Introduction
Diabetic nephropathy (DN) is a characteristic
microvascular complication of diabetes mellitus (DM). In total,
~30% of patients with type 1 DM and 40% of those with type 2 DM
(T2DM) further develop DN (1).
DN is the leading cause of end-stage renal disease and a major
cause of mortality in patients with DM (2). Proteinuria is a typical clinical
feature of DN that is associated with a substantial risk of
progressive kidney damage (2).
Podocytes, which are highly differentiated glomerular epithelial
cells, are critical for maintaining glomerular filtration function.
Podocyte injury is closely associated with glomerular sclerosis,
increased proteinuria and loss of renal function, all of which are
crucial to the progression of DN (3). Although various medications have
been used to control blood glucose and blood pressure, reduce
proteinuria and improve renal function, the increased urinary
albumin excretion and decreased evaluation of glomerular filtration
rate still increase the risk of DN (4). Thus, the underlying mechanisms of
action and treatment strategies for DN need to be further
investigated.
Multiple studies have confirmed that inflammation,
induced by the NLR family pyrin domain containing 3 (NLRP3)
inflammasome, is crucial in the improvement of DN (5,6).
Inflammasomes are macromolecular complexes consisting of a sensor
molecule, the adaptor apoptosis-associated speck-like protein
containing a caspase recruitment domain (ASC) and the effector
protease pro-caspase-1, which trigger central and rapid
inflammatory responses to cytosolic insults (7). The NLRP3 inflammasome is currently
the most characterized inflammasome. Activated NLRP3 can recruit
ASC to promote the conversion of pro-caspase-1 to active caspase-1,
which is crucial for the maturation of interleukin (IL)-1β and
IL-18 (8). The NLRP3
inflammasome activation primarily in renal resident cells including
podocytes is crucial to the establishment of DN (6). Considerable NLRP3 inflammasome
activation has been demonstrated in patients with T2DM, and its
inhibition or NLRP3 deficiency can protect against podocyte injury
and delay the progression of DN (9). IL-1β is a pleiotropic
pro-inflammatory cytokine that can induce other inflammatory
mediators, such as IL-6, tumor necrosis factor (TNF)-α and monocyte
chemoattractant protein-1 (MCP-1), which are also closely
associated with the progression of DN (10,11). Hence, targeting the NLRP3,
caspase-1 and IL-1β pathways could be an effective treatment
strategy for the management of DN.
Astragaloside IV (AS-IV) is a key active component
of Astragalus membranaceus (Fisch.) Bge, which is an
herbaceous perennial widely used in Traditional Chinese medicine.
AS-IV has been used to treat several complications related to
diabetes, including DN, cardiovascular diseases and diabetic
retinopathy (12,13). The protective effects of AS-IV
have been linked to its diverse bioactive properties, including
anti-inflammatory and antioxidant effects (14). A recent study has suggested that
AS-IV can inhibit NLRP3 inflammasome activation, thus protecting
against high glucose-induced endothelial cell injury (15). Thus, AS-IV ameliorates DN likely
by inhibiting NLRP3 inflammasome-mediated inflammation.
The aim of the present study was to evaluate the
protective effects of AS-IV on the progression of DN in
db/db mice, a T2DM mouse model with similar kidney lesions
to those observed in patients with DN (16), and analyze the expression levels
of NLRP3, caspase-1 and IL-1β in vivo and in vitro to
determine whether AS-IV ameliorates DN by inhibiting NLRP3
inflammasome-mediated inflammation.
Materials and methods
Chemicals and reagents
AS-IV (high-performance liquid chromatography ≥98
0%; cat. no. 180408) was purchased from Chengdu King-tiger
Pharm-chem. Tech. Co., Ltd. Benazepril (cat. no. X2689) was
purchased from Novartis International AG. The mouse albumin ELISA
kit (cat. no. CSB-E1387m) was purchased from Cusabio Technology
LLC. The Coomassie Brilliant Blue kit was purchased from Nanjing
Jiancheng Bioengineering Institute. The BCA protein assay kit (cat.
no. P1511) was purchased from Applygen Technologies, Inc. The
rabbit anti-NLRP3 (product code ab214185) antibody was purchased
from Abcam, the caspase-1 (cat. no. 22915-1-AP) antibody from
ProteinTech Group, Inc. and the anti-podocin antibody (cat. no.
BS90960) from Bioworld Technology, Inc. β-Actin (product no. 4970)
and anti-rabbit IgG HRP-linked antibodies (product no. 7074S) were
purchased from Cell Signaling Technology, Inc. Mouse
anti-synaptopodin (cat. no. sc-515842) was purchased from Santa
Cruz Biotechnology, Inc. Fluorescent mounting medium with DAPI
(cat. no. ZLI-9557), Alexa-488-labeled goat anti-rabbit antibody
(cat. no. ZF-0511), and Alexa-594-labeled goat anti-mouse antibody
(cat. no. ZF-0513) were purchased from ZSGB-BIO; OriGene
Technologies. TRIzol® (cat. no. 15596-026) and RevertAid
First Strand cDNA Synthesis kit (cat. no. K1622), and
Alexa-488-labeled donkey anti-mouse antibody (cat. no. A32766) were
purchased from Invitrogen; Thermo Fisher Scientific, Inc. Power
SYBR™ Green PCR Master mix (cat. no. A25742) was purchased from
ABI, Inc.; Thermo Fisher Scientific, Inc. Super ECL detection
reagent (cat. no. 36208ES60) was obtained from Yeasen Biotechnology
(Shanghai) Co., Ltd. and recombinant murine γ-interferon (cat. no.
96-315-05) from PeproTech, Inc.
Animals and treatment
Male 6-week-old BKS-Leprem2Cd479/Nju (db/db;
n=30; weight, 35-40 g) and C57BL/Ksj (wild-type, WT; n=10; weight,
18-20 g) mice were purchased from the Model Animal Research Center
of Nanjing University [Nanjing, China; approval no. SCXK (SU)
2015-0001]. The animals were maintained at the Animal Center of
Guang'anmen Hospital (living conditions, 24±2°C; 60-80% relative
humidity; 12-h light/dark cycle). All mice were given free access
to standard mice chow and drinking water. After 2 weeks of
acclimatization, the db/db mice were randomly divided into
three groups (n=10). Each group was then administered either
vehicle (distilled water, 10 ml/kg), benazepril (10 mg/kg) or AS-IV
(40 mg/kg) (17) once a day via
gavage. A total of 10 WT mice were administered vehicle alone. The
experiment lasted for 12 weeks.
During the experiment, body weight was measured once
a week. Every three weeks, all mice were fasted for 6 h to assess
fasting blood glucose (FBG). Next, 24-h urine samples were
collected using individual metabolic cages on the last 3
experimental days. All mice were anesthetized with isoflurane
inhalation and euthanized by exsanguination after 12 weeks of
administration. Anesthesia was induced by inhalation of isoflurane
overdose (4%) and maintained with isoflurane (2%) inhalation in an
isoflurane vaporizer (18).
Pinch reflex examination was monitored to ensure full anesthesia.
Blood samples (1-2 ml) for serum assessment were collected by
cardiac puncture after confirming anesthesia. Respiratory and
cardiac arrest and the absence of reflexes were used to ensure
death. The kidney was removed quickly after confirming death. The
kidney index was determined as follows: Kidney/body weight (g/kg).
The renal cortex was carefully separated. Renal cortex tissues (1
mm3) were fixed in 4% glutaraldehyde for 4 h at 4°C for
transmission electron microscopy (TEM) assays. Some tissues were
fixed in 4% paraformaldehyde for 24 h at 4°C for histological,
immunohistochemical, and immunofluorometric assays. The other
tissues were frozen in liquid nitrogen and stored at −80°C for
further analyses. The procedures for animal experimentation were in
accordance with the guidelines of the Guang'anmen Hospital, Chinese
Academy of Traditional Chinese Medicine (approval no.
IACUC-GAMH-2019-010), and adhered to the Guide for the Care and Use
of Laboratory Animals published by the United States National
Institutes of Health (NIH Publication no. 85-23, revised 1996).
Construction of lentiviral vector and
lentivirus packaging
The lentiviral vector overexpressing the NLRP3 gene
(NLRP3-ov) and its empty vector (control-ov) were constructed and
provided by Shanghai Genechem, Inc. (cat. no. GXDL0222696). In
brief, chemical synthesis was used to produce DNA full-length of
NLRP3 transcript (NM_001359638.1) and further cloned it into
pLV[Exp]-copGFP:T2A:Puro-CMV>Shuttle lentiviral empty vector
with 5′-flank NheI and 3′-flank NotI single
restriction endonuclease enzymes, and finally generated
overexpressing plasmid pLV[Exp]-copGFP:T2A:Puro-CM
V>mNlrp3[NM_001359638.1]/FLAG. All of the positive clones were
confirmed by DNA sequencing. Additionally, 293T packaging cells
(cat. no. CRL3216; ATCC) from liquid nitrogen were quickly thawed,
seeded into a 10-cm diameter dish and passaged two generations
before transfection. At the transfection step, the NLRP3 plasmid
was mixed with two packaging plasmids psPAX2 (cat. no. 12260) and
pMD2.G (cat. no. 12259; both from Addgene, Inc.) at a ratio of
4:3:3 (4ug:3ug:3ug), and co-transfected into 293T cells at 70%
confluence using jetPRIME® transfection reagent
(reference no. 114-75; Polyplus-transfection SA) according to the
manufacturer's instructions. Each viral supernatant was harvested
~48 h later and centrifuged at 5,000 × g for 1.5 h at 4°C in a
Beckman ultracentrifuge. The negative control lentivirus was
prepared using the same method, respectively.
Cell culture and transfection
The conditional immortal mouse podocytes were kindly
donated by Professor Weijing Liu from Dongzhimen Hospital, Beijing
University of Chinese Medicine and were cultured as previously
described (19). Briefly, after
podocytes were cultured at 33°C in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) containing 100 U/ml recombinant murine
γ-interferon, they were cultured in medium without recombinant
murine γ-interferon to promote differentiation at 37°C for 7 days.
The immunofluorescence of synaptopodin, a marker protein of process
formation differentiation of podocytes, was used to authenticate
the differentiation and maturation of podocytes. Next, podocytes
were divided into five groups: The control (podocytes treated with
5.5 mM glucose), high glucose (HG, podocytes treated with 50 mM
glucose), 10-µM AS-IV, 20-µM AS-IV and 40-µM
AS-IV groups. When podocytes reached 60% confluence, groups were
incubated with HG for 24 h (20)
except for the control group. Drug treatment groups were
pre-treated with different doses of AS-IV (10, 20 and 40 µM)
at 37°C for 24 h prior to HG administration. Finally, podocytes in
each group were collected for the following examination. Next, to
determine the effect of AS-IV on NLRP3 in HG-induced podocyte
injury, podocytes were infected with NLRP3-ov vector at a
multiplicity of infection (MOI) of 20 using
Lipofectamine® 3000 for 4 h. The medium was replaced
with fresh culture medium after transfection, and podocytes were
harvested for subsequent studies at 24 h after transfection.
Subsequently, podocytes were treated with 50 µM D-glucose
for 24 h in the absence or presence of 40 µM AS-IV. The
control-ov vector was also used as aforementioned.
MTT assay
Podocytes were placed in a 96-well plate at a
density of 1×105 cells/well. Podocytes were treated with
vehicle and indicated concentrations of AS-IV (10, 20 and 40
µM) in medium at 37°C for 24 h. Following incubation, the
cell cultures were mixed with MTT solution at 37°C for 4 h. Then
MTT solution was removed and dimethyl sulfoxide was added to
dissolve the formazan crystals and absorption was measured at 570
nm on a spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.).
Biochemical characteristics in the urine
and serum
The urinary albumin concentration was determined
using the mouse albumin ELISA kit, according to the manufacturer's
instructions. Urinary total protein was measured using Coomassie
Brilliant Blue staining reagent. Serum total cholesterol (TC),
triacylglycerol (TG), high-density lipoprotein cholesterol (HDL-C),
low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen
(BUN) and urinary creatinine levels were assessed using an
automatic biochemical analyzer (cat. no. AU5821; Beckman Coulter,
Inc.). Albumin-to-creatinine ratio (ACR) was used to evaluate
urinary albumin excretion (21).
Creatinine clearance rate (Ccr) was used to determine the
glomerular filtration rate (17). Ccr (ml/min) was calculated as
follows: (Urinary creatinine/serum creatinine) x 24-h urine volume
(ml)/1,440.
Histological analysis
Paraffin-embedded tissues were sectioned into
4-µm-thick slices and placed onto slides. The slides were
stained with hematoxylin and eosin, periodic acid-Schiff and
Masson's trichrome staining to evaluate glomerular alteration.
Histological tissue damage to the kidney was observed using a Nikon
ECLIPSE Ti-S inverted light microscope (Nikon Corporation). In each
section, 10 renal glomerular areas were measured using ImageJ
software (version 1.52; National Institutes of Health) to calculate
the mean glomerular cross-sectional area. The glomerular volume was
calculated using the Weibel-Gomez technique with the following
formula: Glomerular volume=Area1.5 × 0.75+0.21 (22). The fibrotic area in the glomeruli
was calculated using Masson's trichrome staining.
TEM
Renal cortex tissues were fixed with 4%
glutaraldehyde solution for 1 h at 4°C followed by 1% osmic acid
for 2 h at 4°C. The samples were then dehydrated in gradient
acetone, embedded in an Epon/Araldite mixture for 2 h at 25°C,
sectioned into 50-nm-thick slices and stained with uranyl acetate
and lead citrate for 30 min at 25°C. A TEM (cat. no. H-7650;
Olympus Corporation) was used to perform TEM analysis. The
ultrastructural changes, including glomerular basement membrane
(GBM) thickness and foot processes width, were determined as
previously described (23).
Luminex assays
The serum levels of IL-1β, IL-6, TNF-α and MCP-1
were detected using Mouse High Sensitivity T Cell Magnetic Bead
Panel with a Luminex 200 system (Luminex Corporation) as previously
described (24) and following
the manufacturer's instructions.
Immunofluorometric analysis
Double immunofluorescence was performed to localize
NLRP3 and caspase-1 within podocytes in the kidney tissues.
Following xylene dewaxing and gradient ethanol hydration,
4-µm paraffin sections were subjected to antigen repair by
heat mediation using citrate buffer (pH 6.0) at 95°C for 20 min and
permeabilized with 0.3% Triton X-100 in Tris-buffered saline at
25°C for 10 min. Following washing thrice with PBS (5 min per
wash), the slides were blocked with 10% goat serum for 2 h at 25°C
and incubated overnight at 4°C with either mouse anti-synaptopodin
(1:50) and rabbit anti-NLRP3 (1:50) antibodies, or mouse
anti-synaptopodin (1:50) and rabbit anti-caspase-1 (1:100)
antibodies. In addition, the slides were incubated overnight at 4°C
with rabbit anti-podocin antibody (1:200) alone to assess podocyte
injury. Following washing thrice with TBS (5 min per wash), these
slides were incubated with Alexa-488-labeled and (or)
Alexa-594-labeled secondary antibodies (1:50) for 1 h at 25°C in a
humidified chamber. In vitro, podocytes were cultured on
glass slides in a 12-well plate for 48 h, fixed with 4%
paraformaldehyde for 15 min at 4°C, and incubated with mouse
anti-synaptopodin (1:50) to authenticate the differentiation and
maturation. The slides were sealed using mounting medium with DAPI
and then examined using the Nikon ECLIPSE Ti-S inverted
fluorescence microscope (Nikon Corporation).
Western blot analysis
Total proteins were extracted from the renal cortex
or mouse podocytes using precooled RIPA and PMSF (Beyotime
Biotechnology) mix (99:1). The BCA protein assay kit was used to
quantify the protein concentration following the manufacturer's
instructions. The protein samples were then mixed with 5X loading
buffer (4:1) and boiled for 5 min at 100°C. In total, 80 µg
protein was separated using 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (0.22 µm; EMD Millipore).
The membranes were blocked using 5% skimmed milk powder in TBS with
0.1% Tween-20 for 1 h at 25°C, and incubated overnight at 4°C with
the primary rabbit anti-NLRP3 (1:1,000), rabbit anti-caspase-1
(1:1,500) or rabbit anti-β-actin (1:1,000) antibodies. Following
washing thrice with TBS with 0.1% Tween-20 (15 min per wash), the
membranes were further incubated with goat anti-rabbit antibody
(1:2,000) for 1 h at 25°C. The super ECL solution was used (for 2
min) to develop and visualize the protein bands. The results were
observed by gel imaging system (Chemi Doc XRS; Bio-Rad
Laboratories, Inc.). Western blots were quantified using ImageJ
software (version 1.52).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the renal cortex was extracted using
TRIzol. RT was performed using the Revert Aid First Strand cDNA
Synthesis kit according to the manufacturer's instructions. RT-qPCR
was performed in the CFX96 Real-Time PCR Detection system (Bio-Rad
Laboratories, Inc.) using Power SYBR™ Green PCR Master mix. The
primers used are listed in Table
I. The thermocycling conditions were as follows: Initial
denaturation at 95°C for 10 min; followed by 40 of cycles of
denaturation at 95°C for 25 sec, annealing at 55°C for 15 sec,
extension at 72°C for 50 sec; and a final extension at 72°C for 5
min. GAPDH was used as an internal control (25). The relative quantitative
expression was calculated according to the 2−ΔΔCq method
(26).
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|
| NLRP3 |
ATTACCCGCCCGAGAAAGG |
TCGCAGCAAAGATCCACACAG |
| Caspase-1 |
TGGCAGGAATTCTGGAGCTT |
GAGGGCAAGACGTGTACGAG |
| IL-1β |
TGCCACCTTTTGACAGTGATG |
TGATGTGCTGCTGCGAGATT |
| GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCC |
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical analysis was performed using Graphpad Prism
8 software (Graphpad Software, Inc.). One-way ANOVA, followed by
Tukey's post hoc test, was used to compare differences among all
groups. Correlations were assessed using the Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of AS-IV on body weight and
metabolic markers
During the experiment, db/db mice exhibited a
significantly increased body weight and hyperglycemia compared with
the normal control mice (Fig. 1A and
B; P<0.001). The bodyweight of vehicle-treated db/db
mice increased continuously. Starting from the 9th week,
AS-IV-treated db/db mice exhibited a noticeable decrease in
body weight (Fig. 1A;
P<0.05). In the 12th week, the AS-IV administration group
exhibited a decreasing trend in FBG compared with the vehicle- and
benazepril-treated groups (Fig.
1B; P<0.05). However, significant hypoglycemia and weight
loss were not recorded following benazepril administration
(Fig. 1A and B; P>0.05).
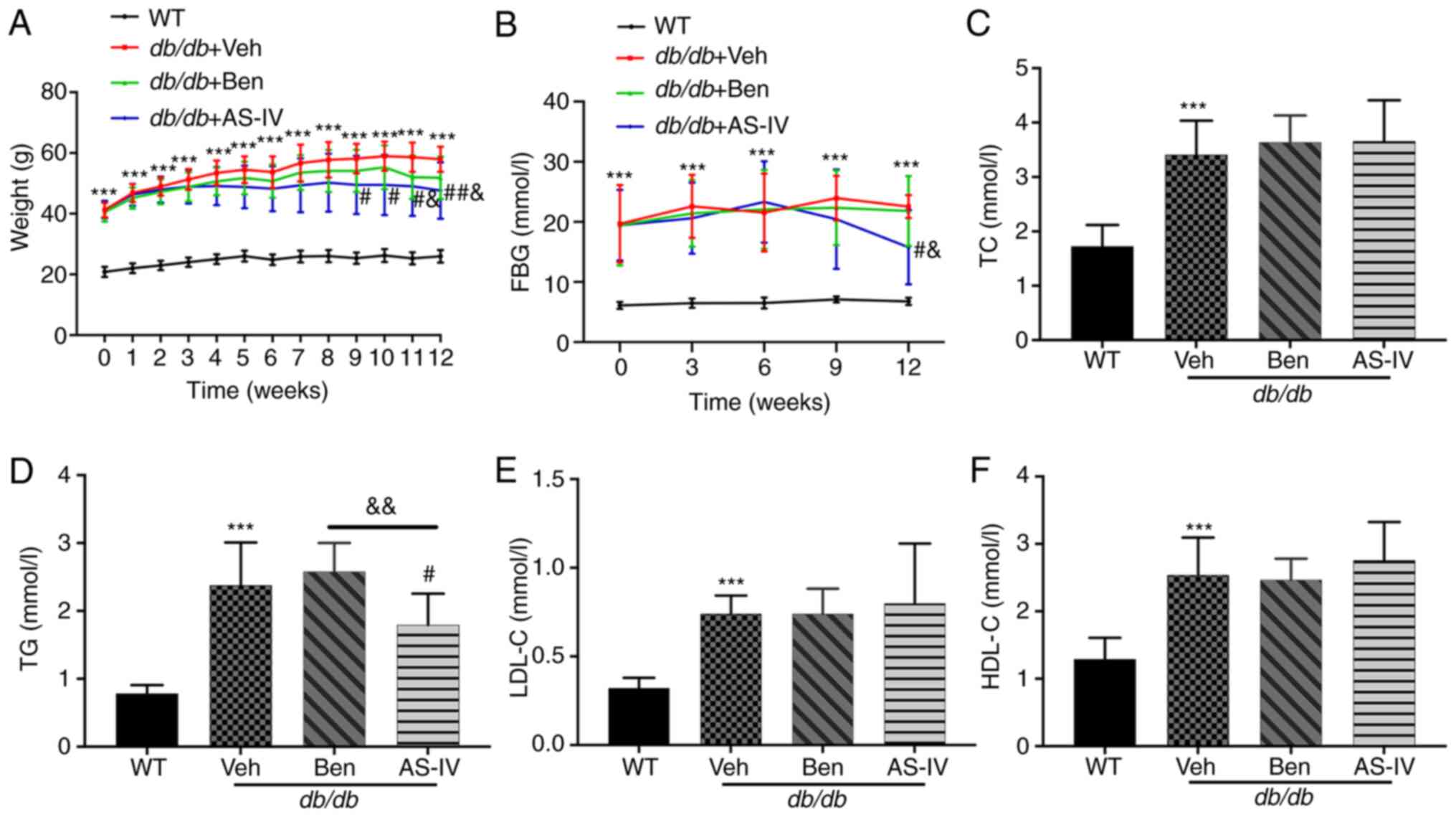 | Figure 1Effect of AS-IV on body weight, FBG
and serum lipid profiles in different groups after 12 weeks of
administration. (A) Weight, (B) FBG, (C) TC, (D) TG, (E) LDL-C, and
(F) HDL-C. Data are presented as the mean ± standard deviation
(n=7-10). ***P<0.001 vs. WT control;
#P<0.05 and ##P<0.01 vs. db/db +
vehicle; and &P<0.05 and
&&P<0.01 vs. db/db + benazepril.
AS-IV, astragaloside IV; WT, wild-type mice; FBG, fasting blood
glucose; TC, total cholesterol; TG, triacylglycerol; LDL-C,
low-density lipoprotein cholesterol; HDL-C, high-density
lipoprotein cholesterol. |
Compared with WT control mice, db/db mice
exhibited a noticeable increase in serum TC, TG, LDL-C and HDL-C
levels (Fig. 1C-F; P<0.001).
AS-IV administration for 12 weeks significantly reduced TG levels
(Fig. 1D; P<0.05), which were
lower than those in benazepril-treated mice (Fig. 1D; P<0.01). In the present
study, AS-IV administration did not cause a noticeable decrease in
serum TC, HDL-C and LDL-C levels (Fig. 1C, E and F; P>0.05).
AS-IV administration protects renal
function
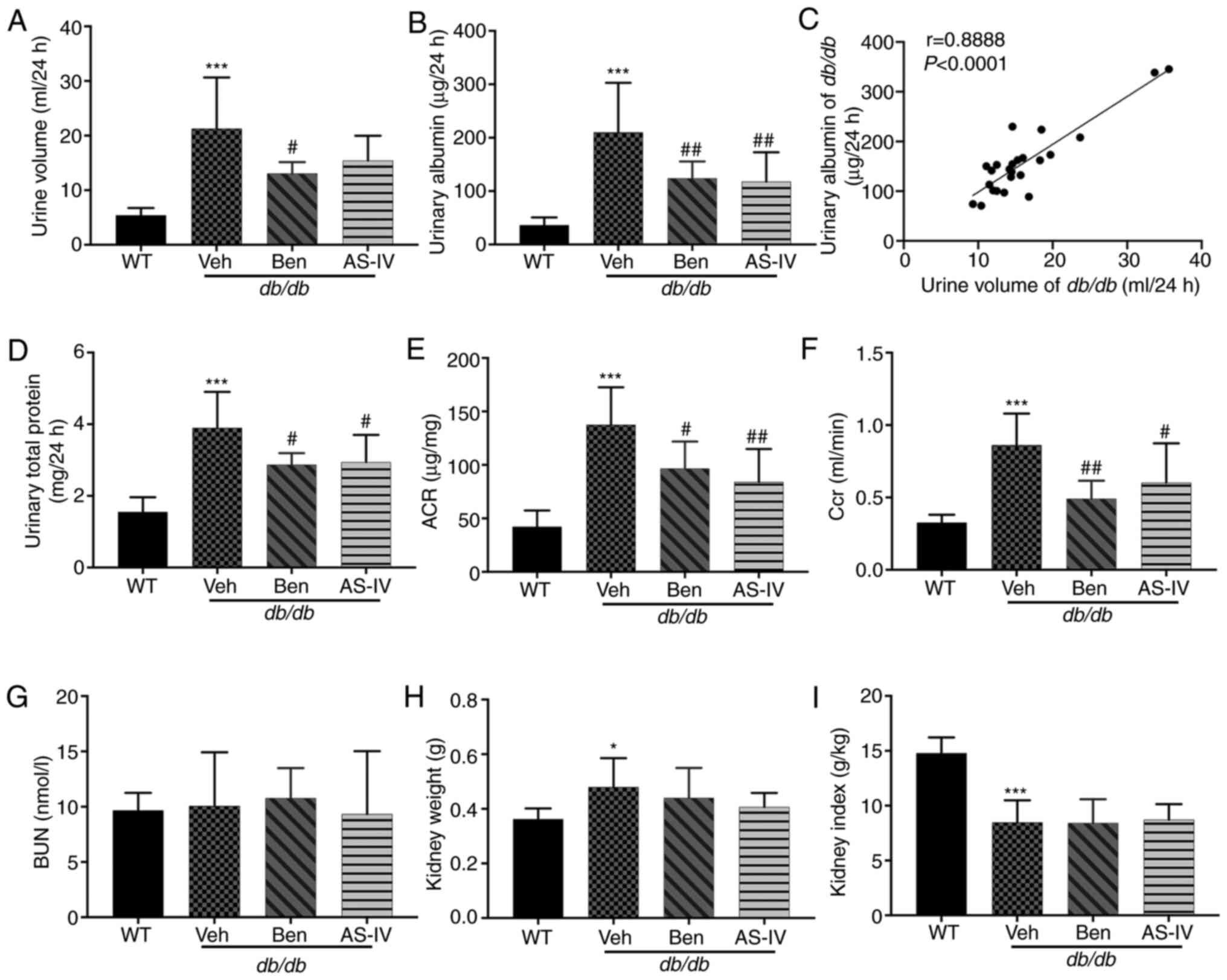
The 24-h urine volume, urinary albumin, urinary
total protein and urinary ACR were higher in db/db mice
compared with those in WT mice; these parameters were markedly
decreased in benazepril-treated mice (Fig. 2A, B, D and E; P<0.05 or
P<0.01). AS-IV administration also significantly decreased
urinary albumin, urinary total protein and ACR in db/db mice
(Fig. 2B, D and E; P<0.05 or
P<0.01); the 24-h urine volume demonstrated a decreasing trend,
but without statistical significance (Fig. 2A; P>0.05). Correlation
analysis between 24-h urine volume and urinary albumin indicated a
significant positive correlation (Fig. 2C; P<0.0001). Benazepril and
AS-IV administration significantly reduced Ccr levels compared with
the Veh group (Fig. 2F;
P<0.05 or P<0.01). The kidney weight of db/db mice was
higher than that of WT mice (Fig.
2H; P<0.05), indicating renal hypertrophy in the former.
However, the reduction in kidney weight and index following AS-IV
administration was not significant (Fig. 2H and I; P>0.05). No changes in
BUN levels were observed among different groups (Fig. 2G; P>0.05).
AS-IV ameliorates renal histopathology
and podocyte injury
Histological examination revealed that db/db
mice displayed glomerular hypertrophy, obvious mesangial
proliferation, increased extracellular matrix (ECM) deposition and
severe renal fibrosis at 20 weeks compared with the WT control mice
(Fig. 3A). The glomerular volume
and fibrotic area in the glomeruli of db/db mice were higher
than those in WT mice (Fig. 3B and
C; P<0.001). AS-IV- and benazepril-treated groups revealed a
significant reduction in the mean glomerular volume and fibrotic
area in the glomeruli compared with db/db mice treated with
vehicle alone (Fig. 3B and C;
P<0.05, P<0.01 or P<0.001).
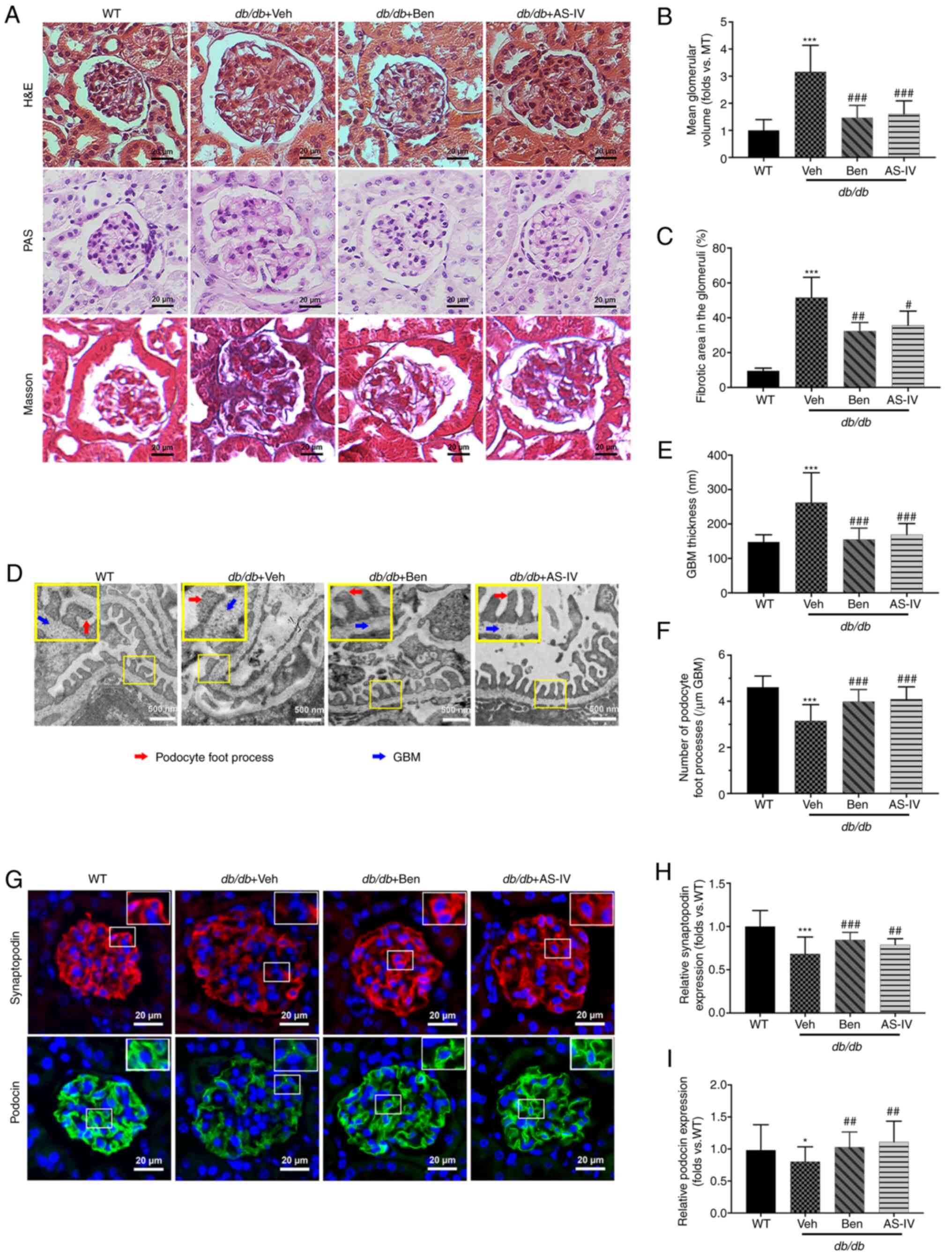 | Figure 3Benazepril and AS-IV ameliorate
kidney lesions and podocyte injury in db/db mice.
Representative images of the pathological structure of the kidneys
after (A) H&E, PAS, and Masson's trichrome staining (scale bar,
20 µm; magnification, ×200). Quantitative analyses of the
results for (B) mean glomerular volume and (C) fibrotic area in the
glomeruli. (D) Representative images of the ultrastructure observed
using TEM (scale, 500 nm; magnification, ×15,000). (E)
Quantification of GBM thickness and (F) number of podocyte foot
processes per µm GBM. (G) Representative immunofluorometric
images of synaptopodin and podocin (scale, 20 µm;
magnification, ×200). Quantification of the (H) relative expression
of synaptopodin and (I) podocin in the glomeruli. Data are
presented as the mean ± standard deviation (n=3).
*P<0.05 and ***P<0.001 vs. WT control;
#P<0.05, ##P<0.01 and
###P<0.001 vs. db/db + vehicle. AS-IV,
Astragaloside IV; WT, wild-type mice; Veh, vehicle; Ben,
benazepril; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff; GBM, glomerular basement membrane; TEM, transmission
electron microscopy. |
TEM images revealed that vehicle-treated
db/db mice presented extensive podocyte foot process fusion,
podocyte foot process widening, podocyte loss and GBM thickening
compared with the control mice (Fig.
3D). GBM thickness was attenuated by AS-IV or benazepril
administration (Fig. 3E;
P<0.001). In addition, the number of podocyte foot processes per
µm of GBM was higher in both AS-IV- and benazepril-treated
mice than in db/db mice treated with vehicle alone (Fig. 3F; P<0.001).
To further evaluate the effect of AS-IV on podocytes
in db/db mice, immunofluorescence staining of synaptopodin
and podocin (the podocyte markers in the glomeruli) was performed.
As revealed in Fig. 3G,
immunofluorescence revealed intense and linear staining of both
podocin and synaptopodin in WT controls. This expression pattern
was weak and discontinuous in vehicle-treated db/db mice.
Moreover, the relative expression of both podocin and synaptopodin
was significantly decreased in vehicle-treated db/db mice
compared with that in WT mice (Fig.
3H and I; P<0.05 or P<0.001); this effect was partly
reversed by AS-IV and benazepril administration (Fig. 3H and I; P<0.01 or
P<0.001).
AS-IV inhibits NLRP3
inflammasome-mediated inflammation in the renal cortex and
serum
To explore the protective mechanism of AS-IV, the
gene expression of NLRP3, caspase-1 and IL-1β in the renal cortex
of vehicle- and AS-IV-treated db/db and WT mice was firstly
determined. The mRNA levels of NLRP3 and caspase-1 were
significantly higher in the vehicle-treated mice than in the WT
mice, as revealed by RT-qPCR (Fig.
4A and B; P<0.001 and P<0.05, respectively). However, the
IL-1β level exhibited an increasing trend, with no significant
difference (Fig. 4C; P=0.0872).
Following treatment with AS-IV for 12 weeks, the gene expression of
NLRP3 and IL-1β was significantly reduced (Fig. 4A and C; P<0.01 and P<0.05,
respectively). Next, the protein expression of NLRP3 and caspase-1
in the renal cortex and IL-1β in the serum were detected (Fig. 4D-I). An increased activation of
NLRP3, pro-caspase-1, caspase-1 and IL-1β was observed in the
vehicle-treated group (Fig.
4E-I; P<0.05, P<0.01 or P<0.001). AS-IV administration
inhibited the levels of NLRP3, caspase-1 and IL-1β and reduced the
caspase-1 to pro-caspase-1 ratio (Fig. 4E and G-I; P<0.05, P<0.01 or
P<0.001); a decreasing trend was observed in pro-caspase-1, but
without statistical significance (Fig. 4F; P>0.05).
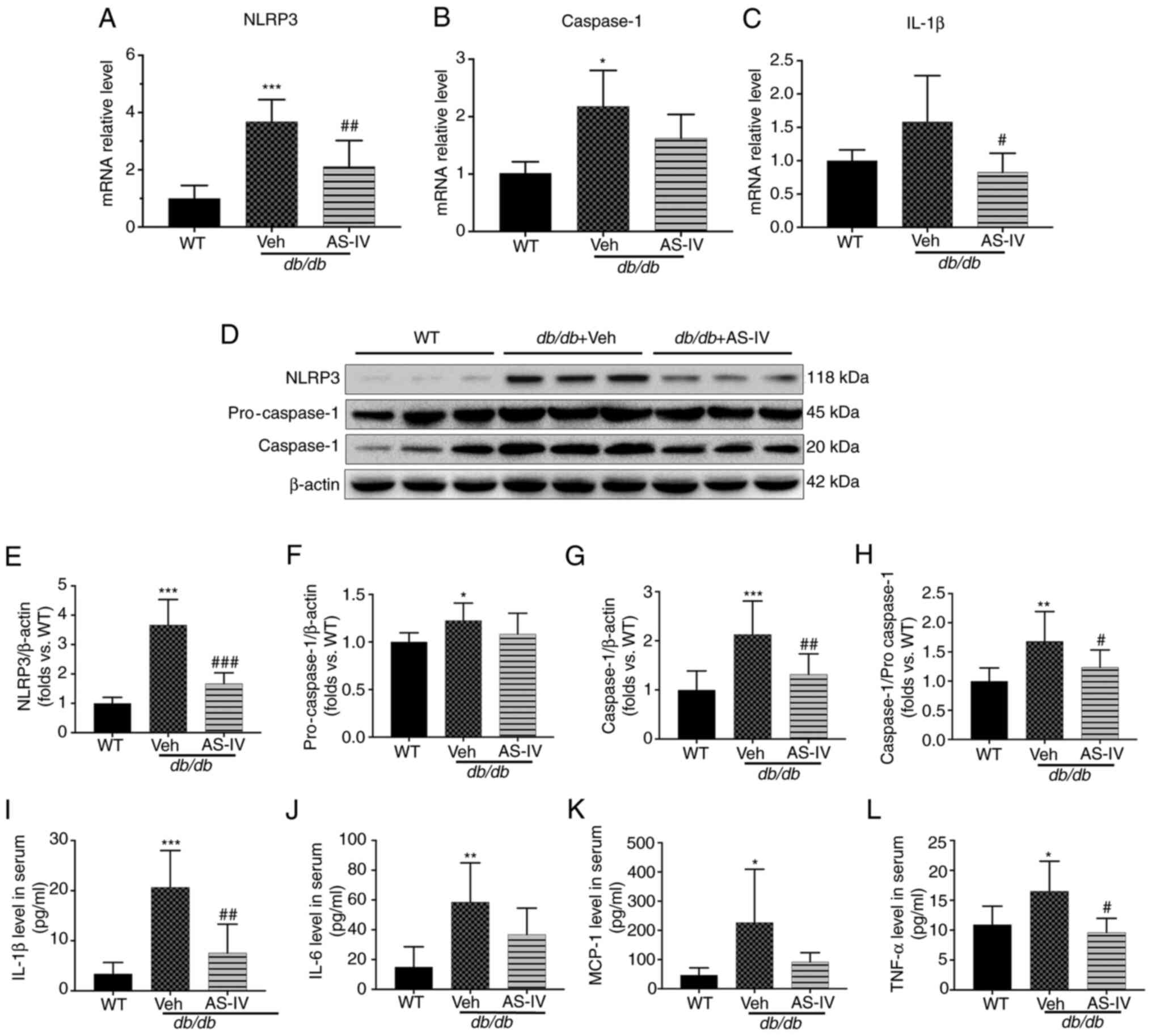 | Figure 4AS-IV inhibits NLRP3 inflammasome
activation in the renal cortex of db/db mice. The mRNA
levels of (A) NLRP3, (B) caspase-1, and (C) IL-1β. (D) Western blot
analysis of NLRP3, pro-caspase-1 and caspase-1. The protein levels
of (E) NLRP3, (F) pro-caspase-1, and (G) caspase-1 were normalized
to β-actin protein level. (H) Ratio of the protein level of
caspase-1 to that of pro-caspse-1. The levels of (I) IL-1β, (J)
IL-6, (K) MCP-1, and (L) TNF-α in serum. Data are presented as the
mean ± standard deviation. (A-C and I-L) n=6-7; (E-H) n=3.
*P<0.05, **P<0.01 and
***P<0.001 vs. WT mice; #P<0.05,
##P<0.01 and ###P<0.001 vs.
db/db + vehicle. WT, wild-type mice; Veh: Vehicle; AS-IV,
Astragaloside IV; NLRP3, NLR family pyrin domain containing 3; IL,
interleukin; TNF, tumor necrosis factor; MCP-1, monocyte
chemoattractant protein-1. |
Furthermore, the expression of certain downstream
inflammatory cytokines of IL-1β, including IL-6, TNF-α and MCP-1
(10,11), was detected in the serum. The
expression of these cytokines increased in vehicle-treated
db/db mice compared with that in WT mice (P<0.05 or
P<0.01; Fig. 4J-L). AS-IV
administration significantly reduced TNF-α levels (P<0.05;
Fig. 4L), but the reduction in
IL-6 and MCP-1 levels was not significant (P=0.1775 and P=0.1119,
respectively; Fig. 4J and
K).
AS-IV inhibits NLRP3 inflammasome
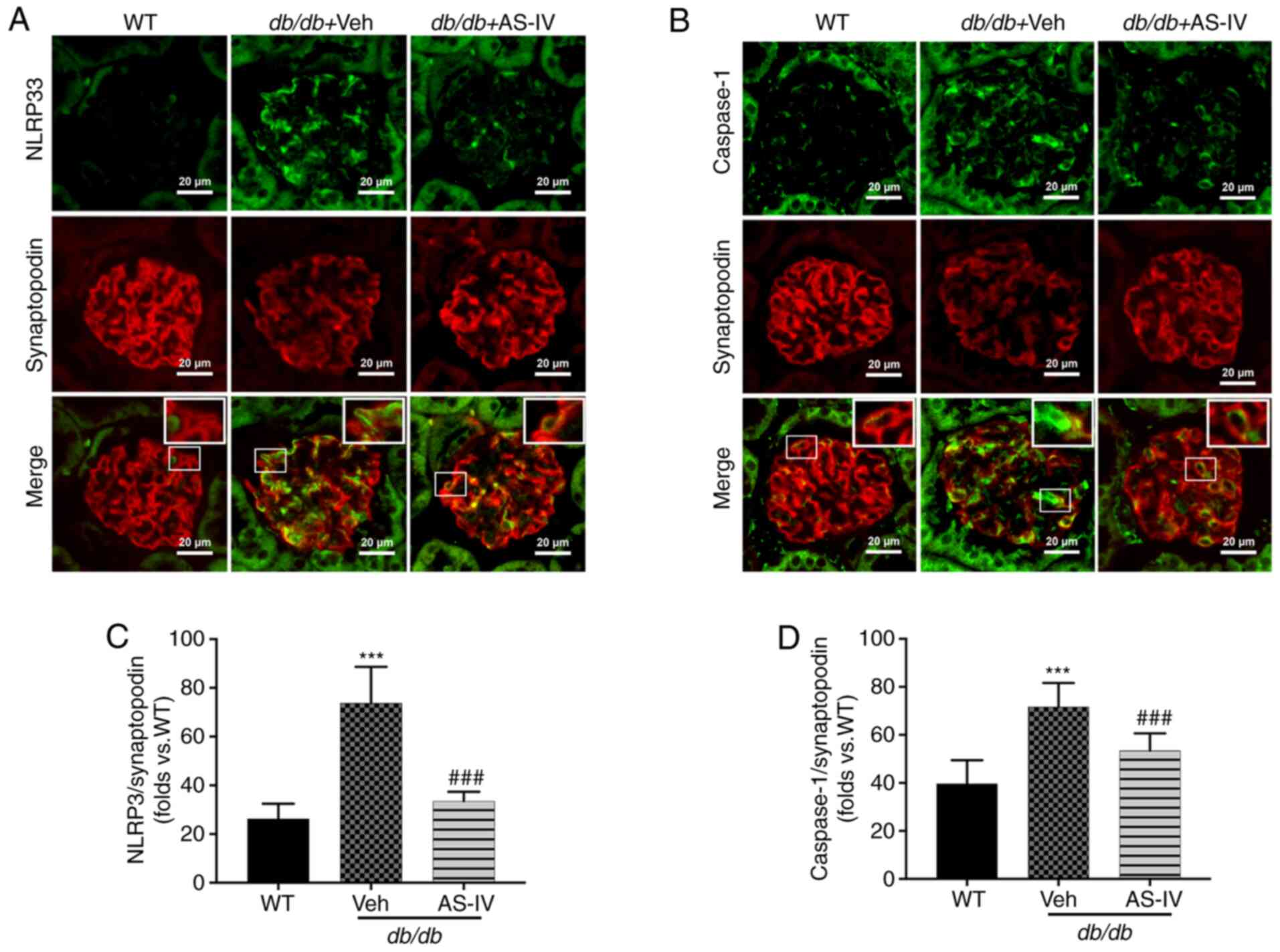
activation in podocytes from db/db mice
NLRP3 and caspase-1 were localized within the
podocytes of mouse renal tissue, as revealed by double
immunofluorescence staining. Synaptopodin was used as the podocyte
marker. The colocalization of NLRP3 and caspase-1 with synaptopodin
was observed using confocal microscopy. Colocalization was
increased in the glomeruli of vehicle-treated db/db mice
compared with that in the glomeruli of WT mice. The expression
levels of NLRP3 and caspase-1 were significantly increased, while
that of synaptopodin was decreased in the glomeruli of
vehicle-treated db/db mice (Fig. 5A and B). However, AS-IV
administration significantly reduced the mean fluorescence
intensity of both NLRP3 with synaptopodin and caspase-1 with
synaptopodin in the glomeruli of db/db mice (P<0.001;
Fig. 5C and D).
AS-IV inhibits the decrease in cell
viability and NLRP3 inflammasome activation in HG-induced
podocytes
Podocytes cultured for 2 days at 37°C in medium
without recombinant murine γ-interferon express no or low levels of
synaptopodin (Fig. S1A), but
the podocytes cultured for 10 days at 37°C express high levels of
synaptopodin (Fig. S1B). On
days 10-14, large, differentiated arborized podocytes with
well-developed prominent processes could be observed and there was
no significant difference in the morphology of podocytes among
different groups (Fig. 6A).
However, the cell viability of podocytes was significantly
decreased after a 24-h stimulation with HG (Fig. 6B, P<0.001), which was reversed
by treatment with 40 µM AS-IV for 24 h (Fig. 6B; P<0.01), while treatment
with 10 and 20 µM AS-IV did not influence cell viability
(Fig. 6B; P>0.05). The
protein expression of NLRP3, pro-caspase-1, and cleaved caspase-1
was also detected (Fig. 6C).
Podocytes in the HG group exhibited higher protein levels of NLRP3,
pro-caspase-1 and caspase-1 than those in the control group
(Fig. 6D-F; P<0.01), which
were significantly decreased by 40 µM AS-IV treatment
(P<0.01). In addition, 10 and 20 µM AS-IV treatment also
significantly inhibited the levels of NLRP3 and pro-caspase-1
(P<0.05 or P<0.01), while the reduction of caspase-1 was not
significant (Fig. 6D-F;
P>0.05).
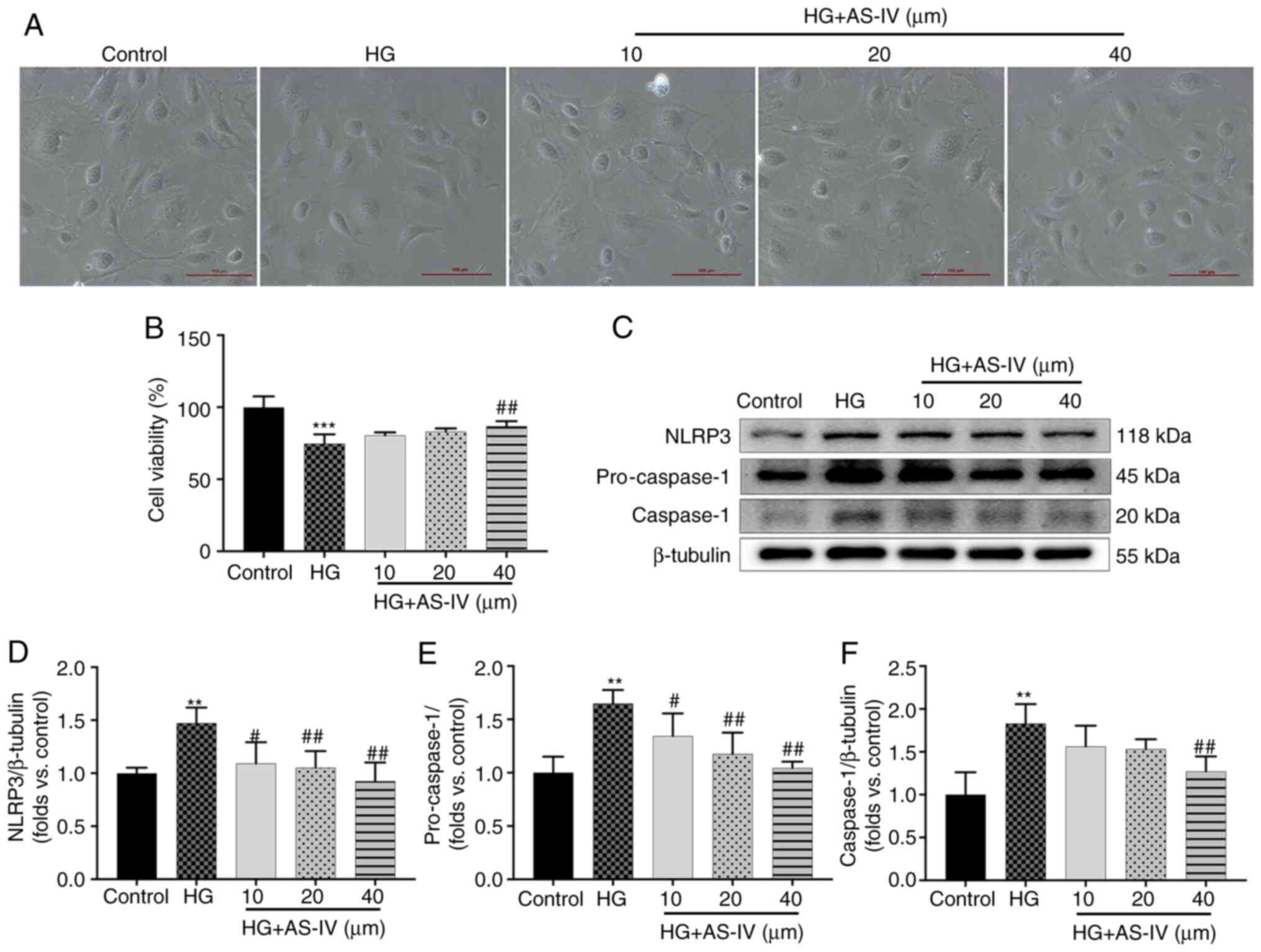 | Figure 6AS-IV inhibits HG-induced decrease in
cell viability and NLRP3 inflammasome activation in podocytes. (A)
Morphological images of each group of podocytes (scale bar, 100
µm; magnification, ×100). (B) Podocytes were cultured with
different concentration of AS-IV (10, 20, or 40 µM) for 24 h
and the cell viability was assessed using MTT assays. (C) Western
blot analysis of NLRP3, pro-caspase-1, and caspase-1. The protein
levels of (D) NLRP3, (E) pro-caspase-1, and (F) caspase-1 were
normalized to β-tubulin protein level. Data are presented as the
mean ± standard deviation (n=3). **P<0.01 and
***P<0.001 vs. the control; #P<0.05 and
##P<0.01 vs. HG. Control, normal glucose; HG, high
glucose; AS-IV, Astragaloside IV; NLRP3, NLR family pyrin domain
containing 3. |
AS-IV attenuates HG-induced podocyte
injury by inhibiting NLRP3 inflammasome activation
To further confirm the role of NLRP3 inflammasome in
the protective effect of AS-IV on HG-induced podocyte injury, NLRP3
was overexpressed in podocytes by the recombinant lentivirus
vectors for NLRP3. The increase of cell viability following AS-IV
administration was revealed to be inhibited by NLRP3 overexpression
(Fig. 7A). The protein
expression levels of NLRP3, pro-caspase-1 and active caspase-1 in
NLRP3-ov groups were significantly increased compared with those in
the control-ov groups (Fig.
7B-E). HG induced an obvious activation of NLRP3, pro-caspase-1
and caspase-1 which were inhibited by AS-IV treatment, while these
inhibitory effects were all abolished by NLRP3 overexpression
(Fig. 7B-E). These results
indicated that AS-IV attenuated HG-induced podocyte injury by
inhibiting NLRP3 inflammasome activation.
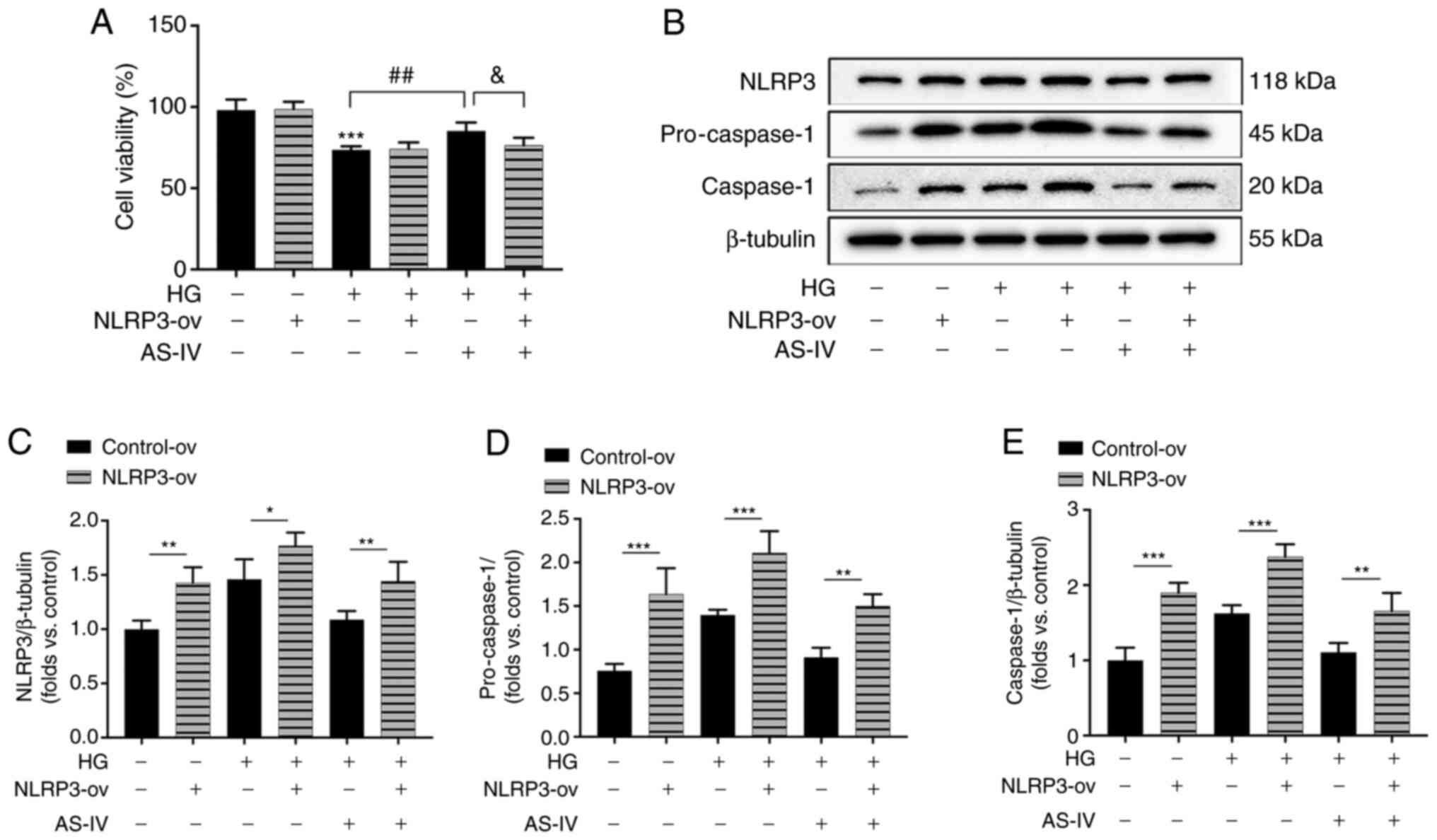 | Figure 7AS-IV inhibits HG-induced cell
viability decrease and NLRP3 inflammasome activation in podocytes.
(A) Podocytes were transfected with the recombinant lentiviral
vectors for NLRP3 and empty vector, and the cell viability was
assessed using an MTT assay. (B) Western blot analysis of NLRP3,
pro-caspase-1, and caspase-1. The protein levels of (C) NLRP3, (D)
pro-caspase-1, and (E) caspase-1 were normalized to the β-tubulin
protein level. Data are presented as the mean ± standard deviation.
(A, C-E) n=3. *P<0.05, **P<0.01 and
***P<0.001; ##P<0.01 and
&P<0.05. AS-IV, Astragaloside IV; HG, high
glucose; NLRP3, NLR family pyrin domain containing 3; NLRP3-ov,
NLRP3 overexpression. |
Discussion
Proteinuria is the primary diagnostic and
post-treatment evaluation marker of progressive kidney damage
(2). ACR is an important marker
for the evaluation of urinary albumin excretion and early renal
function decline in DN (27). A
previous study established that albumin excretion rates were
8-62-fold higher in db/db mice than that in control mice at
the age of 8 weeks (16). In the
present study, the 24-h urine volume, urinary total protein and ACR
levels were higher in db/db mice than in WT mice at 20
weeks. These results confirmed that db/db mice presented
with nephropathy.
Obesity, hyperglycemia and dyslipidemia are
currently considered major risk factors for DN (28). AS-IV was revealed to effectively
reduce weight gain, hyperglycemia and serum TG levels in
db/db mice. It was also revealed that AS-IV could reduce
24-h urinary albumin levels and ACR. In addition, the positive
correlation between urinary albumin and urinary volume indicated
that the mice in the present study exhibited glomerular
hyperfiltration in DN. AS-IV reduced the elevated Ccr levels in
db/db mice, mitigating renal hypertrophy and glomerular
hyperfiltration. However, the BUN level was not significantly
increased. These results demonstrated that 20-week-old db/db
mice were still in the early stage of DN and that AS-IV is an
effective therapeutic option for DN.
Structural lesions, including glomerular
hypertrophy, thickened GBM, increased ECM deposition and
glomerulosclerosis, are involved in progressive proteinuria in DN
(29). In addition, podocyte
injury has been confirmed to be a key event that may lead to
glomerulosclerosis and proteinuria, as it represents renal function
decline in DN (30). Podocytes
are terminally differentiated epithelial cells that are
infrequently able to divide once damaged (31). In that respect, the pathological
changes in glomeruli were firstly observed using various staining
techniques. The results indicated that AS-IV administration
attenuated glomerular injury, including glomerular mesangial cell
proliferation, ECM deposition and renal fibrosis, in db/db
mice. The ultrastructure of glomeruli was further observed by TEM.
Foot process fusion, foot process widening, podocyte loss and GBM
thickening were aggravated in db/db mice. All these changes
were suppressed following AS-IV administration. In addition, the
expression levels of podocyte markers, including podocin and
synaptopodin, were detected and revealed to be significantly
reduced in vehicle-treated db/db mice. These changes
indicated that db/db mice exhibited significant podocyte
injury at 20 weeks. In the present study, the activation of podocin
and synaptopodin was demonstrated in AS-IV-treated db/db
mice, which confirmed that AS-IV prevents the progression of DN by
protecting podocytes.
Several studies have indicated that inflammation
plays an indispensable role in the progression of DN (9,32). The NLRP3 inflammasome, a key
component of innate immunity maintenance, can be activated by
several factors, including chronic hyperglycemia, obesity and
reactive oxygen species, which in turn activate pro-inflammatory
mediators such as IL-1β, resulting in diabetic renal damage
(5). A study has reported that
NLRP3 inflammation activation occurs at an early stage of
nephropathy in db/db mice and is accompanied by podocyte
injury, which is associated with the increase in ALB of
db/db mice (6). In the
present study, the expression of NLRP3 and caspase-1 was higher in
the renal cortex, podocytes of the glomeruli of db/db mice
and mouse podocytes incubated with HG in vitro.
Caspase-1-dependent inflammasome activation plays a crucial role in
DN initiation (33). NLRP3 can
promote IL-1β induction by cleaving caspase-1 (8). The IL-1β level in the serum was
also increased in vehicle-treated mice. AS-IV administration
reduced the expression levels of NLRP3, caspase-1 and IL-1β, and
decreased the colocalization of both NLRP3 with synaptopodin and
caspase-1 with synaptopodin in the glomeruli of db/db mice.
Further experiments revealed that AS-IV administration also
inhibited NLRP3 activation in HG-induced mouse podocytes in
vitro, while these protective effects were blunted by NLRP3
overexpression. These results indicated that AS-IV db/db
exerted its therapeutic effect on DN by inhibiting NLRP3
inflammasome activation in podocytes.
In addition, it was also observed that serum levels
of IL-6, MCP-1 and TNF-α, which are downstream inflammatory
cytokines of IL-1β, were increased in db/db mice treated
with vehicle alone. Multiple studies have confirmed that the levels
of IL-6, TNF-α and MCP-1 are correlated with albuminuria excretion
in patients with DM and DN (34,35). These inflammatory cytokines can
further recruit macrophages to accumulate in the kidney tissues,
which release more pro-inflammatory factors and enhance the
inflammatory response (10,36). AS-IV administration reduced the
serum levels of IL-6, MCP-1 and TNF-α, particularly those of TNF-α.
These results suggested that AS-IV could reduce inflammatory
reaction in the kidney and serum of db/db mice.
In conclusion, it was demonstrated herein that AS-IV
can reduce weight gain, mitigate glucolipid metabolism disorders
and protect the kidney against proteinuria and podocyte injury in
db/db mice, likely through anti-NLRP3 inflammasome-mediated
inflammation. These findings indicated that AS-IV, a natural food
supplement, could become an effective therapeutic option for
T2DM-associated nephropathy. AS-IV may have a wide application
prospect in the field of medicine due to the high prevalence of
dyslipidemia and obesity among patients with T2DM (37). However, experiments in fatty
acid-induced cells were not performed, which was one limitation in
this study. In future studies, the role of AS-IV on podocytes in
high palmitic acid in vitro will further be explored.
Supplementary Data
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
XL, XZ, and HF designed the study. XZ, HF, YT, BK
and SF conducted the experiments. HF analyzed the data and wrote
the manuscript. HF, XZ, YT, BK, and SF reviewed and edited the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved (approval no.
IACUC-GAMH-2019-010) by the Animal Ethics Committee of Guang'anmen
Hospital, Chinese Academy of Traditional Chinese Medicine (Beijing,
China) and the procedures were consistent with the guidelines of
the Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Alicic RZ, Rooney MT and Tuttle KR:
Diabetic kidney disease: Challenges, progress and possibilities.
Clin J Am Soc Nephrol. 12:2032–2045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liew A, Bavanandan S, Prasad N, Wong MG,
Chang JM, Eiam-Ong S, Hao CM, Lim CY, Lim SK, Oh KH, et al: Asian
pacific society of nephrology clinical practice guideline on
diabetic kidney disease. Nephrology (Carlton). 25(Suppl 2):
S12–S45. 2020. View Article : Google Scholar
|
|
3
|
Lin JS and Susztak K: Podocytes: The
weakest link in diabetic kidney disease? Curr Diab Rep. 16:452016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nichols GA, Déruaz-Luyet A, Brodovicz KG,
Kimes TM, Rosales AG and Hauske SJ: Kidney disease progression and
all-cause mortality across estimated glomerular filtration rate and
albuminuria categories among patients with vs. without type 2
diabetes. BMC Nephrol. 21:1672020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hou Y, Lin S, Qiu J, Sun W, Dong M, Xiang
Y, Wang L and Du P: NLRP3 inflammasome negatively regulates
podocyte autophagy in diabetic nephropathy. Biochem Biophys Res
Commun. 521:791–798. 2020. View Article : Google Scholar
|
|
6
|
Shahzad K, Bock F, Dong W, Wang H, Kopf S,
Kohli S, Al-Dabet MM, Ranjan S, Wolter J, Biemann R, et al:
Nlrp3-inflammasome activation in non-myeloid-derived cells
aggravates diabetic nephropathy. Kidney Int. 87:74–84. 2015.
View Article : Google Scholar :
|
|
7
|
Karki R and Kanneganti TD: Diverging
inflammasome signals in tumorigenesis and potential targeting. Nat
Rev Cancer. 19:197–214. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu D, Zeng X, Li X, Cui C, Hou R, Guo Z,
Mehta JL and Wang X: Advances in the molecular mechanisms of NLRP3
inflammasome activators and inactivators. Biochem Pharmacol.
175:1138632020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu M, Han W, Song S, Du Y, Liu C, Chen N,
Wu H, Shi Y and Duan H: NLRP3 deficiency ameliorates renal
inflammation and fibrosis in diabetic mice. Mol Cell Endocrinol.
478:115–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro-Gonzalez JF, Mora-Fernandez C,
Muros de Fuentes M and Garcia-Perez J: Inflammatory molecules and
pathways in the pathogenesis of diabetic nephropathy. Nat Rev
Nephrol. 7:327–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Xuan J, Gu YT, Shi KS, Xie JJ,
Chen JX, Zheng ZM, Chen Y, Chen XB, Wu YS, et al: Celastrol reduces
IL-1β induced matrix catabolism, oxidative stress and inflammation
in human nucleus pulposus cells and attenuates rat intervertebral
disc degeneration in vivo. Biomed Pharmacother. 91:208–219. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ju Y, Su Y, Chen Q, Ma K, Ji T, Wang Z and
Li W and Li W: Protective effects of Astragaloside IV on
endoplasmic reticulum stress-induced renal tubular epithelial cells
apoptosis in type 2 diabetic nephropathy rats. Biomed Pharmacother.
109:84–92. 2019. View Article : Google Scholar
|
|
13
|
Zhang Z, Wang J, Zhu Y, Zhang H and Wang
H: Astragaloside IV alleviates myocardial damage induced by type 2
diabetes via improving energy metabolism. Mol Med Rep.
20:4612–4622. 2019.PubMed/NCBI
|
|
14
|
Li L, Hou X, Xu R, Liu C and Tu M:
Research review on the pharmacological effects of astragaloside IV.
Fundam Clin Pharmacol. 31:17–36. 2017. View Article : Google Scholar
|
|
15
|
Leng B, Zhang Y, Liu X, Zhang Z, Liu Y,
Wang H and Lu M: Astragaloside IV suppresses high glucose-induced
NLRP3 inflammasome activation by inhibiting TLR4/NF-κB and CaSR.
Mediators Inflamm. 2019:10824972019. View Article : Google Scholar
|
|
16
|
Sharma K, McCue P and Dunn SR: Diabetic
kidney disease in the db/db mouse. Am J Physiol Renal Physiol.
284:F1138–F1144. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun H, Wang W, Han P, Shao M, Song G, Du
H, Yi T and Li S: Astragaloside IV ameliorates renal injury in
db/db mice. Sci Rep. 6:325452016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Zhang X, Liu L, Cai M, Guo Z and
Qiu L: Application of red clover isoflavone extract as an adjuvant
in mice. Exp Ther Med. 19:1175–1182. 2020.PubMed/NCBI
|
|
19
|
Jiang L, Cui H and Ding J: Smad3
signalling affects high glucose-induced podocyte injury via
regulation of the cytoskeletal protein transgelin. Nephrology
(Carlton). 25:659–666. 2020. View Article : Google Scholar :
|
|
20
|
Li F, Chen Y, Li Y, Huang M and Zhao W:
Geniposide alleviates diabetic nephropathy of mice through
AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 886:1734492020.
View Article : Google Scholar
|
|
21
|
Ma Y, Li W, Yazdizadeh Shotorbani P,
Dubansky BH, Huang L, Chaudhari S, Wu P, Wang LA, Ryou MG, Zhou Z
and Ma R: Comparison of diabetic nephropathy between male and
female eNOS(-/-) db/db mice. Am J Physiol Renal Physiol.
316:F889–F897. 2019. View Article : Google Scholar
|
|
22
|
Lane PH, Steffes MW and Mauer SM:
Estimation of glomerular volume: A comparison of four methods.
Kidney Int. 41:1085–1089. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Canaud G, Bienaimé F, Viau A, Treins C,
Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, et
al: AKT2 is essential to maintain podocyte viability and function
during chronic kidney disease. Nat Med. 19:1288–1296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhan Y, Zhou Y, Zheng W, Liu W, Wang C,
Lan X, Deng X, Xu Y, Zhang B and Ning Y: Alterations of multiple
peripheral inflammatory cytokine levels after repeated ketamine
infusions in major depressive disorder. Transl Psychiatry.
10:2462020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo CW, Shen CJ, Tung YT, Chen HL, Chen
YH, Chang WH, Cheng KC, Yang SH and Chen CM: Extracellular
superoxide dismutase ameliorates streptozotocin-induced rat
diabetic nephropathy via inhibiting the ROS/ERK1/2 signaling. Life
Sci. 135:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Nowak N, Skupien J, Smiles AM, Yamanouchi
M, Niewczas MA, Galecki AT, Duffin KL, Breyer MD, Pullen N,
Bonventre JV and Krolewski AS: Markers of early progressive renal
decline in type 2 diabetes suggest different implications for
etiological studies and prognostic tests development. Kidney Int.
93:1198–1206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tziomalos K and Athyros VG: Diabetic
nephropathy: New risk factors and improvements in diagnosis. Rev
Diabet Stud. 12:110–118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanwar YS, Sun L, Xie P, Liu FY and Chen
S: A glimpse of various pathogenetic mechanisms of diabetic
nephropathy. Annu Rev Pathol. 6:395–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weil EJ, Lemley KV, Mason CC, Yee B, Jones
LI, Blouch K, Lovato T, Richardson M, Myers BD and Nelson RG:
Podocyte detachment and reduced glomerular capillary endothelial
fenestration promote kidney disease in type 2 diabetic nephropathy.
Kidney Int. 82:1010–1017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barisoni L and Mundel P: Podocyte biology
and the emerging understanding of podocyte diseases. Am J Nephrol.
23:353–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wada J and Makino H: Innate immunity in
diabetes and diabetic nephropathy. Nat Rev Nephrol. 12:13–26. 2016.
View Article : Google Scholar
|
|
33
|
Shahzad K, Bock F, Al-Dabet MM, Gadi I,
Kohli S, Nazir S, Ghosh S, Ranjan S, Wang H, Madhusudhan T, et al:
Caspase-1, but not caspase-3, promotes diabetic nephropathy. J Am
Soc Nephrol. 27:2270–2275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Perlman AS, Chevalier JM, Wilkinson P, Liu
H, Parker T, Levine DM, Sloan BJ, Gong A, Sherman R and Farrell FX:
Serum Inflammatory and immune mediators are elevated in early stage
diabetic nephropathy. Ann Clin Lab Sci. 45:256–263. 2015.PubMed/NCBI
|
|
35
|
Murakoshi M, Gohda T and Suzuki Y:
Circulating tumor necrosis factor receptors: A potential biomarker
for the progression of diabetic kidney disease. Int J Mol Sci.
21:19572020. View Article : Google Scholar :
|
|
36
|
Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols
E, Rollins BJ and Tesch GH: Monocyte chemoattractant
protein-1-induced tissue inflammation is critical for the
development of renal injury but not type 2 diabetes in obese db/db
mice. Diabetologia. 50:471–480. 2007. View Article : Google Scholar
|
|
37
|
Narindrarangkura P, Bosl W, Rangsin R and
Hatthachote P: Prevalence of dyslipidemia associated with
complications in diabetic patients: A nationwide study in Thailand.
Lipids Health Dis. 18:902019. View Article : Google Scholar : PubMed/NCBI
|