Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has
presented an unprecedented challenge to public health, with a
substantial loss of human life worldwide (1). The rate of asymptomatic SARS-CoV-2
infection has been calculated to be between 28 and 31.4% (2). The spectrum of symptomatic infection
ranges from mild to critical. The majority of patients experience
mild, self-limiting upper respiratory tract infection (~80% of
symptomatic patients) (3,4) and only a small number of patients
develop acute respiratory distress syndrome that can rapidly lead
to multiorgan failure and death (5). A study conducted in Mexico reported
that the most common initial symptoms are headache, fatigue,
myalgia, arthralgia, fever, sore throat and cough (4); however, research conducted worldwide
has reported a very heterogeneous clinical symptom frequency
(6). Studies on the time needed by
patients with COVID-19 to achieve a normal state of health (with
standard medical care) have reported that 50–65% of patients
regained their normal state of health 7 days from the date of
diagnosis, whereas 35% of patients had not returned to their normal
state of health 12–14 days after receiving a positive test result
(4,7).
COVID-19 is an acute viral disease whose severity is
associated with the dysregulation of inflammatory immune responses,
which in turn inhibits the development of protective immunity
against infection (8). The most
severe complications of COVID-19 infection are sepsis-like
inflammation, coagulopathy and respiratory or cardiovascular
complications. The therapeutic strategies for the management of
severe symptoms are focused on the control of viremia and/or
inflammation, in addition to providing optimal organ support
(8–14). The use of non-steroidal
anti-inflammatory drugs (NSAIDs) for the treatment of COVID-19 was
considered controversial at the onset of the pandemic, due to
concerns that these types of drugs may increase the risk of
infection or the severity of SARS-CoV-2 (15). However, the World Health
Organization (WHO), as well as a recent meta-analysis, found no
indication to date of any negative association between the use of
NSAIDs, including ibuprofen, and SARS-CoV-2 infection or its
outcomes (15). NSAIDs, such as
ibuprofen, may prove beneficial for the early management of
COVID-19 by ameliorating the suggested inflammatory process leading
to lymphopenia and immunosuppression that has been reported to be a
common feature associated with severe disease (16). Ibuprofen and diclofenac potassium
have been shown to be superior to the currently used paracetamol,
not only with regard to their analgesic and antipyretic effect, but
also in significantly improving the lymphocytic count in patients
with COVID-19, enhancing their immune response and favoring a 5-day
recovery period (15,16).
Mefenamic acid is a NSAID that exhibits a wide range
of anti-inflammatory, antipyretic and analgesic activities
(17). In a previous study on a
series of five cases, the early initiation of treatment with
mefenamic acid provided symptomatic relief in reducing fever in
non-hypoxic patients (18); in
addition, the use of mefenamic acid led to reduced C-reactive
protein (CRP) values, which can also prevent the cytokine storm,
reflecting the significant anti-inflammatory activity of mefenamic
acid in patients with COVID-19 (18). The use of mefenamic acid in
post-COVID myalgia, until the CRP levels decrease to <1, has
also been suggested (17,18). In addition, mefenamic acid exerts
an inhibitory effect on the serine proteases of the RNA virus
(DENV2 NS2B-NS3 protease of the dengue virus) (19). Various members of an expert panel
in India shared their anecdotal experience on the effectiveness of
mefenamic acid as an antipyretic, analgesic and anti-inflammatory
agent in the management of COVID-19 (20). The expert panel recommended the use
of mefenamic acid (500 mg, three times a day, for 7 days) for
treating COVID-19 in adults; however, they stated that further
extensive clinical trials are required to confirm the same findings
in the management of COVID-19 (20).
Therefore, the present study was designed to
randomly select patients with COVID-19 on an outpatient basis and
compare the safety and efficacy of standard medical care combined
with mefenamic acid, vs. standard medical care (mainly treatment
with acetaminophen) (14) plus a
placebo.
Patients and methods
Study design
A prospective, randomized, double-blind, two-arm,
parallel group, phase II clinical trial was conducted between
August and December, 2020, and was performed according to the
‘CONSORT statement’ guidelines for randomized controlled trials
(http://www.consort-statement.org/).
The present study was conducted to evaluate the safety and efficacy
of mefenamic acid for decreasing the time period for patients to
reach an acceptable symptom state [patient acceptable symptom state
(PASS)]. It was approved by the Ethics Committee of the Mexican
Social Security Institute, delegation of the State of Colima,
Mexico (July 29, 2020), and written statements of informed consent
were obtained from all the participants. The trial was performed in
accordance with the principles of the Declaration of Helsinki and
the international conference on harmonization-good clinical
practice guidelines. The present clinical trial was registered as
MEFECOVID-19: RPCEC00000388 in the Cuban Public Registry of
Clinical Trials (RPCEC, the Spanish acronym) database (August 31,
2021).
Study subjects
The inclusion criteria were the following: Males and
non-pregnant women ≥18 years of age, presenting with COVID-19
infection and a positive diagnosis of SARS-CoV-2 provided using
reverse transcription PCR, that had a medical consultation due to
their illness and were indicated for at-home ambulatory treatment.
The women agreed to utilize effective contraceptive measures during
the study period and for at least 15 days after the final drug
administration of the analysis. The exclusion criteria comprised
pregnant or breastfeeding women and patients presenting with any of
the following conditions, prior to the diagnosis of COVID-19:
Cancer, ischemic heart disease, chronic decompensated systemic
disease, creatinine levels 1.25-fold higher than the normal values
or a creatinine clearance <50 ml/min (Cockcroft-Gault method),
blood hemoglobin levels <10 g/dl, drug addiction (use of illegal
drugs), or any known liver disease with a doubling of liver
function test values [aspartate aminotransferase (AST), alanine
aminotransferase (ALT), alkaline phosphatase, or bilirubin).
Additionally, the following elimination criteria were used:
Patients that voluntarily decided to abandon the study, patients
that presented with severe toxicity [grade ≥3, according to the
common terminology criteria for adverse events (CTCAE) v5.0, US
Department of Health and Human Services (21)] attributable to the administration
of the experimental drug, at some point of the study. No
participant was vaccinated against SARS-CoV-2 prior to the
development of the study, given that the vaccines were only
available in Mexico after closing this clinical trial.
The physicians participating in the project
identified candidates at the public secondary healthcare center,
the Zone 1 General Hospital of the IMSS Colima, in the Mexican
State of Colima, and requested their participation in the study.
The patients that agreed to be included were randomly allocated to
the experimental group (oral mefenamic acid + standard medical
care) or the control group (placebo + standard medical care).
Simple randomization was performed using computer-generated random
allocation cards (groups A and B). All the patients were advised
that they would continue to be under the supervision of their
regular physician. The research team would not, under any
circumstances, modify or limit any intervention that their usual
physician, or they themselves, considered pertinent, such as going
to the emergency department if there were alarm symptoms.
Intervention
All the patients received standard medical care and
treatment prescribed by their family physician or specialist, which
consisted of the administration of 500 mg oral paracetamol every
6–8 h, in addition to any other medication indicated by their
treating physician (Table I). The
experimental group was administered mefenamic acid (500 mg, Ponstan
tablets, Pfizer, S.A. de C.V., Mexico) every 8 h for 7 days. The
control group was administered a placebo starch tablet every 8 h
for the same amount of time. The pills were recommended to be taken
with milk or with meals, to reduce gastrointestinal adverse events.
All patients (experimental and control groups) were administered
one 20 mg omeprazole tablet daily during the study. All patients
were instructed to visit the emergency service if they presented
with respiratory difficulty or worsening of symptomatology. The
researchers did not intervene in drug prescription or lifestyle
indications.
 | Table I.Main clinical characteristics of the
participating subjects at the moment of enrollment and standard
prescribed drugs. |
Table I.
Main clinical characteristics of the
participating subjects at the moment of enrollment and standard
prescribed drugs.
| Clinical
characteristic | All (n=36) | Control (n=17) | Experimental
(n=19) | P-value |
|---|
| Women, n (%) | 24 (66.7) | 11 (64.7) | 13 (68.4) | 0.546a |
| Age (years), n
(%) | 39.5±15.4 | 42.5±15.9 | 36.8±14.8 | 0.276b |
| >60 years old, n
(%) | 6
(16.7) | 3
(17.6) | 3
(15.8) | 0.614a |
| Body mass
index | 30.2±6.8 | 31.2±8.5 | 29.3±5.0 | 0.402b |
| Obesity, n (%) | 17 (47.2) | 7
(41.2) | 10 (52.6) | 0.363a |
| Diabetes, n
(%) | 2 (5.6) | 1 (5.9) | 1 (5.3) | 0.729a |
| High blood
pressure, n (%) | 3 (8.3) | 1 (5.9) | 2
(10.5) | 0.543a |
| Asthma, n (%) | 1 (2.8) | 0 (0.0) | 1 (5.3) | 0.543a |
| Smoking, n (%) | 1 (2.8) | 1
(5.9%) | 0 (0.0) | 0.472a |
| Progression
timee | 3.2±2.2 | 2.6±1.7 | 3.7±2.5 | 0.127b |
| Body temperature
(°C) | 36.9±0.8 | 37.0±1.0 | 36.7±0.6 | 0.228b |
| %
SpO2 | 96.3±2.0 | 96.4±1.6 | 96.2±2.3 | 0.831b |
| SpO2
<94%, n (%) | 6
(16.7) | 2
(11.8) | 4
(21.1) | 0.386a |
| Degree of
dyspnea | 1.0±1.8 | 1.3±1.7 | 0.8±1.7 | 0.244c |
| Symptom
severityf | 6.5±2.7 | 6.5±2.6 | 6.6±2.8 | 0.907c |
| No. of
symptomsg | 11.5 (5.0) | 11.7 (4.0) | 11.0 (4.0) | 0.468b |
| Disease
severity |
|
|
| 0.655d |
| (WHO)h,
n (%) |
|
Mild | 32 (88.9) | 15 (88.2) | 17 (89.5) |
|
|
Moderate | 4
(11.1) | 2
(11.8) | 2
(10.5) |
|
|
Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| Number of drugs
used for COVID-19i | 1.7±1.2 | 1.7±1.3 | 1.6±1.1 | 0.743b |
|
Paracetamol, n (%) | 36 (100) | 17
(100.0) | 19
(100.0) | 0.957a |
|
NSAIDsj, n (%) | 4
(11.1) | 2
(11.8) | 2
(10.5) | 0.906a |
|
Ivermectin, n (%) | 3 (8.3) | 1 (5.9) | 2
(10.5) | 0.619a |
|
Antibiotics, n (%) | 4
(11.1) | 2
(11.8) | 2
(10.5) | 0.906a |
|
Antivirals, n (%) | 2 (5.6) | 1 (5.9) | 1 (5.3) | 0.935a |
|
Steroids, n (%) | 1 (2.8) | 1 (5.9) | 0 (0.0) | 0.759a |
|
Vitamins, n (%) | 2 (5.6) | 1 (5.9) | 1 (5.3) | 0.935a |
|
Mucolitic, n (%) | 2 (5.6) | 1 (5.9) | 1 (5.3) | 0.935a |
Outcome measures and follow-up
There were two co-primary endpoints. The first
primary endpoint was the time for reaching PASS, defined as the
value of symptoms the patient considered to be wellbeing thresholds
of pain and function. The study incorporated the most widely used
anchoring question to identify PASS cut-off points, which was the
following: ‘Taking into account all your daily activities, do you
consider your current state satisfactory, in relation to pain level
and functional impairment?’ The response options were ‘Yes’ or ‘No’
(4). Treatment success was defined
as a PASS answered in the affirmative on days 1–14 of
follow-up.
The second endpoint was the last day the patient
presented with each symptom of COVID-19. Fever, fatigue,
arthralgia, myalgia, headache, sore throat, nausea, vomiting,
diarrhea, dizziness, conjunctivitis, rhinorrhea, exanthema, skin
rash, and loss of sense of smell or taste were catalogued as
present or absent for each day of follow-up. Adverse events were
monitored by the researchers through anamnesis. Follow-up was
carried out for at least 14 days or until patient outcome (cure or
death). Daily follow-up was suspended in the hospitalized patients.
From the first day of hospital admission, their registers were
considered lost data and were not considered in the analysis from
that day forward. The exception was the PASS, and its subsequent
registers were reported as a negative acceptable symptom state.
Nevertheless, the general aspects of hospitalization and outcome
(cure or death) of those patients were registered.
The patients also had a baseline disease severity
classification of mild, moderate, severe, or critical disease, as
previously described and as directed by the WHO interim clinical
management guidance (22,23). In addition, the concepts of
asymptomatic patients (0 major symptoms and 0 minor symptoms) and
pauci-symptomatic patients (0 major symptoms and 1–2 minor
symptoms) were considered, as previously defined (major symptoms:
Fever >37.8°C and new persistent cough; minor symptoms:
Hoarseness, non-persistent cough, sore throat, runny or stuffy
nose, shortness of breath, wheezing, headache, muscle aches, nausea
and/or vomiting and/or diarrhea, and loss of sense of taste or
smell) (24). The patients were
also evaluated using a severity score, which was determined by the
response to the question: ‘Considering all the ways in which
illness and health conditions may affect you at this time, please
indicate below how you are doing?’ The response options were
measured on the 0–10 visual analogue scale (VAS), from ‘very well’
(score of 0) to ‘very poorly’ (score of 10), as previously
described (4). That question is
validated in the routine assessment of patient index data 3
(RAPID3) (25), previously used on
non-hospitalized patients with COVID-19, in which 0 indicates ‘no
symptoms’ and 10 indicates ‘severe symptoms’ (4).
Blinding
The patients, the clinicians evaluating them, and
the physicians that performed the statistical analyses were blinded
to the assigned therapy group.
Sample size
The sample size calculation was based on the number
of patients that had a PASS on day 8. It was estimated that 95% of
the patients in the experimental group and 56% of the controls
would reach a PASS on day 8, based on previously published data
that described achieving a PASS on day 5 of treatment in mild
COVID-19 (4). A total of 36
patients were needed to reach the required power (0.8), when the
statistical analysis was performed at the level of the two-tailed
alpha value (0.05) (26). At the
end of the study, the statistical power for detecting a difference
between two distinct groups was calculated, utilizing the number of
patients with a PASS on day 8, resulting in 84.7% (26).
Statistical analysis
Data are presented as the mean ± standard deviation
(for data with normal distribution), and as median with
interquartile range (IQR) for data with a non-normal distribution
or percentages. Normal data distribution was first determined using
the Kolmogorov-Smirnov test, and the equality of variances was
confirmed using Levene's test. Numerical data with normal
distribution (e.g., body mass index or age) were compared between
groups, utilizing an unpaired Student's t-test, whereas ordinal
data were compared using the Mann-Whitney U test. Categorical
values were compared using Fisher's exact test or the likelihood
ratio Chi-squared test. Kaplan-Meier analyses were performed with
the use of log-rank tests. Binary logistic regression analyses were
employed to determine the probability of achieving a PASS on day 8
(binomial outcome of yes or no), in the mefenamic acid group,
compared with the placebo group. Data were summarized as relative
risks (RRs) with 95% confidence intervals (CIs) and P-values,
adjusted for age, sex, obesity, diabetes, hypertension, progression
time and baseline severity.
Statistical analysis was carried out using SPSS
version 20 software (IBM Corp.), with the exception of the number
needed to treat (NNT), which was calculated using MedCalc v17.7.2
software (MedCalc Software Bvba), and sample size and statistical
power, which were calculated using online ClinCalc.com software
(ClinCalc LLC), to compare two proportions: Two-sample, two-sided
(26). A value of P<0.05 was
considered to indicate a statistically significant difference.
Sample size and statistical power were calculated for a one-tailed
test. The remainder of the analyses were two-tailed tests.
Results
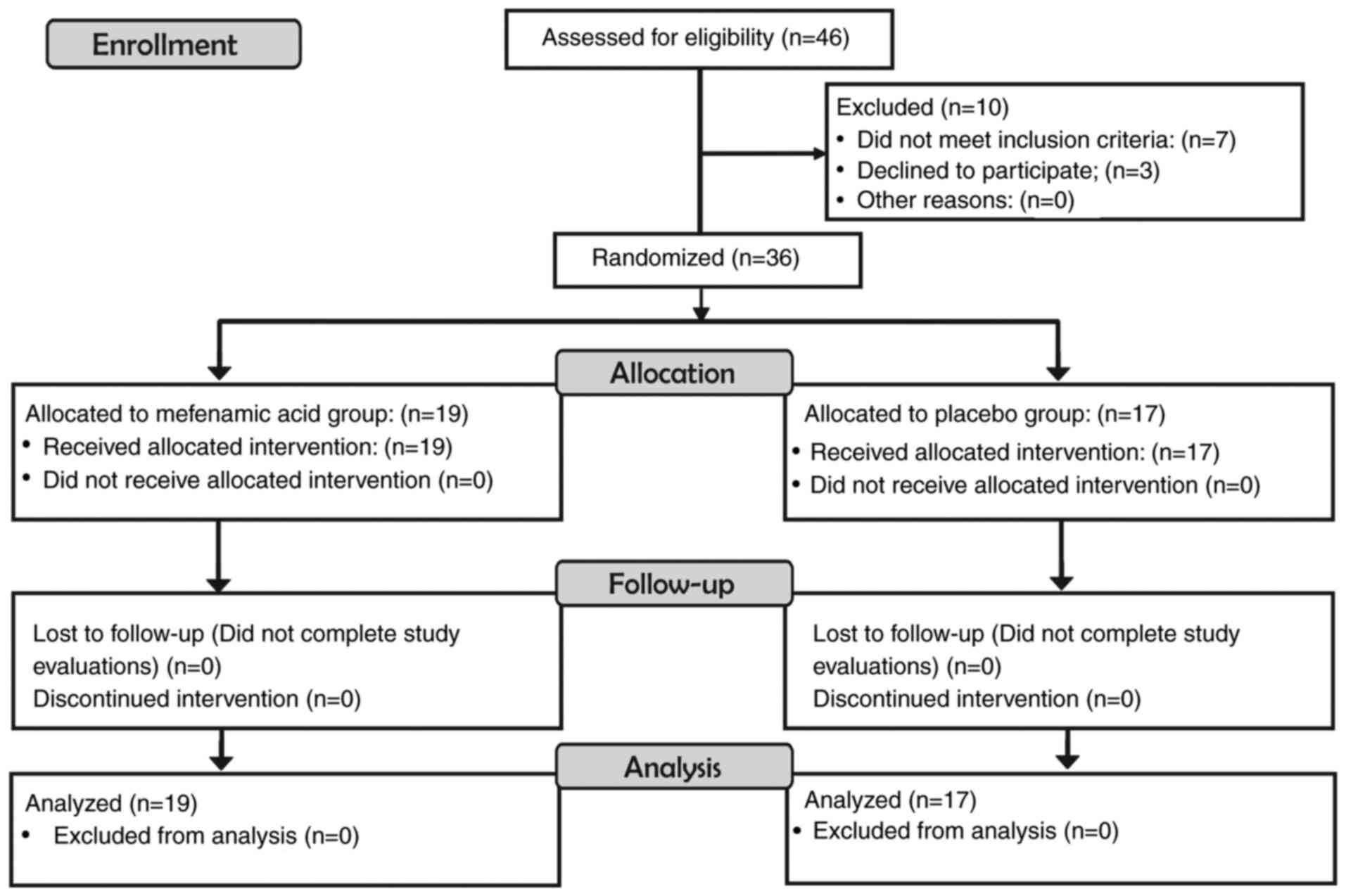
A total of 36 patients were randomized and analyzed.
A total of 19 patients in the experimental group and 17 patients in
the control group agreed to participate in the study. No patient
discontinued the intervention (Fig.
1).
The clinical severity of SARS-CoV-2 infection for
all the patients in the present study was mild (n=32, 88.9%) and
moderate (n=4, 11.1%), according to the WHO interim clinical
management guidance (22,23). The symptom severity score for all
the patients, according to a self-assessment 10-point visual
analogue scale, was 6.5±2.7, whereas the reported median number of
COVID-19-compatible symptoms was 11.5 (IQR, 5). None of the
patients were asymptomatic or pauci-symptomatic as all required
therapy under the guidance of a healthcare professional. Even
though the patients had no clinical or imaging signs suggestive of
pneumonia, they were very symptomatic. The main clinical
characteristics and prescribed drugs are listed in Table I, together with the homogeneous
characteristics between the groups (experimental vs. control) at
the beginning of the study (Table
I).
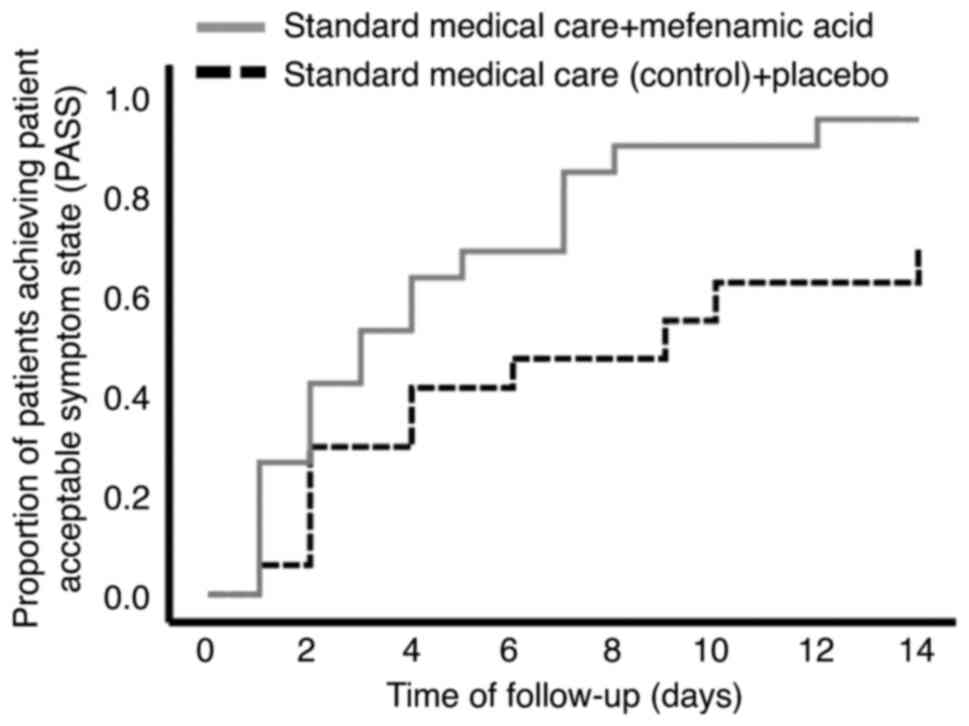
The control group achieved a PASS on day 8.0±1.3,
compared with day 4.4±0.8 in the experimental group (P=0.020,
derived from Kaplan-Meier analyses using log-rank tests) (Table II and Fig. 2). The univariate and multivariate
analyses revealed that patients receiving mefenamic acid plus
standard medical care had an ~16-fold higher probability of
achieving PASS on day 8 (adjusted RR, 15.57; 95% CI, 1.22-198.71;
P=0.035), compared with the standard medical care plus placebo
group (Table III). For 1 patient
to achieve the benefit of PASS on day 8 or earlier, the number of
patients needed to be treated with mefenamic acid was 2 patients
(NNT 2.4; 95% CI, 1.50-5.89; P=0.029). Only 1 patient required
hospitalization and there were no deaths, with no statistically
significant differences between the groups, regarding those
parameters (Table III).
 | Table II.COVID-19 outcomes in the experimental
and control groups. |
Table II.
COVID-19 outcomes in the experimental
and control groups.
| Outcome | Control (n=17) | 95% CI | Experimental
(n=19) | 95% CI |
P-valuea |
|---|
| Days for PASS | 8.0±1.3 | 5.4-10.7 | 4.4±0.8 | 2.5-5.4 | 0.020 |
| PASS on day 8 | 52.9% |
| 94.7% |
| 0.005 |
| Hospitalized | 0.0% |
| 5.3% |
| 0.528 |
| Death | 0.0% |
| 0.0% |
| - |
 | Table III.Relative risk ratio values for
reaching PASS on day 8 in patients with COVID-19 treated with
mefenamic acid, compared with the placebo (control group). |
Table III.
Relative risk ratio values for
reaching PASS on day 8 in patients with COVID-19 treated with
mefenamic acid, compared with the placebo (control group).
|
|
| 95% CI |
|
|---|
|
|
|
|
|
|---|
| PASS on day 8 | Value | Lower | Upper | P-value |
|---|
| Crude
RRa | 16.00 | 1.72 | 148.43 | 0.015 |
| Adjusted
RRb | 15.57 | 1.22 | 198.71 | 0.035 |
| NNTc | 2.4 | 1.50 | 5.89 | 0.029 |
The results with respect to the last day average of
the symptoms analyzed per group are presented in Table IV. All the symptoms lasted fewer
days in the mefenamic acid group, compared with the placebo group
(Table IV). However, as regards
the symptoms, the difference was statistically significant only in
relation to headache, retro-orbital eye pain and sore throat.
Myalgia, dizziness and rhinorrhea exhibited close to statistically
significant values (Table
IV).
 | Table IV.Last day with the presence of
different signs and symptoms of COVID-19 in the control and
experimental groups. |
Table IV.
Last day with the presence of
different signs and symptoms of COVID-19 in the control and
experimental groups.
|
| Last day with the
sign or symptom |
|
|---|
|
|
|
|
|---|
| Symptom | Control (n=17) | 90% CI | Experimental
(n=19) | 90% CI |
P-valuea |
|---|
| Headache | 8.8±4.9 | 6.6-11.1 | 4.7±4.4 | 3.0-6.5 | 0.008 |
| Fatigue | 10.8±5.5 | 8.3-13.2 | 9.7±4.2 | 7.9-11.4 | 0.250 |
| Myalgia | 6.3±2.5 | 4.1-8.4 | 3.9±3.3 | 2.4-5.3 | 0.065 |
| Sore throat | 4.6±4.2 | 2.7-6.5 | 2.3±1.4 | 1.7-2.9 | 0.029 |
| Cough | 8.4±4.6 | 6.4-10.4 | 8.3±5.1 | 6.0-10.5 | 0.471 |
| RO eye pain | 4.6±4.2 | 3.0-6.2 | 2.3±1.4 | 0.8-4.1 | 0.049 |
| Arthralgia | 4.9±4.4 | 2.8-7.0 | 4.0±4.2 | 2.2-5.7 | 0.277 |
| Fever | 2.0±1.2 | 0.9-3.0 | 2.3±2.1 | 0.6-4.0 | 0.375 |
| Chills | 3.1±3.1 | 1.2-5.0 | 2.1±1.4 | 1.2-3.0 | 0.213 |
| Rhinorrhea | 7.6±5.4 | 5.0-10.1 | 4.4±5.3 | 1.3-7.4 | 0.082 |
| Nausea | 6.2±4.5 | 3.7-8.7 | 5.4±3.7 | 3.4-7.5 | 0.344 |
| Conjunctivitis | 5.5±3.5 | 2.6-8.4 | 4.5±6.1 | 0.1-8.9 | 0.375 |
| Anosmia | 11.0±4.1 | 8.7-13.2 | 10.9±4.2 | 8.5-13.3 | 0.478 |
| Ageusia | 5.1±3.6 | 8.4-13.0 | 3.6±3.2 | 5.9-11.7 | 0.184 |
| Dizziness | 6.5±4.3 | 4.1-8.6 | 3.7±4.1 | 1.2-6.2 | 0.083 |
| Vomiting | 2.0±1.0 | 0.3-3.7 | 2.0±0.8 | 1.0-2.9 | 0.500 |
| Diarrhea | 5.1±1.0 | 3.2-7.1 | 3.6±3.2 | 1.5-5.7 | 0.168 |
As regards possible adverse effects related to
mefenamic acid, 2 (10.5%) patients presented with abdominal
pain/discomfort (grade 1 or 2 gastritis) at some point during the
follow-up; however, symptomatology ceased after emphasizing that
the patients take the medication with meals. All the patients were
administered omeprazole to prevent severe acute NSAID-related
gastroduodenal damage. No laboratory tests were indicated by the
patients' treating physicians during the follow-up. No patient
required definitive suspension of mefenamic acid due to adverse
effects.
Discussion
In the present study, in ambulatory patients with
COVID-19 under standard medical care, the additional administration
of mefenamic acid reduced the duration of the symptomatic illness,
compared with ambulatory patients treated with standard medical
care plus the placebo. Various signs and symptoms, such as
headache, sore throat and retro-orbital eye pain improved at a
significantly more rapid rate with the addition of mefenamic acid
to standard medical care, reducing the time to reach PASS (8.0±1.3
to 4.4±0.8 days, P=0.020) (Tables
II–IV and Fig. 2).
The beneficial effects of the administration of
mefenamic acid can be generally associated with several mechanisms.
The first is symptom reduction due to its antipyretic and analgesic
activities. The reduction of inflammatory processes is another
important effect. Based on the pathogenesis of COVID-19, NSAIDs
have been suggested to be beneficial for the early management of
COVID-19, by possibly ameliorating the inflammatory process leading
to lymphopenia and immunosuppression (10), preventing disease progression, or
even reversing lymphocytopenia.
Progression in patients with COVID-19 has been
associated with the presence of the ‘cytokine storm’ induced by the
virus and the hyper-inflammatory immune response of the host. One
of the most critical pro-inflammatory cytokines of the innate
immune response is IL-6. Mefenamic acid has been shown to exert an
inhibitory effect on IL-6 levels (27), which may be beneficial for reducing
disease progression.
The large majority of NSAIDs owe their
anti-inflammatory effects to the inhibition of prostaglandin
synthesis via cyclooxygenase (COX). Mefenamic acid has also another
anti-inflammatory mechanism: Nucleotide-binding oligomerization
domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome
activation intensely induces cytokine production as an inflammatory
response to viral infection (28).
Therefore, the NLRP3 inflammasome may be a potential target for the
treatment of COVID-19. The NLRP3 inflammasome is responsible for
processing the pro-inflammatory cytokine, IL-1β. Mefenamic acid has
been shown to selectively inhibit the NLRP3 inflammasome and the
release of IL-1β, independent of its COX-mediated anti-inflammatory
activity (20,29).
It has also been demonstrated that mefenamic acid
exerts a possible antiviral effect. In a previous study, in
vitro antiviral activity against the Chikungunya virus (CHIKV)
was observed, enhancing the effects of other antivirals, such as
ribavirin (30). Its antiviral
activity has been suggested to be due to interactions with the
viral envelope that lead to the inactivation of the virus,
interfering with its internalization in Vero cells. Such an effect
was not observed with other NSAIDs, including aspirin (30). In addition, mefenamic acid was
previously shown to exert an inhibitory effect on the serine
proteases of an RNA virus (DENV2 NS2B-NS3 protease of the dengue
virus) (19). That antiviral
effect was observed at a concentration of 20–30 mcM (4.8-7.2
mcg/ml) in the in vitro trials, and even at lower
concentrations, including 5 mcM (1.2 mcg/ml), when combined with
ribavirin (19,30). In in vivo experiments
(CHIKV-infected mice), the antiviral effect of mefenamic acid was
observed at a dose of 15 mg/kg (30). To provide context, a maximum plasma
concentration of 3.6-5.9 mcg/ml has been calculated to be reached
in an adult receiving 250 mg oral mefenamic acid, varying in each
individual and depending on the formulation employed (31). At an oral dose of 1 g administered
to an adult, the maximum plasma concentration has been reported at
up to 10 mcg/ml (32). Of note, in
the present clinical trial, the dose employed for patients with
COVID-19 was 500 mg, administered three times a day, for 7 days.
Thus, a probable antiviral effect of mefenamic acid after customary
doses of the drug may be reached, albeit further studies are
required to confirm the present findings. Previous in silico
analyses have demonstrated that mefenamic acid is able to interact
with the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) amino acid
residues via a predominant metal coordination bond and hydrogen
bonding with the active site (33). RdRp is a target protein in
SARS-CoV-2 that has been validated and extensively studied for drug
development, with respect to COVID-19 (33). Given that mefenamic acid has been
shown to potentiate the effect of antiviral drugs, such as
ribavirin (30), perhaps it could
enhance the effect of antivirals used against SARS-CoV-2, such as
remdesivir, molnupiravir or favipiravir (34).
Mefenamic acid is often used in the treatment of
dysmenorrhea and heavy menstrual bleeding, at an oral dose of 1.5 g
per day for 3–5 days; however, it has also been administered for
prolonged periods of up to 6 months (35,36).
The drug is generally well-tolerated. The most common adverse
effects are related to symptoms of gastritis. Therefore, taking the
medication with meals, and receiving omeprazole throughout the
study, was a prime necessity. Contraindications for and precautions
regarding its use should be evaluated in each patient, adhering to
the widely described recommendations in the medical literature
(37).
The present study has several limitations. All the
patients included in the study were unvaccinated and the majority
had mild COVID-19; therefore, other studies on patients with severe
forms of the disease or vaccinated patients are warranted. Of note
however, disease presentation in the majority of symptomatic
patients with COVID-19 is mild or moderate; thus, the results of
the present study are very relevant for community use. The sample
size in the present study was sufficient and had adequate
statistical power to determine the efficacy of mefenamic acid for
more rapidly reaching an acceptable symptom state. However, an
analysis with a greater number of patients and a follow-up
including laboratory parameters, or serial respiratory viral load
(to confirm the possible antiviral effect of mefenamic acid), is
recommended in future investigations. Notably, other NSAIDs, such
as ibuprofen and diclofenac, have been shown to be superior to the
currently used paracetamol, in patients with mild COVID-19
infection (15,16). Thus, further studies comparing the
effects of various NSAIDs are required.
In conclusion, the present study demonstrated that
the administration of mefenamic acid markedly reduced
symptomatology and the time to reach PASS in ambulatory patients
with COVID-19. Its administration was well-tolerated and there were
no notable adverse effects. Given its probable antiviral effects
and potent anti-inflammatory mechanisms, it may prove to be useful
in the treatment of COVID-19, in combination with other drugs,
including the new antivirals, such as remdesivir, molnupiravir or
favipiravir (34). Nevertheless,
future studies are required to analyze these aspects.
Acknowledgements
The authors wish to thank Gusti Gould for the
English language editing of the manuscript. Mefenamic acid was
donated by the Foundation for Cancer Ethics, Education, and
Research of the Cancerology State Institute, Colima 28085,
Mexico.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JGE, IDE, HRGS, IPRS, JDE and MLMF designed the
study and wrote the manuscript. HPGS, ACCV, JAGS, KAMR, CBCA, MWG,
OBG, OGDE, HSGG and DCC visited and evaluated the patients. BAPM,
FRL, VM and IDE designed and performed the statistical analysis.
AGS and EMZ authorized and coordinated the recruitment of patients
in the hospital. JDE and OGDE were the administrative coordinators
of the clinical trial. IDE and HPGS confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Mexican Social Security Institute, delegation of
the State of Colima, Mexico (July 29, 2020), and written informed
consent was obtained from all the participants. All procedures
performed in the present protocol were carried out in accordance
with the Declaration of Helsinki and the clinical trial was
registered as MEFECOVID-19: RPCEC00000388 in the Cuban Public
Registry of Clinical Trials (RPCEC) database (August 31, 2021).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Caldera-Villalobos C, Garza-Veloz I,
Martínez-Avila N, Delgado-Enciso I, Ortiz-Castro Y, Cabral-Pacheco
GA and Martinez-Fierro ML: The coronavirus disease (COVID-19)
challenge in Mexico: A critical and forced reflection as
individuals and society. Front Public Health. 8:3372020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alene M, Yismaw L, Assemie MA, Ketema DB,
Mengist B, Kassie B and Birhan TY: Magnitude of asymptomatic
COVID-19 cases throughout the course of infection: A systematic
review and meta-analysis. PLoS One. 16:e02490902021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in china: Summary of a report of 72314 cases from the
Chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delgado-Enciso I, Paz-Garcia J,
Barajas-Saucedo CE, Mokay-Ramírez KA, Meza-Robles C, Lopez-Flores
R, Delgado-Machuca M, Murillo-Zamora E, Toscano-Velazquez JA,
Delgado-Enciso J, et al: Safety and efficacy of a COVID-19
treatment with nebulized and/or intravenous neutral electrolyzed
saline combined with usual medical care vs. usual medical care
alone: A randomized, open-label, controlled trial. Exp Ther Med.
22:9152021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fagni F, Simon D, Tascilar K, Schoenau V,
Sticherling M, Neurath MF and Schett G: COVID-19 and
immune-mediated inflammatory diseases: Effect of disease and
treatment on COVID-19 outcomes and vaccine responses. Lancet
Rheumatol. 3:e724–e736. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q,
Wu Y, Sun L and Xu Y: Prevalence and severity of corona virus
disease 2019 (COVID-19): A systematic review and meta-analysis. J
Clin Virol. 127:1043712020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tenforde MW, Kim SS, Lindsell CJ, Billig
Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS,
Smithline HA, et al: Symptom duration and risk factors for delayed
return to usual health among outpatients with COVID-19 in a
multistate health care systems network-United States, March-June
2020. MMWR Morb Mortal Wkly Rep. 69:993–998. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Manjili RH, Zarei M, Habibi M and Manjili
MH: COVID-19 as an acute inflammatory disease. J Immunol.
205:12–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vivarelli S, Falzone L, Torino F,
Scandurra G, Russo G, Bordonaro R, Pappalardo F, Spandidos DA,
Raciti G and Libra M: Immune-checkpoint inhibitors from cancer to
COVID-19: A promising avenue for the treatment of patients with
COVID-19 (Review). Int J Oncol. 58:145–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dehelean CA, Lazureanu V, Coricovac D,
Mioc M, Oancea R, Marcovici I, Pinzaru I, Soica C, Tsatsakis AM and
Cretu O: SARS-CoV-2: Repurposed drugs and novel therapeutic
approaches-insights into chemical structure-biological activity and
toxicological screening. J Clin Med. 9:20842020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARS-CoV-2 infection: Mechanistic insights into current
COVID-19 therapies (Review). Int J Mol Med. 46:467–488. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stancioiu F, Papadakis GZ, Kteniadakis S,
Izotov BN, Coleman MD, Spandidos DA and Tsatsakis A: A dissection
of SARS-CoV2 with clinical implications (Review). Int J Mol Med.
46:489–508. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sherren PB, Ostermann M, Agarwal S,
Meadows CIS, Ioannou N and Camporota L: COVID-19-related organ
dysfunction and management strategies on the intensive care unit: A
narrative review. Br J Anaesth. 125:912–925. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsatsakis A, Calina D, Falzone L, Petrakis
D, Mitrut R, Siokas V, Pennisi M, Lanza G, Libra M, Doukas SG, et
al: SARS-CoV-2 pathophysiology and its clinical implications: An
integrative overview of the pharmacotherapeutic management of
COVID-19. Food Chem Toxicol. 146:1117692020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore N, Bosco-Levy P, Thurin N, Blin P
and Droz-Perroteau C: NSAIDs and COVID-19: A systematic review and
meta-analysis. Drug Saf. 44:929–938. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kelleni MT: Early use of non-steroidal
anti-inflammatory drugs in COVID-19 might reverse pathogenesis,
prevent complications and improve clinical outcomes. Biomed
Pharmacother. 133:1109822021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tripathi SK and Kasture PN: Mefenamic
acid: The evolution of a versatile NSAID. Indian J Clin Pract.
32:115–123. 2021.
|
|
18
|
Aggarwal KK: Mefenamic acid as
steroid-sparing anti-inflammatory drug during viral phase of
Covid-19: 5 case reports. Indian J Clin Pract. 31:759–763.
2021.
|
|
19
|
Rothan HA, Buckle MJ, Ammar YA,
Mohammadjavad P, Shatrah O, Noorsaadah AR and Rohana Y: Study the
antiviral activity of some derivatives of tetracycline and
non-steroid anti inflammatory drugs towards dengue virus. Trop
Biomed. 30:681–690. 2013.PubMed/NCBI
|
|
20
|
Aggarwal KK, Chong YW, Sharma R, Pillai M,
Naidu R, Chan AY, Urabe MU, Cavalcanti D, Budhathoky P, Sajjad Q,
et al: Repurposing mefenamic acid in the management of covid-19. J
Indian Med Assoc. 119:16–23. 2021.
|
|
21
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salepci E, Turk B, Ozcan SN, Bektas ME,
Aybal A, Dokmetas I and Turgut S: Symptomatology of COVID-19 from
the otorhinolaryngology perspective: A survey of 223 SARS-CoV-2
RNA-positive patients. Eur Arch Otorhinolaryngol. 278:525–535.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Health Organization, . Clinical
Management of COVID-19: Interim guidance. 27–May. 2020, World
Health Organization; Geneva: 2020
|
|
24
|
Rivett L, Sridhar S, Sparkes D, Routledge
M, Jones NK, Forrest S, Young J, Pereira-Dias J, Hamilton WL,
Ferris M, et al: Screening of healthcare workers for SARS-CoV-2
highlights the role of asymptomatic carriage in COVID-19
transmission. Elife. 9:e587282020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pincus T, Swearingen CJ, Bergman M and
Yazici Y: RAPID3 (routine assessment of patient index data 3), a
rheumatoid arthritis index without formal joint counts for routine
care: Proposed severity categories compared to disease activity
score and clinical disease activity index categories. J Rheumatol.
35:2136–2147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kane SP: Sample size calculator. ClinCalc.
https://clincalc.com/Stats/SampleSize.aspxJanuary
20–2021
|
|
27
|
Kang BS, Chung EY, Yun YP, Lee MK, Lee YR,
Lee KS, Min KR and Kim Y: Inhibitory effects of anti-inflammatory
drugs on interleukin-6 bioactivity. Biol Pharm Bull. 24:701–703.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao N, Di B and Xu LL: The NLRP3
inflammasome and COVID-19: Activation, pathogenesis and therapeutic
strategies. Cytokine Growth Factor Rev. 61:2–15. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daniels MJ, Rivers-Auty J, Schilling T,
Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS, Baldwin AG,
Freeman S, et al: Fenamate NSAIDs inhibit the NLRP3 inflammasome
and protect against Alzheimer's disease in rodent models. Nat
Commun. 7:125042016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rothan HA, Bahrani H, Abdulrahman AY,
Mohamed Z, Teoh TC, Othman S, Rashid NN, Rahman NA and Yusof R:
Mefenamic acid in combination with ribavirin shows significant
effects in reducing chikungunya virus infection in vitro and in
vivo. Antiviral Res. 127:50–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Etman MA, Farid RM, Nada AH and Ebian AA:
In vitro/in vivo correlation of fast release mephenamic acid
microspheres in humans. Med Princ Pract. 21:223–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shinkuma D, Hamaguchi T, Yamanaka Y and
Mizuno N: Correlation between dissolution rate and bioavailability
of different commercial mefenamic acid capsules. Int J Pharm.
21:187–200. 1984. View Article : Google Scholar
|
|
33
|
Baby K, Maity S, Mehta CH, Suresh A, Nayak
UY and Nayak Y: Targeting SARS-CoV-2 RNA-dependent RNA polymerase:
An in silico drug repurposing for COVID-19. F1000Res. 9:11662020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdelnabi R, Foo CS, Kaptein SJ, Zhang X,
Do TN, Langendries L, Vangeel L, Breuer J, Pang J, Williams R, et
al: The combined treatment of molnupiravir and favipiravir results
in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster
infection model. EBioMedicine. 72:1035952021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guzman-Esquivel J, Mendoza-Hernandez MA,
Tiburcio-Jimenez D, Avila-Zamora ON, Delgado-Enciso J,
De-Leon-Zaragoza L, Casarez-Price JC, Rodriguez-Sanchez IP,
Martinez-Fierro ML, Meza-Robles C, et al: Decreased biochemical
progression in patients with castration-resistant prostate cancer
using a novel mefenamic acid anti-inflammatory therapy: A
randomized controlled trial. Oncol Lett. 19:4151–4160.
2020.PubMed/NCBI
|
|
36
|
Melnikov V, Tiburcio-Jimenez D,
Mendoza-Hernandez MA, Delgado-Enciso J, De-Leon-Zaragoza L,
Guzman-Esquivel J, Rodriguez-Sanchez IP, Martinez-Fierro ML,
Lara-Esqueda A, Delgado-Enciso OG, et al: Improve cognitive
impairment using mefenamic acid non-steroidal anti-inflammatory
therapy: Additional beneficial effect found in a controlled
clinical trial for prostate cancer therapy. Am J Transl Res.
13:4535–4543. 2021.PubMed/NCBI
|
|
37
|
Basri NI, Abd Ghani NA, Mahdy ZA, Abdul
Manaf MR and Mohamed Ismail NA: Celecoxib versus mefenamic acid in
the treatment of primary dysmenorrhea. Horm Mol Biol Clin Investig.
41:2020.PubMed/NCBI
|