Introduction
Recent research has demonstrated that fibroblasts
can be reprogrammed into different cell types by altering gene
expression regulation, and can even be induced to differentiate
into terminal cell types, including neurons, cardiac cells and
macrophage-like cells (1,2).
Induced pluripotent stem cells (iPSCs) can be successfully produced
by reprogramming embryonic or adult somatic cells with four
pluripotent transcription factors (3). However, the slow reprogramming
process, low rearrangement efficiency, reprogramming inconsistency
and the potential oncogenic hazards, limit the clinical
applications of iPSCs (4).
Currently, some small molecules can be used to reduce the risk and
improve reprogramming efficiency, and produce unmodified iPSC lines
with improved suitability for downstream applications (5,6).
The transforming growth factor (TGF)-β superfamily
plays an important role in a variety of pathophysiological
processes, as well as in regulating cellular responses, including
differentiation, proliferation, growth, adhesion, migration,
survival and the specification of developmental fate (7). RepSox, a selective inhibitor of the
TGF-β-RI/ALK5 pathway, has been used to replace cMyc and Sox2
during cellular reprogramming of murine embryonic fibroblasts into
iPSCs (8). Moreover, RepSox can
attenuate skin fibrosis (9),
improve the developmental potential of somatic cell nuclear
transfer embryos (10-12), and promote the development of
leukemic stem/progenitor cells (13). RepSox has been demonstrated to
effectively block the phosphorylation of Smads by inhibiting the
TGF-β signaling pathway, and this inhibition may contribute to a
more efficient induction of iPSCs (14,15).
Bone morphogenetic proteins (BMPs) are members of
the TGF-β superfamily, and have multiple functions in promoting the
differentiation of pluripotent stem cells into adipocytes,
osteoblasts, chondrocytes and muscle cells (16). Recent studies have confirmed that
human adipose tissue is an important means of energy storage,
showing important features in energy metabolism, immune function
and blood glucose regulation (17,18). However, to the best of our
knowledge, at present, there is no report available on the direct
differentiation of sheep fibroblasts into adipocytes induced by
small molecule compounds. Given the importance of TGF-β in
adipogenesis and the potential application of RepSox in clinical
medicine (19), the present
study aimed to evaluate the effects of RepSox on adipogenesis in
adult sheep fibroblasts, and analyzed the changes in biological
characteristics during the process of directed differentiation.
Materials and methods
Cell recovery and culture of Mongolian
sheep fibroblasts
A male adult Mongolian sheep was provided by the
Animal Experimental Base of Institute of Beijing Animal Science and
Veterinary, Chinese Academy of Agricultural Sciences. The use of
animals and all experimental procedures were approved by
Institutional Animal Care and Use Committee (IACUC) for Ethics of
Bengbu Medical College (approval no. 2017-016). Following local
anesthesia with 0.5 ml 2% lidocaine hydrochloride subcutaneously,
the ear edge tissue of Mongolian sheep was obtained using an ear
puncher for fibroblast culture.
The Mongolian sheep adult fibroblasts (SAFs) were
cultured using the tissue adherent culturing method and enzyme
digestion, and cryopreserved for >10 years in liquid nitrogen.
To recover the cells, the frozen vials were removed from the liquid
nitrogen and rapidly thawed in a water bath at 42°C, following
which they were transferred into a flask in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and incubated at 37°C in a 5%
CO2 atmosphere.
Effects of RepSox on cell proliferation,
apoptosis and cell cycle
RepSox (cat. no. R0158-25MG) was purchased from
Sigma-Aldrich; Merck KGaA and dissolved in DMSO solution. The SAFs
(below passage 6) in the logarithmic phase were treated with RepSox
(10, 15, 20 and 25 µM) for four days. Negative control
cultures were maintained in the same volume of DMSO without RepSox.
After fixing with 4% paraformaldehyde for 18 min, permeabilization
with 0.1% Triton X-100 (v/v) for 10 min and blocking with 1% bovine
serum albumin (BSA, w/v, Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, the effect of RepSox on the cell actin cytoskeleton
was detected using Alexa Fluor 568 phalloidin labeling staining
(1:200; cat. no. A12380; Invitrogen; Thermo Fisher Scientific,
Inc.). Cell proliferation was detected via immunofluorescence using
a monoclonal antibody against BrdU (1:200; Cell Signaling
Technology, Inc.) for 2 h at room temperature and incubation with
Alexa Fluor 546-labeled secondary antibody (1:500; cat. no. A10036;
Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at 37°C, after
cell fixation and blocking at room temperature (20,21). The results of the
immunofluorescence staining were observed under a fluorescence
microscope (IX71, Olympus, Inc.).
Cells were harvested, and fixed with 70% ethanol at
-20°C overnight. The cells were then stained with propidium iodide
(50 µg/ml) following treatment with RNaseA (100
µg/ml). The analysis of cell cycle distribution of
3×104 cells was carried out using a flow cytometer
(Cytomics FC 500; Beckman Coulter, Inc.). Cell apoptosis was
evaluated using an Annexin V-FITC/PI kit (cat. no. C1062M, Beyotime
Institute of Biotechnology). In brief, ~1×105 cells were
harvested and resuspended in 195 µl of assay buffer.
Subsequently, 5 µl of Annexin V-FITC and 10 µl of PI
were added and mixed gently before incubated at room temperature in
dark for 25 min. Samples were analyzed immediately by a flow
cytometer (Cytomics FC 500; Beckman Coulter, Inc.). Metaphase
chromosome spreads were prepared, fixed and stained with
Giemsa.
Histone phosphorylation and epigenetic
immunofluorescence analysis
The fibroblasts were fixed with 4% paraformaldehyde,
followed by permeabilization with 0.1% Triton X-100 (v/v) for 10
min and blocking with 3% BSA (w/v, Sigma-Aldrich; Merck KgaA).
Cells were incubated with the following primary antibodies for 2 h
at room temperature or overnight at 4°C: Epigenetic markers,
anti-histone acH3K9 (1:200; Santa Cruz Biotechnology, Inc.) and
anti-histone meH3K9 (1:500; Abcam), and the histone phosphorylation
markers, anti-phosphorylated (p)-H3S10 (1:200; Santa Cruz
Biotechnology, Inc.) and anti-p-H3S28 (1:500; Abcam). The catalog
numbers for all primary antibodies are presented in Table SI. Subsequently, the cells were
incubated with 488/-labeled secondary antibody (1:500; cat. no.
A21206, Invitrogen; Thermo Fisher Scientific, Inc.) for 1 h at
37°C. The cell nucleus was counterstained with 10 µg/ml PI
for 10 min. The results of the immunofluorescence staining were
observed under a confocal microscope (TE-2000-E, Nikon, Inc.). In
addition, the effects of RepSox on the TGF-β pathway and histone
modifications were also detected via incubating with anti-Smad1
(1:200; Acris Antibodies; OriGene Technologies, Inc.), anti-p-Smad3
(1:500; BIOSS), anti-acH3K9 (1:200; Santa Cruz Biotechnology, Inc.)
and anti-meH3K9 (1:500; Abcam) antibodies according to the
manufacturer's protocols for direct staining of using flow
cytometry (Cytomics FC 500; Beckman Coulter, Inc.),
respectively.
RepSox treatment and detection of
adipocyte differentiation
For adipogenic differentiation, the fibroblasts were
pre-treated with 15 µM RepSox for three days, and then
further cultured for 14 days in adipocyte-inducing differentiation
(AID) medium supplemented with 1.7 mM insulin, 1 mM dexamethasone
and 0.5 mM 3-isobutyl-1-methylxanthinethe. The formation of lipid
droplets was assessed using Oil Red O staining kit (cat. no.
C0158S, Beyotime Institute of Biotechnology) according to the
manufacturer's instructions, and the expression levels of the
fat-specific genes, lipoprotein lipase (LPL) and peroxisome
proliferator-activated receptor γ (PPARγ), were detected by reverse
transcription-quantitative PCR (RT-qPCR).
Influence of RepSox on the BMP and TGF-β
pathways during adipocyte differentiation
The effects of RepSox on the BMP and TGF-β pathways
during adipocyte differentiation were evaluated by western blot
analysis and RT-qPCR. Total proteins from the different groups were
extracted using the total protein extraction kit according to the
manufacturer's instructions (cat. no. P0028; Beyotime Institute of
Biotechnology) and the concentration of each sample was determined
using the BCA kit (P0010, Beyotime Institute of Biotechnology).
β-actin (1:5,000; Affinity Biosciences, Inc.) was used as internal
reference protein. Equal amounts of protein (40 µg) were
separated with 10% SDS-polyacrylamide gel electrophoresis and
transferred to PVDF membranes. The membranes were blocked with 5%
skimmed milk powder diluted in TBST at room temperature for 1 h,
and incubated with the following primary antibodies overnight at
4°C: Anti-Smad1, anti-Smad2 (1:300; Acris Antibodies; OriGene
Technologies, Inc.), anti-p-Smad3, anti-Smad7, anti-BMP2 and
anti-BMP3 (1:500; BIOSS), pluripotent markers anti-octamer-binding
transcription factor 4 (Oct4), anti-Sox2, anti-L-Myc (1:1,000;
Abcam) and anti-Nanog (1:500; Cell Signaling Technology, Inc.), as
well as cell surface markers anti-E-cadherin, anti-N-cadherin,
anti-CTGF and anti-collagen I (1:500; BIOSS). The blots were
developed using Beyo ECL Plus reagent (cat. no. P0018M, Beyotime
Institute of Biotechnology, Inc.) and analyzed using Bio-Rad
Quantity One software (Bio-Rad Laboratories, Inc.). The catalog
numbers for all primary antibodies are presented in Table SI (22).
A subset of candidate genes specific to the BMP and
TGF-β pathways were used to initially evaluate the effects of
RepSox on the plasticity of sheep fibroblasts by semi-quantitative
PCR. Total RNA was extracted from the different groups treated with
RepSox using TRIzol® reagent (cat. no. 15596026,
Invitrogen; Thermo Fisher Scientific, Inc.), and reverse
transcribed into cDNA using a PrimeScript RT reagent kit (cat. no.
RR037A, Takara Bio, Inc.) as described in the protocol. An Applied
Biosystems QuantStudio 6 Flex thermocycler was employed to perform
quantitative PCR using a TB Green Premix Ex Taq kit (cat. no.
RR42LR, Takara Bio, Inc.). The PCR conditions were as follows:
Denaturation at 94°C for 30 sec, annealing at 55-63.5°C for 30 sec
and elongation at 72°C for 1 min for a total of 33 cycles with an
initial denaturation at 95°C for 4 min and a final extension of 5
min. Amplified fragments were then subjected to electrophoresis on
a 1% agarose gel stained with Gel-Red (cat. no. D0140, Beyotime
Institute of Biotechnology, Inc.) for visualization (23), and the primers used for PCR are
presented in Table SII.
Bioinformatics analysis on
RNA-sequencing
Total RNA was extracted from two samples, DMSO
control (DC) and 15 µM RepSox for 3 days (Rep3), using the
Arcturus PicoPure RNA Isolation kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Two cDNA libraries were constructed at
Sangon Biotech Co., Ltd., subjected to 125 bp end sequencing using
an IlluminaHiSeq™ 2500 platform (Illumina, Inc.). Raw reads were
filtered to obtain high-quality clean reads. Each sample was then
mapped to a reference genome with TopHat2 (version 2.0.3.12)
(24). The expression levels
were normalized using the Fragments Per Kilobase of transcript per
Million mapped reads method. DESeq (an R package, http://www.rproject.org/) was used to evaluate
differentially expressed genes (DEGs) in both groups, of which
genes with a fold-change ≥2 and a false discovery rate <0.05
were deemed to be the significant DEGs (25). The Gene Ontology (GO)
classifications were compared between the upregulated and
downregulated unigenes using the Web Gene Ontology Annotation Plot
method. clusterProfiler (version 3.8.1) from the R software package
was used to annotate the pathways related to the DEGs and compared
against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database
(26). To understand the dynamic
changes and absolute expression magnitude between the two samples,
the genes associated with the top 25 pathways were separated for
further research.
Statistical analysis
All data are expressed as the mean ± SD from at
least three independent experiments. Statistical significance
(P<0.05) was determined using a paired Student's t-test or
one-way ANOVA with Dunnett's for multiple pairwise comparisons.
GraphPad Prism 7.0 software (GraphPad Software Inc.) was used for
statistical analysis and for the generation of graphs.
Results
Effects of RepSox on the morphology of
sheep fibroblasts
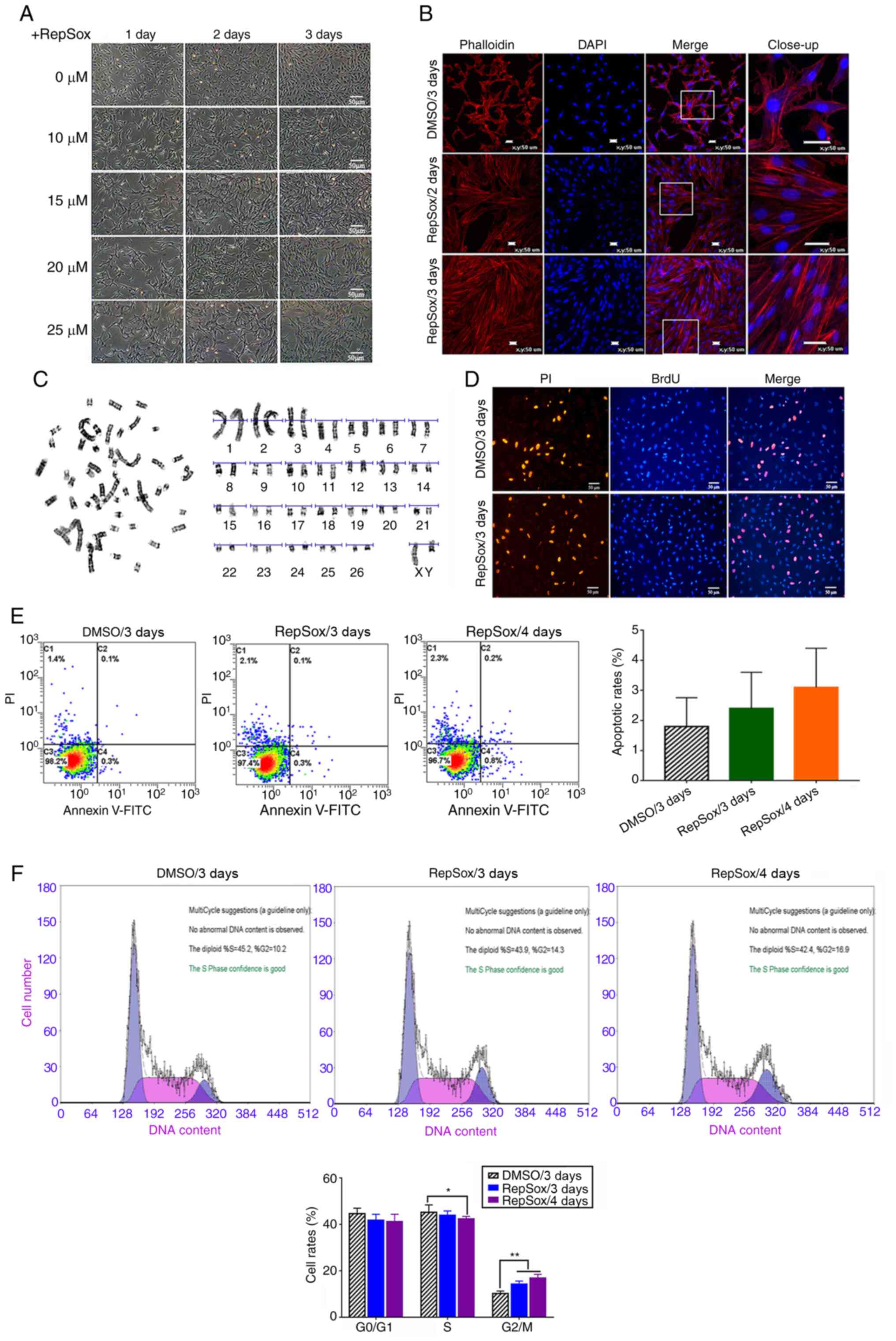
Following RepSox treatment for three days, the sheep
fibroblasts acquired a notably different morphology, and
transformed from a spindle shape into an elongated shape with more
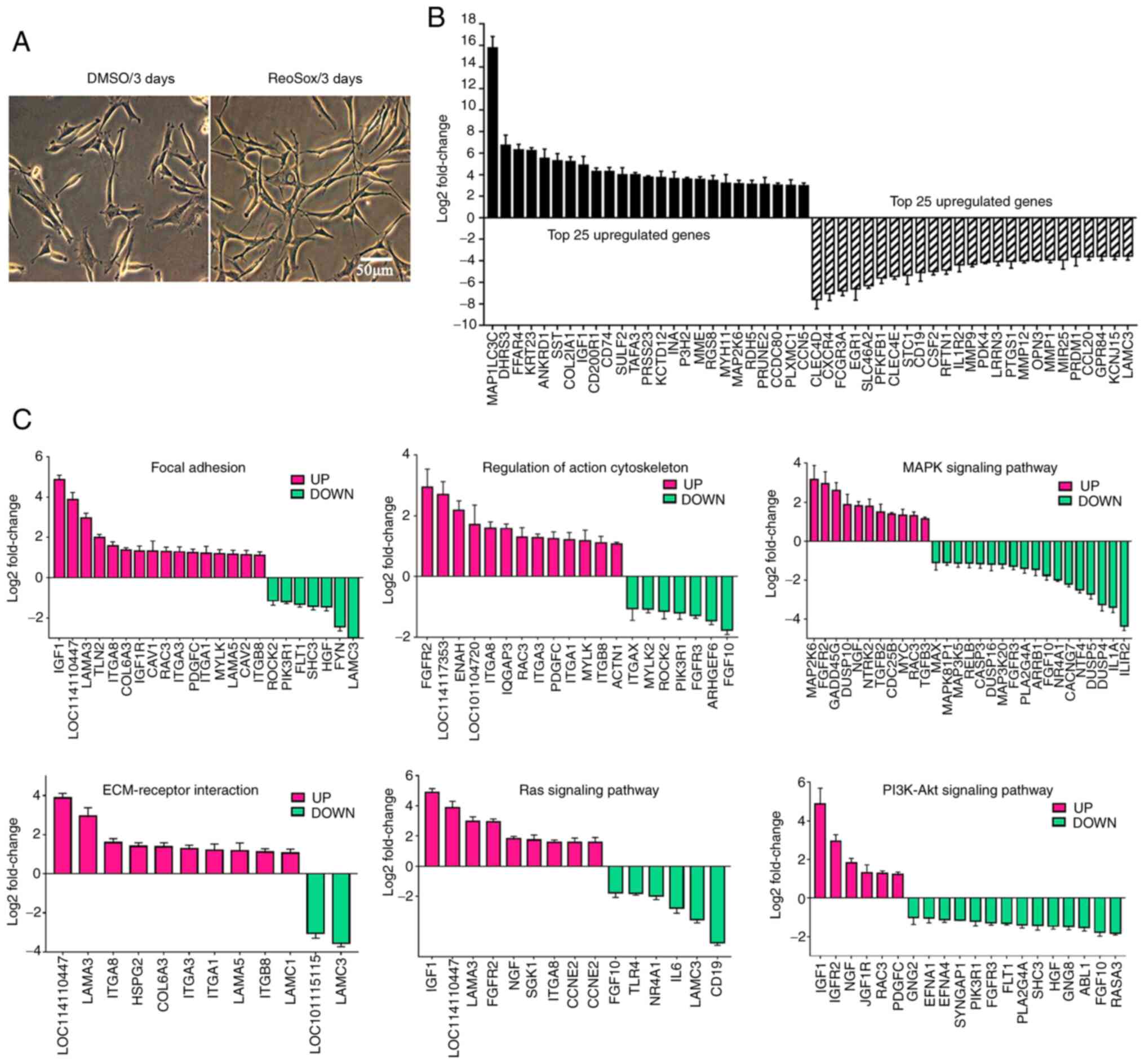
present bunching (Fig. 1A).
Moreover, the fibroblasts treated with 10 and 15 µM RepSox
were more slender in shape, and the microfilament skeleton
gradually changed to a parallel arrangement, and displayed a
notably different actin organization, as detected by phalloidin
labeling staining (Fig. 1B).
However, following treatment with >20 µM RepSox for three
days, the intercellular space was increased, the cells were more
adhesive to the culture plate and formed a poly heap, and cell
morphology gradually became flat and their three-dimensional
structure was lost (Fig.
1A).
Effects of RepSox on cell proliferation
and apoptosis
Cell proliferation was not markedly affected by
RepSox, as determined by BrdU indirect immunofluorescence assay
(Fig. 1D). Moreover, there were
no substantial differences in the rates of cell apoptosis and cell
death between the RepSox-treated groups and the DC (Fig. 1E). However, RepSox treatment
resulted in a significant increase in the number of cells in the
G2/M phase (P<0.05), while the number of cells in the
S phase was slightly lower than that in the DC (Fig. 1F). Chromosome G-Banding analysis
demonstrated that the percentage of RepSox-treated diploid cells
(2n=60) was 93.6% (Fig. 1C).
Furthermore, no chromosome aberration was observed, which suggested
that fibroblasts treated with RepSox still possessed genetic
stability.
RepSox regulates histone epigenetic
modifications
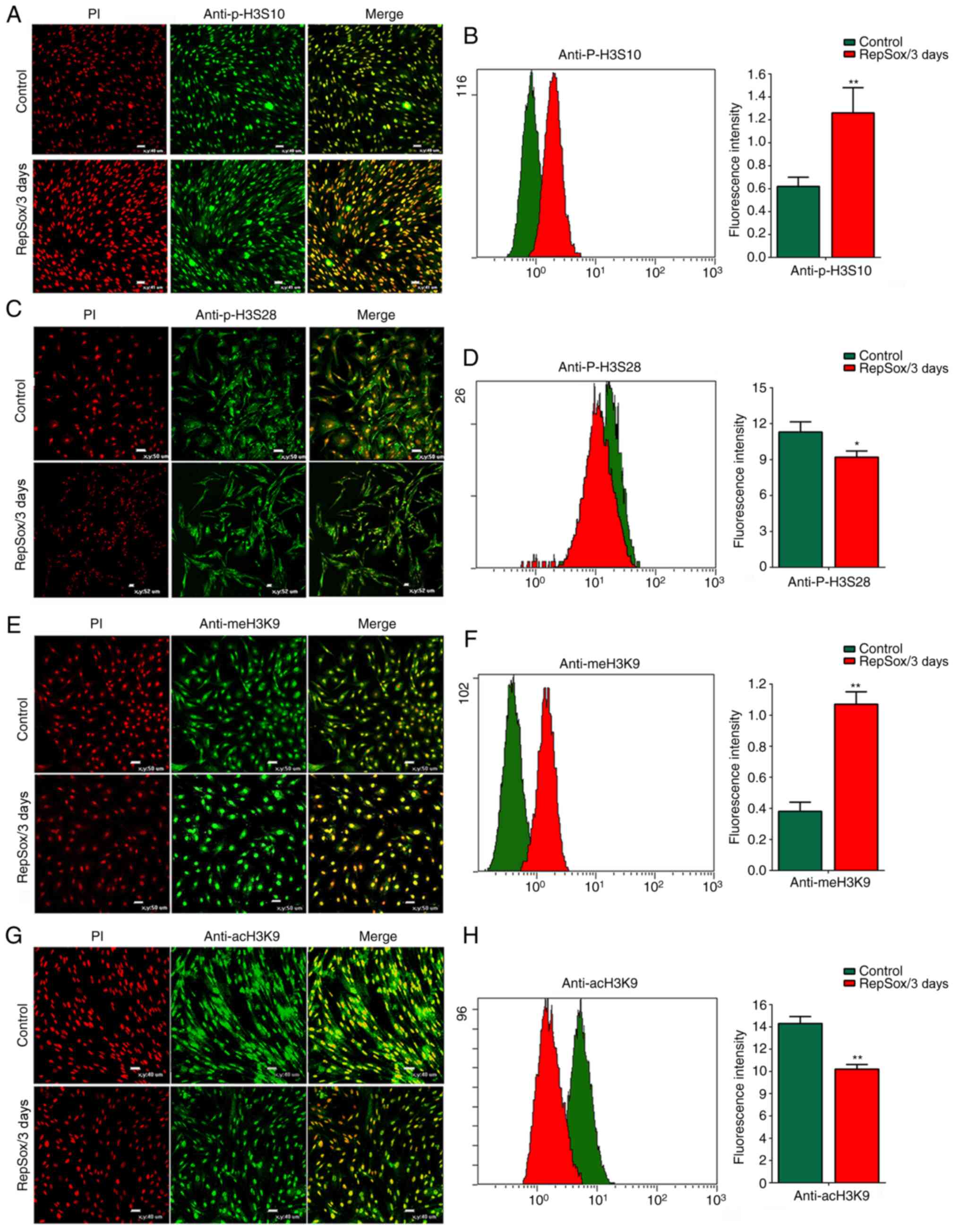
Previous studeis have demonstrated that the
phosphorylation of H3S10 plays an important role in the initiation
of transcription and aggregation of mitotic chromosomes, which is
critical for the initiation of the G2 phase, by
regulating gene transcription activity (27,28). In the present study, compared
with the control group, RepSox led to a considerable increase in
the level of H3S10 phosphorylation (increased by 95.6±3.66%;
Fig. 2A and B), while H3S28
phosphorylation was reduced by 26.3% (Fig. 2C and D). These results confirmed
that RepSox treatment resulted in a marked increase in the number
of cells in the G2/M phase by regulating histone
phosphorylation. By contrast, the meH3K9 level notably increased by
74.7±2.38% (Fig. 2E and F), and
the level of acH3K9 acetylation decreased by 64.8±6.38% (Fig. 2G and H). There was a
substantially negative association between H3K9 acetylation and
methylation.
RepSox pre-treatment enhances the
differentiation potential towards adipocytes
RepSox-treated fibroblasts could differentiate into
mesodermal lineage adipocytes under specific AID conditions. The
induced cells exhibited multipolar projections, the volume became
large and the intracellular lipid droplets in the cytoplasm
gradually increased after induced differentiation for 10 days. Over
time, small lipid droplets gradually gathered into bunches and
formed large lipid droplets, and the nuclei were crowded to the
edge of the cell. Moreover, lipid droplets in induced adipocytes
were confirmed by Oil Red O staining (Fig. 3A). The cells expressed multiple
adipogenic markers, including LPL and PPARγ (Fig. 3B). Previous research has reported
that C/EBPβ is transiently induced during the early phase of
adipocyte differentiation, while C/EBPα is upregulated during the
terminal stages of adipogenesis (29). The present study found that
RepSox treatment for two and four days led to a marked upregulation
in the levels of C/EBPα and C/EBPβ (Fig. 3B). Moreover, the expression of
adipogenic transcription factor 422/aP2 was also substantially
increased, which is regulated by C/EBPα (Fig. 3B).
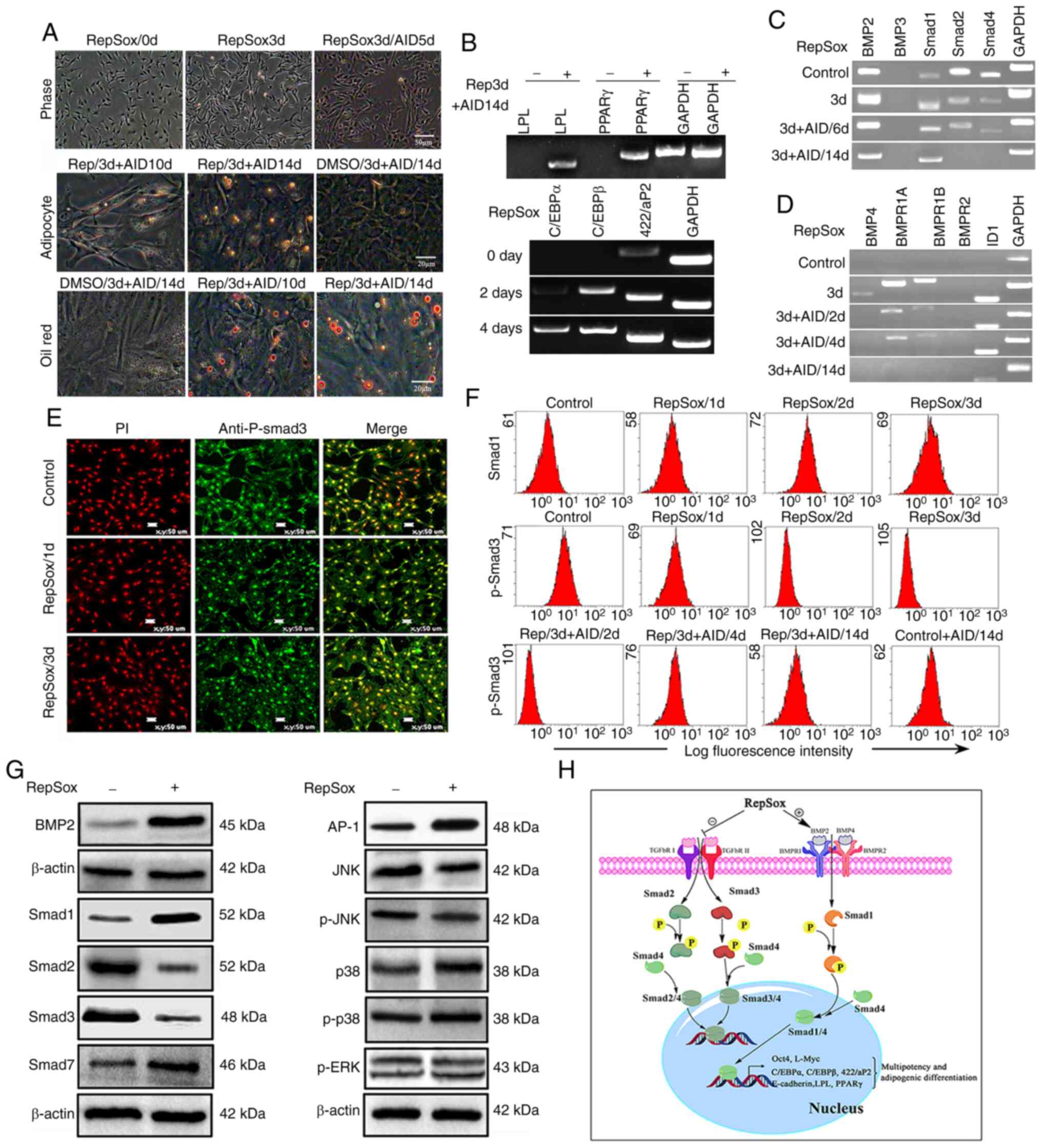 | Figure 3RepSox pretreatment promotes the
differentiation potential of SAFs toward adipocytes. (A)
Fibroblasts were pretreated with 15 µM RepSox for three
days, and then further cultured for 14 days in adipocyte-inducing
differentiation. Lipid droplets were confirmed by Oil Red O
staining. (B) Expression levels of multiple adipogenic cell marker
genes were analyzed via semi-quantitative PCR. (C) Expression
levels of Smad2 and Smad4 were downregulated in response to RepSox
treatment. (D) Expression levels of BMP2, BMP4, BMPR1A, BMPR1B and
ID1 were markedly upregulated following RepSox treatment and at the
early stage of adipogenesis. (E) The expression of p-Smad3 markedly
decreased following prolonged treatment with RepSox. (F) Cells were
co-labeled with Smad and P-Smad3, and analyzed by flow cytometry.
(G) Differential expression of key genes of the TGF-β and BMP
pathways were detected by western blot analysis. (H) RepSox
inhibited TGF-β signaling and activated BMP signaling in SAFs. SAF,
sheep adult fibroblasts; BMP, bone morphogenetic protein; ID1,
DNA-binding protein inhibitor ID-1; TGF-β, transforming growth
factor-β; P-, phosphorylated; AID, adipocyte-inducing
differentiation medium; d, day. |
RepSox treatment inhibits TGF-β and
promotes BMP signaling in SAFs
Following treatment with 15 µM RepSox for
three days, the expression levels of components of the TGF-β
signaling pathway were markedly decreased, including Smad2
(0.06-0.29-fold of control) and Smad3 (0.08-0.25-fold) (Fig. 3C and G). Furthermore, the
expression of p-Smad3 was notably downregulated following prolonged
treatment with RepSox and AID induction (Fig. 3E and F). Additionally, an evident
increase in the levels of Smad1 protein was also observed (Fig. 3G). However, a notable increase in
the expression of BMP2 was also observed, as opposed to BMP3, in
response to RepSox treatment (Fig.
3C). The expression levels of BMP4, BMPR1A,
BMPR1B and DNA-binding protein inhibitor ID-1 (ID1) were
evidently upregulated following RepSox treatment and at the early
stage of adipogenesis (Fig. 3D).
Moreover, the expression levels of BMP pathway-related factors were
notably downregulated, and no expression was observed at the late
stage of adipogenesis (Fig. 3C and
D), indicating that the BMP pathway was not active in the later
stages of adipogenesis.
MAPK signaling pathways, including the ERK, JNK and
p38 sub-pathways, are some of the key pathways involved in
adipogenesis (30). In the
present study, RepSox reduced the phosphorylation of Smad3
(Fig. 3E) and JNK, but not ERK
or p38 activation (Fig. 3G).
Furthermore, the expression of Smad7, one of the I-Smad proteins,
which is able to block TGF-β signaling pathway selectively and
replace Sox2 to enhance reprogramming, was reinforced in
RepSox-treated fibroblasts (Fig.
3G). These results suggested that RepSox regulated adipogenesis
by inhibiting the Smad3 and JNK/AP-1 pathways.
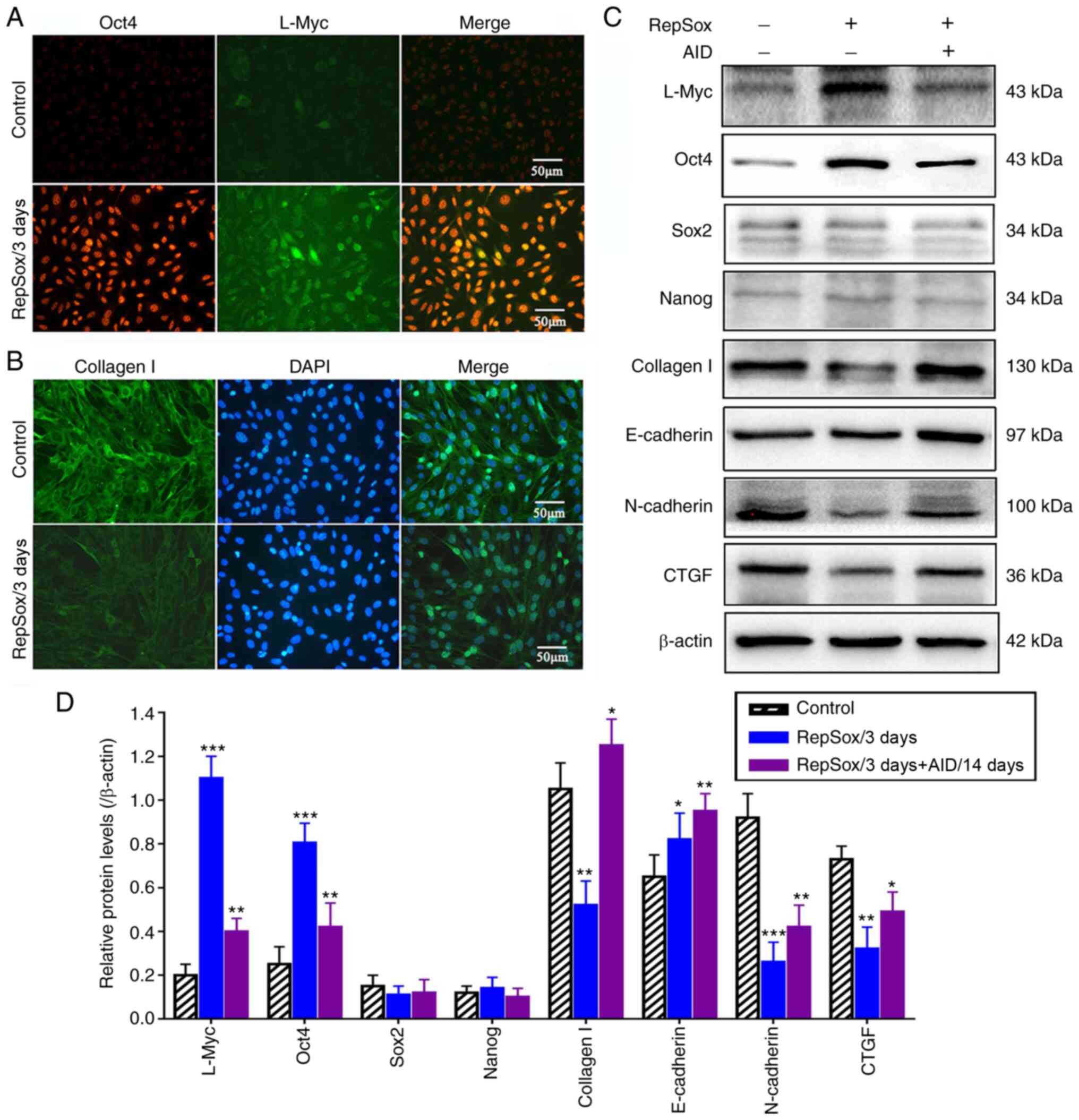
RepSox treatment promotes Oct4 expression
and accelerates adipogenesis by inducing MET
RepSox markedly promoted the expression of Oct4
(4.5-fold, Fig. 4D); however,
Sox2 and Nanog expression were not detected (Fig. 4A and B), which indicated that the
activation of Oct4 may play a crucial role in the acquisition of
cell multipotency. These results also suggested that RepSox did not
replace Sox2 by directly activating endogenous Sox2. By contrast,
it was found that RepSox did increase the expression of L-Myc by
5-fold, which is a close homolog of cMyc that can functionally
replace it in reprogramming. Therefore, although RepSox probably
functions at the level of the initial somatic cell population to
replace Oct4 and cMyc, it does not act by replacing Sox2 and
Nanog.
Previous studies have demonstrated that the process
of EMT is dependent on autocrine TGF-β signaling (31,32). Thus, in the present study, it was
hypothesized that RepSox regulates the expression of E-cadherin and
N-cadherin by regulating the BMP family. In addition, the
expression levels of connective tissue growth factor (CTGF) and
collagen I were markedly decreased following treatment with RepSox
(Fig. 4B-D). Moreover, Smad7
constitutively formed a complex with the TGF-β receptors, and the
inhibitory effect of Smad7 on the promoter activity of collagen I
was enhanced in RepSox-treated fibroblasts. Thus, it was
demonstrated that RepSox exerted the most potent inhibitory effects
on TGF-β-induced expression of target genes (CTGF and collagen I)
to regulate cell morphology and cell junctions.
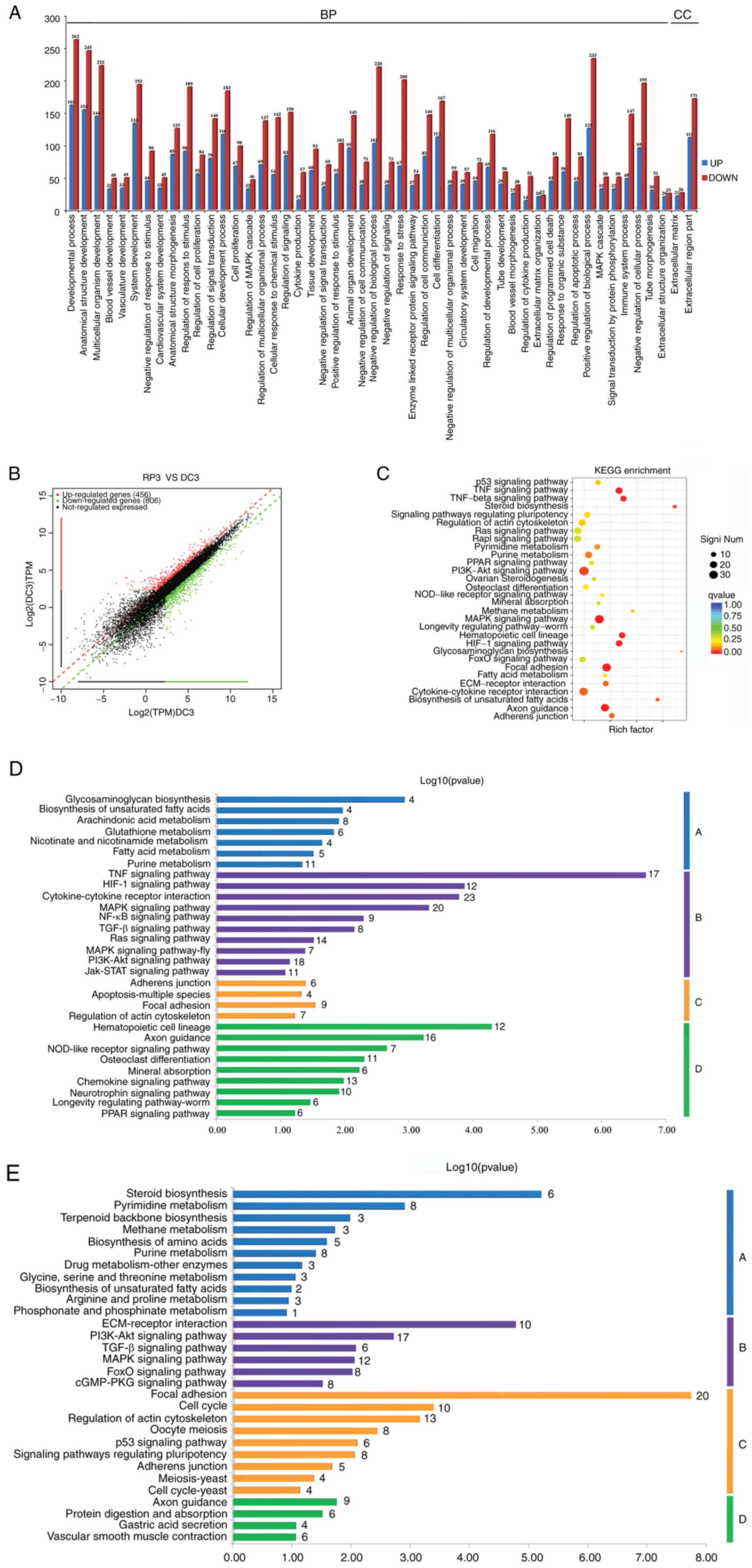
High-throughput RNA sequencing for DEGs
between RepSox treatment and control groups
GO and KEGG analysis revealed that the six most
enriched pathways between the Rep3 and DC groups were associated
with 'Focal adhesion', 'MAPK signaling pathway', 'TGF-β signaling
pathway', 'PI3K/Akt signaling pathway', 'Regulation of actin
cytoskeleton' and 'Ras signaling pathway'. Moreover, upregulated
DEGs were mainly enriched in the PI3K/Akt signaling pathway, while
the MAPK signaling pathway was the most enriched pathway for the
downregulated DEGs. Both the PI3K/Akt and MAPK signaling pathways
participated in the regulation of a number of cellular processes,
such as cell proliferation regulation, cell junction, cell
morphogenesis, cell migration and differentiation. The pathways
labeled with red boxes indicate the most enriched DEGs between the
two groups (Fig. 5C).
A total of 50 enriched GO terms and scatter plots of
DEGs between the Rep3 and DC groups are shown in Fig. 5A and B. Furthermore, the top 25
upregulated and top 25 downregulated genes are presented in
Fig. 6B. Additionally, 30
enriched pathways consisting of upregulated genes were further
classified into four categories that are essential for cell
functions: 'Metabolism', 'Environmental Information Processing',
'Cellular Processes' and 'Organismal Systems' (Fig. 5D). Similarly, 30 enriched
pathways consisting of downregulated genes were also further
classified into the same four categories (Fig. 5E).
The majority of genes involved in the 'Regulation of
actin cytoskeleton', 'MAPK signaling pathway', 'Focal adhesion' and
'ECM-receptor interaction' were significantly up-or downregulated
in the RepSox-treated fibroblasts (Fig. 6C), which suggested that RepSox
markedly promoted the cell morphogenesis of sheep fibroblasts
(Fig. 6A). Moreover, these DEGs
involved in various metabolic processes, such as 'Pyrimidine
metabolism', 'Steroid biosynthesis', 'Methane metabolism' and
'Purine metabolism', were markedly upregulated in RepSox-treated
fibroblasts, which indicates that high concentrations of RepSox
could cause changes in cell metabolism (Fig. 5D). In addition, the DEGs involved
in most signaling pathways, such as PI3K-Akt, MAPK, Ras and TGF-β
signaling pathways, were markedly downregulated in the
RepSox-treated fibroblasts, which suggests that RepSox can affect
cell characteristics by altering multiple signaling pathways
(Fig. 5E).
Discussion
The small molecule, RepSox, can notably improve the
efficiency of mouse fibroblast differentiation into iPSCs by
inducing the expression of Nanog (8). However, the results of the present
study revealed that RepSox markedly increased the expression of
Oct4 (4.5-fold) and L-Myc (5.0-fold), although the expression
levels of Sox2 and Nanog were not markedly different. These results
indicated the importance of Oct4 in the acquisition of cell
multipotency, and RepSox did not directly activate the expression
of endogenous Sox2 and Nanog in the sheep fibroblasts. In addition,
RepSox increased the level of histone H3S10 phosphorylation and
reduced H3S28 phosphorylation. Moreover, the meH3K9 level notably
increased, and the levels of acH3K9 acetylation decreased.
Therefore, the RepSox induced-differentiation of fibroblasts into
multipotent progenitor-type cells may be partly attributed to the
activation of histone methylation, and the reduction of histone
acetylation.
RepSox also exerted a marked effect on the
morphology conversion of sheep fibroblasts. Cell morphology
gradually transformed from a spindle shape into a considerably
elongated state, and the cells gradually became flat, losing their
three-dimensional structure following treatment with increasing
concentrations of RepSox. In addition, the expression levels of
CTGF and collagen I were markedly decreased following treatment
with RepSox. High-throughput RNA sequencing revealed that the DEGs
were mainly involved in 'Regulation of actin cytoskeleton', 'MAPK
signaling pathway', 'Focal adhesion' and 'ECM-receptor
interaction'. Thus, it could be hypothesized that the plasticity of
RepSox-treated fibroblast cells could be tightly related to cell
morphology. Moreover, the DEGs were markedly downregulated in the
majority of signaling pathways of fibroblasts, such as PI3K/Akt,
MAPK, Ras and TGF-β signaling pathways, which could indicate that
RepSox promoted the plasticity of sheep fibroblasts and markedly
upregulated components linked to stem cell maintenance by
regulating multiple signaling pathways. However, no substantial
differences in cell apoptosis and cell death were observed, which
indicated that RepSox treatment did not cause irreversible damage
to fibroblasts. TGF-β is a multifunctional regulator of the TGF-β
superfamily that regulates a variety of cellular functions,
including cell proliferation, differentiation, apoptosis, matrix
synthesis and the immune response (33). Smad proteins have been identified
as intracellular mediators for members of the TGF-β superfamily.
Moreover, the phosphorylation and nuclear export of Smads can be
used as an indicator of TGF-β pathway activation. Previous reports
demonstrated that RepSox inhibited the TGF-β pathway by inhibiting
the phosphorylation of Smad2/3 in murine embryonic fibroblasts.
Similarly, RepSox treatment of SAFs led to an evident decrease in
the expression of components (Smad2, Smad3 and Smad4) of the TGF-β
signaling pathway, particularly the level of Smad3 phosphorylation
(34,35).
In addition, BMP signaling is an alternative pathway
of the TGF-β superfamily signaling and is frequently associated
with the phosphorylation of Smad1/5/8 instead of Smad2/3.
Furthermore, BMPs are associated with stem cell maintenance and
have been demonstrated to promote iPSC production in mice (36). Moreover, the present study found
that there was a notable increase in the expression of BMP2,
instead of BMP3, in response to RepSox treatment. The expression
levels of BMP4, BMPR1A, BMPR1B and ID1 were markedly increased upon
treatment with RepSox and at the early stage of adipogenesis, which
may have further phosphorylated Smad1/5/8 proteins. The process of
differentiation from precursor preadipocytes into mature adipocytes
follows a precise series of events (37). PPARγ and C/EBPα are the most
important factors in the regulation of adipogenesis. Moreover,
C/EBPα is sufficient to trigger the differentiation of
preadipocytes into mature adipocytes via the induction of
adipogenic transcription factors 422/aP2 and PPARγ. In the present
study, there was a marked upregulation in the levels of C/EBPα and
C/EBPβ following RepSox treatment of SAFs for two and four days.
Moreover, it was found that the expression levels of 422/aP2
and PPARγ were also markedly increased, which was regulated
by C/EBPα. These data also demonstrated the pivotal function of
422/aP2 and PPARγ in the regulation of adipogenesis.
Previous research has demonstrated that the
reprogramming of cells is a result of the inhibition of EMT and
facilitation of MET (38). In
the present study, RepSox treatment promoted cell conversion from
fibroblasts to adipocytes and disrupted the EMT. MET is an early
requisite step during the reprogramming of fibroblasts via the
activation of the epithelial program. Additionally, BMP signaling
has been implicated in the induction of MET by reversing
TGF-β-induced EMT during the initiation phase of reprogramming
(39). In the present study,
RepSox substantially increased the expression of BMPs, such as
BMP2, BMP4 and BMP6, induced the expression of epithelial-related
genes (E-cadherin) and inhibited the expression of
mesenchymal-related genes (N-cadherin). These results indicated
that BMPs can enhance Oct4-mediated reprogramming and acceleration
of adipogenesis by inducing MET in RepSox-treated fibroblasts.
In conclusion, in the present study, RepSox was
demonstrated to be a key mediator in promoting the plasticity of
SAFs and facilitating adipocyte differentiation via blocking the
TGF-β/Smad signaling pathway and activating BMP signaling pathways
simultaneously.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. High-throughput RNA sequencing data is available at the
Sequence Read Archive (SRA) database under the accession no.
PRJNA722043.
Authors' contributions
CL, WG and XL designed the experiments and revised
the manuscript. YG, HZ, YL, TS and YW performed the cell
experiments, analyzed the results and drafted the manuscript. CW
and CM performed the statistical analysis. CL and WG confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments were performed according to the
protocol approved by the Institutional Animal Care and Use
Committee (IACUC) for Ethics of Bengbu Medical College (permit no.
2017-016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Zhou Q, Brown J, Kanarek A, Rajagopal J
and Melton DA: In vivo reprogramming of adult pancreatic exocrine
cells to beta-cells. Nature. 455:627–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei X, Chen Y, Xu Y, Zhan Y, Zhang R, Wang
M, Hua Q, Gu H, Nan F and Xie X: Small molecule compound induces
chromatin de-condensation and facilitates induced pluripotent stem
cell generation. J Mol Cell Biol. 6:409–420. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ichida JK, Blanchard J, Lam K, Son EY,
Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et
al: A small-molecule inhibitor of tgf-Beta signaling replaces sox2
in reprogramming by inducing nanog. Cell Stem Cell. 5:491–503.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou P, Li Y, Zhang X, Liu C, Guan J, Li H,
Zhao T, Ye J, Yang W, Liu K, et al: Pluripotent stem cells induced
from mouse somatic cells by small-molecule compounds. Science.
341:651–654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huangfu D, Maehr R, Guo W, Eijkelenboom A,
Snitow M, Chen AE and Melton DA: Induction of pluripotent stem
cells by defined factors is greatly improved by small-molecule
compounds. Nat Biotechnol. 26:795–797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao Y, Zhang R, Dai S, Zhang X, Li X and
Bai C: Role of TGF-β/Smad pathway in the transcription of
pancreas-specific genes during beta cell differentiation. Front
Cell Dev Biol. 7:3512019. View Article : Google Scholar
|
|
8
|
Maherali N and Hochedlinger K: Tgfbeta
signal inhibition cooperates in the induction of iPSCs and replaces
Sox2 and cMyc. Curr Biol. 19:1718–1723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ide M, Jinnin M, Tomizawa Y, Wang Z,
Kajihara I, Fukushima S, Hashizume Y, Asano Y and Ihn H:
Transforming growth factor β-inhibitor Repsox downregulates
collagen expression of scleroderma dermal fibroblasts and prevents
bleomycin-induced mice skin fibrosis. Exp Dermatol. 26:1139–1143.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu HY, Jin L, Guo Q, Luo ZB, Li XC, Zhang
YC, Xing XX, Xuan MF, Zhang GL, Luo QR, et al: RepSox improves
viability and regulates gene expression in rhesus monkey-pig
interspecies cloned embryos. Biotechnol Lett. 39:775–783. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin G, Zhao J and Huang J: RepSox
increases Porcine cloning efficiency by improving pluripotency of
Donor nuclei. Cell Reprogram. 21:181–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo ZB, Jin L, Guo Q, Wang JX, Xing XX,
Xuan MF, Luo QR, Zhang GL, Yin XJ and Kang JD: Cotreatment with
RepSox and LBH589 improves the in vitro developmental competence of
porcine somatic cell nuclear transfer embryos. Reprod Fertil Dev.
30:1342–1351. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jajosky AN, Coad JE, Vos JA, Martin KH,
Senft JR, Wenger SL and Gibson LF: RepSox slows decay of CD34+
acute myeloid leukemia cells and decreases T cell immunoglobulin
mucin-3 expression. Stem Cells Transl Med. 3:836–848. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng M, Liu P, Xiao H, Zhang Y, Wang Y,
Zhao J and Xu J: Improving the osteogenic efficacy of BMP2 with
mechano growth factor by regulating the signaling events in BMP
pathway. Cell Tissue Res. 361:723–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Zhang Q, Gao Y, Tan M, Zheng R,
Zhao L and Zhang X: Induced pluripotent stem cell-conditioned
medium suppresses pulmonary fibroblast-to-myofibroblast
differentiation via the inhibition of TGF-β1/Smad pathway. Int J
Mol Med. 41:473–484. 2018.
|
|
16
|
Zhou X, Tao Y, Liang C, Zhang Y, Li H and
Chen Q: BMP3 alone and together with TGF-β Promote the
differentiation of human mesenchymal stem cells into a Nucleus
Pulposus-Like Phenotype. Int J Mol Sci. 16:20344–20359. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cannon B and Nedergaard J: Cell biology:
Neither brown nor white. Nature. 488:286–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon MY, Kim HJ, Kim MJ, Uhm S, Park JW,
Suk KT, Park JB, Kim DJ and Kim SE: Rap1 regulates hepatic stellate
cell migration through the modulation of RhoA activity in response
to TGF-β1. Int J Mol Med. 44:491–502. 2019.PubMed/NCBI
|
|
19
|
Tu WZ, Fu YB and Xie X: RepSox, a small
molecule inhibitor of the TGFβ receptor, induces brown adipogenesis
and browning of white adipocytes. Acta Pharmacol Sin. 40:1523–1531.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Guo Y, Yao Y, Hua J, Ma Y, Liu C and
Guan W: Reversine increases the plasticity of long-term
cryopreserved fibroblasts to multipotent progenitor cells through
activation of Oct4. Int J Biol Sci. 12:53–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma C, Guo Y, Wen H, Zheng Y, Tan L, Li X,
Wang C, Guan W and Liu C: Identification and multilineage potential
research of a novel type of Adipose-Derived mesenchymal stem cells
from goose inguinal groove. DNA Cell Biol. 37:731–741. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waldsee R, Eftekhari S, Ahnstedt H,
Johnson LE and Edvinsson L: CaMKII and MEK1/2 inhibition
time-dependently modify inflammatory signaling in rat cerebral
arteries during organ culture. J Neuroinflammation. 11:902014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang M, Zhang G, Wang Y, Liu T, Zhang Y,
An Y and Li Y: Crosstalk of mesenchymal stem cells and macrophages
promotes cardiac muscle repair. Int J Biochem Cell Biol. 58:53–61.
2015. View Article : Google Scholar
|
|
24
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu G, Wang LG, Han Y and He QY:
ClusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li W, Wang P, Zhang B, Zhang J, Ming J,
Xie W and Na J: Differential regulation of H3S10 phosphorylation,
mitosis progression and cell fate by Aurora Kinase B and C in mouse
preimplantation embryos. Protein Cell. 8:662–674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sotero-Caio CG, de Souza MJ,
Cabral-de-Mello DC, Brasileiro-Vidal AC and Guerra M:
Phosphorylation of histone H3S10 in animal chromosomes: Is there a
uniform pattern? Cytogenet Genome Res. 135:111–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Linhart HG, Ishimura-Oka K, DeMayo F, Kibe
T, Repka D, Poindexter B, Bick RJ and Darlington GJ: C/EBPalpha is
required for differentiation of white, but not brown, adipose
tissue. Proc Natl Acad Sci USA. 98:12532–12537. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mei L, Sang W, Chen Z, Zheng L, Jin K, Lou
C, Huang W and He D: Small molecule inhibitor RepSox prevented
ovariectomy-induced osteoporosis by suppressing osteoclast
differentiation and bone resorption. J Cell Physiol. 233:9724–9738.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q and Huang Q: Single-cell qPCR
demonstrates that Repsox treatment changes cell fate from endoderm
to neuroectoderm and disrupts epithelial-mesenchymal transition.
PLoS One. 14:e02237242019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao B, Guan H, Liu JQ, Zheng Z, Zhou Q,
Zhang J, Su LL and Hu DH: Hypoxia drives the transition of human
dermal fibroblasts to a myofibroblast-like phenotype via the
TGF-beta1/Smad3 pathway. Int J Mol Med. 39:153–159. 2017.
View Article : Google Scholar
|
|
33
|
Guo M, Zhou JJ and Huang W: Metformin
alleviates endometrial hyperplasia through the
UCA1/miR144/TGFβ1/AKT signaling pathway. Int J Mol Med. 45:623–633.
2020.PubMed/NCBI
|
|
34
|
Han S, Cui C, Wang Y, He H, Liu Z, Shen X,
Chen Y, Li D, Zhu Q and Yin H: Knockdown of CSRP3 inhibits
differentiation of chicken satellite cells by promoting TGF-β/Smad3
signaling. Gene. 707:36–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wen X, Liu Y, Bai Y, Li M, Fu Q and Zheng
Y: LOXL2, a copper-dependent monoamine oxidase, activates lung
fibroblasts through the TGF-β/Smad pathway. Int J Mol Med.
42:3530–3541. 2018.PubMed/NCBI
|
|
36
|
Chen J, Liu J, Yang J, Chen Y, Chen J, Ni
S, Song H, Zeng L, Ding K and Pei D: BMPs functionally replace Klf4
and support efficient reprogramming of mouse fibroblasts by Oct4
alone. Cell Res. 21:205–212. 2011. View Article : Google Scholar
|
|
37
|
Wu L, Cai X, Zhang S, Karperien M and Lin
Y: Regeneration of articular cartilage by adipose tissue derived
mesenchymal stem cells: Perspectives from stem cell biology and
molecular medicine. J Cell Physiol. 228:938–944. 2013. View Article : Google Scholar
|
|
38
|
Zhang Y, Fan K, Xu X and Wang A: The
TGF-β1 Induces the Endothelial-to-Mesenchymal Transition via the
UCA1/miR-455/ZEB1 Regulatory axis in human umbilical vein
endothelial cells. DNA Cell Biol. 39:1264–1273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu YC, Wang YK, Bai SJ, Zha FF, Feng G,
Gao CP and Liu J: Suppression of CIP4/Par6 attenuates
TGF-β1-induced epithelial-mesenchymal transition in NRK-52E cells.
Int J Mol Med. 40:1165–1171. 2017. View Article : Google Scholar : PubMed/NCBI
|