Introduction
Hepatic ischemia-reperfusion (I/R) injury is a
common cause of postoperative complications or liver damage, and is
also considered a major reason for post-transplantation liver-graft
dysfunction (1,2). During I/R injury, oxidative
phosphorylation is inhibited by hypoxia that results in anaerobic
glycolysis (3). The generation
of reactive oxygen species (ROS), lipid peroxidation and dsDNA
damage are prime mechanisms consequently associated to I/R injury
(4,5). ROS generated by mitochondria and
release of inflammatory mediators from the endoplasmic reticulum
result in upregulation of autophagy-related genes (6,7).
Several attempts have been made to control hepatic I/R injury. For
instance, Chen et al reported that dexmedetomidine could
protect the liver from I/R injury by decreasing inflammatory
response associated with NLRC5 (8). In addition, oleanolic acid
(9), l-tetrahydropalmatine
(10), hyperoside (11), cerium oxide nanoparticles
(12) have also been reported to
have a protective role against hepatic I/R injury.
Regenerative medicine based on stem cell therapy is
a new area in medicine that offers treatment of degenerative
disorders and injuries. However, implanted stem cells face certain
challenges such as poor survival particularly in hypoxic conditions
causing poor therapeutic response (13,14). Saidi et al used human
adipose-derived mesenchymal stem cells (hADMSCs) against hepatic
I/R injury in a mouse model (15). They identified that infusion of
1-2 million hADMSCs 30 min prior to ischemia could decrease I/R
injury (15). In another study,
Saat et al determined that C57BL/6 mice-derived MSCs were
ineffective against hepatic I/R injury (with hepatectomy) when
cells were infused 2 h prior or 1 h following ischemia (16). It was observed that within 2 h,
infused cells disappeared, and the remaining cells could not reach
the area of the injury (16).
Additionally, the necessity of phenotypically stable cells, higher
cost, handling complications, risk of rejection following
implantation and potential risk of ectopic tissue/tumor formation
are other challenges in the application of stem cells. To avoid
such drawbacks related to the administration of stem cells,
exosomes have emerged as a new tool to achieve therapeutic outcomes
with prolonged circulatory life. Exosomes are bi-layered vesicles
that are released by cells for intercellular signaling (17). They contain proteins, lipids and
nucleic acids (18). Among them,
microRNAs (miRNAs or miRs) were reported to induce the therapeutic
effect of exosomes (18).
Exosomes derived from stem cells have exhibited various therapeutic
responses such as promotion of wound healing (13), recovery of neurological function
following nerve injury (18),
and protection against kidney injury (19) and erectile dysfunction (20). Recently, their potential for the
treatment of severe COVID-19 was also proposed (21).
Adipose-derived stem cells (ADSCs) were found to
attenuate hepatic I/R injury in swine models by decreasing
oxidative stress (22). Based on
research concerning the therapeutic potential of exosomes which is
similar to stem cells, it was hypothesized that exosomes derived
from ADSCs (ADSCs-exo) may protect hepatic I/R injury and these
exosomes have greater potential for clinical translation
considering drawbacks related to direct stem cell therapy. The
present study aimed to investigate the protective efficacy of
ADSCs-exo against hepatic I/R injury. ADSCs-exo were isolated from
ADSC culture medium through the differential centrifugation
process. These exosomes were evaluated for their efficacy through
pre-treatment via the portal vein of an animal model.
Materials and methods
Animals
Male Sprague-Dawley rats (n=41; 6 weeks old),
weighing 160-200 g, were purchased from Sino-British SIPPR/BK Lab
Animal Ltd. (Shanghai, China). The animals were housed in a
pathogen-free environment with standard conditions of temperature
(27±2°C), humidity (50±5% RH) and a 12-h light/dark cycle. All of
the animals were provided with 24-h free access to food and water.
All procedures were conducted in accordance with the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health and was approved (approval no. 00100097) by the Medical
Ethics Committee of Naval Medical University (Shanghai, China).
Isolation of ADSCs-exo
ADSCs were isolated from the inguinal fat. Briefly,
5 rats were euthanized by intraperitoneal injection of sodium
pentobarbital (100 mg/kg). Following confirmation of death by lack
of pulse, breathing and corneal reflex, the abdomen and back of
rats were disinfected using 75% alcohol and placed under an aseptic
environment. The skin from both sides of the abdomen in the groin
region was removed using scissors to expose fat pads. Fat pads were
clamped using an Allis tissue clamp and removed using ophthalmic
scissors. A total of 4-5 ml of fat tissue from each rat was
obtained under sterilized conditions, and was digested with
collagenase type I (0.25%) for 50 min at 37°C with shaking using a
laboratory shaker. Following digestion, high sugar DMEM containing
10% FBS (both from Life Technologies; Thermo Fisher Scientific,
Inc.) and standard concentrations of penicillin (10 U/ml) and
streptomycin (100 µg/ml; both from Shanghai Yeasen
Biotechnology Co., Ltd.) was added to the fat mixture followed by
filtration through a cell strainer. Subsequently, following
appropriate dilution, the supernatant was centrifuged at 1,000 × g
for 10 min at 25°C to collect cells that were further cultured in
10 cm2 cell culture plates. Following the third passage
of cells, surface markers CD73, CD90, CD105, CD34, CD45 and CD11b
were identified using marker identification kit
Oricell®; cat. no. RAXMX-09011; [Saiye (Guangzhou)
Biotechnology Co., Ltd.] according to the manufacturer's protocol,
and a flow cytometer (BD LSR II; BD Biosciences).
The third generation of ADSCs was cultured at 37°C
and 5% CO2 in a petri dish with complete medium, and
after 80% confluency was reached, the medium was replaced with
serum-free medium that was collected after 48 h. The collected
serum-free medium was centrifuged at 400 × g for 15 min at 4°C to
remove floating cells. The supernatant was collected and
centrifuged at 10,000 × g for 15 min at 4°C to remove cellular
debris. Exosomes were collected through ultracentrifugation at
100,000 × g for 13 h at 4°C. Subsequently, the exosomes were gently
washed with PBS and collected through centrifugation at 14,000 × g
for 10 min at 4°C. Collected exosomes were observed under
transmission electron microscopy (TEM) and surface markers
including TSG101 (1:1,000; product code ab83), CD9 (1:1,000;
product code ab92726) and CD63 (1:1,000; product code ab193349; all
from Abcam) were identified using western blot analysis according
to the manufacturer's protocol.
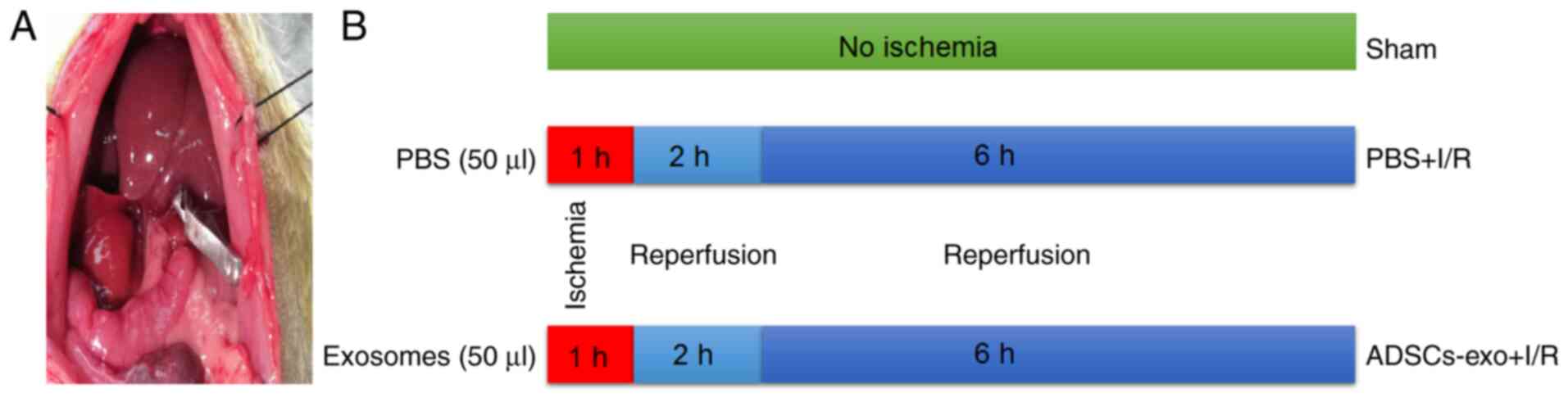
Surgical procedures
A total of 36 SD rats were divided into three
groups: Sham-operated rats (Sham), PBS-treated rats for I/R injury
(PBS + I/R) and exosome-treated rats for I/R injury (ADSCs-exo +
I/R). Rats were fasted 12 h prior to the experiment. For the
surgical procedure, rats were anesthetized by intraperitoneal
(i.p.) injection of 1% pentobarbital sodium (45 mg/kg of body
weight). Following midline laparotomy, the portal vein and portal
artery were clamped to induce 70% hepatic ischemia. A total of 50
µl exosomes (30 µg) were administered via the portal
vein before induction of ischemia in rats included in the ADSCs-exo
+ I/R group while a similar volume of PBS was administered in rats
in the PBS + I/R group (Fig. 1).
Ischemia was maintained for 60 min followed by reperfusion. During
experimental procedures, the rats were placed on a 37°C warmed
surface. Following 2 and 6 h of reperfusion, rats (n=6, for each
time-point) were sacrificed using 100 mg/kg intraperitoneal
injection of pentobarbital sodium and liver and serum samples were
collected for further examination. The death of the rats was
confirmed by lack of pulse, breathing and corneal reflex. For the
sham group, the same procedure was followed without clamping of
hepatic vessels.
Biochemical analysis
Collected blood was centrifuged at 1,000 × g for 15
min at 4°C to obtain serum that was analyzed for quantification of
alanine aminotransferase (ALT), aspartate aminotransferase (AST)
and lactate dehydrogenase (LDH) using commercial kits (cat. no.
ALT000A, AST000S and LDH000S for ALT, AST and LDH, respectively;
from Beijing Autobio Co. Ltd.) following the manufacturer's
protocol on Auto-Analyzer (TBA-120FR; TOSHIBA).
Antioxidant enzymes and lipid
peroxidation analysis
The levels of malondialdehyde (MDA; cat. no.
MBS727531; MyBioSource, Inc.), superoxide dismutase (SOD; cat. no.
MBS266897; MyBioSource, Inc.) and ROS (cat. no. LS-F9759; LifeSpan
BioSciences, Inc.) were determined in liver homogenates using a
commercial testing kits according to the manufacturer's
protocol.
Determination of inflammatory
markers
The expression levels of interleukin (IL)-1β
(product code ab255730; Abcam) and TNF-α (product code ab236712;
Abcam) were detected in rat serum using respective ELISA kits.
cAMP and PGE2 assay levels
The amount of cAMP in serum was quantified using
cAMP Complete ELISA kit (product code ab133051; Abcam).
Furthermore, prostaglandin E2 (PGE2) was also quantified in serum
using PGE2 ELISA kit (product code ab133021; Abcam).
Histological analysis
Following 2 and 6 h of reperfusion, collected liver
tissue samples were fixed in 4% formaldehyde solution for 12 h at
4°C. The tissue was dehydrated and embedded in paraffin (23). Embedded tissues were sliced into
5-µm thick sections. For necrosis evaluation, hematoxylin
and eosin (H&E) staining was performed according to the
manufacturer's protocol (Beyotime Institute of Biotechnology).
Briefly, hematoxylin staining solution was added on specimens for 5
min at room temperature followed by washing with tap water for 1-2
min. The slides were dipped in 1% hydrochloric acid in 70% ethanol
for 10 sec followed by washing with tap water for 10 min. Eosin
staining solution was added on specimens for 30 sec and directly
immersed in 95% ethanol followed by absolute ethanol for 10 sec.
Necrotic areas were quantified using ImageJ software (version
1.51j8; National Institutes of Health). TdT-mediated dUTP nick-end
labeling (TUNEL) staining was also performed to observe apoptotic
cells in liver samples using a commercial kit (cat. no.
11684817910; Roche Diagnostics), following the manufacturer's
protocol. Briefly, TUNEL reaction mixture (50 µl) was added
onto specimens and placed at 37°C for 1 h in a dark humidified box.
After washing with PBS (three times for 3 min each), 50 µl
converter-peroxidase was added to specimens for 30 min at 37°C for
1 h in a dark humidified box followed by DAB staining for 30 min at
25°C. Stained samples were observed using light microscope (Olympus
CX41; Olympus Corporation). The percentage apoptotic cells was
counted using the following formula: (Number of apoptotic
cells/total number of cells) ×100.
For immunohistochemical analysis, liver samples were
fixed using 4% paraformaldehyde (in normal saline) for 10 min at
room temperature, and further sliced into 30-µm-thick
sections. Following washing with PBS, permeabilization was
performed using washing buffer with 0.3% H2O2
and 0.5% Triton X-100. Sections were incubated with respective
primary antibodies including ERK1/2 (1:1,000; product no. 4695),
p-ERK1/2 (1:2,000; product no. 4370), GSK-3β (1:1,000; product no.
5676) and p-GSK-3β (1:1,000; product no. 9322; all from Cell
Signaling Technology, Inc.) at 4°C overnight after blocking
non-specific binding sites with 3% BSA (Shanghai Yeasen
Biotechnology Co., Ltd.) for 1 h at room temperature. Samples were
washed and incubated in secondary antibodies (HRP-goat anti-mouse
IgG, cat. no. GB23301 for ERK1/2 and GSK3β and HRP-goat anti-rabbit
IgG, cat. no. GB23303 for p-ERK1/2 and p-GSK3β; both from
Servicebio) at 1:200 dilution for 1 h at room temperature followed
by staining with DAB (0.05%) for 10 min at room temperature. Images
were analyzed to calculate the positive expression of
phosphorylation of extracellular receptor kinase (ERK) and glycogen
synthase kinase-3b (GSK-3β). Positive area <10% was graded as
(-), positive area between 10-30% was graded as (+) while positive
area >30% was graded as (++).
TEM
Following 6 h of reperfusion, electron microscopic
analysis of liver tissue was performed to observe subcellular
modification. Liver samples were first fixed in a 1% osmic acid
fixative solution for 3 h at 4°C. Fixed samples were dehydrated
with graded ethanol solution followed by ethanol/acetone solutions
and 90% acetone. Following washing with PBS, dehydrated samples
were embedded in acetone: OCT compound (2:1) for 4 h. Tissues were
finally embedded in 100% embedding medium and dried (at 37°C
overnight, 45°C for 12 h and 60°C for 24 h). Dried samples were
sliced into 50-nm sections and observed under a transmission
electron microscope (Crossbeam 550 FE; Carl Zeiss AG) following
staining with 2% uranyl acetate and lead citrate at room
temperature for 10 min. Embedding medium and staining solutions
were provided by Microscopy Core Facility of Westlake
University.
Western blot analysis
Protein expression levels of ERK1/2, phosphorylated
(p)-ERK1/2, GSK-3β, p-GSK-3β, Bcl-2 and Bax were evaluated using
western blotting as per standard protocol. The liver lysate was
obtained by homogenizing the liver tissue in RIPA lysis buffer
(cat. no. P0013B; Beyotime Institute of Biotechnology) followed by
centrifugation at 12,000 × g at 4°C for 15 min. A total of 40
µg of protein sample, following quantification using a BCA
kit, were separated using 10% SDS-PAGE and transferred to PVDF
membranes. Non-specific binding of antibodies was blocked by
incubation in 5% BSA for 32 h at 4°C. Blots were further incubated
at 4°C overnight with respective primary antibodies: ERK1/2
(1:1,000; product no. 4695), p-ERK1/2 (1:2,000; product no. 4370),
GSK-3β (1:1,000; product no. 5676), p-GSK-3β (1:1,000; product no.
9322; all from Cell Signaling Technology, Inc.), Bcl-2 mouse
monoclonal antibody (1:1,000; 60178-1-Ig; ProteinTech Group, Inc.),
Bax rabbit monoclonal antibody (1:1,000; cat. no. 60267-1-Ig;
ProteinTech Group, Inc.) and GAPDH (1:1,000; product no. 5174; Cell
Signaling Technology, Inc.) followed by incubation with goat
anti-rabbit IgG HRP-linked secondary antibody (1:2,000; cat. no.
7074; Cell Signaling Technology, Inc.) for 1.5 h at 25°C. Blots
were washed with TBS with 0.05% Tween-20 (TBST) thrice and a
chemiluminescence reaction was achieved with an ECL reagent (cat.
no. 34076; Thermo Fisher Scientific, Inc.) for visualization.
Images were captured using an image analysis system (model
GIS-1000; Tanon Science and Technology Co., Ltd.). Western blot
images were further analyzed using ImageJ software (version 1.51j8;
National Institutes of Health) to determine the relative expression
of proteins.
Reverse transcription-quantitative
(RT-q)PCR
In addition to immunohistochemistry and western
blotting, RT-qPCR was also performed to analyze the expression of
ERK1/2 and GSK-3β. Total RNA was extracted using TRIzol LS and
TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Subsequently, the concentration and
purity of obtained RNA were determined by Nano-Drop
spectrophotometer (Thermo Fisher Scientific, Inc.), and if the
OD260/280 was in the range of ~1.8-2.0 this was deemed as
acceptable. Then, the expression level of genes was examined by
RT-qPCR with the SYBR Green I (Takara Bio, Inc.) dye detection
method according to the manufacturer's protocol. The thermocycling
conditions were as follows: Denaturation at 95°C for 5 min followed
by annealing at 60°C for 15 sec and extension at 72°C for 25 sec
(40 cycles). A total of 1 µg of total RNA from each sample
was reverse-transcribed using Takara reverse transcription kit
(cat. no. 639505, Takara Bio, Inc.) following the manufacturer's
protocol. β-actin was used as a loading control. The primers were
as follows: β-actin forward, 5′-CCC GCG AGT ACA ACC TTC T-3′ and
reverse, 5′-CGT CAT CCA TGG CGA ACT -3′; ERK1/MAPK3 (248 bp)
forward, 5′-TCC GCC ATG AGA ATG TTA TAG GC-3′ and reverse, 5′-GGT
GGT GTT GAT AAG CAG ATT GG-3′; ERK2/MAPK1 (84 bp) forward, 5′-GGT
TGT TCC CAA ATG CTG ACT -3′ and reverse, 5′-CAA CTT CAA TCC TCT TGT
GAG GG-3′; and GSK-3β (200 bp) forward, 5′-TAT GGT CTG CAG GCT GT G
TG-3′ and reverse, 5′-CCG AAA GAC CTT CGT CCA A-3′. Finally, the
cycle threshold (Cq) of each sample was detected using ABI 7900
thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.),
and the relative expression of genes was calculated using
2−ΔΔCq method (24).
Each experiment was repeated three times.
Statistical analysis
All data were presented as the mean ± standard
deviation (SD) (n=6). Comparison between different groups was
performed using one-way ANOVA followed by Tukey's post hoc test.
Statistical analysis was performed using GraphPad Prism software,
version 8.0.2 (GraphPad Software, Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
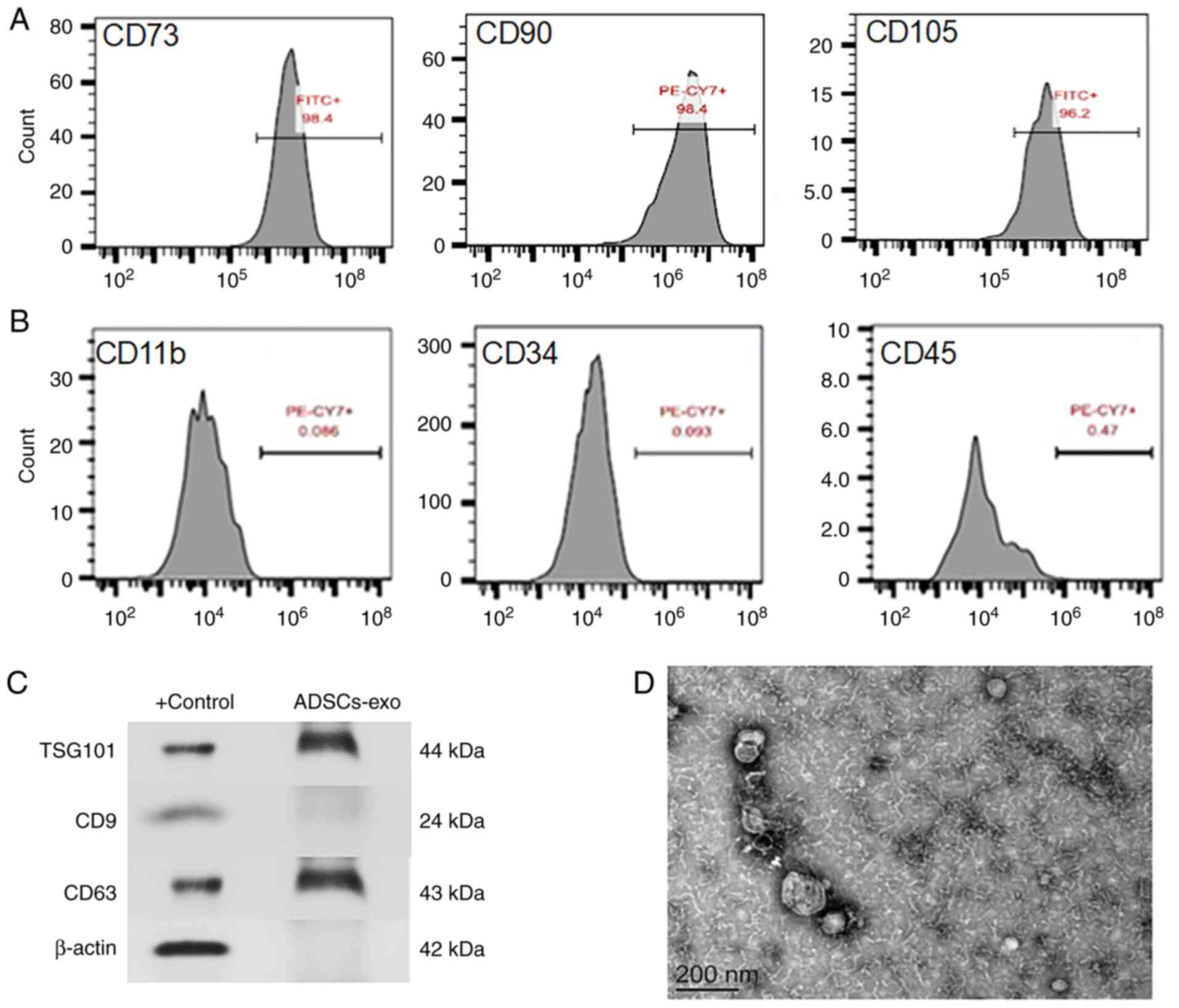
Identification of isolated ADSCs and
ADSCs-exo
Isolated ADSCs were identified using surface
markers. Fig. 2A reveals CD73,
CD90 and CD105 on cell surfaces that are positive markers for
ADSCs. Negative expression of CD34, CD45 and CD11b was also
observed for ADSCs as revealed in Fig. 2B. Furthermore, isolated exosomes
from ADSCs were also confirmed using surface markers such as
TSG101, CD9 and CD63 through western blot analysis as revealed in
Fig. 2C. The morphology and size
of exosomes were evaluated by TEM, and the images obtained revealed
spherical vesicular shape with a size range of 80-150 nm (Fig. 2D). Isolation of exosomes is a
critical step and several methods have been reported. All methods
have advantages and disadvantages; however, no consensus exists
between researchers on a single method. Ultracentrifugation is a
commonly employed method for isolation of exosomes and was used in
the present study as it does not significantly affect the protein
and RNA content of exosomes (25).
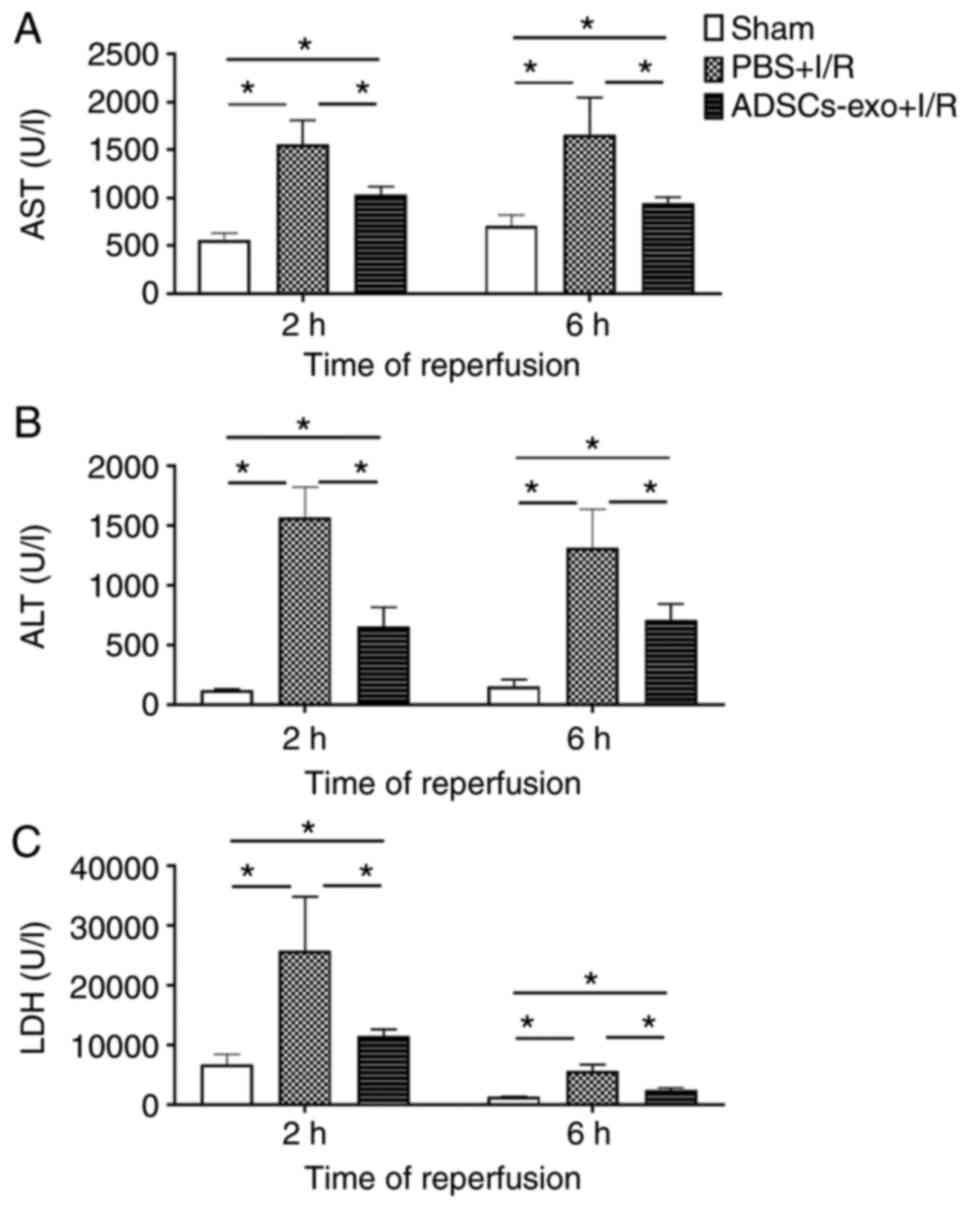
Effect on liver enzymes
Serum concentrations of AST, ALT and LDH were
quantified for three groups as revealed in Fig. 3. The PBS + I/R rats exhibited
higher concentrations of all markers indicating hepatic damage
following 2 and 6 h of perfusion. In the case of exosome-treated
rats, AST, ALT and LDH concentrations were higher than those of the
sham group; however, their concentrations were significantly lower
than the PBS + I/R group (Fig.
3). These results demonstrated that exosome treatment could
prevent hepatocyte damage induced by hepatic I/R injury.
Ultrastructural modification of cellular
components
TEM was performed to observe subcellular structures
of hepatocytes following 6 h reperfusion. Hepatocytes in the sham
group exhibited normal features of nuclei, mitochondria and
endoplasmic reticulum (Fig. 4A).
The PBS + I/R group exhibited typical structural changes of I/R
injury including swelling of mitochondria with visible cristae,
dilation of hepatic sinuses, swelling of the endoplasmic reticulum,
presence of Kupffer cells and the nuclei of hepatocytes exhibited
pyknosis (Fig. 4B). In the
ADSCs-exo + I/R group, nuclei were slightly swollen, the
endoplasmic reticulum was slightly widened, while no noticeable
swelling was observed in mitochondria (Fig. 4C). Collectively, ADSCs-exo
treatment was found to attenuate the I/R injury effect on
subcellular components.
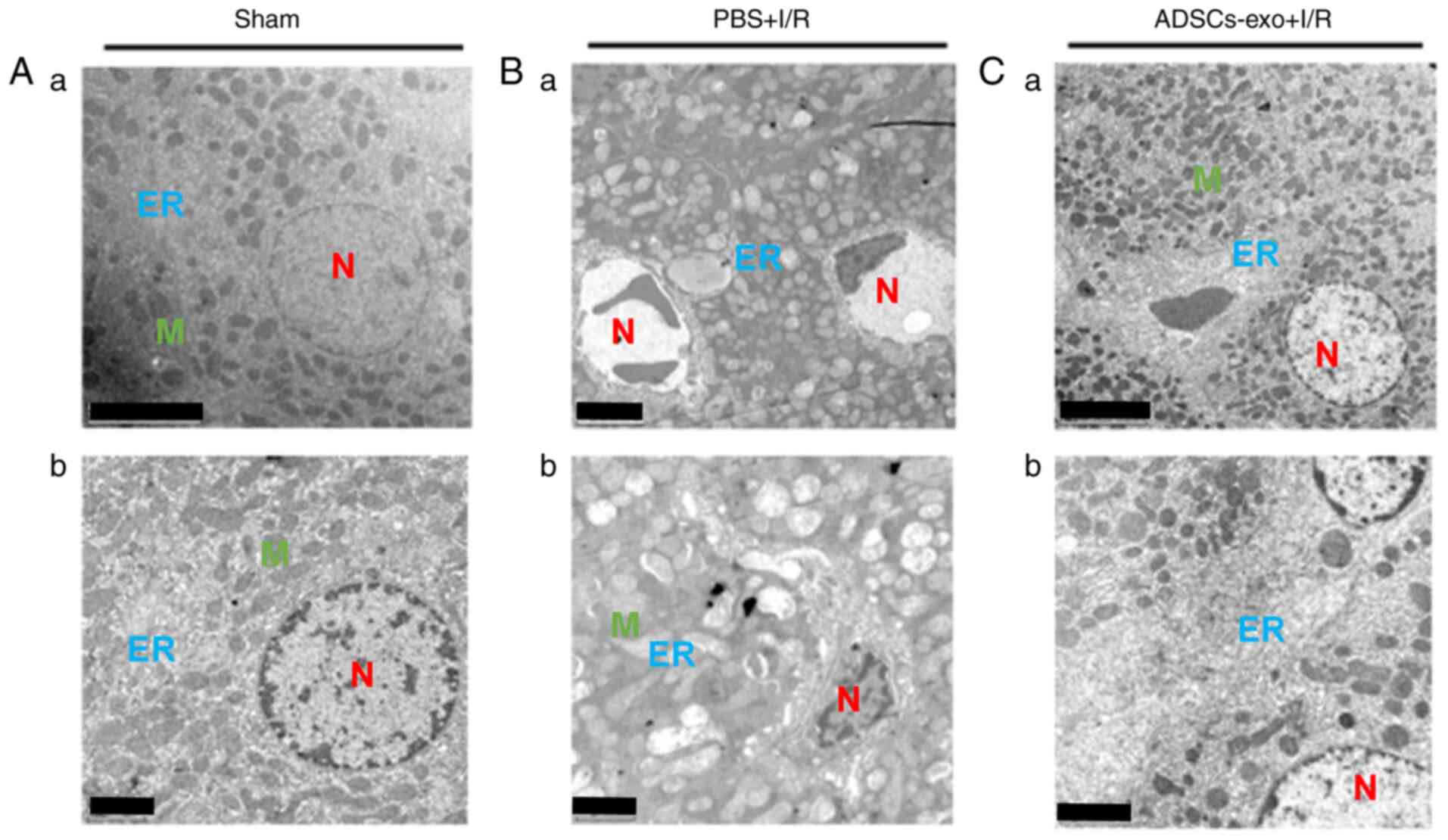 | Figure 4Ultrastructure morphological analysis
of hepatocytes isolated from (A-a) sham rats following 6 h of
reperfusion, (B-a) rats from the PBS + I/R group and (C-a) the
ADSCs-exo + I/R group. (A-b, B-b and C-b) are the magnified images
of A-a, B-a and C-a, respectively. Scale bar, 5 µm for A-a,
B-a and C-a; 2 µm for A-b, B-b and C-b. ER, endoplasmic
reticulum; N, nucleus; K, Kupffer cells; M, mitochondria; I/R,
ischemia-reperfusion; ADSCs-exo, exosomes from adipose-derived stem
cells. |
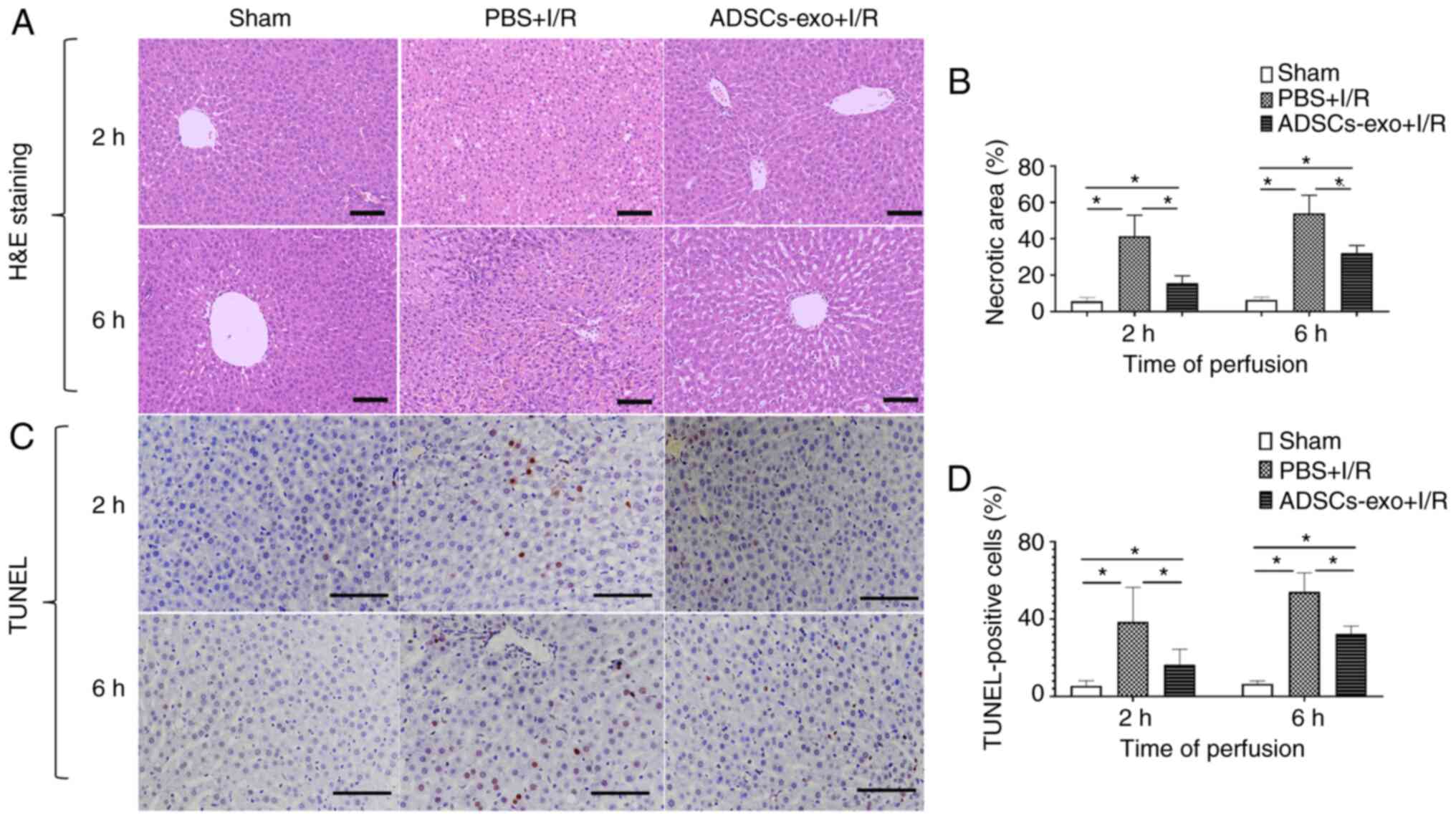
Influence of ADSCs-exo on necrosis and
apoptosis
The protective effect of ADSCs-exo against I/R
injury was further evaluated using necrosis and apoptosis.
H&E-stained images of liver samples of all groups following 2
and 6 h of reperfusion are presented in Fig. 5A. The sham group exhibited no
significant liver tissue necrosis (Fig. 5A and B). The PBS + I/R group
exhibited a significantly higher necrotic area which was evidence
of I/R injury (Fig. 5B). In
addition, with ADSCs-exo treatment, a significant reduction in the
necrotic area was observed which indicated the efficacy of
ADSCs-exo in protecting against I/R injury (Fig. 5A and B). Similarly, the PBS + I/R
group exhibited a higher number of apoptotic cells as determined by
TUNEL staining of liver sections following 2 and 6 h of reperfusion
(Fig. 5C and D). ADSCs-exo
pre-treatment produced a significant reduction in apoptotic cells
as revealed in Fig. 5C and D.
Collectively, it was demonstrated that ADSCs-exo pre-treatment
produced a significant reduction in liver tissue necrosis and
apoptosis caused by I/R injury.
Analysis of oxidative stress and lipid
peroxidation due to I/R injury
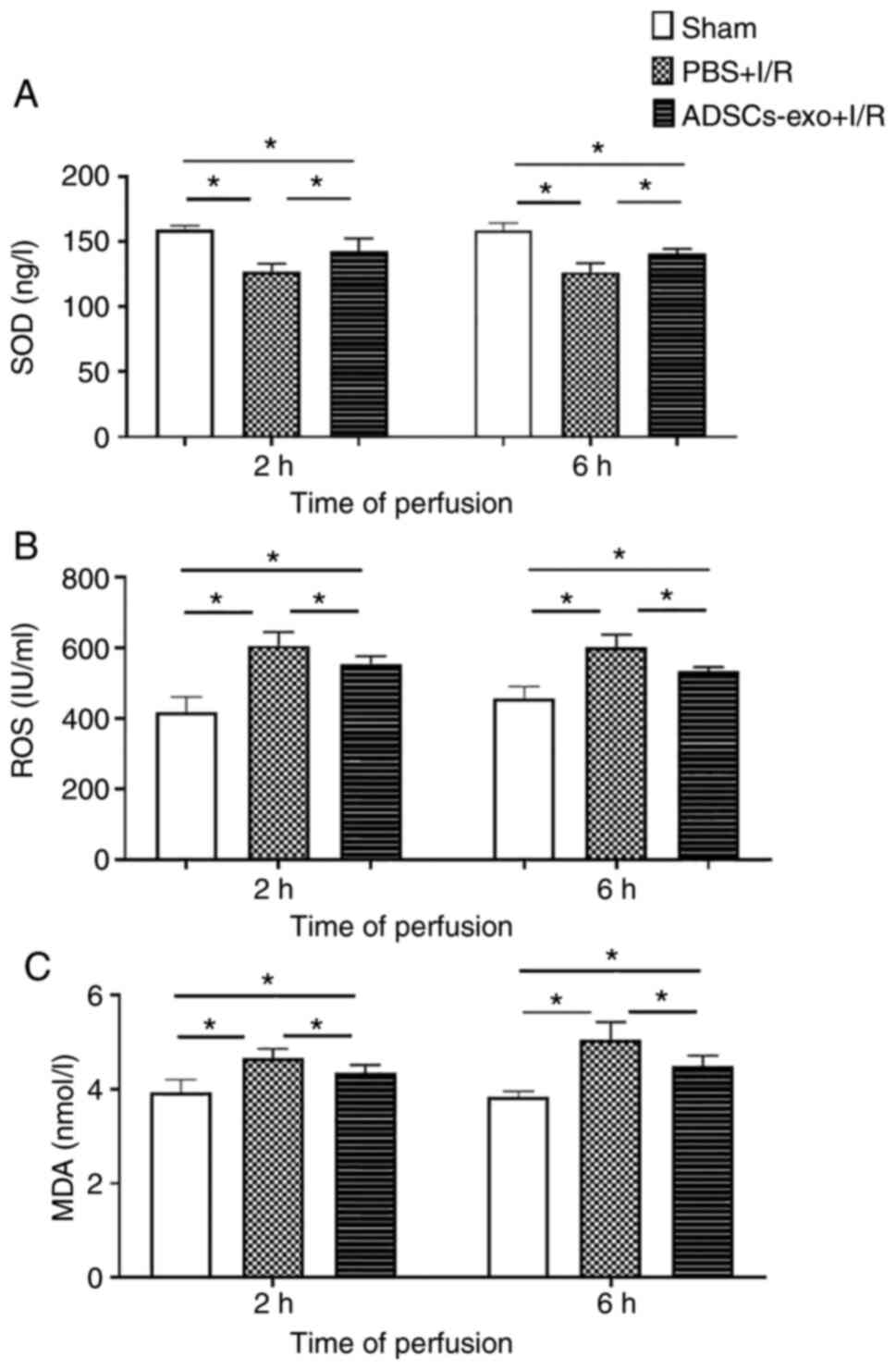
For oxidative stress evaluation, the concentration
of SOD and ROS activity in liver homogenates were quantified. A
lower concentration level of SOD was observed in the PBS + I/R
group following 2 and 6 h of reperfusion compared with the sham
group (Fig. 6A). This reduced
level of SOD also increased ROS in the PBS + I/R group (Fig. 6B). Regarding ADSCs-exo
pre-treatment, a significant increase in SOD was revealed to reduce
the oxidative stress by reducing ROS (Fig. 6A and B). Reduction in ROS may
protect hepatocytes from damage induced by I/R injury. Furthermore,
MDA was also quantified as a marker for lipid peroxidation. Higher
MDA was observed in the PBS + I/R group as compared with the
ADSCs-exo-treated rats indicating that ADSCs-exo could reduce lipid
peroxidation (Fig. 6C). In
summary, ADSCs-exo treatment caused a reduction in oxidative stress
and consequently a reduction in lipid peroxidation.
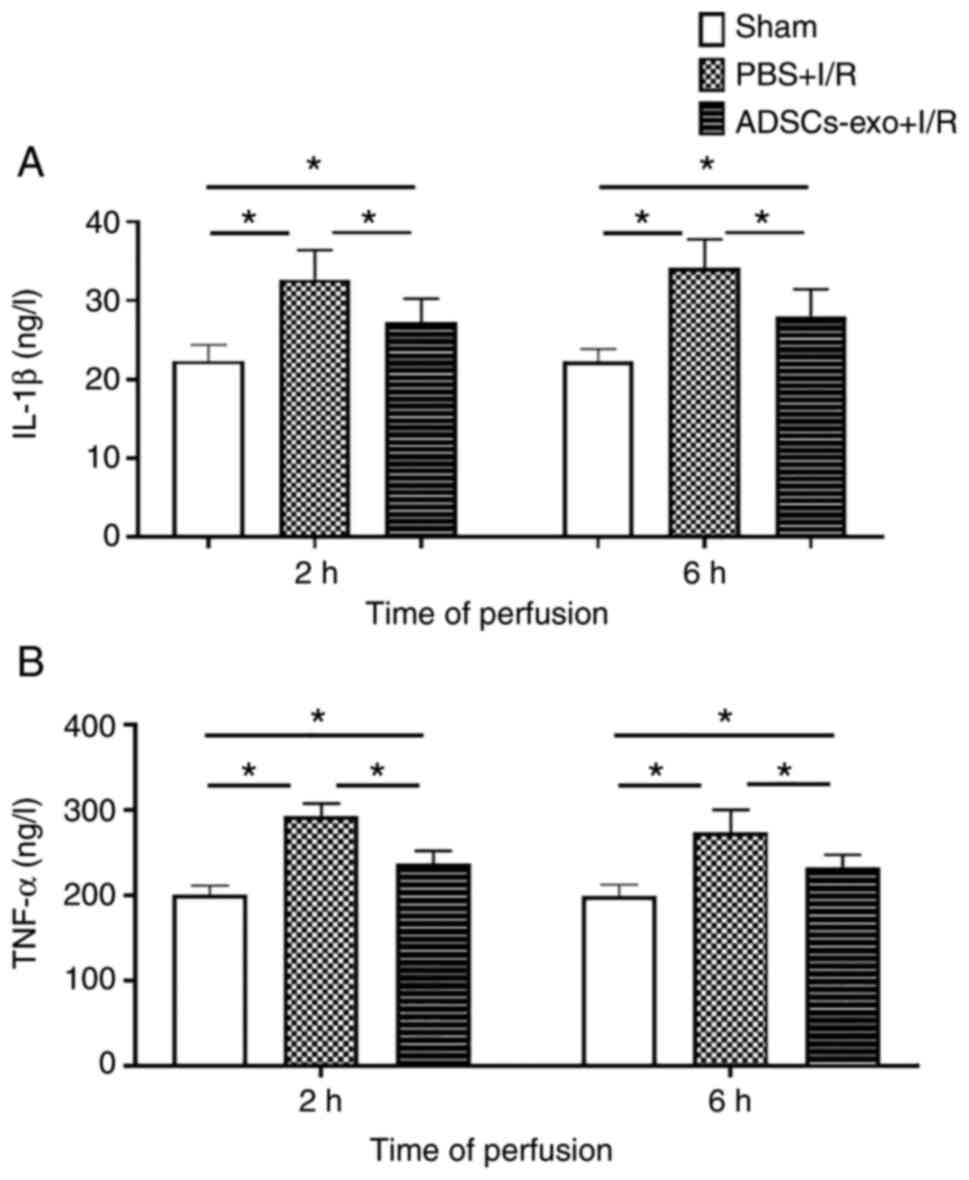
Inflammatory markers
IL-1β and TNF-α (inflammatory cytokines) in serum
are markers of neutrophil activation and reported to be increased
in I/R injury (26). The PBS +
I/R group exhibited a higher concentration of IL-1β and TNF-α than
the ADSC-exo-treated rats following 2 and 6 h of reperfusion as
revealed in Fig. 7. The sham
group showed lower values for inflammatory markers as compared with
both the PBS + I/R and ADSCs-exo + I/R groups (Fig. 7). ADSCs-exo treatment could
reduce inflammatory accumulation of inflammatory cytokines, thus,
protecting the liver damage from such mediators.
Analysis of expression of ERK and
GSK-3β
ERK1/2 and GSK-3β were evaluated using
immunohistochemical analysis as these pathways have been reported
to attenuate ischemic injury by reducing apoptosis and oxidative
stress (27,28). As shown in Fig. 8A, phosphorylation of ERK1/2 and
GSK-3β were revealed to be increased in the ADSCs-exo-treated rats.
Furthermore, p-ERK1/2 and p-GSK-3β upregulation was also confirmed
by western blot analysis following 2 h of reperfusion which
revealed a higher ratio of p-ERK/ERK and p-GSK-3β/GSK-3β in the
ADSCs-exo-treated rats compared with the PBS + I/R group (Fig. 8B and C). In the sham group lower
expression of p-GSK-3β was due to lack of I/R injury. ADSCs-exo
caused phosphorylation of ERK1/2 and GSK-3β in the liver
contributing to a protective effect against damage.
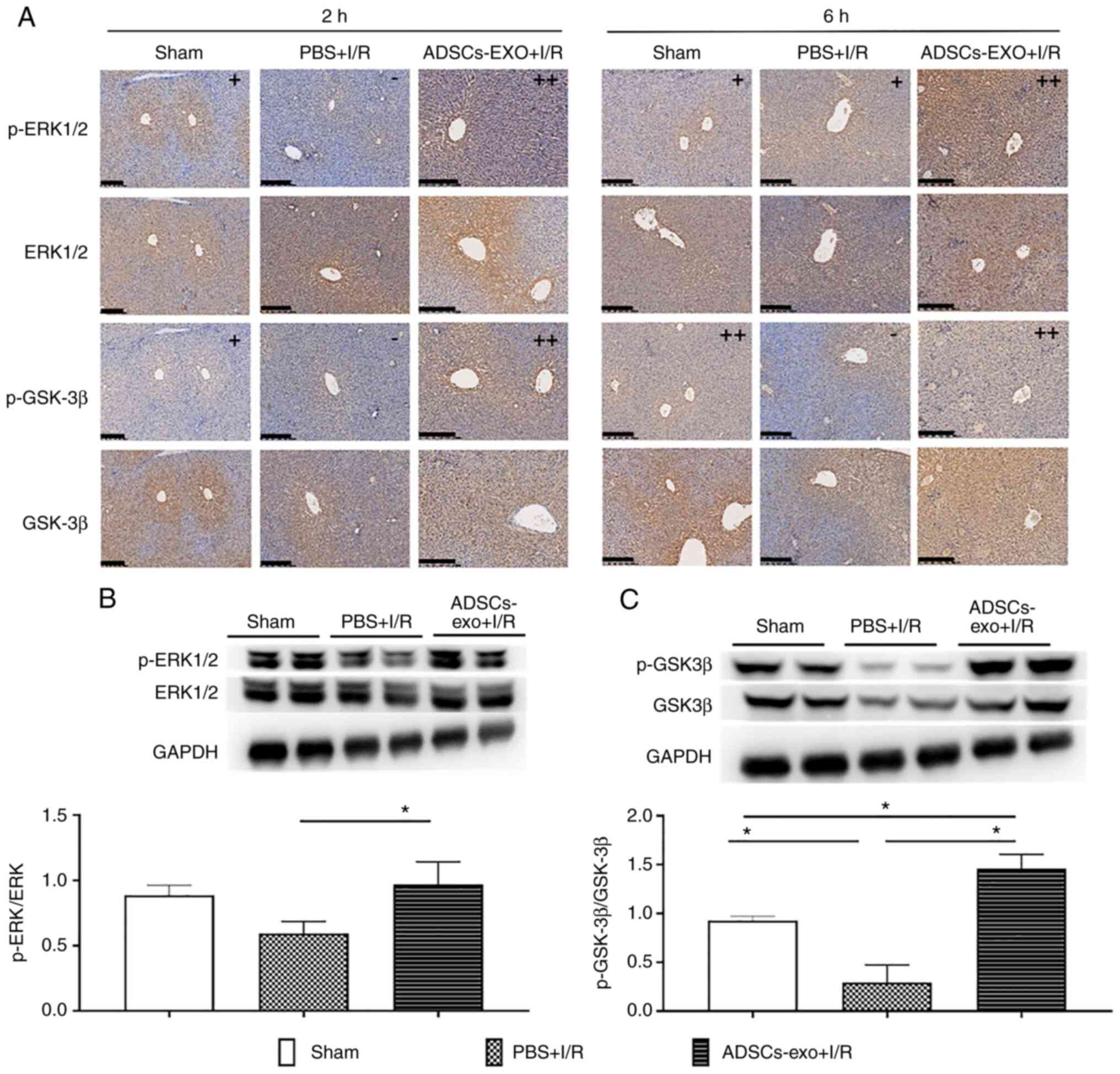 | Figure 8Expression of ERK1/2, p-ERK1/2,
GSK-3β and p-GSK-3β. (A) Immunohistochemical staining of ERK1/2,
p-ERK1/2, GSK-3β and p-GSK-3β after 2 and 6 h of reperfusion (scale
bar, 200 µm). Positive area <10% was graded as (-),
positive area between 10-30% was graded as (+) while positive area
>30% was graded as (++). Expression quantification of relative
(B) p-ERK1/2 and (C) p-GSK-3β using western blot analysis.
*P<0.05. ERK, extracellular receptor kinase; p-,
phosphorylated; GSK-3β, glycogen synthase kinase-3b; I/R,
ischemia-reperfusion; ADSCs-exo, exosomes from adipose-derived stem
cells. |
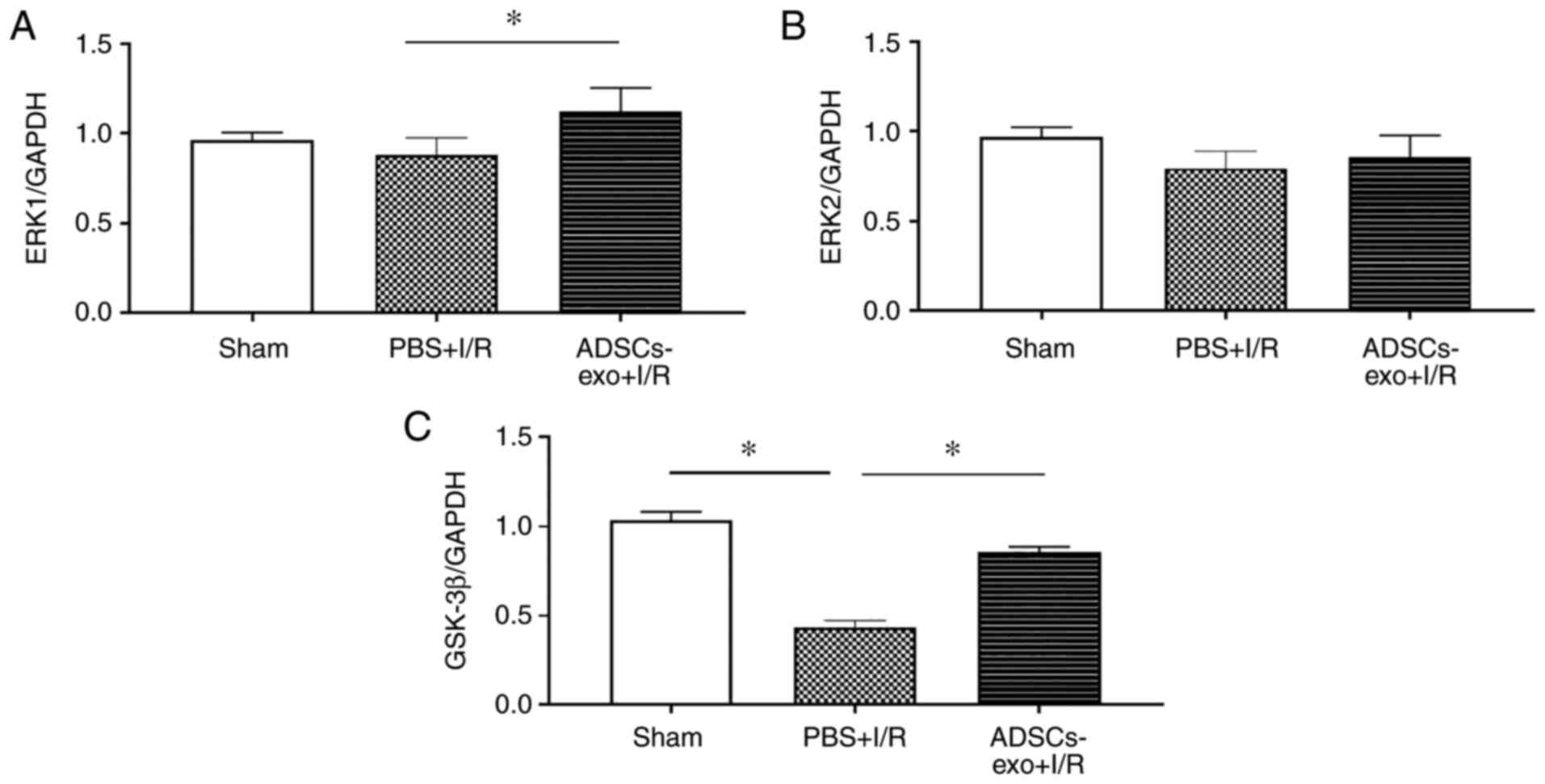
For further validation of results, RT-qPCR was also
performed which revealed significantly higher expression levels of
ERK1 and GSK-3β in the ADSCs-exo-treated rats compared with the PBS
+ I/R group. However, no significant increase was observed in the
expression of ERK2 (Fig. 9).
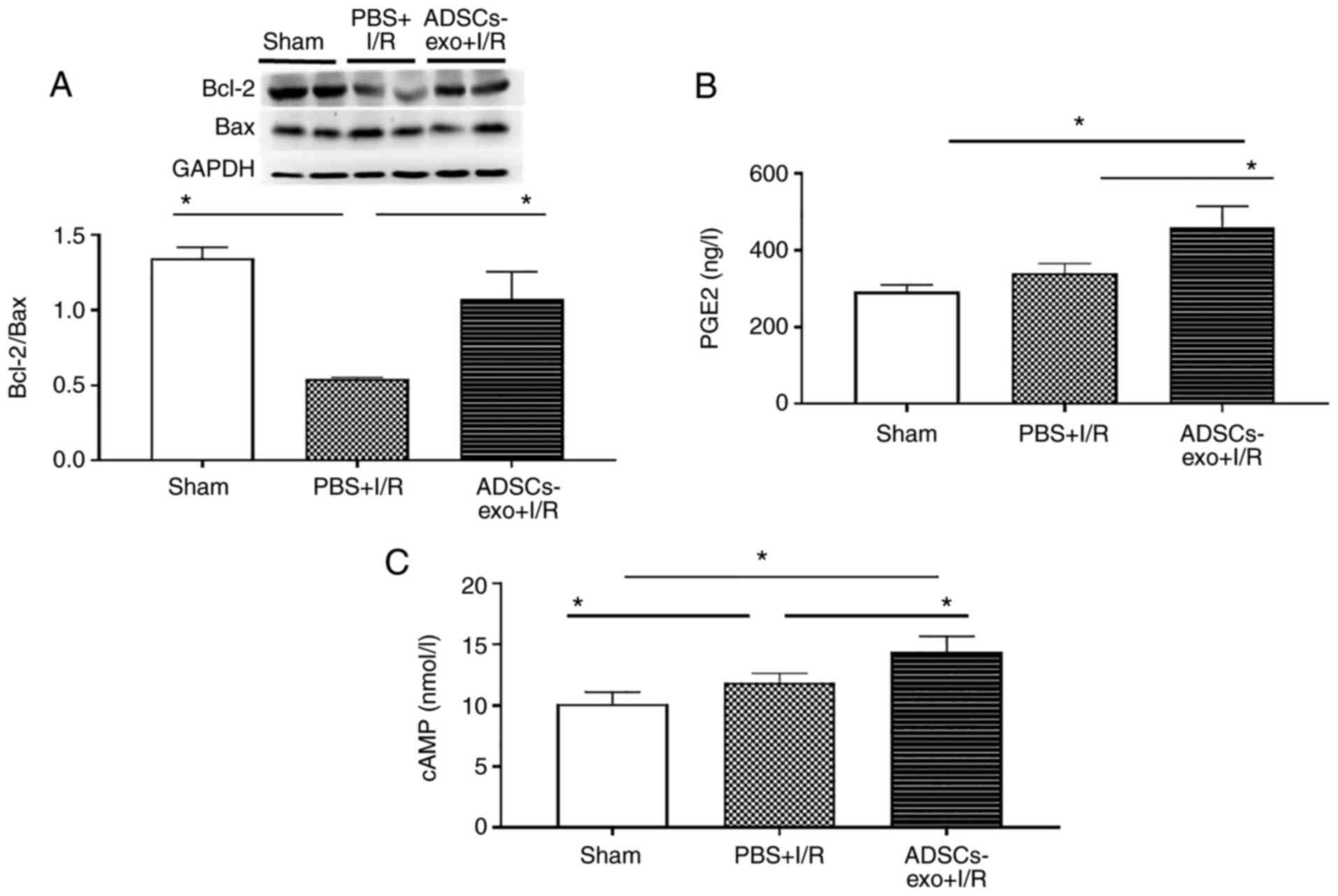
Bax and Bcl-2
Relative expression of Bax and Bcl-2 is important to
determine apoptotic behavior. As revealed in Fig. 10A, higher Bcl-2 expression was
observed in the ADSCs-exo-treated group compared with the PBS + I/R
group.
PGE2 and cAMP levels
PGE2 levels in the sham, PBS + I/R and ADSCs-exo +
I/R groups are presented in Fig.
10B, which revealed a significantly higher concentration of
PGE2 in the ADSCs-exo + I/R group after 6 h of reperfusion compared
to both the sham and PBS + I/R groups. Furthermore, a similar
behavior was observed in the case of cAMP levels (Fig. 10C).
Discussion
ADSCs may easily be obtained through minimally
invasive surgical procedures and have been explored in various
therapeutic applications (29).
However, several drawbacks exist concerning the application of stem
cells as a therapeutic tool (13,14). Recently, ADSCs-exo or components
of ADSCs-exo have exhibited protective potential against a number
of health issues including peripheral nerve injury (30), erectile dysfunction (31,32), ischemic stroke (33), wound healing (34), stress urinary incontinence
(35) and cardiomyocyte
apoptosis caused by ischemia (36). Based on these studies, it was
hypothesized that ADSCs-exo may potentially protect hepatic I/R
injury through reduction of apoptosis by countering oxidative
stress caused by hypoxia.
The largest parenchymal organ in mammals is the
liver which performs several important biological functions
including metabolism of biomolecules (lipids, proteins and
carbohydrates), secretion of bile to aid digestion and
detoxification of endo/exogenous toxins (37). Hepatic I/R injury is a common
cause of postoperative morbidity/mortality during hepatectomy,
transplantation or trauma (38).
Hypoxia and generation of ROS are considered major contributors to
hepatic damage by I/R injury (39). ROS may cause DNA damage, lipid
peroxidation and apoptosis of hepatocytes (40,41). Additionally, the release of
inflammatory mediators further exacerbates apoptosis and tissue
damage (42). In the present
study, pre-treatment with ADSCs-exo revealed a reduction of ROS, an
increase in SOD concentration and decreases in IL-1β and TNF-α
expression levels, thus reducing apoptosis/necrosis. Electron
microscopy revealed stable subcellular structures of hepatocytes
for ADSCs-exo-treated rats as compared with the PBS + I/R group.
Furthermore, high expression of p-ERK and p-GSK-3β was revealed
with ADSCs-exo treatment.
GSK-3β inactivation (phosphorylation) has been
reported to protect organs against I/R injury as a self-regulatory
mechanism (43,44). The inactivation of GSK-3β may
cause accumulation of β-catenin via the GSK-3β/β-catenin signaling
pathway, which has been revealed to increase anti-apoptotic protein
expression (Bcl-2 and survivin) in cells (28). GSK-3β was also revealed to be
involved in inducing mitochondrial permeability transition pore
(MPTP) that leads to mitochondria-mediated cell death (45,46). Phosphorylation (inactivation) of
GSK-3β inhibited the induction of MPTP via ANT interaction which is
also considered a protective effect of inactivation of GSK-3β
against I/R injury. In the present study, an increase in Bcl-2 was
revealed following 6 h of reperfusion due to overexpression of
p-GSK-3β.
In addition to GSK-3β inactivation, ADSCs-exo
treatment was also found to upregulate ERK1/2 which was also a
contributor to the protective effect of exosomes against I/R
injury. Activation (phosphorylation) of ERK was reported to occur
in I/R injury of different organs including the liver. This
upregulation of ERK1/2 with ADSCs-exo treatment was revealed to be
consistent with a recent study (47). ERKs are considered to induce an
anti-apoptotic effect via reduction of Bax protein and an increase
of Bcl-2 (48). Recently, Cai
et al reported a prostaglandin E receptor subtype
(EP4)-mediated hepatoprotective effect against I/R injury in rat
model via ERK1/2 activation (49). In another recent study, Gao et
al used a limb ischemic post-conditioning technique to
counteract hepatic I/R injury (50). They identified activation of
ERK1/2 as a key mechanism in the prevention of hepatic I/R injury.
In addition, ERK1/2 activation-mediated cell survival was also
reported by overexpression of Ets-I and Elk-I transcription
factors, which promoted cell growth and proliferation (51,52). In the present study, an increase
in the expression of ERK1/2 was revealed as an ADSCs-exo-mediated
protective mechanism. The downregulation of Bax mediated by ERK1/2
following 6 h of reperfusion in the ADSCs-exo-treated rats as
compared with the PBS + I/R group, produced fewer apoptotic
cells.
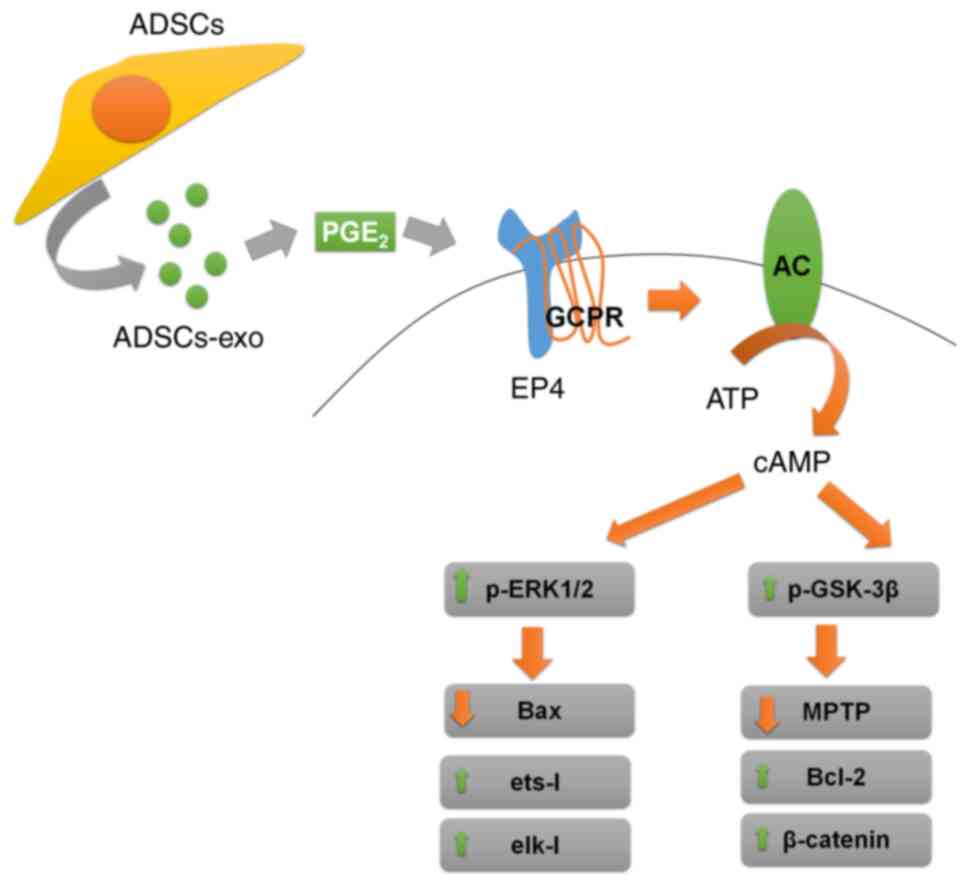
In our previous study, EP4 activation was revealed
to ameliorate hepatic I/R injury via ERK1/2 and GSK-3β-mediated
MPTP inhibition (49). Stem
cell-derived exosomes were reported to carry PGE2 which is a ligand
of EP4 (53,54). From this perspective, it is
proposed that activation of ERK1/2 and inactivation GSK-3β mediated
by ADSCs-exo was due to PGE2 via secondary messenger-cAMP. In the
present study, higher PGE2 and cAMP levels were also found in
ADSCs-exo-treated rats compared with the PBS + I/R group, which
validated the PGE2-mediated protective effect of ADSCs-exo. A
schematic diagram of the mechanism involved in the protective
effect of exosomes against hepatic I/R injury is presented in
Fig. 11. There may be other
components of exosomes such as heat shock protein-70 (HSP70) which
could also be responsible for the protective effect of exosomes
against hepatic I/R injury (55). In addition, another component of
exosomes that may play an important role in the protection from I/R
injury is miR-126 which was reported to be effective against acute
myocardial infarction (56).
However, the present study proposed PGE2 as an important factor in
I/R injury. Further study may be conducted to evaluate the
hepatoprotective effect of these other components. ADSCs-exo were
established as a potential therapeutic tool in the protection
against hepatic I/R; however, general limitations such as lack of
homogeneity, batch-to-batch variation and quality control for
isolated exosomes still need to be addressed.
In conclusion, the present study revealed the
potential of ADSCs-exo in ameliorating hepatic I/R injury in a rat
model. ERK1/2 activation and GSK-3β inactivation was observed via
an increase in G-protein receptor-mediated cAMP, and these
signaling pathways were found to reduce the effects of I/R injury.
The present study was based on single dose model via portal vein
administration. Further study for detailed exploration of the
protective mechanism of ADSCs-exo with different administration
routes and dosages should be considered in future research.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, YL and QW performed the experiments, analyzed
the data and drafted the manuscript. DZ, XF, WZ, LC and QZ helped
to conduct the experiments and analysis. HF and HX conceived and
designed the study and edited the final manuscript. YZ and QW
confirmed the authenticity of raw data. All authors read and
approved the final version of the manuscript.
Ethical approval and consent to
participate
All procedures in the present study were conducted
in accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health and was approved
(approval no. 00100097) by the Medical Ethics Committee of Naval
Medical University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81670564 and 81470847) and the
Youth Project of the National Natural Science Foundation of China
(grant no. 81800554).
References
|
1
|
Cannistrà M, Ruggiero M, Zullo A, Gallelli
G, Serafini S, Maria M, Naso A, Grande R, Serra R and Nardo B:
Hepatic ischemia reperfusion injury: A systematic review of
literature and the role of current drugs and biomarkers. Int J
Surg. 33(Suppl 1): S57–S70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rampes S and Ma D: Hepatic
ischemia-reperfusion injury in liver transplant setting: Mechanisms
and protective strategies. J Biomed Res. 33:221–234. 2019.
|
|
3
|
Ding W, Duan Y, Qu Z, Feng J, Zhang R, Li
X, Sun D, Zhang X and Lu Y: Acidic microenvironment aggravates the
severity of hepatic ischemia/reperfusion injury by modulating
M1-polarization through regulating PPAR-γ signal. Front Immunol.
12:6973622021. View Article : Google Scholar
|
|
4
|
Granger DN and Kvietys PR: Reperfusion
injury and reactive oxygen species: The evolution of a concept.
Redox Biol. 6:524–551. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Golen RF, Reiniers MJ, Marsman G,
Alles LK, van Rooyen DM, Petri B, Van der Mark VA, van Beek AA,
Meijer B, Maas MA, et al: The damage-associated molecular pattern
HMGB1 is released early after clinical hepatic
ischemia/reperfusion. Biochim Biophys Acta Mol Basis Dis.
1865:1192–1200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Go KL, Lee S, Zendejas I, Behrns KE and
Kim JS: Mitochondrial dysfunction and autophagy in hepatic
ischemia/reperfusion injury. Biomed Res Int. 2015:1834692015.
View Article : Google Scholar
|
|
7
|
Zhang H, Yan Q, Wang X, Chen X, Chen Y, Du
J and Chen L: The role of mitochondria in liver
ischemia-reperfusion injury: From aspects of mitochondrial
oxidative stress, mitochondrial fission, mitochondrial membrane
permeable transport pore formation, mitophagy, and
mitochondria-related protective measures. Oxid Med Cell Longev.
2021:66705792021.PubMed/NCBI
|
|
8
|
Chen Z, Ding T and Ma CG: Dexmedetomidine
(DEX) protects against hepatic ischemia/reperfusion (I/R) injury by
suppressing inflammation and oxidative stress in NLRC5 deficient
mice. Biochem Biophys Res Commun. 493:1143–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Wu L, Li J, Ji J, Chen K, Yu Q, Li
S, Feng J, Liu T, Zhang J, et al: Alleviation of hepatic ischemia
reperfusion injury by oleanolic acid pretreating via reducing HMGB1
release and inhibiting apoptosis and autophagy. Mediators Inflamm.
2019:32407132019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu Q, Wu L, Liu T, Li S, Feng J, Mao Y,
Fan X, Guo C and Wu J: Protective effects of
levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are
mediated by inhibition of the ERK/NF-κB pathway. Int
Immunopharmacol. 70:435–445. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Qiu X, Dai M, Zhang X and Jin G:
Hyperoside attenuates hepatic ischemia-reperfusion injury by
suppressing oxidative stress and inhibiting apoptosis in rats.
Transplant Proc. 51:2051–2059. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manne NDPK, Arvapalli R, Graffeo VA,
Bandarupalli VVK, Shokuhfar T, Patel S, Rice KM, Ginjupalli GK and
Blough ER: Prophylactic treatment with cerium oxide nanoparticles
attenuate hepatic ischemia reperfusion injury in sprague dawley
rats. Cell Physiol Biochem. 42:1837–1846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Han F, Gu L, Ji P, Yang X, Liu M,
Tao K and Hu D: Adipose mesenchymal stem cell exosomes promote
wound healing through accelerated keratinocyte migration and
proliferation by activating the AKT/HIF-1α axis. J Mol Histol.
51:375–383. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai Y, Han YD, Yan XL, Ren J, Zeng Q, Li
XD, Pei XT and Han Y: Adipose mesenchymal stem cell-derived
exosomes stimulated by hydrogen peroxide enhanced skin flap
recovery in ischemia-reperfusion injury. Biochem Biophys Res
Commun. 500:310–317. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saidi RF, Rajeshkumar B, Shariftabrizi A,
Bogdanov AA, Zheng S, Dresser K and Walter O: Human adipose-derived
mesenchymal stem cells attenuate liver ischemia-reperfusion injury
and promote liver regeneration. Surgery. 156:1225–1231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saat TC, van den Engel S, Bijman-Lachger
W, Korevaar SS, Hoogduijn MJ, IJzermans JN and de Bruin RW: Fate
and effect of intravenously infused mesenchymal stem cells in a
mouse model of hepatic ischemia reperfusion injury and resection.
Stem Cells Int. 2016:57614872016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
G Kugeratski F and Kalluri R: Exosomes as
mediators of immune regulation and immunotherapy in cancer. FEBS J.
288:10–35. 2021. View Article : Google Scholar
|
|
18
|
Zhang ZG, Buller B and Chopp M:
Exosomes-beyond stem cells for restorative therapy in stroke and
neurological injury. Nat Rev Neurol. 15:193–203. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Mi Y, Wu S, You X, Huang Y, Zhu J
and Zhu L: Exosomes from adipose-derived stem cells protect against
high glucose-induced erectile dysfunction by delivery of corin in a
streptozotocin-induced diabetic rat model. Regen Ther. 14:227–233.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sengupta V, Sengupta S, Lazo A, Woods P,
Nolan A and Bremer N: Exosomes derived from bone marrow mesenchymal
stem cells as treatment for severe COVID-19. Stem Cells Dev.
29:747–754. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ge Y, Zhang Q, Jiao Z, Li H, Bai G and
Wang H: Adipose-derived stem cells reduce liver oxidative stress
and autophagy induced by ischemia-reperfusion and hepatectomy
injury in swine. Life Sci. 214:62–69. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeller R: Fixation, embedding, and
sectioning of tissues, embryos, and single cells. Curr Protoc Mol
Biol. 7.14.1.1–14.1.8. 1989.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Yu LL, Zhu J, Liu JX, Jiang F, Ni WK, Qu
LS, Ni RZ, Lu CH and Xiao MB: A comparison of traditional and novel
methods for the separation of exosomes from human samples. Biomed
Res Int. 2018:36345632018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Konishi T and Lentsch AB: Hepatic
ischemia/reperfusion: Mechanisms of tissue injury, repair, and
regeneration. Gene Expr. 17:277–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi DE, Jeong JY, Choi H, Chang YK, Ahn
MS, Ham YR, Na KR and Lee KW: ERK phosphorylation plays an
important role in the protection afforded by hypothermia against
renal ischemia-reperfusion injury. Surgery. 161:444–452. 2017.
View Article : Google Scholar
|
|
28
|
Yan Y, Li G, Tian X, Ye Y, Gao Z, Yao J,
Zhang F and Wang S: Ischemic preconditioning increases
GSK-3β/β-catenin levels and ameliorates liver ischemia/reperfusion
injury in rats. Int J Mol Med. 35:1625–1632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang T, He D, Kleiner G and Kuluz J:
Neuron-like differentiation of adipose-derived stem cells from
infant piglets in vitro. J Spinal Cord Med. 30(Suppl 1): S35–S40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu CY, Yin G, Sun YD, Lin YF, Xie Z,
English AW, Li QF and Lin HD: Effect of exosomes from
adipose-derived stem cells on the apoptosis of Schwann cells in
peripheral nerve injury. CNS Neurosci Ther. 26:189–196. 2020.
View Article : Google Scholar
|
|
31
|
Zhu LL, Huang X, Yu W, Chen H, Chen Y and
Dai YT: Transplantation of adipose tissue-derived stem cell-derived
exosomes ameliorates erectile function in diabetic rats.
Andrologia. 50:e128712018. View Article : Google Scholar
|
|
32
|
Chen F, Zhang H, Wang Z, Ding W, Zeng Q,
Liu W, Huang C, He S and Wei A: Adipose-derived stem cell-derived
exosomes ameliorate erectile dysfunction in a rat model of type 2
diabetes. J Sex Med. 14:1084–1094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang M, Wang H, Jin M, Yang X, Ji H,
Jiang Y, Zhang H, Wu F, Wu G, Lai X, et al: Exosomes from
miR-30d-5p-ADSCs reverse acute ischemic stroke-induced,
autophagy-mediated brain injury by promoting M2
microglial/macrophage polarization. Cell Physiol Biochem.
47:864–878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Xie X, Lian W, Shi R, Han S, Zhang
H, Lu L and Li M: Exosomes from adipose-derived stem cells
overexpressing Nrf2 accelerate cutaneous wound healing by promoting
vascularization in a diabetic foot ulcer rat model. Exp Mol Med.
50:1–14. 2018.
|
|
35
|
Ni J, Li H, Zhou Y, Gu B, Xu Y, Fu Q, Peng
X, Cao N, Fu Q, Jin M, et al: Therapeutic potential of human
adipose-derived stem cell exosomes in stress urinary
incontinence-an in vitro and in vivo study. Cell Physiol Biochem.
48:1710–1722. 2018. View Article : Google Scholar
|
|
36
|
Liu L, Zhang H, Mao H, Li X and Hu Y:
Exosomal miR-320d derived from adipose tissue-derived MSCs inhibits
apoptosis in cardiomyocytes with atrial fibrillation (AF). Artif
Cells Nanomed Biotechnol. 47:3976–3984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dolganiuc A: Role of lipid rafts in liver
health and disease. World J Gastroenterol. 17:2520–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai L, Li Y, Zhang Q, Sun H, Yan X, Hua T,
Zhu Q, Xu H and Fu H: Salidroside protects rat liver against
ischemia/reperfusion injury by regulating the GSK-3β/Nrf2-dependent
antioxidant response and mitochondrial permeability transition. Eur
J Pharmacol. 806:32–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen R, Lai UH, Zhu L, Singh A, Ahmed M
and Forsyth NR: Reactive oxygen species formation in the brain at
different oxygen levels: The role of hypoxia inducible factors.
Front Cell Dev Biol. 6:1322018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive oxygen species-induced lipid
peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med
Cell Longev. 2019:50808432019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soares ROS, Losada DM, Jordani MC, Évora P
and Castro-E-Silva O: Ischemia/reperfusion injury revisited: An
overview of the latest pharmacological strategies. Int J Mol Sci.
20:50342019. View Article : Google Scholar :
|
|
43
|
Wang Y, Ge C, Chen J, Tang K and Liu J:
GSK-3β inhibition confers cardioprotection associated with the
restoration of mitochondrial function and suppression of
endoplasmic reticulum stress in sevoflurane preconditioned rats
following ischemia/reperfusion injury. Perfusion. 33:679–686. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xia Y, Rao J, Yao A, Zhang F, Li G, Wang X
and Lu L: Lithium exacerbates hepatic ischemia/reperfusion injury
by inhibiting GSK-3β/NF-κB-mediated protective signaling in mice.
Eur J Pharmacol. 697:117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu J, Rebecchi MJ, Glass PS, Brink PR and
Liu L: Cardioprotection of the aged rat heart by GSK-3beta
inhibitor is attenuated: Age-related changes in mitochondrial
permeability transition pore modulation. Am J Physiol Heart Circ
Physiol. 300:H922–H930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tanaka T, Saotome M, Katoh H, Satoh T,
Hasan P, Ohtani H, Satoh H, Hayashi H and Maekawa Y: Glycogen
synthase kinase-3β opens mitochondrial permeability transition pore
through mitochondrial hexokinase II dissociation. J Physiol Sci.
68:865–871. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G
and Li D: Exosomes from adipose-derived stem cells (ADSCs)
overexpressing miR-21 promote vascularization of endothelial cells.
Sci Rep. 9:128612019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang M, Lu X, Dong X, Hao F, Liu Z, Ni G
and Chen D: pERK1/2 silencing sensitizes pancreatic cancer BXPC-3
cell to gemcitabine-induced apoptosis via regulating Bax and Bcl-2
expression. World J Surg Oncol. 13:662015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cai LL, Xu HT, Wang QL, Zhang YQ, Chen W,
Zheng DY, Liu F, Yuan HB, Li YH and Fu HL: EP4 activation
ameliorates liver ischemia/reperfusion injury via
ERK1/2GSK3β-dependent MPTP inhibition. Int J Mol Med. 45:1825–1837.
2020.PubMed/NCBI
|
|
50
|
Gao Y, Zhou S, Wang F, Zhou Y, Sheng S, Qi
D, Huang JH, Wu E, Lv Y and Huo X: Hepatoprotective effects of limb
ischemic post-conditioning in hepatic ischemic rat model and liver
cancer patients via PI3K/ERK pathways. Int J Biol Sci.
14:2037–2050. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Q, Eppolito C, Odunsi K and Shrikant
PA: Antigen-induced Erk1/2 activation regulates Ets-1-mediated
sensitization of CD8+ T cells for IL-12 responses. J
Leukoc Biol. 87:257–263. 2010. View Article : Google Scholar
|
|
52
|
Godeny MD and Sayeski PP: ERK1/2 regulates
ANG II-dependent cell proliferation via cytoplasmic activation of
RSK2 and nuclear activation of elk1. Am J Physiol Cell Physiol.
291:C1308–C1317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noël D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7:162142017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu J, Kuwabara A, Kamio Y, Hu S, Park J,
Hashimoto T and Lee JW: Human mesenchymal stem cell-derived
microvesicles prevent the rupture of intracranial aneurysm in part
by suppression of mast cell activation via a PGE2-dependent
mechanism. Stem Cells. 34:2943–2955. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu HH, Huang CC, Chang CP, Lin MT, Niu KC
and Tian YF: Heat shock protein 70 (HSP70) reduces hepatic
inflammatory and oxidative damage in a rat model of liver
ischemia/reperfusion injury with hyperbaric oxygen preconditioning.
Med Sci Monit. 24:8096–8104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Luo Q, Guo D, Liu G, Chen G, Hang M and
Jin M: Exosomes from MiR-126-overexpressing adscs are therapeutic
in relieving acute myocardial ischaemic injury. Cell Physiol
Biochem. 44:2105–2116. 2017. View Article : Google Scholar : PubMed/NCBI
|