Introduction
Rab proteins are a subfamily of the Ras superfamily
of small GTPases that are key regulators of intracellular membrane
trafficking, from the formation of transport vesicles to their
fusion with membranes. These proteins can be detected in an
inactive or active conformation, depending on the nucleotide-bound
status, and are considered switches that cycle between an active,
membrane-associated status and an inactive cytosolic status. To
date, >70 mammalian Rab proteins have been identified (1). Some Rab proteins exhibit a
regulated expression, tissue-specific expression, or
developmental-specific expression, while others are ubiquitously
expressed (1). Each Rab protein
exhibits a characteristic subcellular distribution (2).
Rab1a regulates vesicular protein transport from the
endoplasmic reticulum to the Golgi apparatus (3,4)
and to the cell surface (5). It
also plays a role in secretion of interleukin (IL)-8 and growth
hormones. Rab1a function has been implicated in neuronal
differentiation (6) and cardiac
development (7). The
overexpression of Rab1a in transgenic mice has been shown to be
associated with an increased cardiac mass and cardiac hypertrophy,
leading to cardiomyopathy (7).
Rab1a activity is also targeted by bacterial (8-10) and viral pathogens (11). Additionally, Rab1a regulates the
mTORC1 pathway in glucose homeostasis (12), colorectal cancer (13), liver cancer development (14) and breast cancer cells (15).
The guanosine nucleotide diphosphate (GDP)
dissociation inhibitor (GDI) proteins regulate the Rab family
GTPase function by binding to Rab GTPase in its GDP-bound inactive
form to retrieve it from the cell membrane and to maintain a
soluble pool of inactive proteins ready to be re-used (16). The GDI family includes the GDI1
and GDI2 proteins. GDI1 is expressed primarily in neural and
sensory tissues, whereas GDI2 is ubiquitously expressed (17).
In a recent study, it was demonstrated by the
authors that GDI2 binds to the immunoreceptor tyrosine-based
inhibitory motif (ITIM) domain of sialic acid-binding
immunoglobulin-type lectin G (Siglec-G) in hematopoietic cells,
such as B-1a cells under conditions of normal homeostasis, whereas
Rab1a is recruited to the ITIM domain during bacterial infection
(18). Therefore, it was
hypothesized that GDI2 and Rab1a may regulate the immune response
through interaction with the ITIM domain during bacterial
infection. The present study thus aimed to explore the function of
Rab1a in vivo by generating a Rab1a null mutant model
with a trapped Rab1a gene. The homozygous deletion of the
Rab1a gene resulted in early embryonic lethality. Rab1a
protein was expressed from the trapped gene during early
post-implantation development, suggesting a critical role of Rab1a
in the transport of materials between organelles in eukaryotic
cells.
Materials and methods
Reagents
Rabbit anti-mouse Rab1a antibodies (cat. no. sc-311)
were obtained from Santa Cruz Biotechnology, Inc. and
lipopolysaccharide (LPS; from Escherichia coli (E. coli)
055:B5 strain] were purchased from MillliporeSigma. Goat
anti-mouse β-actin (cat. no. sc-1615) and horse- radish peroxidase
(HRP)-conjugated goat anti-rabbit IgG antibodies (cat. no. sc-2004)
were purchased from Santa Cruz Biotechnology, Inc.
5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-gal) was
obtained from Thermo Fisher Scientific, Inc.
Generation of Rab1a mutant mice
A male chimeric mouse generated from the ES cell
line, XB498, was obtained from Bay Genomics, LLC. The ES cell line,
XB498, was generated by using a gene trap protocol with the
trapping construct pGT0pfs containing the intron from the engrailed
2 homeobox gene upstream of the gene encoding the
β-galactosidase/neomycin-resistance fusion protein (please see
Fig. 1 and https://igtc.org/cgi-bin/annotation.py?cellline=XB498).
Genotyping was determined by the polymerase chain reaction (PCR)
analysis of DNA from tail biopsies, as previously described
(19).
PCR-based genotyping of mice
Aliquots of 0.1 μg (10 μl) DNA were
mixed with 10 μl of 2X GoTaq Green Master Mix buffer
(Promega Corporation) and 10 pmol of each primer, as previously
described (19). PCR
amplification was carried out at 96°C for 2 min, with 35 cycles of
96°C for 10 sec, 55°C for 30 sec, and 72°C for 60 sec. To screen
for the homologous recombination of DNA, the following primers were
used: P1, 5′-ACT GAG TAT CCC TGG CTG GC-3′ and P2, 5′-AAG AGT AGG
CTA GCC AGT CA-3′. The wild-type (WT) allele was not amplified (no
band was detected), while the mutant allele produced a 300-bp band
corresponding to the amplification product. The following primers
were also used to confirm the presence or absence of the WT allele:
P3, 5′-AGC ACA GAC AAG CAC AGT AG-3′ and P4, 5′-GTT ATC AGG CTT GGC
AGC AG-3′. The amplification of the WT allele produced a 485-bp
band, while the mutant allele was not amplified and therefore
produced no band. Therefore, WT mice (Rab1a+/+)
produced a WT 485-band and homozygous mice
(Rab1a−/−) produced a 300-bp band, while
heterozygous mice (Rab1a+/−) produced both a WT
485-bp band and a mutant 300-bp band. The PCR products were
separated by agarose gel electrophoresis, stained with
SYBR® Safe DNA Gel Stain (Thermo Fisher Scientific,
Inc.) and visualized using Axygen Gel Documentation System
(Corning, Inc.).
X-gal staining of mouse tissue and mouse
embryos
The X-gal staining of tissues (frozen sections for
E7 and E15 embryos and adult small intestine samples were prepared
from WT or Rab1a+/− mice) was performed using
standard procedures, as previously described (20). Embryos were embedded in optimal
cutting temperature (OCT) compound and subjected to cryo-sectioning
to generate slices that were 8-μm-thick. The cryosections
were fixed in X-gal fixation buffer (0.1 M phosphate buffer, pH
7.3, 5 mM EGTA, 2 mM MgCl2, 0.2% glutaraldehyde) for 15
min, washed three times with X-gal wash buffer (0.1 M phosphate
buffer, pH 7.3, 2 mM MgCl2), and stained overnight at
37°C in X-gal staining buffer [0.1 M phosphate buffer, pH 7.3, 2 mM
MgCl2, 5 mM K4Fe(CN)6,
3H2O, 5 mM K3Fe(CN)6, 1 mg/ml
X-gal]. The stained sections were washed three times with X-gal
wash buffer and mounted in Aquatex® aqueous mounting
medium (MilliporeSigma). Images were acquired using an EVOS FL Auto
Imaging System (Thermo Fisher Scientific, Inc.).
Experimental animal models
The Rab1a mutant male mouse was from the
Mutant Mouse Regional Resource Center (MMRRC) at the University of
California, Davis (UC Davis). For backcrossing, two WT C57BL/6
female were obtained from Jackson Laboratory. A total of 159 (male,
53; female, 106) adult mice (Rab1a+/+, 16;
Rab1a+/−, 16 for LPS treatment experiments and
Rab1a+/−, 127 for producing pups and embryos)
(6-8 weeks of age; weight, 20-25 g), as well as 250 pups and 77
embryos were produced in the laboratory of Dr GYC and used in the
present study. The mice were maintained in individually ventilated
cages (25°C and 55-65% humidity) with a 12/12-h light/dark cycle
and free access to standard laboratory mouse chow and water. Age-
and sex-matched WT littermates were used as controls for
heterozygous Rab1a+/− or homozygous
Rab1a−/− mice. All procedures involving animals
were approved by the University of Tennessee Health Science Center
(UTHSC) Animal Care and Use Committee (IACUC), protocol nos. 17-117
(approved January 29, 2018) and 20-0211 (approved January 26,
2021). At the end of each experiment, adults and neonates >10
days of age were euthanized with CO2 followed by
cervical dislocation. The CO2 displacement rate for a
euthanasia chamber is 30-70% per min (as a percentage of the
chamber volume per minute). To assess the period of developmental
failure, pregnant mice were euthanized with CO2 followed
by cervical dislocation and embryos from heterozygote hybridization
were collected on embryonic day (E)10.5, E11.5, E12.5 and E14.5,
and genotyped using PCR as stated above. Neonates <10 days of
age were euthanized with 5% isoflurane followed by decapitation
with scissors. For the mouse model of endotoxemia, age, sex and
weight-matched WT and Rab1a+/− mice were injected
(i.p.) with 10 mg/kg LPS in PBS. The mice were monitored for up to
5 days.
Western blot analysis
Embryo lysates were prepared by incubation in lysis
buffer (20 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, pH 7.6,
including protease inhibitors, 1 μg/ml leupeptin, 1
μg/ml aprotinin and 1 mM phenylmethylsulfonyl fluoride),
sonication, centrifugation was performed at 4°C and at 16,200 × g
for 5 min to remove cell debris. Proteins in the lysates were
determined by BCA and separated on 10% SDS-PAGE gels, transferred
to PVDF membranes, and then examined by western blotting, as
previously described (21).
After blocking with 5% skimmed milk in PBS-T (PBS with 0.01%
Tween-20) at room temperature for 1 h, the blots were incubated
with goat anti-mouse β-actin primary antibodies (1:1,000 dilution)
at 4°C overnight. The membranes were then incubated with
horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
secondary antibodies (1:5,000 dilution) at room temperature for 2 h
and the signal was detected using a luminol-based enhanced
chemiluminescence (ECL) kit (Santa Cruz Biotechnology, Inc.).
Histological analysis and
immunohistochemistry
Tissues from WT or mutant mice were fixed in 4%
paraformaldehyde at room temperature for 24 h, dehydrated and
embedded in paraffin according to the standard procedure and as
previously described (19).
Sections at a thickness of 5 μm were cut, dewaxed in xylene,
dehydrated in 100% ethanol and then stained with hematoxylin and
eosin [H&E; hematoxylin (cat. no. SH26-500D, Fisher Chemical;
Thermo Fisher Scientific, Inc.) for 3 min and eosin (cat. no.
S176-16OZ, Poly Scientific R&D Corp.) for 30 sec] at room
temperature or reacted with anti-mouse Rab1a antibody (1:1,000;
cat. no. sc-311, Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. The sections were washed in phosphate-buffered
saline (PBS) and subsequently incubated with HRP-conjugated goat
anti-rabbit secondary antibodies (1:1,000; cat. no. sc-2004, Santa
Cruz Biotechnology, Inc.) at room temperature for 30 min. After
being washed in PBS, slides were developed with
3,3′-diaminobenzidine (DAB) and counterstained with hematoxylin for
10 sec at room temperature. For the control, immunohistochemical
staining was performed by omitting the primary antibody. No
significant staining was observed upon control staining (data not
shown). Images were acquired using an EVOS FL Auto Imaging System
(Thermo Fisher Scientific, Inc.).
Measurement of inflammatory cytokine
levels
Blood samples were obtained at the indicated time
points and cytokines in the serum and measured using a mouse
cytokine bead array designed for inflammatory cytokines (cat. no.
552364; BD Biosciences), as previously described (21-24). The kit quantitatively detects the
levels of the cytokines, IL-6, IL-10, monocyte chemoattractant
protein-1 (MCP-1), interferon-γ (IFN-γ), tumor necrosis factor
(TNF) and the IL-12 heterodimer (IL-12p70).
Statistical analysis
The differences in cytokine concentrations were
analyzed using two-tailed t-tests in single pairwise comparisons
calculated with Excel (Microsoft). Data are presented as the mean ±
SD. A value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Generation of Rab1a mutant mice
To determine the function of Rab1a in vivo,
we obtained a Rab1a mutant mouse from MMRRC which was
generated by the blastocyst injection of a Rab1a trapped
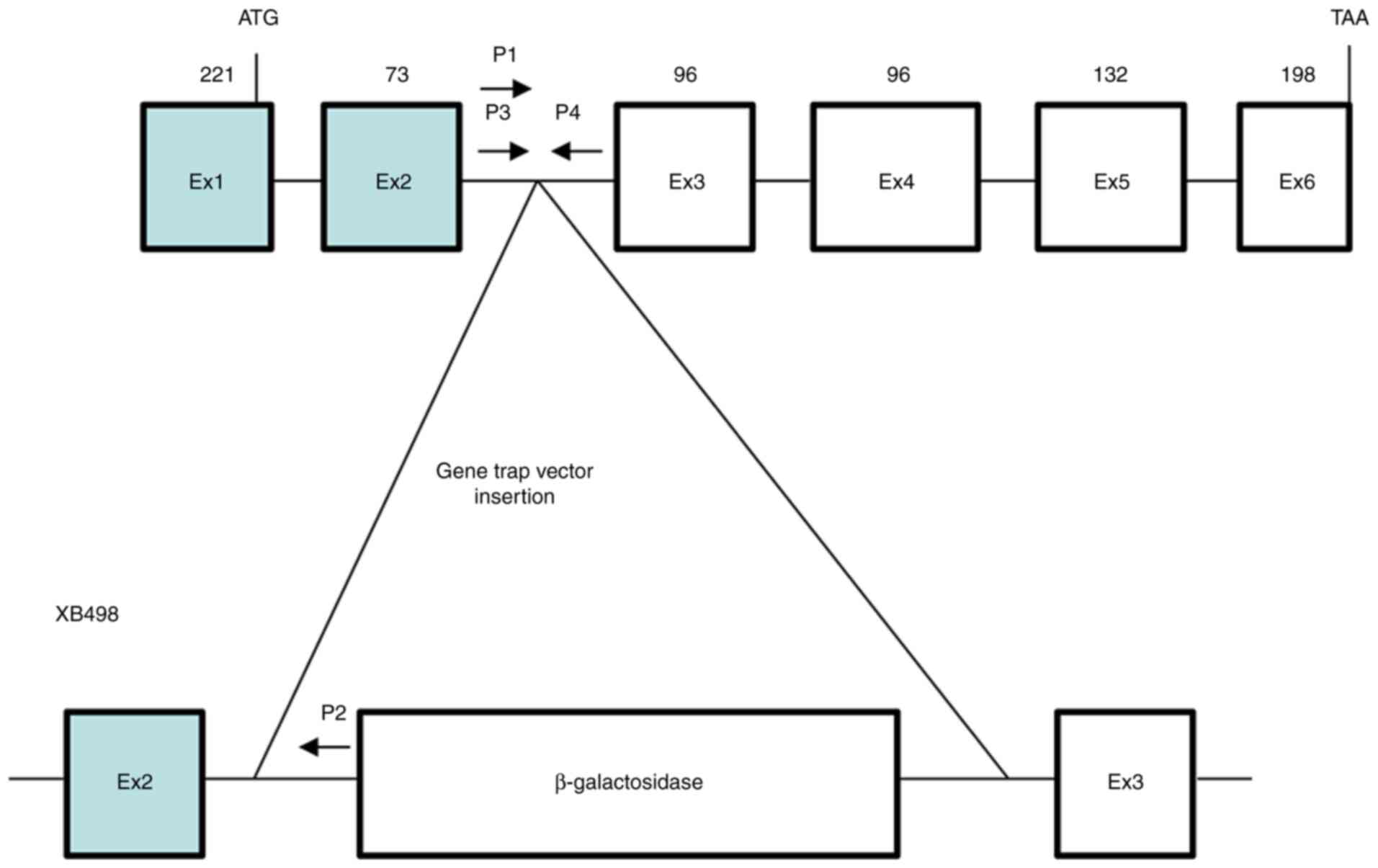
embryonic stem cell clone (XB498, Bay Genomics). Gene disruption
was caused by the insertion of the retroviral gene trap vector
pGT0pfs containing a promoterless β-galactosidase reporter gene.
Selection for the expression of the gene requires transcription
from an endogenous cellular promoter and consequently, a mutation
in a cellular gene. The expression of the tagged gene can be
examined by staining for β-galactosidase. The methods used for gene
trap mutagenesis have been previously reported (25-27). In the XB498 ES cell line, the
gene trap vector pGT0pfs was inserted between exons 2 and 3 of
Rab1a (Fig. 1 and
Data S1); the point of
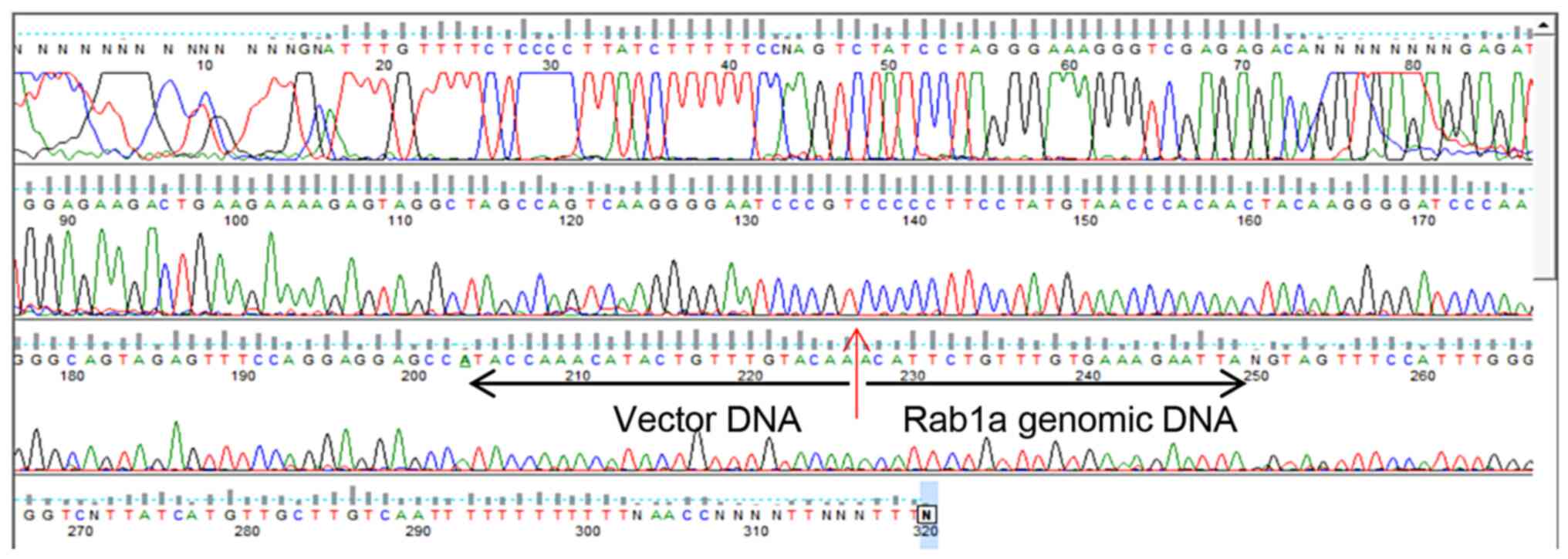
insertion was confirmed by PCR and DNA sequencing (Fig. 2 and Data S2). Offspring were genotyped by
PCR analyses using primers P1 and P2 for the knockout (KO) PCR and
primers P3 and P4 for the WT PCR (Fig. 1).
Chimeric male offspring were mated with WT C57Bl/6
mice to test for the germline transmission of the disrupted
Rab1a allele. Heterozygous Rab1a+/− mice
were viable and displayed no obvious abnormality in weight or
fertility during a 12-month observation period (data not shown). To
remove contaminating background heterozygosity,
Rab1a+/− mice were backcrossed >10 generations
with C57BL/6 mice.
Expression of Rab1a in mice
The expression of β-galactosidase is controlled by
the endogenous Rab1a gene promoter. Thus, β-galactosidase
expression was used in Rab1a+/− mice to document
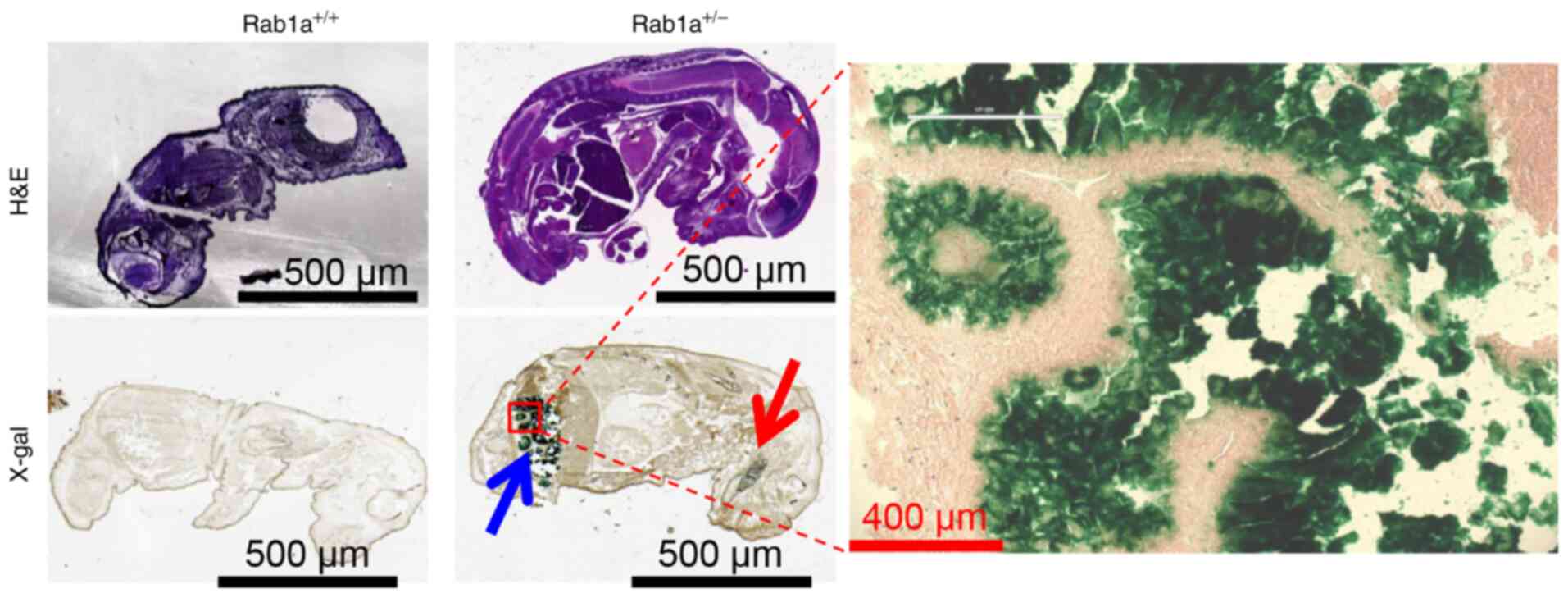
the pattern of Rab1a expression in mouse embryos. To visualize the
expression pattern of Rab1a, X-gal staining of cryosections
of Rab1a+/+ and Rab1a+/− E7
embryos was performed; sections of WT embryos served as the
negative controls. Rab1a was ubiquitously expressed in whole
embryos (Fig. 3). To investigate
endogenous Rab1a protein expression, WT E7 embryos were collected,
sectioned and immunostained with anti-Rab1a antibodies. Similar to
the Rab1a gene, Rab1a protein was ubiquitously expressed in
the whole embryo (Fig. 3). Based
on these findings, it was concluded that Rab1a protein expression
was consistent with Rab1a β-galactosidase activity.
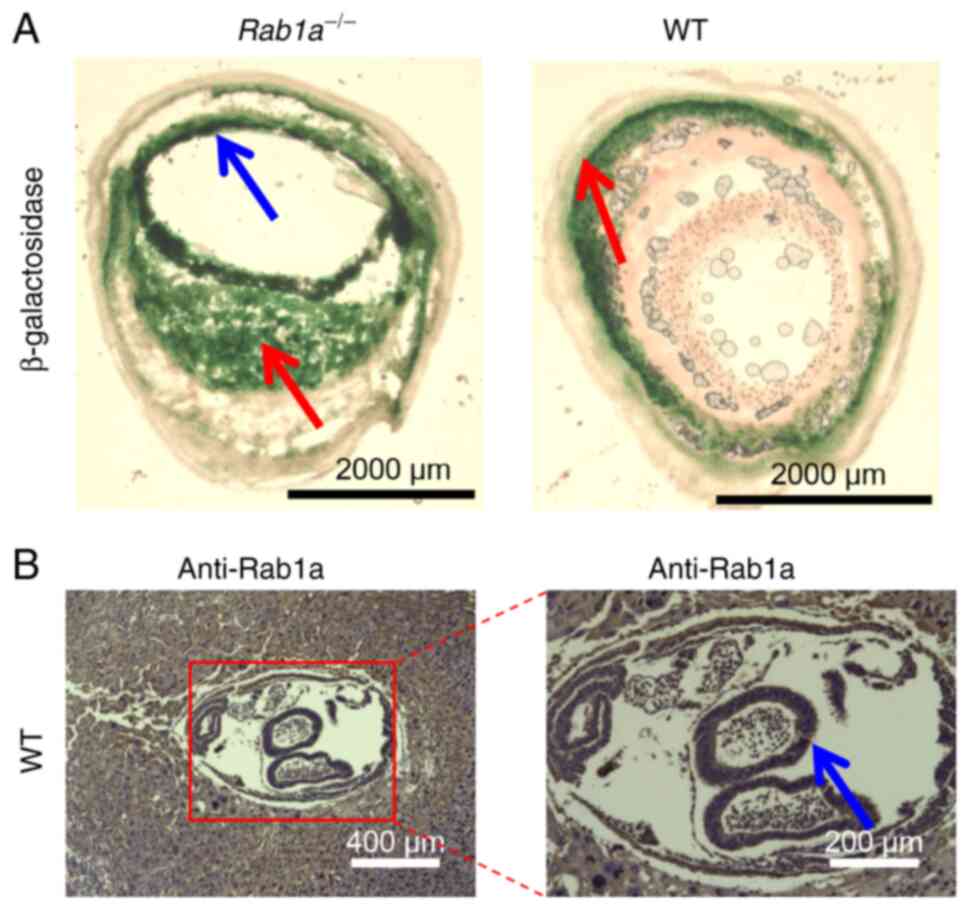
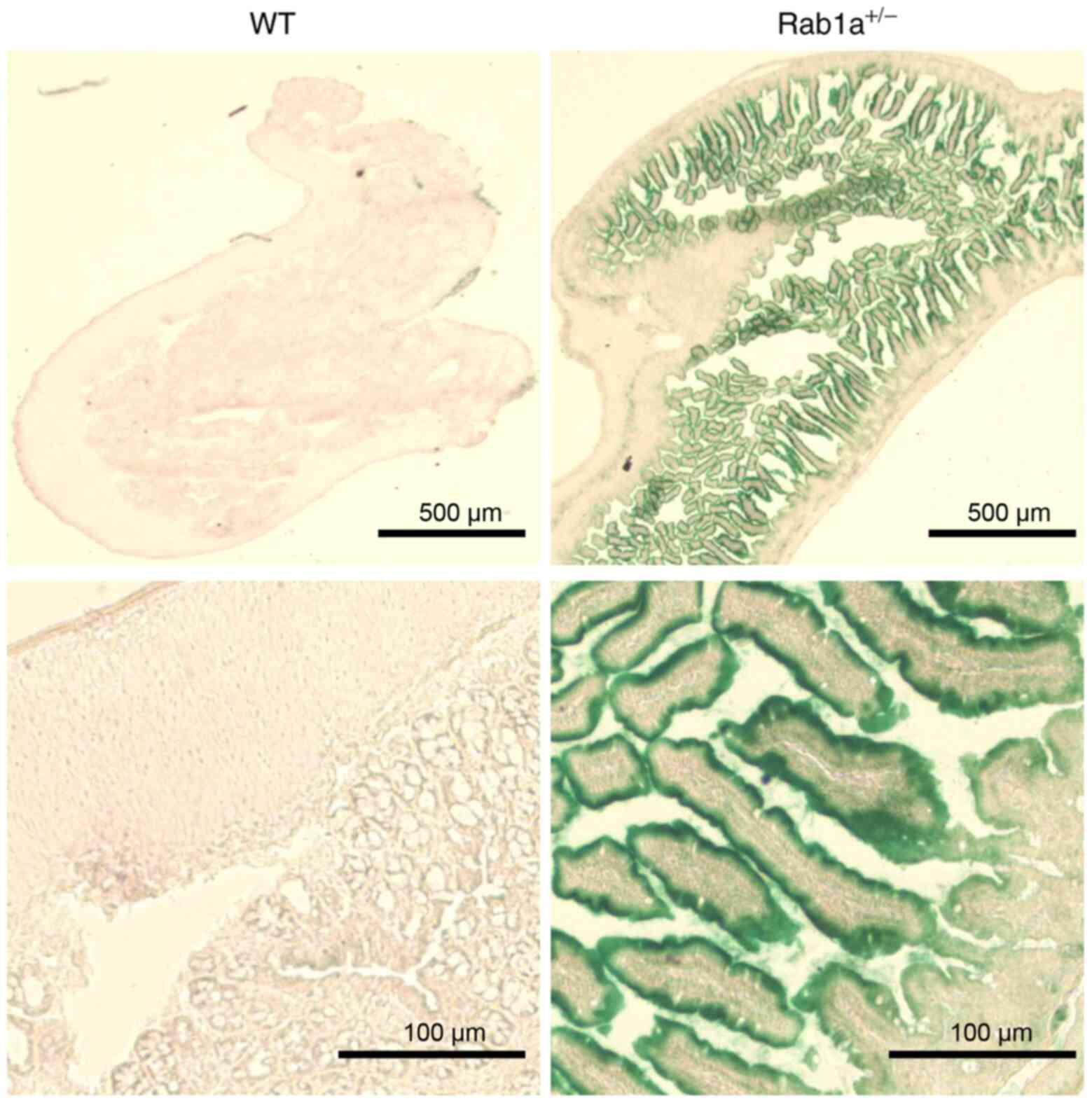
In addition, the expression of Rab1a during
embryo development was examined using X-gal staining. At E15
embryo, Rab1a was mainly expressed in the intestine
(Fig. 4) and a small amount of
Rab1a was also detected in the brain (Fig. 4), as previously reported
(28,29). In adult mice, Rab1a was
expressed in the small intestine (Fig. 5).
Rab1a deficiency causes embryonic
lethality
To generate Rab1a−/− mice,
heterozygous Rab1a+/− mice were intercrossed. The
genotypes of the offspring were identified at 2 weeks after birth.
None of the 250 offspring were homozygous mutants
(Rab1a−/−) (total, 250;
Rab1a+/+, 94; Rab1a+/−, 156;
Rab1a−/−, 0), and no increase in neonatal
mortality was observed in the initial 2 weeks after birth. The
ratio between the WT and heterozygote mice was 0.60, in accordance
with Mendel's law. These results thus suggest that Rab1a is
essential for embryonic development: one functional Rab1a
allele is sufficient for murine embryonic development; however, a
double mutant leads to embryonic lethality.
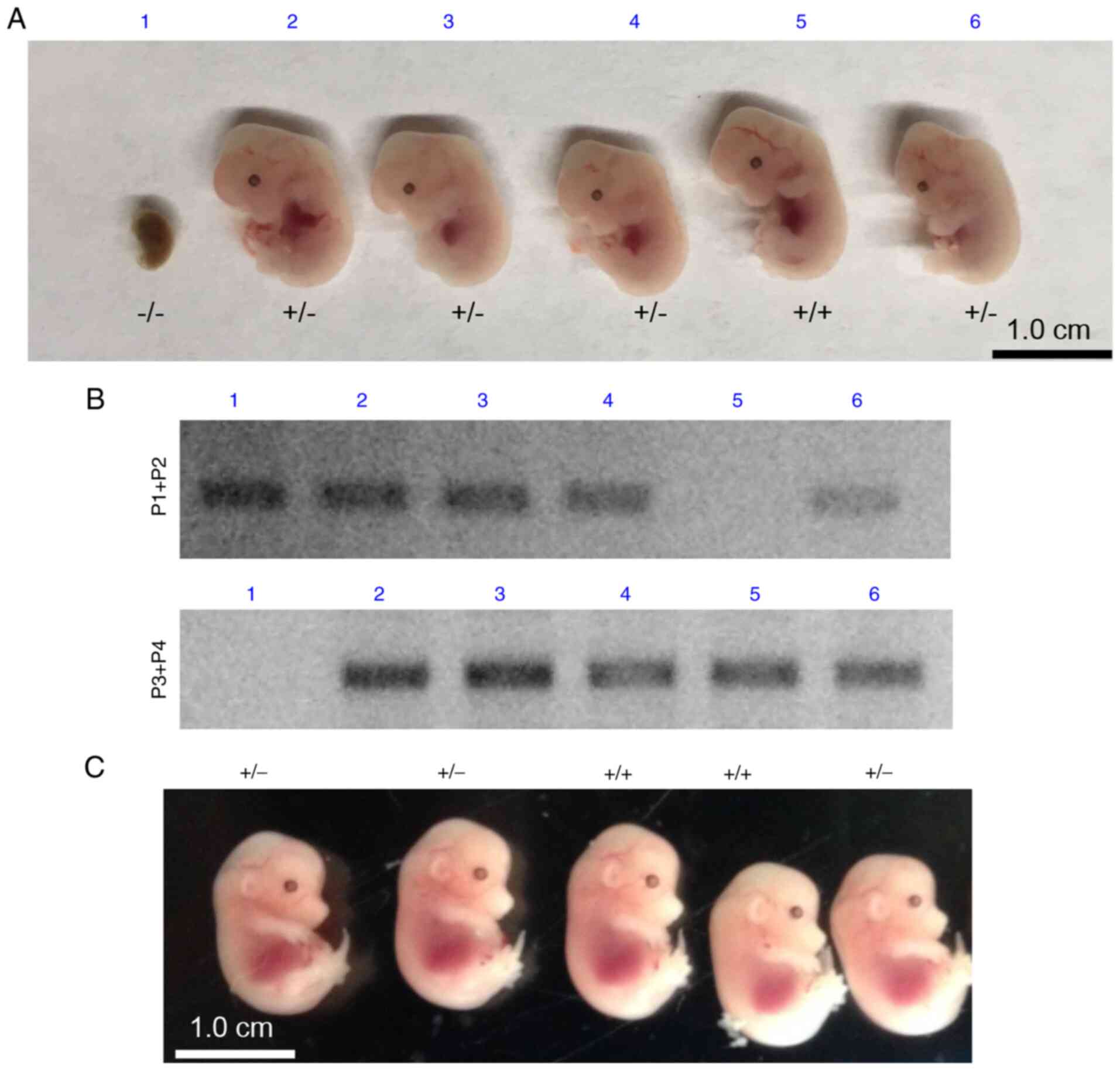
To characterize the effect of Rab1a mutation
on embryonic development, timed matings (breeding we set up at 5
p.m. and the following morning the presence of a copulatory plug
was examined at 7 a.m. If the presence of a copulatory plug was
confirmed, this day was recorded as day 0.5) were performed between
mice heterozygous for Rab1a. Embryos were collected at E12.5
and E14.5 from Rab1a+/− breeding mice and
genotyped using PCR analysis with genomic DNA. No viable
Rab1a−/− embryos were recovered (Fig. 6). The developmental retardation
of Rab1a−/− embryos was apparent at E12.5,
suggesting that embryonic lethality occurred prior to E12.5
(Fig. 6). Embryos at E10.5 and
E11.5 were also collected and it was found that viable
Rab1a−/− embryos were recovered at E10.5, whereas
no viable Rab1a−/− embryos were recovered at
E11.5; the data for viable embryos in different embryonic stages
are summarized in Table I, the
different numbers in the table indicate the viable embryos found in
the different embryonic genotypes. Thus, Rab1a
mutation-induced embryonic lethality occurred between E10.5 and
E11.5.
 | Table IEffects of Rab1a mutation on the
number of viable embryos. |
Table I
Effects of Rab1a mutation on the
number of viable embryos.
| Stage | Total |
Rab1a+/+ |
Rab1a+/− |
Rab1a−/− |
|---|
| E10.5 | 21 | 6 | 10 | 5 |
| E11.5 | 23 | 9 | 14 | 0 |
| E12.5 | 18 | 7 | 11 | 0 |
| E14.5 | 15 | 5 | 10 | 0 |
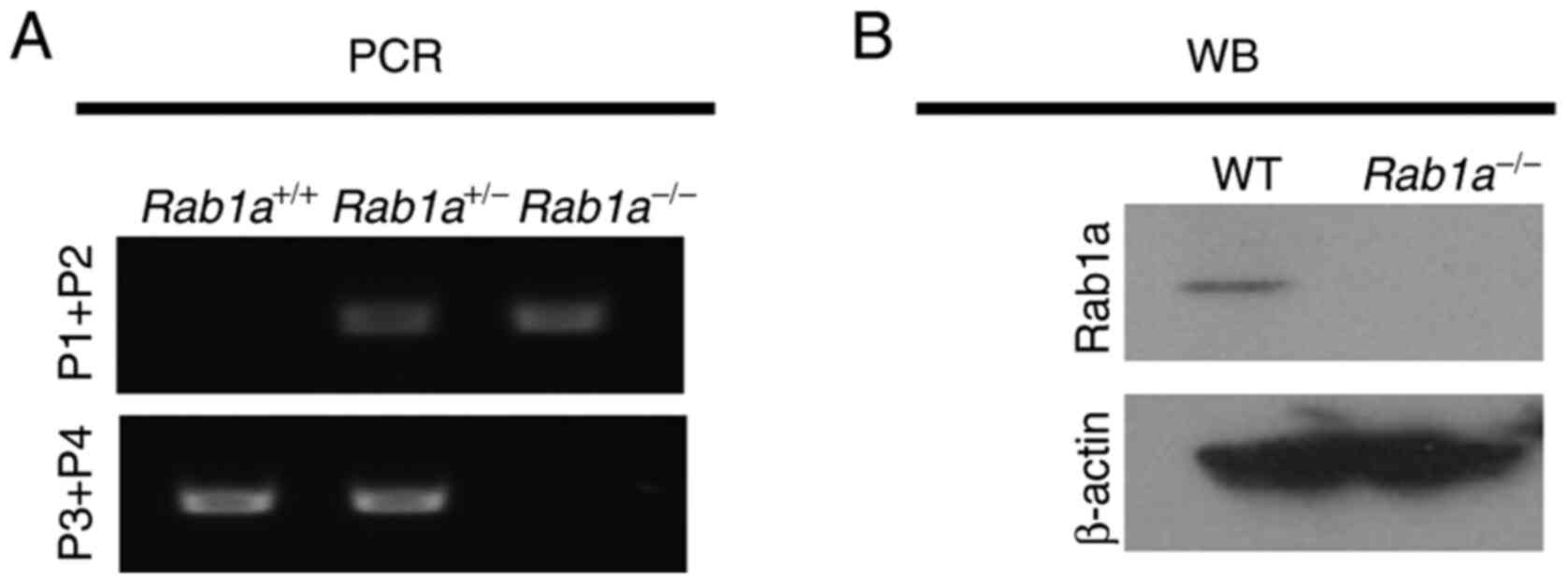
Rab1a protein deficiency in
Rab1a−/− KO mice
WT and mutant alleles were assessed using PCR of
genomic DNA isolated from mice (Fig.
7A). Western blot analysis was also performed to test the
successful disruption of the Rab1a gene in
Rab1a−/− mice. E10.5 embryos were collected and
genotyped using PCR. Rab1a+/+ and
Rab1a−/− embryos were lysed and used for western
blot analysis with anti-Rab1a antibody. As shown in Fig. 7B, Rab1a was completely absent in
Rab1a−/− embryos, indicating the functional loss
of Rab1a; β-actin was used as a loading control.
One Rab1a allele is sufficient for
resistance to LPS-induced sepsis
The loss of GDI2 in tumor cells alters the crosstalk
between tumor cells and tumor-associated macrophages to enhance
both local inflammation and tumor cell invasion and growth,
resulting in inflammatory cytokine secretion by macrophages to
promote metastatic growth (30).
Moreover, Rab1a is required for NLRP3 inflammasome activation and
inflammatory lung injury (31).
Recently, the authors demonstrated that Rab1a bound to the ITIM
domain of Siglec-G under normal homeostasis. By contrast, Rab1a was
recruited to the ITIM domain during bacterial infection, suggesting
that GDI2 and Rab1a may regulate immune response through
interaction with the ITIM domain during bacterial infection
(18). In the present study, to
investigate whether Rab1a plays a role during bacterial infection,
WT and Rab1a+/− mice were challenged with 10
mg/kg LPS and collected serum from the mice as previously described
(21,22,24). As shown in Fig. 8, both WT and
Rab1a+/− mice produced similar levels of
inflammatory cytokines following LPS stimulation. Moreover, after
120 h, 50% (8/16, Rab1a+/+) and 56% (9/16,
Rab1a+/−) of the mice did not survive; from data
pooled from two independent experiments, a similar percentage of
death was observed following LPS treatment (32) (data not shown); no significant
differences were observed between the WT and
Rab1a+/− mice as regards survival following the
LPS challenge.
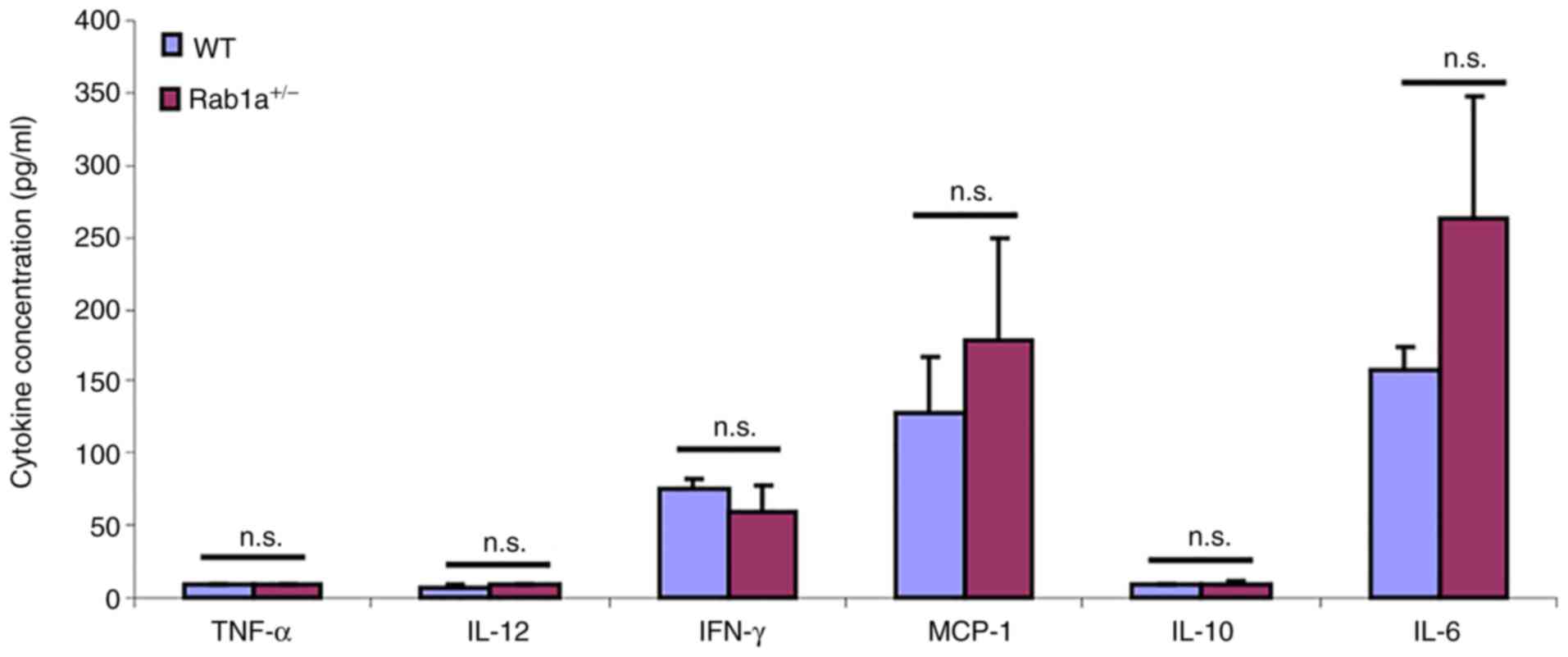 | Figure 8One Rab1a allele is sufficient for
the resistance to LPS challenge. Serum concentrations of cytokines
TNF-α, IL-6, IL-12, IL-10, MCP-1 and IFN-γ at 16 h following the
lipopolysaccharide (LPS) challenge (mean ± SD, n=8) were measured
by using a mouse cytokine bead array designed for inflammatory
cytokines. The experiments in this figure were reproduced twice.
n.s., no significant differences were found. TNF-α, tumor necrosis
factor α; IL, interleukin; MCP-1, monocyte chemoattractant
protein-1; IFN-γ, interferon γ. |
Discussion
The transfer of material between organelles in
eukaryotic cells is predominantly mediated by vesicular transport.
GTP binding proteins play key roles in the regulation of vesicular
traffic at several stages of the exocytic and endocytic transport
pathways. Rab GTPases are small GTP-binding proteins in the Ras
superfamily. Following a vesicle fusion event, Rab is returned to
its membrane of origin by GDI. GDI proteins regulate the GDP-GTP
exchange reaction of Rab family members that are involved in the
vesicular trafficking of molecules between cellular organelles.
GDIs decrease the rate of dissociation of GDP from Rab proteins and
release GDP from membrane bound Rabs (1,33).
The authors have previously demonstrated that Rab1a
may regulate the immune response through interaction with the ITIM
domain during bacterial infection in vitro (18). To further investigate the
function of Rab1a in vivo, the present study generated mice
with a trapped Rab1a gene and uncovered a novel role for
Rab1a during early embryonic development in mice. None of the 250
genotyped pups from Rab1a heterozygous mating pairs
exhibited the homozygous deletion of the Rab1a allele. The
present study was also unable to detect any viable Rab1a
null embryos after E11.5, indicating a severe early embryonic
defect caused by the complete loss of Rab1a function.
However, one functional Rab1a allele is sufficient for
murine embryo development, as the frequency of heterozygote
offspring was as predicted.
Although Rab1a interacts with the ITIM domain during
bacterial infection, there was no significant difference in
cytokine production and survival between the WT and
Rab1a−/− KO mice after the LPS challenge. These
data suggest that one Rab1a allele is sufficient to maintain
function. The conditional KO strategy is a useful method which may
be used to solve the problem of embryonic lethality observed in
conventional gene KOs (34).
Therefore, to explore the function of Rab1a in hematopoietic cells
in bacterial infection and to further investigate the role of Rab1a
in embryonic development, a Rab1a conditional KO mouse model
is needed (34).
Rab8 is reportedly necessary for the proper
localization of apical proteins and the absorption and digestion of
various nutrients in the small intestine (35). Previous research has indicated
that Rab11a is essential for the proper localization of apical
proteins in the intestine and that the loss of Rab11 leads to the
mislocalization of apical proteins in the small intestine, as
demonstrated using Rab11a intestine-specific knockout (IKO) mice
(36). Rab25 KO mice exhibit
increased numbers of intestinal neoplasms when crossed with
APCmin/+ mice (37).
With the use of X-gal staining, the present study found that Rab1a
was mainly expressed in the small intestine in E15 embryos
(Fig. 4) and in adult mice
(Fig. 5). It would be of
interest to determine whether Rab1a also plays an important role in
controlling the proper localization of apical proteins or the
absorption and digestion of various nutrients in the small
intestine. However, intestine specific Rab1a conditional KO
mice are required to further investigate the function of Rab1a in
the small intestine.
Although the present study demonstrates that
Rab1a is essential for embryonic development and homozygotes
die between E10.5 and E11.5, the mechanisms underlying the
regulatory effects of Rab1a on embryonic development remain
unclear. Moreover, while it was found that Rab1a was mainly
expressed in the small intestine in E15 embryos and in adult mice,
it is not yet clear whether Rab1a plays a critical role in the
small intestine. Further experiments using Rab1a conditional
KO mice are thus required to provide further insight into this
matter.
Supplementary Data
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GYC designed the experiments. YW, DY and GYC
conducted the experiments. GC wrote the manuscript. YW and GYC
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving animals were approved by
the University of Tennessee Health Science Center (UTHSC) Animal
Care and Use Committee (IACUC), protocol nos. 17-117 (approved
January 29, 2018) and 20-0211 (approved January 26, 2021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by Grant R01AI137255 from the
National Institutes of Health.
References
|
1
|
Pfeffer SR: Rab GTPases: Specifying and
deciphering organelle identity and function. Trends Cell Biol.
11:487–491. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zerial M and McBride H: Rab proteins as
membrane organizers. Nat Rev Mol Cell Biol. 2:107–117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tisdale EJ, Bourne JR, Khosravi-Far R, Der
CJ and Balch WE: GTP-binding mutants of rab1 and rab2 are potent
inhibitors of vesicular transport from the endoplasmic reticulum to
the Golgi complex. J Cell Biol. 119:749–761. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nuoffer C, Davidson HW, Matteson J,
Meinkoth J and Balch WE: A GDP-bound of rab1 inhibits protein
export from the endoplasmic reticulum and transport between Golgi
compartments. J Cell Biol. 125:225–237. 1994. View Article : Google Scholar
|
|
5
|
Zhuang X, Adipietro KA, Datta S, Northup
JK and Ray K: Rab1 small GTP-binding protein regulates cell surface
trafficking of the human calcium-sensing receptor. Endocrinology.
151:5114–5123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marie M, Dale HA, Sannerud R and Saraste
J: The function of the intermediate compartment in pre-Golgi
trafficking involves its stable connection with the centrosome. Mol
Biol Cell. 20:4458–4470. 2009. View Article : Google Scholar :
|
|
7
|
Wu G, Yussman MG, Barrett TJ, Hahn HS,
Osinska H, Hilliard GM, Wang X, Toyokawa T, Yatani A, Lynch RA, et
al: Increased myocardial Rab GTPase expression: A consequence and
cause of cardiomyopathy. Circ Res. 89:1130–1137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Machner MP and Isberg RR: A bifunctional
bacterial protein links GDI displacement to Rab1 activation.
Science. 318:974–977. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neunuebel MR, Chen Y, Gaspar AH, Backlund
PS Jr, Yergey A and Machner MP: De-AMPylation of the small GTPase
Rab1 by the pathogen Legionella pneumophila. Science. 333:453–456.
2011. View Article : Google Scholar :
|
|
10
|
Tan Y and Luo ZQ: Legionella pneumophila
SidD is a deAM-Pylase that modifies Rab1. Nature. 475:506–509.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sklan EH, Serrano RL, Einav S, Pfeffer SR,
Lambright DG and Glenn JS: TBC1D20 is a Rab1 GTPase-activating
protein that mediates hepatitis C virus replication. J Biol Chem.
282:36354–36361. 2007. View Article : Google Scholar
|
|
12
|
Zhang X, Wang X, Yuan Z, Radford SJ, Liu
C, Libutti SK and Zheng XF: Amino acids-Rab1A-mTORC1 signaling
controls whole-body glucose homeostasis. Cell Rep. 34:1088302021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar
|
|
14
|
Xu BH, Li XX, Yang Y, Zhang MY, Rao HL,
Wang HY and Zheng XF: Aberrant amino acid signaling promotes growth
and metastasis of hepatocellular carcinomas through Rab1A-dependent
activation of mTORC1 by Rab1A. Oncotarget. 6:20813–20828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu H, Qian M, Zhao B, Wu C, Maskey N, Song
H, Li D, Song J, Hua K and Fang L: Inhibition of RAB1A suppresses
epithelial-mesenchymal transition and proliferation of
triple-negative breast cancer cells. Oncol Rep. 37:1619–1626. 2017.
View Article : Google Scholar
|
|
16
|
Takai Y, Sasaki T and Matozaki T: Small
GTP-binding proteins. Physiol Rev. 81:153–208. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar :
|
|
18
|
Wu Y, Yang D and Chen GY: The role of the
Siglec-G ITIM domain during bacterial infection. Cell Mol Biol.
67:163–169. 2021. View Article : Google Scholar
|
|
19
|
Chen GY, Muramatsu H, Kondo M, Kurosawa N,
Miyake Y, Takeda N and Muramatsu T: Abnormalities caused by
carbohydrate alterations in
Ibeta6-N-acetylglucosaminyltransferase-deficient mice. Mol Cell
Biol. 25:7828–7838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bundschu K, Knobeloch KP, Ullrich M,
Schinke T, Amling M, Engelhardt CM, Renné T, Walter U and Schuh K:
Gene disruption of Spred-2 causes dwarfism. J Biol Chem.
280:28572–28580. 2005. View Article : Google Scholar
|
|
21
|
Chen GY, Brown NK, Wu W, Khedri Z, Yu H,
Chen X, van de Vlekkert D, D'Azzo A, Zheng P and Liu Y: Broad and
direct interaction between TLR and Siglec families of pattern
recognition receptors and its regulation by Neu1. Elife.
3:e040662014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Yang D, Liu R, Wang L and Chen GY:
Selective response to bacterial infection by regulating Siglec-E
expression. iScience. 23:1014732020. View Article : Google Scholar :
|
|
23
|
Chen GY, Chen X, King S, Cavassani KA,
Cheng J, Zheng X, Cao H, Yu H, Qu J, Fang D, et al: Amelioration of
sepsis by inhibiting sialidase-mediated disruption of the
CD24-SiglecG interaction. Nat Biotechnol. 29:428–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen GY, Tang J, Zheng P and Liu Y: CD24
and Siglec-10 selectively repress tissue damage-induced immune
responses. Science. 323:1722–1725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen WV and Soriano P: Gene trap
mutagenesis in embryonic stem cells. Methods Enzymol. 365:367–386.
2003.PubMed/NCBI
|
|
26
|
Friedrich G and Soriano P: Insertional
mutagenesis by retro-viruses and promoter traps in embryonic stem
cells. Methods Enzymol. 225:681–701. 1993. View Article : Google Scholar
|
|
27
|
Stryke D, Kawamoto M, Huang CC, Johns SJ,
King LA, Harper CA, Meng EC, Lee RE, Yee A, L'Italien L, et al:
BayGenomics: A resource of insertional mutations in mouse embryonic
stem cells. Nucleic Acids Res. 31:278–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ayala J, Olofsson B, Touchot N, Zahraoui
A, Tavitian A and Prochiantz A: Developmental and regional
expression of three new members of the ras-gene family in the mouse
brain. J Neurosci Res. 22:384–389. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olofsson B, Chardin P, Touchot N, Zahraoui
A and Tavitian A: Expression of the ras-related ralA, rho12 and rab
genes in adult mouse tissues. Oncogene. 3:231–234. 1988.PubMed/NCBI
|
|
30
|
Said N, Sanchez-Carbayo M, Smith SC and
Theodorescu D: RhoGDI2 suppresses lung metastasis in mice by
reducing tumor versican expression and macrophage infiltration. J
Clin Invest. 122:1503–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Y, Wang L, Lv Y, Jiang C, Wu G, Dull
RO, Minshall RD, Malik AB and Hu G: The GTPase Rab1 is required for
NLRP3 inflammasome activation and inflammatory lung injury. J
Immunol. 202:194–206. 2019. View Article : Google Scholar
|
|
32
|
Maitre B, Magnenat S, Heim V, Ravanat C,
Evans RJ, de la Salle H, Gachet C and Hechler B: The P2X1 receptor
is required for neutrophil extravasation during
lipopolysaccharide-induced lethal endotoxemia in mice. J Immunol.
194:739–749. 2015. View Article : Google Scholar
|
|
33
|
Seabra MC and Wasmeier C: Controlling the
location and activation of Rab GTPases. Curr Opin Cell Biol.
16:451–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Le Y and Sauer B: Conditional gene
knockout using cre recombinase. Methods Mol Biol. 136:477–485.
2000.PubMed/NCBI
|
|
35
|
Sato T, Mushiake S, Kato Y, Sato K, Sato
M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al: The Rab8
GTPase regulates apical protein localization in intestinal cells.
Nature. 448:366–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sobajima T, Yoshimura S, Iwano T, Kunii M,
Watanabe M, Atik N, Mushiake S, Morii E, Koyama Y, Miyoshi E and
Harada A: Rab11a is required for apical protein localisation in the
intestine. Biol Open. 4:86–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nam KT, Lee HJ, Smith JJ, Lapierre LA,
Kamath VP, Chen X, Aronow BJ, Yeatman TJ, Bhartur SG, Calhoun BC,
et al: Loss of Rab25 promotes the development of intestinal
neoplasia in mice and is associated with human colorectal
adenocarcinomas. J Clin Invest. 120:840–849. 2010. View Article : Google Scholar : PubMed/NCBI
|