Introduction
Abdominal aortic aneurysm (AAA) represents a fatal
human vascular disease and a chronic degenerative pathological
process in which inflammatory factors and activated matrix
metalloproteinases (MMPs) are highly expressed (1). AAA can be triggered by a family
history of AAA, hypertension, coronary artery disease, myocardial
infarction and peripheral artery injury, and males >65 years
with a history of smoking are the most vulnerable at-risk
population for the development of AAA (2). As a public health concern of utmost
importance and severity, AAA has an incidence of 1.3-12.5% and a
30-day mortality rate of up to 70% (3,4).
A physical examination, ultrasonography and imaging are promising
means for the diagnosis of AAA (2); to date, surgical intervention
remains the only effective treatment method for AAA (5). However, any possible
pharmacological target for AAA therapy remains unavailable
(6). In this context, novel
therapeutic strategies for AAA are urgently required.
As a prevalent neuroprotectant applied in anesthetic
surgeries, dexmedetomidine (Dex) is able to suppress the activities
of neuroendocrine hormones and inflammatory mediators (7). In addition, Dex helps to maintain a
balanced and steady myocardial function and coronary blood flow
(8). The administration of Dex
during aortic vascular treatment can protect patients by reducing
the incidence of myocardial ischemia as it effectively suppresses
alterations in blood pressure and heart rate (9). According to a recent study,
microRNA (miRNA/miR)-340 expression was markedly increased by Dex
administration and in turn, the protective effects of Dex on the
nervous system with inflammatory responses were improved,
indicating the functional system of Dex and miRNAs (10). As small sequences in non-coding
RNAs, miRNAs modulate various cell activities by degrading or
suppressing specific mRNA translation, which connects them to many
diseases including cardiovascular diseases (5). In addition, miRNAs function as
promising biomarkers in relieving inflammatory diseases by
controlling macrophage polarization, which is closely connected to
severe inflammatory responses (11). miR-21 is a pivotal participator
in negatively controlling inflammatory responses, particularly in
macrophages (12). miR-21 is
importantly involved in processes, such as extracellular matrix
remodeling, lipid accumulation and inflammatory responses in
cerebral aneurysms (13). A
previous review article outlined the known effects of relevant
miRNAs, including miR-21, in the development of AAA (14). miR-21 blocks the development of
AAA and nicotine-augmented expansion (15). Moreover, it has been demonstrated
that programmed cell death 4 (PDCD4) is a target gene of miR-21.
PDCD4 is an inflammation- and apoptosis-related gene, and PDCD4
deficiency has been shown to ameliorate left ventricular remodeling
and insulin resistance in a rat model of type 2 diabetic
cardiomyopathy (16). miR-21
confers resistance against CVB3-induced myocarditis by inhibiting
PDCD4-mediated apoptosis (17).
Activation of SIRT1 protects against acute aortic dissection
symptoms by enhancing PDCD4 signaling pathway (18). But there is no research on the
role of PDCD4 in AAA, and on the combined efficacy of Dex and the
miR-21/PDCD4 pathway on AAA. From the above, it is reasonable to
hypothesize that there may be an interaction between Dex and the
miR-21/PDCD4 axis in AAA. Thus, the present study conducted a
series of experiments to verify this hypothesis. The present study
aimed to provide theoretical guidance for the early prevention and
development of AAA.
Materials and methods
Establishment of rat model of AAA
Male Sprague-Dawley rats [n=84, 8-10 weeks old,
weighing 380-450 g; State Key Laboratory of Biotherapy, Sichuan
University, Chengdu, China, SYXK (Sichuan) 2016-178] were fed under
a 12-h light-dark cycle and humidity-controlled clean cage at
24-26°C. Food and water were freely accessible.
The establishment of the rat model of AAA was
performed as previously described (19). As previously reported, the rats
were anesthetized by inhaling isoflurane (4% for induction and 2%
for maintenance; 30% O2 and 70% N2 at a flow
rate of 650 ml/min) during the surgery (20,21). A midline laparotomy was conducted
to expose a 10-mm segment of the infrarenal abdominal aorta. The
rats were then administered an intraluminal injection of 30 U of
porcine pancreatic elastase (135 U/mg; Sigma-Aldrich; Merck KGaA)
via standard silicone tubes into the common femoral artery at the
right side towards the aorta. The aorta was enveloped with gauze
soaked in 0.5 mol CaCl2 (Sigma-Aldrich; Merck KGaA) for
20 min with simultaneous elastase treatment. No rats died during
the surgery. Subsequently, the abdominal cavity was rinsed using
normal saline followed by suturing. The sutured rats were then
placed in the cage again for raising. The health and behaviors of
the rats were monitored every day. All rats survived to the end of
the experiment.
The present study was approved and supervised by the
Ethics Committee of The First Affiliated Hospital of Nanchang
University. All animal experiments complied with the ARRIVE
guidelines and were carried out in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals. Significant efforts were made in order to minimize both
the number of animals used and their suffering.
Animal grouping
These rats were arbitrarily divided into 7 groups,
with 12 rats in each group. The experiment included 3 parts. The
sham-operated (sham) group, AAA group (AAA model rats) and AAA +
Dex group (Dex was intraperitoneally injected into the rats with
AAA) were involved in the first part of the experiment. The Dex +
control group (AAA rats were injected with Dex and
intraperitoneally injected with antagomir (ant)-NC) and Dex +
ant-miR-2 group (AAA rats were injected with Dex and
intraperitoneally injected ant-miR-21) were included in the second
part of the experiment. The lentivirus (LV) + negative control (NC)
group (AAA rats were treated with control lentivirus) and
LV-PDCD4-short hairpin RNA (shRNA) group (AAA rats were injected
with LV-PDCD4-shRNA recombinant lentivirus) were included in the
third part of the experiment.
The target organ of the experiment was the abdominal
aorta. As previously described (22,23), the drug was administered by
intraperitoneal injection. The rats with AAA received an
intraperitoneal injection of 50 µg/kg DEX (24) from the day of AAA modeling to 13
days after the surgery. In order to inhibit the expression of
miR-21 in the abdominal aorta, ant-miR-21 or ant-NC (25) were injected intraperitoneally at
the dose of 65 mg/kg/day on the 1st, 2nd and 3rd day after surgery.
LV-PDCD4-shRNA and its control LV-NC were used to intervene with
the expression of PDCD4 in the rats with AAA by injecting
5×107 infectious lentiviral particles per rat with AAA
via the tail vein, as previously described (26,27). The rats in the sham and AAA
groups were operated in the same manner, but normal saline was used
instead of protease (28).
Cholesterol-conjugated ant-miR-21 (5′-UCA ACA UCA GUC UGA UAA GCU
A-3′) and ant-NC (5′-CAG UAC UUU UGU GUA GUA CAA A-3′) were
obtained from GenePharma, Inc. and were resuspended in sterile
phosphate-buffered saline (PBS) at 37°C and preserved at -20°C.
Recombinant lentivirus LV-NC and LV-PDCD4-shRNA were synthesized
and packaged by GenePharma, Inc. LV-NC was a recombinant lentivirus
containing pLKO.1 empty vector, and LV-PDCD4-shRNA was a
recombinant lentivirus containing pLKO.1 plasmid transfected with
PDCD4 shRNA fragment (5′-GCG GAG ATG TTA GGG ATT TG-3′).
The maximum diameter of the renal inferior abdominal
aorta on the day prior to the surgery was regarded as the baseline.
That of the renal inferior abdominal aorta in the anesthetized rats
was assessed using a diasonograph (Vivid7 Dimenson, Cytiva) on the
7 and 14th day after the surgery. Subsequently, the rats were
injected with excessive pentobarbital sodium [800 mg/kg; (29)]. The disappearance of reflexes,
respiratory arrest and cardiac arrest were observed, which
indicated that euthanasia was successful. The abdominal aorta was
flushed using cold PBS and transected through the left ventricle.
Subsequently, the aorta, from the left renal artery to the
bifurcation, was isolated from connective and fat tissues under a
microscope (Leica Microsystems GmbH) (30). The abdominal aorta at the
perfusion segment and the abdominal aorta approximately 5 mm from
its proximal and distal ends were selected. Samples from each group
were randomly assigned into 2 groups, with 6 samples in each group.
The partial samples were fixed with 4% paraformaldehyde and
reserved for histological analysis and the rest were used for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis.
Histological analysis
The tissues already fixed with 4% paraformaldehyde
were embedded in paraffin. The 5-mm-thick serial cross sections of
the abdominal aortas were placed on microscope slides and measured
with histological analysis and immunohistochemistry. These sections
were then used for hematoxylin and eosin (H&E) staining and
Elastica van Gieson (EVG) staining. Following gradient dewaxing and
dehydration with conventional xylene and ethanol (both from
Sinopharm Chemical Reagent Co., Ltd.), the sections were stained
using H&E staining kit (G1120, Beijing Solarbio Science &
Technology Co., Ltd.) at room temperature, stained with hematoxylin
for 5 min, then washed, differentiated for 30 sec, then soaked in
tap water for 15 min, and stained with eosin for 30 sec. The
sections were then stained with Verhöeff staining solution (G1598,
Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at
room temperature, rinsed with tap water, differentiated with
Verhöeff differentiation solution (G1598, Beijing Solarbio Science
& Technology Co., Ltd.) for 15 sec, then treated with 95%
ethanol for 5 min, and and counterstained with eosin for 30 sec.
After dyeing, the sections were dehydrated and cleared using
ethanol and xylene at room temperature, and then sealed with
neutral gum (G8590; Beijing Solarbio Science & Technology Co.,
Ltd.) and observed under Leica DM 750 optical microscope (Leica
Microsystems GmbH).
Immunohistochemistry was applied on the sections
embedded in paraffin on the 14th day with antibodies (all from
Abcam), including MMP-2 (1:500, ab86607), MMP-9 (1:1,000, ab38898),
nuclear factor-κB (NF-κB) p65 (1:500, ab16502) and cluster of
differentiation 68 (CD68) for macrophages (1:100, ab31630). In
detail, the sections free from paraffin were cultivated with
primary antibodies overnight at 4°C and at room temperature for 10
min cultured with immunoglobulin G secondary antibodies (1:2,000,
ab205719 and ab205718) (28,31). The sections were observed under
Leica DM 750 optical microscope (Leica Microsystems GmbH).
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was employed to extract total RNA from the aortic
tissues at the perfusion segment and approximately 5 mm from the
proximal and distal ends with a firm compliance to its
instructions. The extracted RNA concentration was determined under
a 260-nm wavelength. The total RNA was reverse transcribed into
cDNA. To assess the expression of miR-21 and U6, a One
StepPrimeScript miRNA cDNA Synthesis kit (Takara Biotechnology Co.,
Ltd.) was employed to perform reverse transcription, and the
remaining RNA was reverse transcribed using a PrimeScript RT
Reagent kit with genomic DNA Eraser (Takara Biotechnology Co.,
Ltd.). The experiment cited reverse transcribed cDNA as a standard
and was conducted with a firm compliance to SYBR Premix Ex Taq II
(Takara Biotechnology Co., Ltd.). This RNA was measured on a
LightCycler 480 (Roche Diagnostics). The primer sequences are
listed in Table I. The
thermocycling conditions were as follows: Pre-denaturation at 95°C
for 5 min, and a total of 40 cycles of denaturation at 95°C for 15
sec, annealing at 58°C for 35 sec, and extension at 72°C for 30
sec. The results are displayed in arbitrary units compared with the
mRNA expression of β-actin or U6. The method of 2−ΔΔCq
was used for quantification (32).
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| MMP-2 |
GGAATGCCATCCCTGATAACCT |
CTTCACGCTCTTGAGACTTTGGT |
| MMP-9 |
CTTTGTAGGGTCGGTTCT |
CCTGTGAGTGGGTTGGATT |
| MCP-1 |
CTATGCAGGTCTCTGTCACGCTTC |
CAGCCGACTCATTGGGATCA |
| TNF-α |
TCAGTTCCATGGCCCAGAC |
GTTGTCTTTGAGATCCATGCCATT |
| IFN-γ |
GAAAGCCTAGAAAGTCTGAAGAAC |
GCACCGACTCCTTTTCCGCTTCCT |
| IL-6 |
ACTCACCTCTTCAGAACGAATTG |
CCATCTTTGGAAGGTTCAGGTTG |
| IL-1β |
CATGATCCGAGATGTGGAACTGGC |
CTGGCTCAGCCACTCCAGC |
| PDCD4 |
TTGAGCACGGAGATACGAAC |
GTCCCGCAAAGGTCAGAAAG |
| β-actin |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC |
| miR-21 |
TGTAACAGACACTCCATGTGG |
GCTGTCAACAGTACGCTACG |
| U6 |
ATTGGAACGATACAGAGAAGAT |
GGAACGCTTCACGAATTTG |
Western blot analysis
A total protein extraction kit (Beyotime Institute
of Biotechnology, Inc.) and Nuclear and Cytoplasmic Protein
Extraction kit (Thermo Fisher Scientific, Inc.) were utilized to
extract protein from the abdominal aorta at the perfusion segment
and approximately 5 mm from the proximal and distal ends. The
bicinchoninic acid protein method (Beyotime Institute of
Biotechnology) was introduced to determine the protein
concentration. Proteins (30 µg) were then run on 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and transferred
onto polyvinylidene fluoride (PVDF) membranes (Thermo Fisher
Scientific, Inc.). The membranes were then cultivated in PBS with
50 g/l skim milk powder at room temperature for 4 h and then
cultured with antibodies (all from Abcam), including rabbit
anti-MMP-2 (1:2,000, ab92536)to detect pro MMP2 and active MMP2,
MMP-9 (1:1,000, ab38898) to detect pro MMP9 and active MMP9,
monocyte chemoattractant protein (MCP)-1 (1:2,000, ab25124), tumor
necrosis factor (TNF)-α (1:1,000, ab205587), interferon (IFN)-γ
(1:2,000, ab171081), interleukin (IL)-1β (1:200, ab9722), PDCD4
(1:500, ab194522), NF-κB p65 (1:500, ab16502), histone H3 antibody
(1:1,000, ab176842), glyceraldehyde-3-phosphate dehydrogenase
(1:10,000, ab181602) and mouse anti-IL-6 (1:400, ab9324) at 37°C
for 1 h. Following 3 PBS washes, the PVDF membranes were cultured
with peroxidase-conjugated secondary antibody goat anti-rabbit
(1:5,000, ab205718) or goat anti-mouse (1:5,000, ab205719) at room
temperature for 1 h. Chemiluminescent reagent ECL (PE0010,
Solarbio) was used for development for 3 min. A chemiluminescence
imager ChemiDoc (Bio-Rad Laboratories, Inc.) was used for imaging,
and Image Pro Plus 6.0 software (Media Cybernetics, Inc.) was used
for gray value analysis.
Dual-luciferase reporter gene assay
TargetScan (http://www.targetscan.org/vert_72/) analysis used to
predict the binding site between the targeting gene and miR-21. The
complementary binding sequence and mutant sequence of miR-21 and
PDCD4 were amplified and cloned to pmiR-GLO luciferase vector
(Promega Corporation) to establish a PDCD4 wild-type plasmid and
PDCD4 mutant-type plasmid. Mimic-NC and mimic-miR-21 were
transfected into 293T cells (Shanghai Institute of Biochemistry,
Chinese Academic of Sciences) according to the instructions
provided with Lipofectamine™ 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The dual-luciferase reporter gene assay system
(Promega Corporation) was used to detect the luciferase activity 48
h later. The ratio of firefly luciferase activity to Renilla
luciferase activity was calculated to reflect the relative
activity. All the experiments were performed 3 times. Mimic-miR-21
and its NC were both synthesized by GenePharma, Inc.
Statistical analysis
Statistical analysis was conducted using SPSS21.0
software (IBM Corp.). Normally distributed measurement data are
expressed as the means ± standard deviation. GraphPad Prism 8.0
(GraphPad Software, Inc.) was applied for graphing. The
Kolmogorov-Smirnov test indicated whether the data were normally
distributed. The non-paired t-test was used for comparisons between
2 groups. One-way analysis of variance (ANOVA) was used for
comparing different groups, and Tukey’s test was used as a post hoc
test for these data. A P-value was attained using a two-tailed
test, and P<0.05 was considered to indicate a statistically
significant difference, and P<0.01 a highly statistically
significant difference.
Results
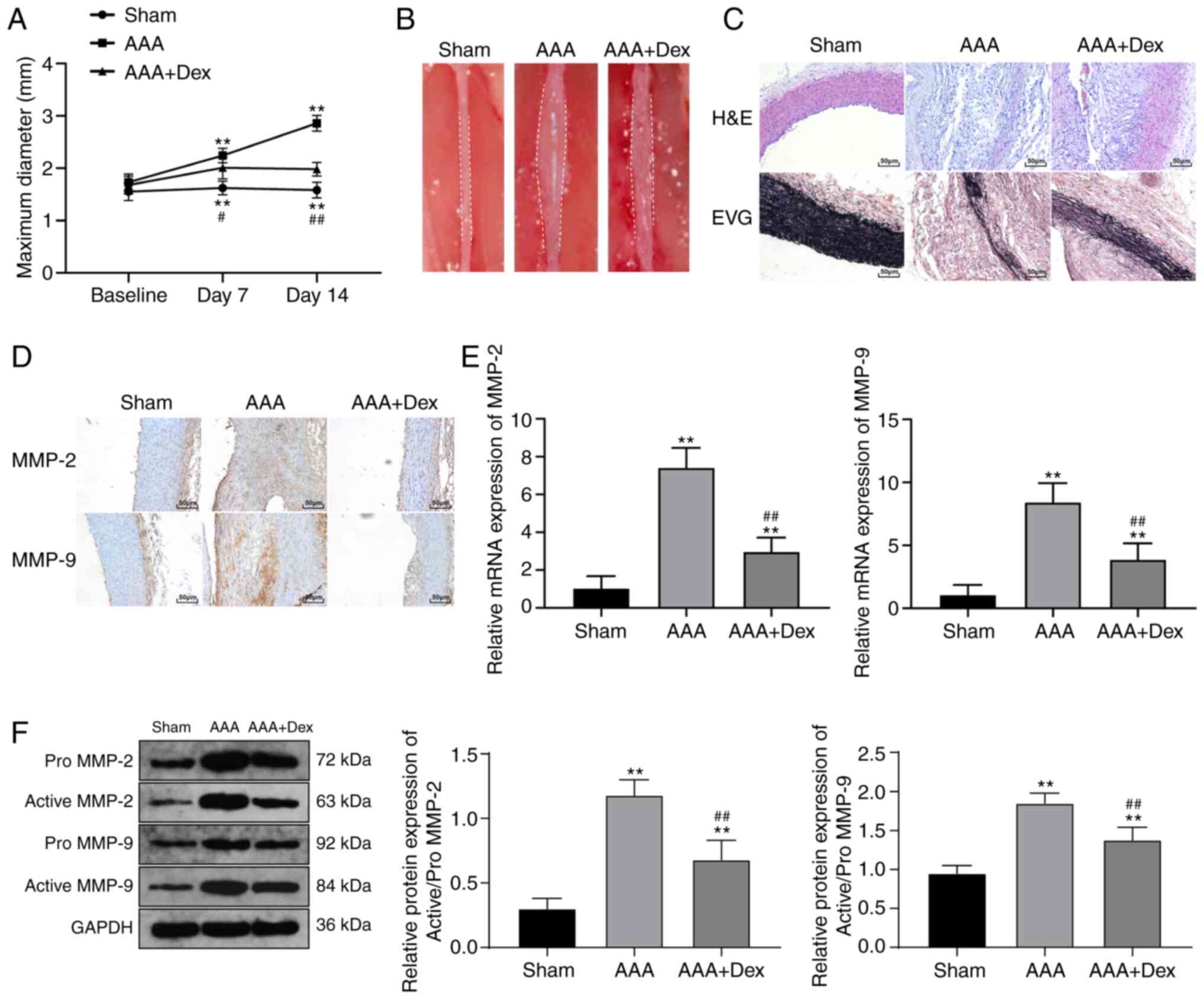
Dex attenuates the development of AAA in
rat models
It was observed that the maximum diameter of the
abdominal aorta in the rats with AAA was increased significantly on
the 7th day, and the maximum diameter was markedly expanded during
AAA modeling 1.8-fold on the 14th day (P<0.01). Compared with
the rats with AAA on the 7 and 14th day, the administration of Dex
alleviated the expanded abdominal aortic diameter (both P<0.05;
Fig. 1A and B). H&E and EVG
staining revealed a smooth outer membrane of the abdominal aorta,
complete structure, dense middle layer cells and a mass of wavy and
lamellar black elastic fibers in the rats from the sham group; in
the rats with AAA, there was a large number of infiltrating
inflammatory cells and a mass of degraded elastic fibers, and the
remaining ones were heavily broken without a wavy arrangement.
However, Dex ameliorated the disrupted abdominal aorta in the rats
with AAA (Fig. 1C). The results
from immunohistochemistry revealed that in the tunica media of the
abdominal aorta of rats with AAA, there were many regions that
positively expressed MMP-2 and MMP-9; these effects were suppressed
by Dex (Fig. 1D). In addition,
the mRNA and protein levels of MMP-2 and MMP-9 in rats with AAA
were decreased when Dex was administered to the rats (all
P<0.01; Fig. 1E and F).
Dex alleviates the inflammatory response
in rats with AAA
The abundant infiltration of CD68+
macrophages in the abdominal aorta was witnessed in rats with AAA,
which was mitigated by the administration of Dex (Fig. 2A). In addition, the upregulated
mRNA and protein levels of MCP-1 in the rats with AAA were markedly
inhibited by Dex (all P<0.01; Fig. 2B and C). Furthermore, the
activated expression of the inflammatory factors, TNF-α, IFN-γ,
IL-6 and IL-1β, triggered by AAA were suppressed by Dex (all
P<0.01; Fig. 2B and C).
Similarly, the same tendency was observed when the distribution of
NF-κB p65 and the protein levels in the abdominal aortic wall
nucleus were assessed (all P<0.01; Fig. 2D and E).
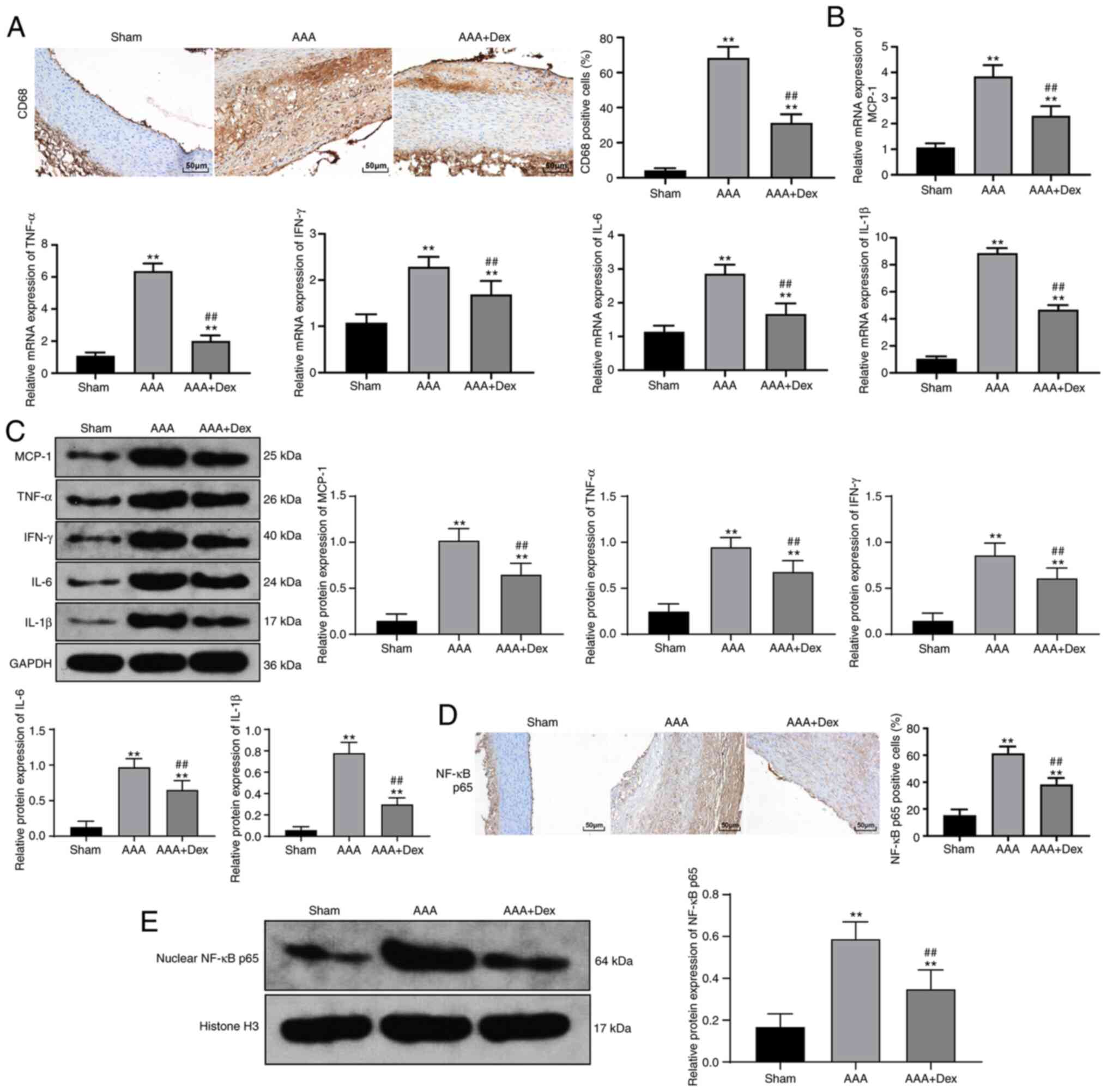 | Figure 2Dex alleviates the inflammatory
reaction in rats with AAA. (A) Immunohistochemistry suggested that
Dex mitigated the infiltrating CD68+ macrophages in the
abdominal aorta, and the macrophage-infiltrated part is displayed
in brown color. (B and C) Upregulated levels of MCP-1, TNF-α,
IFN-γ, IL-6 and IL-1β in the abdominal aortic wall were decreased
following the administration of Dex, as shown by the results of
RT-qPCR and western blot analysis. (D) Upregulated expression of
NF-κB p65 in the abdominal aortic wall nucleus was decreased after
the addition of Dex, as shown by immunohistochemistry, and the
positive expression is displayed in brown color. (E) Upregulated
expression of NF-κB p65 in the abdominal aortic wall nucleus was
decreased following the addition of Dex, as shown by western blot
analysis. Data are expressed as the means ± standard deviation; n=6
in each group. One-way ANOVA was applied to determine the
statistical significance of the data. **P<0.01,
compared with the sham group; ##P<0.01, compared with
the AAA group. Dex, dexmedetomidine; AAA, abdominal aortic
aneurysm; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor
necrosis factor-α; IFN-γ, interferon γ; IL, interleukin. |
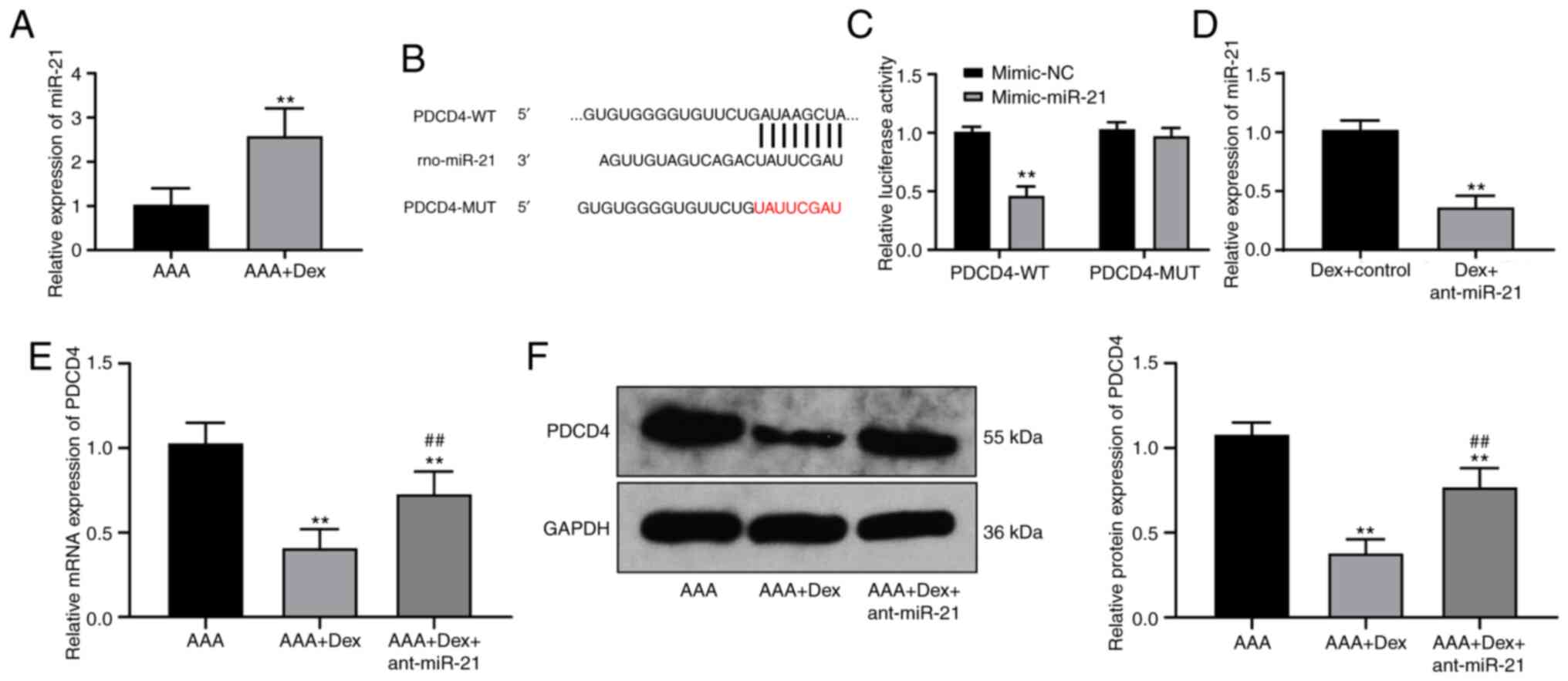
High expression of miR-21 in rats with
AAA treated with Dex targets PDCD4
Dex can affect the occurrence of AAA in rats;
however, its mechanism of action remains unclear. In a previous
study, miR, as an endogenous small noncoding RNA, was found to be a
potential therapeutic target to block the development of AAA
(33). miR-21 can prevent the
expansion of AAA (15). In the
present study, RT-qPCR verified that miR-21 was also highly
expressed in the abdominal aortic wall in the rats in the AAA + Dex
group (P<0.01; Fig. 3A).
TargetScan predicted that PDCD4 may be a target gene downstream of
miR-21, which was verified by dual-luciferase reporter gene assay
(P<0.01; Fig. 3B and C). The
detection of the mRNA and protein levels of PDCD4 in the rats with
AAA treated with the combination of Dex and ant-21 revealed that
the silencing of miR-21 evidently activated PDCD4 expression (all
P<0.05; Fig. 3D and E).
Ant-miR-21 promotes the development of
AAA and inflammatory responses
A combination of Dex and ant-miR-21 injected into
rats with AAA inhibited miR-21 expression, thereby damaging the
abdominal aortic structure and enhancing AAA expansion, which
reversed the favorable results induced by Dex (all P<0.01;
Fig. 4A-C), while promoting the
levels of MMP-2 and MMP-9 (all P<0.01; Fig. 4D). Similarly, ant-miR-21
activated the inflammatory responses and NF-κB p65 expression (all
P<0.01; Fig. 4E and F).
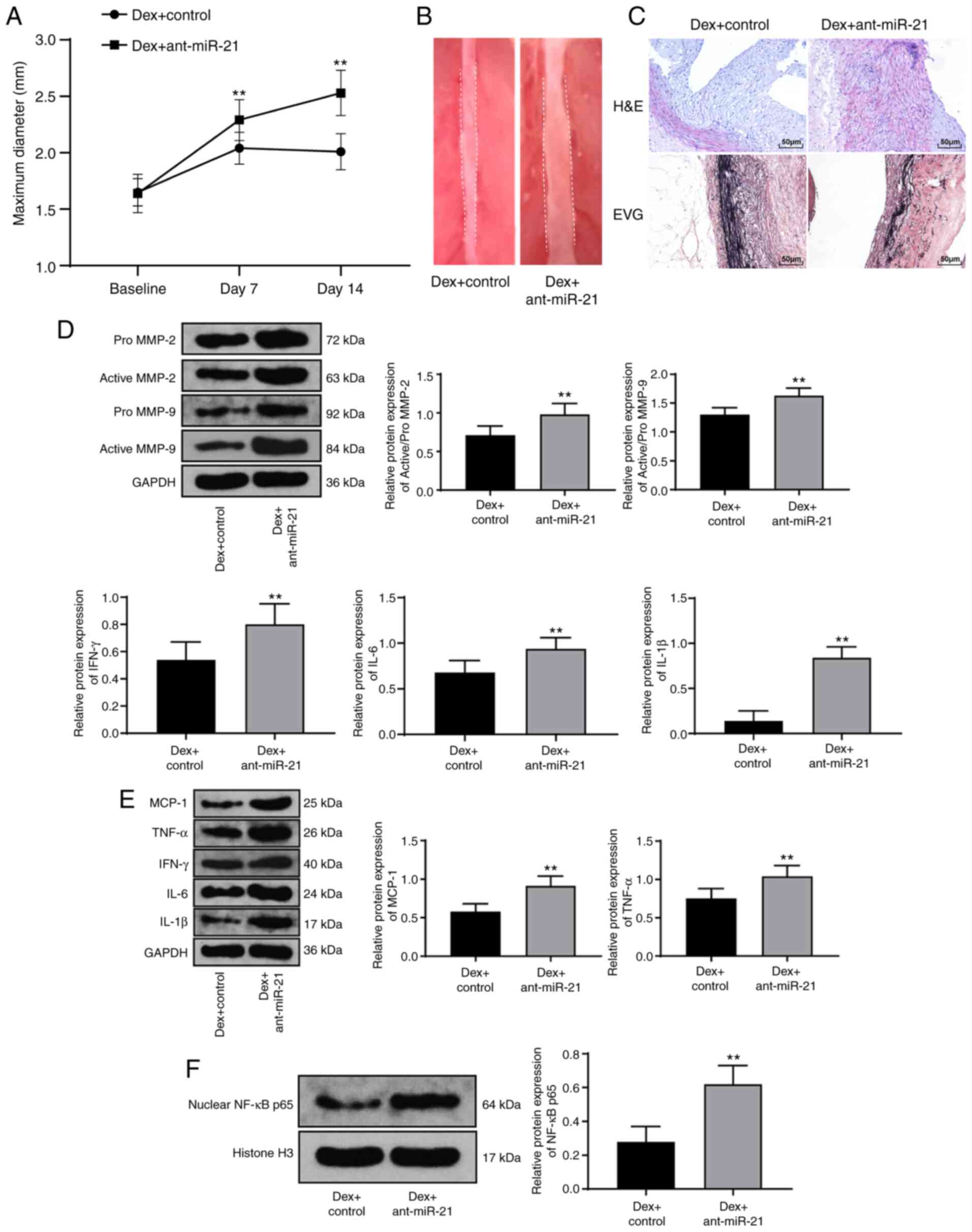 | Figure 4AntagomiR-21 enhances the development
of AAA and inflammatory reactions. (A) The maximum diameter of the
abdominal aorta in rats with AAA varied over time. (B) The
abdominal aortic structure in rats with AAA was disrupted on the
14th day after the surgery. (C) H&E and EVG staining revealed
that AAA deteriorated after the combination of Dex and antagomiR-21
was injected. (D) Western blot analysis revealed that the levels of
MMP-2 and MMP-9 were increased. (E) Western blot analysis indicated
that the levels of MCP-1, TNF-α, IFN-γ, IL-6 and IL-1β were
increased. (F) Western blot analysis suggested that NF-κB p65
expression was increased. Data are expressed as the mean ± standard
deviation; n=6 in each group. (D-F) Data were analyzed using the
t-test. (A) Data were analyzed by one-way ANOVA.
**P<0.01, compared with the Dex + control group. Dex,
dexmedetomidine; AAA, abdominal aortic aneurysm; H&E,
hematoxylin and eosin; EVG, Elastica van Gieson; MMP, matrix
metalloproteinase; MCP-1, monocyte chemoattractant protein 1;
TNF-α, tumor necrosis factor α; IFN-γ, interferon γ; IL,
interleukin. |
Inhibition of PDCD4 expression attenuates
the development of AAA and inflammatory responses
LV-PDCD4-shRNA was utilized to limit PDCD4
expression in rats with AAA, thus attenuating AAA expansion (all
P<0.01; Fig. 5A and B) and
reconstructing the structure and elastic fibers in the abdominal
aorta (both P<0.01; Fig. 5C and
D). Furthermore, the levels of MMP-2 and MMP-9, as well as
those of inflammatory factors were suppressed (all P<0.01;
Fig. 5E and F). Similarly, the
expression of NF-κB p65, the key protein in modulating inflammatory
responses, was also downregulated and inactivated by LV-PDCD4-shRNA
(P<0.01; Fig. 5G).
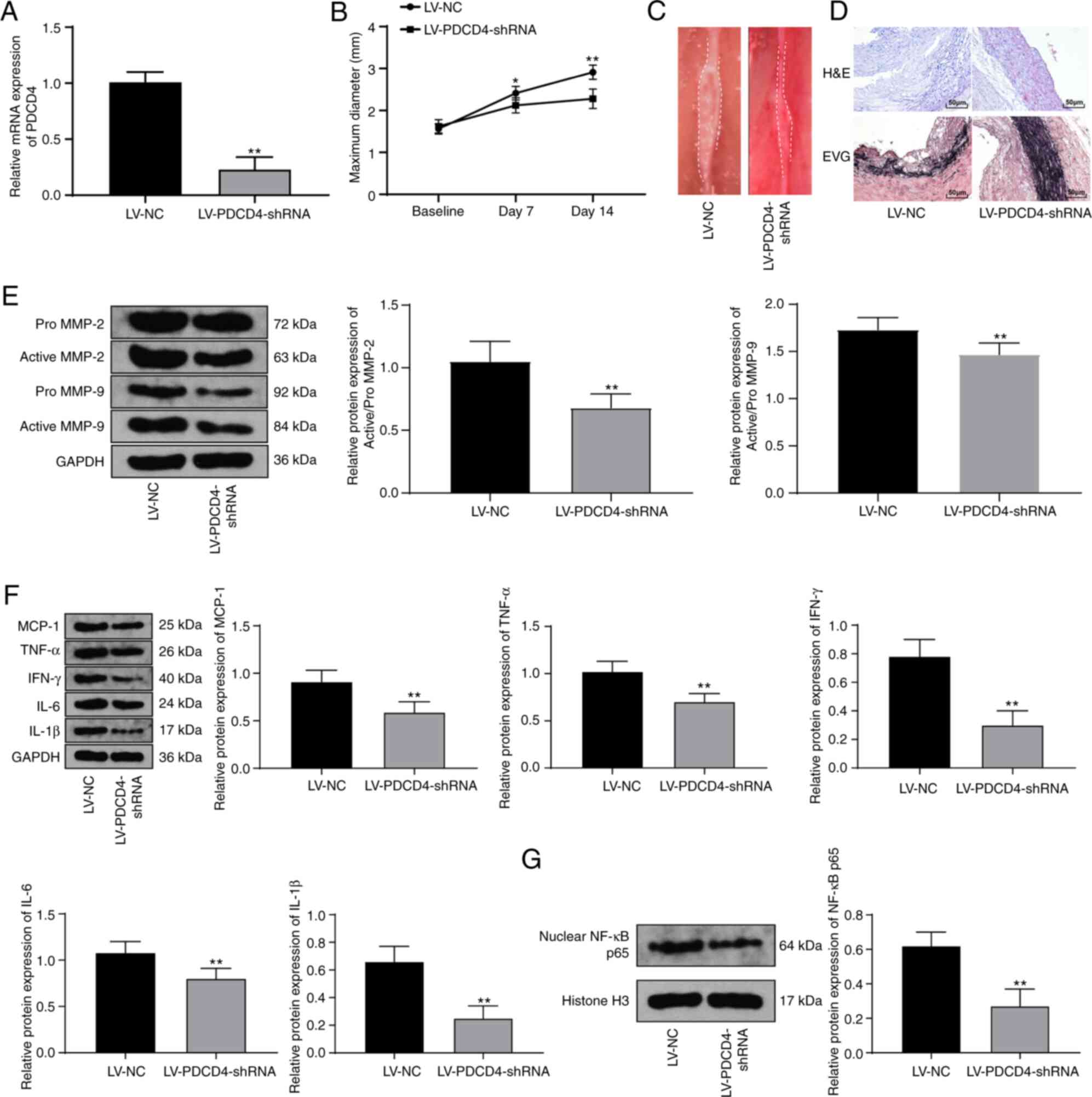 | Figure 5LV-PDCD4-shRNA relieves AAA
development and inflammatory reactions. (A) RT-qPCR revealed PDCD4
mRNA expression in AAA rats following LV-PDCD4-shRNA treatment. (B)
The maximum diameter of the abdominal aorta in rats with AAA varied
over time. (C) Abdominal aortic structure in rats with AAA
recovered on the 14th day after the surgery. (D) The structure and
elastic fibers in the abdominal aorta were improved, as shown by
H&E and EVG staining. (E) Western blot analysis indicated
decreased levels of MMP-2 and MMP-9. (F) Western blot analysis
suggested that the levels of MCP-1, TNF-α, IFN-γ, IL-6 and IL-1β
were decreased. (G) Western blot analysis revealed that NF-κB p65
was downregulated and inactivated. Data are expressed as the means
± standard deviation; n=6 in each group. (A, E, F and G) Data were
analyzed using the t-test. (B) Data were analyzed by one-way ANOVA.
*P<0.05, **P<0.01, compared with the
LV-NC group. Dex, dexmedetomidine; AAA, abdominal aortic aneurysm;
H&E, hematoxylin and eosin; EVG, Elastica van Gieson; MMP,
matrix metalloproteinase; MCP-1, monocyte chemoattractant protein
1; TNF-α, tumor necrosis factor-α; IFN-γ, interferon γ; IL,
interleukin. |
Discussion
AAA involves a series of alterations in the aortic
wall structure, characterized by a thinning adventitia and media
caused by extracellular matrix degradation and the insufficiency of
smooth muscle cells (34). Dex
is a highly selective α2-adrenoceptor agonist that is
conducive in anti-inflammatory functions (35). miR-21 plays a significant role in
the modulation of apoptosis and the proliferation of vascular wall
smooth muscle cells in the progression of AAA, thereby protecting
the progression of AAA (15).
Among the theories on AAA, only a few are related to Dex;
therefore, the present study aimed to explore novel therapies for
AAA based on Dex. Consequently, the findings presented herein
revealed that Dex mitigated AAA by regulating the miR-21/PDCD4
signaling pathway.
First, decreased sizes of the abdominal aorta in the
rats treated with Dex were observed; the decreased expression of
MMP-2 and MMP-9 was also observed. Increasing data have confirmed
that MMPs may be a potential treatment in inflammatory diseases
(36). Among the growing MMPs in
the aortic wall, MMP-2 and MMP-9 are notably increased in AAA
(37). The increased activity of
MMPs can promote the degradation of elastin and collagen in the
arterial wall, resulting in the expansion of the arterial wall, and
the inflammation in the aneurysm wall will also destroy the
extracellular matrix. These mechanisms promote the formation of AAA
(38). Dex has been found to be
able to promote alterations in MMPs to exert anti-inflammatory
effects (39). Of note, the
present study found that Dex reduced the expression of MCP-1 and
that of the inflammatory factors, TNF-α, IFN-γ, IL-6 and IL-1β, in
the rats with AAA. MCP-1 is involved in T-lymphocyte
differentiation and chemokines controlling monocyte chemotaxis,
which are involved in the pathological progression of
atherosclerosis and inflammatory diseases (40). Dex has been shown to exert
suppressive effects on MCP-1 in astrocytes via the
α2-adrenoceptor (41). According to a previous study
using mice with myocardial ischemia/reperfusion, following Dex
treatment, the TNF-α, IL-6 and IL-1β levels were all decreased
(42). Thus, Dex may be helpful
in palliating AAA.
In the present study, miR-21 was found to be
strongly expressed in rats with AAA treated with Dex. The
expression of miR-21 related to the endothelium has been shown to
be markedly increased in atherosclerotic AAA tissues (43). In another study, the
overexpression of miR-21 using a lentivirus promoted aortic
aneurysm cell growth and suppressed cell apoptosis, preventing the
progression of AAA (15). The
results of the present study also verified that the addition of
ant-miR-21 aggravated AAA expansion and inflammatory responses and
activated NF-κB. As a previous study demonstrated, the silencing of
miR-21 led to an evident growth of inflammatory factors, such as
TNF-α, IL-6 and IL-1β in cardiac cells and tissues of mice with
myocardial infarction (44).
NF-κB represents a major activator of immune homeostasis and
inflammation as it regulates various significant anti- or
pro-inflammatory factors in a number of inflammatory diseases,
including autoimmune disease, atherosclerosis, inflammatory bowel
disease and even certain types of cancer with inflammatory
components (45). A previous
study demonstrated that following the attenuation of AAA with
downregulated inflammatory responses, NF-κB was markedly inhibited
(46). Similarly, the silencing
of miR-21 has been shown to aggravate NF-κB expression in
peritonitis (47), indicating a
negative association between miR-21 and NF-κB in inflammatory
diseases. Additionally, it was noted that miR-21 targeted PDCD4.
The highly expression of PDCD4 serves as an engine of mitochondrial
function loss and cardiomyocyte apoptosis caused by hypoxic injury
(48). According to a recent
study on cardiovascular diseases, PDCD4 was the direct target of
miR-21 in vascular smooth muscle cells (49), which is in accordance with the
findings of the present study. In addition, the overexpression of
miR-21 and silencing of PDCD4 can both contribute to a
significantly decreased inflammatory response (50). Furthermore, in the present study,
the inhibition of PDCD4 mitigated AAA expansion and inflammatory
responses, and inactivated NF-κB. It has also been shown that in
macrophage foam cells, which serve as an engine in atherosclerotic
malignancy, the loss of PDCD4 improves cell autophagy, thus
reversing the conversion from macrophages to foam cells and
alleviating atherosclerosis (51). PDCD4 augments inflammatory
responses by upregulating NF-κB expression (52). Another study demonstrated that in
pigs with coronary microembolization, the PDCD4, NF-κB and TNF-α
levels were all increased (53).
Furthermore, the suppression of PDCD4 was conducive to treating
cardiac dysfunction resulting from coronary microembolization
(54). In addition, the
inactivation of NF-κB expression by Dex treatment decreased the
expression of inflammatory factors, including TNF-α, IL-6 and IL-1β
(55). Thus, the downregulation
of the miR-21/PDCD4 axis relieved AAA progression.
In conclusion, the present study demonstrated that
Dex attenuated AAA by overexpressing miR-21 and targeting PDCD4.
These results suggest a novel approach for the treatment of AAA. In
the future, the authors aim to further explore the underlying
mechanisms of other targets of Dex. Further attention also needs to
be paid to identifying reliable therapeutic targets for AAA.
Nevertheless, the present study was a preclinical study; although
the findings presented herein provide therapeutic implications for
the treatment of AAA, the experimental results and effective
application in clinical practice require further validation. In the
present study, a model of AAA was established in rats by elastin
perfusion combined with calcium chloride coating, which effectively
simulated the degradation process of extracellular matrix elastin
and the inflammatory reaction of the arterial wall during the
formation of human AAA. The therapeutic effects of Dex and
downstream miR-21 on the development of AAA in rats were
investigated and a good experimental basis was established for the
further study of Dex as a preventive measure for AAA. Although the
establishment of a model of AAA reflects the role and mechanism of
Dex in the prevention of AAA to a certain extent, the cells
targeted by Dex were not further explored in the present study. In
addition, there are a number of factors that induce AAA clinically,
and there are some differences between patients with AAA and rat
models of AAA. Therefore, Dex needs to be applied in clinical
practice for in-depth study. Due to the limitations of the
experimental conditions and funding, the present study only
discussed the effects of miR-21 and PDCD4 on the occurrence of AAA,
but failed to further examine the localization of its role. In the
future, the authors aim to mainly focus on the study of abdominal
aorta-related cells, and the localization of miR-21 and PDCD4 in
the abdominal aorta will be shown in future studies.
Availability of data and materials
The data that support the findings of this study are
included in this published article or are available from the
corresponding author upon reasonable request.
Authors’ contributions
All the authors are the guarantors of integrity of
the entire study. QY and MD contributed to the conception and
design of the study. XY and JD contributed to the definition of the
intellectual content and the literature research for the study. QLi
and YZ contributed to performing the experiments. QLiu and RH
contributed to data acquisition and analysis. QY contributed to the
preparation of the manuscript. MD contributed to the manuscript
review. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved and supervised by the
Ethics Committee of The First Affiliated Hospital of Nanchang
University. All animal experiments complied with the ARRIVE
guidelines and were carried out in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals. Significant efforts were made in order to minimize both
the number of animals used and their suffering.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Li H, Bai S, Ao Q, Wang X, Tian X, Li X,
Tong H, Hou W and Fan J: Modulation of immune-inflammatory
responses in abdominal aortic aneurysm: Emerging molecular targets.
J Immunol Res. 2018.7213760:2018.
|
|
2
|
Keisler B and Carter C: Abdominal aortic
aneurysm. Am Fam Physician. 91:538–543. 2015.PubMed/NCBI
|
|
3
|
Altobelli E, Rapacchietta L, Profeta VF
and Fagnano R: Risk factors for abdominal aortic aneurysm in
population-based studies: A systematic review and meta-analysis.
Int J Environ Res Public Health. 15:28052018. View Article : Google Scholar
|
|
4
|
Tchana-Sato V, Sakalihasan N and Defraigne
JO: Ruptured abdominal aortic aneurysm. Rev Med Liege. 73:296–299.
2018.In French. PubMed/NCBI
|
|
5
|
Joviliano EE, Ribeiro MS and Tenorio EJR:
MicroRNAs and current concepts on the pathogenesis of abdominal
aortic aneurysm. Braz J Cardiovasc Surg. 32:215–224.
2017.PubMed/NCBI
|
|
6
|
Wang YD, Liu ZJ, Ren J and Xiang MX:
Pharmacological therapy of abdominal aortic aneurysm: An update.
Curr Vasc Pharmacol. 16:114–124. 2018. View Article : Google Scholar
|
|
7
|
Jiang L, Hu M, Lu Y, Cao Y, Chang Y and
Dai Z: The protective effects of dexmedetomidine on ischemic brain
injury: A meta-analysis. J Clin Anesth. 40:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou SZ, Li ZM, Liu XR, Zhou J, Tan XQ,
Yang Y and Wei JC: Bidirectional regulatory effects of
dexmedetomidine on porcine coronary tone in vitro. Med Sci Monit.
23:1621–1626. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soliman R and Zohry G: The myocardial
protective effect of dexmedetomidine in high-risk patients
undergoing aortic vascular surgery. Ann Card Anaesth. 19:606–613.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bao Y, Zhu Y, He G, Ni H, Liu C, Ma L,
Zhang L and Shi D: Dexmedetomidine attenuates neuroinflammation in
LPS-stimulated BV2 microglia cells through upregulation of miR-340.
Drug Des Devel Ther. 13:3465–3475. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Essandoh K, Li Y, Huo J and Fan GC:
MiRNA-Mediated macrophage polarization and its potential role in
the regulation of inflammatory response. Shock. 46:122–131. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheedy FJ: Turning 21: Induction of miR-21
as a key switch in the inflammatory response. Front Immunol.
6:192015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bekelis K, Kerley-Hamilton JS, Teegarden
A, Tomlinson CR, Kuintzle R, Simmons N, Singer RJ, Roberts DW,
Kellis M and Hendrix DA: MicroRNA and gene expression changes in
unruptured human cerebral aneurysms. J Neurosurg. 125:1390–1399.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adam M, Raaz U, Spin JM and Tsao PS:
MicroRNAs in abdominal aortic aneurysm. Curr Vasc Pharmacol.
13:280–290. 2015. View Article : Google Scholar
|
|
15
|
Maegdefessel L, Azuma J, Toh R, Deng A,
Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell
MV, et al: MicroRNA-21 blocks abdominal aortic aneurysm development
and nicotine-augmented expansion. Sci Transl Med. 4:pp.
122ra1222012, View Article : Google Scholar
|
|
16
|
Zhang J, Zhang M, Yang Z, Huang S, Wu X,
Cao L, Wang X, Li Q, Li N and Gao F: PDCD4 deficiency ameliorates
left ventricular remodeling and insulin resistance in a rat model
of type 2 diabetic cardiomyopathy. BMJ Open Diabetes Res Care.
8:pp. e0010812020, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He J, Yue Y, Dong C and Xiong S: MiR-21
confers resistance against CVB3-induced myocarditis by inhibiting
PDCD4-mediated apoptosis. Clin Invest Med. 36:E103–E111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang K, Pan X, Zheng J, Liu Y and Sun L:
SIRT1 protects against aortic dissection by regulating AP-1/decorin
signaling-mediated PDCD4 activation. Mol Biol Rep. 47:2149–2159.
2020PubMed/NCBI
|
|
19
|
Tanaka A, Hasegawa T, Chen Z, Okita Y and
Okada K: A novel rat model of abdominal aortic aneurysm using a
combination of intraluminal elastase infusion and extraluminal
calcium chloride exposure. J Vasc Surg. 50:1423–1432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du C, Hu R, Csernansky CA, Hsu CY and Choi
DW: Very delayed infarction after mild focal cerebral ischemia: A
role for apoptosis? J Cereb Blood Flow Metab. 16:195–201. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeon AR and Kim JE: PDI knockdown inhibits
seizure activity in acute seizure and chronic epilepsy rat models
via S-nitrosylation-independent thiolation on NMDA receptor. Front
Cell Neurosci. 12:4382018. View Article : Google Scholar :
|
|
22
|
Lai CH, Chang JY, Wang KC, Lee FT, Wu HL
and Cheng TL: Pharmacological inhibition of cathepsin S suppresses
abdominal aortic aneurysm in mice. Eur J Vasc Endovasc Surg.
59:990–999. 2020PubMed/NCBI
|
|
23
|
Zhang Y, Yuan H, Bu P, Shen YH, Liu T,
Song S and Hou X: Recombinant leptin attenuates abdominal aortic
aneurysm formation in angiotensin II-infused apolipoprotein
E-deficient mice. Biochem Biophys Res Commun. 503:1450–1456. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chi X, Wei X, Gao W, Guan J, Yu X, Wang Y,
Li X and Cai J: Dexmedetomidine ameliorates acute lung injury
following orthotopic autologous liver transplantation in rats
probably by inhibiting toll-like receptor 4-nuclear factor kappa B
signaling. J Transl Med. 13:1902015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zampetaki A, Attia R, Mayr U, Gomes RS,
Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, et
al: Role of miR-195 in aortic aneurysmal disease. Circ Res.
115:857–866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li P, Yin YL, Guo T, Sun XY, Ma H, Zhu ML,
Zhao FR, Xu P, Chen Y, Wan GR, et al: Inhibition of aberrant
microRNA-133a expression in endothelial cells by statin prevents
endothelial dysfunction by targeting GTP cyclohydrolase 1 in vivo.
Circulation. 134:1752–1765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu YL, Peng XE, Zhu YB, Yan XL, Chen WN
and Lin X: Hepatitis B virus X protein induces hepatic steatosis by
enhancing the expression of liver fatty acid binding protein. J
Virol. 90:1729–1740. 2016. View Article : Google Scholar :
|
|
28
|
Setozaki S, Minakata K, Masumoto H, Hirao
S, Yamazaki K, Kuwahara K, Ikeda T and Sakata R: Prevention of
abdominal aortic aneurysm progression by oral administration of
green tea polyphenol in a rat model. J Vasc Surg. 65:1803–1812.
2017. View Article : Google Scholar
|
|
29
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res.
13:602017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: miR-24 limits aortic vascular inflammation and murine abdominal
aneurysm development. Nat Commun. 5:52142014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurobe H, Matsuoka Y, Hirata Y, Sugasawa
N, Maxfield MW, Sata M and Kitagawa T: Azelnidipine suppresses the
progression of aortic aneurysm in wild mice model through
anti-inflammatory effects. J Thorac Cardiovasc Surg. 146:1501–1508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Stather PW, Sylvius N, Sidloff DA, Dattani
N, Verissimo A, Wild JB, Butt HZ, Choke E, Sayers RD and Bown MJ:
Identification of microRNAs associated with abdominal aortic
aneurysms and peripheral arterial disease. Br J Surg. 102:755–766.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakalihasan N, Michel JB, Katsargyris A,
Kuivaniemi H, Defraigne JO, Nchimi A, Powell JT, Yoshimura K and
Hultgren R: Abdominal aortic aneurysms. Nat Rev Dis Primers.
4:342018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Wang H, Dong B, Ma J and Wu X:
Adding dexmedetomidine to ropivacaine for femoral nerve block
inhibits local inflammatory response. Minerva Anestesiol.
83:590–597. 2017.PubMed/NCBI
|
|
36
|
Vandenbroucke RE and Libert C: Is there
new hope for therapeutic matrix metalloproteinase inhibition? Nat
Rev Drug Discov. 13:904–927. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rabkin SW: The role matrix
metalloproteinases in the production of aortic aneurysm. Prog Mol
Biol Transl Sci. 147:239–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duellman T, Warren CL, Peissig P, Wynn M
and Yang J: Matrix metalloproteinase-9 genotype as a potential
genetic marker for abdominal aortic aneurysm. Circ Cardiovasc
Genet. 5:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J, Chen Q, He X, Alam A, Ning J, Yi B,
Lu K and Gu J: Dexmedetomidine attenuates lung apoptosis induced by
renal ischemia-reperfusion injury through α2AR/PI3K/akt
pathway. J Transl Med. 16:782018. View Article : Google Scholar
|
|
40
|
Bianconi V, Sahebkar A, Atkin SL and Pirro
M: The regulation and importance of monocyte chemoattractant
protein-1. Curr Opin Hematol. 25:44–51. 2018. View Article : Google Scholar
|
|
41
|
Liu H, Davis JR, Wu ZL and Abdelgawad AF:
Dexmedetomidine attenuates lipopolysaccharide induced MCP-1
expression in primary astrocyte. Biomed Res Int.
2017.6352159:2017.
|
|
42
|
Sun Y, Jiang C, Jiang J and Qiu L:
Dexmedetomidine protects mice against myocardium
ischaemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS
pathway. Clin Exp Pharmacol Physiol. 44:946–953. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kin K, Miyagawa S, Fukushima S, Shirakawa
Y, Torikai K, Shimamura K, Daimon T, Kawahara Y, Kuratani T and
Sawa Y: Tissue- and plasma-specific MicroRNA signatures for
atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 1:pp.
e0007452012, View Article : Google Scholar
|
|
44
|
Yang L, Wang B, Zhou Q, Wang Y, Liu X, Liu
Z and Zhan Z: MicroRNA-21 prevents excessive inflammation and
cardiac dysfunction after myocardial infarction through targeting
KBTBD7. Cell Death Dis. 9:7692018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mitchell JP and Carmody RJ: NF-κB and the
transcriptional control of inflammation. Int Rev Cell Mol Biol.
335:41–84. 2018. View Article : Google Scholar
|
|
46
|
Liu YF, Bai YQ and Qi M: Daidzein
attenuates abdominal aortic aneurysm through NF-κB, p38MAPK and
TGF-β1 pathways. Mol Med Rep. 14:955–962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Barnett RE, Conklin DJ, Ryan L, Keskey RC,
Ramjee V, Sepulveda EA, Srivastava S, Bhatnagar A and Cheadle WG:
Anti-Inflammatory effects of miR-21 in the macrophage response to
peritonitis. J Leukoc Biol. 99:361–371. 2016. View Article : Google Scholar
|
|
48
|
Xu H, Cao H, Zhu G, Liu S and Li H:
Overexpression of microRNA-145 protects against rat myocardial
infarction through targeting PDCD4. Am J Transl Res. 9:5003–5011.
2017.PubMed/NCBI
|
|
49
|
Liu K, Liu C and Zhang Z: lncRNA GAS5 acts
as a ceRNA for miR-21 in suppressing PDGF-bb-induced proliferation
and migration in vascular smooth muscle cells. J Cell Biochem.
120:15233–15240. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhu Y, Liu L, Hu L, Dong W, Zhang M, Liu Y
and Li P: Effect of celastrus orbiculatus in inhibiting
helicobacter pylori induced inflammatory response by regulating
epithelial mesenchymal transition and targeting miR-21/PDCD4
signaling pathway in gastric epithelial cells. BMC Complement
Altern Med. 19:912019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang L, Jiang Y, Song X, Guo C, Zhu F,
Wang X, Wang Q, Shi Y, Wang J, Gao F, et al: Pdcd4 deficiency
enhances macrophage lipoautophagy and attenuates foam cell
formation and atherosclerosis in mice. Cell Death Dis. 7:pp.
e20552016, View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liang X, Xu Z, Yuan M, Zhang Y, Zhao B,
Wang J, Zhang A and Li G: MicroRNA-16 suppresses the activation of
inflammatory macrophages in atherosclerosis by targeting PDCD4. Int
J Mol Med. 37:967–975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Su Q, Li L, Zhao J, Sun Y and Yang H:
Effects of trimetazidine on PDCD4/NF-κB/TNF-α pathway in coronary
microembolization. Cell Physiol Biochem. 42:753–760. 2017.
View Article : Google Scholar
|
|
54
|
Su Q, Li L, Liu Y, Zhou Y, Wang J and Wen
W: Ultrasound-Targeted microbubble destruction-mediated microRNA-21
transfection regulated PDCD4/NF-κB/TNF-α pathway to prevent
coronary microembolization-induced cardiac dysfunction. Gene Ther.
22:1000–1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen Z, Ding T and Ma CG: Dexmedetomidine
(DEX) protects against hepatic ischemia/reperfusion (I/R) injury by
suppressing inflammation and oxidative stress in NLRC5 deficient
mice. Biochem Biophys Res Commun. 493:1143–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|