Introduction
Aging is a complex process characterized by a
progressive loss of functions; in particular, learning and memory
impairments (1). In women,
decrease in estrogen levels is an important component of the aging
following menopause, and brain and endocrine aging occur
simultaneously and are closely associated with cognitive impairment
and pathological states (2-6).
Inflammation was discovered to be a biomarker of
both normal and accelerated aging (7). The cells and tissues of older
organisms tend to have higher levels of inflammatory markers, which
can lead to low, aseptic, and chronic inflammatory conditions
(8,9). This phenomenon has been defined as
immune aging and is associated with numerous types of age-related
diseases, such as cancer, cardiovascular disease, and
neurodegenerative diseases (10-15). Neuroinflammation is a common
feature of the majority of central nervous system (CNS) diseases,
and has been discovered to cause cognitive impairments (16,17). Aging was reported to influence
neuroinflammatory responses by promoting the release of a large
number of neuroinflammatory cytokine levels, such as IL-1, IL-6,
IL-8, transforming growth factor-β (TGFβ), TNF-α, and activating
immune system cells involved in the regulation of neurogenesis,
synaptic plasticity, neuronal survival and other critical
processes. These changes, in turn, were demonstrated to affect
cognitive functions (18-20).
The effect of estrogen replacement therapy on
aging-related changes in cognition remains controversial (2). Therefore, a variety of natural
polysaccharides from functional and medicinal foods have attracted
considerable attention due to their significant pharmacological
activities (21). For example,
the polysaccharides of Pleurotus bisporus were reported to
have antioxidant and anti-aging effects (22), Sargassum polysaccharide
exerted numerous pharmacological activities, including
antioxidative proinflammatory, anti-aging and anti-fatigue effects
(23) and Cordyceps
sinensis polysaccharides were demonstrated to exert significant
antioxidant and anti-aging activities (24). As an edible Chinese herbal
medicine, Lycium barbarum polysaccharides (LBPs) have been
reported to exert antioxidant and neuroimmune regulatory functions
(25,26), in addition to anti-inflammatory
and anti-aging effects (26-28). However, although the benefits of
LBPs have been reported, their effects on learning and memory
during the aging process induced by estrogen deficiency are poorly
understood, at least to the best of our knowledge.
Ovariectomized (OVX) rodents are useful models for
neurocognitive impairments caused by decreased estrogen levels in
women (29-31). Hence, the present study aimed to
investigate the therapeutic effects of LBP on neuroinflammation and
the potential mechanisms underlying its effects using an
ovariectomy (OVX) mouse model.
Materials and methods
Animals studies
A total of 45 female ICR mice (age, 12 weeks;
weight, 22-25 g) were purchased from the Laboratory Animal Services
Center of Ningxia Medical University [Yinchuan, China; animal
license no. SCXK (Ning) 2015-0001]. Mice were housed under standard
laboratory conditions at room temperature (22±2°C) and 30-60%
humidity, with a 12-h light/dark cycle. Food and water were
available ad libitum. Body weight (BW), food and water
intake were measured weekly. The present study was conducted in
accordance with the 2011 Guidelines for the Protection and Use of
Experimental Animals (National Research Council of the National
Academies), and approved by the Animal Ethics Committee of General
Hospital of Ningxia Medical University, Ningxia, China (approval
no. 2020-449).
OVX model induction and LBP
treatment
Mice were randomly divided into sham (15 mice) and
OVX (30 mice) groups. After adaptive feeding for 1 week, OVX was
performed in mice in the OVX group as previously described
(32). Briefly, mice were deeply
anesthetized with an intraabdominal injection of 50 mg/kg sodium
pentobarbital prior to the operation, and an ~4×4-cm2
area of hair was removed from the middle of the back. A 2-3-cm
midline incision was made through the skin along the dorsal
midline, both ovaries were removed and then the muscle and skin
incisions were sutured. Sham mice received anesthesia and underwent
the skin and abdominal incision without OVX. Mice were allowed to
recover for 7 days prior to further experimentation. OVX mice were
subsequently randomly divided into the OVX (15 mice) and OVX + LBP
(15 mice; 100 mg/kg/d) (cat. no. RL190914; Xi'an Realin
Biotechnology Co., Ltd.; http://ch.xarealin.com/) groups. The mice in the OVX +
LBP group received daily intragastric feeding with 10 ml/kg LBP
solution (10 mg/ml). Both the sham and OVX groups were administered
with 10 ml/kg/day distilled water. The administration period of
this experiment lasted for 24 weeks.
Open field test
All mice were individually placed in the open field
(length x width x height: 50×50×50 cm) for 10 min. The total time
taken to enter the central area, the total distance of open land
covered and the total number of times the mouse crossed the central
square were recorded using an automatic tracking system (Smart 3.0;
Panlab; https://www.rwdls.com/). Throughout the
experiment, the environmental noise level was <50 dB.
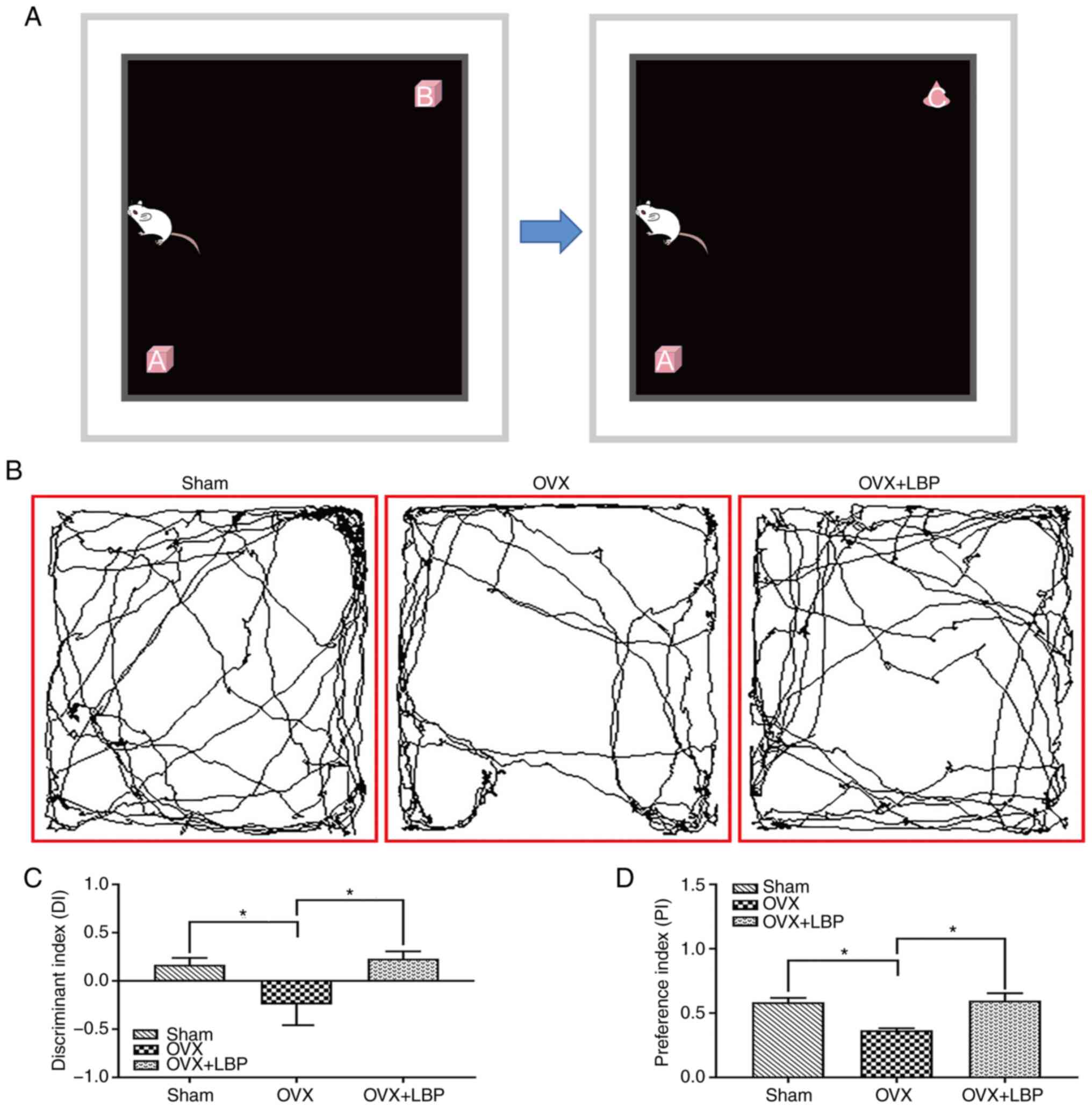
Novel object recognition test
The experiment was divided into three stages, and
each mouse performed the experiment alone. In the first stage, mice
were able to move freely in the new object recognition test box for
10 min and no objects were placed in the test box. In the second
stage, the mice were placed into the test box with two identical
square objects (A and B), and in the third stage, a square object
(B) was replaced with a conical object (C) to recorded as EA (A)
and EB (B) as the total exploration time of the two objects
(Fig. 1A). The discriminant
index (DI) was calculated using the following formula: DI = EB −
EA/(EA + EB), in which the DI value was >0.5 if the mice showed
tendencies of exploring novel objects. The preference index (PI),
which reflects the ability of mice to explore new objects, was
calculated using the following formula: PI = EB/(EA + EB).
Preparation of mouse brain samples
At 36 weeks old, all mice were euthanized with 200
mg/kg sodium pentobarbital. Then brains were immediately dissected
and the hippocampus was isolated. Then, half the brains from each
group were fixed in 4% paraformaldehyde (LEAGENE®;
http://www.leagene.com/) for 24 h at 4°C,
dehydrated in an ascending series of alcohol and embedded in
paraffin. Paraffin-embedded tissues were cut into 4-µm-thick
sections, washed with 1X PBS at room temperature and deparaffinized
in xylene before undergoing routine histology and
immunohistochemical analysis. The remaining brains from each group
were preserved in liquid nitrogen for mRNA sequencing, western
blotting and reverse transcription-quantitative PCR (RT-qPCR)
analysis.
ELISA
Blood was collected from mice in each group into
1.5-ml centrifuge tubes, maintained at room temperature for 2 h and
centrifuged at 1,000 × g for 20 min at 4°C to obtain the serum for
ELISA analysis. The concentration of estrogen in the serum of mice
was measured using an ELISA detection kit (cat. no. SU-B20462;
Quanzhou Konodi Biotechnology Co., Ltd.; http://www.qzkndbio.com/), according to the
manufacturer's protocol. The optical density was measured at a
wavelength of 450 nm using a microplate reader (Varioskan™ LUX;
Thermo Scientific™; Thermo Fisher Scientific, Inc.). Linear
regression equations were used to analyze the results.
mRNA library construction and RNA
sequencing
Total RNA was isolated and purified using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The concentration
and purity of RNA from each sample were quantified using NanoDrop
ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.). RNA
integrity was assessed using a Bioanalyzer 2100 (Agilent
Technologies, Inc.) with an RNA integrity of >7.0, and the
findings were confirmed via electrophoresis with denaturing agarose
gel. Poly(A) RNA was purified from 1 µg of total RNA using
Dynabeads Oligo (dT)25-61005 (Thermo Fisher Scientific, Inc.)
following two rounds of purification. Then, the poly (A) RNA was
fragmented into small pieces using a NEBNext® Magnesium
RNA Fragmentation Module (cat. no. e6150; New England BioLabs,
Inc.) for 5-7 min at 94°C. The cleaved RNA fragments were
subsequently reverse transcribed into cDNA using SuperScript™ II
Reverse Transcriptase (cat. no. 18064014; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, which
was then used to synthesize U-labeled second-stranded DNA with DNA
Polymerase I (E. coli) (cat. no. m0209; New England BioLabs,
Inc.), RNase H (cat. no. m0297; New England BioLabs, Inc.) and dUTP
solution (cat. no. R0133; Thermo Fisher Scientific, Inc.). An
A-base was then added to the blunt ends of each strand to prepare
them for ligation to the 18064014 indexed adapters; each adapter
contained a T-base overhang for ligating the adapter to the
A-tailed fragmented DNA. Single- or dual-index adapters were
ligated to the fragments, and size selection was performed using
AMPureXP beads (cat. no. A63881; Beckman Coulter, Inc.). After the
U-labeled second-stranded DNA was treated with a heat-labile UDG
enzyme (cat. no. m0280; New England BioLabs, Inc.), the ligated
products were amplified via PCR using the following thermocycling
conditions: Initial denaturation at 95°C for 3 min, followed by
eight cycles of denaturation at 98°C for 15 sec, annealing at 60°C
for 15 sec and extension at 72°C for 30 sec; and a final extension
at 72°C for 5 min. The average insert size for the final cDNA
library was 300±50-bp. Finally, the 2×150-bp paired-end sequencing
(PE150) was performed on an Novaseq™ 6000 system (Illumina, Inc.)
according with the manufacturer's protocol.
Cutadapt software (https://cutadapt.readthedocs.io/en/stable/,
version:cutadapt-1.9) was used to remove reads that contained
adaptor contamination (command line: cutadapt -a ADAPT1 -A ADAPT2
-o out1.fastq-p out2.fastq in1.fastq in2.fastq -O 5-m100). After
removing low-quality and undetermined bases, HISAT2 software
(https://daehwan-kimlab.github.io/hisat2/; version,
hisat2-2.0.4) was used to map reads to the genome (for example,
Homo sapiens Ensembl v96; command line: ~hisat2 -1
R1.fastq.gz -2 R1.fastq.gz -S sample_mapped.sam). The mapped reads
of each sample were assembled using StringTie (http://ccb.jhu.edu/software/stringtie/;
version, stringtie-1.3. 4d.Linux_x86_64) with default parameters
(command line: ~stringtie-p4-G genome. gtf-o output. gtf-l sample
input. bam). Then, transcriptomes from all samples were merged to
reconstruct a comprehensive transcriptome using gffcompare software
(http://ccb.jhu.edu/software/stringtie/gffcompare.shtml;
version, gffcompare-0.9.8. Linux_x86_64). After the final
transcriptome was generated, StringTie and ballgown (http://www.biocon-ductor.org/packages/release/bioc/html/ballgown.html)
were used to estimate the expression levels of all transcripts and
to determine mRNA expression based on fragments per kilobase of
exon (FPKM) using the following equation: FPKM =
[total_exon_fragments/mapped_reads (millions) x exon_length (kB);
command line: ~stringtie- -B-p4-G merged.gtf-osamples.gtf
samples.bam]. Differentially expressed genes (DEGs) with fold
changes of >2 or <0.5 and P<0.05 were selected using the R
package, edgeR (https://bioconductor.org/packages/release/bioc/html/edgeR.
html) or DESeq2 (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html)
(33,34). Gene Ontology (GO) (35) functional term and Kyoto
Encyclopedia of Genes and Genomes (KEGG) (36) signaling pathway enrichment
analyses were then used to evaluate DEGs.
RT-qPCR
Total RNA from hippocampal tissues was extracted
using TRIzol® reagent (Invitrogen™; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using a PrimeScript™ RT reagent kit (cat. no. RR047A; Takara
Biotechnology Co., Ltd.) at 37°C for 15 min and 85°C for 5 sec, and
then maintained at 4°C. qPCR was performed using a PerfectStart™
Green qPCR Super Mix (cat. no. AQ601; Beijing TransGen Biotech Co.,
Ltd.) on a CFX96™ Real-Time PCR detection system (Bio-Rad
Laboratories, Inc.). The following thermocycling conditions were
used for the qPCR: Initial denaturation at 94°C for 30 sec,
followed by 40 cycles of annealing at 94°C for 5 sec, and extension
at 60°C for 30 sec. GAPDH was used as the endogenous reference gene
for normalizing Toll-like receptor 4 (TLR4), myeloid
differentiation factor 88 (MyD88), NF-κB, IL-1β, IL-6 and TNF-α
expression in the mouse hippocampus. The sequences of the primers
used for the qPCR are presented in Table I. The expression levels were
quantified using the 2−∆∆Cq method (37).
 | Table ISequences of the primers used for
reverse transcription-quantitative PCR (5′-3′). |
Table I
Sequences of the primers used for
reverse transcription-quantitative PCR (5′-3′).
| Primer | Forward | Reverse |
|---|
| GAPDH |
ACAACTTTGGCATTGTGGAA |
GATGCAGGGATGATGTTCTG |
| TLR4 |
CCGCTTTCACCTCTGCCTTCAC |
ACCACAATAACCTTCCGGCTCTTG |
| MyD88 |
AGCAGAACCAGGAGTCCGAGAAG |
GGGCAGTAGCAGATAAAGGCATCG |
| NF-κB p65 |
GACACGACAGAATCCTCAGCATCC |
CCACCAGCAGCAGCAGACATG |
| TNF-α |
GCCTCTTCTCATTCCTGCTTGTGG |
GTGGTTTGTGAGTGTGAGGGTCTG |
| IL-1β |
TCGCAGCAGCACATCAACAAGAG |
AGGTCCACGGGAAAGACACAGG |
| IL-6 |
CTTCTTGGGACTGATGCTGGTGAC |
AGGTCTGTTGGGAGTGGTATCCTC |
Histopathological analysis
The paraffin sections were cut to a thickness of
4-µm, stored at 60°C for 2 h and then deparaffinized with
xylene at room temperature. The sections were subsequently
rehydrated with a descending alcohol series at room temperature
(anhydrous ethanol I, 5 min; anhydrous ethanol II, 5 min; 95%
alcohol, 5 min; 85% alcohol, 5 min; and 75% alcohol, 5 min). The
sections were hydrated with tap water for 3 min and then stained
with a hematoxylin and eosin (H&E) staining kit (cat. no.
DH0006; LEAGENE®; http://www.leagene.com/). Briefly, the sections were
stained with hematoxylin for 5 min at room temperature, washed with
tap water for 10 min, differentiated with 1% hydrochloric acid
alcohol differentiation for 2-3 sec, washed with water for 15 min
and stained with eosin dye solution for 5 min at room temperature.
The sections were then dehydrated with an ascending gradient
alcohol series at room temperature (70% alcohol, 1 min; 80%
alcohol, 1 min; 90% alcohol, 1 min; 95% alcohol, 5 min; anhydrous
ethanol I, 10 min; anhydrous ethanol II, 10 min; and ethanol III, 5
min) and deparaffinized with xylene (CAS no. 1330-20-7; Tianjin
Damao Chemical Reagent Factory) at room temperature (xylene I, 10
min; xylene II, 10 min; and xylene III, 10 min). The slices were
sealed with neutral balsam (CAS no. 96949-21-2; Beijing Solarbio
Science & Technology Co., Ltd.) and dried in a ventilation
cabinet. A 200-fold image of CA1 and CA3 was captured (DP73;
Olympus Corporation) to view the organization and structure.
Neuronal damage was rated in accordance with this scoring criteria
proposed by Shi et al (38).
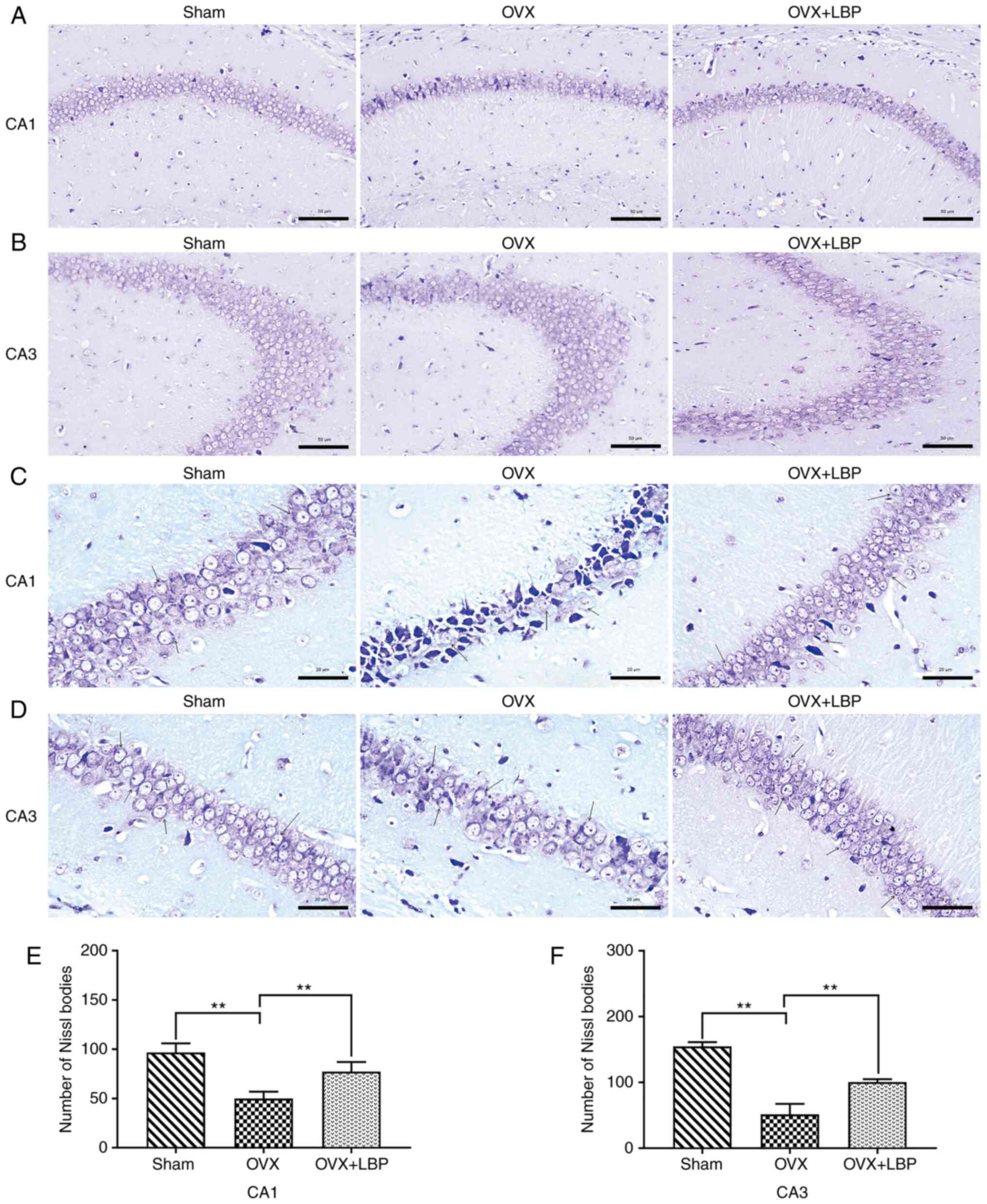
Nissl staining
Nissl staining was performed to evaluate the extent
of neuronal damage in the hippocampus. The paraffin sections of the
brain were cut into 4-µm thick sections and the methods of
deparaffinization, rehydration and hydration were performed as
described for H&E staining. Then, after rinsing the sections
with 1X PBS, the sections were stained with 0.1% cresol purple dye
(CAS no. 10510-54-0; Beijing Solarbio Science & Technology Co.,
Ltd.) for 30 min at room temperature, washed with distilled water
and differentiated with 0.5% acetic acid solution for 2-3 sec (CAS
no. 64-19-7; Xiya Reagent®: http://www.xiyashiji.com/) at room temperature.
Subsequent steps, including dehydration, xylene transparency and
neutral gum sealing were performed as described for H&E
staining. Images were captured of CA1 and CA3 (200 and 400-fold)
(DP73; Olympus Corporation) to view the organization and structure.
The Nissl body counts in the hippocampal CA1 and CA3 regions from
each animal were calculated using Image Oro-Plus 6.0 software
(Media Cybernetics, Inc.).
Immunohistochemical analysis
Immunohistochemical analyses were performed to
analyze the expression of TLR4, MyD88, NF-κB, TNF-α, IL-6 and IL-1β
in paraffin-embedded brain tissue sections (4-µm thick). The
methods of deparaffinization, rehydration and hydration of the
paraffin sections were performed as described for H&E staining,
and sections were subsequently washed with 1X PBS. Antigen
retrieval was performed via incubation with citrate buffer (pH 6.0;
cat. no. s8220; Beijing Solarbio Science & Technology Co.,
Ltd.) in a microwave oven for 10 min, the endogenous peroxidase
activity was blocked with 3% H2O2 (CAS no.
7722-84-1; Aikeshiji) for 10 min at room temperature and blocking
for non-specific binding was subsequently performed with 10% goat
serum (cat. no. SL038; Beijing Solarbio Science & Technology
Co., Ltd.) for 1 h at room temperature. The brain tissues were then
incubated with the following primary antibodies at 4°C overnight:
Anti-MyD88 (cat. no. WL02494; 1:200), anti-NF-κB (cat. no. WL01980;
1:200), anti-IL-6 (cat. no. WL02841; 1:200), anti-TNF-α (cat. no.
WL01896; 1:200), anti-IL-1β (cat. no. WLH3903; 1:200) (all
Wanleibio Co., Ltd.) and anti-TLR4 (cat. no. ab22048; Abcam;
1:100). Following the primary antibody incubation, the sections
were incubated with HRP-conjugated secondary antibodies (code nos.
115-035-003, 1:200; and 111-035-003, 1:500; Jackson ImmunoResearch
Laboratories, Inc.) for 2 h at room temperature. The sections were
then incubated with a DAB staining kit (cat. no. DAB-1031; MXB
Biotechnology) and counterstained with hematoxylin for 60 sec at
room temperature. Finally, the sections were rinsed in purified
water for 60 sec and observed under a light microscope (400-fold)
(DP73; Olympus Corporation). Protein expression was calculated
using Image pro-Plus 6.0 software.
Western blotting
Total protein was extracted from mouse hippocampal
tissue using a total protein extraction kit (cat. no. KGP2100;
Nanjing KeyGen Biotech Co., Ltd.). Total protein was quantified
using a BCA kit (cat. no. KGP902; Nanjing KeyGen Biotech Co., Ltd.)
and 30-50 µg protein per lane was separated via SDS-PAGE
using a 10% PAGE Gel Fast Preparation kit (cat. no. PG112; Shanghai
EpiZyme Biotech Co., Ltd.; http://www.epizyme.cn/). The separated proteins were
subsequently transferred onto a polyvinylidene fluoride membrane
(cat. no. IPVH00010; Immobilon®-P; Merck KGaA) and
blocked with 5% skimmed milk powder at room temperature for 2 h.
The membranes were incubated with the following primary antibodies
overnight at 4°C on a shaker: Anti-TLR4 (cat. no. WL00196;
Wanleibio Co., Ltd.; 1:1,000), anti-NF-κB (1:1,000) and anti-MyD88
(1:1,000). Following the primary antibody incubation, the membranes
were incubated with an HRP-conjugated goat anti-rabbit IgG
secondary antibody (1:5,000; cat. no. A21030; Abbkine Scientific
Co., Ltd.) for 1 h at room temperature. Protein bands were
visualized using an enhanced chemiluminescence reagent (cat. no.
34094; Pierce; Thermo Fisher Scientific, Inc.) and a 600
ultra-sensitive multifunctional imager (Amersham; Cytiva). ImageJ
v1.8.0 software was used for densitometric analysis (National
Institutes of Health).
Statistical analysis
Each experiment was repeated at least 3 times or
using three mice. Statistical analysis was performed using GraphPad
8.0 software (GraphPad Software, Inc.). Statistical differences
between groups were determined using a one-way ANOVA and an LSD
post hoc test. Data were presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
LBP attenuates OVX-induced learning and
memory impairments in mice
The novel object recognition test was used to assess
non-spatial learning, recognition, and memory function in OVX mice
treated with LBP (Fig. 1).
Although all three groups (sham, OVX and OVX + LBP) exhibited DI
values of <0.5, the OVX group exhibited a negative DI value.
Mice in the OVX group also displayed a significantly decreased
ability to explore new objects and a decreased preference for new
objects (therefor, PI) compared with the other two groups.
Collectively, these findings indicated that OVX may decrease the
ability of the mouse to recognize and remember new objects and that
LBP treatment may repair these deficits (Fig. 1B-D).
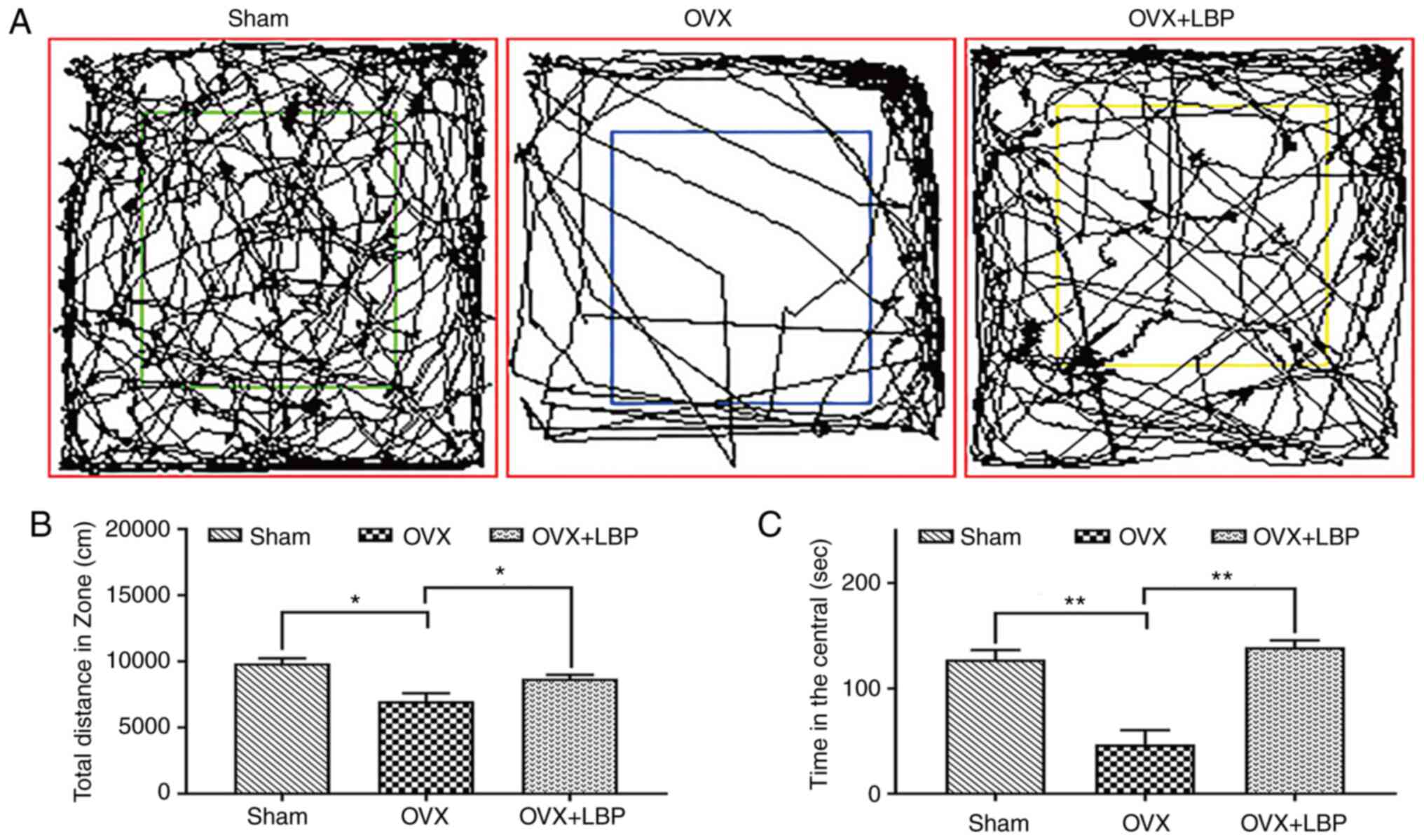
An open-field test was used to evaluate the effect
of LBP on the motor ability of mice in OVX and OVX + LBP groups.
The findings revealed that both the number of crossings and total
distance traveled were significantly decreased in the OVX group
compared with the sham group, suggesting that mice experienced
decreased motor ability and an increased fear of the open
environment following OVX. By contrast, the number of central
crossings and total distance traveled were significantly increased
in the OVX + LBP group compared with the OVX group, indicating that
LBP treatment may increase motor ability and decrease fear of the
open environment in the OVX model mice (Fig. 2B and C).
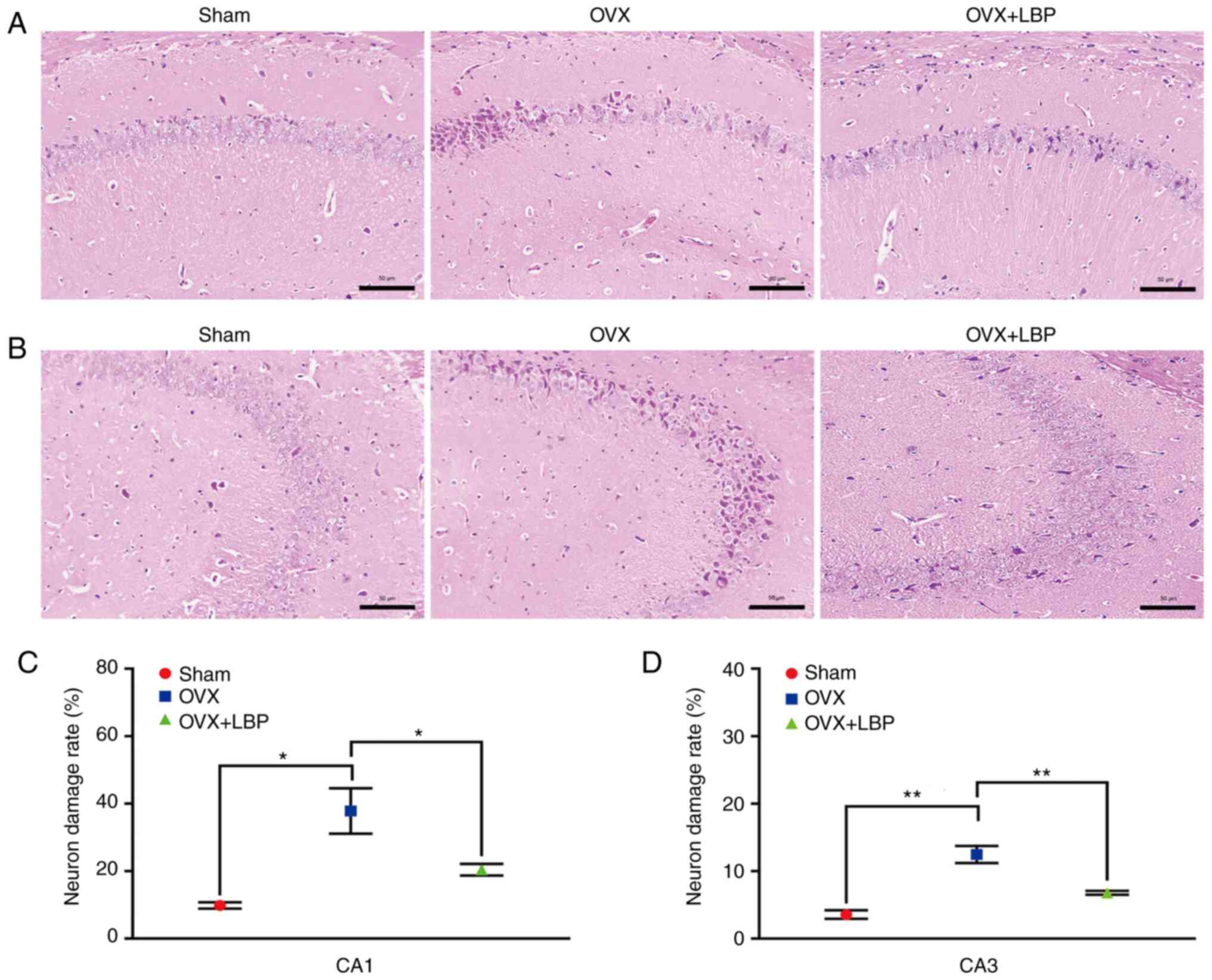
LBP treatment inhibits hippocampal
neuronal damage in OVX model mice
To determine the effects of LBP on OVX-induced
hippocampal neuronal injury, H&E and Nissl staining were
performed. Overt signs of neuronal injury were observed in the
hippocampal CA1 and CA3 regions of the OVX model mice; however,
these changes were significantly attenuated following LBP treatment
(Fig. 3A-D). In addition, Nissl
staining revealed significant decreases in the number of
Nissl-positive bodies in the hippocampal CA1 and CA3 regions of the
OVX model group compared with the sham group. However, the number
of Nissl bodies in the hippocampal CA1 and CA3 regions was
significantly increased following LBP treatment (Fig. 4A-F).
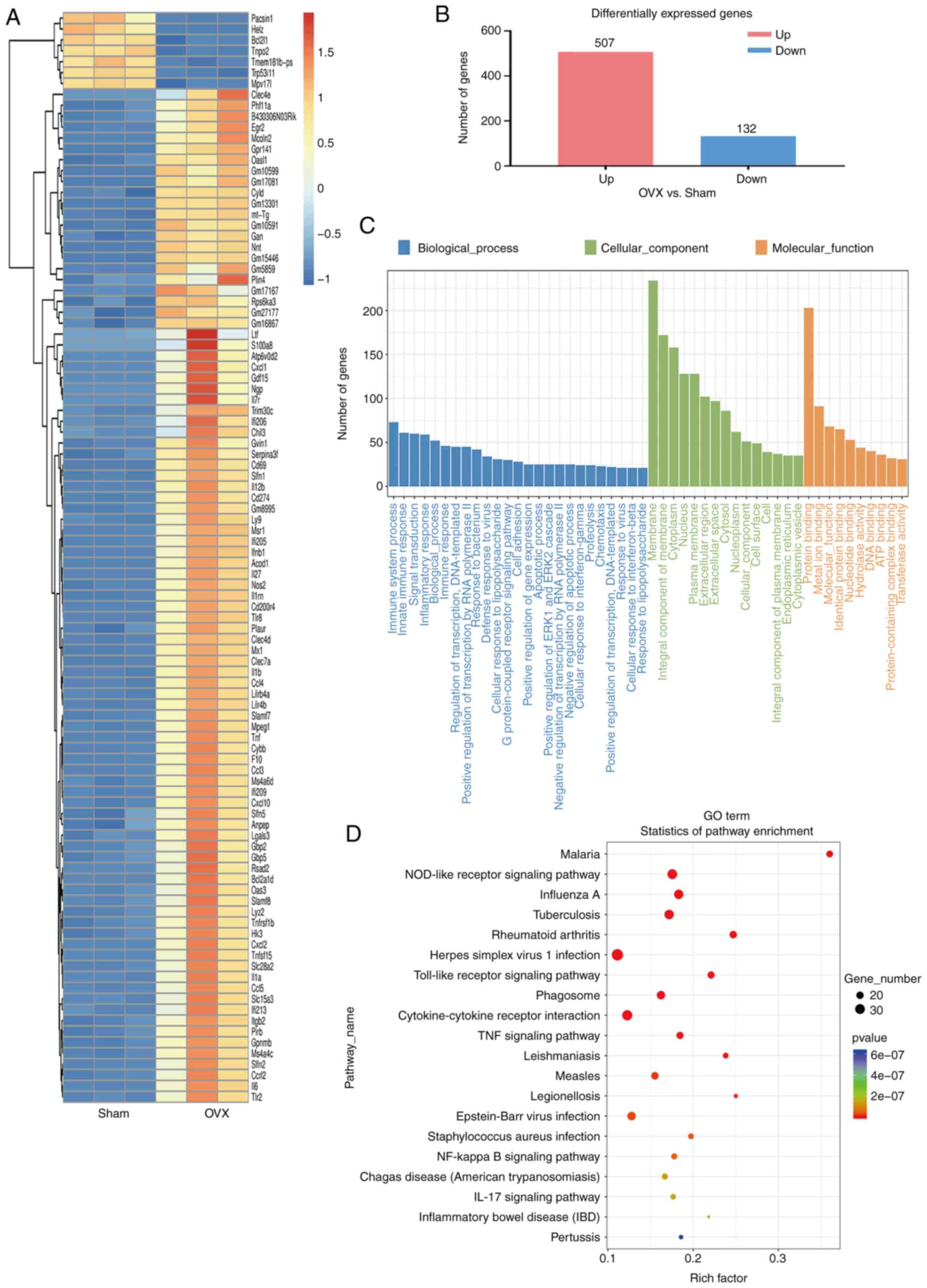
RNA sequencing transcriptional analysis
the possible effect of OVX on the hippocampus of mice
To determine the effects of the OVX surgery, RNA
sequencing analysis on hippocampal tissue was performed. Cluster
analysis (Fig. 5A), column chart
(Fig. 5B), GO functional term
enrichment (Fig. 5C) and KEGG
signaling pathway enrichment (Fig.
5D) analyses of DEGs were conducted, and the results revealed
that the most significant changes were in inflammatory genes and
inflammation-related pathways (Fig.
5). GO functional enrichment analysis revealed that the number
of DEGs in the 'membrane', 'membrane components' and 'cytoplasm'
was increased in OVX model mice compared with the sham mice
(Fig. 5C). Among molecular
functions, 'protein binding-related gene enrichment' was the most
enriched. However, among biological processes, those associated
with 'immune system processes', 'intracellular immune responses',
'signal transduction' and 'inflammatory response-related genes'
exhibited more significant changes compared with other processes
(Fig. 5C). KEGG signaling
pathway enrichment analysis revealed that the most significant DEGs
were those involved in 'nucleotide-binding', 'oligomerization
domain (NOD)-like receptor signaling pathway', 'Toll-like receptor
signaling pathway', 'cytokine-cytokine receptor interaction', 'TNF
signaling pathway', 'NF-κB signaling pathway' and 'Epstein-Barr
virus infection'(Fig. 5D).
Effect of LBP treatment on the expression
levels of TLR4, MyD88 and NF-κB in OVX model mice
To investigate the role of TLR4 signaling pathway in
OVX-induced cognitive impairment, the mRNA expression levels of
TLR4, MyD88 and NF-κB in the hippocampus of sham, OVX and OVX + LBP
mice were analyzed. The mRNA expression levels of TLR4, MyD88 and
NF-κB in the hippocampus were significantly upregulated in the OVX
group compared with the sham group; however, the expression levels
were significantly downregulated in the OVX + LBP group compared
with the OVX group (Fig. 6A).
The results of the ELISAs revealed that the serum estrogen levels
were significantly reduced in mice following bilateral resection of
the ovaries compared with the sham group. After oral LBP treatment,
no significant difference was observed in the estrogen levels
compared with the OVX group (P>0.05; Fig. 6B). These results indicated that
the OVX was successful. In addition, the results of the western
blotting revealed that the protein expression levels of TLR4, MyD88
and NF-κB were significantly upregulated in the hippocampus of OVX
model mice compared with the sham group; however, LBP treatment
attenuated these increases (Fig.
6C).
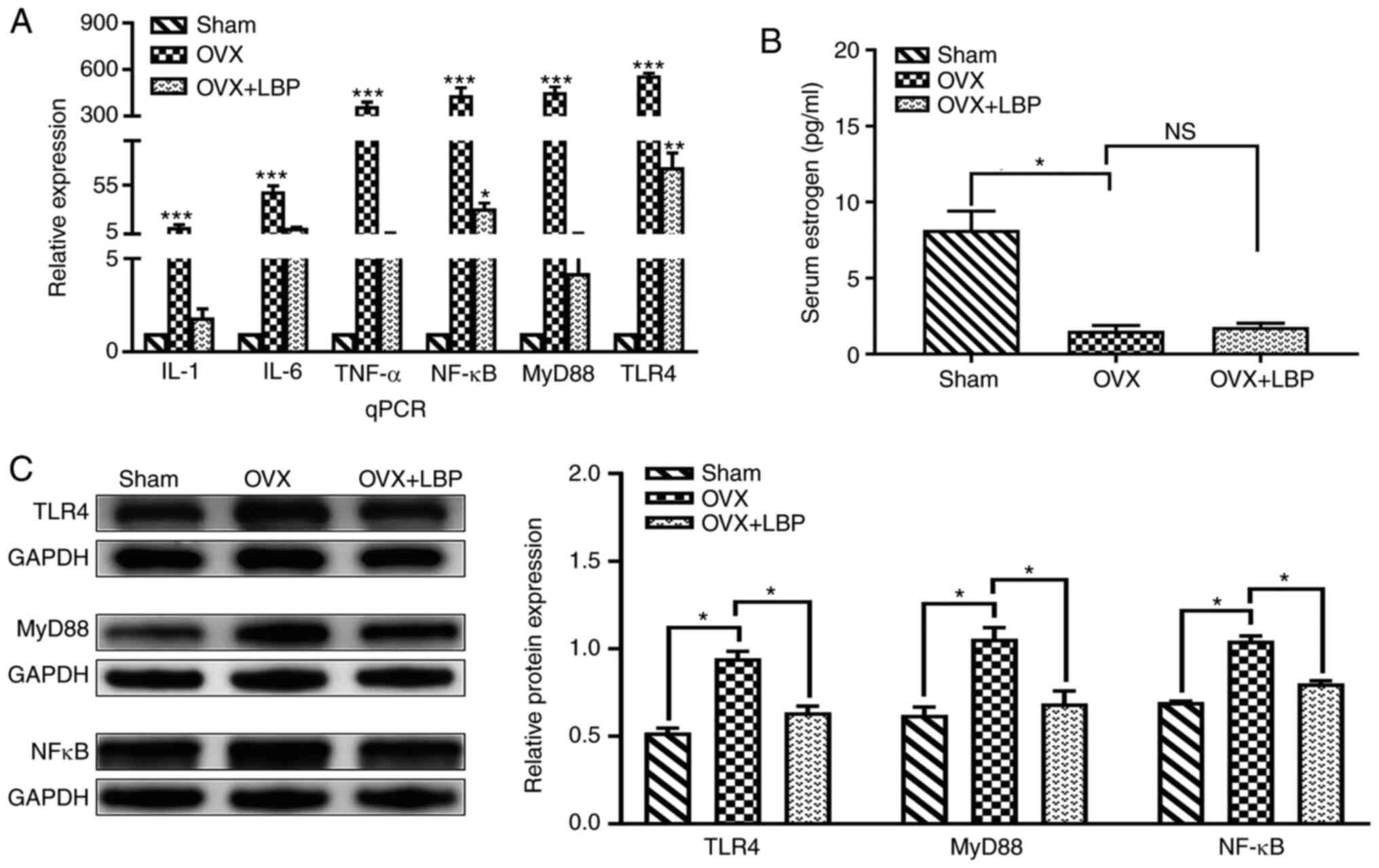 | Figure 6TLR4/NF-κB signaling pathway is
closely associated with the progression of neuroinflammation in the
brain. (A) Reverse transcription-quantitative PCR analysis of TLR4,
MyD88, NFκB, TNF-α, IL-1β and IL-6 mRNA expression levels in
hippocampal CA1 and CA3 regions. Data were analyzed using a one-way
ANOVA and an LSD post hoc test. *P<0.05,
**P<0.01, ***P<0.001 vs. the Sham
group. (B) ELISA analysis of serum estrogen levels. Data were
analyzed using a one-way ANOVA and an LSD post hoc test.
*P<0.05 vs. the OVX group; NSP>0.05 vs.
the OVX group. (C) Western blot analysis of TLR4, MyD88 and NF-κB
protein expression levels in the hippocampus. Data were analyzed
using a one-way ANOVA and an LSD post hoc test.
*P<0.05, vs. the OVX group. TLR, Toll-like receptor;
MyD88, myeloid differentiation primary response 88; OVX,
ovariectomy; LBP. Lycium barbarum polysaccharide; NS, not
significant. |
Statistical analysis of the immunohistochemical
findings (Fig. 7A and B) for
TLR4, MyD88, and NF-κB expression revealed that the number of
positive cells in the hippocampal CA1 and CA3 regions was
significantly increased in the OVX group compared with the sham
group. Notably, the number of positive cells in the OVX + LBP group
was lower compared with the OVX group (Fig. 7C).
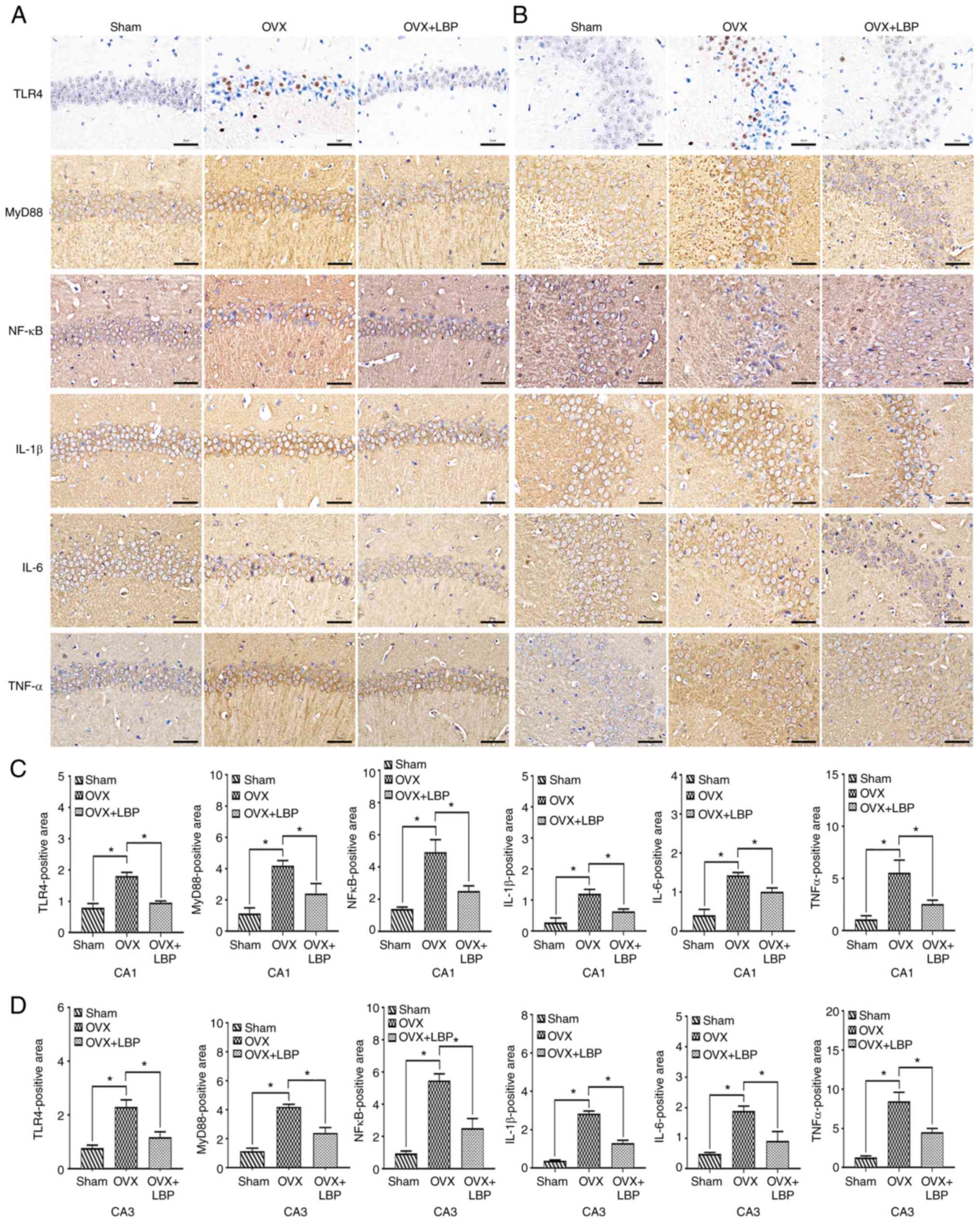 | Figure 7LBP inhibits the release of
inflammatory factors and reduces neuroinflammation in OVX model
mice. (A and B) Immunohistochemical staining was used to analyze
the expression of TLR4, MyD88, NF-κB, IL-6, IL-1β and TNF-α in the
hippocampal CA1 and CA3 regions. Scale bar, 20 µm. (C and D)
Positive staining area in the CA1 and CA3 regions for TLR4, MyD88,
NF-κB, IL-6, IL-1β and TNF-α expression in OVX model mice. Data
were analyzed using a one-way ANOVA and an LSD post hoc test.
*P<0.05 vs. the OVX group (n=5/group). LBP, Lycium
barbarum polysaccharide; OVX, ovariectomy; TLR, Toll-like
receptor; MyD88, myeloid differentiation primary response 88. |
Effect of LBP treatment on the expression
of inflammatory factors in OVX model mice
To further clarify the effect of LBP on brain
inflammation in OVX model mice, the expression of key inflammatory
proteins (IL6, IL-1β and TNF-α) in the hippocampus were analyzed.
As revealed in Fig. 7D, the
expression levels of these three factors in the hippocampal CA1 and
CA3 regions were significantly upregulated in the OVX group
compared with the sham group. Compared with the OVX group mice,
those treated with LPS exhibited downregulated expression levels of
IL-6, IL-1β and TNF-α. These findings were consistent with those
obtained from RT-qPCR analysis.
Discussion
Aging has been associated with a wide range of
effects in the CNS, including decreased cognitive ability,
increased oxidative stress, and chronic inflammation (39). In the present study, an
OVX-induced model was established to investigate the therapeutic
effect of LBP on learning and memory impairments associated with
estrogen deficiency, and the potential mechanisms underlying the
observed effects were determined. The presents results suggested
that LBP may attenuate learning and memory impairments and protect
against hippocampal neuron injury. Furthermore, LBP could suppress
the release of inflammatory factors and reduce neuroinflammation by
downregulating the expression levels of mRNA and proteins
associated with the TLR4/NF-κB signaling pathway and decreasing the
release of the proinflammatory cytokines IL-6, IL-1β and TNF-α.
Impairments in cognition, learning and memory are
the main clinical manifestations of neurodegenerative diseases
(40). Cognitive decline caused
by estrogen deficiency and other aging-related processes has become
increasingly common given the rapid aging of the global population
(41-44). Moreover, decreases in cognitive
function were demonstrated to severely impact the quality of life
of patients (45). Although
effective interventions to address these impairments and their
underlying mechanisms remain to be identified (3,46), accumulating evidence has
suggested that neuroinflammation in the brain may play a vital role
in the pathophysiology of cognitive decline (47). To determine whether the
anti-inflammatory properties of LBP exerted a protective effect
against neurodegenerative disease, an OVX-induced cognitive
impairment in vivo model was established, which is widely
accepted as an experimental model for the study of
neurodegenerative diseases (47,48).
The results of the present study revealed that serum
estrogen levels were significantly reduced following bilateral
ovarian ablation and that this could not be attenuated by LBP
treatment, which suggested that the OVX operation was successful
and that oral LBP treatment did not affect serum estrogen levels.
Subsequently, a novel target recognition testing method was used to
evaluate spatial learning and memory in experimental animals. The
present results indicated that the DI values were negative and the
PI values were significantly decreased in OVX mice. These findings
were consistent with previous data regarding the effects of OVX on
spatial learning ability of mice (48). LBP treatment significantly
increased PI and DI values and enhanced the spatial learning
ability. In addition, the findings of the open-field test
demonstrated that LBP treatment could improve OVX-induced
impairments on motor ability. These results indicated that LBP
could attenuate the effects of estrogen deficiency on spatial
learning and memory in aging mice.
Damage to neurons in the hippocampal CA1 and CA3
regions was reported to induce impairments in learning and memory
(49,50). Notably, the hippocampus has been
identified as a target for estrogen (51); however, to be the best of our
knowledge, the reason for the estrogen-related neurochemical
changes in the hippocampus remains unknown. The results of the
present study indicated that the effect of LBP on learning and
memory impairment may be associated with its protective effect on
hippocampal neurons. To verify this hypothesis, H&E and Nissl
staining were used to evaluate the effect of LBP on hippocampal
neurons. The results indicated that oral LBP exerted a
neuroprotective effect, significantly reducing the atrophy and loss
of hippocampal neurons. In addition, the number of living neurons
was negatively correlated with cognitive impairment. These findings
suggest that LBP treatment may attenuate OVX-induced injury to
hippocampal neurons and protect against impairments in learning and
memory.
OVX modeling was previously revealed to induce
neuroinflammation in the rodent brain, leading to impairments in
learning, memory and cognition (32,52,53). Further research indicated that
OVX increased TLR2, TL4R and proinflammatory cytokine IL-6 levels,
in addition to the activity of NF-κB in the hippocampus (54). Notably, several studies have
reported that inhibiting the activation of TLR4 in the hippocampus
could alleviate cognitive impairment (55-59). In addition, LBP could inhibit
lipopolysaccharide-induced inflammation by blocking the TLR4/NF-κB
signaling pathway in RAW264.7 cells (60). LBP also reduced liver
inflammation induced by CCl4 in Wistar rats by
downregulating the expression levels of components of the
TLR4/NF-κB signaling pathway (61). To further investigate whether LBP
plays a neuroprotective role in OVX model mice by regulating the
TLR4/NF-κB signaling pathway, the present study analyzed the
expression levels of TLR4, MyD88 and NF-κB in the CA1 and CA3
regions of the hippocampus using immunohistochemistry, RT-qPCR and
western blotting. These analyses revealed that LBP downregulated
the expression levels of all three of these factors, suggesting
that LBP may reduce hippocampal inflammation by inhibiting the
TLR4/NF-κB signaling pathway.
Perivascular edema appeared in the images of certain
figures, possibly caused by the immersion-fixation methodology that
was used for immunohistochemistry. The use of immersion-fixation of
the brain may not provide a thorough and suitable fixation which
frequently leads to false results due to the loss and/or decrease
and/or change of localization of the proteins and antigens in
question. However, the mice brains are considerably small compared
to the other experimental animals. In addition, the brain was fixed
in 4% paraformaldehyde for 24 h at 4°C which may also facilitate
the fixation to an extent. However, in the future study,
perfusion-fixation will be considered to avoid artifacts.
In addition to inducing neuronal damage in the
brain, OVX was revealed to activate astrocytes and microglia in the
hippocampus and increased the release of inflammatory cytokines
such as IL-6, IL-1β and TNF-α, leading to decreases in cognitive
function (47,54,62). The present experimental results
revealed that IL-6, IL-1β and TNF-α levels were significantly
decreased following LBP treatment, further supporting the theory
that LBP may reduce neuroinflammation. However, further studies are
required to determine whether LBP exerts its effects via astrocytes
or microglia.
In conclusion, the findings of the present study
suggested that oral LBP may significantly attenuate OVX-induced
learning and memory impairments in mice by blocking injury to
hippocampal neurons via reducing neuroinflammation. Therefore, LBP
may represent a potential therapeutic agent for the prevention and
treatment of learning and memory impairments in patients with
accelerated aging caused by ovarian dysfunction.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, ZM and YuL designed the study. XZ, JW, FB, JX
and ZC performed the research. XZ and YiL analyzed the data. YW and
ZM wrote and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Guidelines for the Protection and Use of
Experimental Animals (National Research Council of the National
Academies), and approved by the Animal Ethics Committee of General
Hospital of Ningxia Medical University, Ningxia, China (approval
no. 2020-449).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82060223 and 81460220), and the
Ningxia Natural Science Foundation (grant nos. 2020AAC03143 and
2020AAC03150).
References
|
1
|
Kumar A: Editorial: Neuroinflammation and
cognition. Front Aging Neurosci. 10:4132018. View Article : Google Scholar
|
|
2
|
Russell JK, Jones CK and Newhouse PA: The
role of estrogen in brain and cognitive aging. Neurotherapeutics.
16:649–665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Tang Y, Liu A, Jin X, Zhu J and Lu
X: Oral administration of Grifola Frondosa polysaccharides improves
memory impairment in aged rats via antioxidant action. Mol Nutr
Food Res. 61:17003132017. View Article : Google Scholar
|
|
4
|
Ryan J, Carriere I, Scali J, Ritchie K and
Ancelin ML: Life-time estrogen exposure and cognitive functioning
in later life. Psychoneuroendocrino. 34:287–298. 2009. View Article : Google Scholar
|
|
5
|
Lamar M, Resnick SM and Zonderman AB:
Longitudinal changes in verbal memory in older adults:
Distinguishing the effects of age from repeat testing. Neurology.
60:82–86. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hohman TJ, Beason-Held LL, Lamar M and
Resnick SM: Subjective cognitive complaints and longitudinal
changes in memory and brain function. Neuropsychology. 25:125–130.
2011. View
Article : Google Scholar :
|
|
7
|
Campisi J, Kapahi P, Lithgow GJ, Melov S,
Newman JC and Verdin E: From discoveries in ageing research to
therapeutics for healthy ageing. Nature. 571:183–192. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garbers C, Kuck F, Aparicio-Siegmund S,
Konzak K, Kessenbrock M, Sommerfeld A, Häussinger D, Lang PA,
Brenner D, Mak TW, et al: Cellular senescence Or EGFR signaling
induces interleukin 6 (IL-6) receptor expression controlled by
mammalian target of rapamycin (mTOR). Cell Cycle. 12:3421–3432.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Franceschi C, Garagnani P, Parini P,
Giuliani C and Santoro A: Inflammaging: A new immune-metabolic
viewpoint for age-related diseases. Nat Rev Endocrinol. 14:576–590.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renz H, Holt PG, Inouye M, Logan AC,
Prescott SL and Sly PD: An exposome perspective: Early-life events
and immune development in a changing world. J Allergy Clin Immunol.
140:24–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sly PD, Carpenter DO, Van den Berg M,
Stein RT, Landrigan PJ, Brune-Drisse MN and Suk W: Health
consequences of environmental exposures: Causal thinking in global
environmental epidemiology. Ann Glob Health. 82:3–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Floreani A, Leung PS and Gershwin ME:
Environmental basis of autoimmunity. Clin Rev Allergy Immunol.
50:287–300. 2016. View Article : Google Scholar
|
|
13
|
Ferrucci L and Fabbri E: Inflammageing:
Chronic inflammation in ageing, cardiovascular disease, and
frailty. Nat Rev Cardiol. 15:505–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liberale L, Montecucco F, Tardif JC, Libby
P and Camici GG: Inflamm-ageing: The role of inflammation in
age-dependent cardiovascular disease. Eur Heart J. 41:2974–2982.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franceschi C and Campisi J: Chronic
inflammation (inflammaging) and its potential contribution to
age-associated diseases. J Gerontol A Biol Sci Med Sci. 69(Suppl
1): S4–S9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang RP, Ho YS, Leung WK, Goto T and Chang
RC: Systemic inflammation linking chronic periodontitis to
cognitive decline. Brain Behav Immun. 81:63–73. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baulch JE, Acharya MM, Allen BD, Ru N,
Chmielewski NN, Martirosian V, Giedzinski E, Syage A, Park AL,
Benke SN, et al: Cranial grafting of stem cell-derived
microvesicles improves cognition and reduces neuropathology in the
irradiated brain. Proc Natl Acad Sci USA. 113:4836–4841. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JY, Joo B, Nam JH, Nam HY, Lee W, Nam
Y, Seo Y, Kang HJ, Cho HJ, Jang YP, et al: An aqueous extract of
herbal medicine ALWPs enhances cognitive performance and inhibits
LPS-induced neuroinflammation via FAK/NF-kappaB signaling pathways.
Front Aging Neurosci. 10:2692018. View Article : Google Scholar
|
|
19
|
Jacobs AH and Tavitian B: Noninvasive
molecular imaging of neuroinflammation. J Cereb Blood Flow Metab.
32:1393–1415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chandrasekaran A, Idelchik M and Melendez
JA: Redox control of senescence and age-related disease. Redox
Biol. 11:91–102. 2017. View Article : Google Scholar
|
|
21
|
Zeng P, Li J, Chen Y and Zhang L: The
structures and biological functions of polysaccharides from
traditional chinese herbs. Prog Mol Biol Transl Sci. 163:423–444.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Liu H, Wang W, Wang X, Zhang C,
Zhang J, Jing H, Ren Z, Gao Z, Song X and Jia L: Antioxidant and
anti-aging effects of acidic-extractable polysaccharides by
agaricus bisporus. Int J Biol Macromol. 106:1297–1306. 2018.
View Article : Google Scholar
|
|
23
|
Zhang R, Zhang X, Tang Y and Mao J:
Composition, isolation, purification and biological activities of
Sargassum Fusiforme Polysaccharides: A review. Carbohydr Polym.
228:1153812020. View Article : Google Scholar
|
|
24
|
Zhu Y, Yu X, Ge Q, Li J, Wang D, Wei Y and
Ouyang Z: Antioxidant and anti-aging activities of polysaccharides
from Cordyceps cicadae. Int J Biol Macromol. 157:394–400. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Li Z, Peng L, Jiang N, Liu Q, Zhang
E, Liang B, Li R and Zhu H: Lycium barbarum polysaccharide protects
human keratinocytes against UVB-induced photo-damage. Free Radic
Res. 51:200–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian X, Liang T, Liu Y, Ding G, Zhang F
and Ma Z: Extraction, structural characterization, and biological
functions of Lycium Barbarum Polysaccharides: A Review.
Biomolecules. 9:3892019. View Article : Google Scholar :
|
|
27
|
Chang RC and So KF: Use of anti-aging
herbal medicine, Lycium barbarum, against aging-associated
diseases. What do we know so far? Cell Mol Neurobiol. 28:643–652.
2008. View Article : Google Scholar
|
|
28
|
Zhang Z, Zhou Y, Fan H, Billy KJ, Zhao Y,
Zhan X, Yang L and Jia Y: Effects of Lycium barbarum
polysaccharides on health and aging of C. Elegans depend On
Daf-12/Daf-16. Oxid Med Cell Longev. 2019:63794932019.
|
|
29
|
Blair JA, Bhatta S and Casadesus G: CNS
luteinizing hormone receptor activation rescues ovariectomy-related
loss of spatial memory and neuronal plasticity. Neurobiol Aging.
78:111–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bohm-Levine N, Goldberg AR, Mariani M,
Frankfurt M and Thornton J: Reducing luteinizing hormone levels
after ovariectomy improves spatial memory: Possible role of
brain-derived neurotrophic factor. Horm Behav. 118:1045902020.
View Article : Google Scholar
|
|
31
|
Monthakantirat O, Sukano W, Umehara K,
Noguchi H, Chulikhit Y and Matsumoto K: Effect of miroestrol on
ovariectomy-induced cognitive impairment and lipid peroxidation in
mouse brain. Phytomedicine. 21:1249–1255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sbisa AM, Gogos A and van den Buuse M:
Spatial working memory in the touchscreen operant platform is
disrupted in female rats by ovariectomy but not estrous cycle.
Neurobiol Learn Mem. 144:147–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robinson MD, McCarthy DJ and Smyth GK:
EdgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar
|
|
34
|
Pertea M, Pertea GM, Antonescu CM, Chang
TC, Mendell JT and Salzberg SL: StringTie enables improved
reconstruction of a transcriptome from RNA-seq reads. Nat
Biotechnol. 33:290–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
The Gene Ontology Resource: 20 years and
still GOing strong. Nucleic Acids Res. 47:D330–D338. 2019.
View Article : Google Scholar :
|
|
36
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar :
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Shi Z, Zhu L, Li T, Tang X, Xiang Y, Han
X, Xia L, Zeng L, Nie J, Huang Y, et al: Neuroprotective mechanisms
of Lycium barbarum polysaccharides against ischemic insults by
regulating NR2B and NR2A containing NMDA receptor signaling
pathways. Front Cell Neurosci. 11:2882017. View Article : Google Scholar :
|
|
39
|
Fjell AM, McEvoy L, Holland D, Dale AM and
Walhovd KB: What is normal in normal aging? Effects of aging,
amyloid and Alzheimer's disease on the cerebral cortex and the
hippocampus. Prog Neurobiol. 117:20–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stanojlovic M, Pallais JP, Lee MK and Kotz
CM: Pharmacological and chemogenetic orexin/hypocretin intervention
ameliorates hipp-dependent memory impairment in the A53T mice model
of Parkinson's disease. Mol Brain. 12:872019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
No authors listed. 2020 Alzheimer's
disease facts and figures. Alzheimers Dement. Mar 10–2020.Epub
ahead of print.
|
|
42
|
Boyle PA, Yu L, Wilson RS, Leurgans SE,
Schneider JA and Bennett DA: Person-specific contribution of
neuropathologies to cognitive loss in old age. Ann Neurol.
83:74–83. 2018. View Article : Google Scholar :
|
|
43
|
Nascimento C, Di Lorenzo Alho AT, Bazan
Conceição Amaral C, Leite REP, Nitrini R, Jacob-Filho W,
Pasqualucci CA, Hokkanen SRK, Hunter S, Keage H, et al: Prevalence
of transactive response DNA-binding Protein 43 (TDP-43)
proteinopathy in cognitively normal older adults: systematic review
and meta-analysis. Neuropathol Appl Neurobiol. 44:286–297. 2018.
View Article : Google Scholar
|
|
44
|
Morgan KN, Derby CA and Gleason CE:
Cognitive changes with reproductive aging, perimenopause, and
menopause. Obstet Gynecol Clin North Am. 45:751–763. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bettio L, Rajendran L and Gil-Mohapel J:
The effects of aging in the hippocampus and cognitive decline.
Neurosci Biobehav Rev. 79:66–86. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rehman SU, Shah SA, Ali T, Chung JI and
Kim MO: Anthocyanins reversed D-galactose-induced oxidative stress
and neuroinflammation mediated cognitive impairment in adult rats.
Mol Neurobiol. 54:255–271. 2017. View Article : Google Scholar
|
|
47
|
Huang C, Irwin MG, Wong G and Chang R:
Evidence of the impact of systemic inflammation on
neuroinflammation from a non-bacterial endotoxin animal model. J
Neuroinflammation. 15:1472018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang J, Wu Y, Yuan H, Yang Y, Xiong Q,
Liang C, Li Z, Li C, Zhang G, Lai X, et al: Dendrobium officinale
polysaccharides attenuate learning and memory disabilities via
anti-oxidant and anti-inflammatory actions. Int J Biol Macromol.
126:414–426. 2019. View Article : Google Scholar
|
|
49
|
Zhang X, Bai L, Zhang S, Zhou X, Li Y and
Bai J: Trx-1 ameliorates learning and memory deficits in
MPTP-induced Parkinson's disease model in mice. Free Radic Biol
Med. 124:380–387. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Arroyo-García LE, Tendilla-Beltrán H,
Vázquez-Roque RA, Jurado-Tapia EE, Díaz A, Aguilar-Alonso P,
Brambila E, Monjaraz E, De La Cruz F, Rodríguez-Moreno A and Flores
G: Amphetamine sensitization alters hippocampal neuronal morphology
and memory and learning behaviors. Mol Psychiatry. Jun 17–2020.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ramani M, Kumar R, Halloran B, Lal CV,
Ambalavanan N and McMahon LL: Supraphysiological levels of oxygen
exposure during the neonatal period impairs signaling pathways
required for learning and memory. Sci Rep. 8:99142018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu J, Xu Y, Hu W, Gao Y, Ni X, Sheng H and
Liu Y: Exercise ameliorates depression-like behavior and increases
hippocampal BDNF level in ovariectomized rats. Neurosci Lett.
573:13–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kouhestani S, Jafari A and Babaei P:
Kaempferol attenuates cognitive deficit via regulating oxidative
stress and neuroinflammation in an ovariectomized rat model of
sporadic dementia. Neural Regen Res. 13:1827–1832. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saied NM, Georgy GS, Hussien RM and Hassan
WA: Neuromodulatory effect of curcumin on catecholamine systems and
inflammatory cytokines in ovariectomized female rats. Clin Exp
Pharmacol Physiol. 48:337–346. 2021. View Article : Google Scholar
|
|
55
|
Lu SM, Gui B, Dong HQ, Zhang X, Zhang SS,
Hu LQ, Liu HL, Sun J and Qian YN: Prophylactic lithium alleviates
splenectomy-induced cognitive dysfunction Possibly by inhibiting
hippocampal TLR4 activation in aged rats. Brain Res Bull.
114:31–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhong Q, Zou Y, Liu H, Chen T, Zheng F,
Huang Y, Chen C and Zhang Z: Toll-like receptor 4 deficiency
ameliorates β2-microglobulin induced age-related cognition decline
due to neuroinflammation in mice. Mol Brain. 13:202020. View Article : Google Scholar
|
|
57
|
Wang S, Zhang X, Zhai L, Sheng X, Zheng W,
Chu H and Zhang G: Atorvastatin attenuates cognitive deficits and
neuroinflammation induced by Aβ1-42 involving modulation
of TLR4/TRAF6/NF-κB pathway. J Mol Neurosci. 64:363–373. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Q, Wu HH, Wang Y, Gu GJ, Zhang W and
Xia R: Neural stem cell transplantation decreases neuroinflammation
in a transgenic mouse model of Alzheimer's disease. J Neurochem.
136:815–825. 2016. View Article : Google Scholar
|
|
59
|
He P, Yan S, Zheng J, Gao Y, Zhang S, Liu
Z, Liu X and Xiao C: Eriodictyol attenuates LPS-induced
neuroinflammation, amyloidogenesis, and cognitive impairments via
the inhibition of NF-κB in male C57BL/6J mice and BV2 microglial
cells. J Agric Food Chem. 66:10205–10214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu Y, Sheng H, Bao Q, Wang Y, Lu J and Ni
X: NLRP3 inflammasome activation mediates estrogen
deficiency-induced depression- and anxiety-like behavior and
hippocampal inflammation in mice. Brain Behav Immun. 56:175–186.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peng Q, Liu H, Shi S and Li M: Lycium
ruthenicum polysaccharide attenuates inflammation through
inhibiting TLR4/NF-κB signaling pathway. Int J Biol Macromol.
67:330–335. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gan F, Liu Q, Liu Y, Huang D, Pan C, Song
S and Huang K: Lycium barbarum polysaccharides improve CCl4-induced
liver fibrosis, inflammatory response and TLRs/NF-kB signaling
pathway expression in wistar rats. Life Sci. 192:205–212. 2018.
View Article : Google Scholar
|