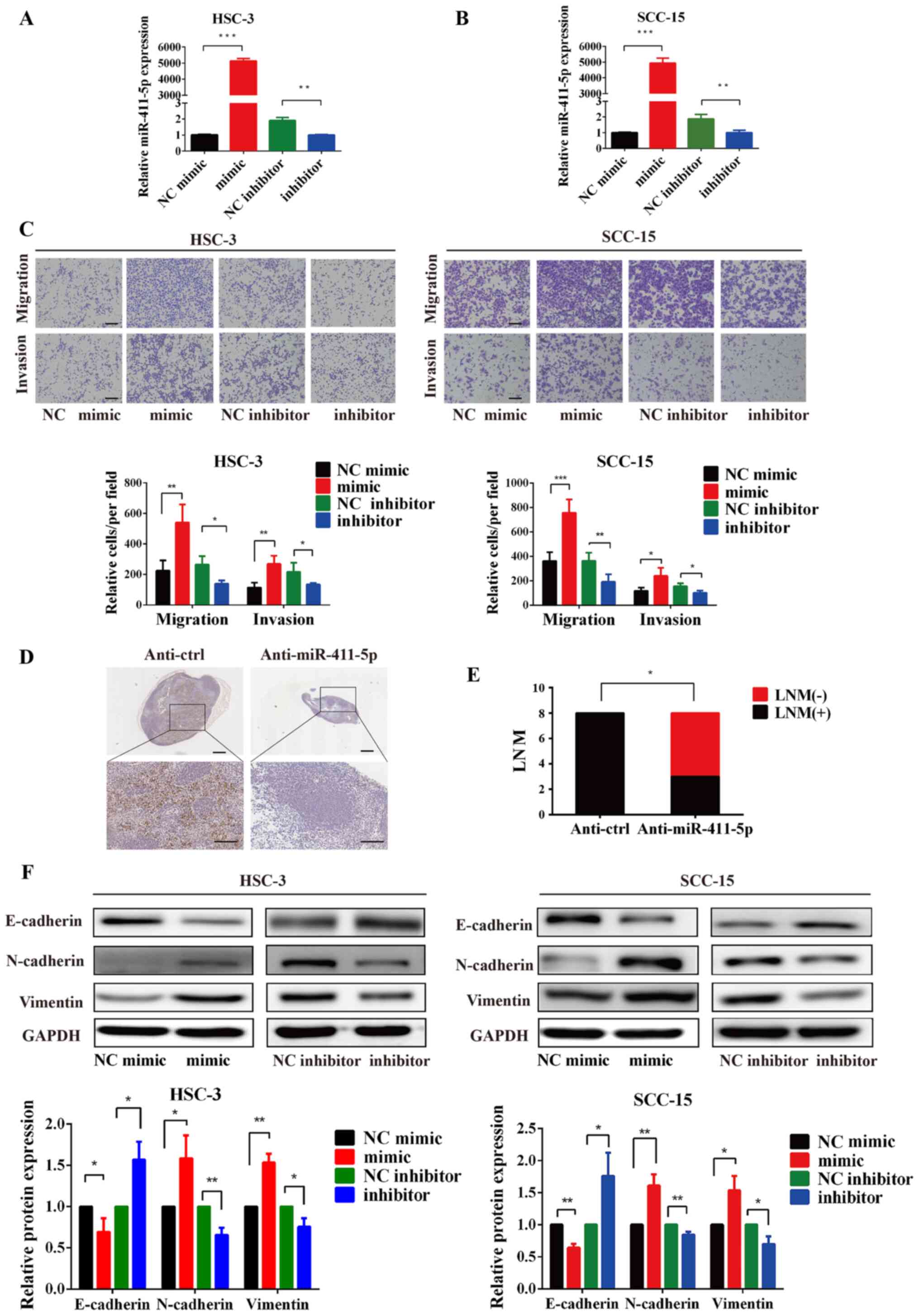 | Figure 3miR-411-5p promotes HNSCC cell
migration, invasion and EMT in vitro and metastasis in
vivo. (A and B) Transfection efficiency of miR-411-5p was
verified using reverse transcription-quantitative PCR. (C) Effects
of miR-411-5p mimics and inhibitors on the invasion and migration
of HNSCC cells were analyzed using Transwell assays. (D)
Representative staining of metastatic tumor cells in the cervical
lymph nodes of mice following the injection of 2x106
SCC-15 cells with or without miR-411-5p inhibitor using
anti-pan-cytokeratin. (E) Metastatic ratio of cervical lymph nodes.
(F) Overexpression of miR-411-5p downregulated the expression
levels of E-cadherin, and upregulated N-cadherin and vimentin
expression levels in HNSCC cells. By contrast, the knockdown of
miR-411-5p upregulated the expression levels of E-cadherin, and
downregulated vimentin and N-cadherin expression levels. The
expression levels of the EMT-related markers, E-cadherin,
N-cadherin and vimentin were analyzed using western blotting. GAPDH
was used as the internal loading control. Scale bar, 100 μm.
*P<0.05, **P<0.01,
***P<0.001. miR, microRNA; HNSCC, head and neck
squamous cell carcinoma; EMT, epithelial-mesenchymal transition;
NC, negative control; LNM, lymph node metastasis. |
It is well-established that the induction of EMT is
a major event that promotes the motility of cancer cells in the
invasion-metastasis cascade. Therefore, the effect of miR-411-5p on
EMT was determined by analyzing the expression levels of
EMT-related markers. The overexpression of miR-411-5p downregulated
the expression levels of the epithelial marker, E-cadherin, and
upregulated the expression levels of the mesenchymal markers,
N-cadherin and vimentin, in HNSCC cells (Fig. 3F). By contrast, the genetic
knockdown of miR-411-5p upregulated the expression levels of
N-cadherin and vimentin and downregulated E-cadherin expression
levels (Fig. 3F). These results
indicated that miR-411-5p may promote the migration, invasion and
EMT of HNSCC cells. Furthermore, CCK-8 and colony formation assays
were conducted to analyze the proliferation of HNSCC cells. The
results revealed that the overexpression and knockdown of
miR-411-5p did not affect the proliferation of HNSCC cells
(Fig. S2A and B).
RYBP is a direct target gene of
miR-411-5p in HNSCC
The bioinformatics analysis demonstrated that the
seed sequence of miR-411-5p was complementary to the 3'-UTR of RYBP
(Fig. 4A), which suggested that
RYBP may be a potential target of miR-411-5p. Dual-luciferase
reporter assays confirmed that miR-411-5p overexpression
significantly suppressed the relative luciferase activity of the
RYBP-3'-UTR-Wt reporter gene, but had no effect on the
RYBP-3'-UTR-Mut reporter gene (Fig.
4B). Moreover, RT-qPCR and western blotting confirmed that the
overexpression of miR-411-5p significantly downregulated RYBP
expression levels, while the silencing of miR-411-5p expression
upregulated RYBP expression levels (Fig. 4C and D). Clinically, a negative
correlation was identified between miR-411-5p and RYBP expression
levels in HNSCC tissues (Fig.
4E). Collectively, these results suggested that RYBP may be a
direct downstream target of miR-411-5p in HNSCC cells.
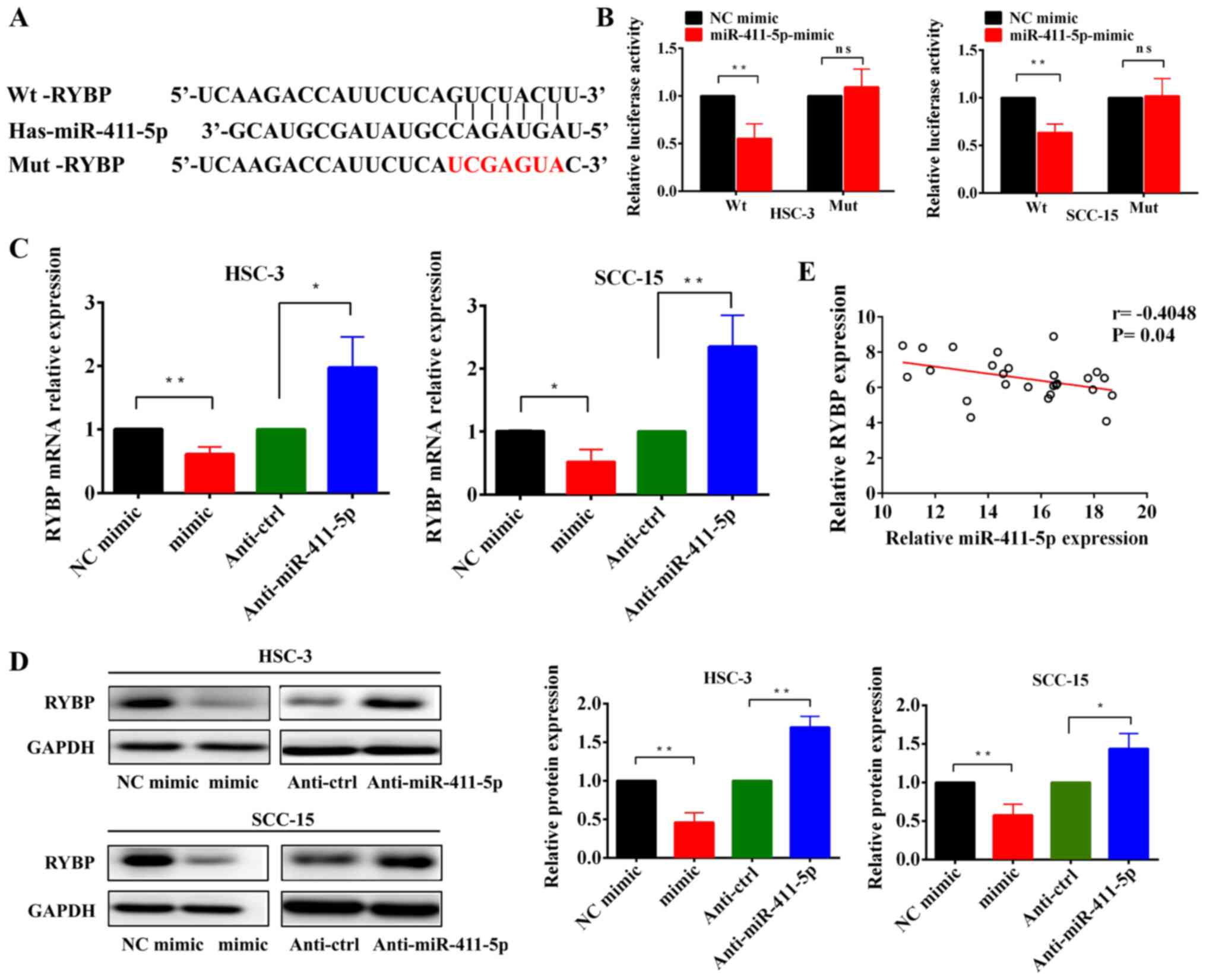 | Figure 4RYBP is a direct target gene of
miR-411-5p in HNSCC cells. (A) Predicted and mut binding sites of
miR-411-5p in the 3'-untranslated region of RYBP. (B) Relative
luciferase activity was analyzed in HSC-3 and SCC-15 cells
transfected with miR-411-5p mimic or NC and Wt or Mut reporter
genes. (C and D) Reverse transcription-quantitative PCR and western
blotting of RYBP mRNA and protein expression levels, respectively,
following the overexpression and knockdown of miR-411-5p in HNSCC
cells. (E) Spearman's correlation analysis was used to determine
the correlation between RYBP and miR-411-5p expression levels in
HNSCC tissues. *P<0.05, **P<0.01. RYBP,
RING1 and YY1 binding protein; miR, microRNA; Wt, wild-type; Mut,
mutant; NC, negative control; HNSCC, head and neck squamous cell
carcinoma; ns, not significant. |
Knockdown of RYBP promotes HNSCC cell
migration, invasion and EMT
As expected, the expression levels of RYBP were
downregulated in patients with HNSCC with LNM compared with
patients without LNM (Fig. 5A).
To analyze the effects of RYBP on HNSCC cells, si-RYBP was
transfected into HSC-3 and SCC-15 cells. The genetic knockdown of
RYBP resulted in the significant downregulation of RYBP protein
expression levels (Fig. 5B). The
results of the Transwell assays showed that the knockdown of RYBP
significantly promoted HNSCC cell migration and invasion compared
with the control siRNA (Fig. 5C and
D). Furthermore, the knockdown of RYBP upregulated the
expression levels of N-cadherin and vimentin, and downregulated
E-cadherin expression levels in HSC-3 and SCC-15 cells (Fig. 5E and F).
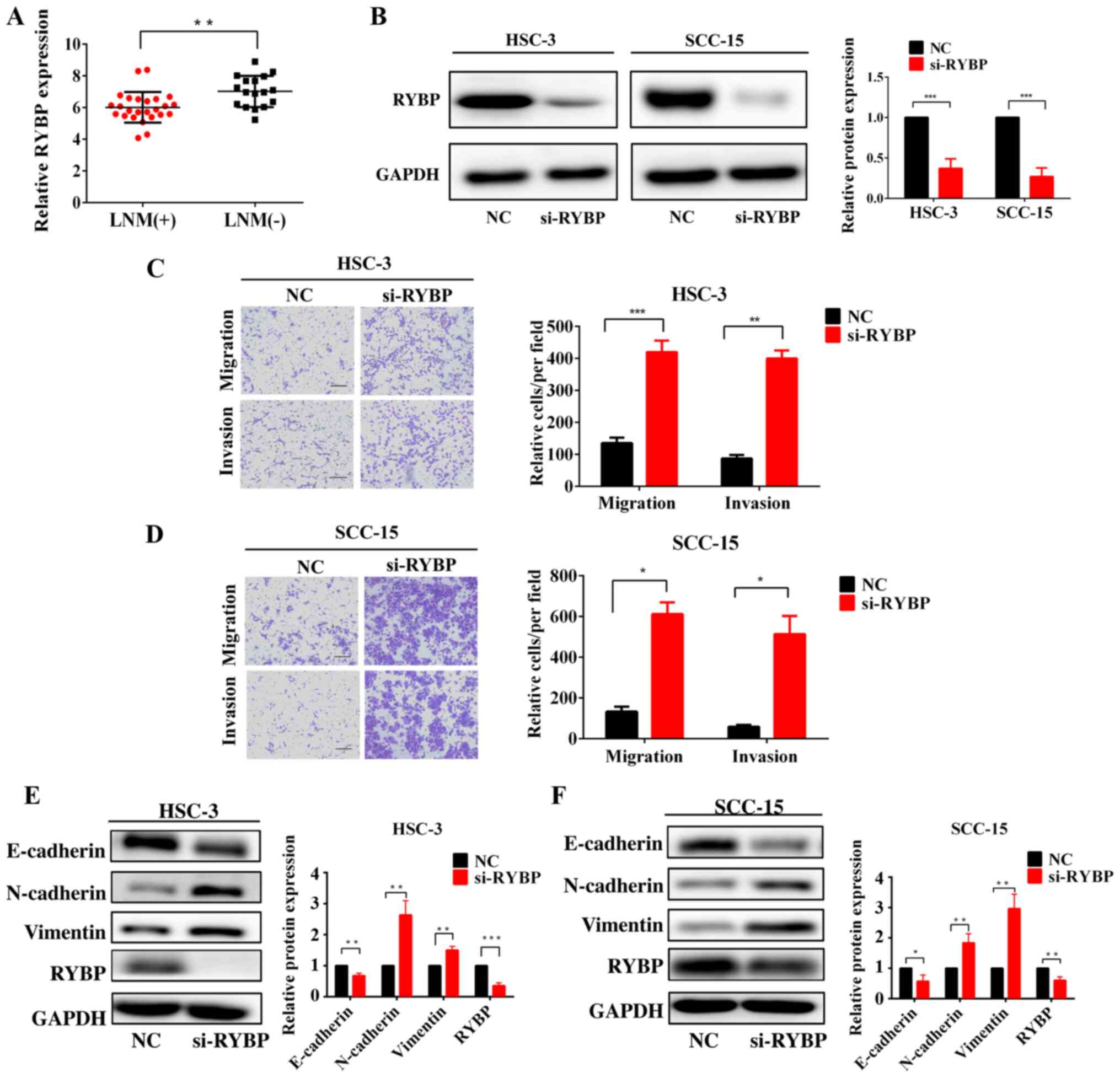 | Figure 5Knockdown of RYBP promotes HNSCC cell
migration, invasion and epithelial-mesenchymal transition. (A)
Expression levels of RYBP in patients with HNSCC with or without
LNM were analyzed using reverse transcription-quantitative PCR. (B)
Western blotting of the protein expression levels of RYBP following
the silencing of RYBP in HNSCC cells. (C and D) Effects of the
silencing of RYBP on HNSCC cell invasion and migration. (E and F)
Western blotting of the effect of RYBP on the expression levels of
E-cadherin, N-cadherin and vimentin. GAPDH was used as the internal
loading control. Scale bar, 100 μm. *P<0.05,
**P<0.01, ***P<0.001. RYBP, RING1 and
YY1 binding protein; HNSCC, head and neck squamous cell carcinoma;
LNM, lymph node metastasis; si-, small interfering RNA; NC,
negative control. |
Knockdown of RYBP restores
miR-411-5p-induced increases in HNSCC cell migration and
invasion
To determine whether the knockdown of RYBP
expression was able to rescue HNSCC cell migration and invasion in
the anti-miR-411-5p HNSCC cell line, si-RYBP was transfected into
the anti-miR-411-5p HNSCC cell line. The results of the Transwell
assays revealed that the genetic silencing of RYBP in the
anti-miR-411-5p HNSCC cell line increased cell migration and
invasion compared with the NC group (Fig. 6A and B). Moreover, the silencing
of RYBP rescued the miR-411-5p knockdown-induced downregulation of
the expression levels of mesenchymal markers and upregulation of
the expression levels of epithelial markers (Fig. 6C and D). Taken together, these
results indicated that miR-411-5p may regulate HNSCC cell invasion,
migration and EMT by targeting RYBP.
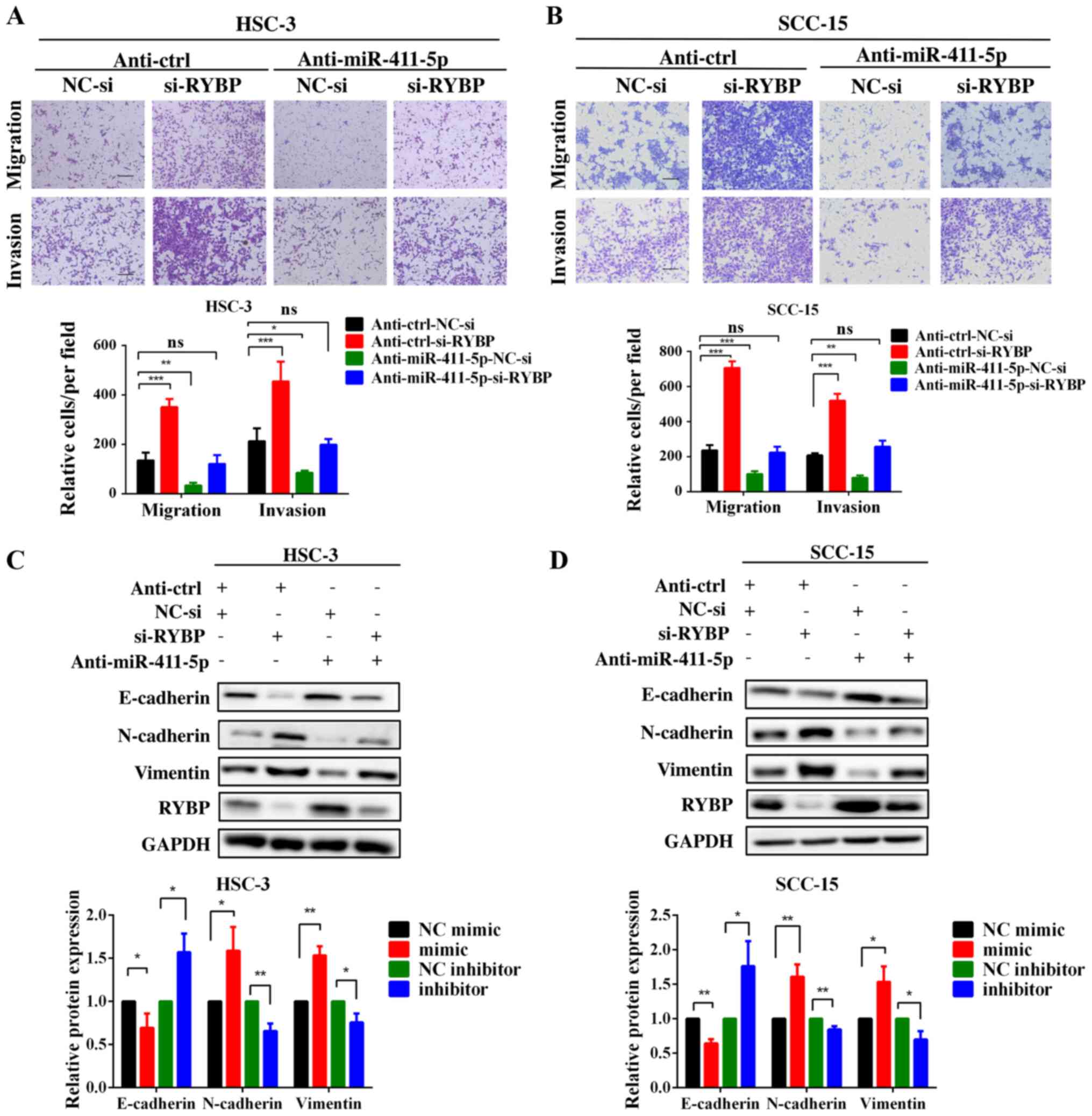 | Figure 6RYBP, as a direct target, may be
responsible for miR-411-5p-induced head and neck squamous cell
carcinoma cell invasion, migration and epithelial-mesenchymal
transition. (A and B) Transwell assays were performed following the
co-transfection of HSC-3 and SCC-15 cells with the miR-411-5p
inhibitor and si-RYBP. (C and D) Western blotting of E-cadherin,
N-cadherin and vimentin expression levels following the
co-transfection of HSC-3 and SCC-15 cells with the miR-411-5p
inhibitor and si-RYBP. GAPDH was used as the internal loading
control. Scale bar, 100 μm. *P<0.05,
**P<0.01, ***P<0.001. RYBP, RING1 and
YY1 binding protein; miR, microRNA; si-, small interfering RNA; ns,
not significant; NC, negative control. |
Discussion
Metastasis is a major obstacle for the effective
treatment of HNSCC (28).
Previously, it has emerged that miRNAs play important roles in
cancer initiation, development, drug resistance and metastasis
(5,10). Previous studies have demonstrated
that miR-411-5p exhibits both tumor suppressive and oncogenic
properties in different types of cancer (29-31). However, to the best of our
knowledge, few studies have focused on the association between
miR-411-5p expression levels and metastasis in HNSCC. The present
study first used bioinformatics analysis to analyze the expression
levels of miR-411-5p in patients with or without LNM. The results
revealed that miR-411-5p expression levels were upregulated in
patients with HNSCC with LNM. Similarly, data obtained from patient
tissues further validated that miR-411-5p expression levels were
upregulated in patients with HNSCC with LNM. Based on these
observations, it was hypothesized that miR-411-5p may be associated
with the aggressiveness and metastasis of HNSCC. Consistent with
this hypothesis, the present results demonstrated that the
upregulated expression levels of miR-411-5p were positively
associated with the metastatic potential of HNSCC, and therefore,
may serve as a potential indicator of poor prognosis in patients
with HNSCC.
To investigate the effect of miR-411-5p on HNSCC
migration and invasion in the present study, miR-411-5p was
overexpressed and knocked down. miR-411-5p was discovered to
significantly promote HNSCC cell migration and invasion, which was
consistent with the reported oncogenic role of miR-411-5p in NSCLC
(17). However, miR-411-5p has
been reported to inhibit proliferation and migration in bladder and
gastric cancer (32,33), which suggests that miR-411-5p may
have opposing roles in different types of cancer and the mechanism
requires further investigation. In addition, to further determine
the effects on cell proliferation, CCK-8 assays were also performed
in the current study. However, the results revealed that the
changes in the expression levels of miR-411-5p did not affect the
levels of cell proliferation. Therefore, it was suggested that
miR-411-5p may have an oncogenic role that impacts HNSCC metastasis
rather than cell proliferation.
EMT serves an important role in the progression of
tumor metastasis, but was first described as a feature of
embryogenesis in the 1980s (34). EMT is a biological process in
which epithelial cells acquire a mesenchymal phenotype or
fibroblast-like properties, with an increased invasive potential
(35). miRNAs are well-studied
in the context of EMT plasticity (36). For example, Lin et al
(37) reported that the
downregulation of miR-639 expression levels was associated with
TGF-β-induced EMT in tongue oral squamous cell carcinoma (OSCC),
which may represent a possible target for the treatment of OSCC
metastasis. Another previous study reported that the miR-200 family
suppressed the EMT process and the metastatic ability of tongue
OSCC cells (38). The findings
of the present study demonstrated that miR-411-5p promoted EMT,
indicating that miR-411-5p may affect the metastatic ability of
HNSCC by promoting EMT.
miRNAs are known to regulate gene expression by
binding to the 3'-UTR of target mRNAs and either inhibiting their
translation or inducing the degradation of the mRNA (4). To further understand the mechanism
of action of miR-411-5p in HNSCC, bioinformatics analysis was
performed. The results revealed that miR-411-5p bound to the 3'-UTR
of RYBP. Dual-luciferase reporter assays validated that RYBP was a
direct downstream target of miR-411-5p. Further analysis confirmed
that the overexpression of miR-411-5p downregulated RYBP expression
levels, whereas the knockdown of miR-411-5p upregulated RYBP
expression levels at both the mRNA and protein levels. RYBP is a
member of the PcG of proteins (18). Dinglin et al (39) previously reported that RYBP
inhibited lung cancer metastasis by reversing EMT. Another previous
study reported that RYBP expression levels were negatively
correlated with the upregulated expression levels of the
EMT-related transcription factors, zinc finger E-box binding
homeobox (ZEB)1 and ZEB2 (22).
The present study revealed that RYBP expression levels were
downregulated in patients with HNSCC with LNM. The genetic
silencing of RYBP promoted the metastasis, invasion and EMT of
HNSCC cells. In addition, the knockdown of RYBP expression restored
the stimulatory effects of miR-411-5p on HNSCC cell migration,
invasion and EMT, thus supporting the contribution of
miR-411-5p-regulated RYBP in HNSCC metastasis and EMT. These
results suggested that miR-411-5p may have the potential to be a
prognostic marker and potential predictor of cervical LNM in
HNSCC.
In conclusion, the findings of the present study
suggested that the upregulated expression levels of miR-411-5p were
associated with a poor prognosis in patients with HNSCC with LNM.
Therefore, the miR-411-5p/RYBP axis may serve a significant role in
promoting HNSCC metastasis and EMT.
Supplementary Data
Funding
This study was supported by the National Nature
Science Foundation of China (grant no. 81870769) and Guangdong
Financial Fund for High-Caliber Hospital Construction (grant no.
174-2018-XMZC-0001-03-0125/D-05).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JX and BC conceived and designed the experiments. CZ
and HFW performed the experiments and collected important
background information. MD, LHH and FP were involved in data
acquisition and analysis. YH and ZNF drafted the manuscript and
acquired data. JX and BC confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Sun Yat-Sen University (Guangzhou, China). Informed consent was
obtained from all participants. All included patients fulfilled
criteria and completed the study.
Patient consent for publication
All participants provided written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inglehart RC, Scanlon CS and Silva NJ:
Reviewing and reconsidering invasion assays in head and neck
cancer. Oral Oncol. 12:1137–1143. 2014. View Article : Google Scholar
|
|
4
|
Shukla GC, Singh J and Barik S: MicroRNAs:
Processing, maturation, target recognition and regulatory
functions. Mol Cellular Pharmacol. 3:83–92. 2011.
|
|
5
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hui ABY, Lenarduzzi M, Krushel T, Waldron
L, Pintilie M, Shi W, Perez-Ordonez B, Jurisica I, O'Sullivan B,
Waldron J, et al: Comprehensive MicroRNA profiling for head and
neck squamous cell carcinomas. Clin Cancer Res. 16:1129–1139. 2010.
View Article : Google Scholar
|
|
7
|
Liu X, Bi L, Wang Q, Wen M, Li C, Ren Y,
Jiao Q, Mao JH, Wang C, Wei G and Wang Y: MiR-1204 targets VDR to
promotes epithelial-mesenchymal transition and metastasis in breast
cancer. Oncogene. 37:3426–3439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiiba M, Uzawa K and Tanzawa H: MicroRNAs
in head and neck squamous cell carcinoma (HNSCC) and oral squamous
cell carcinoma (OSCC). Cancers (Basel). 2:653–669. 2010. View Article : Google Scholar
|
|
9
|
Li Y, He Q, Wen X, Hong X, Yang X, Tang X,
Zhang P, Lei Y, Sun Y, Zhang J, et al: EZH2-DNMT1-mediated
epigenetic silencing of miR-142-3p promotes metastasis through
targeting ZEB2 in nasopharyngeal carcinoma. Cell Death Differ.
26:1089–1106. 2019. View Article : Google Scholar :
|
|
10
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong Y, Zheng Y, Wang C, Ding X, Du Y, Liu
L, Zhang W, Zhang W, Zhong Y, Wu Y and Song X: MiR-876-5p modulates
head and neck squamous cell carcinoma metastasis and invasion by
targeting vimentin. Cancer Cell Int. 18:1212018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shiah SG, Hsiao JR, Chang WM, Chen YW, Jin
YT, Wong TY, Huang JS, Tsai ST, Hsu YM, Chou ST, et al:
Downregulated miR329 and miR410 promote the proliferation and
invasion of oral squamous cell carcinoma by targeting Wnt-7b.
Cancer Res. 74:7560–7572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Carvalho AC, Scapulatempo-Neto C, Maia
DC, Evangelista AF, Morini MA, Carvalho AL and Vettore AL: Accuracy
of microRNAs as markers for the detection of neck lymph node
metastases in patients with head and neck squamous cell carcinoma.
BMC Med. 13:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nadal E, Zhong J, Lin J, Reddy RM, Ramnath
N, Orringer MB, Chang AC, Beer DG and Chen G: A MicroRNA cluster at
14q32 drives aggressive lung adenocarcinoma. Clin Cancer Res.
20:3107–3117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Xu G, Liu G, Ye Y, Zhang C, Fan
C, Wang H, Cai H, Xiao R, Huang Z and Luo Q: MiR-411-5p inhibits
proliferation and metastasis of breast cancer cell via targeting
GRB2. Biochem Bioph Res Commun. 476:607–613. 2016. View Article : Google Scholar
|
|
16
|
Zhang X, Zhang M, Cheng J, Lv Z, Wang F
and Cai Z: MiR-411 functions as a tumor suppressor in renal cell
cancer. Int J Biol Markers. 32:e454–e460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang C, Wang H, Liu X, Hu Y, Ding L,
Zhang X, Sun Q and Li Y: Oncogenic microRNA-411 promotes lung
carcinogenesis by directly targeting suppressor genes SPRY4 and
TXNIP. Oncogene. 38:1892–1904. 2019. View Article : Google Scholar :
|
|
18
|
Garcia E, Marcos-Gutierrez C, del Mar
Lorente M, Moreno JC and Vidal M: RYBP, a new repressor protein
that interacts with components of the mammalian Polycomb complex,
and with the transcription factor YY1. EMBO J. 18:3404–3418. 1999.
View Article : Google Scholar
|
|
19
|
Zhao Q, Cai W, Zhang X, Tian S, Zhang J,
Li H, Hou C, Ma X, Chen H, Huang B and Chen D: RYBP expression is
regulated by KLF4 and Sp1 and is related to hepatocellular
carcinoma prognosis. J Biol Chem. 292:2143–2158. 2017. View Article : Google Scholar :
|
|
20
|
Zhou H, Li J, Zhang Z, Ye R, Shao N,
Cheang T and Wang S: RING1 and YY1 binding protein suppresses
breast cancer growth and metastasis. Int J Oncol. 49:2442–2452.
2016. View Article : Google Scholar
|
|
21
|
Voruganti S, Xu F, Qin JJ, Guo Y, Sarkar
S, Gao M, Zheng Z, Wang MH, Zhou J, Qian B, et al: RYBP predicts
survival of patients with non-small cell lung cancer and regulates
tumor cell growth and the response to chemotherapy. Cancer Lett.
369:386–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Yan M, Luo W, Liu W, Ren Y, Bei C,
Tang G, Chen R and Tan S: Expression and clinical significanceof
PcG-associated protein RYBP in hepatocellular carcinoma. Oncol
Lett. 13:141–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luan PB, Jia XZ and Yao J: MiR-769-5p
functions as an oncogene by down-regulating RYBP expression in
gastric cancer. Eur Rev Med Pharmacol Sci. 24:6699–6706.
2020.PubMed/NCBI
|
|
24
|
Zhao G, Li Q, Wang A and Jiao J: YY1
regulates melanoma tumorigenesis through a miR-9 ~ RYBP axis. J Exp
Clin Cancer Res. 34:662015. View Article : Google Scholar
|
|
25
|
Pérez Sayáns M, Chamorro Petronacci CM,
Lorenzo Pouso AI, PadíIruegas E, Blanco Carrión A, Suárez Peñaranda
JM and García García A: Comprehensive genomic review of TCGA head
and neck squamous cell carcinomas (HNSCC). J Clin Med. 8:18962019.
View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
National Research Council (US) Committee
for the Update of the Guide for the Care and use of Laboratory
Animals: Guide for the care and use of laboratory animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
28
|
Loberg RD, Bradley DA, Tomlins SA,
Chinnaiyan AM and Pienta KJ: The lethal phenotype of cancer: The
molecular basis of death due to malignancy. CA Cancer J Clin.
57:225–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu N, Yang W, Liu Y, Yan F and Yu Z:
MicroRNA-411 promoted the osteosarcoma progression by suppressing
MTSS1 expression. Environ Sci Pollutr Res Int. 25:12064–12071.
2018. View Article : Google Scholar
|
|
30
|
Zhao J, Xu J and Zhang R: MicroRNA-411
inhibits malignant biological behaviours of colorectal cancer cells
by directly targeting PIK3R3. Oncol Rep. 39:633–642. 2018.
|
|
31
|
Jin H, Sun W, Zhang Y, Yan H, Liufu H,
Wang S, Chen C, Gu J, Hua X, Zhou L, et al: MicroRNA-411
downregulation enhances tumor growth by upregulating MLLT11
expression in human bladder cancer. Mol Ther Nucleic Acids.
11:312–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Liu T, Jin H, Yin L, Yu H and Bi J:
MiR-411 suppresses the development of bladder cancer by regulating
ZnT1. Onco Targets Ther. 11:8695–8704. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bai TL, Liu YB and Li BH: MiR-411 inhibits
gastric cancer proliferation and migration through targeting SETD6.
Eur Rev Med Pharmaco. 23:3344–3350. 2019.
|
|
34
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7:re82014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Bio. 15:178–196. 2014. View Article : Google Scholar
|
|
36
|
Domingues CSDC, Serambeque BP, Laranjo
Cândido MS, Marto CMM, Veiga FJB, Sarmento Antunes Cruz Ribeiro AB,
Figueiras ARR, Botelho MFR and Dourado MARF: Epithelial-mesenchymal
transition and microRNAs: Challenges and future perspectives in
oral cancer. Head Neck. 40:2304–2313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin Z, Sun L, Chen W, Liu B, Wang Y, Fan
S, Li Y and Li J: MiR-639 regulates transforming growth factor
beta-induced epithelial-mesenchymal transition in human tongue
cancer cells by targeting FOXC1. Cancer Sci. 105:1288–1298. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamagawa S, Beder LB, Hotomi M, Gunduz M,
Yata K, Grenman R and Yamanaka N: Role of miR-200c/miR-141 in the
regulation of epithelial-mesenchymal transition and migration in
head and neck squamous cell carcinoma. Int J Mol Med. 33:879–886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dinglin X, Ding L, Li Q, Liu Y, Zhang J
and Yao H: RYBP inhibits progression and metastasis of lung cancer
by suppressing EGFR signaling and epithelial-mesenchymal
transition. Transl Oncol. 10:280–287. 2017. View Article : Google Scholar : PubMed/NCBI
|