Introduction
Myocardial ischemia disease remains a major cause of
death (~1.72%) and disability (~2.45) in most countries in the
world (1). Apoptosis of
myocardiocytes can promote myocardial ischemia,
ischemia/reperfusion (I/R) injury, post-ischemia cardiac remodeling
and coronary atherosclerosis (2). Evidence has indicated that
myocardial ischemia presents relatively high lethality, which is
closely associated with metabolism disorders in endothelial cells
of heart vessels (3). Numerous
studies have indicated that the increase of myocardiocyte apoptosis
contributes to the development of cardiovascular diseases (4-6).
Foundationally, exploring drugs which protect against myocardial
ischemia and reperfusion injury plays crucial a role in modulating
myocardial apoptosis and levels of inflammation (7,8).
In addition, inhibition of myocardial ischemia injury-induced
apoptosis of cardiomyocytes could significantly improve cardiac
function (9). Furthermore,
myocardial ischemia-reperfusion injury has been revealed to induce
a sterile inflammatory response and apoptosis of myocardial tissue,
which further contributes to the final infarct size (10). Therefore, developing new
therapies for myocardial injury represents an urgent and
significant research interest (11).
Tanshinone-IIA (Tan-IIA) has been revealed to
possess anti-atherosclerosis effects and is widely used in
treatment of cardiovascular and cerebrovascular diseases (12). A previous study has reported that
Tan-IIA demonstrates rich cardioprotective activities for clinical
applications (13). Another
study reported that Tan-IIA could inhibit the myocardial apoptosis
in a heart failure rat model by upregulating the microRNA (miR)-133
level (14). In addition,
Tan-IIA has been demonstrated to be an effective and safe agent for
the treatment of patients with coronary heart disease (15). Furthermore, Tan-IIA has presented
a significant protection of cardiomyocytes against apoptosis via
decreasing oxidative stress and inflammatory responses (16). Although the anti-apoptotic effect
of Tan-IIA has been well explored in animal models of myocardial
ischemia (17), the molecular
mechanism has not been clearly documented. Therefore, the effects
and molecular mechanisms of Tan-IIA on cardiomyocytes were
evaluated both in vitro and in vivo.
Oxidative stress is enhanced in chronic heart
failure and is key to providing some suggestions for the treatment
of heart diseases (18).
Oxidative stress plays an important role in the pathophysiology of
myocardial ischemia and improved understanding of the role of
oxidative stress in myocardial ischemia resulted in novel
therapeutic options for patients with myocardial ischemia (19). In addition, the mitochondrial
pathway of apoptosis is activated in atrial fibrillation of heart
failure patients, which contributes to the understanding of atrial
contractile dysfunction (20).
Furthermore, oxidative stress and the mitochondrial apoptotic
pathway were revealed to be associated with apoptosis of cardiac
myocytes induced by osteopontin (21). However, the precise mechanisms by
which oxidative stress induces the mitochondrial apoptotic pathway
in myocardiocytes remain unknown.
The purpose of this study was to investigate whether
Tan-IIA had a protective effect on apoptosis of myocardial tissue
in an animal model of myocardial ischemia. The potential
anti-apoptotic mechanism of Tan-IIA in myocardiocytes was also
investigated.
Materials and methods
Animals and drug treatment
The present study was approved (approval no.
20160512C10) by the Ethics Committee of Shenzhen Nanshan People's
Hospital (Shenzhen, China). A total of 20 male Sprague Dawley (SD)
rats (10 weeks old; 320-340 g body weight) were purchased from the
Animal Experiment Center of Tongji University (Shanghai, China).
Myocardial ischemia was established as previously described
(22). Briefly, SD rats were
anaesthetized by intraperitoneal injection of sodium pentobarbital
(30 mg/kg body weight) and ventilated with oxygen using a small
animal ventilator. After an incision in the left thorax at the
level between the fourth and fifth ribs, the heart was exposed, and
a 6-0 silk suture slipknot was placed around the proximal left
anterior descending coronary artery (LAD) approximately 2 mm below
the left auricle. Successful myocardial infarction injury was
identified by the blanched appearance of the ligation region and
marked arrhythmia. Sham-operated rats underwent the same surgical
procedures except for the suture around the LAD which was not
ligated. Experimental rats were randomly divided into 2 groups.
Experimental rats were subjected to intragastric oral
administration (p.o.) Tan-IIA (10 mg/kg; n=10) or PBS (n=10). The
treatment continued 10 times twice a day for a total 20-day
therapeutic period. All the rats in the study were housed in an
environment at 23±1.0°C and 50±5% humidity with 12-h light/dark
circadian cycle and ad libitum access to food and water. The
mice were caged for 24 h since the last injection and the
myocardial tissues were collected after cardiac perfusion. No rats
succumbed during the experiments. On day 21, experimental animals
were euthanized under intravenous injection of pentobarbital (40
mg/kg), and efforts were made to minimize the suffering of the
rats. Cervical dislocation was used as the euthanasia method. The
myocardial tissues were used for immunohistochemical analysis.
Analysis of cardiac function and
myocardial infarct size
Experimental rats were anesthetized with isoflurane
and following procedures were processed according to a previous
study (23). The parameters of
left ventricular end-diastolic diameter (LVEDD), left ventricular
end-systolic diameter (LVESD), left ventricular ejection fraction
(LVEF) and left ventricular fractional shortening (LVES) were
evaluated using the Sequoia 512 echocardiography system (Siemens
Healthineers) following the manufacturer's instructions. The size
of the myocardial infarction was assessed by
2,3,5-triphenyltetrazolium chloride (TTC) and Evan blue double
staining assay as previously described (24). The infarct size in tissue
sections was assessed by computerized planimetry and quantitated
using ImageJ v2.0 (National Institutes of Health).
Immunohistochemical analysis
On day 21, myocardial tissues were obtained from
experimental rats, immediately excised and placed in a 4%
paraformaldehyde solution overnight at 4°C, followed by
dehydration, washing with PBS, and paraffin embedding.
Paraffin-embedded myocardial tissues were cut into 4-µm
sections, subjected to hydrogen peroxide (3%) for 10 min, and
blocked with BSA (5%) for 2 h at 37°C. Myocardial tissue sections
were incubated with rabbit anti-rat antibodies Bcl-2 (1:1,000;
product code ab182858), Bcl-xL (1:1,000; product code ab32370),
caspase-3 (1:1,000; product code ab184787), cytoplasmic cytochrome
c (Cyto c; 1:1,000; product code ab133504), Apaf-1
(1:1,000; product code ab234436; all from Abcam) overnight at 4°C.
All sections were washed 3 times and incubated with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:2,000; product
code ab205718; Abcam) for 1 h at 37°C. The myocardial sections were
stained with a 3,3-diaminobenzidine substrate system (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Images were captured
using a light microscope (BX51; Olympus Corporation) under a
magnification of ×100.
Cells and reagents
Tan-IIA (purity >99.2%) was purchased from
Herbasin (Shenyang) Co., Ltd.; Dasherb Corp. and dissolved in
dimethyl sulfoxide (DMSO). Myocardiocytes were isolated from
experimental rats with myocardial ischemia as previously described
(25). Briefly, after
dissection, heart tissues were washed, rinsed with HEPES-buffered
saline solution and then incubated at 37°C for 2 h with
HEPES-buffered saline solution containing 1.2 mg/ml pancreatin and
0.14 mg/ml collagenase (Gibco; Thermo Fisher Scientific, Inc.).
After centrifugation (2,000 × g for 10 min at 4°C), cells were
resuspended in Dulbecco's modified Eagle's medium/Ham's F-12
(DMEM/F12; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 5% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), 0.1 mM
ascorbate, insulin-transferring-sodium selenite media supplement
(Sigma-Aldrich; Merck KGaA), 100 µg/ml streptomycin, 100
U/ml penicillin and 0.1 mM bromodeoxyuridine. Cells were then
diluted to 1×105 cells/ml and cultured in DMEM/F12
supplemented with 10% FBS. Myocardiocytes were treated with
H2O2 (1 µM) and/or concentrations of
Tan-IIA (0-40 µM) and/or tunicamycin (TM; 1 µM;
Sigma-Aldrich; Merck KGaA) for 24, 48 and 72 h at 37°C. PBS buffer
containing 10% DMSO (pH 7.2) was used as the control. All cells
were maintained at 37°C in a humidified atmosphere containing 5%
CO2.
Counting Kit-8 (CCK-8) assay
The regulatory effects of Tan-IIA on proliferative
ability of myocardiocytes were examined by CCK-8 assay as
previously described (26).
Briefly, the treated myocardiocytes were seeded into 96-well plates
(1×103 cells/well) and cultured at 37°C in a humidified
incubator containing 5% CO2. Then, 10 µl of CCK-8
reagent (Beijing Solarbio Science & Technology Co., Ltd.) was
added to the cells followed by incubation at 37°C for 2 h according
to the manufacturer's instructions. Cell viability was determined
by a microplate reader (Eon BioTech, Pte Ltd.) at 450 nm. Each
experiment was repeated for 3 times.
Caspase-3 overexpression
The regulatory effects of caspase-3 overexpression
on Tan-IIA-regulated apoptotic factors in myocardiocytes were
examined by stable transfection. Expression plasmid pRK5-caspase-3
(casp-3OP) with a Flag tag (cat. no. PPL00180-2a; Public
Protein/Plasmid Library) at the C-terminus was constructed by
Invitrogen; Thermo Fisher Scientific, Inc. Briefly, myocardiocytes
(1×105 cells/ml) were cultured at 37°C in DMEM/F12
(Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Sigma-Aldrich; Merck KGaA) in 6-well plates. After 24 h,
myocardiocytes were transfected with plasmid containing either
pRK5-caspase-3 (0.5 µg) or pRK5-vector (0.5 µg) by
using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.) at 37°C
for 72 h according to the manufacturer's instructions. After 72 h
of transfection, expression of caspase-3 was evaluated using
western blot analysis and cells were used for further
experiments.
TUNEL analysis
The terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end labeling (TUNEL) assay (Roche
Diagnostics GmbH) was used to detect apoptosis in myocardial tissue
and myocardiocytes. Briefly, heart tissues were immediately excised
and placed in a 4% paraformaldehyde solution for 12 h at 4°C. This
was followed by dehydration, washing with PBS, and paraffin
embedding. The paraffin blocks were cut into 4-µm sections
and stained with hematoxylin and eosin (H&E; Beijing Solarbio
Science & Technology Co., Ltd.) for 15 min at room temperature
according to the manufacturer's instructions. For myocardiocytes,
the treated cells (1×105 cells) were cultured in 6-well
plates for 12 h at 37°C, washed with PBS and fixed with 4%
paraformaldehyde for 12 h at 4°C. The cells were washed with PBS
and stained using a TUNEL kit for 30 min at 25°C according to the
manufacturer's protocols. Finally, the cells were counterstained
with 5% DAPI (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. Images from 6 randomly selected fields of view were
captured using a fluorescence microscope at a magnification of ×50
(DMI3000B; Leica Microsystems, Inc.).
Western blot analysis
The treated myocardiocytes were lysed using RIPA
lysis buffer (Sigma-Aldrich; Merck KGaA). The cells were
centrifuged at 12,000 × g for 10 min at 4°C. The concentration of
protein was determined using a bicinchoninic acid assay (Thermo
Fisher Scientific, Inc.). A total of 30 µg of protein/lane
was separated using 12% SDS-PAGE, blocked with 5% BSA
(Sigma-Aldrich; Merck KGaA) overnight at 4°C, and then transferred
to a PVDF (EMD Millipore) membrane. Membranes were incubated with
appropriate primary antibodies: Bcl-2 (1:1,000), Bak (1:1,000;
product code ab32371; Abcam,), caspase-3 (1:1,000), Cyto c
(1:1,000), Apaf-1 (1:1,000), Bim (1:1,000; product code ab32158;
Abcam), CHOP (1:1,000; product code ab11419; Abcam) and β-actin
(1:1,000; product code ab8227; Abcam) overnight at 4°C, washed with
PBS and incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (1:2,000, product code ab7090; Abcam) for 2 h
at 25°C. Protein expression was evaluated using enhanced
chemiluminescence (product no. CPS1A300; Sigma-Aldrich; Merck
KGaA). The expression of protein was quantified using LabWorks™
Image Acquisition (version 4.0; UVP, LLC).
Confocal laser microscopy
The treated myocardiocytes were cultured in 6-well
plates for 12 h at 37°C, washed with PBS, fixed with 4%
paraformaldehyde for 15 min at room temperature, and then incubated
with 0.1% Triton X-100 and 1% BSA for 30 min at room temperature.
Myocardiocytes were incubated with primary anti-mouse antibodies
against Bim (1:1,000), CHOP (1:1,000), activating transcription
factor 4 (ATF4; 1:1,000; product code ab216839; Abcam),
inositol-requiring enzyme 1α (IRE1α; 1:1,000; product code ab37073;
Abcam), thiobarbituric acid reactive substances (TBARS; 1:1,000;
ab118970; Abcam), reactive oxygen species (ROS; 1:1,000; ab186027;
Abcam), H2O2 (1:1,000; ab138874; Abcam)
overnight at 4°C. Myocardiocytes were washed with PBS and incubated
with corresponding anti-rabbit secondary antibody (1:2,000, product
code ab7090; Abcam) for 2 h at 25°C. Subsequently, myocardiocytes
were stained with 5% DAPI for 30 min at room temperature. Images of
myocardiocytes were captured using a Zeiss confocal spectral
microscope (Carl Zeiss AG) at a magnification of ×100.
Statistical analysis
All data are expressed as the mean ± SD of
triplicate dependent experiments. All data were analyzed using SPSS
Statistics 22.0 (IBM Corp.) Paired Student's test was used to
assess the significant differences between two groups. One-way
variance analysis (ANOVA) followed by Tukey's HSD test were used to
assess the significant differences among multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protective effect of Tan-IIA in
myocardial ischemia-reperfusion rat model
To determine the therapeutic effect of Tan-IIA in
myocardial ischemia, a myocardial ischemia-reperfusion rat model
was established and received treatment with Tan-IIA, or PBS. As
demonstrated in Fig. 1A, Tan-IIA
attenuated the myocardial infarction sizes after
ischemia-reperfusion compared with the PBS group (P<0.01). In
addition, the myocardial functions LVEDD, LVESD, LVEF and LVES were
significantly ameliorated during reperfusion in the Tan-IIA group
compared with the PBS group (Fig.
1B-E). Tan-IIA treatment exhibited a significant elevation in
dp/dt(max) throughout the reperfusion period compared with the PBS
(P<0.01; Fig. 1F). These data
indicated that Tan-IIA played a protective role in a myocardial
ischemia-reperfusion rat model.
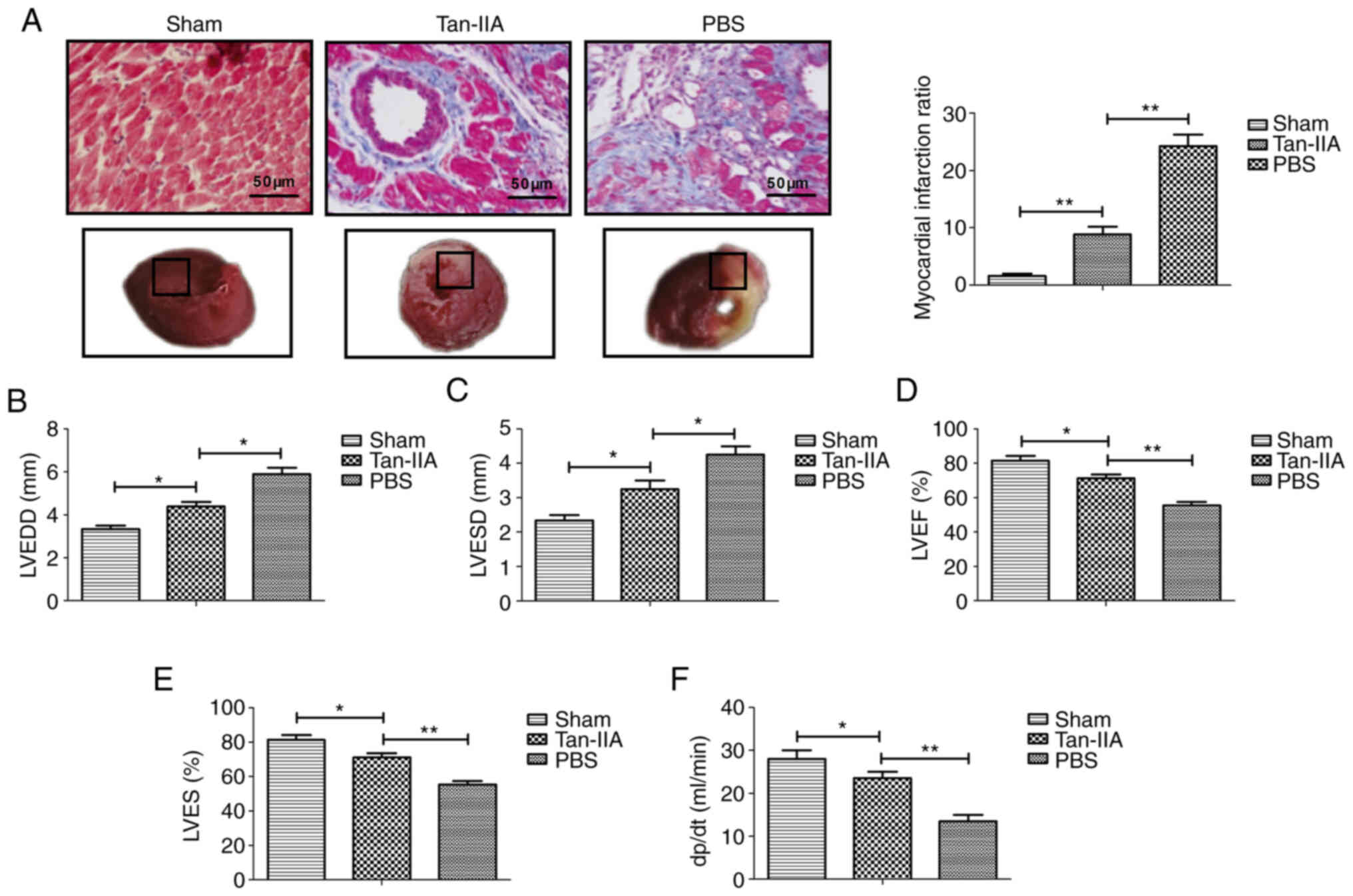 | Figure 1Therapeutic effect of Tan-IIA on a
myocardial ischemia-reperfusion rat model. (A) TTC and Evan blue
double staining for myocardial tissue and the myocardial infarct
ratio of the PBS, Tan-IIA and sham groups. (B-E) Assessment of (B)
LVEDD, (C) LVESD, (D) LVEF and (E) LVES in experimental rat PBS,
Tan-IIA and sham groups. (F) Coronary flow in experimental rat PBS,
Tan-IIA and sham groups. *P<0.05,
**P<0.01. n=6 animals in each group. The results are
expressed as the mean ± SD. Tan-IIA, tanshinone-IIA; TTC,
2,3,5-triphenyltetrazolium chloride; LVEDD, left ventricular
end-diastolic diameter; LVESD, left ventricular end-systolic
diameter; LVEF, left ventricular ejection fraction; LVES, left
ventricular fractional shortening. |
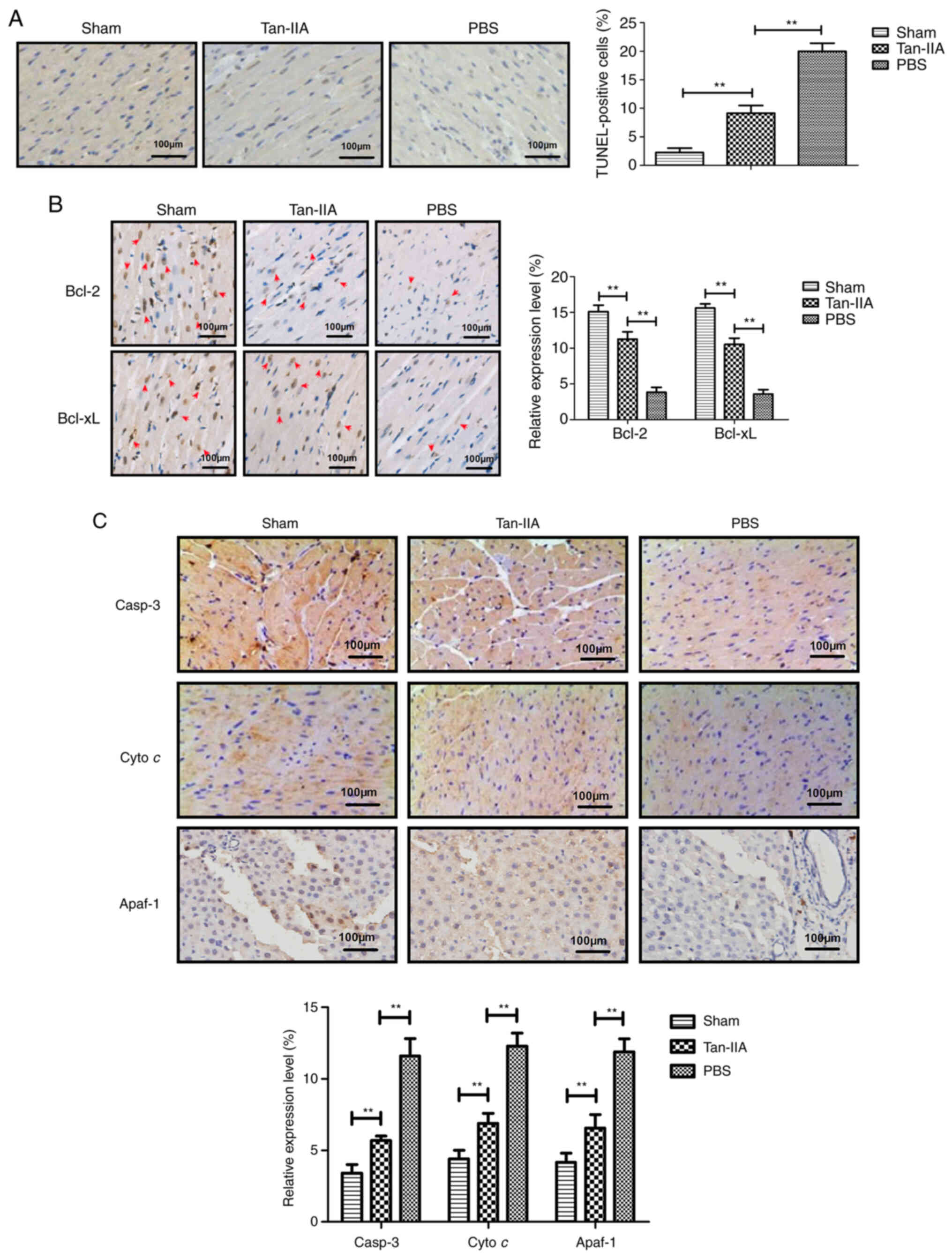
Tan-IIA treatment inhibits myocardiocyte
apoptosis in a rat model of myocardial ischemia
The anti-apoptotic role of Tan-IIA in a rat model of
myocardial ischemia was next analyzed. A TUNEL assay demonstrated
that Tan-IIA decreased apoptosis of myocardial tissue compared with
PBS (Fig. 2A).
Immunohistochemical analysis revealed that Tan-IIA upregulated the
anti-apoptotic protein Bcl-2 and Bcl-xL expression in myocardial
tissue compared with PBS (Fig.
2B). The data also demonstrated that Tan-IIA downregulated
pro-apoptotic protein caspase-3, Cyto c and Apaf-1
expression in myocardial tissue compared with PBS (Fig. 2C). These data indicated that
Tan-IIA could inhibit apoptosis of myocardial tissue in rat model
of myocardial ischemia.
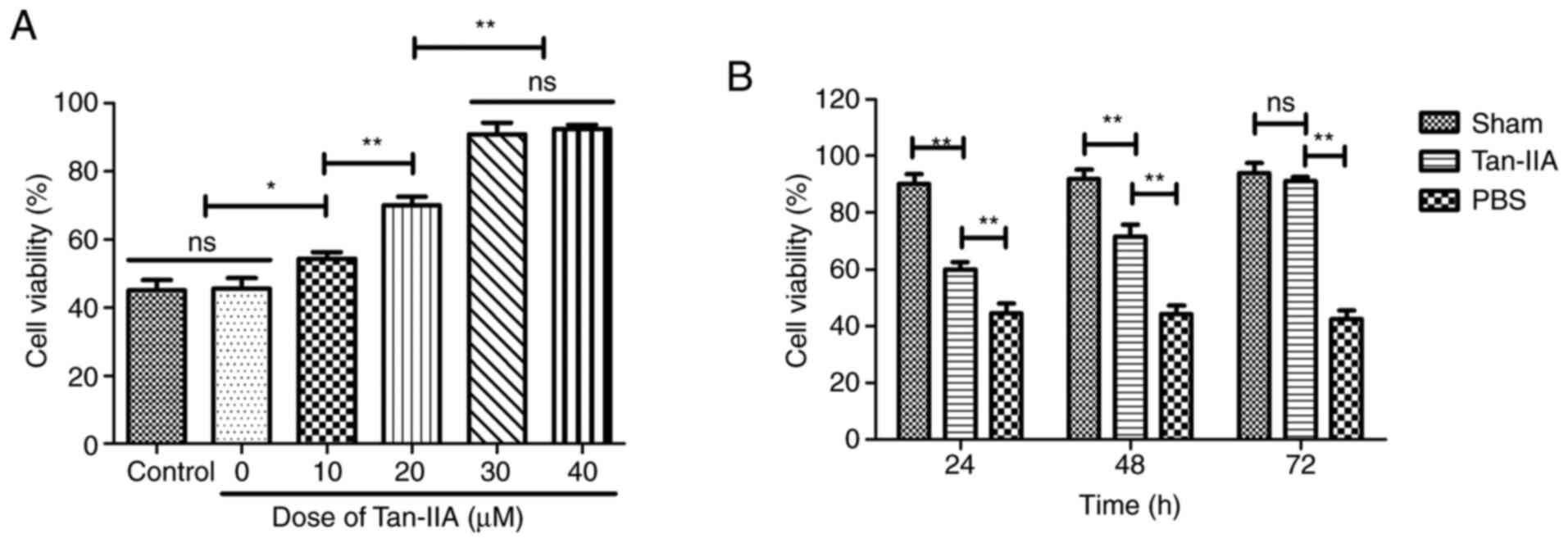
Effect of Tan-IIA on protection in
H2O2-induced myocardiocytes
To verify the protective effect of Tan-IIA on
myocardiocytes, viability of myocardiocytes was analyzed in
vitro. As demonstrated in Fig.
3A, 30 µM of Tan-IIA presented the optimal protective
effect on viability of myocardiocytes. As demonstrated in Fig. 3B, Tan-IIA (30 µM)
increased the viability of myocardiocytes in a time-dependent
manner compared with PBS. These data indicated that Tan-IIA could
increase the viability of myocardiocytes.
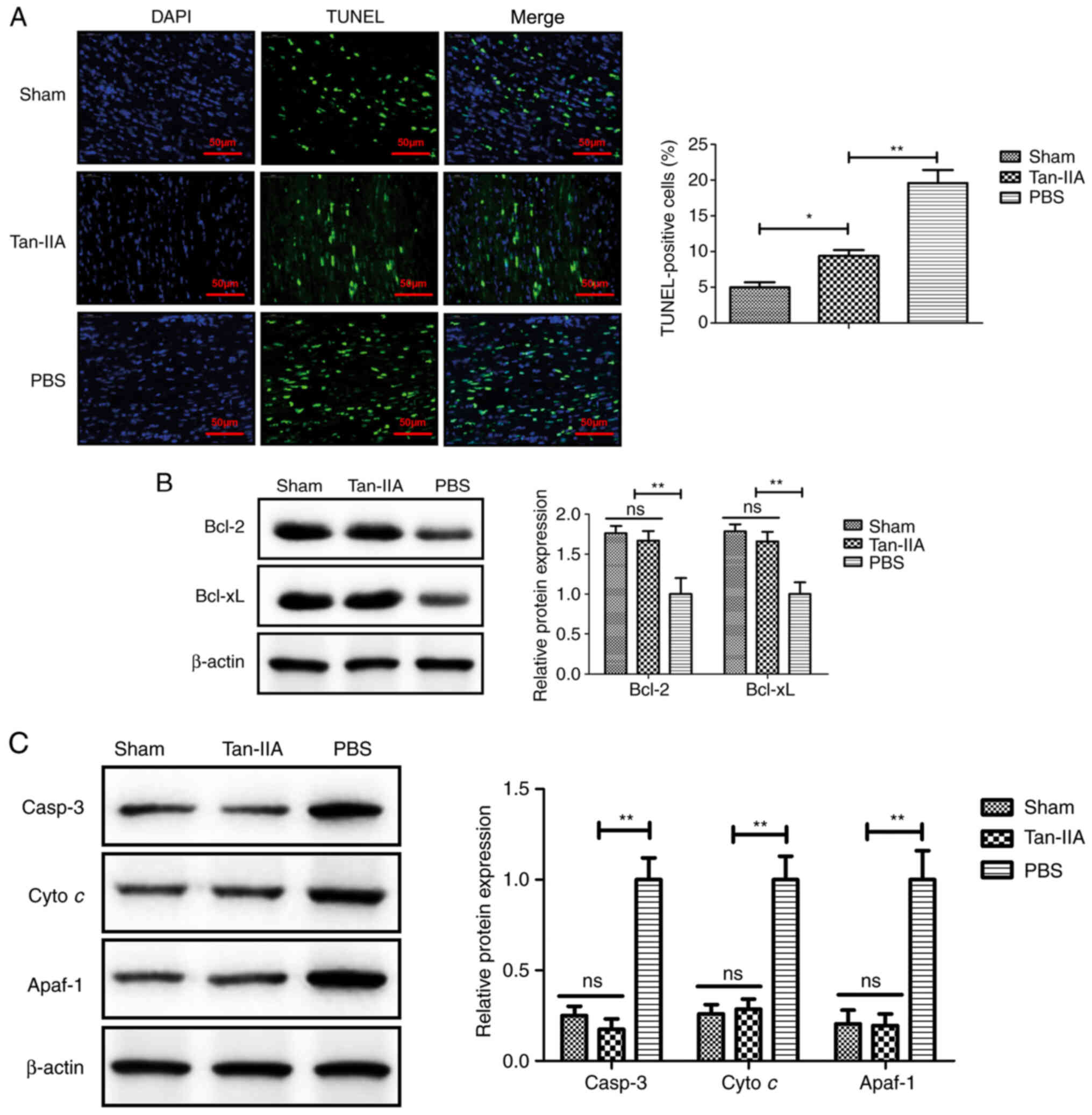
Tan-IIA treatment inhibits myocardiocyte
apoptosis in myocardiocytes in vitro
The anti-apoptotic effect of Tan-IIA was analyzed in
myocardiocytes in vitro. As revealed in Fig. 4A, Tan-IIA produced a significant
reduction of apoptosis of myocardiocytes compared with PBS
treatment. The results revealed that expression levels of Bcl-2 and
Bcl-xl were upregulated by treatment with Tan-IIA in myocardiocytes
compared with PBS treatment (Fig.
4B). Western blot analysis demonstrated that apoptotic factors
including cleaved caspase-3 (casp-3), Cyto c and Apaf-1 in
the mitochondrial apoptotic pathway were downregulated by Tan-IIA
in myocardiocytes compared with treatment by PBS (Fig. 4C). These data indicated that
Tan-IIA may inhibit myocardiocyte apoptosis via the mitochondrial
apoptotic pathway.
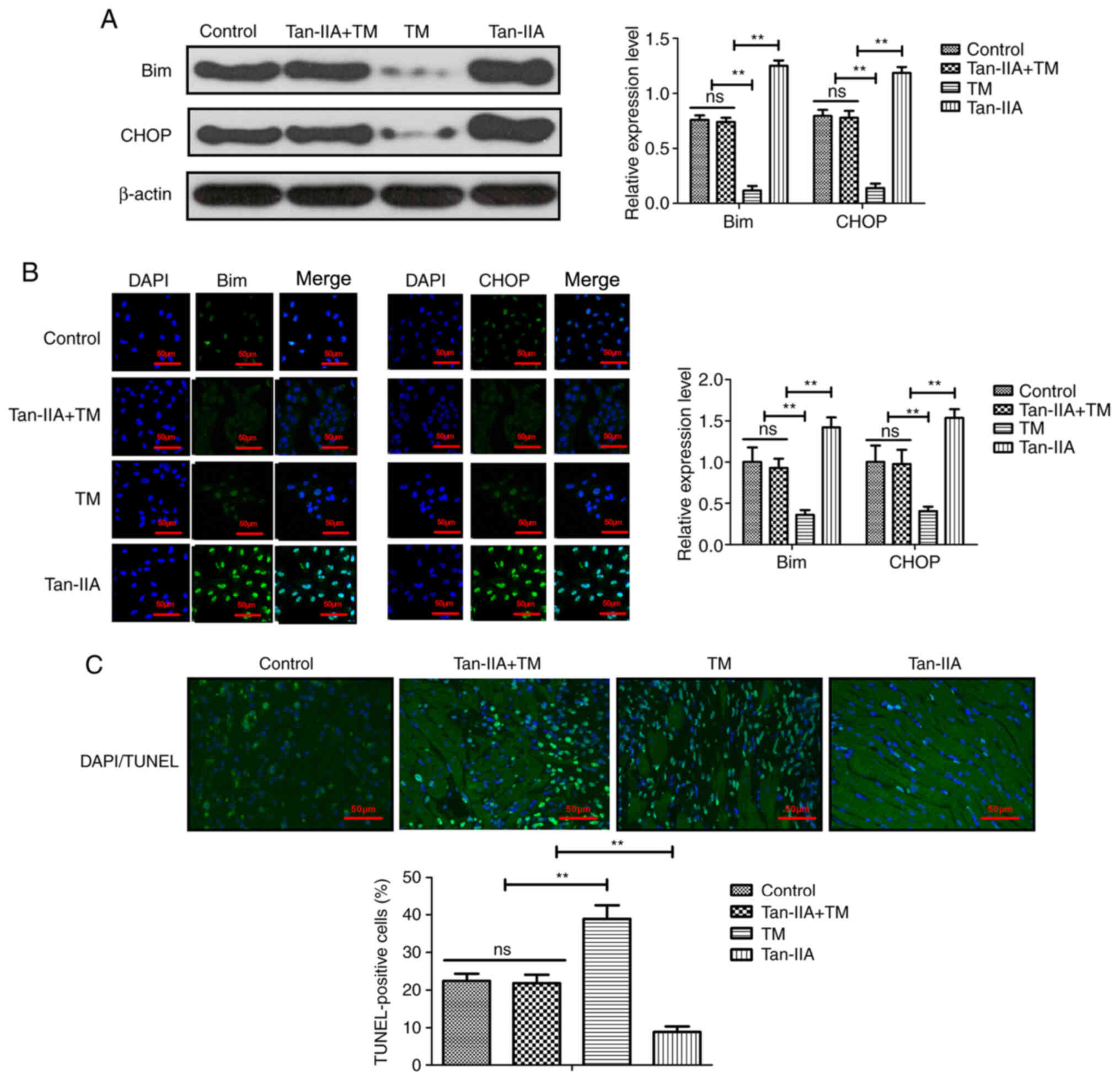
Tan-IIA improves myocardial ischemia by
regulating oxidative stress
The effect of Tan-IIA on oxidative stress was
analyzed in myocardiocytes. As demonstrated in Fig. 5A and B, Tan-IIA treatment
upregulated the expression of endoplasmic reticulum stress-related
proteins Bim and CHOP in myocardiocytes compared with the control
group. However, the oxidative stress stimulator TM abolished the
Tan-IIA-increased Bim and CHOP expression in myocardiocytes
compared with the control group. The data also demonstrated that
myocardial injury-induced apoptosis was inhibited by Tan-IIA
pretreatment compared with the control group and this effect was
abolished by TM treatment in myocardiocytes (Fig. 5C). These results indicated that
Tan-IIA could protect myocardiocytes against apoptosis by
modulating oxidative stress.
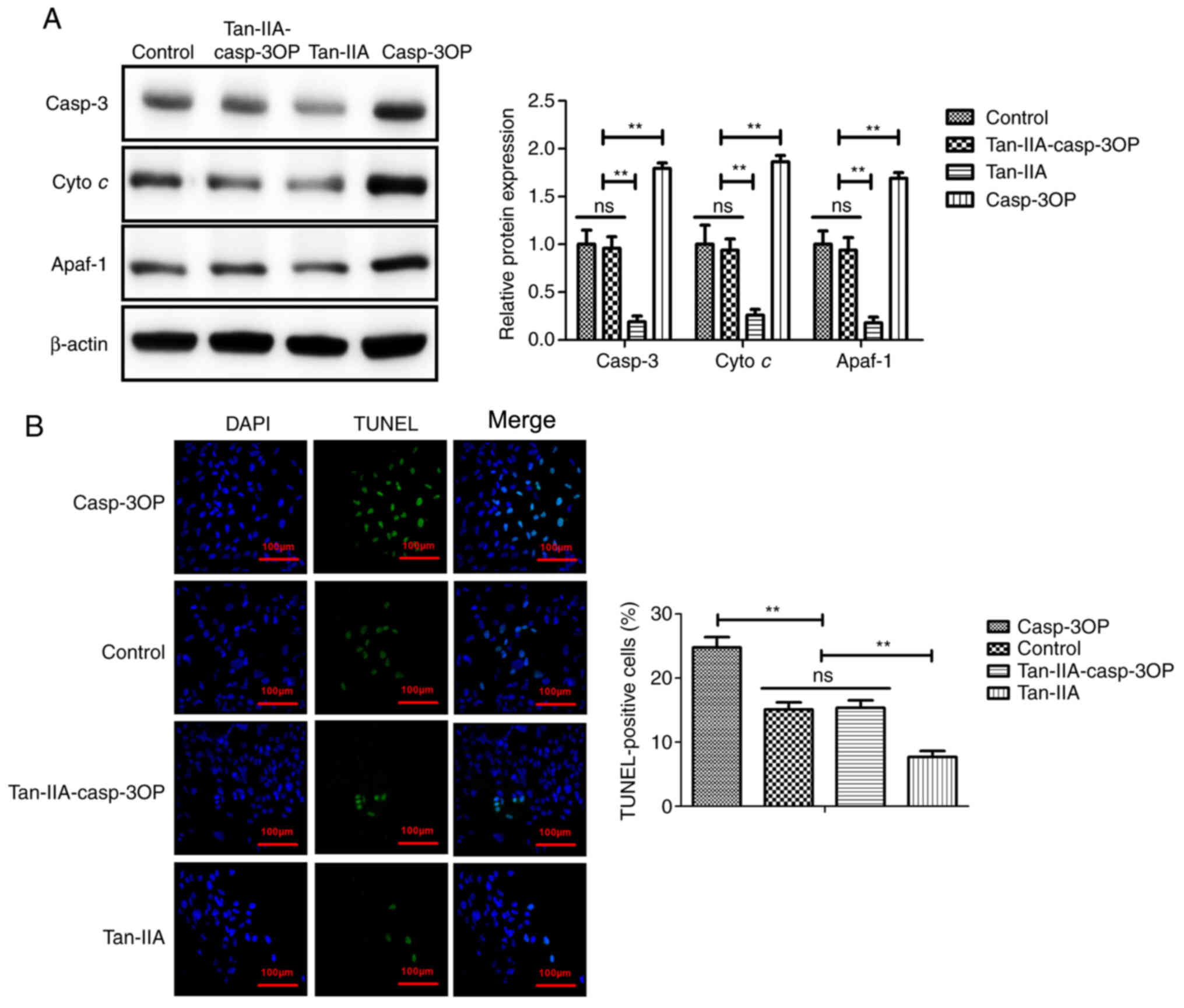
Tan-IIA improves myocardial ischemia by
decreasing apoptosis of myocardiocytes via inhibition of the
mitochondrial apoptotic signaling pathway
To identify the possible mechanism of Tan-IIA in
myocardiocytes, caspase-3-overexpressed (casp-3OP) myocardiocytes
were established. As demonstrated in Fig. 6A, caspase-3 overexpression
reversed Tan-IIA-decreased Casp-3, Cyto c, and Apaf-1 in
myocardiocytes. The results in Fig.
6B demonstrated that Casp-3 overexpression reversed
Tan-IIA-decreased apoptosis of myocardiocytes compared with the
control group. These results indicated that Tan-IIA could decrease
apoptosis of myocardiocytes via inhibition of the mitochondrial
apoptotic signaling pathway.
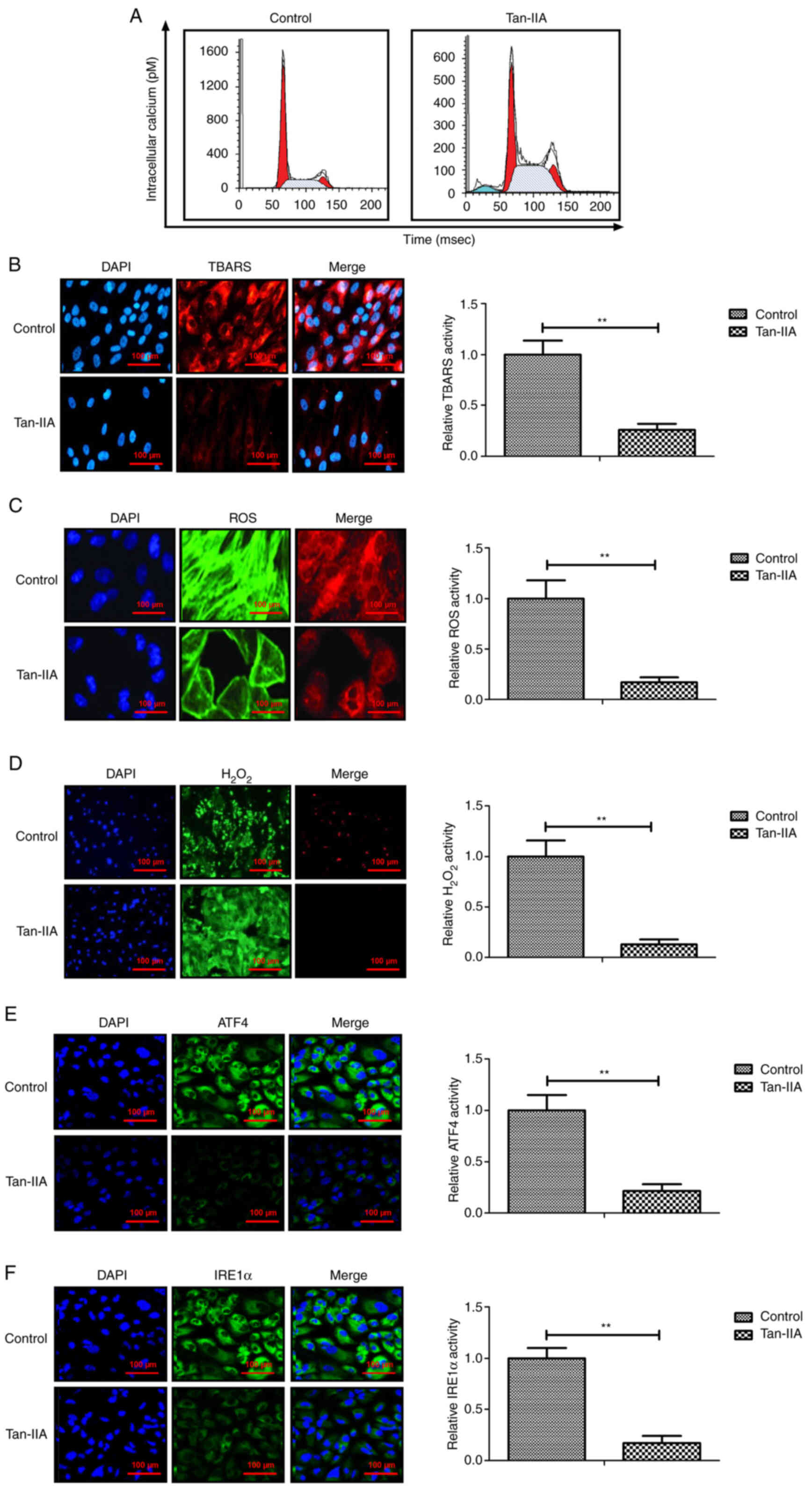
Effects of Tan-IIA on intracellular
calcium and oxidative stress in myocardiocytes
Previous data has revealed that the intracellular
calcium level and oxidative stress are increased during apoptosis
of myocardiocytes (21). It has
been demonstrated that I/R injury induces oxidative and
inflammatory responses, and further ultimately damages cardiac
function in patients suffering myocardial ischemia (27). To investigate whether the
protective effect of Tan-IIA on myocardiocytes in myocardial
ischemia rats is related with intracellular calcium and oxidative
stress, the intracellular calcium and oxidative stress levels were
analyzed. As demonstrated in Fig.
7A, Tan-IIA decreased the level of intracellular calcium in
myocardiocytes compared with the control. The results revealed that
production of TBARS, ROS and H2O2 was
inhibited by Tan-IIA compared with the control groups (Fig. 7B-D). The data also demonstrated
that Tan-IIA decreased expression of ATF4 and IRE1α expression in
myocardiocytes (Fig. 7E and F).
Collectively, these results indicated that Tan-IIA could decrease
intracellular calcium and oxidative stress in myocardiocytes.
Discussion
Tan-IIA is an effective drug for the treatment of
cardiovascular diseases (28). A
previous study revealed that Tan-IIA injection was effective and
safe in improving clinical outcomes in patients with coronary heart
disease (15). Tan-IIA-decreased
apoptosis of myocardiocytes has been revealed to contribute to the
recovery of myocardial function (17). However, the idiographic mechanism
of Tan-IIA remains unknown in myocardiocytes. The therapeutic
effect of Tan-IIA on inhibition of myocardial tissue apoptosis in
an experimental rat model of myocardial ischemia and the possible
mechanism of Tan-IIA in myocardiocytes were investigated in the
present study. The results provided insights on Tan-IIA-induced
cellular mechanisms for anti-apoptotic activities in impaired
myocardiocytes undergoing the oxidative stress-dependent pathway
and mitochondrial signaling pathway, which demonstrated the
potential value of using Tan-IIA for cardiovascular disease
therapy.
Apoptosis of myocardiocytes has been revealed to be
induced by myocardial ischemia caused by hypoxia, while reperfusion
aggravates the apoptotic process during the preceding ischemic
period (29). A previous study
revealed that apoptosis of myocardiocytes leads to increasing
intracellular calcium and oxidative stress, which aggravates the
inflammatory response and activation of proapoptotic signaling
proteins during the reperfusion period (30). Our results demonstrated that
Tan-IIA improved myocardial infarction size, myocardial functions,
such as dp/dt, coronary flow and LVDP. The data also revealed that
Tan-IIA could inhibit apoptosis of myocardial tissue in a rat model
of myocardial ischemia, indicating that Tan-IIA has clinical
value.
A previous study reported that apoptosis mediated by
endoplasmic reticulum stress partly depended on signaling through
activation of PERK and EIF2α expression (31). The data in this previous study
identified that Tan-IIA decreased PERK and EIF2α expression in
myocardiocytes, which relieved endoplasmic reticulum stress and
further led to reduction of apoptosis of myocardiocytes. In
addition, intracellular calcium dynamics have been demonstrated to
be important in promoting triggered activity during acetylcholine
infusion in patients with heart failure (32). Furthermore, maintaining the
balance between intracellular oxidants and antioxidants has been
revealed to have a protective effect against myocardial (I/R)
injury (33). The results of the
present study revealed that Tan-IIA decreased intracellular calcium
and downregulated TBARS, ROS and H2O2
production in myocardiocytes. Importantly, no side effects were
observed with Tan-IIA treatment such as thrombotic phenomena,
ulcerating atherosclerotic lesion or micronecrosis in experimental
animals during experimentation.
Tan-IIA has cardioprotective function through
multiple targets related with NO production, such as eNOS
phosphorylation, L-arginine uptake and CAT expression, which may
have major clinical implications (34). Inhibition of the mitochondrial
apoptotic signaling pathway has been revealed to contribute to the
improvement of cardiac function and energy metabolism in mice after
myocardial ischemia injury (35). The present study revealed that
Tan-IIA downregulated protein expression levels, such as cleaved
caspase-3, Cyto c and Apaf-1 in the mitochondria-mediated
internal signaling pathway. A previous study reported that
upregulation of Bcl-2 and Bak expression could inhibit apoptosis of
cells during myocardial I/R injury (36). Although Bim protein is considered
to localize in mitochondria and acts as an anti-apoptosis protein,
it is also found on the endoplasmic reticulum and nuclear membranes
(37). Additionally, Bak is
downregulated during ischemia and/or reperfusion injury in
myocardial infarction (38). In
the present study, the results revealed that Bcl-2 and Bak
expression levels were upregulated in myocardiocytes, which were
associated with the inhibition of the mitochondrial apoptotic
signaling pathway during myocardial ischemia injury. The data
revealed that Tan-IIA decreased apoptosis of myocardiocytes in a
rat myocardial injury model and this was mediated by the
mitochondrial signaling pathway. Wang et al reported that
Bim is involved in protection of myocardial apoptosis after I/R
injury (39). In the present
study, our in vivo and in vitro data demonstrated
that Tan-IIA not only increased Bim expression in myocardial
tissue, but also upregulated Bim expression in myocardial cells. Yu
et al reported that naringenin treatment protects against
myocardial I/R injury by reducing oxidative stress and CHOP
expression (40). The present
study is the first, to the best of our knowledge, to elucidate the
relationship between Tan-IIA and CHOP during ischemic heart
disease. Collectively, Tan-IIA treatment may lead to the inhibition
of downstream caspase-3 in apoptosis in myocardiocytes, which may
be a potential drug for the treatment of patients with myocardial
ischemia.
Certain limitations of the present study should be
noted. Firstly, this study did not analyze the endoplasmic
reticulum stress-dependent pathway. Notably, the main mechanism of
Tan-IIA in myocardiocytes is intricate. Nevertheless, further
experiments and data analysis should be conducted in future
studies. Secondly, the effects of Tan-IIA on loss of the
mitochondrial membrane potential and the content of Cyto c
in mitochondria and the cytoplasm in myocardiocytes were not
analyzed. Thirdly, the specific anti-apoptotic pathway by which
Tan-IIA protected against myocardial infarction was not confirmed.
Finally, the sample size was small, therefore, the effect of
Tan-IIA on myocardial infarct and apoptosis of myocardiocytes
should be identified in a larger sample size to draw conclusions in
our future study.
In conclusion, data in the present study indicated
the therapeutic effect in a rat model of myocardial ischemia and
provided a possible mechanism responsible for the anti-apoptotic
effect of Tan-IIA in myocardiocytes. Our analysis revealed that
Tan-IIA ameliorated apoptosis of myocardiocytes through the
mitochondrial signaling pathway and improved myocardial function
via oxidative stress-dependent pathways. The present study further
demonstrated the anti-apoptotic effects of Tan-IIA in infarct
expansion after myocardial ischemia, which provides a significant
clinical reference for the treatment of patients with myocardial
ischemia.
Availability of data and materials
All datasets generated or analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
YF, CD, SC, ZL and WA collected and interpreted the
data and wrote the manuscript. LW, PX, BJ and YF designed and
performed the experiments. HF conceived the study, reviewed and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
20160512C10) by the Ethics Committee of Shenzhen Nanshan People's
Hospital (Shenzhen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Dong Y, Chen H, Gao J, Liu Y, Li J and
Wang J: Molecular machinery and interplay of apoptosis and
autophagy in coronary heart disease. J Mol Cell Cardiol. 136:27–41.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yndestad A, Sandanger Ø, Jong WMC, Aukrust
P and Zuurbier CJ: Response to letter from Toldo et al on 'NLRP3
inflammasome activation during myocardial ischemia reperfusion is
cardioprotective'. Biochem Biophys Res Commun. 474:328–329. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Z, Zhang J, Ren T and Dong Z:
Targeted metabolomic profiling of cardioprotective effect of Ginkgo
biloba L. extract on myocardial ischemia in rats. Phytomedicine.
23:621–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Niu X, Hu J, Xing H, Sun M, Wang
J, Jian Q and Yang H: After myocardial ischemia-reperfusion,
miR-29a, and Let7 could affect apoptosis through regulating IGF-1.
Biomed Res Int. 2015:2454122015. View Article : Google Scholar
|
|
5
|
Wakiyama H, Cowan DB, Toyoda Y, Federman
M, Levitsky S and McCully JD: Selective opening of mitochondrial
ATP-sensitive potassium channels during surgically induced
myocardial ischemia decreases necrosis and apoptosis. Eur J
Cardiothorac Surg. 21:424–433. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Elsässer A, Suzuki K, Lorenz-Meyer S, Bode
C and Schaper J: The role of apoptosis in myocardial ischemia: A
critical appraisal. Basic Res Cardiol. 96:219–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niermann C, Gorressen S, Klier M, Gowert
NS, Billuart P, Kelm M, Merx MW and Elvers M: Oligophrenin1
protects mice against myocardial ischemia and reperfusion injury by
modulating inflammation and myocardial apoptosis. Cell Signal.
28:967–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo CX, Jiang X, Zeng XJ, Wang HX, Li HH,
Du FH and Chen BX: Soluble receptor for advanced glycation
end-products protects against ischemia/reperfusion-induced
myocardial apoptosis via regulating the ubiquitin proteasome
system. Free Radic Biol Med. 94:17–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song T, Yao Y, Wang T, Huang H and Xia H:
Tanshinone IIA ameliorates apoptosis of myocardiocytes by
up-regulation of miR-133 and suppression of caspase-9. Eur J
Pharmacol. 815:343–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inserte J, Cardona M, Poncelas-Nozal M,
Hernando V, Vilardosa Ú, Aluja D, Parra VM, Sanchis D and
Garcia-Dorado D: Studies on the role of apoptosis after transient
myocardial ischemia: Genetic deletion of the executioner caspases-3
and -7 does not limit infarct size and ventricular remodeling.
Basic Res Cardiol. 111:182016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dongó E, Hornyák I, Benko Z and Kiss L:
The cardioprotective potential of hydrogen sulfide in myocardial
ischemia/reperfusion injury (review). Acta Physiol Hung.
98:369–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar
|
|
13
|
Mao C, Zhang Y, Zhang Y, Cao L, Shao H,
Wang L, Zhu L and Xu Z: The effect of tanshinone IIA on the
cardiovascular system in ovine fetus in utero. Am J Chin Med.
37:1031–1044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Li SS and Liang QS: Effects of
Tanshinone II A on the myocardial apoptosis and the miR-133 levels
in rats with heart failure. Zhongguo Zhong Xi Yi Jie He Za Zhi.
32:930–933. 2012.In Chinese. PubMed/NCBI
|
|
15
|
Yu ML, Li SM, Gao X, Li JG, Xu H and Chen
KJ: Sodium tanshinone II A sulfonate for coronary heart disease: A
systematic review of randomized controlled trials. Chin J Integr
Med. 26:219–226. 2020. View Article : Google Scholar
|
|
16
|
Yang R, Liu A, Ma X, Li L, Su D and Liu J:
Sodium tanshinone IIA sulfonate protects cardiomyocytes against
oxidative stress-mediated apoptosis through inhibiting JNK
activation. J Cardiovasc Pharmacol. 51:396–401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao S, Li L, Li L, Ni J, Guo R, Mao J and
Fan G: Effects of the combination of tanshinone IIA and puerarin on
cardiac function and inflammatory response in myocardial ischemia
mice. J Mol Cell Cardiol. 137:59–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aimo A, Castiglione V, Borrelli C, Saccaro
LF, Franzini M, Masi S, Emdin M and Giannoni A: Oxidative stress
and inflammation in the evolution of heart failure: From
pathophysiology to therapeutic strategies. Eur J Prev Cardiol.
27:494–510. 2020. View Article : Google Scholar
|
|
19
|
van der Pol A, Gil A, Tromp J, Silljé HHW,
van Veldhuisen DJ, Voors AA, Hoendermis ES, Grote Beverborg N,
Schouten EM, de Boer RA, et al: OPLAH ablation leads to
accumulation of 5-oxoproline, oxidative stress, fibrosis, and
elevated fillings pressures: A murine model for heart failure with
a preserved ejection fraction. Cardiovasc Res. 114:1871–1882. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang JP, Chen MC, Liu WH, Lin YS, Huang
YK, Pan KL, Ho WC, Fang CY, Chen CJ and Chen HC: Mitochondrial
apoptotic pathway activation in the atria of heart failure patients
due to mitral and tricuspid regurgitation. Exp Mol Pathol.
99:65–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dalal S, Zha Q, Singh M and Singh K:
Osteopontin-stimulated apoptosis in cardiac myocytes involves
oxidative stress and mitochondrial death pathway: Role of a
pro-apoptotic protein BIK. Mol Cell Biochem. 418:1–11. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuznetsov G, Bush KT, Zhang PL and Nigam
SK: Perturbations in maturation of secretory proteins and their
association with endoplasmic reticulum chaperones in a cell culture
model for epithelial ischemia. Proc Natl Acad Sci USA.
93:8584–8589. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang HZ, Kim MH, Lim JH and Bae HR:
Time-dependent expression patterns of cardiac aquaporins following
myocardial infarction. J Korean Med Sci. 28:402–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Wu G, Liu H, Xing N, Sun Y, Zhai
Y, Yang B, Kong AT, Kuang H and Wang Q: Cardioprotective effect of
the xanthones from Gentianella acuta against myocardial
ischemia/reperfusion injury in isolated rat heart. Biomed
Pharmacother. 93:626–635. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JT, Chung HJ, Seo JY, Yang YI, Choi
MY, Kim HI, Yang TH, Lee WJ, Youn YC, Kim HJ, et al: A
fibrin-supported myocardial organ culture for isolation of cardiac
stem cells via the recapitulation of cardiac homeostasis.
Biomaterials. 48:66–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai M, Pan CL, Jiang GX, Zhang YM and
Zhang Z: CircHIPK3 aggravates myocardial ischemia-reperfusion
injury by binding to miRNA-124-3p. Eur Rev Med Pharmacol Sci.
23:10107–10114. 2019.PubMed/NCBI
|
|
27
|
Wallert M, Ziegler M, Wang X, Maluenda A,
Xu X, Yap ML, Witt R, Giles C, Kluge S, Hortmann M, et al:
α-Tocopherol preserves cardiac function by reducing oxidative
stress and inflammation in ischemia/reperfusion injury. Redox Biol.
26:1012922019. View Article : Google Scholar
|
|
28
|
Wei B, Li WW, Ji J, Hu QH and Ji H: The
cardioprotective effect of sodium tanshinone IIA sulfonate and the
optimizing of therapeutic time window in myocardial
ischemia/reperfusion injury in rats. Atherosclerosis. 235:318–327.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakayoshi T, Sasaki K, Kajimoto H, Koiwaya
H, Ohtsuka M, Ueno T, Chibana H, Itaya N, Sasaki M, Yokoyama S, et
al: Correction: FOXO4-knockdown suppresses oxidative stress-induced
apoptosis of early pro-angiogenic cells and augments their
neovascularization capacities in ischemic limbs. PLoS One.
10:e01272452015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagy T, Kovács V, Hardi P, Veres TG,
Takács I, Jancsó G, Sinay L, Fazekas G, Pintér Ö and Arató E:
Inhibition of glutathione S-transferase by ethacrynic acid augments
ischemia-reperfusion damage and apoptosis and attenuates the
positive effect of ischemic postconditioning in a bilateral acute
hindlimb ischemia rat model. J Vasc Res. 52:53–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hassan M, Selimovic D, Hannig M, Haikel Y,
Brodell RT and Megahed M: Endoplasmic reticulum stress-mediated
pathways to both apoptosis and autophagy: Significance for melanoma
treatment. World J Exp Med. 5:206–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lim YC, Budin SB, Othman F, Latip J and
Zainalabidin S: Roselle polyphenols exert potent negative inotropic
effects via modulation of intracellular calcium regulatory channels
in isolated rat heart. Cardiovasc Toxicol. 17:251–259. 2017.
View Article : Google Scholar
|
|
33
|
Tao L, Huang K, Wang J, Xue Y, Zhou Y, He
F, Shen Y, Wang J, Gu X, Ji K, et al: Retinol palmitate protects
against myocardial ischemia/reperfusion injury via reducing
oxidative stress and inhibiting apoptosis. Am J Transl Res.
11:1510–1520. 2019.PubMed/NCBI
|
|
34
|
Pan C, Lou L, Huo Y, Singh G, Chen M,
Zhang D, Wu A, Zhao M, Wang S and Li J: Salvianolic acid B and
tanshinone IIA attenuate myocardial ischemia injury in mice by NO
production through multiple pathways. Ther Adv Cardiovasc Dis.
5:99–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li S, Wu H, Han D, Zhang M, Li N, Yu W,
Sun D, Sun Z, Ma S, Gao E, et al: ZP2495 protects against
myocardial ischemia/reperfusion injury in diabetic mice through
improvement of cardiac metabolism and mitochondrial function: The
possible involvement of AMPK-FoxO3a signal pathway. Oxid Med Cell
Longev. 2018:64519022018.PubMed/NCBI
|
|
36
|
Bhuiyan MS, Shibuya M, Shioda N, Moriguchi
S, Kasahara J, Iwabuchi Y and Fukunaga K: Cytoprotective effect of
bis(1-oxy-2-pyridinethiolato)oxovanadiun(IV) on myocardial
ischemia/reperfusion injury elicits inhibition of Fas ligand and
Bim expression and elevation of FLIP expression. Eur J Pharmacol.
571:180–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shukla S, Sharma A, Pandey VK, Raisuddin S
and Kakkar P: Concurrent acetylation of FoxO1/3a and p53 due to
sirtuins inhibition elicit Bim/PUMA mediated mitochondrial
dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol
Appl Pharmacol. 291:70–83. 2016. View Article : Google Scholar
|
|
38
|
Babu PP, Suzuki G, Ono Y and Yoshida Y:
Attenuation of ischemia and/or reperfusion injury during myocardial
infarction using mild hypothermia in rats: An immunohistochemical
study of Bcl-2, Bax, Bak and TUNEL. Pathol Int. 54:896–903. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang D, Hu X, Lee SH, Chen F, Jiang K, Tu
Z, Liu Z, Du J, Wang L, Yin C, et al: Diabetes exacerbates
myocardial ischemia/reperfusion injury by down-regulation of
microRNA and up-regulation of O-GlcNAcylation. JACC Basic Transl
Sci. 3:350–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu LM, Dong X, Zhang J, Li Z, Xue XD, Wu
HJ, Yang ZL, Yang Y and Wang HS: Naringenin attenuates myocardial
ischemia-reperfusion injury via cGMP-PKGIα signaling and in vivo
and in vitro studies. Oxid Med Cell Longev. 2019:76708542019.
View Article : Google Scholar
|