Introduction
Pulmonary arterial hypertension (PAH) is a complex
and progressive disease characterized by persistent pulmonary
vasoconstriction, vascular remodeling, in situ thrombosis
and perivascular inflammatory infiltration. These pathological
features lead to vascular stenosis or occlusion, causing pulmonary
blood vessel resistance and a progressive increase in pulmonary
artery (PA) pressure, resulting in right heart failure and death
(1). Vascular remodeling is a
key process in PAH pathogenesis, and abnormal proliferation of
vascular smooth muscle cells (VSMCs) plays a key role in this
context (2-4). Although great progress has been
made in the treatment and understanding of the pathophysiological
mechanism underlying PAH, this disease is not preventable and
cannot be treated. The overall survival of patients with PAH
remains limited (5), and the
pathogenesis of this disease has not been fully clarified.
Previous studies have suggested that diabetes (DM)
and insulin resistance (IR) are associated with PAH (6-8).
DM and IR can specifically affect right ventricular afterload and
remodeling in patients with PAH, which have recently been
identified as risk factors for PAH deterioration and can reduce
survival rate (7). DM and PAH
also share common pathological mechanisms, such as chronic
inflammation (8). A recent study
suggested that these two diseases share a common molecular target,
microRNA (miRNA)-7110, which acts as an intermediate molecule
linking these two diseases (6).
Metformin (MET) is the most widely used, first-line,
oral hypoglycemic agent in clinical practice. Although MET has been
used clinically for >50 years, its mechanism of action in DM has
not yet been fully elucidated (9). Epidemiological and clinical studies
have revealed that MET reduces the risk of cancer in patients with
DM (10-12). It targets the key pathways of
cancer cell proliferation and angiogenesis, such as the mTORC1
signaling pathway, and inhibits the proliferation and angiogenesis
of cancer cells (10-12). However, the role of MET in PAH
pathogenesis remains unclear. Previous studies have confirmed that
MET reverses hypoxia-induced and monocrotaline (MCT)-induced PAH in
rats and inhibits pulmonary vascular remodeling (13-15).
Long non-coding RNA (lncRNAs) are a class of RNA
molecules that regulate numerous biological processes, including
genome imprinting, chromosome inactivation, differentiation,
carcinogenesis, transcriptional and post-transcriptional regulation
(16,17). Abnormal expression of lncRNAs is
associated with several human diseases, including various types of
cancer, cardiovascular diseases and respiratory diseases (18-20). The mechanism of action of lncRNAs
in PAH has attracted increased attention (21,22). For example, metastasis associated
lung adenocarcinoma transcript 1 (MALAT1) is highly expressed in
the lungs of hypoxic PAH model mice (23), while TCONS_00034812 is expressed
at low levels in a rat model of PAH and hypoxic PA smooth muscle
cells (PASMCs) (3).
Additionally, maternally expressed 3 (MEG3) is expressed at low
levels in the lung, PAs and hypoxic human PASMCs of patients with
PAH (4). Hoxaas3 is highly
expressed in the lung vessels and PASMCs of hypoxic PAH mice
(24), while H19 is also highly
expressed in the lungs of PAH model rats treated with MCT (25). Moreover, urothelial cancer
associated 1 (UCA1) is highly expressed in hypoxic human PASMCs
(26).
NONRATT015587.2 is a novel lncRNA with no reported
biological function. Our previous study confirmed that
NONRATT015587.2 could promote the proliferation of PASMCs via p53
and hypoxia-inducible factor-1α, as well as pulmonary vascular
remodeling (22). The exact
mechanism through which NONRATT015587.2 promotes the proliferation
of PASMCs remains unclear. Previous bioinformatics analysis has
revealed that p21 is located at the downstream target of
NONRATT015587.2. p21 is regulated by MCT and MET, and its
expression is negatively correlated with that of NONRATT015587.2
(22). p21 is a strong
cyclin-dependent kinase inhibitor that plays a key role in cell
cycle progression and in the control of proliferation (27,28). p21 is a downstream target gene of
p53, which is regulated through p53-dependent and -independent
mechanisms (29,30).
The current authors hypothesized that p21 may be the
target gene of NONRATT015587.2. Thus, NONRATT015587.2 may exert its
effects on the proliferation of PASMCs by targeting p21, and, in
turn, MET may target p21 through NONRATT015587.2, thus blocking the
cell cycle at G0/G1 and inhibiting the
proliferation of PASMCs and cell cycle progression. In the present
study, NONRATT015587.2 and p21 silencing were performed to explore
the pathogenesis of PAH and the mechanism of action of MET in the
prevention and treatment of this disease, in the hope that the
findings may highlight new strategies for the prevention and
treatment of PAH.
Materials and methods
Animal model
All animal experiments were approved by the Animal
Care and Use Institutional Committee of China Pharmaceutical
University prior to the initiation of the present study (approval
no. YKD-20180207001). A total of 30 female Sprague-Dawley rats
(age, 8 weeks; weight range, 200-220 g) were purchased from the
Experimental Animal Center of Nantong University (Nantong, China).
The rats were housed in groups of five at 23°C under a 12 h
light/dark cycle and a relative humidity of 45%, with free access
to food and water. The rats were randomly divided into three groups
as follows: i) The control group, which were administered normal
saline; ii) the MCT group, which were administered a single
subcutaneous injection of 60 mg/kg MCT (31); and iii) the MCT + MET, which were
administered a single subcutaneous injection of 60 mg/kg MCT and
MET (100 mg/kg/day via intragastrical injection; Sigma-Aldrich;
Merck KGaA). An injection of sodium chloride 0.9% (5 ml/kg) was
given to control and MCT groups every day for a total of 30 days by
intragastric administration. MET was administered intragastrically
at a dose of 100 mg/kg/day every day for 30 days, starting on the
day of MCT injection (day 0). After the model was successfully
constructed, the rats were anesthetized using 3% sodium
pentobarbital (45 mg/kg) injected into the abdominal cavity. After
anesthesia, the chest cavity was opened along the ribs on both
sides with tissue scissors to expose the heart and lungs. Surgical
scissors were used to remove the heart and lung for subsequent
experiments.
Hemodynamic experiments
Right ventricular systolic pressure (RVSP) and right
ventricle/left ventricle + septum (RV/LV+S) were analyzed as
previously described (22). The
lung tissue samples were then fixed with 4%paraformaldehyde at 4°C
for 24 h and embedded in paraffin for subsequent
immunohistochemistry experiments.
Immunohistochemistry
Tissue samples were cut into 4-µm sections
for immunohistochemistry. The slices were baked in an oven at 60°C
for 2 h. Dewaxing, rehydration and antigen retrieval were then
performed. Slices were placed in 0.01 M sodium citrate buffer
solution (pH 6.0) and heated in the microwave for 4 min until
boiling. Sections were then heated four times for 6 min each,
replenishing the liquid at each interval to prevent the slices from
drying out. Lung tissue sections were subsequently incubated with
5% goat serum for 30 min at room temperature. Platelet-derived
growth factor subunit B (PDGFB; cat. no. AF0240; 1:200; Affinity
Biosciences) and p21 (cat. no. AF6290; 1:200; Affinity Biosciences)
primary antibodies were then added at 4°C for 12 h. After overnight
incubation, the sections were washed three times with PBS and then
exposed to HRP-conjugated Affinipure goat anti-rabbit IgG(H+L)
secondary antibodies (cat. no. SA00001-2; 1:200; ProteinTech Group,
Inc.) for 60 min at room temperature. Positivity for PDGFB and p21
was reflected by brown staining. An IX73 light microscope
(magnification, ×10; Olympus Corporation) was used for the
acquisition of light images.
Rat PASMC culture
Rat PASMCs were isolated from the lung tissue of
healthy Sprague-Dawley rats. The lung tissue was placed in sterile
PBS to wash away blood, then placed under a microscope in order to
strip the PA vessels. Microscissors and forceps were used to remove
the adventitia and intima of the PAs. The PAs were then cut into
1-2 mm pieces with microscissors and digested with a mixture
containing 2 mg/ml collagenase, 0.5 mg/ml elastase and 1.5 mg/ml
BSA (Nanjing KeyGen Biotech Co., Ltd.) for 1-2 h until the tissue
mass became a cell mass. DMEM (Hyclone; Cytiva) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) was
added to the cell pellets to stop the digestion by pipetting, after
which the supernatant was removed by centrifugation at 2,000 x g at
room temperature for 5 min. PASMCs were resuspended in DMEM
containing 10% FBS in a cell culture flask and maintained in an
incubator at 37°C with 5% CO2. The purity of PASMCs was
determined using a monoclonal antibody specific for α-smooth muscle
actin (cat. no. AH11074812; 1:200; BIOSS) at 4°C for 12 h.
Platelet-derived growth factor-BB (PDGF-BB) (cat. no. 100-14B;
PeproTech, Inc.) was used to stimulate cell proliferation. Cells
from passage 2-5 were used for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
RT-qPCR was performed as previously described
(22). Total RNA was extracted
from PAs and PASMCs using Trizol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). The yield of RNA was assessed
using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.), after which the integrity was evaluated using 1% agarose gel
electrophoresis stained with ethidium bromide. RT was performed
using a miScript® II RT kit (Qiagen, Inc.; cat. no.
218160). Reactions were analyzed in GeneAmp® PCR System
9700 (Applied Biosystems; Thermo Fisher Scientific, Inc.) under the
following temperature protocol: 25°C for 10 min, 50°C for 30 min
and at 85°C for 5 min. The 10 µl RT reaction mix was then
diluted 10× in nuclease-free water and held at -20°C. qPCR was
performed using a LightCycler® 480 II Real-time PCR
Instrument (Roche Diagnostics) with 10 µl PCR reaction
mixture that included 1 µl cDNA, 5 µl 2×
QuantiFast® SYBR® Green PCR Master Mix
(Qiagen GmbH), 0.2 µl forward primer, 0.2 µl reverse
primer and 3.6 µl nuclease-free water. Reactions were
incubated in 96-well plates (Roche Diagnostics) under the following
thermocycling conditions: 95°C for 5 min, followed by 40 cycles of
95°C for 10 sec and 60°C for 30 sec. Each sample was run in
triplicate for analysis. At the end of PCR cycling, melting curve
analysis was performed to validate the specific generation of the
expected PCR product. The primer sequences were designed in the
laboratory and were synthesized by Generay Biotech (Generay Biotech
Co., Ltd.) based on the mRNA sequences obtained from the NCBI
database. The sequences of the primers are presented in Table I. The expression levels of
NONRATT015587.2 were normalized to those of β-actin and were
calculated using the 2−ΔΔCq method (32).
 | Table ISpecific primer sequences used in
PCR. |
Table I
Specific primer sequences used in
PCR.
|
Oligonucleotides | Sequence
(5′-3′) |
|---|
|
NONRATT015587.2 | |
| Forward |
ACATGACCTCTAAAGATTCTGC |
| Reverse |
GACCATTCCTACATCTCCCA |
| β-actin | |
| Forward |
CCACCATGTACCCAGGCATT |
| Reverse |
CGGACTCATCGTACTCCTGC |
Western blotting
RIPA protein lysis buffer (cat. no. KGP702-100;
Nanjing KeyGen Biotech Co., Ltd.) was added to PA homogenate or
PASMCs to extract protein. The protein concentration was quantified
using bicinchoninic acid protein concentration kit (Nanjing KeyGen
Biotech Co., Ltd.). Samples containing 20 µg total protein
were added to 5X loading buffer for denaturation at 95°C, then
resolved using 10% SDS-PAGE, transferred to a nitrocellulose
membrane, blocked with 5% skimmed milk for 1 h at room temperature
and incubated with primary antibodies at 4°C overnight. The primary
antibodies used were as follows: p21 (cat. no. AF6290; 1:1,000;
Affinity Biosciences), proliferating cell nuclear antigen (PCNA;
cat. no. 10205-2-AP; 1:5,000; ProteinTech Group, Inc.), cyclin A
(cat. no. 13295-1-AP; 1:1,000; ProteinTech Group, Inc.), cyclin E
(cat. no. 11554-1-AP; 1:2,000; ProteinTech Group, Inc.), GAPDH
(cat. no. 60004-1-l g; 1:5,000; ProteinTech Group, Inc.) and
β-actin (cat. no. 60008-1-1 g; 1:10,000; ProteinTech Group, Inc.).
The membranes were then incubated with HRP-conjugated anti-mouse
(cat. no. SA00001-1; 1:20,000; ProteinTech Group, Inc.) or
anti-rabbit IgG (cat. no. SA00001-2; 1:20,000; ProteinTech Group,
Inc.) secondary antibodies for 1 h at room temperature. The
membranes were washed with TBS +0.1% Tween for 40 min at room
temperature, after which electrochemiluminescence (Nanjing KeyGen
Biotech Co., Ltd.) solution was added for 5 min. The bands were
analyzed using a VersaDoc™ MP 4000 instrument (Bio-Rad
Laboratories, Inc.) with PDQuest Advanced 2D analysis software
(version 8.0; Bio-Rad Laboratories, Inc.).
Transfection
The sequences of small interfering RNA (siRNA)
targeting NONRATT015587.2 and the non-targeted negative control
(NC) were synthesized by Shanghai GenePharma Co., Ltd. The
sequences were as follows: si-NONRATT015587.2 sense, 5′-GCC UGG UUC
AUU GUU GGU UTT-3′ and antisense, 5′-AAC CAA CAA UGA ACC AGG
CTT-3′; si-p21 (33) sense,
5′-GGA ACA AGG AGU CAG ACA UTT-3′ and antisense, 5′-AUG UCU GAC UCC
UUG UUC CTT-3′; NC sense, 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and
antisense, 5′-ACG UGA CAC GUU CGG AGA ATT-3′. The NONRATT015587.2
overexpression plasmid (pcDNA-NONRATT015587.2) was constructed by
Shanghai GenePharma Co., Ltd. After_6 h of transfection, subsequent
experiments were performed. A total of 2 µg siRNA and 10
µl Lipofectamine® 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) were diluted in
serum-free Opti-MEM-1 (Gibco; Thermo Fisher Scientific, Inc.)
medium and mixed together. The mixture (siRNA/Transfection Reagent)
was then incubated at room temperature for 20 min and added
directly onto cells. After 6 h of exposure to siRNA, the
transfection reagents were removed and PASMCs were cultured in DMEM
containing 5% FBS for a further 24 h in an incubator at 37°C with
5% CO2. Cells treated with transfection reagent alone
(mock) and untreated PASMCs (blank) from the same isolation served
as additional negative controls.
Cell Counting Kit-8 (CCK-8) analysis
PASMCs were digested with trypsin and collected by
centrifugation at 2,000 x g at room temperature for 5 min to
prepare a cell suspension, then seeded in 96-well plates at a
density of 1×104 cells/well. PASMCs were treated
according to different experimental groups. After incubation at
37°C in 5% CO2 for 24 h, 20 µl CCK-8 was added to
the cells for 4 h at 37°C. Absorbance was analyzed using a
Synergic2 multiplate reader (Synergy H1; BioTek) at 450 nm.
Cell cycle analysis
Propidium iodide single staining was used to analyze
the cell cycle. PASMCs were digested with trypsin and centrifuged
at 2,000 x g at room temperature for 5 min. The cells were washed
three times by centrifugation at 2,000 x g at room temperature for
5 min with PBS, then fixed with 70% ethanol for 24 h at 4°C. After
washing with ethanol, the cells were incubated with 200 µl
PBS. Solution A was added according to the instructions of the flow
cytometry cycle detection kit (BD Biosciences), and the cells were
then incubated for 10 min at room temperature. Solution B was then
added. Finally, solution C was added to the cells, which were then
incubated for 10 min in the dark at room temperature. DNA
fluorescence measurements were analyzed using a flow cytometer
(FACSCanto II; BD Biosciences). The results were analyzed using the
ModFit software (version 4.1; Verity Software House, Inc.).
PASMC RNA fluorescence in situ
hybridization (FISH)
Fluorescence-conjugated lncRNA NONRATT015587.2
probes were synthesized by Guangzhou RiboBio Co., Ltd. and used for
RNA FISH. Hybridization was performed using an RNA FISH kit
(Guangzhou RiboBio Co., Ltd.) according to the manufacturer's
instructions. The FISH sections were then incubated with DAPI for 5
min at room temperature. An IX73 fluorescence microscope
(magnification, ×10; Olympus Corporation) was used for the
acquisition of fluorescent images.
Statistical analysis
Data are presented as the mean ± SD of at least
three independent experiments and were analyzed using one-way
ANOVA. Tukey's post-hoc test was used for pairwise comparisons.
Statistical analysis was carried out using data analysis tool (PAST
v2.17) software (WinAll Application Platform). P<0.05 was
considered to indicate a statistically significant difference.
Results
NONRATT015587.2 and p21 expression and
intracellular localization of NONRATT015587.2 in PAH model
rats
To determine whether MET reversed MCT-induced PAH,
in vivo RVSP and RV/(LV+S) were calculated. The results
demonstrated that MET attenuated the increase in RVSP and RV/(LV+S)
induced by MCT (Fig. 1A and B).
Our previous study suggested that the expression of lncRNA
NONRATT015587.2 was significantly upregulated in the MCT group and
significantly downregulated following MET intervention. The
expression levels of the p21 gene were negatively correlated with
those of NONRATT015587.2 (MCT group, r=−0.894 and P=0.016; MCT +
MET group, r=−0.957 and P=0.0028) (22). In the present study, the
expression levels of NONRATT015587.2 and p21 protein were analyzed
in the PAs of each group of rats. The expression levels of
NONRATT015587.2 were significantly upregulated in the MCT group
compared with those of the control group; however, NONRATT015587.2
was significantly downregulated following MET treatment (Fig. 1C). Moreover, p21 protein
expression levels were significantly downregulated in the MCT group
and upregulated after MET treatment (Fig. 1D and E). These results are
consistent with those included in our previous bioinformatics
analysis (22). In addition,
PDGFB was highly expressed in the MCT group, but markedly
downregulated following treatment with MET (Fig. 1D).
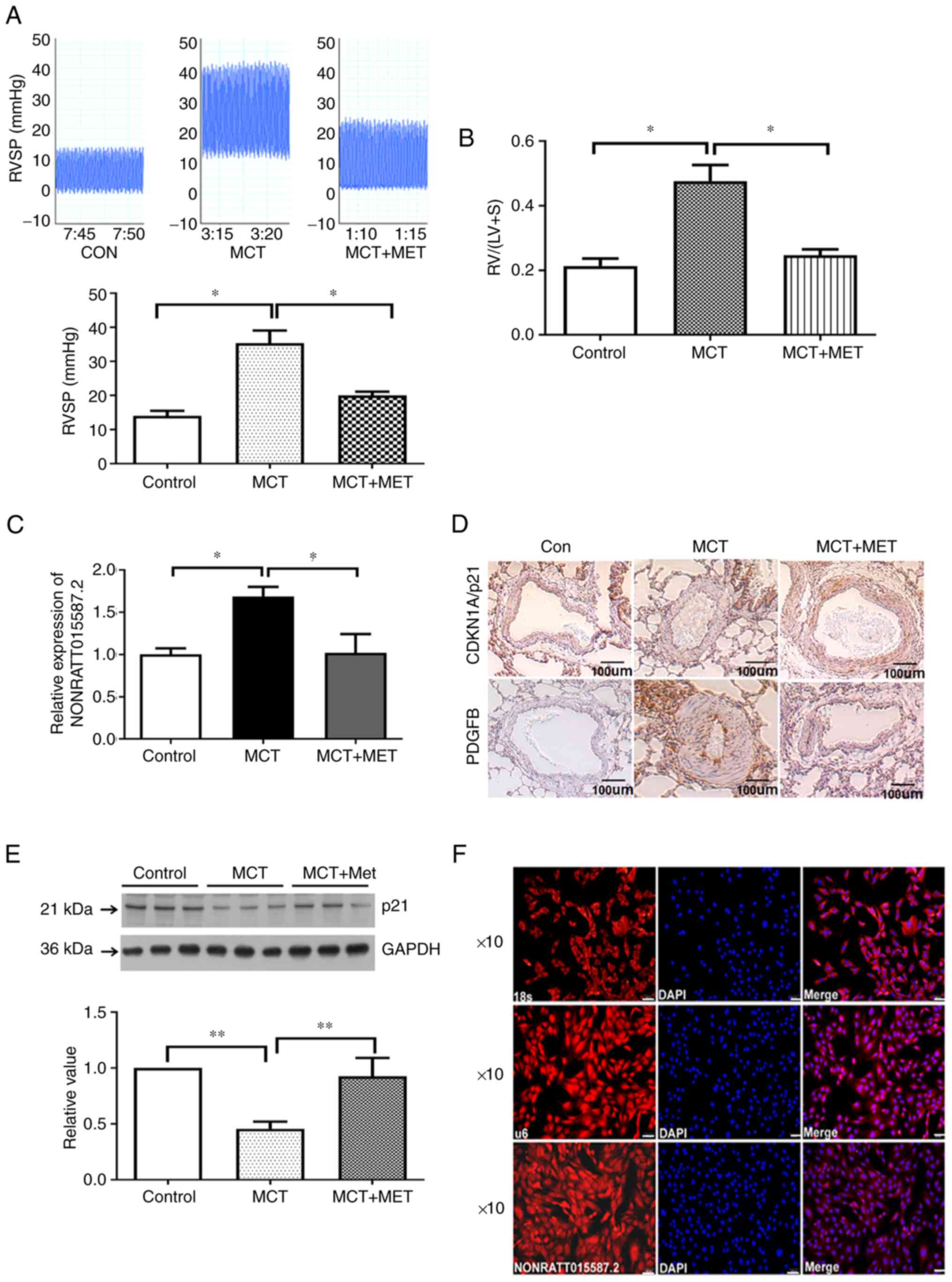 | Figure 1Expression of NONRATT015587.2 and p21
in MCT-induced pulmonary hypertension rats. (A) Assessment of RVSP
in MCT-induced PAH rats, controls and following MCT+MET treatment.
(B) Changes in the RV/(LV+S) ratio. (C) NONRATT015587.2 gene
expression was calculated in each group. (D) The expression of
CDKN1A/p21 and PDGFB in pulmonary artery tissue was analyzed by
immunohistochemistry staining (scale bars, 100 µm). (E) p21
protein expression was determined via western blotting and
subsequently quantified. (F) Fluorescence in situ
hybridization was performed to analyze the subcellular location of
NONRATT015587.2. In this experiment, 18s was used as the
cytoplasmic reference gene and u6 was used as the nuclear reference
gene. DAPI was used for nuclear staining (scale bars, 50
µm). Data represent mean ± standard deviation (SD).
*P<0.05 and **P<0.01 as indicated (n=
≥3). Con, control; MCT, monocrotaline; MET, metformin; PDGFB,
platelet-derived growth factor subunit B; RV/(LV+S), right
ventricle/left ventricle plus septum; RVSP, right ventricular
systolic pressure. |
In order to further analyze the relationship between
NONRATT015587.2 and p21, FISH fluorescent probes were used to
analyze the subcellular localization of NONRATT015587.2. The
results indicated that NONRATT015587.2 was located in the nucleus
(Fig. 1F).
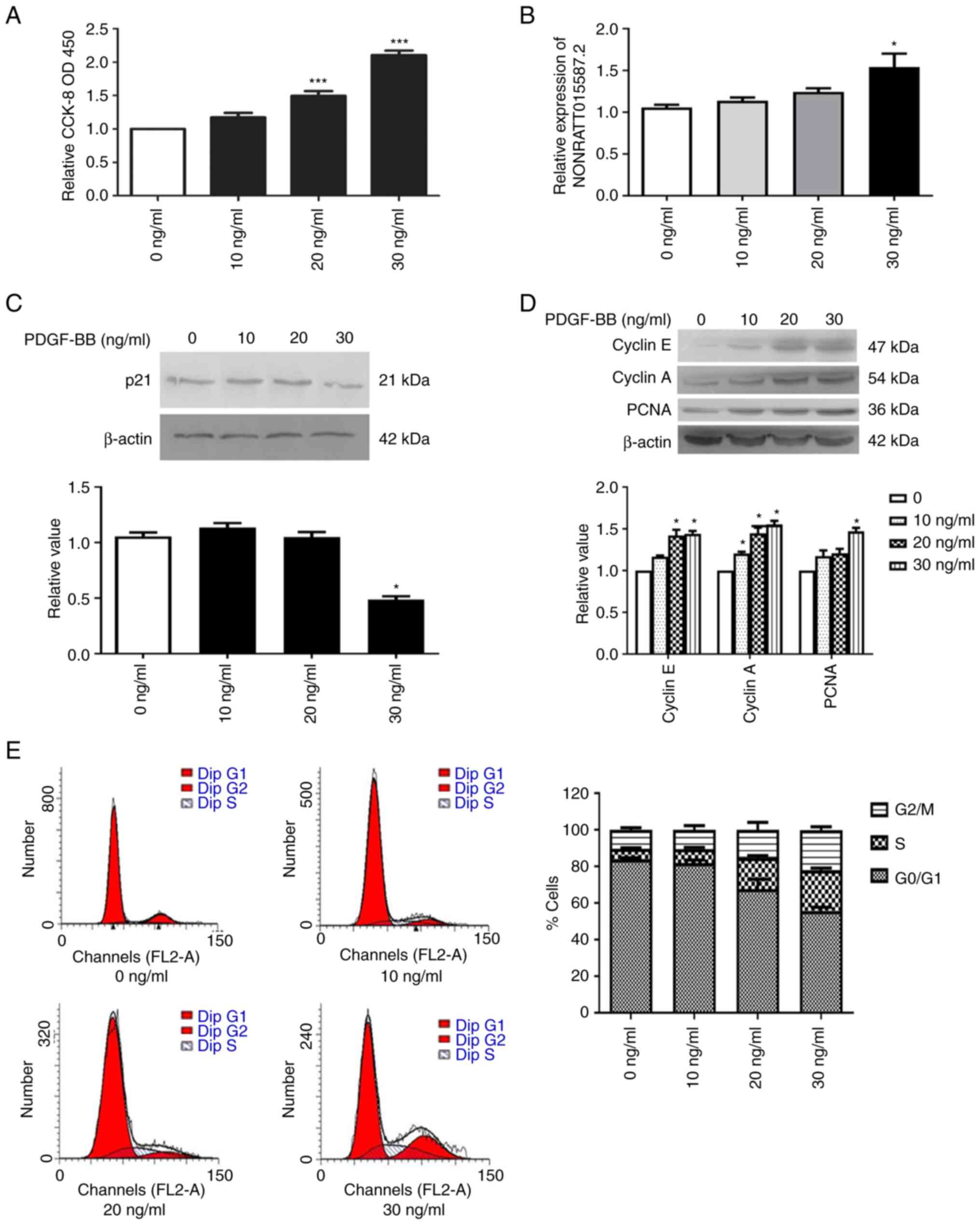
PDGF-BB promotes the proliferation of
PASMCs
A concentration gradient of PDGF-BB was used to
stimulate the cell viability of PASMCs in vitro in order to
observe its effect on these cells. The results suggested that
PASMCs cell viability gradually increased (Fig. 2A) and that the expression of
NONRATT015587.2 significantly increased with increasing PDGF-BB
concentrations (Fig. 2B),
reaching a maximum at a concentration of 30 ng/ml. However, p21
exhibited the opposite trend, and its protein expression levels
decreased with increasing PDGF-BB concentrations, reaching its
lowest at 30 ng/ml (Fig. 2C).
Moreover, the protein expression levels of cyclin E, cyclin A and
PCNA increased in a dose dependent manner (Fig. 2D).
The results of flow cytometry indicated that PDGF-BB
treatment resulted in a gradual decrease in the proportion of cells
in the G0/G1 phase. By contrast, the
proportion of cells in the S phase gradually increased, indicating
that transition through the G0/G1 cell cycle
phase was accelerated, indicative of enhanced proliferation and
replication (Fig. 2E). These
observations suggested that PDGF-BB stimulation promoted the
proliferation of PASMCs in a concentration-dependent manner,
reaching a maximum at a concentration of 30 ng/ml. Based on the
aforementioned results, the concentration of 30 ng/ml was used in
subsequent experiments.
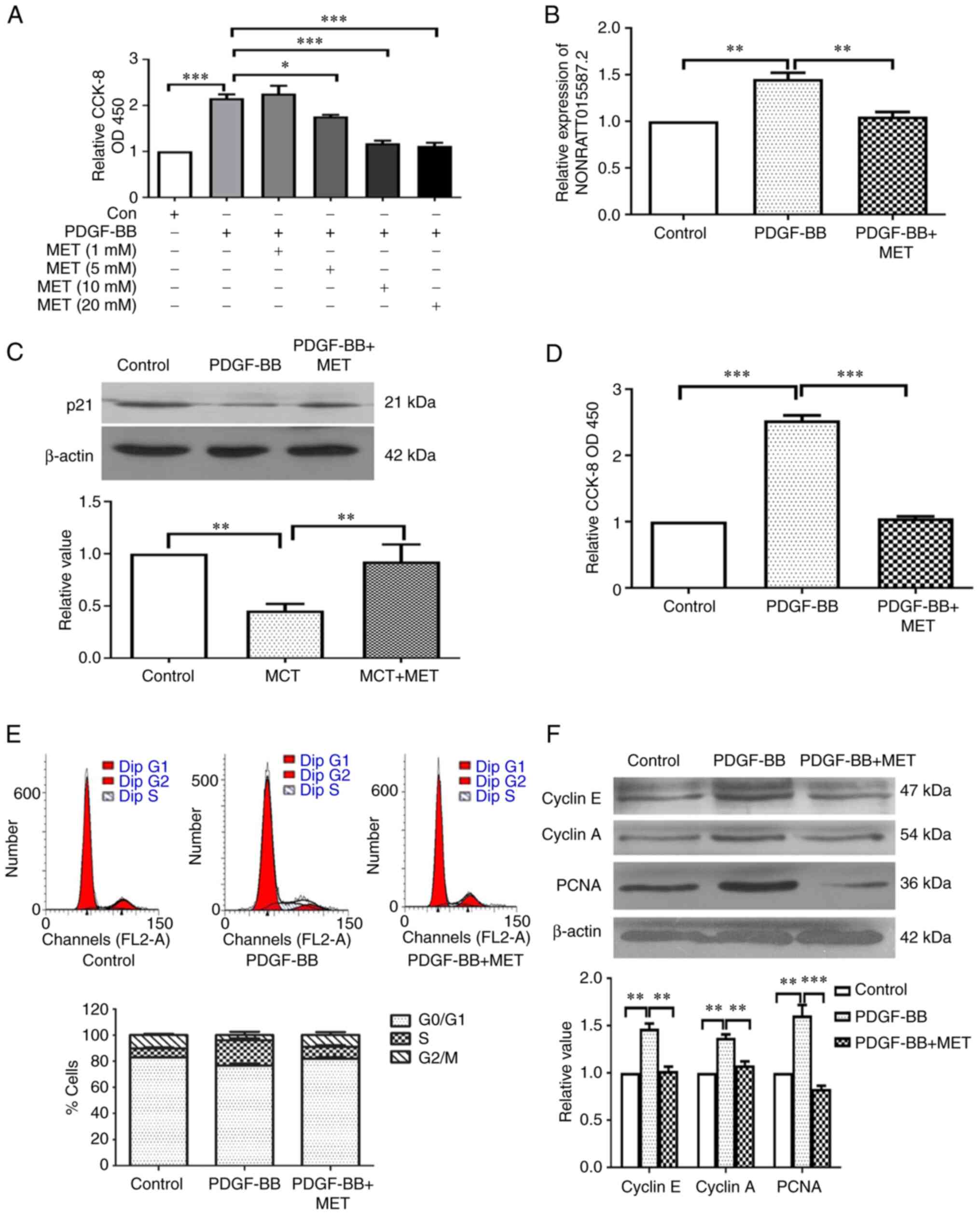
MET inhibits PDGF in proliferating PASMCs
and inhibits its effect on the expression of NONRATT015587.2 and
p21
Our previous study suggested that MET treatment
could reverse MCT-induced PAH and vascular remodeling (22). In addition, Song et al
(34) revealed that MET could
reduce PDGF-induced PASMC proliferation. In the present study, the
effect of MET on the proliferation of PASMCs was examined, in order
to select the appropriate concentration for MET. PDGF-BB-induced
proliferation of PASMCs was reversed by MET and was gradually
reduced with increasing MET concentrations, reaching its lowest at
20 mM (Fig. 3A). A number of
previous reports have indicated that MET inhibits tumor cell
proliferation. The pathological features of pulmonary hypertension
are very similar to tumors, a common feature of which is the
abnormal proliferation of cells. Therefore, when selecting the
appropriate concentration of MET in vitro, previously
reported literature (35-38)
and our previous published article (39) was referred to. Following MET
treatment, the expression of NONRATT015587.2 was significantly
downregulated (Fig. 3B), while
that of the p21 protein was significantly upregulated (Fig. 3C). In addition, the observed
effects on cell proliferation (Fig.
3D) and cell cycle progression (Fig. 3E), as well as cyclin E, cyclin A
and PCNA protein expression (Fig.
3F), were reversed by MET.
NONRATT015587.2 promotes the
proliferation of PASMCs
In our previous study, PASMC proliferation was
examined following overexpression of NONRATT015587.2 (22). In the present study, the effect
of NONRATT015587.2 overexpression on PASMC proliferation and the
possible underlying mechanism were investigated (Fig. 4). Flow cytometry results revealed
that the proportion of cells in the S phase following
NONRATT015587.2 overexpression was increased; however, this was
reduced to the levels of the pcDNA group following MET treatment
(Fig. 4A). Moreover, PASMC
proliferation, as well as cyclin E, cyclin A and PCNA protein
expression levels, were significantly increased following
NONRATT015587.2 overexpression, although MET could reverse these
effects (Fig. 4C and E).
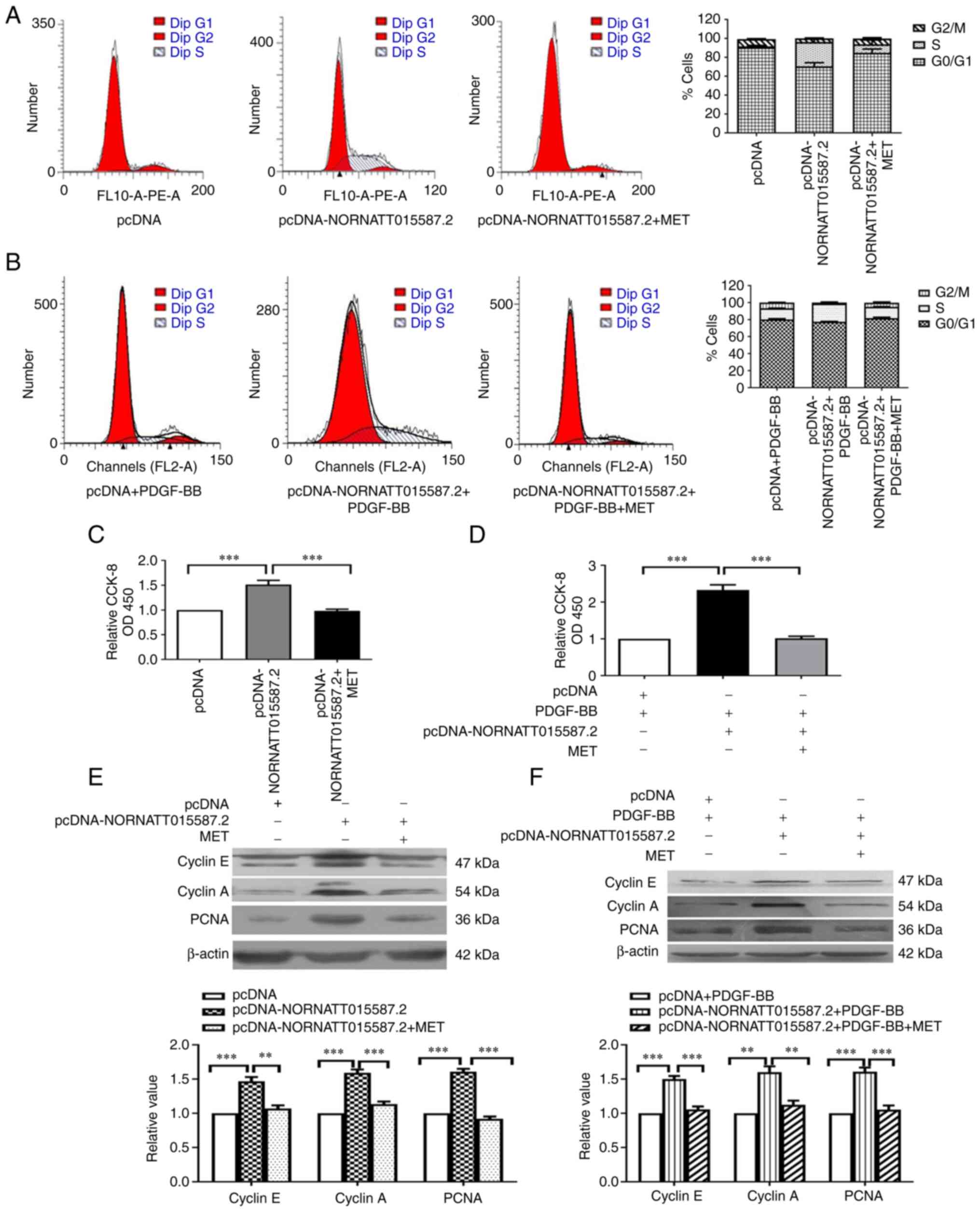 | Figure 4Effect of NONRATT015587.2
overexpression on the proliferation of PASMCs. Flow cytometry was
performed to analyze the effect of NONRATT015587.2 overexpression
on the proliferation of PASMCs in the (A) absence or (B) presence
of the external stimulus, PDGF-BB. A CCK-8 assay was performed to
analyze the proliferation of NONRATT015587.2 overexpression on the
proliferation of PASMCs in the (C) absence or (D) presence of the
external stimulus, PDGF-BB. Western blotting was conducted to
determine the protein expression of PCNA, Cyclin A and Cyclin E
after NONRATT015587.2 overexpression in the (E) absence or (F)
presence of the external stimulus, PDGF-BB. **P<0.01
and ***P<0.001 as indicated (n= ≥3). CCK-8, Cell
Counting Kit-8; MET, metformin; OD, optical density; PASMCs,
pulmonary artery smooth muscle cells; PCNA, proliferating cell
nuclear antigen; PDGF-BB, platelet-derived growth factor-BB. |
The above results indicated that endogenous
NONRATT015587.2 regulated PASMC proliferation. Therefore, the next
experiment aimed to determine whether NONRATT015587.2 promoted
PASMC proliferation in the presence of PDGF external stimulation.
The results of flow cytometry revealed that the proportion of cell
cycle S phase (DNA synthesis phase) was increased after
overexpressing NONRATT015587.2 in the presence of exogenous
PDGF-BB. Furthermore, MET intervention reversed the proportion of S
phase and restored the cell cycle distribution to the level of
pcDNA+PDGF-BB group (Fig. 4B).
PASMCs proliferation and Cyclin E, Cyclin A, PCNA protein
expression were significantly increased after overexpression of
NONRATT015587.2, and MET could reverse these results in the
presence of exogenous PDGF-BB (Fig.
4D and F). The results indicated that NONRATT015587.2 was a
pro-proliferation factor in PASMCs, regardless of the presence or
absence of PDGF-BB as an external stimulus.
Inhibitory effect of MET on
PDGF-BB-stimulated PASMC proliferation is abolished by silencing
p21, and NONRATT015587.2 targets p21 to promote PASMC
proliferation
PDGF negatively regulates p21 expression. In
addition, PDGF-stimulated PASMC proliferation was inhibited by MET.
The transfection efficiency of p21 silencing was verified using
western blotting (Fig. 5A). The
effect of p21 silencing following PDGF-BB and MET stimulation on
the cell cycle progression and proliferation of PASMCs was then
examined. The results suggested that p21 silencing could abolish
the inhibitory effect of MET on PDGF-BB-induced PASMC
proliferation. In addition, an increase in cell proliferation was
observed, together with upregulation of cyclin E, cyclin A and PCNA
proteins (Fig. 5B-D). This
suggested that p21 was targeted by MET to inhibit the proliferation
of PASMCs and that silencing p21 could abolish the inhibitory
effects of MET.
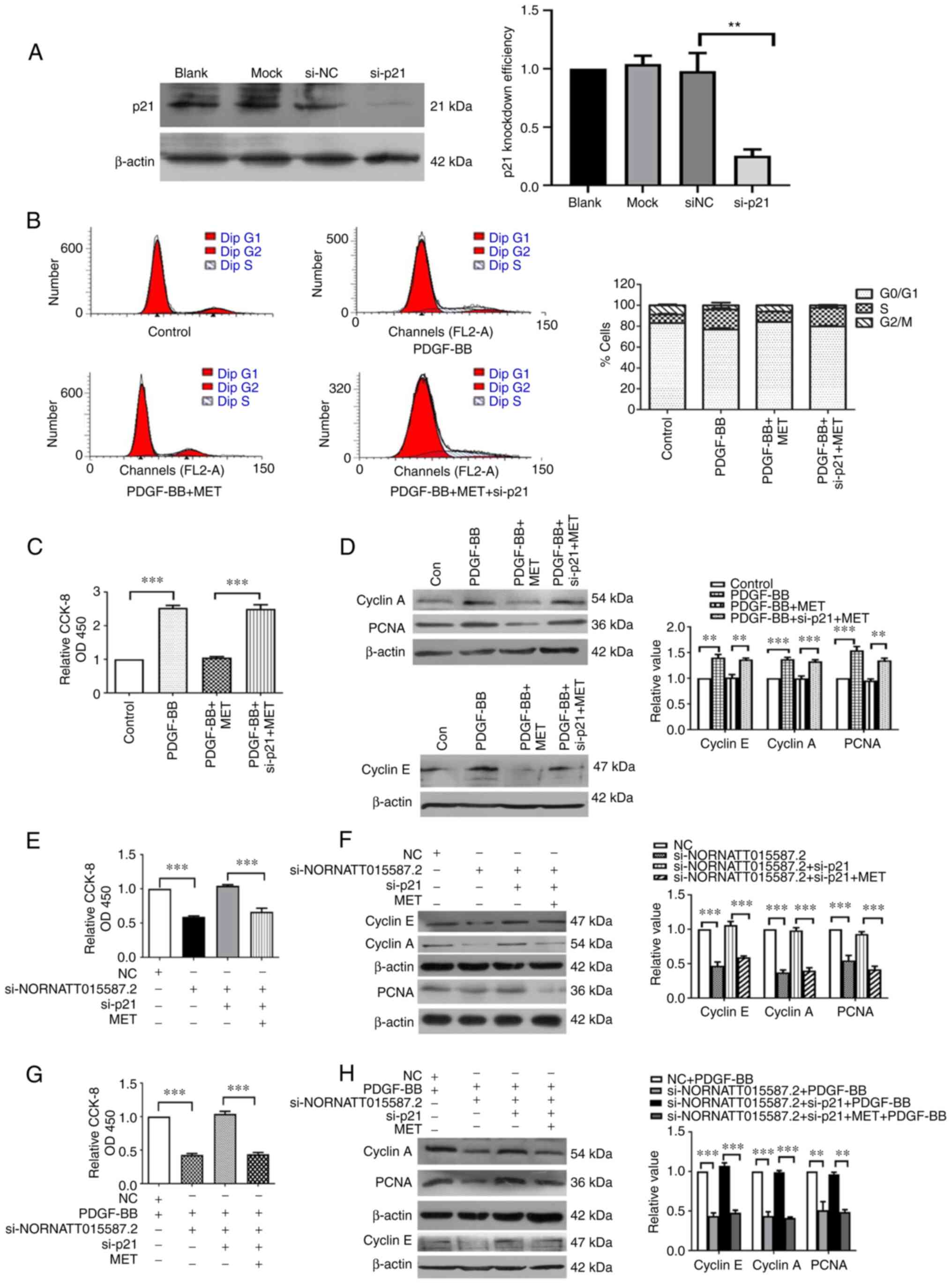 | Figure 5NONRATT015587.2 targets p21 to
promote the proliferation of PASMCs, and metformin reverses this
effect. (A) The efficiency of p21 silencing was assessed by western
blotting. (B) Flow cytometry was performed to detect the number of
cells in each phase of the cell cycle in PASMCs. (C) A CCK-8 assay
was performed to determine the proliferation of PASMCs after
control, PDGF-BB, PDGF-BB+MET, and PDGF-BB+si-p21+MET treatments.
(E) A CCK-8 assay was performed to determine the proliferation of
PASMCs after NC, si-NONRATT015587.2, si-NONRATT015587.2+si-p21 and
si-NONRATT015587.2+si-p21+MET treatment. (G) A CCK-8 assay was
performed to determine the proliferation of PASMCs after
NC+PDGF-BB, si-NONRATT015587.2+
PDGF-BB,si-NONRATT015587.2+si-p21+PDGF-BB and
si-NONRATT015587.2+si-p21+ MET+PDGF-BB treatment. (D, F and H) The
protein expression of PCNA, Cyclin A and Cyclin E was determined in
PASMCs under different conditions. **P<0.01 and
***P<0.001 as indicated (n= ≥3). CCK-8, Cell Counting
Kit-8; Con, control; MET, metformin; NC, negative control; OD,
optical density; PASMCs, pulmonary artery smooth muscle cells;
PCNA, proliferating cell nuclear antigen; PDGF-BB, platelet-derived
growth factor-BB; si, small interfering RNA. |
The next experiments were carried out to determine
whether p21 was the regulatory target of NONRATT015587.2.
NONRATT015587.2 silencing was used to test this hypothesis. The
results revealed that PASMC proliferation and the protein
expression of cyclin E, cyclin A and PCNA were significantly
decreased following the NONRATT015587.2 knockdown (Fig. 5E and F). However, p21 silencing
could rescue the inhibitory effect of NONRATT015587.2 silencing on
the proliferation of PASMCs, as well as the expression of cyclin E,
cyclin A and PCNA proteins to the levels of the NC group. However,
after treatment with MET, the proliferation of PASMC was inhibited
when NONRATT015587.2 and p21 were silenced at the same time. These
results suggested that p21 was the target of NONRATT015587.2. Thus,
MET may inhibit the proliferation of PASMCs via the
NONRATT015587.2/p21 axis.
The aforementioned results suggested that endogenous
NONRATT015587.2 regulated the proliferation of PASMCs by targeting
p21. Therefore, the next experiments were designed to determine
whether NONRATT015587.2 would promote PASMC proliferation in the
presence of PDGF-BB external stimulation. PDGF-BB stimulated PASMC
proliferation and PCNA, cyclin A and cyclin E protein expression
was significantly reduced after NONRATT015587.2 silencing, and cell
proliferation was inhibited. Silencing p21 reversed the effects on
cell proliferation and the expression of cyclin E, cyclin A and
PCNA proteins to the levels of PDGF-BB treatment alone. Following
treatment with MET, the proliferation of PASMCs was reduced
(Fig. 5G and H). Therefore,
NONRATT015587.2 may regulate the proliferation of PASMCs by
targeting p21 in the presence or absence of external stimuli.
Discussion
Abnormal proliferation of PASMCs is an important
feature of PAH vascular remodeling and is also the main reason for
the progression of PAH, leading to thickening of the pulmonary
blood vessels, narrowing of the lumen and increased tension, which
ultimately results in increased PA pressure, right heart failure
and death (3,24,40). Pathophysiological stimuli, such
as hypoxia, inflammation and oxidative stress can promote the
proliferation of PASMCs (3,24,40). The proliferation of PASMCs is
controlled by the cell cycle, which is in turn regulated by various
signaling pathways (41).
Indeed, the cell cycle is strictly regulated by cell cycle
regulators, which involves a complex cascade of cellular events
(42,43). These regulators include cyclins,
cyclin-dependent kinases (CDKs), cyclin-dependent kinase inhibitors
(CKIs), retinoblastoma proteins (Rb) and PCNA (42,43). Positive regulators of the cell
cycle (cyclin, CDKs, Rb and PCNA) are also known as cell cycle
engines, which help cells transition through cell cycle
checkpoints, thereby promoting cell proliferation and
transformation (34). The
activity of CDKs is controlled by cyclin regulatory subunits. These
subunits bind to specific cyclins to form complexes and are
regulated by CKIs at specific stages of the cell cycle. The
functional activation of cyclin/CDK complexes is necessary for cell
cycle progression (27,43,44). PASMCs are usually in a static
state in the middle PA with low-index proliferation and are in the
G0/G1 phase of the cell cycle (43). Cells enter the cell cycle from a
resting state, activate the cyclin/CDK complex (such as cyclin
E/CDK2, cyclin A/CDK2 and cyclin D/CDK4) in the G1
phase, then trigger the transition from the
G0/G1 to the S phase (42,43). PCNA is a gene product that is
modulated by the phosphorylation of Rb. It is synthesized during
the G0/G1 and S phases of the cell cycle and
plays an important role in the transition from the G1 to
the S phase (42,43). p21, a CKI, is a negative
regulator of cell cycle progression (27,28). p21 can bind to CDK2 or CDK4
complexes and inhibit their activity (27,28,34), especially in the case of the CDK2
complex (cyclin E/CDK2, cyclin A/CDK2) (44). p21 can also interact with PCNA
and play a regulatory role in S-phase DNA replication and DNA
damage repair (28). Therefore,
p21 inhibits cell proliferation by inhibiting G1/S cell
cycle checkpoints (cyclin E/CDK2, cyclin A/CDK2 and PCNA) (34,43).
The PDGF family includes four heterogeneous
polypeptide chains (A-C and D), which can assemble into five
different dimeric forms (PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC and
PDGF-DD), among which the PDGF-BB homodimer is the strongest
inducer of VSMC proliferation identified to date (1). PDGF is necessary for VSMC
proliferation (1,43). PDGF expression is upregulated in
MCT-induced and hypoxia-induced PAH rats, as well as in the lung
tissues and PASMCs of patients with PAH (25,40,45). The current results also suggested
that PDGF is highly expressed in pulmonary vessels of MCT induced
PAH rats. PDGF-mediated VSMC proliferation is a tightly regulated
process involving Ras/MAPK, PI3K/Akt, phospholipase C (PLC)-γ and
JAK/STAT signaling pathways (1,34,42,43). The phosphorylation of PLC-γ, Akt,
ERK1/2, p38 and JNK in PDGF-BB-stimulated VSMCs has been
demonstrated to be significantly increased (42,43). These signals represent early cues
for the proliferation of VSMCs and are eventually brought together
into the common regulatory pathway in the cell cycle through a
complex signaling cascade (1,42). PDGF promotes cell cycle
transition from the G0/G1 phase to the S
phase by upregulating the expression of positive cell cycle
regulators (cyclin E, cyclin D, CDK2, CDK4, PCNA and S-phase kinase
associated protein 2) and downregulating the expression of negative
regulators (p21 and p27) to induce VSMC proliferation (34,40,42,43). In the present study, PDGF-BB
stimulation significantly increased the expression of cyclin E,
cyclin A and PCNA protein at the G1/S cell cycle
checkpoints, downregulated the expression of p21, accelerated the
cell cycle transition from G0/G1 phase to S
phase and promoted cell proliferation. Previous studies, including
our own, confirmed that MET could inhibit MCT-induced pulmonary
vascular remodeling in PAH rats (14,15,22) and PDGF-induced PASMC
proliferation (34). The
majority of these studies focused on MET targeting PI3K/Akt/mTOR
signaling. In another study focusing on the inhibitory effect of
MET on tumor cell proliferation, it was observed that MET acted as
a G1/S cell cycle checkpoint inhibitor, which could
suppress tumor cell proliferation by upregulating p21 to block the
cell cycle at G0/G1 phase (29). To the best of our knowledge, the
effect of MET on PASMC proliferation and cell cycle progression has
not yet been reported. In the present study, MET could
significantly inhibit the expression of G1/S
checkpoint-related proteins (cyclin E, cyclin A and PCNA) and
induce p21 expression. Cell cycle progression was blocked at the
G1 phase, thereby inhibiting the proliferation of
PASMCs.
The regulation of lncRNAs in the pathogenesis of PAH
has been attracting increasing attention. Several lncRNA molecules
that regulate the proliferation of PASMCs have been identified,
such as TCONS_00034812 (3), MEG3
(4), MALAT1 (23), Hoxaas3 (24), H19 (25) and UCA1 (26). NONRATT015587.2 is a novel lncRNA
whose biological function has not yet been fully characterized. The
present study confirmed that NONRATT015587.2 promoted the
proliferation of PASMCs in a PAH rat model. This lncRNA was
upregulated in the MCT group and downregulated in the MET + MCT
group. By contrast, p21 expression was downregulated in the MCT
group and upregulated in the MCT + MET group. In addition, the
expression of NONRATT015587.2 was significantly upregulated in
PASMCs stimulated with PDGF-BB, which was accompanied by the
downregulation of p21 expression. This induced the upregulation of
G1/S phase checkpoint-related proteins and promoted the
proliferation of PASMCs. In vitro, following MET treatment,
the expression of NONRATT015587.2 was downregulated, and that of
p21 was upregulated, which inhibited the expression of
G1/S checkpoint-related proteins and the proliferation
of PASMCs. Thus, the expression patterns of NONRATT015587.2 and p21
and the results of MET intervention were found to be consistent
between the in vivo and the in vitro experiments. In
addition, our previous study demonstrated that the expression of
p21 was negatively correlated with that of NONRATT015587.2, which
is located downstream, and is jointly regulated by MCT and MET
(22). In the current study, MET
inhibited the expression of G1/S checkpoint-related
proteins (cyclin E, cyclin A and PCNA), blocked the cell cycle at
G0/G1 and inhibited PDGF-induced PASMC
proliferation by upregulating p21. Silencing p21 abolished the
effects of MET, upregulating the expression of G1/S
phase checkpoint-related proteins, promoting the cell cycle entry
into the S phase and restoring PASMC proliferation. This suggests
that p21 is a key target for MET and that MET inhibits the
proliferation of PASMCs by upregulating p21 to block the cell cycle
at the G0/G1 phase. Furthermore,
NONRATT015587.2 and p21 silencing experiments were used to verify
the interaction between these two molecules. Following
NONRATT015587.2 silencing, the expression of G1/S phase
checkpoint-related proteins was downregulated and PASMC
proliferation was inhibited. In addition, p21 silencing could
rescue the inhibitory effect of NONRATT015587.2 silencing on PASMC
proliferation, induce the expression of G1/S
checkpoint-related proteins and promote the proliferation of
PASMCs. Following treatment with MET, the expression levels of
G1/S-phase checkpoint-related proteins were reduced and
PASMC proliferation was inhibited. These data suggest that p21 is
the target of NONRATT015587.2. Targeting p21 to interfere with the
cell cycle may represent a new research direction for PAH therapy
(1). In summary, MET inhibits
PASMC proliferation through NONRATT015587.2-mediated p21
upregulation, which provides insight into the pathogenesis of PAH
and mechanism of action of MET.
The subcellular localization of lncRNAs is
significant and is an important determinant of their functional
characteristics (46). In the
present study, RNA FISH analysis demonstrated that NONRATT015587.2
was located in the nucleus. Generally, lncRNAs localized in the
nucleus mainly serve a role in chromatin regulatory modification
and transcriptional regulation, whereas cytoplasmic lncRNAs are
involved in competing endogenous RNA network regulation by
adsorbing miRNAs, affecting mRNA stability and translation
regulation, thus playing a regulatory role at both the
transcriptional and the post-transcriptional level (23). Nuclear lncRNA molecules can bind
to specific chromatin sites and act as epigenetic regulators,
regulating basic cellular processes, such as proliferation or
apoptosis (46). In addition,
the function of lncRNAs also depends on their sequence, which
determines their interaction with transcription factors and their
ability to regulate target mRNA transcripts (46). Our previous study determined that
p53 was involved in the regulation of the proliferation mechanism
of NONRATT015587.2 (22). p53 is
located in the nucleus and cytoplasm, accounting for ~50%
separately (42). Therefore, it
may be hypothesized that NONRATT015587.2 interacts with p53 in the
nucleus. Activated p53 could translocate to the nucleus or shuttle
between the cytoplasm and the nucleus, where it exerts its
regulatory effect (4). In
addition, p21 is a downstream target gene of p53 that is regulated
in a p53-dependent and -independent manner (29,30,44). p21 also has p53 and PCNA binding
sequences, which regulate cell cycle progression and proliferation
(29,30,44).
The lncRNA NONRATT015587.2 has not been further
verified in humans, which was a limitation to the current study.
The main reason is that the conservation of lncRNA is a difficult
point in current research, and its conservation is significantly
lower than that of mRNA. In the absence of a corresponding genome
database, its completeness and accuracy vary significantly among
different laboratories. With the continuous in-depth study of
lncRNA conservation and the continuous improvement of
bioinformatics screening tools, a future constructed lncRNA
reference database would be more comprehensive and accurate, and it
would also have more reference value. The present study was
primarily concerned with the therapeutic effect of MET on pulmonary
hypertension and its mechanism. The main finding of the study was
that MET reduced pulmonary vascular remodeling by regulating the
expression of lncRNA. This provides a new theoretical basis for the
next step in studying the therapeutic effects and pathological
mechanisms of MET in patients with pulmonary hypertension. In
addition, there are some newly discovered lncRNAs, such as
NONRATT018084.2, NONRATT009275.2, NONRATT007865.2 and
NONRATT026300.2 which require (21). Although these were not identified
in our previous microarray analysis (22), further study surrounding the PA
tissues of MCT and MCT+MET rats should be performed to elucidate
novel regulatory mechanisms in which MET reverses pulmonary
vascular remodeling. In summary, lncRNA NONRATT015587.2 could
promote the expression of PASMC proliferation genes by targeting
p21, while MET could upregulate p21 through NONRATT015587.2 to
inhibit PDGF-BB-induced PASMC proliferation. These findings may
also improve our understanding of the pathogenesis of PAH and the
mechanisms underlying the action of MET.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
ZS and YaL contributed substantially to the
conception and design of the experiments. YaL conducted all
experiments and wrote the manuscript. NL conducted data analyses
and modified the manuscript draft. RH and TW performed in
vivo experiments. YuL was performed PCR experiments. ZS and YuL
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was supervised and approved by
the Ethics Committee of China Pharmaceutical University (approval
no. 202021) and performed in accordance with the Regulations on the
Administration of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
This study was supported by National Natural Science Foundation
of China (grant no. 31871155), the Key Laboratory Open Project
(grant no. KFKT-2103), the project of Jiangsu Provincial Commission
of Health and Family Planning (grant no. QNRC2016505) and the Six
Big Talent Peak C Projects (grant no. YY-110).
References
|
1
|
Wang D, Uhrin P, Mocan A, Waltenberger B,
Breuss JM, Tewari D, Mihaly-Bison J, Huminiecki Ł, Starzyński RR,
Tzvetkov NT, et al: Vascular smooth muscle cell proliferation as a
therapeutic target. Part 1: Molecular targets and pathways.
Biotechnol Adv. 36:1586–1607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimoda LA and Laurie SS: Vascular
remodeling in pulmonary hypertension. J Mol Med (Berl). 91:297–309.
2013. View Article : Google Scholar
|
|
3
|
Liu Y, Sun Z, Zhu J, Xiao B, Dong J and Li
X: LncRNA-TCONS_00034812 in cell proliferation and apoptosis of
pulmonary artery smooth muscle cells and its mechanism. J Cell
Physiol. 233:4801–4814. 2018. View Article : Google Scholar
|
|
4
|
Sun Z, Nie X, Sun S, Dong S, Yuan C, Li Y,
Xiao B, Jie D and Liu Y: Long non-coding RNA MEG3 downregulation
triggers human pulmonary artery smooth muscle cell proliferation
and migration via the p53 signaling pathway. Cell Physiol Biochem.
42:2569–2581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thenappan T, Ormiston ML, Ryan JJ and
Archer SL: Pulmonary arterial hypertension: Pathogenesis and
clinical management. BMJ. 360:j54922018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson J, Lakshmanan G, M B, R M V,
Kalimuthu K and Sekar D: Computational identification of MiRNA-7110
from pulmonary arterial hypertension (PAH) ESTs: A new microRNA
that links diabetes and PAH. Hypertens Res. 43:360–362. 2020.
View Article : Google Scholar
|
|
7
|
Whitaker ME, Nair V, Sinari S, Dherange
PA, Natarajan B, Trutter L, Brittain EL, Hemnes AR, Austin ED,
Patel K, et al: Diabetes mellitus associates with increased right
ventricular afterload and remodeling in pulmonary arterial
hypertension. Am J Med. 131:702.e7–702.e13. 2018. View Article : Google Scholar
|
|
8
|
Hemnes AR, Luther JM, Rhodes CJ, Burgess
JP, Carlson J, Fan R, Fessel JP, Fortune N, Gerszten RE, Halliday
SJ, et al: Human PAH is characterized by a pattern of lipid-related
insulin resistance. JCI Insight. 4:e1236112019. View Article : Google Scholar :
|
|
9
|
Zhou T, Xu X, Du M, Zhao T and Wang J: A
preclinical overview of metformin for the treatment of type 2
diabetes. Biomed Pharmacother. 106:1227–1235. 2018. View Article : Google Scholar
|
|
10
|
Li M, Li X, Zhang H and Lu Y: Molecular
mechanisms of metformin for diabetes and cancer treatment. Front
Physiol. 9:10392018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zi F, Zi H, Li Y, He J, Shi Q and Cai Z:
Metformin and cancer: An existing drug for cancer prevention and
therapy. Oncol Lett. 15:683–690. 2018.PubMed/NCBI
|
|
12
|
Ikhlas S and Ahmad M: Metformin: Insights
into its anticancer potential with special reference to AMPK
dependent and independent pathways. Life Sci. 185:53–62. 2017.
View Article : Google Scholar
|
|
13
|
Agard C, Rolli-Derkinderen M,
Dumas-de-La-Roque E, Rio M, Sagan C, Savineau JP, Loirand G and
Pacaud P: Protective role of the antidiabetic drug metformin
against chronic experimental pulmonary hypertension. Br J
Pharmacol. 158:1285–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Han D, Zhang Y, Xie X, Ke R, Zhu Y,
Liu L, Song Y, Yang L and Li M: Activation of AMPK prevents
monocrotaline-induced extracellular matrix remodeling of pulmonary
artery. Med Sci Monit Basic Res. 22:27–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhai C, Shi W, Feng W, Zhu Y, Wang J, Li
S, Yan X, Wang Q, Zhang Q, Chai L, et al: Activation of AMPK
prevents monocrotaline-induced pulmonary arterial hypertension by
suppression of NF-κB-mediated autophagy activation. Life Sci.
208:87–95. 2018. View Article : Google Scholar
|
|
16
|
Chaumeil J, Le Baccon P, Wutz A and Heard
E: A novel role for Xist RNA in the formation of a repressive
nuclear compartment into which genes are recruited when silenced.
Genes Dev. 20:2223–2237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling H, Vincent K, Pichler M, Fodde R,
Berindan-Neagoe I, Slack FJ and Calin GA: Junk DNA and the long
non-coding RNA twist in cancer genetics. Oncogene. 34:5003–5011.
2015. View Article : Google Scholar :
|
|
19
|
Greco S, Gorospe M and Martelli F:
Noncoding RNA in age-related cardiovascular diseases. J Mol Cell
Cardiol. 83:142–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vencken SF, Greene CM and McKiernan PJ:
Non-coding RNA as lung disease biomarkers. Thorax. 70:501–503.
2015. View Article : Google Scholar
|
|
21
|
Hou S, Chen D, Liu J, Chen S, Zhang X,
Zhang Y, Li M, Pan W, Zhou D, Guan L and Ge J: Profiling and
molecular mechanism analysis of long non-coding RNAs and mRNAs in
pulmonary arterial hypertension rat models. Front Pharmacol.
12:7098162021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Z, Liu Y, Yu F, Xu Y, Yanli L and Liu
N: Long non-coding RNA and mRNA profile analysis of metformin to
reverse the pulmonary hypertension vascular remodeling induced by
monocrotaline. Biomed Pharmacother. 115:1089332019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brock M, Schuoler C, Leuenberger C,
Bühlmann C, Haider TJ, Vogel J, Ulrich S, Gassmann M, Kohler M and
Huber LC: Analysis of hypoxia-induced noncoding RNAs reveals
metastasis-associated lung adenocarcinoma transcript 1 as an
important regulator of vascular smooth muscle cell proliferation.
Exp Biol Med (Maywood). 242:487–496. 2017. View Article : Google Scholar
|
|
24
|
Zhang H, Liu Y, Yan L, Wang S, Zhang M, Ma
C, Zheng X, Chen H and Zhu D: Long noncoding RNA Hoxaas3
contributes to hypoxia-induced pulmonary artery smooth muscle cell
proliferation. Cardiovasc Res. 115:647–657. 2019. View Article : Google Scholar
|
|
25
|
Su H, Xu X, Yan C, Shi Y, Hu Y, Dong L,
Ying S, Ying K and Zhang R: LncRNA H19 promotes the proliferation
of pulmonary artery smooth muscle cells through AT1R via sponging
let-7b in monocrotaline-induced pulmonary arterial hypertension.
Respir Res. 19:2542018. View Article : Google Scholar
|
|
26
|
Zhu TT, Sun RL, Yin YL, Quan JP, Song P,
Xu J, Zhang MX and Li P: Long noncoding RNA UCA1 promotes the
proliferation of hypoxic human pulmonary artery smooth muscle
cells. Pflugers Arch. 471:347–355. 2019. View Article : Google Scholar
|
|
27
|
Zhang Y, Wang Y, Wang X, Zhang Y, Eisner
GM, Asico LD, Jose PA and Zeng C: Insulin promotes vascular smooth
muscle cell proliferation via microRNA-208-mediated downregulation
of p21. J Hypertens. 29:1560–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bi M, Yu H, Huang B and Tang C: Long
non-coding RNA PCAT-1 over-expression promotes proliferation and
metastasis in gastric cancer cells through regulating CDKN1A. Gene.
626:337–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi A, Kimura F, Yamanaka A,
Takebayashi A, Kita N, Takahashi K and Murakami T: Metformin
impairs growth of endometrial cancer cells via cell cycle arrest
and concomitant autophagy and apoptosis. Cancer Cell Int.
14:532014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fornari F, Milazzo M, Chieco P, Negrini M,
Marasco E, Capranico G, Mantovani V, Marinello J, Sabbioni S,
Callegari E, et al: In hepatocellular carcinoma miR-519d is
up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21,
PTEN, AKT3 and TIMP2. J Pathol. 227:275–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dean A, Nilsen M, Loughlin L, Salt IP and
MacLean MR: Metformin reverses development of pulmonary
hypertension via aromatase inhibition. Hypertension. 68:446–454.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Xu X, Gu J, Ding X, Ge G, Zang X, Ji R,
Shao M, Mao Z, Zhang Y, Zhang J, et al: LINC00978 promotes the
progression of hepatocellular carcinoma by regulating EZH2-mediated
silencing of p21 and E-cadherin expression. Cell Death Dis.
10:7522019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song Y, Wu Y, Su X, Zhu Y, Liu L, Pan Y,
Zhu B, Yang L, Gao L and Li M: Activation of AMPK inhibits
PDGF-induced pulmonary arterial smooth muscle cells proliferation
and its potential mechanisms. Pharmacol Res. 107:117–124. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elgendy M, Cirò M, Hosseini A, Weiszmann
J, Mazzarella L, Ferrari E, Cazzoli R, Curigliano G, DeCensi A,
Bonanni B, et al: Combination of hypoglycemia and metformin impairs
tumor metabolic plasticity and growth by modulating the
2A-GSK3β-MCL-1 axis. Cancer Cell. 35:798–815.e5. 2019. View Article : Google Scholar
|
|
36
|
Li B, Zhou P, Xu K, Chen T, Jiao J, Wei H,
Yang X, Xu W, Wan W and Xiao J: Metformin induces cell cycle
arrest, apoptosis and autophagy through ROS/JNK signaling pathway
in human osteosarcoma. Int J Biol Sci. 16:74–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia C, Liu C, He Z, Cai Y and Chen J:
Metformin inhibits cervical cancer cell proliferation by modulating
PI3K/Akt-induced major histocompatibility complex class I-related
chain A gene expression. J Exp Clin Cancer Res. 39:1272020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zahra MH, Afify SM, Hassan G, Nawara HM,
Kumon K, Seno A and Seno M: Metformin suppresses self-renewal and
stemness of cancer stem cell models derived from pluripotent stem
cells. Cell Biochem Funct. 39:896–907. 2021. View Article : Google Scholar
|
|
39
|
Liu Y, Xu Y, Zhu J, Li H, Zhang J, Yang G
and Sun Z: Metformin prevents progression of experimental pulmonary
hypertension via inhibition of autophagy and activation of
adenosine monophosphate-activated protein kinase. J Vasc Res.
56:117–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhuang W, Lian G, Huang B, Du A, Gong J,
Xiao G, Xu C, Wang H and Xie L: CPT1 regulates the proliferation of
pulmonary artery smooth muscle cells through the AMPK-p53-p21
pathway in pulmonary arterial hypertension. Mol Cell Biochem.
455:169–183. 2019. View Article : Google Scholar
|
|
41
|
Lee J and Kang H: Hypoxia promotes
vascular smooth muscle cell proliferation through microRNA-mediated
suppression of cyclin-dependent kinase inhibitors. Cells.
8:8022019. View Article : Google Scholar :
|
|
42
|
Kwon H, Lee JJ, Lee JH, Cho WK, Gu MJ, Lee
KJ and Ma JY: Cinnamon and its components suppress vascular smooth
muscle cell proliferation by up-regulating cyclin-dependent kinase
inhibitors. Am J Chin Med. 43:621–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yu JY, Lee JJ, Jung JK, Kim TJ, Yoo HS,
Yun YP and Lee JC: JY0691, a newly synthesized obovatol derivative,
inhibits cell cycle progression of rat aortic smooth muscle cells
through up-regulation of p21(cip1). Eur J Pharmacol. 624:23–30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Poon RY, Jiang W, Toyoshima H and Hunter
T: Cyclin-dependent kinases are inactivated by a combination of p21
and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J
Biol Chem. 271:13283–13291. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Perros F, Montani D, Dorfmüller P,
Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E,
Mussot S, Mercier O, et al: Platelet-derived growth factor
expression and function in idiopathic pulmonary arterial
hypertension. Am J Respir Crit Care Med. 178:81–88. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jandl K, Thekkekara Puthenparampil H,
Marsh LM, Hoffmann J, Wilhelm J, Veith C, Sinn K, Klepetko W,
Olschewski H, Olschewski A, et al: Long non-coding RNAs influence
the transcriptome in pulmonary arterial hypertension: The role of
PAXIP1-AS1. J Pathol. 247:357–370. 2019. View Article : Google Scholar :
|