Introduction
Glaucoma is a progressive optic neuropathy
characterized by the damage of optic nerve and visual function,
which can lead to irreversible vision loss (1,2).
Over 70 million individuals worldwide are affected by glaucoma
(3). Elevated intraocular
pressure (IOP) is the primary risk factor for glaucoma, and
reducing IOP is an effective therapy for glaucoma (4,5).
The high IOP results from the imbalance of the aqueous humor inflow
and outflow. Trabecular meshwork (TM), responsible for the
extracellular matrix (ECM) production, plays an important role in
aqueous humor outflow (6,7).
It has been suggested that the excessive deposition of ECM in TM
cells causes the resistance of outflow (8,9).
Thus, it is of great significance to clarify the potential
mechanism underlying ECM deposition in TM cells for glaucoma
treatment.
MicroRNAs (miRNAs/miRs), a family of small
non-coding RNAs with ~22 nucleotides in length, play crucial roles
in the regulation of posttranscriptional gene silencing (10,11). miRNAs serve as key regulators in
various cellular processes, including cell growth, metabolism,
migration and apoptosis in a number of human diseases (12,13). Previously, increasing number of
miRNAs are reported to be involved in the progression of glaucoma.
miR-21a-5p exerts neuroprotective effects on mesenchymal stem cells
by targeting programmed cell death protein 4 in acute glaucoma
(14). miR-1298 protects human
trabecular meshwork (HTM) cells against chronic oxidative injury by
targeting eukaryotic translation initiation factor 4E type 3
(15). miR-483-3p targeting
Smad4 has a suppressive effect on ECM production in HTM cells
(16). Moreover, the functions
of miR-29b-3p in a variety of diseases have been elucidated. For
example, miR-29b-3p expression is upregulated in patients with
congenital heart disease and miR-29b-3p silencing promotes
cardiomyocyte proliferation via targeting NOTCH2 (17). miR-29b-3p contributes to the
inflammatory response of human bronchial epithelial cells
stimulated by particulate matter by suppressing the AMPK pathway
(18). miR-29b-3p promotes the
apoptosis of retinal microvascular endothelial cells via
downregulating sirtuin-1 in an in vitro model of diabetic
retinopathy (19).
The aim of the present study was to investigate the
role and regulatory mechanism of miR-29b-3p in HTM cells under
oxidative stress in order to find potential novel therapeutic
targets for glaucoma treatment.
Materials and methods
Laboratory animals
A total of 18 Wistar rats (8 weeks old, male,
weighing 220~260 g) were provided by Beijing Vital River Laboratory
Animal Technology Co., Ltd. Before the experiments, all rats
received 1 week of adaptive feeding, with four to six rats in each
cage. Rats were raised with ad libitum access to water and
food in a temperature-controlled room (22±2°C) with a 12 h
light/dark cycle and a relative humidity of 40-60%. All animal
studies were performed following the animal guidelines of the
International Association for the Study of Pain (20), and approved by the Ethics
Committee of the Second Hospital of Anhui Medical University
(approval no. 2019-051; Hefei, China).
Establishment of glaucoma models and IOP
measurement
For establishment of glaucoma models, rats were
anesthetized with intraperitoneal injection of 3% pentobarbital
sodium (30 mg/kg; Sigma-Aldrich; Merck KGaA). The right eyes of
rats underwent operation. 0.1 ml aqueous fluid was extracted from
the anterior temporal horn of iris through a cannula, and 14 rats
were slowly injected with 3% compound carbomer solution through the
cannula, while 4 rats were treated with the same amount of normal
saline. After administration, all rats were treated with 0.5%
moxifloxacin three times a day for 3 days. IOP measurement was
operated using a tonometer (Mentor O&O, Inc.). IOP was measured
at 10 AM and 10 PM every day. A mean IOP was calculated from five
automatically averaged measurements. A total of 12 rats with high
IOP >30 mmHg were chosen. When the steadily increased IOP was
achieved, four glaucoma model rats were injected with 5 µl
antagomiR-29b-3p (Shanghai GenePharma Co., Ltd.), whereas the
control rats were injected with 5 µl antagomiR-negative
control (NC) (Shanghai GenePharma Co., Ltd.). Rats were
anesthetized with an intraperitoneal injection of 30 mg/kg
pentobarbital sodium and were sacrificed by decapitation.
Hematoxylin and eosin (H&E)
staining
The right eyeballs of rats were isolated after the
rats were sacrificed, and then fixed in 4% paraformaldehyde for 24
h at room temperature. The cornea was incised along the sclera and
the iris and lens were removed 30 min later. The retinal tissues
were paraffin-embedded and cut into 5-µm sections for
H&E staining assay. The sections were stained with hematoxylin
for 2 min and stained with eosin for 10 min at room temperature
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.). After
dehydration with gradient alcohol, the sections were sealed using
neutral resin. The morphology of retinal tissues was observed under
a light microscope at ×20 magnification (Olympus Corporation), and
the results were quantified using ImageJ software (version 1.46;
National Institutes of Health). The mean value was obtained from
five randomly selected fields.
HTM cell culture and treatment
HTM cells (ScienCell Research Laboratories, Inc.)
were cultured in Dulbecco's modified Eagle's medium (DMEM)
containing 15% fetal bovine serum, 2 mM L-glutamine, 0.05%
gentamicin, 1 ng/ml FGF-2 and 0.25 µg/ml amphotericin B (all
from ScienCell Research Laboratories, Inc.) at 37°C with 5%
CO2. The medium was replaced every day when the culture
reached 70% confluence. To induce oxidative stress, cells were
incubated in DMEM supplemented with H2O2
(Beyotime Institute of Biotechnology) at concentrations of 100,
200, 300, 400 µM for 24 h after reaching 85% confluence.
Cell transfection
The mature miR-29b-3p mimic (for miR-29b-3p
overexpression) and its NC mimic, miR-29b-3p inhibitor (for
miR-29b-3p silencing) and its NC inhibitor, small interfering
(si)RNA against E3 ubiquitin-protein ligase RNF138 (RNF138;
siRNF138, for RNF138 knockdown) and its NC (si-NC) were constructed
by Shanghai GenePharma Co., Ltd. The following sequences were
included in the present study: NC mimic, 5′-CAG UAC UUU UGU GUA GUA
CAA A-3′; miR-29b-3p mimic, 5′-UAG CAC CAU UUG AAA UCA GUG U-3′; NC
inhibitor, 5′-UUU GUA CUA CAC AAA AGU ACU G-3′; miR-29b-3p
inhibitor, 5′-UAG CAC CAU UUG AAA UCA GUG U-3′; si-NC, 5′-UAG AAG
UUA ACU UCA CAG CAU -3′; and siRNF138, 5′-ACA UUU UCU ACA GAA AAC
GUG -3′. HTM cells were seeded into the 6-well plates at a density
of 1×106 and subsequently transfected with miR-29b-3p
mimic/inhibitor (50 nM), NC mimic/inhibitor (50 nM), siRNF138 and
si-NC (100 ng) using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h. The HTM cells were
assigned into the following groups: i) Control group, cells without
H2O2 stimulation; ii)
H2O2 group, cells with 300 µM
H2O2 stimulation; iii)
H2O2 + NC group, cells with 300 µM
H2O2 stimulation + NC; iv)
H2O2 + miR-29b-3p mimic group, cells with 300
µM H2O2 stimulation + miR-29b-3p
mimic; v) H2O2 + miR-29b-3p inhibitor group,
cells with 300 µM H2O2 stimulation +
miR-29b-3p inhibitor; vi) H2O2 + siRNF138
group, cells with 300 µM H2O2
stimulation + siRNF138; and v) H2O2 +
miR-29b-3p inhibitor + siRNF138 group, cells with 300 µM
H2O2 stimulation + miR-29b-3p inhibitor +
siRNF138. The time interval between transfection and subsequent
experimentation was 48 h.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was employed for the extraction of total RNA from
HTM cells. First-strand cDNA synthesis was performed using M-MLV
reverse transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. qPCR was conducted using
the TaqMan™ Universal Master Mix II (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For quantification of
miRNAs, cDNA was obtained from 3 µg RNA using a specific
stem-loop primer. The 2−ΔΔCq method (21) was used for quantification and
miRNA expression was normalized to U6, while GAPDH acted as the
endogenous reference gene for mRNA. The following primers were used
for RT-qPCR: miR-29b-3p forward, 5′-UAG CAC CAU UUG AAA UC-3′ and
reverse, 5′-GTG CAG GTC CGA GGT -3′; U6 forward, 5′-CGCTTC GGC AGC
ACA TAT AC-3′ and reverse, 5′-TTC ACG AAT TTG CGT GTC AT-3′; RNF138
forward, 5′-TAT AGT TCT GGC TCT CTG AA-3′ and reverse, 5′-CTG ACT
TGG GTA AAT GCT CAA T-3′; collagen α-1(I) chain (COL1A1) forward,
5′-CCT GTC TGC TTC CTG TAA AC-3′ and reverse, 5′-ATG TTC GGT TGG
TCA AAG ATA AAT -3′; collagen α-2(I) chain (COL1A2) forward, 5′-CAG
TCG TAT GCG CGT ATA GC-3′ and reverse, 5′-CGT AGT CGT AGC TAG CTA
GAG A-3′; and GAPDH forward, 5′-GGA GCG AGA TCC CTC CAA AAT-3′ and
reverse, 5′-GGC TGT TGT CAT ACT TCT CAT GG-3′. The thermocycling
conditions used were as follows: 94°C for 5 min; followed by 40
cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 1 min.
Western blot analysis
The treated HTM cells were collected and washed with
pre-cooling PBS. After centrifugation at 600 × g for 5 min at 4°C,
the cells were lysed with radio immunoprecipitation assay buffer
(Sigma-Aldrich; Merck KGaA). Then, cell lysates were centrifuged at
12,000 × g for 12 min at 4°C, followed by measurement of proteins
in the supernatant using the BCA protein assay (Pierce; Thermo
Fisher Scientific, Inc.). After proteins (20 µg per lane)
were separated via sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 10% gel, proteins were transferred to
polyvinylidene difluoride membranes. Afterwards, the membranes were
blocked with 5% non-fat dry milk at 4°C overnight, and incubated
with the following primary antibodies at 4°C overnight: Anti-RNF138
(cat. no. K107111P; 1:5,000; Beijing Solarbio Science &
Technology Co., Ltd.), anti-Bcl-2 (cat. no. ab185002; 1:1,000;
Abcam), anti-Bax (cat. no. ab32503; 1:1,000; Abcam), anti-COL1A1
(cat. no. ab210966; 1:1,000; Abcam), anti-COL1A2 (cat. no. ab96723;
1:1,000; Abcam), anti-ERK (cat. no. ab115799; 1:1,000; Abcam),
anti-phosphorylated (p)-ERK (cat. no. ab214036; 1:1,000; Abcam) and
anti-β-actin (cat. no. ab179467; 1:1,000; Abcam). The membranes
were subsequently cultured with the corresponding secondary
antibodies (cat. no. ab205718; 1:5,000; Abcam) for 1.5 h at room
temperature. The proteins were detected using the chemiluminescence
detection (ECL Plus; Pierce; Thermo Fisher Scientific, Inc.).
Semi-quantification was performed using ImageJ software (version
1.46; National Institutes of Health).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
HTM cell viability was assessed using an MTT assay.
After transfection and H2O2 stimulation,
cells (1,000 cells per well) were seeded in the 96-well plates,
followed by incubation with 50 µl MTT (5 mg/ml;
Sigma-Aldrich; Merck KGaA) for 4 h. Then, 150 µl
dimethylsulfoxide (Sigma-Aldrich; Merck KGaA) was used to dissolve
the formazan deposited in cells for 10 min. The absorbance at 490
nm was detected using a microplate reader (Bio-Rad Laboratories,
Inc.).
Cell apoptosis assay
After transfection and H2O2
stimulation, HTM cells were washed in PBS and digested using
EDTA-free trypsin. Then, cells were suspended in binding buffer at
a density of 1×106 cells/ml. Following which, 5
µl Fluorescein isothiocyanate-labeled Annexin V and 100 ng
propidium iodide were added to the 500 µl suspended cells,
followed by incubation for 15 min at room temperature in the dark.
Cell apoptosis was determined using the Attune™ NxT Flow Cytometer
(Invitrogen; Thermo Fisher Scientific, Inc.) and the apoptosis rate
was analyzed with FlowJo software (version 10.0; FlowJo LLC).
Apoptosis rate = apoptosis rate in quadrant (Q)2 + apoptosis rate
in Q3.
Detection of reactive oxygen species
(ROS)
ROS levels in HTM cells were examined using a ROS
assay kit (cat. no. S0033M; Beyotime Institute of Biotechnology).
After transfection and H2O2 stimulation, HTM
cells were washed in PBS and subsequently cultured in DMEM
containing 10 µM 2,7-dichlorodihydrofluorescein diacetate
(DCFHDA) for 20 min at 37°C. After washing with PBS twice, the
cells were treated with 0.25% trypsin-EDTA, followed by
centrifugation at 800 × g for 6 min at 37°C. Then, cells were
resuspended in 500 µl PBS. The fluorescence intensity,
regarded as ROS generation, was measured by an Infinite M200
microplate reader (485 and 525 nm emission).
Bioinformatics analysis
Targets of miR-29b-3p were predicted using miRanda
version 3.3a (http://cbio.mskcc.org/microrna_data/miRanda-aug2010.tar.gz),
microT-CDS DIANA Tools version 5.0 (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index)
and RNA22 version 2.0 (https://cm.jefferson.edu/rna22/Interactive/).
Luciferase reporter assay
The wild-type (Wt) and mutated (Mut) sequences of
the 3′UTR of RNF138 complementary to miR-29b-3p were subcloned into
the pmirGLO-luciferase plasmids (Promega Corporation) to generate
Wt RNF138 (RNF138-Wt) and Mut type RNF138 (RNF138-Mut). RNF138-Wt
and RNF138-Mut reporters were co-transfected with NC or miR-29b-3p
mimic into 293T cells (American Type Culture Collection) using
Lipofectamine 3000. The interaction between miR-29b-3p and 3′-UTR
of RNF138 was detected using a Luciferase Assay system (Promega
Corporation) 48 h post-transfection. Relative luciferase activity
was defined as the ratio of firefly luciferase activity to
Renilla luciferase activity.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments and analyzed using the SPSS 21.0
software (IBM Corp.). Comparisons between 2 groups were analyzed
using unpaired Student's t-test, while comparisons among ≥3 groups
were analyzed using one-way analysis of variance. Dunnett's or
Tukey's post hoc tests were used following one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-29b-3p is upregulated in a rat
glaucoma model and antagomiR-29b-3p alleviates the symptoms of
glaucoma
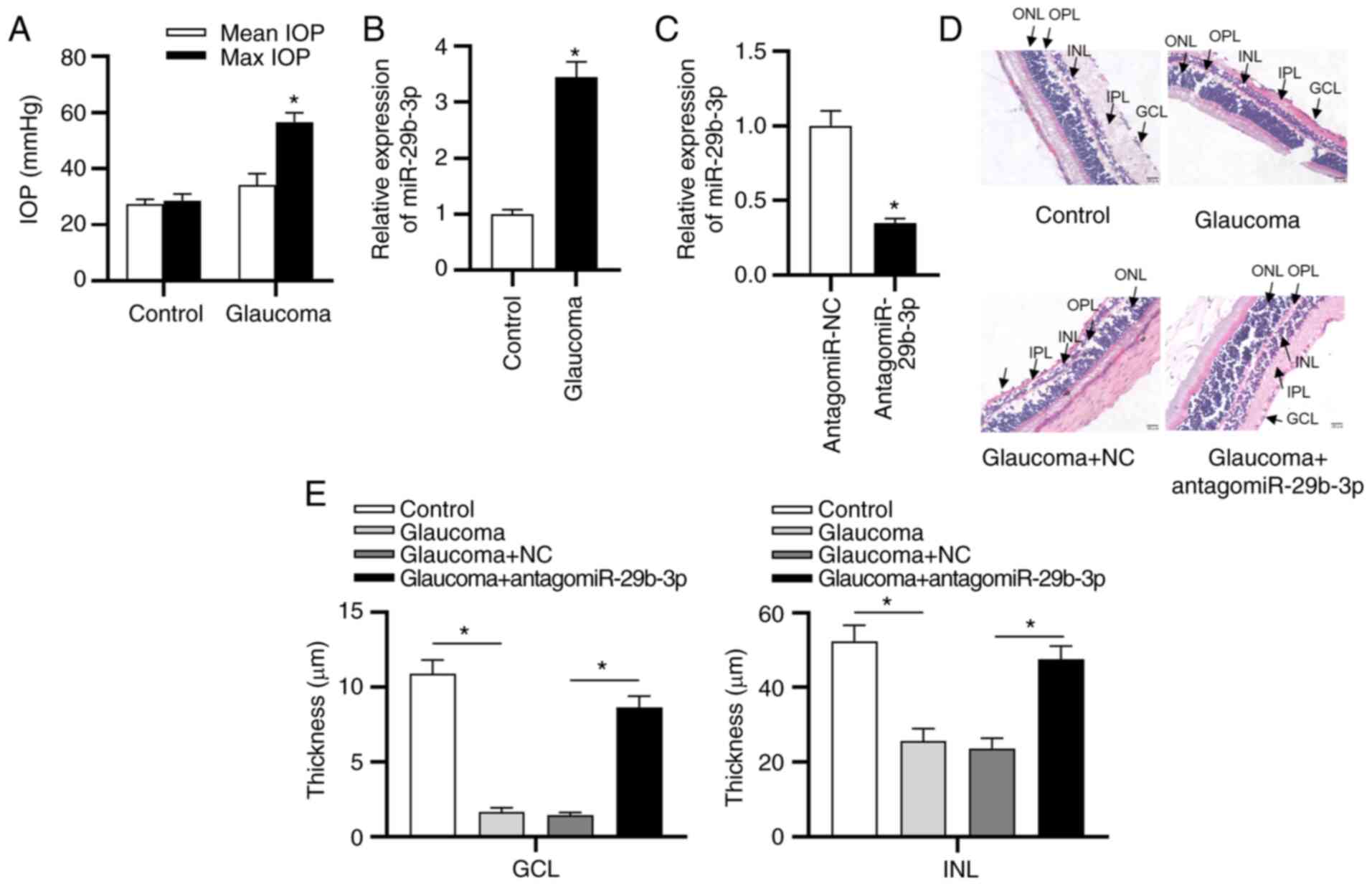
As shown in Fig.
1A, in the control group, there was no significant difference
between the mean and max IOP; however, the max IOP was
significantly higher than the mean IOP in the glaucoma group.
Moreover, RT-qPCR revealed a higher expression of miR-29b-3p in the
glaucoma group compared with the control group (Fig. 1B). As presented in Fig. 1C, miR-29b-3p expression levels
were decreased in rats following injection with antagomiR-29b-3p
compared with the NC group. Next, the functions of antagomiR-29b-3p
in a glaucoma model were investigated. H&E staining revealed
that the thickness of each layer of the retinal, including the
outer nuclear layer (ONL), inner nuclear layer (INL) and ganglion
cell layer (GCL), were significantly thinner in the glaucoma model
compared with the control group (Fig. 1D and E), and transfection with
antagomiR-29b-3p increased the thickness.
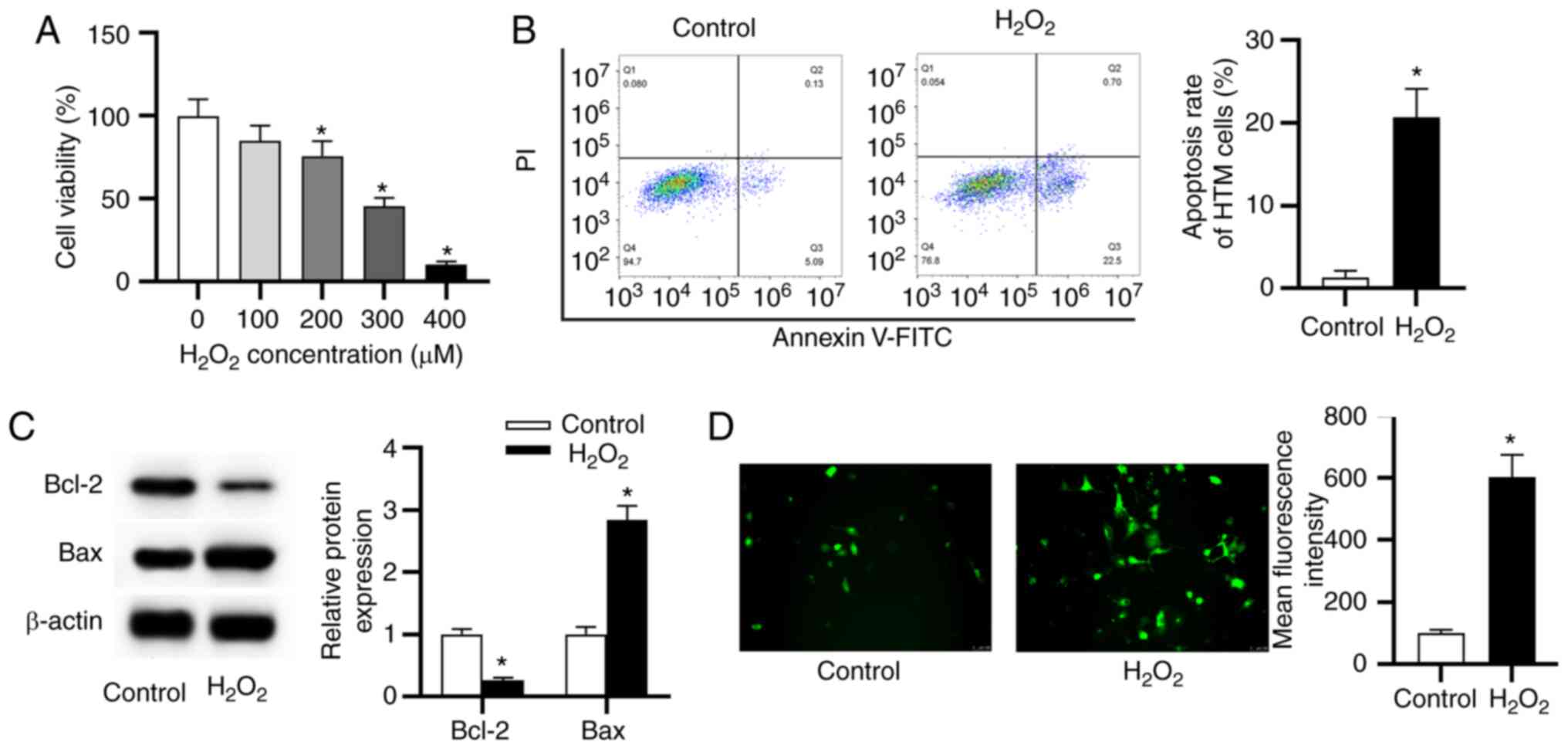
H2O2 induces
oxidative damage in HTM cells
HTM cells were treated with different concentrations
of H2O2 (100, 200, 300 and 400 µM) for
24 h and concentration of H2O2 at 0 µM
was used as the control. The data from MTT assay revealed that
100-400 µM of H2O2 inhibited cell
viability in a concentration-dependent manner compared with the
control (Fig. 2A). Since 300
µM of H2O2 decreased >50% of cell
viability, 300 µM H2O2 was used for
induction of oxidative damage in further assays. Subsequently, the
apoptosis rate and ROS generation of HTM cells were detected. Flow
cytometry results showed that the H2O2 group
exhibited a significant increase of cell apoptosis (Fig. 2B). Similarly, the reduced level
of Bcl-2 (anti-apoptotic marker) and the elevated level of Bax
(pro-apoptotic marker) were revealed in the
H2O2 group (Fig. 2C), implying that the stimulation
of H2O2 promoted HTM cell apoptosis. As
presented in Fig. 2D,
H2O2 stimulation increased the level of ROS
compared with the control.
miR-29b-3p facilitates
H2O2-induced injury and promotes ECM
deposition while silencing of miR-29b-3p exerted the opposite
effects
As presented in Fig.
3A, miR-29b-3p expression was at a higher level in the
H2O2 group than in the control group.
miR-29b-3p expression was increased by transfection with miR-29b-3p
mimic compared with the control group. Under transfection with
miR-29b-3p inhibitor, miR-29b-3p expression was 34% of the control
group. MTT assay revealed that the H2O2 +
miR-29b-3p mimic group displayed decreased cell viability, while
the H2O2 + miR-29b-3p inhibitor group showed
the opposite result (Fig. 3B).
Flow cytometry showed that the apoptosis rate of HTM cells was
higher in the H2O2 + miR-29b-3p mimic group,
and lower in the H2O2 + miR-29b-3p inhibitor
group, compared with the H2O2 + NC group
(Fig. 3C). Likewise, Bcl-2
expression was downregulated and Bax was upregulated in the
H2O2 + miR-29b-3p mimic group, whereas the
miR-29b-3p inhibitor exerted opposite effects (Fig. 3D). Furthermore, the
H2O2 + miR-29b-3p mimic group exhibited an
increased level of ROS and the H2O2 +
miR-29b-3p inhibitor group presented the opposite trend (Fig. 3E). Additionally, the expression
levels of COL1A1 and COL1A2 were determined by RT-qPCR and western
blotting. As presented in Fig. 3F
and G, the mRNA and protein levels of COL1A1 and COL1A2 were
higher in the H2O2 groups than in the control
group. miR-29b-3p mimics increased COL1A1 and COL1A2 levels,
whereas the miR-29b-3p inhibitor decreased COL1A1 and COL1A2 levels
in HTM cells under oxidative injury.
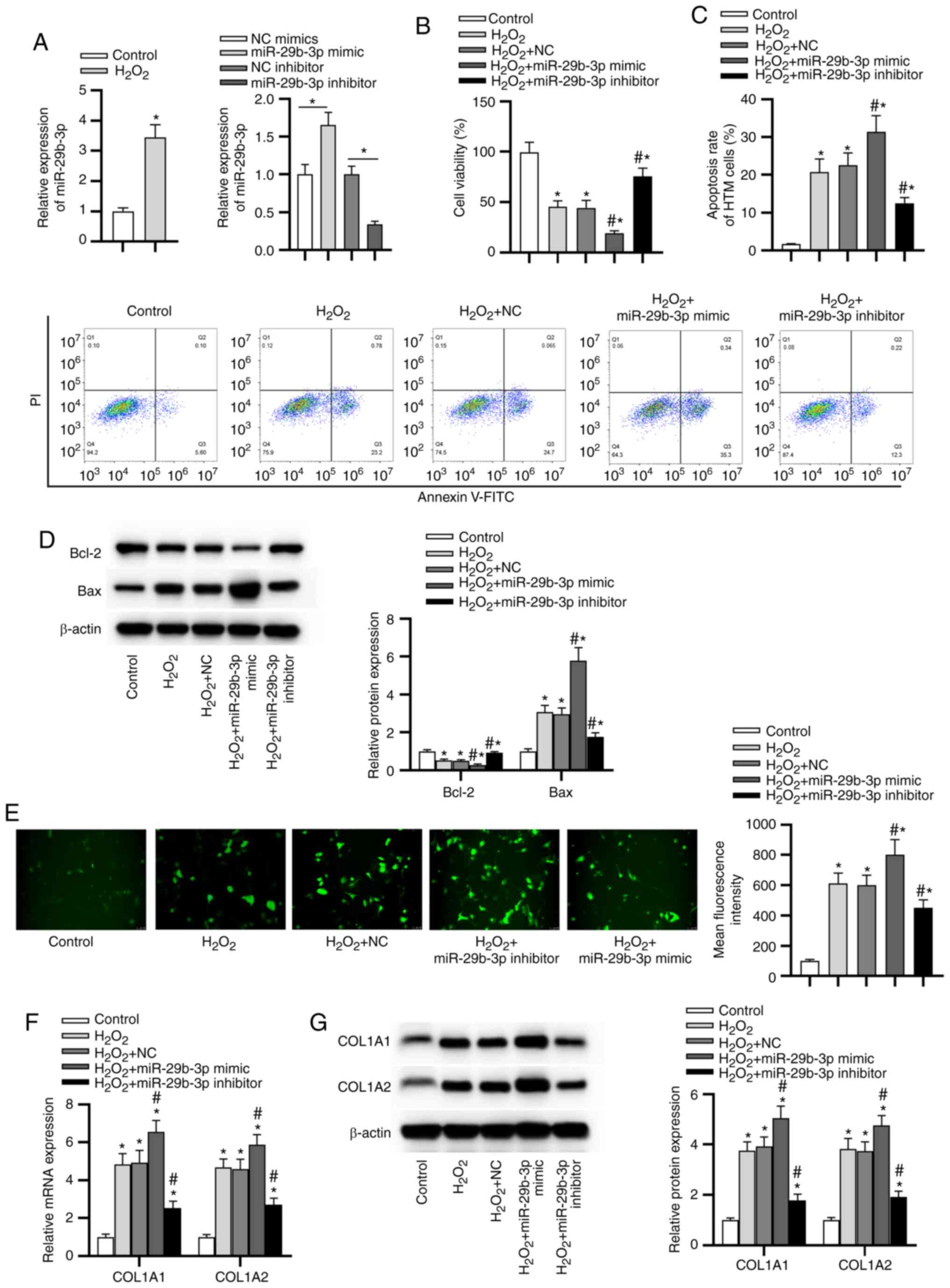 | Figure 3miR-29b-3p facilitates
H2O2-induced injury and promotes ECM
deposition, whereas silencing of miR-29b-3p exerts the opposite
effects. (A) The expression of miR-29b-3p in HTM cells following
different treatments was determined by RT-qPCR. (B) HTM cell
viability in each group was assessed by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
(C) The apoptotic rate of HTM cells in each group was evaluated
using flow cytometry. (D) The levels of apoptosis-related proteins
in HTM cells in each group were measured by western blotting. (E)
Reactive oxygen species generation in HTM cells in each group was
detected by 2,7-dichlorodihydrofluorescein diacetate staining. (F)
The mRNA expression of COL1A1 and COL1A2 in HTM cells was detected
by RT-qPCR. (G) The protein levels of ECM-related genes in HTM
cells were evaluated by western blotting. *P<0.05 vs.
control group; #P<0.05 vs. H2O2
and H2O2 + NC groups. miR, microRNA; HTM,
human trabecular meshwork; RT-qPCR, reverse transcription
quantitative polymerase chain reaction; ECM, extracellular matrix;
NC, negative control; COL1A1, collagen α-1(I) chain; COL1A2,
collagen α-2(I) chain. |
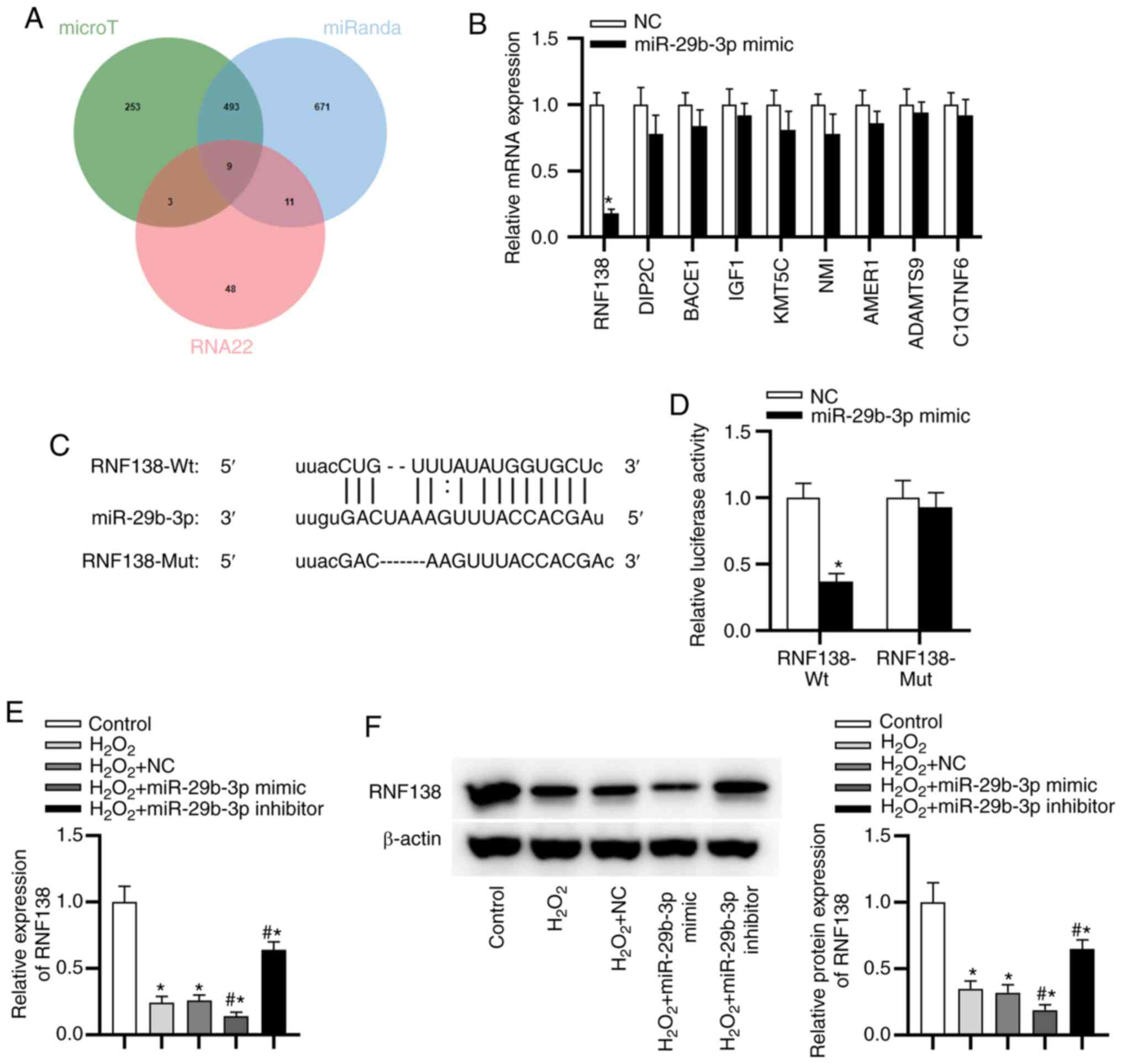
RNF138 is a target gene of
miR-29b-3p
As displayed in Fig.
4A, there were 9 potential targets of miR-29b-3p. RNF138
expression showed the most significant downregulation after
miR-29b-3p overexpression using miR-29b-3p mimics (Fig. 4B). The predicted binding sites
between miR-29b-3p and RNF138 are exhibited in Fig. 4C. To confirm the direct binding
between miR-29b-3p and RNF138, a luciferase reporter assay was
performed. The luciferase activity of RNF138-Wt was reduced by
miR-29b-3p mimic, while that of RNF138-Mut reporters was not
significantly altered in response to the overexpression of
miR-29b-3p (Fig. 4D), indicating
that RNF138 3′ UTR was directly targeted by miR-29b-3p. In
addition, the downregulated expression of RNF138 in HTM cells was
found in the H2O2 group compared with control
group (Fig. 4E). RNF138
expression was decreased in the H2O2 +
miR-29b-3p mimic group and elevated in the
H2O2 + miR-29b-3p inhibitor group (Fig. 4E). Western blotting showed that
miR-29b-3p overexpression decreased the level of RFN138 protein,
whereas miR-29b-3P downregulation had the opposite result (Fig. 4F).
Silencing of RNF138 promotes
H2O2-induced injury and silencing of
miR-29b-3p ameliorates it by upregulating RNF138
The silencing efficiency of RNF138 was confirmed via
RT-qPCR and western blotting (Fig.
5A). Knockdown of RNF138 significantly inhibited viability
(Fig. 5B), promoted apoptosis
(Fig. 5C and D), increased ROS
level (Fig. 5E) and ECM
deposition (Fig. 5F) in HTM
cells under oxidative injury. Moreover, siRNF138 significantly
rescued the effects of miR-29b-3p inhibitor on
H2O2-stimulated HTM cells.
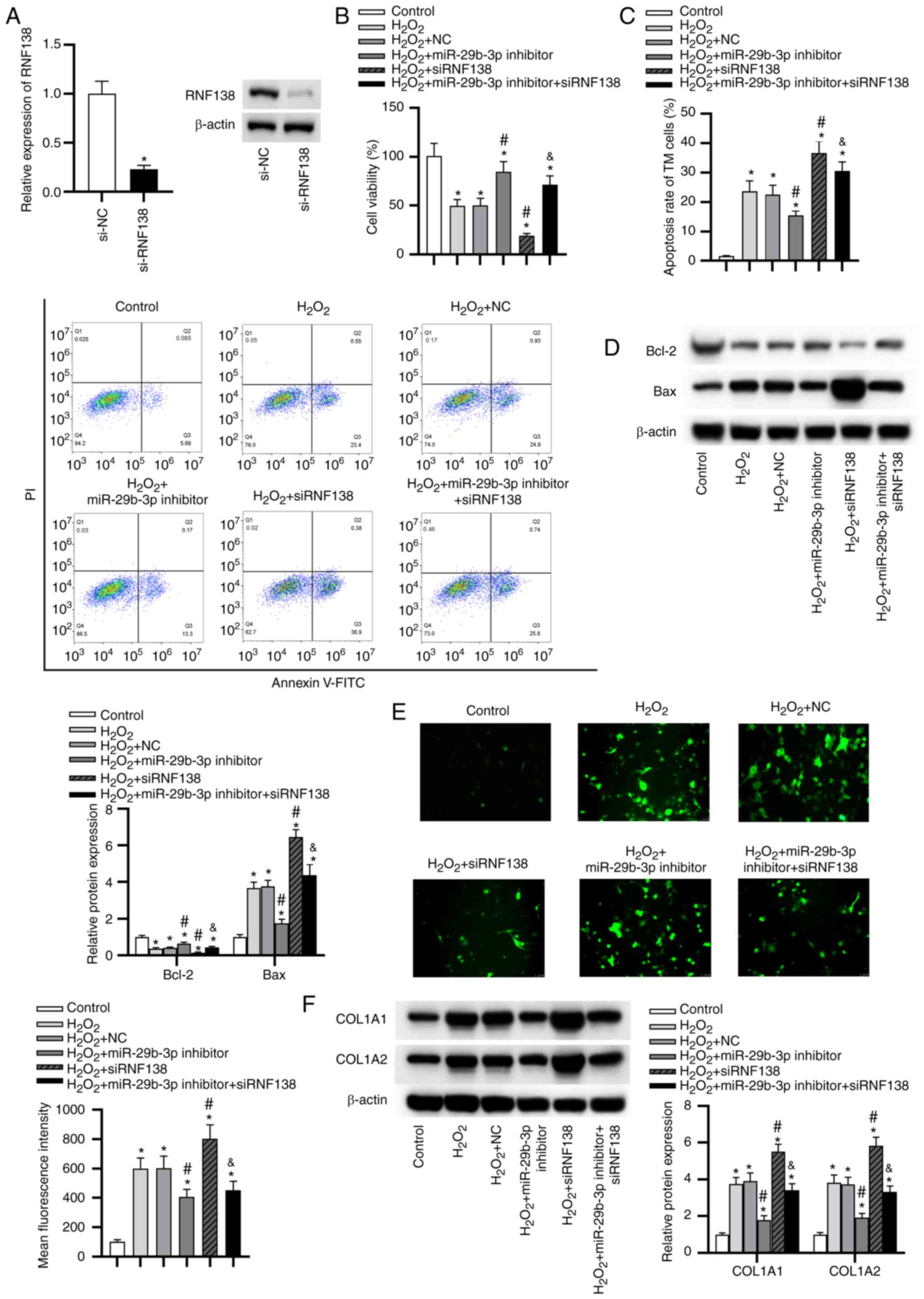 | Figure 5Silencing RNF138 expression promotes
H2O2-induced injury and silencing of
miR-29b-3p ameliorates it by upregulating RNF138. (A) The silencing
efficiency of RNF138 in HTM cells was verified by reverse
transcription quantitative polymerase chain reaction and western
blotting. *P<0.05 vs. si-NC group. (B) HTM cell
viability in each group was assessed by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
assays. (C) The apoptotic rate of HTM cells in each group was
evaluated by flow cytometry analysis. (D) The levels of
apoptosis-related proteins in HTM cells of each group were
determined by western blotting. (E) Reactive oxygen species
generation in HTM cells of each group was detected via
2,7-dichlorodihydrofluorescein diacetate staining. (F) The protein
levels of extracellular matrix-related genes in HTM cells of each
group were evaluated by western blot analysis.
*P<0.05 vs. control group; #P<0.05 vs.
H2O2 and H2O2 + NC
groups; &P<0.05 vs. H2O2 +
miR-29b-3p inhibitor group. RNF138, E3 ubiquitin-protein ligase
RNF138; miR, microRNA; HTM, human trabecular meshwork; si-, small
interfering RNA; NC, negative control; COL1A1, collagen α-1(I)
chain; COL1A2, collagen α-2(I) chain. |
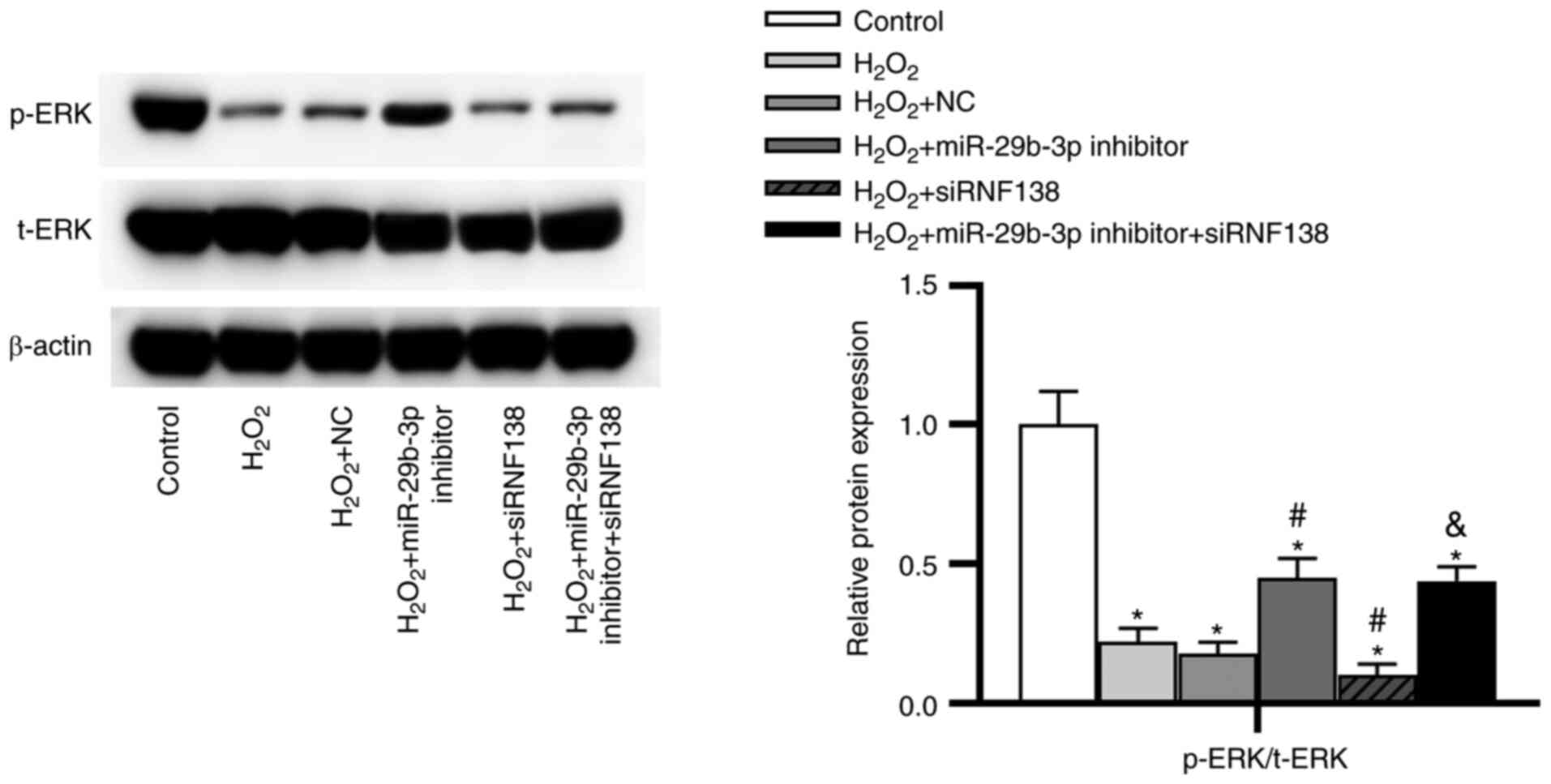
Downregulation of miR-29b-3p activates
the ERK pathway by upregulating RNF138
As presented in Fig.
6, the protein level of p-ERK was lower in the
H2O2 group than in the control group.
Compared with the H2O2 + NC group, the
H2O2 + miR-29b-3p inhibitor group displayed
increased ratio of p-ERK/t-ERK, whereas the
H2O2 + siRNF138 group showed the opposite
results. Moreover, the effect of miR-29b-3p inhibitor on the ERK
pathway was significantly rescued by siRNF138.
Discussion
The loss of vision caused by glaucoma is
irreversible. Multiple miRNAs are related to retinal damage,
retinal homeostasis and retinogenesis (22,23). Previous reports identified a
series of miRNAs that may be potential biomarkers in glaucoma
(24,25). Additionally, oxidative stress is
a key pathophysiological mechanism in glaucoma (26). The high IOP results from the
imbalance of the aqueous humor inflow and outflow, and the
oxidative injury of TM is responsible for aqueous humor outflow
(6,7). Therefore, the present study
explored the role of miR-29b-3p in the oxidative injury of TM
cells. miRNAs act as key regulators of TM cells in the progression
of glaucoma (15,27). The present study found that
miR-29b-3p expression was increased in glaucoma model rats and
antagomiR-29b-3p alleviated the symptoms of glaucoma. Moreover,
miR-29b-3p expression was significantly upregulated in
H2O2-stimulated HTM cells. Downregulation of
miR-29b-3p alleviated H2O2-induced oxidative
injury in HTM cells by promoting cell viability, and inhibiting
cell apoptosis and ROS generation as well as ECM production.
Previous studies have reported that oxidative damage aggravates
glaucoma progression by increasing TM cell apoptosis and ECM
production (28,29). These findings suggested that
inhibition of miR-29b-3p may play a protective role in
glaucoma.
Based on bioinformatics analysis, RNF138 was
predicted as a downstream target of miR-29b-3p. RNF138 serves as an
anti-apoptotic gene in cancers. Upregulation of RNF138 promotes
cell proliferation and inhibits apoptosis in cisplatin-sensitive
gastric cancer cells (30).
Additionally, downregulation of RNF138 induces apoptosis of
spermatogenic cells in mice (31). In the present study, RNF138 was
confirmed to be the functional downstream gene of miR-29b-3p.
RNF138 expression was downregulated in HTM cells with
H2O2 stimulation. Silencing of RNF138
inhibited viability, promoted apoptosis, ROS generation and ECM
production in HTM cells under oxidative injury. In addition, RNF138
knockdown significantly reversed the protective effects of
miR-29b-3p inhibitor on the oxidative injury in HTM cells, which
was consistent with the anti-apoptotic role of RNF138 identified in
previous literatures.
The ERK signaling pathway is an important
intracellular pathway, which has the ability to promote
proliferation in various cell types (32,33). The activation of the ERK pathway
induced by Vitamin D attenuates the
H2O2-stimulated oxidative injury in human
endothelial cells (34). The ERK
pathway activated by myeloid cell leukemia1 protects rat
pheochromocytoma cells from H2O2 oxidant
injury (35). RNF138 silencing
suppresses the development of glioma by repressing the ERK pathway
(36). Therefore, the present
study detected the changes of key proteins in the ERK pathway in
HTM cells stimulated by H2O2. The ERK pathway
was significantly suppressed in HTM cells under
H2O2 stimulation, and this suppression was
rescued by miR-29b-3p suppression. Additionally, RNF138 knockdown
inhibited the activation of ERK pathway induced by miR-29b-3p
knockdown. Therefore, miR-29b-3p regulated the ERK pathway by
targeting RNF138 in HTM cells under oxidative injury.
In conclusion, the present study revealed that
silencing of miR-29b-3p alleviated glaucoma and suppressed
apoptosis, ROS generation, ECM deposition, as well as promoting the
viability of HTM cells under oxidative injury. Mechanistically,
miR-29b-3p was demonstrated to target RNF138 3′UTR and
downregulates its expression to inactive the ERK pathway. The
protective role of silencing of miR-29b-3p in HTM cells may offer a
novel therapeutic strategy for glaucoma.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, YX, QZ and LT performed the experiments. HL, QZ,
QW, YX and LT contributed to data analysis and wrote the paper. HL
and LT made substantial contributions to the design of the present
study and acquired experimental materials. All authors read and
approved the final manuscript. HL and LT confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
All animal studies were performed following the
animal guidelines of the International Association for the Study of
Pain, and approved by the Ethics Committee of the Second Hospital
of Anhui Medical University (approval no. 2019-051; Hefei,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by the Research Fund of Anhui Medical
University (grant no. 2020xkj202).
References
|
1
|
Yanagi M, Kawasaki R, Wang JJ, Wong TY,
Crowston J and Kiuchi Y: Vascular risk factors in glaucoma: A
review. Clin Exp Ophthalmol. 39:252–258. 2011. View Article : Google Scholar
|
|
2
|
Liao Q, Wang DH and Sun HJ: Association of
genetic polymorphisms of eNOS with glaucoma. Mol Vis. 17:153–158.
2011.PubMed/NCBI
|
|
3
|
Williams PA, Harder JM, Foxworth NE,
Cochran KE, Philip VM, Porciatti V, Smithies O and John SW: Vitamin
B3 modulates mitochondrial vulnerability and prevents glaucoma in
aged mice. Science. 355:756–760. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heijl A, Leske MC, Bengtsson B, Hyman L,
Bengtsson B and Hussein M; Early Manifest Glaucoma Trial Group:
Reduction of intraocular pressure and glaucoma progression: Results
from the early manifest glaucoma trial. Arch Ophthalmol.
120:1268–1279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hysi PG, Cheng CY, Springelkamp H,
Macgregor S, Bailey JNC, Wojciechowski R, Vitart V, Nag A, Hewitt
AW, Höhn R, et al: Genome-wide analysis of multi-ancestry cohorts
identifies new loci influencing intraocular pressure and
susceptibility to glaucoma. Nat Genet. 46:1126–1130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villarreal G Jr, Oh DJ, Kang MH and Rhee
DJ: Coordinated regulation of extracellular matrix synthesis by the
microRNA-29 family in the trabecular meshwork. Invest Ophthalmol
Vis Sci. 52:3391–3397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vidal-Sanz M, Salinas-Navarro M,
Nadal-Nicolás FM, Alarcón-Martínez L, Valiente-Soriano FJ, de
Imperial JM, Avilés-Trigueros M, Agudo-Barriuso M and
Villegas-Pérez MP: Understanding glaucomatous damage: Anatomical
and functional data from ocular hypertensive rodent retinas. Prog
Retin Eye Res. 31:1–27. 2012. View Article : Google Scholar
|
|
8
|
Vranka JA, Kelley MJ, Acott TS and Keller
KE: Extracellular matrix in the trabecular meshwork: Intraocular
pressure regulation and dysregulation in glaucoma. Exp Eye Res.
133:112–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medina-Ortiz WE, Belmares R, Neubauer S,
Wordinger RJ and Clark AF: Cellular fibronectin expression in human
trabecular meshwork and induction by transforming growth factor-β2.
Invest Ophthalmol Vis Sci. 54:6779–6788. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schanen BC and Li X: Transcriptional
regulation of mammalian miRNA genes. Genomics. 97:1–6. 2011.
View Article : Google Scholar :
|
|
11
|
Wahid F, Khan T and Kim YY: MicroRNA and
diseases: Therapeutic potential as new generation of drugs.
Biochimie. 104:12–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen K and Rajewsky N: The evolution of
gene regulation by transcription factors and microRNAs. Nat Rev
Genet. 8:93–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su W, Li Z, Jia Y, Zhu Y, Cai W, Wan P,
Zhang Y, Zheng SG and Zhuo Y: MicroRNA-21a-5p/PDCD4 axis regulates
mesenchymal stem cell-induced neuroprotection in acute glaucoma. J
Mol Cell Biol. 9:289–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ruibin W, Zheng X, Chen J, Zhang X, Yang X
and Lin Y: Micro RNA-1298 opposes the effects of chronic oxidative
stress on human trabecular meshwork cells via targeting on EIF4E3.
Biomed Pharmacother. 100:349–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen W, Han Y, Huang B, Qi Y, Xu L, Guo R,
Wang X and Wang J: MicroRNA-483-3p inhibits extracellular matrix
production by targeting Smad4 in human trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 56:8419–8427. 2015. View Article : Google Scholar
|
|
17
|
Yang Q, Wu F, Mi Y, Wang F, Cai K, Yang X,
Zhang R, Liu L, Zhang Y, Wang Y, et al: Aberrant expression of
miR-29b-3p influences heart development and cardiomyocyte
proliferation by targeting NOTCH2. Cell Prolif. 53:e127642020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Zhu M, Ye L, Chen C, She J and
Song Y: MiR-29b-3p promotes particulate matter-induced inflammatory
responses by regulating the C1QTNF6/AMPK pathway. Aging (Albany
NY). 12:1141–1158. 2020. View Article : Google Scholar
|
|
19
|
Zeng Y, Cui Z, Liu J, Chen J and Tang S:
MicroRNA-29b-3p promotes human retinal microvascular endothelial
cell apoptosis via blocking SIRT1 in diabetic retinopathy. Front
Physiol. 10:16212019. View Article : Google Scholar
|
|
20
|
Orlans FB: Ethical decision making about
animal experiments. Ethics Behav. 7:163–171. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Izzotti A, Ceccaroli C, Longobardi MG,
Micale RT, Pulliero A, La Maestra S and Saccà SC: Molecular damage
in glaucoma: From anterior to posterior eye segment. Microrna.
4:3–17. 2015. View Article : Google Scholar
|
|
23
|
Genini S, Guziewicz KE, Beltran WA and
Aguirre GD: Altered miRNA expression in canine retinas during
normal development and in models of retinal degeneration. BMC
Genomics. 15:1722014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hindle A, Thoonen R, Jasien JV, Grange
RMH, Amin K, Wise J, Ozaki M, Ritch R, Malhotra R and Buys ES:
Identification of candidate miRNA biomarkers for glaucoma. Invest
Ophthalmol Vis Sci. 60:134–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Romano G, Platania CB, Forte S, Salomone
S, Drago F and Bucolo C: MicroRNA target prediction in glaucoma.
Prog Brain Res. 220:217–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KY, Perkins GA, Shim MS, Bushong E,
Alcasid N, Ju S, Ellisman MH, Weinreb RN and Ju WK: DRP1 inhibition
rescues retinal ganglion cells and their axons by preserving
mitochondrial integrity in a mouse model of glaucoma. Cell Death
Dis. 6:e18392015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Li F and Wang S: MicroRNA-93 is
overexpressed and induces apoptosis in glaucoma trabecular meshwork
cells. Mol Med Rep. 14:5746–5750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ueda J, Wentz-Hunter K and Yue BY:
Distribution of myocilin and extracellular matrix components in the
juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci.
43:1068–1076. 2002.PubMed/NCBI
|
|
29
|
Fatma N, Kubo E, Toris CB, Stamer WD,
Camras CB and Singh DP: PRDX6 attenuates oxidative stress- and
TGFbeta-induced abnormalities of human trabecular meshwork cells.
Free Radic Res. 43:783–795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu Y, Han D, Liu W, Huang R, Ou J, Chen X,
Zhang X, Wang X, Li S, Wang L, et al: RNF138 confers cisplatin
resistance in gastric cancer cells via activating Chk1 signaling
pathway. Cancer Biol Ther. 19:1128–1138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Lu Y, Han D, Yao R, Wang H, Zhong S,
Luo Y, Han R, Li K, Fu J, et al: Rnf138 deficiency promotes
apoptosis of spermatogonia in juvenile male mice. Cell Death Dis.
8:e27952017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. 89:867–882.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Y, Liu WZ, Liu T, Feng X, Yang N and
Zhou HF: Signaling pathway of MAPK/ERK in cell proliferation,
differentiation, migration, senescence and apoptosis. J Recept
Signal Transduct Res. 35:600–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polidoro L, Properzi G, Marampon F,
Gravina GL, Festuccia C, Di Cesare E, Scarsella L, Ciccarelli C,
Zani BM and Ferri C: Vitamin D protects human endothelial cells
from H2O2 oxidant injury through the
Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res. 6:221–231.
2013. View Article : Google Scholar
|
|
35
|
Li R, Yin F, Guo YY, Zhao KC, Ruan Q and
Qi YM: Knockdown of ANRIL aggravates
H2O2-induced injury in PC-12 cells by
targeting microRNA-125a. Biomed Pharmacother. 92:952–961. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu H, Li X, Feng M, Yao L, Deng Z, Zao G,
Zhou Y, Chen S and Du Z: Downregulation of RNF138 inhibits cellular
proliferation, migration, invasion and EMT in glioma cells via
suppression of the Erk signaling pathway. Oncol Rep. 40:3285–3296.
2018.PubMed/NCBI
|