Introduction
Glucosamine (GlcN) is an aminosaccharide that acts
as a preferred substrate for the biosynthesis of glycosaminoglycans
and, subsequently, for the production of aggrecan and other
proteoglycans in the connective and cartilage tissues (1). GlcN supports joint structure
function by serving as a building block of the cartilage matrix,
and maintains joint health by preventing tissue degradation,
reducing inflammation and oxidative stress, improving the autophagy
response of chondrocytes and increasing the chondrogenic potential
of mesenchymal stem cells resident in the niche (1,2).
Additionally, GlcN is an essential substrate for the synthesis of
glycosylated proteins and lipids (3). For its biological properties, GlcN
is prescribed as a drug or a dietary supplement in the management
of one of the most common joint disorders, osteoarthritis (OA), to
delay the progression of tissue degeneration and to attenuate the
symptoms in humans (1,4,5).
Furthermore, GlcN is recommended for joint health to prevent
sports-related cartilage injuries in athletes (6). At present, GlcN preparations are
the most widely used nutraceutical for OA (7,8).
There are three common forms of GlcN supplements on the market:
GlcN hydrochloride, GlcN sulfate, and N-acetyl GlcN. The
chondroprotective action of these GlcN compound, is supported both
by evidence obtained using different in vitro and in
vivo experimental models, and also clinical trials (1). Currently, the prescription of
crystalline GlcN sulfate (1,500 mg once daily) is recommended by
the majority of clinical practice guidelines in the management of
OA (9).
During the evolution of OA and disease progression,
there are substantial subchondral bone metabolic alterations and
remodeling (10); as OA in the
elderly is often accompanied by osteoporosis (11), it is critical to also consider
how bone tissue may be affected by GlcN. At present, there are
limited data available concerning the effects of GlcN on human
osteoclasts and osteoblasts that populate the bone microenvironment
(12-14). It would be beneficial to obtain
information concerning this in order to broaden the pharmacological
relevance and potential therapeutic efficacy of GlcN in skeletal
diseases.
The primary aim of the present pilot study was to
examine the effects of GlcN on human primary osteoclasts (hOCs)
cultured in conventional two-dimensional (2D) monolayer, as well as
those in a more complex culture system that more closely models the
in vivo bone microenvironment, consisting of an
osteoclast/osteoblast 3D dynamic co-culture system (15). The employment of this in
vitro model mimicking the process of bone matrix deposition and
remodeling provides simultaneous information on osteoclast and
osteoblast cell populations. The effects of crystalline GlcN
sulfate on osteoclastogenesis were investigated, which was
performed both by treatment with osteoclastogenic inducers or by
the presence of osteoblasts. As a source of osteoclast progenitors,
human primary monocytes (hMCs) from the peripheral blood of donors
(healthy controls or patients with OA) were used.
Materials and methods
Reagents
DONA® (crystalline GlcN sulfate) was
obtained from Mylan Italia S.r.l., resuspended at 50 mg/ml and
stored at 4°C. Histopaque®-1077, ascorbic
acid-2-phosphate, β-glycerophosphate, dexamethasone, MTT, Alizarin
Red S (ARS), paraformaldehyde, Triton X-100, tartrate-resistant
acid phosphatase (TRAP) kit (cat. no. 386), fetal calf serum (FCS),
L-glutamine and antibiotics (penicillin and streptomycin) were
purchased from Sigma-Aldrich (Merck KGaA). A High Capacity cDNA
Reverse Transcription kit, TaqMan Gene Expression assays, Universal
Master Mix II and Alexa Fluor® 488 Phalloidin (cat. no.
A12379) were purchased from Thermo Fisher Scientific, Inc.
Antibodies for human runt-related transcription factor 2 (Runx2;
cat. no. sc-10758), collagen type 1α (COL1a1; cat. no. sc-28657),
nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1; cat.
no. sc-13033) and cathepsin K (cat. no. sc-48353) were purchased
from Santa Cruz Biotechnology, Inc., and osteopontin (OPN; clone
LF-123) was a generous gift from Dr Larry Fisher (National
Institutes of Health). High-glucose Dulbecco's modified Eagle's
medium (DMEM), Ham's F12 and PBS were purchased from Euroclone
SpA.
Cell isolation and culture
Female patients with OA (n=7; 50-74 years) and
healthy volunteers (n=4; 43-48 years) were enrolled between May
2019 and December 2019 during routine medical check-ups at Centro
di Medicina (Ferrara, Italy) after obtaining written informed
consent; the study was approved by the Centro di Medicina's
research committee (approval no. 172201). Briefly, peripheral blood
mononuclear cells (PBMCs) were obtained from 20 ml peripheral blood
and separated using Histopaque-1077 as previously described
(16). hMCs were purified from
PBMCs via adhesion selection on polystyrene plates. PBMCs
(1×106/cm2) were plated and allowed to settle
for 4 h at 37°C, and flasks were then rinsed to remove non-adherent
cells. In order to confirm the ability of isolated hMCs to
differentiate into mature osteoclasts (hOCs), macrophage
colony-stimulating factor (25 ng/ml) and receptor activator of
NF-κB ligand (RANKL; 30 ng/ml; PeproTech EC Ltd.) were added to the
culture medium; after 14 days, TRAP staining was performed. The
expression levels of the osteoclast-specific markers cathepsin K
and NFATc1 were assessed via immunocytochemistry.
Human osteoblasts (hOBs) were obtained from
vertebral laminae discarded during spinal surgery to remove lumbar
herniated discs (Pfirrmann grade 2). Bone fragments were obtained
between September 2019 and December 2019, after obtaining written
informed consent from 4 donors with no comorbidity (43-48 years; 2
males and 2 females) using research protocols approved by the
Ethics Committee of the University of Ferrara and St. Anna Hospital
(approved on November 17, 2016). Briefly, bone fragments were
placed in sterile PBS at 4°C and dissected within 16 h after
removal. Bone chips were minced into smaller pieces as previously
reported (17), washed twice
with PBS, plated in T-25 culture flasks (Sarstedt, Inc.) and
cultured in high-glucose DMEM/Ham's F12 (1:1) supplemented with 10%
FCS, 1 mM L-glutamine and antibiotics [(penicillin (100
µg/ml) and streptomycin, (10 µg/ml)]. From each
patient, a primary cell culture was obtained. Upon detection of a
cell colony from the bone fragments (after 7 days), the cells were
expanded until confluent [passage (P)0)]. The cells were then
harvested after treatment with 0.05% trypsin EDTA for 2 min at 37°C
(Sigma-Aldrich; Merck KGaA), washed, counted via hemocytometric
analysis and used for further experiments (P1-3). During the
culture period, cells were incubated at 37°C in a humidified
atmosphere of 5% CO2, and the medium was changed every 3
days. hOBs (P0) were characterized for the presence of OPN, Runx2
and COL1a1 via immunostaining.
Based on previous studies, osteogenic
differentiation was performed by culturing hOBs for up to 14 days
in osteogenic medium (OM) (18,19) consisting of high-glucose DMEM,
10% FCS, 10 mM β-glycerophosphate, 100 nM dexamethasone and 100
µM ascorbic acid-2-phosphate.
TRAP staining
TRAP staining of cells was performed as previously
described (20). Briefly, the
cells were fixed in 4% PFA with 0.1 M cacodilic buffer, pH 7.2 (0.1
M sodium cacodilate, 0.0025% CaCl2) for 15 min at room
temperature, extensively washed in the same buffer, and stained for
TRAP according to the manufacturer's protocols. After washing with
distilled water and drying, samples were observed under a Leica
microscope (Leica Microsystems GmbH). Mature TRAP-positive
multinucleated cells containing >3 nuclei were counted as
osteoclasts in 10 randomly selected optical fields for each sample
(magnification, ×20).
Immunocytochemistry
Immunocytochemical analysis was performed using an
ImmPRESS Universal Reagent kit (Vector Laboratories, Inc.). hOCs or
hOBs (1×106/cm2 and
1×104/cm2 cells, respectively) were seeded in
24-well plates, fixed in cold 100% methanol at room temperature for
10 min and permeabilized with 0.2% (v/v) Triton X-100 in TBS (1X).
Then, the cells were treated in 0.3% H2O2 in
TBS (1X) for 10 min at room temperature, and subsequently incubated
with ready-to-use (2.5%) normal horse serum blocking solution
(ImmPRESS Universal Reagent kit) for 15 min at room
temperature.
After the incubation in blocking serum, cells were
incubated at 4°C overnight following addition of the following
rabbit anti-human polyclonal primary antibodies: Runx2 (1:200);
COL1a1 (1:100); NFATc1 (1:300); cathepsin K (1:200); and OPN
(1:200). After rinsing in 1X TBS, the cells were incubated for 30
min at room temperature with ImmPRESS reagent and then stained with
substrate/chromogen mix (ImmPACT™ DAB). After washing, the cells
were mounted in glycerol/PBS (9:1), counterstained with hematoxylin
and observed with a Nikon Eclipse 50i optical microscope
(magnification, ×20; Nikon Corporation).
MTT assay
The effect of GlcN on hOC and hOB viability was
assessed using MTT colorimetric assays. The cells were seeded in
96-well plates, treated with increasing concentrations of GlcN (10,
100 and 200 µg/ml) maintained at 37°C. After 72 h of
treatment, a solution of MTT in PBS was added to each well and the
plate was incubated for 3 h at 37°C. The MTT crystals were
solubilized with 200 µl lysis buffer (10% SDS).
Spectrophotometric absorbance of each sample was then measured at
570 nm by using a microplate reader (Sunrise™ Absorbance Reader;
Tecan Group, Ltd.). Live cells were calculated as a percentage of
the control (untreated cells).
Apoptosis (TUNEL assay)
At the end of osteoclastogenic induction, mature
hOCs were treated with GlcN (100 and 200 µg/ml) for 72 h.
The cells were then rinsed twice with PBS and fixed for 25 min in
4% PFA at room temperature. Apoptotic cells were detected using a
DeadEnd Colorimetric Apoptosis Detection system (Promega
Corporation) according to the manufacturer's instructions.
Moreover, all cells were subjected to hematoxylin staining to
reveal nuclei. The cells were mounted in glycerol/PBS (9:1) and
observed under a Leica microscope (magnification, ×20; Leica
Microsystems GmbH). The apoptotic rate was calculated as the
percentage of apoptotic nuclei (dark brown nuclei) compared with
the total number of nuclei of osteoclasts, evaluated in triplicate
from each experimental sample (10 randomly selected optical
fields/sample).
Phalloidin staining
For analysis of F-actin organization, hOCs were
fixed with 4% PFA for 10 min at room temperature, permeabilized
with 0.1% Triton X-100 for 15 min and stained with Alexa Fluor 488
Phalloidin (1:500 in PBS) for 30 min at room temperature. Nuclei
were counterstained with DAPI for 2 min at room temperature.
Fluorescent images were obtained using a fluorescence microscope,
evaluated by two independent investigators in 10 randomly selected
optical fields (magnification, ×40; Nikon Eclipse 50i).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from hOBs [2D culture in OM
in the presence or absence of GlcN (200 µg/ml)] by using an
RNeasy Micro kit (Qiagen GmbH) according to the manufacturer's
instructions. RNA concentration and quality were measured using a
NanoDrop™ ND1000 UV-VIS spectrophotometer (Isogen Life Science
B.V.). cDNA was synthesized from total RNA in a 20 µl
reaction volume using a High Capacity cDNA RT kit, according to the
manufacturer's instructions. Finally, 100 ng cDNA was used for qPCR
analysis. TaqMan Universal Master Mix II and probes for human
alkaline phosphatase (ALP; assay no. Hs01029144_m1), Runx2 (assay
no. Hs00231692_m1), OPN (assay no. Hs00959010_ m1), COL1A1 (assay
no. Hs00164004_m1), osteocalcin (OCN; assay no. Hs01587813_g1),
bone sialoprotein (BSP; assay no. Hs00913377_m1) were used
according to the manufacturer's instructions. Thermocycling
conditions for qPCR were as follows: Initial activation at 95°C for
10 min, followed by 40 cycles of thermal denaturation at 95°C for
15 sec and annealing/elongation at 60°C for 1 min. RPL13a (assay
no. Hs04194366_g1) was used for normalization of mRNA expression.
Gene expression was assessed using a CFX96TM PCR detection system
(Bio-Rad Laboratories, Inc.), and relative gene expression was
calculated using the comparative 2−ΔΔCq method (21) and expressed as fold change. All
reactions were performed in triplicate (n=4).
hOBs/hOCs cultured in 3D dynamic
system
The 3D dynamic culture conditions were set up using
an RCCS-4™ bioreactor (Synthecon, Inc.), with a High Aspect Ratio
Vessel™ (HARV; Synthecon, Inc.). The HARV consists of a
horizontally rotated culture chamber where the cells are suspended
and a perfusion system with media continuously flowing through the
culture chamber. The culture chamber can rotate in the X-axis at
certain speeds (rpm); higher rpm values are associated with lower
gravity. The rotation speed applied for the experiments was 4 rpm,
corresponding to ground based dynamic culture in which aggregates
are in continuous falling rotation close to the bottom of the
vessel (3D-DycC conditions) (15).
hMCs from healthy donors were used as source of
osteoclast progenitors and combined with hOBs from vertebral
laminae to create a 3D culture system. Each aggregate was generated
with unpooled cells from four different donors of hOBs and hMCs.
hOB/hMC aggregates were generated in the absence of exogenous
scaffolds. 3D-DycC dynamic co-culture conditions were applied as
previously reported (15,20).
Briefly, 1-2×106 hOBs and 0.5-1×106 hMCs were
inoculated into HARVs filled with high-glucose DMEM containing 10%
FCS (2 ml); all air bubbles were removed from the culture chamber.
Before treatment, the formation of spontaneously generated cell
aggregates was verified at different cell ratios (1:1, 1:2, 1:3 and
conversely). The 2:1 hOBs/hMCs cell ratio was selected as the most
effective condition to generate mature hOCs and applied for the
following experiments.
HARV was then inserted into the RCCS-4 rotary
bioreactor and placed in an incubator at 37°C with 5%
CO2. After 24 h, the presence of aggregates was
observed, and the vessels were filled with osteogenic medium alone
(OM) or in the presence of 200 µg/ml GlcN (OM/GlcN).
Osteogenic medium and treatment with GlcN was refreshed twice a
week. After 14 days, the aggregates were collected, fixed in 4% PFA
(15 min, room temperature) and embedded in paraffin for further
analysis.
Histology
Immunohistochemistry was performed using the
ImmPRESS Universal Reagent kit. Histological sections (5 µm)
of aggregates were subjected to immunohistochemistry.
Non-consecutive sections were deparaffinized, rehydrated and
enzymatically treated with 1 mg/ml protease K for 10 min at 37°C
(Sigma-Aldrich) for antigen retrieval and permeabilization. Slides
were then immunostained overnight with primary antibodies against
OPN (1:100) in a humid chamber at 4°C, followed by treatment with
ImmPRESS reagent (ImmPRESS reagent kit; Vector Laboratories, Inc.)
for 30 min. The reaction were developed using DAB solution (Vector
Laboratories, Inc.); the sections were counterstained with
hematoxylin, mounted in glycerol and observed using a Nikon Eclipse
50i optical microscope (magnification, ×10).
For ARS staining, the sections were deparaffinized
and stained with 40 mM ARS solution (pH 4.2) at room temperature
for 20 min. TRAP staining was conducted using the TRAP kit
according to the manufacturer's protocols. Staining was quantified
using a computerized video camera-based image analysis system
ImageJ v1.51 software (http://rsb.info.nih.gov/nih-image/; National
Institutes of Health) under light microscopy (magnification, ×20;
Nikon Eclipse 50i). Color TIFF file images were converted to 32-bit
images and inverted so that the background could be set to the
lower threshold limit. After applying the image threshold, the
background was removed and not counted toward mean pixel intensity.
Mean pixel intensity per area was used to quantify OPN staining
(five sections/sample; n=3). The percentage positive area was used
to quantify ARS and TRAP staining, accounting for tears/holes
within the matrix of samples.
Statistical analysis
Results are presented as the mean ± SD. Statistical
significance was analyzed using GraphPad Prism 5 (GraphPad
Software, Inc.) via one-way ANOVA followed by Tukey's post hoc test
or Student's t-test. P<0.05 was considered to indicate a
significantly significant difference.
Results
GlcN induces apoptosis and decreases
differentiation of osteoclasts from patients with OA
hMCs from peripheral blood of healthy controls or
patients with OA were used as a source of osteoclast progenitors.
The ability of hMCs to differentiate into mature multinucleated
hOCs was demonstrated by analyzing the presence of established
osteoclast markers, such as TRAP, cathepsin K and NFATc1, during
osteoclastogenic induction. Exposure to different GlcN
concentrations (10-200 µg/ml) did not affect osteoclast
viability (Fig. S1A).
Consistent with previous evidence (12,22,23), it was selected to treat the cells
with GlcN concentrations of 100 and 200 µg/ml.
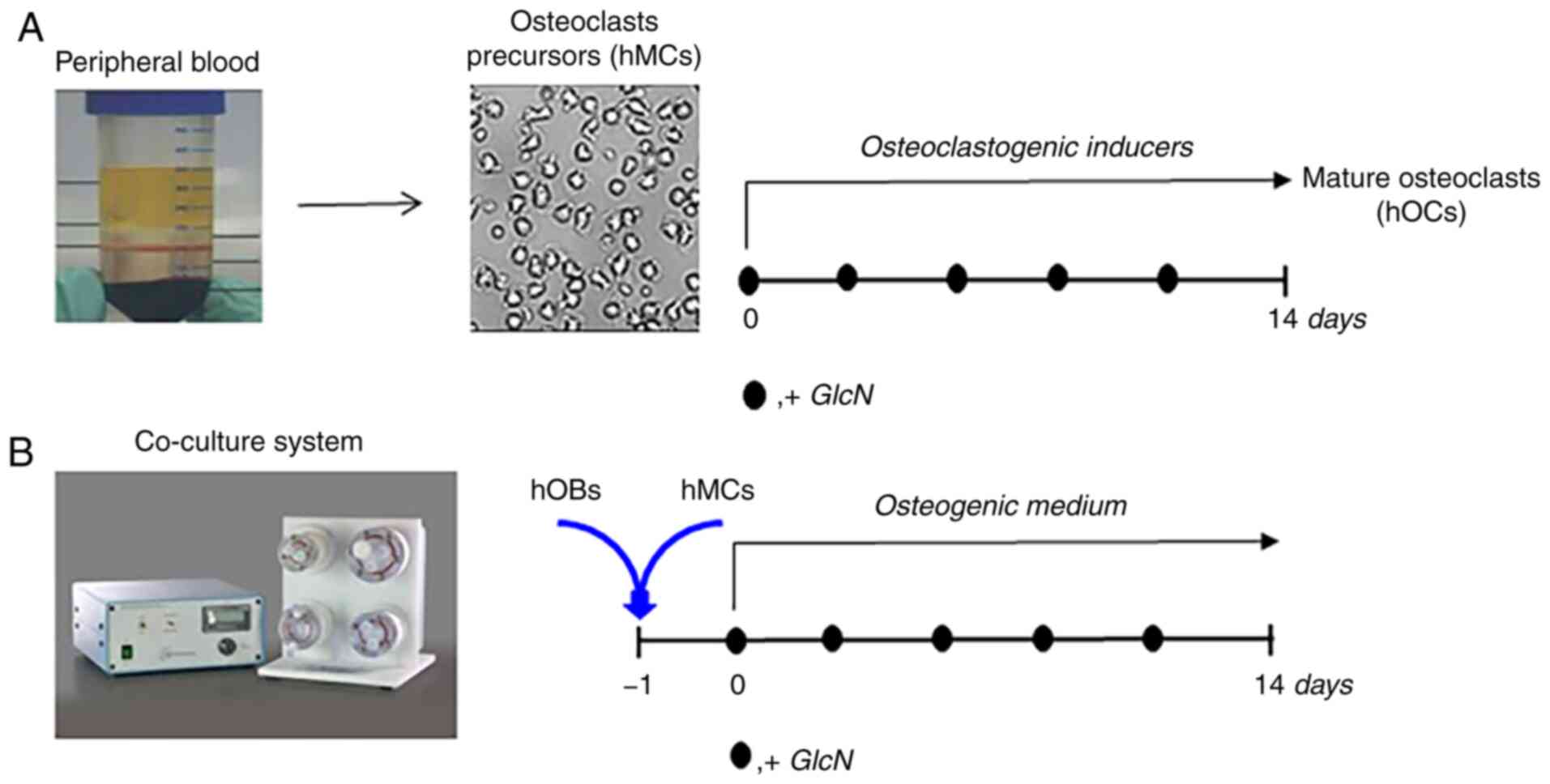
To investigate the effects of GlcN on hOCs, an
experimental strategy was designed (Fig. 1), accounting for the low number
of cells available from each patient sample that limited the
experimental analysis that could be performed. Microscopic
observations revealed that the number of multinuclear hOCs both
from healthy donors or patients with OA was not significantly
altered following GlcN treatment (Fig. S1B). After differentiation was
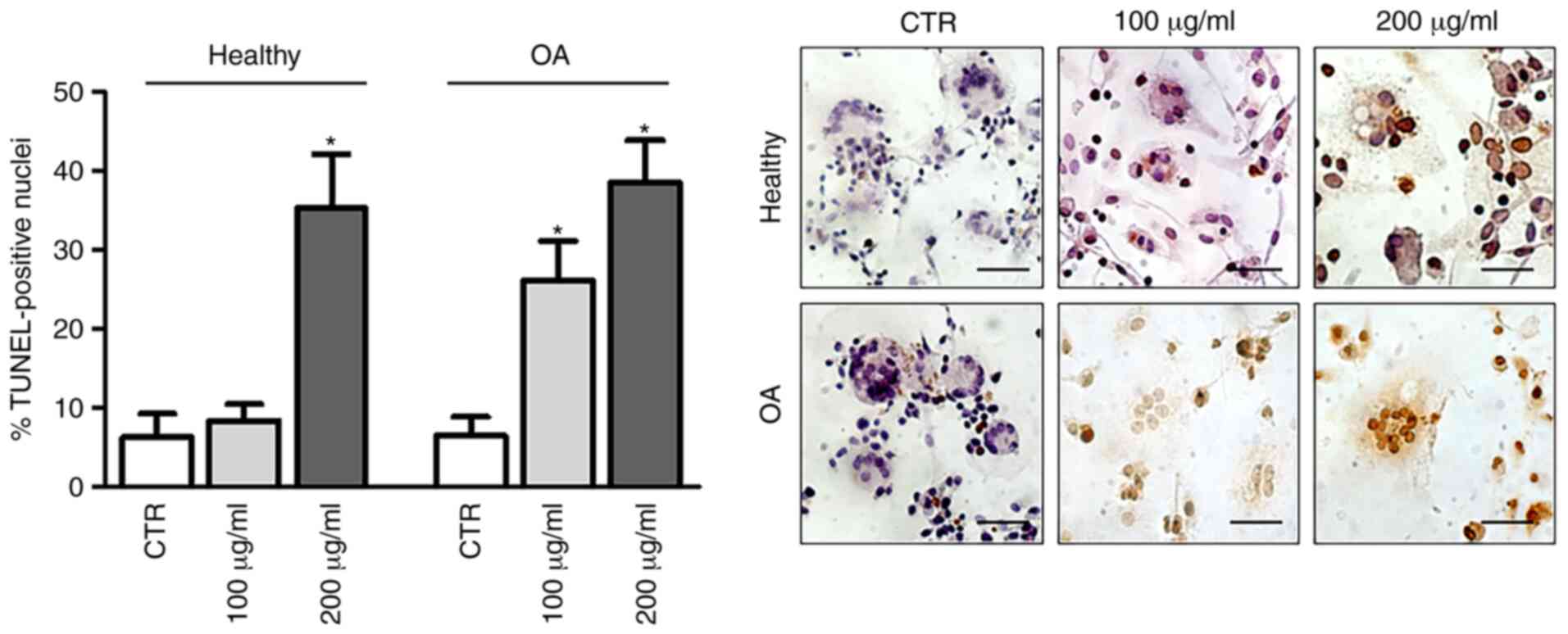
completed, apoptosis was assessed using a TUNEL assay (Fig. 2). The results demonstrated that
GlcN treatment induced dose-dependent cell apoptosis in hOCs from
patients with OA, whereas hOCs from healthy donors underwent
GlcN-induced DNA fragmentation only after exposure to 200
µg/ml.
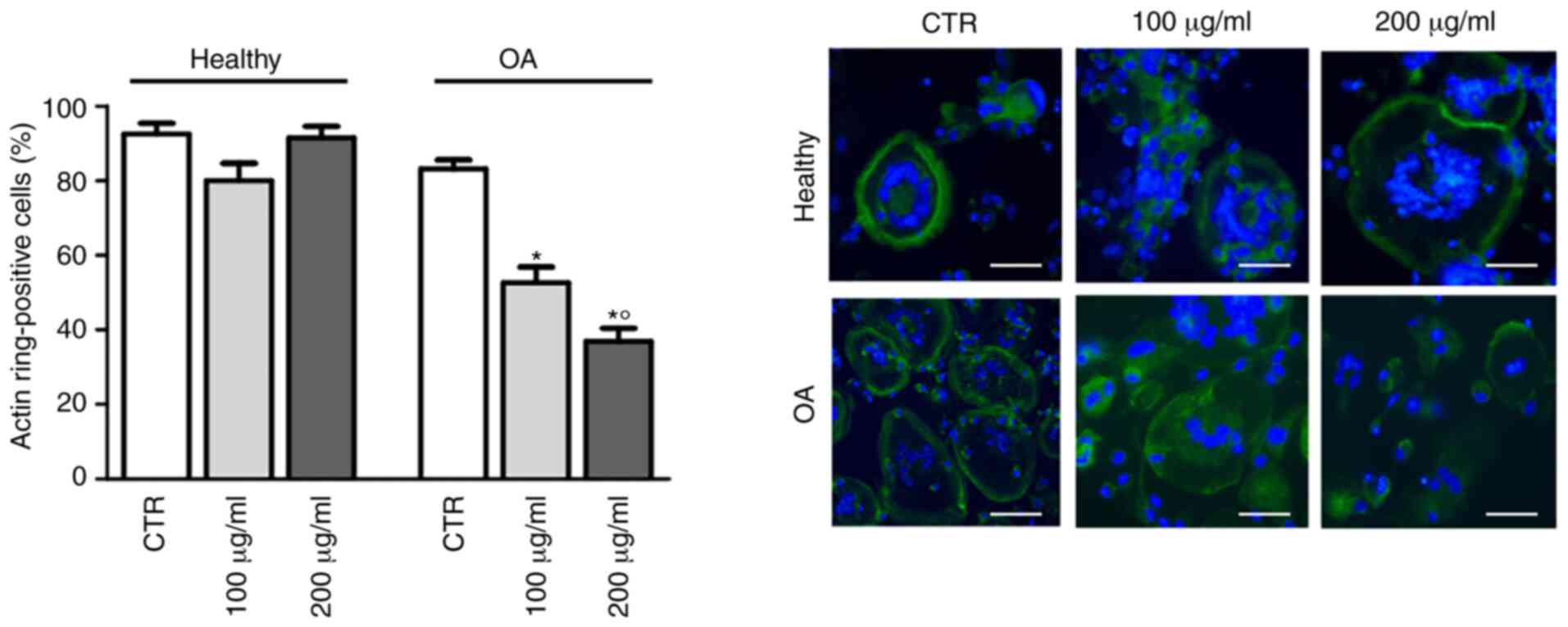
Considering that cytoskeletal rearrangements are a
prerequisite for bone resorption by osteoclasts (24), the effects of GlcN on hOC
differentiation were subsequently investigated by staining with
FITC-conjugated phalloidin to evaluate actin ring formation. As
shown in Fig. 3, GlcN treatment
significantly decreased the polymerization of F-actin in a circular
manner in hOCs from patients with OA, but not in hOCs from healthy
donors.
GlcN positively affects osteoblast
activity in hOC/hOB 3D co-culture systems
Subsequent experiments investigated hOC responses to
GlcN when combined with osteoblasts (hOBs) in a 3D co-culture
system. The aim was to validate the hOC responsiveness to GlcN in
an experimental condition that more closely resembles the in
vivo bone microenvironment whilst also attempting to understand
if hOBs could represent a GlcN target. The quality of the cells was
assessed; only those hOB samples expressing conventional
osteoblastic markers, such as OPN, COL1a1 and Runx2 (Fig. S1C) were selected. When subjected
to GlcN treatment up to 200 µg/ml, hOBs did not exhibit any
change in viability (Fig. S1C).
Therefore, this concentration was selected for the subsequent
experiments. hMC osteoclast precursors from healthy donors were
then combined with hOBs in a 3D dynamic co-culture system in
presence of OM without osteoclastogenic inducers, based on a
previous protocol (20). Under
these conditions, osteoclastogenesis was supported by hOBs and the
cells were able to produce sizeable self-assembling aggregates
(Fig. 4). After exposure to
GlcN, it was observed that the relative TRAP-positive area
significantly decreased (Fig.
4). Of note, GlcN exhibited a positive effect on osteoblast
activity; a significant increase of both mineral matrix deposition
(ARS-positive areas) and OPN expression was found in GlcN-treated
cellular aggregates (Fig.
4).
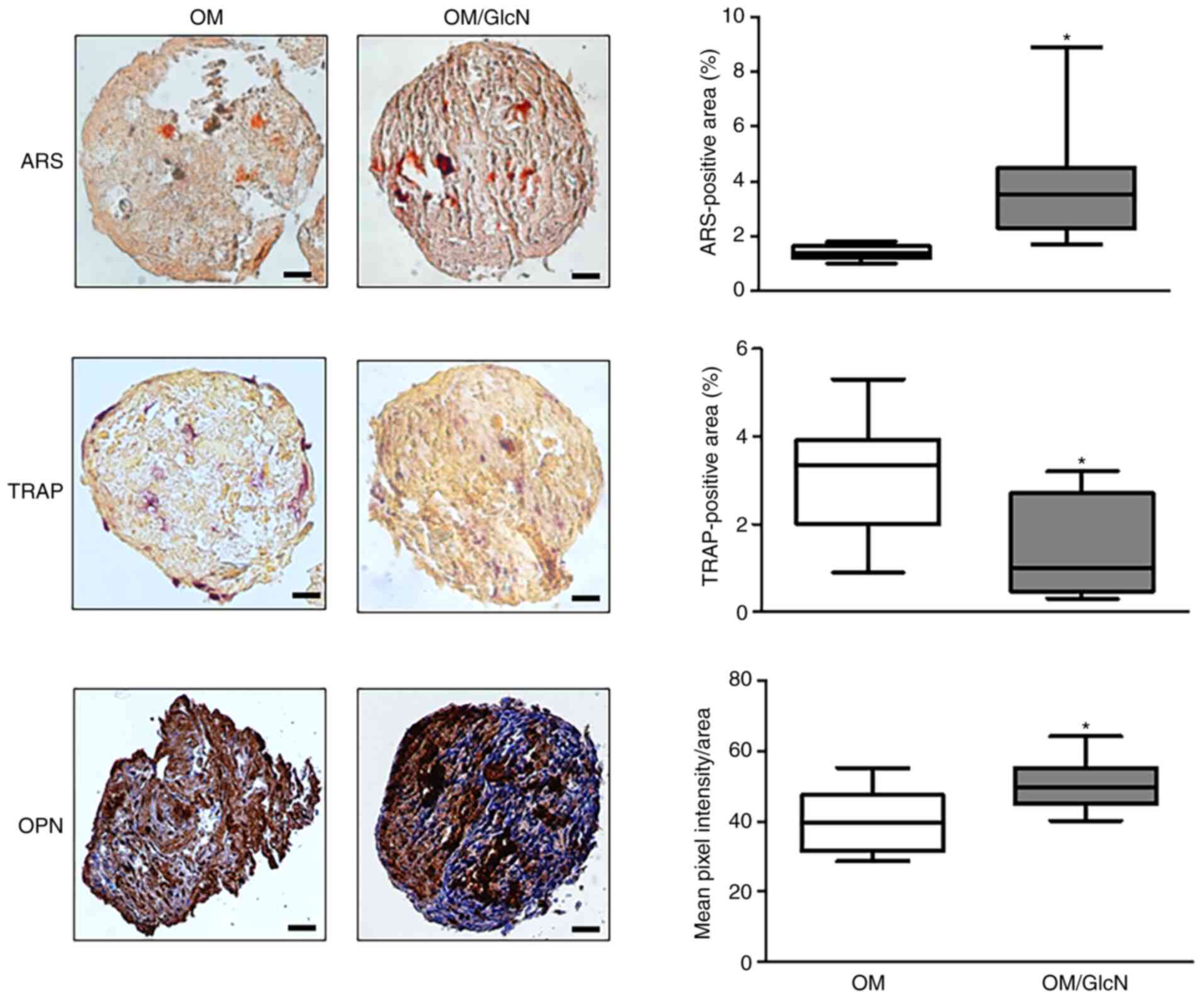 | Figure 4Responsiveness of hOCs and hOBs to
GlcN in the 3D dynamic co-culture system. Human primary monocytes
from healthy donors were co-cultured with hOBs for 14 days in a 3D
dynamic system. Cells were cultured in OM or OM/GlcN. GlcN
treatment was repeated every 3 days. Representative
microphotographs of ARS, TRAP and OPN staining are reported. Scale
bars, 50 µm. TRAP activity and ARS were quantified by ImageJ
software and expressed as the percentage positive area (mean ± SD,
five sections/sample, n=4). OPN levels were quantified by ImageJ
software and expressed as the mean pixel intensity/area (mean value
± SD, five sections/sample, n=3). *P<0.05 vs. OM. 3D,
three-dimensional; ARS, Alizarin Red S; GlcN, glucosamine; hOC,
human primary osteoclast; hOB, human primary osteoblast; OM,
osteogenic medium; OM/GlcN, OM with 200 µg/ml GlcN; OPN,
osteopontin; TRAP, tartrate-resistant acid phosphatase. |
Although some aspects of OPN function in bone
homeostasis remain to be determined, migration, adhesion and
activation of osteoclasts in an OPN-dependent manner have been
demonstrated (25). However, it
was hypothesized that the increase in OPN expression in the hOC/hOB
3D co-culture system is to be attributed to the hOBs, as GlcN
significantly increased ARS and decreased TRAP staining. Therefore,
these results suggested that GlcN was effective not only in
inhibiting the activity of hOBs, but also in enhancing the activity
of hOCs.
This was further explored, as a number of osteogenic
markers were analyzed via RT-qPCR after expanding the hOBs in 2D
conventional culture. As shown in Fig. 5, GlcN induced a general increase
in early and middle stage osteogenic markers such as Runx2, COL1a1,
ALP, OPN and BSP (26). In
particular, a significant increase in expression was observed for
Runx2, which is considered the master regulator of osteogenesis
(26), and OPN. No significant
changes in expression were observed for OCN, the late
differentiation marker.
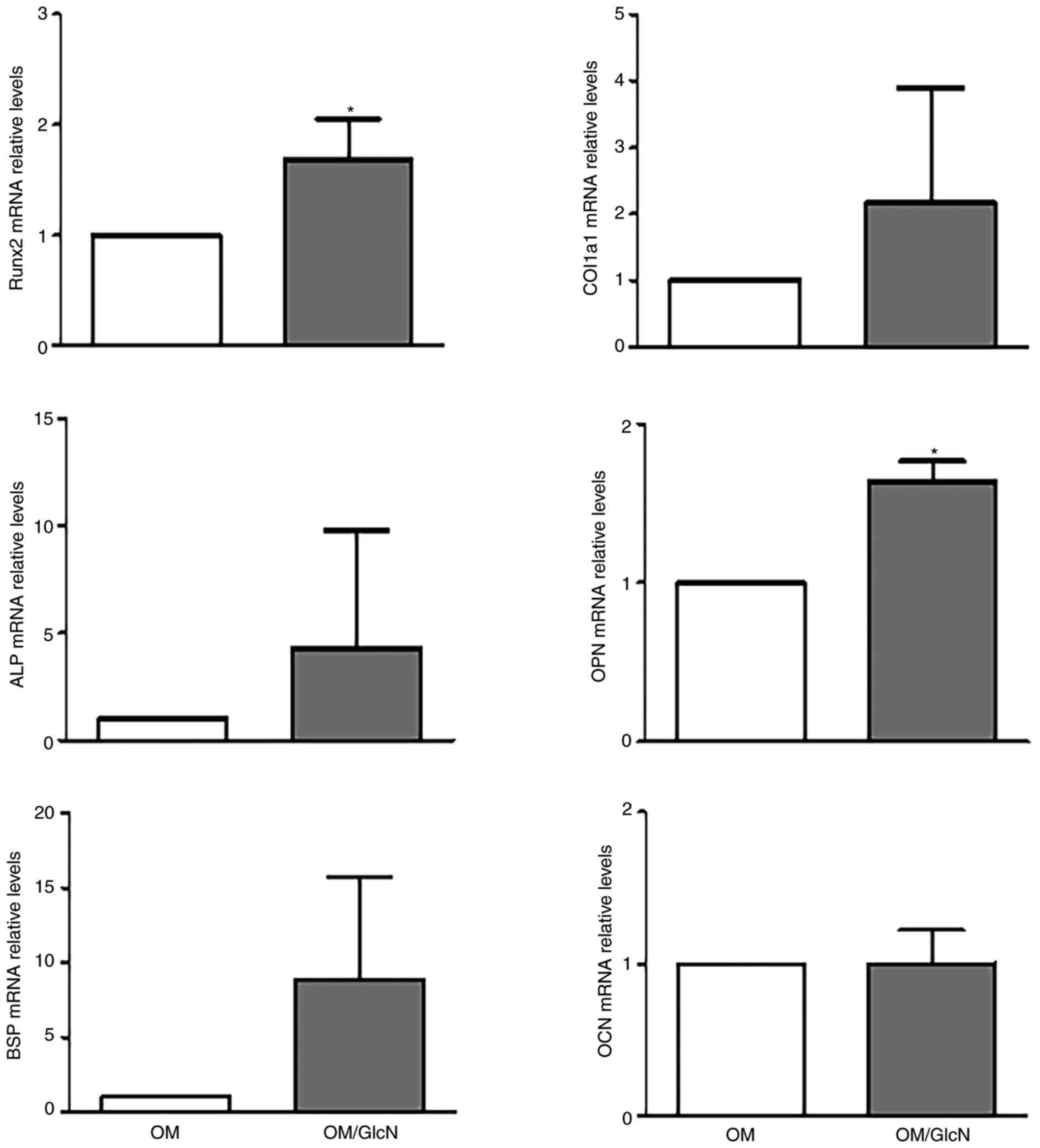 | Figure 5Effect of GlcN on hOBs in
two-dimensional conventional cell culture. The expression of
typical osteogenic markers was analyzed in hOBs cultured in OM or
OM/GlcN for 14 days. GlcN treatment was repeated every 3 days.
Total RNA was purified, and the mRNA expression levels of Runx2,
COL1a1, ALP, OPN, BSP and OCN were evaluated via reverse
transcription-quantitative PCR. Relative expression levels were
normalized to OM. All reactions were performed in triplicate. Data
are presented as the mean ± SD (n=4). *P<0.05 vs. OM.
ALP, alkaline phosphatase; BSP, bone sialoprotein; COL1a1, collagen
type 1α; GlcN, glucosamine; hOB, human primary osteoblast; OCN,
osteocalcin; OM, osteogenic medium; OM/GlcN, OM with 200
µg/ml GlcN; OPN, osteopontin; Runx2, runt-related
transcription factor 2. |
Discussion
Despite considerable knowledge of the biological
activities of GlcN, including chondroprotective and
anti-inflammatory actions (1-7),
its role in osteogenesis and bone tissue remains to be investigated
in detail. The evidence collected so far on bone cells is mainly
based on the use of monolayered non-human cell lines, such as mouse
MC3T3-E1 (14) or RAW264.7
(22), the fetal osteoblastic
cell line hFOB1.19 (23), or
animal models such as rats, mice or rabbits receiving GlcN oral
administration (27-29).
The present study focused on cells from human bone
microenvironments. Initial experiments involved peripheral blood
samples from patients with OA; as these patients were outpatients
who did not require surgery, it was only possible to obtain a
limited amount of peripheral blood, although this was sufficient to
produce the hOC precursors for a comparative study with hOCs from
healthy donors. This may be a limitation of this study; in the near
future, there are plans to enroll patients with OA that require
orthopedic surgery, so that both endogenous osteoclasts and
osteoblasts can be obtained to conduct a greater number of
analysis. Nevertheless, the present data demonstrated that OA and
healthy osteoclasts were differentially susceptible to GlcN
treatment, which inhibited the differentiation and function of OA
osteoclasts.
These findings led to subsequent investigations into
the effects of GlcN in a more complex culture system one step
closer to the in vivo bone microenvironment, consisting of
an hOC/hOB 3D dynamic co-culture system. With this approach, the
effect of GlcN on osteoclast behavior was validated, revealing a
decrease in TRAP activity, but the responsiveness of human
osteoblasts was also investigated. After GlcN treatment,
osteoblasts increased mineral matrix deposition and the expression
of specific differentiation markers, such as OPN, demonstrating the
ability of GlcN to exert anabolic effects. This is an encouraging
proof of concept that needs to be validated in the future through
the use of a larger number of cells, which will allow for analysis
of a larger number of osteogenic markers. The reduced amount of
cells harvestable from patients and issues during the aggregate
post-culturing process had narrowed the number of experimental
analyses performed.
When GlcN treatment was performed on 2D conventional
osteoblast culture and RT-qPCR analysis of differentiation markers
was conducted, the aforementioned findings were validated,
demonstrating that GlcN supported favorable conditions for
osteogenic differentiation and maintenance of osteoblastic
phenotypes.
The opposing responses of the different bone cell
populations merits further study; however, the present findings
suggest that GlcN may be a candidate as a broader
treatment/therapeutic aimed at resolving both cartilage and
skeletal diseases. Additionally, it is proposed that the results of
research conducted in this area will help clinicians with providing
a broader and more targeted prescription of GlcN, whilst providing
benefits to patients and their bone tissues.
It is important to underline that identifying
molecules capable of simultaneously modulating the activity of
osteoblasts and osteoclasts is an important benefit for patients
affected by bone loss, as it provides the opportunity to control a
complex balance (30,31). It is well known that bone
deposition by osteoblasts and resorption by osteoclasts are tightly
coupled, and their balance defines both the mass and quality of
bone tissue (30). Using culture
conditions to the in vivo bone microenvironment such as
those reported in the present study provides a novel perspective,
both by generating informative data on the still-controversial
efficacy of biological agents such as GlcN and by conducting
patient-oriented research. This last aspect is based on the
possibility of generating autologous osteoclast/osteoblast 3D
co-cultures with cells from the same patient, who, in addition to
peripheral blood, may provide bone fragments during orthopedic
surgery. Therefore, the employment of such an approach may further
improve understanding of the role of GlcN in bone tissue
homeostasis, as well as the development of patient-tailored
nutraceutical and pharmaceutical treatments (32,33).
The effects of GlcN reported in the present study
are consistent with the only other study, to the authors'
knowledge, into the human bone microenvironment, namely that by Tat
et al (12). In this
paper, the authors studied the effect of chondroitin sulfate, GlcN
sulfate and vitamin D3 on osteoblast metabolism in the subchondral
bone of patients with OA, demonstrating that GlcN decreased
osteoblast pro-resorptive activity by modulating
osteoprotegerin/RANKL signaling (12).
At present, understanding how the altered bone
remodeling that supports the development of both osteoarthritis and
osteoporosis can be counteracted by adequate GlcN treatment in
terms of dose and intake remains an open question. For this reason,
clinical studies have to be accompanied by the development of
suitable preclinical experimental models that provide useful
information on exact mechanisms of action underlying the beneficial
effects of GlcN.
It is worth mentioning that, in addition to the
well-known role of GlcN in the synthesis of components of the
extra-cellular matrix (1,34),
it is the precursor of N-acetyl-GlcN, which is added to the serine
and threonine residues of nuclear and cytoplasmic proteins in the
O-GlcNAcylation post-translational modification (35). O-GlcNAcylation plays a critical
role in the regulation of cellular homeostasis in response to
nutritional or hormonal cues, and also in response to stress or
damage (36). A previous study
reported that an increase of global O-GlcNAc glycosylation occurs
during the early stages of osteoblast differentiation in MC3T3-E1
cells, but not during the osteoclastic differentiation of RAW264
cells (37). Considering that
acute and chronic alterations in the amount of O-GlcNAcylated
proteins have been associated with different human diseases
(38), a key point that requires
further investigation will be to clarify the involvement of
O-GlcNAc glycosylation in altered bone metabolism and the
modulation of osteogenic gene expression in human bone cells.
Collectively, the present findings provided evidence
that compounds such as GlcN that are positioned between
pharmaceuticals and nutraceuticals merit further investigation for
developing novel approaches for bone health maintenance and
treatment of bone diseases.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LP designed the study, performed the experiments and
analyzed the data. EL designed the study, performed the experiments
and analyzed the data. AP analyzed the data and reviewed the
manuscript. DM analyzed the data and reviewed the manuscript. VS
designed and coordinated the study, and helped with the
interpretation of data. RP designed the study, and wrote and edited
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval for the study was obtained from the Centro
di Medicina (Ferrara, Italy) and from the Ethics Committee of the
University of Ferrara and St. Anna Hospital (protocol approved on
November 17, 2016). Written informed consent was obtained from each
patient. No animals were involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
We wish to thank Dr Francesco Nicoli (Department of
Chemical and Pharmaceutical Sciences-University of Ferrara-Italy)
and Dr Leticia Scussel Bergamin (Department of Neuroscience and
Rehabilitation, University of Ferrara-Italy) for technical
assistance, and Professor Pasquale De Bonis (Department of
Translational Medicine and for Romagna-University of Ferrara-Italy)
for providing bone surgical fragments.
References
|
1
|
Nagaoka I, Igarashi M and Sakamoto K:
Biological activities of glucosamine and its related substances.
Adv Food Nutr Res. 65:337–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varghese S, Theprungsirikul P, Sahani S,
Hwang N, Yarema KJ and Elisseeff JH: Glucosamine modulates
chondrocyte proliferation, matrix synthesis, and gene expression.
Osteoarthritis Cartilage. 15:59–68. 2007. View Article : Google Scholar
|
|
3
|
Reily C, Stewart TJ, Renfrow MB and Novak
J: Glycosylation in health and disease. Nat Rev Nephrol.
15:346–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Block JA, Oegema TR, Sandy JD and Plaas A:
The effects of oral glucosamine on joint health: Is a change in
research approach needed? Osteoarthritis Cartilage. 18:5–11. 2010.
View Article : Google Scholar
|
|
5
|
Agiba AM: Nutraceutical formulations
containing glucosamine and chondroitin sulphate in the treatment of
osteoarthritis: Emphasis on clinical efficacy and formulation
challenges. Int J Curr Pharm Res. 9:1–7. 2017. View Article : Google Scholar
|
|
6
|
Ostojic SM, Arsic M, Prodanovic S, Vukovic
J and Zlatanovic M: Glucosamine administration in athletes: Effects
on recovery of acute knee injury. Res Sports Med. 15:113–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Adamo S, Cetrullo S, Panichi V, Mariani
E, Flamigni F and Borzì RM: Nutraceutical activity in
osteoarthritis biology: A focus on the nutrigenomic role. Cells.
9:12322020. View Article : Google Scholar :
|
|
8
|
Ragle RM and Sawitzke AD: Nutraceuticals
in the management of osteoarthritis: A critical review. Drugs
Aging. 29:717–731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rovati LC, Girolami F and Persiani S:
Crystalline glucosamine sulfate in the management of knee
osteoarthritis: Efficacy, safety, and pharmacokinetic properties.
Ther Adv Musculoskelet Dis. 4:167–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldring SR and Goldring MB: Changes in
the osteochondral unit during osteoarthritis: Structure, function
and cartilage-bone crosstalk. Nat Rev Rheumatol. 12:632–644. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Im GI and Kim MK: The relationship between
osteoarthritis and osteoporosis. J Bone Miner Metab. 32:101–109.
2014. View Article : Google Scholar
|
|
12
|
Tat SK, Pelletier JP, Vergés J, Lajeunesse
D, Montell E, Fahmi H, Lavigne M and Martel-Pelletier J:
Chondroitin and glucosamine sulfate in combination decrease the
pro-resorptive properties of human osteoarthritis subchondral bone
osteoblasts: A basic science study. Arthritis Res Ther. 9:R1172007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anastassiades T, Rees-Milton K, Xiao H,
Yang X, Willett T and Grynpas M: N-acylated glucosamines for bone
and joint disorders: Effects of N-butyryl glucosamine on
ovariectomized rat bone. Transl Res. 162:93–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Igarashi M, Sakamoto K and Nagaoka I:
Effect of glucosamine, a therapeutic agent for osteoarthritis, on
osteoblastic cell differentiation. Int J Mol Med. 28:373–379.
2011.PubMed/NCBI
|
|
15
|
Penolazzi L, Lolli A, Sardelli L,
Angelozzi M, Lambertini E, Trombelli L, Ciarpella F, Vecchiatini R
and Piva R: Establishment of a 3D-dynamic osteoblasts-osteoclasts
co-culture model to simulate the jawbone microenvironment in vitro.
Life Sci. 152:82–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Penolazzi L, Pocaterra B, Tavanti E,
Lambertini E, Vesce F, Gambari R and Piva R: Human osteoclasts
differentiated from umbilical cord blood precursors are less prone
to apoptotic stimuli than osteoclasts from peripheral blood.
Apoptosis. 13:553–561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lambertini E, Penolazzi L, Angelozzi M,
Grassi F, Gambari L, Lisignoli G, De Bonis P, Cavallo M and Piva R:
The expression of cystathionine gamma-lyase is regulated by
estrogen receptor alpha in human osteoblasts. Oncotarget.
8:101686–101696. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wrobel E, Leszczynska J and Brzoska E: The
characteristics of human bone-derived cells (HBDCS) during
osteogenesis in vitro. Cell Mol Biol Lett. 21:262016. View Article : Google Scholar
|
|
19
|
Choudhary S, Sun Q, Mannion C, Kissin Y,
Zilberberg J and Lee WY: Hypoxic three-dimensional cellular network
construction replicates ex vivo the phenotype of primary human
osteocytes. Tissue Eng Part A. 24:458–468. 2018. View Article : Google Scholar :
|
|
20
|
Mandatori D, Penolazzi L, Pipino C, Di
Tomo P, Di Silvestre S, Di Pietro N, Trevisani S, Angelozzi M, Ucci
M, Piva R and Pandolfi A: Menaquinone-4 enhances osteogenic
potential of human amniotic fluid mesenchymal stem cells cultured
in 2D and 3D dynamic culture systems. J Tissue Eng Regen Med.
12:447–459. 2018. View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Takeuchi T, Sugimoto A, Imazato N, Tamura
M, Nakatani S, Kobata K and Arata Y: Glucosamine suppresses
osteoclast differentiation through the modulation of glycosylation
including O-GlcNAcylation. Biol Pharm Bull. 40:352–356. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv C, Wang L, Zhu X, Lin W, Chen X, Huang
Z, Huang L and Yang S: Glucosamine promotes osteoblast
proliferation by modulating autophagy via the mammalian target of
rapamycin pathway. Biomed Pharmacother. 99:271–277. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsubara T, Kinbara M, Maeda T, Yoshizawa
M, Kokabu S and Yamamoto TT: Regulation of osteoclast
differentiation and actin ring formation by the cytolinker protein
plectin. Biochem Biophys Res Commun. 489:472–476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luukkonen J, Hilli M, Nakamura M, Ritamo
I, Valmu L, Kauppinen K, Tuukkanen J and Lehenkari P: Osteoclasts
secrete osteopontin into resorption lacunae during bone resorption.
Histochem Cell Biol. 151:475–487. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chapurlat RD and Confavreux CB: Novel
biological markers of bone: From bone metabolism to bone
physiology. Rheumatology (Oxford). 55:1714–1725. 2016. View Article : Google Scholar
|
|
27
|
Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu
W and Peng Y: Dietary natural N-Acetyl-d-Glucosamine prevents bone
loss in ovariectomized rat model of postmenopausal osteoporosis.
Molecules. 23:23022018. View Article : Google Scholar :
|
|
28
|
Ivanovska N and Dimitrova P: Bone
resorption and remodeling in murine collagenase-induced
osteoarthritis after administration of glucosamine. Arthritis Res
Ther. 13:R442011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang SX, Laverty S, Dumitriu M, Plaas A
and Grynpas MD: The effects of glucosamine hydrochloride on
subchondral bone changes in an animal model of osteoarthritis.
Arthritis Rheum. 56:1537–1548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng X and McDonald JM: Disorders of bone
remodeling. Annu Rev Pathol. 6:121–145. 2011. View Article : Google Scholar
|
|
31
|
Kim BJ and Koh JM: Coupling factors
involved in preserving bone balance. Cell Mol Life Sci.
76:1243–1253. 2019. View Article : Google Scholar
|
|
32
|
Nasri H, Baradaran A, Shirzad H and
Rafieian-Kopaei M: New concepts in nutraceuticals as alternative
for pharmaceuticals. Int J Prev Med. 5:1487–1499. 2014.
|
|
33
|
Daliu P, Santini A and Novellino E: From
pharmaceuticals to nutraceuticals: Bridging disease prevention and
management. Expert Rev Clin Pharmacol. 12:1–7. 2019. View Article : Google Scholar
|
|
34
|
Felson DT: Concerns about report
suggesting glucosamine and chondroitin protect against cartilage
loss. Ann Rheum Dis. 74:e382015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang X and Qian K: Protein
O-GlcNAcylation: Emerging mechanisms and functions. Nat Rev Mol
Cell Biol. 18:452–465. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herrero-Beaumont G and Largo R:
Glucosamine and O-GlcNAcylation: A novel immunometabolic
therapeutic target for OA and chronic, low-grade systemic
inflammation? Ann Rheum Dis. 79:1261–1263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koyama T and Kamemura K: Global increase
in O-linked N-acetylglucosamine modification promotes osteoblast
differentiation. Exp Cell Res. 338:194–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|