Introduction
Stroke is one of the leading causes of human
mortality and long-term disability worldwide (1), and its annual incidence is
gradually increasing. Approximately 80% of stroke cases involve
ischemic stroke, which affects the daily life and work of patients,
and places a heavy burden on family and society (2). Brain ischemia is caused by blood
vessel obstruction (insufficiency) and brain damage is caused by
vascular occlusion (3). The
greater the decrease in cerebral blood flow, the more severe the
brain damage (4). The
pathophysiological mechanisms are complex and include inflammation,
apoptosis, excitotoxicity and periinfarct depolarization (5). The reduction of inflammation and
elimination of inflammatory genes exerts neuroprotective effects
against brain damage following ischemic stroke (6). In addition, cerebral ischemia can
affect the innate and adaptive immune systems, and can induce
neuronal apoptosis (7). Although
drugs have been developed to target inflammation, oxidative stress,
apoptosis and other pathophysiological mechanisms, there remains a
lack of effective neuroprotectors to prevent cerebral ischemia
(8).
Thrombolytic therapy is one of the strategies
recommended for the treatment of brain ischemia; however, its use
is limited by a very narrow time window and adverse effects
(9). Recombinant tissue
plasminogen activator, a thrombolytic agent, is the only approved
thrombolytic therapy, and it must be administered within a limited
time frame in order for a clinical benefit to be achieved (10). Therefore, the identification of
novel treatment strategies for stroke is essential. Notably,
traditional Chinese medicine (TCM) has a long history in the
treatment of ischemic stroke.
Chuanxinlian is the dry, aerial part of
Andrographis paniculata, and one of its main components is
andrographolide (Andro). In a previous study, a rat model of
permanent middle cerebral artery occlusion (pMCAO) was established
to investigate the potential therapeutic effects of Andro in
cerebral ischemia; it was found that after Andro was administered
intraperitoneally to rats with pMCAO, the neurological behavior
scores and infarction area were decreased (11). Andro may exert therapeutic
effects against hippocampal neuron injury and cognitive dysfunction
induced by chronic cerebral hypoperfusion (CCH), and it also exerts
a potential neuroprotective effect in cases of hippocampal neuronal
damage and cognitive impairment from CCH due to the suppression of
astrocyte activation (12). The
present study aimed to further explore the effects of Andro on
cerebral ischemia-reperfusion through both network analysis and
in vivo experiments.
Network pharmacology is a novel drug research method
that combines systems biology, multi-direction pharmacology,
network analysis and computer technology. The association between
drugs and disease has been investigated by combining drug component
targets and disease targets from multiple perspectives (13,14). In the present study, the active
components and disease targets of Chuanxinlian against ischemic
stroke were summarized using the database of network pharmacology,
and the association between the active components of Chuanxinlian,
targets and pathways was explored through multi-directional systems
biology. A TCM-based network pharmacology method was used to
establish and analyze compound-target-disease and function-pathway
networks in order to elucidate the possible mechanisms through
which Andrographis paniculata exerts its protective effects
against cerebral ischemia-reperfusion injury (CIRI). The classical
pharmacology of Andro was also studied in vivo to verify the
results of network pharmacology. Our study aims to provide a
scientific basis for the clinical application of Andro in stroke.
In addition, this study provides theoretical support for subsequent
experimental studies.
Materials and methods
Animals
A total of 90 male 6-week-old specific pathogen-free
(SPF) Kunming mice weighing 18-22 g were obtained from Jinan
Pengyue Experimental Animal Breeding Co., Ltd. (Laboratory animal
certificate: SCXK 2014-0007). The animals were housed in cages with
an ambient temperature of 23-25°C and a humidity of 35-45%, under a
12-h light/dark cycle beginning at 08:00; standard mouse chow and
water were provided ad libitum. The present study was
performed in accordance with the recommendations of the Ministry of
Science and Technology of China's Guidance for the Care and Use of
Laboratory Animals. All procedures were approved by the Laboratory
Animal Ethics Committee of Henan University of Chinese Medicine
(the number of approval ethics code: DWLL201903026).
Drug administration
Using high-performance liquid chromatography, the
purity of Andro provided by Shanghai Yuanye Bio-Technology Co.,
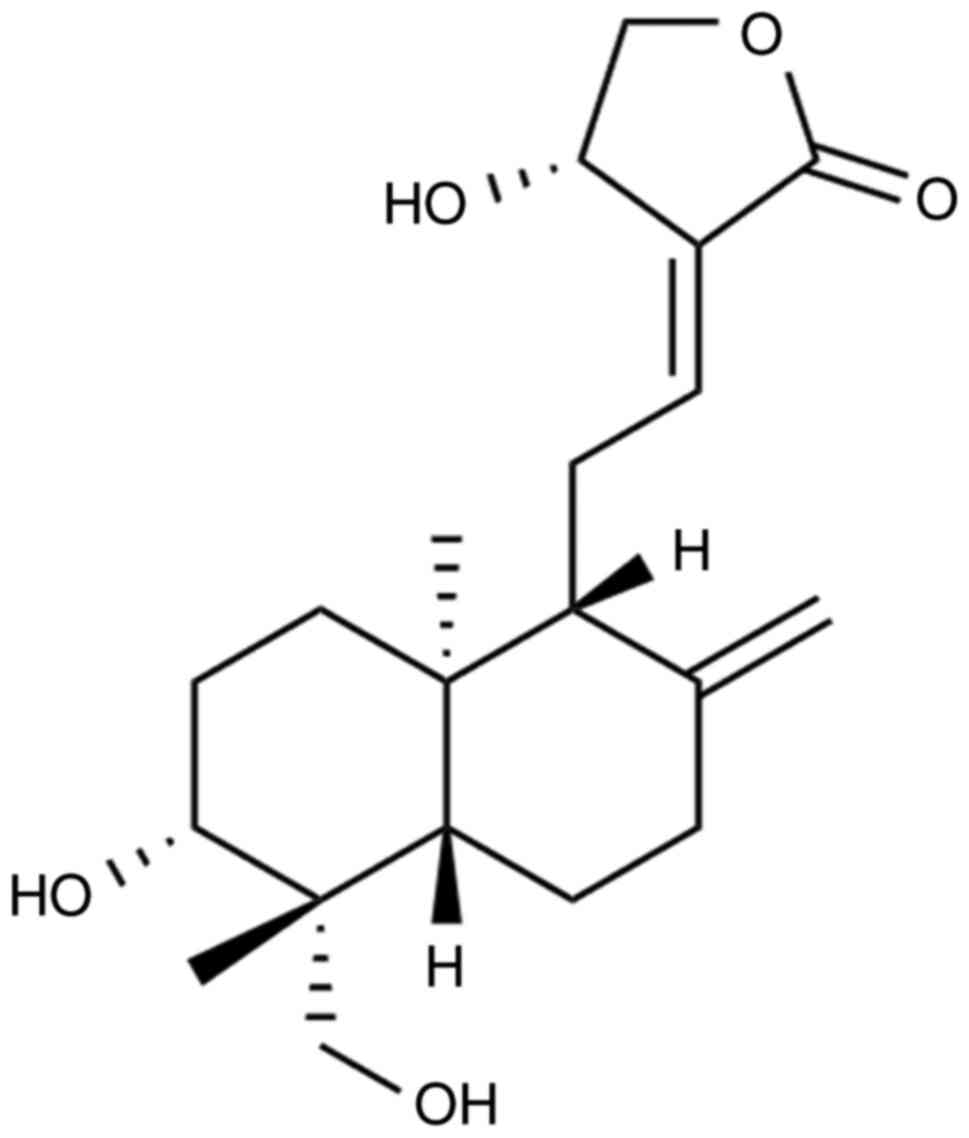
Ltd. (chemical structure presented in Fig. 1) was determined to be 98%. The
maximum dose of Andro used in the present study (120 mg/kg; 12
mg/ml) was selected based on a previous study (15) and preliminary experiments (data
not shown). A 200 mg/ml solution of Andro dissolved in dimethyl
sulfoxide was prepared and diluted in distilled water to the
appropriate concentration prior to use. The animals were randomly
assigned to 5 groups as follows: The sham-operated group (n=10),
repeated CIRI group (n=20) and 3 Andro groups (30, 60 and 120
mg/kg) (n=20). Following randomization, the corresponding drugs
were administered orally, with the sham-operated group and CIRI
group being treated with an equal volume of normal saline. At 1 h
after the final administration on the 14th day, the mice in each
group were subjected to repeated CIRI surgery.
Repeated CIRI in mice
Repeated CIRI was performed through bilateral common
carotid artery occlusion (BCCAO) according to a previously
published method (16). In
brief, anesthesia was initialized with 4% isoflurane inhalation and
then maintained with 1.5% isoflurane in 70% nitrous oxide
(N2O)/30% oxygen (O2) using a laboratory
anesthesia system (Matrix VIP 3000; Midmark Corp.). The bilateral
common carotid arteries (BCCAs) were exposed, the carotid artery
was lifted with 4-0 silk sutures, and the BCCAs were ligated with
artery clamps for 10 min. The artery clamps were then removed and
the BCCAs were visually inspected for reperfusion. After the blood
flow had been restored for 10 min, the BCCAs were again ligated for
10 min. The sham-operated mice received the same procedure, but
without carotid artery ligation. Wounds were sutured with a 4-0
silk suture. During the surgery, the temperature was maintained at
37±0.5°C. To ameliorate the suffering of animals observed
throughout the experimental period, the animals were euthanized
with 4% isoflurane inhalation followed by the dislocation of the
cervical vertebra. Animal death was confirmed by respiratory and
cardiac arrest, and no righting reflex. The brain tissues were
removed (n=3), then fixed in 4% paraformaldehyde and embedded in
paraffin prior to hematoxylin and eosin (H&E), Nissl and
immunofluorescence staining. The other brain tissues of the mice
were cryopreserved in liquid nitrogen for western blot analysis,
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and biochemical detection.
H&E staining
Mouse brains were excised and fixed overnight in 4%
paraformaldehyde at 4°C, embedded in paraffin and serial
5-µm-thick sections were prepared. The sections were stained
with H&E (C0105, Beyotime Institute of Biotechnology) to assess
brain histology. Briefly, the sections were stained with
hematoxylin for 5 min, then washed with running water for 5 min and
differentiated in 1% acid alcohol for 10 sec at room temperature.
The sections were then stained with 1% eosin for 1 min, then washed
with running water and dehydrated in a decreasing concentration of
alcohol (70, 80, 90 and 100%) and cleared in xylene. Finally, the
sections were mounted with neutral resin and observed under a
microscope (Olympus, BX63; Olympus Corporation).
Nissl staining
The brain tissue sections were stained with Nissl
staining solution (C0117, Beyotime Institute of Biotechnology) for
30 min at 37°C. The brain sections were then washed with 95% ethyl
alcohol and observed under a microscope (Olympus, BX63; Olympus
Corporation). Large cell bodies with abundant cytoplasm and
significant levels of Nissl bodies represent normal neurons. Cells
with pyknosis or blurring of Nissl bodies represent damaged
cells.
Immunofluorescence staining
Serial 5-µm-thick paraffinembedded sections
were baked at 60°C for 30 min, deparaffinized with xylene and
rehydrated. The sections were then submerged into the citrate
antigen retrieval buffer, heated in a microwave for antigen
retrieval, treated with 3% hydrogen peroxide to quench endogenous
peroxidase activity and incubated with 1% bovine serum albumin to
block non-specific binding. The sections were incubated with
primary antibody [glial fibrillary acidic protein (GFAP), 1:500,
GB12096, Wuhan Servicebio Technology Co., Ltd.; neuronal nuclei
(NeuN), 1:500, GB11138, Wuhan Servicebio Technology Co., Ltd.]
overnight at 4°C. After washing, the tissue sections were treated
with fluorescence-conjugated secondary antibody (G1213-100UL, Wuhan
Servicebio Technology Co., Ltd.) in the dark (37°C) for 2 h. After
washing with PBS, the sections were immediately examined under a
fluorescence microscope (Olympus, BX63; Olympus Corporation).
Cytokine analysis
The infarct sides of brain tissues following
repeated CIRI surgery were used for assessing expression
brain-derived neurotrophic factor (BDNF) and pro-inflammatory
cytokines. The levels of BDNF and pro-inflammatory cytokines in the
supernatant of the brain homogenates or serum were detected by
enzyme-linked immunosorbent assay (ELISA).
Western blot analysis
The brain tissues of the mice were removed from the
liquid nitrogen and placed in a pre-cooled mortar that had been
cleaned and dried at high temperature in advance. The liquid
nitrogen was poured into the mortar, and the tissues were ground
into a powder in the liquid nitrogen. Approximately 100 mg of brain
tissue powder were weighed and placed in a 5-ml EP tube, and 1 ml
of RIPA lysis buffer containing 1% PMSF and 1% phosphatase
inhibitor was added. Extraction with a syringe was performed until
the tissue was completely fragmented completely and in full contact
with the lysis buffer. The lysate was then placed on ice for 30
min. Lysates were centrifuged at 12,000 × g for 15 min in a
high-speed centrifuge at 4°C, and the supernatant was collected to
obtain total protein. The protein concentration was measured using
a BCA protein assay kit (Beijing Solarbio Science & Technology
Co., Ltd.). The samples were then separated on 10% SDS-PAGE gels
and electro-transferred to polyvinylidene fluoride (PVDF)
membranes. The membranes were blocked in Tris-buffered saline (TBS)
with 5% non-fat milk for 2 h at room temperature, then incubated
with primary antibodies [tropomyosin receptor kinase B (TrkB),
1:800, GB11295-1, Wuhan Servicebio Technology Co., Ltd.; GFAP,
1:1,000, GB12096, Wuhan Servicebio Technology Co., Ltd.; NeuN,
1:800, GB11138, Wuhan Servicebio Technology Co., Ltd.; p-PI3K,
1:1,000, 17366, Cell Signaling Technology, Inc.; PI3K, 1:800, 4257,
Cell Signaling Technology, Inc.; Akt, 1:800, 4685, Cell Signaling
Technology, Inc.; p-Akt, 1:1,000, 4058, Cell Signaling Technology,
Inc.] at 4°C overnight. Subsequently, the membranes were washed
with Tris-buffered saline/Tween-20 (TBST) and incubated with
secondary antibodies, then washed with TBS. The secondary antibody
used was IRDye800CW goat anti-rabbit antibody (1:20,000; LI-COR
Biosciences). Finally, labeled proteins were detected using a
near-infrared imaging system (LI-COR Biosciences) and analyzed
using an image analyzer (Image Studio™ Software). The densitometric
values were normalized to the corresponding GAPDH densitometry
values.
RT-qPCR
Total RNA was extracted from the brain tissues using
the TRIzol reagent (R0016, Beyotime Institute of Biotechnology).
RNA concentration of the samples was determined by Natodrop 2000
(Thermo Fisher Scientific, Inc.). The total RNA reverse
transcription was performed using the PrimeScript® RT
Master Mix Perfect Real-Time kit (RR036A, Takara Bio, Inc.). In the
following, cDNA was taken as the template for real-time PCR with TB
Green® Premix Ex Taq (RR420A, Takara Bio, Inc.). The
steps for qPCR included: Initial denaturation by incubating in 95°C
for 30 sec; 40 qPCR cycles by 95°C for 5 sec and then 60°C for 30
sec in each cycle. Each sample was determined triply and relative
expression levels were analyzed using the 2−ΔΔCq method
(17). β-actin was used for
normalization. The sequences of the primers are presented in
Table I.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Gene | Sense primer
(5′-3′) | Antisense primer
(5′-3′) |
|---|
| β-actin |
GTGACGTTGACATCCGTAAAGA |
GTAACAGTCCGCCTAGAAGCAC |
| BDNF |
GCCCATGAAAGAAGTAAACGTCC |
AGTGTCAGCCAGTGATGTCGTC |
| IL-6 |
TTCTTGGGACTGATGCTGGTG |
GCCATTGCACAACTCTTTTCTC |
| IL-1β |
ACAGGCTCCGAGATGAACAAC |
GTGGGTGTGCCGTCTTTCAT |
| TNF-α |
ACCCTCACACTCACAAACCA |
ATAGCAAATCGGCTGACGGT |
Network pharmacology analyses
Network pharmacology analyses included the
following:
i) Establishment of
the drug database. Traditional Chinese Medicine Systems
Pharmacology (TCMSP) was used to screen, predict and collect the
active ingredients of Chuanxinlian using oral bioavailability (OB)
and drug-likeness (DL) as the limiting conditions.
ii) Targets of CXL
active ingredients. The simplified molecular-input line-entry
system (SMILES) chemical formulas of all active ingredients of
Chuanxinlian obtained in the current screen were downloaded from
the PubChem database and the SMILES chemical formulas corresponding
to the active ingredients were then entered into the similarity
ensemble approach (SEA; http://sea.bkslab.org/) database to obtain therapeutic
targets. Finally, the UniProtKB search function in the protein
database (http://www.uniprot.org/uniprot/) was applied, and
targets and UniProt numbers related to active ingredients were
obtained after all retrieved targets were associated to their
official symbol by entering the targets name and qualifying the
species as human.
iii) Potential disease
targets. Multiple databases (TTD, Drugbank and DisGeNET) were
searched to collect therapeutic targets related to ischemic stroke
using the terms 'Cerebral ischemic stroke', 'Cerebral infarction',
'Ischemic stroke' and 'Acute ischemic stroke'. The database of
targets related to ischemic stroke was constructed by comparing and
de-weighting the 3 databases.
iv) Protein
interaction. The targets of the active ingredients of
Chuanxinlian were intersected with the targets implicated in
ischemic stroke to obtain the targets of Chuanxinlian potentially
involved in treating ischemic stroke, referred to as
Chuanxinlian-target-cerebral ischemia. The String database
(https://string-db.org/) (17), a database containing known and
predicted protein-protein interactions, was used.
The protein targets of Chuanxinlian were imported
into the String database to obtain their interaction associations,
qualifying the species as human. The results were saved in TSV
format, and the nodes (node 1, node 2) and combined score
information in the file were reserved, then imported into Cytoscape
3.2.1 to draw the interaction network, and the network results were
analyzed and saved. Using the 'generate style from statistics' tool
in Cytoscape, the node size and color were set to reflect the
number of components connected to the target (degree), and the
thickness of edge setting was set to reflect the size of the
combined core. Finally, the ultimate protein interaction network
was obtained.
v) Construction of
active ingredient-target network diagram. The active
ingredients and targets of Cuanxinlian were imported into Cytoscape
3.2.1 software and the ingredient-target network diagram of
Chuanxinlian was constructed to predict the main targets and active
ingredients of Chuanxinlian involved in treating cerebral
ischemia.
vi) Analysis of
biological processes and pathways. The targets of Chuanxinlian
were input into the DAVID database (18), with the selected Identifier set
to the OFFICIAL GENE SYMBOL, List Type set to the GENE List, and
species limited to human. Gene ontology (GO) functional enrichment
analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analysis were carried out for the targets of Andro. Its
mechanism was investigated and the results were saved. The top
biological processes and signaling pathways were obtained by
setting P<0.05.
Statistical analysis
All data are expressed as the means ± standard
deviation and analyzed using SPSS 17.0 (SPSS, Inc.). One-way
analysis of variance and the Bonferroni post hoc test were used to
evaluate statistical significance. Results were considered
statistically significant when P<0.05.
Results
Components of Chuanxinlian and their
corresponding target proteins
Through the TCMSP database and literature
investigation, 49 chemical components of Chuanxinlian were
obtained. Using OB ≥30% and DL ≥0.18 as the filter criteria, 13
active ingredients were found to meet the requirements. Basic
information on the ingredients is presented in Table II.
 | Table IIBasic information on ingredients of
Chuanxinlian. |
Table II
Basic information on ingredients of
Chuanxinlian.
| Ingredient
code | Ingredient
name | OB (%) | DL |
|---|
| MOL000173 | Wogonin | 30.68 | 0.23 |
| MOL002928 | Oroxylin A | 41.37 | 0.23 |
| MOL002932 | Panicolin | 76.26 | 0.29 |
| MOL008203 |
Andrographolide | 57.06 | 0.34 |
| MOL008206 |
Moslosooflavone | 44.09 | 0.25 |
| MOL008218 | 1-Monoolein | 34.13 | 0.3 |
| MOL008219 |
3-[2-[(1R,4aS,5R,8aS)-5,8a-dimethyl-2-methylene-5-methylol-decalin-1-yl]ethyl]-5H-furan-2-one | 51.78 | 0.28 |
| MOL008228 | Andrographin | 37.57 | 0.33 |
| MOL008229 | Andrographin F | 33.34 | 0.85 |
| MOL008230 | Andrographidine
F_qt | 77.13 | 0.45 |
| MOL008232 |
(3Z,4S)-3-[2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5,8a-dimethyl-2-methylene-5-methylol-decalin-1-yl]ethylidene]-4-hydroxy-tetrahydrofuran-2-one | 46.96 | 0.36 |
| MOL008238 |
3-[2-[(1S,4aR,5S,8aR)-5,8a-dimethyl-2-methylene-5-methylol-decalin-1-yl]ethyl]-5H-furan-2-one | 63.54 | 0.28 |
| MOL008239 | Quercetin
tetramethyl(3′,4′,5,7) ether | 31.57 | 0.41 |
Target prediction
All targets were obtained from the TCMSP, SEA and
SIA databases, and the duplicates and false positives were removed.
Subsequently, 428 targets of Chuanxinlian were integrated; 421
ischemic stroke symptom targets were obtained from the DisGeNET,
Drugbank and TTD databases; in addition, 68 potential targets were
obtained from the intersection of Chuanxinlian targets and ischemic
stroke targets (Table II).
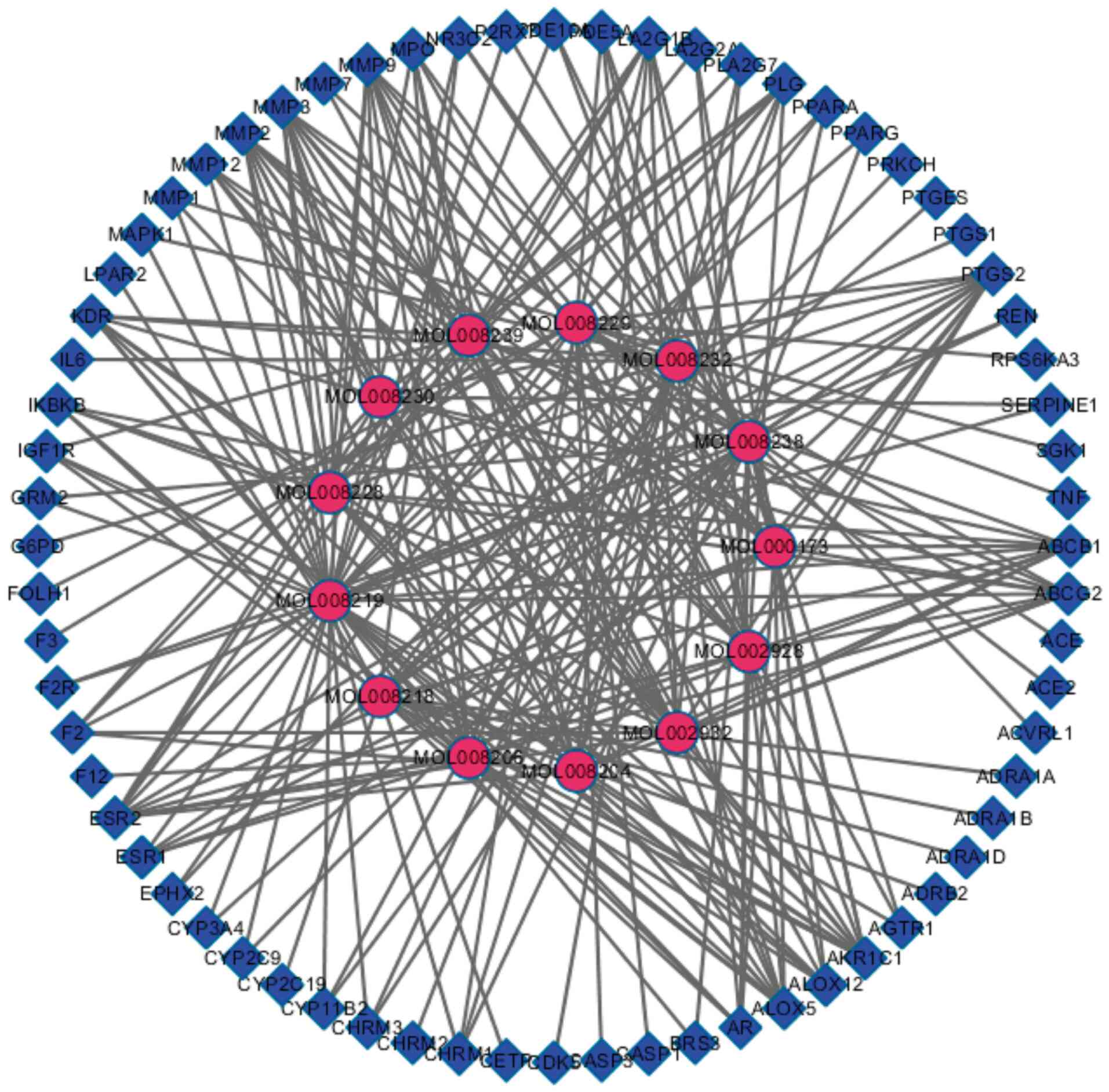
Active ingredient-target network
Cytoscape 3.2.1 was used to construct the
Andrographis paniculata active ingredient-anti-ischemic
target map (Fig. 2), which
contained 81 nodes and 249 edges. The rose-red-colored circles
represent the active ingredients of Chuanxinlian, while the
blue-colored squares represent the targets of these active
ingredients against cerebral ischemia, and the edge represents the
interaction between the active ingredient and anti-cerebral
ischemia targets.
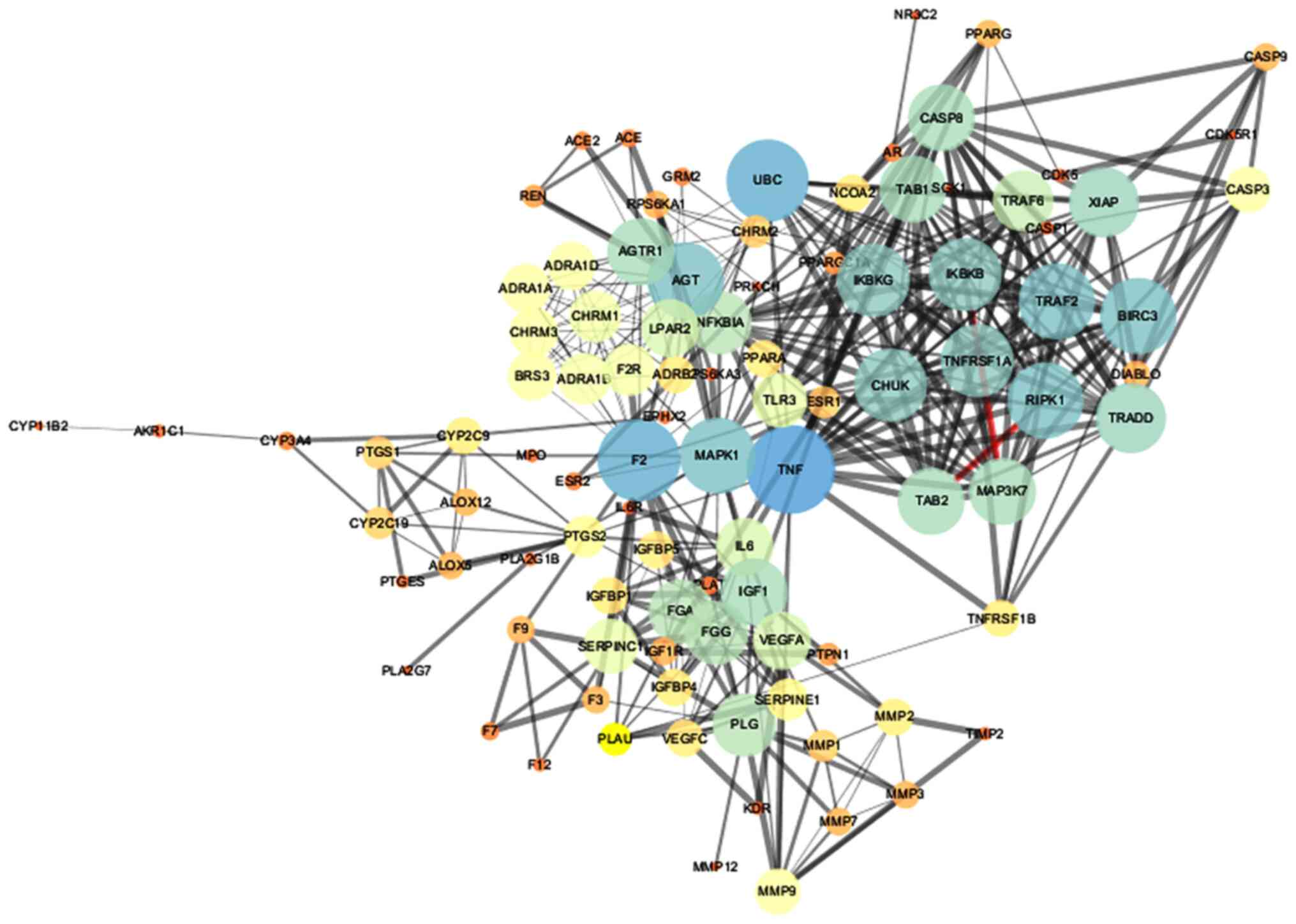
Protein interaction network diagram
Target interactions were obtained from the String
database, using Cytoscape 3.2.1 to draw the network diagram
(Fig. 3). The 'edge' represents
the correlation between the targets, and the thickness of the edge
corresponds to the combined score: The thicker the edge, the
greater the value of the combined score and the greater the degree
of association. The node represents the target, and the value of
'degree' represents the effect intensity. The larger the degree
value, the larger the node, and the larger the degree value
corresponding to the color ranged from blue to red. There were 98
nodes and 1,099 edges in the figure. The average node degree was
20.4 and the average local clustering coefficient was 0.624.
Finally, nodes with degree ≥5 and betweenness ≥0.00262056 were
selected as the main nodes; thus, there were a total of 22 main
nodes, as listed in Table
III.
 | Table IIIInformation on potential targets and
topological properties of Chuanxinlian against cerebral
ischemia. |
Table III
Information on potential targets and
topological properties of Chuanxinlian against cerebral
ischemia.
| No. | UniProt ID | Gene name | Protein name | Degree | Betweenness |
|---|
| 1 | O14920 | IKBKB | Inhibitor of
nuclear factor kappa-B kinase subunit beta | 18 | 0.00467253 |
| 2 | P00734 | F2 | Coagulation factor
II, thrombin | 11 | 0.00838562 |
| 3 | P00747 | PLG | Plasminogen | 15 | 0.05091082 |
| 4 | P01375 | TNF | Tumor necrosis
factor | 23 | 0.19812513 |
| 5 | P03372 | ESR1 | Estrogen receptor
1 | 6 | 0.01264776 |
| 6 | P05121 | SERPINE1 | Serpin family E
member 1 | 8 | 0.00336983 |
| 7 | P07550 | ADRB2 | Adrenoceptor beta
2 | 7 | 0.01043808 |
| 8 | P08069 | IGF1R | Insulin-like growth
factor 1 receptor | 5 | 0.00441784 |
| 9 | P08172 | CHRM2 | Muscarinic
acetylcholine receptor M2 | 6 | 0.00606293 |
| 10 | P08253 | MMP2 | Matrix
metallopeptidase 2 | 7 | 0.01000518 |
| 11 | P11712 | CYP2C9 | Cytochrome P450
family 2 subfamily C member 9 | 7 | 0.04508112 |
| 12 | P13726 | F3 | Coagulation factor
III, tissue factor | 5 | 0.00583017 |
| 13 | P14780 | MMP9 | Matrix
metallopeptidase 9 | 9 | 0.04962157 |
| 14 | P25116 | F2R | Coagulation factor
II thrombin receptor | 11 | 0.00838562 |
| 15 | P28482 | MAPK1 | Mitogen-activated
protein kinase 1 | 19 | 0.21744713 |
| 16 | P30556 | AGTR1 | Angiotensin II
receptor type 1 | 16 | 0.0567444 |
| 17 | P33261 | CYP2C19 | Cytochrome P450
family 2 subfamily C member 19 | 6 | 0.0237056 |
| 18 | P35354 | PTGS2 |
Prostaglandin-endoperoxide synthase 2 | 8 | 0.12466034 |
| 19 | P37231 | PPARG | Peroxisome
proliferator-activated receptor gamma | 5 | 0.04858176 |
| 20 | P42574 | CASP3 | Caspase-3 | 9 | 0.00262056 |
| 21 | Q07869 | PPARA | Peroxisome
proliferator-activated receptor alpha | 7 | 0.02624386 |
| 22 | Q9HBW0 | LPAR2 | Lysophosphatidic
acid receptor 2 | 13 | 0.01747176 |
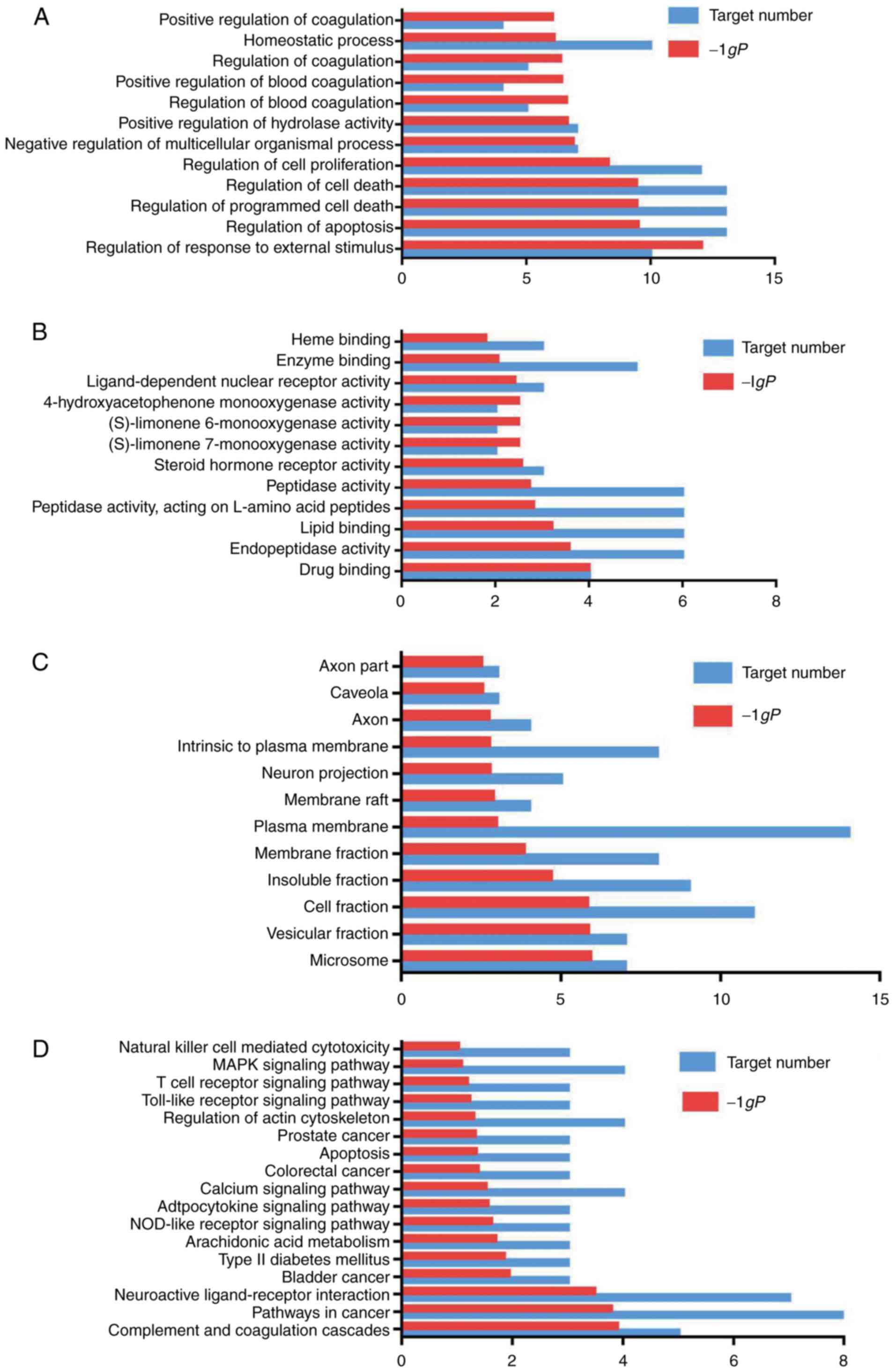
Gene function and pathway analysis
Gene function and pathway analysis revealed the
following results:
i) GO enrichment
analysis. The GO enrichment function and the KEGG pathway in
DAVID were used to analyze the 22 selected targets. GO enrichment
analysis includes 3 branches: Biological processes, molecular
function and cellular component. At the P<0.05 setting, 298
biological processes or pathways were obtained. Biological
processes or pathways with a higher P-value were screened, and
GraphPad Prism7.0 was used for mapping.
As shown in Fig.
4A, the main biological processes of the targets were the
regulation of response to an external stimulus, regulation of
apoptosis and the regulation of programmed cell death. As shown in
Fig. 4B, the main molecular
functions of the targets were drug binding, endopeptidase activity,
lipid binding and peptidase activity. Plasminogen (PLG) is a
single-chain glycoprotein that plays an important role in
thrombolytic therapy for acute cardiogenic cerebral infarction.
When stimulated, vascular endothelial cells secrete a large amount
of tissue-type plasminogen activator, which converts PLG into
fibrinase and this activates the fibrinolytic system in
vivo. Some autosomal genes associated with ischemic heart
disease, including the gene encoding PLG, have been identified
(19). PLG is also a key
molecule involved in the thrombolytic treatment of cerebral
ischemia. As shown in Fig. 4C,
the cell components of the targets included cell fraction, plasma
membrane and neuron projection.
ii) Pathway
analysis. The results of KEGG pathway enrichment analysis are
shown in Fig. 4D. The targets
identified were associated with the NOD-like receptor,
mitogen-activated protein kinase (MAPK) and phosphoinositide
3-kinase (PI3K)/Akt and Toll-like receptor signaling pathways. The
results revealed that the main active ingredients of Chuanxinlian
were largely distributed into different metabolic pathways. Thus,
the mechanisms through which Chuanxinlian protects against damage
following ischemic stroke may involve multiple ingredients,
multiple targets and multiple pathways.
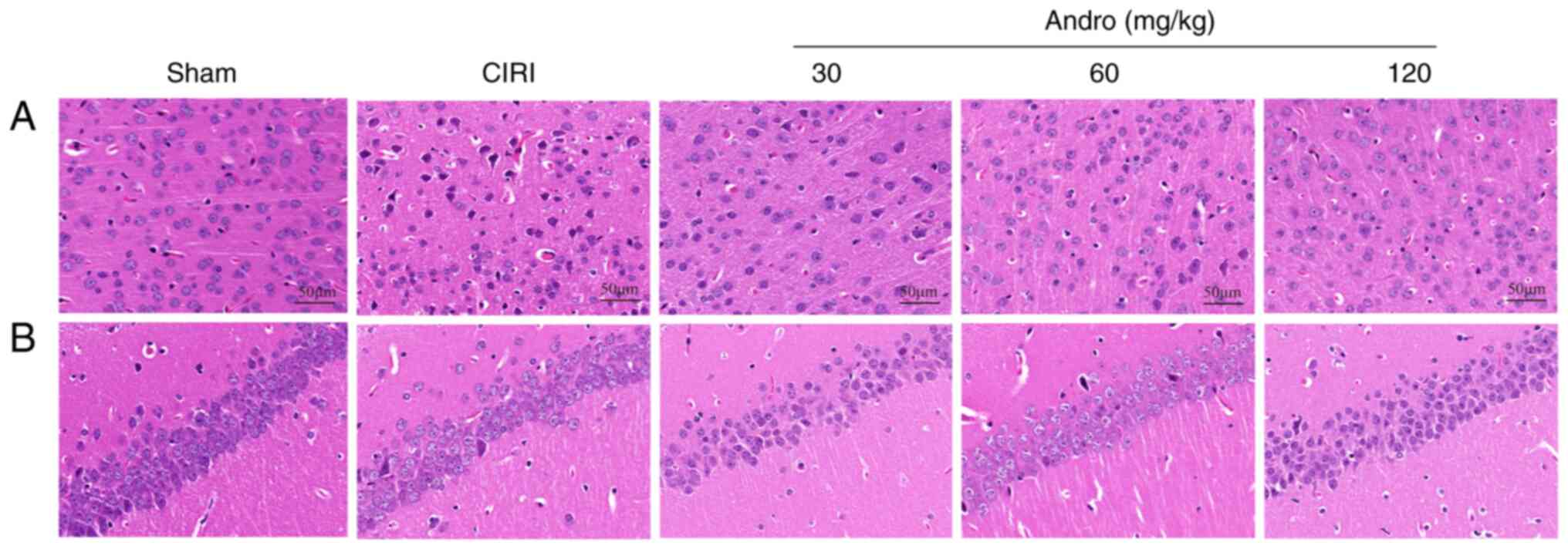
Andro attenuates the pathological
abnormalities caused by CIRI as shown by H&E staining
The brain sections of the mice were stained with
H&E (Fig. 5), and the
results revealed that neurons in the cerebral cortex and
hippocampal CA1 region in the sham group were normal. In these
mice, brain tissue was not damaged and no obvious vacuolar space
was observed. The nerve cells were densely and evenly arranged,
with normal structure and morphology, clear cell contour, complete
normal morphology and clearly visible nucleoli. By contrast, in the
CIRI group, ischemic necrosis occurred in the cerebral cortex, with
the shrinkage of neuronal nuclei; some cells exhibited spot-like
necrosis, with evident infiltration of microglial cells, neuronal
disorder and tissue edema in the hippocampus.
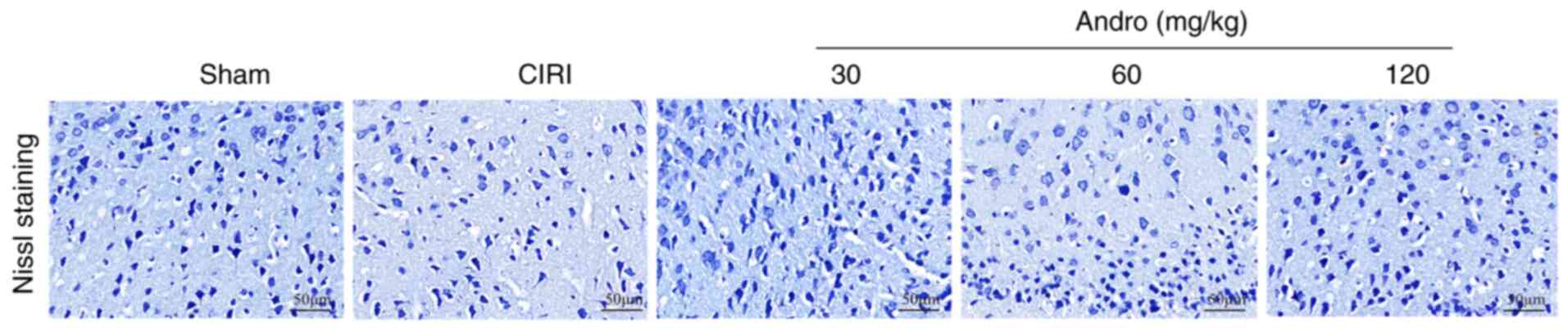
Andro increases Nissl positive
staining
The mouse brain sections were stained with Nissl
(Fig. 6), and the results
revealed that, compared with the sham group, the numbers of
Nissl-positive cells in the CIRI group were markedly decreased.
Following pre-treatment with Andro, the numbers of Nissl-positive
cells were significantly increased compared to the CIRI group.
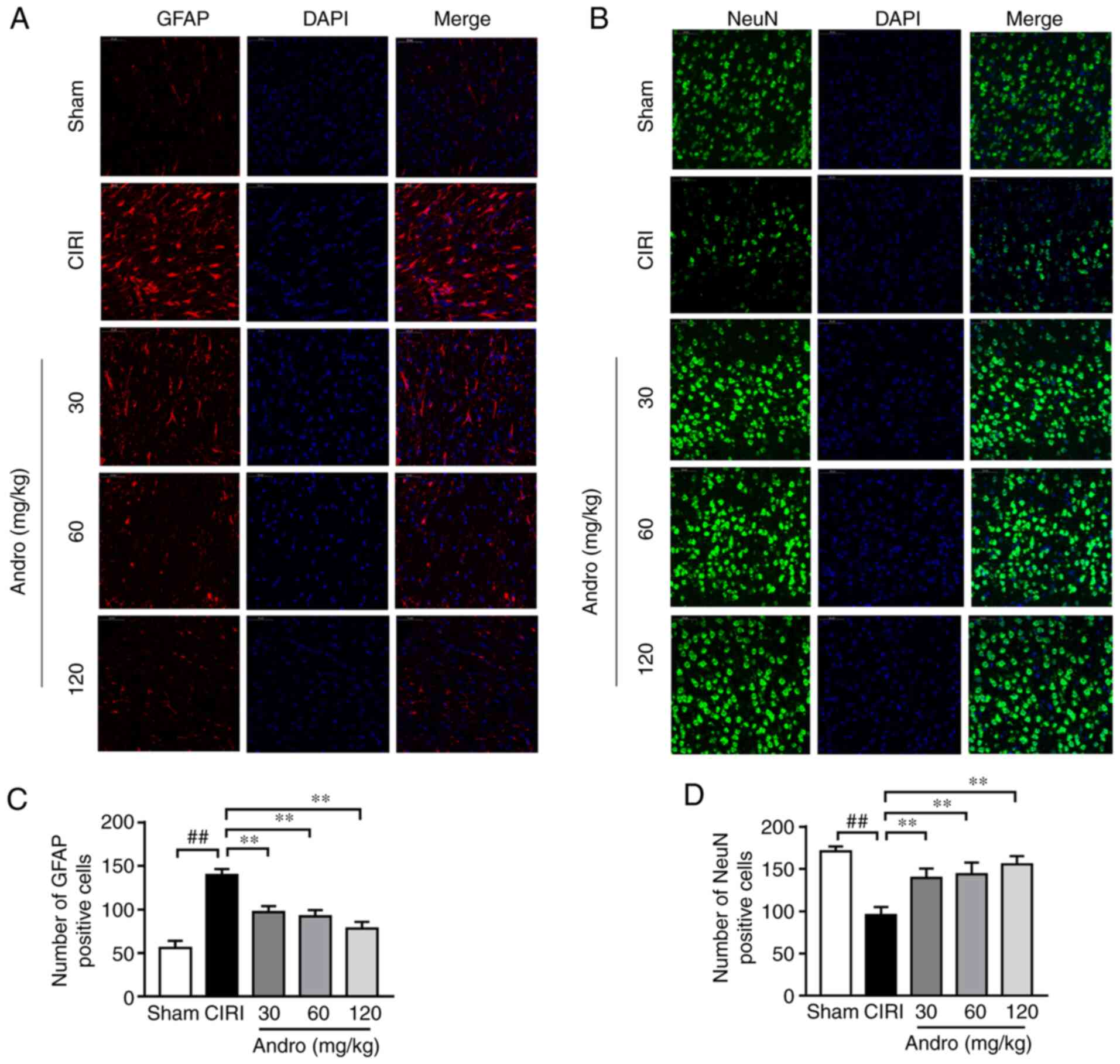
Andro decreases GFAP and increases NeuN
expression, as shown by immunofluorescence staining
The results of immunofluorescence staining
illustrated that, compared with the sham group, the expression of
GFAP was significantly increased (Fig. 7A and C) and the expression of
NeuN was significantly decreased (Fig. 7B and D) in the brains of the mice
subjected to CIRI. Following pre-treatment with Andro, the
expression of GFAP significantly decreased, while the expression of
NeuN significantly increased.
Andro increases the levels of BDNF
BDNF is involved in the development of the nervous
system, and CIRI promotes the release of BDNF. Compared with the
sham group, the BDNF content in the serum of mice in the CIRI group
was significantly increased (P<0.05), and the BDNF mRNA
level in the brain tissues also exhibited an increasing tendency
(Fig. 8A and B). This indicates
that cerebral ischemia-reperfusion can stimulate the release of
BDNF in a short period of time. Compared with the CIRI group, the
serum BDNF levels were significantly increased in mice pre-treated
with Andro (P<0.05), and the BDNF mRNA levels in the
brain tissues of mice receiving CIRI also exhibited an increasing
trend. In addition, Andro pre-treatment enhanced the transcription
of BDNF in mice subjected to CIRI.
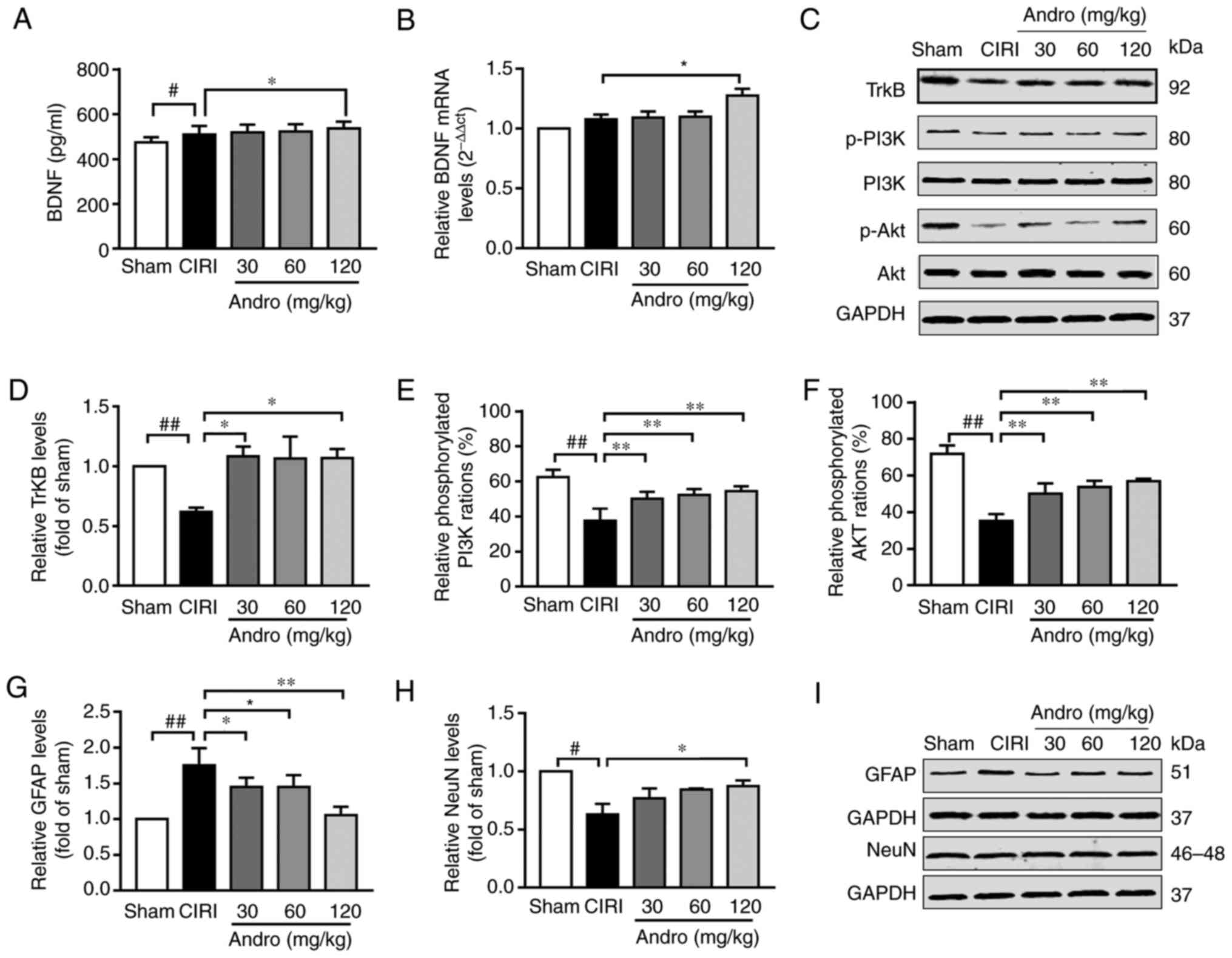 | Figure 8Influence of Andro on BDNF, TrkB,
GFAP, NeuN, p-PI3K and p-Akt in mouse brains following CIRI was
examined. Mice were treated with Andro (30, 60, or 120 mg/kg) for
14 days prior to CIRI, and repeated CIRI was then performed. BDNF
was detected by (A) enzyme-linked immunosorbent assay and (B)
RT-qPCR. (C-I) Protein levels of TrkB, p-PI3K, PI3K, p-Akt, Akt,
GFAP and NeuN in brains of CIRI mice were assessed by western blot
analysis. #P<0.05, ##P<0.01 vs. sham
group; *P<0.05, **P<0.01 vs. CIRI group
BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin receptor
kinase B; GFAP, glial fibrillary acidic protein; NeuN, neuronal
nuclei; p-PI3K, phosphorylated phosphoinositide 3-kinase; Sham,
sham-operated group; CIRI, cerebral ischemia-reperfusion injury;
Andro, andrographolide. |
Andro increases the levels of TrkB, and
downstream proteins
TrkB is a primary receptor of BDNF. Compared with
the sham group, TrkB protein expression in the brain tissues of
mice in the CIRI group was significantly decreased (P<0.01), and
compared with the CIRI group, the mice that received Andro (120
mg/kg) pre-administration exhibited a significantly upregulated
TrkB protein expression in the brain tissue (P<0.01; Fig. 8C and D).
The levels of proteins downstream of TrkB [including
phosphorylated PI3K (p-PI3K) and phosphorylated Akt (p-Akt) were
reduced in the mice in the CIRI group, while Andro pre-treatment
markedly increased the phosphorylation levels of these proteins
compared to the CIRI group (Fig. 8C,
E and F).
GFAP is a specific marker of activated astrocytes,
and NeuN is a specific marker of mature neurons. CIRI increased the
expression of GFAP and inhibited the expression of NeuN, while
Andro administration markedly reduced the expression of GFAP and
enhanced the expression of NeuN (Fig. 8G-I).
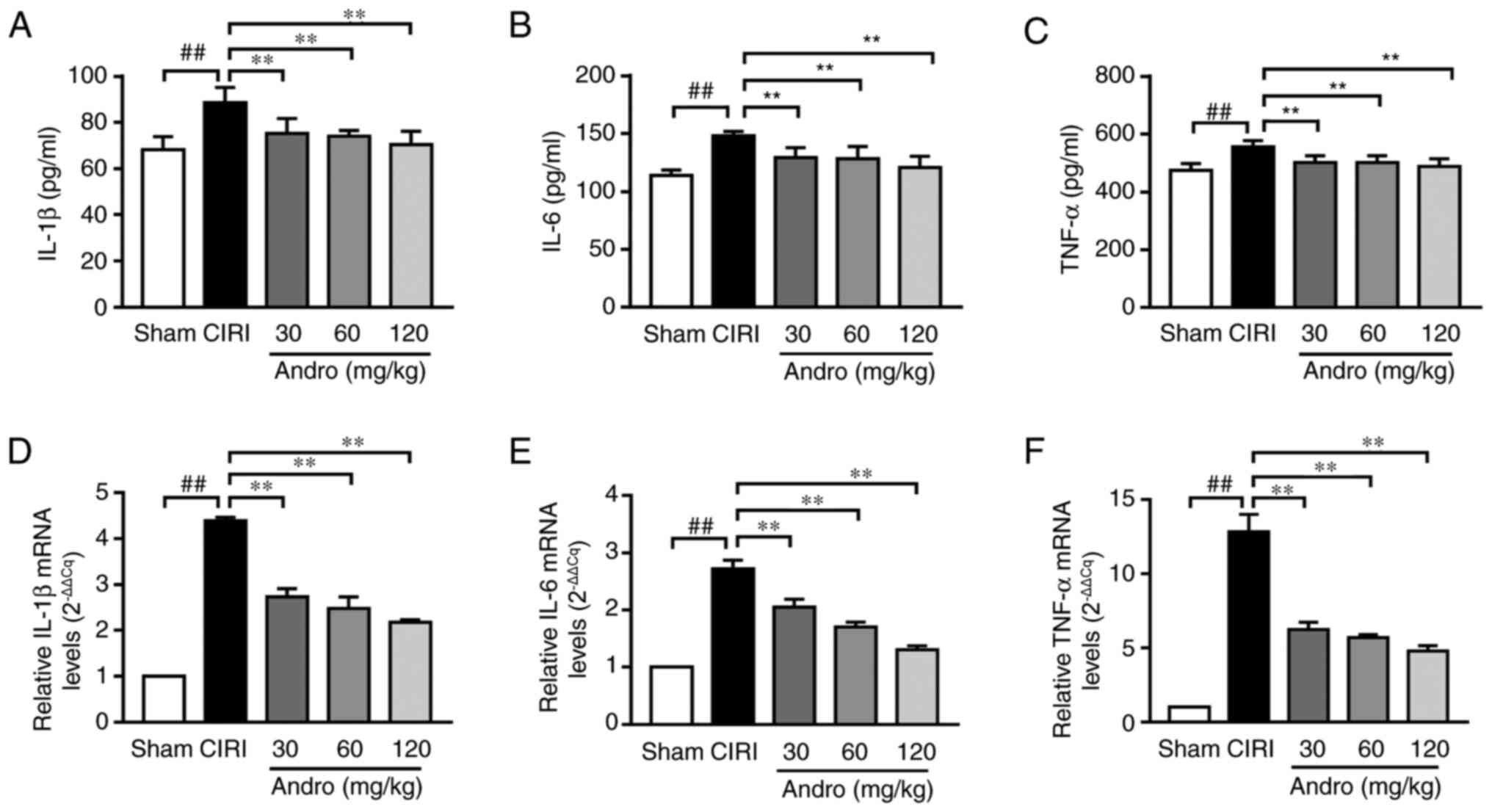
Andro reduces the levels of
pro-inflammatory cytokines
ELISA was used to determine the effects of Andro on
the levels of the inflammatory cytokines, IL-1β, IL-6
and TNF-α in serum. The serum levels of IL-1β and
TNF-α were significantly increased in the CIRI group
compared to the sham group. However, compared with the CIRI group,
the serum levels of IL-1β, IL-6 and TNF-α were
markedly decreased in the Andro groups (Fig. 9A-C).
In addition, the IL-1β mRNA, IL-6 mRNA
and TNF-α mRNA levels in brain samples were analyzed by
RT-qPCR (Fig. 9D-F). Compared
with the sham group, the IL-1β mRNA, IL-6 mRNA and
TNF-α mRNA levels were significantly increased in the CIRI
group. In addition, pre-treatment with Andro significantly
downregulated the IL-1β mRNA, IL-6 mRNA and
TNF-α mRNA levels (Fig.
9D-F).
Discussion
Stroke is the third-leading cause of mortality in
several developed countries and is associated with high morbidity,
disability and mortality (20).
Inflammation and apoptosis, as the critical factors involved in
CIRI, can interact with each other and further create a destructive
cascade effect (21). When brain
tissue is damaged, the levels of pro-inflammatory cytokines
(IL-1β, IL-6 and TNF-α) increase. Due to the
high incidence and severe consequences of ischemic stroke, new
drugs are urgently required for the treatment of ischemic stroke
(22).
The present study mainly aimed to predict and
analyze the target and pathway of Andrographis paniculata
through network pharmacology. From previous studies (6,11,12), Andro has been demonstrated to
possess potent neuroprotective action in the treatment of
neurological injury and cognitive dysfunction. Andro is one of the
main components of Andrographis paniculata. Therefore, Andro
was used to further verify the results, and other components could
also be selected for subsequent experiments.
Andrographis paniculata, an agent used in
TCM, has the effect of clearing heat and removing toxicity, and
clearing heat and removing toxicity is one of the 8 treatment
methods of TCM. Andro is a terpene that has been used extensively
in China for the treatment of a range of diseases. Previous studies
have illustrated that Andro significantly inhibits free radical
formation, blood-brain barrier destruction and cerebral infarction
in rats subjected to MCAO, and the heme oxygenase 1 (HO-1)
inhibitor zinc protoporphyrin IX reverses these effects, indicating
that Andro increases Nrf2-HO-1 expression through the regulation of
p38 MAPK, confirming that it protects against MCAO-induced brain
injury (23). Moreover, Andro
reduces the expression of NADPH oxidase 2 (NOX2) and inducible
nitric oxide synthase (iNOS), possibly by impairing
PI3K/Akt-dependent NF-κB and hypoxia-inducible factor (HIF)-1α
activation. This affects microglial activation, which in turn
mediates the protective effects of Andro in mice subjected to CIRI
(24).
The present study aimed to explore the
neuroprotective effects of Andro on CIRI. The data demonstrated
that the administration of Andro attenuated pathological changes
and increased the number of Nissl-positive cells. Moreover,
immunofluorescence staining confirmed that Andro effectively
reduced the expression of GFAP and increased the expression of NeuN
in mice following CIRI. Nissl bodies, which exist in the cytoplasm
of neurons, are characteristic structures of neurons, and their
role is to synthesize structural proteins, enzymes, and nerve
modulators for neurons. When neurons undergo pathological changes,
Nissl bodies begin to dissolve and become dust-like particles that
spread out from the nucleus until they disappear (25). NeuN is a specific marker of
mature neuronsand is expressed only after the neuron matures, with
its expression increasing gradually. Therefore, the expression of
NeuN can reflect the maturity of neurons (26). GFAP is a specific marker of
activated astrocytes (27).
Astrocytes, which are a substantial component of nerve cells, are
less sensitive to ischemic hypoxia (28). They are activated when cerebral
ischemia and other injuries occur (28).
The results of the present study illustrate that
Andro protects the brain from CIRI through a reduction in the
levels of apoptosis-related proteins by activating the
BDNF-TrkB/PI3K/Akt signaling pathway. It has been demonstrated that
BDNF promotes post-CIRI neurological recovery, prevents neuron
death and supports the survival of multiple neurons (29). BDNF is mainly synthesized and
secreted by presynaptic and postsynaptic neurons and may mediate
axonal growth and brain plasticity (30). BDNF activates downstream
signaling pathways such as the PI3K/Akt pathway through binding to
its cell surface receptor TrkB after being secreted into the
extracellular space (31). It
has been confirmed that the PI3K/Akt signaling pathway, a
significant cell signal transduction pathway, plays an essential
role in regulating cell survival, apoptosis, necrosis and other
pathophysiological processes (32). The suppression of Akt activity
has been associated with neuronal cell death following I/R injury
(33), and a previous study
demonstrated that Akt was activated by Ser473 phosphorylation
(34). The results of the
present study demonstrated that following CIRI, the phosphorylation
of both PI3K and Akt was increased, indicating that this signal
transduction pathway was activated.
The findings of the present study provide novel
insight into a potential method of protection against cerebral
ischemia, as well as the mechanism through which Andro exerts its
neuroprotective effects. Multiple factors and pathways may be
responsible for the therapeutic effects of Andro, including
inhibiting inflammation and mediating cell apoptosis. Future
studies are required to focus on other pathways that are likely
involved in this process. It is difficult to identify the true
target proteins of Andro and to measure binding affinity. Future
studies may focus on verifying the effects reported herein,
particularly through the use of knockout mice. Moreover, proteomics
and transcriptomics techniques can also be used to find
differential targets. The PI3K/Akt pathway is an important
neuroprotective and anti-apoptotic pathway. These findings provide
novel insight into a potential method of protection against
cerebral infarction, as well as the mechanisms through which Andro
exerts its neuroprotective effects.
In the present study, the protective effects of
Andro were assessed in a mouse model of CIRI, and as shown in the
pathological section, the CIRI of neurons was significantly
attenuated. Pre-treatment with Andro upregulated the protein
expression of TrkB, p-PI3K and p-Akt. It also decreased the
expression of GFAP and increased the expression of NeuN.
Furthermore, Andro attenuated inflammation significantly by
reducing the serum levels of IL-1β, IL-6 and
TNF-α.
In conclusion, the present study demonstrates that
Andro can protect brain tissue from ischemia-reperfusion injury in
mice by reducing the inflammatory response and apoptosis of brain
tissue cells. It was found that Andro exerts this neuroprotective
effect by regulating the expression of important proteins in the
PI3K/Akt pathway, indicating that this signaling pathway may be the
mechanism behind this protective effect. The results of the present
study suggest that Andro may be an effective agent for the
treatment of CIRI. Moreover, the findings presented herein provide
a potential explanation for the clinical anti-apoptotic and
neuroprotective effects of Andro.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YL, LLX, JXM, CW and MSM conceived and designed the
all experiments. YL, LLX and JXM performed the experiments. YL, LLX
and CW analyzed the data. YL wrote and edited the manuscript. JXM
and MSM revised the manuscript. YL and LLX confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the recommendations of the Ministry of Science and Technology of
China's Guidance for the Care and Use of Laboratory Animals. All
procedures were approved by the Laboratory Animal Ethics Committee
of Henan University of Chinese Medicine. (the number of approval
ethics code: DWLL201903026).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the National
Science and Technology Major Project of 'Development Program of
Significant New Drug' (no. 2017ZX09301071), the Scientific and
Technological Project of Henan Province (no. 17210231004), the Key
Scientific Research Projects of Colleges and Universities in Henan
Province (no. 21A360021), Natural Science Foundation of Henan
Province (no. 202300410259) and the Henan Province Postdoctoral
Research Startup Project (no. 202001043).
Abbreviations:
|
Andro
|
andrographolide
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
CCH
|
chronic cerebral hypoperfusion
|
|
CIRI
|
cerebral ischemia-reperfusion
injury
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
DL
|
drug-likeness
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GFAP
|
glial fibrillary acidic protein
|
|
GO
|
gene ontology
|
|
H&E
|
hematoxylin and eosin
|
|
IL-1β
|
interleukin 1β
|
|
IL-6
|
interleukin 6
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
MCAO
|
middle cerebral artery occlusion
|
|
NeuN
|
neuronal nuclei
|
|
OB
|
oral bioavailability
|
|
PI3K
|
phosphoinositide 3-kinase
|
|
PVDF
|
polyvinylidene fluoride
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TBST
|
Tris-buffered saline/Tween-20
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Krishnamurthi RV, Feigin VL, Forouzanfar
MH, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson
LM, Truelsen T, et al: Global and regional burden of first-ever
ischaemic and haemorrhagic stroke during 1990-2010: Findings from
the global burden of disease study 2010. Lancet Glob Health.
1:e259–e281. 2013. View Article : Google Scholar
|
|
2
|
Miralbell J, López-Cancio E, López-Oloriz
J, Arenillas JF, Barrios M, Soriano-Raya JJ, Galán A, Cáceres C,
Alzamora M, Pera G, et al: Cognitive patterns in relation to
biomarkers of cerebrovascular disease and vascular risk factors.
Cerebrovasc Dis. 36:98–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doyle KP, Simon RP and Stenzel-Poore MP:
Mechanisms of ischemic brain damage. Neuropharmacology. 55:310–318.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song W, Zhao J, Yan XS, Fang X, Huo DS,
Wang H, Jia JX and Yang ZJ: Mechanisms associated with protective
effects of ginkgo biloba leaf extracton in rat cerebral ischemia
reperfusion injury. J Toxicol Environ Health A. 82:1045–1051. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Becker KJ and Buckwalter M: Stroke,
inflammation and the immune response: Dawn of a new era.
Neurotherapeutics. 13:659–660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu T, Yang K, Li G, Zhou K, Tan J, Chen
J, Li T, Yu Y and Ning W: Experimental evidence and network
pharmacology identify the molecular targets of Tong Sheng tablets
in cerebral ischemia reperfusion injury. Am J Transl Res.
11:3301–3316. 2019.PubMed/NCBI
|
|
7
|
Iadecola C and Anrather J: The immunology
of stroke: From mechanisms to translation. Nat Med. 17:796–808.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman
M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C,
et al: Heart disease and stroke statistics-2017 update: A report
from the American heart association. Circulation. 135:e146–e603.
2017. View Article : Google Scholar
|
|
9
|
Powers WJ and Rabinstein AA: Response by
powers and rabinstein to letter regarding article, '2018 guidelines
for the early management of patients with acute ischemic stroke: A
guideline for healthcare professionals from the American heart
association/American stroke association'. Stroke. 50:e277–e278.
2019. View Article : Google Scholar
|
|
10
|
Muresanu DF, Strilciuc S and Stan A:
Current drug treatment of acute ischemic stroke: Challenges and
opportunities. CNS Drugs. 33:841–847. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan SJ, Wong WS, Wong PT and Bian JS:
Neuroprotective effects of andrographolide in a rat model of
permanent cerebral ischaemia. Br J Pharmacol. 161:668–679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang DP, Yin H, Lin Q, Fang SP, Shen JH,
Wu YF, Su SH and Hai J: Andrographolide enhances hippocampal BDNF
signaling and suppresses neuronal apoptosis, astroglial activation,
neuroinflammation, and spatial memory deficits in a rat model of
chronic cerebral hypoperfusion. Naunyn Schmiedebergs Arch
Pharmacol. 392:1277–1284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W
and Piao G: How can synergism of traditional medicines benefit from
network pharmacology? Molecules. 22:11352017. View Article : Google Scholar
|
|
14
|
Ning K, Zhao X, Poetsch A, Chen WH and
Yang J: Computational molecular networks and network pharmacology.
Biomed Res Int. 2017:75739042017. View Article : Google Scholar
|
|
15
|
Zhang JJ, Gao TT, Wang Y, Wang JL, Guan W,
Wang YJ, Wang CN, Liu JF and Jiang B: Andrographolide exerts
significant antidepressant-like effects involving the hippocampal
BDNF system in mice. Int J Neuropsychopharmacol. 22:585–600. 2019.
View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Fan M, Song C, Wang T, Li L, Dong Y, Jin W
and Lu P: Protective effects of lithium chloride treatment on
repeated cerebral ischemia-reperfusion injury in mice. Neurol Sci.
36:315–321. 2015. View Article : Google Scholar
|
|
18
|
Huang DW, Sherman BT, Tan Q, Kir J, Liu D,
Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC and Lempicki RA:
DAVID bioinformatics resources: Expanded annotation database and
novel algorithms to better extract biology from large gene lists.
Nucleic Acids Res. 35(Web Server Issue): W169–W175. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schooling CM, Huang JV, Zhao JV, Kwok MK,
Au Yeung SL and Lin SL: Disconnect between genes associated with
ischemic heart disease and targets of ischemic heart disease
treatments. EBioMedicine. 28:311–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Hoof RHM, Schreuder FHBM, Nelemans P,
Truijman MTB, van Orshoven NP, Schreuder TH, Mess WH, Heeneman S,
van Oostenbrugge RJ, Wildberger JE and Kooi ME: Ischemic stroke
patients demonstrate increased carotid plaque microvasculature
compared to (Ocular) transient ischemic attack patients.
Cerebrovasc Dis. 44:297–303. 2017. View Article : Google Scholar
|
|
21
|
Zhang Q, An R, Tian X, Yang M, Li M, Lou
J, Xu L and Dong Z: β-Caryophyllene pretreatment alleviates focal
cerebral ischemia-reperfusion injury by activating PI3K/Akt
signaling pathway. Neurochem Res. 42:1459–1469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Du Q, Yang Y, Wang J, Liu Y, Zhao
Z, Zhu Y and Liu C: Salidroside alleviates ischemic brain injury in
mice with ischemic stroke through regulating BDNK mediated PI3K/Akt
pathway. Biochem Pharmacol. 156:99–108. 2018. View Article : Google Scholar
|
|
23
|
Yen TL, Chen RJ, Jayakumar T, Lu WJ, Hsieh
CY, Hsu MJ, Yang CH, Chang CC, Lin YK, Lin KH and Sheu JR:
Andrographolide stimulates p38 mitogen-activated protein
kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1
signaling in primary cerebral endothelial cells for definite
protection against ischemic stroke in rats. Transl Res. 170:57–72.
2016. View Article : Google Scholar
|
|
24
|
Chern CM, Liou KT, Wang YH, Liao JF, Yen
JC and Shen YC: Andrographolide inhibits PI3K/AKT-dependent NOX2
and iNOS expression protecting mice against
hypoxia/ischemia-induced oxidative brain injury. Planta Med.
77:1669–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar
|
|
26
|
Yu ZH, Cai M, Xiang J, Zhang ZN, Zhang JS,
Song XL, Zhang W, Bao J, Li WW and Cai DF: PI3K/Akt pathway
contributes to neuroprotective effect of Tongxinluo against focal
cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol.
181:8–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao Y, Qin L, Kim E, Bhosle S, Guo H,
Febbraio M, Haskew-Layton RE, Ratan R and Cho S: CD36 is involved
in astrocyte activation and astroglial scar formation. J Cereb
Blood Flow Metab. 32:1567–1577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freeman MR: Specification and
morphogenesis of astrocytes. Science. 330:774–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanfranconi S, Locatelli F, Corti S,
Candelise L, Comi GP, Baron PL, Strazzer S, Bresolin N and Bersano
A: Growth factors in ischemic stroke. J Cell Mol Med. 15:1645–1687.
2011. View Article : Google Scholar
|
|
30
|
Erpolat S, Celik HT and Bozkurt B:
Brain-derived neurotrophic factor is increased in serum levels of
patients with symptomatic dermographism. Postepy Dermatol Alergol.
34:346–349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ya BL, Liu Q, Li HF, Cheng HJ, Yu T, Chen
L, Wang Y, Yuan LL, Li WJ, Liu WY and Bai B: Uric acid protects
against focal cerebral ischemia/reperfusion-induced oxidative
stress via activating Nrf2 and regulating neurotrophic factor
expression. Oxid Med Cell Longev. 2018:60691502018. View Article : Google Scholar :
|
|
32
|
Wang J, Ma W and Liu Y: Long non-coding
RNA HULC promotes bladder cancer cells proliferation but inhibits
apoptosis via regulation of ZIC2 and PI3K/AKT signaling pathway.
Cancer Biomark. 20:425–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar
|
|
34
|
Calleja V, Alcor D, Laguerre M, Park J,
Vojnovic B, Hemmings BA, Downward J, Parker PJ and Larijani B:
Intramolecular and intermolecular interactions of protein kinase B
define its activation in vivo. PLoS Biol. 5:e952007. View Article : Google Scholar
|