Introduction
Gastric cancer is the fifth most common malignancy
and the third most common cause of cancer-related mortality
worldwide, with over 1 million estimated new cases annually and
784,000 deaths globally in 2018, prompting the World Health
Organization to declare it a public health concern (1,2).
Gastric cancer is a multi-step and multi-factorial disease. The
efficacy of gastric cancer treatment is dependent on the stage of
the tumor. Patients with early-stage gastric cancer, who receive
radical surgery, have a favorable 5-year survival rate; however,
the majority of patients are diagnosed at an advanced stage due to
the inability of early detection, and the 5-year survival rate of
these patients is generally poor (3). Hence, a novel therapeutic agent, as
well as an improved understanding of the molecular mechanisms
underlying gastric cancer, are urgently required in order to
improve patient prognosis and the survival rate.
Taxifolin (Tax), also known as dihydroquercetin
(3,5,7,3,4-pentahydroxy flavanone), is a flavonoid naturally
occurring in milk thistle, onion, Douglas fir bark and French
maritime pine bark, which has been reported to possess multiple
biological activities in the management of oxidative stress,
inflammation, microbial infections, liver and cardiovascular
disorders, as well as tumors (4). Tax has also been identified as a
potential antitumor agent in different types of cancer, such as
osteosarcoma, colorectal, breast and lung cancer (5-8).
For example, Razak et al (6) demonstrated that Tax induced
cytotoxicity and cell cycle arrest in colorectal cancer cells and
hampered the tumor growth of HCT116-derived xenografts in mice. Li
et al (5) reported that
Tax not only had the potential to inhibit the proliferation,
migration and invasion of breast cancer cells in vitro, but
also hindered the growth of primary tumors and reduced the lung
metastasis of breast cancer in vivo. However, to the best of
the authors' knowledge, no research performed to date has reported
the antitumor effects of Tax in gastric cancer.
In the present study, the effects of Tax were
examined on two gastric cancer cell lines, AGS and NCI-N87 cells
in vitro, and tumor-bearing mice in vivo, and the
potential regulatory mechanisms of Tax were further investigated.
The present study was conducted in accordance with the ARRIVE
guidelines checklist (9).
Materials and methods
Cell culture and treatment
Two human gastric cancer cell lines, AGS and
NCI-N87, obtained from the American Type Culture Collection were
cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientifc, Inc.)
and 1% penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) in a humidified incubator with 5% CO2 and 95% air
at 37°C. For treatment, Tax was obtained from Shanghai Huicheng
Technology, Ltd. The AGS and NCI-N87 cells were treated with
increasing concentrations of Tax (1, 3, 10, 30 and 100 µM)
for 48 h. In addition, the aryl hydrocarbon receptor (AhR) agonist
SB203580 (10 µM; Sigma-Aldrich; Merck KGaA) was applied for
further treatment.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed using a CCK-8 assay
(Beyotime Institute of Biotechnology). The AGS and NCI-N87 cells
were cultured in 96-well plates (1×104 cells/well) for
24 h, and were then treated with various concentrations of Tax (1,
3, 10, 30 and 100 µM) for a further 48 h. Then, 10 µl
CCK-8 reagent was added to each well, and the cells were incubated
at 37°C for 3 h. The absorbance at 450 nm was detected using a
microplate reader (ELx800; BioTek Instruments, Inc.). The results
are presented as a relative percentage of the untreated control
cells.
Colony formation assay
The cell proliferative ability was assessed using a
colony formation assay. Cells were seeded into six-well plates at a
density of 500 cells/well. After being subjected to the Tax (100
µM) treatment with or without SB203580 (10 µM)
treatment, cells were incubated in a 5% CO2 incubator at
37°C for 2 weeks. Thereafter, the cells were fixed with methanol
for 10 min at room temperature and stained with 0.5% crystal violet
for a further 10 min at room temperature. Images were captured
under a light microscope (magnification, ×10), and colonies
containing >50 cells were counted.
Wound-healing assay
The cell migratory ability was assessed using a
wound-healing assay. The cells were re-suspended with serum-free
medium and added to 24-well plates for a 24-h incubation at 37°C.
Upon reaching 100% confluency, a scratch was subsequently generated
in the cell monolayer using a sterile micropipette tip. The cells
were then incubated in serum-free medium containing Tax (100
µM) with or without SB203580 (10 µM) at 37°C for 24
h. The wound width at 0 and 24 h was captured using a light
microscope (magnification, ×100).
Transwell assay
The cell invasive ability was assessed using a
Transwell assay with a 24-well Transwell plate with pore size of
8-µm (EMD Millipore) precoated with BD Matrigel (BD
Biosciences) at 37°C for 1 h. The cells were suspended in
serum-free medium and added to the upper chamber (3×104
cells/well) of the 24-well Transwell plate, followed by a 24-h
incubation of Tax (100 µM) with or without SB203580 (10
µM). Complete medium containing 10% FBS was added to the
lower chamber. Following 24 h of incubation at 37°C, the
non-invasive cells were removed using cotton swabs, and the
invasive cells were fixed with 100% methanol for 10 min and stained
with 0.1% crystal violet for 20 min at room temperature. Images
were captured under a light microscope (magnification, ×100).
Western blot analysis
Cells were lysed using RIPA lysis (Wuhan Boster
Biological Technology, Ltd.) containing 1 mM phenylmethylsulfonyl
fluoride (PMSF). A BCA assay was used to determine the protein
concentration. Equal amounts of protein (30 µg/lane) were
separated by a 12% SDS-PAGE gel and transferred to PVDF membranes
(EMD Millipore). Subsequently, the membranes were blocked in 5%
skimmed milk for 2 h at room temperature, and then incubated with
corresponding primary antibodies against matrix metalloproteinase
(MMP)2 (1:1,000; product code ab92536), MMP9 (1:1,000; product code
ab38898), E-cadherin (1:1,000; product code ab231303), Zonula
occludens-1 (ZO-1; 1:1,000; product code ab96587), N-cadherin
(1:5,000; product code ab76011), Snail (1:1,000; product code
ab180714), AhR (1:1,000; product code ab108518), cytochrome P450
1A1 (CYP1A1; 1:1,000; product code ab126887), Ki67 (1:1,000;
product code ab16667), proliferating cell nuclear antigen, (PCNA;
1:1,000; product code ab92552) and GAPDH (1:1,000; product code
ab8245) from Abcam at 4°C overnight. On the second day, the
membranes were washed three times and incubated with HRP-conjugated
goat anti-mouse (1:2,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) or goat anti-rabbit (1:2,000; product code
ab97051; Abcam) antibodies for 2 h at room temperature. Bands were
exposed by an enhanced chemiluminescence (ECL) kit (Beyotime
Institute of Biotechnology) and analyzed using ImageJ software
version 1.50 (National Institutes of Health).
In vivo experiments
A total of 24 male 6-week-old BALB/c null nude mice
(22±2 g) were obtained from HFK Bioscience Co., Ltd. and housed at
the Animal Care Facility of West China Hospital, Sichuan University
(Chengdu, China) under a controlled temperature (22±°C) and
humidity (55±5%), with a 12-h light/dark cycle and free access to
water and food. Prior to the operation, all mice were acclimatized
for 1 week. Tumor xenografts in mice were established by injecting
1×106 AGS or NCI-N87 cells subcutaneously into the right
flank region. After 5 days, the mice were randomly assigned into
two groups (n=6 for each group) and intraperitoneally injected with
25 mg/kg Tax twice weekly or an equal volume of saline,
respectively. During this period, the tumor size and body weight of
the mice were observed and recorded every 3 days. The allowed
maximum diameter of the tumors was 1.5 cm. At the end of the
experiment (the 21st day), all the 24 mice were sacrificed by
cervical dislocation under deep anesthesia (sodium pentobarbital
intraperitoneal injection, 50 mg/kg). After the cessation of the
heartbeat and respiratory arrest of the mice was confirmed, the
tumors were collected for measuring the weight and size, and frozen
at -80°C for use in subsequent western blot analysis. All animal
experiments were performed in accordance with the Care and Use of
Laboratory Animals established by the US National Institutes of
Health (10), and were approved
by the Ethics Committee of West China Hospital, Sichuan University
(approval no. 2021-05).
Immunohistochemistry
The tumor specimens were dissected and fixed in 4%
paraformaldehyde at 37°C for 48 h. Then, the tissues were
paraffin-embedded, and sectioned into 4-µm-thick slices. The
slices were deparaffinized, rehydrated and subjected to antigen
retrieval. Subsequently, the slices were blocked with 3% bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) at room temperature
for 30 min, and incubated with anti-Ki67 antibody (1:200; product
code ab16667; Abcam) at 4°C overnight. After washing with PBS, the
slices were incubated with HRP-conjugated goat anti-rabbit antibody
(1:1,000; product code ab97051; Abcam). Slices were counterstained
with hematoxylin for 2 min at room temperature and visualized with
DAB (ZSJQ-BIO) under a light microscope (magnification, ×200).
Statistical analysis
SPSS 17.0 software (SPSS, Inc.) was used for
statistical analysis and data are presented as the mean ± SD. All
data were determined from at least three independent experiments. A
Student's unpaired t-test was performed for comparisons between two
groups, and a one-way AVONA followed by a Tukey's post hoc test was
performed for comparisons among more than two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Tax on the viability and
proliferation of gastric cancer cells
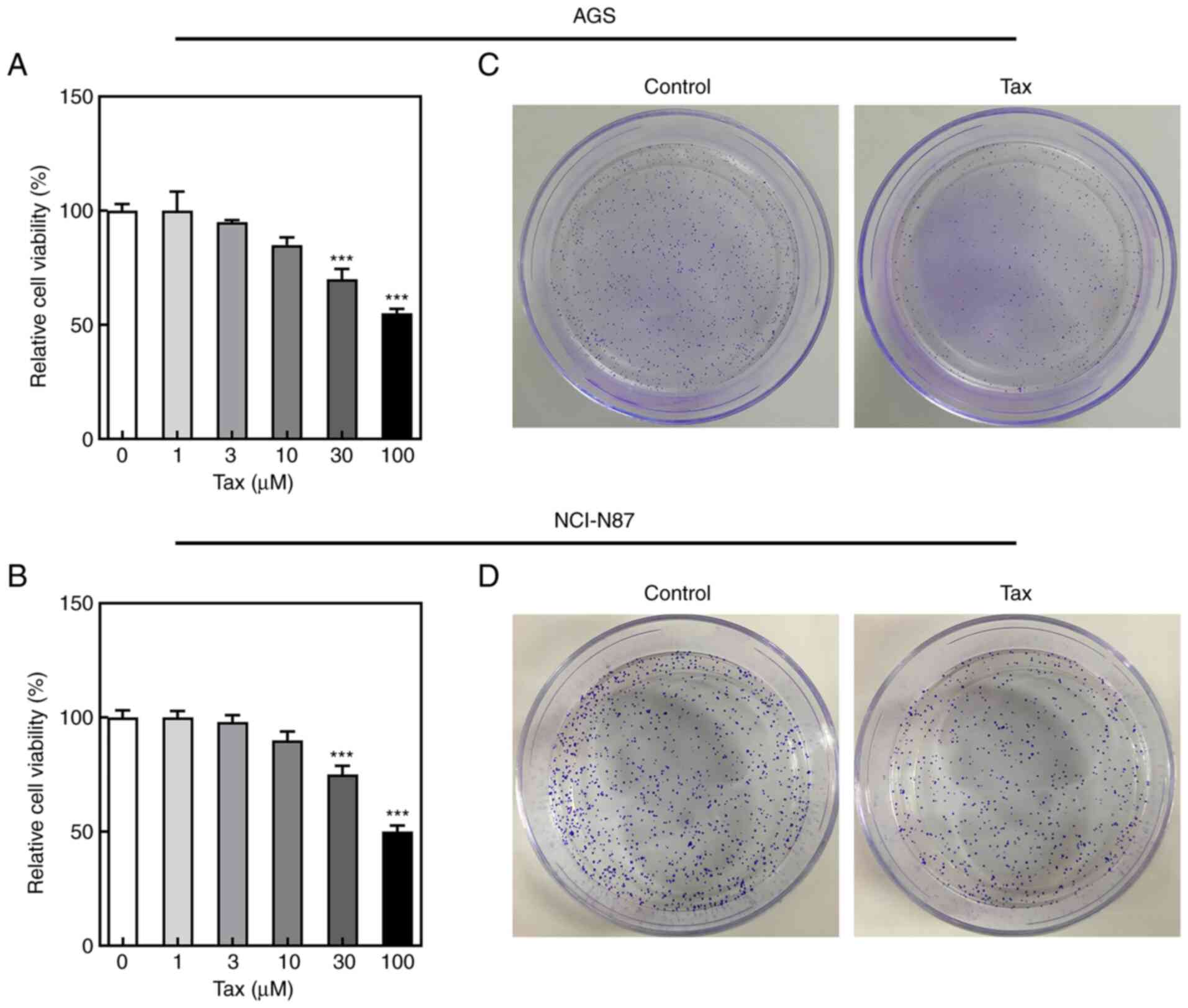
To examine the antitumor effects of Tax, two gastric
cancer cell lines, AGS and NCI-N87, were treated with various
concentrations of Tax (1, 3, 10, 30 and 100 µM). It was
observed that treatment of the gastric cancer cells with Tax
suppressed cell viability in a concentration-dependent manner
(Fig. 1A and B). In subsequent
experiments, 100 µM Tax was selected to treat the AGS and
NCI-N87 cells. A colony formation assay was performed to detect the
changes in colony formation following Tax treatment. As revealed in
Fig. 1C, Tax treatment decreased
the number of cell colonies formed compared with the control group
in both AGS and NCI-N87 cells. These results indicated that Tax had
the ability to hinder cell proliferation.
Effects of Tax on the migratory and
invasive abilities of gastric cancer cells
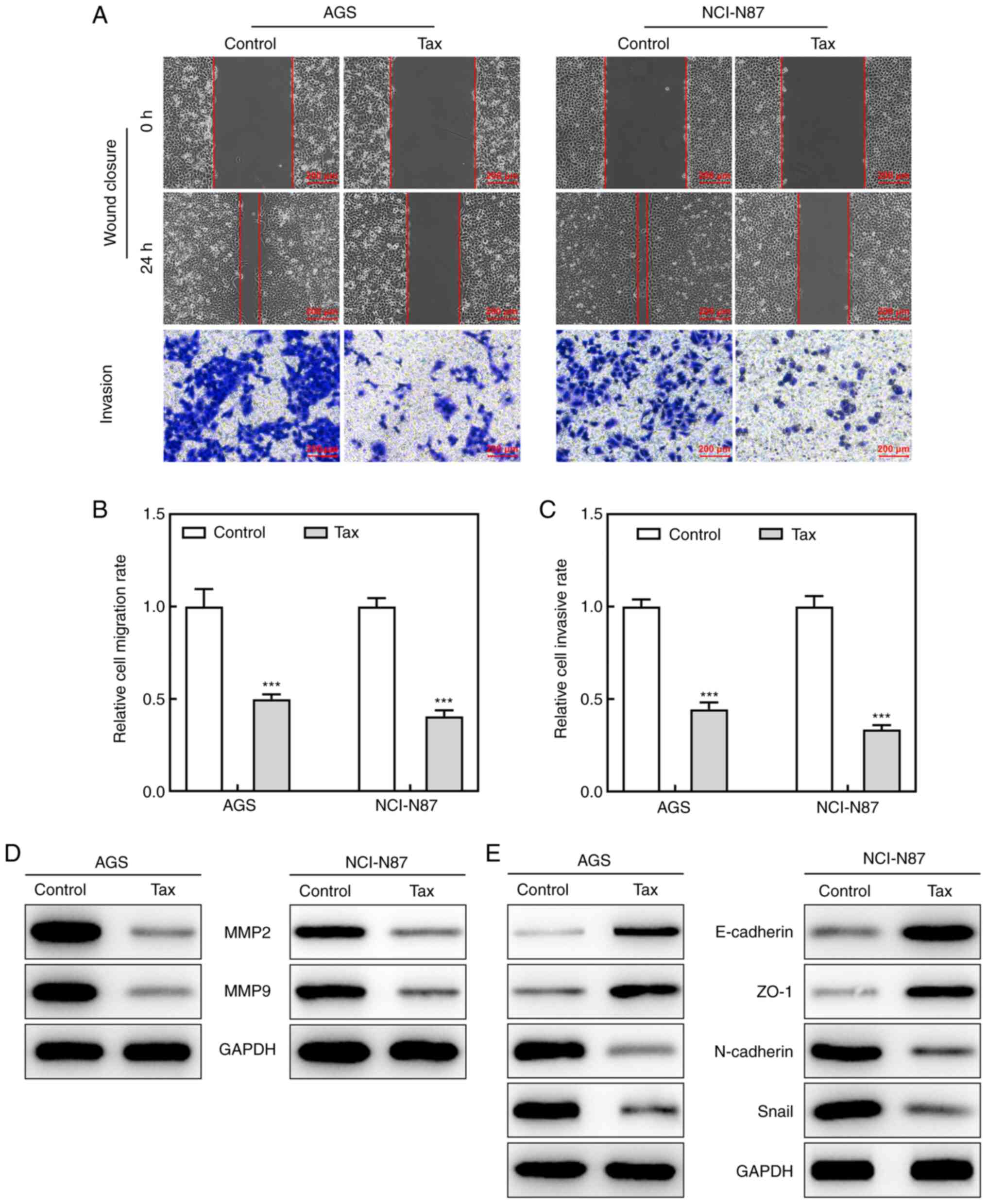
After demonstrating the inhibitory effects of Tax on
the cell proliferative ability, the present study then determined
whether Tax suppressed the cell migratory and invasive abilities.
In a wound-healing assay, Tax treatment led to a lower 'healing'
ability at 24 h in both the AGS and NCI-N87 cells. In addition, a
Transwell assay revealed that Tax hindered the invasive ability of
not only the AGS cells, but also the NCI-N87 cells (Fig. 2A-C). Moreover, the downregulated
protein expression levels of MMP2 and MMP9 upon Tax treatment
further demonstrated the role of Tax in gastric cancer (Fig. 2D). Furthermore, Tax treatment
increased the protein expression levels of E-cadherin and ZO-1, and
reduced the protein expression levels of N-cadherin and Snail
(Fig. 2E), indicating a
potential inhibitory role of Tax in epithelial-mesenchymal
transition (EMT) in gastric cancer. Therefore, these results
indicated that Tax impeded the migratory and invasive abilities of
gastric cancer cells by regulating EMT.
Effect of Tax on the AhR/CYP1A1 signaling
pathway in gastric cancer cells
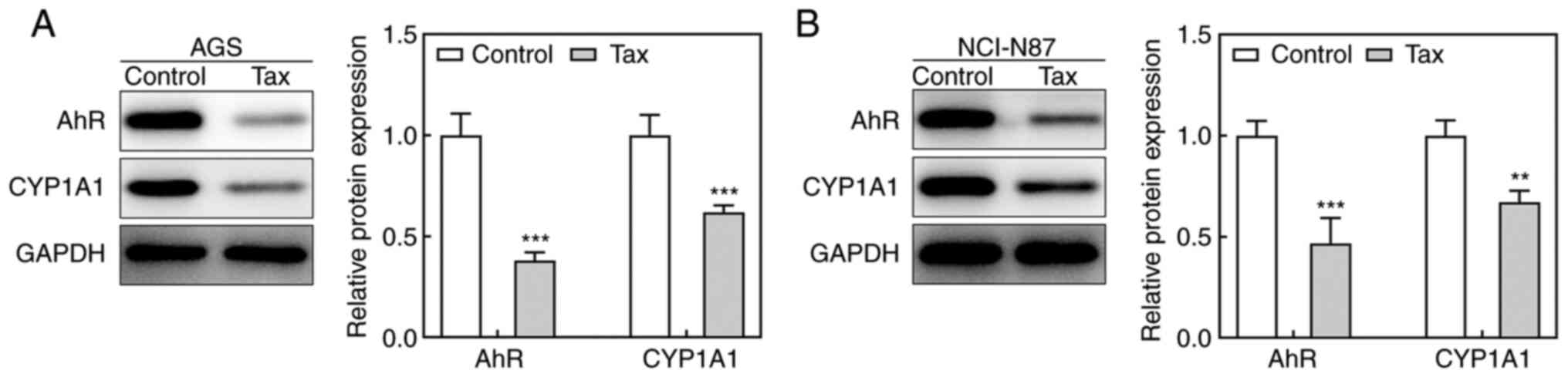
To elucidate the potential mechanisms underlying the
protective role of Tax in gastric cancer, the effects of Tax on the
AhR/CYP1A1 signaling pathway were examined. As revealed in Fig. 3A, Tax treatment significantly
decreased the protein expression levels of AhR and CYP1A1 in AGS
cells. A similar trend was observed in NCI-N87 cells (Fig. 3B). Therefore, these results
indicated that Tax suppressed the activation of the AhR/CYP1A1
signaling pathway.
Effects of the AhR agonist, SB203580, on
Tax-treated gastric cancer cells
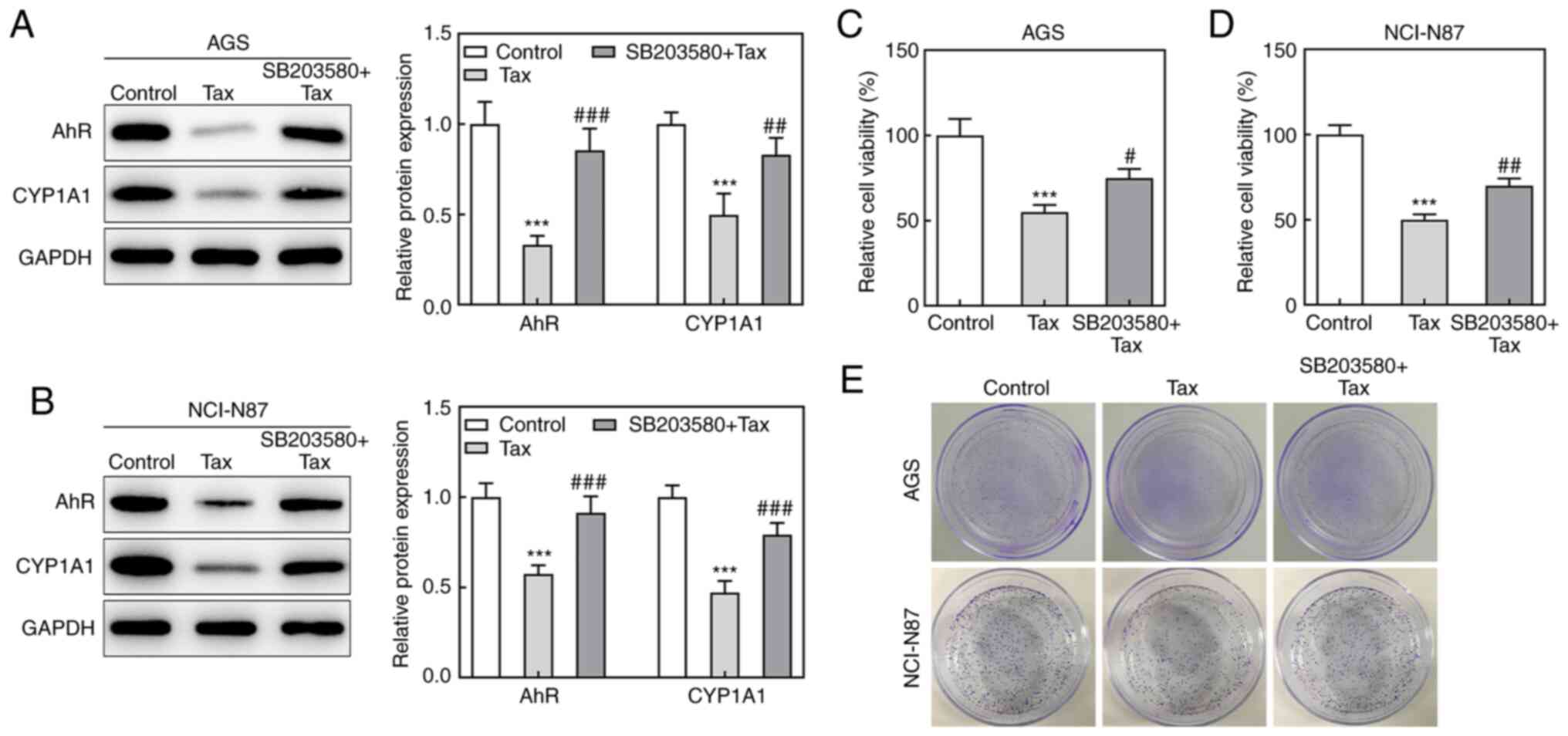
Subsequently, to identify whether the protective
role of Tax in gastric cancer was mediated via AhR/CYP1A1
signaling, the effects of the AhR agonist, SB203580, on Tax-treated
gastric cancer cells were examined. Firstly, SB203580 was revealed
to increase the protein expression levels of AhR and CYP1A1 in
Tax-treated AGS or NCI-N87 cells (Fig. 4A and B). Subsequently, a series
of cell biological behaviors were examined, as aforementioned. On
the one hand, the addition of SB203580 increased the viability of
AGS and NCI-N87 cells, and increased the colony formation number
compared with Tax treatment alone (Fig. 4C-E). On the other hand, the
hindered migratory and invasive abilities of Tax were partly
restored by SB203580, which was further verified by the upregulated
protein expression levels of MMP2 and MMP9 in the SB203580 + Tax
group (Fig. 5A-D). Furthermore,
SB203580 diminished the expression levels of E-cadherin and ZO-1,
whereas it elevated the expression levels of N-cadherin and Snail
in Tax-treated AGS and NCI-N87 cells (Fig. 5E). Therefore, these results
indicated that the inhibition of AhR/CYP1A1 signaling partly
attenuated the effects of Tax on gastric cancer cells.
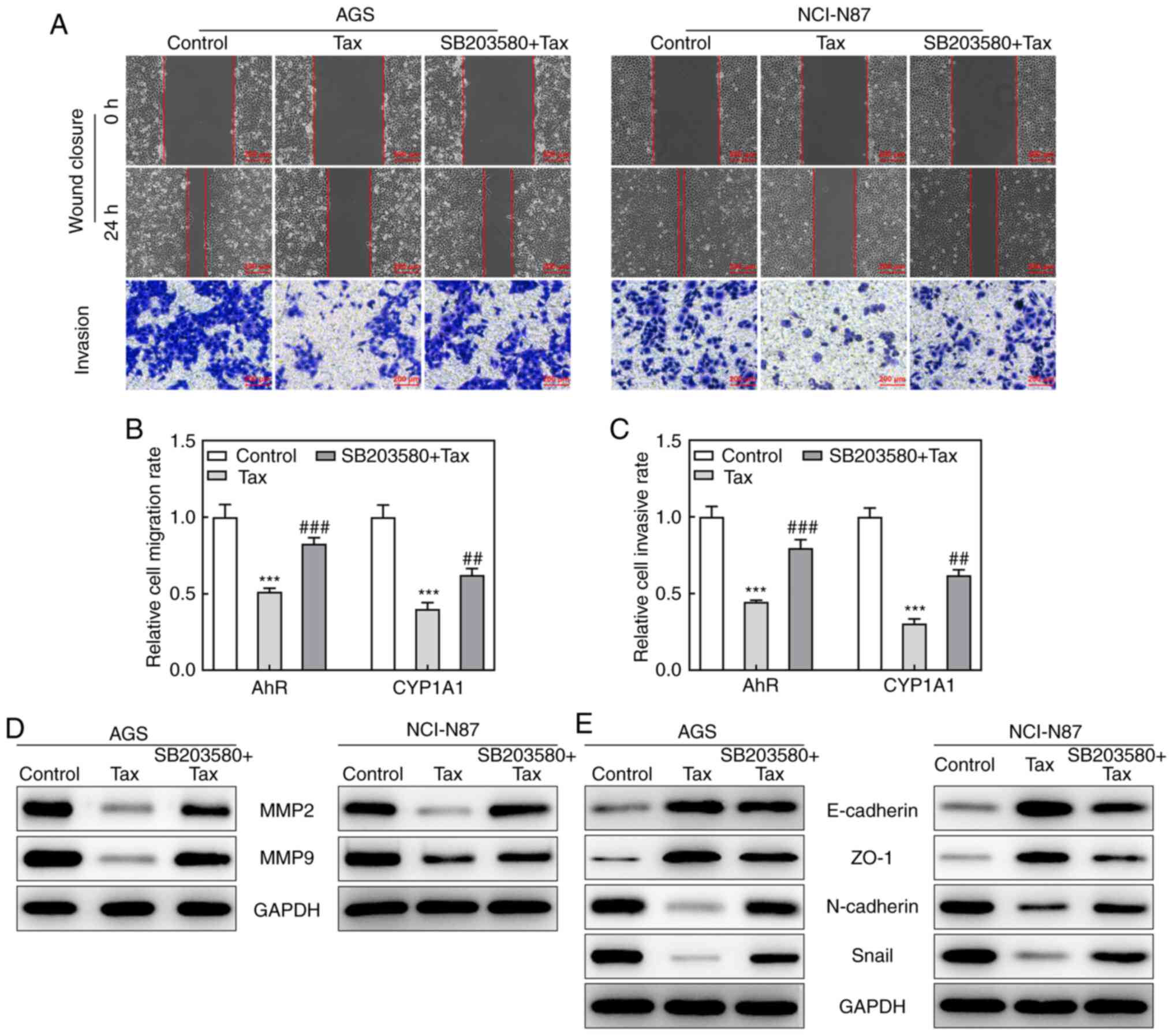 | Figure 5Effects of AhR agonist, SB203580, on
cell migration and invasion in Tax-treated gastric cancer cells.
(A) Wound-healing and Transwell assays were conducted to observe
cell migration and invasion abilities, respectively. (B) The
migration rate of each group was quantified. (C) The invasion rate
of each group was quantified. (D and E) The protein expression of
MMP2, MMP9, E-cadherin, ZO-1, N-cadherin and Snail was assessed by
western blotting. ***P<0.001 vs. the control;
##P<0.01 and ###P<0.001 vs. Tax. Tax,
taxifolin; AhR, aryl hydrocarbon receptor; CYP1A1, cytochrome P450
1A1; MMP, matrix metalloproteinase; ZO-1, Zonula occludens-1. |
Effects of Tax on gastric cancer in
vivo
Finally, the antitumor effects of Tax were further
examined in vivo. A mouse tumor model was established by
injecting AGS or NCI-N87 cells subcutaneously into the right flank
region. Following sacrifice, the tumors were removed and weighed.
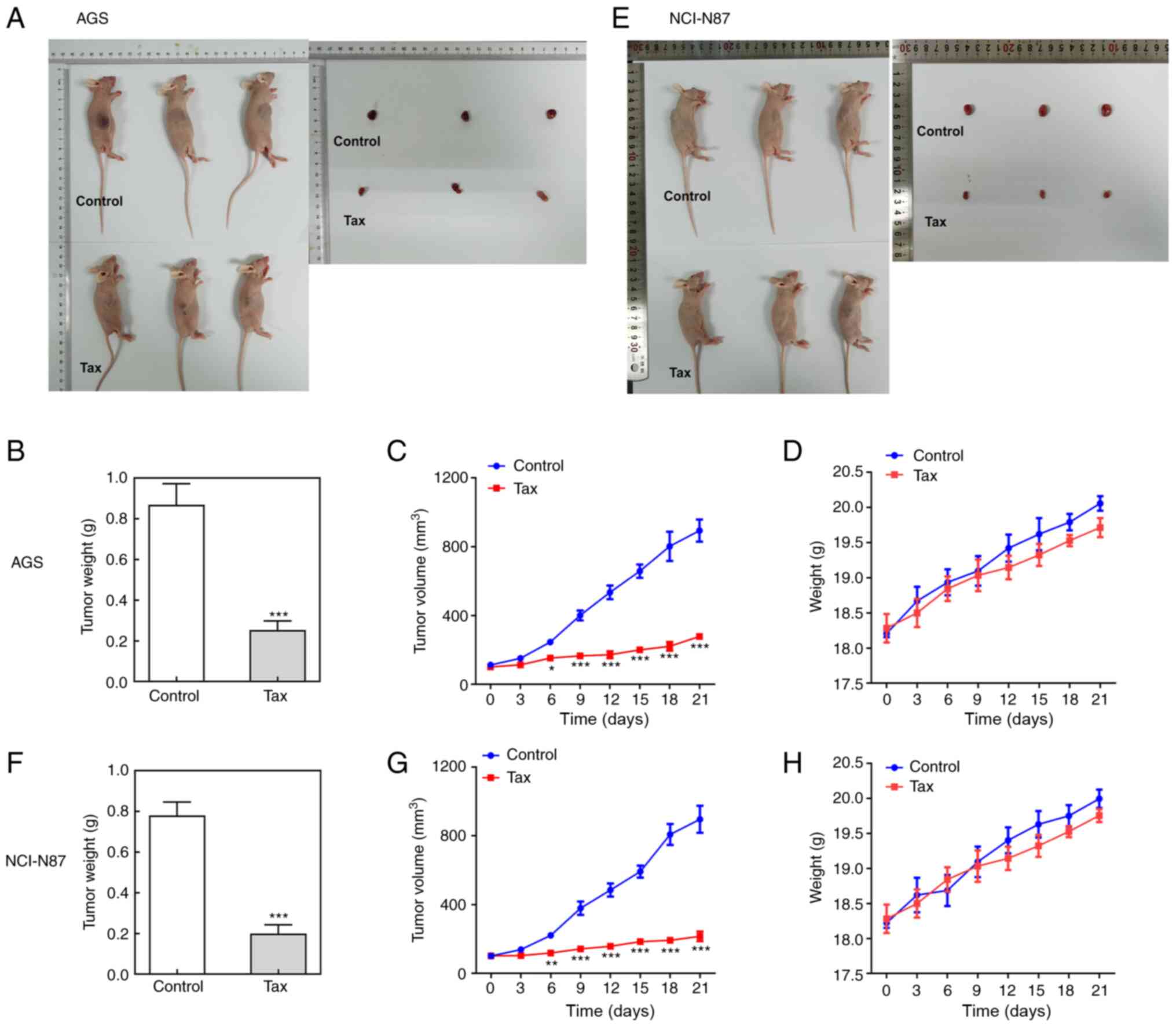
As revealed in Fig. 6A and B,
compared with the controls, the size and weight of tumors from mice
injected with AGS cells and treated with Tax were significantly
decreased. During the process of tumor growth, tumor size was
recorded every 3 days. The curve presented in Fig. 6C illustrates a continuous
inhibition of tumor size by Tax treatment. The body weights of mice
were also monitored every three days, but there were no significant
differences between these two groups (Fig. 6D). Similar results were obtained
from mice injected with NCI-N87 cells (Fig. 6E-H). These results demonstrated
that Tax suppressed the growth of gastric cancer in vivo. In
addition, immunohistochemical analysis revealed that Tax treatment
greatly reduced the expression level of Ki67 of tumor tissues from
mice injected with AGS or NCI-N87 cells (Fig. 7A and B). The protein expression
levels of Ki67, PCNA, MMP2 and MMP9 in the tumor tissues were
markedly reduced by Tax treatment (Fig. 7C and D). Concurrently, the
protein expression levels of E-cadherin and ZO-1 were upregulated,
whereas the protein expression levels of N-cadherin and Snail were
downregulated by Tax treatment (Fig.
7C and D); these results were consistent with those obtained
in vitro. Furthermore, AhR/CYP1A1 signaling was reduced, as
evidenced by the significantly downregulated protein expression
levels of AhR and CYP1A1 following Tax treatment (Fig. 7E and F). Therefore, the in
vivo experiments further demonstrated the antitumor activity of
Tax in gastric cancer and its potential mechanisms of action.
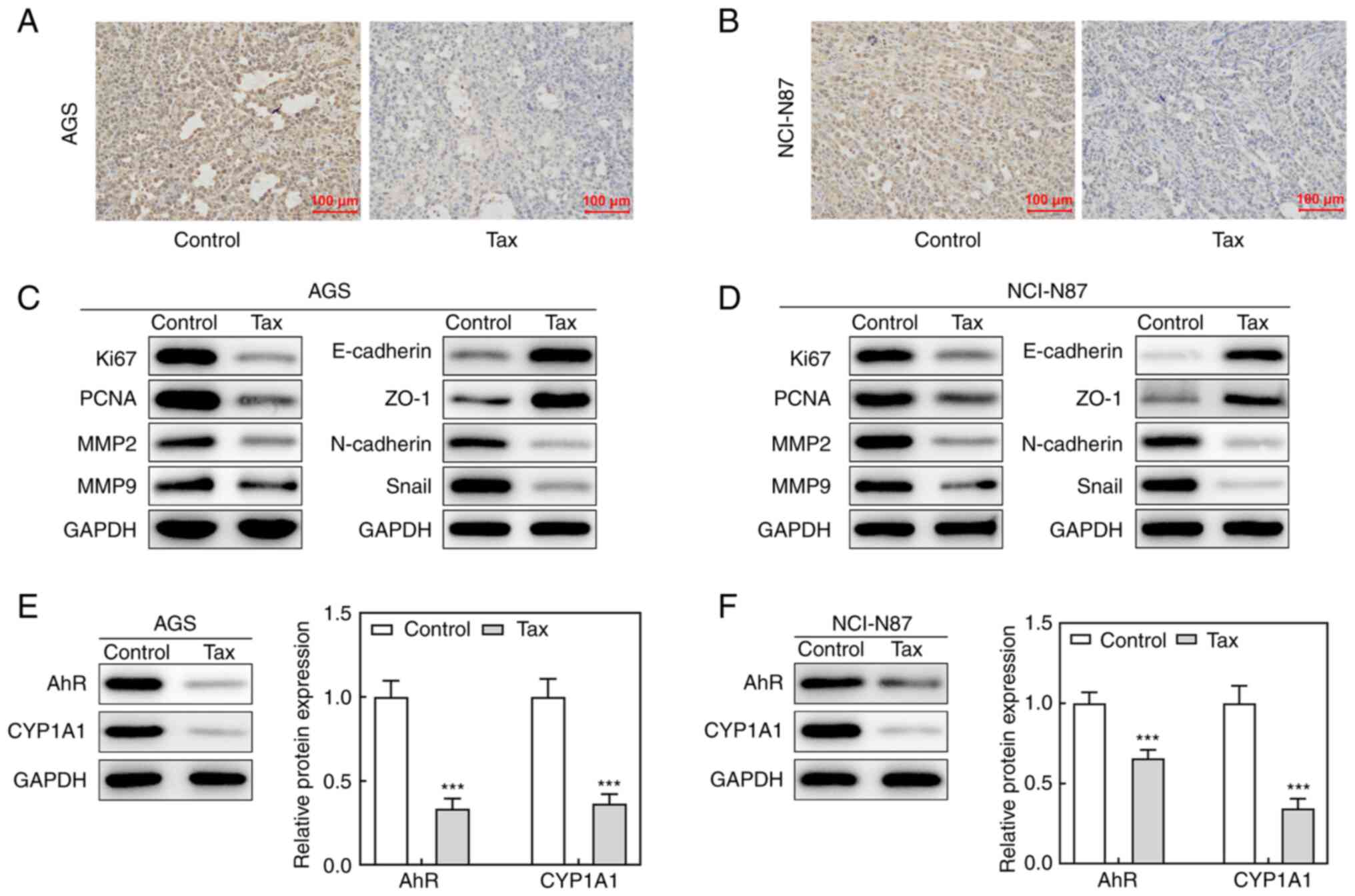 | Figure 7Effects of Tax on tumor growth of
gastric cancer in vivo. (A and B) BALB/c null nude mice were
administered AGS/NCI-N87 cell suspension injection to establish a
gastric cancer animal model, and were treated with Tax. After
sacrifice, immunohistochemical analysis was conducted to
investigate the expression level of Ki67 of tumor tissues from mice
injected with AGS or NCI-N87 cells. (C and D) The protein
expression levels of Ki67, PCNA, MMP2, MMP9, E-cadherin,
N-cadherin, ZO-1 and Snail were assessed using western blotting. (E
and F) The protein expression levels of AhR and CYP1A1 were
assessed using western blotting. ***P<0.001 vs. the
control. Tax, taxifolin; PCNA, proliferating cell nuclear antigen;
MMP, matrix metalloproteinase; ZO-1, Zonula occludens-1; AhR, aryl
hydrocarbon receptor; CYP1A1, cytochrome P450 1A1. |
Discussion
Gastric cancer is one of the most common
malignancies and the third leading cause of cancer-related
mortality worldwide (11).
Patients with gastric cancer are characterized as 'three low and
three high', whereby the 5-year survival rate, early diagnosis rate
and radical resection are low, and the morbidity, mortality and
metastatic rate are high (12).
Thus, discovery of effective therapeutics is crucial to overcome
this problem. Tax has been reported to possess certain antitumor
properties. However, its effects on gastric cancer have not yet
been explored, at least to the best of our knowledge.
The inhibition of uncontrolled cell proliferation is
crucial to hindering tumor progression. In the present study, it
was revealed that Tax inhibited the viability of two gastric cancer
cell lines (AGS and NCI-N87) in a concentration-dependent manner.
On the basis of these findings, the suitable Tax concentration was
used in subsequent experiments to assess the effects of Tax on
cellular biological behaviors. The results revealed that Tax
significantly reduced cell colony formation, indicating that Tax
exerted a suppressive effect on the cell proliferative ability. In
addition, Tax significantly diminished the cell migratory and
invasive abilities of AGS and NCI-N87 cells. To explore these
inhibitory activities in further detail, the protein expression of
MMPs and EMT markers was determined. In malignancies, MMPs promote
a large range of cellular processes, including cell proliferation,
migration and invasion, as well as facilitating EMT (13). During the process of EMT,
polarized epithelial cells complete multifaceted changes and
acquire mesenchymal cell phenotypes. In particular, the loss of
E-cadherin, a marker of epithelial cells, and the gain of
N-cadherin, a marker of mesenchymal cells, are the principal
characteristics of EMT (14).
Coenzyme Q0 has been reported to inhibit MMP9 expression and
increase the expression of E-cadherin in breast cancer tumors, and
to prevent tumor cell metastasis (15). Sinulariolide has also been
revealed to exhibit antitumor activity in gastric cancer by
inhibiting cell migration and invasion through the downregulation
of the EMT process (16). In the
present study, the upregulation of E-cadherin and ZO-1, and the
downregulation of N-cadherin and Snail, were also observed
following Tax treatment, indicating that Tax significantly
inhibited the EMT process of gastric cancer cells. Thus, Tax
exhibited potent anti-tumor activity in gastric cancer by
inhibiting cell proliferation, migration, invasion and EMT.
AhR is a cytosolic ligand-activated transcriptional
factor and plays an important role in the regulation of cancer
development. Once activated, AhR can initiate the transcriptional
regulation of a range of genes, such as CYP1A1, which is involved
in chemically-induced carcinogenesis (17-21). Thus, the AhR/CYP1A1 signaling
pathway has been widely researched in various types of cancer, and
plays a crucial role in the regulation of cancer progression.
Al-Dhfyan et al (21)
demonstrated that the AhR/CYP1A1 signaling pathway controlled the
proliferation, self-renewal ability and chemoresistance of breast
cancer stem cells. Maayah et al (19) revealed that the inhibition of the
AhR/CYP1A1 pathway exerted protective effects against breast cancer
initiation in human epithelial breast cells. Yin et al
(22) indicated that AhR and
CYP1A1 were upregulated in colorectal cancer tissues and
keratinocyte growth factor promoted cell proliferation via AhR
signaling in colorectal cancer cells. In addition, it was reported
that knockdown of AhR effectively decreased cell proliferation,
migration and invasion abilities in gastric cancer cells (23), demonstrating an important
regulatory role of AhR/CYP1A1 in gastric cancer; however, the
effects of Tax on the AhR/CYP1A1 signaling pathway in gastric
cancer have not been reported thus far, and whether Tax exerted its
anti-tumor function through the AhR/CYP1A1 signaling pathway in
gastric cancer remains unclear, and requires clarification. Of
note, a recent study demonstrated that Tax inhibited breast
carcinogenesis by inhibiting the AhR/CYP1A1 signaling pathway
(24). Thus, it was hypothesized
that Tax may exert its antitumor effects through AhR/CYP1A1
signaling pathway in gastric cancer. As was anticipated, Tax
treatment reduced the protein expression levels of AhR and CYP1A1
in gastric cancer in vitro and in vivo. SB203580 was
then used to activate AhR/CYP1A1 signaling. Further experiments
revealed that the activation of AhR/CYP1A1 signaling partly
abolished the suppressive effects of Tax on gastric cancer cell
proliferation, migration and invasion, indicating that Tax exerted
its anti-tumor effects partly via inhibiting the AhR/CYP1A1
signaling pathway.
However, certain limitations remain to be addressed
in the present study. Firstly, the potential mechanism of Tax in
gastric cancer via the AhR/CYP1A1 signaling pathway was focused on,
and the results revealed that the antitumor effects of Tax in
gastric cancer could be partly abolished by SB203580, an AhR
agonist; however, knocking down AhR and CYP1A1 in mice is also a
direct experiment which is necessary to be conducted in the future
to verify the critical role of AhR/CYP1A1 signaling underlying the
protective role of Tax in gastric cancer. Secondly, RNA-sequencing
is another effective way to verify our conclusion or further
analyze the mechanism of Tax in gastric cancer. In addition,
RNA-sequencing will provide much information about the differential
genes before or after Tax treatment, which is necessary to be
further investigated in our future work.
In conclusion, the present study determined for the
first time, to the best of our knowledge, that Tax inhibited
gastric cancer cell proliferative, migratory and invasive
abilities, and inhibited tumor growth. In addition, Tax exerted its
antitumor effects partly by inhibiting the AhR/CYP1A1 signaling
pathway. These findings provide a promising strategy for the
treatment of gastric cancer.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW conceptualized and designed the study. JX and YP
performed the experiments and acquired the data. XW and JX analyzed
and interpreted the data. All authors wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the Care and Use of Laboratory Animals established by the US
National Institutes of Health and were approved by the Ethics
Committee of West China Hospital, Sichuan University (Chengdu,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Cooperative Fund of
Nanchong Government and North Sichuan Medical College (grant no.
18SXHZ0357) and the Scientific fund of North Sichuan Medical
College (grant no. CBY15-A-ZD008).
References
|
1
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
3
|
Song Z, Wu Y, Yang J, Yang D and Fang X:
Progress in the treatment of advanced gastric cancer. Tumour Biol.
39:10104283177146262017. View Article : Google Scholar
|
|
4
|
Sunil C and Xu B: An insight into the
health-promoting effects of taxifolin (dihydroquercetin).
Phytochemistry. 166:1120662019. View Article : Google Scholar
|
|
5
|
Li J, Hu L, Zhou T, Gong X, Jiang R and Li
H, Kuang G, Wan J and Li H: Taxifolin inhibits breast cancer cells
proliferation, migration and invasion by promoting mesenchymal to
epithelial transition via β-catenin signaling. Life Sci.
232:1166172019. View Article : Google Scholar
|
|
6
|
Razak S, Afsar T, Ullah A, Almajwal A,
Alkholief M, Alshamsan A and Jahan S: Taxifolin, a natural
flavonoid interacts with cell cycle regulators causes cell cycle
arrest and causes tumor regression by activating Wnt/β-catenin
signaling pathway. BMC Cancer. 18:10432018. View Article : Google Scholar
|
|
7
|
Wang R, Zhu X, Wang Q, Li X, Wang E, Zhao
Q, Wang Q and Cao H: The anti-tumor effect of taxifolin on lung
cancer via suppressing stemness and epithelial-mesenchymal
transition in vitro and oncogenesis in nude mice. Ann Transl Med.
8:5902020. View Article : Google Scholar
|
|
8
|
Chen X, Gu N, Xue C and Li BR: Plant
flavonoid taxifolin inhibits the growth, migration and invasion of
human osteosarcoma cells. Mol Med Rep. 17:3239–3245. 2018.
|
|
9
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18:e30004102020. View Article : Google Scholar
|
|
10
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press; Washington, DC: 2011
|
|
11
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar
|
|
12
|
Wu H, Wang W, Tong S and Wu C:
Nucleostemin regulates proliferation and migration of gastric
cancer and correlates with its malignancy. Int J Clin Exp Med.
8:17634–17643. 2015.
|
|
13
|
Scheau C, Badarau IA, Costache R, Caruntu
C, Mihai GL, Didilescu AC, Constantin C and Neagu M: The role of
matrix metalloproteinases in the epithelial-mesenchymal transition
of hepatocellular carcinoma. Anal Cell Pathol (Amst).
2019:94239072019.
|
|
14
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar
|
|
15
|
Yang HL, Thiyagarajan V, Shen PC, Mathew
DC, Lin KY, Liao JW and Hseu YC: Anti-EMT properties of CoQ0
attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through
ROS-mediated apoptosis. J Exp Clin Cancer Res. 38:1862019.
View Article : Google Scholar
|
|
16
|
Wu YJ, Lin SH, Din ZH, Su JH and Liu CI:
Sinulariolide inhibits gastric cancer cell migration and invasion
through downregulation of the EMT process and suppression of
FAK/PI3K/AKT/mTOR and MAPKs signaling pathways. Mar Drugs.
17:6682019. View Article : Google Scholar
|
|
17
|
Kamenickova A and Dvorak Z: Effects of
flavored mineral waters on AhR-CYP1A1 signaling pathway in primary
human hepatocytes and in human hepatic and intestinal cancer cells.
Food Chem Toxicol. 50:1933–1939. 2012. View Article : Google Scholar
|
|
18
|
Haarmann-Stemmann T, Abel J, Fritsche E
and Krutmann J: The AhR-Nrf2 pathway in keratinocytes: On the road
to chemoprevention? J Invest Dermatol. 132:7–9. 2012. View Article : Google Scholar
|
|
19
|
Maayah ZH, Ghebeh H, Alhaider AA, El-Kadi
AO, Soshilov AA, Denison MS, Ansari MA and Korashy HM: Metformin
inhibits 7,12-dimethylbenz[a]anthracene-induced breast
carcinogenesis and adduct formation in human breast cells by
inhibiting the cytochrome P4501A1/aryl hydrocarbon receptor
signaling pathway. Toxicol Appl Pharmacol. 284:217–226. 2015.
View Article : Google Scholar
|
|
20
|
Vondracek J, Umannova L and Machala M:
Interactions of the aryl hydrocarbon receptor with inflammatory
mediators: Beyond CYP1A regulation. Curr Drug Metab. 12:89–103.
2011. View Article : Google Scholar
|
|
21
|
Al-Dhfyan A, Alhoshani A and Korashy HM:
Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates
breast cancer stem cells expansion through PTEN inhibition and
beta-Catenin and Akt activation. Mol Cancer. 16:142017. View Article : Google Scholar
|
|
22
|
Yin J, Sheng B, Pu A, Han B, Yang K, Wang
Q, Sun L and Yang H: Keratinocyte growth factor regulation of aryl
hydrocarbon receptor activation in colorectal cancer cells. Dig Dis
Sci. 61:444–452. 2016. View Article : Google Scholar
|
|
23
|
Yin XF, Chen J, Mao W, Wang YH and Chen
MH: Downregulation of aryl hydrocarbon receptor expression
decreases gastric cancer cell growth and invasion. Oncol Rep.
30:364–370. 2013. View Article : Google Scholar
|
|
24
|
Haque MW and Pattanayak SP: Taxifolin
inhibits 7,12-dimethylbenz(a)anthracene-induced breast
carcinogenesis by regulating AhR/CYP1A1 signaling pathway.
Pharmacogn Mag. 13(Suppl 4): S749–S755. 2018.
|