Nitric oxide (NO) is a water-soluble, gaseous,
short-lived free radical molecule that plays multifaceted roles in
a broad range of physiological and pathological processes in
mammals (1-3). NO is produced by NO synthase (NOS)
as a consequence of the process of L-arginine (L-arg) conversion
into L-citrulline with the participation of oxygen and nicotinamide
adenine dinucleotide phosphate. Three isoforms of NOS have been
identified: Neuronal NOS (nNOS) and endothelial NOS (eNOS) are
constitutively expressed calcium-dependent enzymes, characterized
by the rapid production of a small amount of NO; inducible NOS
(iNOS) is a calcium-independent enzyme that is upregulated at the
transcriptional level during inflammation, causing a relatively
slow yet increased-output NO production (2,3).
The most common target of NO is soluble guanylate cyclase (sGC),
which generates the second messenger cyclic guanosine monophosphate
(cGMP) from guanosine-5′-triphosphate within the cell (4,5).
cGMP mainly acts on protein kinase G (PKG) and can be degraded by
phosphodiesterase (PDE), such as PDE5, 6 and 9 (2,6).
The effect of NO on bone mass regulation and bone metabolism has
been well investigated and reviewed elsewhere; however, studies on
the involvement of NO in orthodontic tooth movement are limited
(7-9).
Tooth movement induced by orthodontic force is
achieved through bone remodeling, as a result of the sequential
transduction of molecular signals and changes in cellular behaviors
(10,11). It is of utmost significance to
determine the underlying mechanism of orthodontic tooth movement,
in order to reduce possible side-effects and shorten the duration
of therapy. NO is extensively involved in orthodontic-related
biological events, such as aseptic inflammation, mechanical signal
transduction and bone remodeling. Furthermore, the regulatory
effect of NO on bone remodeling has been demonstrated to be
cGMP-related (12,13). In the present review, the
regulatory effects of NO on the functional states of related cells
and tissues during orthodontic tooth movement, as well as the
possible mechanisms involved are discussed, with the aim of
providing helpful insight towards the application of effective
therapeutic interventions in orthodontics.
Orthodontic tooth movement relies upon periodontal
ligament (PDL) and alveolar bone remodeling. The PDL is a dense
connective tissue that plugs the tooth to the adjacent alveolar
bone (14,15). It contains collagen fiber bundle,
blood vessel, nerves, interstitial fluids and multiple cell types,
including fibroblasts, osteoclasts, osteoblasts and macrophages
(10,14). The alveolar bone consists of bone
cells (osteoclasts, osteoblasts and osteocytes) and the mineralized
matrix (14,16). The force applied to the tooth
triggers cell-signaling cascades in the PDL and the alveolar bone,
leading to tissue remodeling and tooth movement (11,17).
Orthodontic tooth movement can be organized into
three phases: i) The initial phase; ii) lag phase; and iii)
post-lag phase (18). In the
initial phase, tooth movement occurs due to the deformation of PDL
and tooth displacement within the alveolar socket 24 to 48 h after
the application of force to the teeth. The lag phase follows the
initial phase, during which little or no tooth movement is observed
due to PDL hyalinization in the compression region. This phase
lasts 20-30 days. Following the removal of necrotic tissue by
macrophages, tooth movement resumes in the post-lag phase (19,20). This phase usually occurs 40 days
after the initial application of force.
The pressure-tension theory describes orthodontic
tooth movement as an outcome of bone resorption in the compression
region and bone formation in the tension region (21). On the pressure side, the
reduction of blood flow and the distortion of nerve endings in PDL
may cause hypoxia and the release of vasoactive neurotransmitters,
including substance P, calcitonin gene-related peptide (CGRP), and
vasoactive intestinal polypeptide. As a result, vasodilatation and
the aggregation of circulating leukocytes, monocytes, macrophages,
lymphocytes and mast cells has been observed (22-26). Growth factors, chemokines and
other cytokines also contribute to these processes (23,27,28).
Osteoclasts are multinucleated cells, that initially
differentiate from multipotential hematopoietic precursors in the
monocyte/macrophage lineage, upon macrophage-colony stimulating
factor (M-CSF) and receptor activator of nuclear factor-κB ligand
(RANKL) stimulation, which are secreted primarily by cells of the
osteoblast lineage (29-35). M-CSF promotes the proliferation,
adhesion and migration of osteoclast precursor cells (36-38). RANKL promotes the fusion,
differentiation and bone resorptive function of osteoclasts through
the activation of RANK on the surface of osteoclast precursors
(33,39,40). OPG, a decoy receptor for RANKL,
suppresses osteoclastogenesis through the blockage of the
RANK/RANKL signaling pathway (41,42).
The aseptic inflammatory response caused by
orthodontic forces is indispensable for tooth movement (11,43). Interleukin (IL)-1β, IL-6, tumor
necrosis factor (TNF)-α and prostaglandin E2 (PGE2) can induce the
release of RANKL and MCS-F to stimulate osteoclast precursor
differentiation (41,44-47). In addition to the enhancement of
osteoclastogenic factor expression, TNF-α also activates osteoclast
precursors directly through it binding to TNF receptor (32,48,49). PGE2 enhances the bone-resorbing
activity of osteoclasts through the increase of intracellular
cyclic adenosine monophosphate (cAMP) levels or the partial
mediation of TNF-α (50). Mature
osteoclasts occupy small cavities termed Howship's lacunae, in
which hydrogen ions and proteolytic enzymes are released, including
cathepsin K and matrix metalloproteinases (MMPs), in order to
degrade the bone matrix (39,51,52). When the magnitude of the force
decreases, osteoclasts become inactive and detach from the bone
(53).
Bone deposition induced by osteoblasts presents is
the predominant event on the tension side (20,54). Derived from bone marrow
mesenchymal stem cells, osteoblasts secrete an organic matrix known
as the osteoid, which is then incorporated further into the mature
bone (55). During bone
formation, some osteoblasts transform into bone lining cells on the
bone surface, or osteocytes embedded in the bone matrix. Osteocytes
are connected and communicate through cytoplasmic processes in tiny
canals, called canaliculi (56,57).
Transcription factor runt-related transcription
factor 2 (Runx2), also known as ore-binding factor subunit alpha-1
(Cbfa1) and the Wnt/β-catenin pathway provide the initial and
essential stimulus for osteoblast differentiation (34,58). Bone morphogenetic protein (BMP),
as a member of the transforming growth factor β (TGF-β)
superfamily, induces the differentiation of osteoprogenitor cells
and promotes osteoblast function through the stimulation of Runx2
expression via the small mother against decapentaplegic or p38
mitogen-activated protein kinase (MAPK) pathways (59-62). In addition, TGF-β also suppresses
bone resorption activity through the upregulation of the tissue
inhibitor of metalloproteinases expression (43,63). IL-10 induces an overall reduction
in RANK signaling, through the facilitation of OPG expression and
the reduction of RANKL production (43,64,65).
Regional hypoxia caused by orthodontic force induces
hypoxia-inducible factor (HIF)-1 expression and upregulates the
transcription of vascular endothelial growth factor (VEGF) in PDL
fibroblasts and osteoblasts. VEGF is associated with osteogenic
differentiation and matrix mineralization under the regulation of
BMP, corroborating the concept that angiogenesis and osteogenesis
are combined (66,67). Furthermore, HIF-1 and VEGF also
stimulate osteoclast differentiation via the upregulation of RANKL,
contributing to the combination of bone resorption and bone
formation (68-70).
Some molecules that regulate the response of PDL
fibroblasts to the orthodontic forces have been identified in
previous studies, such as CC chemokine receptor 5 (CCR5) and CCR5
ligands axis (71), relaxin
(Rln) and Rln family peptides (Rxfps) axis (72), and secretory leucocyte peptidase
inhibitor (73). The expression
levels of these molecules were upregulated in the PDL, due to
compression and tension force; however, their downstream effects
were different. Another consequence was the upregulation of the
osteoclastogenesis-relating factors, including RANKL, MCSF and
MMPs, on the compression side, and osteoclast activity inhibiting
factors, including Runx2, IL-6, and IL-12, that may induce
osteoblast differentiation on the tension side.
Osteocytes are critical for the transduction of
mechanical stimuli into biochemical signals (74-76). When a force is exerted on the
tooth, the squeeze of the interstitial fluid causes fluid shear
stress (FSS) in the extracellular matrix (ECM) (77). The fluid flow hypothesis
describes the response of osteocytes to FSS as an essential
mechanism during orthodontic treatment. FSS stimulates an increase
in the intracellular calcium concentration and the release of
intercellular molecules in osteocytes through the activation of
integrin, a transmembrane protein that connects ECM macromolecules
to the internal cytoskeleton (78-80). The FSS-related up-regulation of
NO, PGE2, TFG-β, and insulin-like growth factor alters the
osteocyte metabolic state and osteoblast/osteoclast functions
(81,82). Gap junctions formed by connexin
(Cx) also participate in the osteocyte-osteoblast communication
(83,84). For example, Cx is involved in the
release of PGE2, which enhances Runx2 DNA binding activity through
the simultaneous activation of the cAMP/cAMP-dependent protein
kinase and MAPK pathways and the subsequent stimulation of RANKL
expression in osteoblasts (85-88).
The inhibitory effect of osteocytes on osteoblastic
activity can be induced by the secretion of sclerostin, which
antagonizes BMP effect and blocks canonical Wnt signaling (89-91). Osteocytes regulate osteoclastic
differentiation via the alternation of major osteoclast regulators,
namely RANKL and M-CSF (92-94). Moreover, osteocyte apoptosis
induction is an important event in the recruitment and
differentiation of osteoclasts (95-97). These findings confirm that
osteocytes play a key role in the response to biomechanical stimuli
and controlling bone remodeling by coordinating the activity of
osteoblasts and osteoclasts.
Fibroblasts are involved in mechanosensation and
mechanotransduction in connective tissues. The application of
mechanical stretching activates integrin and causes conformational
changes in focal adhesion kinase (FAK), inducing a signaling
cascade that modulates cytoskeletal dynamics and gene transcription
in fibroblasts (98,99).
Three NOS isoforms in total are expressed in
osteoblasts, osteoclasts and osteocytes (100-102). iNOS and eNOS are expressed in
human PDL stem cells (103,104). Previous studies revealed the
presence of sGC and cGMP in mouse bone marrow macrophages (105), osteoclasts (105-107), and osteocytes (108). Davidovitch et al
(109,110) performed immunohistochemistry
(IHC) on alveolar bone sections obtained from cats and revealed
that cGMP expression was increased in the PDL fibroblast cells
stained intensely for; however, most cGMP expression was not
detected through IHC staining in osteoblasts. However, cGMP
expression increased due to the subjection of the alveolar bone to
mechanical force (111,112). The application of electric
currents to the bone, also led to the upregulation of cGMP in
osteoblast and PDL fibroblast cells, accompanied by bone deposition
near the cathode (113-115). Since a piezoelectric current
can be generated by mechanical stress, the above findings suggest
that NO/cGMP is an important signaling pathway, which mediates bone
cell response to mechanical force (116).
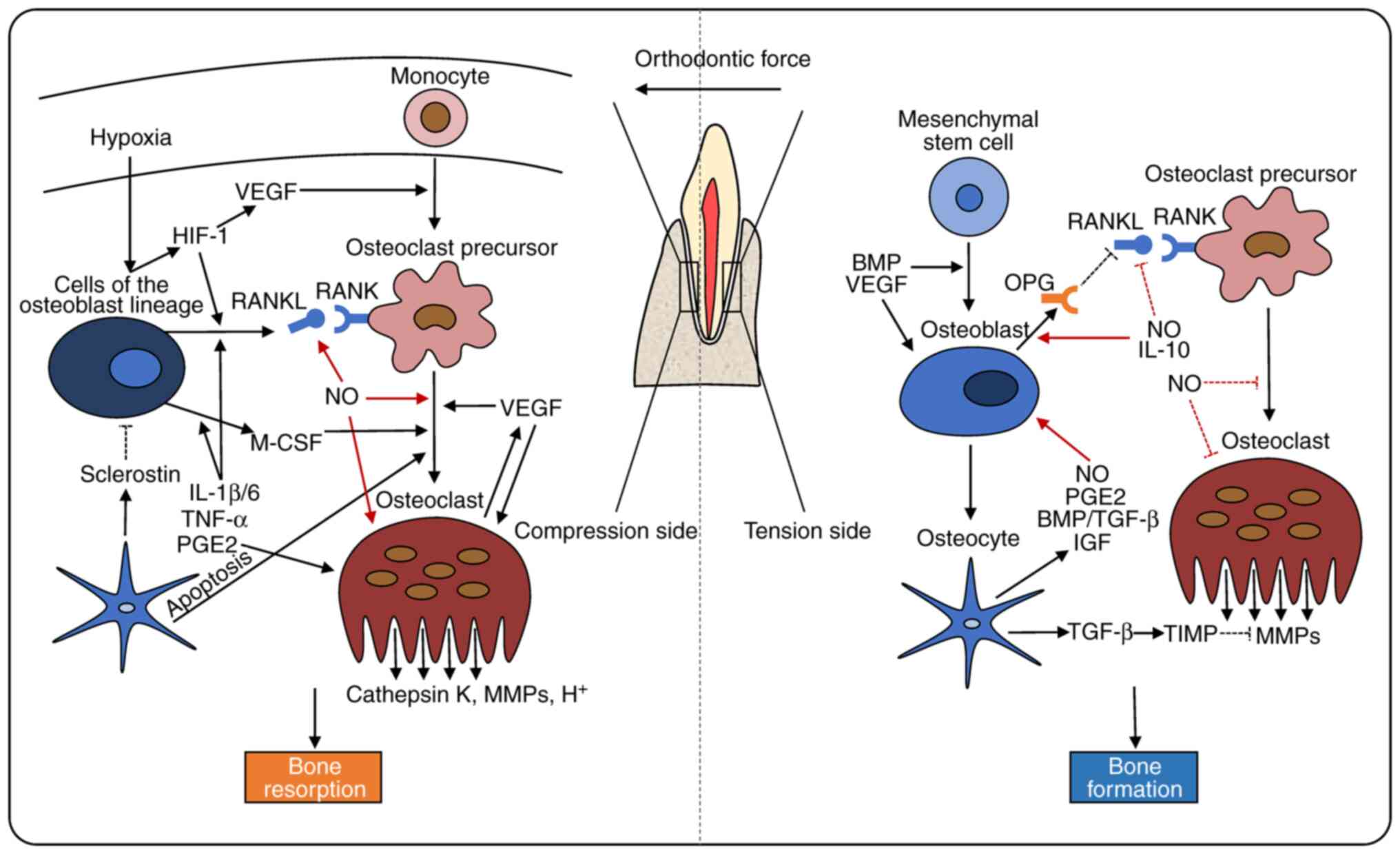
Mounting evidence indicates that NO regulates
multiple cellular behaviors related to orthodontic movement
(Fig. 1 and Table I).
A number of studies have demonstrated that NO exerts
biphasic effects on osteoclast formation and function. In several
cases, NO promotes osteoclastogenesis and bone resorption. NO
mediates pre-osteoclasts fusion through the upregulation of actin
cytoskeleton remodeling (117).
Histopathological studies have demonstrated that osteoclasts,
Howship's lacunae and new capillaries were increased in rats that
received an injection of the NO precursor L-arg during tooth
movement (118-120).
iNOS is an important regulator of osteoclast
differentiation under bacterial infection-induced inflammatory
conditions (121-123). iNOS was previously found to
mediate alveolar bone loss and periapical infectious bone
resorption following the oral administration of Porphyromonas
gingi-valis (124) or
lipopolysaccharide (122). In
another study, histochemical analysis revealed that the osteoclast
number in iNOS(-/-) mice in comparison to wild-type mice was
considerably decreased (123).
Tooth eruptions are similar to tooth movement in terms of monocyte
recruitment and osteoclast differentiation. Evidence indicates that
increased levels of iNOS are associated with a greater number of
osteoclasts in mice with accelerated tooth eruption, indicating
that iNOS may be a bone resorption modulator candidate (125).
As previously demonstrated, M1-like macrophage
polarization and an enhanced M1/M2 macrophage ratio increase the
number of osteoclasts in rats or mice, accompanied by an increase
in M1 macrophage marker expression (TNF-α and iNOS) on the
compression side, during tooth movement (126,127). TNF-α stimulates the survival of
differentiated osteoclasts through the induction of iNOS-dependent
NO generation (128). In the
rheumatism inflammatory environment, the TNF-α promoting effect on
alveolar bone resorption is partly mediated through the activation
of iNOS and the resulting production of NO (129).
It has been observed that the promoting effect of
iNOS on osteoclasts is mediated through the NO/cGMP pathway. Kaneko
et al (105) revealed
that 8-nitro-cGMP, a NO-dependent derivative of cGMP in mammals,
increased RANKL mRNA expression, and enhanced osteoclast
differentiation. The reduction in cGMP levels due to the inhibition
of NOS caused RANKL-induced osteoclast differentiation
suppression.
By contrast, evidence has revealed an inhibitory
effect of NO on osteoclasts at low concentrations. NO has been
reported to increase osteoclast and osteoclast precursor cell
apoptosis (101,130-132). A novel NO donor,
nitrosyl-cobinamide (NO-Cbi), has been found to reduce the
RANKL/OPG gene expression ratio or directly inhibit osteoclast
differentiation in vitro and in vivo (133). Nicorandil, an agent that can
increase NO production in osteoclasts, was previously shown to
suppress osteoclast differentiation via activating sGC (134). NO causes osteoclast detachment
and downregulates osteoclast bone-resorbing activity via the
NO/cGMP/PKG pathway in vitro (101,107,135-137). Of note, the selective
inhibition of iNOS was previously found to markedly promote bone
resorption in vivo. In an iNOS(−/−) mouse model of apical
periodontitis, enhanced osteoclast differentiation and increased
bone resorption were observed in comparison with the control group,
accompanied by increased IL-1β, TNF-α, RANK, RANKL and monocyte
chemoattractant protein-1 (MCP-1) levels (138,139). These results suggest that NO
deficiency is associated with an imbalance in the host inflammatory
response, resulting in severe bone loss.
Moreover, iNOS exerts an inhibitory effect on
osteoclast differentiation through other pathways. Zheng et
al (140) demonstrated that
iNOS was a RANKL-induced autocrine negative feedback inhibitor of
RANKL-mediated osteoclastogenesis. RANKL triggered iNOS expression
and NO release, and subsequently inhibited RANKL-induced osteoclast
formation in a cGMP-independent manner.
The inconsistent effects of NO on osteoclastogenesis
may be attributed to the differences in NO synthesis quantity, cell
types and development states. NO action is also affected by the
cytokines in the microenvironment. Multiple factors influence the
downstream signaling of NO, and further studies are required to
elucidate the specific mechanism of NO regulation.
NO is also involved in the bidirectional regulation
of osteoblasts. Decreased NO concentrations promote osteoblast
proliferation, differentiation and survival (133,141-143). Mineralized nodule formations
and mRNA expression levels of osteoblastic genes, such alkaline
phosphatase, osteocalcin and collagen-1 genes, have been shown to
be enhanced by NO donors and 8-Br-cGMP, an analog of cGMP (141-143). This effect was blocked by
1H-(1,2,4)oxadiazolo-(4,3-a)quinoxalin-1-one
(ODQ), a competitive blocker that prevents sGC activation and
lowers cGMP/PKG activity. It has been recently stated that PDE5
inhibitors, which can significantly increase intracellular cGMP
levels, induce osteoblast differentiation and enhance bone
regeneration in osteopenic mice via the cGMP/VEGF pathway (144). These findings further support
the involvement of NO/cGMP/PKG pathway in the regulation of
osteoblast activity (133,143,145).
Increased iNOS expression and NO levels have been
observed during osteoblast differentiation in vitro. iNOS
has been reported to mediate the regulation of Runx2 translocation
and downstream events (146).
In eNOS knockout mice, osteoblast growth has been shown to be
inhibited (147). Evidence
suggests that eNOS activation promotes cell survival and enhances
osteoblastic gene expression in osteoblasts via pathway cascades
involving Src/extracellular signal-regulated kinase (ERK),
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and
Wnt/β-catenin (148,149).
NO mediates the action of several local and systemic
factors, including mechanical stimulation, hormones and other
signaling molecules in osteoblasts (13,150). It has also been revealed that
1,25-dihydroxyvitamin D(3)
regulates bone mass via the upregulation of iNOS expression and NO
production (151). CGRP has
been found to promote mandibular bone fracture healing in
vivo and stimulate the eNOS activity through the increase of
intracellular calcium concentrations in osteoblasts in vitro
(152,153). Furthermore, it has been
observed that 17 β-estradiol, a major endogenous estrogen, may
promote eNOS expression and osteoblast differentiation through Akt
phosphorylation in a dose-dependent manner (154). It has been previously
demonstrated that the bone-protective effects of estrogen rely upon
the NO/cGMP pathway (147,150). High concentrations of NO
negatively impact osteoblast proliferation and survival (145). NO simultaneously induces cell
death and autophagy in osteoblasts (155).
The effect of NO on osteocytes is similar to that of
osteoblasts. Parathyroid hormone and 17β-estradiol levels increase
the expression of cGMP expression in osteocytes (108). Cinaciguat, an activator of sGC
that has been declared as a potential drug target for osteoporosis,
was previously found to reverse osteocyte apoptosis and enhance
bone formation in mice subjected to ovariectomy (156). NO/cGMP/PKG signaling mediated
17β-estradiol anti-apoptotic effect on osteocytes through either
the activation of the pro-survival kinases, ERK and Akt, mediated
by type II PKG, or direct phosphorylation of protein related to
cell death by type I (PKG) (157,158). However, inflammation-induced
iNOS activation and elevated concentrations of NO can lead to
osteocyte apoptosis (132).
NO/cGMP/PKG signaling has been shown to regulate
human PDL fibroblast proliferation and differentiation, with the
involvement of MAPK and nuclear factor κ-light-chain-enhancer of
activated B cells pathways (103,159-161). However, the effect of NO on
cell proliferation in PDL has not yet been fully clarified. A
previous study revealed that NO did not influence PDL stem cell
proliferation (103). In other
studies, it has been revealed that exogenous NO inhibits
proliferation and induces apoptosis of PDL fibroblasts (159,162). This discrepancy could be
attributed to differences in the cellular differentiation levels
and varying applied agent concentrations.
The PDL and the alveolar bone are developed from the
dental follicle during tooth development. The literature was
reviewed and it was observed that studies of NO regulation impact
upon the dental follicle during tooth development has not been
reported yet, to the best of our knowledge. It was surmised that
the exploration of the underlying mechanism of NO on the
development of the PDL and alveolar bone may provide novel insights
into the role of NO in the tissue remodeling observed during
orthodontic tooth movement.
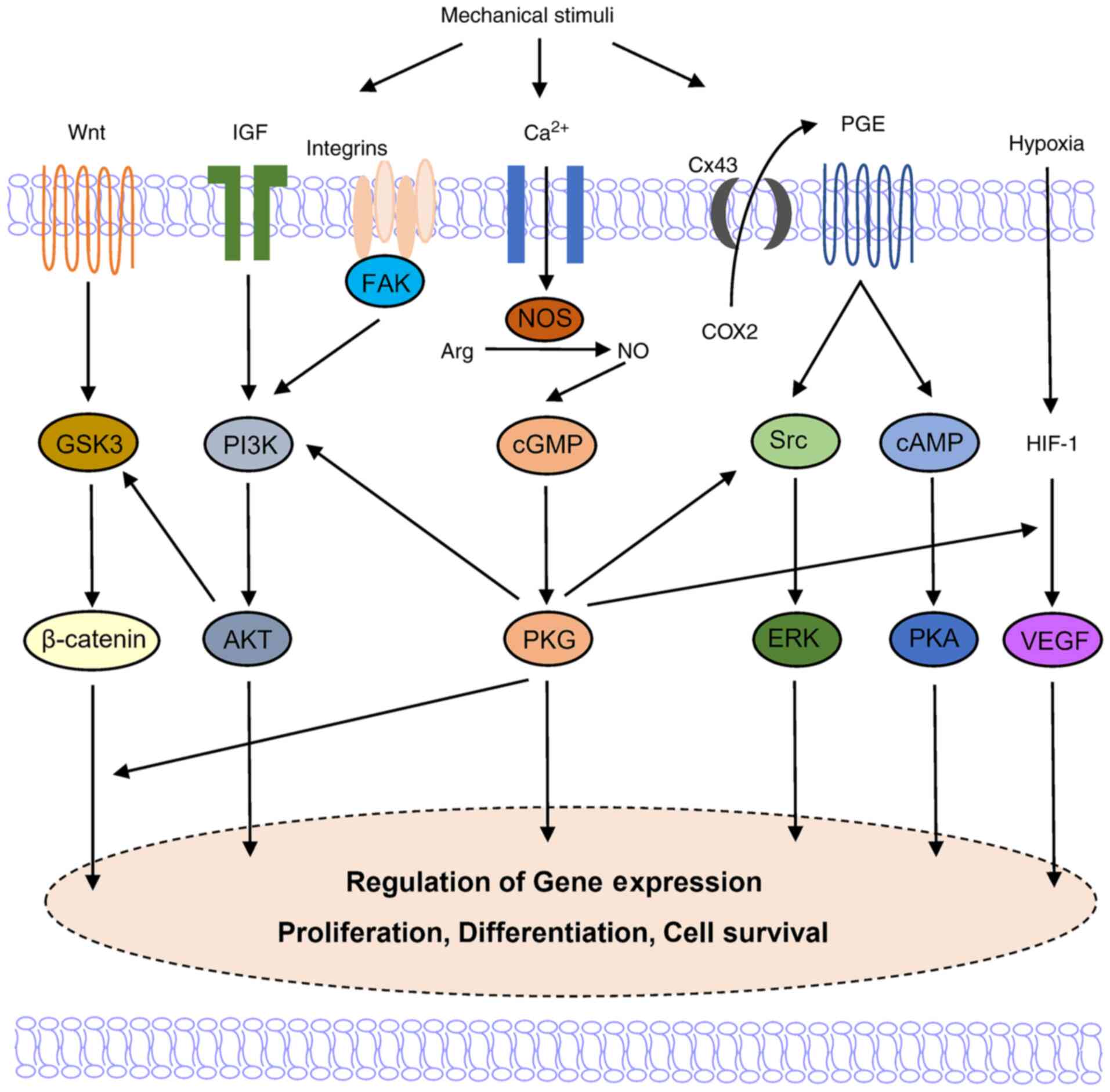
The mechanical loading-induced activation of the
Wnt/β-catenin pathway is an important signaling event in
osteoblasts, osteocytes, and PDL fibroblasts, and is mediated by a
NO-dependent mechanism involving the FAK, Src/ERK and PI3K/Akt
signaling pathways (169,173,174). PFF increases NO synthesis in
osteoblasts, resulting in PKG II-dependent activation of Src and
PI3K-dependent phosphorylation of Akt. The nuclear translocation of
β-catenin is induced and the gene expression of c-fos is
upregulated, initiating a proliferative response in mechanically
stimulated osteoblasts (175-177). When the osteoblast and
osteocyte cytoskeleton system of disrupted, PFF-induced NO
production is affected (178).
PFF induces the release of multiple soluble factors
that promote osteogenesis and inhibits bone resorption. This
process is partially dependent on the generation of NO (74,179-181). PFF-induced NO inhibited
osteocyte apoptosis through the downregulation of B-cell lymphoma-2
(Bcl-2) and caspase-3 (182).
NO also modulates mechanically induced VEGF expression,
contributing to angiogenesis during bone remodeling (183,184).
The main NOS isoform that produces NO in osteoblasts
and osteocytes under the mechanical force action has not yet been
elucidated. The activation of eNOS is associated with the
phosphorylation or dephosphorylation at several functional sites on
eNOS, which may be induced by FSS, estrogens, VEGF and insulin
(185-187). Several studies have revealed
that FSS-induced NO production is attributed to the
calcium-dependent eNOS activation in bone cells (13,185,188,189). It has been revealed that the
occlusal force led to iNOS and eNOS increased expression in
hypofunctional and normal PDL fibroblasts (100,104).
Additionally, it has been suggested that eNOS may be
not indispensable for mechanically-induced NO synthesis in cultured
osteoblasts or eNOS (−/−) mice (190,191). It has also been mentioned that
ultrasound-induced bone formation may be mediated through nNOS and
iNOS upregulation in osteoblasts (82,163,192,193). Furthermore, osteopontin has
been shown to suppress the osteoblast response to ultrasound by
inhibiting the expression of nNOS and iNOS through FAK
downregulation (194). This
inconsistency may be explained in view of the possibility of an
alternative way of NO production induction by other NOS isoforms
and through a non-enzymatic NO production manner (including
reduction of nitrite and denitrosylation of some proteins), in case
a specific NOS isoform is absent (195-197). The aforementioned ultrasound
results can only prove the role of nNOS or iNOS in
ultrasound-induced promotion on osteoblasts; however, those results
do not contradict the involvement of eNOS.
These findings suggest that NO plays a complex role
in mechanotransduction under stress in the periodontal tissue, and
further research on this topic is required.
Many studies have focused on the differential
expression of NOS isoforms between areas of compression and tension
during orthodontic tooth movement. Experiments in rats revealed
that the changes in NOS activity in the PDL could be detected as
soon as 1 h after teeth were subjected to orthodontic force
(198). The increased
expression of iNOS on the pressure side and eNOS on the tension
side was observed 24 h after initiating mechanical loading, while
increased nNOS expression mainly occurred after 3 h (199). An increase of iNOS-positive
osteocytes in the compression area was detected 6 h after force
application, while eNOS-positive osteocytes in the tension area
increased after 24 h (200). As
indicated above, it is generally accepted that iNOS dominates bone
resorption at the compression site while eNOS mediates the
osteogenic effect in the tension area (200,201).
The availability of studies related to the changes
in NO levels in human periodontal tissues before and after
orthodontic treatment is limited. Analysis of gingival tissue
collected from orthodontic patients revealed that eNOS and iNOS
levels increased dramatically 2 weeks after the appliance placement
(202). A variety of biomarkers
in gingival crevicular fluid (GCF) are often analyzed, in order to
facilitate the improvement of clinical treatment. In various
studies, many of which recent, it has been mentioned that NO
expression levels in GCF is related to orthodontic treatment
(203-206). Ford et al (203) revealed that NO concentration in
GCF on the compression side of the central incisor increased
significantly 1 h after the application of fixed orthodontic
appliances. In patients who received rapid maxillary expansion
therapy, the NO levels in GCF were elevated on day 1 and 10 and
were still elevated after 3 months of retention (204,205). However, no significant
difference was detected in NO levels in GCF on the tension side,
during the above treatment. These results further support the
different regulatory effects of NO on the tension and pressure
side, which are related to the presence of different NOS isoforms
on different sides.
The role of NO in orthodontic treatment has also
been confirmed in animal experiments. Tooth movement was markedly
promoted in rats that received L-arg injection, whereas a
significant reduction of tooth movement was observed in the L-NAME
(eNOS inhibitor) group. Histological results also revealed a
greater number of osteoclasts in the group with greater tooth
movement (119,120,207). Notably, decreased force-induced
root resorption was noted in this group in comparison with the
control group, although the number of osteoclasts increased in the
L-arg injection group (119).
Influences of NO and oral microbiota on the
orthodontic tooth movement are also notable. In addition to being
synthesized by the body, NO can be produced by oral bacteria under
hypoxic conditions through the transformation of saliva nitrate
into nitrite (208-210). It has been observed that NO
production is upregulated during the deposition of dental plaque
(211). In diseases related to
plaque accumulation, including periodontitis, an increase in NO
levels in both blood and saliva was reported (212-214). Additionally, apart from the
oral bacteria-originating NO production, this has also been
ascribed to the inflammatory response of the body. It has been
previously demonstrated that an enhanced osteoclast formation and
accelerated orthodontic tooth movement may be observed in patients
with periodontitis (215). It
is reasonable to speculate that NO may be involved in this process,
but more direct evidence is necessary in order to confirm this
(203-207).
NO is widely involved in the biomechanical response
of the periodontium to orthodontic forces. NO exerts dose-dependent
and biphasic effects on the functional status and cell fate
determination of osteoblasts, osteoclasts, osteocytes, and PDL
fibroblasts, and has been shown to promote the proliferation,
differentiation, or inhibition of survival and function of cells.
As an inflammatory factor and a key second messenger in mechanical
transduction, NO is differentially expressed on the tension and
compression side during tooth movement, suggesting its complex
involvement in bone remodeling. The facilitation of NO precursor
and the inhibition of NOS inhibitor in orthodontic tooth movement
have also been confirmed in animal experiments. Additional studies
are required, in order to evaluate the role and impact of NO on
tooth movement in clinical practice. As NO exerts complex effects
on both osteoblastic and osteoclastic activities, the
spatiotemporal generation of NO may determine its specific
biological effect on bone remodeling. The precise and controlled
delivery of NO to periodontal tissue via NO-releasing polymeric
nanomaterials may be a promising approach for the acceleration of
orthodontic tooth movement.
Not applicable.
TY, YX and FH conceived the review. TY performed
literature search and manuscript writing. YX contributed to the
manuscript writing and the preparation of figures and tables. HH,
WF and FH revised the manuscript. TY and FH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
|
1
|
Loscalzo J: Nitric oxide and vascular
disease. N Engl J Med. 333:251–253. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837.
837a–837d. 2012. View Article : Google Scholar :
|
|
3
|
Snyder SH: Nitric oxide. No endothelial
NO. Nature. 377:196–197. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moncada S and Higgs A: The
L-arginine-nitric oxide pathway. N Engl J Med. 329:2002–2012. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sawa T, Ihara H, Ida T, Fujii S, Nishida M
and Akaike T: Formation, signaling functions, and metabolisms of
nitrated cyclic nucleotide. Nitric Oxide. 34:10–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francis SH, Busch JL, Corbin JD and Sibley
D: cGMP-dependent protein kinases and cGMP phosphodiesterases in
nitric oxide and cGMP action. Pharmacol Rev. 62:525–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van't Hof RJ and Ralston SH: Nitric oxide
and bone. Immunology. 103:255–261. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wimalawansa SJ: Nitric oxide and bone. Ann
NY Acad Sci. 1192:391–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Evans DM and Ralston SH: Nitric oxide and
bone. J Bone Miner Res. 11:300–305. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Jacox LA, Little SH and Ko CC:
Orthodontic tooth movement: The biology and clinical implications.
Kaohsiung J Med Sci. 34:207–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krishnan V and Davidovitch Z: On a path to
unfolding the biological mechanisms of orthodontic tooth movement.
J Dent Res. 88:597–608. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalyanaraman H, Schall N and Pilz RB:
Nitric oxide and cyclic GMP functions in bone. Nitric Oxide.
76:62–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klein-Nulend J, van Oers RF, Bakker AD and
Bacabac RG: Nitric oxide signaling in mechanical adaptation of
bone. Osteoporos Int. 25:1427–1437. 2014.
|
|
14
|
Nanci A and Bosshardt DD: Structure of
periodontal tissues in health and disease. Periodontol. 40:11–28.
2006. View Article : Google Scholar
|
|
15
|
Hassell TM: Tissues and cells of the
periodontium. Periodontol. 3:9–38. 1993. View Article : Google Scholar
|
|
16
|
Bartold PM and McCulloch CA: Information
generation and processing systems that regulate periodontal
structure and function. Periodontol. 63:7–13. 2013. View Article : Google Scholar
|
|
17
|
Antoun JS, Mei L, Gibbs K and Farella M:
Effect of orthodontic treatment on the periodontal tissues.
Periodontol. 74:140–157. 2017. View Article : Google Scholar
|
|
18
|
Burstone C: The biomechanics of tooth
movement. Kraus BS and Riedel BA: Vistas in orthodontics. Lea,
Febiger; Philadelphia: pp. 197–213. 1962
|
|
19
|
Dhenain T, Côté F and Coman T: Serotonin
and orthodontic tooth movement. Biochimie. 161:73–79. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asiry MA: Biological aspects of
orthodontic tooth movement: A review of literature. Saudi J Biol
Sci. 25:1027–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin Schwarz A: Tissue changes incident
to orthodontic tooth movement. Int J Orthodontia Oral Surg
Radiography. 18:331–352. 1932. View Article : Google Scholar
|
|
22
|
Norevall LI, Forsgren S and Matsson L:
Expression of neuropeptides (CGRP, substance P) during and after
orthodontic tooth movement in the rat. Eur J Orthod. 17:311–325.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Middleton J, Patterson AM, Gardner L,
Schmutz C and Ashton BA: Leukocyte extravasation: Chemokine
transport and presentation by the endothelium. Blood.
100:3853–3860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SK, Pi SH, Kim SH, Min KS, Lee HJ,
Chang HS, Kang KH, Kim HR, Shin HI, Lee SK and Kim EC: Substance P
regulates macrophage inflammatory protein 3alpha/chemokine C-C
ligand 20 (CCL20) with heme oxygenase-1 in human periodontal
ligament cells. Clin Exp Immunol. 150:567–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi M, Kojima T, Kanekawa M, Aihara
N, Nogimura A and Kasai K: Neuropeptides stimulate production of
interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha
in human dental pulp cells. Inflamm Res. 53:199–204. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kvinnsland I and Kvinnsland S: Changes in
CGRP-immunoreactive nerve fibres during experimental tooth movement
in rats. Eur J Orthod. 12:320–329. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ren Y, Hazemeijer H, de Haan B, Qu N and
de Vos P: Cytokine profiles in crevicular fluid during orthodontic
tooth movement of short and long durations. J Periodontol.
78:453–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapoor P, Kharbanda OP, Monga N, Miglani R
and Kapila S: Effect of orthodontic forces on cytokine and receptor
levels in gingival crevicular fluid: A systematic review. Prog
Orthod. 15:652014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie R, Kuijpers-Jagtman AM and Maltha JC:
Osteoclast differentiation during experimental tooth movement by a
short-term force application: An immunohistochemical study in rats.
Acta Odontol Scand. 66:314–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
32
|
Azuma Y, Kaji K, Katogi R, Takeshita S and
Kudo A: Tumor necrosis factor-alpha induces differentiation of and
bone resorption by osteoclasts. J Biol Chem. 275:4858–4864. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Udagawa N, Takahashi N, Jimi E, Matsuzaki
K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, et
al: Osteoblasts/stromal cells stimulate osteoclast activation
through expression of osteoclast differentiation factor/RANKL but
not macrophage colony-stimulating factor: Receptor activator of
NF-kappa B ligand. Bone. 25:517–523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katagiri T and Takahashi N: Regulatory
mechanisms of osteoblast and osteoclast differentiation. Oral Dis.
8:147–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thirunavukkarasu K, Halladay DL, Miles RR,
Yang X, Galvin RJ, Chandrasekhar S, Martin TJ and Onyia JE: The
osteoblast-specific transcription factor Cbfa1 contributes to the
expression of osteoprotegerin, a potent inhibitor of osteoclast
differentiation and function. J Biol Chem. 275:25163–25172. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suda T, Takahashi N and Martin TJ:
Modulation of osteoclast differentiation. Endocr Rev. 13:66–80.
1992.PubMed/NCBI
|
|
37
|
Takahashi N, Udagawa N, Akatsu T, Tanaka
H, Shionome M and Suda T: Role of colony-stimulating factors in
osteoclast development. J Bone Miner Res. 6:977–985. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liggett W Jr, Shevde N, Anklesaria P,
Sohoni S, Greenberger J and Glowacki J: Effects of macrophage
colony stimulating factor and granulocyte-macrophage colony
stimulating factor on osteoclastic differentiation of hematopoietic
progenitor cells. Stem Cells. 11:398–411. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tanaka S, Nakamura K, Takahasi N and Suda
T: Role of RANKL in physiological and pathological bone resorption
and therapeutics targeting the RANKL-RANK signaling system. Immunol
Rev. 208:30–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aubin JE and Bonnelye E: Osteoprotegerin
and its ligand: A new paradigm for regulation of osteoclastogenesis
and bone resorption. Medscape Womens Health. 11:905–913. 2000.
|
|
42
|
Udagawa N, Takahashi N, Yasuda H, Mizuno
A, Itoh K, Ueno Y, Shinki T, Gillespie MT, Martin TJ, Higashio K
and Suda T: Osteoprotegerin produced by osteoblasts is an important
regulator in osteoclast development and function. Endocrinology.
141:3478–3484. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garlet TP, Coelho U, Silva JS and Garlet
GP: Cytokine expression pattern in compression and tension sides of
the periodontal ligament during orthodontic tooth movement in
humans. Eur J Oral Sci. 115:355–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu XH, Kirschenbaum A, Yao S and Levine
AC: Cross-talk between the interleukin-6 and prostaglandin E(2)
signaling systems results in enhancement of osteoclastogenesis
through effects on the osteoprotegerin/receptor activator of
nuclear factor-{kappa B} (RANK) ligand/RANK system. Endocrinology.
146:1991–1998. 2005. View Article : Google Scholar
|
|
45
|
Zhang YH, Heulsmann A, Tondravi MM,
Mukherjee A and Abu-Amer Y: Tumor necrosis factor-alpha (TNF)
stimulates RANKL-induced osteoclastogenesis via coupling of TNF
type 1 receptor and RANK signaling pathways. J Biol Chem.
276:563–568. 2001. View Article : Google Scholar
|
|
46
|
Tani-Ishii N, Tsunoda A, Teranaka T and
Umemoto T: Autocrine regulation of osteoclast formation and bone
resorption by IL-1 alpha and TNF alpha. J Dent Res. 78:1617–1623.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miyaura C, Inada M, Matsumoto C, Ohshiba
T, Uozumi N, Shimizu T and Ito A: An essential role of cytosolic
phospholipase A2alpha in prostaglandin E2-mediated bone resorption
associated with inflammation. J Exp Med. 197:1303–1310. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kitaura H, Yoshimatsu M, Fujimura Y,
Eguchi T, Kohara H, Yamaguchi A and Yoshida N: An anti-c-Fms
antibody inhibits orthodontic tooth movement. J Dent Res.
87:396–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abu-Amer Y, Erdmann J, Alexopoulou L,
Kollias G, Ross FP and Teitelbaum SL: Tumor necrosis factor
receptors types 1 and 2 differentially regulate osteoclastogenesis.
J Biol Chem. 275:27307–27310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamasaki K: The role of cyclic AMP,
calcium, and prostaglandins in the induction of osteoclastic bone
resorption associated with experimental tooth movement. J Dent Res.
62:877–881. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Domon S, Shimokawa H, Matsumoto Y,
Yamaguchi S and Soma K: In situ hybridization for matrix
metalloproteinase-1 and cathepsin K in rat root-resorbing tissue
induced by tooth movement. Arch Oral Biol. 44:907–915. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Apajalahti S, Sorsa T, Railavo S and
Ingman T: The in vivo levels of matrix metalloproteinase-1 and -8
in gingival crevicular fluid during initial orthodontic tooth
movement. J Dent Res. 82:1018–1022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bonafe-Oliveira L, Faltin RM and
Arana-Chavez VE: Ultrastructural and histochemical examination of
alveolar bone at the pressure areas of rat molars submitted to
continuous orthodontic force. Eur J Oral Sci. 111:410–416. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Krishnan V and Davidovitch Z: Cellular,
molecular, and tissue-level reactions to orthodontic force. Am J
Orthod Dentofacial Orthop. 129:469.e1–432. 2006. View Article : Google Scholar
|
|
55
|
Ducy P, Schinke T and Karsenty G: The
osteoblast: A sophisticated fibroblast under central surveillance.
Science. 289:1501–1504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Franz-Odendaal TA, Hall BK and Witten PE:
Buried alive: How osteoblasts become osteocytes. Dev Dyn.
235:176–190. 2006. View Article : Google Scholar
|
|
57
|
Capulli M, Paone R and Rucci N: Osteoblast
and osteocyte: Games without frontiers. Arch Biochem Biophys.
561:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kundu M, Javed A, Jeon JP, Horner A, Shum
L, Eckhaus M, Muenke M, Lian JB, Yang Y, Nuckolls GH, et al:
Cbfbeta interacts with Runx2 and has a critical role in bone
development. Nat Genet. 32:639–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Canalis E, Economides AN and Gazzerro E:
Bone morphogenetic proteins, their antagonists, and the skeleton.
Endocr Rev. 24:218–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar :
|
|
61
|
Wu M, Chen G and Li YP: TGF-β and BMP
signaling in osteoblast, skeletal development, and bone formation,
homeostasis and disease. Bone Res. 4:160092016. View Article : Google Scholar
|
|
62
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Leivonen SK, Lazaridis K, Decock J,
Chantry A, Edwards DR and Kähäri VM: TGF-β-elicited induction of
tissue inhibitor of metalloproteinases (TIMP)-3 expression in
fibroblasts involves complex interplay between Smad3, p38α, and
ERK1/2. PLoS One. 8:e574742013. View Article : Google Scholar
|
|
64
|
Park-Min KH, Ji JD, Antoniv T, Reid AC,
Silver RB, Humphrey MB, Nakamura M and Ivashkiv LB: IL-10
suppresses calcium-mediated costimulation of receptor activator
NF-kappa B signaling during human osteoclast differentiation by
inhibiting TREM-2 expression. J Immuno. 183:2444–2455. 2009.
View Article : Google Scholar
|
|
65
|
Zhang L, Ding Y, Rao GZ and Miao D:
Effects of IL-10 and glucose on expression of OPG and RANKL in
human periodontal ligament fibroblasts. Braz J Med Biol Res.
49:e43242016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kusumbe AP, Ramasamy SK and Adams RH:
Coupling of angiogenesis and osteogenesis by a specific vessel
subtype in bone. Nature. 507:323–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sivaraj KK and Adams RH: Blood vessel
formation and function in bone. Development. 143:2706–2715. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Park HJ, Baek KH, Lee HL, Kwon A, Hwang
HR, Qadir AS, Woo KM, Ryoo HM and Baek JH: Hypoxia inducible
factor-1α directly induces the expression of receptor activator of
nuclear factor-κB ligand in periodontal ligament fibroblasts. Mol
Cells. 31:573–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dandajena TC, Ihnat MA, Disch B, Thorpe J
and Currier GF: Hypoxia triggers a HIF-mediated differentiation of
peripheral blood mononuclear cells into osteoclasts. Orthod
Craniofac Res. 15:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Knowles HJ and Athanasou NA:
Hypoxia-inducible factor is expressed in giant cell tumour of bone
and mediates paracrine effects of hypoxia on monocyte-osteoclast
differentiation via induction of VEGF. J Pathol. 215:56–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee SY, Yoo HI and Kim SH: CCR5-CCL Axis
in PDL during orthodontic biophysical force application. J Dent
Res. 94:1715–1723. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang SY, Kim JW, Lee SY, Kang JH,
Ulziisaikhan U, Yoo HI, Moon YH, Moon JS, Ko HM, Kim MS and Kim SH:
Upregulation of relaxin receptors in the PDL by biophysical force.
Clin Oral Investig. 19:657–665. 2015. View Article : Google Scholar
|
|
73
|
Lee SY, Moon JS, Yang DW, Yoo HI, Jung JY,
Kim OS, Kim MS, Koh JT, Chung HJ and Kim SH: SLPI in periodontal
Ligament is not sleepy during biophysical force-induced tooth
movement. J Clin Periodontol. 48:528–540. 2021. View Article : Google Scholar
|
|
74
|
Dallas SL, Prideaux M and Bonewald LF: The
osteocyte: An endocrine cell and more. Endocr Rev. 34:658–690.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Tresguerres FGF, Torres J, López-Quiles J,
Hernández G, Vega JA and Tresguerres IF: The osteocyte: A
multifunctional cell within the bone. Ann Anat. 227:1514222020.
View Article : Google Scholar
|
|
76
|
Tatsumi S, Ishii K, Amizuka N, Li M,
Kobayashi T, Kohno K, Ito M, Takeshita S and Ikeda K: Targeted
ablation of osteocytes induces osteoporosis with defective
mechanotransduction. Cell Metab. 5:464–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Goulet GC, Cooper DM, Coombe D and
Zernicke RF: Influence of cortical canal architecture on
lacunocanalicular pore pressure and fluid flow. Comput Methods
Biomech Biomed Engin. 11:379–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang Y, McNamara LM, Schaffler MB and
Weinbaum S: A model for the role of integrins in flow induced
mechanotransduction in osteocytes. Proc Natl Acad Sci USA.
104:15941–15946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Phillips JA, Almeida EA, Hill EL, Aguirre
JI, Rivera MF, Nachbandi I, Wronski TJ, van der Meulen MC and
Globus RK: Role for beta1 integrins in cortical osteocytes during
acute musculoskeletal disuse. Matrix Biol. 27:609–618. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liedert A, Kaspar D, Blakytny R, Claes L
and Ignatius A: Signal transduction pathways involved in
mechanotransduction in bone cells. Biochem Biophys Res Commun.
349:1–5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Heino TJ, Hentunen TA and Väänänen HK:
Conditioned medium from osteocytes stimulates the proliferation of
bone marrow mesenchymal stem cells and their differentiation into
osteoblasts. Exp Cell Res. 294:458–468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li L, Yang Z, Zhang H, Chen W, Chen M and
Zhu Z: Low-intensity pulsed ultrasound regulates proliferation and
differentiation of osteoblasts through osteocytes. Biochem Biophys
Res Commun. 418:296–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yellowley CE, Li Z, Zhou Z, Jacobs CR and
Donahue HJ: Functional gap junctions between osteocytic and
osteoblastic cells. J Bone Miner Res. 15:209–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Taylor AF, Saunders MM, Shingle DL,
Cimbala JM, Zhou Z and Donahue HJ: Mechanically stimulated
osteocytes regulate osteoblastic activity via gap junctions. Am J
Physiol Cell Physiol. 292:C545–C552. 2007. View Article : Google Scholar
|
|
85
|
Cherian PP, Cheng B, Gu S, Sprague E,
Bonewald LF and Jiang JX: Effects of mechanical strain on the
function of Gap junctions in osteocytes are mediated through the
prostaglandin EP2 receptor. J Biol Chem. 278:43146–43156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Cheng B, Kato Y, Zhao S, Luo J, Sprague E,
Bonewald LF and Jiang JX: PGE(2) is essential for gap
junction-mediated intercellular communication between
osteocyte-like MLO-Y4 cells in response to mechanical strain.
Endocrinology. 142:3464–3473. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kanno T, Takahashi T, Tsujisawa T,
Ariyoshi W and Nishihara T: Mechanical stress-mediated Runx2
activation is dependent on Ras/ERK1/2 MAPK signaling in
osteoblasts. J Cell Biochem. 101:1266–1277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Franceschi RT and Xiao G: Regulation of
the osteoblast-specific transcription factor, Runx2: Responsiveness
to multiple signal transduction pathways. J Cell Biochem.
88:446–454. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sapir-Koren R and Livshits G: Osteocyte
control of bone remodeling: Is sclerostin a key molecular
coordinator of the balanced bone resorption-formation cycles?
Osteoporos Int. 25:2685–2700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
ten Dijke P, Krause C, de Gorter DJ, Löwik
CW and van Bezooijen RL: Osteocyte-derived sclerostin inhibits bone
formation: Its role in bone morphogenetic protein and Wnt
signaling. J Bone Joint Surg Am. 90(Suppl 1): S31–S35. 2008.
View Article : Google Scholar
|
|
91
|
Galli C, Passeri G and Macaluso GM:
Osteocytes and WNT: The mechanical control of bone formation. J
Dent Res. 89:331–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kitaura H, Marahleh A, Ohori F, Noguchi T,
Shen WR, Qi J, Nara Y, Pramusita A, Kinjo R and Mizoguchi I:
Osteocyte-related cytokines regulate osteoclast formation and bone
resorption. Int J Mol Sci. 21:51692020. View Article : Google Scholar :
|
|
93
|
Plotkin LI, Gortazar AR, Davis HM, Condon
KW, Gabilondo H, Maycas M, Allen MR and Bellido T: Inhibition of
osteocyte apoptosis prevents the increase in osteocytic receptor
activator of nuclear factor κB ligand (RANKL) but does not stop
bone resorption or the loss of bone induced by unloading. J Biol
Chem. 290:18934–18942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang
J, Li Y, Feng G, Gao X and He L: Sclerostin mediates bone response
to mechanical unloading through antagonizing Wnt/beta-catenin
signaling. J Bone Miner Res. 24:1651–1661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cheung WY, Simmons CA and You L: Osteocyte
apoptosis regulates osteoclast precursor adhesion via osteocytic
IL-6 secretion and endothelial ICAM-1 expression. Bone. 50:104–110.
2012. View Article : Google Scholar
|
|
96
|
Al-Dujaili SA, Lau E, Al-Dujaili H, Tsang
K, Guenther A and You L: Apoptotic osteocytes regulate osteoclast
precursor recruitment and differentiation in vitro. J Cell Biochem.
112:2412–2423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Jilka RL, Noble B and Weinstein RS:
Osteocyte apoptosis. Bone. 54:264–271. 2013. View Article : Google Scholar :
|
|
98
|
Wang JH, Thampatty BP, Lin JS and Im HJ:
Mechanoregulation of gene expression in fibroblasts. Gene.
391:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang HB, Dembo M, Hanks SK and Wang Y:
Focal adhesion kinase is involved in mechanosensing during
fibroblast migration. Proc Natl Acad Sci USA. 98:11295–11300. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Warita H, Watarai H and Soma K: Nitric
oxide synthase expression is increased by occlusal force in rat
periodontal ligament. Orthod Craniofac Res. 7:122–126. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Brandi ML, Hukkanen M, Umeda T,
Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM and MacIntyre I:
Bidirectional regulation of osteoclast function by nitric oxide
synthase isoforms. Proc Natl Acad Sci USA. 92:2954–2958. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Helfrich MH, Evans DE, Grabowski PS,
Pollock JS, Ohshima H and Ralston SH: Expression of nitric oxide
synthase isoforms in bone and bone cell cultures. J Bone Miner Res.
12:1108–1115. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Yang S, Guo L, Su Y, Wen J, Du J, Li X,
Liu Y, Feng J, Xie Y, Bai Y, et al: Nitric oxide balances
osteoblast and adipocyte lineage differentiation via the JNK/MAPK
signaling pathway in periodontal ligament stem cells. Stem Cell Res
Ther. 9:1182018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Watarai H, Warita H and Soma K: Effect of
nitric oxide on the recovery of the hypofunctional periodontal
ligament. J Dent Res. 83:338–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kaneko K, Miyamoto Y, Tsukuura R, Sasa K,
Akaike T, Fujii S, Yoshimura K, Nagayama K, Hoshino M, Inoue S, et
al: 8-Nitro-cGMP is a promoter of osteoclast differentiation
induced by RANKL. Nitric Oxide. 72:46–51. 2018. View Article : Google Scholar
|
|
106
|
Korkmaz Y, Baumann MA, Schröder H,
Behrends S, Addicks K, Raab WH and Bloch W: Localization of the
NO-cGMP signaling pathway molecules, NOS III-phosphorylation sites,
ERK1/2, and Akt/PKB in osteoclasts. J Periodontol. 75:1119–1125.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Dong SS, Williams JP, Jordan SE, Cornwell
T and Blair HC: Nitric oxide regulation of cGMP production in
osteoclasts. J Cell Biochem. 73:478–487. 1999. View Article : Google Scholar
|
|
108
|
Nagayama K, Miyamoto Y, Kaneko K,
Yoshimura K, Sasa K, Akaike T, Fujii S, Izumida E, Uyama R, Chikazu
D, et al: Production of 8-nitro-cGMP in osteocytic cells and its
upregulation by parathyroid hormone and prostaglandin
E2. In Vitro Cell Dev Biol Anim. 55:45–51. 2019.
View Article : Google Scholar
|
|
109
|
Davidovitch Z, Montgomery PC and Shanfeld
JL: Cellular localization and concentration of bone cyclic
nucleotides in response to acute PTE administration. Calcif Tissue
Res. 24:81–91. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Davidovitch Z, Montgomery PC and Shanfeld
JL: Guanosine 3′,5′-monophosphate in bone: Microscopic
visualization by an immuno-histochemical technique. Calcif Tissue
Res. 24:73–79. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Davidovitch Z, Montgomery PC, Yost RW and
Shanfeld JL: Immuno-histochemical localization of cyclic
nucleotides in mineralized tissues: Mechanically-stressed
osteoblasts in vivo. Anat Rec. 192:363–373. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Graziani E, Zelent ME and Pelliccioni GA:
Biochemical aspects of orthodontic movement: Recent findings and
trends. Mondo Ortod. 16:77–84. 1991.In Italian. PubMed/NCBI
|
|
113
|
Davidovitch Z, Finkelson MD, Steigman S,
Shanfeld JL, Montgomery PC and Korostoff E: Electric currents, bone
remodeling, and orthodontic tooth movement. II. Increase in rate of
tooth movement and periodontal cyclic nucleotide levels by combined
force and electric current. Am J Orthod. 77:33–47. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Davidovitch Z, Finkelson MD, Steigman S,
Shanfeld JL, Montgomery PC and Korostoff E: Electric currents, bone
remodeling, and orthodontic tooth movement. I. The effect of
electric currents on periodontal cyclic nucleotides. Am J Orthod.
77:14–32. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Davidovitch Z, Steigman S, Finkelson MD,
Yost RW, Montgomery PC, Shanfeld JL and Korostoff E:
Immunohistochemical evidence that electric currents increase
periosteal cell cyclic nucleotide levels in feline alveolar bone in
vivo. Arch Oral Biol. 25:321–327. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Karanth HS and Shetty KS: Orthodontic
tooth movement and bioelectricity. Indian J Dent Res. 12:212–221.
2001.
|
|
117
|
Nilforoushan D, Gramoun A, Glogauer M and
Manolson MF: Nitric oxide enhances osteoclastogenesis possibly by
mediating cell fusion. Nitric Oxide. 21:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Erez A, Nagamani SC, Shchelochkov OA,
Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK,
et al: Requirement of argininosuccinate lyase for systemic nitric
oxide production. Nat Med. 17:1619–1626. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Shirazi M, Nilforoushan D, Alghasi H and
Dehpour AR: The role of nitric oxide in orthodontic tooth movement
in rats. Angle Orthod. 72:211–215. 2002.PubMed/NCBI
|
|
120
|
Akin E, Gurton AU and Olmez H: Effects of
nitric oxide in orthodontic tooth movement in rats. Am J Orthod
Dentofacial Orthop. 126:608–614. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Armour KE, Van'T Hof RJ, Grabowski PS,
Reid DM and Ralston SH: Evidence for a pathogenic role of nitric
oxide in inflammation-induced osteoporosis. J Bone Miner Res.
14:2137–2142. 1999. View Article : Google Scholar
|
|
122
|
Lin SK, Kok SH, Kuo MY, Lee MS, Wang CC,
Lan WH, Hsiao M, Goldring SR and Hong CY: Nitric oxide promotes
infectious bone resorption by enhancing cytokine-stimulated
interstitial collagenase synthesis in osteoblasts. J Bone Miner
Res. 18:39–46. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cuzzocrea S, Mazzon E, Dugo L, Genovese T,
Di Paola R, Ruggeri Z, Vegeto E, Caputi AP, Van De Loo FA, Puzzolo
D and Maggi A: Inducible nitric oxide synthase mediates bone loss
in ovariectomized mice. Endocrinology. 144:1098–1107. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gyurko R, Shoji H, Battaglino RA, Boustany
G, Gibson FC III, Genco CA, Stashenko P and Van Dyke TE: Inducible
nitric oxide synthase mediates bone development and P.
gingivalis-induced alveolar bone loss. Bone. 36:472–479. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Graves DT, Alsulaimani F, Ding Y and Marks
SC Jr: Developmentally regulated monocyte recruitment and bone
resorption are modulated by functional deletion of the monocytic
chemoattractant protein-1 gene. Bone. 31:282–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
He D, Kou X, Luo Q, Yang R, Liu D, Wang X,
Song Y, Cao H, Zeng M, Gan Y and Zhou Y: Enhanced M1/M2 macrophage
ratio promotes orthodontic root resorption. J Dent Res. 94:129–139.
2015. View Article : Google Scholar
|
|
127
|
He D, Kou X, Yang R, Liu D, Wang X, Luo Q,
Song Y, Liu F, Yan Y, Gan Y and Zhou Y: M1-like macrophage
polarization promotes orthodontic tooth movement. J Dent Res.
94:1286–1294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lee SK, Huang H, Lee SW, Kim KH, Kim KK,
Kim HM, Lee ZH and Kim HH: Involvement of iNOS-dependent NO
production in the stimulation of osteoclast survival by TNF-alpha.
Exp Cell Res. 298:359–368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kaur S, White S and Bartold M: Periodontal
disease as a risk factor for rheumatoid arthritis: A systematic
review. JBI Libr Syst Rev. 10(Suppl 42): S1–S12. 2012. View Article : Google Scholar
|
|
130
|
Wang JW, Yeh CB, Chou SJ, Lu KC, Chu TH,
Chen WY, Chien JL, Yen MH, Chen TH and Shyu JF: YC-1 alleviates
bone loss in ovariectomized rats by inhibiting bone resorption and
inducing extrinsic apoptosis in osteoclasts. J Bone Miner Metab.
36:508–518. 2018. View Article : Google Scholar
|
|
131
|
van't Hof RJ and Ralston SH:
Cytokine-induced nitric oxide inhibits bone resorption by inducing
apoptosis of osteoclast progenitors and suppressing osteoclast
activity. J Bone Miner Res. 12:1797–1804. 1997. View Article : Google Scholar
|
|
132
|
Kitaura H, Fujimura Y, Yoshimatsu M,
Kohara H, Morita Y, Aonuma T, Fukumoto E, Masuyama R, Yoshida N and
Takano-Yamamoto T: IL-12- and IL-18-mediated, nitric oxide-induced
apoptosis in TNF-α-mediated osteoclastogenesis of bone marrow
cells. Calcif Tissue Int. 89:65–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Kalyanaraman H, Ramdani G, Joshua J,
Schall N, Boss GR, Cory E, Sah RL, Casteel DE and Pilz RB: A novel,
direct no donor regulates osteoblast and osteoclast functions and
increases bone mass in ovariectomized mice. J Bone Miner Res.
32:46–59. 2017. View Article : Google Scholar
|
|
134
|
Iwaki F, Amano H and Ohura K: Nicorandil
inhibits osteoclast differentiation in vitro. Eur J Pharmacol.
793:14–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yaroslavskiy BB, Li Y, Ferguson DJ, Kalla
SE, Oakley JI and Blair HC: Autocrine and paracrine nitric oxide
regulate attachment of human osteoclasts. J Cell Biochem.
91:962–972. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yaroslavskiy BB, Zhang Y, Kalla SE, García
Palacios V, Sharrow AC, Li Y, Zaidi M, Wu C and Blair HC:
NO-dependent osteoclast motility: Reliance on cGMP-dependent
protein kinase I and VASP. J Cell Sci. 118:5479–5487. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yaroslavskiy BB, Turkova I, Wang Y,
Robinson LJ and Blair HC: Functional osteoclast attachment requires
inositol-1,4,5-trisphosphate receptor-associated cGMP-dependent
kinase substrate. Lab Invest. 90:1533–1542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fukada SY, Silva TA, Saconato IF, Garlet
GP, Avila-Campos MJ, Silva JS and Cunha FQ: iNOS-derived nitric
oxide modulates infection-stimulated bone loss. J Dent Res.
87:1155–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Silva MJ, Sousa LM, Lara VP, Cardoso FP,
Júnior GM, Totola AH, Caliari MV, Romero OB, Silva GA,
Ribeiro-Sobrinho AP and Vieira LQ: The role of iNOS and PHOX in
periapical bone resorption. J Dent Res. 90:495–500. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zheng H, Yu X, Collin-Osdoby P and Osdoby
P: RANKL stimulates inducible nitric-oxide synthase expression and
nitric oxide production in developing osteoclasts. An autocrine
negative feedback mechanism triggered by RANKL-induced
interferon-beta via NF-kappaB that restrains osteoclastogenesis and
bone resorption. J Biol Chem. 281:15809–15820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Otsuka E, Hirano K, Matsushita S, Inoue A,
Hirose S, Yamaguchi A and Hagiwara H: Effects of nitric oxide from
exogenous nitric oxide donors on osteoblastic metabolism. Eur J
Pharmacol. 349:345–350. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hikiji H, Shin WS, Oida S, Takato T,
Koizumi T and Toyo-oka T: Direct action of nitric oxide on
osteoblastic differentiation. FEBS Lett. 410:238–242. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Inoue A, Hiruma Y, Hirose S, Yamaguchi A
and Hagiwara H: Reciprocal regulation by cyclic nucleotides of the
differentiation of rat osteoblast-like cells and mineralization of
nodules. Biochem Biophys Res Commun. 215:1104–1110. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Pal S, Rashid M, Singh SK, Porwal K, Singh
P, Mohamed R, Gayen JR, Wahajuddin M and Chattopadhyay N: Skeletal
restoration by phosphodiesterase 5 inhibitors in osteopenic mice:
Evidence of osteoanabolic and osteoangiogenic effects of the drugs.
Bone. 135:1153052020. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Mancini L, Moradi-Bidhendi N, Becherini L,
Martineti V and MacIntyre I: The biphasic effects of nitric oxide
in primary rat osteoblasts are cGMP dependent. Biochem Biophys Res
Commun. 274:477–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zaragoza C, López-Rivera E, García-Rama C,
Saura M, Martínez-Ruíz A, Lizarbe TR, Martín-de-Lara F and Lamas S:
Cbfa-1 mediates nitric oxide regulation of MMP-13 in osteoblasts. J
Cell Sci. 119:1896–1902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Armour KE, Armour KJ, Gallagher ME,
Gödecke A, Helfrich MH, Reid DM and Ralston SH: Defective bone
formation and anabolic response to exogenous estrogen in mice with
targeted disruption of endothelial nitric oxide synthase.
Endocrinology. 142:760–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ma P, Gu B, Xiong W, Tan B, Geng W, Li J
and Liu H: Glimepiride promotes osteogenic differentiation in rat
osteoblasts via the PI3K/Akt/eNOS pathway in a high glucose
microenvironment. PLoS One. 9:e1122432014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Almeida M, Han L, Bellido T, Manolagas SC
and Kousteni S: Wnt proteins prevent apoptosis of both uncommitted
osteoblast progenitors and differentiated osteoblasts by
beta-catenin-dependent and -independent signaling cascades
involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol
Chem. 280:41342–41351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wimalawansa SJ: Rationale for using nitric
oxide donor therapy for prevention of bone loss and treatment of
osteoporosis in humans. Ann N Y Acad Sc. 1117:283–297. 2007.
View Article : Google Scholar
|
|
151
|
Willems HM, van den Heuvel EG, Carmeliet
G, Schaafsma A, Klein-Nulend J and Bakker AD: VDR dependent and
independent effects of 1,25-dihydroxyvitamin D3 on nitric oxide
production by osteoblasts. Steroids. 77:126–131. 2012. View Article : Google Scholar
|
|
152
|
Yan L, Yinghui T, Bo Y, Gang Z, Xian X and
Lu Z: Effect of calcitonin gene-related peptide on nitric oxide
production in osteoblasts: An experimental study. Cell Biol Int.
35:757–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Li Y, Tan Y, Zhang G, Yang B and Zhang J:
Effects of calcitonin gene-related peptide on the expression and
activity of nitric oxide synthase during mandibular bone healing in
rabbits: An experimental study. J Oral Maxillofac Surg. 67:273–279.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
O'Shaughnessy MC, Polak JM, Afzal F,
Hukkanen MV, Huang P, MacIntyre I and Buttery LD: Nitric oxide
mediates 17beta-estradiol-stimulated human and rodent osteoblast
proliferation and differentiation. Biochem Biophys Res Commun.
277:604–610. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Yang JY, Park MY, Park SY, Yoo HI, Kim MS,
Kim JH, Kim WJ and Jung JY: Nitric oxide-induced autophagy in
MC3T3-E1 cells is associated with cytoprotection via ampk
activation. Korean J Physiol Pharmacol. 19:507–514. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Joshua J, Schwaerzer GK, Kalyanaraman H,
Cory E, Sah RL, Li M, Vaida F, Boss GR and Pilz RB: Soluble
guanylate cyclase as a novel treatment target for osteoporosis.
Endocrinology. 155:4720–4730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Marathe N, Rangaswami H, Zhuang S, Boss GR
and Pilz RB: Pro-survival effects of 17β-estradiol on osteocytes
are mediated by nitric oxide/cGMP via differential actions of
cGMP-dependent protein kinases I and II. J Biol Chem. 287:978–988.
2012. View Article : Google Scholar
|
|
158
|
Joshua J, Kalyanaraman H, Marathe N and
Pilz RB: Nitric oxide as a mediator of estrogen effects in
osteocytes. Vitam Horm. 96:247–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Lee SK, Choi HI, Yang YS, Jeong GS, Hwang
JH, Lee SI, Kang KH, Cho JH, Chae JM, Lee SK, et al: Nitric oxide
modulates osteoblastic differentiation with heme oxygenase-1 via
the mitogen activated protein kinase and nuclear factor-kappaB
pathways in human periodontal ligament cells. Biol Pharm Bull.
32:1328–1334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
d'Alessandro L, Petrini M, Ferrante M, Di
Marco S, Trubiani O and Spoto G: Cyclic nucleotide
phosphodiesterase activity in stem cells of human periodontal
ligament (PDL-MSCs) before and after osteogenic induction. Oral
Surg Oral Med Oral Pathol Oral Radiol. 116:e317–e323. 2013.
View Article : Google Scholar
|
|
161
|
Tang J, Wu T, Xiong J, Su Y, Zhang C, Wang
S, Tang Z and Liu Y: Porphyromonas gingivalis lipopolysaccharides
regulate functions of bone marrow mesenchymal stem cells. Cell
Prolif. 48:239–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Seo T, Cha S, Woo KM, Park YS, Cho YM, Lee
JS and Kim TI: Synergic induction of human periodontal ligament
fibroblast cell death by nitric oxide and N-methyl-D-aspartic acid
receptor antagonist. J Periodontal Implant Sci. 41:17–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Reher P, Harris M, Whiteman M, Hai HK and
Meghji S: Ultrasound stimulates nitric oxide and prostaglandin E2
production by human osteoblasts. Bone. 31:236–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wittkowske C, Reilly GC, Lacroix D and
Perrault CM: In vitro bone cell models: Impact of fluid shear
stress on bone formation. Front Bioeng Biotechnol. 4:872016.
View Article : Google Scholar :
|
|
165
|
Shibata K, Yoshimura Y, Kikuiri T,
Hasegawa T, Taniguchi Y, Deyama Y, Suzuki K and Iida J: Effect of
the release from mechanical stress on osteoclastogenesis in
RAW264.7 cells. Int J Mol Med. 28:73–79. 2011.PubMed/NCBI
|
|
166
|
van der Meijden K, Bakker AD, van Essen
HW, Heijboer AC, Schulten EA, Lips P and Bravenboer N: Mechanical
loading and the synthesis of 1,25(OH)2D in primary human
osteoblasts. J Steroid Biochem Mol Biol. 156:32–39. 2016.
View Article : Google Scholar
|
|
167
|
Diniz P, Soejima K and Ito G: Nitric oxide
mediates the effects of pulsed electromagnetic field stimulation on
the osteoblast proliferation and differentiation. Nitric Oxide.
7:18–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Pathak JL, Bravenboer N, Luyten FP,
Verschueren P, Lems WF, Klein-Nulend J and Bakker A: Mechanical
loading reduces inflammation-induced human osteocyte-to-osteoclast
communication. Calcif Tissue Int. 97:169–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Premaraj S, Souza I and Premaraj T: Focal
adhesion kinase mediates β-catenin signaling in periodontal
ligament cells. Biochem Biophys Res Commun. 439:487–492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Nakago-Matsuo C, Matsuo T and Nakago T:
Basal nitric oxide production is enhanced by hydraulic pressure in
cultured human periodontal ligament fibroblasts. Am J Orthod
Dentofacial Orthop. 117:474–478. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Kraft DC, Bindslev DA, Melsen B, Abdallah
BM, Kassem M and Klein-Nulend J: Mechanosensitivity of dental pulp
stem cells is related to their osteogenic maturity. Eur J Oral Sci.
118:29–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Kraft DC, Bindslev DA, Melsen B and
Klein-Nulend J: Human dental pulp cells exhibit bone cell-like
responsiveness to fluid shear stress. Cytotherapy. 13:214–226.
2011. View Article : Google Scholar
|
|
173
|
Santos A, Bakker AD, Zandieh-Doulabi B, de
Blieck-Hogervorst JM and Klein-Nulend J: Early activation of the
beta-catenin pathway in osteocytes is mediated by nitric oxide,
phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase.
Biochem Biophys Res Commun. 391:364–369. 2010. View Article : Google Scholar
|
|
174
|
Santos A, Bakker AD, Zandieh-Doulabi B,
Semeins CM and Klein-Nulend J: Pulsating fluid flow modulates gene
expression of proteins involved in Wnt signaling pathways in
osteocytes. J Orthop Res. 27:1280–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Rangaswami H, Schwappacher R, Tran T, Chan
GC, Zhuang S, Boss GR and Pilz RB: Protein kinase G and focal
adhesion kinase converge on Src/Akt/β-catenin signaling module in
osteoblast mechanotransduction. J Biol Chem. 287:21509–21519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Rangaswami H, Schwappacher R, Marathe N,
Zhuang S, Casteel DE, Haas B, Chen Y, Pfeifer A, Kato H, Shattil S,
et al: Cyclic GMP and protein kinase G control a Src-containing
mechanosome in osteoblasts. Sci Signal. 3:ra912010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Rangaswami H, Marathe N, Zhuang S, Chen Y,
Yeh JC, Frangos JA, Boss GR and Pilz RB: Type II cGMP-dependent
protein kinase mediates osteoblast mechanotransduction. J Biol
Chem. 284:14796–14808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Willems HM, van den Heuvel EG, Castelein
S, Buisman JK, Bronckers AL, Bakker AD and Klein-Nulend J: Fluoride
inhibits the response of bone cells to mechanical loading.
Odontology. 99:112–118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Vezeridis PS, Semeins CM, Chen Q and
Klein-Nulend J: Osteocytes subjected to pulsating fluid flow
regulate osteoblast proliferation and differentiation. Biochem
Biophys Res Commun. 348:1082–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Tan SD, de Vries TJ, Kuijpers-Jagtman AM,
Semeins CM, Everts V and Klein-Nulend J: Osteocytes subjected to
fluid flow inhibit osteoclast formation and bone resorption. Bone.
41:745–751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Bakker AD, Soejima K, Klein-Nulend J and
Burger EH: The production of nitric oxide and prostaglandin E(2) by
primary bone cells is shear stress dependent. J Biomech.
34:671–677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Tan SD, Bakker AD, Semeins CM,
Kuijpers-Jagtman AM and Klein-Nulend J: Inhibition of osteocyte
apoptosis by fluid flow is mediated by nitric oxide. Biochem
Biophys Res Commun. 369:1150–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Juffer P, Jaspers RT, Lips P, Bakker AD
and Klein-Nulend J: Expression of muscle anabolic and metabolic
factors in mechanically loaded MLO-Y4 osteocytes. Am J Physiol
Endocrinol Metab. 302:E389–E395. 2012. View Article : Google Scholar
|
|
184
|
Furumatsu T, Shen ZN, Kawai A, Nishida K,
Manabe H, Oohashi T, Inoue H and Ninomiya Y: Vascular endothelial
growth factor principally acts as the main angiogenic factor in the
early stage of human osteoblastogenesis. J Biochem. 133:633–639.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Fisslthaler B, Loot AE, Mohamed A, Busse R
and Fleming I: Inhibition of endothelial nitric oxide synthase
activity by proline-rich tyrosine kinase 2 in response to fluid
shear stress and insulin. Circ Res. 102:1520–1528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Ghimire K, Altmann HM, Straub AC and
Isenberg JS: Nitric oxide: What's new to NO? Am J Physiol Cell
Physiol. 312:C254–C262. 2017. View Article : Google Scholar
|
|
187
|
Zaman G, Pitsillides AA, Rawlinson SC,
Suswillo RF, Mosley JR, Cheng MZ, Platts LA, Hukkanen M, Polak JM
and Lanyon LE: Mechanical strain stimulates nitric oxide production
by rapid activation of endothelial nitric oxide synthase in
osteocytes. J Bone Miner Res. 14:1123–1131. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Dimmeler S, Fleming I, Fisslthaler B,
Hermann C, Busse R and Zeiher AM: Activation of nitric oxide
synthase in endothelial cells by Akt-dependent phosphorylation.
Nature. 399:601–605. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
189
|
Klein-Nulend J, Helfrich MH, Sterck JG,
MacPherson H, Joldersma M, Ralston SH, Semeins CM and Burger EH:
Nitric oxide response to shear stress by human bone cell cultures
is endothelial nitric oxide synthase dependent. Biochem Biophys Res
Commun. 250:108–114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Bakker AD, Huesa C, Hughes A, Aspden RM,
van't Hof RJ, Klein-Nulend J and Helfrich MH: Endothelial nitric
oxide synthase is not essential for nitric oxide production by
osteoblasts subjected to fluid shear stress in vitro. Calcif Tissue
Int. 92:228–239. 2013. View Article : Google Scholar
|
|
191
|
Das-Gupta V, Williamson RA and Pitsillides
AA: Expression of endothelial nitric oxide synthase protein is not
necessary for mechanical strain-induced nitric oxide production by
cultured osteoblasts. Osteoporos Int. 23:2635–2647. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Hou CH, Lin J, Huang SC, Hou SM and Tang
CH: Ultrasound stimulates NF-kappaB activation and iNOS expression
via the Ras/Raf/MEK/ERK signaling pathway in cultured
preosteoblasts. J Cell Physiol. 220:196–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Padilla F, Puts R, Vico L and Raum K:
Stimulation of bone repair with ultrasound: A review of the
possible mechanic effects. Ultrasonics. 54:1125–1145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Kusuyama J, Bandow K, Ohnishi T, Hisadome
M, Shima K, Semba I and Matsuguchi T: Osteopontin inhibits
osteoblast responsiveness through the down-regulation of focal
adhesion kinase mediated by the induction of low-molecular weight
protein tyrosine phosphatase. Mol Biol Cell. 28:1326–1336. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Aggarwal H, Pathak P, Singh P, Gayen JR,
Jagavelu K and Dikshit M: Systemic insulin resistance and metabolic
perturbations in chow fed inducible nitric oxide synthase knockout
male mice: Partial reversal by nitrite supplementation.
Antioxidants (Basel). 9:7362020. View Article : Google Scholar
|
|
196
|
Foster MW, Hess DT and Stamler JS: Protein
S-nitrosylation in health and disease: A current perspective.
Trends Mol Med. 15:391–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Huang B, Chen SC and Wang DL: Shear flow
increases S-nitrosylation of proteins in endothelial cells.
Cardiovasc Res. 83:536–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Yoo SK, Warita H and Soma K: Duration of
orthodontic force affecting initial response of nitric oxide
synthase in rat periodontal ligaments. J Med Dent Sci. 51:83–88.
2004.PubMed/NCBI
|
|
199
|
Nilforoushan D and Manolson MF: Expression
of nitric oxide synthases in orthodontic tooth movement. Angle
Orthod. 79:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tan SD, Xie R, Klein-Nulend J, van Rheden
RE, Bronckers AL, Kuijpers-Jagtman AM, Von den Hoff JW and Maltha
JC: Orthodontic force stimulates eNOS and iNOS in rat osteocytes. J
Dent Res. 88:255–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Baloul SS: Osteoclastogenesis and
osteogenesis during tooth movement. Front Oral Bio. 18:75–79. 2016.
View Article : Google Scholar
|
|
202
|
D'Attillio M, Di Maio F, D'Arcangela C,
Filippi MR, Felaco M, Lohinai Z, Festa F and Perinetti G: Gingival
endothelial and inducible nitric oxide synthase levels during
orthodontic treatment: A cross-sectional study. Angle Orthod.
74:851–858. 2004.
|
|
203
|
Ford H, Suri S, Nilforoushan D, Manolson M
and Gong SG: Nitric oxide in human gingival crevicular fluid after
orthodontic force application. Arch Oral Biol. 59:1211–1216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Topal SC, Tuncer BB, Elgun S, Erguder I
and Ozmeric N: Levels of cytokines in gingival crevicular fluid
during rapid maxillary expansion and the subsequent retention
period. J Clin Pediatr Dent. 43:137–143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Ozel N, Aksoy A, Kırzıoglu FY, Doguc DK
and Aksoy TA: Evaluation of interleukin-1β level and oxidative
status in gingival crevicular fluid during rapid maxillary
expansion. Arch Oral Biol. 90:74–79. 2018. View Article : Google Scholar
|
|
206
|
AtuğÖzcan SS, Ceylan I, Ozcan E, Kurt N,
Dağsuyu IM and Canakçi CF: Evaluation of oxidative stress
biomarkers in patients with fixed orthodontic appliances. Dis
Markers. 2014:5978922014.
|
|
207
|
Hayashi K, Igarashi K, Miyoshi K, Shinoda
H and Mitani H: Involvement of nitric oxide in orthodontic tooth
movement in rats. Am J Orthod Dentofacial Orthop. 122:306–309.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Rausch-Fan X and Matejka M: From plaque
formation to periodontal disease, is there a role for nitric oxide?
Eur J Clin Invest. 31:833–835. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
de Farias JO, de Freitas Lima SM and
Rezende TMB: Physiopathology of nitric oxide in the oral
environment and its biotechnological potential for new oral
treatments: A literature review. Clin Oral Investig. 24:4197–4212.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ and
Wang SL: From Nitrate to Nitric Oxide: The role of salivary glands
and oral bacteria. J Dent Res. 95:1452–1456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Carossa S, Pera P, Doglio P, Lombardo S,
Colagrande P, Brussino L, Rolla G and Bucca C: Oral nitric oxide
during plaque deposition. Eur J Clin Invest. 31:876–879. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Khodaii Z, Mehrabani M, Rafieian N,
Najafi-Parizi GA, Mirzaei A and Akbarzadeh R: Altered levels of
salivary biochemical markers in periodontitis. Am J Dent.
32:183–186. 2019.PubMed/NCBI
|
|
213
|
Sundar NM, Krishnan V, Krishnaraj S,
Hemalatha VT and Alam MN: Comparison of the salivary and the serum
nitric oxide levels in chronic and aggressive periodontitis: A
biochemical study. J Clin Diagn Res. 7:1223–1227. 2013.PubMed/NCBI
|
|
214
|
Parwani SR, Chitnis PJ and Parwani RN:
Salivary nitric oxide levels in inflammatory periodontal disease-a
case-control and interventional study. Int J Dent Hyg. 10:67–73.
2012. View Article : Google Scholar
|
|
215
|
Sokos D, Everts V and de Vries TJ: Role of
periodontal ligament fibroblasts in osteoclastogenesis: A review. J
Periodontal Res. 50:152–159. 2015. View Article : Google Scholar
|