Introduction
Persistent pulmonary hypertension of the newborn
(PPHN) is the most common severe pulmonary vascular disease in the
neonatal intensive care unit (NICU), and mortality rates in US
centers range from 4-33% (1).
The pathological changes include an increase in the pulmonary
artery contraction response and excessive remodeling of the distal
pulmonary arterioles (including thickening of the vessel walls and
obstruction of small blood vessels), particularly proliferation of
pulmonary artery smooth muscle cells (PASMCs) (2-4).
Based on the above changes, the current treatment of PPHN utilizes
a comprehensive strategy, including inhaled nitric oxide (iNO),
mechanical ventilation to improve oxygenation and drugs inducing
dilation of the pulmonary vasculature, such as prostaglandins
(iloprost and treprostinil), endothelin receptor antagonists
(bosentan and ambrisentan) and phosphodiesterase 5 inhibitors (such
as sildenafil and tadalafil) (5-8).
Sildenafil is currently the most widely used
pulmonary vasoactive drug. Clinical studies have confirmed that
sildenafil results in vasodilation comparable to that caused by iNO
in patients with pulmonary hypertension, and that it is beneficial
for patients with idiopathic pulmonary hypertension and other
primary subtypes. However, the detailed mechanism of action
requires further elucidation (1,9-11).
Sildenafil is a selective and potent
phosphodiesterase 5 inhibitor that can relax distal pulmonary
arterioles through the classic iNO/cGMP/PKG pathway to achieve
pulmonary hypertension (10,12-14).
Peroxisome proliferator-activated receptor γ
(PPARγ), a member of one of three related nuclear receptor
superfamilies (PPARα, PPARγ and PPARβ/δ), is a ligand-activated
transcription factor (15) that
regulates lung and alveolar development, regulates pulmonary
vascular tone and decreases PASMC proliferation, thus inhibiting
vascular remodeling (16). PPARγ
also mediates the protective effects of sildenafil against acute
kidney injury (AKI) (17)
induced by ischemia-reperfusion in rats and Adriamycin-induced
nephropathy (14).
The transient receptor potential canonical (TRPC)
gene family is known to be the molecular basis of store-operated
calcium channels (SOCCs) and receptor-operated calcium channels
(ROCCs) in PASMCs. Previous studies have shown that chronic hypoxia
selectively upregulates the expression of TRPC1 and TRPC6 in PASMCs
(18). In addition, PPARγ can
inhibit the expression of store-operated calcium entry (SOCE)
channels and TRPCs in PASMCs by inhibiting caveolin-1 (19). These results indicate that PPARγ
acts as an upstream signal of TRPCs and affects the proliferation
and migration of PASMCs through TRPCs.
In the preliminary work of the present study, a PPHN
rat model was established via hypoxia exposure and indomethacin
treatment. PPARγ expression was decreased, whereas TRPC1 and TRPC6
expression were significantly increased in the model (20), suggesting that activation of
PPARγ expression and reductions in TRPC1 and TRPC6 expression may
play roles in dilating pulmonary vessels and improving pulmonary
vascular remodeling.
Based on the establishment of this model of
pulmonary hypertension in neonatal rats, conventional techniques,
such as hematoxylin and eosin (H&E) staining,
immunofluorescence (IF), western blotting, and PCR were used to
explore the possible mechanisms of action of sildenafil in addition
to the classical pathway.
Materials and methods
Animals
This study was approved by the Ethics Committee of
Shengjing Hospital of China Medical University (Shenyang, China;
approval no. 2019PS026K). All experimental procedures and protocols
were performed in accordance with the guidelines for the care and
use of experimental animals. A total of 39 newborn specific
pathogen-free (SPF) Sprague-Dawley (SD) rats (male: female, 1:1;
body weight 5-7 g) were provided by the SPF-level laboratory of the
Animal Experimental Center of Shengjing Hospital affiliated with
China Medical University. A total of 15 newborn rats were used for
the preliminary experiments, and 24 newborn rats were used for the
formal experiments. All the animals were fed with sufficient food
and water, and kept in a natural environment (12-h light/dark
cycles; temperature range: 25-27°C; and humidity range,
50-70%).
Reagents and materials
Sildenafil citrate (Selleck Chemicals; cat. no.
S1431), GW9662 (Abcam; cat. no. ab141125), PPARγ antibody (Abcam;
cat. no. ab19481), TRPC1 antibody (Sigma-Aldrich; Merck KGaA; cat.
no. T8276), TRPC6 antibody (ProteinTech Group, Inc.; cat. no.
18236-1-AP), Ki67 antibody (Cell Signaling Technology, Inc.; cat.
no. 9129), β-actin antibody (Abcam; cat. no. ab8226), donkey
anti-mouse IgG H&L (Alexa Fluor® 488) (Abcam; cat.
no. ab150105), anti-rabbit IgG H&L (Alexa Fluor®
594) (Abcam; cat. no. ab150076), a small animal ventilator
(ALC-V8D; Shanghai Alcott Biotech, Co.), and an
electrophysiological recorder (BL-420F; Biosignal Acquisition and
Analysis System, Chengdu Taimeng Software, Co., Ltd.) were used in
the present study.
Model preparation
According to our previously established method
(21,22), newborn rats were fed by maternal
rats after full-term delivery and maintained under normal
atmospheric conditions for 48 h. In the pre-experiment, 15 neonatal
rats were randomly divided into 5 groups according to their body
weight: i) Normoxic control group [fraction of inspired oxygen
(FiO2) 0.21±0.05; temperature 25-27°C; humidity 50-70%;
n=3]; ii) hypoxia group (FiO2 0.1±0.05; temperature
25-27°C; humidity 50-70%; n=3); iii) hypoxia + 5 mg/kg/day
sildenafil group (FiO2 0.1±0.05; temperature 25-27°C;
humidity 50-70%; sildenafil 5 mg/kg/day; n=3); iv) hypoxia + 10
mg/kg/day sildenafil group (FiO2 0.1±0.05; temperature
25-27°C; humidity 50-70%; sildenafil 10 mg/kg/day; n=3); and v)
hypoxia + 25 mg/kg/day sildenafil group (FiO2 0.1±0.05;
temperature 25-27°C; humidity 50-70%; sildenafil 25 mg/kg/day;
n=3). The oxygen concentration and air humidity were continuously
monitored. In the formal experiments, 24 neonatal rats were
randomly divided into 3 groups according to their body weight: i)
Control group, in which the rats were kept in a normal-air
environment (FiO2 0.21±0.05; temperature 25-27°C;
humidity 50-70%; n=8); ii) PPHN model group, in which the rats were
placed in a hypoxic glass box for 14 days (FiO2
0.1±0.05; temperature 25–27°C; humidity 50-70%; n=8); and iii)
sildenafil treatment group, in which the rats were subjected to
conditions equivalent to those of the PPHN group and were placed in
the hypoxic glass box on the first day of intragastric
administration (25 mg/kg/day sildenafil was administered by gavage
with a no. 8 gavage needle; the first dose was administered
immediately prior to placement in the hypoxic glass box; n=8)
(23). The mother rats were
exchanged between the experimental group/sildenafil treatment group
and the control group every 24 h to avoid a decrease in feeding
ability due to hypoxia. The litter was replaced when the mother was
replaced, and the sildenafil group was administered the drug
treatment. The operation was completed within 15 min.
Hemodynamic evaluations
After 14 days, mice were anesthetized with sodium
pentobarbital (50 mg/kg, intraperitoneal injection). The mice were
fixed, intubated and connected to a small animal ventilator. The
heart was exposed through a sternotomy, and the trocar tip
connected to the multichannel physiological recorder was inserted
into the right ventricle (RV) to monitor the RV pressure (an
indirect response to pulmonary artery pressure).
Specimen collection
Following hemodynamic evaluations, the animals were
euthanized by an intraperitoneal injection of 50 mg/kg body weight
pentobarbital, followed by exsanguination. The heart and lungs were
rapidly removed en bloc from the thoracic cavity. The RV and
left ventricle + ventricular septum (LV+S) were carefully separated
under the microscope and weighed separately. The right ventricular
hypertrophy index (RVHI) was calculated as the RV weight/LV+S
weight. The lower lobe of the right lung was fixed in 4%
paraformaldehyde for 48-72 h at room temperature, embedded in
paraffin, and 4.5 µm sections were prepared for H&E
staining, immunohistochemistry (IHC) and IF staining.
H&E staining
The paraffin blocks were cut into 4-µm-thick
sections. The sections were stained with hematoxylin for 5 min at
room temperature and then washed with running water for 30 min. The
slices were next stained with eosin for 1 min at room temperature
and then washed with water.
IF staining to observe the localization
and expression of α-SMA, PPARγ, TRPC1, TRPC6 and Ki67
Paraffin sections of lung tissue were dewaxed in a
gradient of alcohol solutions and xylene. After the samples were
immersed in PBS and placed in Tris-EDTA repair solution for
microwave antigen retrieval, they were washed three times with PBS,
soaked in PBS containing 0.3% Triton X for 10 min, and blocked with
goat serum for 40 min at room temperature. Primary antibodies
(α-SMA, 1:40; PPARγ, 1:2.5; TRPC1, 1:50; TRPC6, 1:20; and Ki67,
1:80) were added, and the sections were incubated overnight at 4°C.
The following day (after 14-16 h), the samples were rewarmed at
room temperature for 30 min, washed three times with PBS, and
incubated with a fluorescent secondary antibody mixture [Alexa
Fluor 488 (green) donkey anti-mouse IgG or Alexa Fluor 594 (red)
anti-rabbit IgG; both at 1:100]. The samples were incubated for 4 h
at room temperature in the dark, washed three times with PBS,
stained with DAPI for nuclear staining, washed again three times
and imaged with a confocal microscope (magnification, ×40; scale
bar, 50 µm; NIKON C1, Nikon Corporation).
Cell IF
The cells were fixed with 4% paraformaldehyde for 20
min, and then 0.3% Triton X-100 was used for cell permeabilization;
all aforementioned steps were performed at room temperature. The
sections were washed with PBS three times, immunostained with
primary antibodies (α-SMA, 1:100) and incubated in a wet chamber
overnight at 4°C. The sections were stained with the corresponding
secondary antibody (1:100) and incubated at room temperature for 30
min. The nucleus was stained for 5 min with DAPI at room
temperature.
Real-time PCR detection of PPARγ, TRPC1
and TRPC6 gene expression in lung tissue
A total of 8 samples from each group were obtained,
and total RNA was extracted using TRIzol® reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The RNA purity and concentration were determined based on
the optical density (OD) 260/280 nM ratio. The mRNA was
reverse-transcribed into cDNA using a PrimeScript RT Reagent kit
(Takara Bio, Inc.). A SYBR Green PCR kit (Takara Bio, Inc.) was
used according to the manufacturer's instructions with an Applied
Biosystems 7500 Real-Time PCR system (Applied Biosystems 7500;
Thermo Fisher Scientific, Inc.), and the final volume of the
reaction mixture for PCR was 20 µl.
The PCR thermocycling conditions were: 95°C for 30
sec; followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
The gene expression levels were calculated using the
2−ΔΔCq method (24).
β-actin was used as an internal control. The primer sequences are
listed in Table I.
 | Table IGene-specific primer sequences. |
Table I
Gene-specific primer sequences.
| Gene | Sequence,
5′-3′ |
|---|
| PPARγ | |
| Forward |
ACCACAGTTGATTTCTCCAG |
| Reverse |
TGTTGTAGAGCTGGGTCTTT |
| TRPC1 | |
| Forward |
AGCCTCTTGACAAACGAGGA |
| Reverse |
ACCTGACATCTGTCCGAACC |
| TRPC6 | |
| Forward |
TACTGGTGTGCTCCTTGCAG |
| Reverse |
GAGCTTGGTGCCTTCAAATC |
| Ki67 | |
| Forward |
CCTGCCCGACCCTACAAAAT |
| Reverse |
TCCGCCGTCTTAAGGTAGGA |
| β-actin | |
| Forward |
ACAACCTTCTTGCAGCTCCT |
| Reverse |
CAGTTGGTGACAATGCCGTG |
Western blot detection of PPARγ, TRPC1
and TRPC6 protein levels in lung tissue
Total protein was extracted from lung tissue; the
protein concentration was determined using a BCA assay; 30
µg protein samples were separated on an 8% SDS-gel using
SDS-PAGE and transferred to PVDF membranes; the membranes were
blocked using 5% skimmed milk at room temperature in TBST (10%
Tween-20) for 2 h to block nonspecific binding. The membranes were
then incubated overnight at 4°C with primary antibodies against
PPARγ (1:400), TRPC1 (1:200), TRPC6 (1:500) and β-actin (1:2,000).
After the membranes were washed three times with TBST, they were
incubated with a goat anti-rabbit secondary antibody or a goat
anti-mouse secondary antibody for 2 h at room temperature. After
another wash, signals were visualized using enhanced
chemiluminescence reagent, and an imaging system was used for
imaging. ImageJ version 1.8.0 (National Institutes of Health) was
used to analyze the density of the protein bands and to normalize
the protein levels to those of β-actin; the values are expressed in
arbitrary units.
Culture of human PASMCs (HPASMCs)
The HPASMCs were derived from ScienCell primary
cells (purchased from Guangzhou Huatuo Biotechnology Co., Ltd.).
Cells were cultured in high-glucose DMEM (Hyclone) containing 10%
FBS (CLARK), 1% penicillin and streptomycin (Biological
Industries). When the cells grew to 80-90% confluence, they were
subcultured every 3-5 days, and cells at passage 4-8 were used for
follow-up experiments. To simulate the pathological process of
hypoxic pulmonary hypertension in vitro, HPASMCs were
cultured under normal and hypoxic conditions. The conventional
culture conditions were 37°C, 5% CO2 and 21%
O2, and the hypoxic conditions were 37°C, 5%
CO2 and 4% O2.
The cells were divided into three groups: i) the
normoxic control group (21% O2, 60 h, n=3); ii) the
hypoxic group (4% O2, 60 h, n=3) and iii) the hypoxia +
sildenafil group (4% O2, 60 h, n=3), to investigate the
role of sildenafil on HPASMCs under hypoxic conditions.
Then HPASMCs were exposed to hypoxia and treated
with sildenafil and GW9662 (PPAR-γ antagonist). The cells were
divided into four groups: i) the hypoxic control group (n=3), ii)
the hypoxia + sildenafil group (n=3), iii) the hypoxia + GW9662
group (n=3) and iv) the hypoxia + sildenafil + GW9662 group
(n=3).
Small interfering (si)RNA
transfection
Hanheng Biotechnology (Shanghai) Co., Ltd., siRNA
targeting the PPARγ gene and negative control siRNA. Cells were
seeded at 2×105 cells/well in a 6-well plate and
transfected the following day with 3 µl of the indicated
siRNAs (20 µM) or controls using a Lipofectamine™ 2000
transfection kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. After culturing at
37°C for 48 h, the cells were counted and lysed for RNA and protein
extraction.
Three siRNA sequences (siR-PP1, siR-PP2 and siR-PP3)
targeting the PPARγ gene were designed. The sequences of the siRNAs
are listed in Table II. The
cells were divided into four groups: si-NC, siR-PP1, siR-PP2 and
siR-PP3.
 | Table IISequences of the siRNAs targeting the
PPARγ genes used in this study. |
Table II
Sequences of the siRNAs targeting the
PPARγ genes used in this study.
| siRNA | Sequence |
|---|
| siR-PP1 | |
| Sense |
GGUUGCAGAUUACAAGUAUdTdT |
| Antisense |
AUACUUGUAAUCUGCAACCdTdT |
| siR-PP2 | |
| Sense |
CAAGAGUACCAAAGUGCAAdTdT |
| Antisense |
UUGCACUUUGGUACUCUUGdTdT |
| siR-PP3 | |
| Sense |
GGAGAACAAUCAGAUUGAAdTdT |
| Antisense |
UUCAAUCUGAUUGUUCUCCdTdT |
The effects of sildenafil and PPARγ siRNA in
cultured HPASMCs under hypoxic conditions were further
investigated. The cells were divided into four groups: i) The
hypoxic control group (60 h, 4% O2), ii) the hypoxia +
sildenafil group (60 h, 4% O2), iii) the hypoxia +
siR-PP1 group (60 h, 4% O2), and iv) the hypoxia +
sildenafil + siR-PP1 group.
Statistical analysis
The data are expressed as the mean ± the standard
error of the mean. Statistical analysis was performed using SPSS
version 23 (IBM Corp.). Differences between groups were compared
using an one-way ANOVA followed by a post hoc LSD test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Sildenafil reverses the increases in the
RV mean pressure and RVHI induced by hypoxia and attenuates
pulmonary arterial remodeling
At 48 h after birth, neonatal rats were randomly
divided into 5 groups for a pre-experiment (Fig. 1A): i) the normoxic control group
(n=3); ii) the hypoxia group (10% O2, n=3); iii) the
hypoxia + 5 mg/kg/day sildenafil group (10% O2,
sildenafil 5 mg/kg/day, n=3), iv) the hypoxia + 10 mg/kg/day
sildenafil group (10% O2, sildenafil 10 mg/kg/day, n=3),
and v) the hypoxia + 25 mg/kg/day sildenafil group (10%
O2, sildenafil 25 mg/kg/day, n=3). The RV mean pressure
and RVHI were measured after 14 days. The results showed that 25
mg/kg/day sildenafil significantly attenuated the increase in RV
pressure and RVHI caused by hypoxia-induced pulmonary hypertension
in newborn rats, so 25 mg/kg/day was selected for subsequent
tests.
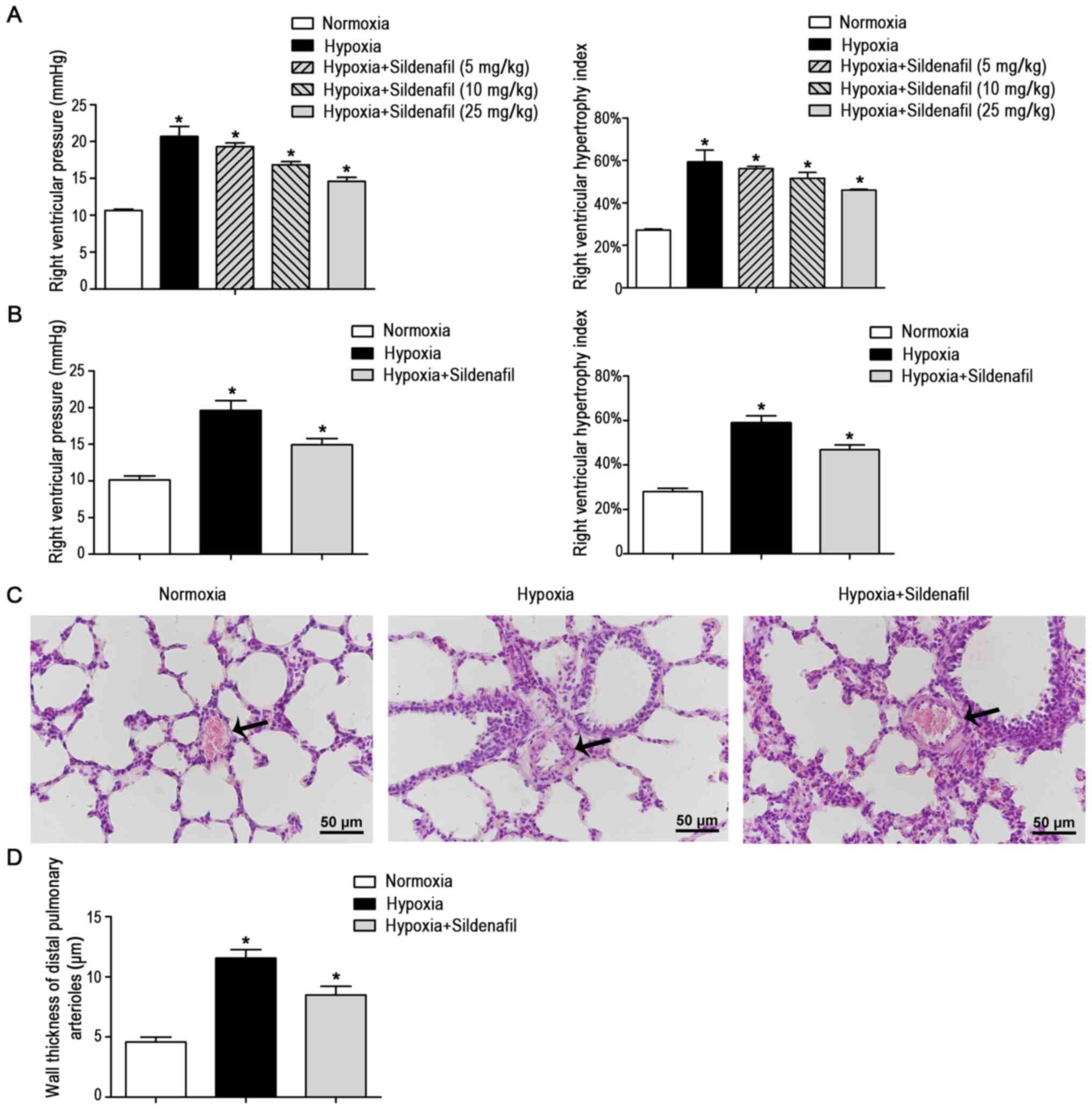 | Figure 1Sildenafil attenuated the increase in
the RV mean pressure and RVHI induced by hypoxia and attenuated
pulmonary arterial remodeling. (A) Different concentrations of
sildenafil attenuated the increase in the RV mean pressure in
neonatal rats induced by hypoxia. There were five groups: i) the
normoxic control group (14 days), ii) the hypoxia group (14 days,
10% O2), iii) the hypoxia + 5 mg/kg/day sildenafil group
(14 days, 10% O2, 5 mg/kg/days sildenafil), iv) the
hypoxia + 10 mg/kg/day sildenafil group (14 days, 10%
O2, 10 mg/kg/days sildenafil), and v) the hypoxia + 25
mg/kg/day sildenafil group (14 day, 10% O2, 25 mg/kg/day
sildenafil). Different concentrations of sildenafil attenuated the
increase in the RVHI in neonatal rats induced by hypoxia. n=3. (B)
Sildenafil attenuated the increase in the RV mean pressure in
neonatal rats induced by hypoxia. There were three groups: i) the
normoxic control group (14 days), ii) the hypoxia group (14 days,
10% O2), and iii) the hypoxia + sildenafil group (14
days, 10% O2, 25 mg/kg/day sildenafil). Sildenafil
attenuated the increase in the RVHI in neonatal rats induced by
hypoxia + sildenafil group. n=8. (C) Representative H&E-stained
photomicrographs of the distal pulmonary artery. Sildenafil
treatment reduced hypoxia-induced pulmonary arterial wall
thickening. The arrows indicate the distal pulmonary arteriolar
wall. Magnification, ×40; scale bar, 50 µm. (D) Quantitative
analysis of the wall thickness of the distal pulmonary artery. Data
are presented as the mean ± standard deviation. Sildenafil
treatment reduced hypoxia-induced pulmonary arterial wall
thickening. n=8. *P<0.05 vs. control. RV, right
ventricular; RVHI, RV hypertrophy index; H&E, hematoxylin and
eosin. |
In the formal experiment, neonatal rats were
randomly divided into 3 groups: i) the normoxic control group
(n=8), ii) the hypoxia group (10% O2, n=8), and iii) the
hypoxia + sildenafil group (10% O2, sildenafil 25
mg/kg/day, n=8). A total of 14 days later, the RV pressure and RVHI
were measured, and lung tissue samples were collected. As shown in
Fig. 1B, the RV mean pressure
measured in the hypoxia group was 19.63±1.35 mmHg, which was
significantly higher than that in the normoxic control group
(10.13±0.58 mmHg, P<0.05), and the RVHI measured in the hypoxia
group was 58.91±3.22%, which was significantly higher than that in
the normoxic control group (28.01±1.48%, P<0.05). However, the
increases in the RV mean pressure and the RVHI were significantly
inhibited by intragastric administration of sildenafil. The data
showed that the RV mean pressure and RVHI decreased significantly
to 14.93±0.88 mmHg (P<0.05) and 46.85%±2.18% (P<0.05),
respectively (Fig. 1B).
With regard to morphology, H&E staining
(Fig. 1C) of lung tissue
sections was performed to observe the remodeling of distal
pulmonary arterioles. Compared with those in the normoxic control
group, the distal pulmonary arteriolar walls (Fig. 1D) in the hypoxia group were
significantly thickened and the lumina were narrowed, whereas
sildenafil treatment alleviated the hypoxia-induced arterial wall
thickening and lumen narrowing.
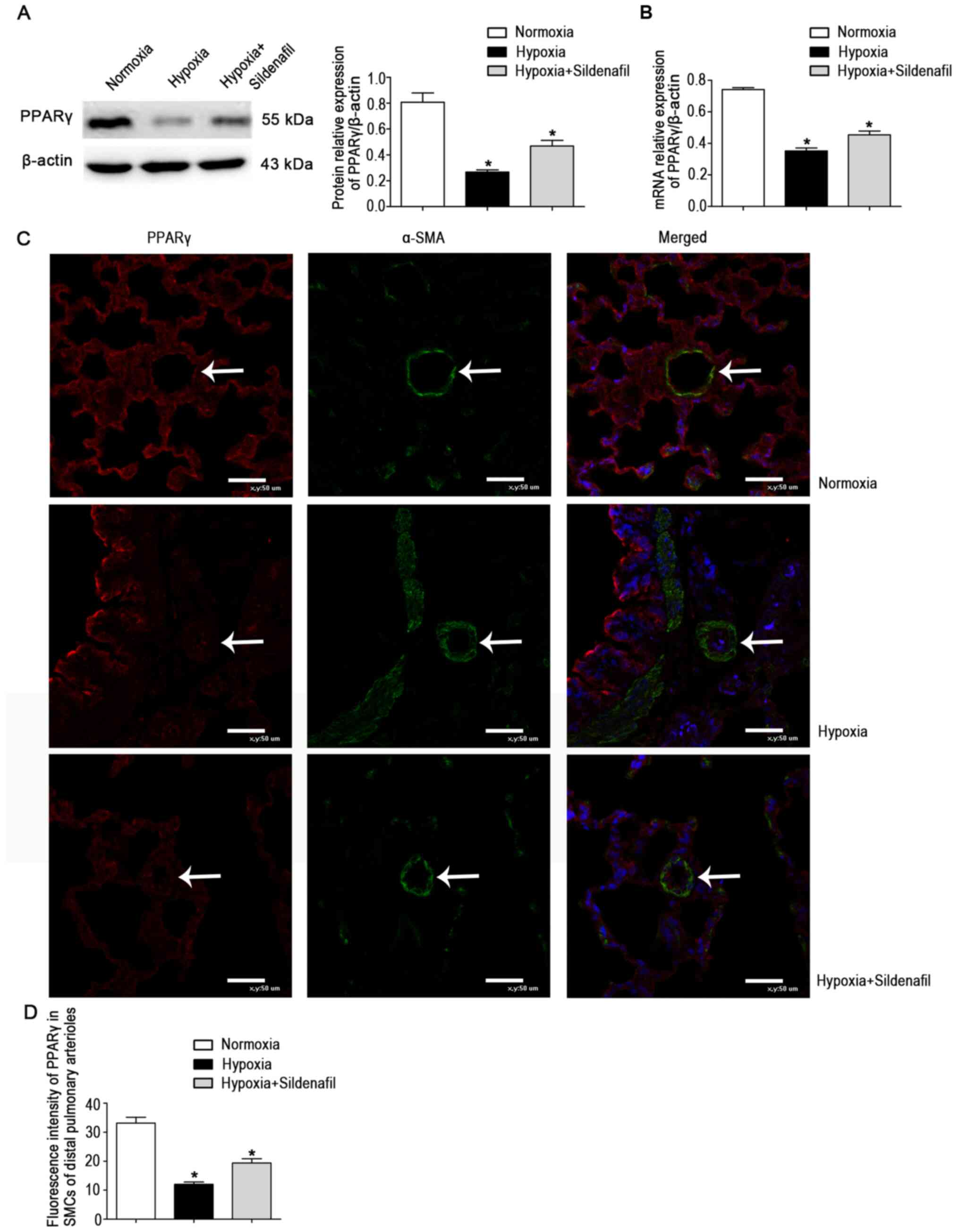
Sildenafil attenuates hypoxia-induced
downregulation of PPARγ expression and inhibits hypoxia-induced
upregulation of TRPC expression in neonatal rats
The protein levels in lung tissue homogenates were
detected (Fig. 2A). The results
showed a significant decrease in PPARγ protein expression in the
hypoxia group. This change was attenuated by sildenafil, similar to
the change in the mRNA levels (Fig.
2B). However, the expression levels in lung homogenates may not
be representative of the expression levels in distal pulmonary
small vessels. Subsequently, double IF staining for α-SMA and PPARγ
was performed on lung tissue sections to further clarify the
localization and expression levels of PPARγ in lung tissue
(Fig. 2C). The results of IF
double staining showed that the fluorescence of PPARγ colocalized
with α-SMA was significantly decreased in the hypoxia group.
Compared with that in the hypoxia group, the fluorescence of PPARγ
was enhanced in the hypoxia + sildenafil group (Fig. 2D). The fluorescence trend of
PPARγ was the same as the protein and mRNA expression trends in
lung homogenates.
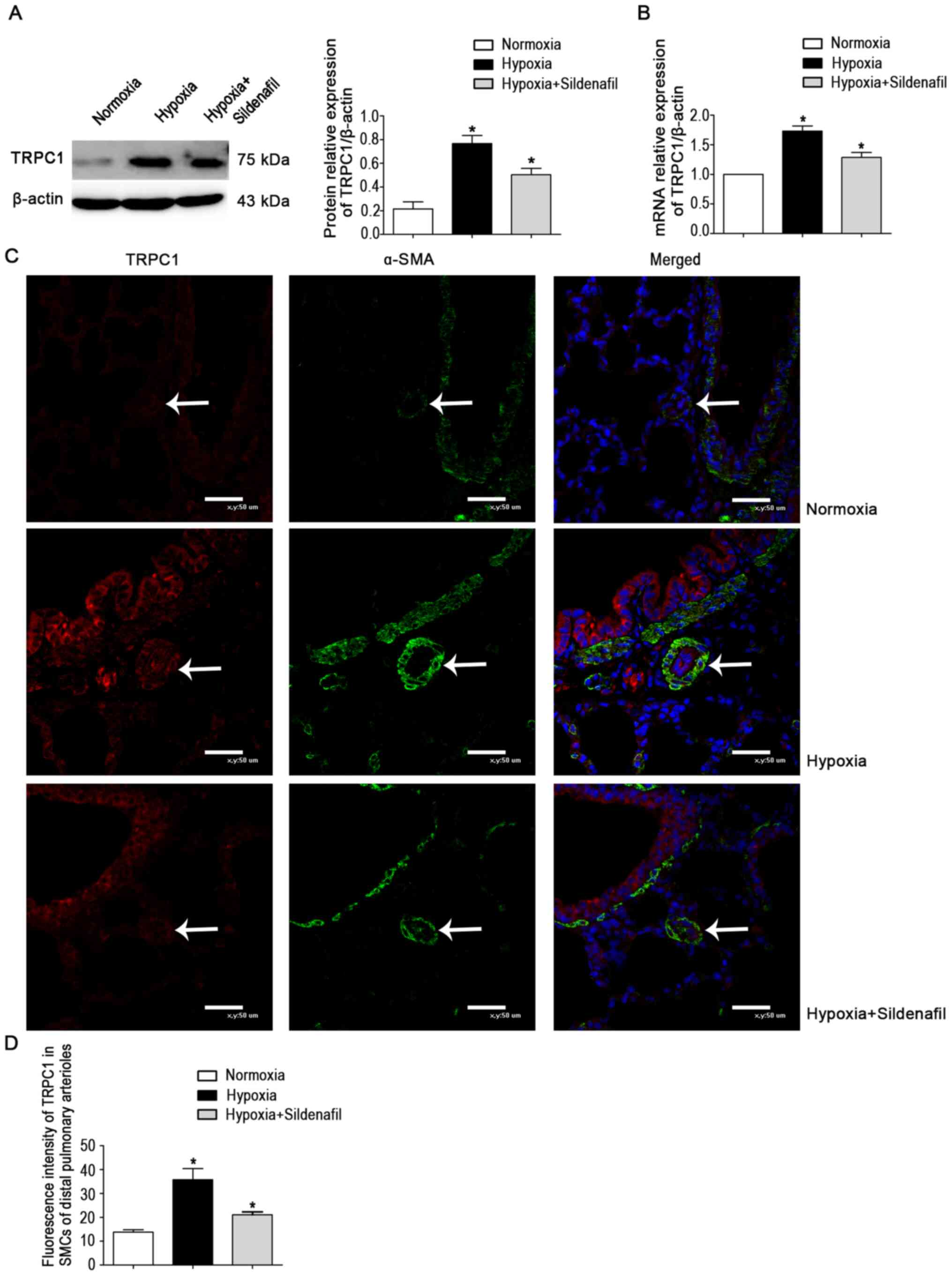
The data showed a marked increase in TRPC1 protein
expression in the hypoxia group (Fig. 3A). The change was attenuated by
sildenafil, similar to the change in mRNA levels (Fig. 3B). The fluorescence trend of
TRPC1 (Fig. 3C and D) was the
same as the protein and mRNA expression trends in lung
homogenates.
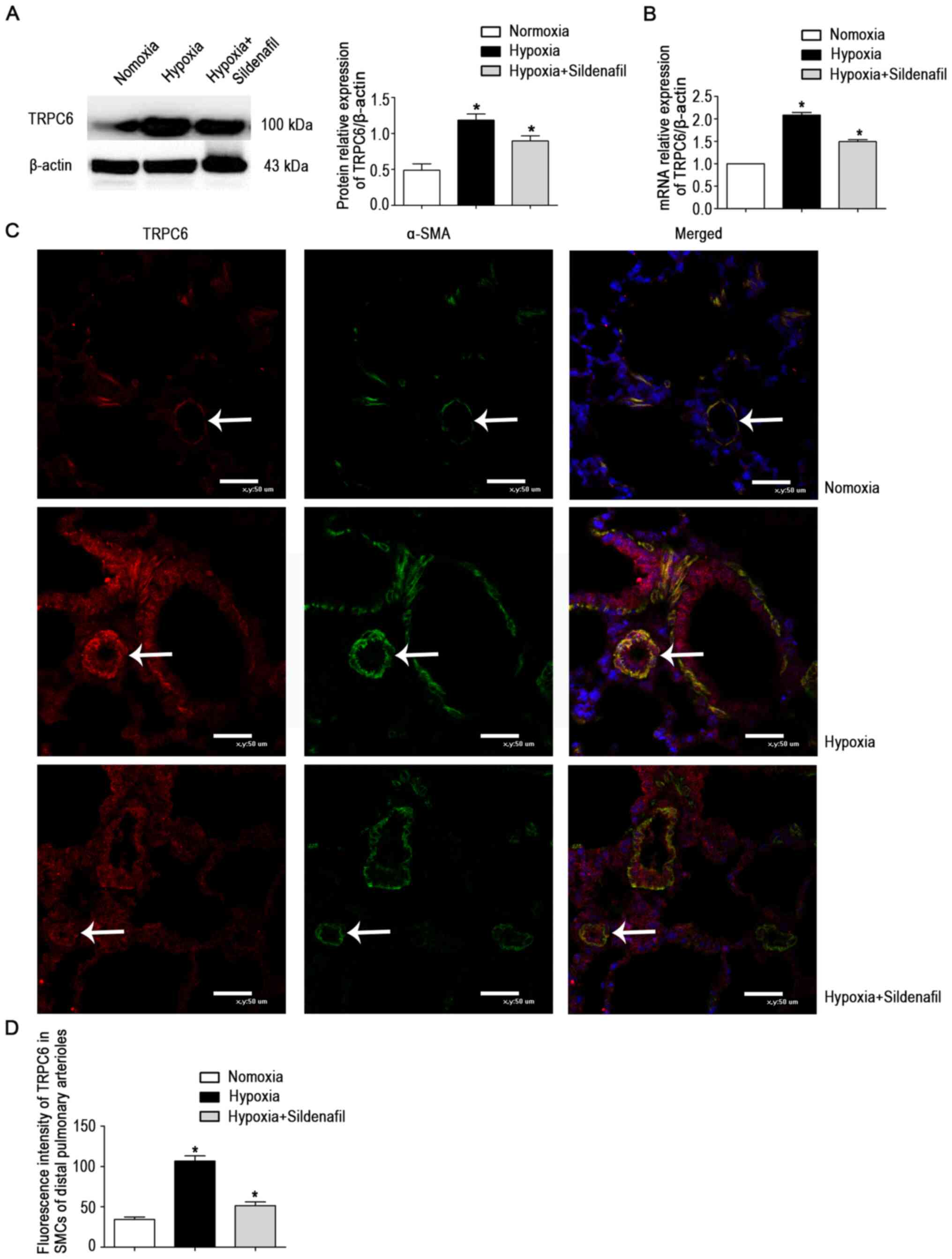
The data showed a marked increase in TRPC6 protein
expression in the hypoxia group (Fig. 4A). The changes were attenuated by
sildenafil, similar to the changes in mRNA levels (Fig. 4B) and fluorescence levels
(Fig. 4C and D).
Sildenafil attenuates hypoxia-induced
downregulation of PPARγ expression and inhibits hypoxia-induced
upregulation of TRPC expression and cell proliferation in HPASMCs
during prolonged hypoxia
HPASMCs derived from ScienCell primary cells were
used for the in vitro experiments. Under normoxic
conditions, IF staining of α-SMA in HPASMCs showed that green
fluorescence was present throughout the HPASMCs, and the cell
morphology was as described in previous literature (Fig. 5A) (25,26).
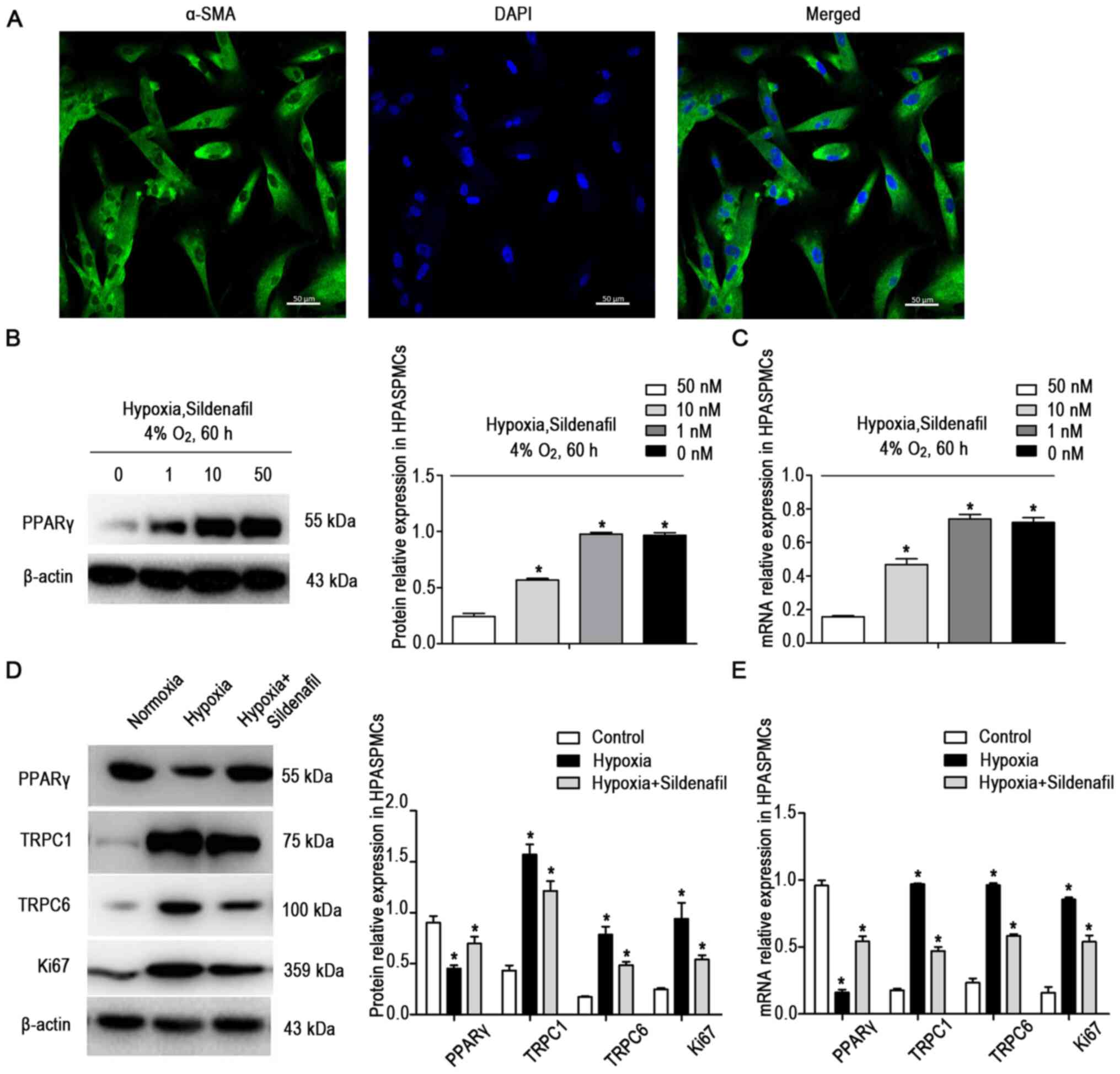 | Figure 5Sildenafil attenuated the
hypoxia-induced downregulation of PPARγ expression and inhibited
the hypoxia-induced upregulation of TRPC and Ki67 expression in
HPASMCs. (A) HPASMCs were identified by immunofluorescence staining
for α-SMA in cells grown under normoxic conditions. (B) Western
blot and (C) RT-qPCR analysis of the increase in PPARγ protein and
mRNA expression in HPASMCs induced by different concentrations of
sildenafil under hypoxic conditions. Each of the four groups were
treated with 0, 1, 10 or 50 nM sildenafil. (D) Western blot and (E)
RT-qPCR analysis of the sildenafil-mediated attenuation of
hypoxia-induced downregulation of PPARγ expression and
sildenafil-mediated inhibition of hypoxia-induced upregulation of
TRPC and Ki67 expression in HPASMCs. There were three experimental
groups: i) The normoxic control group (60 h), ii) the hypoxia group
(60 h, 4% O2), and iii) the hypoxia + sildenafil group
(60 h, 4% O2, 50 nM sildenafil). Data are presented as
the mean ± standard deviation of three repeats.
*P<0.05 vs. control. PPARγ, peroxisome
proliferator-activated receptor γ; HPASMC, human pulmonary artery
smooth muscle cell; SMA, smooth muscle actin; TRPC, transient
receptor potential canonical; RT-qPCR, reverse
transcription-quantitative PCR. |
The HPASMCs were cultured under normoxic or hypoxic
conditions. To determine the optimal concentration of sildenafil,
four concentrations of sildenafil, 0, 1, 10 and 50 nM, were applied
to HPASMCs in a hypoxic state. The protein (Fig. 5B) and mRNA (Fig. 5C) expression levels of PPARγ were
detected after the cells were cultured for 60 h. The changes in
PPARγ were not obvious with the 10 nM treatment. Therefore, 10 nM
was selected as the sildenafil dose for the follow-up
experiment.
The cells were divided into three groups: i) the
normoxic control group (21% O2, 60 h, n=3); ii) the
hypoxic group (4% O2, 60 h, n=3) and iii) the hypoxia +
sildenafil group (4% O2, 60 h, n=3). The protein
(Fig. 5D) and mRNA (Fig. 5E) expression levels of PPARγ,
TRPC1 and TRPC6 were detected. Compared with the normoxic control
cells, HPASMCs exposed to prolonged hypoxia exhibited decreases in
PPARγ expression and increases in TRPC1 and TRPC6 expression, and
these changes were attenuated by sildenafil.
In the in vitro experiments, the expression
of Ki67 protein (Fig. 5D) and
mRNA (Fig. 5E) was detected. The
results showed that the expression of Ki67 was significantly
increased in the hypoxic group, and that the increase was
attenuated by sildenafil.
Sildenafil inhibits hypoxia-induced
upregulation of TRPC expression and cell proliferation in HPASMCs
by activating PPARγ
HPASMCs were treated with GW9662, an inhibitor of
PPARγ. Three concentrations of GW9662 (0, 1 and 10 nM) were used
during exposure to hypoxia (4%) for 60 h. The expression of PPARγ
was detected and found to be significantly inhibited by a
concentration of 10 nM (Fig. 6A and
B).
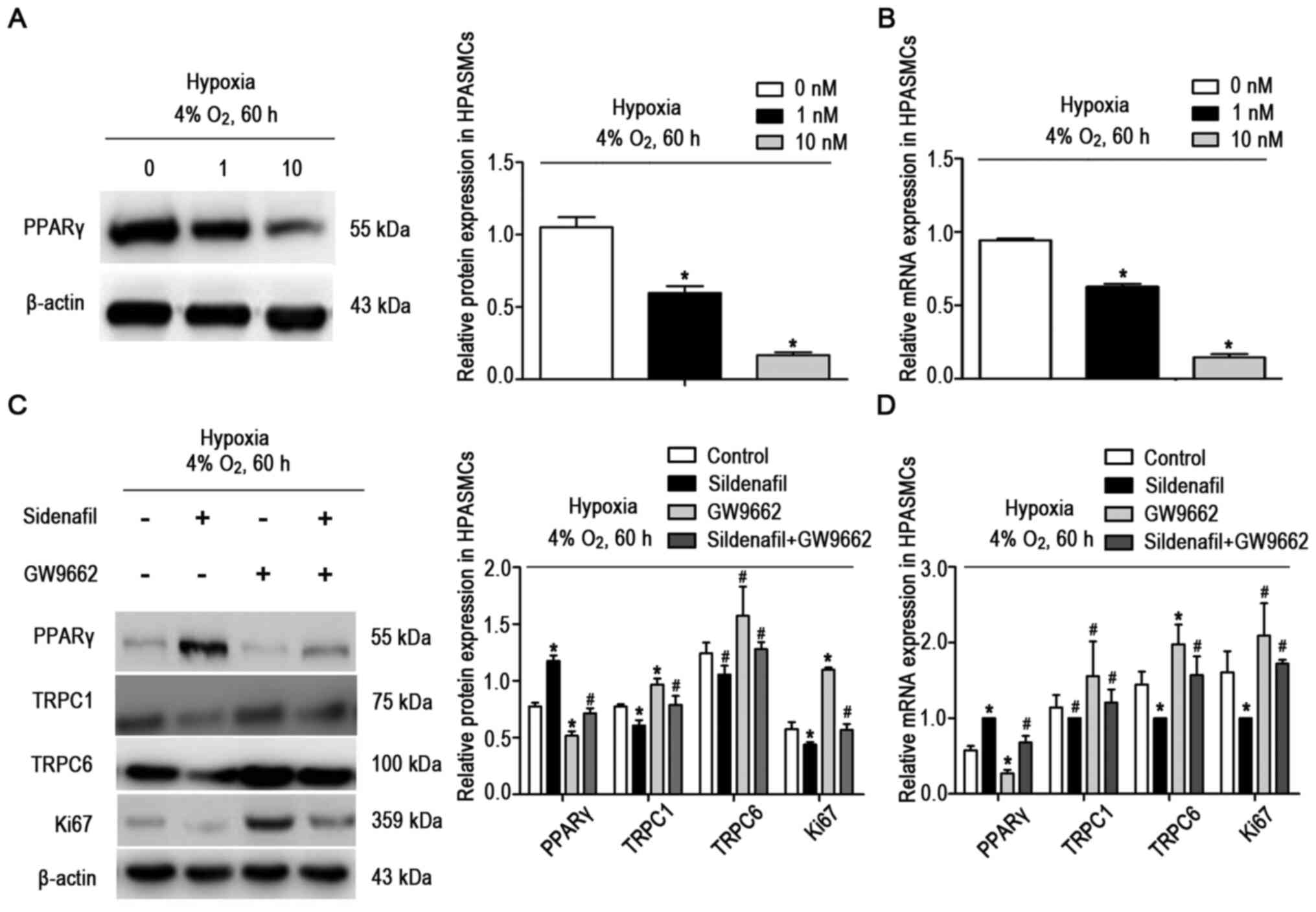 | Figure 6A PPARγ inhibitor (GW9662) inhibited
the sildenafil-induced downregulation of TRPC and Ki67 protein
expression in HPASMCs under hypoxic conditions. (A) Western blot
and (B) RT-qPCR analysis of the inhibitory effects of different
concentrations of a PPARγ inhibitor (GW9662) on PPARγ protein
expression in HPASMCs under hypoxic conditions. Cells were treated
with either 0, 1 or 10 nM GW9662. (C) Western blot and (D) RT-qPCR
analysis of the inhibitory effect of a PPARγ inhibitor (GW9662) on
the sildenafil-induced downregulation of TRPC and Ki67 protein
expression in HPASMCs under hypoxic conditions. There were four
groups: i) The hypoxic control group (60 h, 4% O2), ii)
the hypoxia + sildenafil group (60 h, 4% O2), iii) the
hypoxia + GW9662 group (60 h, 4% O2, 10 nM), and (iv)
the hypoxia + sildenafil + GW9662 group (60 h, 4% O2, 10
nM). Data are presented as the mean ± standard deviation of three
repeats. *P<0.05 vs. control, #P>0.05
vs. control. PPARγ, peroxisome proliferator-activated receptor γ;
HPASMC, human pulmonary artery smooth muscle cell; TRPC, transient
receptor potential canonical; RT-qPCR, reverse
transcription-quantitative PCR. |
HPASMCs were exposed to hypoxia and treated with
sildenafil and GW9662. The cells were divided into four groups: i)
the hypoxic control group (n=3), ii) the hypoxia + sildenafil group
(n=3), iii) the hypoxia + GW9662 group (n=3) and iv) the hypoxia +
sildenafil + GW9662 group (n=3). The results showed that sildenafil
increased the expression of PPARγ and decreased the expression of
TRPC1, TRPC6 and Ki67 under hypoxic conditions (4% O2,
60 h; Fig. 6C and D). However,
when sildenafil and GW9662 were used simultaneously, GW9662
attenuated the upregulation of PPARγ and the inhibition of TRPC1,
TRPC6 and Ki67 expression induced by sildenafil.
The effects of sildenafil and PPARγ siRNA on the
expression of TRPCs and Ki67 in cultured HPASMCs under hypoxic
conditions were further investigated. HPASMCs were transfected with
siRNA (Fig. 7A and B), the
expression levels of PPARγ decreased significantly, indicating that
PPARγ had been successfully knocked down. As illustrated in
Fig. 7C and D, the expression
levels of TRPC1, TRPC6 and Ki67 were significantly increased after
transfection with PPARγ siRNA, but sildenafil did not notably
downregulate the expression levels of TRPC1 and TRPC6.
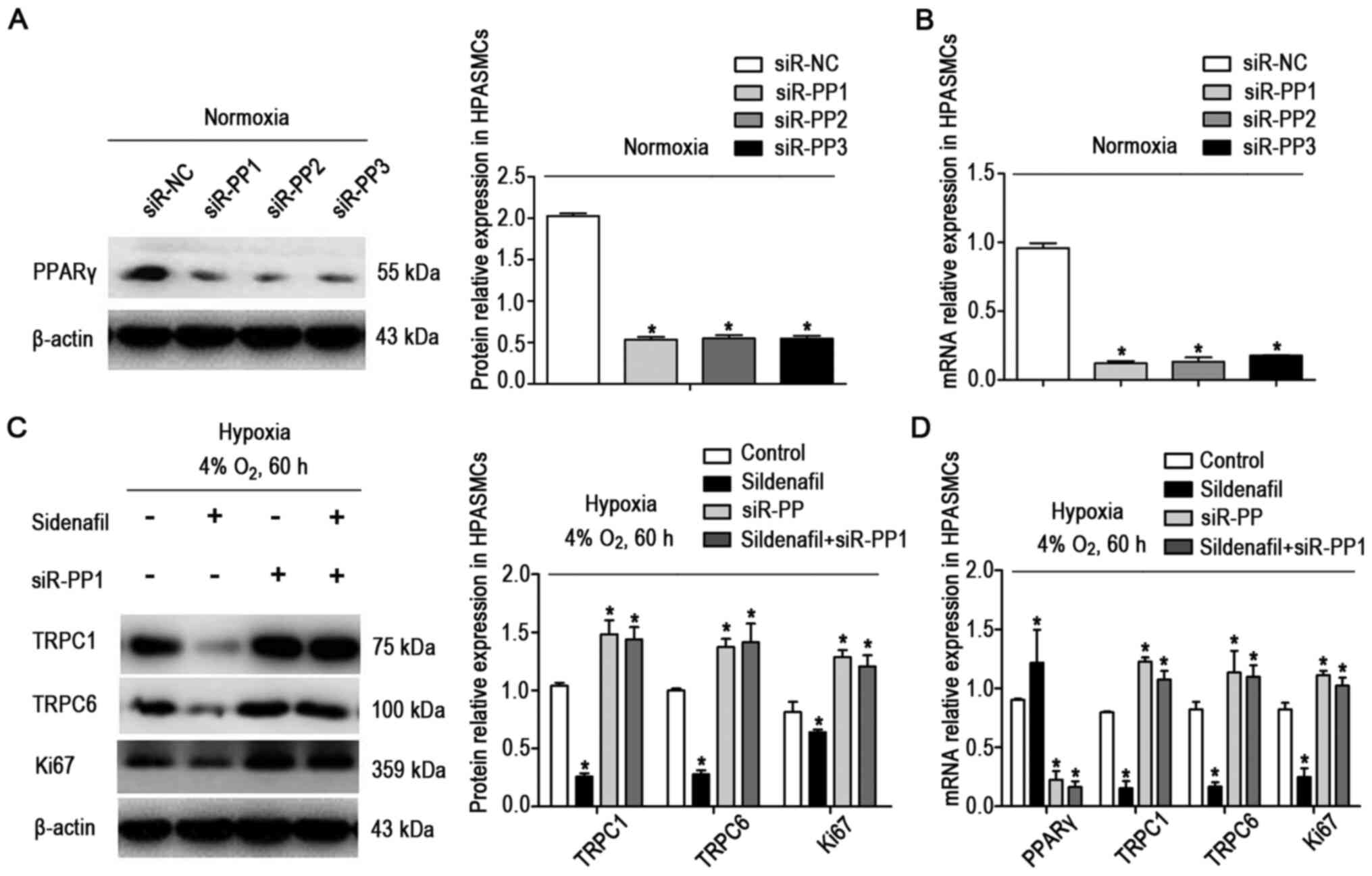 | Figure 7si-PPARγ reversed the
sildenafil-induced downregulation of TRPC and Ki67 expression under
hypoxic conditions. (A) Western blot and (B) RT-qPCR analysis of
the downregulation of PPARγ protein expression in HPASMCs
transfected with si-PPARγ under normoxic conditions. There were
four groups: si-NC, siR-PP1, siR-PP2 and siR-PP3. (C) Western blot
and (D) RT-qPCR analysis of the reversal of the sildenafil-induced
downregulation of TRPC and Ki67 protein expression in HPASMCs
induced by PPARγ siRNA under hypoxic conditions. There were four
groups: i) The hypoxic control group (60 h, 4% O2), ii)
the hypoxia + sildenafil group (60 h, 4% O2), iii) the
hypoxia + siR-PP1 group (60 h, 4% O2), and iv) the
hypoxia + sildenafil + siR-PP1 group. Data are presented as the
mean ± standard deviation of three repeats. *P<0.05
vs. control. PPARγ, peroxisome proliferator-activated receptor γ;
HPASMC, human pulmonary artery smooth muscle cell; siRNA, small
interfering RNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR. |
Discussion
PPHN is the most common severe pulmonary vascular
disease in the NICU, with an incidence of ~2/1,000 live births
(27) and a mortality rate of
4-33% in US centers (1). In
addition to a continuously increased pulmonary artery pressure,
excessive remodeling of the distal pulmonary arterioles (including
thickening of the pulmonary vascular wall and obstruction of small
blood vessels) and right ventricular hypertrophy are major features
of this disease.
Despite significant improvements in health care,
~1.9% of newborns are exposed to intermittent or chronic hypoxia
during the perinatal period and are at risk of developing PPHN.
Compared with adult pulmonary vessels, neonatal pulmonary vessels
are more vulnerable to hypoxic injury. In this study, hypoxic
conditions were used to induce pulmonary hypertension in neonatal
rats (28).
There are several animal models used for the study
of neonatal pulmonary hypertension. For example, in fetal lambs,
contraction of the ductus arteriosus can produce fetal and neonatal
pulmonary hypertension. After delivery, the lambs have persistent
hypoxemia and increased pulmonary vascular resistance, and thus can
be used as an important experimental model for human infant PPHN.
This model of PPHN is associated with significant pulmonary
vascular remodeling and elevated levels of reactive oxygen species
in the lungs and pulmonary arteries (29). Hyperoxia exposure can also lead
to vascular remodeling and pulmonary hypertension, so this model is
often used to study bronchopulmonary dysplasia. Hyperoxia exposure
interferes with the signaling pathways required for lung
development and repair in premature and newborn mice and interrupts
alveolar formation and pulmonary angiogenesis (30).
At present, PPHN treatment is based on a
comprehensive strategy involving inhaled nitric oxide (iNO);
high-frequency ventilation; and application of pulmonary
dilation-inducing vascular drugs, including prostaglandins,
endothelin receptor antagonists and phosphodiesterase 5 inhibitors
(such as sildenafil and tadalafil) (27).
At present, the phosphodiesterase 5 inhibitor
sildenafil, a selective and potent vasodilator, has been developed
as an alternative therapy or as an adjuvant for PPHN treatment with
iNO, and is also the most widely used pulmonary vasoactive drugs in
the clinic (10). Clinical
studies have shown that sildenafil significantly reduces mortality
and that it is more effective than placebo in improving oxygenation
(1,9). In previous animal studies, large
experimental animals, such as newborn sheep and newborn pigs, have
been used (31-34). Sildenafil has rarely been used in
neonatal rat models of pulmonary hypertension to the best of our
knowledge, but the use of such small animals can reduce the cost
and increase the repeatability of the experiments.
The pathogenesis of PPHN is complex and still not
fully understood. Currently, proliferation and migration disorders
of PASMCs are suggested to be the main causes of the abnormal
excessive remodeling of distal pulmonary arterioles that occurs
during the pathogenesis of pulmonary hypertension (35). α-SMA is a commonly used vascular
smooth muscle cell marker in part because it is the first protein
expressed in smooth muscle cell differentiation during development
and because it shows specificity for smooth muscle cells (35). In addition, α-SMA is required for
force contraction in fully differentiated smooth muscle cells, and
is the most abundant protein in differentiated smooth muscle cells,
accounting for 40% of total cellular proteins (35). Therefore, α-SMA-labeled pulmonary
vascular smooth muscle cells were used in the present study. To
identify the effects of sildenafil on PPARγ, TRPCs and Ki67 in the
smooth muscle cells of distal pulmonary arterioles, α-SMA was
double-stained with PPARγ, TRPCs and Ki67.
An increasing number of studies have shown that
PPARγ plays an important role in the pathogenesis of pulmonary
hypertension (36). PPARγ, a
member of one of three related nuclear receptor superfamilies
(PPARα, PPARγ and PPARβ/δ), is a ligand-activated transcription
factor (15). Research has shown
that PPARγ plays important roles in glucose homeostasis and fatty
acid metabolism and that it can also attenuate vascular dysfunction
(37-39). In addition to being highly
expressed in adipocytes, cardiomyocytes, macrophages and renal
podocytes (14), PPARγ is highly
expressed in cell types in the pulmonary vascular wall, including
vascular endothelial cells and smooth muscle cells (15,35,40). In the present study, the
expression levels of PPARγ were significantly decreased in the
distal pulmonary arterioles and lung tissues of the PPHN group,
consistent with observations in the literature. Studies have shown
that PPARγ can inhibit the proliferation and migration of smooth
muscle cells by blocking the expression of platelet-derived growth
factor. In addition, PPARγ induces smooth muscle cell apoptosis by
mediating phosphorylation of the retinoblastoma gene and by
increasing the expression of the proapoptotic protein Gadd45
(5). Treatment with the PPARγ
agonists rosiglitazone (37) and
pioglitazone (2) attenuated the
progression of hypoxia-induced pulmonary hypertension and pulmonary
vascular remodeling.
Sildenafil can promote fat formation by increasing
the expression of PPARγ in adipocytes. PPARγ also mediates the
protective effect of sildenafil against acute kidney injury
(17) induced by
ischemia-reperfusion in rats and Adriamycin-induced nephropathy
(14). In the present study, the
application of sildenafil significantly inhibited the decreases in
PPARγ expression in distal PASMCs and lung tissue caused by chronic
hypoxia, producing an effect similar to that of PPARγ agonists;
thus, sildenafil may reduce hypoxia-induced pulmonary artery
pressure changes and pulmonary vascular remodeling by activating
PPARγ expression. The precise mechanism by which sildenafil
upregulates PPARγ remains entirely unknown. Sonneveld et al
(14) proposed a mechanism in
which sildenafil-induced increases in cGMP levels lead to further
activation of PKG-1 and then PPARγ.
Increased free calcium concentrations in PASMCs are
major contributors to pulmonary vasoconstriction and can stimulate
the proliferation and migration of PASMCs, all of which can cause
pulmonary vascular remodeling (18,41). There are three main
Ca2+ channels on PASMCs: L-type voltage-dependent
Ca2+ channels, ROCCs and calcium pool-related SOCCs.
Amongst them, ROCCs and SOCCs play an important role in the
pathogenesis of pulmonary hypertension (5). The typical TRPC gene family members
are known to form the molecular basis for SOCCs and ROCCs in
PASMCs, and it has previously been shown that chronic hypoxia
selectively upregulates the expression of TRPC1 and TRPC6 in PASMCs
(18). In the present study,
TRPC1 and TRPC6 expression was significantly increased in the
distal PASMCs of the PPHN group. The high expression levels of
TRPC1 and TRPC6 were significantly attenuated after the application
of sildenafil, indicating that sildenafil may also reduce the
intracellular Ca2+ concentration, reduce pulmonary
vasoconstriction, and inhibit pulmonary vascular remodeling by
reducing the expression of TRPC1 and TRPC6. These changes can
improve pulmonary hypertension.
Studies have shown that PPARγ can inhibit the
expression of SOCE channels and TRPCs in PASMCs by inhibiting
caveolin-1 (19). These results
indicate that PPARγ acts as an upstream signal of TRPCs, and
affects the proliferation and migration of PASMCs through TRPCs. In
addition, Sonneveld et al (14) found that treatment with PPARγ
agonists in vitro and in vivo downregulated the
expression levels of TRPC6, reduced podocyte damage caused by
Adriamycin and improved the associated proteinuria. Further
chromatin immunoprecipitation analysis showed that PPARγ can bind
to the TRPC6 promoter and regulate the expression and activity of
TRPC6 through a direct mechanism of action (14).
In conclusion, the present study demonstrated that
sildenafil may attenuate pulmonary vasoconstriction by activating
PPARγ and downregulating TRPC1 and TRPC6, thereby reducing
pulmonary hypertension and inhibiting the thickening of the distal
pulmonary arteriole wall, in neonatal rats exposed to hypoxia.
However, further studies are needed to validate the roles of PPARγ,
TRPC1 and TRPC6 in pulmonary hypertension in neonatal rats.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH performed the experiments and participated in the
design, statistics, drafting and revising of the manuscript. NL, XT
and YD performed the experiments and analyzed the data. All authors
read and approved the final manuscript. WH and NL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments performed in the present
study were approved by the institutional review board of Shengjing
Hospital of China Medical University.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
Abbreviations:
|
PPHN
|
persistent pulmonary hypertension of
the newborn
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
TRPC
|
transient receptor potential
canonical
|
|
iNO
|
inhaled nitric oxide
|
|
PASMC
|
pulmonary artery smooth muscle
cell
|
|
IHC
|
immunohistochemistry
|
|
IF
|
immunofluorescence
|
|
RV mean pressure
|
right ventricular mean pressure
|
|
RVHI
|
right ventricular hypertrophy
index
|
References
|
1
|
Kelly LE, Ohlsson A and Shah PS:
Sildenafil for pulmonary hypertension in neonates. Cochrane
Database Syst Rev. 8:CD0054942017.PubMed/NCBI
|
|
2
|
Behringer A, Trappiel M, Berghausen EM,
Ten Freyhaus H, Wellnhofer E, Odenthal M, Blaschke F, Er F,
Gassanov N, Rosenkranz S, et al: Pioglitazone alleviates cardiac
and vascular remodelling and improves survival in monocrotaline
induced pulmonary arterial hypertension. Naunyn Schmiedebergs Arch
Pharmacol. 389:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tajsic T and Morrell NW: Smooth muscle
cell hypertrophy, proliferation, migration and apoptosis in
pulmonary hypertension. Compr Physiol. 1:295–317. 2011.PubMed/NCBI
|
|
4
|
Zhang Y, Cui Y, Deng W, Wang H, Qin W,
Huang C, Li C, Zhang J, Guo Y, Wu D, et al: Isoquercitrin protects
against pulmonary hypertension via inhibiting PASMCs proliferation.
Clin Exp Pharmacol Physiol. 44:362–370. 2017. View Article : Google Scholar
|
|
5
|
Afdal P and AbdelMassih AF: Is pulmonary
vascular disease reversible with PPAR γ agonists? Microcirculation.
25:e124442018. View Article : Google Scholar
|
|
6
|
Kahveci H, Yilmaz O, Avsar UZ, Ciftel M,
Kilic O, Laloglu F and Ozturk K: Oral sildenafil and inhaled
iloprost in the treatment of pulmonary hypertension of the newborn.
Pediatr Pulmonol. 49:1205–1213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kraemer U, Cochius-den Otter S, Snoek KG
and Tibboel D: Pharmacodynamic considerations in the treatment of
pulmonary hypertension in infants: Challenges and future
perspectives. Expert Opin Drug Metab Toxicol. 12:1–19. 2016.
View Article : Google Scholar
|
|
8
|
Yamamura A, Fujitomi E, Ohara N, Tsukamoto
K, Sato M and Yamamura H: Tadalafil induces antiproliferation,
apoptosis, and phosphodiesterase type 5 downregulation in
idiopathic pulmonary arterial hypertension in vitro. Eur J
Pharmacol. 810:44–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barst RJ, Beghetti M, Pulido T, Layton G,
Konourina I, Zhang M and Ivy DD; STARTS-2 Investigators: STARTS-2:
Long-term survival with oral sildenafil monotherapy in
treatment-naive pediatric pulmonary arterial hypertension.
Circulation. 129:1914–1923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iacovidou N, Syggelou A, Fanos V and
Xanthos T: The use of sildenafil in the treatment of persistent
pulmonary hypertension of the newborn: A review of the literature.
Curr Pharm Des. 18:3034–3045. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RC, Jiang FM, Zheng QL, Li CT, Peng
XY, He CY, Luo J and Liang ZA: Efficacy and safety of sildenafil
treatment in pulmonary arterial hypertension: A systematic review.
Respir Med. 108:531–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Francis SH, Busch JL, Corbin JD and Sibley
D: cGMP-dependent protein kinases and cGMP phosphodiesterases in
nitric oxide and cGMP action. Pharmacol Rev. 62:525–563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu W, Ran P, Zhang D, Peng G, Li B, Zhong
N and Wang J: Sildenafil inhibits chronically hypoxic upregulation
of canonical transient receptor potential expression in rat
pulmonary arterial smooth muscle. Am J Physiol Cell Physiol.
298:C114–C123. 2010. View Article : Google Scholar :
|
|
14
|
Sonneveld R, Hoenderop JG, Isidori AM,
Henique C, Dijkman HB, Berden JH, Tharaux PL, van der Vlag J and
Nijenhuis T: Sildenafil prevents podocyte injury via
PPAR-γ-mediated TRPC6 inhibition. J Am Soc Nephrol. 28:1491–1505.
2017. View Article : Google Scholar
|
|
15
|
Chandra M, Miriyala S and Panchatcharam M:
PPARγ and its role in cardiovascular diseases. PPAR Res.
2017:64046382017. View Article : Google Scholar
|
|
16
|
Martinho S, Adão R, Leite-Moreira AF and
Brás-Silva C: Persistent pulmonary hypertension of the newborn:
Pathophysiological mechanisms and novel therapeutic approaches.
Front Pediatr. 8:3422020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohey V, Singh M, Puri N, Kaur T, Pathak D
and Singh AP: Sildenafil obviates ischemia-reperfusion
injury-induced acute kidney injury through peroxisome
proliferator-activated receptor γ agonism in rats. J Surg Res.
201:69–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia Y, Yang XR, Fu Z, Paudel O, Abramowitz
J, Birnbaumer L and Sham JS: Classical transient receptor potential
1 and 6 contribute to hypoxic pulmonary hypertension through
differential regulation of pulmonary vascular functions.
Hypertension. 63:173–180. 2014. View Article : Google Scholar
|
|
19
|
Yang K, Lu W, Jiang Q, Yun X, Zhao M,
Jiang H and Wang J: Peroxisome proliferator-activated receptor
γ-mediated inhibition on hypoxia-triggered store-operated calcium
entry. A Caveolin-1-dependent mechanism. Am J Respir Cell Mol Biol.
53:882–892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Du Y, Fu J, Yao L, Qiao L, Liu N, Xing Y
and Xue X: Altered expression of PPAR γ and TRPC in neonatal rats
with persistent pulmonary hypertension. Mol Med Rep. 16:1117–1124.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu YP, Zhu JJ, Cheng F, Jiang KW, Gu WZ,
Shen Z, Wu YD, Liang L and Du LZ: Ghrelin ameliorates
hypoxia-induced pulmonary hypertension via phospho-GSK3 β/β-catenin
signaling in neonatal rats. J Mol Endocrinol. 47:33–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du Y, Fu J, Yao L, Zhang D, Liu N and Xue
X: Effects of FHL1 and P21 on hypoxia-induced pulmonary vascular
remodeling in neonatal rats. Exp Ther Med. 14:4245–4253.
2017.PubMed/NCBI
|
|
23
|
Jasińska-Stroschein M, Owczarek J, Łuczak
A and Orszulak-Michalak D: The beneficial impact of fasudil and
sildenafil on monocrotaline-induced pulmonary hypertension in rats:
A hemodynamic and biochemical study. Pharmacology. 91:178–184.
2013. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Li Y, Liu G, Cai D, Pan B, Lin Y, Li X, Li
S, Zhu L, Liao X and Wang H: H2S inhibition of chemical
hypoxia-induced proliferation of HPASMCs is mediated by the
upregulation of COX-2/PGI2. Int J Mol Med. 33:359–366. 2014.
View Article : Google Scholar
|
|
26
|
Lin C, Li X, Luo Q, Yang H, Li L, Zhou Q,
Li Y, Tang H and Wu L: RELM-β promotes human pulmonary artery
smooth muscle cell proliferation via FAK-stimulated surviving. Exp
Cell Res. 351:43–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hilgendorff A, Apitz C, Bonnet D and
Hansmann G: Pulmonary hypertension associated with acute or chronic
lung diseases in the preterm and term neonate and infant. The
European Paediatric Pulmonary Vascular Disease Network, endorsed by
ISHLT and DGPK. Heart. 102(Suppl 2): ii49–ii56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YP, He Q, Shen Z, Shu XL, Wang CH, Zhu
JJ, Shi LP and Du LZ: miR-126a-5p is involved in the
hypoxia-induced endothelial-to-mesenchymal transition of neonatal
pulmonary hypertension. Hypertens Res. 40:552–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gong J, Feng Z, Peterson AL, Carr JF, Vang
A, Braza J, Choudhary G, Dennery PA and Yao H: Endothelial to
mesenchymal transition during neonatal hyperoxia-induced pulmonary
hypertension. J Pathol. 252:411–422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Menon RT, Shrestha AK, Reynolds CL,
Barrios R, Caron KM and Shivanna B: Adrenomedullin is necessary to
resolve hyperoxia-induced experimental bronchopulmonary dysplasia
and pulmonary hypertension in mice. Am J Pathol. 190:711–722. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Makker K, Afolayan AJ, Teng RJ and Konduri
GG: Altered hypoxia-inducible factor-1α (HIF-1α) signaling
contributes to impaired angiogenesis in fetal lambs with persistent
pulmonary hypertension of the newborn (PPHN). Physiol Rep.
7:e139862019. View Article : Google Scholar
|
|
32
|
Cohen SS, Powers BR, Lerch-Gaggl A, Teng
RJ and Konduri GG: Impaired cerebral angiogenesis in the fetal lamb
model of persistent pulmonary hypertension. Int J Dev Neurosci.
38:113–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amer R, Elsayed YN, Graham MR, Sikarwar
AS, Hinton M and Dakshinamurti S: Effect of vasopressin on a
porcine model of persistent pulmonary hypertension of the newborn.
Pediatr Pulmonol. 54:319–332. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blasina F, Vaamonde L, Silvera F, Solla G,
Abin-Carriquiry JA, Gutiérrez C, Beltramo P, Garcia-Gabay I and
Martell M: Efficacy and safety of a novel nitric oxide generator
for the treatment of neonatal pulmonary hypertension: Experimental
and clinical studies. Pulm Pharmacol Ther. 54:68–76. 2019.
View Article : Google Scholar
|
|
35
|
Fernandez RA, Wan J, Song S, Smith KA, Gu
Y, Tauseef M, Tang H, Makino A, Mehta D and Yuan JX: Upregulated
expression of STIM2, TRPC6, and Orai2 contributes to the transition
of pulmonary arterial smooth muscle cells from a contractile to
proliferative phenotype. Am J Physiol Cell Physiol. 308:C581–C593.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tseng V, Sutliff RL and Hart CM: Redox
biology of peroxisome proliferator-activated receptor-γ in
pulmonary hypertension. Antioxid Redox Signal. 31:874–897. 2019.
View Article : Google Scholar :
|
|
37
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications - a review. Nutr J. 13:172014. View Article : Google Scholar
|
|
38
|
Wang L, Waltenberger B, Pferschy-Wenzig
EM, Blunder M, Liu X, Malainer C, Blazevic T, Schwaiger S,
Rollinger JM, Heiss EH, et al: Natural product agonists of
peroxisome proliferator-activated receptor gamma (PPARγ): A review.
Biochem Pharmacol. 92:73–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ajith TA and Jayakumar TG: Peroxisome
proliferator-activated receptors in cardiac energy metabolism and
cardiovascular disease. Clin Exp Pharmacol Physiol. 43:649–658.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Lu W, Yang K, Wang Y, Zhang J, Jia
J, Yun X, Tian L, Chen Y, Jiang Q, et al: Peroxisome
proliferator-activated receptor γ inhibits pulmonary hypertension
targeting store-operated calcium entry. J Mol Med (Berl).
93:327–342. 2015. View Article : Google Scholar
|
|
41
|
Malczyk M, Veith C, Fuchs B, Hofmann K,
Storch U, Schermuly RT, Witzenrath M, Ahlbrecht K, Fecher-Trost C,
Flockerzi V, et al: Classical transient receptor potential channel
1 in hypoxia-induced pulmonary hypertension. Am J Respir Crit Care
Med. 188:1451–1459. 2013. View Article : Google Scholar : PubMed/NCBI
|