Introduction
Glioma is the most prevalent and malignant primary
intracranial tumor with high morbidity and mortality due to the
particularity of its location, seriously affecting the health of
global patients (1). The annual
incidence of glioma in the population is 6.13 per 100,000 in the
United States (2), with >70%
of patients dying of the disease within 2 years of diagnosis
worldwide (3). At present, with
the application of multiple therapies, including surgical
resection, radiation, chemotherapy, immunotherapy and photodynamic
therapy, the average overall survival of patients with high-grade
glioma is less than 15 months with a 5-year survival rate of 9.8%
and the current treatment status and surgical prognosis methods are
not optimistic (4). Hence, it is
of great necessity to explore the potential mechanisms underlying
glioma development in order to find novel and promising targets for
the treatment of this disease.
Microrchidia family CW-type zinc finger 2 (MORC2), a
member of the MORC family of proteins, is a recently identified
chromatin modifier with an emerging role in cancer metastasis
(5). As is a ubiquitously
expressed protein, abnormal elevated MORC2 expression is observed
in multiple cancer tissues, such as colorectal, liver and lung
cancer (6). A study has
demonstrated that MORC2 expression is notably enhanced in glioma
tissues compared with the adjacent tissues, and the higher the
level of glioma differentiation, the higher the expression of MORC2
is (6). Additionally, MORC2 was
found to promote the proliferation, invasion and migration of
colorectal cancer cells by binding with the promoter region of
N-myc downstream regulated gene 1 (NDRG1), a well-characterized
metastasis suppressor that has been demonstrated to have the
potential to be developed as a target for antimetastatic therapy
(7,8). In human cholangiocarcinoma, MORC2
is reported to accelerate the growth and metastasis of
cholangiocarcinoma cells via activation of PI3K/Akt signaling
(9). Overexpression of NDRG1
targets PTEN and suppresses PI3K signaling in human pancreatic
cancer (10). A number of
studies have validated that PTEN/PI3K/Akt signaling participates in
the occurrence and development of glioma (11,12). However, the effect and mechanism
of MORC2 on NDRG1 and PTEN/PI3K/Akt pathways in glioma are not
fully understood and need to be further explored.
The present study aimed to explore the expression of
MORC2 in several glioma cells and the effects of MORC2 in the
proliferation, invasion, migration and epithelialmesenchymal
transition (EMT) of glioma cells. To investigate the potential
mechanisms of MORC2 in the regulation of glioma development, the
present study focused on the NDRG1 and PTEN/PI3K/Akt signaling
pathways. The findings of the present study may provide novel
insight into understanding the pathogenesis of glioma.
Materials and methods
Cell lines and culture
Human glioma cell lines U251 (cat. no. TCHu 58),
SHG44 (cat. no. TCHu 48), as well as normal human astrocyte (NHA)
cells were provided by Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). LN229 (cat. no.
ATCC® CRL-2611) and T98G (cat. no. ATCC®
CRL-1690) were obtained from the American Type Culture Collection.
Cells were cultured and preserved in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific Inc.)
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 IU/ml penicillin and 100
µg/ml streptomycin) in a cell incubator at 37°C in the
presence of 5% CO2.
Cell transfection
A lentiviral expression vector (pLVX) containing two
short hairpin (sh)RNAs targeting MORC2 (shRNA-MORC2-1 and
sh-MORC2-2; 40 nM), shRNA negative control (shRNA-NC; 40 nM), the
MORC2 plasmid (pc-MORC2; 4 µg), NDRG1 plasmid (pc-NDRG1; 4
µg) and empty vector plasmid (pc-NC; 4 µg) were
designed and synthesized by Shanghai GeneChem Co. Ltd. U251 cells
were plated into 6-well plates (1×106 cells per well).
At 80% confluence, the transfection procedure was carried out using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h according to the manufacturer's
protocol. The transfection efficiency was tested using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) or
western blotting analysis after 48 h of transfection. The
untransfected U251 cells were used as the control group.
Cell viability assay
Cell viability was determined by means of a cell
counting kit-8 kit (CCK-8) purchased from Shanghai Yi Sheng
Biotechnology Co. Ltd. Briefly, U251 cells were collected and the
concentration was adjusted to 5×103 cells/well before
they were cultivated in a humidified cell incubator at 37°C with 5%
CO2. At 24, 48 and 72 h after transfection, 10 µl
CCK-8 solution was added to the medium and incubated for an
additional 3 h. Absorbance at 450 nm was determined with a
microplate reader (Bio-Rad Laboratories, Inc.).
Transwell invasion assay
U251 cell invasion was assessed with an 8-µm
pore insert precoated with Matrigel (BD Biosciences) at 37°C for 6
h. A total of 5×104 transfected U251 cells suspended in
100 µl of serum-free medium were placed into the upper
chamber. A total of 600 µl of DMEM medium containing 10% FBS
was added into the lower compartment as a chemoattractant.
Following 24 h incubation at 37°C, cells that invaded the lower
surface of the membrane were fixed with 4% paraformaldehyde for 30
min at room temperature and stained with 0.1% crystal violet for 30
min at room temperature. Images were photographed using an inverted
light microscope (Olympus Corporation; magnification, ×100). The
cell numbers were counted using Image J software version 1.52r
(National Institutes of Health).
Wound scratch healing assay
To evaluate cell migration, U251 cells were seeded
on 6-well plates at the density of 5×105 cells/well and
cultured until they reached 90% confluence. Serum-free medium was
utilized to incubate overnight at 37°C prior to initiating the
experiment. Following this, scratches were made using a 200
µl micro tip. After removing the cell debris by washing
three times with PBS, cells were continued to be cultured in
serum-free medium for 72 h at 37°C. Images of the wound areas were
captured by a light microscope (Olympus Corporation; magnification,
×100). The average distance of cells migrating into the wound areas
was analyzed with ImageJ software version 1.52r (National
Institutes of Health).
Immunofluorescence staining
U251 cells were grown on 4-well sterile glass slides
for 24 h in 6-well plates to reach 80-90% confluence at 37°C.
Following serum starvation for 12 h, cells were washed with PBS
three times, immobilized with 4% paraformaldehyde at room
temperature for 20 min, permeabilized with 0.1% Triton X-100 at
room temperature for 10 min and blocked with 3% bovine serum
albumin (BSA; Sigma-Aldrich; Merck KGaA) at 37°C for 90 min. Then,
cells were incubated with primary antibodies recognizing Ki67 (cat.
no. 11882S; 1:200; Cell Signaling Technology, Inc.), E-cadherin
(E-cad; cat. no. 3195T; 1:200; Cell Signaling Technology, Inc.) and
N-cadherin (N-cad; cat. no. sc-8424; 1:200; Santa Cruz
Biotechnology, Inc.) at 4°C overnight followed by probing with the
DyLight™ 488-conjugated secondary antibody (cat. no. ab96899;
1:250; Abcam) at room temperature for 2 h. DAPI (Roche Diagnostics)
was used to stain the nuclei in the dark at room temperature for 5
min. Images were taken under an Olympus fluorescent microscope
(Olympus Corporation; magnification, ×200).
Luciferase reporter assay
NDRG1 promoter-driven luciferase reporter plasmid
pGL3-basic (Promega, Inc.) was constructed by Shanghai GenePharma
Co., Ltd. The sequence of the NDRG1 promoter used were (-446 to
-213): 5′-GAT CGA TAG TGT CAA AGA CAG GCC TGA AAC ACA GAT GTC CTG
GGT CCT AGA GGT GCT GTT TGC CCC TCT CCA TAT TTC TTT TGT TCC AGA AAA
CCC TTC TCC AAA ACT GGC CCT AAT AAT CAG AGG GGA AAG CCA TGG CCC CTG
CCT TGGGGA CAG CAT GGG TTG GCA CAG AAA AGA GGT TTA CAA TTC AG CAG
GAA GTG TTG TGC GTG CGC GCG TGT GTG TCT GTG GAG GCG C-3′. The
indicated reporter and Renillaencoding plasmids were co-transfected
into U251 cells through applying Lipofectamine 3000®
reagent (Invitrogen; Thermo Fisher Scientific Inc.) following the
manufacturer's instructions. After 48 h, the luciferase activities
were quantified by the Dual-Luciferase Reporter Assay System
(Promega, Inc.). Renilla luciferase activity was used for
normalization.
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay kit (cat. no. P2078; Beyotime Institute
of Biotechnology) was used to corroborate the binding of MORC2 to
NDRG1 promoter according to the manufacturer's instructions. A
total of 1% formaldehyde was added into cultured U251 cells for 10
min at room temperature to produce cross-linked protein and DNA and
then chromatin fragments were obtained using sonication (10 sec per
time; 800 Hz) at intervals of 10 sec (15 cycles) at 4°C, followed
by centrifugation at 6,500 × g for 1 min at 4°C. The
immunoprecipitation of crosslinked 100 µg DNA/protein was
performed using 2 µg anti-MORC2 (cat. no. ab14429; Abcam) or
anti-IgG (cat. no. sc-69786; Santa Cruz Biotechnology, Inc.)
antibody. IgG was used as the blank control group to exclude the
influence of other factors on ChIP assay. Protein Agarose/Sepharose
(40 µl) was supplemented to precipitate the endogenous
DNA-protein complex. Subsequent to transient centrifugation at
6,500 × g for 1 min at 4°C, the supernatant was discarded and the
non-specific complexes were washed. The complex was de-crosslinked
at 65°C and the DNA fragment was recovered by phenol/chloroform
extraction and purification. The recuperated DNA fragments were
evaluated by RT-qPCR.
Construction of xenograft models
A total of 9 male BALB/c nude mice (5-6 weeks old,
weighing 20±2 g, ~18-22 g) were obtained from the Shanghai Slac
Animal Laboratory (Shanghai, China) and housed under pathogen-free
conditions with a 12-h light/dark cycle, constant temperature of
25-27°C and constant humidity of 45-50% with free access to food
and water. Animal experiments were approved by the Ethics Committee
of Beijing Friendship Hospital, Capital Medical University
(Beijing, China). Animals were randomly allocated into 3 groups,
with 3 mice in each group. To assess tumorigenicity, U251 cells
transfected with pc-NC, pc-NDRG1, pc-NDRG1 and pc-MORC2 for 48 h
were subcutaneously injected into the right flank of nude mice (a
total of 5×106 cells suspended in 200 µl PBS used
to inject 3 mice in each group). Then, the mice were maintained for
2 weeks before being sacrificed. Tumor volumes were recorded every
2 days. After 2 weeks, mice were sacrificed and tumor weight was
measured. Tumor volume was calculated based on the formula: Tumor
volume (mm3)=(width)x(height)2/2. All animals
were sacrificed with an intraperitoneal injection of 200 mg/kg
sodium pentobarbital (body weight). After death verification by
cessation of the heartbeat, the tumor tissues were obtained for the
further investigation.
Immunohistochemistry
Tumor tissues were fixed in 10% buffered formalin 24
h at room temperature and then embedded in paraffin.
Paraffin-embedded sections (4 µm thick) of tissues used for
immunohistochemistry were deparaffinized with 100% xylene, then
treated with graded descending series of alcohol (100, 95 and 80%)
for rehydration. Following antigen retrieval with a 10-mM citrate
buffer, slides were incubated with 0.3% hydrogen peroxide at room
temperature for 10 min to block endogenous peroxidase. Then, the
slides were incubated with the first rabbit anti-Ki67 antibody
(cat. no. A00254, 1:100; Wuhan Boster Biological Technology, Ltd.)
overnight at 4°C, with the second antibody against
HRP-conjugated-rabbit Ig (cat. no. ab181658; 1:1,000; Abcam) for 2
h at room temperature. Lastly, the tissue sections were treated
with 3,3′-diaminobenzidine solution. After counterstaining with
hematoxylin at room temperature for 5 min, an optical microscope
(Olympus Corporation; magnification, ×200) was adopted for the
evaluation of the degree of staining for each image. The images
were analyzed using ImageJ software (version 1.52r; National
Institutes of Health).
RT-qPCR
Total RNA from NHA, U251, SHG44, LN229 and T98G
cells was extracted with TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
To prepare cDNA, a RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) was used to perform the experiment
according to the manufacturer's protocol. The PCR reactions was
carried out using a PCR 7500 System and Power SYBR-Green PCR master
mix (both Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The following
thermocycling conditions were used: Initial denaturation at 95°C
for 7 min; and 40 cycles of 95°C for 15 sec and 60°C for 30 sec;
and a final extension at 72°C for 30 sec. Primers sequences used in
were as follows: MORC2 forward, 5′-GGA GGT TCC TTC TCC CAA AGT
C-3′, reverse 5′-CAG AAA CTG CGA CAC TCC GCT T-3′; NDRG1 forward,
5′-CTC CTG CAA GAG TTT GAT GTC C-3′, reverse, 5′-TCA TGC CGA TGT
CAT GGT AGG-3′; and GAPDH forward, 5′-AAT GAA GGG GTC ATT GAT
GG-3′, reverse, 5′-AAG GTG AAG GTC GGA GTC AA-3′. Comparative
quantification was determined with the 2−ΔΔCq method
(13) with GAPDH used as the
endogenous control.
Western blotting
Total proteins in U251 cell extracts and tumor
tissues from mice were prepared using radio immunoprecipitation
assay (RIPA) buffer containing a protease inhibitor cocktail tablet
(Sigma-Aldrich; Merck KGaA). A bicinchoninic acid (BCA) assay kit
(Beyotime Institute of Biotechnology) was used to evaluate the
concentrations of total proteins. The same amount of protein (50
µg/lane) was subjected to 10% SDS-PAGE electrophoresis.
After electrophoresis for 1.5 h, the proteins were transferred onto
a polyvinylidene difluoride membrane (MilliporeSigma) followed by
blocking with 5% non-fat milk for 1.5 h at room temperature.
Subsequently, these blots were incubated at 4°C overnight with the
following primary antibodies: Anti-MORC2 (cat. no. CAB17641,
1:1,000; Bethyl Laboratories, Inc.), anti-proliferating cell
nuclear antigen (PCNA; cat. no. 13110T; 1:1,000; Cell Signaling
Technology, Inc.), anti-cyclin-dependent kinase 2 (CDK2; cat. no.
18048T; 1:1,000; Cell Signaling Technology, Inc.), anti-GAPDH (cat.
no. 5174T; 1:1,000; Cell Signaling Technology, Inc.), anti-cyclin
E1 (cat. no. A00543-2; 1:1,000; Wuhan Boster Biological Technology,
Inc.), anti-E-cad (cat. no. PB9561; 1:1,000; Wuhan Boster
Biological Technology, Inc.), anti-N-cad (cat. no. BA0673; 1:1,000;
Wuhan Boster Biological Technology, Inc.), anti-Vimentin (cat. no.
PB9359; 1:1,000; Wuhan Boster Biological Technology, Inc.)
anti-Slug (cat. no. PB9439; 1:1,000; Wuhan Boster Biological
Technology, Inc.), anti-matrix metalloproteinase (MMP)2 (cat. no.
sc-13594; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-MMP9 (cat.
no. sc-393859; 1:1,000; Santa Cruz Biotechnology, Inc.), anti-NDRG1
(cat. no. sc-398291; 1:1,000; Santa Cruz Biotechnology, Inc.),
anti-PTEN (cat. no. sc-7974; 1:1,000; Santa Cruz Biotechnology,
Inc.), anti-phosphorylated (p)-PI3K (cat. no. ab278545; 1:1,000;
Abcam), anti-PI3K (cat. no. ab140307; 1:1,000; Abcam), anti-p-AKT
(cat. ab38449; 1:1,000; Abcam) and anti-AKT (cat. no. ab8805;
1:1,000; Abcam). After incubation with goat anti-rabbit
HRP-conjugated secondary antibody (cat. no. 7074S; 1:3,000; Cell
Signaling Technology, Inc.) or horse anti-mouse HRP-conjugated
secondary antibody (cat. no. 7076S; 1:3,000; Cell Signaling
Technology, Inc.) for 1.5 h at room temperature, the immunoreactive
protein bands were visualized using the enhanced chemiluminescence
kit (Amersham; Cytiva). The relative intensity of target bands were
quantified by Image J software version 1.52r (National Institutes
of Health) and GAPDH was used as the loading control.
Statistical analysis
Data are presented as the mean ± standard deviation
from at least 3 independent experiments. The statistical analysis
was performed by GraphPad Prism version 8.0 (GraphPad Software,
Inc.) software. Comparisons among multiple groups were analyzed
using one-way ANOVA followed by the post hoc Tukey's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
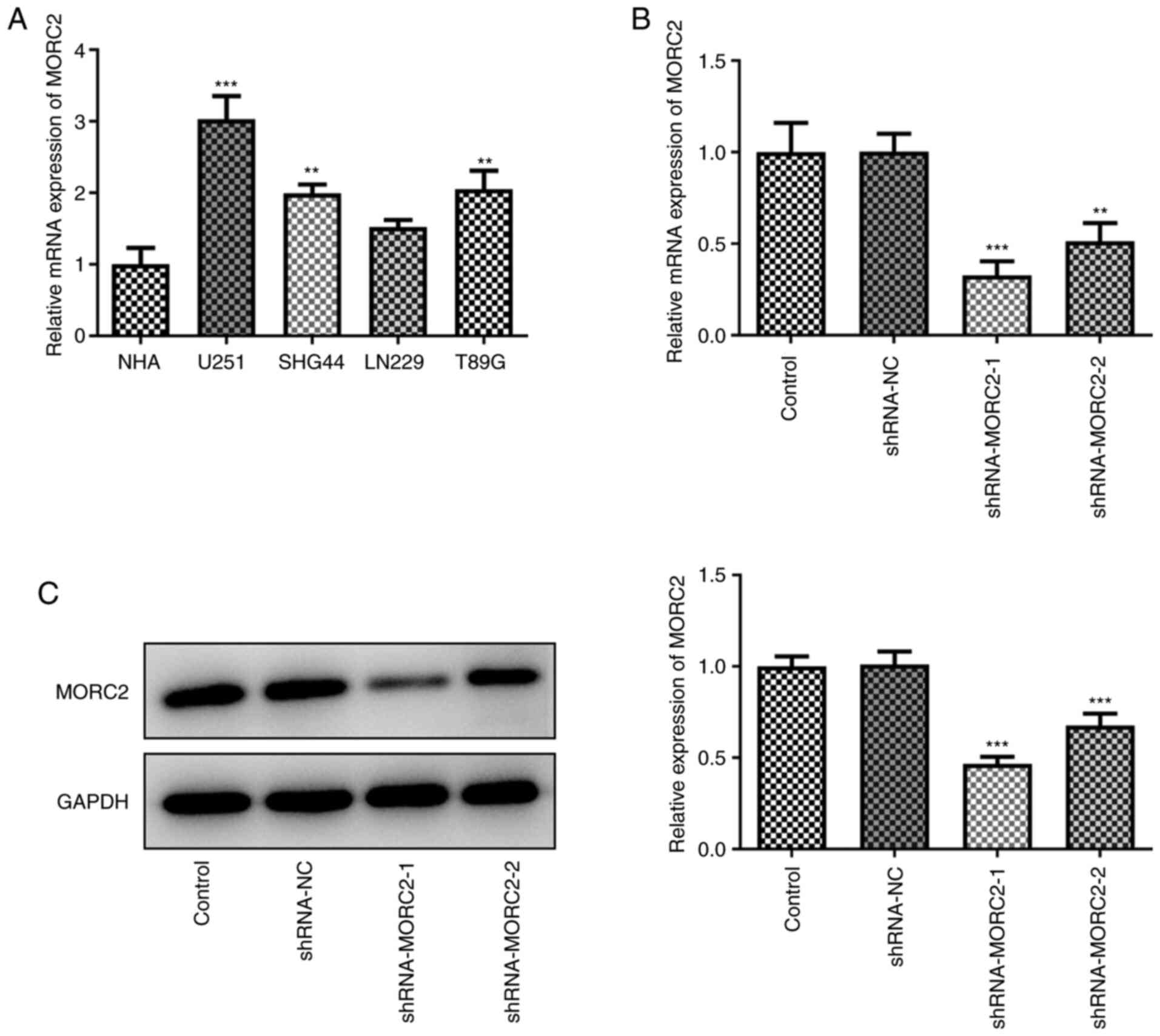
MORC2 is highly expressed in human glioma
cells compared with normal glial cells
Human glioma cell lines have been used widely in
studies of glioma pathogenesis (14-16). First of all, the expression of
MORC2 in several human glioma cell lines (U251, SHG44, LN229 and
T98G) was determined using RT-qPCR. MORC2 level was notably
elevated in glioma cells compared with the human astroglial cell
line NHA (Fig. 1A). The highest
MORC2 expression was observed in U251 cells (Fig. 1A), hence it was chosen for
further experiments in the present study. Subsequently, U251 cells
were transfected with shRNA-MORC2 to silence MORC2 expression.
shRNA-MORC2 transfection led to significant downregulation in the
expression levels of MORC2 when compared with the shRNA-NC group
(Fig. 1B and C). Cells
transfected with shRNA-MORC2-1 were used to conduct further
experiments due to the lower MORC2 expression compared with
shRNA-MORC2-2. These data implied the abnormal high MORC2
expression in human glioma cells.
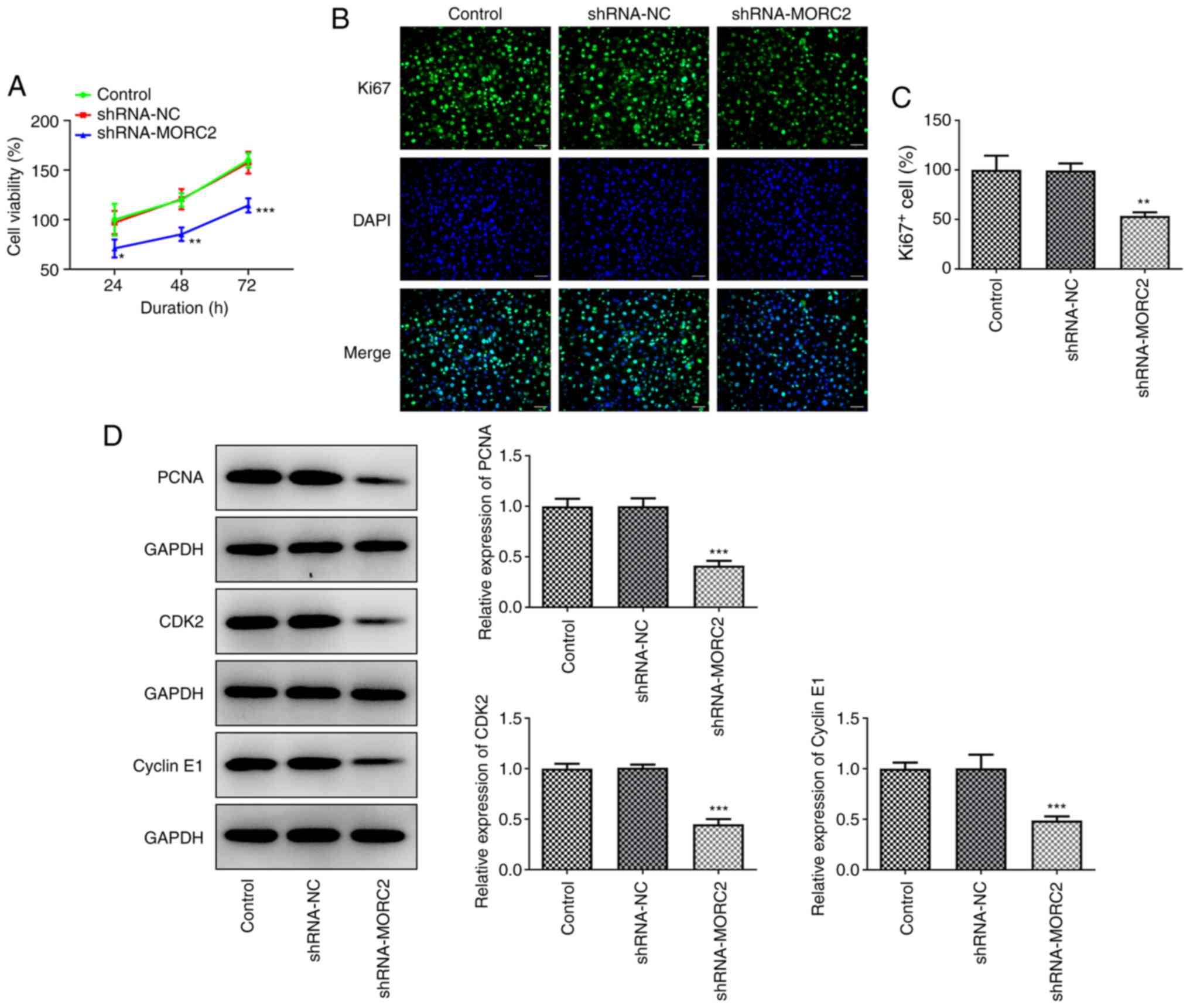
MRC2 silencing inhibits the
proliferation, invasion, migration and EMT process of glioma
cells
Subsequently, the effects of MORC2 silencing on the
progression of glioma was detected. MORC2-knockdown resulted in a
notable decrease in cell viability relative to the shRNA-NC group
(Fig. 2A). Meanwhile,
significantly reduced expression of proliferation-related proteins
including Ki67, PCNA, CDK2 and Cyclin E1 was found following MORC2
silencing (Fig. 2B-D) (17,18). MORC2 silencing remarkably
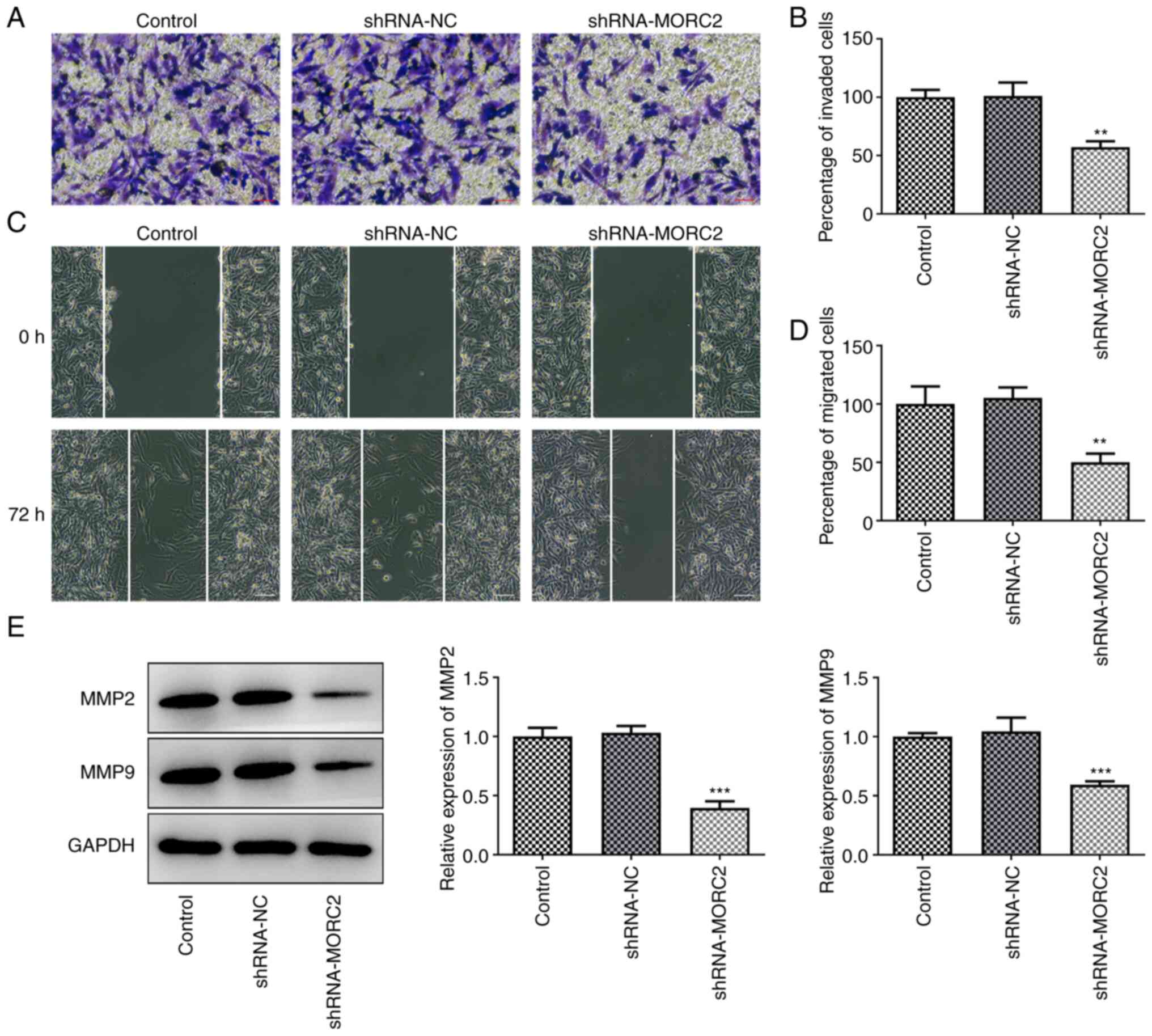
inhibited the abilities of U251 cell invasion and migration when
compared with the negative control group (Fig. 3A-D). The expression of MMP2 and
MMP9 in U251 cells was suppressed by MORC2-knockdown (Fig. 3E). EMT, a process of epithelial
phenotype transition to mesenchymal phenotype is closely implicated
in invasion and migration of tumor cells (19). Results of western blotting and
immunofluorescence staining shown in Fig. 4A-C suggested that MORC2 silencing
notably upregulated E-cad, which is a crucial epithelial marker,
but downregulated N-cad, Vimentin and Slug expression, which are
important mesenchymal marker genes, compared with the shRNA-NC
group (20,21). These results suggested the
inhibitory effects of MORC2-knockdown on the progression of
glioma.
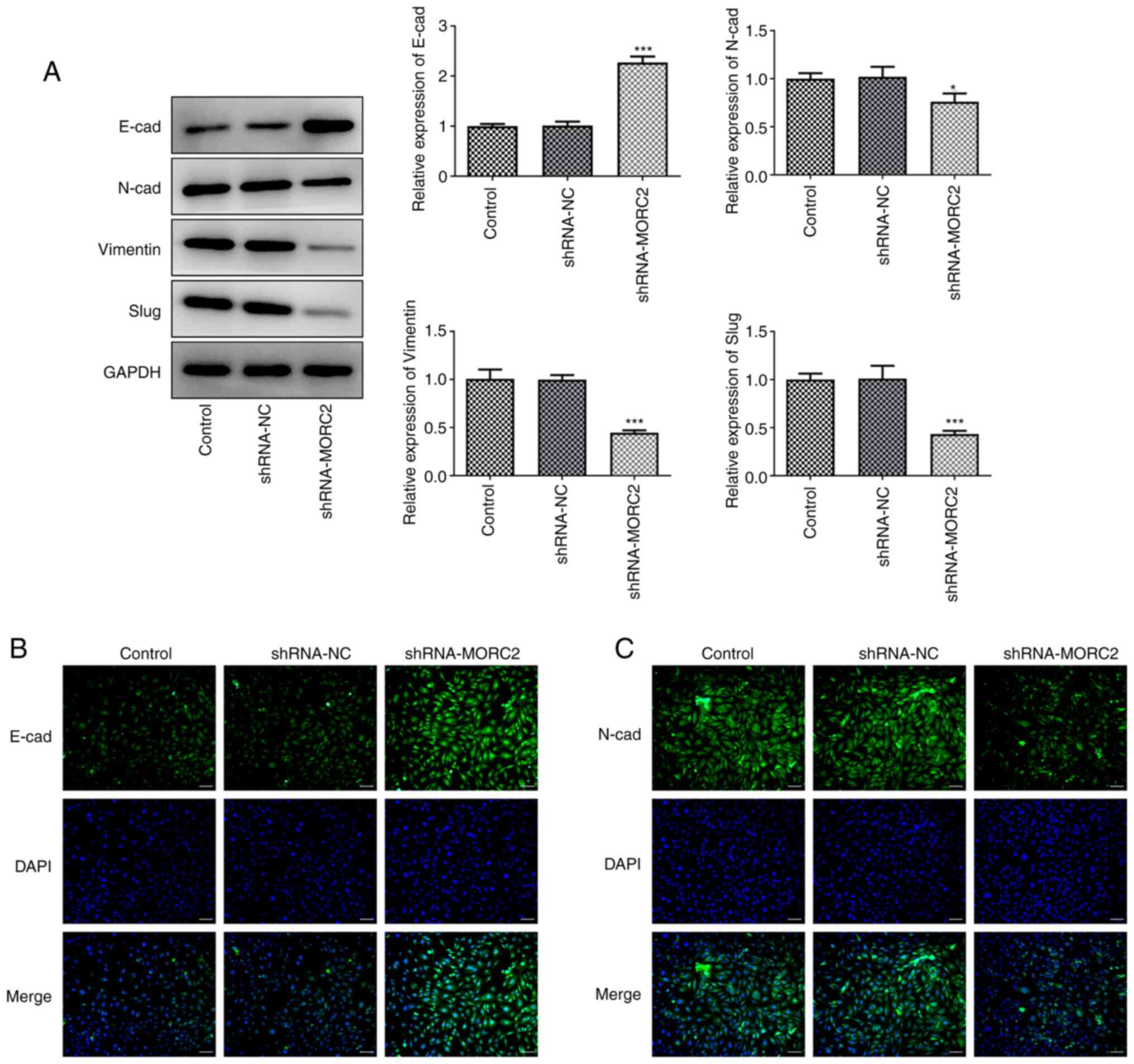 | Figure 4MORC2-knockdown suppresses the EMT
process of U251 cells. (A) E-cad, N-cad, Vimentin and Slug
expression was determined by western blotting. (B and C) E-cad and
N-cad expression was detected by immunofluorescence staining. Scale
bar, 50 µm. *P<0.05 and
***P<0.001 vs. shRNA-NC. Sh, short hairpin; MORC2,
Microrchidia family CW-type zinc finger 2; E-cad, E-cadherin;
N-cad, N-cadherin; NC, negative control; EMT,
epithelial-mesenchymal transition; control, untransfected U251
cells. |
MORC2 silencing upregulates NDRG1
expression and inactivates the PTEN/PI3K/AKT signaling pathway
To study the potential mechanisms of MORC2
downregulation in glioma progression, the expression of NDRG1 and
PTEN/PI3K/AKT signaling related proteins was measured by western
blotting. Notably, MORC2 silencing increased the expression levels
of NDRG1 when compared with shRNA-NC (Fig. 5). Additionally, PTEN expression
was significantly upregulated and p-PI3K/PI3K and p-AKT/AKT
expression was downregulated compared with the shRNA-NC group
(Fig. 5). These findings
revealed that MORC2 regulated NDRG1 and PTEN/PI3K/AKT signaling,
which may be related to the progression of glioma.
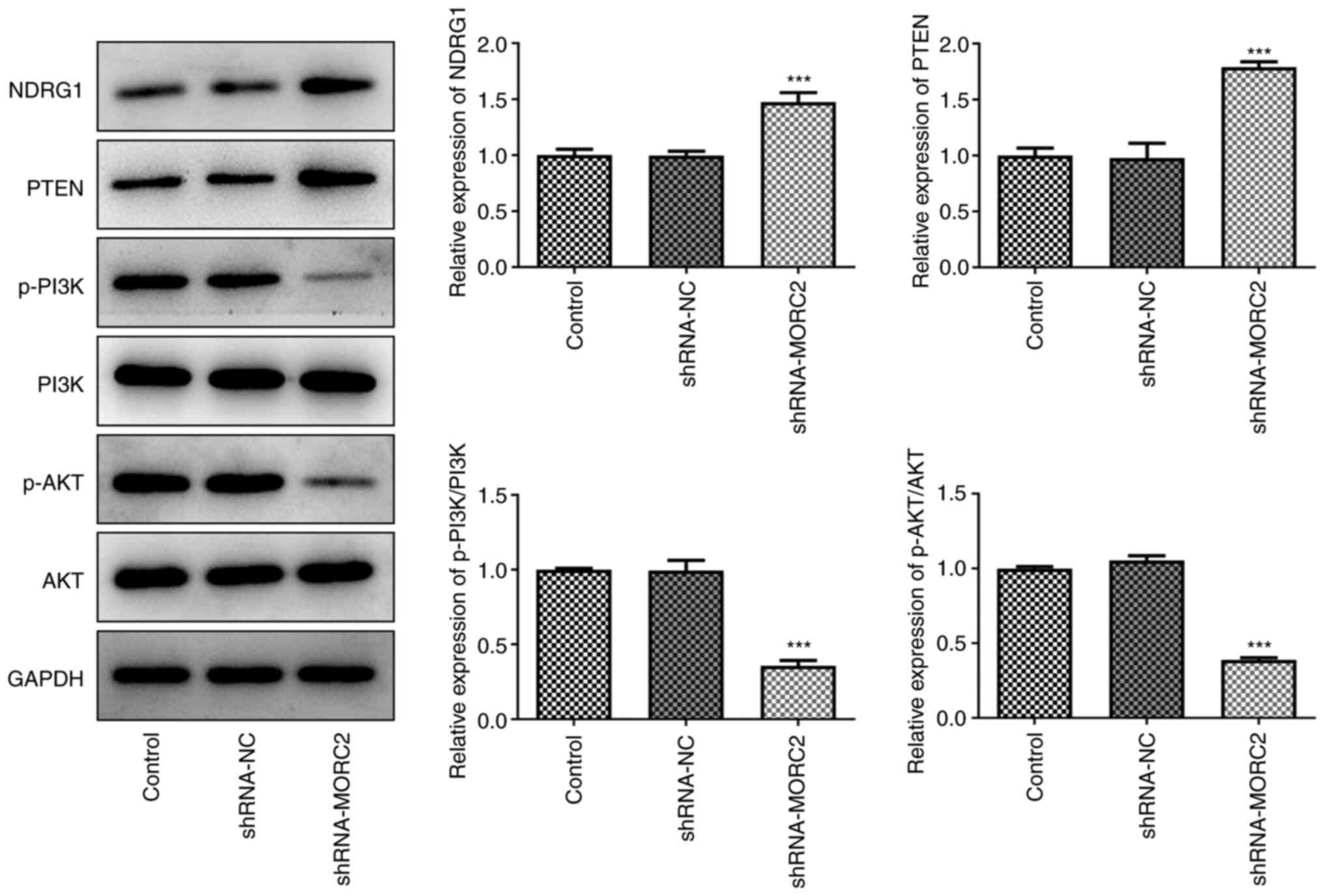 | Figure 5MORC2 silencing upregulates NDRG1
expression and inactivates the PI3K/Akt signaling pathway. NDRG1,
PTEN, p-PI3K, p-AKT, AKT, PI3K, expression was evaluated with
western blotting. ***P<0.001 vs. shRNA-NC. Sh, short
hairpin; MORC2, Microrchidia family CW-type zinc finger 2; NC,
negative control; NDRG1, N-myc downstream regulated gene 1; p,
phosphorylated; control, untransfected U251 cells. |
MORC2 binds to the NDRG1 promoter and
MORC2 overexpression restores the effects of NDRG1 upregulation on
the progression of glioma
Subsequently, to elucidate whether the increase in
NDRG1 protein is dependent on MORC2 as a regulator of
transcription, the binding of MORC2 to NDRG1 promotor was tested.
The NDRG1 promotor activity was significantly decreased in the
pc-MORC2 group compared with the pc-NC group in NDRG1 promotor site
(-466 to -213) (Fig. 6A).
Additionally, MORC2 could bind to the -446 to -213 bp region of
NDRG1 promoter (Fig. 6B). These
results demonstrated that MORC2 bound to the NDRG1 promoter and
suppressed the activity of NDRG1 promoter. Then, NDRG1 and MORC2
were overexpressed by transfection with plasmids. Significantly
upregulated NDRG1 and MORC2 expression was observed compared with
the pc-NC groups (Fig. 6C and
D). NDRG1 upregulation significantly inhibited U251 cell
viability compared with the pc-NC group, which was partly blocked
by co-transfection with MORC2 and NDRG1 plasmids (Fig. 6E). In concert, the inhibitory
effects of NDRG1 overexpression on the expression of Ki67, PCNA,
CDK2 and Cyclin E1 was reversed after the addition of MORC2 plasmid
(Fig. 6F-H). In addition, it was
found that gain-function of NDRG1 significantly decreased invasion
and migration of U251 cells when compared with the pc-NC group,
which was abrogated by MORC2 overexpression (Fig. 7A-D). In concert, the same
findings were observed for the expression of MMP2 and MMP9
(Fig. 7E). NDRG1 overexpression
led to significantly upregulated expression of E-cad and
downregulated expression of N-cad, Vimentin and Slug compared with
the pc-NC group (Fig. 8A-C).
However, MORC2 plasmid partially counteracted the regulatory impact
of NDRG1 upregulation on the expression of aforementioned
EMT-related proteins. Overall, these data suggested that MORC2
bound to the NDRG1 promoter and MORC2 overexpression restored the
effects of NDRG1 upregulation on the progression of glioma.
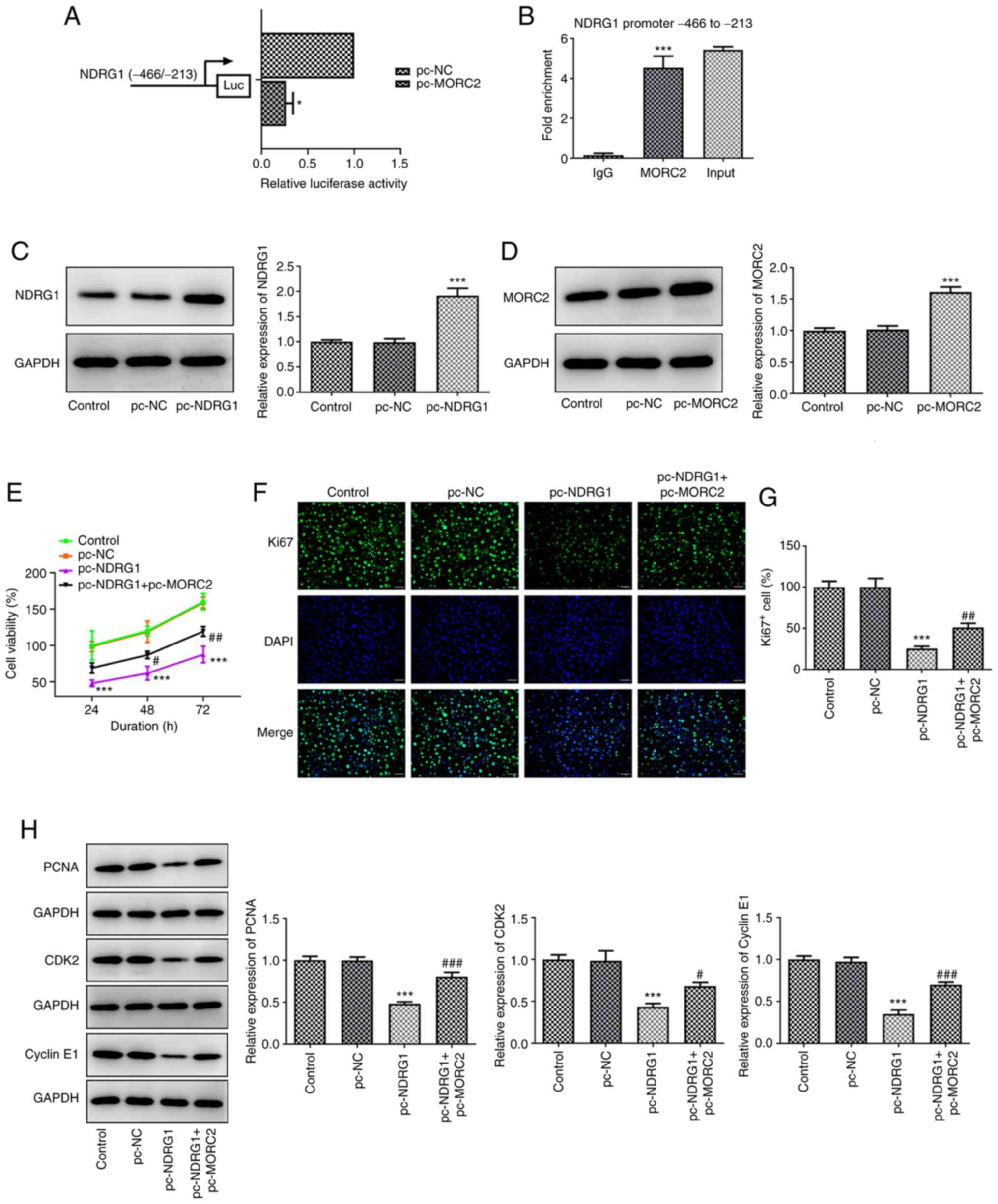 | Figure 6MORC2 binds to the NDRG1 promoter and
MORC2 overexpression attenuates the effects of NDRG1upregulation on
the proliferation of glioma. (A) Bond between MORC2 and promoter
region was examined using luciferase reporter assay.
*P<0.05 vs. pc-NC. (B) Direct binding of MORC2 to
NDRG1 promoter in U251 cells was confirmed by means of ChIP assay.
***P<0.001 vs. IgG. (C and D) NDRG1 and MORC2
expression after transfection was tested using western blotting.
***P<0.001 vs. pc-NC. (E) Cell viability was assessed
using a CCK-8 assay. (F and G) Ki67 expression was detected by
immunofluorescence staining. Scale bar, 50 µm. (H) PCNA,
CDK2 and Cyclin E1 expression was examined with western blotting.
The grouping of images from different parts of the same gel.
***P<0.001 vs. pc-NC; #P<0.05,
##P<0.01 and ###P<0.001 vs. pc-NDRG1.
MORC2, Microrchidia family CW-type zinc finger 2; NC, negative
control; NDRG1, N-myc downstream regulated gene 1; pc-NC, empty
vector; CDK2, cyclin-dependent kinase 2; pc-MORC2, overexpression
plasmid of MORC2; pc-NDRG1, overexpression plasmid of NRDG1; ChIP,
chromatin immunoprecipitation; PCNA, proliferating cell nuclear
antigen; control, untransfected U251 cells. |
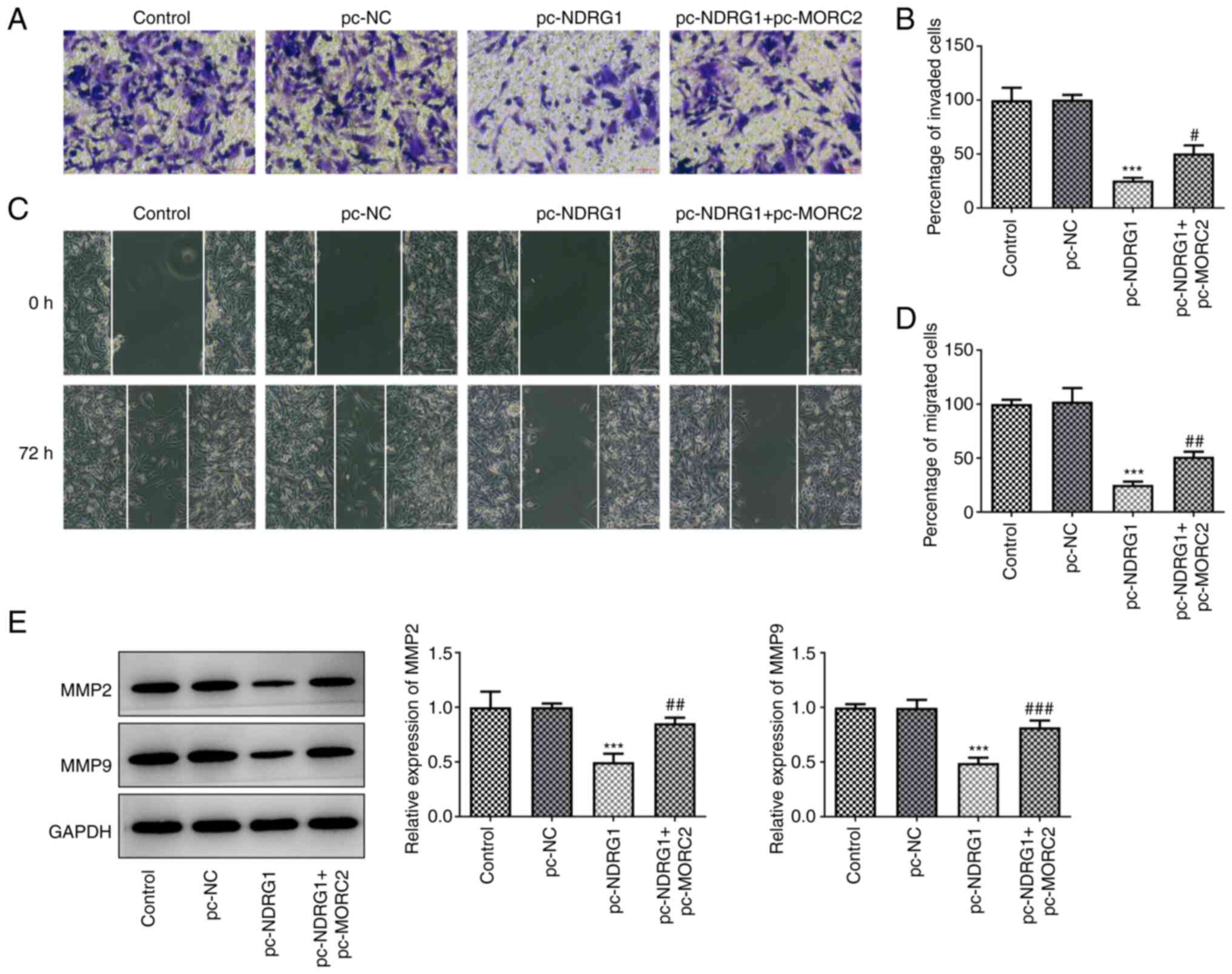 | Figure 7MORC2 overexpression blocks the
effects of NDRG1 upregulation on the invasion and migration of
glioma. (A and B) Cell invasion was examined with the Transwell
assay. Scale bar, 100 µm. (C and D) U251 cell migration was
tested by the wound scratch healing assay. Scale bar, 100
µm. (E) Western blotting was performed to evaluate the
expression of MMP2 and MMP9. ***P<0.001 vs. pc-NC;
#P<0.05, ##P<0.01 and
###P<0.001 vs. pc-NDRG1. MORC2, Microrchidia family
CW-type zinc finger 2; MMP, matrix metalloproteinase; NDRG1, N-myc
downstream regulated gene 1; pc-NC, empty vector; NC, negative
control; pc-MORC2, overexpression plasmid of MORC2; pc-NDRG1,
overexpression plasmid of NRDG1; control, untransfected U251
cells. |
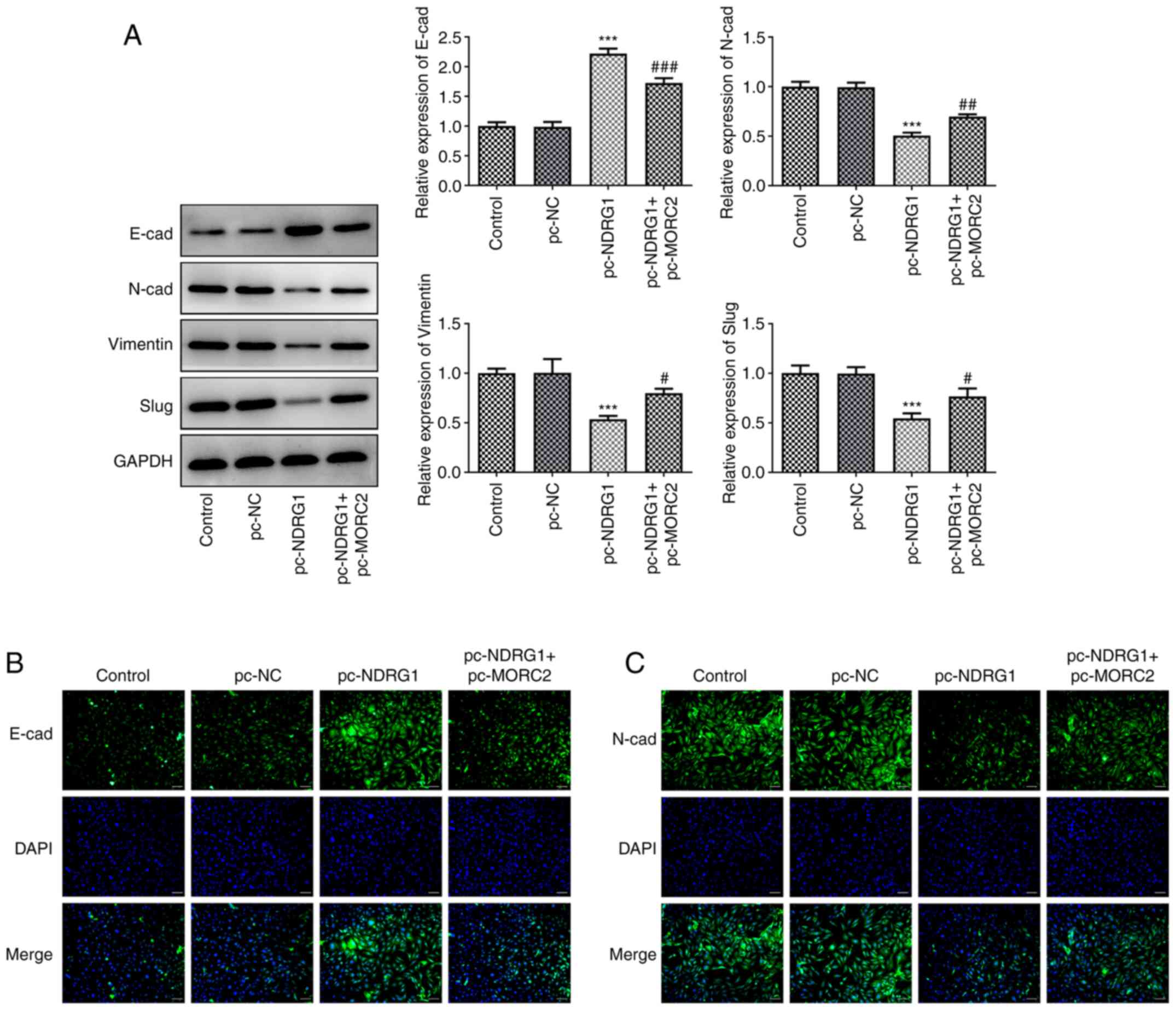 | Figure 8MORC2 overexpression mitigates the
effects of NDRG1 upregulation on the EMT process of glioma. (A)
E-cad, N-cad, Vimentin and Slug expression was determined by
western blotting. (B and C) E-cad and N-cad expression was detected
by immunofluorescence staining. Scale bar, 50 µm.
***P<0.001 vs. pc-NC; #P<0.05,
##P<0.01 and ###P<0.001 vs. pc-NDRG1.
MORC2, Microrchidia family CW-type zinc finger 2; E-cad,
E-cadherin; N-cad, N-cadherin; NC, negative control; EMT,
epithelial-mesenchymal transition; pc-NC, empty vector; pc-MORC2,
overexpression plasmid of MORC2; pc-NDRG1, overexpression plasmid
of NRDG1; CDK2, cyclin-dependent kinase 2; PCNA, proliferating cell
nuclear antigen; control, untransfected U251 cells. |
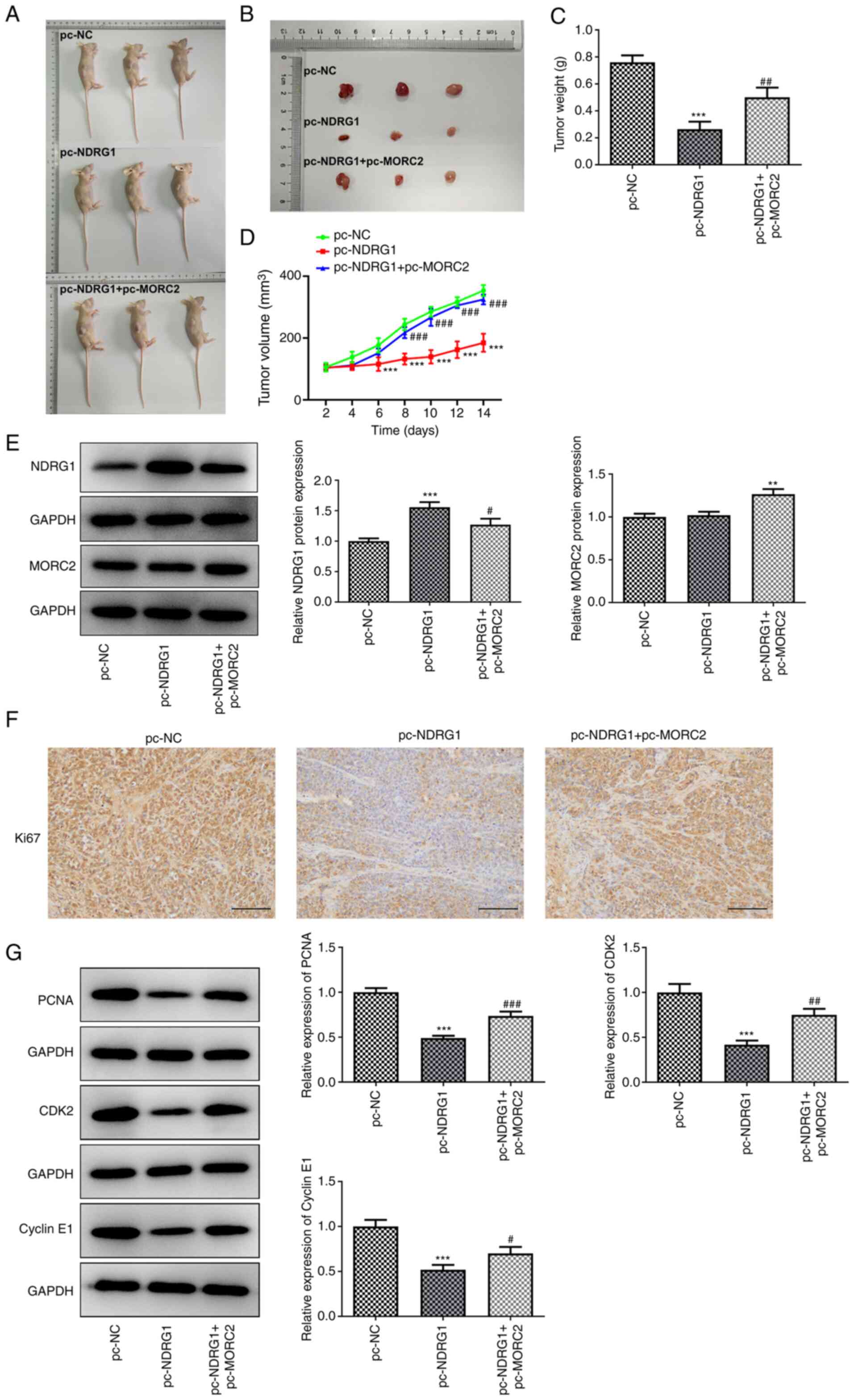
MORC2 overexpression reverses the
inhibitory effects of NDRG1 upregulation on the growth of glioma in
U251 tumor-bearing mice
Subsequently, the effects of MORC2 and NDRG1 on the
growth of glioma in vivo were investigated in U251
tumor-bearing mice (Fig. 9A and
B). The xenograft results demonstrated that NDRG1 upregulation
significantly inhibited tumor weight and volume compared with the
pc-NC group, whereas MORC2 upregulation alleviated this inhibitory
effects (Fig. 9C and D). The
expression of NDRG1 and MORC2 in tumor tissues was determined by
western blotting. NDRG1 expression was significantly upregulated in
the pc-NDRG1 group compared with the pc-NC group, which was
partially attenuated by MORC2 overexpression (Fig. 9E). There was no significant
difference between pc-NC and pc-NDRG1 groups, whereas
pc-NDRG1+pc-MORC2 markedly elevated MORC2 expression (Fig. 9E). Additionally, significantly
reduced expression levels of proliferation-related proteins
including Ki67, PCNA, CDK2 and Cyclin E1 were observed in tumorous
tissue in U251 tumor-bearing mice with NDRG1 overexpression, which
was restored by MORC2 upregulation (Fig. 9E-G). Gain-of-function of NDRG1
significantly downregulated MMP2, MMP9, N-cad, Vimentin and Slug
expression, but upregulated E-cad expression compared with the
pc-NC group, which was reversed by MORC2-upregulation (Fig. 10A-C). These data provided
evidence that overexpression of MORC2 alleviates the inhibitory
effects of NDRG1upregulation on the growth of glioma in U251
tumor-bearing mice.
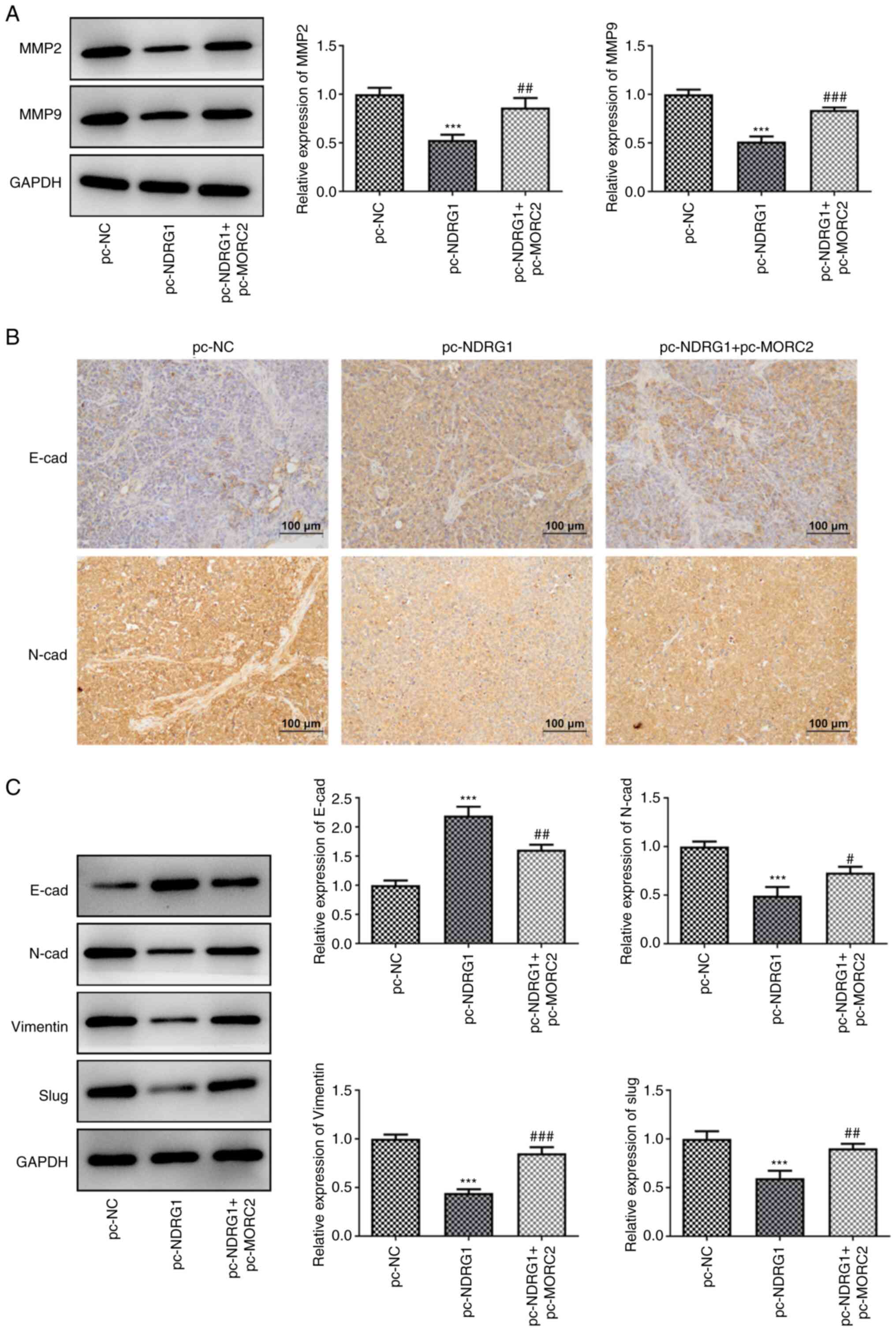 | Figure 10MORC2 overexpression alleviates the
inhibitory effects of NDRG1upregulation on the migration and EMT
process of glioma in U251 tumor-bearing mice. (A) MMP2 and MMP9
expression was tested by western blotting. (B) E-cad and N-cad
expression in tumorous tissue in U251 tumor-bearing mice was
evaluated using immunohistochemistry analysis. (C) Western blot
analysis was performed for the evaluation of E-cad, N-cad, Vimentin
and Slug expression. ***P<0.001 vs. pc-NC;
#P<0.05, ##P<0.01 and
###P<0.001 vs. pc-NDRG1. MORC2, Microrchidia family
CW-type zinc finger 2; E-cad, E-cadherin; N-cad, N-cadherin; NC,
negative control; EMT, epithelial-mesenchymal transition; pc-NC,
empty vector; pc-MORC2, overexpression plasmid of MORC2; pc-NDRG1,
overexpression plasmid of NRDG1; MMP, matrix metalloproteinase. |
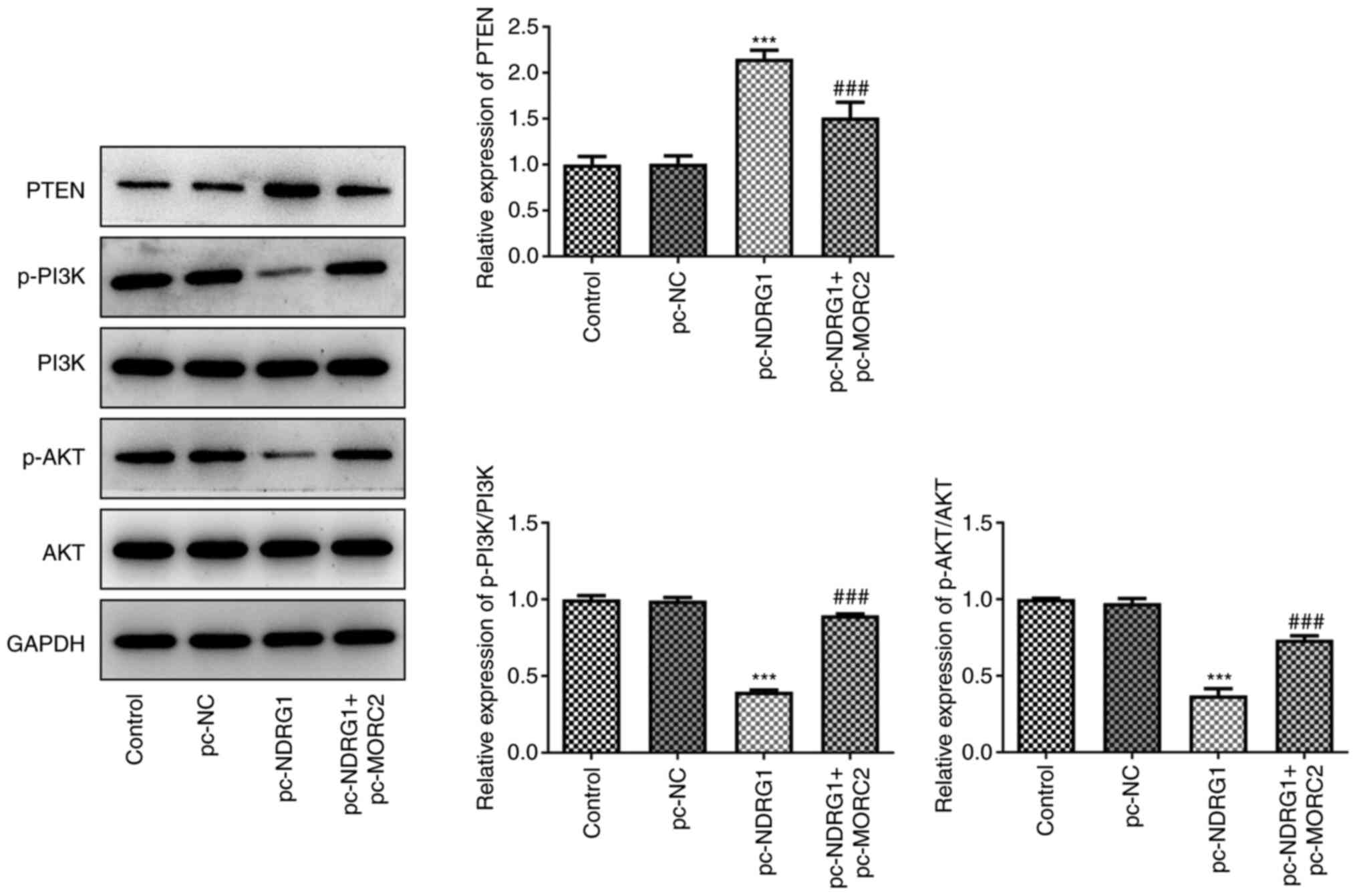
MORC2 regulates the PTEN/PI3K/AKT
signaling via binding to the NDRG1 promoter in glioma
Subsequently, the expression of proteins of
PTEN/PI3K/AKT signaling in tumorous tissue in U251 tumor-bearing
mice was evaluated by western blotting. NDRG1 overexpression
notably elevated PTEN expression and reduced p-PI3K/PI3K and
p-AKT/AKT expression compared with the pc-NC group (Fig. 11). In contrast, MORC2
upregulation reversed the impact of NDRG1 overexpression on the
expression of aforementioned proteins (Fig. 11). Collectively, these findings
suggested that MORC2 modulated PTEN/PI3K/AKT signaling via binding
to the NDRG1 promoter in glioma.
Discussion
MORC2, also known as ZCWCC1, ZCW3, KIAA0852 and
AC004542.C22.1, is an important member of the MORC family of
proteins (22,23). MORC2 serves important roles in
multiple biological processes (proliferation, migration and
invasion) and aberrant high expression level of MORC2 is a common
character in multiple cancers, such as non-small cell lung cancer
and breast cancer (6). A recent
study reported that MORC2 severs as a novel oncogene in liver
cancer and highly expressed MORC2 contributes to the proliferation
and metastasis of this disease (24). MORC2 upregulation drives lung
cancer growth by promoting angiogenesis (25). Liao et al (26) demonstrated that MORC2 can
accelerate the invasion and migration of breast cancer cells. A
study demonstrated that MORC2 can bind with histone deacetylase 4
and function as a transcriptional repressor by mediating the
deacetylation of histone H3 (27). It is noteworthy that MORC2
expression is notably enhanced in glioma tissues and the higher the
level of glioma differentiation, the higher the expression of MORC2
is (6). In the present study to
the best of our knowledge for the first time, the roles of MORC2 in
functional assays of glioma cells were assessed. The present study
demonstrated that MORC2 was notably upregulated in human glioma
cells compared with the human astroglial NHA cell line, and MORC2
knockdown significantly inhibited proliferation, invasion,
migration and the EMT process of glioma cells suggesting the potent
antitumor effects of MORC2 silencing in glioma.
Notably, MORC2 has been demonstrated to promote the
proliferation, invasion and migration of colorectal cancer cells by
binding with the promoter region of NDRG1 (7). NDRG1, a member of the N-myc
down-regulated gene family which belongs to the alpha/beta
hydrolase superfamily has been found to be involved in different
aspects of carcinogenesis and development of various cancers
(28). Studies have proposed
that NDRG1 is highly expressed in cervical cancer, bladder cancer
and hepatocellular carcinoma and this was found to be related to
tumor metastasis (29-31). Paradoxically, a considerable body
of evidence implicates the inhibitory functions of NDRG1 in the
progression of cancer. For example, NDRG1 deficiency is related to
regional metastasis in oral cancer via inducing EMT (32). NDRG1 was negatively associated
with poor prognosis by inhibiting vasculogenic mimicry and tumor
aggressiveness in gastric carcinoma (33). NDRG1 suppresses EMT-induced
metastasis in metastatic colorectal cancer (34). Ki67 is an antigen associated with
proliferating cells that is closely related to mitosis and is
indispensable during the cell proliferation of tumors (35). Cyclin E/CDK2 is a key complex
associated with the initiation of DNA replication during cell
proliferation (36). The process
of EMT is commonly characterized by downregulation of E-cad, a key
epithelial marker, accompanied by upregulation of N-cad, Vimentin
and Slug, which are crucial mesenchymal marker genes (19,37). Compelling evidence has indicated
that the 4 glioma cell lines (U251, SHG44, LN229 and T98G) used in
the present study display different mesenchymal and epithelial
characteristics and U251 cells expressed particularly low levels,
but SHG44 cells displayed the highest levels of epithelial markers
(38-41). Additionally, SHG44 cells
demonstrated the lowest levels, but LN229 cells exhibited the
highest levels of mesenchymal markers (39). NDRG1 mRNA and protein expression
is lower in glioma tissues compared with the adjacent tissues, and
reduced NDRG1 level is associated with tumor progression and
survival of patients (42). The
present study demonstrated that NDRG1 overexpression inhibited the
proliferation, invasion, migration and EMT of glioma cells and
tumor-bearing mice. Luciferase reporter assay and CHIP experiments
verified the binding of MORC2 to NDRG1 promotor in the present
study. Notably, gain-of-function of MORC2 restored the inhibitory
effects of NDRG1 upregulation on the progression of glioma in
vitro and in vivo, which further revealed that MORC2
regulates the progression of glioma by binding to the NDRG1
promoter.
Numerous studies have validated that the
PTEN/PI3K/AKT signaling pathway participates in the development of
multiple cancers (43-45). For glioma, inhibition of PTEN
expression promotes glioma cell proliferation via regulating
PI3K/AKT pathway (46,47). Upregulation of PTEN in glioma
cells prevents migration by downregulation of PI3K/AKT signaling
(48). A study has demonstrated
that MORC2 accelerates the growth and metastasis of
cholangiocarcinoma cells via activation of PI3K/AKT signaling
(9). It has been reported that
NDRG1 can interact with several key oncogenic pathways involved in
oncogenesis, such as NF-κB, PI3K/AKT/mTOR and Ras/Raf/MEK/ERK
pathways (49). In human
pancreatic cancer, overexpression of NDRG1 targeted PTEN and
suppressed PI3K signaling (10).
In the present study, MORC2 knockdown and NDRG1 upregulation
markedly elevated PTEN expression and reduced the phosphorylation
levels of PI3K and AKT in glioma cells. In contrast, in the present
study, MORC2 upregulation reversed the impact of NDRG1
overexpression on the expression of aforementioned PTEN/PI3K/AKT
signaling proteins, suggesting that MORC2 could regulate the
PTEN/PI3K/AKT signaling via binding to NDRG1 promoter in glioma.
However, whether the results of the present study are applicable to
patient derived glioma cells, clinical samples and clinical
application on predicting the prognosis of patients with glioma
should be explored by further studies. Additionally, the lack of
results about the difference between cells with high expression of
mesenchymal markers and those with high expression of epithelial
markers or not is also a limitation of the present study. A
comprehensive analysis of this is required in future studies.
Taken together, the findings of the present study to
the best of our knowledge provide evidence for the first time that
MORC2 is upregulated in glioma cells and interference of MORC2
prevents the proliferation, invasion, migration and EMT of glioma
cells. The present study also identified MORC2 as a crucial
transcription factor that promoted the growth and metastasis of
glioma by PTEN/PI3K/AKT signaling via binding to NDRG1 promoter.
Hence, the findings of the present study provide researchers with
valuable additional insights into an in-depth understanding of the
underlying mechanisms of MORC2 and NDRG1 in glioma, which may
provide a basis for the development of targeted treatments for
patients with glioma.
Availability of data and materials
Data used to support the results of this study can
be obtained from the corresponding authors as required.
Authors' contributions
JZ, YY and YD searched the literature, and designed
and performed the experiments. JZ and CL analyzed and interpreted
the data, and wrote the manuscript. YY revised the manuscript. All
authors have read and approved the final manuscript. JZ and YY
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study protocols were approved by the Ethics
Committee of Beijing Friendship Hospital, Capital Medical
University (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Wen PY and Reardon DA: Neuro-oncology in
2015: Progress in glioma diagnosis, classification and treatment.
Nat Rev Neurol. 12:69–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Cote DJ, Ascha M, Kruchko C and
Barnholtz-Sloan JS: Adult glioma incidence and survival by race or
ethnicity in the United States from 2000 to 2014. JAMA Oncol.
4:1254–1262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson DR and Galanis E: Incorporation of
prognostic and predictive factors into glioma clinical trials. Curr
Oncol Rep. 15:56–63. 2013. View Article : Google Scholar
|
|
4
|
Molinaro AM, Taylor JW, Wiencke JK and
Wrensch MR: Genetic and molecular epidemiology of adult diffuse
glioma. Nat Rev Neurol. 15:405–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li DQ, Nair SS, Ohshiro K, Kumar A, Nair
VS, Pakala SB, Reddy SD, Gajula RP, Eswaran J, Aravind L and Kumar
R: MORC2 signaling integrates phosphorylation-dependent,
ATPase-coupled chromatin remodeling during the DNA damage response.
Cell Rep. 2:1657–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding QS, Zhang L, Wang BC, Zeng Z, Zou XQ,
Cao PB, Zhou GM, Tang M, Wu L, Wu LL, et al: Aberrant high
expression level of MORC2 is a common character in multiple
cancers. Hum Pathol. 76:58–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Shao Y, He Y, Ning K, Cui X, Liu F,
Wang Z and Li F: MORC2 promotes development of an aggressive
colorectal cancer phenotype through inhibition of NDRG1. Cancer
Sci. 110:135–146. 2019. View Article : Google Scholar :
|
|
8
|
Sahni S, Krishan S and Richardson DR:
NDRG1 as a molecular target to inhibit the epithelial-mesenchymal
transition: The case for developing inhibitors of metastasis.
Future Med Chem. 6:1241–1244. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liao G, Liu X, Wu D, Duan F, Xie X, Wen S,
Li Y and Li S: MORC2 promotes cell growth and metastasis in human
cholangiocarcinoma and is negatively regulated by miR-186-5p. Aging
(Albany NY). 11:3639–3649. 2019. View Article : Google Scholar
|
|
10
|
Kovacevic Z, Chikhani S, Lui GY,
Sivagurunathan S and Richardson DR: The iron-regulated metastasis
suppressor NDRG1 targets NEDD4L, PTEN, and SMAD4 and inhibits the
PI3K and ras signaling pathways. Antioxid Redox Signal. 18:874–887.
2013. View Article : Google Scholar
|
|
11
|
Guo LP, Zhang ZJ, Li RT, Li HY and Cui YQ:
Influences of LncRNA SNHG20 on proliferation and apoptosis of
glioma cells through regulating the PTEN/PI3K/AKT signaling
pathway. Eur Rev Med Pharmacol Sci. 23:253–261. 2019.PubMed/NCBI
|
|
12
|
Chai C, Song LJ, Han SY, Li XQ and Li M:
MicroRNA-21 promotes glioma cell proliferation and inhibits
senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT
signaling pathway. CNS Neurosci Ther. 24:369–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Bi L, Liu Y, Yang Q, Zhou X, Li H, Liu Y,
Li J, Lu Y and Tang H: Paris saponin H inhibits the proliferation
of glioma cells through the A1 and A3 adenosine receptormediated
pathway. Int J Mol Med. 47:302021. View Article : Google Scholar
|
|
15
|
Zhang Q, Xu B, Hu F, Chen X, Liu X, Zhang
Q and Zuo Y: Tenascin C promotes glioma cell malignant behavior and
inhibits chemosensitivity to paclitaxel via activation of the
PI3K/AKT signaling pathway. J Mol Neurosc. 71:1636–1647. 2021.
View Article : Google Scholar
|
|
16
|
Wang X and Zhu Y: Circ_0000020 elevates
the expression of PIK3CA and facilitates the malignant phenotypes
of glioma cells via targeting miR-142-5p. Cancer Cell Int.
21:792021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li B, Wang F, Liu N, Shen W and Huang T:
Astragaloside IV inhibits progression of glioma via blocking
MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 491:98–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Quan Y, Lv J, Dong Q and Gong S:
LncRNA IDH1-AS1 suppresses cell proliferation and tumor growth in
glioma. Biochem Cell Biol. 98:556–564. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelialmesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Zheng H, Li L, Zhou C, Chen X, Zhou
X and Cao Y: KIF3C promotes proliferation, migration, and invasion
of glioma cells by activating the PI3K/AKT pathway and inducing
EMT. Biomed Res Int. 2020:63493122020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Cai H, Sun L, Zhan P, Chen M,
Zhang F, Ran Y and Wan J: LGR5, a novel functional glioma stem cell
marker, promotes EMT by activating the wnt/β-catenin pathway and
predicts poor survival of glioma patients. J Exp Clin Cancer Res.
37:2252018. View Article : Google Scholar
|
|
22
|
Li DQ, Nair SS and Kumar R: The MORC
family: New epigenetic regulators of transcription and DNA damage
response. Epigenetics. 8:685–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GL, Wang CY, Cai XZ, Chen W, Wang XH
and Li F: Identification and expression analysis of a novel CW-type
zinc finger protein MORC2 in cancer cells. Anat Rec (Hoboken).
293:1002–1009. 2010. View
Article : Google Scholar
|
|
24
|
Pan Z, Ding Q, Guo Q, Guo Y, Wu L, Wu L,
Tang M, Yu H and Zhou F: MORC2, a novel oncogene, is upregulated in
liver cancer and contributes to proliferation, metastasis and
chemoresistance. Int J Oncol. 53:59–72. 2018.PubMed/NCBI
|
|
25
|
Liu M, Sun X and Shi S: MORC2 enhances
tumor growth by promoting angiogenesis and tumor-associated
macrophage recruitment via wnt/β-catenin in lung cancer. Cell
Physiol Biochem. 51:1679–1694. 2018. View Article : Google Scholar
|
|
26
|
Liao XH, Zhang Y, Dong WJ, Shao ZM and Li
DQ: Chromatin remodeling protein MORC2 promotes breast cancer
invasion and metastasis through a PRD domain-mediated interaction
with CTNND1. Oncotarget. 8:97941–97954. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shao Y, Li Y, Zhang J, Liu D, Liu F, Zhao
Y, Shen T and Li F: Involvement of histone deacetylation in
MORC2-mediated down-regulation of carbonic anhydrase IX. Nucleic
Acids Res. 38:2813–2824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kovacevic Z and Richardson DR: The
metastasis suppressor, Ndrg-1: A new ally in the fight against
cancer. Carcinogenesis. 27:2355–2366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishio S, Ushijima K, Tsuda N, Takemoto S,
Kawano K, Yamaguchi T, Nishida N, Kakuma T, Tsuda H, Kasamatsu T,
et al: Cap43/NDRG1/Drg-1 is a molecular target for angiogenesis and
a prognostic indicator in cervical adenocarcinoma. Cancer Lett.
264:36–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li A, Zhu X, Wang C, Yang S, Qiao Y, Qiao
R and Zhang J: Upregulation of NDRG1 predicts poor outcome and
facilitates disease progression by influencing the EMT process in
bladder cancer. Sci Rep. 9:51662019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng J, Xie HY, Xu X, Wu J, Wei X, Su R,
Zhang W, Lv Z, Zheng S and Zhou L: NDRG1 as a biomarker for
metastasis, recurrence and of poor prognosis in hepatocellular
carcinoma. Cancer Lett. 310:35–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Lima JM, Morand GB, Macedo CCS, Diesel
L, Hier MP, Mlynarek A, Kowalski LP, Maschietto M, Alaoui-Jamali MA
and da Silva SD: NDRG1 deficiency is associated with regional
metastasis in oral cancer by inducing epithelial-mesenchymal
transition. Carcinogenesis. 41:769–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong X, Hong Y, Sun H, Chen C, Zhao X and
Sun B: NDRG1 suppresses vasculogenic mimicry and tumor
aggressiveness in gastric carcinoma. Oncol Lett. 18:3003–3016.
2019.PubMed/NCBI
|
|
34
|
Ma J, Gao Q, Zeng S and Shen H: Knockdown
of NDRG1 promote epithelial-mesenchymal transition of colorectal
cancer via NF-κB signaling. J Surg Oncol. 114:520–527. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao SP, Wang F, Yang M, Wang XY, Jin CL,
Ji QK, Li S and Zhao XL: CBX3 promotes glioma U87 cell
proliferation and predicts an unfavorable prognosis. J Neurooncol.
145:35–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Terano T, Tanaka T, Tamura Y, Kitagawa M,
Higashi H, Saito Y and Hirai A: Eicosapentaenoic acid and
docosahexaenoic acid inhibit vascular smooth muscle cell
proliferation by inhibiting phosphorylation of Cdk2-cyclinE
complex. Biochem Biophys Res Commun. 254:502–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang Y and Massague J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Lv QL, Huang YT, Zhang LH and
Zhou HH: Akt/FoxM1 signaling pathway-mediated upregulation of MYBL2
promotes progression of human glioma. J Exp Clin Cancer Res.
36:1052017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tan Y, Hu X, Deng Y, Yuan P, Xie Y and
Wang J: TRA2A promotes proliferation, migration, invasion and
epithelial mesenchymal transition of glioma cells. Brain Res Bull.
143:138–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao C, Wang XB, Zhang YH, Zhou YM, Yin Q
and Yao WC: MicroRNA-424 inhibits cell migration, invasion and
epithelial-mesenchymal transition in human glioma by targeting
KIF23 and functions as a novel prognostic predictor. Eur Rev Med
Pharmacol Sci. 22:6369–6378. 2018.PubMed/NCBI
|
|
41
|
Chen Z, Wei X, Shen L, Zhu H and Zheng X:
20(S)-ginsenoside-Rg3 reverses temozolomide resistance and
restrains epithelial-mesenchymal transition progression in
glioblastoma. Cancer Sci. 110:389–400. 2019. View Article : Google Scholar
|
|
42
|
Sun B, Chu D, Li W, Chu X, Li Y, Wei D and
Li H: Decreased expression of NDRG1 in glioma is related to tumor
progression and survival of patients. J Neurooncol. 94:213–219.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zi Y, Zhang Y, Wu Y, Zhang L, Yang R and
Huang Y: Downregulation of microRNA-25-3p inhibits the
proliferation and promotes the apoptosis of multiple myeloma cells
via targeting the PTEN/PI3K/AKT signaling pathway. Int J Mol Med.
47:102021.
|
|
44
|
Ni J, Chen Y, Fei B, Zhu Y, Du Y, Liu L,
Guo L and Zhu W: MicroRNA-301a promotes cell proliferation and
resistance to apoptosis through PTEN/PI3K/akt signaling pathway in
human ovarian cancer. Gynecol Obstet Invest. 86:108–116. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang XY and Mao L: Circular RNA
Circ_0000442 acts as a sponge of MiR-148b-3p to suppress breast
cancer via PTEN/PI3K/Akt signaling pathway. Gene. 766:1451132021.
View Article : Google Scholar
|
|
46
|
Liu CJ, Wu HB, Li YY, Shen L, Yu R, Yin H,
Sun T, Sun C, Zhou Y and Du Z: SALL4 suppresses PTEN expression to
promote glioma cell proliferation via PI3K/AKT signaling pathway. J
Neurooncol. 135:263–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moon SH, Kim DK, Cha Y, Jeon I, Song J and
Park KS: PI3K/Akt and stat3 signaling regulated by PTEN control of
the cancer stem cell population, proliferation and senescence in a
glioblastoma cell line. Int J Oncol. 42:921–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dasari VR, Kaur K, Velpula KK, Gujrati M,
Fassett D, Klopfenstein JD, Dinh DH and Rao JS: Upregulation of
PTEN in glioma cells by cord blood mesenchymal stem cells inhibits
migration via downregulation of the PI3K/Akt pathway. PLoS One.
5:122010. View Article : Google Scholar
|
|
49
|
Sun J, Zhang D, Bae DH, Sahni S, Jansson
P, Zheng Y, Zhao Q, Yue F, Zheng M, Kovacevic Z and Richardson DR:
Metastasis suppressor, NDRG1, mediates its activity through
signaling pathways and molecular motors. Carcinogenesis.
34:1943–1954. 2013. View Article : Google Scholar : PubMed/NCBI
|