Colorectal cancer (CRC) remains the third most
commonly diagnosed type of cancer globally, only following breast
and lung cancer. In addition, it is now the second most common
cause of cancer-related mortality following lung cancer (1). Metastatic spread, notably to the
liver, accounts for ~70% of deaths, and ~25% of cases are
metastatic at the time of diagnosis (2,3).
While the majority of primary tumors can be removed, only a small
fraction of those diagnosed with metastatic CRC are eligible for
upfront surgery. The five-year survival rate for patients with
metastatic CRC is dismal at only 12%, compared to 64% for CRC in
general (4). Furthermore, a
considerable proportion of those with localized disease may also
have recurrence as distant metastases (5), due to residual cancer cells that
migrate out of the localized tumor towards distant sites. Arguably,
metastasis is the major cause of mortality among patients with CRC,
and understanding this multi-step process may provide insight on
the steps where therapeutic intervention could be possible.
The journey of metastatic cells from the primary
tumor to distant sites of metastases is an arduous one and requires
the breaking and breaching of barriers before a new site is
colonized. Up until the last decade, a number of the mechanisms
that contribute to metastatic dissemination remained obscure. The
epithelial-to-mesenchymal (EMT) program is now considered key to
understanding invasion and metastasis and explains the physical and
ultrastructural changes cells have to undergo to break away from
the primary tumor and migrate towards a new metastatic niche. More
importantly, it is now recognized that a tumor has to modify its
microenvironment for it to thrive and colonize distant sites
(6).
The discovery of exosomes as purveyors of metastatic
spread helped explain the incompletely understood concept of
metastatic organotropism and the preparation of a pre-metastatic
niche (7). Guided by connexins
and integrins (7,8) and through their cargo of functional
biomolecules, they can instigate an entire program of angiogenesis
and immune suppression to recipient cells in the new site via
horizontal transfer (9), thus
ensuring survival of metastasizing cells. In recent years, it has
become apparent that the role of exosomes is not limited to
organotropism of primary tumor cells. They affect both local and
distant environments and are involved in multiple steps of the
metastatic cascade. Among their multiple cargoes, microRNAs (miRNAs
or miRs) play critical roles in metastatic success. Exosomal miRNAs
(exomiRs), released by both tumor and stromal cells, have been
shown to affect multiple hallmarks of cancer.
In the present review, key processes contributing to
metastatic spread, including EMT, organotropism and the preparation
of the pre-metastatic niche are discussed. Furthermore, a
discussion of exosome biogenesis and cargo sorting is included to
link the initial steps of the metastatic cascade with those of
colonization of distant sites. Lastly, the role of exosomal miRNAs
identified from CRC tumor and stromal cells in the metastatic
continuum is discussed along with insight on the mechanisms through
which they ensure the survival of metastasizing tumors.
EMT is a biological process wherein adherent
epithelial cells convert to a migratory mesenchymal phenotype.
Epithelial cells are characterized by cell-cell and cell-matrix
connections that allow them to function as barriers by forming
organized interconnected sheets of cells. These intercellular
adhesions prevent movement and establish apical, lateral and basal
membrane domains, with cellular components displaying apico-basal
polarity. On the other hand, mesenchymal cells lack intercellular
junctions and therefore have no clear apical and lateral membranes,
no apico-basal polarized distribution of cell components, and are
essentially loose motile cells that are capable of invasion
(10).
While EMT is required for key developmental
processes, such as embryogenesis and organ formation (type 1 EMT),
as well as wound healing and tissue regeneration (type 2 EMT), it
is also known to play a role in tumor dissemination and metastasis
in epithelium-derived carcinomas (type 3 EMT) (11). EMT provides tumor cells with the
ability to dissociate from the primary tumor to ultimately
metastasize to distant organs. In fact, EMT, as evidenced by
significant changes in the levels of EMT-related markers, has been
shown to be associated with a poor prognosis in several types of
cancer, including lung (12),
esophageal (13), pancreatic
(14), breast (15) and CRC (16,17).
The term 'transition' in EMT refers to its transient
and reversible nature, with the converse process termed
mesenchymal-to-epithelial transition (MET). The plasticity of
epithelial cells allows them to transition into mesenchymal cells
then back to an epithelial phenotype, either fully at either end of
the spectrum or only partially, wherein they express both
mesenchymal and epithelial characteristics (18). Core phenotypic changes in EMT
include the loss of intercellular junctions and apical-basal
polarity, cytoskeletal remodeling, the acquisition of cell
protrusions and extracellular matrix degradation. Gene expression
and signaling pathways are also reprogrammed, further contributing
to the EMT process.
Epithelial cells are initially attached to their
neighboring cells through cell-cell adhesion complexes mediated by
E-cadherin (19,20). EMT begins with the dissolution of
epithelial cell-cell contacts essential for basement membrane
integrity, such as tight junctions (at the apical surface of
cells), adherens junctions (basal to the apical tight junctions),
desmosomes (anchored to the intermediate filaments in the
cytoskeleton) and gap junctions (channels between cells for passage
of ions and small molecules), resulting in the downregulation of
their associated proteins, claudins and occludins, cadherins,
desmoplakin and plakophilin and connexins, respectively (21,22). The loss of E-cadherin leads to
the translocation of β-catenin to the nucleus, where it can
contribute to EMT through wingless-type MMTV integration site
family (Wnt) signaling (23,24).
The disintegration of cell-cell junctions is also
accompanied by the disruption of cell polarity complexes,
consequently resulting in epithelial cells losing their
apical-basal polarity and acquiring a front-rear polarity (25). In mammals, three protein
complexes establish apical-basal cell polarity and membrane
identity through their mutually antagonistic interactions (26). Partitioning-defective (PAR)
complex [PAR6, PAR3 and atypical protein kinase C (aPKC)] (27) and Crumbs (CRB) complex [CRB,
protein associated with Lin-7 1 (Pals 1) and Pals 1-associated
tight-junction protein (PATJ)] (28) localize at the apical domain.
Here, the PAR complex, which is required for tight junction
formation (29), is stabilized
by the CRB complex. The Scribble (Scrib) complex [Scrib, Disc large
(dlg) and lethal giant larvae (lgl)] localize at the basolateral
domain, opposite the PAR complex (30). During EMT, inducers such as Zinc
finger E-box-binding homeobox (ZEB) and Zinc finger protein SNAI1
(commonly known as Snail) transcription factors disrupt the
assembly and suppress the expression of these polarity complexes
(26). This is enhanced by the
downregulation of E-cadherin, which prevents the recruitment of
Scrib complexes to the basolateral membrane (31). The overall loss of contact with
the basement membrane reduces epithelial cell adhesion to
eventually facilitate movement.
Cells reorganize their cytoskeletal architecture to
enable migration. Peripheral F-actin fibers are replaced by stress
fibers, and extracellular matrix (ECM) adhesion molecules such as
integrins, paxillin, and focal adhesion kinase localize at the tips
(32-34). Intermediate filaments, such as
cytokeratins are replaced by vimentin, changing the cellular
morphology from cuboidal or columnar to fibroblastic or
spindle-shaped (35,36). The cell membrane projections
[lamellipodia (thin sheet-like region at the leading edge of
migrating cells) and filopodia (spike-like extensions at the edge
of lamellipodia)] permit directional movement of the cell across
its surroundings. Invadopodia, together with the increased
expression of matrix metalloproteinases (MMP-1, -2, -3, -7, -9, -13
and -14), degrade the ECM, allowing the cells to detach from each
other, penetrate the basement membrane, and invade the stroma and
surrounding tissues (25,32,37).
Once mesenchymal cells reach the metastatic site,
they may undergo EMT reversal or MET. Cells revert to an epithelial
state, resulting in secondary tumors that resemble the
histopathological phenotype of the primary tumor (11). MET may occur in the local
environment of the distant organ, due to the absence of heterotypic
signals that induced EMT at the primary tumor site (11). Additionally, tumor cells would
need to induce signaling pathways to resume proliferation during
colonization of the foreign environment (38,39).
EMT is driven by a marked change in transcriptional
programming. The modulation of gene expression during EMT involves
several signal transduction pathways and transcription factors that
reprogram the cell towards a mesenchymal phenotype. The changes in
gene expression are responsible for phenotypic manifestations, such
as the loss of adhesion and polarity, and an increase in migration
and invasiveness.
Several transcription factors that control EMT are
dysregulated or aberrantly expressed in CRC. These include
SNAI1/SNAI2 (Snail and Slug), ZEB1/ZEB2 (40,41) in the ZEB family, twist family
basic helix-loop-helix transcription factors (TWIST) Twist1/Twist2
(42,43) and the forkhead box (FOX) family
(44-46) of transcription factors. SNAIL and
SLUG are responsible for the repression of E-cadherin (47,48). Slug is also required for
Twist1-mediated EMT (48).
Twist1 and Zeb1 are also known to drive the loss of E-cadherin and
a pro-metastatic cellular phenotype of enhanced invasion (49). These transcription factors have
been shown to be associated with cell migration, tumor progression
and metastatic potential in CRC (25,50,51).
Multiple signal transduction pathways have been
implicated as modulators of the EMT transcriptional program.
Transforming growth factor β (TGF-β) is one of the key inducers of
EMT in CRC, as well as other types of cancer. Mothers against
decapentaplegic homolog (SMAD) family member 4 (SMAD4) is a
pro-epithelial factor whose loss leads to heightened EMT through
aberrant signal transducer and activator of transcription 3 (STAT3)
activation and increased signaling via the TGF-β/bone morphogenetic
protein (BMP) pathways (52).
Apart from the SMAD pathways, TGF-β also signals via
SMAD-independent pathways, such as the Ras/mitogen-activated
protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/Akt
pathways (53,54).
The Wnt/β-catenin pathway is another crucial driver
of EMT in CRC. The canonical Wnt pathway leads to SNAIL
upregulation and SLUG stabilization. Consequently, Wnt3a expression
is associated with mesenchymal markers in clinical samples, and Wnt
inhibition can partially reverse EMT expression. In addition, the
non-canonical Wnt pathway has also been implicated in metastatic
CRC (55).
Transcriptional reprogramming during EMT drives the
characteristic switch towards mesenchymal marker expression. The
common markers of EMT are typically proteins involved in the
architecture of cell junctions and the cytoskeleton, controlling
cell morphology and interactions with neighboring cells, as well as
the extracellular environment, key physical features that are
altered in EMT. Thus, the EMT transcriptional program capacitates
the cancer cell for metastatic spread by altering the expression of
key structural proteins to facilitate pro-metastatic
characteristics such as loss of adhesion, cytoskeletal remodeling,
migration and invasion.
One hallmark of EMT is the cadherin switch from
E-cadherin to N-cadherin. The epithelial marker, E-cadherin, is a
cell-surface protein that mediates cell-cell adhesion, and also
binds β-catenin at its cytoplasmic domain. The downregulation of
E-cadherin results in the loss of adhesion, an important phenotype
for cells progressing towards metastasis, and also frees β-catenin
for activation of its signaling cascade. On the other hand,
N-cadherin, which is upregulated in mesenchymal cells, facilitates
dynamic adhesion contacts crucial for migration, and confers an
affinity for other N-cadherin-expressing mesenchymal cells. Thus,
the E-to-N-cadherin switch results in the loss of adhesion and
increased migration (25).
Claudins and occludins are crucial cell-cell
adhesion proteins found in tight junctions, and are subsequently
downregulated in EMT to dissolve epithelial apical-basal polarity
(56). Conversely, the
N-cadherin ligand, neural cell adhesion molecule (NCAM), is
upregulated in EMT, modulating the activity of
N-cadherin-associated receptor tyrosine kinases, such as Fyn to
facilitate focal adhesions (57).
As regards cytoskeletal proteins, EMT is marked by
the downregulation of cytokeratin and the upregulation of vimentin,
which are both components of intermediate filaments (IFs). The
altered composition of IFs facilitates differential trafficking of
organelles and proteins, as well as enhanced cellular motility
(56). Finally, cell-matrix
interactions are also modulated via the altered regulation of
integrins, upregulation of fibronectin, and increased expression
and secretion of matrix metalloproteinases MMP2 and MMP9 (25).
Similar to normal stem cells, CSCs also have the
capacity for self-renewal and differentiation, and can initiate new
tumors, such as in seeding metastatic lesions, or in cancer
recurrence following therapy. CSCs are distinguishable by specific
expression marker profiles, and can apparently arise from the
acquisition of oncogenic alterations in normal stem cells, or from
the dedifferentiation of differentiated cancer cells (58). In fact, pro-stemness factors are
often proto-oncogenic molecules that are dysregulated in cancer.
Thus, CSCs are often viewed as an important component of emerging
therapeutic resistance and subsequent progression and metastasis,
as CSCs that escape treatment are able to expand and initiate new
lesions at a later stage.
EMT has also been implicated in the emergence and
maintenance of CSCs. Cancer cells that have undergone EMT often
exhibit a CSC phenotype, and conversely, CSCs often express EMT
markers, signifying a mesenchymal shift. The close association
between EMT and CSCs underscores the important role of both in the
overall process of metastatic spread. TGF-β/Smad signaling is a key
link, as it modulates both EMT and stemness phenotypes (59).
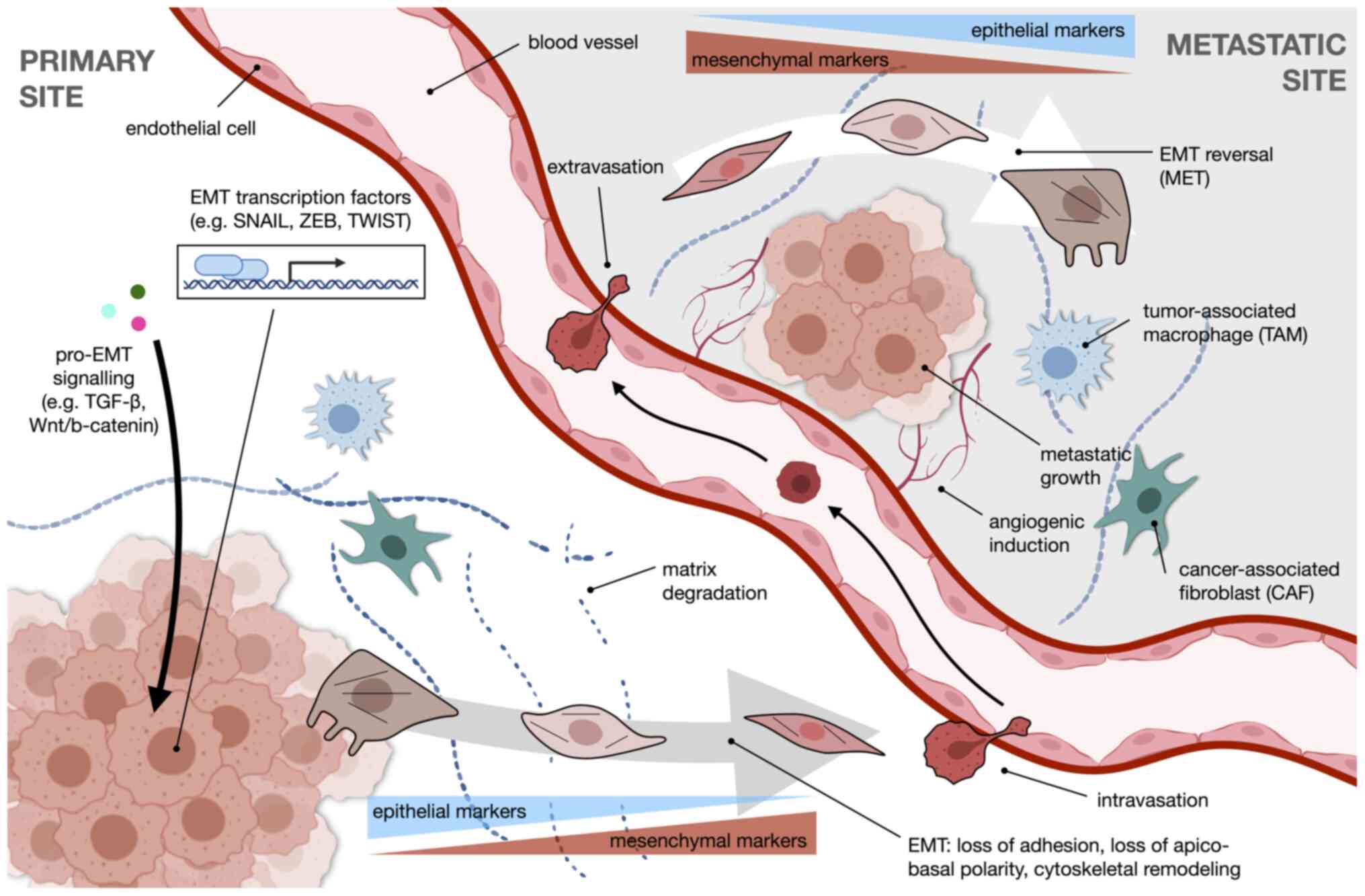
A summary of the overall process of EMT in the
context of metastasis is illustrated in Fig. 1. Pro-EMT signaling, such as
through the TGF-β or Wnt/β-catenin pathways, drives transcriptional
reprogramming facilitated by EMT transcription factors which
include SNAIL, ZEB and TWIST. The cancer cell undergoes changes in
morphology towards a more motile mesenchymal phenotype, accompanied
by a switch in expression from epithelial to mesenchymal markers.
With enhanced ECM degradation, the cell is free to intravasate into
the circulation and extravasate at a distant metastatic site. Once
there, the cell reverts to an epithelial-like phenotype. Angiogenic
induction and the action of stromal cells, such as tumor-associated
macrophages (TAMs) or cancer-associated fibroblasts (CAFs) further
modify the niche to favor metastatic growth.
Tumors are known to have a predisposition to
metastasize to specific organs. This phenomenon, however, remained
poorly understood for almost a century. The intrinsic properties of
cancer cells, including genes and pathways implicated in the
colonization of new metastatic niches, were invoked and constituted
the predominant explanation for the phenomenon known as metastatic
organotropism (60-62). Indeed, EMT itself has been shown
to mediate metastatic colonization and metastatic organotropism
both directly and indirectly. In pancreatic ductal adenocarcinoma
(PDAC) models, the loss of p120ctn, binding partner and stabilizer
of E-cadherin at adherens junctions, has been shown to cause a
shift in the metastatic load from the liver to the lung.
Furthermore, this could be reversed by the transfection of PDAC
cells with p120ctn isoform 1A (63). EMT has also been shown to affect
organ-specific metastasis by influencing metabolic reprogramming
(64,65) and by regulating the tumor immune
microenvironment (66). Whether
these can be extrapolated to other epithelial tumors, such as CRC
warrants further investigation.
While events that render the pre-metastatic niche
favorable for dissemination and the growth of tumor cells upon
arrival were being characterized, among these being angiogenesis
and immunosuppression (67-69), the question of what dictates
metastasis to specific organs remained unanswered. The discovery
that exosomal integrins in tumor-released exosomes directed
organ-specific colonization helped explain the induction of the
pre-metastatic niche (7).
Together with integrins, connexins, proteins that function as an
integral component of channels at gap junctions, were found to be
embedded in exosomal membranes (70). Furthermore, resident stromal
cells in metastatic target organs have been shown to internalize
these exosomes and their cargo, further influencing expression of
genes implicated in the preparation of the pre-metastatic niche
(7).
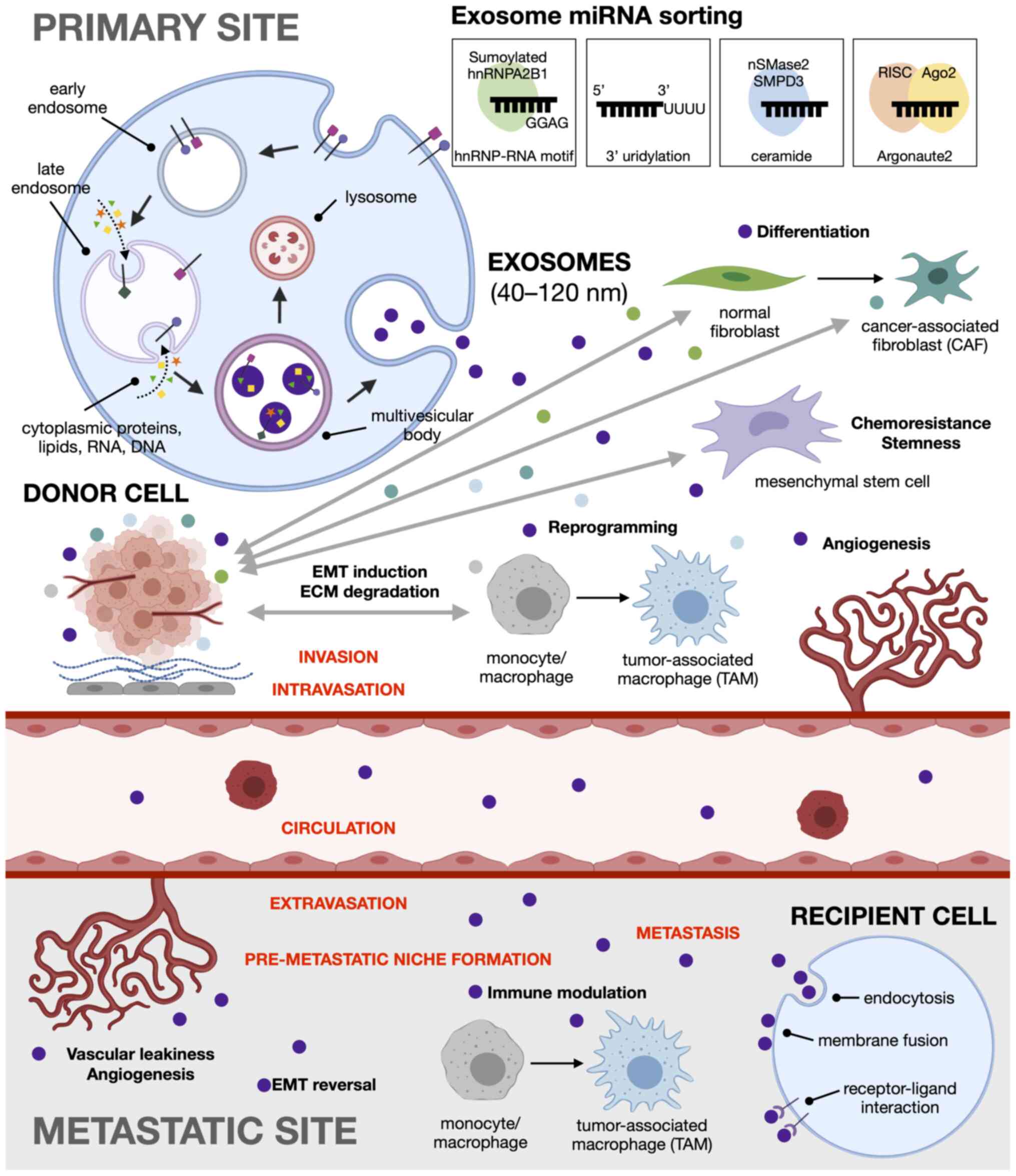
Exosomes are spherical membrane-enclosed
nanovesicles secreted by all types of living cells into the
extracellular milieu, notably in abundance by tumor cells. They
have been detected in a range of biological fluids, including
blood, urine, saliva, breast milk and pleural effusions (71-73), with a substantial amount of up to
1012 exosomes/ml of body fluid (~0.1% volume) in
physiological conditions (74).
Exosomes are important mediators of intercellular communications,
capable of transferring functional genetic cargo to modify
neighboring and distant recipient cells (75-78).
Exosomes can be differentiated from other
extracellular vesicles based on their mode of biogenesis:
microvesicles (100 to 1,000 nm) originate from the cell surface and
are formed through the direct outward budding of the plasma
membrane (79), while apoptotic
bodies (800 to 5,000 nm) are generated from the outward blebbing
and fragmentation of the cell membrane as cells undergo apoptosis
(80). Exosomes (40-120 nm in
diameter) are initially formed as intraluminal vesicles (ILVs)
within the endosomal system. In this pathway, the invagination of
early endosomes during maturation sequesters cytoplasmic genetic
material, such as RNA, DNA, lipids and proteins into ILVs. The late
endosomes, now referred to as multivesicular bodies (MVBs), are
either targeted to the lysosomes for degradation, serve as
temporary storage compartments, or fuse with the plasma membrane,
releasing ILVs into the extracellular space as exosomes (81-83).
The formation of intraluminal vesicles occurs via
multiple mechanisms. The most well-described pathway is dependent
on the endosomal sorting complex required for transport (ESCRT)
budding machinery, where ESCRT-0 recognizes and forms domains of
ubiquitylated proteins, which are later confined when the endosomal
membrane is deformed by the ESCRT-I and ESCRT-II complexes.
ESCRT-III then cleaves the bud neck to form intraluminal vesicles
(84). On the other hand, the
syndecan-syntenin-ALG-2-interacting protein X (ALIX) pathway
recognizes any material bound to the heparan sulfate of syndecan.
Syndecans are transmembrane proteins whose cytosolic domain can
bind to and recruit syntenin, a soluble cytoplasmic protein, to the
plasma membrane. Syntenin also binds to ALIX, an accessory protein
to the ESCRT-III, in turn linking the syndecans to the ESCRT
complexes for ILV formation (85,86). Alternatively, membrane budding
can occur through an ESCRT-independent pathway involving the
sphingolipid ceramide. Ceramide induces the coalescence of lipid
microdomains found in the endosomal membrane, and its cone-shaped
structure causes spontaneous negative curvature between the
membrane leaflets, resulting in the inward budding of endosomes to
produce ILVs (87). A recent
study also reported another ESCRT-independent pathway using Rab31,
which prevents the fusion of the MVB to the lysosome to instead
promote ILV secretion (88). In
these pathways, Rab guanosine triphosphatases (Rab GTPases) mediate
the fusion of MVBs with the plasma membrane. Rab27a and Rab27b
influence the localization (89), whereas Rab35 regulates the
docking and tethering (90) of
the MVBs at the plasma membrane for subsequent secretion of the
intraluminal vesicles into the extracellular fluid as exosomes.
The major cargo residing within exosomes are
proteins, lipids, RNA and a minimal amount of DNA. The contents of
exosomes are highly heterogeneous, depending on the tissue, cell
type and physiological or pathological context. Notably, exosome
profiles do not fully reflect that of parent cells, signifying a
selective mechanism for sorting cellular genetic material into
exosomes (9,91,92). There is also evidence to indicate
that some RNA transcripts can be found exclusively in exosomes and
not in the cells of origin (9,92) which suggests that certain genes
are destined for export and communication with other cells
(93).
Deep sequencing has revealed a variety of exosomal
RNA cargo, including mRNAs, miRNAs, long non-coding RNAs (lncRNAs),
ribosomal RNAs, transfer RNAs, piwi-interacting RNAs, small nuclear
RNAs, small nucleolar RNAs and even circular RNAs, each present at
varying levels of abundance (74,91,94-96). Among these, miRNAs are generally
the most predominant RNAs encapsulated in exosomes (91,96). Several mechanisms regulating the
selective export of miRNAs into exosomes have been proposed, from
intrinsic sequences present in the 3′ end of miRNAs to cellular
lipids and proteins that guide their incorporation into exosomes.
First, heterogeneous nuclear ribonucleoproteins (hnRNPs) directly
bind to 4-bp sequence motifs in the 3′ end of miRNA sequences to
package them into exosomes. Sumoylated hnRNP A2/B1 can recognize
the RNA motif GGAG in miR-198 and miR-601 (97), while hnRNP-Q can recognize the
GGCU motif in miR-3470a, miR-194-2-3p, miR-6981-5p, miR-690 and
miR-365-2-5p (98). Other
members of the hnRNP family, such as hnRNPA1 and hnRNPC (97) and miRNAs enriched with the GUUG
motif (99) were also sorted
into exosomes, although their cognate sequence motifs and RNA
binding proteins, respectively, have not yet been identified.
Second, 3′ end post-transcriptional modifications can also
influence cargo loading. The RNA sequencing of B cells and urine
samples has revealed that adenylated miRNAs are retained in the
cells, while those that are uridylated are preferentially sorted
into exosomes (100). Third,
membrane lipids involved in EV biogenesis also control the loading
of miRNAs into exosomes, indicative of the ceramide-dependent
secretory pathway independent of the ESCRT machinery (101). The overexpression of neural
sphingomyelinase 2 (nSMase2), which regulates ceramide
biosynthesis, has been shown to increase the quantity of miRNA
exported into exosomes, whereas nSMase2 inhibition reduces the
quantity of exosomal miRNAs (102). Similarly, the inhibition of
sphingomyelin phosphodiesterase 3 (SMPD3), which catalyzes the
hydrolysis of sphingomyelin to ceramide, has been shown to result
in a concentration-dependent increase in cellular miR-638 levels
and a decrease in exosomal miR-638 levels, using the SW480 human
colorectal and HuH-7 human hepatocellular cancer cell line
(103). In patients with CRC,
the expression levels of miR-638 have also been found to be
downregulated in serum exosomes and to be associated with a poor
overall and disease-free survival (104). Fourth, Argonaute 2 (Ago2), a
key component of the RNA-induced silencing complex (RISC) appears
to play a role in exosomal miRNA secretion. The knockdown of Ago2
has been shown to decrease the expression of miRNAs (miR-451,
miR-150 and miR-142-3p) that are most commonly exported from
different cell types (105). In
this regard, KRAS mutations have also been implicated in miRNA
export, negatively regulating the secretion of miRNAs into
exosomes, since downstream MEK/ERK signaling inhibits the
association of Ago2 with multivesicular bodies (106). Notably, all essential RISC
components, such as Dicer, transactivation response RNA-binding
protein (TRBP) and Ago2 are present in cancer-derived exosomes and
this enables processing of pre-miRNAs into mature miRNAs within the
nanovesicles, unlike exosomes derived from normal cells (107).
Apart from cell state, exosome secretion is
influenced by several factors, such as cellular stress, heat,
ischemia, pH, the loss of cellular attachment and the accumulation
of intracellular calcium (108). Of note, low pH levels and
hypoxia are common features of solid tumors. The low pH of the
tumor microenvironment results in increased exosome release and
subsequent uptake by recipient cells, which explains the abundance
and effects of exosomes in cancer (109). Moreover, hypoxia increases the
release of exosomes, which can potentially facilitate angiogenesis
and metastasis, further sustaining the growth and survival of
cancer cells (110).
Exosome internalization by recipient cells
highlights the critical role of exosomes in cell-to-cell crosstalk,
with the unique advantage of targeting specific locations compared
to cytokines and hormones in the systemic circulation. Exosomes can
transfer genetic information to neighboring or distant cells
through three principal mechanisms: i) Direct fusion of the
exosomal lipid membrane with the cellular membrane of recipient
cells, releasing the exosome cargo into the cytosol (111); ii) proteins on the exosomal
membrane serve as ligands for receptors on the surface of recipient
cells; and iii) endocytosis either mediated by the clathrin
protein, which is associated with engulfment of partner receptors,
or micropinocytosis, which is associated with membrane ruffles that
are induced by receptor tyrosine kinases (112). The specificity of these
receptor-ligand interactions suggests that exosomes are targeted to
particular cells (7).
Exosomes were once considered to serve only as a
means of cellular waste disposal when they were first described for
removal of plasma membrane proteins during reticulocyte maturation
(81,113). Later on, Raposo et al
(114) isolated exosomes from B
lymphocytes and demonstrated their involvement in antigen
presentation, capable of inducing an immune response. Exosomal
messenger RNAs were then found to be internalized and translated
into functional proteins (9,92), while exosomal miRNAs and lncRNAs
can regulate the translation of target mRNAs (75,107,115,116) in recipient cells, concretely
establishing a role in intercellular communication. This links
exosomes to several biological processes as well as disease
pathogenesis.
In the context of cancer, cumulative evidence
suggests that exosomes can promote tumorigenesis through the
horizontal transfer of oncogenic material to recipient cells.
Likewise, cancer cells can utilize exosomes to discard
tumor-suppressive genetic material not beneficial for tumor growth
so as to increase their own oncogenicity (117). For instance, in CRC, miR-100 is
a tumor suppressor that inhibits cellular migration and invasion by
targeting Leucine-rich repeat-containing G protein-coupled receptor
5 (Lgr5) (118). Mutant KRAS
CRC cells have been reported to secrete exosomes enriched in
miR-100 as a strategy to sustain low intracellular levels (117). Tumor-derived exosomes have been
shown to induce (119) or
suppress (120) the immune
response, promote the formation of a pre-metastatic tumor niche
(7,121), regulate angiogenesis (122), enhance migration (76) and cell proliferation (77), and induce epithelial to
mesenchymal transition (123),
among others (Fig. 2).
Exosomes can also promote tumor resistance by
encapsulating drugs and their metabolites into exosomes for export,
as a drug efflux mechanism (124). Similarly, drug-resistant cancer
cells can induce chemoresistance in other cancer cells through
exosome-mediated transfer of efflux transporters (78).
Various miRNAs can inhibit EMT progression by
directly targeting components of the EMT regulatory pathways. CRC
exosomes may be enriched with oncogenic miRNAs that downregulate
EMT inhibitors. Alternatively, tumor suppressive miRNAs that
downregulate inducers of EMT may themselves be downregulated or
disposed of in CRC exosomes. Exosomal cargo can both originate from
and be delivered to either tumor cells or cells in the tumor
microenvironment, enhancing the capacity for metastasis by both
driving EMT in tumor cells and influencing the properties of the
microenvironment. Moreover, miRNAs carried by serum exosomes can be
delivered to sites distant from the originating tumor, further
extending metastatic potential. The promotion of EMT by altered
regulation of exosomal miRNAs results in expression of
characteristic mesenchymal markers and enhanced phenotypic features
of pro-metastatic cells.
CAFs are essential components of the TME, with roles
in matrix deposition and remodeling (125). Up to 80% of stromal fibroblasts
in a tumor are believed to acquire an activated phenotype and
become CAFs (126). These cells
can modulate tumor progression by releasing cytokines such as
TGF-β, tumor necrosis factor α (TNF-α), and interleukin (IL)-6 and
IL-8 (127). Tumor cells and
CAFs in the TME maintain extensive reciprocal signaling
interactions (125). Recently,
primary CRC cells, as well as HCT116 cells were shown to release
exosomes containing miR-10b which are taken up by surrounding
fibroblasts. miR-10b was found to increase TGF-β and α-smooth
muscle actin (α-SMA) through the inhibition of PI3K activity, which
is indicative of fibroblast activation into pro-tumorigenic CAFs
(127). In a similar manner,
CAFs have been shown to deliver exosomal miRNA to tumor cells. In
patients with CRC, CAF-derived exosomes have been found to be
enriched with miR-92a, which is delivered to CRC cells (128). The uptake of exosomal miR-92a
enhances EMT and metastatic capability by downregulating F-Box and
WD repeat domain containing 7 (FBXW7) and modulator of apoptosis 1
(MOAP1), and by activating the Wnt/β-catenin pathway (128). Of note, cellular miR-92a has
also been characterized as an oncogenic miRNA promoting migration,
F-actin remodeling and EMT marker expression in CRC cells via the
downregulation of neurofibromin (NF2)/Merlin, a tumor suppressor
gene that controls contact-dependent inhibition, adhesion and
migration (129). Other miR-92a
targets include Dickkopf-3 (Dkk-3) and claudin-11, resulting in
enhanced angiogenesis and disruption of tight junctions,
respectively (130). In
patients with CRC, the loss of claudin-11 has been shown to be
associated with increased metastasis and a poor prognosis (131). In the CRC microenvironment,
CAFs were previously shown to deliver miR-21 to cancer cells in
exosomes. miR-21 was identified as the most abundant and most
enriched miRNA in an exosomal cancer-associated fibroblast
signature that also includes miRNAs 329, 181a, 199b, 382 and 215.
Orthotopic xenografts derived from fibroblasts overexpressing
miR-21 exhibited an increased liver metastases relative to the
controls (132).
The exosomal cargo of CAFs in CRC may also include
the lncRNA H19. H19 can attenuate the inhibitory effects of miR-141
and can then activate the Wnt/β-catenin pathway to promote stemness
(133). Thus, exosomal delivery
of miRNAs and/or lncRNAs from CAFs may drive EMT through redundant
mechanisms. This is in addition to other forms of stromal-tumor
crosstalk that may exist in parallel, notably the secretion of
CAF-derived soluble factors such as TGF-β and juxtacrine signaling
(134,135). Increasing evidence suggests
that CAFs are heterogenous and may assume different functional
roles (136,137). It is highly likely that
different groups of CAFs are involved in exosomal miRNA exchange
with tumor cells.
In patients with metastatic CRC, serum exosomal
miR-106b-3p has been found to be upregulated and correlated with
metastatic progression (138).
Cellular and exosomal miR-106b-3p has been found to enhance
migration and invasion, and drive pro-EMT expression, as well as
promote lung metastasis in a mouse xenograft model, by targeting
the tumor suppressor, deleted in liver cancer 1 (DLC-1) (138). DLC-1 is a GTPase activating
protein (GAP), thus a negative regulator, for Rho GTPases, which
regulate actin remodeling and thus cellular motility (139).
Exosomal miR-106b-3p from highly metastatic CRC
cells also has profound effects on cell adhesion. They are released
to less metastatic CRC cells and are then able to upregulate
N-cadherin and downregulate E-cadherin protein expression. This is
also achieved via the downregulation of DLC-1, effectively
promoting metastasis in situ via coordinated effects on both
cell adhesion and cytoskeletal rearrangement (138). Exosomes harboring miR-210 are
also secreted by adherent HCT-8 colon cancer cells and cause them
to detach and grow in suspension, indicative of metastatic capacity
and anoikis resistance. Furthermore, these detached cells have been
shown to be E-cadherin-negative and vimentin-positive, in contrast
to adherent colonies that are E-cadherin-positive and
vimentin-negative (123).
Exosomal miR-1246 in CRC, on the other hand, has
been linked to the degradation of the ECM. Gain-of-function
mutations in the p53 gene found in CRC cells have been shown to
increase miR-1246 levels in exosomes, which are in turn able to
reprogram macrophages into TAMs, the major component of
tumor-infiltrating immune cells (140-142). The TAM phenotype is a classic
phenotype of solid tumors with poor prognosis, and is characterized
by heightened stimulation of ECM degradation, as well as enhanced
migratory and invasive capacities (142).
Tumor-derived exosomal miRNAs may promote EMT
reversal or MET by suppressing effectors, inducers, transcription
factors, and other players involved in EMT. An increased expression
of miR-200c and miR-141 in exosomes is indicative of MET in CRC
cells. In a previous study, upon treatment with the drug
decitabine, SW480 (primary CRC) cells did not exhibit any
significant differences, while the metastatic cell lines SW620
(derived from lymph node metastasis) and SW620/OxR (derived from
lymph node metastasis with acquired resistance to oxaliplatin)
exhibited decreased migration and invasion properties. This was
accompanied by the upregulation of E-cadherin and exosomal miR-200c
and miR-141, which together suggest the acquisition of epithelial
characteristics through the reversal of EMT (143).
CRC-derived exosomal miR-200c, miR-141 and miR-429
can also inhibit EMT by directly targeting the ZEB family in
endothelial cells. In a previous study, in 3D spheroid models,
co-culture with naïve CRC cells did not demonstrate disruption of
lymphatic (exomiR-200c) (144)
and blood (exomiR-200c, -141 and -429) (145) endothelial cell layers. By
contrast, co-culture with 5-fluorouracil-resistant CRC cells which
release exosomes without these miRNAs, resulted in enhanced
disruption of endothelial cell layers. This suggests that exosomal
miR-200c, -141 and -429 contribute to maintaining epithelial
barrier integrity and prevents EMT, which explains the increased
metastasis in chemoresistant CRC (144,145).
Another tumor suppressor exosomal miRNA is
miR-1255b-5p, which has been found to be an EMT inhibitory miRNA
downregulated in serum exosomes of CRC patients (146). CRC-derived exosomal
miR-1255b-5p, which downregulates human telomerase reverse
transcriptase (hTERT) and inhibits Wnt/β-catenin signaling, has
been found to be downregulated under hypoxic conditions, providing
a link between hypoxic regulation, telomere maintenance and EMT
(146).
CSC and EMT plasticity can also be modulated by
miRNA action, involving the deregulation of key tumor suppressor
miRNAs, such as miR-200c, miR-203 and miR-183 repressed by
TGF-β/Zeb1 (147); and the
let-7 downregulation of high mobility group A2 (HMGA2), implicated
in TGF-β/Smad/SNAIL, SLUG transcriptional activation (148).
The downregulation of tumor suppressive exosomal
miRNAs, as well as disposal via cargo sorting are also resorted to
by CRC cells to promote invasion and metastasis. The tumor
suppressive miR-149 and miR-96-5p are both downregulated in
exosomes of CRC cells, while expression levels of glypican-1
(GPC1), its direct in vitro and in vivo target, and
which induces EMT and promotes invasion, is increased (131).
The tumor suppressive miR-486-5p is downregulated in
CRC tissues, in part because of promoter hypermethylation.
miR-486-5p is a negative regulator of pleiomorphic adenoma
gene-like 2 (PLAGL2), a transcription factor for β-catenin and
insulin-like growth factor 2 (IGF2) with roles in promoting
proliferation, cell survival and metastasis, as well as decreasing
E-cadherin and increasing N-cadherin expression. Interestingly,
miR-486-5p was found to be particularly enriched in plasma exosomes
(154), suggesting that
preferential exomiR cargo loading may be at play to evade its tumor
suppressive effects.
Similar observations have been reported for the
tumor suppressive miR-8073 and miR-193a. While miR-8073 has
invariant expression intracellularly and in exosomes of normal
colorectal cells, it is preferentially sorted into exosomes at up
to 60 times the concentration found inside CRC cells. Thus, its
oncogenic mRNA targets are effectively de-repressed. These include,
among others, FOXM1, which is involved in cancer growth and
metastasis, as well as Methyl-CpG-binding domain protein 3 (MBD3)
which is known to induce pluripotent stem cells (155). In metastatic colon cancer in
the liver, tumor cells selectively sort miR-193a out of cells via
exosomes. Thus, its direct target inside the cell, Caprin1, is
de-repressed and leads to G1 cell cycle arrest, and consequently,
cell proliferation. Likewise, miR-193a has been found to be
enriched in the exosomal fraction of serum from patients with CRC,
particularly in advanced stages of the disease with higher risks of
metastasis (156).
The journey of metastatic cells continues way after
they have detached from the primary tumor, remodeled their
cytoskeletal architecture, and breached tissue boundaries. The
remaining steps of the metastatic cascade are fraught with further
hurdles that include intravasation into the circulation,
extravasation into the secondary site, and preparation of the
pre-metastatic niche prior to colonization. The latter entails
forming new blood vessels as well as immune-proofing of the new
microenvironment.
Tumor growth and metastasis depend on blood vessels
for the supply of oxygen and nutrients, for the removal of waste
products, and as routes for cancer cells to be able to migrate to a
different site (157). However,
to stimulate new vessel growth, there must be a balance between
activators and inhibitors of angiogenesis (158). Tumor-derived exosomes can
shuttle such cargo, including miRNAs which can target
anti-angiogenic and pro-angiogenic genes, between cancer cells and
endothelial cells (159).
Among the pro-angiogenic exosomal miRNAs in CRC are
miR-25-3p, miR-92a, miR-1229, miR-183-5p and miR-1246. CRC cells
are able to transfer the metastasis-promoting miR-25-3p to
endothelial cells via exosome transfer. By targeting Kruppel-like
factor (KLF)2, vascular endothelial growth factor (VEGF) receptor 2
(VEGFR2) expression is upregulated, promoting angiogenesis
(160). Targeting KLF4, on the
other hand, regulates endothelial integrity through the activation
of tight junction proteins, such as claudin, occludin and zonula
occludens-1 (ZO-1) (161). CRC
exosome-mediated transfer of miR-25-3p is thus able to induce
vascular leakiness and can promote pre-metastatic niche formation
in secondary sites, such as the liver and lungs. miR-25-3p has also
been shown to be enriched in the circulating exosomes of CRC
patients with metastasis when compared to those from patients with
non-metastatic CRC (162).
Exosomal miR-92a-3p facilitates tumor angiogenesis
by inducing partial EMT in endothelial cells (130). Exosomes derived from colon
cancer cells and from plasma derived from murine xenograft models
which were enriched with miR-92a-3p have been found to stimulate
tube formation in human umbilical vein endothelial cells (HUVECs)
upon transfer. miR-92a-3p promotes angiogenesis through the
downregulation of Dkk-3 and claudin-11 (130,163).
CRC-derived exosomal miR-1229 promotes tube
formation in HUVECs through the inhibition of
homeodomain-interacting protein kinase 2 (HIPK2) and the subsequent
activation of the VEGF pathway. In patients with CRC, serum
exosomes harbor increased levels of miR-1229 which correlate with
tumor size, lymphatic metastasis, 'tumor, nodes, metastases' (TNM)
stage and poor survival (164).
The upregulation of miR-183-5p in CRC cell-derived exosomes has
been found to enhance angiogenesis by the repression of FOXO1
(165). A pro-angiogenic role
has also been demonstrated for miR-1246, which has been found to be
contained in microvesicles secreted by CRC cells, and activates
Smad1/5/8 signaling via the direct targeting of promyelocytic
leukemia (PML) mRNA (166).
Anti-angiogenic exosomal miRNAs in CRC include
miR-126, miR-125a-3p and miR-125a-5p. miR-126 has been reported to
target VEGF, an activator of angiogenesis (167) as well as its negative
regulators, such as Sprouty-related, EVH1 domain-containing protein
1 (SPRED1) (168,169). However, in vitro studies
on CRC have yielded contradicting results, wherein both the
overexpression (170) and
silencing (171) of miR-126
have been shown to lead to neo-vessel formation. Nonetheless,
patients with metastatic CRC exhibit low miR-126 expression levels
which are associated with poor survival, confirming the tumor
suppressive role of miR-126 in CRC (167). Increased levels of
extracellular miR-126 in the plasma of patients with metastatic CRC
could also be a predictive biomarker for resistance to
anti-angiogenic treatment using bevacizumab, a monoclonal anti-VEGF
antibody (172).
miR-125a-3p targets fucosyltransferase (FUT)5 and
FUT6, which regulate the PI3K/Akt signaling pathway. The
overexpression of miR-125a-3p has been shown to result in the
downregulation of FUT5 and FUT6, subsequently inhibiting the
proliferation, migration, invasion and angiogenesis of CRC cells
(173). The significant
upregulation of miR-125a-3p levels in plasma exosomes is being
considered as a useful biomarker for the detection of early-stage
colon cancer (174).
miR-125a-5p is a known tumor suppressor in CRC,
since it directly targets: i) VEGFA, resulting in reduced tube
formation in HUVECs and a suppressed cell proliferation, migration
and invasion in CRC (175); ii)
Tafazzin (TAZ), a key transducer of the Hippo tumor-suppressor
pathway, resulting in inhibited migration, invasion and EMT
(176); and iii) B-cell
lymphoma 2 (Bcl-2), Bcl-2-like protein 12 (BCL2L12) and myeloid
cell leukemia 1 (Mcl-1), resulting in the inhibition of cell
proliferation and the promotion of apoptosis (177) in colon cancer cells. However,
as a biomarker, miR-125a-5p is barely detectable in plasma exosomes
due to its low expression in CRC tissues (174).
Tumor-derived exosomes act as intercellular
messengers between cancer cells and immune cells to either activate
or inhibit immune response and/or escape recognition by the immune
system. In tumors, an immunosuppressive microenvironment is mainly
induced by inflammation (178).
CRC-derived exosomes harboring miR-203 have been shown to
differentiate monocytes into TAMs following internalization
(121). It has been
hypothesized that tumor-derived exosomal miR-203 targets suppressor
of cytokine signaling 3 (SOCS3) (179), which is crucial for the
activation of M1 macrophages characterized by a pro-inflammatory
phenotype. Consequently, the loss of SOCS3 expression in the
macrophages results in their anti-inflammatory M2 TAM
characteristics (180). In line
with this finding, circulating miR-203 has been found to promote
liver metastasis in murine xenograft models, suggesting their role
in hepatic pre-metastatic niche formation. In patients with CRC, a
high miR-203 expression in serum exosomes and a low miR-203
expression in tumor tissues has been shown to be associated with
metastasis and a poor prognosis (121).
CRC cell-derived exosomal miR-934 has been found to
induce M2 polarization in macrophages, enabling the induced TAMs to
promote liver metastasis via secretion of the chemokine C-X-C motif
chemokine ligand 13 (CXCL13) to remodel the premetastatic niche
(181). Exosomal miR-25,
miR-130b and miR-425 have been similarly implicated in TAM
polarization and C-X-C motif chemokine 12 (CXCL12)/C-X-C motif
chemokine receptor 4 (CXCR4)-dependent liver metastasis (182). On the other hand, CRC
cell-derived exosomes have been found to diminish the migration of
THP-1 monocytes in vitro via the delivery of let-7d, which
can downregulate the chemokine C-C motif chemokine ligand 7 (CCL7)
(183), suggesting that
exosomal miRNAs can aid immune evasion by interfering with immune
cell chemotaxis. A list of exosomal miRNAs that have been
implicated in different steps of the metastatic cascade is
presented in Table I.
Much has been achieved in terms of identifying the
morphological and structural changes that accompany EMT, the
signaling pathways involved, and the transcriptional reprogramming
that has to take place to bring about these changes. Investigations
into the contributions of the tumor microenvironment to cancer
progression has provided further insight on the mechanisms through
which tumor cells can modify stromal cells, and vice versa, to
break free from the primary site en route to its metastatic
destination. More importantly, the discovery of exosomes and their
cargo was pivotal in elucidating the mechanisms of tumor-stroma
crosstalk, regulation of genes involved in metastatic
dissemination, metastatic organotropism, and preparation of the
pre-metastatic niche. The functional elucidation of individual
exomiRs, in particular, clarified the mechanisms through which
invading and metastasizing cells are able to breach barriers and
overcome sheer stress in the circulation to enable metastasis to
distant sites.
Exosomes are an ideal source of disease biomarkers
since they contain genetic material representative of the parental
tumor (92,140) and the lipid bilayer membrane
protects exosome cargo from nuclease degradation and unfavorable
storage conditions (184,185). Cancer cells also secrete
significantly more exosomes than normal cells (186), resulting in the enrichment of
cancer-derived exosomes in all types of biological fluids, making
them easy to obtain in a non-invasive or minimally invasive manner.
For instance, serum exosome levels of seven miRNAs (let-7a,
miR-1229, miR-1246, miR-150, miR-21, miR-223 and miR-23a) have been
found to be increased in patients with CRC compared with the
healthy controls, and the expression levels significantly decreased
following the surgical resection of tumors (140). Exosomal miR-19a has also been
identified as a serum biomarker for recurrence of CRC. Compared
with healthy individuals, circulating exosomal miR-19a was more
abundant in patients with CRC, regardless of stage of the disease,
and was associated with a poorer patient prognosis (187). Additionally, tumor-derived
exosomes may be useful biomarkers to predict future sites of organ
metastasis. Integrins on the exosome surface have been reported to
bind to specific cell types to prepare them as pre-metastatic
niches (7).
Given their role in disease pathogenesis, exosomes
can serve as therapeutic targets, either by inhibiting exosome
formation, release, and uptake or by targeting bioactive cargo that
can contribute to tumor metastasis. Exosomes can also serve as
therapeutic agents, as unlike common drug delivery vehicles, such
as liposomes and polymer nanoparticles, exosomes have minimal
immunogenicity and toxicity and can be modified with synthetic
peptides to carry small molecule drugs for targeting specific cells
and tissues. Furthermore, unlike monoclonal antibodies (mAbs),
which are used as targeted drug delivery vehicles, smaller
iterations of therapeutic mAbs, such as fragment antibodies, domain
antibodies and nanobodies can themselves be part of the exosomal
cargo that can be internalized by recipient cells. Lastly, the
exomiRs implicated in metastasis can themselves be potential
targets for antagomiRs loaded onto exosomes.
Not applicable.
All three authors (JMCD, AGGU and RLG) contributed
equally in organizing and writing the present review article. JMCD,
AGGU and RLG confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
The figures presented herein were created with
BioRender. com on a student plan premium license.
The present study was funded by grants from the Philippine
Council for Health Research and Development (grant no. FP150025),
the University of the Philippines System (OVPAA-EIDR code 06-008),
the University of the Philippines Diliman Office of the Vice
Chancellor for Research and Development (grant project no. 181809
PNSE), and the National Institute of Molecular Biology and
Biotechnology, University of the Philippines Diliman (in-house
grant).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Augestad KM, Bakaki PM, Rose J, Crawshaw
BP, Lindsetmo RO, Dørum LM, Koroukian SM and Delaney CP: Metastatic
spread pattern after curative colorectal cancer surgery. A
retrospective, longitudinal analysis. Cancer Epidemiol. 39:734–744.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Cutsem E, Cervantes A and Nordlinger
B: Metastatic colorectal cancer: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 25(Suppl 3):
iii1–iii9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nozawa H, Kawai K, Hata K, Tanaka T,
Nishikawa T, Otani K, Sasaki K, Kaneko M, Emoto S and Murono K:
High-risk stage II colorectal cancers carry an equivalent risk of
peritoneal recurrence to stage III. In Vivo. 32:1235–1240. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutchinson L: Understanding metastasis.
Nat Rev Clin Oncol. 12:2472015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soares AR, Martins-Marques T,
Ribeiro-Rodrigues T, Ferreira JV, Catarino S, Pinho MJ, Zuzarte M,
Isabel Anjo S, Manadas BPG, Sluijter J, et al: Gap junctional
protein Cx43 is involved in the communication between extracellular
vesicles and mammalian cells. Sci Rep. 5:132432015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fristrom D: The cellular basis of
epithelial morphogenesis. A review Tissue Cell. 20:645–690. 1988.
View Article : Google Scholar
|
|
11
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soltermann A, Tischler V, Arbogast S,
Braun J, Probst-Hensch N, Weder W, Moch H and Kristiansen G:
Prognostic significance of epithelial-mesenchymal and
mesenchymal-epithelial transition protein expression in non-small
cell lung cancer. Clin Cancer Res. 14:7430–7437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Chen L, Deng H, Xu B, Li M, Zheng
X, Wu C and Jiang J: Epithelial-to-mesenchymal transition in human
esophageal cancer associates with tumor progression and patient's
survival. Int J Clin Exp Pathol. 7:6943–6949. 2014.PubMed/NCBI
|
|
14
|
Handra-Luca A, Hong SM, Walter K, Wolfgang
C, Hruban R and Goggins M: Tumour epithelial vimentin expression
and outcome of pancreatic ductal adenocarcinomas. Br J Cancer.
104:1296–1302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
DiMeo TA, Anderson K, Phadke P, Feng C,
Perou CM, Naber S and Kuperwasser C: A novel lung metastasis
signature links Wnt signaling with cancer cell self-renewal and
epithelial-mesenchymal transition in basal-like breast cancer.
Cancer Res. 69:5364–5373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shioiri M, Shida T, Koda K, Oda K, Seike
K, Nishimura M, Takano S and Miyazaki M: Slug expression is an
independent prognostic parameter for poor survival in colorectal
carcinoma patients. Br J Cancer. 94:1816–1822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spaderna S, Schmalhofer O, Hlubek F, Berx
G, Eger A, Merkel S, Jung A, Kirchner T and Brabletz T: A
transient, EMT-Linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshida-Noro C, Suzuki N and Takeichi M:
Molecular nature of the calcium-dependent cell-cell adhesion system
in mouse teratocarcinoma and embryonic cells studied with a
monoclonal antibody. Dev Biol. 101:19–27. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frixen UH, Behrens J, Sachs M, Eberle G,
Voss B, Warda A, Löchner D and Birchmeier W: E-cadherin-mediated
cell-cell adhesion prevents invasiveness of human carcinoma cells.
J Cell Biol. 113:173–185. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci. 116(Pt 10):
1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vandewalle C, Comijn J, De Craene B,
Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F and
Berx G: SIP1/ZEB2 induces EMT by repressing genes of different
epithelial cell-cell junctions. Nucleic Acids Res. 33:6566–6578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maretzky T, Reiss K, Ludwig A, Buchholz J,
Scholz F, Proksch E, de Strooper B, Hartmann D and Saftig P: ADAM10
mediates E-cadherin shedding and regulates epithelial cell-cell
adhesion, migration, and -catenin translocation. Proc Natl Acad Sci
USA. 102:9182–9187. 2005. View Article : Google Scholar
|
|
24
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a0029152010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moreno-Bueno G, Portillo F and Cano A:
Transcriptional regulation of cell polarity in EMT and cancer.
Oncogene. 27:6958–6969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki A, Yamanaka T, Hirose T, Manabe N,
Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T and Ohno S:
Atypical protein kinase C is involved in the evolutionarily
conserved par protein complex and plays a critical role in
establishing epithelia-specific junctional structures. J Cell Biol.
152:1183–1196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roh MH, Makarova O, Liu CJ, Shin K, Lee S,
Laurinec S, Goyal M, Wiggins R and Margolis B: The Maguk protein,
Pals1, functions as an adapter, linking mammalian homologues of
Crumbs and Discs Lost. J Cell Biol. 157:161–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X and Macara IG: Par-3 controls tight
junction assembly through the Rac exchange factor Tiam1. Nat Cell
Biol. 7:262–269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bilder D, Li M and Perrimon N: Cooperative
regulation of cell polarity and growth by drosophila tumor
suppressors. Science. 289:113–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Navarro C, Nola S, Audebert S, Santoni MJ,
Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le
Bivic A, et al: Junctional recruitment of mammalian Scribble relies
on E-cadherin engagement. Oncogene. 24:4330–4339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haynes J, Srivastava J, Madson N, Wittmann
T and Barber DL: Dynamic actin remodeling during
epithelial-mesenchymal transition depends on increased moesin
expression. Mol Biol Cell. 22:4750–4764. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Webb DJ, Donais K, Whitmore LA, Thomas SM,
Turner CE, Parsons JT and Horwitz AF: FAK-Src signalling through
paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell
Biol. 6:154–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pagan R, Martı́n I, Alonso A, Llobera M
and Vilaró S: Vimentin filaments follow the preexisting cytokeratin
network during epithelial-mesenchymal transition of cultured
neonatal rat hepatocytes. Exp Cell Res. 222:333–344. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen WT: Proteolytic activity of
specialized surface protrusions formed at rosette contact sites of
transformed cells. J Exp Zool. 251:167–185. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang GJ, Zhou T, Tian HP, Liu ZL and Xia
SS: High expression of ZEB1 correlates with liver metastasis and
poor prognosis in colorectal cancer. Oncol Lett. 5:564–568. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kahlert C, Lahes S, Radhakrishnan P, Dutta
S, Mogler C, Herpel E, Brand K, Steinert G, Schneider M,
Mollenhauer M, et al: Overexpression of ZEB2 at the invasion front
of colorectal cancer is an independent prognostic marker and
regulates tumor invasion in vitro. Clin Cancer Res. 17:7654–7663.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gomez I, Peña C, Herrera M, Muñoz C,
Larriba MJ, Garcia V, Dominguez G, Silva J, Rodriguez R, Garcia de
Herreros A, et al: TWIST1 is expressed in colorectal carcinomas and
predicts patient survival. PLoS One. 6:e180232011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu H, Jin GZ, Liu K, Dong H, Yu H, Duan
JC, Li Z, Dong W, Cong WM and Yang JH: Twist2 is a valuable
prognostic biomarker for colorectal cancer. World J Gastroenterol.
19:2404–2411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y,
Li D and Cai S: Overexpression of forkhead Box C2 promotes tumor
metastasis and indicates poor prognosis in colon cancer via
regulating epithelial-mesenchymal transition. Am J Cancer Res.
5:2022–2034. 2015.PubMed/NCBI
|
|
45
|
Weng W, Okugawa Y, Toden S, Toiyama Y,
Kusunoki M and Goel A: FOXM1 and FOXQ1 are promising prognostic
biomarkers and novel targets of tumor-suppressive miR-342 in human
colorectal cancer. Clin Cancer Res. 22:4947–4957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li D, Li Q, Zhuo C, Xu Y and Cai S:
Contribution of FOXC1 to the progression and metastasis and
prognosis of human colon cancer. J Clin Oncol. 33(Suppl 3):
S6362015. View Article : Google Scholar
|
|
47
|
Peinado H, Ballestar E, Esteller M and
Cano A: Snail Mediates E-cadherin repression by the recruitment of
the Sin3A/Histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell
Biol. 24:306–319. 2004. View Article : Google Scholar :
|
|
48
|
Bolos V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116(Pt 3): 499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Caramel J, Papadogeorgakis E, Hill L,
Browne GJ, Richard G, Wierinckx A, Saldanha G, Osborne J,
Hutchinson P, Tse G, et al: A switch in the expression of embryonic
EMT-inducers drives the development of malignant melanoma. Cancer
Cell. 24:466–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vu T and Datta P: Regulation of EMT in
colorectal cancer: A culprit in metastasis. Cancers (Basel).
9:1712017. View Article : Google Scholar
|
|
51
|
Stemmler MP, Eccles RL, Brabletz S and
Brabletz T: Non-redundant functions of EMT transcription factors.
Nat Cell Biol. 21:102–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao S, Venkatasubbarao K, Lazor JW,
Sperry J, Jin C, Cao L and Freeman JW: Inhibition of STAT3 Tyr705
Phosphorylation by Smad4 suppresses transforming growth factor
beta-mediated invasion and metastasis in pancreatic cancer cells.
Cancer Res. 68:4221–4228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zavadil J, Bitzer M, Liang D, Yang YC,
Massimi A, Kneitz S, Piek E and Bottinger EP: Genetic programs of
epithelial cell plasticity directed by transforming growth
factor-beta. Proc Natl Acad Sci USA. 98:6686–6691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wilkes MC, Mitchell H, Penheiter SG, Doré
JJ, Suzuki K, Edens M, Sharma DK, Pagano RE and Leof EB:
Transforming growth factor-beta activation of phosphatidylinositol
3-Kinase is independent of Smad2 and Smad3 and regulates fibroblast
responses via p21-Activated Kinase-2. Cancer Res. 65:10431–10440.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A Noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang RY, Guilford P and Thiery JP: Early
events in cell adhesion and polarity during epithelial-mesenchymal
transition. J Cell Sci. 125(Pt 19): pp. 4417–4422. 2012, View Article : Google Scholar
|
|
57
|
Lehembre F, Yilmaz M, Wicki A, Schomber T,
Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, et
al: NCAM-induced focal adhesion assembly: A functional switch upon
loss of E-cadherin. EMBO J. 27:2603–2615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, et al: Molecular definition of
breast tumor heterogeneity. Cancer Cell. 11:259–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cox TR, Rumney RMH, Schoof EM, Perryman L,
Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al:
The hypoxic cancer secretome induces pre-metastatic bone lesions
through lysyl oxidase. Nature. 522:106–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reichert M, Bakir B, Moreira L, Pitarresi
JR, Feldmann K, Simon L, Suzuki K, Maddipati R, Rhim AD, Schlitter
AM, et al: Regulation of epithelial plasticity determines
metastatic organotropism in pancreatic cancer. Dev Cell.
45:696–711.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shaul YD, Freinkman E, Comb WC, Cantor JR,
Tam WL, Thiru P, Kim D, Kanarek N, Pacold ME, Chen WW, et al:
Dihydropyrimidine accumulation is required for the
epithelial-mesenchymal transition. Cell. 158:1094–1109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kim NH, Cha YH, Lee J, Lee SH, Yang JH,
Yun JS, Cho ES, Zhang X, Nam M, Kim N, et al: Snail reprograms
glucose metabolism by repressing phosphofructokinase PFKP allowing
cancer cell survival under metabolic stress. Nat Commun.
8:143742017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-Induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kaplan RN, Riba RD, Zacharoulis S, Bramley
AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et
al: VEGFR1-positive haematopoietic bone marrow progenitors initiate
the pre-metastatic niche. Nature. 438:820–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hiratsuka S, Nakamura K, Iwai S, Murakami
M, Itoh T, Kijima H, Shipley JM, Senior RM and Shibuya M: MMP9
induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shimaoka M, Kawamoto E, Gaowa A, Okamoto T
and Park E: Connexins and integrins in exosomes. Cancers.
11:1062019. View Article : Google Scholar :
|
|
71
|
Liu T, Zhang Q, Zhang J, Li C, Miao YR,
Lei Q, Li Q and Guo AY: EVmiRNA: A database of miRNA profiling in
extracellular vesicles. Nucleic Acids Res. 47(D1): D89–D93. 2019.
View Article : Google Scholar :
|
|
72
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Simpson RJ, Jensen SS and Lim JWE:
Proteomic profiling of exosomes: Current perspectives. Proteomics.
8:4083–4099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Li M, Zeringer E, Barta T, Schageman J,
Cheng A and Vlassov AV: Analysis of the RNA content of the exosomes
derived from blood serum and urine and its potential as biomarkers.
Philos Trans R Soc Lond B Biol Sci. 369:201305022014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Baroni S, Romero-Cordoba S, Plantamura I,
Dugo M, D'Ippolito E, Cataldo A, Cosentino G, Angeloni V, Rossini
A, Daidone MG and Iorio MV: Exosome-mediated delivery of miR-9
induces cancer-associated fibroblast-like properties in human
breast fibroblasts. Cell Death Dis. 7:e23122016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Demory Beckler M, Higginbotham JN,
Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M,
Liebler DC and Coffey RJ: Proteomic analysis of exosomes from
mutant KRAS colon cancer cells identifies intercellular transfer of
mutant KRAS. Mol Cell Proteomics. 12:343–355. 2013. View Article : Google Scholar :
|
|
77
|
Lucchetti D, Calapà F, Palmieri V, Fanali
C, Carbone F, Papa A, De Maria R, De Spirito M and Sgambato A:
Differentiation affects the release of exosomes from colon cancer
cells and their ability to modulate the behavior of recipient
cells. Am J Pathol. 187:1633–1647. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lv MM, Zhu XY, Chen WX, Zhong SL, Hu Q, Ma
TF, Zhang J, Chen L, Tang JH and Zhao JH: Exosomes mediate drug
resistance transfer in MCF-7 breast cancer cells and a probable
mechanism is delivery of P-glycoprotein. Tumor Biol.
35:10773–10779. 2014. View Article : Google Scholar
|
|
79
|
Heijnen HF, Schiel AE, Fijnheer R, Geuze
HJ and Sixma JJ: Activated platelets release two types of membrane
vesicles: Microvesicles by surface shedding and exosomes derived
from exocytosis of multivesicular bodies and alpha-granules. Blood.
94:3791–3799. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Crescitelli R, Lässer C, Szabó TG, Kittel
A, Eldh M, Dianzani I, Buzás EI and Lötvall J: Distinct RNA
profiles in subpopulations of extracellular vesicles: Apoptotic
bodies, microvesicles and exosomes. J Extracell Vesicles.
2:206772013. View Article : Google Scholar
|
|
81
|
Pan BT, Teng K, Wu C, Adam M and Johnstone
RM: Electron microscopic evidence for externalization of the
transferrin receptor in vesicular form in sheep reticulocytes. J
Cell Biol. 101:942–948. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Théry C, Zitvogel L and Amigorena S:
Exosomes: Composition, biogenesis and function. Nat Rev Immunol.
2:569–579. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
83
|
Stoorvogel W, Kleijmeer MJ, Geuze HJ and
Raposo G: The biogenesis and functions of exosomes. Traffic.
3:321–330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wollert T and Hurley JH: Molecular
mechanism of multivesicular body biogenesis by ESCRT complexes.
Nature. 464:864–869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Baietti MF, Zhang Z, Mortier E, Melchior
A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C,
Vermeiren E, et al: Syndecan-syntenin-ALIX regulates the biogenesis
of exosomes. Nat Cell Biol. 14:677–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Roucourt B, Meeussen S, Bao J, Zimmermann
P and David G: Heparanase activates the syndecan-syntenin-ALIX
exosome pathway. Cell Res. 25:412–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Trajkovic K, Hsu C, Chiantia S, Rajendran
L, Wenzel D, Wieland F, Schwille P, Brügger B and Simons M:
Ceramide triggers budding of exosome vesicles into multivesicular
endosomes. Science. 319:1244–1247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wei D, Zhan W, Gao Y, Huang L, Gong R,
Wang W, Zhang R, Wu Y, Gao S and Kang T: RAB31 marks and controls
an ESCRT-independent exosome pathway. Cell Res. 31:157–177. 2021.
View Article : Google Scholar :
|
|
89
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et
al: Rab27a and Rab27b control different steps of the exosome
secretion pathway. Nat Cell Biol. 12(19-30): 1–13. 2010. View Article : Google Scholar
|
|
90
|
Hsu C, Morohashi Y, Yoshimura S,
Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M,
Möbius W, Rhee J, et al: Regulation of exosome secretion by Rab35
and its GTPase-activating proteins TBC1D10A-C. J Cell Biol.
189:223–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huang X, Yuan T, Tschannen M, Sun Z, Jacob
H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, et al:
Characterization of human plasma-derived exosomal RNAs by deep
sequencing. BMC Genomics. 14:3192013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Skog J, Würdinger T, van Rijn S, Meijer
DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky
AM and Breakefield XO: Glioblastoma microvesicles transport RNA and
proteins that promote tumour growth and provide diagnostic
biomarkers. Nat Cell Biol. 10:1470–1476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Pérez-Boza J, Lion M and Struman I:
Exploring the RNA landscape of endothelial exosomes. RNA.
24:423–435. 2018. View Article : Google Scholar :
|
|
94
|
Amorim MG, Valieris R, Drummond RD, Pizzi
MP, Freitas VM, Sinigaglia-Coimbra R, Calin GA, Pasqualini R, Arap
W, Silva IT, et al: A total transcriptome profiling method for
plasma-derived extracellular vesicles: Applications for liquid
biopsies. Sci Rep. 7:143952017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Jenjaroenpun P, Kremenska Y, Nair VM,
Kremenskoy M, Joseph B and Kurochkin IV: Characterization of RNA in
exosomes secreted by human breast cancer cell lines using
next-generation sequencing. PeerJ. 1:e2012013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yuan T, Huang X, Woodcock M, Du M, Dittmar
R, Wang Y, Tsai S, Kohli M, Boardman L, Patel T and Wang L: Plasma
extracellular RNA profiles in healthy and cancer patients. Sci Rep.
6:194132016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Villarroya-Beltri C, Gutiérrez-Vázquez C,
Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N,
Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M and
Sánchez-Madrid F: Sumoylated hnRNPA2B1 controls the sorting of
miRNAs into exosomes through binding to specific motifs. Nat
Commun. 4:29802013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Santangelo L, Giurato G, Cicchini C,
Montaldo C, Mancone C, Tarallo R, Battistelli C, Alonzi T, Weisz A
and Tripodi M: The RNA-Binding Protein SYNCRIP is a component of
the hepatocyte exosomal machinery controlling MicroRNA sorting.
Cell Rep. 17:799–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gao T, Shu J and Cui J: A systematic
approach to RNA-associated motif discovery. BMC Genomics.
19:1462018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Koppers-Lalic D, Hackenberg M, Bijnsdor p
IV, van Eijndhoven MAJ, Sadek P, Sie D, Zini N, Middeldorp JM,
Ylstra B, de Menezes RX, et al: Nontemplated Nucleotide additions
distinguish the small RNA composition in cells from exosomes. Cell
Rep. 8:1649–1658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kosaka N, Iguchi H, Yoshioka Y, Takeshita
F, Matsuki Y and Ochiya T: Secretory mechanisms and intercellular
transfer of MicroRNAs in living cells. J Biol Chem.
285:17442–17452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kosaka N, Iguchi H, Hagiwara K, Yoshioka
Y, Takeshita F and Ochiya T: Neutral Sphingomyelinase 2
(nSMase2)-dependent exosomal transfer of angiogenic MicroRNAs
regulate cancer cell metastasis. J Biol Chem. 288:10849–10859.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kubota S, Chiba M, Watanabe M, Sakamoto M
and Watanabe N: Secretion of small/microRNAs including miR-638 into
extracellular spaces by sphingomyelin phosphodiesterase 3. Oncol
Rep. 33:67–73. 2015. View Article : Google Scholar
|
|
104
|
Yan S, Dang G, Zhang X, Jin C, Qin L, Wang
Y, Shi M, Huang H and Duan Q: Downregulation of circulating
exosomal miR-638 predicts poor prognosis in colon cancer patients.
Oncotarget. 8:72220–72226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Guduric-Fuchs J, O'Connor A, Camp B,
O'Neill CL, Medina RJ and Simpson DA: Selective extracellular
vesicle-mediated export of an overlapping set of microRNAs from
multiple cell types. BMC Genomics. 13:3572012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
McKenzie AJ, Hoshino D, Hong NH, Cha DJ,
Franklin JL, Coffey RJ, Patton JG and Weaver AM: KRAS-MEK signaling
controls Ago2 sorting into exosomes. Cell Rep. 15:978–987. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Melo SA, Sugimoto H, O'Connell JT, Kato N,
Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, et
al: Cancer exosomes perform cell-independent MicroRNA biogenesis
and promote tumorigenesis. Cancer Cell. 26:707–721. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Savina A, Furlán M, Vidal M and Colombo
MI: Exosome release is regulated by a calcium-dependent mechanism
in K562 cells. J Biol Chem. 278:20083–20090. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Parolini I, Federici C, Raggi C, Lugini L,
Palleschi S, De Milito A, Coscia C, Iessi E, Logozzi M, Molinari A,
et al: Microenvironmental pH is a key factor for exosome traffic in
tumor cells. J Biol Chem. 284:34211–34222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Park JE, Tan HS, Datta A, Lai RC, Zhang H,
Meng W, Lim SK and Sze SK: Hypoxic tumor cell modulates its
microenvironment to enhance angiogenic and metastatic potential by
secretion of proteins and exosomes. Mol Cell Proteomics.
9:1085–1099. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Fitzner D, Schnaars M, van Rossum D,
Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK and Simons
M: Selective transfer of exosomes from oligodendrocytes to
microglia by macropinocytosis. J Cell Sci. 124(Pt 3): 447–458.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Tian T, Zhu YL, Zhou YY, Liang GF, Wang
YY, Hu FH and Xiao ZD: Exosome uptake through clathrin-mediated
endocytosis and macropinocytosis and mediating miR-21 delivery. J
Biol Chem. 289:22258–22267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Harding C, Heuser J and Stahl P:
Receptor-mediated endocytosis of transferrin and recycling of the
transferrin receptor in rat reticulocytes. J Cell Biol. 97:329–339.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chiba M, Kimura M and Asari S: Exosomes
secreted from human colorectal cancer cell lines contain mRNAs,
microRNAs and natural antisense RNAs, that can transfer into the
human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep.
28:1551–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Takahashi K, Yan IK, Kogure T, Haga H and
Patel T: Extracellular vesicle-mediated transfer of long non-coding
RNA ROR modulates chemosensitivity in human hepatocellular cancer.
FEBS Open Bio. 4:458–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Cha DJ, Franklin JL, Dou Y, Liu Q,
Higginbotham JN, Demory Beckler M, Weaver AM, Vickers K, Prasad N,
Levy S, et al: KRAS-dependent sorting of miRNA to exosomes. Elife.
4:e071972015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhou MK, Liu XJ, Zhao ZG and Cheng YM:
MicroRNA-100 functions as a tumor suppressor by inhibiting Lgr5
expression in colon cancer cells. Mol Med Rep. 11:2947–2952. 2015.
View Article : Google Scholar
|
|
119
|
Gastpar R, Gehrmann M, Bausero MA, Asea A,
Gross C, Schroeder JA and Multhoff G: Heat shock protein 70
surface-positive tumor exosomes stimulate migratory and cytolytic
activity of natural killer cells. Cancer Res. 65:5238–5247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Huber V, Fais S, Iero M, Lugini L, Canese
P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, et al:
Human colorectal cancer cells induce T-Cell death through release
of proapoptotic microvesicles: Role in immune escape.
Gastroenterology. 128:1796–1804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Takano Y, Masuda T, Iinuma H, Yamaguchi R,
Sato K, Tobo T, Hirata H, Kuroda Y, Nambara S, Hayashi N, et al:
Circulating exosomal microRNA-203 is associated with metastasis
possibly via inducing tumor-associated macrophages in colorectal
cancer. Oncotarget. 8:78598–78613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Huang Z and Feng Y: Exosomes derived from
hypoxic colorectal cancer cells promote angiogenesis through
Wnt4-Induced β-catenin signaling in endothelial cells. Oncol Res.
25:651–661. 2017. View Article : Google Scholar
|
|
123
|
Bigagli E, Luceri C, Guasti D and Cinci L:
Exosomes secreted from human colon cancer cells influence the
adhesion of neighboring metastatic cells: Role of microRNA-210.
Cancer Biol Ther. 17:1062–1069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shedden K, Xie XT, Chandaroy P, Chang YT
and Rosania GR: Expulsion of small molecules in vesicles shed by
cancer cells: Association with gene expression and chemosensitivity
profiles. Cancer Res. 63:4331–4337. 2003.PubMed/NCBI
|
|
125
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sappino AP, Skalli O, Jackson B, Schürch W
and Gabbiani G: Smooth-muscle differentiation in stromal cells of
malignant and non-malignant breast tissues. Int J Cancer.
41:707–712. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Dai G, Yao X, Zhang Y, Gu J, Geng Y, Xue F
and Zhang J: Colorectal cancer cell-derived exosomes containing
miR-10b regulate fibroblast cells via the PI3K/Akt pathway. Bull
Cancer. 105:336–349. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS,
Yan YR, Song FY, Wang FF, Zhu XH, Liao WJ, et al: CAFs secreted
exosomes promote metastasis and chemotherapy resistance by
enhancing cell stemness and epithelial-mesenchymal transition in
colorectal cancer. Mol Cancer. 18:912019. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Alcantara KMM and Garcia RL: MicroRNA-92a
promotes cell proliferation, migration and survival by directly
targeting the tumor suppressor gene NF2 in colorectal and lung
cancer cells. Oncol Rep. 41:2103–2116. 2019.PubMed/NCBI
|
|
130
|
Yamada NO, Heishima K, Akao Y and Senda T:
Extracellular vesicles containing MicroRNA-92a-3p facilitate
partial endothelial-mesenchymal transition and angiogenesis in
endothelial cells. Int J Mol Sci. 20:44062019. View Article : Google Scholar :
|
|
131
|
Li J, Zhou C, Ni S, Wang S, Ni C, Yang P
and Ye M: Methylated claudin-11 associated with metastasis and poor
survival of colorectal cancer. Oncotarget. 8:96249–96262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Bhome R, Goh RW, Bullock MD, Pillar N,
Thirdborough SM, Mellone M, Mirnezami R, Galea D, Veselkov K, Gu Q,
et al: Exosomal microRNAs derived from colorectal cancer-associated
fibroblasts: Role in driving cancer progression. Aging (Albany NY).
9:2666–2694. 2017. View Article : Google Scholar
|
|
133
|
Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen
S, Wang Y, Wang T and Hou Y: Carcinoma-associated fibroblasts
promote the stemness and chemoresistance of colorectal cancer by
transferring exosomal lncRNA H19. Theranostics. 8:3932–3948. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kojima Y, Acar A, Eaton EN, Mellody KT,
Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg
RA and Orimo A: Autocrine TGF- and stromal cell-derived factor-1
(SDF-1) signaling drives the evolution of tumor-promoting mammary
stromal myofibroblasts. Proc Natl Acad Sci USA. 107:20009–20014.
2010. View Article : Google Scholar
|
|
136
|
Philippeos C, Telerman SB, Oulès B, Pisco
AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD
and Watt FM: Spatial and single-cell transcriptional profiling
identifies functionally distinct human dermal fibroblast
subpopulations. J Invest Dermatol. 138:811–825. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Croft AP, Campos J, Jansen K, Turner JD,
Marshall J, Attar M, Savary L, Wehmeyer C, Naylor AJ, Kemble S, et
al: Distinct fibroblast subsets drive inflammation and damage in
arthritis. Nature. 570:246–251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Liu H, Liu Y, Sun P, Leng K, Xu Y, Mei L,
Han P, Zhang B, Yao K, Li C, et al: Colorectal cancer-derived
exosomal miR-106b-3p promotes metastasis by down-regulating DLC-1
expression. Clin Sci (Lond). 134:419–434. 2020. View Article : Google Scholar
|
|
139
|
Kim TY, Vigil D, Der CJ and Juliano RL:
Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in
regulation of the cytoskeleton and cell motility. Cancer Metastasis
Rev. 28:77–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ogata-Kawata H, Izumiya M, Kurioka D,
Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H,
et al: Circulating exosomal microRNAs as biomarkers of colon
cancer. PLoS One. 9:e929212014. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wei C, Li Y, Huang K, Li G and He M:
Exosomal miR-1246 in body fluids is a potential biomarker for
gastrointestinal cancer. Biomark Med. 12:1185–1196. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cooks T, Pateras IS, Jenkins LM, Patel KM,
Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG and Harris
CC: Mutant p53 cancers reprogram macrophages to tumor supporting
macrophages via exosomal miR-1246. Nat Commun. 9:7712018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tanaka S, Hosokawa M, Ueda K and Iwakawa
S: Effects of decitabine on invasion and exosomal expression of
miR-200c and miR-141 in oxaliplatin-resistant colorectal cancer
cells. Biol Pharm Bull. 38:1272–1279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Senfter D, Holzner S, Kalipciyan M,
Staribacher A, Walzl A, Huttary N, Krieger S, Brenner S, Jäger W,
Krupitza G, et al: Loss of miR-200 family in 5-fluorouracil
resistant colon cancer drives lymphendothelial invasiveness in
vitro. Hum Mol Genet. 24:3689–3698. 2015.PubMed/NCBI
|
|
145
|
Holzner S, Senfter D, Stadler S,
Staribacher A, Nguyen CH, Gaggl A, Geleff S, Huttary N, Krieger S,
Jäger W, et al: Colorectal cancer cell-derived microRNA200
modulates the resistance of adjacent blood endothelial barriers in
vitro. Oncol Rep. 36:3065–3071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhang X, Bai J, Yin H, Long L, Zheng Z,
Wang Q, Chen F, Yu X and Zhou Y: Exosomal miR-1255b-5p targets
human telomerase reverse transcriptase in colorectal cancer cells
to suppress epithelial-to-mesenchymal transition. Mol Oncol.
14:2589–2608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J and Song E: let-7 regulates
self renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Xu J, Liao K and Zhou W: Exosomes regulate
the transformation of cancer cells in cancer stem cell homeostasis.
Stem Cells Int. 2018:48373702018. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Li H and Li F: Exosomes from BM-MSCs
increase the population of CSCs via transfer of miR-142-3p. Br J
Cancer. 119:744–755. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Farace C, Pisano A, Griñan-Lison C,
Solinas G, Jiménez G, Serra M, Carrillo E, Scognamillo F, Attene F,
Montella A, et al: Deregulation of cancer-stem-cell-associated
miRNAs in tissues and sera of colorectal cancer patients.
Oncotarget. 11:116–130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Liu T, Zhang X, Du L, Wang Y, Liu X, Tian
H, Wang L, Li P, Zhao Y, Duan W, et al: Exosome-transmitted
miR-128-3p increase chemosensitivity of oxaliplatin-resistant
colorectal cancer. Mol Cancer. 18:432019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Hu Y, Yan C, Mu L, Huang K, Li X, Tao D,
Wu Y and Qin J: Fibroblast-derived exosomes contribute to
chemoresistance through priming cancer stem cells in colorectal
cancer. PLoS One. 10:e01256252015. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Liu X, Chen X, Zeng K, Xu M, He B, Pan Y,
Sun H, Pan B, Xu X, Xu T, et al: DNA-methylation-mediated silencing
of miR-486-5p promotes colorectal cancer proliferation and
migration through activation of PLAGL2/IGF2/β-catenin signal
pathways. Cell Death Dis. 9:10372018. View Article : Google Scholar
|
|
155
|
Mizoguchi A, Takayama A, Arai T, Kawauchi
J and Sudo H: MicroRNA-8073: Tumor suppressor and potential
therapeutic treatment. PLoS One. 13:e02097502018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Teng Y, Ren Y, Hu X, Mu J, Samykutty A,
Zhuang X, Deng Z, Kumar A, Zhang L, Merchant ML, et al:
MVP-mediated exosomal sorting of miR-193a promotes colon cancer
progression. Nat Commun. 8:144482017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Dameron KM, Volpert OV, Tainsky MA and
Bouck N: Control of angiogenesis in fibroblasts by p53 regulation
of thrombospondin-1. Science. 265:1582–1584. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Soheilifar MH, Grusch M, Neghab HK, Amini
R, Maadi H, Saidijam M and Wang Z: Angioregulatory microRNAs in
colorectal cancer. Cancers (Basel). 12:712019. View Article : Google Scholar
|
|
160
|
Bhattacharya R, SenBanerjee S, Lin Z, Mir
S, Hamik A, Wang P, Mukherjee P, Mukhopadhyay D and Jain MK:
Inhibition of vascular permeability factor/vascular endothelial
growth factor-mediated angiogenesis by the kruppel-like factor
KLF2. J Biol Chem. 280:28848–28851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Ma J, Wang P, Liu Y, Zhao L, Li Z and Xue
Y: Krüppel-Like Factor 4 regulates blood-tumor barrier permeability
via ZO-1, Occludin and Claudin-5. J Cell Physiol. 229:916–926.
2014. View Article : Google Scholar
|
|
162
|
Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J,
Zhou K, Liu X, Ren X, Wang F, et al: Cancer-derived exosomal
miR-25-3p promotes pre-metastatic niche formation by inducing
vascular permeability and angiogenesis. Nat Commun. 9:53952018.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yamada N, Nakagawa Y, Tsujimura N,
Kumazaki M, Noguchi S, Mori T, Hirata I, Maruo K and Akao Y: Role
of intracellular and extracellular MicroRNA-92a in colorectal
cancer. Transl Oncol. 6:482–492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hu HY, Yu CH, Zhang HH, Zhang SZ, Yu WY,
Yang Y and Chen Q: Exosomal miR-1229 derived from colorectal cancer
cells promotes angiogenesis by targeting HIPK2. Int J Biol
Macromol. 132:470–477. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Shang A, Wang X, Gu C, Liu W, Sun J, Zeng
B, Chen C, Ji P, Wu J, Quan W, et al: Exosomal miR-183-5p promotes
angiogenesis in colorectal cancer by regulation of FOXO1. Aging
(Albany NY). 12:8352–8371. 2020. View Article : Google Scholar
|
|
166
|
Yamada N, Tsujimura N, Kumazaki M,
Shinohara H, Taniguchi K, Nakagawa Y, Naoe T and Akao Y: Colorectal
cancer cell-derived microvesicles containing microRNA-1246 promote
angiogenesis by activating Smad 1/5/8 signaling elicited by PML
down-regulation in endothelial cells. Biochim Biophys Acta.
1839:1256–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Ebrahimi F, Gopalan V, Wahab R, Lu CT,
Smith RA and Lam AK: Deregulation of miR-126 expression in
colorectal cancer pathogenesis and its clinical significance. Exp
Cell Res. 339:333–341. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
MiR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Nicoli S, Standley C, Walker P, Hurlstone
A, Fogarty KE and Lawson ND: MicroRNA-mediated integration of
haemodynamics and Vegf signalling during angiogenesis. Nature.
464:1196–1200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Hansen TF, Andersen CL, Nielsen BS,
Spindler KL, Sørensen FB, Lindebjerg J, Brandslund I and Jakobsen
A: Elevated microRNA-126 is associated with high vascular
endothelial growth factor receptor 2 expression levels and high
microvessel density in colorectal cancer. Oncol Lett. 2:1101–1106.
2011. View Article : Google Scholar
|
|
171
|
Zhang Y, Wang X, Xu B, Wang B, Wang Z,
Liang Y, Zhou J, Hu J and Jiang B: Epigenetic silencing of miR-126
contributes to tumor invasion and angiogenesis in colorectal
cancer. Oncol Rep. 30:1976–1984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Hansen TF, Carlsen AL, Heegaard NH,
Sørensen FB and Jakobsen A: Changes in circulating microRNA-126
during treatment with chemotherapy and bevacizumab predicts
treatment response in patients with metastatic colorectal cancer.
Br J Cancer. 112:624–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Liang L, Gao C, Li Y, Sun M, Xu J, Li H,
Jia L and Zhao Y: MiR-125a-3p/FUT5-FUT6 axis mediates colorectal
cancer cell proliferation, migration, invasion and pathological
angiogenesis via PI3K-Akt pathway. Cell Death Dis. 8:e29682017.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Wang J, Yan F, Zhao Q, Zhan F, Wang R,
Wang L, Zhang Y and Huang X: Circulating exosomal miR-125a-3p as a
novel biomarker for early-stage colon cancer. Sci Rep. 7:41502017.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Yang X, Qiu J, Kang H, Wang Y and Qian J:
MiR-125a-5p suppresses colorectal cancer progression by targeting
VEGFA. Cancer Manag Res. 10:5839–5853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Tang L, Zhou L, Wu S, Shi X, Jiang G, Niu
S and Ding D: MiR-125a-5p inhibits colorectal cancer cell
epithelial-mesenchymal transition, invasion and migration by
targeting TAZ. Onco Targets Ther. 12:3481–3489. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Tong Z, Liu N, Lin L, Guo X, Yang D and
Zhang Q: MiR-125a-5p inhibits cell proliferation and induces
apoptosis in colon cancer via targeting BCL2, BCL2L12 and MCL1.
Biomed Pharmacother. 75:129–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Katoh H, Wang D, Daikoku T, Sun H, Dey SK
and DuBois RN: CXCR2-expressing myeloid-derived suppressor cells
are essential to promote colitis-associated tumorigenesis. Cancer
Cell. 24:631–644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Ru P, Steele R, Hsueh EC and Ray RB:
Anti-miR-203 Upregulates SOCS3 expression in breast cancer cells
and enhances cisplatin chemosensitivity. Genes Cancer. 2:720–727.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Liu Y, Stewart KN, Bishop E, Marek CJ,
Kluth DC, Rees AJ and Wilson HM: Unique expression of suppressor of
cytokine signaling 3 is essential for classical macrophage
activation in rodents in vitro and in vivo. J Immunol.
180:6270–6278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu
Y, Zhang Z, Cai S, Xu Y, Li X, et al: Tumor-derived exosomal
miR-934 induces macrophage M2 polarization to promote liver
metastasis of colorectal cancer. J Hematol Oncol. 13:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Wang D, Wang X, Si M, Yang J, Sun S, Wu H,
Cui S, Qu X and Yu X: Exosome-encapsulated miRNAs contribute to
CXCL12/CXCR4-induced liver metastasis of colorectal cancer by
enhancing M2 polarization of macrophages. Cancer Lett. 474:36–52.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Noh GT, Kwon J, Kim J, Park M, Choi DW,
Cho KA, Woo SY, Oh BY, Lee KY and Lee RA: Verification of the role
of exosomal microRNA in colorectal tumorigenesis using human
colorectal cancer cell lines. PLoS One. 15:e02420572020. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Cheng L, Sharples RA, Scicluna BJ and Hill
AF: Exosomes provide a protective and enriched source of miRNA for
biomarker profiling compared to intracellular and cell-free blood.
J Extracell Vesicles. 3:237432014. View Article : Google Scholar
|
|
185
|
Ge Q, Zhou Y, Lu J, Bai Y, Xie X and Lu Z:
MiRNA in plasma exosome is stable under different storage
conditions. Molecules. 19:1568–1575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Rabinowits G, Gerçel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal MicroRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Matsumura T, Sugimachi K, Iinuma H,
Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano
Y, et al: Exosomal microRNA in serum is a novel biomarker of
recurrence in human colorectal cancer. Br J Cancer. 113:275–281.
2015. View Article : Google Scholar : PubMed/NCBI
|