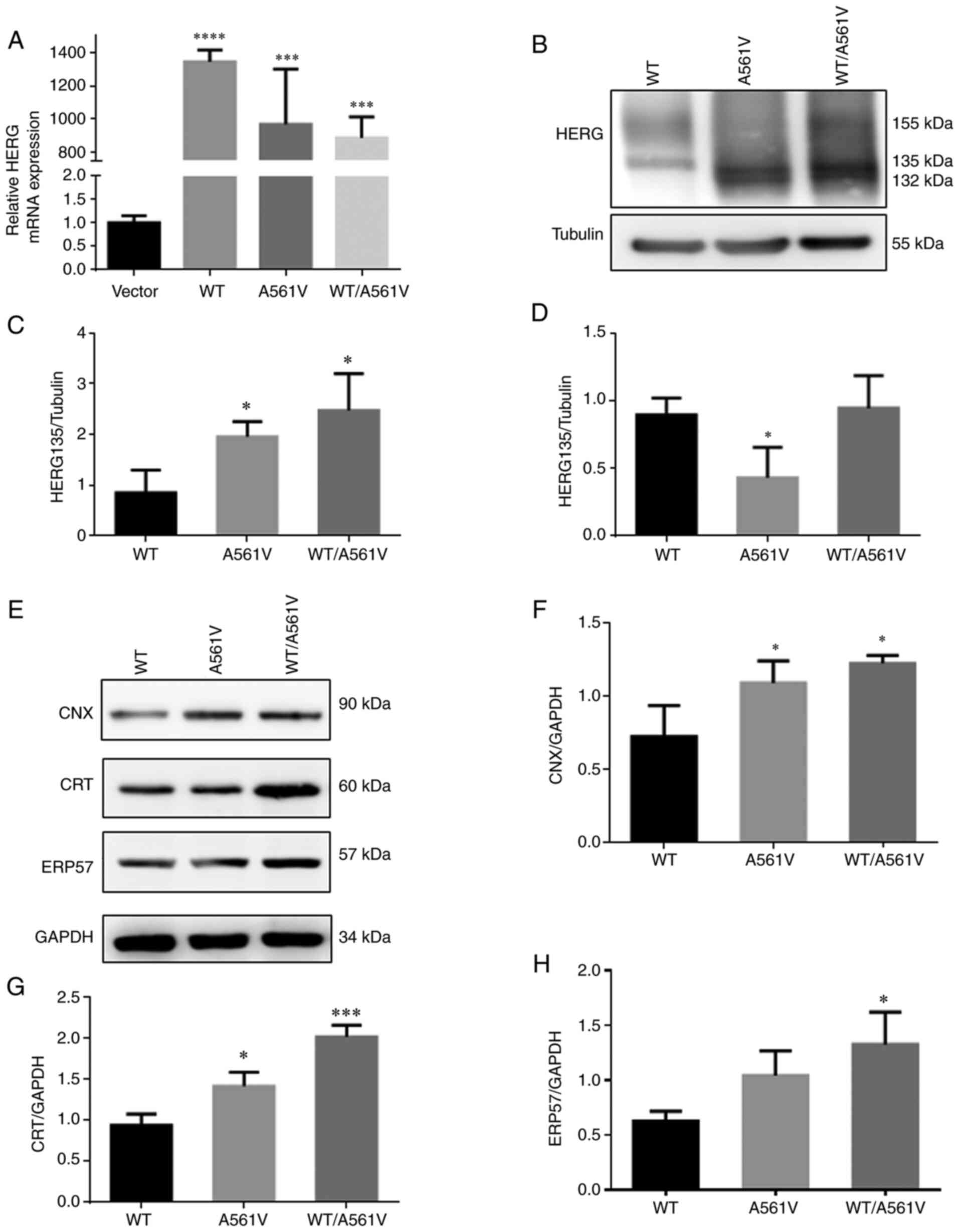
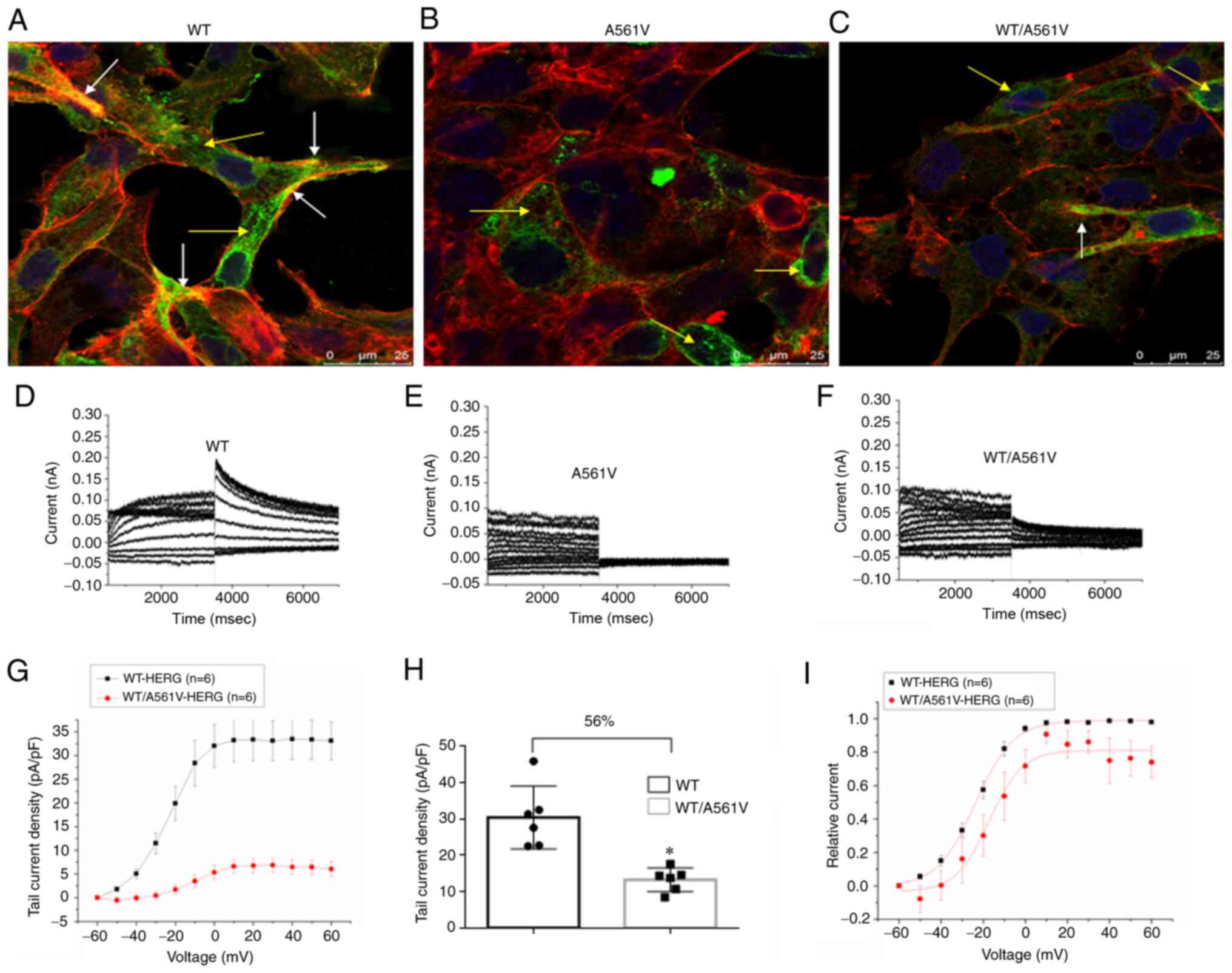
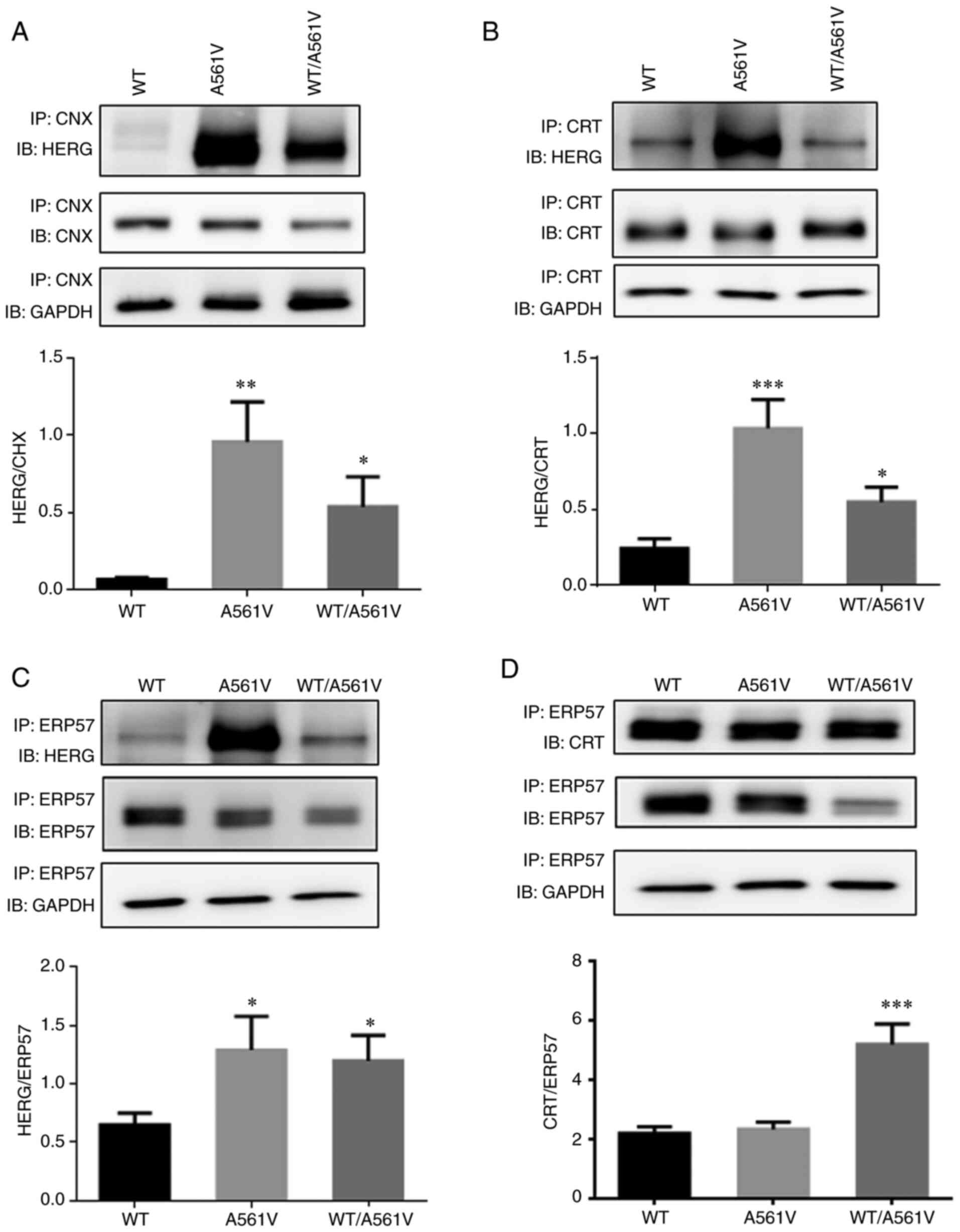
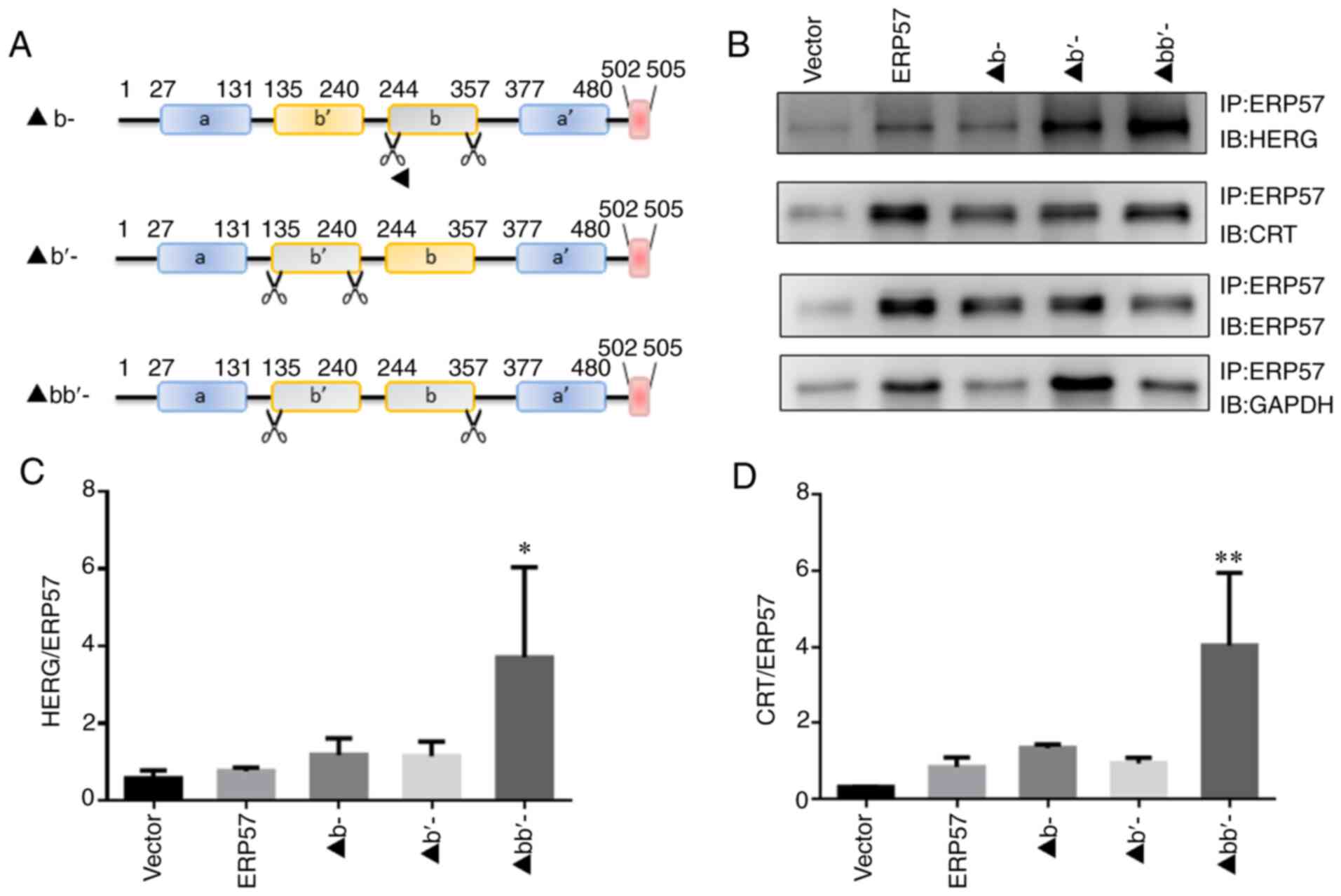
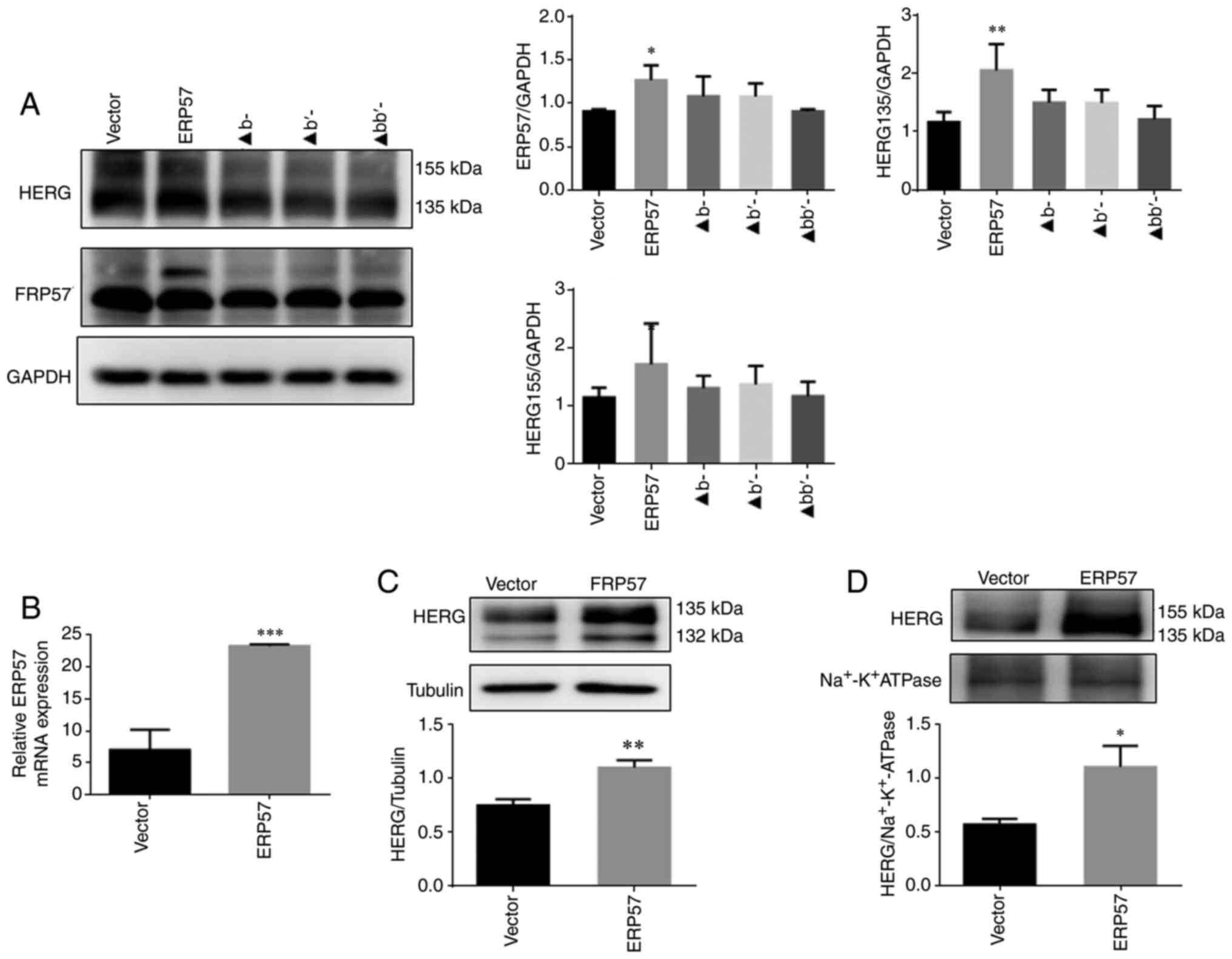
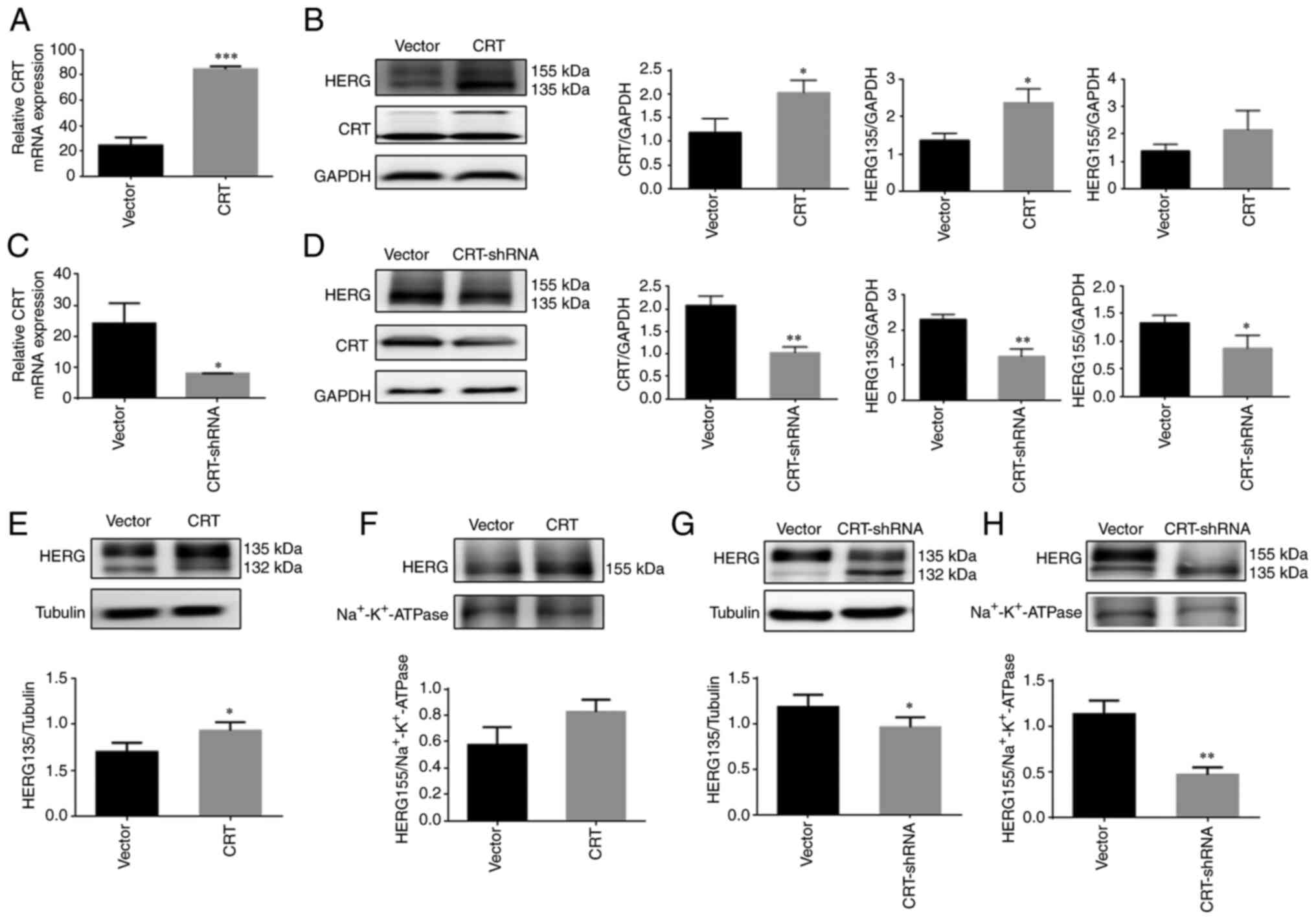
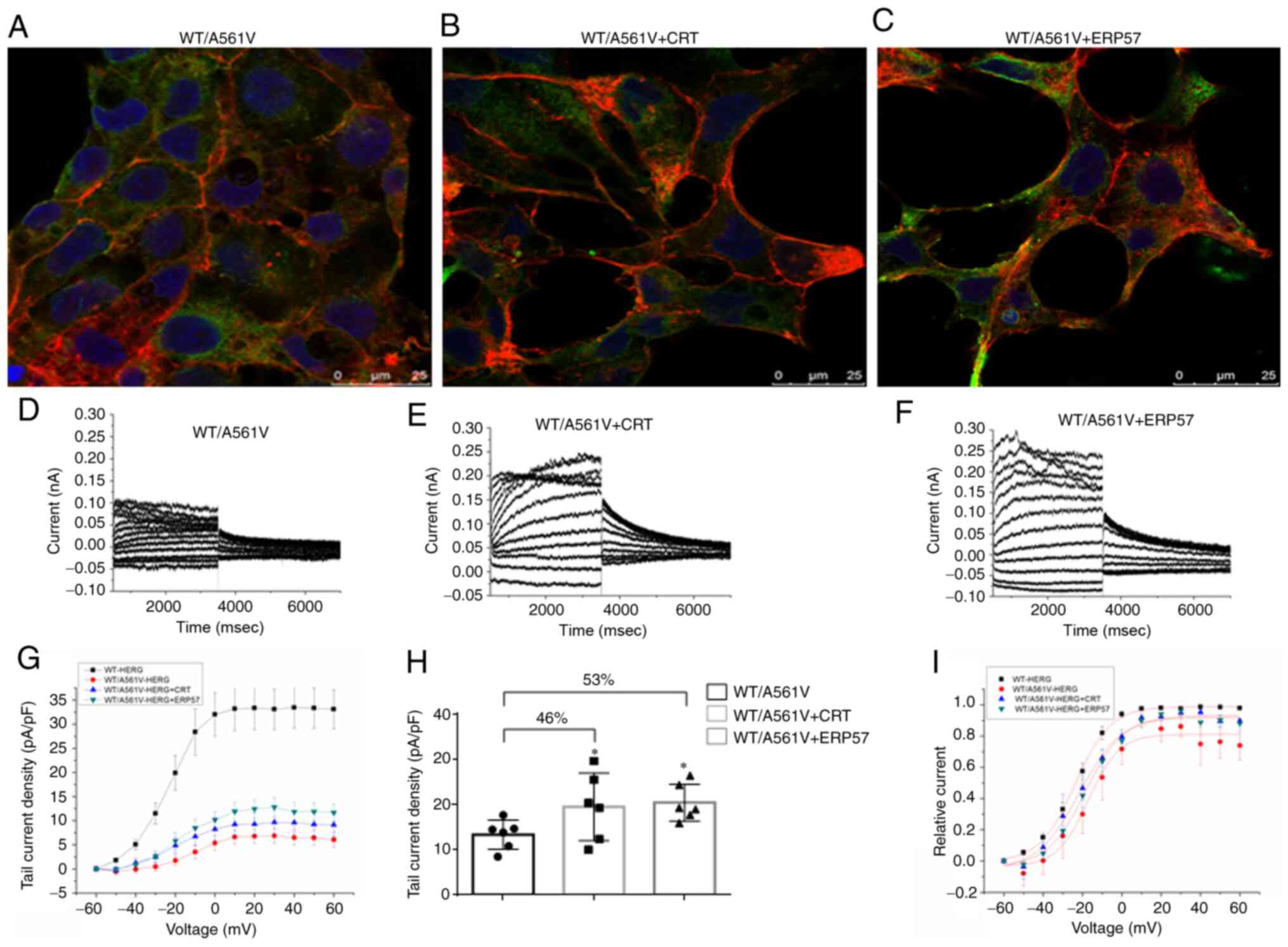
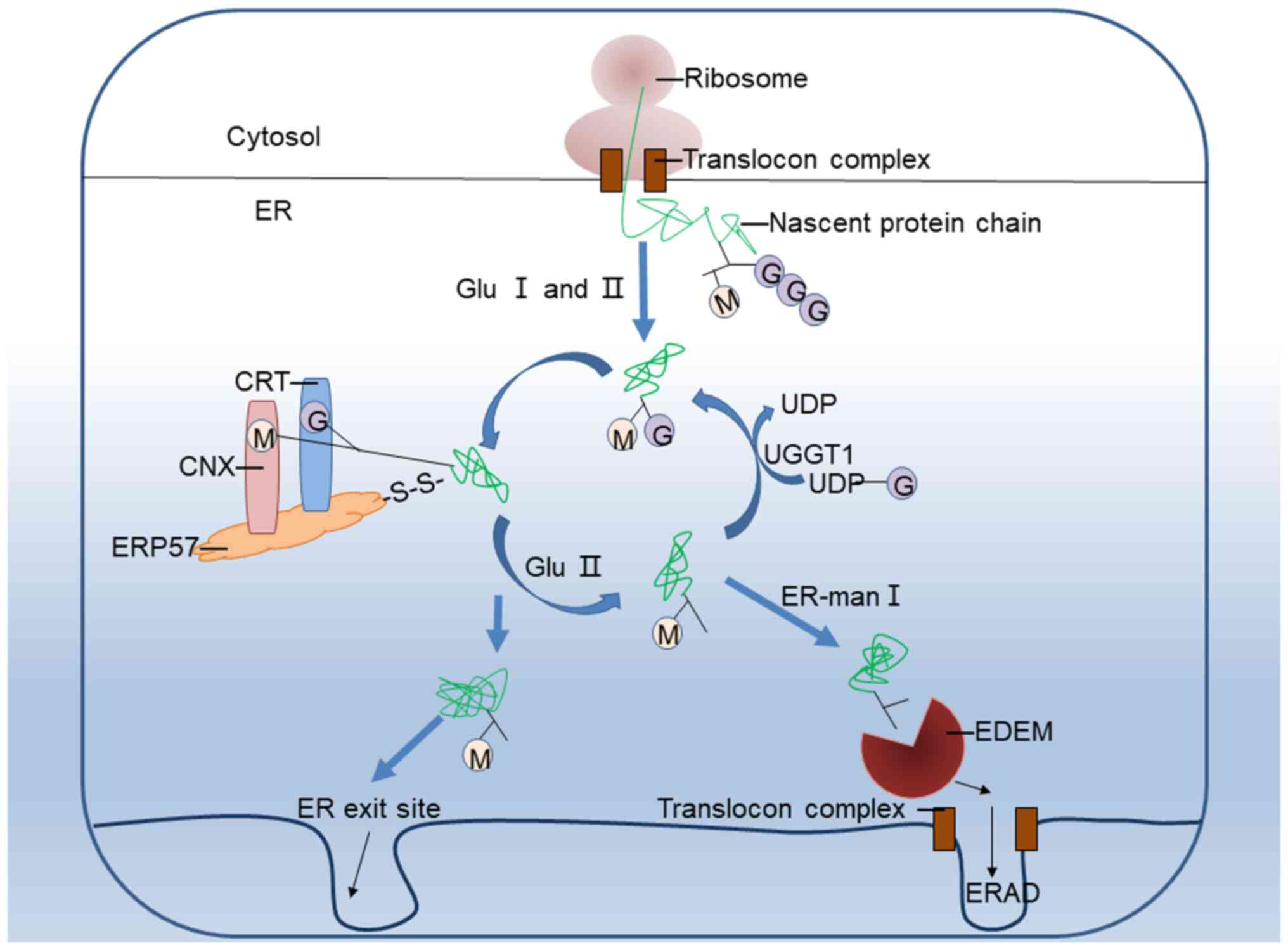
|
1
|
Wallace E, Howard L, Liu M, O'Brien T,
Ward D, Shen S and Prendiville T: Long QT syndrome: Genetics and
future perspective. Pediatr Cardiol. 40:1419–1430. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanner SA, Jain A and Colecraft HM:
Development of a high-throughput flow cytometry assay to monitor
defective trafficking and rescue of long QT2 mutant hERG channels.
Front Physiol. 9:3972018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butler A, Helliwell MV, Zhang Y, Hancox JC
and Dempsey CE: An update on the structure of hERG. Front
Pharmacol. 10:15722020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perissinotti L, Guo J, Kudaibergenova M,
Lees-Miller J, Ol'khovich M, Sharapova A, Perlovich GL, Muruve DA,
Gerull B, Noskov SY and Duff HJ: The pore-lipid interface: Role of
amino-acid determinants of lipophilic access by ivabradine to the
hERG1 pore domain. Mol Pharmacol. 96:259–271. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang KP, Yang BF and Li BX: Translational
toxicology and rescue strategies of the hERG channel dysfunction:
Biochemical and molecular mechanistic aspects. Acta Pharmacol Sin.
35:1473–1484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li G, Shi R, Wu J, Han W, Zhang A, Cheng
G, Xue X and Sun C: Association of the hERG mutation with long-QT
syndrome type 2, syncope and epilepsy. Mol Med Rep. 13:2467–2475.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foo B, Williamson B, Young JC, Lukacs G
and Shrier A: hERG quality control and the long QT syndrome. J
Physiol. 594:2469–2481. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Araki K and Nagata K: Protein folding and
quality control in the ER. Cold Spring Harb Perspect Biol.
3:a0075262011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marti L, Lia A, Reca IB, Roversi P,
Santino A and Zitzmann N: In planta preliminary screening of ER
glycoprotein folding quality control (ERQC) modulators. Int J Mol
Sci. 19:21352018. View Article : Google Scholar :
|
|
10
|
Lamothe SM, Hulbert M, Guo J, Li W, Yang T
and Zhang S: Glycosylation stabilizes hERG channels on the plasma
membrane by decreasing proteolytic susceptibility. FASEB J.
32:1933–1943. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patel C, Saad H, Shenkman M and
Lederkremer GZ: Oxidoreductases in glycoprotein glycosylation,
folding, and ERAD. Cells. 9:21382020. View Article : Google Scholar :
|
|
12
|
Tannous A, Pisoni GB, Hebert DN and
Molinari M: N-linked sugar-regulated protein folding and quality
control in the ER. Semin Cell Dev Biol. 41:79–89. 2015. View Article : Google Scholar :
|
|
13
|
Foo B, Barbier C, Guo K, Vasantharuban J,
Lukacs GL and Shrier A: Mutation-specific peripheral and ER quality
control of hERG channel cell-surface expression. Sci Rep.
9:60662019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi YQ, Yan M, Liu LR, Zhang X, Wang X,
Geng HZ, Zhao X and Li BX: High glucose represses hERG K+ channel
expression through trafficking inhibition. Cell Physiol Biochem.
37:284–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hettinghouse A, Liu R and Liu CJ:
Multifunctional molecule ERp57: From cancer to neurodegenerative
diseases. Pharmacol Ther. 181:34–48. 2018. View Article : Google Scholar
|
|
16
|
Song D, Liu H, Wu J, Gao X, Hao J and Fan
D: Insights into the role of ERp57 in cancer. J Cancer.
12:2456–2464. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kozlov G and Gehring K: Calnexin
cycle-structural features of the ER chaperone system. FEBS J.
287:4322–4340. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pollock S, Kozlov G, Pelletier MF, Trempe
JF, Jansen G, Sitnikov D, Bergeron JJ, Gehring K, Ekiel I and
Thomas DY: Specific interaction of ERp57 and calnexin determined by
NMR spectroscopy and an ER two-hybrid system. EMBO J. 23:1020–1029.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Shen T, Fang P, Zhou J, Lou K, Cen
Z, Qian H, Zhou J, Liu N and Lian J: The role and mechanism of
chaperones calnexin/calreticulin in which ALLN selectively rescues
the trafficking defective of HERG-A561V mutation. Biosci Rep. Sep
7–2018.Epub ahead of print.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Smith JL, Anderson CL, Burgess DE, Elayi
CS, January CT and Delisle BP: Molecular pathogenesis of long QT
syndrome type 2. J Arrhythm. 32:373–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jenewein T, Kanner SA, Bauer D, Hertel B,
Colecraft HM, Moroni A, Thiel G and Kauferstein S: The mutation
L69P in the PAS domain of the hERG potassium channel results in
LQTS by trafficking deficiency. Channels (Austin). 14:163–174.
2020. View Article : Google Scholar
|
|
23
|
Zhan G, Wang F, Ding YQ, Li XH, Li YX,
Zhao ZR, Li JX, Liu Y, Zhao X, Yan CC and Li BX: Rutaecarpine
targets hERG channels and participates in regulating
electrophysiological properties leading to ventricular arrhythmia.
J Cell Mol Med. 25:4938–4949. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Huang X, Zhou J, Yang X, Li D, Mao
H, Sun HH, Liu N and Lian J: Trafficking-deficient G572R-hERG and
E637K-hERG activate stress and clearance pathways in endoplasmic
reticulum. PLoS One. 7:e298852012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ono M, Burgess DE, Schroder EA, Elayi CS,
Anderson CL, January CT, Sun B, Immadisetty K, Kekenes-Huskey PM
and Delisle BP: Long QT syndrome type 2: Emerging strategies for
correcting class 2 KCNH2 (hERG) mutations and identifying new
patients. Biomolecules. 10:11442020. View Article : Google Scholar :
|
|
26
|
Anderson CL, Delisle BP, Anson BD, Kilby
JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ and January CT:
Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2
(trafficking-deficient) mechanism. Circulation. 113:365–373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du H, Zheng C, Aslam M, Xie X, Wang W,
Yang Y and Liu X: Endoplasmic reticulum-mediated protein quality
control and endoplasmic reticulum-associated degradation pathway
explain the reduction of N-glycoprotein level under the lead
stress. Front Plant Sci. 11:5985522021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito Y, Takeda Y, Seko A, Izumi M and
Kajihara Y: Functional analysis of endoplasmic reticulum
glucosyltransferase (UGGT): Synthetic chemistry's initiative in
glycobiology. Semin Cell Dev Biol. 41:90–98. 2015. View Article : Google Scholar
|
|
29
|
Tannous A, Patel N, Tamura T and Hebert
DN: Reglucosylation by UDP-glucose: Glycoprotein
glucosyltransferase 1 delays glycoprotein secretion but not
degradation. Mol Biol Cell. 26:390–405. 2015. View Article : Google Scholar :
|
|
30
|
Ferris SP, Jaber NS, Molinari M, Arvan P
and Kaufman RJ: UDP-glucose: Glycoprotein glucosyltransferase
(UGGT1) promotes substrate solubility in the endoplasmic reticulum.
Mol Biol Cell. 24:2597–2608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerriero CJ and Brodsky JL: The delicate
balance between secreted protein folding and endoplasmic
reticulum-associated degradation in human physiology. Physiol Rev.
92:537–576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shenkman M, Ron E, Yehuda R, Benyair R,
Khalaila I and Lederkremer GZ: Mannosidase activity of EDEM1 and
EDEM2 depends on an unfolded state of their glycoprotein
substrates. Commun Biol. 1:1722018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Benyair R, Ogen-Shtern N, Mazkereth N,
Shai B, Ehrlich M and Lederkremer GZ: Mammalian ER mannosidase I
resides in quality control vesicles, where it encounters its
glycoprotein substrates. Mol Biol Cell. 26:172–184. 2015.
View Article : Google Scholar :
|
|
34
|
Ogen-Shtern N, Avezov E, Shenkman M,
Benyair R and Lederkremer GZ: Mannosidase IA is in quality control
vesicles and participates in glycoprotein targeting to ERAD. J Mol
Biol. 428:3194–3205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chung H, Cho H, Perry C, Song J, Ylaya K,
Lee H and Kim JH: Downregulation of ERp57 expression is associated
with poor prognosis in early-stage cervical cancer. Biomarkers.
18:573–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choe MH, Min JW, Jeon HB, Cho DH, Oh JS,
Lee HG, Hwang SG, An S, Han YH and Kim JS: ERp57 modulates STAT3
activity in radioresistant laryngeal cancer cells and serves as a
prognostic marker for laryngeal cancer. Oncotarget. 6:2654–2666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kozlov G, Pocanschi CL, Rosenauer A,
Bastos-Aristizabal S, Gorelik A, Williams DB and Gehring K:
Structural basis of carbohydrate recognition by calreticulin. J
Biol Chem. 285:38612–38620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiuchi T, Izumi M, Mukogawa Y, Shimada A,
Okamoto R, Seko A, Sakono M, Takeda Y, Ito Y and Kajihara Y:
Monitoring of glycoprotein quality control system with a series of
chemically synthesized homogeneous native and misfolded
glycoproteins. J Am Chem Soc. 140:17499–17507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ito Y, Kajihara Y and Takeda Y:
Chemical-synthesis-based approach to glycoprotein functions in the
endoplasmic reticulum. Chemistry. 26:15461–15470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghemrawi R and Khair M: Endoplasmic
reticulum stress and unfolded protein response in neurodegenerative
diseases. Int J Mol Sci. 21:61272020. View Article : Google Scholar :
|
|
41
|
Wang SB, Shi Q, Xu Y, Xie WL, Zhang J,
Tian C, Guo Y, Wang K, Zhang BY, Chen C, et al: Protein disulfide
isomerase regulates endoplasmic reticulum stress and the apoptotic
process during prion infection and PrP mutant-induced cytotoxicity.
PLoS One. 7:e382212012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Perri E, Parakh S and Atkin J: Protein
disulphide isomerases: Emerging roles of PDI and ERp57 in the
nervous system and as therapeutic targets for ALS. Expert Opin Ther
Targets. 21:37–49. 2017. View Article : Google Scholar
|
|
43
|
Turano C, Gaucci E, Grillo C and
Chichiarelli S: ERp57/GRP58: A protein with multiple functions.
Cell Mol Biol Lett. 16:539–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sepulveda M, Rozas P, Hetz C and Medinas
DB: ERp57 as a novel cellular factor controlling prion protein
biosynthesis: Therapeutic potential of protein disulfide
isomerases. Prion. 10:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hoffstrom BG, Kaplan A, Letso R, Schmid
RS, Turmel GJ, Lo DC and Stockwell BR: Inhibitors of protein
disulfide isomerase suppress apoptosis induced by misfolded
proteins. Nat Chem Biol. 6:900–906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grek C and Townsend DM: Protein disulfide
isomerase superfamily in disease and the regulation of apoptosis.
Endoplasmic Reticulum Stress Dis. 1:4–17. 2014.PubMed/NCBI
|
|
47
|
Han A, Li C, Zahed T, Wong M, Smith I,
Hoedel K, Green D and Boiko AD: Calreticulin is a critical cell
survival factor in malignant neoplasms. PLoS Biol. 17:e30004022019.
View Article : Google Scholar : PubMed/NCBI
|