Introduction
Lung cancer, a malignant tumour originating from the
bronchial mucosa or gland, has the highest incidence rate of all
cancer types (1). Being a major
threat to human health and life, lung cancer is the main cause of
cancer-related mortality worldwide (2). It is estimated that 1.8 million
individuals are diagnosed with lung cancer, while 1.6 million die
of this disease every year worldwide (3,4).
Lung cancer includes non-small cell lung cancer (NSCLC) and
small-cell lung cancer. The former accounts for >85% of lung
squamous cell carcinomas, lung adenocarcinomas (LUAD) and large
cell lung cancer (5). Over the
past 20 years, various treatments, including chemotherapy, targeted
therapy and immunotherapy, have been effective for some patients
with advanced NSCLC (6).
However, the overall survival (OS) and cure rates of NSCLC remain
very low, with a 5-year survival rate of ~15% (7). Thus, there is a need to further
study the disease biology and malignant proliferation mechanism to
enhance the understanding of NSCLC progression and improve the
survival rate.
The human immunoglobulin superfamily containing
leucine-rich repeat (ISLR) gene (2.4-kb transcript) is
located on human chromosome 15q23-q24, which is associated with
multiple genetic disorders, as evidenced using linkage analysis
(8). In particular, the
ISLR protein is involved in various biological events, such
as embryonic development (9),
Gaucher's disease (10) and
replicative senescence of fibroblasts in some organs, including the
heart, pancreas and bone marrow (11). ISLR, also known as meflin,
is a newly discovered marker of mesenchymal stem cells, which is
involved in pathological fibrosis and the cancer microenvironment
(12,13). In particular, it has recently
been shown that the absence or low expression of ISLR can
cause straightening of stromal collagen fibres in mouse and human
pancreatic ductal adenocarcinoma tissues, respectively; such
straightening is a hallmark of aggressive tumours (14). Moreover, ISLR expression
is upregulated in the stroma of colorectal cancers and gastric
carcinomas; high ISLR expression is considered to be an
independent prognostic indicator for disease-free survival of
patients (9,15).
However, at present, a detailed understanding of the
potential functioning of the ISLR gene in NSCLC is lacking.
Thus, the present study aimed to investigate the potential
molecular mechanisms of action of the ISLR gene and examined
whether it could inhibit tumour progression and glycolysis in
NSCLC. This knowledge may provide novel prospects for guiding the
treatment of NSCLC.
Materials and methods
The Cancer Genome Atlas (TCGA) data for
LUAD
TCGA-LUAD data on ISLR expression in patients
with LUAD were retrieved from the UALCAN web tool (http://ualcan.path.uab.edu/index.html).
The UALCAN web tool was also used to plot the gene expression
figures by using 'TCGA Gene analysis' panel (16). The potential effect of
ISLR on the OS rates of patients with NSCLC was analysed
using the Kaplan-Meier method with Kaplan-Meier Plotter web tools
(https://kmplot.com/analysis/) in 'Lung
cancer' panel (17,18).
Cell culture
Human NSCLC cell lines (H1299, H23 and A549) and
normal immortalised lung epithelial cell lines (16HBE) were
obtained from the China Infrastructure of Cell Line Resources,
Institute of Basic Medical Sciences, Chinese Academy of Medical
Sciences. The 16HBE cells were maintained in DMEM/high glucose
medium (HyClone; Cytiva) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin and streptomycin.
Moreover, H1299, H23 and A549 cell lines were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) with 10% FBS and
placed in a constant-temperature incubator (Thermo Fisher
Scientific, Inc.) with 5% CO2 at 37°C.
Human recombinant IL-6 (cat. no. I1395-50UG;
Sigma-Aldrich; Merck KGaA) was dissolved in PBS to a concentration
of 100 µg/ml as a stock solution and stored at temperature
of -20°C. The cells were pre-treated with 100 ng/ml IL-6 for 48 h
at room temperature.
Cell transfection
ISLR small interfering RNA (siRNA/si)
packaged in lentivirus was purchased from Shanghai GenePharma Co.,
Ltd., and pcDNA3.1-ISLR plasmid was synthesised by
Genomeditech Biotechnology. Human NSCLC cell lines were seeded and
cultured in 24-well plates for 1 day. After reaching 80%
confluency, according to the manufacturer's instructions for
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), A549 cells (1×105 cells) were
transfected with si-negative control (NC) or si-ISLR (10
µl; 2×108 TU/ml) to construct the ISLR
knockdown cell model, and H1299 cells (1×105cells)
transfected with pcDNA3.1-ISLR plasmid (4 µg), vector
only (pcDNA3.1-NC) or blank control to construct the ISLR
overexpression cell model at 37°C for 48 h. The 24-well plates were
then placed in an incubator. After 6 h, the medium in each well was
replaced with the fresh RPMI-1640 medium supplemented with 10% FBS.
At 48 h after transfection, the cells were used for the subsequent
experiments. The following sequences (from 5′→3′) were used:
si-ISLR-1, CAGCCACAATCTCATCTCTGACTTT; si-NC-1,
CAGACACTAACTTCTGTCCACCTTT; si-ISLR-2,
TGCACAACCTCAGTGCCCTCCAATT; and si-NC-2, TGC
CAACTCTGACCGCTCACACATT.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted using an RNA kit (Takara Bio,
Inc.) and reverse transcribed into cDNA using a PrimeScript RT
reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.). Then,
cDNA was amplified using a real-time PCR kit (Takara Bio, Inc.) in
a reaction mixture containing 7 µl dH2O, 1
µl cDNA, 2 µl ISLR primers (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and 10 µl
SYBR-Green qPCR mix according to the manufacturer's instructions.
The conditions for RT were as follows: 25°C for 5 min, 55°C for 20
min and 85°C for 5 min. qPCR was performed using a 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The reaction conditions were as follows: Initial denaturation at
96°C for 3 min; followed by 40 cycles of denaturation at 96°C for
15 sec; annealing and extension, both at 58°C for 30 sec; final
extension at 72°C for 50 sec. The final relative expression of
ISLR was calculated using the 2−ΔΔCq method
(19) and normalised to that of
GAPDH. The primers were synthesised by Genomeditech Biotechnology.
The sequences (from 5′→3′) of primers of ISLR were
TGTTGCTGCAGAGAAGCAGT (forward) and CCTGCATGGTGCCTCCTTCA (reverse);
and GAPDH, TCCTCTGACTTCAACAGCGACAC (forward) and
CACCCTGTTGCTGTAGCCAAATTC (reverse).
Cell viability
Cell viability was estimated using the Cell Counting
Kit (CCK)-8 assay (Shanghai GenePharma Co., Ltd.) according to the
manufacturer's instructions. In total, ~104 NSCLC cells
were seeded in the 96-well plates with 100 µl medium in each
well and incubated at 37°C for 0, 24, 48, 72 and 96 h. Then, 10
µg CCK-8 solution was added to each well and incubated at
37°C for 4 h. Finally, the absorbance at 450 nm was measured using
a Thermomax microplate reader (Molecular Devices, LLC).
Soft agar colony formation
Cell proliferation was detected using a colony
formation assay. After 48 h of transfection, the cells (500 cells)
were seeded in 6-well plates with complete medium for 2 weeks,
which was replaced every 3 days. Colonies with >50 cells were
then fixed with 4% formaldehyde for 15 min at 37°C, stained with
crystal violet for 10-20 min at 37°C and counted under a light
microscope (Olympus Corporation; magnification, ×10). Finally, the
data obtained from five stochastic fields were used for the
statistical analysis.
Wound healing assay
Cell motility was confirmed using a wound healing
assay. After 48 h of transfection, A549 and H1299 cells were
digested with trypsin, and the cell suspension (50 µl;
4.5×105 cells/ml) was prepared and seeded into each well
of a 6-well plate using serum-free medium. When the confluency of
cells reached 80%, a horizontal line was drawn on each well with a
200 µl pipette tip, and cells were flushed twice with PBS.
Cell movement and migration in the entire wound area were observed
using an inverted optical microscope (Olympus Corporation;
magnification, ×100). Finally, to evaluate healing, the cells were
imaged using a microscope (Olympus Corporation; magnification,
×100) at 48 h after the scratch. The migration rate was quantified
as the average distance travelled by the cells to the original
wound surface.
Transwell assay
Cell invasive abilities were evaluated using
Transwell chambers with 8-µm porous membranes coated with
Matrigel (BD Biosciences) at 37°C for 48 h. According to the
manufacturer's protocols, A549 and H1299 cells (5×105
cells/ml) in RPMI-1640 medium without FBS were seeded into the
upper chamber of a Transwell insert. RPMI-1640 medium with 10% FBS
was added to the lower chamber. After 24 h, cells on the upper side
of the membrane were removed using a cotton swab, followed by
washing with PBS three times. The cells on the lower surface of the
membrane were fixed with 4% paraformaldehyde for 10 min at room
temperature and stained with 0.2% crystal violet (Biotechnology)
for 10 min at room temperature. Finally, the images were captured
using an inverted microscope (Nikon Corporation; magnification,
×100).
Flow cytometry analysis of apoptosis
The Annexin V-FITC Apoptosis Detection kit (BD
Biosciences) was used for staining, and flow cytometry was used to
detect cell apoptosis. Briefly, the harvested NSCLC cells were
flushed with PBS and resuspended in 500 µl conjugate buffer.
Then, 10 µl Annexin V-FITC and 10 µl PI (BD
Pharmingen; BD Biosciences) were added to the buffer at 37°C for 15
min. A FACScan flow cytometer (BD Biosciences) was used to analyse
cell apoptosis after 1 h (early + late apoptosis). FlowJo (version
10.6.2; FlowJo LLC) was used to analyse the data.
Western blotting
Cells were lysed using RIPA buffer (Thermo Fisher
Scientific, Inc.) containing protease and phosphatase inhibitors
for 30 min on ice. The density of all proteins extracted from the
supernatant of the cell lysate was detected using a BCA Protein
Assay kit (Takara Bio, Inc.). Next, the proteins (40 µg per
lane) were separated with 10% SDS-polyacrylamide gels, and the
specific proteins were transferred to PVDF membranes (Thermo Fisher
Scientific, Inc.). The membrane was blocked with 5% non-fat dry
milk for 1 h at 37°C and incubated with primary antibodies (all
from Cell Signaling Technology, Inc.) against vimentin (cat. no.
5741; 1:1,000), N-cadherin (cat. no. 13116; 1:1,000), E-cadherin
(cat. no. 3195; 1:1,000), glucose transporter 1 (Glut-1; cat. no.
73015; 1:1,000), hexokinase 2 (HK-2; cat. no. 2867; 1:1,000),
lactate dehydrogenase A (LDHA; cat. no. 3582; 1:1,000), Bcl-2 (cat.
no. 4223; 1:1,000), Bax (cat. no. 2774; 1:1,000), cleaved caspase-3
(cat. no. 9654; 1:1,000), Janus kinase (JAK)2 (cat. no. 3230;
1:1,000), phosphorylated (p)-JAK2 (Tyr1,007/1,008; cat. no. 3776;
1:1,000), STAT3 (cat. no. 12640; 1:1,000), p-STAT3 (Tyr705; cat.
no. 9145; 1:1,000) and GADPH (cat. no. 5174; 1:1,000) overnight at
4°C. After three washes with TBS-0.1% Tween-20, the membranes were
incubated with HRP-conjugated secondary antibody (cat. no. 7076;
1:3,000; Cell Signaling Technology, Inc.) for 45 min at room
temperature. GAPDH served as the control. The intensity of protein
expression was measured using an ECL reagent (Thermo Fisher
Scientific, Inc.). ImageJ software (version 6.0; National
Institutes of Health) was used to semi-quantify the protein
expression.
Glycolysis analysis
The glucose uptake colorimetric assay, lactate
colorimetric assay and ATP colorimetric assay kits (Biovision,
Inc.) were used to examine glucose consumption and lactate and ATP
production in A549 and H1299 cells, according to the manufacturer's
protocols.
ELISA
A human IL-6 ELISA kit (cat. no. PI330; Beyotime
Institute of Biotechnology) was used to detect the concentrations
of human IL-6 in the cell supernatant according to the
manufacturer's instructions. The cell supernatant was harvested via
centrifugation (500 × g; 4°C; 10 min). Cells were seeded in 6-well
plates and cultured for 48 h, and 200 µl culture supernatant
was used for detection.
Tissue samples and
immunohistochemistry
Non-tumorous lung tissue and primary lung cancer
tissues (2 cm away from the tumour margin) were collected from 38
patients (21 men and 17 women; age range, 27-81 years; median age,
54 years) who underwent surgical resection at The Shandong Second
Provincial General Hospital between June 2020 and March 2021. The
inclusion criteria were as follows: i) None of the patients
received neoadjuvant therapy; ii) study patients were diagnosed
with lung cancer via histopathology; and iii) diagnosis was
confirmed by two independent pathologists. The exclusion criteria
were as follows: i) Lung cancer cases with unconfirmed pathology;
ii) lung cancer cases with incomplete data records; and iii)
patients receiving chemotherapy and radiotherapy prior to the
surgery. The use of patient samples was approved by the Ethics
Committee of The Shandong Second Provincial General Hospital
(approval no. XYK20200511).
Tissue samples were fixed in 4% formaldehyde (Thermo
Fisher Scientific, Inc.) at 25°C for 24 h, dehydrated and made
transparent using gradient alcohol, following which they were
paraffin embedded. The sections (thickness, 5 µm) separated
from lung tissue were soaked in citrate buffer solution (pH 6.0)
and heated in a 850 W power microwave oven for 10 min to conduct
antigen repair. Tissue sections were incubated in 3%
H2O2 for 15 min at room temperature and
blocked in 10% normal goat serum (Sigma-Aldrich; Merck KGaA) for 30
min at room temperature. Then, the tissues were incubated with
primary antibody against ISLR (cat. no. ab232986; Abcam)
overnight at 4°C. Next the sections were incubated with goat
anti-rabbit IgG H&L secondary antibody (1:1,000; Abcam; cat.
no. ab150077) at room temperature for 30 min. The sections were
stained with haematoxylin at room temperature for 30 sec
(Sigma-Aldrich; Merck KGaA), dried in an oven at 65°C, rinsed in
water, then mixed with alcohol (Sigma-Aldrich; Merck KGaA) and
xylene (Sigma-Aldrich; Merck KGaA) and naturally dried. Finally,
the sections were observed under a stereomicroscope (magnification,
×200; Thermo Fisher Scientific, Inc.).
Bioinformatics analysis
Gene set enrichment analysis (GSEA) (20) was performed on TCGA database
datasets (LUAD; https://cancergenome.nih.gov/) of ISLR
expression using the R package 'clusterProfiler' (21). For GSEA, ISLR expression
was treated as a numeric variable. The Pearson correlation
coefficients of other genes and ISLR expression were
calculated, and the genes were sequenced according to the
correlation coefficients. The hallmark gene sets were deposited in
the GSEA Molecular Signatures Database resource (h.all.
v7.1.symbols.gmt; https://www.gsea-msigdb.org/gsea/index.jsp), the
differential pathways between the high- and low-ISLR
expression specimens were identified. The number of permutations
was 1,000. Normalised enrichment score >1 and false discovery
rate q-value <0.05 were established as cut-offs for significant
enrichment.
Statistical analysis
All the data were analysed using SPSS software
(version 18.0; SPSS, Inc.). Survival curves were analysed using
Kaplan-Meier survival curves and log-rank tests. All the
experiments were conducted in triplicate, and all data are
expressed as the mean ± SD. An unpaired Student's t-test was used
to compare differences between two groups, while one-way ANOVA and
Tukey's post hoc test were used to compare differences between
multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
ISLR expression is elevated both in NSCLC
tissues and cell lines
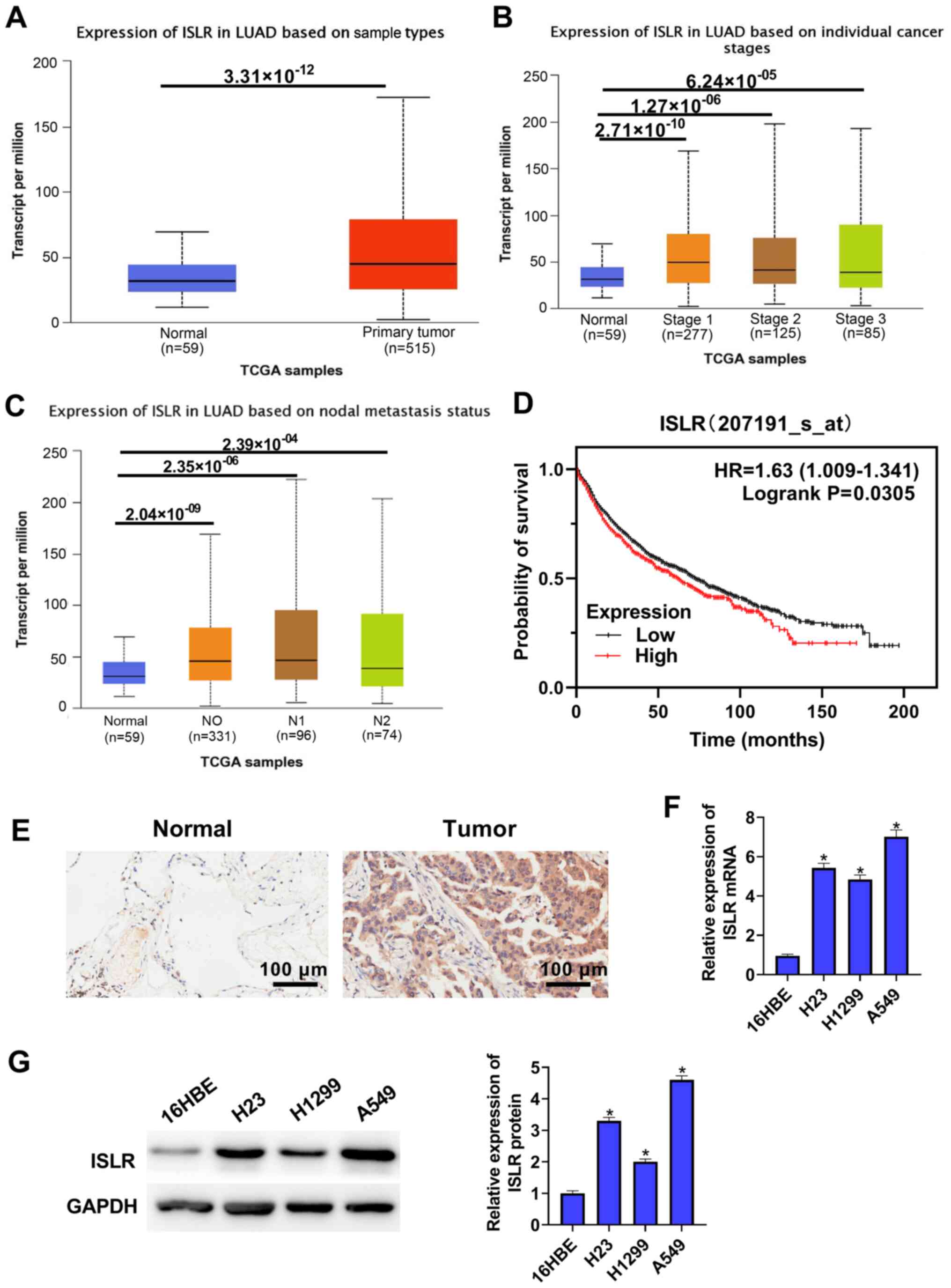
To identify ISLR expression in NSCLC,
TCGA-LUAD cohort datasets were analysed and it was found that
ISLR expression was upregulated in the NSCLC tumour tissues
compared with that in the normal tissues (Fig. 1A). TCGA cohort data-sets showed
that ISLR expression in both NSCLC (at different stages) and
nodal metastasis cancers were significantly higher compared with
those in normal tissues (Fig. 1B and
C). Kaplan-Meier analysis demonstrated that NSCLC cases with
high expression of ISLR had poorer OS compared with patients
with low ISLR expression (Fig. 1D). The immunohistochemical
results identified that ISLR expression in lung cancer
tissues was higher than that in non-tumorous lung tissues (Fig. 1E).
ISLR expression was also detected in several
NSCLC cell lines and normal immortalised lung epithelial cell lines
via RT-qPCR and western blotting. As shown in Fig. 1F and G, compared with 16HBE
cells, ISLR expression was significantly upregulated in all
evaluated NSCLC cell lines, with that in A549 cells being the most
pronounced, followed by H23 and H1299 cells exhibiting the least
upregulation. These results indicated that the abnormal expression
of ISLR may be associated with NSCLC progression. The
selection of cell types was determined according to the expression
of ISLR. A549 cells expressed relatively high levels of
ISLR, whereas H1299 cells expressed relatively low levels of
ISLR; therefore, H1299 and A549 cells were used in
subsequent experiments with pcDNA3.1-ISLR vectors and siRNA,
respectively.
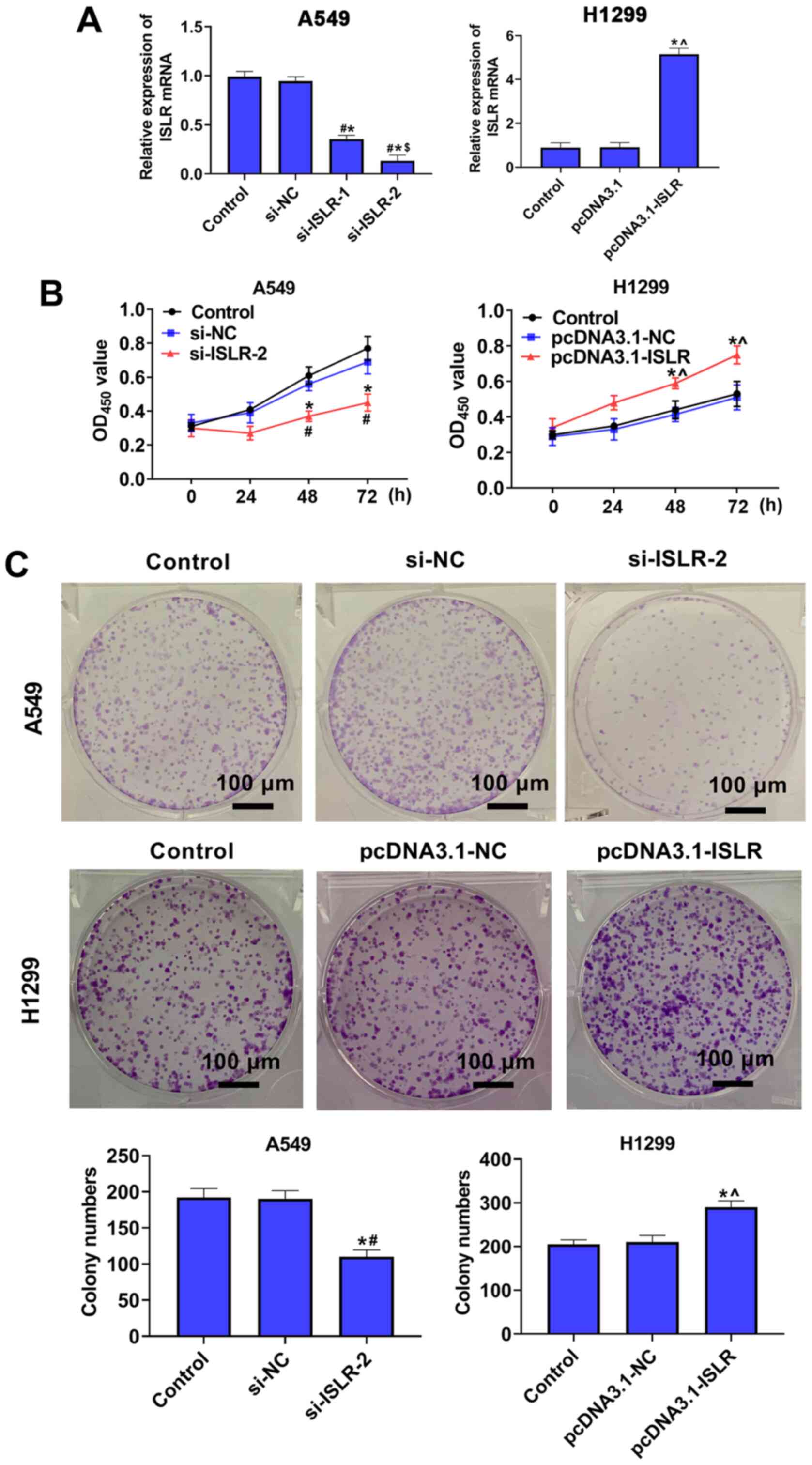
ISLR promotes the proliferation of NSCLC
cells
To further investigate the role of ISLR in
the progression of NSCLC, A549 and H1299 cells were selected for
the follow-up experiments. Subsequently, ISLR was knocked
down in A549 cells and overexpressed in H1299 cells, and the
expression levels were verified using RT-qPCR (Fig. 2A). Cell viability and
proliferation were detected using the CCK-8 and colony formation
assays, respectively. The results demonstrated that ISLR
knockdown suppressed proliferation and colony number in A549 cells,
whereas ISLR overexpression promoted the proliferation of
H1299 cells (Fig. 2B and C),
suggesting that si-ISLR inhibited the proliferation of NSCLC
cells.
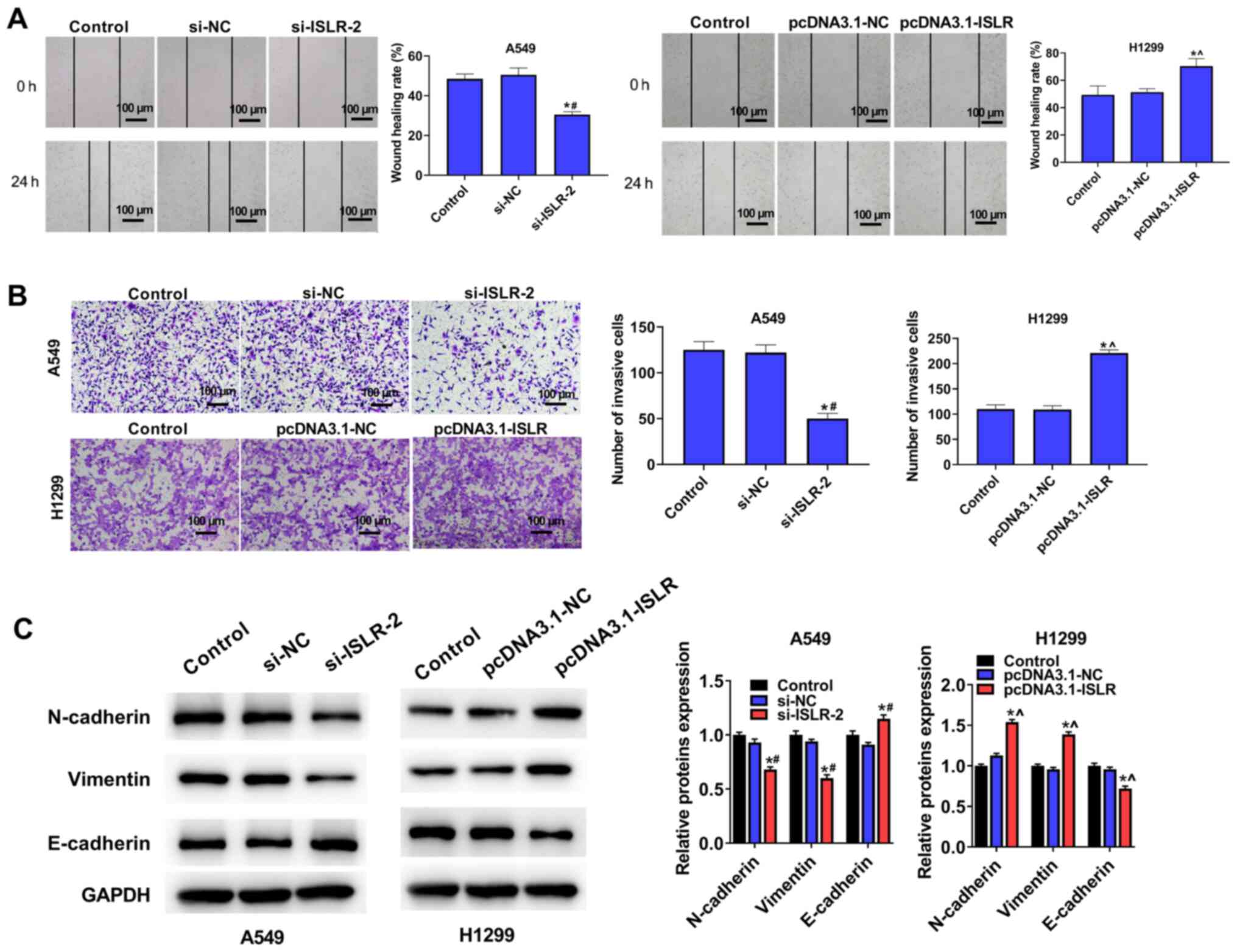
ISLR promotes the migration and invasion
of NSCLC cells
Wound healing and Transwell assays were performed to
investigate the effect of ISLR on cell migration and
invasiveness. As shown in Fig.
3A, ISLR knockdown significantly decreased A549 cell
adhesion ability and the number of migrated cells; however,
ISLR overexpression produced contrary effects on H1299
cells. Moreover, the invasion assays indicated that the ISLR
knockdown significantly inhibited the invasion of A549 cells,
whereas the opposite effect was observed when ISLR was
overexpressed in H1299 cells (Fig.
3B).
The expression levels of proteins involved in
epithelial-mesenchymal transition (EMT) were evaluated using
western blotting. Compared with the respective control cells,
ISLR silencing increased the expression level of the
epithelial marker E-cadherin and reduced the expression levels of
mesenchymal markers, such as N-cadherin and vimentin, in A549 cells
(Fig. 3C). Conversely,
ISLR overexpression produced the opposite effect on the
EMT-related proteins in H1299 cells. These results indicated that
ISLR silencing suppressed the EMT, migration and invasion of
NSCLC cells.
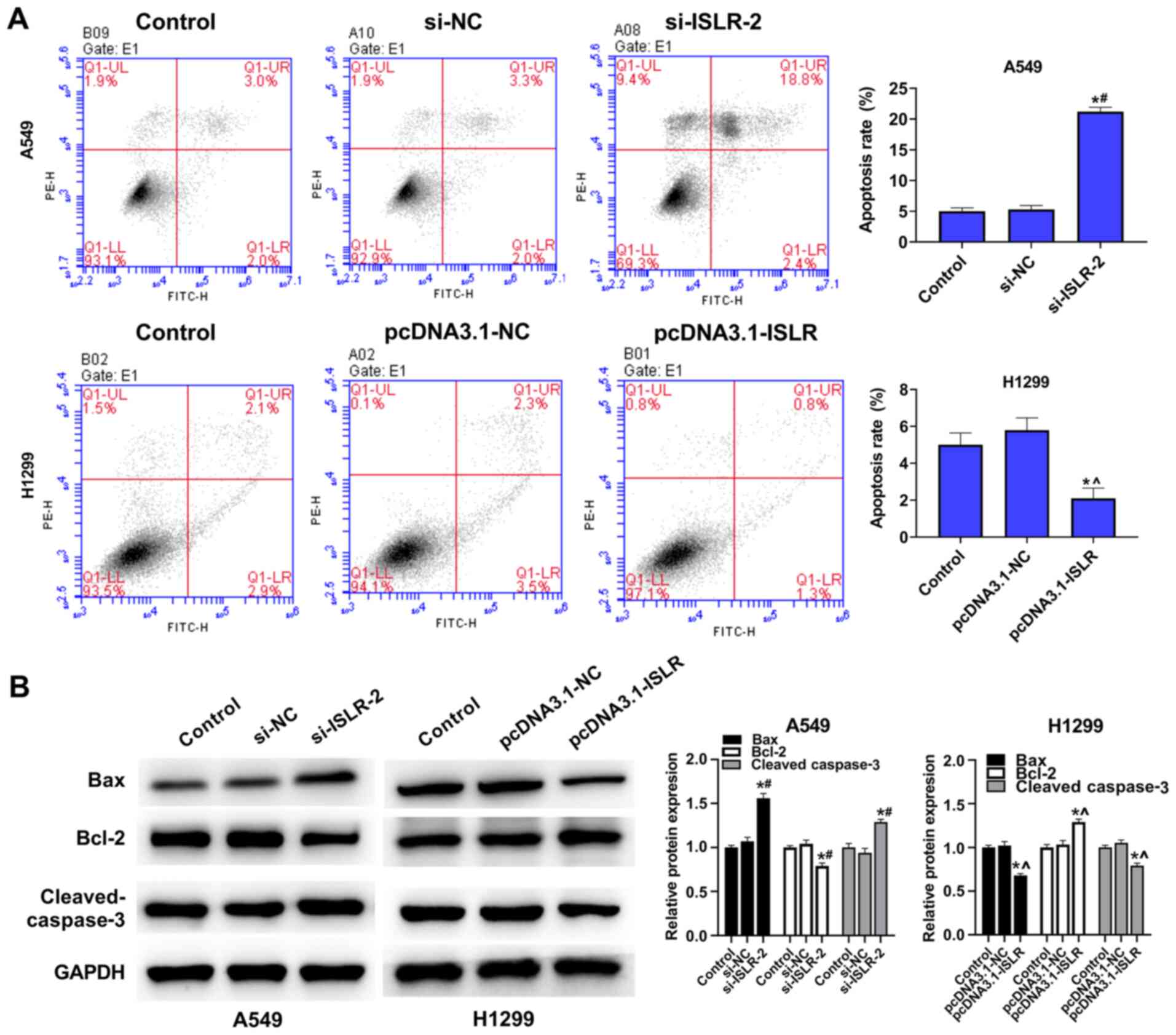
ISLR suppresses the apoptosis of NSCLC
cells
The present results demonstrated that ISLR
silencing increased the apoptosis of A549 cells, while
overexpression of ISLR suppressed the apoptosis of H1299
cells, compared with the corresponding control group (Fig. 4A). To further determine the
mechanism involving ISLR in the apoptosis of NSCLC cells,
western blotting was performed to detect the expression levels of
related genes. Interestingly, ISLR silencing decreased the
expression levels of Bcl-2, but increased the expression levels of
Bax and cleaved caspase-3 in A549 cells. By contrast, ISLR
overexpression exhibited the opposite effect on the expression
levels of Bcl-2, Bax and cleaved caspase-3 in H1299 cells (Fig. 4B). Collectively, these results
demonstrated that ISLR silencing may promote the apoptosis
of NSCLC cells.
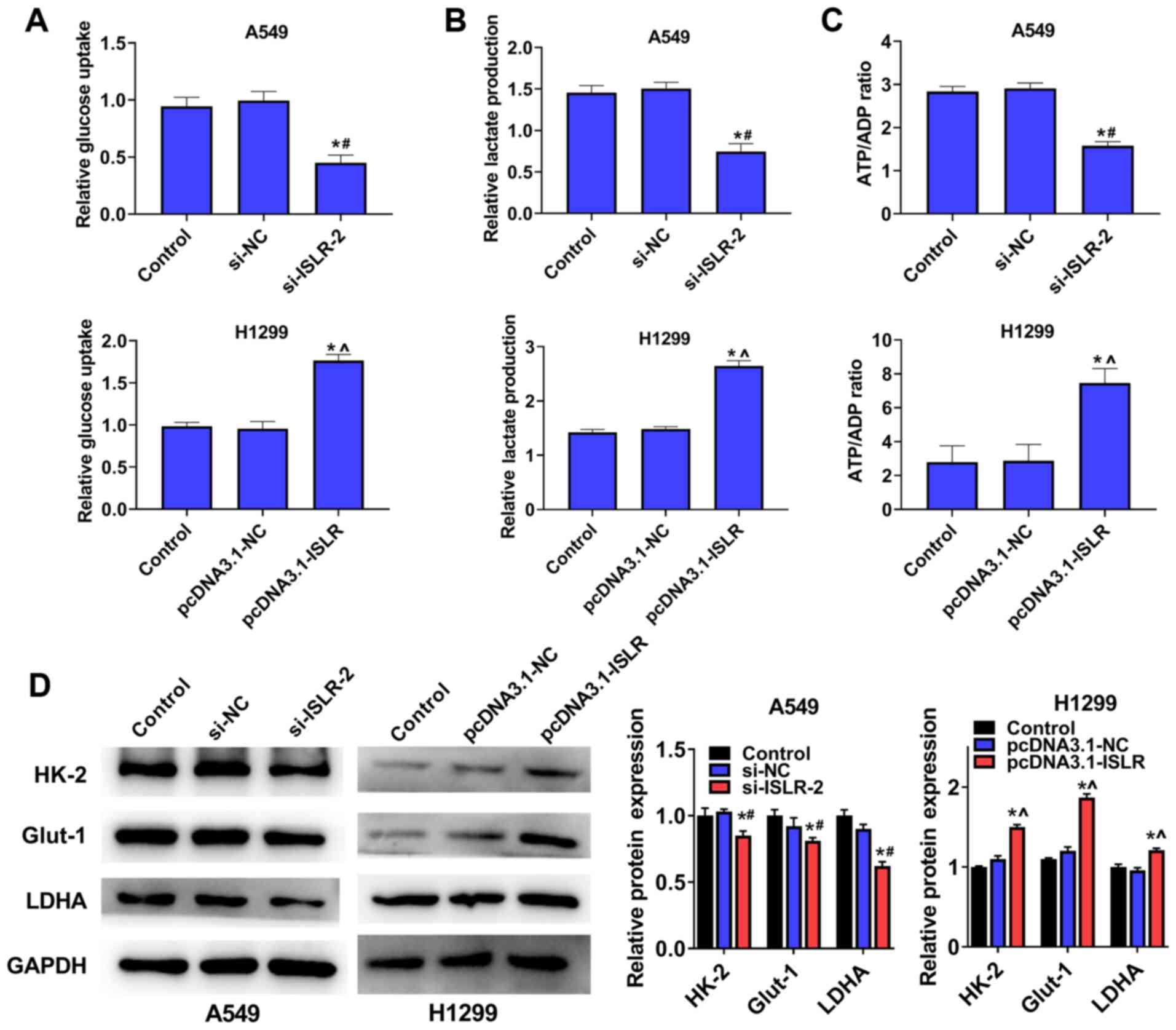
ISLR promotes aerobic glycolysis of NSCLC
cells
To examine the potential role of ISLR in
NSCLC glycolysis, A549 and H1299 cells were transfected with
si-ISLR or pcDNA3.1-ISLR; glucose consumption,
lactate production and ATP production were subsequently analysed.
The results demonstrated that the silencing of ISLR
expression in A549 cells inhibited cellular glucose uptake
(Fig. 5A), lactate consumption
(Fig. 5B) and ATP/ADP ratios
(Fig. 5C), while ISLR
overexpression produced the opposite effects on glycolysis
progression. The western blotting results indicated that the
expression levels of glycolytic target proteins (including HK-2,
Glut-1 and LDHA) were downregulated in A549 cells with ISLR
knockdown, whereas they were upregulated in H1299 cells with
ISLR overexpression (Fig.
5D). Overall, these results indicated that the silencing of
ISLR could inhibit aerobic glycolysis in NSCLC cells.
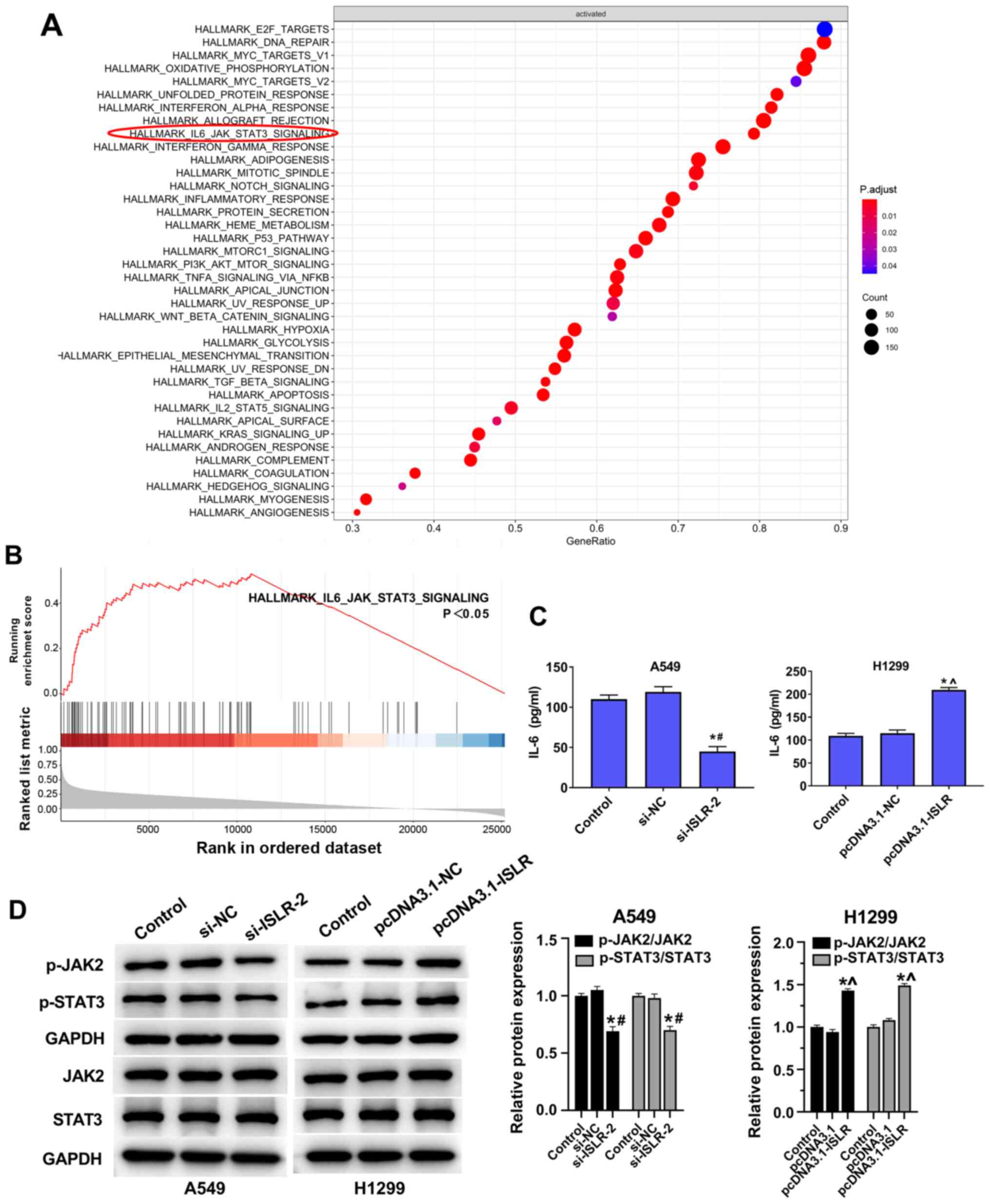
ISLR promotes tumour progression and
glycolysis in NSCLC cells by inactivating the IL-6/JAK/STAT3
pathway
The GSEA results suggested that the IL-6/JAK/STAT3
pathway was enriched in ISLR-related NSCLC (Fig. 6A and B). ELISA results
demonstrated that IL-6 levels in the culture supernatants of NSCLC
cell lines were reduced by knockdown of ISLR (Fig. 6C). The knockdown of ISLR
decreased the phosphorylation levels of JAK2 and STAT3, while the
overexpression of ISLR increased these phosphorylation
levels (Fig. 6D).
Treatment with IL-6 counteracts the ISLR
knockdown-induced facilitation of tumour progression and glycolysis
in NSCLC cells
To further determine the molecular mechanism of
action of ISLR in the IL-6/JAK/STAT3 pathway, IL-6 (pathway
activator) was used to treat si-ISLR-transfected cells.
Notably, the knockdown of ISLR reversed the promotion
effects on cell proliferation (Fig.
7A), invasion (Fig. 7B),
apoptosis (Fig. 7C) and
glycolysis (Fig. 7D) caused by
IL-6. Collectively, it was suggested that the IL-6/JAK/STAT3
signalling pathway may be involved in the effect of ISLR on
NSCLC cell progression (Fig.
7E).
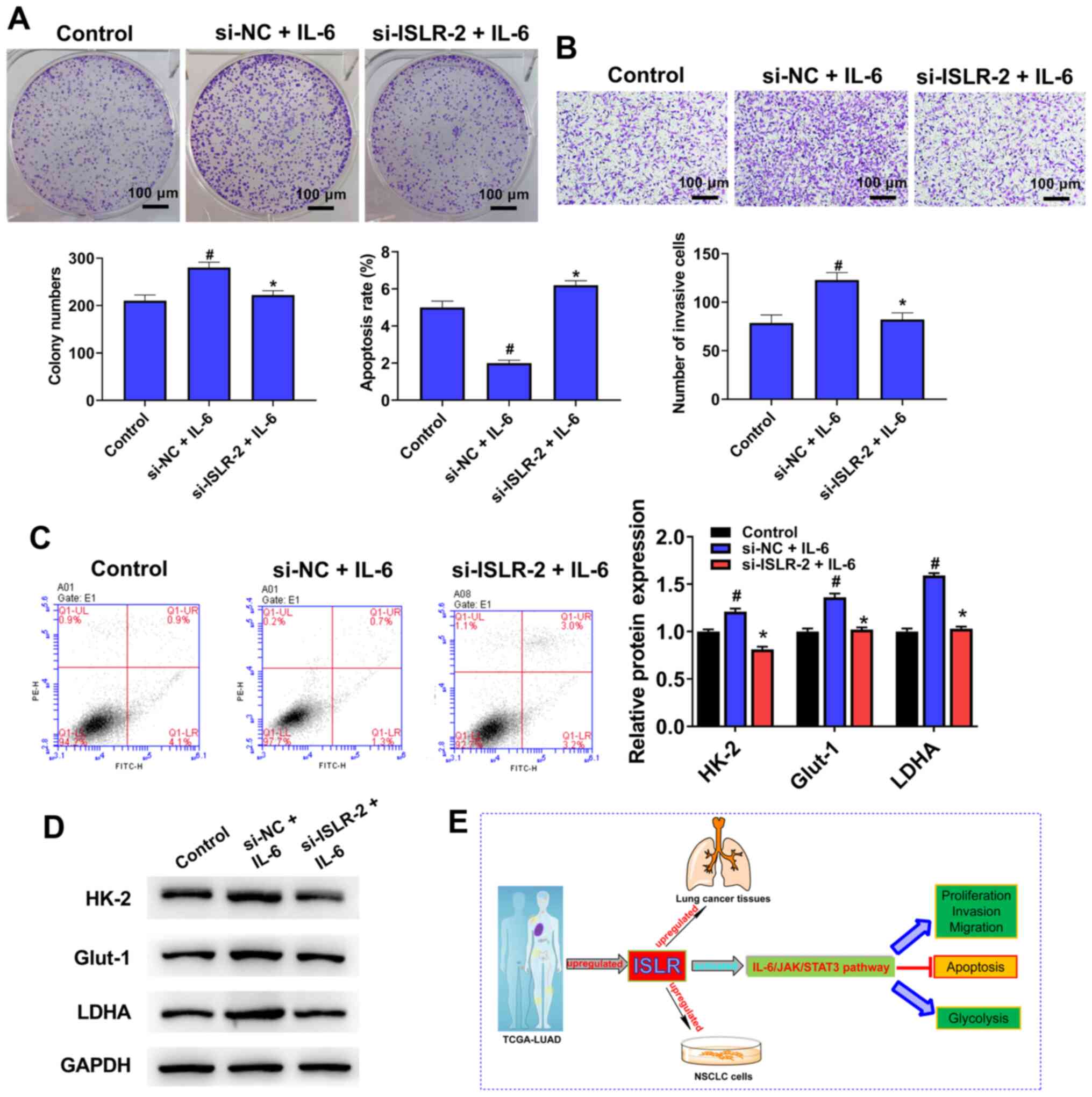 | Figure 7Treatment with IL-6 counteracts the
ISLR knockdown-induced facilitating effects on the tumour
progression and glycolysis of NSCLC cells. After pre-treatment with
IL-6 (100 ng/ml) for 48 h, (A) proliferation, (B) invasion, (C)
apoptosis and (D) glycolysis were determined colony formation,
Transwell, flow cytometry and western blotting assays. (E) A
summary figure for this study: ISLR promotes tumour progression and
glycolysis of NSCLC cells by inactivating the IL-6/JAK/STAT3
pathway. *P<0.05 vs. si-NC + IL-6 group;
#P<0.05 vs. control group. ISLR, immunoglobulin
superfamily containing leucine-rich repeat; NC, negative control;
si, small interfering RNA; NSCLC, non-small cell lung cancer; HK-2,
hexokinase 2; Glut-1, glucose transporter 1; LDHA, lactate
dehydrogenase A; JAK, Janus kinase. |
Discussion
There is currently significant interest in
furthering the understanding of the genetic changes that drive
NSCLC (22). New strategies for
molecular diagnosis and targeted therapy of NSCLC are being
explored, given the potential operability of numerous
molecular-defined subtypes. A large number of molecular markers,
such as microRNAs, oncogenic non-coding RNAs and long non-coding
RNAs, have been recently proven to regulate various cellular
processes, including glycolysis metabolism, proliferation,
invasion, apoptosis and differentiation, resulting in the
progression and tumorigenesis of NSCLC (23-25). The present study provided
evidence on cell proliferation, invasion, apoptosis and aerobic
glycolysis to support the effect of ISLR expression on the
behaviour of NSCLC, which could be used as a biomarker to guide the
diagnosis and treatment of NSCLC.
ISLR is a marker of mesenchymal stromal
cells, and as a secretory protein, it maintains the
undifferentiated features of stromal cells (26). Exogenous ISLR expression
in the tumour matrix inhibits the expression of α-smooth muscle
actin in cancer-associated fibroblasts, as well as tumour
progression. For instance, in human pancreatic ductal
adenocarcinoma, ISLR inhibits α-smooth muscle actin
expression (myofibroblast differentiation) and extracellular matrix
remodelling in cancer-associated fibroblasts, which is crucial for
cancer progression (14). In
recent years, researchers have also confirmed that ISLR is
essential for stromal cells to regulate epithelial cell
proliferation during intestinal regeneration and tumour
development, especially in colorectal cancer (26,27).
However, to the best of our knowledge, the mechanism
of action of ISLR in NSCLC had not been previously
investigated. To alleviate this gap, the current study
characterised the expression pattern and clinical significance of
ISLR in NSCLC tissues by utilising publicly available
TCGA-LUAD cohort datasets. The results demonstrated that
ISLR expression was upregulated in NSCLC with different
stages and nodal metastasis, and was associated with poor OS. In
further cellular experiments, the silencing of ISLR
significantly decreased proliferation, limited migration and
invasion, and increased apoptosis in NSCLC cell lines, thus
indicating weakened NSCLC cell malignancy. Moreover, the
overexpression of ISLR in NSCLC cells had the reverse effect
on these cellular processes.
EMT is a key step in intercellular adhesion,
invasion and metastasis (28). A
hallmark of EMT is the reduced expression of the epithelial marker
E-cadherin, and elevated expression of mesenchymal markers, such as
vimentin and N-cadherin, along with increasing cell migration and
invasion (29). Caspase-3 is
known as the executioner of apoptosis due to its role in
coordinating the destruction of cell structure (30). Bcl-2 belongs to the Bcl-2 family
and is one of the important factors regulating apoptosis (31). Therefore, the expression levels
of proteins involved in EMT and apoptosis were evaluated using
western blotting. The silencing of ISLR increased the
expression of E-cadherin and cleaved caspase-3, and reduced the
expression of N-cadherin, vimentin and Bcl-2 in NSCLC cells.
However, ISLR overexpression had the opposite effect on
these proteins in NSCLC cells. Thus, to the best of our knowledge,
the present study demonstrated for the first time that ISLR
was a vital contributor to NSCLC progression.
Glucose metabolism supplies energy to the cellular
processes of normal cells, as well as those of cancer cells.
Abnormal acceleration of glucose metabolism can be used to
differentiate cancer cells from normal tissue cells (32). The majority of cancer cells
employ aerobic glycolysis as an approach to energy production,
whether they are under normoxic or hypoxic conditions (33). The inhibition of glucose
metabolism is the main subject of clinical treatment for
heterogeneous cancer types. In the current study, ISLR
silencing inhibited cellular glucose uptake, lactate consumption
and ATP/ADP ratios in NSCLC cells, while ISLR overexpression
had the opposite effect on glycolysis progression. Glut-1 belongs
to one of the dominant members of the glucose transporter family,
and it has been shown to be upregulated and to facilitate glucose
metabolism in cancer cells (34). Due to its reversible binding to
mitochondria, HK-2 is a key regulator of glycolysis in several
cancer types, and it can also prevent cancer cells from undergoing
apoptosis (35). LDHA, as a
redox cofactor, is activated during anaerobic glycolysis in various
organisms and promotes glycolysis and cell proliferation (36). The present study identified that
the protein expression levels of HK-2, Glut-1 and LDHA were
downregulated in A549 cells with silenced ISLR, but were
upregulated in H1299 cells with overexpressed ISLR.
Collectively, these results indicated that the silencing of
ISLR inhibited aerobic glycolysis in NSCLC cells.
IL-6 can also induce cellular oxidative stress,
leading to cancer progression (37). IL-6, a proinflammatory cytokine
in the tumour microenvironment, has been regarded as the main
factor involved in EMT and contributes to tumour growth and
metastasis (38). IL-6 leads to
activation of the JAK/STAT signalling pathway, particularly STAT3,
to promote cancer cell proliferation, invasion, apoptosis,
angiogenesis, differentiation and stemness (39). Inhibition of JAK2/STAT3
significantly suppresses IL-6-induced EMT, cell migration and
invasion in HCC and pancreatic cancer (40,41). Furthermore, monoamine oxidase A
promotes tumour cell proliferation via IL-6/STAT3 signalling
(42). Lee et al
(43) reported that monoamine
oxidase A was downregulated as a result of IL-6/IL-6R/STAT3
signalling, and this may be attributed to Epstein-Barr virus
infection in nasopharyngeal carcinoma cells. The present study
found that knockdown of ISLR inactivated the JAK/STAT
signalling pathway, as well as reversed the NSCLC cell
proliferation and glycolysis induced by IL-6, suggesting that
ISLR may be involved in NSCLC progression and prognosis.
Limitations of the present study included the sole
use of in vitro cell experiments. Therefore, in vivo
experiments are required to assess the role of ISLR in NSCLC
progression, as well as the underlying mechanism.
In conclusion, ISLR expression was
significantly upregulated in NSCLC tissue and cells with different
staging and nodal metastasis, and was associated with poor OS. The
silencing of ISLR promoted apoptosis and inhibited the
proliferation, migration, invasion and glycolysis of NSCLC cells by
inactivating the IL-6/JAK/STAT3 pathway. These factors could be
used as molecular targets in crucial applications such as NSCLC
diagnosis and treatment.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and GY designed the study. PZ, ZL and GY
performed the experiments. GY analyzed the data. PZ and GY
confirmed the authenticity of all the raw data. PZ wrote the paper,
while ZL revised the paper. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The protocol of this research has been approved by
the Ethics Committee of Shandong Second Provincial General Hospital
(approval no. XYK20200511). All patients have signed written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Abbreviations:
|
CCK-8
|
Cell Counting Kit-8
|
|
EMT
|
epithelial-mesenchymal transition
|
|
Glut-1
|
glucose transporter 1
|
|
GSEA
|
gene set enrichment analysis
|
|
HK-2
|
hexokinase 2
|
|
ISLR
|
immunoglobulin superfamily containing
leucine-rich repeat
|
|
LDHA
|
lactate dehydrogenase A
|
|
LUAD
|
lung adenocarcinoma
|
|
NC
|
negative control
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
siRNA
|
small interfering RNA
|
|
TGCA
|
The Cancer Genome Atlas
|
References
|
1
|
Bade BC and Dela Cruz CS: Lung Cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
5
|
Coudray N, Ocampo PS, Sakellaropoulos T,
Narula N, Snuderl M, Fenyö D, Moreira AL, Razavian N and Tsirigos
A: Classification and mutation prediction from non-small cell lung
cancer histopathology images using deep learning. Nat Med.
24:1559–1567. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akhtar-Danesh N, Akhtar-Danseh GG, Seow
HY, Shakeel S and Finley C: Trends in survival based on treatment
modality in non-small cell lung cancer patients: A population-based
study. Cancer Invest. 37:355–366. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lind L, Sandström H, Wahlin A, Eriksson M,
Nilsson-Sojka B, Sikström C and Holmgren G: Localization of the
gene for congenital dyserythropoietic anemia type III, CDAN3, to
chromosome 15q21-q25. Hum Mol Genet. 4:109–112. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Homma S, Shimada T, Hikake T and Yaginuma
H: Expression pattern of LRR and Ig domain-containing protein
(LRRIG protein) in the early mouse embryo. Gene Expr Patterns.
9:1–26. 2009. View Article : Google Scholar
|
|
10
|
Ługowska A, Hetmańczyk-Sawicka K,
Iwanicka-Nowicka R, Fogtman A, Cieśla J, Purzycka-Olewiecka JK,
Sitarska D, Płoski R, Filocamo M, Lualdi S, et al: Gene expression
profile in patients with Gaucher disease indicates activation of
inflammatory processes. Sci Rep. 9:60602019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon IK, Kim HK, Kim YK, Song IH, Kim W,
Kim S, Baek SH, Kim JH and Kim JR: Exploration of replicative
senescence-associated genes in human dermal fibroblasts by cDNA
microarray technology. Exp Gerontol. 39:1369–1378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagasawa A, Kudoh J, Noda S, Mashima Y,
Wright A, Oguchi Y and Shimizu N: Human and mouse ISLR
(immunoglobulin superfamily containing leucine-rich repeat) genes:
Genomic structure and tissue expression. Genomics. 61:37–43. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagasawa A, Kubota R, Imamura Y, Nagamine
K, Wang Y, Asakawa S, Kudoh J, Minoshima S, Mashima Y, Oguchi Y, et
al: Cloning of the cDNA for a new member of the immunoglobulin
superfamily (ISLR) containing leucine-rich repeat (LRR). Genomics.
44:273–279. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizutani Y, Kobayashi H, Iida T, Asai N,
Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, et al:
Meflin-positive cancer-associated fibroblasts inhibit pancreatic
carcinogenesis. Cancer Res. 79:5367–5381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Zhao W and Sun M: An analysis
regarding the association between the ISLR gene and gastric
carcinogenesis. Front Genet. 11:6202020. View Article : Google Scholar :
|
|
16
|
Chandrashekar DS, Bashel B, Balasubramanya
SA, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Győrffy B: Survival analysis across the
entire transcriptome identifies biomarkers with the highest
prognostic power in breast cancer. Comput Struct Biotechnol J.
19:4101–4109. 2021. View Article : Google Scholar
|
|
18
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
20
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jonna S and Subramaniam DS: Molecular
diagnostics and targeted therapies in non-small cell lung cancer
(NSCLC): An update. Discov Med. 27:167–170. 2019.PubMed/NCBI
|
|
23
|
Liao H, Liang Y, Kang L, Xiao Y, Yu T and
Wan R: miR4543p inhibits nonsmall cell lung cancer cell
proliferation and metastasis by targeting TGFB2. Oncol Rep.
45:672021. View Article : Google Scholar
|
|
24
|
Li C, Zhang L, Meng G, Wang Q, Lv X, Zhang
J and Li J: Circular RNAs: Pivotal molecular regulators and novel
diagnostic and prognostic biomarkers in non-small cell lung cancer.
J Cancer Res Clin Oncol. 145:2875–2889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu H and Li SB: Role of LINC00152 in
non-small cell lung cancer. J Zhejiang Univ Sci B. 21:179–191.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Tang Y, Sheng X, Tian Y, Deng M, Du
S, Lv C, Li G, Pan Y, Song Y, et al: Secreted stromal protein ISLR
promotes intestinal regeneration by suppressing epithelial Hippo
signaling. EMBO J. 39:e1032552020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi H, Gieniec KA, Wright JA, Wang
T, Asai N, Mizutani Y, Lida T, Ando R, Suzuki N, Lannagan TR, et
al: The balance of stromal BMP signaling mediated by GREM1 and ISLR
drives colorectal carcinogenesis. Gastroenterology. 160:1224–1239.
2021. View Article : Google Scholar
|
|
28
|
Milano A, Mazzetta F, Valente S, Ranieri
D, Leone L, Botticelli A, Onesti CE, Lauro S, Raffa S, Torrisi MR,
et al: Molecular detection of EMT markers in circulating tumor
cells from metastatic non-small cell lung cancer patients:
Potential role in clinical practice. Anal Cell Pathol (Amst).
2018:35068742018.
|
|
29
|
Xu G, Meng L, Yuan D, Li K, Zhang Y, Dang
C and Zhu K: MEG3/miR-21 axis affects cell mobility by suppressing
epithelial mesenchymal transition in gastric cancer. Oncol Rep.
40:39–48. 2018.PubMed/NCBI
|
|
30
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jarskog LF, Selinger ES, Lieberman JA and
Gilmore JH: Apoptotic proteins in the temporal cortex in
schizophrenia: High Bax/Bcl-2 ratio without caspase-3 activation.
Am J Psychiatry. 161:109–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun LY, Li XJ, Sun YM, Huang W, Fang K,
Han C, Chen ZH, Luo XQ, Chen YQ and Wang WT: LncRNA ANRIL regulates
AML development through modulating the glucose metabolism pathway
of AdipoR1/AMPK/SIRT1. Mol Cancer. 17:1272018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lunt SY and Vander Heiden MG: Aerobic
glycolysis: Meeting the metabolic requirements of cell
proliferation. Annu Rev Cell Dev Biol. 27:441–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zambrano A, Molt M, Uribe E and Salas M:
Glut 1 in cancer cells and the inhibitory action of resveratrol as
a potential therapeutic strategy. Int J Mol Sci. 20:33742019.
View Article : Google Scholar :
|
|
35
|
Kang F, Ma W, Ma X, Shao Y, Yang W, Chen
X, Li L and Wang J: Propranolol inhibits glucose metabolism and
18F-FDG uptake of breast cancer through posttranscriptional
downregulation of hexokinase-2. J Nucl Med. 55:439–445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao X, Huang X, Ye F, Chen B, Song C, Wen
J, Zhang Z, Zheng G, Tang H and Xie X: The miR-34a-LDHA axis
regulates glucose metabolism and tumor growth in breast cancer. Sci
Rep. 6:217352016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Yan W, Collins MA, Bednar F,
Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD and di Magliano
MP: Interleukin-6 is required for pancreatic cancer progression by
promoting MAPK signaling activation and oxidative stress
resistance. Cancer Res. 73:6359–6374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Browning L, Patel MR, Horvath EB, Tawara K
and Jorcyk CL: IL-6 and ovarian cancer: Inflammatory cytokines in
promotion of metastasis. Cancer Manag Res. 10:6685–6693. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Y, Li W, Liu R, Guo Q, Li J, Bao Y,
Zheng H, Jiang S and Hua B: Norcantharidin inhibits IL-6-induced
epithelial mesenchymal transition via the JAK2/STAT3/TWIST
signaling pathway in hepatocellular carcinoma cells. Oncol Rep.
38:1224–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen J, Wang S, Su J, Chu G, You H, Chen
Z, Sun H, Chen B and Zhou M: Interleukin-32α inactivates JAK2/STAT3
signaling and reverses interleukin-6-induced epithelial-mesenchymal
transition, invasion, and metastasis in pancreatic cancer cells.
OncoTargets Ther. 9:4225–4237. 2016. View Article : Google Scholar
|
|
42
|
Li J, Pu T, Yin L, Li Q, Liao CP and Wu
BJ: MAOA-mediated reprogramming of stromal fibroblasts promotes
prostate tumorigenesis and cancer stemness. Oncogene. 39:3305–3321.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee HM, Sia AP, Li L, Sathasivam HP, Chan
MS, Rajadurai P, Tsang CM, Tsao SW, Murray PG, Tao Q, et al:
Monoamine oxidase A is down-regulated in EBV-associated
nasopharyngeal carcinoma. Sci Rep. 10:61152020. View Article : Google Scholar : PubMed/NCBI
|