Introduction
Coronary artery disease (CAD) is the most common
type of organ lesion caused by atherosclerosis and affects the life
quality of patients (1).
Atherosclerosis is a major issue globally, since an estimated 31%
of deaths worldwide are caused by cardiovascular disease, and the
number is steadily increasing (2). The cause of CAD remains
incompletely understood, as it may be the result of a combination
of multiple factors. Risk factors for CAD include age, sex,
hypertension, diabetes mellitus, smoking, dyslipidemia and obesity
(3). Currently, effective
treatment strategies include secondary prevention, plasma oxidized
low-density lipoprotein (LDL) reduction, antiplatelet agents,
anticoagulants, blood pressure and diet control, exercise and
percutaneous coronary intervention, or coronary artery bypass
surgery selectively when the major coronary artery exhibits >50%
stenosis (4). However, the
mortality rate of AMI remains high due to the difficulty of
diagnosing CAD at early stages, and the development of new
noninvasive methods for the early diagnosis of CAD is crucial for
improving its prognosis (5).
Since the molecular mechanisms underlying these pathological and
pathophysiological processes are not completely understood, the
identification of key regulatory molecules of cardiovascular
disease is necessary to develop effective preventive measures and
treatments (6).
It has been demonstrated that >75% of the human
genome encodes noncoding RNAs (ncRNAs). Long noncoding RNAs
(lncRNAs; ncRNAs >200 nucleotides) have emerged as notable
contributors in the regulation of chromatin modification, gene
expression, cell proliferation, differentiation and human diseases
such as cancer, neurological disorders and immunological disorders
(7,8). The expression patterns, tumor
specificity and stability of lncRNAs in circulating body fluids
(plasma and urine) have provided a basis for the diagnosis and
treatment of cancer (9,10). Previous studies have demonstrated
that lncRNAs are involved in the development of various CADs
through the regulation of cell differentiation, proliferation,
apoptosis, necrosis and autophagy (11-13). In addition, lncRNAs and microRNAs
(miRNAs) regulate the initiation and progression of CAD by
regulating various molecular mechanisms (6). For example, lncRNA nuclear enriched
abundant transcript 1 increases cell viability and affects CAD by
activating miR-140-3p (14).
High plasma levels of H19 and mitochondrial lncRNA
uc022bqs1 are associated with a high risk of CAD, and these
molecules have the potential to become novel biomarkers of CAD
(15). The interaction of
metastasis-associated lung adenocarcinoma transcript 1
(MALAT1), miR-22 and CXCR2/AKT in the
regulation of endothelial cell function demonstrates the complex
roles of lncRNA-miRNA-mRNA cascades in the progression of CAD
(16,17).
Previous studies have also revealed that cell
proliferation and migration are associated with the occurrence and
development of CAD. For example, variations in 9p21 affect the
expression of cyclin-dependent kinase 2A (CDKN2A) and 2B
(CDKN2A) in vascular smooth muscle cells (VSMCs) as well as
their proliferation, which may be an important mechanism underlying
the association between 9p21 and CAD susceptibility (18). In vascular cells, T-cadherin
(T-CAD) promotes cell proliferation by regulating the cell cycle,
indicating that this protein may promote the progression of
proliferative vascular diseases, such as atherosclerosis,
restenosis and tumor angiogenesis (19). Downregulation of MALAT1 is
associated with numerous pathological processes, including CAD, as
it inhibits the proliferation of vascular endothelial cells and
VSMCs, reduces myocardial cell apoptosis and improves left
ventricular function (20). In
addition, the lncRNA maternally expressed gene 3 suppresses
endothelial cell proliferation and migration by regulating
miR-22 (21).
In our previous study, a previously unreported
lncRNA uc003pxg.1 was identified (22); the genomic location and sequence
of uc003pxg.1 are presented in Data S1. The aim of the present study
was to determine whether the expression levels of uc003pxg.1
differed in peripheral blood mononuclear cells (PBMCs) isolated
from patients with CAD and healthy control subjects, and to
evaluate the interactions between miRNAs and uc003pxg.1 in
human umbilical vein endothelial cells (HUVECs).
Materials and methods
Study subjects
Between August 2017 and December 2019, 160 subjects
(80 patients with CAD and 80 controls) aged 40-85 years were
enrolled in the present study at the Department of Cardiology of
The Affiliated Suzhou Hospital of Nanjing Medical University
(Suzhou, China). CAD was confirmed by angiographic evidence in at
least one segment of a major coronary artery, including the left
anterior descending artery, left circumflex artery or right
coronary artery, with >50% stenosis (23). Patients with severe primary
disease, acute infection, trauma, myocardial infarction, cerebral
infarction, cancer and alcohol or drug addiction were excluded from
the study. The 80 control subjects were selected from individuals
undergoing routine health examinations. The reference intervals of
the routine blood tests were as follows: Blood pressure,
90-140/60-90 mmHg; blood glucose, 4-6.1 mmol/l; total cholesterol,
2.8-5.17 mmol/l; triglycerides, 0.56-1.7 mmol/l; and cholesterol
lipids, 2.8-5.17 mmol/l. The experiments conducted in the present
study were approved by the Ethics Committee of Nanjing Medical
University (KL901117). Written informed consent was obtained from
all patients or their families.
Cell culture and transfection
Immortalized HUVECs (cat. no. CW2919S) were
purchased from CoWin Biosciences and cultured in DMEM (HyClone;
Cytiva) containing 10% fetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml streptomycin and 100 U/ml
penicillin at 37°C in a humidified incubator under an atmosphere
with 5% CO2. Prior to use, the cells were washed with
phosphate-buffered saline (PBS; HyClone; Cytiva) and digested with
a Trypsin-EDTA solution (MilliporeSigma).
For transfection, HUVECs were seeded at ~50%
confluence in a 24-well at 24 h pre-transfection. Subsequently, the
cells were transfected with 100 nM small interfering (si)RNA
targeting lncRNA uc003pxg.1 (si-uc003pxg.1), negative
control (NC) siRNA (si-NC), mir-25-5p mimic,
mir-25-5p inhibitor or corresponding NC (Guangzhou RiboBio
Co., Ltd.) using a riboFECT™ CP Transfection kit (Guangzhou
RiboBio Co., Ltd.). A total of 2.5 µl siRNA was diluted with
30 µl 1X riboFECT™ CP Buffer and gently mixed.
Subsequently, 3 µl riboFECT™ CP Reagent was added and
mixed gently, incubated for ≤15 min at room temperature, and the
transfection complexes were added into the medium and incubated in
an incubator for 24-72 h at 37°C with 5% CO2. The cells
were collected at 24 and 48 h post-transfection for use in reverse
transcription-quantitative (RT-q)PCR and western blotting analyses,
respectively. The oligomer sequences were as follows:
si-uc003pxg.1, 5′-GCA ATG TAG TCA CCA AT AA-3′; si-NC,
5′-GGC TCT AGA AAA GCC TAT GC-3′; miRNA mimic NC, 5′-UUU GUA CUA
CAC AAA AGU ACU G-3′; miRNA inhibitor NC, 5′-CAG UAC UUU UGU GUA
GUA CAA A-3′; has-miR-25-5p mimics sense, 5′-AGG CGG AGA CUU GGG
CAA UUG-3′ and antisense, 5′-CAA UUG CCC AAG UCU CCG CCU-3′;
has-miR-25-5p inhibitor, 5′-CAA UUG CCC AAG UCU CCG CCU-3′.
PBMC isolation, RNA isolation and
RT-qPCR
Blood samples (4-6 ml) from each participant were
collected in EDTA vacuum anticoagulant blood vessels. The plasma
was collected following centrifugation at 1,600 × g for 10 min at
4°C to remove the debris. PBMCs were isolated from the blood cells
via Ficoll-Paque PLUS (GE Healthcare) density-gradient
centrifugation as previously described (24,25) and washed twice with saline.
Total RNA was extracted from PBMCs and HUVECs with
TRIzol® Reagent (Ambion; Thermo Fisher Scientific,
Inc.). Reverse transcription of 500 ng of RNA into cDNA was
performed with 2 µl 5X PrimeScript™ RT Master Mix (cat. no.
RR036A; Takara Bio, Inc.), and the reaction was adjusted to 10
µl with RNase-free dH2O. The thermocycling
conditions were as follows: 37°C for 15 min; 85°C for 5 sec; and
hold at 4°C. RT-qPCR was performed using SYBR® Premix Ex
Taq™ II (Takara Bio, Inc.) on a LightCycler® 480
Instrument II Real-Time PCR Detection system (Roche Diagnostics),
and β-actin was used as the internal reference. The thermocycling
conditions were as follows: 95°C for 30 sec; 40 cycles of 95°C for
5 sec and 60°C for 20 sec; and 65°C for 15 sec. The primers used in
this experiment were as follows: uc003pxg.1 forward, 5′-GTT
ACC AGA AAG CGT TGC CA-3′ and reverse, 5′-TAT ACT CAG TCC AGC AGC
CC-3′; cyclin D1 forward, 5′-GCT GCG AAG TGG AAA CCA TC-3′ and
reverse, 5′-CCT CCT TCT GCA CAC ATT TGA A-3′; and CDK6 forward,
5′-CAT ACC CTC TCT GCT GCT TT-3′ and reverse, 5′-TGC TAC TCA TTT
TGC TCA CCT-3′; β-actin forward, 5′-CAC GAA ACT ACC TTC AAC TCC-3′
and reverse, 5′-CAT ACT CCT GCT TGC TGA TC-3′ and were synthesized
by Genewiz, Inc. For miRNA quantification, bulge-loop RT primers
and qPCR primers specific for miR-25-5p were designed and
synthesized by Guangzhou RiboBio Co., Ltd. (product no.
MQPS0000882-1-100; RT miR-25-5p, cat. no. ssD809231465;
miR-25-5p forward, cat. no. ssD809231628; and
miR-25-5p reverse, cat. no. ssD089261711); U6
(Bulge-Loop U6 qPCR Primer Set; cat. no. MQPS0000002-1-100) was
used as the internal control, and the experiments were performed in
triplicate. The relative expression data were analyzed using the
2−∆∆Cq method (26)
following normalization to the β-actin (ACTB) or U6
levels.
High-content screening (HCS)
The differentially expressed lncRNAs
ENST00000583122, ENST00000606540, N R _ 027387, uc 0 03pxg.1, E NST
0 0 0 0 0 6 02 8 45, ENST00000601618 were selected from the
lncRNA-seq (accession no. GSE171551). siRNA mixes of these lncRNAs
were designed and synthesized by RiboBio Co., Ltd. EdU staining was
performed after 100-nM lncRNA siRNA mixes were transfected into
adherent HUVECs. The siRNA mix sequences were as follows:
si-ENST00000583122, 5′-AGA GAT ACA AGA AGG TCA A-3′, 5′-CCG ACA AAA
TGG GCT TAA T-3′ and 5′-CAG ATG TCA CAA TGT GA TA-3′;
si-ENST00000606540, 5′-AGG CGA AAC TGT CCT CA AA-3′, 5′-CTC CCA ATT
TGG AAA GTA A-3′ and 5′-GGA GAG GCT GAT TTG AAA A-3′; si-NR_027387,
5′-TGA CCA ACT CAT CTA CTC T-3′, 5′-ATC ACT CCT TGA CCA TT AT-3′
and 5′-GCA AAT GTC ATG TTC TGT T-3′; si-uc003pxg.1, 5′-GCA ATG TAG
TCA CCA ATA A-3′, 5′-CAC TAA CCT ACA ACC TTC A-3′ and 5′-CCA AGC
AAT AAC CCT TGT A-3′; si-ENST00000602845, 5′-CCC TGG CG CAA ACA TTT
AA-3′, 5′-AGC GAT TTA GAG ACG CCT T-3′ and 5′-CGA TTT AGA GAC GCC
TTC A-3′; and si-ENST00000601618, 5′-GCG AAA TGC TTG ACA ATC A-3′,
5′-TGC TTG ACA ATC ACA AT CA-3′ and 5′-TGACAGCCAGTAGCATTTA-3′.
Subsequently, the cells were rapidly detected with the GE IN CELL
Analyzer 6500HS high-content screening platform (Cytiva) by
Guangzhou RiboBio Co., Ltd.. The cell proliferation rate was
calculated based on the proportion of proliferating cells among the
total cells, and functional target lncRNAs were identified based on
differences in proliferation.
miRNA-seq high-throughput sequencing and
the selection of target miRNA
High-throughput sequencing was performed by
Guangzhou RiboBio Co., Ltd. siRNA (100 nM) was transfected into
HUVECs, and RNA was extracted from HUVECS in the NC and
si-uc003pxg.1 groups using the MagZol (Magen BioSciences).
The quantity and integrity of the RNA yield were assessed by K5500
and the Agilent 2200 TapeStation (Agilent Technologies, Inc.). The
total RNAs were ligated with a 3′ RNA adaptor, and followed by 5′
adaptor ligation. Subsequently, the adapter-ligated RNAs were
subjected to RT-PCR and amplified with a low cycle: Initial
denaturation at 94°C for 30 sec; 12-15 cycles of denaturation at
94°C for 15 sec, annealing at 62°C for 30 sec and extension at 70°C
for 15 sec; final extension at 70°C for 5 min; hold at 4°C. The PCR
products were size-selected by PAGE. The adaptors, primers, enzymes
and buffers required to convert small RNAs into indexed libraries
for next-generation sequencing on the Illumina platform were
included in the NEBNext® Multiplex Small RNA Library
Prep Set for Illumina® (Index Primers 1-48, cat. no. NEB
#E7560S; Illumina, Inc.), and all procedures were performed
according to the manufacturer's instructions. The purified library
products were evaluated using an Agilent 2200 TapeStation. The
libraries were sequenced on a HiSeq 2500 instrument (Illumina,
Inc.) to generate single-end 50-bp reads by Guangzhou RiboBio Co.,
Ltd.. The miRNA expression was calculated as the reads per million
(RPM) values as follows: RPM=(no. of reads mapping to miRNA/no. of
reads in clean 50 data) ×106. The expression levels were
calculated, and the miRNA expression clusters were analyzed to
identify miRNAs that were differentially expressed. The expression
of miRNAs were verified by RT-qPCR in 25 patients with CAD and 25
control subjects selected from the 160 clinical samples.
RNA fluorescence in situ hybridization
(FISH)
HUVECs were cultured in 24-well plates with round
coverslips at the bottom overnight. FISH assays were performed
using a Ribo™ Fluorescent In Situ Hybridization kit
(Guangzhou RiboBio Co., Ltd.). The Cy 3-labeled DNA probes of
uc003pxg.1, 18S and U6 (RiboTMh-lnc uc003_FISH-probe Mix;
product no. lnc1101634; Red, 20T) were designed by Guangzhou
RiboBio Co., Ltd.. After the cells reached 60-70% confluence, they
were washed with PBS and fixed with 4% paraformaldehyde for 10 min
at room temperature. Subsequently, 1 ml precooled permeabilization
solution was added to each well and incubated for 5 min at 4°C.
Following washing with PBS, 200 µl prehybridization solution
was added to each well and incubated at 37°C for 30 min. Then, 100
µl probe hybridization solution was added to each well and
hybridized overnight at 37°C in the dark. The cells were washed
with saline sodium citrate and PBS, and DAPI was added and
incubated for 10 min at room temperature. Images (≥3) were captured
using confocal fluorescence microscopy at ×63 magnification.
Dual-luciferase reporter assay
A uc003pxg.1 fragment containing the
miR-25-5p-binding site was constructed and cloned into the
target pmirGLO dual-luciferase reporter gene vector (Guangzhou
RiboBio Co., Ltd.) to generate the construct
pmirGLO-uc003pxg.1-wild-type (wt). A uc003pxg.1
sequence containing a mutated miR-25-5p-binding site was
cloned into the vector to generate the construct
pmirGLO-uc003pxg.1-mutant (mut). The binding target
prediction of miR-25-5p and uc003pxg.1 are presented
in Data S1. The vectors were
transfected into E. coli DH5α competent cells (cat. no.
CS01010, Anhui General Biosystems Co., Ltd.), cultured overnight at
37°C, and the plasmids were extracted from the positive clones. The
miR-25-5p mimics and miR-NC were transfected into
HUVECs, which were subsequently transfected with
pmirGLO-uc003pxg.1-wt or pmirGLO-uc003pxg.1-mut.
HUVECs were seeded in 6-well plate the day before transfection. A
total of 2 µg plasmid and or miRNAs were diluted with 100
µl OPTI-MEM medium and incubated for 5 min at room
temperature. Subsequently, Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the
diluted plasmids, and X-tremeGENE siRNA Transfection Reagent
(Guangzhou RiboBio Co., Ltd.) was added to the diluted miRNAs,
incubated for 15 min at room temperature, added into 6-well plates
and incubated in an incubator at 37°C with 5% CO2. The
luciferase activity was measured at 24 h post-transfection with a
Dual-Luciferase Reporter Assay system (cat. no. E1910; Promega
Corporation), and the firefly luciferase activity relative to
Renilla luciferase activity for each construct was compared
with that of the control group.
Cell viability
Cell Counting Kit-8 (CCK-8; Dojindo Laboratories,
Inc.) was used to assess cell proliferation. At 12-24 h
post-transfection with 100 nM siRNA, 1,000 HUVECs (100 µl
cell suspension) were seeded in each well of a 96-well plate and
cultured in an incubator for 3-4 h until the cells adhered to the
well. Subsequently, 10 µl CCK-8 solution was added to each
well every 24 h for 3 days and incubated in an incubator at 37°C
under an atmosphere with 5% CO2 for 2-4 h. Absorbance at
450 nm was determined by a microplate reader.
Cell cycle analysis
At 48 h post-transfection, ~5×104
adherent HUVECs in 6-well plates were digested with trypsin and
washed with PBS. Then, the cells were fixed with 70% cold ethanol
for >24 h at -20°C, after which they were treated with 10
µl RNase A and 25 µl propidium using a Cell Cycle and
Apoptosis Analysis kit (Beyotime Institute of Biotechnology) for 30
min at 37°C. Subsequently, the cells were washed with cold PBS, and
fluorescence at 488 nm was detected by flow cytometry (BD
FACSCalibur™; Becton Dickinson and Company). The data were analyzed
using FlowJo V7.6 software (BD Biosciences).
5-Ethynyl-2′-deoxyuridine (EdU)
assay
A Cell-Light EdU Apollo 567 In Vitro Flow
Cytometry kit (Guangzhou RiboBio Co., Ltd.) was used for the EdU
assay. HUVECs were seeded at 40-60% confluence in 24-well plates
with round coverslips and incubated overnight. Subsequently, at 48
h post-transfection with 100 nM siRNA, the cells were washed with
PBS, fixed with 4% paraformaldehyde for 30 min, and then treated
with a 0.5% Triton X-100/PBS solution for 10 min at room
temperature. The cells were incubated with 1X Apollo reagent for 30
min in the dark at room temperature according to the manufacturer's
instructions, followed by staining with 1X Hoechst for 30 min at
room temperature. Images (≥3 per sample) were captured by confocal
fluorescence microscopy at ×40 magnification (LSM900; Zeiss AG).
The percentage of EdU-positive cells was quantified using ImageJ
v1.8.0 (National Institutes of Health).
Transwell assay
For the Transwell migration assays, 24-well
8.0-µm pore membranes (Corning, Inc.) were used. At 24 h
post-transfection with 100 nM siRNA, 4×104 cells were
seeded in the upper chamber with 200 µl serum-free medium,
and 600-800 µl culture medium containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was added to the lower chamber.
Following a 24 h incubation at 37°C, the cells on the upper surface
of the membrane were gently removed with cotton swabs, and the
cells on the lower surface were fixed with 4% paraformaldehyde for
30 min and stained with 800 µl crystal violet solution for
15-30 min at room temperature. The cells that passed through the
membranes were imaged in ≥3 fields of view with an inverted
fluorescence microscope at ×20 magnification (Leica Microsystems
GmbH).
Western blotting
At 48 h post-transfection with 100 nM siRNA in a
6-well plate, proteins were extracted from HUVECs using RIPA buffer
(Beyotime Institute of Biotechnology) supplemented with protease
inhibitors. The protein concentrations were determined with an
Ultramicro Nucleic Acid Analyzer (Hangzhou Allsheng Instruments
Co., Ltd.), and 20-40 µg protein/lane was separated using
10% SDS-polyacrylamide gels by electrophoresis, following which the
proteins were transferred to 0.2-µm
Immobilon®-PSQ PVDF membranes. Subsequently,
the membranes were blocked with 5% skim milk and incubated with
rabbit anti-cyclin D1 (1:1,000; cat. no. 2978T), anti-cyclin B1
(1:1,000; cat. no. 4138T), anti-CDK4 (1:1,000; cat. no. 12790T),
anti-CDK6 (1:1,000; cat. no. 1333T), anti-proliferating cell
nuclear antigen (PCNA; 1:1,000; cat. no. 13110T), anti-vimentin
(1:1,000; cat. no. 5741T), mouse anti-cyclin E1 (1:1,000; cat. no.
4129T) (all Cell Signaling Technology, Inc.) and anti-GAPDH
(1:5,000; cat. no. 60004-1-lg; Proteintech Group, Inc.) antibodies
overnight at 4°C. The goat anti-mouse lgG (H+L) (1:50,000; cat. no.
A00615) and goat anti-rabbit lgG (H+L) (1:50,000; cat. no. A00834)
secondary antibodies (both Multisciences Biotech Co., Ltd.) were
diluted with 5% skim milk and added to the membranes for 1 h at
room temperature. Following washing the membrane three times for 10
min each with TBS-0.1% Tween-20 washing buffer, the expression
levels of the proteins were detected with an automatic Tanon 5200
Multi chemiluminescence image analyzer using Tanon™ High-sig ECL
western blotting Substrate (both Tanon Science and Technology Co.,
Ltd.). The gray area was determined using ImageJ v1.8.0 (National
Institutes of Health).
Bioinformatics analysis
The original sequencing data of lncRNA-seq from our
previous study (22) and
miRNA-seq from the current study were uploaded to the GEO database
of NCBI (accession nos. lncRNA-seq, GSE171551; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE171551;
miRNA-seq, GSE171715; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE171715).
The upregulated miRNAs were selected with P-value <0.05 and
fold-change >1. Differential expression between two sets of
miRNA-seq samples was calculated with the 'DEseq (V 1.32.0)'
algorithm (27) according to the
criteria of |log2 (fold change)|≥1 and P-value <0.05. miRNA
target genes were predicted, TargetScan, miRDB (http://mirdb.org), miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/) and miRWalk
(http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
were used to predict the target genes of the selected miRNAs. Gene
ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analyses were performed using the target genes
and KOBAS (http://kobas.cbi.pku.edu.cn/kobas3) was used for GO
and KEGG pathway analyses. The target genes involved in KEGG
analyses are listed in Table
SI. miRanda (http://www.microrna.org/microrna/home.do) was used for
the binding target prediction of miR-25-5p and
uc003pxg.1, whereas TargetScan (http://www.targetscan.org/vert_71/) was used to
predict the target genes of miRNAs.
Statistical analyses
Statistical analysis was conducted using SPSS 21.0
(IBM Corp.), and GraphPad Prism 7 (GraphPad Software, Inc.) was
used for data analysis. Data are presented as the mean ± SD of
three independent experiments. Two-tailed Student's t-test was used
for comparison between two groups of normally distributed
variables, and one-way ANOVA with Tukey's post-hoc test was
performed for analysis among multiple groups. Discrete variables
were compared by contingency table analysis of the χ2
test. Receiver operating characteristic (ROC) curves and the area
under the ROC curve (AUC) were used to assess the feasibility of
uc003pxg.1 as a biomarker for the diagnosis of CAD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Verification of uc003pxg.1 expression in
clinical samples and statistical analysis of clinical
indicators
PBMCs from 80 patients with CAD and 80 controls were
isolated for analysis. The clinicopathological characteristics of
the patients and controls are listed in Table I. The hypertension (86.3%),
diabetes (36.3%) and smoking (37.5%) rates were significantly
higher in patients with CAD compared with those in the control
group, and the HDL levels exhibited a decreasing but not
significant trend in the CAD group compared to the control
group.
 | Table IClinical characteristics of the study
subjects. |
Table I
Clinical characteristics of the study
subjects.
| Variable | CAD (n=80) | CON (n=80) | P-value |
|---|
| Age, years | 67.4±17.6 | 62.5±18.5 | 0.786 |
| Sex, male, n
(%) | 48 (60.0%) | 45 (56.3%) | 0.374 |
| BMI,
kg/m2 | 25.39±3.72 | 25.68±3.13 | 0.916 |
| Smoking, n (%) | 30 (37.5%) | 15 (18.8%) | 0.119 |
| Drinking, n
(%) | 12 (15.0%) | 10 (12.5%) | 0.409 |
| Diabetes, n
(%) | 29 (36.3%) | 12 (15.0%) | 0.002a |
| Hypertension, n
(%) | 69 (86.3%) | 26 (32.5%) | <0.001a |
| TC, mmol/l | 4.27 | 4.33 | 0.731 |
| TG, mmol/l | 1.66 | 1.68 | 0.930 |
| HDL, mmol/l | 1.04 | 1.12 | 0.090 |
| LDL, mmol/l | 2.64 | 2.70 | 0.616 |
| VLDL, mmol/l | 0.67 | 0.52 | 0.107 |
High-content screening, selection and
cellular localization of target lncRNAs
The lncRNA uc003pxg.1 was discovered by
lncRNA-seq high-throughput sequencing performed in our previous
study (22). In the present
study, the relative expression levels of uc003pxg.1 in PBMCs
from 80 patients with CAD and 80 controls were determined by
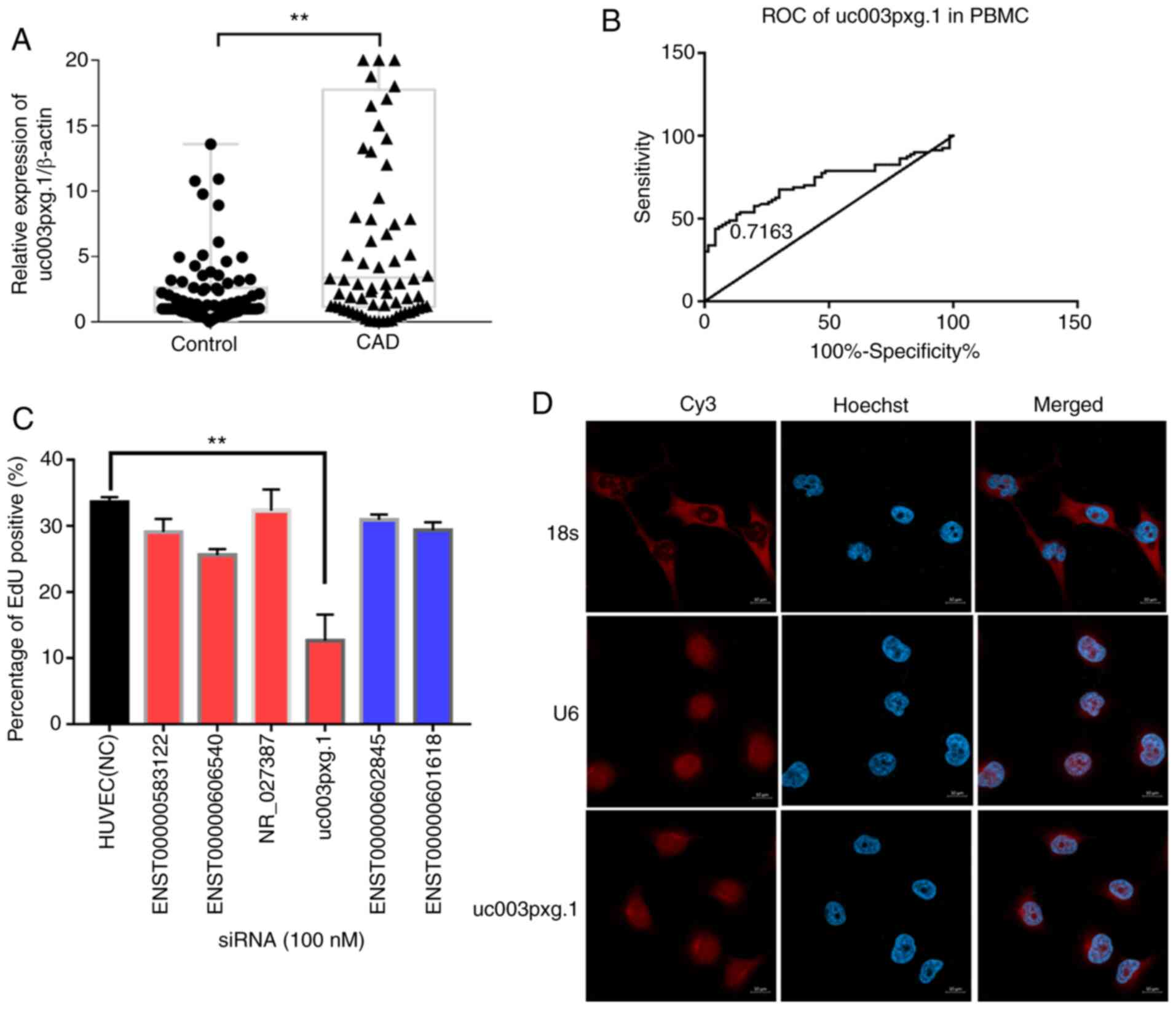
RT-qPCR. The results demonstrated that uc003pxg.1 was
significantly upregulated (~4.6-fold) in PBMCs isolated from
patients with CAD compared with the control group (P<0.01;
Fig. 1A), and the area under the
ROC curve was 0.7163 (Fig. 1B),
suggesting that uc003pxg.1 may be a potential biomarker for
the clinical diagnosis of CAD. A total of six lncRNAs were selected
for cell proliferation high-content screening to identify lncRNAs
associated with biological functions. The percentage of
EdU-positive cells in the si-uc003pxg.1-transfected group
was significantly decreased compared with that in the
control-transfected group (Figs.
1C and S1), indicating that
uc003pxg.1 may serve biological functions in cell
proliferation. The FISH results demonstrates that uc003pxg.1
was primarily localized to the nucleus (Fig. 1D), with a limited amount detected
in the cytoplasm. The competing endogenous RNA regulatory mechanism
is involved in the post-transcriptional regulation in the cytoplasm
(28); thus, cytoplasmic
uc003pxg.1 was used for the subsequent competing endogenous
RNA network analysis.
uc003pxg.1 promotes cell proliferation
and migration
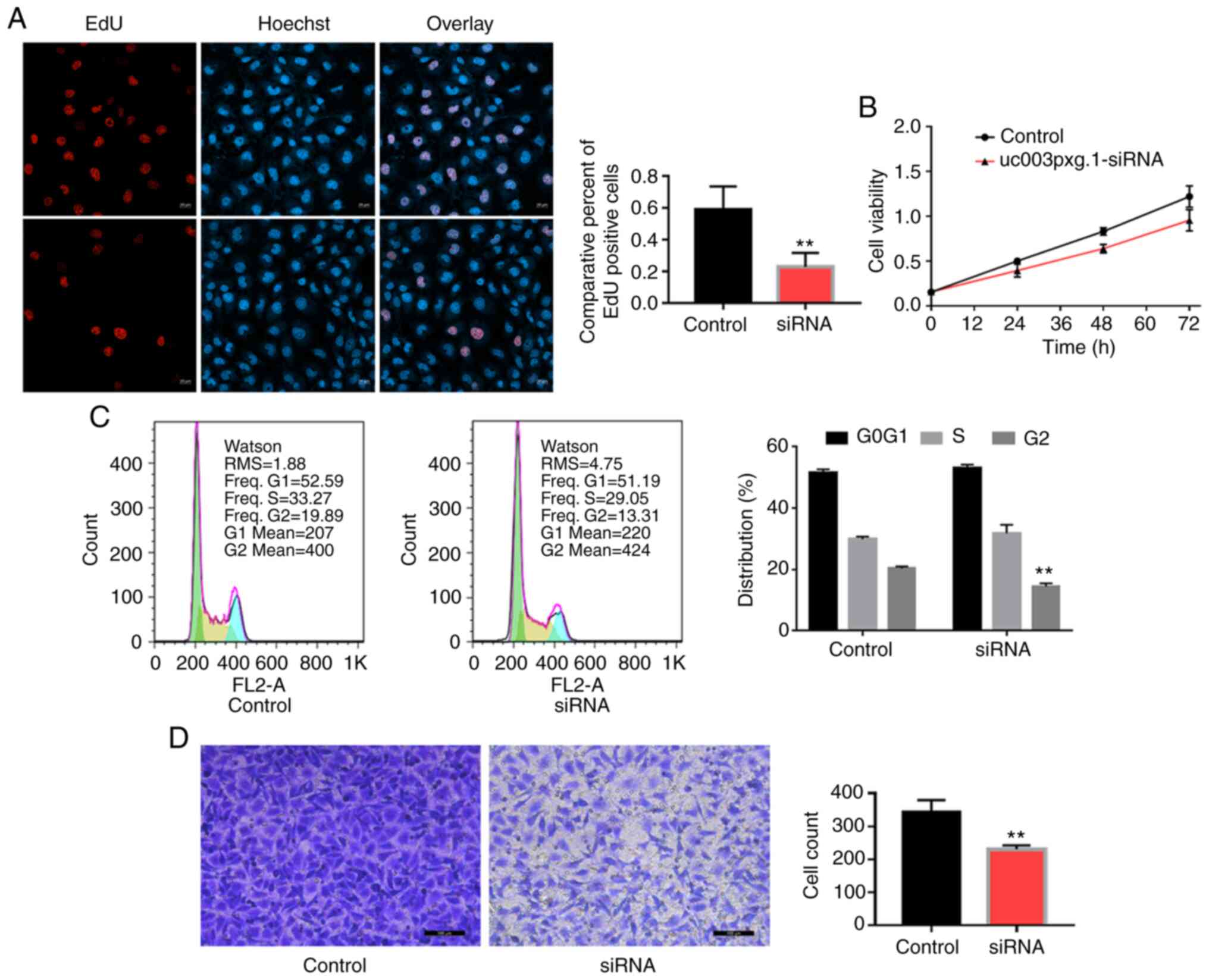
Following HUVEC transfection with
si-uc003pxg.1, cell proliferation was significantly
inhibited, as demonstrated by the results of the EdU (Fig. 2A), CCK-8 (Fig. 2B) and cell cycle (Fig. 2C) assays. Transwell assay results
also revealed that the migratory ability of the cells in the
si-uc003pxg.1 group was significantly reduced compared with
that of the control group (Fig.
2D). These results suggested that uc003pxg.1 promoted
cell proliferation and migration.
uc003pxg.1 promotes the expression of
cell cycle-associated proteins
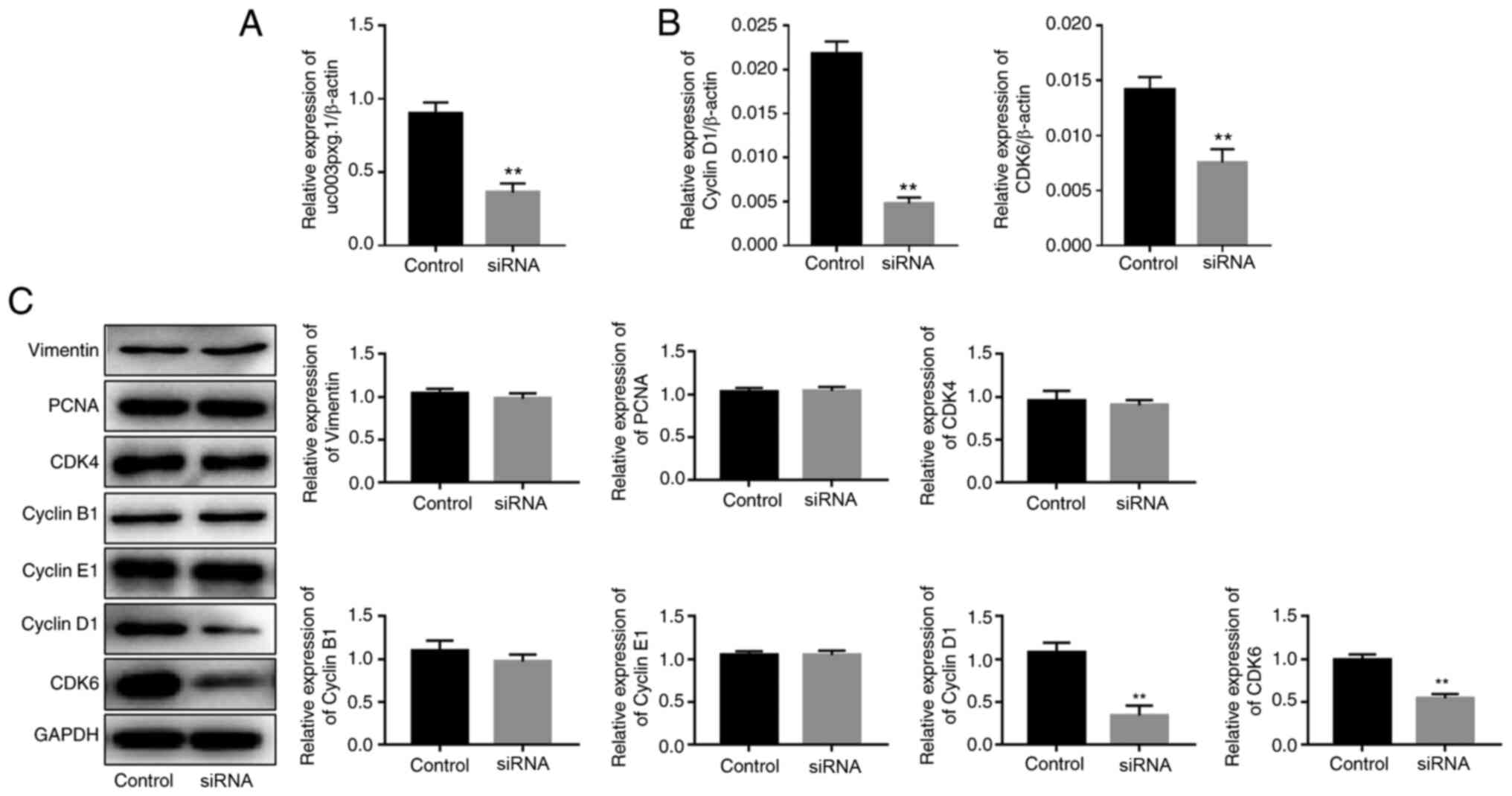
uc003pxg.1 expression in HUVECs was knocked
down by ~60% following transfection with si-uc003pxg.1
(Fig. 3A). The RT-qPCR results
demonstrated that the expression levels of cyclin D1 and
CDK6 were significantly decreased following
uc003pxg.1 knockdown compared with those in the control
group (Fig. 3B). Western
blotting was performed to assess the expression levels of proteins
associated with the cell cycle (vimentin, PCNA, CDK4, CDK6, cyclin
D1, cyclin B1 and cyclin E1), and the results demonstrated that the
levels of CDK6 and cyclin D1 protein expression were significantly
decreased following uc003pxg.1 knockdown compared with those
in the control group (Fig. 3C).
These results suggested that uc003pxg.1 promoted the
expression of cell cycle-related proteins and that
uc003pxg.1 may be associated with the cyclin D1/CDK6
signaling pathway, which is involved in the G1/S phase
of the cell cycle.
Target miRNAs of uc003pxg.1 identified by
high-throughput sequencing
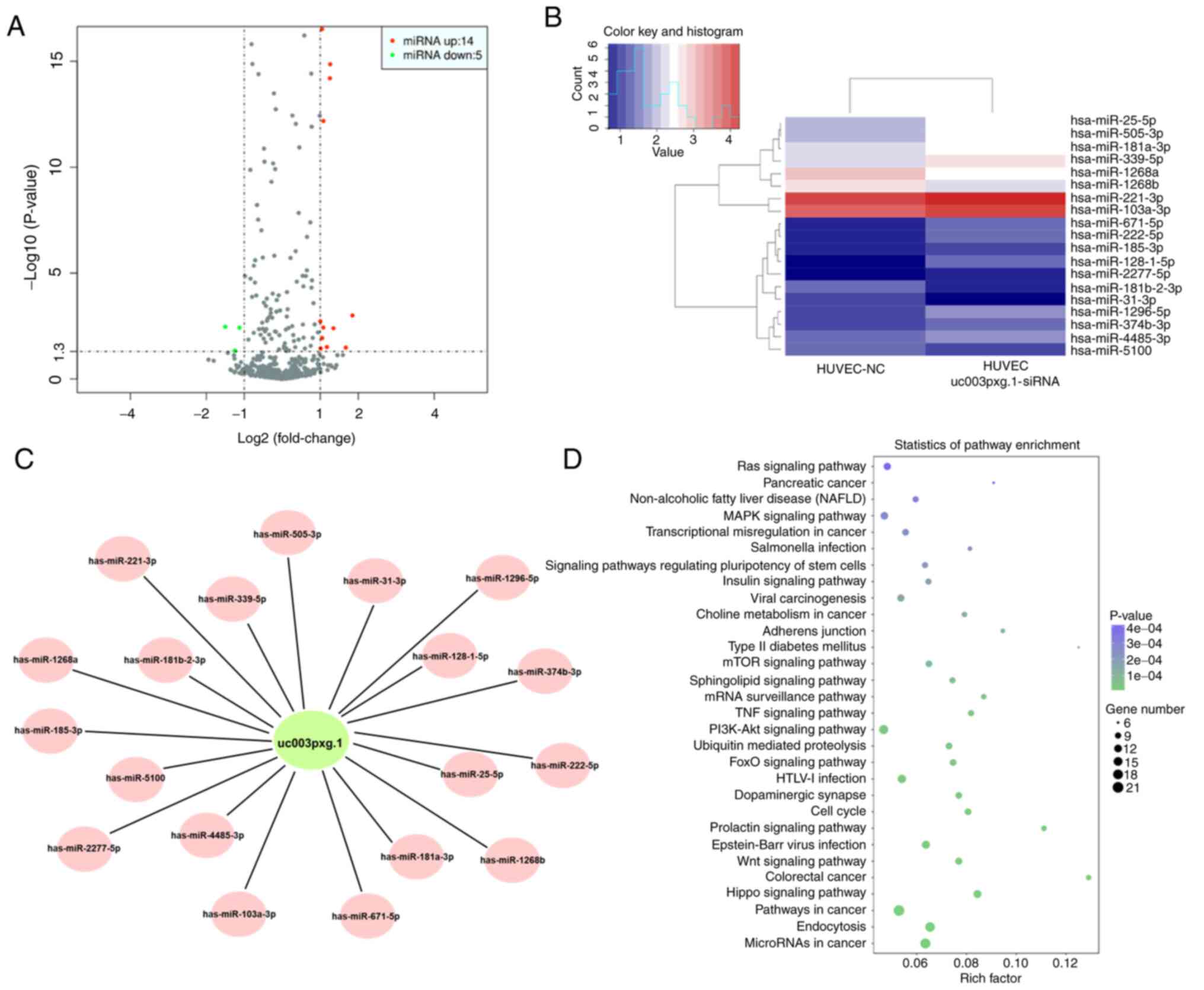
After HUVECs were transfected with siRNA, RNA was
extracted for high-throughput sequencing. Differential expression
between two sets of samples was calculated, and the most
differentially expressed miRNAs (14 upregulated and 5
downregulated) were selected for further analysis (Fig. 4A-C). The results demonstrated
that the Ras and MAPK signaling pathways were enriched with the
differentially expressed miRNAs (Fig. 4D).
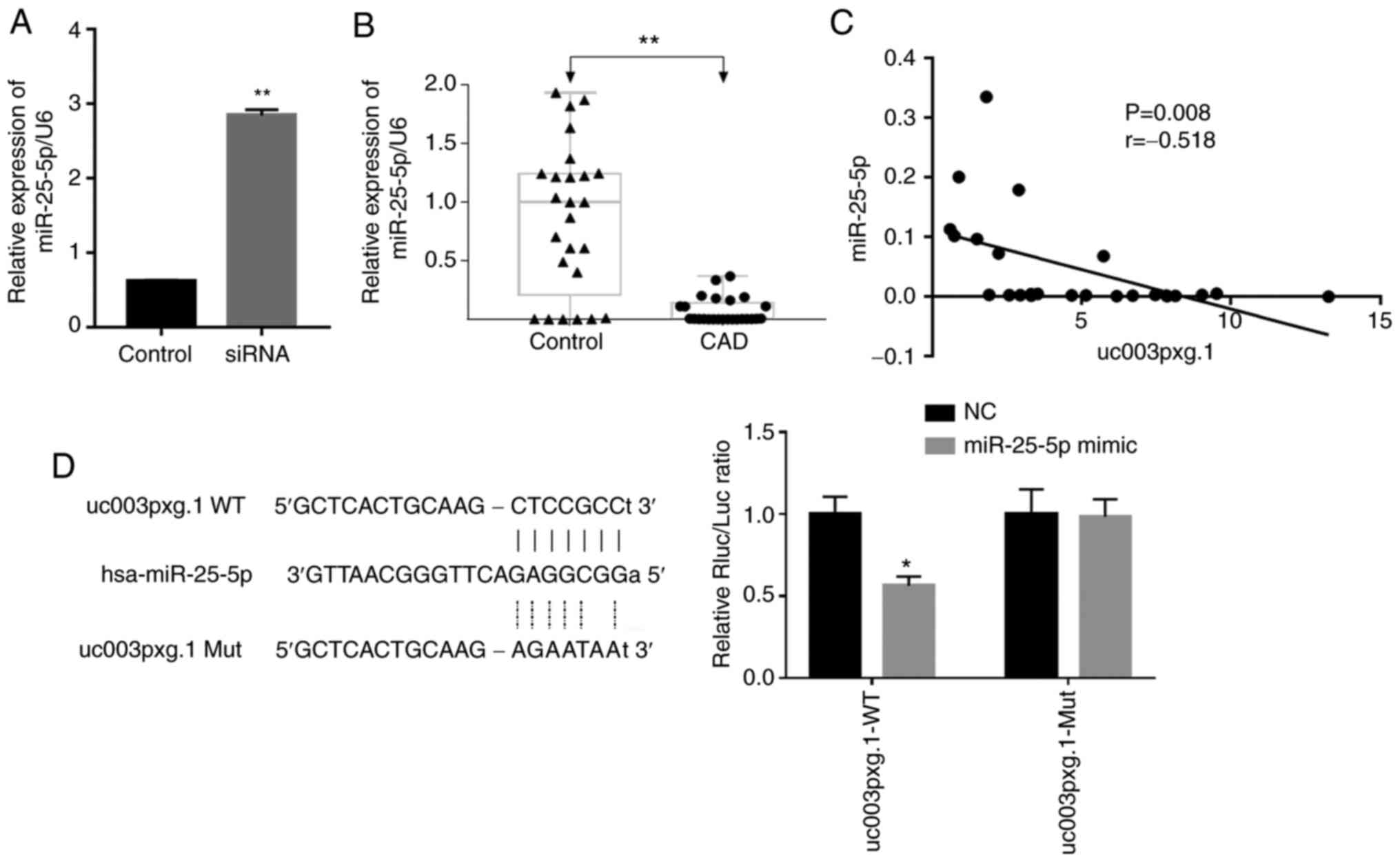
miR-25-5p levels are negatively
correlated with those of uc003pxg.1
The expression levels of miRNAs were verified in
HUVECs following transfection with si-uc003pxg.1, as well as
in PBMCs from clinical samples by RT-qPCR. miR-25-5p, which
is associated with cell proliferation and migration, was selected
for further analysis according to previous studies (29,30). The results demonstrated that the
expression levels of miR-25-5p were significantly
upregulated following uc003pxg.1 knockdown compared with
those in the control cells (Fig.
5A). The expression levels of miR-25-5p were
significantly downregulated in samples from 25 patients with CAD
compared with those in 25 control samples (Fig. 5B), and miR-25-5p
expression levels were significantly negatively correlated with
those of uc003pxg.1 in clinical PBMC samples (Fig. 5C). The luciferase activity of the
miR-25-5p mimic + uc003pxg.1-wt group was
significantly decreased compared with that of the NC group, whereas
no significant differences in the luciferase activities were
observed between the miR-25-5p mimic and NC samples in the
mut group (Fig. 5D). Taken
together, these results suggested that uc003pxg.1 interacted
with miR-25-5p.
miR-25-5p downregulates cyclin D1 and
CDK6 expression and inhibits cell proliferation
HUVECs were transfected with si-uc003pxg.1,
miR-25-5p mimics or miR-25-5p inhibitor. The relative
expression levels of miR-25-5p were significantly increased
in HUVECs following transfection with the miR-25-5p mimics
compared with those in cells transfected with the mimic NC, whereas
a miRNA inhibitor that functionally combined the endogenous miRNA
competitively and thus regulated the suppression on downstream
genes was used, which had no notable effects on the expression
levels of miR-25-5p (Fig.
6A). Subsequently, cell proliferation was analyzed by the EdU
assay. The results demonstrated that the miR-25-5p mimics
and si-uc003pxg.1 reduced cell proliferation compared with
that in the control group, whereas the miR-25-5p inhibitor
increased cell proliferation compared with that in the control
group, although the difference was not statistically significant
(Fig. 6B). In addition,
si-uc003pxg.1 and the miR-25-5p mimics inhibited the
protein expression levels of cyclin D1 and CDK6 compared with those
in the control group (Fig. 6C).
These results suggested that miR-25-5p exerted the opposite
function to uc003pxg.1 in the inhibition of cell
proliferation.
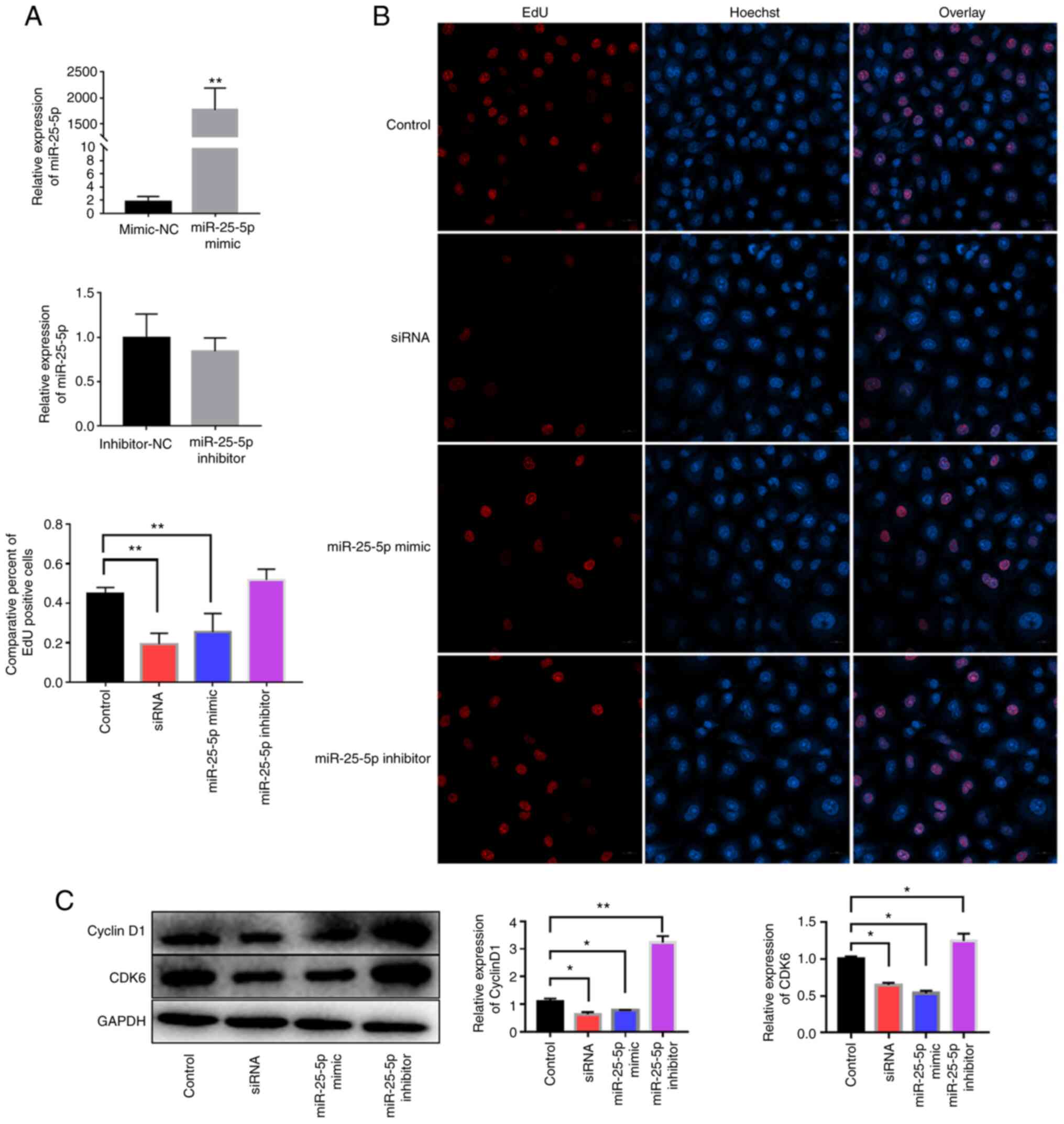 | Figure 6Effects of uc003pxg.1 and
miR-25-5p on cell proliferation and related protein
expression. (A) The relative expression of miR-25-5p in
HUVECs after transfection with the miR-25-5p mimics, mimic-NC,
miR-25-5p inhibitor and inhibitor-NC. **P<0.01 vs.
mimics-NC. (B) The association between miR-25-5p levels and
cell proliferation was analyzed by the EdU assay. Red, EdU; blue,
nuclear staining. **P<0.01. (C) Cyclin D1 and CDK6
expression was determined in HUVECs after transfection with
mimic-NC, siRNA, miR-25-5p mimic and miR-25-5p
inhibitor. *P<0.05 and **P<0.01. miR,
microRNA; HUVECs, human umbilical vein endothelial cells; NC,
negative control; EdU, 5-ethynyl-2′-deoxyuridine; control,
mimic-NC; siRNA, small interfering RNA targeting
uc003pxg.1. |
miR-25-5p inhibits HUVEC viability, cell
cycle and migration
The roles of miR-25-5p in cell proliferation
and migration were further verified by cell cycle (Fig. 7A), CCK-8 (Fig. 7B) and Transwell migration
(Fig. 7C) assays. The CCK-8 and
cell cycle assay results demonstrated that cell proliferation was
significantly reduced at 48 h with no difference at 72 h in the
miR-25-5p mimic group compared with that in the control
group, which was consistent with the results obtained with cells
transfected with si-uc003pxg.1. The cell proliferative
ability was significantly enhanced in the miR-25-5p
inhibitor group at 48 h compared with that in the control group.
Furthermore, the Transwell assay results revealed that the
miR-25-5p mimic and si-uc003pxg.1 significantly
reduced cell migration compared with that in the control group,
whereas the miR-25-5p inhibitor enhanced cell migration
compared with that in the control group.
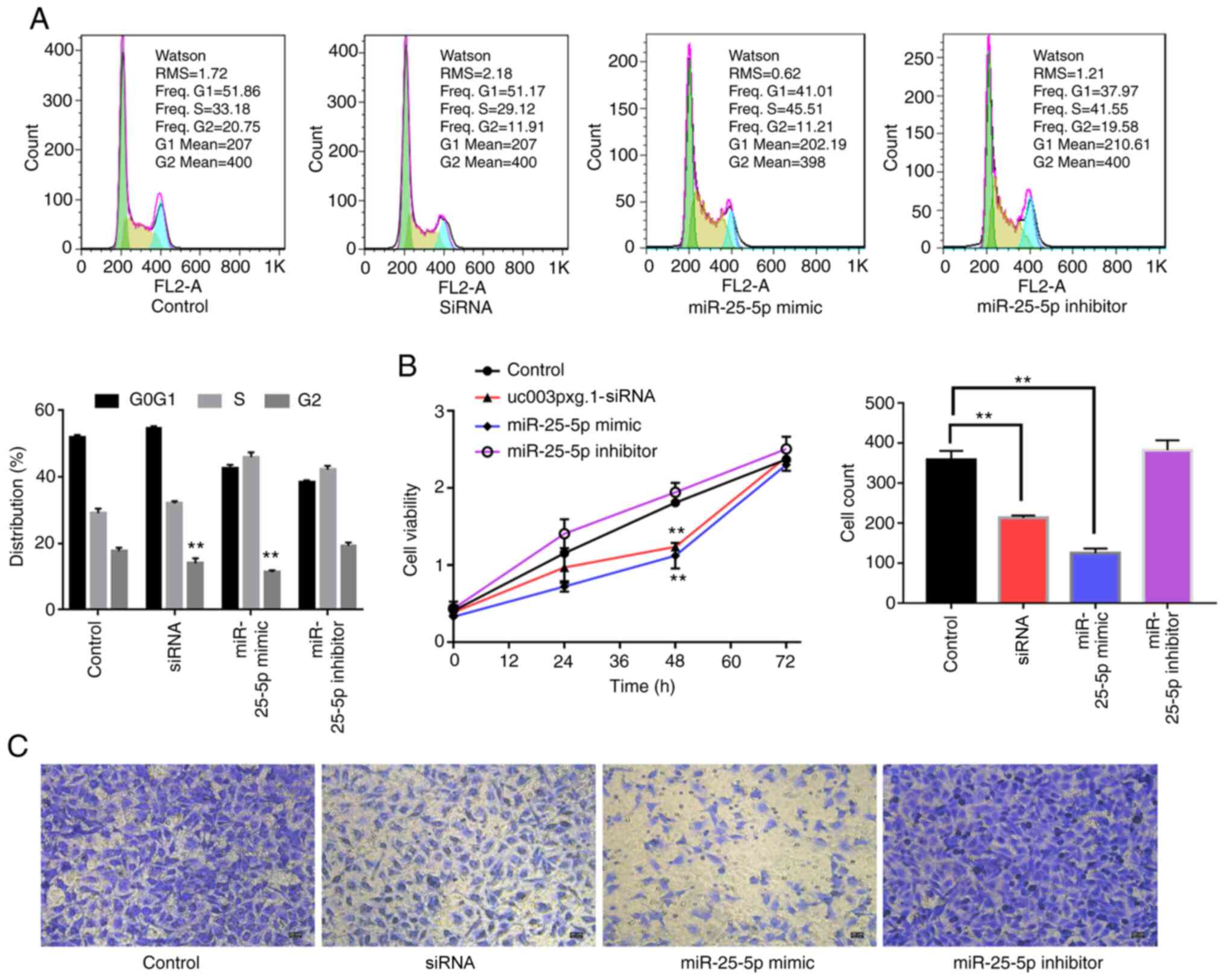 | Figure 7Association between uc003pxg.1
and miR-25-5p during cell proliferation and migration. (A)
The cell cycle progression of HUVECs transfected with mimic-NC,
siRNA, miR-25-5p mimic and miR-25-5p inhibitor was
analyzed by flow cytometry. (B) The effects of HUVEC transfection
with siRNA, miR-25-5p mimic and miR-25-5p inhibitor
on cell proliferation were analyzed by Cell Counting Kit-8 assay.
(C) The migration of HUVECs transfected with mimic-NC, siRNA,
miR-25-5p mimic and miR-25-5p was analyzed by
Transwell assay. **P<0.01. miR, microRNA; HUVECs,
human umbilical vein endothelial cells; NC, negative control;
control, mimic-NC; siRNA, small interfering RNA targeting
uc003pxg.1. |
Discussion
CAD is a chronic inflammatory disease that leads to
the formation of medium to large arterial plaques (31). As arterial plaques gradually
increase and cause narrowing of the arterial lumen, they can block
the blood flow and lead to ischemia of the heart (32). Previous studies have reported
that circulating PBMCs, 5-10% of which are peripheral blood white
blood cells, serve important roles in the development of CAD by
promoting cell migration to the arterial wall and increasing the
extent of atherosclerosis (33,34). Furthermore, lncRNAs have emerged
as regulatory factors in the development of atherosclerosis
(12,13). Thus, it is necessary to study the
differential expression of lncRNAs in patients with CAD to develop
noninvasive strategies for the early diagnosis of CAD.
In our previous study, a number of lncRNAs were
identified as differentially expressed in patients with CAD
compared with healthy controls through high-throughput sequencing
(22). Among these lncRNAs, the
functional lncRNA uc003pxg.1 was demonstrated to function in
cell proliferation and migration through high-content analysis in
the present study. Clinical samples from 80 patients with CAD and
80 control subjects were evaluated, and the results revealed that
the expression levels of uc003pxg.1 were significantly
upregulated in patients with CAD compared with those in the control
subjects, with the area under the ROC curve of 0.7163, suggesting
that uc003pxg.1 may be a potential biomarker for clinical
CAD diagnosis. In addition, the results of the EdU, CCK-8 and cell
cycle assays in HUVECs demonstrated that cell proliferation was
significantly reduced following uc003pxg.1 knockdown.
Transwell experiments revealed a reduction in the cell migratory
ability after HUVECs were transfected with si-uc003pxg.1.
Taken together, these results suggested that uc003pxg.1 may
promote cell proliferation and migration. Additionally, analysis of
the expression of cell cycle-related genes and proteins
demonstrated that cyclin D1 and CDK6 levels were significantly
downregulated after uc003pxg.1 knockdown, further suggesting
that uc003pxg.1 may be associated with the cell cycle and
promote cell proliferation.
The present study also identified differentially
expressed miRNAs in si-uc003pxg.1-transfected and control
HUVECs via sequencing. The results demonstrated that 14 miRNAs were
upregulated and 5 were downregulated following transfection. Among
them, the levels of miR-25-5p were significantly upregulated
in HUVECs following uc003Pxg1 knockdown, but significantly
downregulated in clinical CAD samples compared with those in the
control samples, demonstrating a negative correlation between
miR-25-5p and uc003pxg.1. miR-25-5p has been
reported to be involved in cell proliferation and migration in
various diseases. For example, miR-22 inhibits the
proliferation and cell cycle progression of non-small cell lung
cancer cells by targeting cell division cycle 42 expression
(35) and also enhances the
proliferation, invasion and migration of liver cancer cells by
inhibiting Rho GDP dissociation inhibitor α expression (36). Furthermore, mir-25-5p
regulates the proliferation, migration and apoptosis of oxidized
low-density lipoprotein-treated human brain microvessel endothelial
cells by targeting neuronal growth regulator 1 (30), whereas treatment with melatonin
and pterostilbene has been demonstrated to promote the apoptosis of
colon cancer cells through miR-25-5p (37). The combination of PBMC
miR-19b-5p, miR-221, miR-25-5p and
hypertension is associated with an increased heart failure risk in
patients with coronary heart disease (38). In another study, the expression
levels of miR-25-3p and miR-25-5p in the myocardium
of rats with heart failure have been reported to be upregulated
(39). In the present study, the
effects of miR-25-5p on cell proliferation and migration
were also evaluated. The EdU, cell cycle and CCK-8 assay results
demonstrated that the miR-25-5p mimics exerted similar
effects to those of si-uc003pxg.1 and significantly reduced
the proliferation of HUVECs, whereas the miR-25-5p inhibitor
promoted cell proliferation. Transwell assay results also revealed
that the miR-25-5p mimic, similar to si-uc003pxg.1,
reduced the migration of HUVECs, whereas the miR-25-5p
inhibitor promoted the migratory ability of HUVECs.
Due to their cell and tissue specificity, lncRNAs
can be used as cancer biomarkers and therapeutic targets (10). lncRNAs are secreted or released
from necrotic and apoptotic cells in the blood and urine (40), and have been demonstrated to
serve important roles in inflammation, vascular smooth muscle cell
proliferation, apoptosis and fat metabolism (13,41). lncRNAs are also associated with
the occurrence and development of CAD (42). The results of the present study
revealed that the levels of lncRNA uc003pxg.1 were
significantly upregulated in patients with CAD compared with those
in the control subjects, and that uc003pxg.1 promoted cell
proliferation, migration and cell cycle-related gene and protein
(cyclin D1 and CDK6) expression. In addition, an interaction
between miR-25-5p and uc003pxg.1 was identified
through high-throughput sequencing and dual-luciferase reporter
gene assays. For the first time, the present study demonstrated
that uc003pxg.1-targeted miR-25-5p regulates cell
proliferation and migration. The high expression levels of
uc003pxg.1 in circulating peripheral blood cells of patients
with CAD may increase the proliferation and migration of vascular
endothelial cells and promote the development of CAD by sponging
miR-25-5p. These results may provide a new lncRNA target in
the study of CAD and a potential biomarker for the early diagnosis
of CAD. However, to confirm these findings, a larger number of
clinical samples, additional in vivo experiments, including
those to assess the role of uc003pxg.1 in inflammation and
other pathways, need to be performed, which will be carried out in
our future study.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
KS and GX designed the study and obtained funding.
PL performed the experiments and drafted the manuscript. YL, LC and
XM collected and interpretated the data. XY, MY, BQ, FW, JX and JY
conducted literature search and data interpretation. All authors
reviewed the results. All authors read and approved the final
manuscript. XY and JY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Nanjing Medical University (KL901117). Informed consent was
obtained from the patients and their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Huang L, Zheng Y, Yuan X, Ma Y, Xie G,
Wang W, Chen H and Shen L: Decreased frequencies and impaired
functions of the CD31+ subpopulation in Treg
cells associated with decreased FoxP3 expression and enhanced
Treg cell defects in patients with coronary heart
disease. Clin Exp Immunol. 187:441–454. 2017. View Article : Google Scholar
|
|
2
|
Jokinen E: Coronary artery disease in
patients with congenital heart defects. J Intern Med. 288:383–389.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malakar AK, Choudhury D, Halder B, Paul P,
Uddin A and Chakraborty S: A review on coronary artery disease, its
risk factors, and therapeutics. J Cell Physiol. 234:16812–16823.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valgimigli M, Bueno H, Byrne RA, Collet
JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et
al: 2017 ESC focused update on dual antiplatelet therapy in
coronary artery disease developed in collaboration with EACTS: The
Task Force for dual antiplatelet therapy in coronary artery disease
of the European Society of Cardiology (ESC) and of the European
association for cardio-thoracic surgery (EACTS). Eur Heart J.
39:213–260. 2018. View Article : Google Scholar
|
|
5
|
Cai Y, Yang Y, Chen X, Wu G, Zhang X, Liu
Y, Yu J, Wang X, Fu J, Li C, et al: Circulating 'lncRNA
OTTHUMT00000387022' from monocytes as a novel biomarker for
coronary artery disease. Cardiovasc Res. 112:714–724. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y: The novel regulatory role of
lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med.
22:5768–5775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
8
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sanfilippo PG and Hewitt AW: Translating
the ENCyclopedia Of DNA elements project findings to the clinic:
ENCODE's implications for eye disease. Clin Exp Ophthalmol.
42:78–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaikkonen MU, Lam MT and Glass CK:
Non-coding RNAs as regulators of gene expression and epigenetics.
Cardiovasc Res. 90:430–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greco S, Gorospe M and Martelli F:
Noncoding RNA in age-related cardiovascular diseases. J Mol Cell
Cardiol. 83:142–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Wang L, Li H, Han X, Chen S, Yang B,
Hu Z, Zhu H, Cai C, Chen J, et al: Characterization of LncRNA
expression profile and identification of novel LncRNA biomarkers to
diagnose coronary artery disease. Atherosclerosis. 275:359–367.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Ji N, Gong X, Ni S and Wang Y:
NEAT1/miR-140-3p/MAPK1 mediates the viability and survival of
coronary endothelial cells and affects coronary atherosclerotic
heart disease. Acta Biochim Biophys Sin (Shanghai). 52:967–974.
2020. View Article : Google Scholar
|
|
15
|
Zhang Z, Gao W, Long QQ, Zhang J, Li YF,
Liu DC, Yan JJ, Yang ZJ and Wang LS: Increased plasma levels of
lncRNA H19 and LIPCAR are associated with increased risk of
coronary artery disease in a Chinese population. Sci Rep.
7:74912017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Y, Jin X, Xiang Y, Chen Y, Shen CX,
Zhang YC and Li YG: The lncRNA MALAT1 protects the endothelium
against ox-LDL-induced dysfunction via upregulating the expression
of the miR-22-3p target genes CXCR2 and AKT. FEBS Lett.
589:3189–3196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michalik KM, You X, Manavski Y,
Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W,
Uchida S, et al: Long noncoding RNA MALAT1 regulates endothelial
cell function and vessel growth. Circ Res. 114:1389–1397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motterle A, Pu X, Wood H, Xiao Q, Gor S,
Ng FL, Chan K, Cross F, Shohreh B, Poston RN, et al: Functional
analyses of coronary artery disease associated variation on
chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet.
21:4021–4029. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivanov D, Philippova M, Allenspach R, Erne
P and Resink T: T-cadherin upregulation correlates with cell-cycle
progression and promotes proliferation of vascular cells.
Cardiovasc Res. 64:132–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang G, Li Y, Peng Y, Tang J and Li H:
Association of polymorphisms in MALAT1 with risk of coronary
atherosclerotic heart disease in a Chinese population. Lipids
Health Dis. 17:752018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Z, He Y, Li D, Fang X, Shang T, Zhang H
and Zheng X: Long noncoding RNA MEG3 suppressed endothelial cell
proliferation and migration through regulating miR-21. Am J Transl
Res. 9:3326–3335. 2017.PubMed/NCBI
|
|
22
|
Li P, Yan X, Xu G, Pang Z, Weng J, Yin J,
Li M, Yu L, Chen Q and Sun K: A novel plasma lncRNA ENST00000416361
is upregulated in coronary artery disease and is related to
inflammation and lipid metabolism. Mol Med Rep. 21:2375–2384.
2020.PubMed/NCBI
|
|
23
|
Wenger NK: 2011 ACCF/AHA focused update of
the guidelines for the management of patients with unstable
angina/Non-ST-elevation myocardial infarction (updating the 2007
guideline): Highlights for the clinician. Clin Cardiol. 35:3–8.
2012. View Article : Google Scholar
|
|
24
|
Jia Y, Xu H, Li Y, Wei C, Guo R, Wang F,
Wu Y, Liu J, Jia J, Yan J, et al: A modified ficoll-paque gradient
method for isolating mononuclear cells from the peripheral and
umbilical cord blood of humans for biobanks and clinical
laboratories. Biopreserv Biobank. 16:82–91. 2018. View Article : Google Scholar
|
|
25
|
Trapnell C, Hendrickson DG, Sauvageau M,
Goff L, Rinn JL and Pachter L: Differential analysis of gene
regulation at transcript resolution with RNA-seq. Nat Biotechnol.
31:46–53. 2013. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Wang L, Feng Z, Wang X, Wang X and Zhang
X: DEGseq: An R package for identifying differentially expressed
genes from RNA-seq data. Bioinformatics. 26:136–138. 2010.
View Article : Google Scholar
|
|
28
|
Liu H, Lei C, He Q, Pan Z, Xiao D and Tao
Y: Nuclear functions of mammalian MicroRNAs in gene regulation,
immunity and cancer. Mol Cancer. 17:642018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Tao B, Li J, Mao X, He W and Chen
Q: Melatonin inhibits the progression of oral squamous cell
carcinoma via inducing miR-25-5p expression by directly targeting
NEDD9. Front Oncol. 10:5435912020. View Article : Google Scholar :
|
|
30
|
Zhang Q, Liu C, Li Q, Li J, Wu Y and Liu
J: MicroRNA-25-5p counteracts oxidized LDL-induced pathological
changes by targeting neuronal growth regulator 1 (NEGR1) in human
brain micro-vessel endothelial cells. Biochimie. 165:141–149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niccoli G, Montone RA, Sabato V and Crea
F: Role of allergic inflammatory cells in coronary artery disease.
Circulation. 138:1736–1748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knorr M, Münzel T and Wenzel P: Interplay
of NK cells and monocytes in vascular inflammation and myocardial
infarction. Front Physiol. 5:2952014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jaipersad AS, Shantsila A, Lip GY and
Shantsila E: Expression of monocyte subsets and angiogenic markers
in relation to carotid plaque neovascularization in patients with
pre-existing coronary artery disease and carotid stenosis. Ann Med.
46:530–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang T, Chen T, Li Y, Gao L, Zhang S, Wang
T and Chen M: Downregulation of miR-25 modulates non-small cell
lung cancer cells by targeting CDC42. Tumour Biol. 36:1903–1911.
2015. View Article : Google Scholar
|
|
36
|
Wang C, Wang X, Su Z, Fei H, Liu X and Pan
Q: MiR-25 promotes hepatocellular carcinoma cell growth, migration
and invasion by inhibiting RhoGDI1. Oncotarget. 6:36231–36244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung JH, Shin EA, Kim JH, Sim DY, Lee H,
Park JE, Lee HJ and Kim SH: NEDD9 inhibition by miR-25-5p
activation is critically involved in co-treatment of melatonin- and
pterostilbene-induced apoptosis in colorectal cancer cells. Cancers
(Basel). 11:16842019. View Article : Google Scholar
|
|
38
|
Yao Y, Song T, Xiong G, Wu Z, Li Q, Xia H
and Jiang X: Combination of peripheral blood mononuclear cell
miR-19b-5p miR-221, miR-25-5p and hypertension correlates with an
increased heart failure risk in coronary heart disease patients.
Anatol J Cardiol. 20:100–109. 2018.PubMed/NCBI
|
|
39
|
Wu JB, Ye XH, Xian SX and Dong MG:
Expressions of SERCA2a and miR-25-3p/5p in myocardium of rats with
heart failure and therapeutic effects of Xiefei Lishui recipe.
Zhongguo Ying Yong Sheng Li Xue Za Zhi. 33:146–150. 2017.In
Chinese. PubMed/NCBI
|
|
40
|
Meseure D, Drak Alsibai K, Nicolas A,
Bieche I and Morillon A: Long noncoding RNAs as new architects in
cancer epigenetics, prognostic biomarkers, and potential
therapeutic targets. Biomed Res Int. 2015:3202142015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rizzacasa B, Amati F, Romeo F, Novelli G
and Mehta JL: Epigenetic modification in coronary atherosclerosis:
JACC review topic of the week. J Am Coll Cardiol. 74:1352–1365.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang YH, Pan X, Zeng T, Chen L, Huang T
and Cai YD: Identifying the RNA signatures of coronary artery
disease from combined lncRNA and mRNA expression profiles.
Genomics. 112:4945–4958. 2020. View Article : Google Scholar : PubMed/NCBI
|