Introduction
Diabetes is a metabolic disease and glucose
metabolism disorder is the main clinical manifestation (1). Previous studies have indicated
that, due to the popularity of high-sugar and high-fat diets and
the sedentary lifestyle of individuals, the incidence of diabetes
is anticipated to continue to increase in the next few years
(2,3). The onset of diabetes is often
accompanied by damage to several organs and patients with long-term
diabetes are often diagnosed with severe renal failure (4,5).
A previous study reported that 30% of patients with type 1 diabetes
and 25% of patients with type 2 diabetes developed diabetic
nephropathy (6). Furthermore,
the occurrence and development of diabetic nephropathy led to an
increase in the mortality of patients with diabetes. Diabetic
nephropathy-induced renal podocyte and capillary damage is the main
factor affecting the survival of patients (7). Therefore, developing a treatment
for diabetic nephropathy is crucial for improving the survival rate
of patients with diabetes.
Apelin is located at the chromosomal region
Xq25-26.1 and encoded by the APLN gene (8) and is the endogenous ligand of the G
protein-coupled receptor angiotensin domain type 1
receptor-associated protein (APJ), which is usually secreted and
produced by white adipose tissue (9). Apelin and its receptor APJ are
widely expressed in various tissues, including cerebellar
endothelial cells, heart and blood vessels, especially in the
cardiovascular system (10).
Apelin was identified as an adipokine associated with lipid
metabolism in a recent study (11). However, previous study has
indicated that apelin plays a crucial role in regulating the energy
homeostasis of the body, glucose and water metabolism as well as
other biological functions (12). Apelin expression has been
previously revealed to improve insulin resistance, atherosclerosis
and diabetes-induced vascular damage (13). However, a different study
revealed that apelin expression induced the occurrence and
development of diabetic retinopathy (14). Therefore, the role of apelin in
the occurrence and development of diabetes-related complications
remains unclear.
Furthermore, apelin is present as different subtypes
(apelin-36, apelin-17 and apelin-13) in the body through the shear
of the endoplasmic reticulum. It was revealed that the shorter the
peptide chain of apelin, the stronger its biological activity
(15). A previous study
indicated that apelin improved insulin sensitivity and induced
lower blood sugar levels by reducing chronic inflammation in
patients with type 2 diabetes, thereby enhancing glucose and lipid
metabolism (16). However, the
effect of apelin on diabetic nephropathy is currently
controversial. A previous study has reported that the levels of
apelin/APJ in the serum of patients with diabetic nephropathy are
elevated, promoting the occurrence and development of diabetic
nephropathy (17). On the other
hand, a different study revealed that apelin-13 could inhibit the
morphological changes of renal tubular epithelial cells in the
high-glucose environment, thereby inhibiting the occurrence and
development of the epithelial-mesenchymal transition (EMT) process
in glomerular cells, and ultimately delaying the occurrence of
diabetic nephropathy (18).
Therefore, the effect of apelin on diabetes-induced renal
complications remains unclear.
In the present study, the role of apelin-13 in the
process of diabetic nephropathy was further investigated by
constructing a rat model of diabetic nephropathy.
Materials and methods
Animal assays
A total of 30 Sprague Dawley male rats (6-8 weeks
old; 200-230 g) were obtained from the Beijing Vital River
Laboratory Animal Technology Co., Ltd. and housed in a
temperature-controlled (25°C) and specific pathogen-free room with
constant humidity (40-50%) in a 12-h light/dark cycle and received
food and water ad libitum for 1 week. These rats were then
equally divided into six groups (Control, Model, Ap-E40, Ap-E400,
Ap-L40 and Ap-L400). The rats in the control group were treated
with nothing. The rats of the other groups were fed with a
high-sugar and high-fat diet (Thermo Fisher Scientific, Inc.) and
received an intravenous injection of streptozocin (STZ; 40 mg/kg;
product no. S0130; Merck KGaA) every week. After 3 weeks, the
Ap-E40 and Ap-E400 rats were treated with apelin-13 (40 and 400
pmol/kg, respectively; product no. A6469; Merck KGaA). A total of 6
weeks after the STZ injection, the rats of the Ap-L40 and Ap-L400
groups were treated with apelin-13 (40 and 400 pmol/kg,
respectively). After eight STZ injections, the rats were euthanized
with sodium pentobarbital (160 mg/kg, i.p.). Following euthanasia,
the serum and organs were collected. The weight of the rats was
detected using electronic scales every week, from 1 week prior to
the STZ injection and until euthanasia. Blood glucose levels were
detected every week using a blood glucose meter (Thermo Fisher
Scientific, Inc.). Urine protein levels were also detected using a
BCA assay kit (cat. no. P0012S; Beyotime Institute of
Biotechnology) every week. The fasting serum insulin was also
detected every week using a commercial kit (cat. no. RAB0904; Merck
KGaA) according to the manufacturer's instructions. The index of
insulin resistance was calculated using the following formula:
(Insulin levels in the blood x blood glucose levels)/22.5. All the
experiments were performed according to the Guide for the Care and
Use of Laboratory Animals 8th edition (19) and approved (approval no.
2020-79-PJ01) by the Ethics Committee of the Air Force Medical
Center (Beijing, China).
Detection of blood urea nitrogen (BUN)
and serum creatinine (Scr)
The levels of BUN (cat. no. ml076479) and Scr (cat.
no. ml059158) in the blood were determined using commercial kits
(Shanghai Enzyme-linked Biotechnology Co., Ltd.) after 3 and 8
weeks of STZ injection, respectively. All steps of this assay were
performed according to the manufacturers' instructions.
Detection of nitric oxide (NO),
angiotensin II (Ang II) and endothelin-1 (ET-1)
The levels of NO, Ang II and ET-1 in the serum and
nephridial tissues were detected using NO kit (cat. no. S0021;
Beyotime Institute of Biotechnology), Ang II kit (product no.
RAB0010; Merck KGaA) and ET-1 kit (cat. no. E-EL-R1458c;
Elabscience Biotechnology, Inc.). All steps of the assays were
performed according to the manufacturers' instructions.
Hematoxylin and eosin (H&E)
staining
Following euthanasia, kidney tissues were collected
and washed with pre-cooled phosphate buffer saline (PBS). The
kidney was then separated and fixed with 4% paraformaldehyde
overnight at room temperature. The paraffin-embedded kidney tissues
were cut into 5-μm sections. Next, these sections were
dehydrated in 70 and 90% ethanol solution for 10 min each. Finally,
H&E staining at room temperature for 3 min was performed to
detect pathological changes. All steps of this assay were performed
according to the manufacturer's instructions of the H&E
staining kit (cat. no. C0105S; Beyotime Institute of
Biotechnology).
Immunohistochemistry
Immunohistochemistry was carried out using the
standard peroxidase/DAB method and hematoxylin counterstaining
according to the manufacturer's instructions. After being blocked
with 5% bovine serum albumin (BSA; Beyotime Institute of
Biotechnology) for 20 min at room temperature, sections were
incubated with anti-APJ primary antibody (cat. no. PA5-114830;
Thermo Fisher Scientific, Inc.) overnight at 4°C. After PBS
washing, sections were incubated with goat anti-rabbit horseradish
peroxidase-conjugated secondary antibody (product code ab6721;
1:1,000; Abcam) at 4°C for 50 min, followed by the PBS washing.
Then, sections were treated with DAB solution and counterstained
with hematoxylin at room temperature for 5 min. Finally, the images
were obtained using a light microscope (Olympus Corporation).
Cell culture and treatment
A human renal glomerular microvascular endothelial
cell line (hRGEC) was obtained from the BioVector NTCC, Inc. and
cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (FBS) in humidified air at
37°C with 5% CO2. These cells were divided into the
control, model, Ap-L (Apelin-low) and Ap-H (Apelin-high) groups.
Model, Ap-L and Ap-H group cells were cultured in high-glucose (25
mmol/l; Cytiva) RPMI-1640 medium and control group cells were
cultured in RPMI-1640 medium with normal glucose content (5.5
mmol/l). Next, Ap-L group cells were treated with low levels of
apelin-13 (0.1 μmol/l) and Ap-H group cells were treated
with high levels of apelin-13 (1 μmol/l).
Tube formation experiment
Tube formation in hRGEC cells was determined using a
commercial kit (product no. K905-50; AmyJet Scientific, Inc.). All
images were obtained using a light microscope (magnification, x4;
Olympus Corporation). All steps of this assay were performed
according to the manufacturer's instructions.
Cell Counting Kit-8 (CCK-8) assay
hRGEC cells from various groups were plated into
three 96-well plates (2.5x104/well). After 24, 48 and 72
h of incubation at 37°C, 10 μl CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) was diluted with the culture medium
and added to the 96-well plates. Next, these cells were placed in
an incubator for 1 h. Finally, the absorbance of these cells was
detected using a spectrophotometer (Thermo Fisher Scientific, Inc.)
at a wavelength of 450 nm.
Western blotting
Total proteins were collected from nephridial tissue
and hRGEC cells using RIPA lysis buffer (Santa Cruz Biotechnology,
Inc.). Protein concentration was determined using a BCA assay kit.
Next, equal amounts of protein (40 μg) were separated via
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) (Beyotime Institute of Biotechnology) and transferred to
a polyvinylidene fluoride (PVDF) membrane (EMD Millipore), which
was blocked with 5% bovine serum albumin (BSA) (Beyotime Institute
of Biotechnology) at 37°C for 1.5 h. The membranes were then
incubated with the following primary antibodies at 4°C overnight:
Ang II type 1 receptor (AT1R; 1:1,000; product code ab124734;
Abcam), endothelial NO synthase (eNOS; 1:1,000; product ocde
ab5589; Abcam), APJ (1:1,000; cat. no. PA5-114830; Thermo Fisher
Scientific, Inc.), transforming growth factor β receptor (TGBR;
1:1,000; product code ab97459; Abcam), E-cadherin (1:1,000; product
code ab212059; Abcam), α-smooth muscle actin (α-SMA; 1:1,000;
product no. 19245; Cell Signaling Technology, Inc.) and GAPDH
(1:2,500; product code ab9485; Abcam). Following the primary
antibody incubation, the membranes were washed with PBS-Tween-20
(0.1%) and incubated with HRP-conjugated antibody (1:2,000; product
code ab6721; Abcam) for 2 h at room temperature (7) Finally, the immunoreactive signals
were assessed using a Pierce™ ECL Western Blotting Substrate kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The relative intensities of the immunoblots were
measured with a Versa Doc™ MP 5000 molecular imager and Quantity
One software (version 4.6) (both from Bio-Rad Laboratories,
Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.0 (GraphPad Software, Inc.). Unpaired Student's t-tests
were used to compare two groups. Comparisons among different groups
were assessed using one-way ANOVA followed by Tukey's post-hoc
test. All data are presented as the mean ± standard deviation (SD)
and all experiments were repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
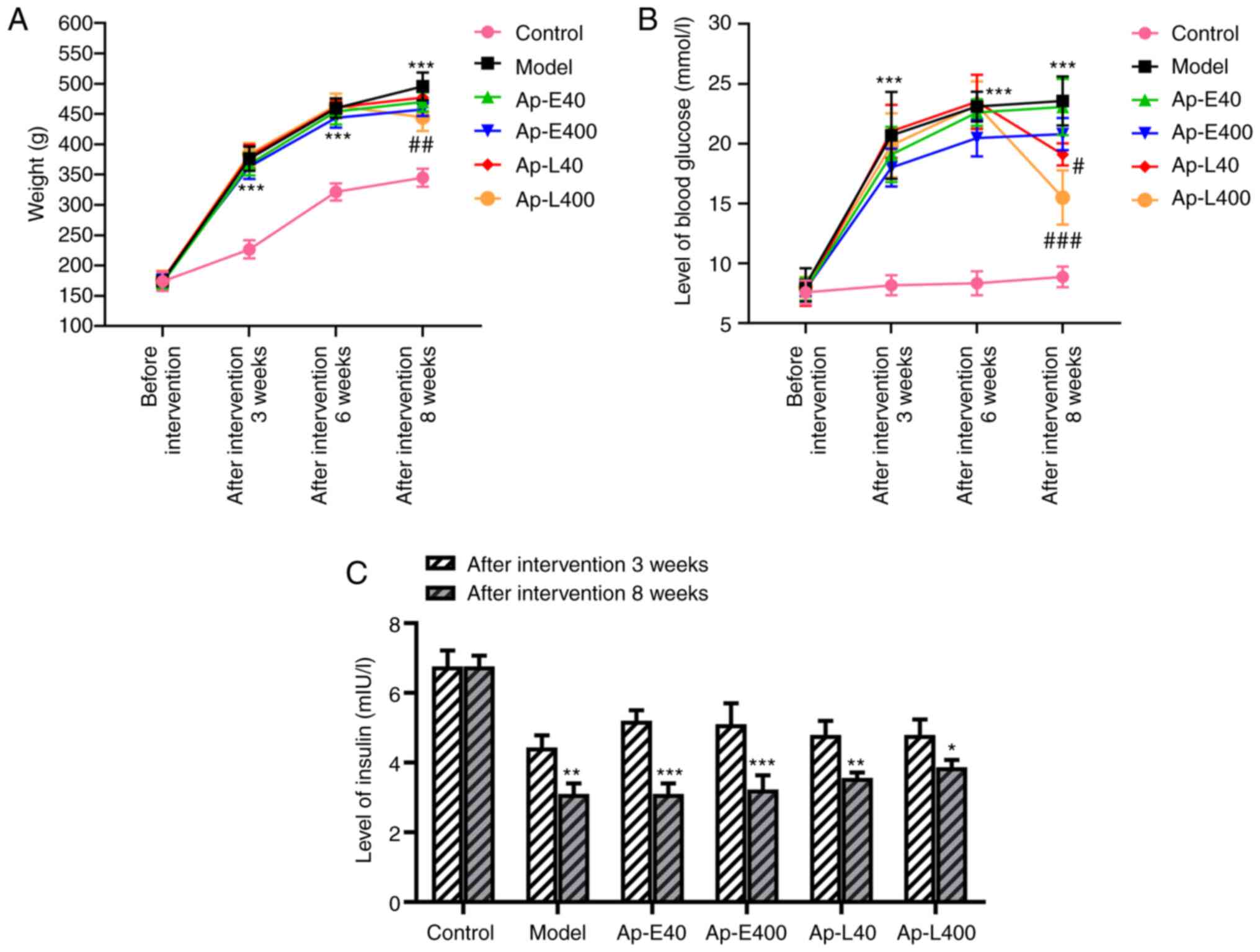
Apelin-13 decreases the weight and blood
glucose levels, and increases insulin levels of diabetic
nephropathy rats at 8 weeks
The weight and blood glucose levels of rats were
detected weekly during the process of raising them. The results
(Fig. 1A and B) revealed that
the weight and blood glucose levels of the rats in the Ap-E40 and
Ap-E400 groups were not significantly altered after the injection
of apelin-13 at 3 weeks. The same parameters in the rats of the
Ap-L40 and Ap-L400 groups were decreased after the apelin-13
injection at 8 weeks compared with that at 3 weeks. The levels of
insulin in the blood were also decreased after the apelin-13
injection at 8 weeks compared with that at 3 weeks (Fig. 1C).
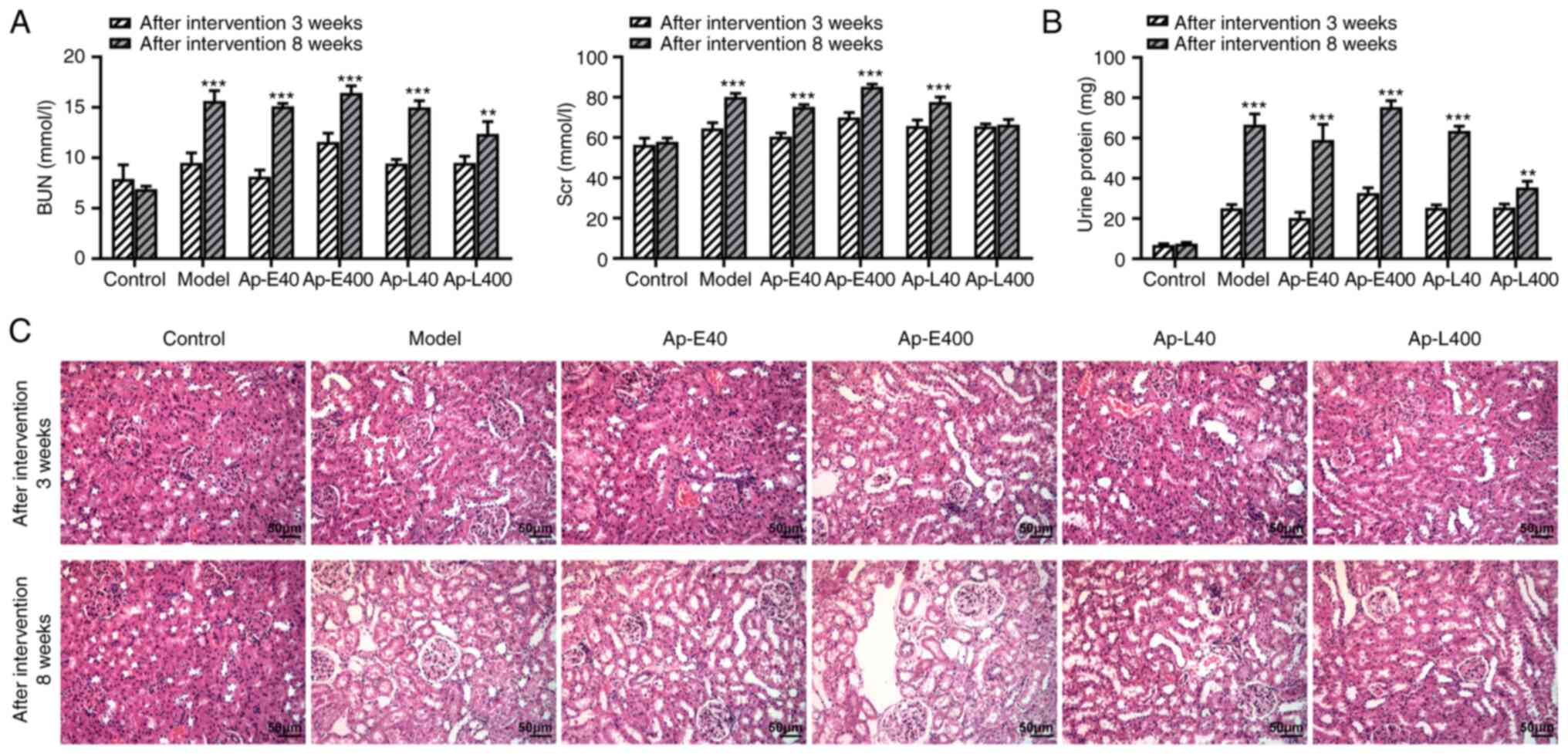
Apelin-13 therapy improves renal function
of diabetic nephropathy rats
The increased BUN and Scr levels indicated the
occurrence and development of renal injury (20). BUN and Scr levels were revealed
to be higher after the apelin-13 injection at 8 weeks compared with
that at 3 weeks (Fig. 2A).
Similarly, the urinary protein content and extent of pathological
injury of renal tissues also revealed the same trends (Fig. 2B and C).
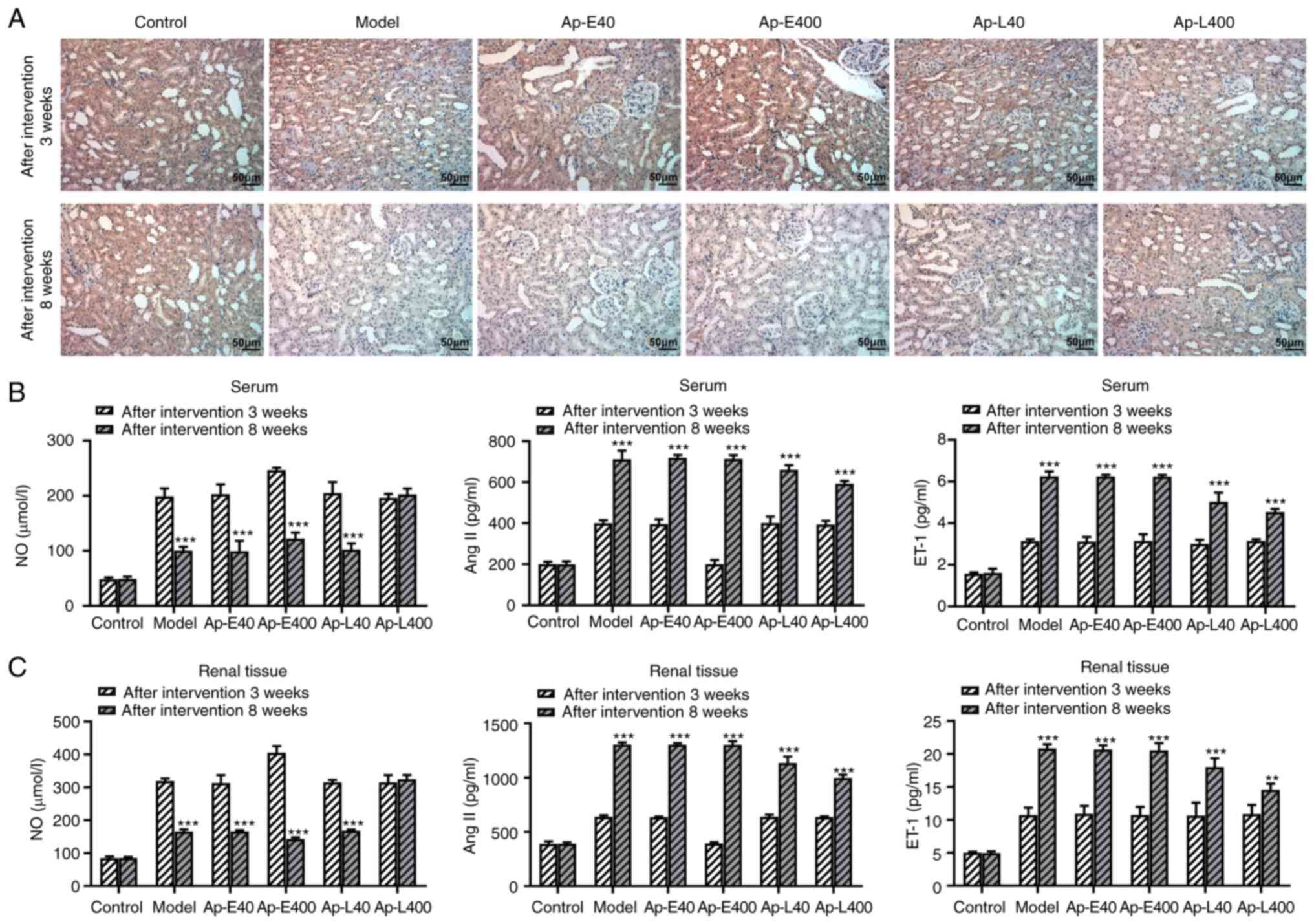
Apelin-13 alleviates diabetic nephropathy
by modulating the production of NO, AngⅡ and ET-1
The expression of APJ was detected and the results
(Fig. 3A) revealed that the
expression of APJ in the renal tissues of rats was decreased after
the apelin-13 injection at 8 weeks compared with that at 3 weeks.
NO production could relieve the diabetic nephropathy-induced
vascular damage in the renal tissue (21). Therefore, the production of NO,
Ang II and ET-1 was detected in the renal tissues. The results
revealed that the levels of NO were decreased after the injection
of apelin-13 at 8 weeks compared with that at 3 weeks. However, the
levels of Ang II and ET-1 were increased after the injection of
apelin-13 at 8 weeks compared with that at 3 weeks (Fig. 3B and C). Next, the expression of
eNOS and AT1R was detected by western blotting. As revealed in
Fig. 4A, the expression of AT1R
was suppressed after the injection of apelin-13 at 3 and 8 weeks
compared with the model group, respectively. The levels of eNOS
were increased after the injection of apelin-13 at 3 and 8 weeks
compared with the model group.
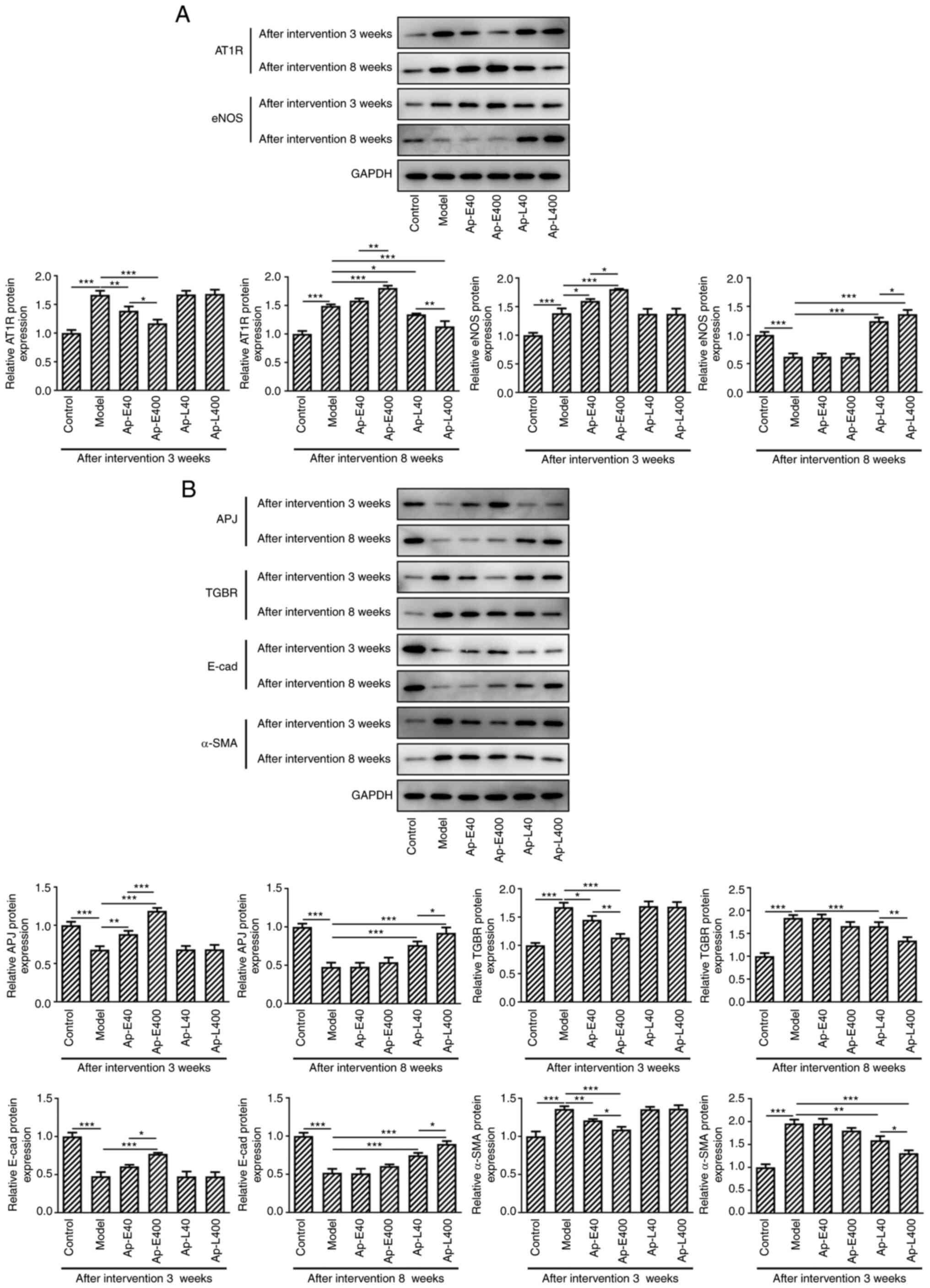
Apelin-13 alleviates diabetic nephropathy
by modulating the expression of AT1R, endothelial nitric eNOS and
renal fibrosis-related proteins in APJ pathway
The expression of APJ was increased and that of TGBR
was decreased after the injection of apelin-13 at 3 and 8 weeks
compared with the model group. The expression levels of the
fibrosis-related proteins, E-cadherin and α-SMA, were detected, and
the results revealed that the expression of E-cadherin was enhanced
and the levels of α-SMA were decreased after the injection of
apelin-13 at 3 and 8 weeks compared with the model group (Fig. 4B).
Apelin-13 promotes proliferation and tube
formation, and modulates the expression of renal fibrosis-related
proteins in the APJ pathway in short-term high glucose-induced
renal vascular endothelial cells
hRGEC cells were cultured in high-glucose medium for
24, 48 and 72 h. Next, a CCK-8 assay was performed to detect cell
viability. The results (Fig. 5A)
revealed that treatment with apelin-13 promoted cell viability. The
application of apelin-13 also promoted lumen formation (Fig. 5B). At 24 h of apelin-13
treatment, it was revealed that the expression levels of APJ, eNOS
and E-cadherin were increased and the levels of TGBR, AT1R and
α-SMA were decreased compared with the model group (Fig. 5C).
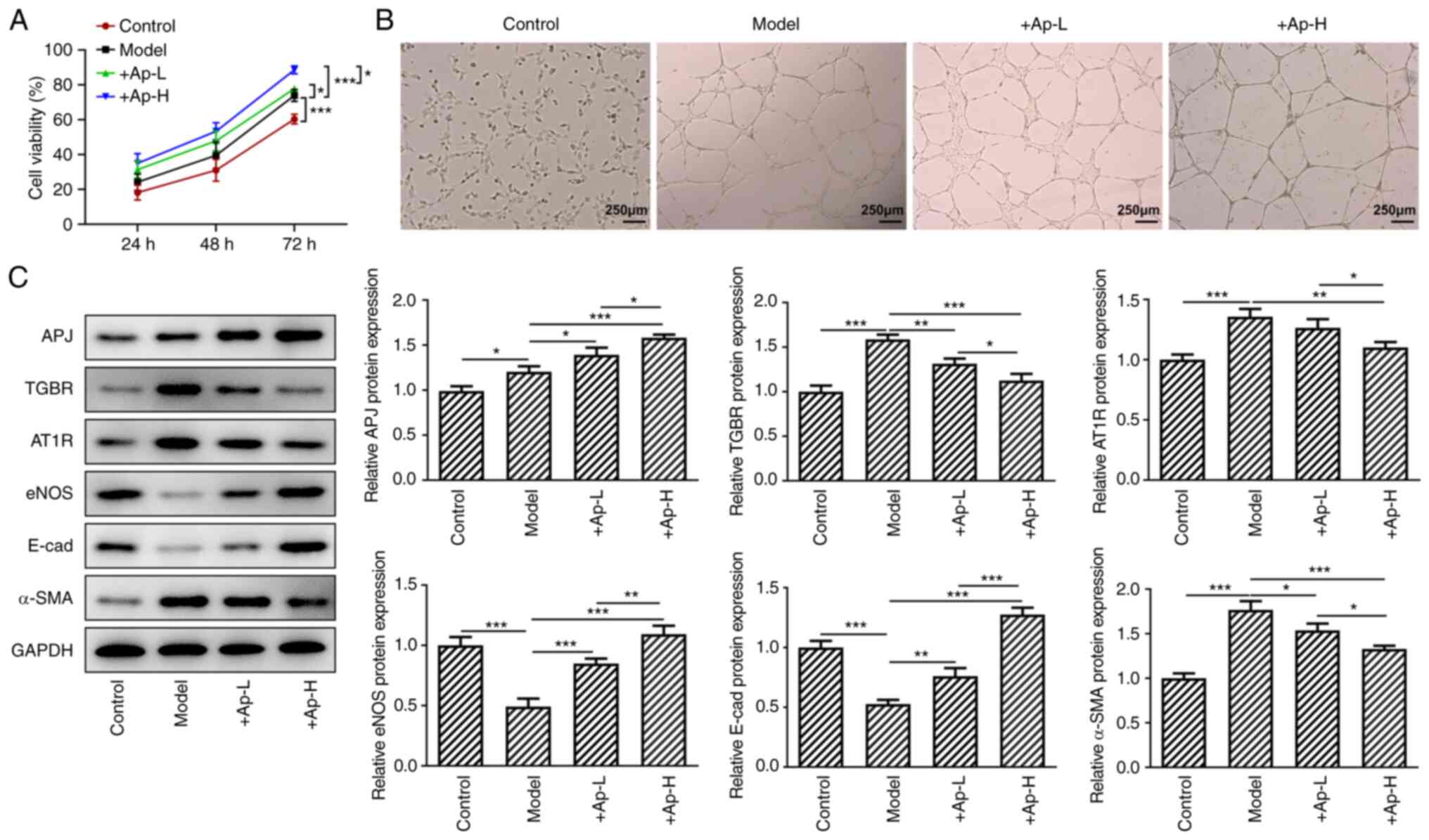 | Figure 5Apelin-13 alleviates the higher
glucose-induced injury of endothelial cells of glomerular vessels.
(A) Cell viability was detected with the Cell Counting Kit-8 assay.
(B) hRGECs were detected with the lumen formation experiments
(magnification, x4). (C) The expression of AT1R, eNOS, APJ, TGBR,
E-cadherin and α-SMA in these cells was determined with the western
blotting. *P<0.05, **P<0.01 and
***P<0.001. AT1R, Ang II type 1 receptor; eNOS,
endothelial nitric oxide synthase; APJ, angiotensin domain type 1
receptor-associated proteins; TGBR, transforming growth factor β
receptor; α-SMA, α-smooth muscle actin. |
Apelin-13 suppresses proliferation and
tube formation, and modulates the expression of renal
fibrosis-related proteins in the APJ pathway in long-term high
glucose-induced renal vascular endothelial cells
Cells were treated with high-glucose medium for 72
and 96 h, and interference with apelin-13 began from 72 h. After 24
h, the CCK-8 assay results revealed that the application of
apelin-13 supressed cell viability compared with the model group
(Fig. 6A). Lumen formation was
inhibited following treatment with apelin-13 (Fig. 6B). In addition, treatment with
apelin-13 also promoted the expression of APJ, eNOS and E-cadherin
compared with the model group. The application of apelin-13 also
suppressed the expression of TGBR, AT1R and α-SMA (Fig. 6C) compared with the model group
in a dose-dependent manner.
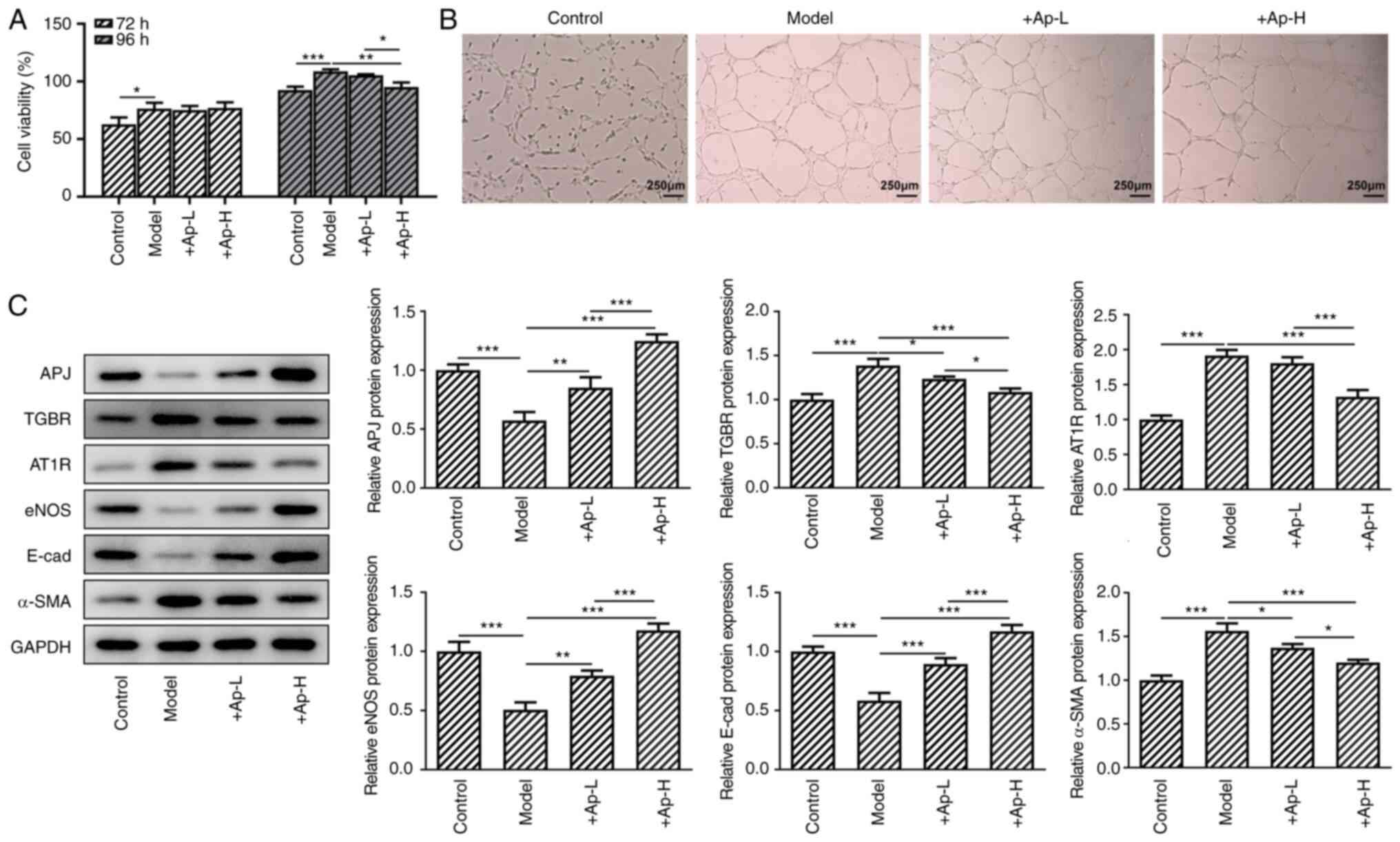 | Figure 6Apelin-13 relieves the injury of
endothelial cells of glomerular vessels by suppressing fibrosis.
(A) Cell viability of endothelial cells of glomerular vessels was
determined with Cell Counting Kit-8 assay. (B) hRGECs were detected
with the lumen formation experiments (magnification, x4). (C) The
levels of AT1R, eNOS, APJ, TGBR, E-cadherin and α-SMA in these
cells were determined with western blotting. *P<0.05,
**P<0.01 and ***P<0.001. AT1R, Ang II
type 1 receptor; eNOS, endothelial nitric oxide synthase; APJ,
angiotensin domain type 1 receptor-associated proteins; TGBR,
transforming growth factor β receptor; α-SMA, α-smooth muscle
actin. |
Discussion
Diabetes is a metabolic disease and glucose
metabolism disorder is its main clinical manifestation. In recent
years, the incidence of this disease has been steadily increasing
(22). Diabetes leads to the
damage and dysfunction of several organs. Diabetes-induced kidney
damage is one of the most prominent causes of mortality in patients
with diabetes (23). The main
clinical manifestations of diabetic nephropathy have been revealed
to be proteinuria and persistent decline in renal function
(24). In addition, the
occurrence and development of diabetic nephropathy have been
revealed to promote the development of cardiovascular disease
(25). Therefore, the need to
develop a new therapy to suppress the damage caused by diabetic
nephropathy in patients is urgent.
Apelin is a fat factor in adipose tissues. A recent
study reported that apelin could relieve the clinical
manifestations of patients with diabetes by regulating blood
glucose levels (26). Another
recent study reported that apelin-36 could inhibit insulin
secretion induced by high-concentration glucose (16.7 mmol/l), but
had no effect on insulin secretion induced by low-concentration
glucose (2.8 mmol/l) (27). In
the bodies of obese and insulin-resistant mice, apelin could
relieve insulin resistance and restore the use of glucose in the
body (28). However, in the
field of diabetic nephropathy, a recent study reported that the
levels of apelin and its receptor APJ in the serum of patients with
diabetic nephropathy were increased, and higher levels of apelin
and APJ promoted blood vessel formation and induced the
proliferation of glomerular capillaries, thereby promoting the
occurrence and development of diabetic nephropathy (29). However, a different study also
indicated that apelin-13 could inhibit the transformation of renal
tubular epithelial cells in a high-glucose environment, thereby
inhibiting the occurrence and development of the EMT process in
glomerular cells, ultimately delaying the occurrence of diabetic
nephropathy (30). In the
present study, it was demonstrated that the use of apelin-13
decreased the levels of blood glucose and relieved insulin
resistance in these rats, as well as reduced the urinary protein
content. Apelin-13 treatment also alleviated the considerable
glucose-induced damage in the endothelial cells of glomerular
vessels. Collectively, these results indicated that the use of
apelin-13 relieved the manifestations of diabetic nephropathy.
A previous study revealed that apelin could reduce
blood glucose content by regulating the expression of eNOS and
activating the Akt pathway (31). The expression of apelin could
also produce NO by activating APJ, therefore relieving the clinical
manifestations of diabetic nephropathy (32). APJ is the receptor of apelin-13,
and apelin-13 could exert its biological effects by binding to the
APJ (33). In the present study,
the production of NO and expression of eNOS and APJ were also
detected. It was revealed that the expression levels of eNOS and
APJ were enhanced in renal tissues and endothelial cells of
glomerular vessels following treatment with apelin-13. NO
production was also enhanced in the renal tissues of rats following
treatment with apelin-13. These results indicated that apelin-13
promoted the expression of eNOS by activating APJ. Therefore,
higher levels of eNOS enhanced NO production, ultimately promoting
vascular permeability and renal filtration.
Recent studies have indicated that renal fibrosis is
a characteristic of diabetic nephropathy (34,35). The results of the present study
revealed that the expression of E-cadherin was enhanced in cells
and renal tissues following treatment with apelin-13, while that of
α-SMA was decreased. Apelin-13 treatment was also revealed to
alleviate diabetic nephropathy by suppressing the fibrosis of renal
tissues and endothelial cells of glomerular vessels.
In conclusion, the present study reported the
effects of apelin-13 on the development of diabetic nephropathy.
The results of the present study revealed that apelin-13 alleviated
diabetic nephropathy by promoting the production of NO and
relieving fibrosis in renal tissues. However, the present study
also has a limitation. Oxidative stress and inflammation play
important roles in the development of diabetic nephropathy
(36-40). Whether apelin-13 improved
diabetic nephropathy by reducing inflammation and oxidative stress
warrants investigation in our future study.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZG conceived the study and revised the manuscript.
ZG and XZ performed the experiments and were responsible for
drafting manuscript. All authors (ZG, XZ, YXT and DL) performed
the data analysis and organized the figures. ZG, XZ
and YXT confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All the experiments were performed according to the
Guide for the Care and Use of Laboratory Animals 8th edition and
approved (approval no. 2020-79-PJ01) by the Ethics Committee of the
Air Force Medical Center (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
References
|
1
|
Yu SY, Dong B, Fang ZF, Hu XQ, Tang L and
Zhou SH: Knockdown of lncRNA AK139328 alleviates myocardial
ischaemia/reperfusion injury in diabetic mice via modulating
miR-204-3p and inhibiting autophagy. J Cell Mol Med. 22:4886–4898.
2018. View Article : Google Scholar
|
|
2
|
Rowley WR and Bezold C: Creating public
awareness: State 2025 diabetes forecasts. Popul Health Manag.
15:194–200. 2012. View Article : Google Scholar
|
|
3
|
Rowley WR, Bezold C, Arikan Y, Byrne E and
Krohe S: Diabetes 2030: Insights from yesterday, today, and future
trends. Popul Health Manag. 20:6–12. 2017. View Article : Google Scholar
|
|
4
|
American Diabetes Association: Standards
of medical care in diabetes-2014. Diabetes Care. 37(Suppl 1):
S5–S13. 2014. View Article : Google Scholar
|
|
5
|
Ahlqvist E, van Zuydam NR, Groop LC and
McCarthy MI: The genetics of diabetic complications. Nat Rev
Nephrol. 11:277–287. 2015. View Article : Google Scholar
|
|
6
|
Navarro-González JF, Jarque A, Muros M,
Mora C and García J: Tumor necrosis factor-alpha as a therapeutic
target for diabetic nephropathy. Cytokine Growth Factor Rev.
20:165–173. 2009. View Article : Google Scholar
|
|
7
|
Alvarez ML and Distefano JK: The role of
non-coding RNAs in diabetic nephropathy: Potential applications as
biomarkers for disease development and progression. Diabetes Res
Clin Pract. 99:112013. View Article : Google Scholar
|
|
8
|
Del Ry S, Cabiati M, Raucci S, Simioniuc
A, Caselli C, Prescimone T and Giannessi D: Sequencing and cardiac
expression of Apelin in Sus Scrofa. Pharmacol Res. 60:314–319.
2009. View Article : Google Scholar
|
|
9
|
Fasshauer M and Blüher M: Adipokines in
health and disease. Trends Pharmacol Sci. 36:461–470. 2015.
View Article : Google Scholar
|
|
10
|
Lv D, Li H and Chen L: Apelin and APJ, a
novel critical factor and therapeutic target for atherosclerosis.
Acta Biochim Biophys Sin (Shanghai). 45:527–533. 2013. View Article : Google Scholar
|
|
11
|
Wysocka MB, Pietraszek-Gremplewicz K and
Nowak D: The role of apelin in cardiovascular diseases, obesity and
cancer. Front Physiol. 9:5572018. View Article : Google Scholar
|
|
12
|
Sentinelli F, Capoccia D, Bertoccini L,
Barchetta I, Incani M, Coccia F, Manconi E, Lenzi A, Cossu E,
Leonetti F, et al: Search for genetic variant in the apelin gene by
resequencing and association study in European subjects. Genet Test
Mol Biomarkers. 20:98–102. 2016. View Article : Google Scholar
|
|
13
|
Onalan E, Yakar B, Barım AO and Gursu MF:
Serum apelin and resistin levels in patients with impaired fasting
glucose, impaired glucose tolerance, type 2 diabetes, and metabolic
syndrome. Endokrynol Pol. 71:319–324. 2020.
|
|
14
|
Adki KM and Kulkarni YA: Potential
biomarkers in diabetic retinopathy. Curr Diabetes Rev. 16:971–983.
2020. View Article : Google Scholar
|
|
15
|
Wu R, Zhu Z and Zhou D: VEGF, apelin and
HO-1 in diabetic patients with retinopathy: A correlation analysis.
BMC Ophthalmol. 20:3262020. View Article : Google Scholar
|
|
16
|
Yasir M, Senthilkumar GP, Jayashree K,
Ramesh Babu K, Vadivelan M and Palanivel C: Association of serum
omentin-1, apelin and chemerin concentrations with the presence and
severity of diabetic retinopathy in type 2 diabetes mellitus
patients. Arch Physiol Biochem. Nov 5–2019.Epub ahead of print.
View Article : Google Scholar
|
|
17
|
Sabouri M, Norouzi J, Zarei Y, Sangani MH
and Hooshmand Moghadam B: Comparing high-intensity interval
training (HIIT) and continuous training on Apelin, APJ, NO, and
cardiotrophin-1 in cardiac tissue of diabetic rats. J Diabetes Res.
2020:14725142020. View Article : Google Scholar
|
|
18
|
Dawood AF, Sabry MM, Estaphan SA, Mohamed
EA, Younes SF, Rashed LA and Elzainy AW: Cross-talk between apelin
and vasopressin in response to different osmotic stimuli in type 2
diabetic rats. J Biol Regul Homeost Agents. 32:1117–1127. 2018.
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
20
|
Chen Y, Lin L, Tao X, Song Y, Cui J and
Wan J: The role of podocyte damage in the etiology of
ischemia-reperfusion acute kidney injury and post-injury fibrosis.
BMC Nephrol. 20:1062019. View Article : Google Scholar
|
|
21
|
Xue M, Shi Y, Pang A, Men L, Hu Y, Zhou P,
Long G, Tian X, Wang R, Zhao Y, et al: Corin plays a protective
role via upregulating MAPK and downregulating eNOS in diabetic
nephropathy endothelial dysfunction. FASEB J. 34:95–106. 2020.
View Article : Google Scholar
|
|
22
|
Rossi L and Gesualdo L: Diabetic
nephropathy and cardiovascular risk. G Ital Nefrol. 34(Suppl 69):
S104–S118. 2017.In Italian.
|
|
23
|
Van Krieken R and Krepinsky JC: Caveolin-1
in the pathogenesis of diabetic nephropathy: Potential therapeutic
target? Curr Diab Rep. 17:192017. View Article : Google Scholar
|
|
24
|
Saran R, Robinson B, Abbott KC, Agodoa
LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A,
Eggers PW, et al: US renal data system 2017 annual data report:
Epidemiology of kidney disease in the United States. Am J Kidney
Dis. 71(3 Suppl 1): A72018. View Article : Google Scholar
|
|
25
|
Khan NU, Lin J, Liu X, Li H, Lu W, Zhong
Z, Zhang H, Waqas M and Shen L: Insights into predicting diabetic
nephropathy using urinary biomarkers. Biochim Biophys Acta Proteins
Proteom. 1868:1404752020. View Article : Google Scholar
|
|
26
|
Castan-Laurell I, El Boustany R, Pereira
O, Potier L, Marre M, Fumeron F, Valet P, Gourdy P, Velho G and
Roussel R: Plasma Apelin and risk of type 2 diabetes in a cohort
from the community. Diabetes Care. 43:e15–e16. 2020. View Article : Google Scholar
|
|
27
|
Guo YY, Li T, Liu H, Tang L, Li YC, Hu HT,
Su YF, Lin Y, Wang YY, Li C, et al: Circulating levels of Elabela
and Apelin in the second and third trimesters of pregnancies with
gestational diabetes mellitus. Gynecol Endocrinol. 36:890–894.
2020. View Article : Google Scholar
|
|
28
|
Tanday N, Irwin N, Moffett RC, Flatt PR
and O'Harte FPM: Beneficial actions of a long-acting apelin
analogue in diabetes are related to positive effects on islet cell
turnover and transdifferentiation. Diabetes Obes Metab. 22. pp.
2468–2478. 2020, View Article : Google Scholar
|
|
29
|
Bae JH, Kwak SE, Lee JH, Yangjie Z and
Song W: Does exercise-induced apelin affect sarcopenia? A
systematic review and meta-analysis. Hormones (Athens). 18:383–393.
2019. View Article : Google Scholar
|
|
30
|
Sabry MM, Mahmoud MM, Shoukry HS, Rashed
L, Kamar SS and Ahmed MM: Interactive effects of apelin,
renin-angiotensin system and nitric oxide in treatment of
obesity-induced type 2 diabetes mellitus in male albino rats. Arch
Physiol Biochem. 125:244–254. 2019. View Article : Google Scholar
|
|
31
|
Ji B, Shang L, Wang C, Wan L, Cheng B and
Chen J: Roles for heterodimerization of APJ and B2R in promoting
cell proliferation via ERK1/2-eNOS signaling pathway. Cell Signal.
73:1096712020. View Article : Google Scholar
|
|
32
|
Nagib AM, El-Diasty A, El Husseny MA,
El-Gamal EM, Abbas MH, Refaie AF and Foudas MA: Apelin and
new-onset diabetes after transplant in living kidney allograft
recipients. Exp Clin Transplant. 13:319–323. 2015.
|
|
33
|
Huang Z, Luo X, Liu M and Chen L: Function
and regulation of apelin/APJ system in digestive physiology and
pathology. J Cell Physiol. 234:7796–7810. 2019. View Article : Google Scholar
|
|
34
|
Wakisaka M, Kamouchi M and Kitazono T:
Lessons from the trials for the desirable effects of sodium glucose
Co-transporter 2 inhibitors on diabetic cardiovascular events and
renal dysfunction. Int J Mol Sci. 20:56682019. View Article : Google Scholar
|
|
35
|
Li A, Peng R, Sun Y, Liu H, Peng H and
Zhang Z: LincRNA 1700020I14Rik alleviates cell proliferation and
fibrosis in diabetic nephropathy via miR-34a-5p/Sirt1/HIF-1α
signaling. Cell Death Dis. 9:4612018. View Article : Google Scholar
|
|
36
|
Zhou X, Ma L, Habibi J, Whaley-Connell A,
Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG and Sowers JR:
Nebivolol improves diastolic dysfunction and myocardial remodeling
through reductions in oxidative stress in the Zucker obese rat.
Hypertension. 55:880–888. 2010. View Article : Google Scholar
|
|
37
|
Wada J and Makino H: Innate immunity in
diabetes and diabetic nephropathy. Nat Rev Nephrol. 12:13–26. 2016.
View Article : Google Scholar
|
|
38
|
Niewczas MA, Pavkov ME, Skupien J, Smiles
A, Md Dom ZI, Wilson JM, Park J, Nair V, Schlafly A, Saulnier PJ,
et al: A signature of circulating inflammatory proteins and
development of end-stage renal disease in diabetes. Nat Med.
25:805–813. 2019. View Article : Google Scholar
|
|
39
|
Mou Z, Feng Z, Xu Z, Zhuang F, Zheng X, Li
X, Qian J and Liang G: Schisandrin B alleviates diabetic
nephropathy through suppressing excessive inflammation and
oxidative stress. Biochem Biophys Res Commun. 508:243–249. 2019.
View Article : Google Scholar
|
|
40
|
Al Hroob AM, Abukhalil MH, Alghonmeen RD
and Mahmoud AM: Ginger alleviates hyperglycemia-induced oxidative
stress, inflammation and apoptosis and protects rats against
diabetic nephropathy. Biomed Pharmacother. 106:381–389. 2018.
View Article : Google Scholar
|