Introduction
Diabetic nephropathy (DN) is a common and
debilitating complication of diabetes (1). According to the United States renal
data system, DN is the leading cause of end-stage renal disease and
a major contributor to morbidity and mortality in patients with
diabetes worldwide (2).
Glomerular mesangial cell (MC) proliferation and extracellular
matrix deposition are prominent features of DN (3).
Under physiological conditions, MCs are located
between glomerular capillary loops and secrete extracellular
matrix, produce cytokines, support the glomerular capillary plexus,
phagocytize and clear macromolecular substances and contract
(4). In the presence of
hyperglycemia and other stimulating factors, MCs proliferate
abnormally and secrete a large amount of extracellular matrix,
which is deposited in the mesangial area (5). MCs can migrate to the gap between
glomerular capillary endothelial cells and the basement membrane
and even protrude into the surrounding capillary cavity, resulting
in capillary occlusion and ultimately leading to glomerulosclerosis
(6). MC proliferation serves a
key role in the process of DN, but the mechanism of MC
proliferation in diabetic patients is complex. It is generally
believed that the kidneys of diabetic patients produce too much
reactive oxygen species (ROS) due to stimulation of the
mitochondrial respiratory chain by long-term hyperglycemia
(7). Low levels of ROS serve
roles in signal transduction and proliferation, which may be one of
the important mechanisms of the abnormal proliferation of MCs. In
addition, transforming growth factor β, connective tissue growth
factor, angiotensin II and other cellular growth factors are also
involved in the abnormal proliferation of MCs (8,9).
Studies have also shown that a decrease in the level of hydrogen
sulfide (H2S) serves an important role in the
proliferation of MCs (10).
H2S has been traditionally viewed as a
toxic gas. However, few individuals realize that H2S is
also a biological gas that is endogenously produced by the
cytosolic enzymes cystathionine-β-synthase (CBS) and
cystathionine-γ-lyase (CSE), which use homocysteine and L-cysteine
as substrates (11).
H2S can not only regulate inflammation, oxygen sensing,
cell growth and ageing but also protect a variety of organs from
ischemia-reperfusion injury, serving an important biological role
in vivo (12). There is
abundant endogenous H2S in kidney tissues (13). H2S can increase the
glomerular filtration rate, inhibit the reabsorption of sodium by
the renal tubules and inhibit the proliferation of glomerular MCs
(10,14).
Dopamine receptors (DRs) have seven transmembrane
domains and belong to the G protein coupled receptor superfamily
(15). According to their
different effects on adenylate cyclase, DRs are divided into two
subfamilies: DR1 (D1, D5) and DR2 (D2, D3, D4) (16). DR1 binds to Gs protein to
activate adenylate cyclase and stimulate phospholipase C, resulting
in an increase in the intracellular free calcium concentration
[(Ca2+)i]; DR2 inhibits adenylate cyclase and
calcium channels (17). DRs are
mainly distributed in the central nervous system and in the
cardiovascular system, kidneys and adrenal glands. DR1 is also
expressed and serves an important role in glomerular MCs (18).
It has been reported that H2S can inhibit
the proliferation of MCs (10).
Yang et al (19) found
that CSE is physiologically activated by calcium-calmodulin, which
increases the production of endogenous H2S in vascular
endothelial cells. Our research group reported that DR1 activation
increases the (Ca2+)i in myocardial
ischemia-reperfusion injury (20). However, it is not clear whether
DR1 activation inhibits MC proliferation by increasing endogenous
H2S. To examine this possibility, the present study
performed animal and cellular experiments and measured changes in
related factors and signaling pathways.
Materials and methods
Materials and drugs
Mouse renal MC lines (SV40-MES13) were purchased
from Shanghai Cell Bank of Chinese Academy of Science. SKF38393
(DR1 agonist), sodium hydrogen sulfide (NaHS), PPG (a CSE
inhibitor), PD98059 (an ERK1/2 inhibitor), streptozocin (STZ) and
7-Azido-4-Methylcoumarin (H2S probe) were purchased from
Sigma-Aldrich (Merck KGaA). The primary antibodies for anti-CSE,
cyclin D1, PCNA, P21, COL1, α-smooth muscle actin (α-SMA), and MMP9
were from ProteinTech Group, Inc. DR1 antibody was purchased from
GeneTex, Inc. The total (t)-ERK1/2 and phosphorylated (p)-ERK1/2
antibodies were obtained from Cell Signaling Technology, Inc. The
Cell Counting Kit-8 (CCK-8) kit and the primary antibody for
β-actin were obtained from Wuhan Boster Biological Technology, Ltd.
The EdU kit was obtained from Guangzhou RiboBio Co., Ltd. The BCA
Protein Assay kit and Enhanced Chemiluminescence (ECL) reagent were
purchased from Beyotime Institute of Biotechnology.
Animal use and experimental design
At total of 48 C57BL/6J (male:female 50:50;
6-weeks-old; 20-22 g) mice were provided by the Experimental Animal
Center of Second Affiliated Hospital in Harbin Medical University.
All experiments were approved by the Animal Care Committee of
Harbin Medical University for the use of experimental animals
(approval no. SCXK2013-001). All animals were given free access to
normal chow and water and were housed in cages at room temperature
with 40-70% humidity and a fixed 12-h light/dark cycle.
After animal adaptation to the environment for 1
week, male and female C57BL/6J (6-weeks-old, 20-22g) mice were made
diabetic by a single injection of streptozotocin (STZ; 150 mg/kg,
intraperitoneally). The STZ was dissolved in 100 µM citrate
buffer (citric acid: sodium citrate=1:1.32). After 3 days, the mice
with glucose levels >16.67 mmol/l were considered hyperglycemic
(diabetic) (21). The daily
water intake and food intake of these diabetic mice were
simultaneously recorded and the weekly weight change monitored. At
the same time, blood glucose was monitored weekly to eliminate mice
whose blood glucose had returned to <16.67 mmol/l.
Briefly, the experimental groups were as follows
(n=8): i) Control group (Control); the mice were injected with the
same volume of physiological saline solution daily. ii) Diabetes
group (T1D); the diabetic mice were injected with the same volume
of physiological saline solution daily. iii) Diabetes + SKF38393
group (T1D + SKF38393); the diabetic mice were injected with
SKF38393 (50 µg/kg) daily. iv) Diabetes + NaHS group (T1D +
NaHS); the diabetic mice were injected with NaHS (100 µM/kg)
daily. According to the results of pre-experiment and published
papers, a dose of 100 µM/kg/day NaHS was used in present
study (11,22). All drugs were dissolved in
physiological saline solution and all the above mice were injected
intraperitoneally. After the treatment reached 4, 8 and 12 weeks,
the mice were anesthetized by intraperitoneal injection of 1%
pentobarbital sodium (60 mg/kg) and then the chest was opened and
the kidneys, hearts, livers and arteries of mice were removed for
related experimental research.
Renal morphology assessment
Kidneys were dehydrated in a series of alcohols and
then embedded. Tissue sections from paraffin-embedded kidneys with
a 4 µm thickness were stained with hematoxylin and eosin and
Masson's trichrome at room temperature for 3 min following
deparaffinization. Staining was conducted to assess renal
morphology and collagen accumulation to estimate the progression of
diabetic nephropathy. Images of random fields under a microscope
(Olympus Corporation) were captured by an individual blinded to the
grouping.
Transmission electron microscopy
Renal tissues (1 mm3) were immersed
immediately in fixative (2.5% glutaraldehyde buffered in 0.1 M
sodium cacodylate, pH 7.2). Following 2-3 days of storage at 4°C,
specimens were rinsed in PBS, postfixed in cacodylate-buffered 1%
osmium tetroxide, dehydrated in an ethanol series, and embedded in
polybed 812. Ultra-thin (90 nm) sections were made with microtome,
and ultrastructural changes of glomerular basement membrane were
observed under an electron microscope. The thickness of glomerular
basement membrane was quantified using ImageJ software (National
Institutes of Health; version 1.48).
Measurement of H2S levels in
the kidney
H2S production rate was measured as
described previously (23). In
brief, following different treatments, the kidney tissues were
collected and homogenized in 50 mM ice-cold potassium phosphate
buffer (pH 6.8). The flasks containing the reaction mixture (100 mM
potassium phosphate buffer, 10 mM l-cysteine, 2 mM pyridoxal
5-phosphate and 10% cell homogenates) and center wells containing
0.5 ml 1% zinc acetate and a piece of filter paper (2×2.5 cm) were
flushed with N2 gas and incubated at 37°C for 90 min.
The reaction was stopped by adding 0.5 ml of 50% trichloroacetic
acid and the flasks were incubated at 37°C for another 60 min. The
contents of the center wells were transferred to test tubes, each
containing 3.5 ml of water. Then 0.5 ml of 20 mM N,
N-dimethyl-p-phenylenediamine sulfate in 7.2 M HCl
and 0.5 ml 30 mM FeCl3 in 1.2 M HCl were added. The
absorbance of the resulting solution at 670 nm was measured 20 min
later with an ultraviolet spectrophotometer.
Cell culture and treatment
The SV40-MES13 were cultured in DMEM/F-12 medium
(3:1) (cat. no. PM150323; Procell Life Science & Technology
Co., Ltd., and cat. no. 31600; Beijing Solarbio Science &
Technology Co., Ltd.) supplemented with 14 mM HEPES, 5% fetal
bovine serum, 1% penicillin and streptomycin in a 5% CO2
humidified atmosphere at 37°C. For the following experiments, the
SV40-MES13 were incubated with serum-free DMEM/F-12 for 12-16 h
(the cells reached 70-80% confluence) and then the cells were
randomly divided into six groups: i) normal-glucose control group
(Control); incubated in DMEM/F-12 medium containing 5.6 mM glucose.
ii) high glucose group (HG); incubated in DMEM/F-12 medium
containing 30 mM glucose for 48 h. iii) SKF38393 treated group (HG
+ SKF38393); 10 µM SKF38393 was added to the medium 1 h
before HG induction. iv) NaHS treated group (HG + NaHS): 100
µM NaHS was added to the medium 1 h before HG condition. v)
PPG and SKF38393 treatment group (HG + PPG + SKF38393); 2 mM PPG
and 10 µM SKF38393 were added to the medium 1 h before HG
condition. vi) PD98059 treatment group (HG + PD98059): 10 µM
PD98059 (an ERK1/2 inhibitor) was added to the medium 1 h before HG
condition.
Cell viability assay
Cell viability was measured by Cell Counting Kit-8.
Cells were seeded in 96-well plates at a concentration of
1×103 cells/well. Prior to different treatments, the
cells were serum starved for 12 h. Subsequently, they were
incubated in 10 µl of CCK-8 reagent at 37°C for 1 h. A
microplate reader was used to determine the optical density at 450
nm.
Measurement of cell proliferation
5-Ethynyl-20-Deoxyuridine (EdU) incorporation assay
was performed with the Cell-Light EdU In Vitro Imaging kit
to detect the proliferation rates of SV40-MES13 (21). Briefly, cells subjected to
different treatment were incubated with 20 mM EdU in complete
growth medium at 37°C for 2 h before fixation in 4%
paraformaldehyde at room temperature for 30 min. After EdU
staining, cell nuclei were stained with Hoechst 33342 at room
temperature for 30 min in a darkroom and visualized under
fluorescence microscopy (magnification, ×200). The percentage of
EdU-positive cells was calculated from six random fields in three
wells.
Detection of H2S by
7-Azido-4-Methylcoumarin
The fluorescence response of H2S in MCs
was detected using 7-Azido-4-Methylcoumarin (C-7Az; Sigma-Aldrich;
Merck KGaA), as described previously (24,25). MCs were incubated with 1 ml PBS
(containing 12 µl C-7Az) for 30 min at room temperature and
then the cells were washed with PBS. The fluorescence activation
response of C-7Az to H2S in MCs were visualized using a
microscope (Olympus Corporation) and six fields (magnification,
×200) were randomly selected for statistical analysis.
Western blotting analysis
Western blotting assay was conducted as described
previously (12). In brief,
cells were lysed and extracted total protein with RIPA lysis buffer
pre-mixed with 1 mM PMSF. The protein concentration in each sample
was determined using the BCA Protein Assay kit. In each western
blot analysis, the same amount of total protein (25-50 µg)
from each group was separated by 8-12% SDS-PAGE and
electro-transferred to PVDF membranes. The membranes were blocked
for 1.5 h at room temperature in TBST (Tris-buffered saline with
0.05% Tween-20, pH 7.4) plus 5% non-fat milk and incubated
overnight at 4°C with diluted primary antibodies against DR1 (cat.
no. GTX100354, 1:1,000; Gene Tex, Inc.), CSE (cat. no. 12217-1-AP,
1:1,000; ProteinTech Group, Inc.), cyclin D1 (cat. no. 60186-1-Ig,
1:5,000; ProteinTech Group, Inc.), PCNA (cat. no. 10205-2-AP,
1:5,000; ProteinTech Group, Inc.), P21 (cat. no. 27296-1-AP, 1:500;
ProteinTech Group, Inc.), α-SMA (cat. no. 14395-1-AP, 1:1,000;
ProteinTech Group, Inc.), COL1 (cat. no. 14695-1-AP, 1:1,000;
ProteinTech Group, Inc.), MMP9 (cat. no. 10375-2-AP, 1:1,1000;
ProteinTech Group, Inc.), total (t)-ERK1/2 (cat. no. 4695, 1:5,000;
Cell Signaling Technology, Inc.), phosphorylated (p)-ERK1/2 (cat.
no. 4370, 1:5,000; Cell Signaling Technology, Inc.), or β-actin
(cat. no. BM0627, 1:1,000; Boster Biological Technology, Ltd.).
Secondary goat anti-rabbit antibody and goat anti-mouse antibody
were used respectively (diluted 1:5,000, cat. no. BA1054 and cat.
no. BA1050; Boster Biological Technology, Ltd.). The secondary
antibodies were incubated for 1.5 h at room temperature (25°C). The
signals were detected by an ECL kit and a multiplex fluorescent
imaging system (ProteinSimple). The integrated optical density of
the analyzed bands on the film was quantified using ImageJ software
(National Institutes of Health; version 1.48). The levels of
analyzed proteins were normalized to the internal control
(β-actin).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and represent at least three independent experiments.
Statistical comparisons were made using one-way ANOVA followed by a
post hoc analysis (Tukey test) where applicable. P<0.05 was
considered to indicate a statistically significant difference.
Results
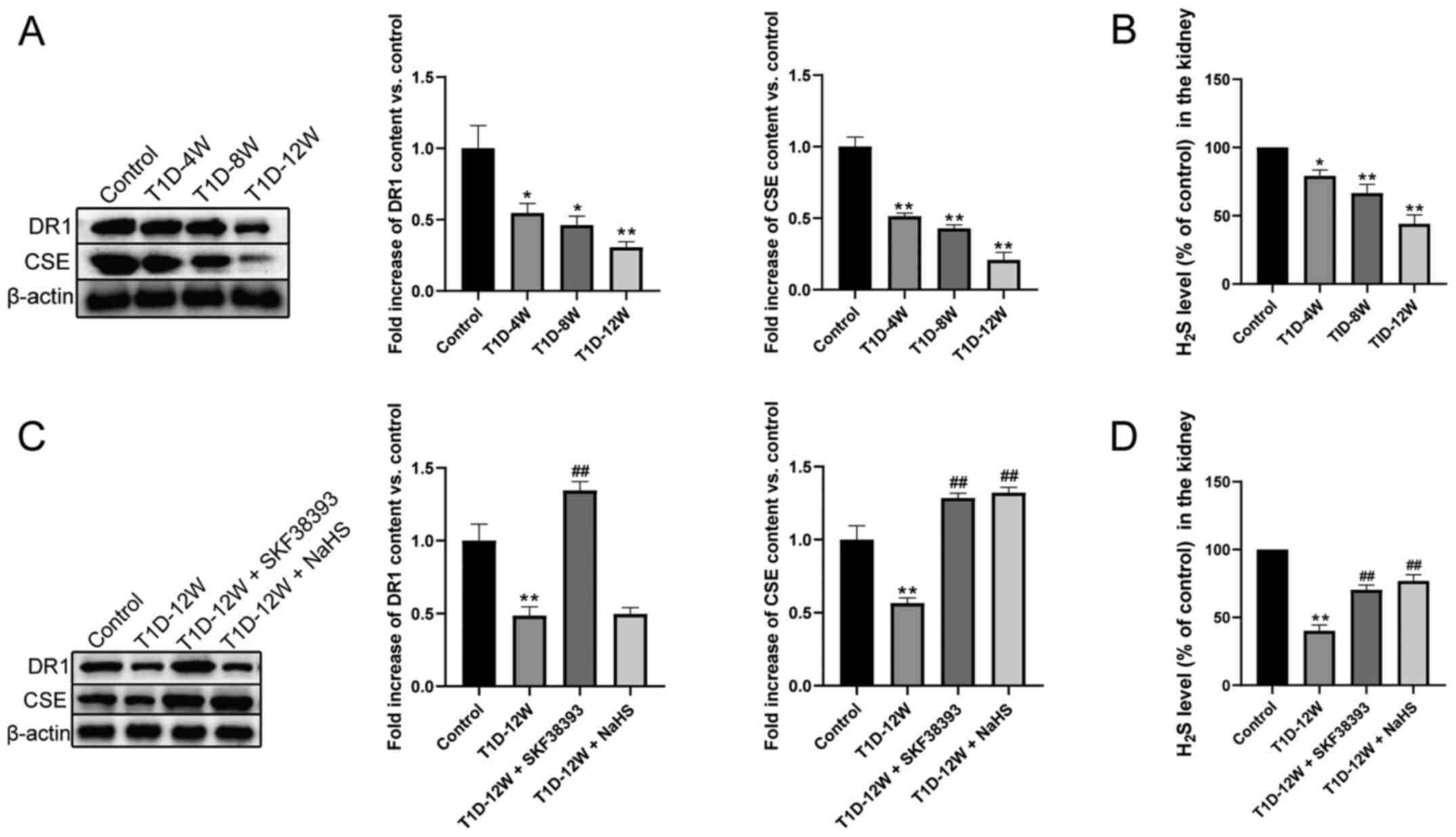
DR1 activation upregulates the
CSE/H2S pathway in the kidneys of T1D mice
As shown in Fig.
1, hyperglycemia downregulated DR1 and CSE expression and
H2S production in the T1D-4 W, T1D-8 W and T1D-12 W
groups compared with the control group. Compared with that in the
control group, this effect was most evident in the T1D-12 W group.
Compared with the T1D-12 W group, SKF38393 (DR1 agonist) treatment
significantly increased the expression of DR1 and CSE and the
production of H2S. NaHS (an exogenous H2S
donor) treatment only increased the expression of CSE and the
production of H2S but had no significant effect on the
expression of DR1.
DR1 activation inhibits cell
proliferation and collagen deposition by upregulating the
CSE/H2S pathway in the kidneys of T1D mice
The data showed that compared with those in the
control group, renal injury was aggravated, including glomerular
hypertrophy, collagen deposition in interstitial kidney tissue and
the thickness of the glomerular basement membrane were increased in
the T1D-4 W, T1D-8 W and T1D-12 W groups. These changes were most
significant in the T1D-12 W group. Pretreatment with SKF38393 or
NaHS significantly reduced these pathological changes in the T1D-12
W group (Fig. 2).
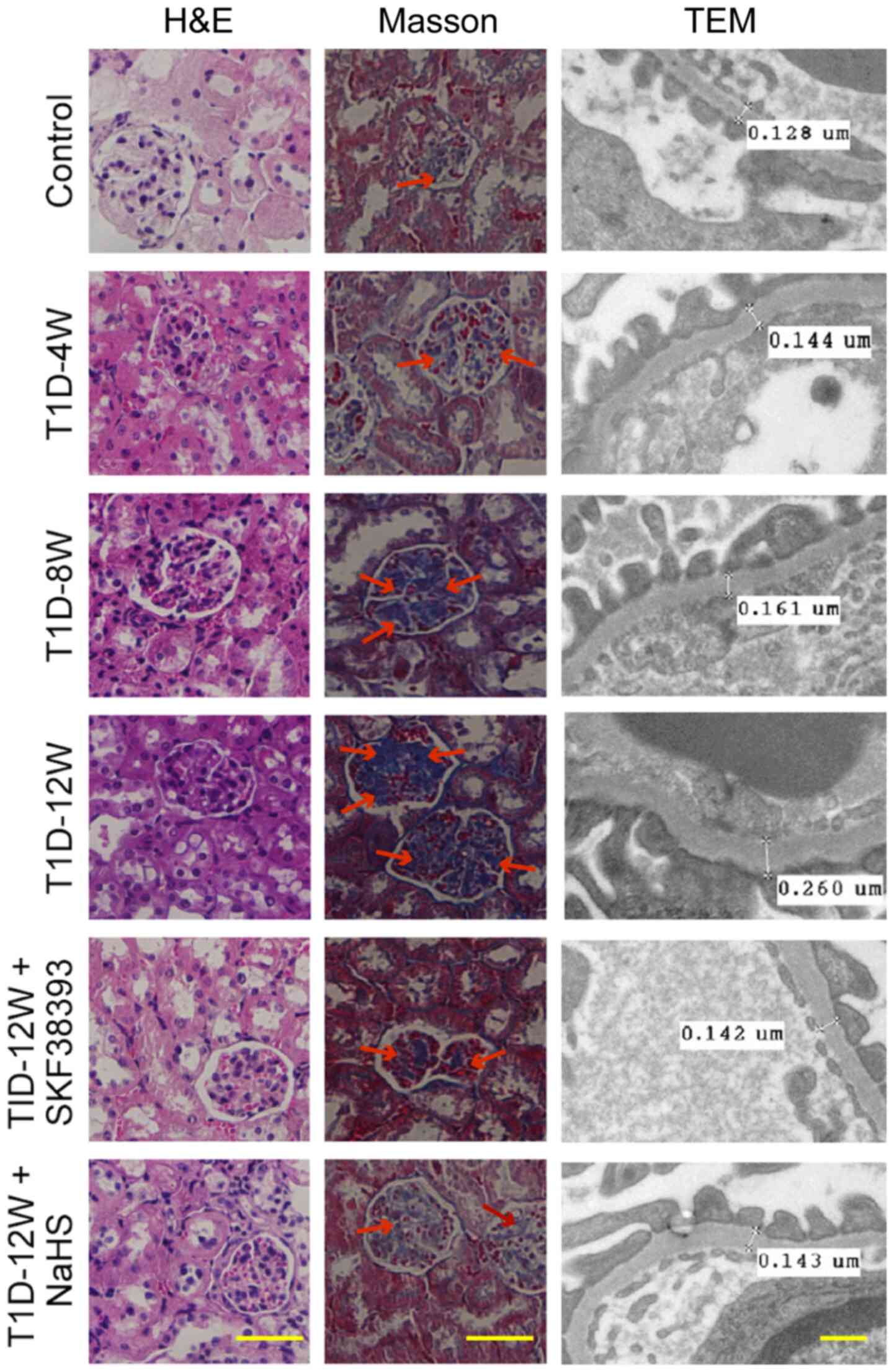 | Figure 2DR1 activation inhibits cell
proliferation and collagen deposition by upregulates the
CSE/H2S pathway in the kidneys of T1D mice. Morphology
of kidney by hematoxylin and eosin staining (magnification, ×400;
scale bars, 100 µm), collagen deposition by Masson staining
(magnification, ×400; scale bars, 100 µm, the blue stain by
the arrow indicated is collagens) and thickness of renal basement
membrane by transmission electron microscope (magnification,
×20,000; scale bars, 2 µm). Renal injury was aggravated,
including glomerular hypertrophy, collagen deposition in
interstitial kidney tissue and the thickness of the glomerular
basement membrane were increased in the T1D-4 W, T1D-8 W and T1D-12
W groups. These changes were most significant in the T1D-12 W
group. Pretreatment with SKF38393 or NaHS significantly reduced
these pathological changes in the T1D-12 W group. All data were
from at least 4 independent experiments. DR1, dopamine 1 receptors;
CSE, cystathionine-γ-lyase; T1D, diabetes group; W, week. |
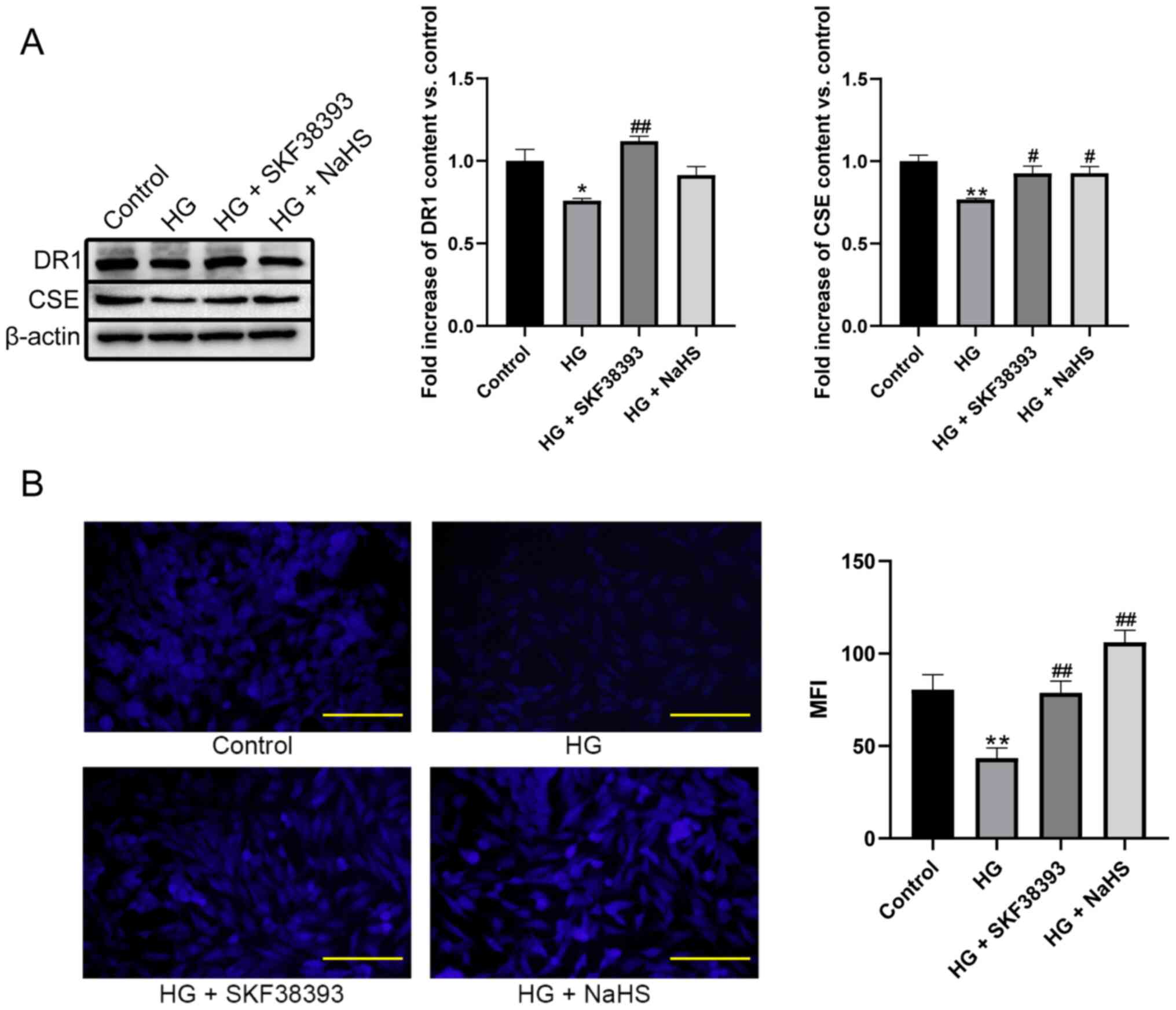
DR1 activation increases the
CSE/H2S pathway in HG-induced MCs
As shown in Fig.
3, the levels of DR1, CSE and H2S were lower in the
HG group compared with the control group. Compared with those in
the HG group, the levels of DR1, CSE and H2S were
significantly increased in cells treated with HG + SKF38393.
Treatment with HG + NaHS only increased the levels of CSE and
H2S but had no significant effect on DR1 levels. These
results were consistent with the results of the animal
experiments.
Activation of the DR1-CSE/H2S
pathway attenuates HG-induced MC proliferation
Cell viability, the proliferation rate and the
expression of cyclin D1 and PCNA were increased and the expression
of P21 was decreased in the HG group compared with the control
group. Compared with the HG group, HG + SKF38393 and HG + NaHS
reduced cell viability, the proliferation rate and the expression
of cyclin D1 and PCNA and increased the expression of P21. PPG (an
inhibitor of CSE) abrogated the inhibitory effect of SKF38393 on MC
proliferation. In addition, the effect of HG + SKF38393 on these
indicators was similar to that of HG + PD98059 (an ERK1/2
inhibitor; Fig. 4).
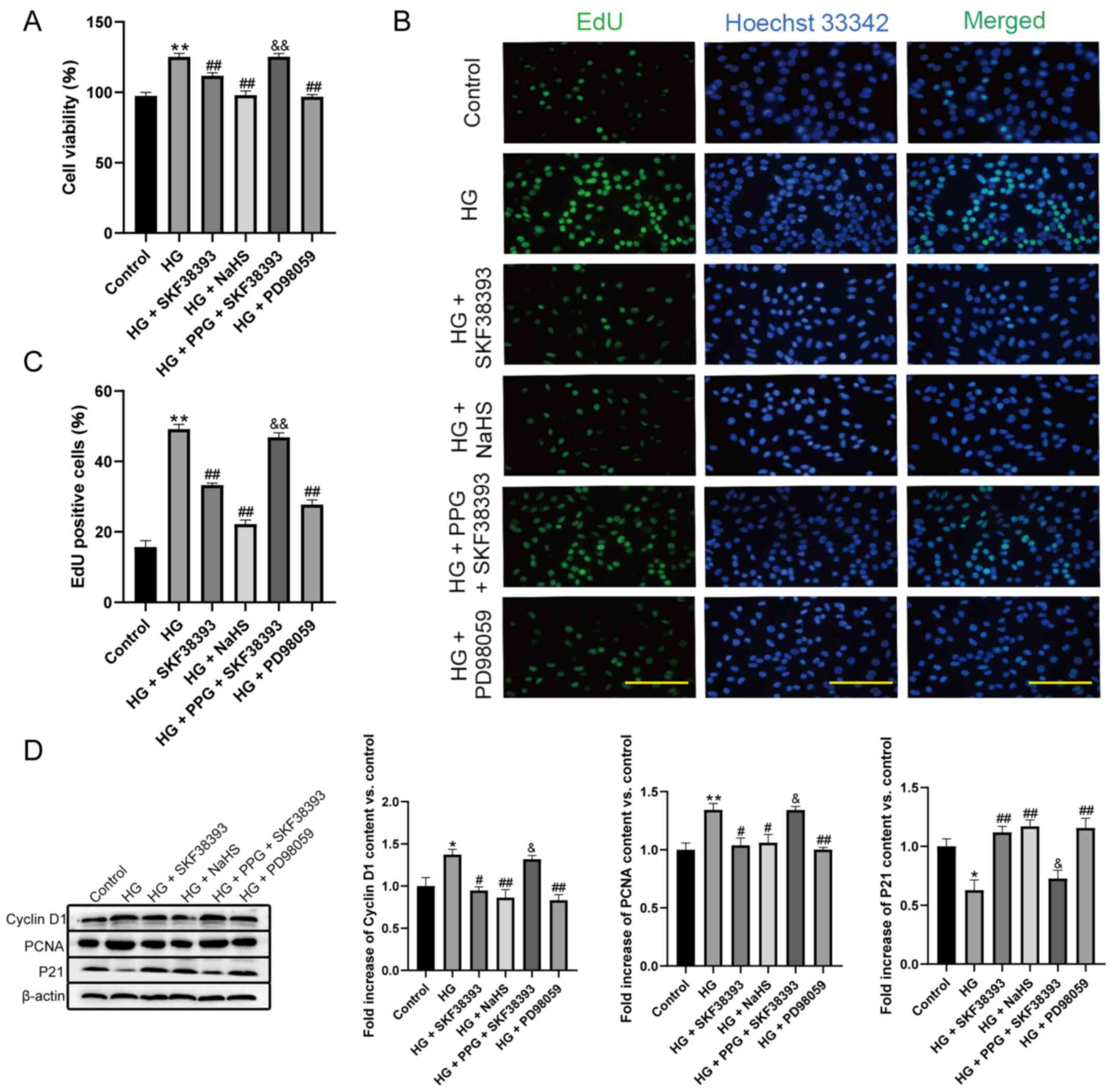 | Figure 4Activation of the
DR1-CSE/H2S pathway attenuates HG-stimulated MC
proliferation. (A) Cell viability was measured by CCK-8 assay
(n=8). (B) Cell proliferation rate was measured by EdU assay (n=6,
magnification, ×200; scale bars, 200 µm). (C) Quantify the
results of (B) with a histogram (n=6). (D) The expression of cyclin
D1, PCNA and P21 in the HG-induced MCs was determined by western
blotting (n=3). The results were expressed as the mean ± standard
error of the mean. *P<0.05, **P<0.01
vs. control group; #P<0.05, ##P<0.01
vs. HG group; &P<0.05,
&&P<0.01 vs. HG + SKF38393 group. DR1,
dopamine 1 receptors; CSE, cystathionine-γ-lyase; HG, high glucose;
MC, mesangial cell. |
Activation of the DR1-CSE/H2S
pathway inhibits HG-induced collagen deposition in MCs
α-smooth muscle actin (α-SMA) and collagen 1 (COL1)
expression was significantly upregulated and MMP9 expression was
downregulated in the HG group compared with the control group.
Compared with that in the HG group, the expression of α-SMA and
COL1 was markedly decreased and the expression of MMP9 was
significantly increased in the HG + SKF38393 and HG + NaHS groups.
These beneficial effects of SKF38393 were blocked by PPG. The
effect of SKF38393 on collagen deposition was similar to that of
PD98059 (Fig. 5).
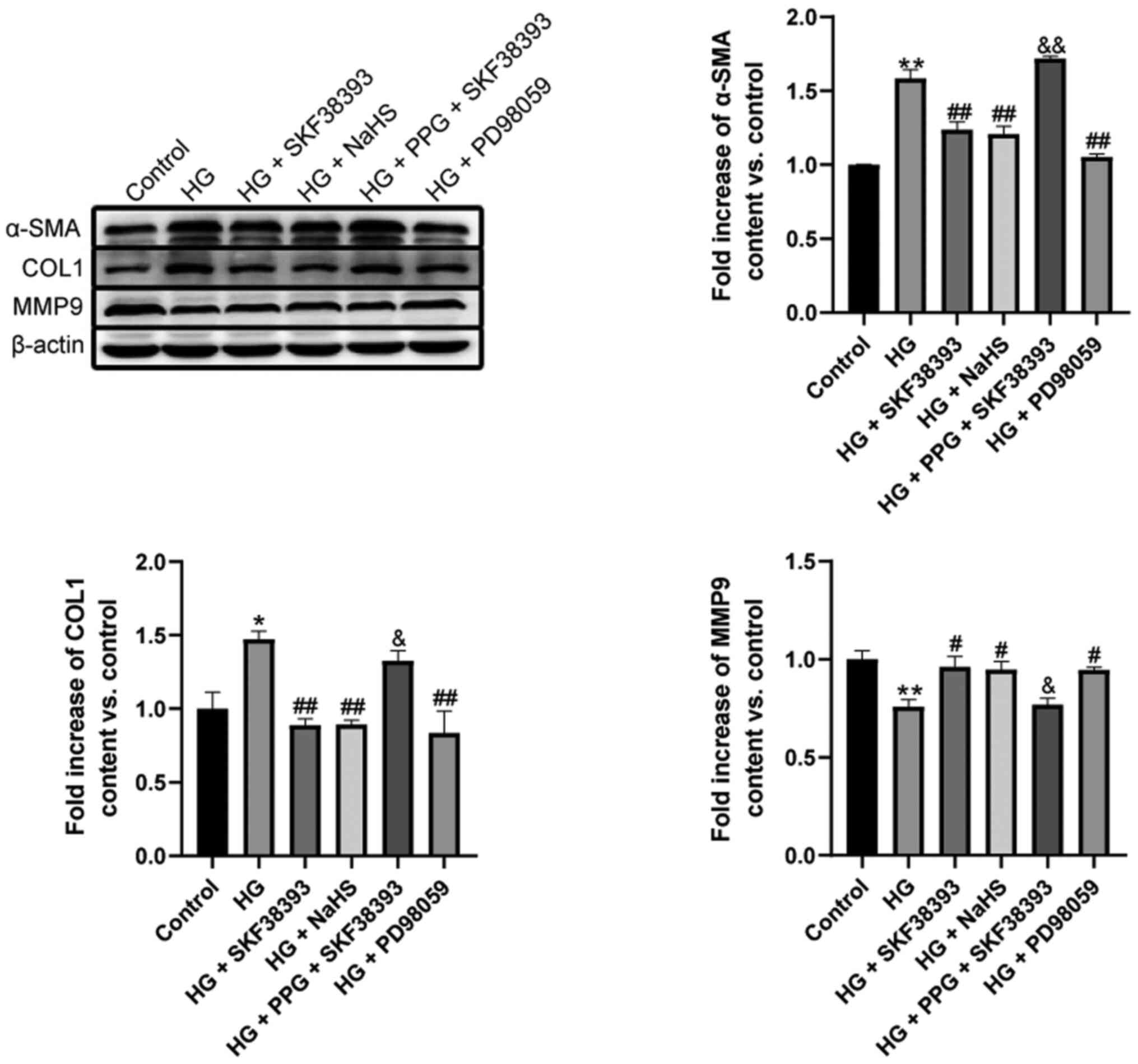 | Figure 5Activation of the
DR1-CSE/H2S pathway inhibits HG-induced collagen
deposition in MCs. The expression of COL1, α-SMA and MMP9 in the
HG-induced MCs was determined by western blotting. The experiments
were repeated at least three times. The results were expressed as
the mean ± standard error of the mean. *P<0.05,
**P<0.01 vs. control group; #P<0.05,
##P<0.01 vs. HG group; &P<0.05,
&&P<0.01 vs. HG + SKF38393 group. DR1,
dopamine 1 receptors; CSE, cystathionine-γ-lyase; HG, high glucose;
MC, mesangial cell; COL1, collagen 1; α-SMA, α-smooth muscle
actin. |
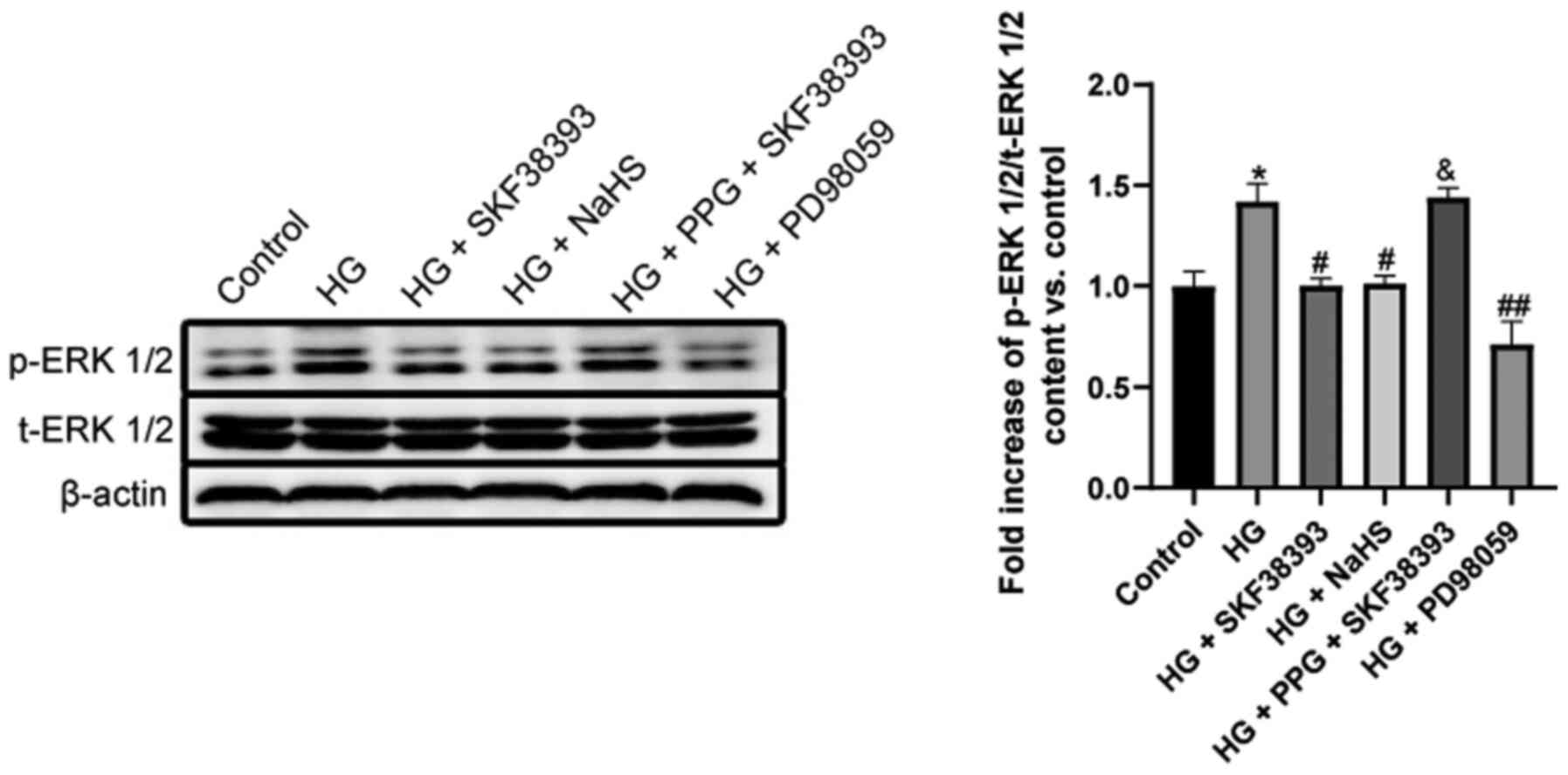
Activation of the DR1-CSE/H2S
pathway downregulates the ERK1/2 pathway in HG-induced MCs
The activity of p-ERK1/2 was significantly higher in
the HG group than in the control group. HG + SKF38393 or HG + NaHS
significantly decreased p-ERK1/2 activity and similar suppression
was observed in the HG + PD98059 group. The inhibitory effect of
SKF38393 on p-ERK1/2 activity was abolished by PPG. The activity of
t-ERK1/2 remained unchanged in different treatments (Fig. 6).
Discussion
Diabetic nephropathy is one of the leading causes of
mortality in individuals with diabetes and is characterized by
diffuse or nodular glomerulosclerosis (26). MC proliferation and extracellular
matrix deposition are the main causes of glomerulosclerosis
(3). Proliferating MCs secrete a
large amount of extracellular matrix, resulting in glomerular
basement membrane thickening and mesangial expansion (27). Inhibiting the abnormal
proliferation of MCs is critical for diabetic patients. The results
of the present study showed that the level of H2S and
the expression of DR1 and CSE were decreased in vivo and
in vitro (Figs. 1 and
3). This finding suggests that
the proliferation of MCs is associated with a decrease in DR1
expression and the downregulation of the CSE/H2S
pathway. To test this hypothesis and examine the relationship
between these factors, SKF38393 (a DR1 agonist) and NaHS (an
exogenous H2S donor) were administered. Our previous
research showed that SKF38393 could increase the expression and
activity of DR1 (20). It has
been reported that 120 µM exogenous H2S increased
the transcription and expression of CSE, while at concentrations
>160 µM, the transcription and expression of CSE were
completely inhibited, suggesting that higher levels of
H2S may be toxic (28,29). The experimental results of the
present study showed that SKF38393 increased the expression of DR1
and CSE/H2S pathway factors, but NaHS only increased
CSE/H2S pathway factors and did not affect the
expression of DR1. In addition, it was also found that PPG (a CSE
inhibitor) abolished SKF38393-mediated enhancement of the
CSE/H2S pathway in the pre-experiment (data not shown).
These results indicated that DR1 activation inhibited the
proliferation of MCs by upregulating the CSE/H2S pathway
and that DR1 is an upstream regulatory factor of the
CSE/H2S pathway (Figs.
1 and 3).
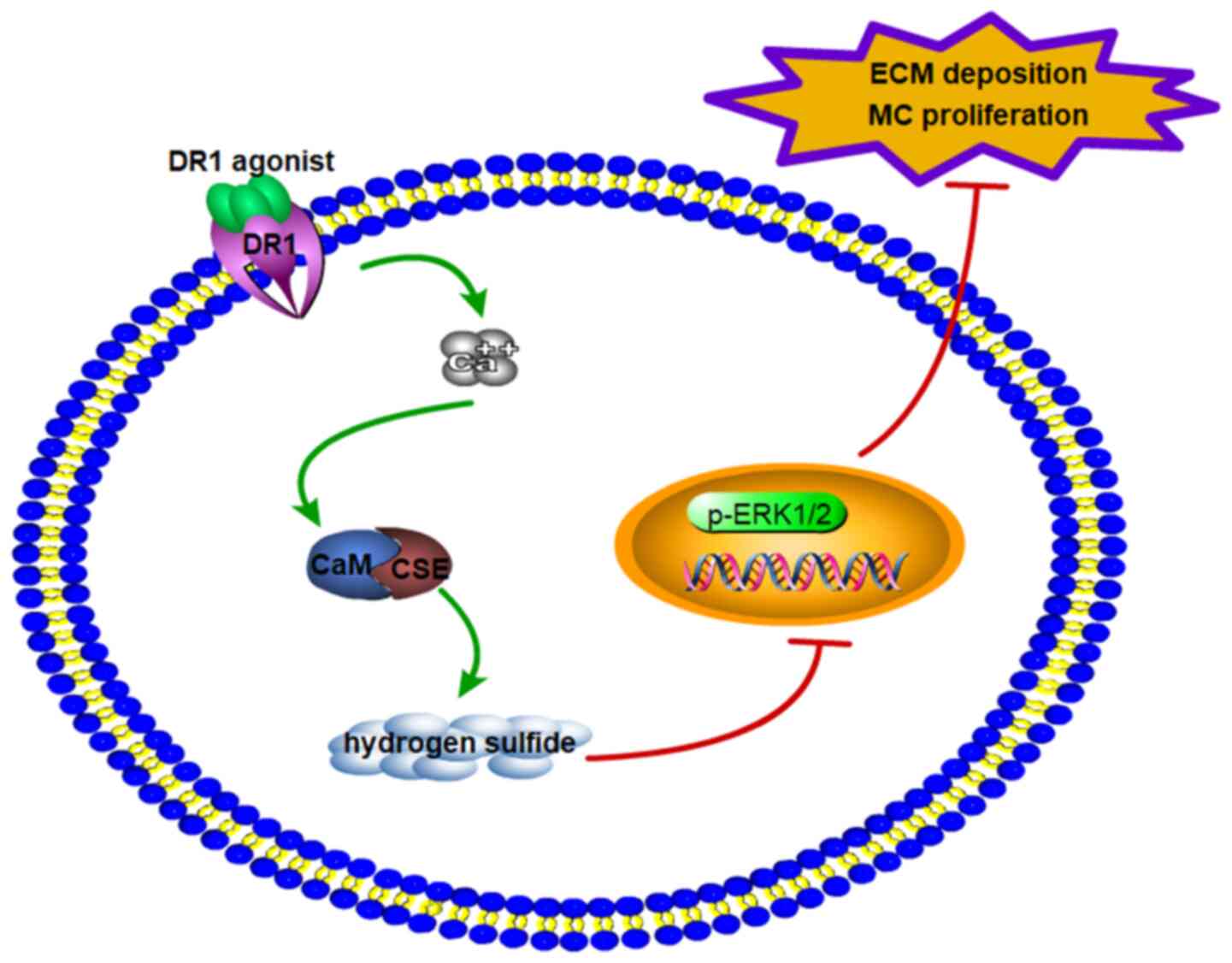
How can DR1 regulate the CSE/H2S pathway
to inhibit MC proliferation? DR1, with seven transmembrane domains,
belongs to the G protein-coupled receptor superfamily, which can
increase (Ca2+)i (17). A previous study by our research
group has also shown that DR1 activation promotes myocardial
ischemia-reperfusion injury by increasing
(Ca2+)i (20). It has been reported that an
increase in (Ca2+)i can activate calmodulin
(CaM), upregulate the expression of CSE and promote the production
of endogenous H2S (19). Therefore, DR1 increases
endogenous H2S production by increasing
(Ca2+)i, which inhibits MC proliferation.
The cell cycle is precisely controlled by cell cycle
regulatory proteins. Cell cycle regulatory proteins can be divided
into positive and negative regulatory proteins according to their
effects on the cell cycle. Positive regulatory proteins include
cyclins and cyclin-dependent kinases; the negative regulatory
proteins are mainly cyclin-dependent kinase inhibitors (30). Cyclins bind with CDK to form
cyclin/CDK active complexes, which regulate cell cycle initiation
and phase transition. CKIs include the INK4 family and CIP/Kip
family. The former includes p16INK4a,
p15INK4B, p18INK4C and p19INK4D,
which can inhibit the activity of cyclin/CDK complexes in G1 and S
phases; the latter includes p21WAF1/CIP1,
p27kip1 and p57kip2, which inhibit most
cyclin/CDK complexes (31-33). Proliferation-related proteins
were measured to explore what caused the inhibition of MC
proliferation. The data showed that HG increased the thickness of
the glomerular basement membrane, cell viability, proliferation and
cyclin D1 and PCNA expression and decreased P21 expression.
SKF38393 reduced the thickness of the glomerular basement membrane,
cell viability, proliferation and cyclin D1 and PCNA expression and
increased P21 expression. This was the same as the effect of
directly using NaHS to supplement exogenous H2S in MCs.
PPG (a CSE inhibitor) abolished the effect of SKF38393 (Figs. 2 and 4). These results suggested that the
DR1-CSE/H2S pathway suppresses cell proliferation by
affecting the expression of cell proliferation-related proteins
(cyclin D1, PCNA and P21).
Renal fibrosis is the common pathway of chronic
kidney disease that leads to end-stage renal failure (26). The deposition of extracellular
matrix is the main cause of renal fibrosis. Collagen is the most
abundant protein in the extracellular matrix and provides
structural support to cells (34). α-SMA is an important marker of
the phenotypic transformation of MCs. Positive α-SMA expression in
diabetic glomeruli is a sign of impaired MC activation (35). Under physiological conditions,
MCs have a contractile phenotype, maintaining a balance between the
synthesis and degradation of mesangial matrix (36). However, under continuous
hyperglycemia, MCs transform to a synthetic phenotype, secrete a
large amount of mesangial matrix such as COL1 to resist traumatic
stimulation and reduce the expression of MMP9 (37). To examine the protective effect
of the DR1-CSE/H2S pathway on renal fibrosis, changes in
the relevant indicators (COL1, α-SMA and MMP9) of renal fibrosis
were measured and it was found that HG increased the expression of
COL1 and α-SMA and decreased the expression of MMP9. SKF38393 and
NaHS reduced COL1 and α-SMA expression and increased the expression
of MMP9. PPG abrogated the effect of SKF38393 (Fig. 5). The results of the present
study demonstrated that the DR1-CSE/H2S pathway improved
renal fibrosis by inhibiting the deposition of collagen and
increasing the degradation of extracellular matrix. MMP2 and
membrane-type matrix metalloproteinase-1 (MT1-MMP) are also
fibrosis markers of diabetic nephropathy (34-37) and these two indicators will be
detected in future studies.
A number of signaling pathways can regulate the
proliferation of MCs, such as ERK1/2, TGF-β, Wnt/β-Catenin and
PI3K-AKT (38-40). ERK1/2 is a major member of the
MAPK family that is involved in a number of physiological
processes, such as gene expression, mitosis, cell metabolism,
survival, apoptosis and differentiation (41). The ERK1/2 pathway activates the
cyclin D1 gene, which leads to cell growth, proliferation and
differentiation (42). The
ERK1/2 pathway becomes activated in MCs cultured under HG
conditions (38). The results of
the present study showed that HG increased the activity of
p-ERK1/2. SKF38393 and that NaHS reduced the activity of p-ERK1/2.
PPG reversed the effect of SKF38393 (Fig. 6). The effect of SKF38393 on cell
viability, proliferation, proliferation-associated protein
expression, fibrosis-associated protein expression and p-ERK1/2
activity was similar to that of PD98059 (Figs. 1-6). This finding suggested that the
DR1-CSE/H2S pathway inhibited MC dysfunction by
inhibiting ERK1/2 activation. However, it remains to be explored
whether the DR1-CSE/H2S pathway also affects other
signaling pathways, such as Wnt/β-Catenin, PI3K-AKT, TGF-β and
others.
Notably, the roles of SKF38393 (a DR1 agonist), NaHS
(an exogenous H2S donor) and PD98059 (an ERK1/2
inhibitor) in MC proliferation were similar, suggesting that these
factors inhibit MC proliferation by regulating the ERK1/2 pathway.
Whether there are other mechanisms involved will be explored in the
future.
In conclusion (Fig.
7), the findings of the present study suggested that: i) MC
proliferation and extracellular matrix deposition were associated
with decreased DR1 expression and the CSE/H2S pathway.
ii) DR1 activation upregulated the CSE/H2S pathway in
HG-induced kidney tissues and MCs. iii) The DR1-CSE/H2S
pathway inhibited MC proliferation and extracellular matrix
deposition by downregulating the ERK1/2 signaling pathway. The
present study explored the potential protective mechanism of the
DR1-CSE/H2S pathway in diabetic nephropathy and provided
a new therapeutic target for antifibrosis strategies against
diabetic nephropathy.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL conceived and designed the research. FS conducted
cell culture. FS and GC performed animal breeding. RW, YW, JH and
SB provided technical support. YX, XW and FS conducted sample
processing and sample extraction. AZ analyzed and interpreted the
data. FS and SB wrote and revised the manuscript. HL and FS confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care Committee of Harbin Medical University for the use of
experimental animals (approval no. SCXK2013-001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81770486 and 81200160).
References
|
1
|
Adeshara KA, Diwan AG and Tupe RS:
Diabetes and complications: Cellular signaling pathways, current
understanding and targeted therapies. Curr Drug Targets.
17:1309–1328. 2016. View Article : Google Scholar
|
|
2
|
Yuan CM, Nee R, Ceckowski KA, Knight KR
and Abbott KC: Diabetic nephropathy as the cause of end-stage
kidney disease reported on the medical evidence form CMS2728 at a
single center. Clin Kidney J. 10:257–262. 2017.PubMed/NCBI
|
|
3
|
Chen B, Li Y, Liu Y and Xu Z: circLRP6
regulates high glucose-induced proliferation, oxidative stress, ECM
accumulation, and inflammation in mesangial cells. J Cell Physiol.
234:21249–21259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marciano DK: Mesangial Cells: The tuft
guys of glomerular development. J Am Soc Nephrol. 30:1551–1553.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abboud HE: Mesangial cell biology. Exp
Cell Res. 318:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Zhao Q, Jin X, Li Y and Song J:
Silencing of LncRNA PVT1 inhibits the proliferation, migration and
fibrosis of high glucose-induced mouse mesangial cells via
targeting microRNA-93-5p. Biosci Rep. 40:402020.
|
|
7
|
Zhang L, Liu J, Zhou F, Wang W and Chen N:
PGC-1α ameliorates kidney fibrosis in mice with diabetic kidney
disease through an antioxidative mechanism. Mol Med Rep.
17:4490–4498. 2018.PubMed/NCBI
|
|
8
|
Zhao JH: Mesangial cells and renal
fibrosis. Adv Exp Med Biol. 1165:165–194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buhl EM, Djudjaj S, Klinkhammer BM, Ermert
K, Puelles VG, Lindenmeyer MT, Cohen CD, He C, Borkham-Kamphorst E,
Weiskirchen R, et al: Dysregulated mesenchymal PDGFR-β drives
kidney fibrosis. EMBO Mol Med. 12:e110212020. View Article : Google Scholar
|
|
10
|
Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z,
Ni J, Yu C, Yao T, Huang Y, et al: Rescue of mesangial cells from
high glucose-induced over-proliferation and extracellular matrix
secretion by hydrogen sulfide. Nephrol Dial Transplant.
26:2119–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R: Physiological implications of
hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev.
92:791–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu M, Li Y, Liang B, Li Z, Jiang Z, Chu C
and Yang J: Hydrogen sulfide attenuates myocardial fibrosis in
diabetic rats through the JAK/STAT signaling pathway. Int J Mol
Med. 41:1867–1876. 2018.
|
|
13
|
Dugbartey GJ: Diabetic nephropathy: A
potential savior with 'rotten-egg' smell. Pharmacol Rep.
69:331–339. 2017. View Article : Google Scholar
|
|
14
|
Feliers D, Lee HJ and Kasinath BS:
Hydrogen sulfide in renal physiology and disease. Antioxid Redox
Signal. 25:720–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Missale C, Nash SR, Robinson SW, Jaber M
and Caron MG: Dopamine receptors: From structure to function.
Physiol Rev. 78:189–225. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beaulieu JM, Espinoza S and Gainetdinov
RR: Dopamine receptors -IUPHAR Review 13. Br J Pharmacol. 172:1–23.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beaulieu JM and Gainetdinov RR: The
physiology, signaling, and pharmacology of dopamine receptors.
Pharmacol Rev. 63:182–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bryson SE, Drew GM, Hall AS, Ball SG and
Balmforth AJ: Characterization of the dopamine receptor expressed
by rat glomerular mesangial cells in culture. Eur J Pharmacol.
225:1–5. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang G, Wu L, Jiang B, Yang W, Qi J, Cao
K, Meng Q, Mustafa AK, Mu W, Zhang S, et al: H2S as a
physiologic vaso-relaxant: Hypertension in mice with deletion of
cystathionine gamma-lyase. Science. 322:587–590. 2008. View Article : Google Scholar
|
|
20
|
Li HZ, Han LP, Jiang CM, Li H, Zhao YJ,
Gao J, Lin Y, Ma SX, Tian Y, Yang BF, et al: Effect of dopamine
receptor 1 on apoptosis of cultured neonatal rat cardiomyocytes in
simulated ischaemia/reperfusion. Basic Clin Pharmacol Toxicol.
102:329–336. 2008. View Article : Google Scholar
|
|
21
|
Zhou X, Feng Y, Zhan Z and Chen J:
Hydrogen sulfide alleviates diabetic nephropathy in a
streptozotocin-induced diabetic rat model. J Biol Chem.
289:28827–28834. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waz S, Heeba GH, Hassanin SO and
Abdel-Latif RG: Nephroprotective effect of exogenous hydrogen
sulfide donor against cyclophosphamide-induced toxicity is mediated
by Nrf2/HO-1/NF-κB signaling pathway. Life Sci. 264:1186302021.
View Article : Google Scholar
|
|
23
|
Yang G, Tang G, Zhang L, Wu L and Wang R:
The pathogenic role of cystathionine γ-lyase/hydrogen sulfide in
streptozotocin-induced diabetes in mice. Am J Pathol. 179:869–879.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen B, Li W, Lv C, Zhao M, Jin H, Jin H,
Du J, Zhang L and Tang X: Fluorescent probe for highly selective
and sensitive detection of hydrogen sulfide in living cells and
cardiac tissues. Analyst (Lond). 138:946–951. 2013. View Article : Google Scholar
|
|
25
|
Olson KR, Gao Y, Arif F, Patel S, Yuan X,
Mannam V, Howard S, Batinic-Haberle I, Fukuto J, Minnion M, et al:
Manganese porphyrin-based SOD mimetics produce polysulfides from
hydrogen sulfide. Antioxidants. 8:82019. View Article : Google Scholar
|
|
26
|
Papadopoulou-Marketou N, Paschou SA,
Marketos N, Adamidi S, Adamidis S and Kanaka-Gantenbein C: Diabetic
nephropathy in type 1 diabetes. Minerva Med. 109:218–228. 2018.
View Article : Google Scholar
|
|
27
|
Drummond K and Mauer M; International
Diabetic Nephropathy Study Group: The early natural history of
nephropathy in type 1 diabetes: II. Early renal structural changes
in type 1 diabetes. Diabetes. 51:1580–1587. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bithi N, Link C, Henderson YO, Kim S, Yang
J, Li L, Wang R, Willard B and Hine C: Dietary restriction
transforms the mammalian protein persulfidome in a tissue-specific
and cystathionine γ-lyase-dependent manner. Nat Commun.
12:17452021. View Article : Google Scholar
|
|
29
|
Wang M, Guo Z and Wang S: The effect of
certain conditions in the regulation of cystathionine γ-lyase by
exogenous hydrogen sulfide in mammalian cells. Biochem Genet.
51:503–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shankland SJ: Cell cycle regulatory
proteins in glomerular disease. Kidney Int. 56:1208–1215. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee MH and Yang HY: Negative regulators of
cyclin-dependent kinases and their roles in cancers. Cell Mol Life
Sci. 58:1907–1922. 2001. View Article : Google Scholar
|
|
32
|
Thullberg M, Welcker M, Bartkova J,
Kjerulff AA, Lukas J, Högberg J and Bartek J: Monoclonal antibody
probes for p21WAF1/CIP1 and the INK4 family of cyclin-dependent
kinase inhibitors. Hybridoma. 19:63–72. 2000. View Article : Google Scholar
|
|
33
|
Chen JH, Tseng TH, Ho YC, Lin HH, Lin WL
and Wang CJ: Gaseous nitrogen oxides stimulate cell cycle
progression by retinoblastoma phosphorylation via activation of
cyclins/Cdks [correction]. Toxicol Sci. 76:83–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chow MJ, Turcotte R, Lin CP and Zhang Y:
Arterial extracellular matrix: A mechanobiological study of the
contributions and interactions of elastin and collagen. Biophys J.
106:2684–2692. 2014. View Article : Google Scholar :
|
|
35
|
Johnson RJ, Floege J, Yoshimura A, Iida H,
Couser WG and Alpers CE: The activated mesangial cell: A glomerular
'myofibroblast'? J Am Soc Nephrol. 2(Suppl 10): S190–S197. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kreisberg JI, Venkatachalam M and Troyer
D: Contractile properties of cultured glomerular mesangial cells.
Am J Physiol. 249:F457–F463. 1985.PubMed/NCBI
|
|
37
|
Ohtomo S, Nangaku M, Izuhara Y, Yamada N,
Dan T, Mori T, Ito S, van Ypersele de Strihou C and Miyata T: The
role of megsin, a serine protease inhibitor, in diabetic mesangial
matrix accumulation. Kidney Int. 74:768–774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Gao H, Wang L, Ma X, Tian L, Zhao
W, Li K, Zhang Y, Ma F, Lu J, et al: Farrerol alleviates high
glucose-induced renal mesangial cell injury through the
ROS/Nox4/ERK1/2 pathway. Chem Biol Interact. 316:1089212020.
View Article : Google Scholar
|
|
39
|
Mao Q, Chen C, Liang H, Zhong S, Cheng X
and Li L: Astragaloside IV inhibits excessive mesangial cell
proliferation and renal fibrosis caused by diabetic nephropathy via
modulation of the TGF-β1/Smad/miR-192 signaling pathway. Exp Ther
Med. 18:3053–3061. 2019.PubMed/NCBI
|
|
40
|
Qian X, He L, Hao M, Li Y, Li X, Liu Y,
Jiang H, Xu L, Li C, Wu W, et al: YAP mediates the interaction
between the Hippo and PI3K/Akt pathways in mesangial cell
proliferation in diabetic nephropathy. Acta Diabetol. 58:47–62.
2021. View Article : Google Scholar
|
|
41
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar :
|
|
42
|
Bramanti V, Grasso S, Tibullo D, Giallongo
C, Raciti G, Viola M and Avola R: Modulation of extracellular
signal-related kinase, cyclin D1, glial fibrillary acidic protein,
and vimentin expression in estradiol-pretreated astrocyte cultures
treated with competence and progression growth factors. J Neurosci
Res. 93:1378–1387. 2015. View Article : Google Scholar
|