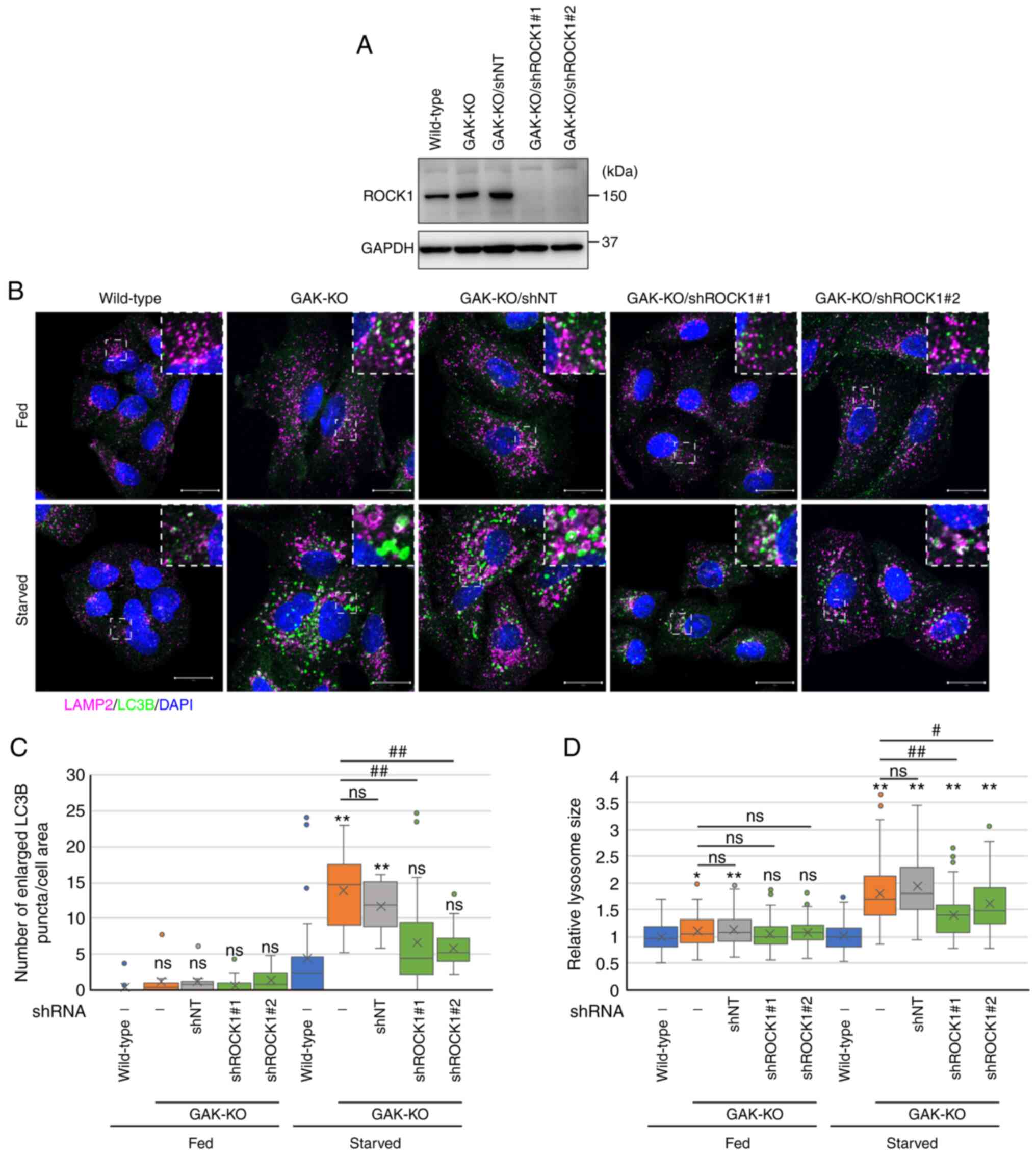
|
1
|
Morishita H and Mizushima N: Diverse
cellular roles of autophagy. Annu Rev Cell Dev Biol. 35:453–475.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
King KE, Losier TT and Russell RC:
Regulation of autophagy enzymes by nutrient signaling. Trends
Biochem Sci. 46:687–700. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hara T, Nakamura K, Matsui M, Yamamoto A,
Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I,
Okano H and Mizushima N: Suppression of basal autophagy in neural
cells causes neurodegenerative disease in mice. Nature.
441:885–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alers S, Löffler AS, Wesselborg S and
Stork B: Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy:
Cross talk, shortcuts, and feedbacks. Mol Cell Biol. 32:2–11. 2012.
View Article : Google Scholar :
|
|
5
|
Galluzzi L, Baehrecke EH, Ballabio A, Boya
P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P,
Colombo MI, et al: Molecular definitions of autophagy and related
processes. EMBO J. 36:1811–1836. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TJ, Gubas A and Tooze SA: A
molecular perspective of mammalian autophagosome biogenesis. J Biol
Chem. 293:5386–5395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishimura T and Tooze SA: Emerging roles
of ATG proteins and membrane lipids in autophagosome formation.
Cell Discov. 6:322020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parzych KR and Klionsky DJ: Vacuolar
hydrolysis and efflux: Current knowledge and unanswered questions.
Autophagy. 15:212–227. 2019. View Article : Google Scholar :
|
|
9
|
Yim WW and Mizushima N: Lysosome biology
in autophagy. Cell Discov. 6:62020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawabata T and Yoshimori T: Autophagosome
biogenesis and human health. Cell Discov. 6:332020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N and Levine B: Autophagy in
human diseases. N Engl J Med. 383:1564–1576. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ballabio A and Bonifacino JS: Lysosomes as
dynamic regulators of cell and organismal homeostasis. Nat Rev Mol
Cell Biol. 21:101–118. 2020. View Article : Google Scholar
|
|
13
|
Pu J, Guardia CM, Keren-Kaplan T and
Bonifacino JS: Mechanisms and functions of lysosome positioning. J
Cell Sci. 129:4329–4339. 2016.PubMed/NCBI
|
|
14
|
de Araujo ME, Liebscher G, Hess MW and
Huber LA: Lysosomal size matters. Traffic. 21:60–75. 2020.
View Article : Google Scholar :
|
|
15
|
Chen Y and Yu L: Recent progress in
autophagic lysosome reformation. Traffic. 18:358–361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korolchuk VI, Saiki S, Lichtenberg M,
Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M,
Menzies FM, et al: Lysosomal positioning coordinates cellular
nutrient responses. Nat Cell Biol. 13:453–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dalle Pezze P, Ruf S, Sonntag AG,
Langelaar-Makkinje M, Hall P, Heberle AM, Razquin Navas P, van
Eunen K, Tölle RC, Schwarz JJ, et al: A systems study reveals
concurrent activation of AMPK and mTOR by amino acids. Nat Commun.
7:132542016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, McPhee CK, Zheng L, Mardones GA,
Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, et al: Termination of
autophagy and reformation of lysosomes regulated by mTOR. Nature.
465:942–946. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kruppa AJ, Kendrick-Jones J and Buss F:
Myosins, actin and autophagy. Traffic. 17:878–890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kast DJ and Dominguez R: The
cytoskeleton-autophagy connection. Curr Biol. 27:R318–R326. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greener T, Zhao X, Nojima H, Eisenberg E
and Greene LE: Role of cyclin G-associated kinase in uncoating
clathrin-coated vesicles from non-neuronal cells. J Biol Chem.
275:1365–1370. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee DW, Wu X, Eisenberg E and Greene LE:
Recruitment dynamics of GAK and auxilin to clathrin-coated pits
during endocytosis. J Cell Sci. 119:3502–3512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanaoka Y, Kimura SH, Okazaki I, Ikeda M
and Nojima H: GAK: A cyclin G associated kinase contains a
tensin/auxilin-like domain. FEBS Lett. 402:73–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimizu H, Nagamori I, Yabuta N and Nojima
H: GAK, a regulator of clathrin-mediated membrane traffic, also
controls centrosome integrity and chromosome congression. J Cell
Sci. 122:3145–3152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naito Y, Shimizu H, Kasama T, Sato J,
Tabara H, Okamoto A, Yabuta N and Nojima H: Cyclin G-associated
kinase regulates protein phosphatase 2A by phosphorylation of its
B'γ subunit. Cell Cycle. 11:604–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fukushima K, Wang M, Naito Y, Uchihashi T,
Kato Y, Mukai S, Yabuta N and Nojima H: GAK is phosphorylated by
c-Src and translocated from the centrosome to chromatin at the end
of telophase. Cell Cycle. 16:415–427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yabuno Y, Uchihashi T, Sasakura T, Shimizu
H, Naito Y, Fukushima K, Ota K, Kogo M, Nojima H and Yabuta N:
Clathrin heavy chain phosphorylated at T606 plays a role in proper
cell division. Cell Cycle. 18:1976–1994. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao X, Greener T, Al-Hasani H, Cushman
SW, Eisenberg E and Greene LE: Expression of auxilin or AP180
inhibits endocytosis by mislocalizing clathrin: Evidence for
formation of nascent pits containing AP1 or AP2 but not clathrin. J
Cell Sci. 114:353–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DW, Zhao X, Zhang F, Eisenberg E and
Greene LE: Depletion of GAK/auxilin 2 inhibits receptor-mediated
endocytosis and recruitment of both clathrin and clathrin adaptors.
J Cell Sci. 118:4311–4321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang CX, Engqvist-Goldstein AE, Carreno
S, Owen DJ, Smythe E and Drubin DG: Multiple roles for cyclin
G-associated kinase in clathrin-mediated sorting events. Traffic.
6:1103–1113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Gjoerup O and Roberts TM: The
serine/threonine kinase cyclin G-associated kinase regulates
epidermal growth factor receptor signaling. Proc Natl Acad Sci USA.
101:10296–10301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kametaka S, Moriyama K, Burgos PV,
Eisenberg E, Greene LE, Mattera R and Bonifacino JS: Canonical
interaction of cyclin G associated kinase with adaptor protein 1
regulates lysosomal enzyme sorting. Mol Biol Cell. 18:2991–3001.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beilina A, Rudenko IN, Kaganovich A,
Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe
K, et al: Unbiased screen for interactors of leucine-rich repeat
kinase 2 supports a common pathway for sporadic and familial
Parkinson disease. Proc Natl Acad Sci USA. 111:2626–2631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Plowey ED, Cherra SJ III, Liu YJ and Chu
CT: Role of autophagy in G2019S-LRRK2 -associated neurite
shortening in differentiated SH-SY5Y cells. J Neurochem.
105:1048–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gómez-Suaga P, Luzón-Toro B, Churamani D,
Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC and
Hilfiker S: Leucine-rich repeat kinase 2 regulates autophagy
through a calcium-dependent pathway involving NAADP. Hum Mol Genet.
21:511–525. 2012. View Article : Google Scholar :
|
|
37
|
Madureira M, Connor-Robson N and
Wade-Martins R: LRRK2: Autophagy and lysosomal activity. Front
Neurosci. 14:4982020. View Article : Google Scholar
|
|
38
|
Susa M, Choy E, Liu X, Schwab J, Hornicek
FJ, Mankin H and Duan Z: Cyclin G-associated kinase is necessary
for osteosarcoma cell proliferation and receptor trafficking. Mol
Cancer Ther. 9:3342–3350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rhodes SL, Sinsheimer JS, Bordelon Y,
Bronstein JM and Ritz B: Replication of GWAS associations for GAK
and MAPT in Parkinson's disease. Ann Hum Genet. 75:195–200.
2011.
|
|
40
|
Dumitriu A, Pacheco CD, Wilk JB,
Strathearn KE, Latourelle JC, Goldwurm S, Pezzoli G, Rochet JC,
Lindquist S and Myers RH: Cyclin-G-associated kinase modifies
α-synuclein expression levels and toxicity in Parkinson's disease:
Results from the GenePD Study. Hum Mol Genet. 20:1478–1487. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma ZG, He F and Xu J: Quantitative
assessment of the association between GAK rs1564282 C/T
polymorphism and the risk of Parkinson's disease. J Clin Neurosci.
22:1077–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cong L, Ran FA, Cox D, Lin S, Barretto R,
Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA and Zhang F:
Multiplex genome engineering using CRISPR/Cas systems. Science.
339:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ran FA, Hsu PD, Wright J, Agarwala V,
Scott DA and Zhang F: Genome engineering using the CRISPR-Cas9
system. Nat Protoc. 8:2281–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanaka H, Hino H, Moriya S, Kazama H,
Miyazaki M, Takano N, Hiramoto M, Tsukahara K and Miyazawa K:
Comparison of autophagy inducibility in various tyrosine kinase
inhibitors and their enhanced cytotoxicity via inhibition of
autophagy in cancer cells in combined treatment with azithromycin.
Biochem Biophys Rep. 22:1007502020.PubMed/NCBI
|
|
45
|
Kaizuka T, Morishita H, Hama Y, Tsukamoto
S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T and
Mizushima N: An autophagic flux probe that releases an internal
control. Mol Cell. 64:835–849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Spangle JM, Ghosh-Choudhury N and Munger
K: Activation of cap-dependent translation by mucosal human
papillomavirus E6 proteins is dependent on the integrity of the
LXXLL binding motif. J Virol. 86:7466–7472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yokota A, Hiramoto M, Hino H, Tokuhisa M,
Miyazaki M, Kazama H, Takano N and Miyazawa K: Sequestosome 1 (p62)
accumulation in breast cancer cells suppresses progesterone
receptor expression via argonaute 2. Biochem Biophys Res Commun.
531:256–263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lamb CA, Joachim J and Tooze SA:
Quantifying autophagic structures in mammalian cells using confocal
microscopy. Methods Enzymol. 587:21–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
51
|
Poole B and Ohkuma S: Effect of weak bases
on the intralysosomal pH in mouse peritoneal macrophages. J Cell
Biol. 90:665–669. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Maclean KH, Dorsey FC, Cleveland JL and
Kastan MB: Targeting lysosomal degradation induces p53-dependent
cell death and prevents cancer in mouse models of lymphomagenesis.
J Clin Invest. 118:79–88. 2008. View Article : Google Scholar
|
|
54
|
Rong Y, Liu M, Ma L, Du W, Zhang H, Tian
Y, Cao Z, Li Y, Ren H, Zhang C, et al: Clathrin and
phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome
reformation. Nat Cell Biol. 14:924–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chang J, Lee S and Blackstone C: Spastic
paraplegia proteins spastizin and spatacsin mediate autophagic
lysosome reformation. J Clin Invest. 124:5249–5262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
McGrath MJ, Eramo MJ, Gurung R, Sriratana
A, Gehrig SM, Lynch GS, Lourdes SR, Koentgen F, Feeney SJ, Lazarou
M, et al: Defective lysosome reformation during autophagy causes
skeletal muscle disease. J Clin Invest. 131:e1351242021. View Article : Google Scholar :
|
|
57
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
58
|
Julian L and Olson MF: Rho-associated
coiled-coil containing kinases (ROCK): Structure, regulation, and
functions. Small GTPases. 5:e298462014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kast DJ, Zajac AL, Holzbaur EL, Ostap EM
and Dominguez R: WHAMM Directs the Arp2/3 Complex to the ER for
autophagosome biogenesis through an Actin comet tail mechanism.
Curr Biol. 25:1791–1797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mathiowetz AJ, Baple E, Russo AJ, Coulter
AM, Carrano E, Brown JD, Jinks RN, Crosby AH and Campellone KG: An
Amish founder mutation disrupts a PI(3)P-WHAMM-Arp2/3
complex-driven autophagosomal remodeling pathway. Mol Biol Cell.
28:2492–2507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai A, Yu L and Wang HW: WHAMM initiates
autolysosome tubulation by promoting actin polymerization on
autolysosomes. Nat Commun. 10:36992019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nakamura S, Hasegawa J and Yoshimori T:
Regulation of lysosomal phosphoinositide balance by INPP5E is
essential for autophagosome-lysosome fusion. Autophagy.
12:2500–2501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nakamura S and Yoshimori T: New insights
into autophagosome-lysosome fusion. J Cell Sci. 130:1209–1216.
2017.PubMed/NCBI
|
|
64
|
Henry KR, D'Hondt K, Chang JS, Nix DA,
Cope MJ, Chan CS, Drubin DG and Lemmon SK: The actin-regulating
kinase Prk1p negatively regulates Scd5p, a suppressor of clathrin
deficiency, in actin organization and endocytosis. Curr Biol.
13:1564–1569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sekiya-Kawasaki M, Groen AC, Cope MJ,
Kaksonen M, Watson HA, Zhang C, Shokat KM, Wendland B, McDonald KL,
McCaffery JM and Drubin DG: Dynamic phosphoregulation of the
cortical actin cytoskeleton and endocytic machinery revealed by
real-time chemical genetic analysis. J Cell Biol. 162:765–772.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Toshima J, Toshima JY, Martin AC and
Drubin DG: Phosphoregulation of Arp2/3-dependent actin assembly
during receptor-mediated endocytosis. Nat Cell Biol. 7:246–254.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Paisán-Ruíz C, Jain S, Evans EW, Gilks WP,
Simón J, van der Brug M, López de Munain A, Aparicio S, Gil AM,
Khan N, et al: Cloning of the gene containing mutations that cause
PARK8-linked Parkinson's disease. Neuron. 44:595–600. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zimprich A, Biskup S, Leitner P, Lichtner
P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB,
et al: Mutations in LRRK2 cause autosomal-dominant parkinsonism
with pleomorphic pathology. Neuron. 44:601–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zimprich A, Müller-Myhsok B, Farrer M,
Leitner P, Sharma M, Hulihan M, Lockhart P, Strongosky A, Kachergus
J, Calne DB, et al: The PARK8 locus in autosomal dominant
parkinsonism: Confirmation of linkage and further delineation of
the disease-containing interval. Am J Hum Genet. 74:11–19. 2004.
View Article : Google Scholar
|
|
70
|
Usmani A, Shavarebi F and Hiniker A: The
Cell Biology of LRRK2 in Parkinson's disease. Mol Cell Biol.
41:e00660–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Clark EH, Vázquez de la Torre A, Hoshikawa
T and Briston T: Targeting mitophagy in Parkinson's disease. J Biol
Chem. 296:1002092020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Malpartida AB, Williamson M, Narendra DP,
Wade-Martins R and Ryan BJ: Mitochondrial dysfunction and mitophagy
in Parkinson's disease: From mechanism to therapy. Trends Biochem
Sci. 46:329–343. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Plotegher N and Duchen MR: Crosstalk
between Lysosomes and Mitochondria in Parkinson's disease. Front
Cell Dev Biol. 5:1102017. View Article : Google Scholar
|
|
74
|
Nguyen M, Wong YC, Ysselstein D, Severino
A and Krainc D: Synaptic, Mitochondrial, and Lysosomal dysfunction
in Parkinson's disease. Trends Neurosci. 42:140–149. 2019.
View Article : Google Scholar
|
|
75
|
Vidyadhara DJ, Lee JE and Chandra SS: Role
of the endolysosomal system in Parkinson's disease. J Neurochem.
150:487–506. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Oxnard GR, Janjigian YY, Arcila ME, Sima
CS, Kass SL, Riely GJ, Pao W, Kris MG, Ladanyi M, Azzoli CG and
Miller VA: Maintained sensitivity to EGFR tyrosine kinase
inhibitors in EGFR-mutant lung cancer recurring after adjuvant
erlotinib or gefitinib. Clin Cancer Res. 17:6322–6328. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Han W, Pan H, Chen Y, Sun J, Wang Y, Li J,
Ge W, Feng L, Lin X, Wang X, et al: EGFR tyrosine kinase inhibitors
activate autophagy as a cytoprotective response in human lung
cancer cells. PLoS One. 6:e186912011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sugita S, Ito K, Yamashiro Y, Moriya S,
Che XF, Yokoyama T, Hiramoto M and Miyazawa K: EGFR-independent
autophagy induction with gefitinib and enhancement of its cytotoxic
effect by targeting autophagy with clarithromycin in non-small cell
lung cancer cells. Biochem Biophys Res Commun. 461:28–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brehmer D, Greff Z, Godl K, Blencke S,
Kurtenbach A, Weber M, Müller S, Klebl B, Cotton M, Kéri G, et al:
Cellular targets of gefitinib. Cancer Res. 65:379–382.
2005.PubMed/NCBI
|
|
80
|
Yamamoto N, Honma M and Suzuki H:
Off-target serine/threonine kinase 10 inhibition by erlotinib
enhances lymphocytic activity leading to severe skin disorders. Mol
Pharmacol. 80:466–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ray MR, Wafa LA, Cheng H, Snoek R, Fazli
L, Gleave M and Rennie PS: Cyclin G-associated kinase: A novel
androgen receptor-interacting transcriptional coactivator that is
overexpressed in hormone refractory prostate cancer. Int J Cancer.
118:1108–1119. 2006. View Article : Google Scholar
|
|
82
|
Sooro MA, Zhang N and Zhang P: Targeting
EGFR-mediated autophagy as a potential strategy for cancer therapy.
Int J Cancer. 143:2116–2125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu M and Zhang P: EGFR-mediated autophagy
in tumourigenesis and therapeutic resistance. Cancer Lett.
469:207–216. 2020. View Article : Google Scholar
|
|
84
|
Rehman SK, Haynes J, Collignon E, Brown
KR, Wang Y, Nixon AM, Bruce JP, Wintersinger JA, Singh Mer A, Lo
EB, et al: Colorectal cancer cells enter a Diapause-like DTP state
to survive chemotherapy. Cell. 184:226–242.e221. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Nagle MW, Latourelle JC, Labadorf A,
Dumitriu A, Hadzi TC, Beach TG and Myers RH: The 4p163 Parkinson
disease risk locus is associated with GAK expression and genes
involved with the synaptic vesicle membrane. PLoS One.
11:e01609252016. View Article : Google Scholar
|
|
86
|
Tumbarello DA, Kendrick-Jones J and Buss
F: Myosin VI and its cargo adaptors-linking endocytosis and
autophagy. J Cell Sci. 126:2561–2570. 2013.PubMed/NCBI
|
|
87
|
Bilanges B, Posor Y and Vanhaesebroeck B:
PI3K isoforms in cell signalling and vesicle trafficking. Nat Rev
Mol Cell Biol. 20:515–534. 2019. View Article : Google Scholar : PubMed/NCBI
|