Introduction
Human coronaviruses have long been known to the
scientific community since their first discovery in the 1960s;
however, severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) is a new member of the Coronaviridae family
(1). These viruses have a
zoonotic source, with bats being the presumed reservoirs (2,3).
The disease caused by SARS-CoV-2 is known as the corona virus
disease-19 (COVID-19) and was officially described in December,
2019 in Wuhan, China. Following intensive research on SARS-CoV-2
biology, it is now known that it expresses four structural
proteins: Spike (S), nucleo-capsid (N), envelope (E) and membrane
(M) proteins (4). It has been
proven that among these four proteins, the S protein plays the most
crucial role in SARS-CoV-2 infection, since it mediates viral
attachment, fusion and entry into host cells. The S protein
consists of two domains, S1 and S2, from which the S1 domain has
been proven to mediate the initial binding to the
angiotensin-converting enzyme 2 (ACE2) receptor via its receptor
binding domain (RBD) (5), while
the C-terminal subunit (S2 domain) entails the fusion of virus with
the cellular membranes (6). It
has been demonstrated that polyclonal antibodies that specifically
targeted SARS-CoV-2 S protein were able to inhibit SARS-CoV-2 entry
into target human cells that expressed ACE2 (7). ACE2 is expressed on the cellular
membrane of a variety of organs, including the lungs, kidneys,
heart, arteries and cerebral cortex. Even though the primary
manifestations of COVID-19 arise from the respiratory system
(namely pneumonia and acute respiratory distress syndrome)
(8), it is well known that
COVID-19 also affects other systems, the majority of which express
ACE2. Such extra-respiratory manifestations may include
neurological, gastrointestinal and cardiovascular dysfunctions
(9-12). Even though the majority of
infected individuals will develop mild-to-moderate disease, a
fraction of patients will be hospitalized in order to receive
oxygen supplementation and supportive therapy (13,14). In addition, some of these
patients will require intensive care unit (ICU) admission and
mechanical ventilation due to cardiorespiratory decompensation. In
fact, it has been well established that such cases are associated
with a higher mortality rate (15).
As part of the global effort to halt the progression
of SARS-CoV2 and its negative impact on health, various vaccines
have been developed, and under certain legislation, have been
distributed for mass use. In Greece, the BNT162b2 COVID-19 mRNA
vaccine was the first vaccine available and healthcare
professionals were prioritized for vaccination. Since the vaccine
was developed and authorized under a conditional market
authorization regime, safety and efficacy parameters, such as the
duration of immunity, need to be closely monitored and examined
(16). The aim of the present
study was to examine the immune response to the BNT162b2 COVID-19
mRNA vaccine among healthcare professionals and to identify
determinants of antibody titers.
In order to measure antibody titers [immunoglobulin
(Ig)A, IgM and IgG], serological tests on whole blood, serum,
plasma or saliva are usually performed. Some of these are
qualitative, while others are semi-quantitative and others
quantitative (11,17,18). The present study utilized
semi-quantitative ELISA in order to evaluate IgG titers in the
serum of vaccinated individuals who have received two doses of the
BNT162b2 COVID-19 mRNA vaccine.
Subjects and methods
Population characteristics and sample
collection
Healthcare professionals working at the Venizeleion
General Hospital of Heraklion and the General Hospital of
Ierapetra, (both located in Crete, Greece) were invited to
participate in the present study through public announcements in
each hospital. Sample collection was performed from March to June,
2021. The study was approved by the Ethics Committees and
Scientific Councils of University Hospital of Heraklion (PAGNI),
Venizeleion General Hospital of Heraklion, General Hospital of
Ierapetra, and written informed consent was obtained from all
participants. All samples generated by the present study were
anonymized, and personal data were managed according to the EU
General Data Protection Regulation (GDPR; https://gdpr-info.eu/). Demographics and
anthropometric characteristics were recorded using a questionnaire.
The collected information included age, sex, weight, height and
smoking habits. Additionally, the nutritional status of the
participants was recorded based on a self-report of any known
deficiencies in vitamins (vitamin D, vitamin B12 and folic acid)
and iron (Fe), since such deficiencies may be linked to an
inadequate immune response (19,20). Furthermore, the use of
immunomodulatory substances (such as corticosteroids) and a medical
history of any chronic disease were also recorded. Participants
were asked to keep a diary of any adverse effects, using a
predetermined list of 27 adverse effects that were stratified into
five categories (localized symptoms, allergic reactions,
neurological/sensory symptoms, cardio-pulmonary and systemic
symptoms). The exclusion criteria were the following: i)
Individuals who had not received any dose of the BNT162b2 COVID-19
mRNA vaccine; ii) individuals who have received only the first dose
of the vaccine; and iii) the unwillingness of the individual to
participate in the study. In total, 517 individuals were included,
of which 319 reported the exact dates of their vaccinations.
According to the medical history of the participants, no one had
been affected by SARS-CoV-2 at the time of blood sampling.
Blood sampling was performed by obtaining 3 ml of
venous blood. The sample was placed in vials and centrifuged for 10
min at 2,900 x g at room temperature in order to separate serum.
All samples were kept at −20°C until further analysis.
Sample analysis
The analysis of the samples was performed using
ELISA with a semi-quantitative ELISA test kit (BIO-SHIELD,
2019-nCoV IgG; cat. no. C1148/C1196; Prognosis Biotech SA) for the
determination of IgG anti-bodies against SARS-CoV-2 in the serum of
individuals who had been fully vaccinated (two doses) with the
BNT162b2 COVID-19 mRNA vaccine, as per the manufacturer's
instructions. The wells of the microtiter strips were coated with a
mixture of different recombinant epitopes of SARS-CoV-2 S protein,
including the SARS-CoV-2 S protein. The diluted serum specimens,
positive controls, cut-off controls and negative controls provided
with the ELISA assay were added to the wells of the microtiter
plate. The antibody isotypes against SARS-CoV-2 in the specimens or
controls (if present) bind to the coated recombinant S protein
epitopes. Any unbound immunoglobulin was removed with a washing
step. A detection solution with the same mixture of recombinant
epitopes of SARS-CoV-2 S protein, conjugated to HRP, provided with
the ELISA assay was added and a double antigen sandwich system was
formed. The detection reagent that did not react was removed by
washing. A chromogen substrate was added to the wells resulting in
the progressive development of a blue coloured complex with the
detection reagent.
Colour development was then terminated by the
addition of stop solution. The measurement was performed
photometrically at 450 nm and the intensity of the produced
coloured complex was proportional to antibodies present in the
specimen. The presence of IgG against SARS-CoV-2 S protein in an
individual specimen was determined by comparing the optical density
of the specimen to the optical density of the cut-off control.
According to the instructions provided with the
assay, the recorded intra-assay precision (% CV values) for one
negative and three positive samples that were assayed 42 times were
6.7% for the negative specimen and from 3.7 to 4.2% for the
positive specimen, and the recorded inter-assay precision (% CV
values) for one negative and 3 positive samples assayed in 10
separate runs over 14 days were 7.1% for the negative and from 3.1
to 4.4% for the positive samples (according the assay brochure,
version 2020-04-03/rev. 03, BIO-SHIELD 2019-nCoV IgG). The results
from the specimens are expressed by the ratio of specimen
OD/cut-off control mean OD. A specimen ratio <0.8 was considered
as negative, a ratio between 0.8 and 1.0 was considered to be
'equivocal' and ratios r≥1 were considered as positive.
Statistical analysis
In order to evaluate the induced immune response
from the two doses of the vaccine, antibody titers were evaluated
in 517 individuals. Categorical variables were expressed as number
(n) and percentage (%), while continuous variables as the mean ± SD
and median. Due to the non-normal distribution of SARS-2-COVID19
antibodies, a Mann-Whitney test was applied for comparing antibody
levels between two groups and a Kruskal-Wallis test was applied for
comparisons between more than two groups. The correlation between
two variables was examined using Spearman's Rho. Multivariate
linear regression analysis was applied to establish correlates of
SARS-CoV-2 antibody titers, with demographics, time of sampling
relative to vaccination and adverse/side effects being used as
independent variables in the model. Bar charts and scatterplots
were applied for data presentation. IBM SPSS Statistics 26.0 was
used for data analysis (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Due to the self-reported completion of the
questionnaire, certain data on demographics, such as age (508 valid
cases, 98.2%) and body mass index (BMI; 481 valid cases, 93.0%)
were not provided. Moreover, there was a marked number of missing
values in the date of vaccination or in the question-naire
completion, with 319 participants providing complete data for
vaccination dates.
The demographics and anthropometric characteristics
of the study participants are presented in Table I. The mean age was 47.7 years,
ranging from 23 to 87 years, while the majority of the participants
were 51-60 years of age. Approximately two-thirds of the
participants were females. The distribution of BMI revealed that
4.9% of the individuals were underweight (<20 kg/m2),
36.6% were of normal weight (BMI ≥20 and <25 kg/m2),
32.4% were overweight (23%, ranging from 25 up to 28
kg/m2 and 12% ranging from 28 to 30 kg/m2),
while 23.2% were obese (≥30 kg/m2). For the remaining
6.9% of the participants, no data for BMI were provided by the
participants. Smokers comprised 34.4% of the participants.
 | Table IDemographic and somatometric measures
of the 517 participants. |
Table I
Demographic and somatometric measures
of the 517 participants.
| Characteristic | Measurement
values |
|---|
| Sex, n (%) | |
| Female | 343 (66.3) |
| Male | 174 (33.7) |
| Smoking status
(yes), n (%) | 178 (34.4) |
| Age groups, divided
in years, n (%) | |
| ≤30 | 56 (11.0) |
| 31-40 | 75 (14.8) |
| 41-50 | 130 (25.6) |
| 51-60 | 198 (39.0) |
| ≥61 | 49 (9.6) |
| Age, years; mean ±
SD (range) | 47.7±11.6
(23-87) |
| BMI groups
(kg/m2), n (%) | |
| <20 | 24 (4.9) |
| ≥20 and
<25 | 176 (36.6) |
| ≥25 and
<28 | 111 (23.1) |
| ≥28 and
<30 | 58 (12.1) |
| ≥30 | 112 (23.2) |
| BMI
(kg/m2); mean ± SD (range) | 26.7±5.0
(17.4-59.3) |
| Weight (kg) | 75.4±16.9
(45.5-190) |
| Height (m) | 1.68±0.09
(1.49-2.00) |
The data on the self-reported nutritional status and
the use of immunomodifiers are presented in Table SI. Vitamin D deficiency was the
most prevalent deficiency reported, followed by Fe deficiency. The
use of immunomodifiers was reported by 9.1% of the participants,
from which 4.8% reported the use of corticosteroids. As regards the
presence of chronic diseases among the participants, this was
reported in only 0.9% (Table
SI).
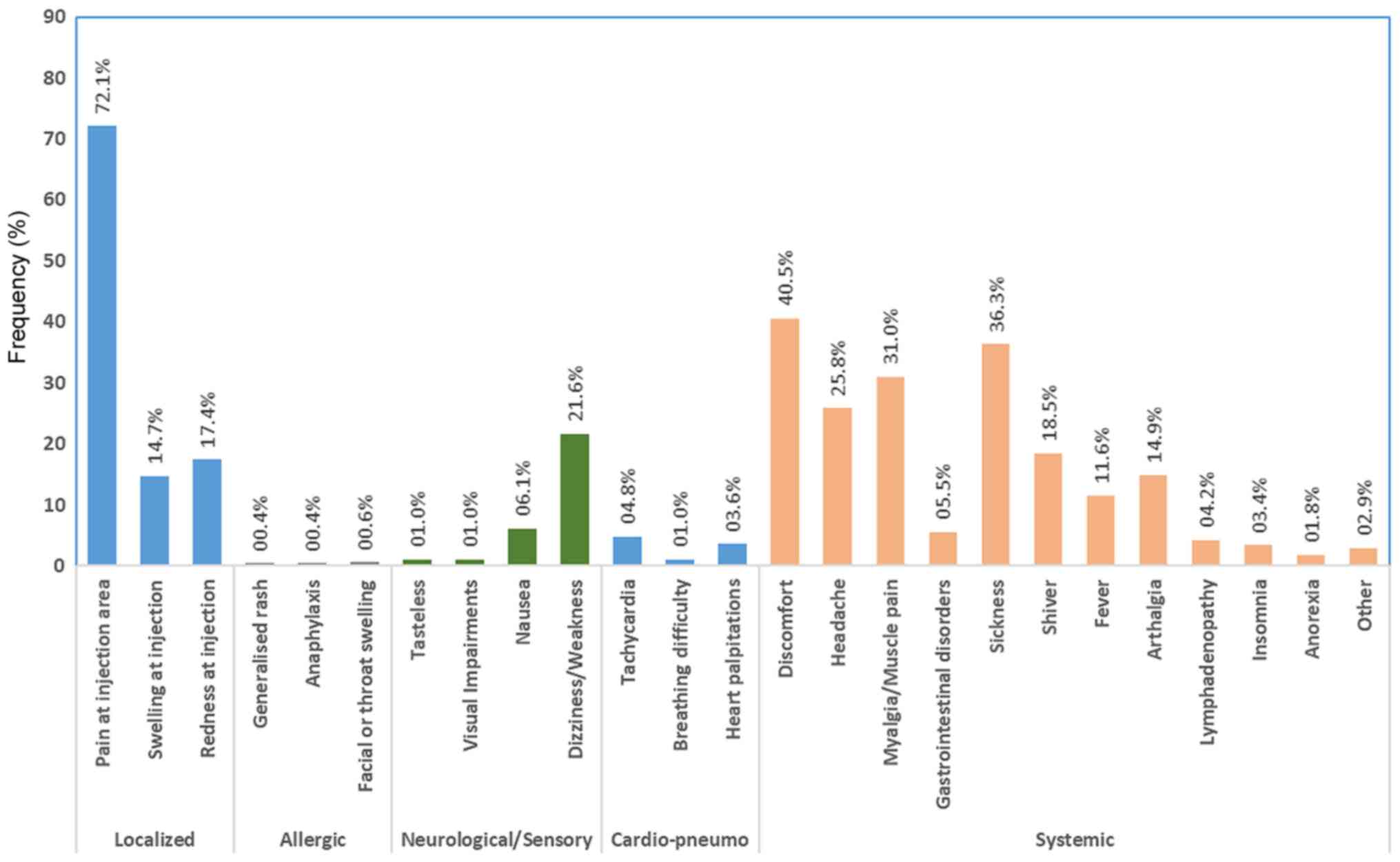
The six most prevalent adverse effects were pain at
the injection site (72.1%), generalized fatigue/discomfort (40.5%),
malaise/sickness (36.3%), myalgia (31.0%), headache (25.8%) and
dizziness/weakness (21.6%) (Fig.
1). Of note, less than one fifth of the participants did not
report any adverse effects. The prevalence of adverse effects among
participants, both separately for each effect and for each category
of effects is presented in Table
SII.
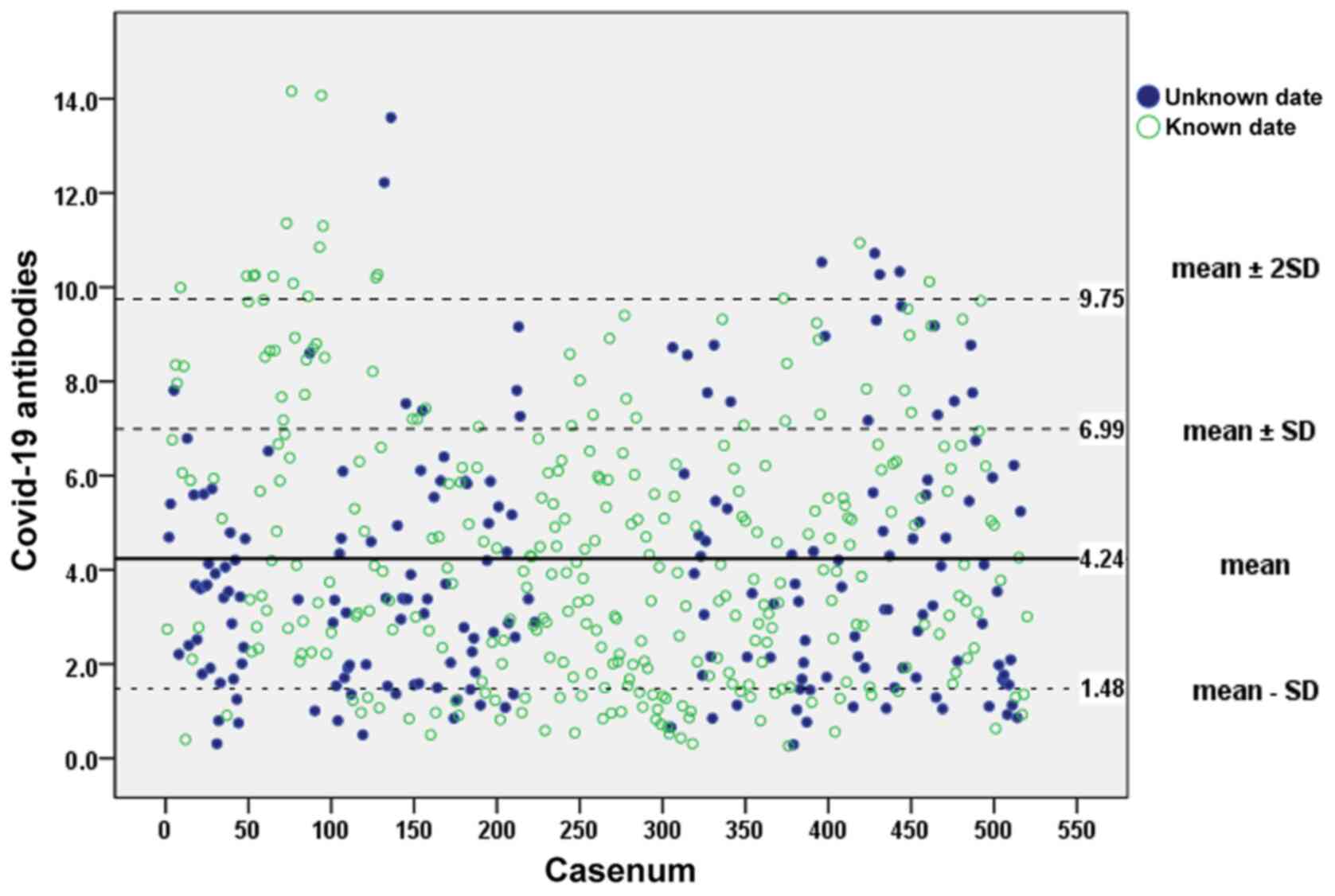
The SARS-CoV-2 antibody titers ranged from 0.26 to
14.16, with a mean value of 4.23±2.76. The scatterplot illustrated
in Fig. 2 presents the
measurements of the whole study group (517 participants) performed
using ELISA. The mean ± 2SD value indicates the limits of estimated
SARS-CoV-2 anti-bodies at 9.75. Out of the 517 individuals that
were included, 198 (38.3%) did not report one or both dates of
their vaccination with the BNT162b2 COVID-19 mRNA vaccine. However,
a comparison of the mean value of the IgG titer of the group with
known vaccination dates (319 participants) (4.43±2.84) labelled
with green non-solid dots vs. the mean value of the whole group
(all 517 participants) did not exhibit any statistically
significant difference (P=0.339).
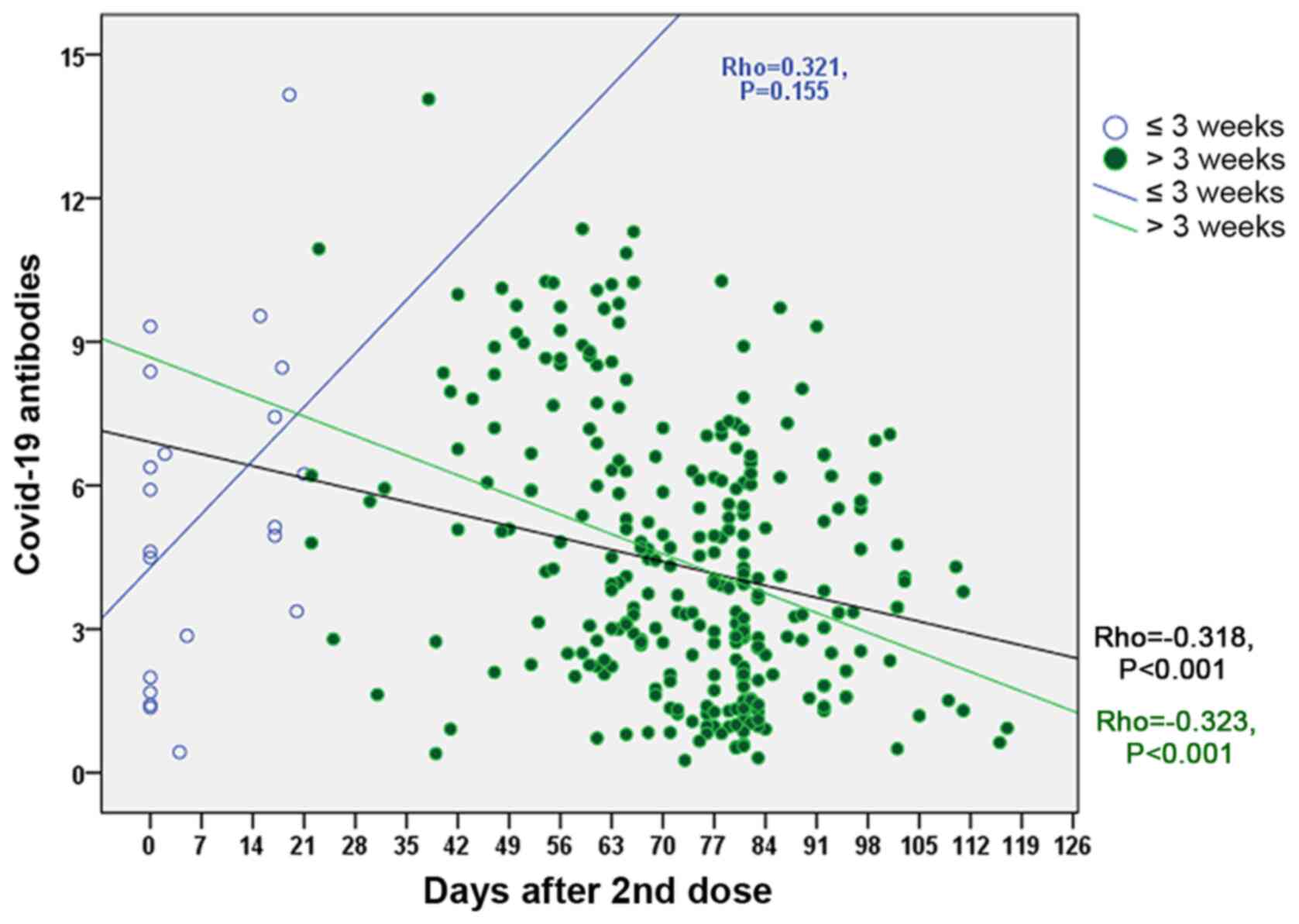
The time (days) of blood sampling post-vaccination
ranged from 0 to 117 days with a mean period of 69.0±23.5 days.
statistically significant negative correlation was found between
the antibody titers from all cases (Rho=−0.318, P<0.001; black
line), while for the time post-vaccination in 319 cases exhibited a
similar association (Rho=−0.323, P<0.001; green line). However,
no statistically significant correlation was found between the
antibody titers in participants whose blood samples were obtained
<3 weeks post-vaccination after the final dose of the vaccine
(Rho=0.321, P=0.155) (blue line) (Fig. 3).
The SARS-CoV-2 antibody titers according to
demo-graphics, anthropometric variables and smoking status are
presented in Table II. Females
had higher antibody titers (4.44±2.70) compared with males
(3.89±2.84; P=0.007). Statistically significant differences were
also observed between non-smokers (4.48±2.79) and smokers
(3.80±2.64; P=0.003). An older age was also associated with lower
anti-body titers (P<0.001). No statistically significant
differences in antibody titers was observed among the different BMI
groups. As regards the self-reported vitamin D, B12, folic acid and
Fe deficiency, no significant differences were observed in the
antibody titers. In addition, the titers of patients who used
immunomodifiers did not markedly differ from that of the general
population titer (all P>0.050) (Table III).
 | Table IISARS-CoV-2 antibody titers according
to demographics, anthropometric variables and smoking status. |
Table II
SARS-CoV-2 antibody titers according
to demographics, anthropometric variables and smoking status.
| Characteristic | SARS-CoV-2 antibody
titers
| P-value |
|---|
| Mean | SD | Median |
|---|
| Sex | | | | 0.007a |
| Female | 4.44 | 2.70 | 3.97 | |
| Male | 3.89 | 2.84 | 3.28 | |
| Age groups, divided
in years | | | | <0.001b |
| ≤30 | 5.45 | 2.97 | 5.42 | |
| 31-40 | 4.43 | 2.91 | 3.68 | |
| 41-50 | 4.42 | 2.70 | 3.70 | |
| 51-60 | 3.94 | 2.65 | 3.37 | |
| ≥61 | 3.23 | 2.42 | 2.57 | |
| BMI groups
(k/m2) | | | | 0.063b |
| <20 | 4.84 | 3.16 | 3.90 | |
| ≥20 and
<25 | 4.41 | 2.56 | 4.06 | |
| ≥25 and
<28 | 3.95 | 2.62 | 3.45 | |
| ≥28 and
<30 | 3.53 | 2.69 | 2.89 | |
| ≥30 | 4.15 | 2.92 | 3.63 | |
| Smoking status | | | | 0.003a |
| No | 4.48 | 2.79 | 4.01 | |
| Yes | 3.80 | 2.64 | 2.98 | |
 | Table IIIAntibody titers according to
nutritional status, the use of immunosuppressants and
corticosteroids (data retrieved from self-reported
questionnaire). |
Table III
Antibody titers according to
nutritional status, the use of immunosuppressants and
corticosteroids (data retrieved from self-reported
questionnaire).
| Variables | SARS-CoV-2 antibody
titers
| P-value |
|---|
No
| Yes
|
|---|
| Mean | SD | Median | Mean | SD | Median |
|---|
| Deficiency | | | | | | | |
| Fe | 4.20 | 2.76 | 3.64 | 4.66 | 2.87 | 4.00 | 0.180 |
| Vitamin D | 4.35 | 2.86 | 3.79 | 4.06 | 2.57 | 3.52 | 0.489 |
| Vitamin B12 | 4.29 | 2.84 | 3.60 | 4.16 | 2.44 | 3.90 | 0.947 |
| Folic acid | 4.31 | 2.79 | 3.73 | 4.07 | 2.87 | 3.33 | 0.536 |
| Use of | | | | | | | |
|
Immunosuppressants | 4.27 | 2.76 | 3.68 | 3.56 | 3.05 | 2.78 | 0.134 |
|
Corticosteroids | 4.29 | 2.78 | 3.68 | 3.21 | 2.30 | 2.44 | 0.064 |
The antibody titers according to the self-reported
adverse effects post-vaccination are presented in Table IV. Participants reporting any
adverse effects had higher titers compared with those reporting no
effects (P=0.002). A similar observation was made for participants
with systemic adverse effects compared with those having no
systemic adverse effects (P=0.001). Higher antibody titers were
found in participants who experienced redness at the injection site
(4.72±2.78, P=0.047, discomfort (4.57±2.85 P=0.015), headache
(4,87±2,86, P=0.001), myalgia 4.81±2.99, P=0.003) and
sickness/malaise (4.58±2.69; P=0.007), chilling and fever compared
with those who reported none of the aforementioned symptoms.
 | Table IVAntibody titers according to
self-reported adverse effects post-vaccination. |
Table IV
Antibody titers according to
self-reported adverse effects post-vaccination.
| Variables | SARS-CoV-2 antibody
titers
| P-value |
|---|
No
| Yes
|
|---|
| Mean | SD | Mean | SD |
|---|
| Any symptom | 3.47 | 2.47 | 4.41 | 2.79 | 0.002 |
| Regional | 3.94 | 2.77 | 4.37 | 2.76 | 0.087 |
| Neuro/sensory | 4.17 | 2.78 | 4.54 | 2.73 | 0.146 |
|
Cardio/-respiratory | 4.28 | 2.77 | 3.97 | 2.79 | 0.492 |
| Systemic | 3.79 | 2.61 | 4.57 | 2.82 | 0.001 |
| Allergic reactions
(generalized rash, anaphylaxis, facial or throat swelling) | 4.27 | 2.77 | 3.61 | 2.54 | 0.621 |
| | | | |
| Regional (at
injection site) | | | | | |
| Pain | 3.93 | 2.76 | 4.37 | 2.78 | 0.074 |
| Swelling | 4.11 | 2.68 | 4.76 | 3.05 | 0.104 |
| Redness | 4.16 | 2.76 | 4.72 | 2.78 | 0.047 |
| Neuro/sensory | | | | | |
| Nausea | 4.14 | 2.72 | 5.19 | 2.99 | 0.053 |
|
Dizziness/weakness | 4.15 | 2.75 | 4.44 | 2.74 | 0.297 |
|
Cardio-respiratory | | | | | |
| Tachycardia | 4.24 | 2.74 | 3.62 | 2.89 | 0.181 |
| Heart
palpitations | 4.25 | 2.77 | 4.60 | 2.79 | 0.526 |
| Systemic
reactions | | | | | |
| Discomfort | 3.96 | 2.65 | 4.57 | 2.85 | 0.015 |
| Headache | 3.98 | 2.67 | 4.87 | 2.86 | 0.001 |
| Myalgia/muscle
pain | 3.95 | 2.59 | 4.81 | 2.99 | 0.003 |
| Gastrointestinal
disorders | 4.17 | 2.73 | 4.93 | 3.07 | 0.214 |
| Malaise | 4.00 | 2.76 | 4.58 | 2.69 | 0.007 |
| Chills | 3.98 | 2.62 | 5.19 | 3.07 | 0.001 |
| Fever | 4.08 | 2.71 | 5.21 | 2.86 | 0.004 |
| Arthralgia | 4.14 | 2.72 | 4.60 | 2.88 | 0.186 |
|
Lymphadenopathy | 4.17 | 2.75 | 5.10 | 2.61 | 0.085 |
| Insomnia | 4.18 | 2.74 | 4.92 | 2.94 | 0.316 |
From the multivariate linear regression analysis
(Table V), age and time (days)
of sampling post-vaccination were negatively associated with
antibody titers. Myalgia was positively associated with antibody
titers.
 | Table VMultivariate linear regression
analysis of antibody titer sampling post-vaccination. |
Table V
Multivariate linear regression
analysis of antibody titer sampling post-vaccination.
| Variables | SARS-CoV-2 antibody
titers
|
|---|
Unstandardised
| Standardised
| P-value |
|---|
| β | 95% LL | 95% UL | β |
|---|
| Age | −0.064 | −0.091 | −0.038 | −0.268 | <0.001 |
| Sex | −0.074 | −0.750 | 0.601 | −0.012 | 0.829 |
| Smoking status | −0.574 | −1.211 | 0.063 | −0.096 | 0.077 |
| Days after 2nd
dose | −0.040 | −0.053 | −0.027 | −0.331 | <0.001 |
| Redness | 0.132 | −0.694 | 0.957 | 0.017 | 0.754 |
| Discomfort | −0.194 | −1.015 | 0.626 | −0.034 | 0.641 |
| Headache | −0.056 | −0.775 | 0.662 | −0.009 | 0.878 |
| Myalgia/muscle
pain | 0.821 | 0.004 | 1.638 | 0.134 | 0.049 |
| Malaise | −0.038 | −0.852 | 0.775 | −0.006 | 0.926 |
| Chills | 0.010 | −0.907 | 0.927 | 0.001 | 0.983 |
| Fever | 0.606 | −0.496 | 1.708 | 0.068 | 0.280 |
Discussion
SARS-CoV-2 has become one of the most pronounced
health challenges of the 21st century, affecting not only the
global healthcare systems, but the socioeconomic systems as well.
In order to halt its progression and protect those individuals who
are at a high risk of developing severe COVID-19 infection,
national authorities implemented a series of emergency measures
among which were restrictions in transportation, socialization and
periodic lockdowns (21,22). Despite certain transient
improvements in infection rates, these measures had major
side-effects, such as the economic stagnation and
social/psychological impairment that numerous individuals
experienced (23). In order to
address these issues, considerable investments in human and
financial resources have been made in order to identify a solution
that will allow a safe, rapid and permanent return to normality
(24).
In the absence of specific pharmacotherapy (25-29), vaccines are expected to play an
important role in suppressing the COVID-19 pandemic (30). Several vaccine platforms have
been tested, such as those containing protein subunits, virus-like
particles, DNA or RNA sequences, non-replicating viral vectors,
replicating viral vectors, inactivated SARS-CoV-2 and live
attenuated SARS-CoV-2 (31). To
date, 297 vaccines are in development; 112 are currently undergoing
clinical testing (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines)
(45 in phase 3 clinical trials https://covid19.trackvaccines.org/vaccines/) and 22
have received authorization by at least one country (https://covid19.trackvaccines.org/trialsvaccines-by-country/#trials);
however, only four are widely authorized for emergency use
(https://www.covid-19vaccinetracker.org/). One of the
first vaccines that was authorized for emergency use was the
BNT162b2 COVID-19 mRNA vaccine (32). It contains mRNA that encodes the
S protein, a trimeric trans-membrane glycoprotein located on the
surface of the virus that plays a key role in viral entry into host
cells. The mRNA is encapsulated in a lipid nanoparticle in order to
protect it from degradation before entering the target cells.
Following injection, the nanoparticles fuse with the target cell
membrane and release the mRNA in the cytoplasm where it is
translated into S protein. Subsequently, the cell projects the
protein on to its surface, which results in the development of an
immune response and the production of antibodies (33).
In Greece, the vaccination program against
SARS-CoV-2 was initiated on December 27, 2020 with the first
vaccine available for use being the BNT162b2 COVID-19 mRNA vaccine.
Since then, three more vaccines, namely Ad26.COV2.S (34), mRNA-1273 (35), ChAdOx1 nCoV-19 (36), became available, accelerating the
progress of the vaccination program and achieving >5.5 million
vaccinations as of July, 2021 (https://emvolio.gov.gr/; accessed August 20, 2021).
However, a major issue of anti-SARS-CoV-2 vaccination that is not
yet fully understood is the levels of anti-SARS-CoV-2 antibody
titers following complete vaccination, and the various parameters
that may affect this. The present study demonstrates that the IgG
antibody titers induced by the BNT162b2 COVID-19 mRNA vaccine are
significantly dependent on and correlated with both modifiable and
non-modifiable parameters. Non-modifiable parameters include: i)
Age, with younger adults developing higher IgG titers than older
adults; and ii) sex, with females developing higher titers than
males. Moreover, the presence of any systemic or non-systemic
symptoms following inoculation was associated with higher antibody
titers. On the other hand, among the modifiable parameters that
were proven to affect antibody titers is the smoking habit, with
non-smokers having higher IgG titers than smokers (37). In addition, the time of sampling
after the second vaccine dose appeared to negatively correlate with
antibody titers starting from the third week post-vaccination. This
suggests that monitoring for a potential decrease in the immune
response should be performed at 3 weeks post-vaccination. These
findings are of utmost importance, since they suggest that those
individuals who are at a greater risk of developing severe COVID-19
infection (older individuals, smokers) are in fact the same
individuals who will not develop high titers of IgG antibodies.
These individuals may thus be vulnerable for SARS-CoV-2 infection
despite immunization or their immunization may last as long as that
of other individuals. However, even though some studies have
suggested that IgG titers in vaccinated individuals decline over
time, the exact rate of this decline in IgG titers or the critical
titer level up to which they can still be considered as adequately
protected from infection and disease, remain unclear (38). At this point, it should be
emphasized that the immune system may produce a series of antibody
variants, each with a different neutralizing capacity, depending on
the exact target-epitope (39-42).
Notably, a multicenter, perspective study (named
CRO-VAX HCP) was conducted recently in Belgium on 200 subjects
having received the BNT162b2 mRNA COVID-19 vaccine, aiming to
assess the antibody response (41). To this end, antibodies against
the SARS-CoV-2 nucleocapsid and SARS-CoV-2 S protein were measured
in different time points, ranging from 14 to 90 days. Of note, a
significant decrease in antibody titers was observed at 3 months
compared with the peak response, with the antibody response being
higher in seropositive compared with initially seronegative
participants (41). A second
study was also performed, enrolling a homogenous healthcare workers
group in Romania, in an attempt to investigate the immunity status
of subjects having received the BNT162b2 mRNA COVID-19 vaccine as
well (42). In this framework,
it was found that following the first vaccination dose, the IgG
levels in non-infected subjects exhibited an increase of ~12-fold
in males and one of ~11-fold in females; however, following the
second round of vaccination, the IgG levels increased 1.33-fold in
males and 2.11-fold in females, when compared with those after the
first dose (42). Importantly,
in that study, the most prevalent adverse effects observed were
pain at the injection site and flu-like symptoms, which are in
accordance with the findings of the present study. Moreover, in
another study that involved 203 patients who had recovered from
SARS-CoV-2 infection in Denmark, broad serological profiles within
the cohort were reported, with the majority of patients having
robust adaptive immune responses regardless of their disease
severity (43). Of note, the
viral surface spike protein was identified as the dominant target
for both neutralizing antibodies and CD8+ T-cell
responses.
In conclusion, crucial questions remain to be
answered as regards the duration of immunity post-vaccination. The
findings of the present study highlight the need for more effective
and detailed immunosurveillance programs. Such programs are
required to focus on those individuals that the present study
identified and to closely monitor their IgG titers in order to
identify in an early stage when they should receive a third dose of
the vaccine.
Supplementary Data
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AT conceived and designed the study. EV and MT
conducted the experiments, interpreted the data and wrote the
manuscript. MF performed the statistical analysis and interpreted
the data. KP, TKN, GP, EH, NCP, ND, EI, GNG, MK, GL, SK and AA,
drafted the manuscript, interpreted the data and critically revised
the article. KF conceived and designed, and critically revised the
study, and also provided the laboratory infrastructure and was
responsible for the critical revision of the article for important
intellectual content. DAS edited the manuscript, was involved in
the conception and design of the study, and was responsible for the
critical revision of the article for important intellectual
content. AT and AA confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Blood and information of the study participants were
obtained with written informed consent. Procedures involving
participants in the present study were approved by the Human Ethics
Committee at the University Hospital of Heraklion on July, 2021 and
by the Scientific Council of Venizeleion General Hospital of
Heraklion on March, 2021 as well as by the Scientific Council of
Ierapetra on April, 2021.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank the nursing and
medical personnel of the Venizeleion General Hospital of Heraklion
and the General Hospital of Ierapetra for their valuable work in
blood sampling.
Funding
The present study was funded by Spin-Off Toxplus S.A. and
supported by the Special Research Account of University of Crete
(ELKE nos. 4602, 4920 and 3963).
Abbreviations:
|
SARS-CoV-2
|
severe acute respiratory syndrome
coronavirus 2
|
|
COVID-19
|
coronavirus disease 2019
|
|
IgG
|
immunoglobulin G
|
|
ACE2
|
angiotensin-converting enzyme 2
|
|
RBD
|
receptor binding domain
|
|
ICU
|
intensive care unit
|
|
WHO
|
World Health Organization
|
References
|
1
|
Docea AO, Tsatsakis A, Albulescu D,
Cristea O, Zlatian O, Vinceti M, Moschos SA, Tsoukalas D, Goumenou
M, Drakoulis N, et al: A new threat from an old enemy: Re-emergence
of coronavirus (Review). Int J Mol Med. 45:1631–1643. 2020.
|
|
2
|
Neagu M, Calina D, Docea AO, Constantin C,
Filippini T, Vinceti M, Drakoulis N, Poulas K, Nikolouzakis TK,
Spandidos DA, et al: Back to basics in COVID-19: Antigens and
antibodies-Completing the puzzle. J Cell Mol Med. 25:4523–4533.
2021. View Article : Google Scholar
|
|
3
|
Latinne A, Hu B, Olival KJ, Zhu G, Zhang
L, Li H, Chmura AA, Field HE, Zambrana-Torrelio C, Epstein JH, et
al: Origin and cross-species transmission of bat coronaviruses in
China. Nat Commun. 11:42352020. View Article : Google Scholar
|
|
4
|
Mittal A, Manjunath K, Ranjan RK, Kaushik
S, Kumar S and Verma V: COVID-19 pandemic: Insights into structure,
function, and hACE2 receptor recognition by SARS-CoV-2. PLoS
Pathog. 16:e10087622020. View Article : Google Scholar
|
|
5
|
Walls AC, Park YJ, Tortorici MA, Wall A,
McGuire AT and Veesler D: Structure, Function, and Antigenicity of
the SARS-CoV-2 Spike Glycoprotein. Cell. 181:281–292.e6. 2020.
View Article : Google Scholar
|
|
6
|
Hoffmann M, Kleine-Weber H, Schroeder S,
Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH,
Nitsche A, et al: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2
and Is Blocked by a Clinically Proven Protease Inhibitor. Cell.
181:271–280.e8. 2020. View Article : Google Scholar
|
|
7
|
Min L and Sun Q: Antibodies and Vaccines
Target RBD of SARS-CoV-2. Front Mol Biosci. 8:6716332021.
View Article : Google Scholar
|
|
8
|
Calina D, Hartung T, Mardare I, Mitroi M,
Poulas K, Tsatsakis A, Rogoveanu I and Docea AO: COVID-19 pandemic
and alcohol consumption: Impacts and interconnections. Toxicol Rep.
8:529–535. 2021. View Article : Google Scholar
|
|
9
|
Pennisi M, Lanza G, Falzone L, Fisicaro F,
Ferri R and Bella R: Sars-cov-2 and the nervous system: From
clinical features to molecular mechanisms. Int J Mol Sci. 21:1–21.
2020. View Article : Google Scholar
|
|
10
|
Silva FAFD, Brito BB, Santos MLC, Marques
HS, Silva Júnior RTD, Carvalho LS, Vieira ES, Oliveira MV and Melo
FF: COVID-19 gastrointestinal manifestations: A systematic review.
Rev Soc Bras Med Trop. 53:e202007142020. View Article : Google Scholar
|
|
11
|
Falzone L, Gattuso G, Tsatsakis A,
Spandidos DA and Libra M: Current and innovative methods for the
diagnosis of COVID-19 infection (Review). Int J Mol Med. 47:472021.
View Article : Google Scholar
|
|
12
|
Thakkar S, Arora S, Kumar A, Jaswaney R,
Faisaluddin M, Ammad Ud Din M, Shariff M, Barssoum K, Patel HP,
Nirav A, et al: A Systematic Review of the Cardiovascular
Manifestations and Outcomes in the Setting of Coronavirus-19
Disease. Clin Med Insights Cardiol. 14:11795468209771962020.
View Article : Google Scholar
|
|
13
|
Tsatsakis A, Calina D, Falzone L, Petrakis
D, Mitrut R, Siokas V, Pennisi M, Lanza G, Libra M, Doukas SG, et
al: SARS-CoV-2 pathophysiology and its clinical implications: An
integrative overview of the pharmacotherapeutic management of
COVID-19. Food Chem Toxicol. 146:1117692020. View Article : Google Scholar
|
|
14
|
Sidiropoulou P, Docea AO, Nikolaou V,
Katsarou MS, Spandidos DA, Tsatsakis A, Calina D and Drakoulis N:
Unraveling the roles of vitamin D status and melanin during
COVID-19 (Review). Int J Mol Med. 47:92–100. 2021. View Article : Google Scholar
|
|
15
|
Skalny AV, Rink L, Ajsuvakova OP, Aschner
M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos
DA, Aaseth J, et al: Zinc and respiratory tract infections:
Perspectives for COVID-19 (Review). Int J Mol Med. 46:17–26.
2020.
|
|
16
|
Kostoff RN, Kanduc D, Porter AL, Shoenfeld
Y, Calina D, Briggs MB, Spandidos DA and Tsatsakis A: Vaccine-and
natural infection-induced mechanisms that could modulate vaccine
safety. Toxicol Rep. 7:1448–1458. 2020. View Article : Google Scholar
|
|
17
|
Moura DTH, McCarty TR, Ribeiro IB, Funari
MP, Oliveira PVAG, Miranda Neto AA, Monte Júnior ESD, Tustumi F,
Bernardo WM, Moura EGH, et al: Diagnostic characteristics of
serological-based COVID-19 testing: A systematic review and
meta-analysis. Clinics (São Paulo). 75:e22122020. View Article : Google Scholar
|
|
18
|
Kubina R and Dziedzic A: Molecular and
serological tests for COVID-19 A comparative review of SARS-CoV-2
coronavirus laboratory and point-of-care diagnostics. Diagnostics
(Basel). 10:4342020. View Article : Google Scholar
|
|
19
|
Childs CE, Calder PC and Miles EA: Diet
and immune function. Nutrients. 11:19332019. View Article : Google Scholar
|
|
20
|
Mikkelsen K and Apostolopoulos V: Vitamin
B12, Folic Acid, and the Immune System. Nutrition and Immunity.
Springer International Publishing; New York, NY: pp. 103–114. 2019,
View Article : Google Scholar
|
|
21
|
The Lancet Respiratory Medicine: COVID-19
transmission -up in the air. Lancet Respir Med. 8:11592020.
View Article : Google Scholar
|
|
22
|
Farsalinos K, Poulas K, Kouretas D,
Vantarakis A, Leotsinidis M, Kouvelas D, Docea AO, Kostoff R,
Gerotziafas GT, Antoniou MN, et al: Improved strategies to counter
the COVID-19 pandemic: Lockdowns vs. primary and community
healthcare. Toxicol Rep. 8:1–9. 2021. View Article : Google Scholar
|
|
23
|
Tsatsakis A, Petrakis D, Nikolouzakis TK,
Docea AO, Calina D, Vinceti M, Goumenou M, Kostoff RN, Mamoulakis
C, Aschner M, et al: COVID-19, an opportunity to reevaluate the
correlation between long-term effects of anthropogenic pollutants
on viral epidemic/pandemic events and prevalence. Food Chem
Toxicol. 141:1114182020. View Article : Google Scholar
|
|
24
|
Goumenou M, Sarigiannis D, Tsatsakis A,
Anesti O, Docea AO, Petrakis D, Tsoukalas D, Kostoff R, Rakitskii
V, Spandidos DA, et al: COVID-19 in Northern Italy: An integrative
overview of factors possibly influencing the sharp increase of the
outbreak (Review). Mol Med Rep. 22:20–32. 2020.
|
|
25
|
Pott-Junior H, Paoliello MMB, Miguel AQC,
da Cunha AF, de Melo Freire CC, Neves FF, da Silva de Avó LR,
Roscani MG, Dos Santos SS and Chachá SGF: Use of ivermectin in the
treatment of Covid-19: A pilot trial. Toxicol Rep. 8:505–510. 2021.
View Article : Google Scholar
|
|
26
|
Vivarelli S, Falzone L, Torino F,
Scandurra G, Russo G, Bordonaro R, Pappalardo F, Spandidos DA,
Raciti G and Libra M: Immune-checkpoint inhibitors from cancer to
COVID-19: A promising avenue for the treatment of patients with
COVID-19 (Review). Int J Oncol. 58:145–157. 2021. View Article : Google Scholar
|
|
27
|
Lam S, Lombardi A and Ouanounou A:
COVID-19: A review of the proposed pharmacological treatments. Eur
J Pharmacol. 886:1734512020. View Article : Google Scholar
|
|
28
|
Hashem AM, Alghamdi BS, Algaissi AA,
Alshehri FS, Bukhari A, Alfaleh MA and Memish ZA: Therapeutic use
of chloroquine and hydroxychloroquine in COVID-19 and other viral
infections: A narrative review. Travel Med Infect Dis.
35:1017352020. View Article : Google Scholar
|
|
29
|
Vivarelli S, Falzone L, Grillo CM,
Scandurra G, Torino F and Libra M: Cancer Management during
COVID-19 Pandemic: Is Immune Checkpoint Inhibitors-Based
Immunotherapy Harmful or Beneficial? Cancers (Basel). 12:1–22.
2020. View Article : Google Scholar
|
|
30
|
Calina D, Docea AO, Petrakis D, Egorov A
M, Ishmukhametov AA, Gabibov AG, Shtilman MI, Kostoff R, Carvalho
F, Vinceti M, et al: Towards effective COVID-19 vaccines: Updates,
perspectives and challenges (Review). Int J Mol Med. 46:3–16. 2020.
View Article : Google Scholar
|
|
31
|
Calina D, Sarkar C, Arsene AL, Salehi B,
Docea AO, Mondal M, Islam MT, Zali A and Sharifi-Rad J: Recent
advances, approaches and challenges in targeting pathways for
potential COVID-19 vaccines development. Immunol Res. 68:315–324.
2020. View Article : Google Scholar
|
|
32
|
Polack FP, Thomas SJ, Kitchin N, Absalon
J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED,
Zerbini C, et al C4591001 Clinical Trial Group: Safety and Efficacy
of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 383:2603–2615.
2020. View Article : Google Scholar
|
|
33
|
Centers for Disease Control and Prevention
(CDC): Understanding mRNA COVID-19 Vaccines. CDC; Atlanta, GA:
2021, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
Updated March 4, 2021.
|
|
34
|
Sadoff J, Gray G, Vandebosch A, Cárdenas
V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H,
Spiessens B, et al; ENSEMBLE Study Group. Safety and Efficacy of
Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med.
384:2187–2201. 2021. View Article : Google Scholar
|
|
35
|
Baden LR, El Sahly HM, Essink B, Kotloff
K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB,
et al; COVE Study Group. Efficacy and Safety of the mRNA-1273
SARS-CoV-2 Vaccine. N Engl J Med. 384:403–416. 2021. View Article : Google Scholar
|
|
36
|
Voysey M, Clemens SAC, Madhi SA, Weckx LY,
Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE,
et al: Oxford COVID Vaccine Trial Group: Safety and efficacy of the
ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim
analysis of four randomised controlled trials in Brazil, South
Africa, and the UK. Lancet. 397:99–111. 2021. View Article : Google Scholar
|
|
37
|
Alexandris N, Lagoumintzis G, Chasapis CT,
Leonidas DD, Papadopoulos GE, Tzartos SJ, Tsatsakis A, Eliopoulos
E, Poulas K and Farsalinos K: Nicotinic cholinergic system and
COVID-19: In silico evaluation of nicotinic acetylcholine receptor
agonists as potential therapeutic interventions. Toxicol Rep.
8:73–83. 2020. View Article : Google Scholar
|
|
38
|
Hernández AF, Calina D, Poulas K, Docea AO
and Tsatsakis AM: Safety of COVID-19 vaccines administered in the
EU: Should we be concerned? Toxicol Rep. 8:871–879. 2021.
View Article : Google Scholar
|
|
39
|
Gerbase-DeLima M, de Marco R, Monteiro F,
Tedesco-Silva H, Medina-Pestana JO and Mine KL: Impact of
Combinations of Donor and Recipient Ages and Other Factors on
Kidney Graft Outcomes. Front Immunol. 11:9542020. View Article : Google Scholar
|
|
40
|
Petrakis D, Margină D, Tsarouhas K, Tekos
F, Stan M, Nikitovic D, Kouretas D, Spandidos DA and Tsatsakis A:
Obesity -a risk factor for increased COVID-19 prevalence, severity
and lethality (Review). Mol Med Rep. 22:9–19. 2020. View Article : Google Scholar
|
|
41
|
Favresse J, Bayart J-L, Mullier F, Elsen
M, Eucher C, Van Eeckhoudt S, Roy T, Wieers G, Laurent C, Dogné JM,
et al: Antibody titres decline 3-month post-vaccination with
BNT162b2. Emerg Microbes Infect. 10:1495–1498. 2021. View Article : Google Scholar
|
|
42
|
Zurac S, Nichita L, Mateescu B, Mogodici
C, Bastian A, Popp C, Cioplea M, Socoliu C, Constantin C and Neagu
M: COVID-19 vaccination and IgG and IgA antibody dynamics in
healthcare workers. Mol Med Rep. 24:5782021. View Article : Google Scholar
|
|
43
|
Nielsen SS, Vibholm LK, Monrad I, Olesen
R, Frattari GS, Pahus MH, Højen JF, Gunst JD, Erikstrup C,
Holleufer A, et al: SARS-CoV-2 elicits robust adaptive immune
responses regardless of disease severity. EBioMedicine.
68:1034102021. View Article : Google Scholar
|