1. Introduction
Osteoarthritis (OA) is the most common joint disease
globally and it primarily affects the elderly. It can be defined as
a degenerative disease of articular cartilage, characterized by
destruction of the articular cartilage, synovial tissue
inflammation, subchondral bone alterations and formation of bony
outgrowths (called osteophytes), which causes joint stiffness,
chronic pain and eventually disability (1). OA is a complex disease the
development of which involves genetic and acquired factors.
Multiple risk factors such as ageing, injury, innate genetic
variations and environmental factors contribute to the progression
of OA (2,3).
Articular cartilage is an avascular tissue covering
joint surfaces that facilitates movement and is responsible for
shock absorbance. Articular cartilage consists of chondrocytes
which are embedded by an extracellular matrix (ECM) (4). ECM consists of collagen,
proteoglycans, hyaluronic acid and other less common components
such as gelatin, a matrix glycoprotein through which collagen
imparts tensile strength and shape to the tissue (5). Type II collagen is the main
structural protein in cartilage and it is responsible for building
the ECM network structure with aggrecan and other proteoglycans
(6).
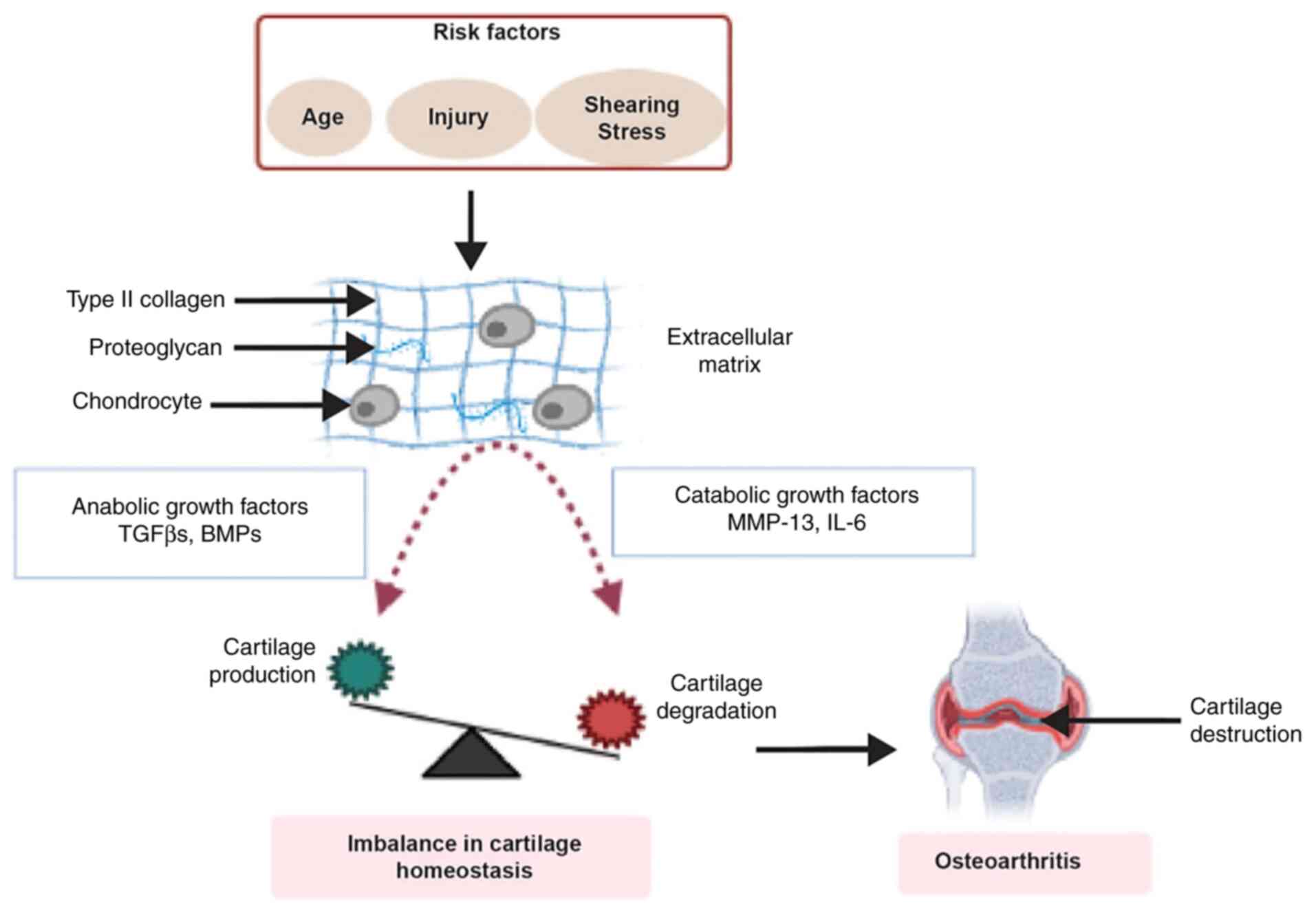
Disruption of healthy cartilage, which is
characterized by the balance between anabolic and catabolic process
of ECM production and degradation, may lead to cartilage loss
(Fig. 1). Chondrocyte governs
joint health by controlling the balance reaction. A number of other
factors such as tensile strain, proinflammatory cytokine and growth
factors are also involved in modulating chondrocyte homeostasis.
The TGF-β superfamily, which involves TGF-β and bone morphogenetic
proteins (BMPs), consists of anabolic growth factors (7,8).
Catabolic factors such as matrix metalloprotease (MMP)-13 and
inflammatory cytokines such as interleukin (IL)-6 are involved in
the destruction of the collagen network and the structure of the
ECM (9,10). These catabolic factors target
cartilage for the degradation of types II and IV collagen,
proteoglycan and aggrecan (11).
The past two decades of extensive research work has
focused on unravelling the disease mechanism and associated
enhancing factors; however, the full understanding of disease has
not been acquired. Current understanding of the disease mechanism
is insufficient with regard to early diagnosis or providing
optimized treatment for OA patients. However, based on recent
findings, the TGF-β signaling pathway role in OA development and
progression may represent a potential therapeutic target for OA
therapy.
2. TGF-β signaling and osteoarthritis
Previous studies demonstrate a crucial role of TGF-β
members in multiple cellular processes such as cell proliferation;
therefore, any deterioration in TGF-β signaling pathways can have
an immense impact on numerous human diseases, including OA
(2,3). The TGF-β family consists of 35
members, which includes TGF-βs, BMPs, activins and fibroblast
growth factor (FGF)-18, all of which are essential in regulating
cell proliferation, inflammation and tissue repair (12).
Three isoforms of TGF-β, TGF-β1, TGF-β2 and TGF-β3,
exist in mammalian tissue and exhibit a high degree of homology;
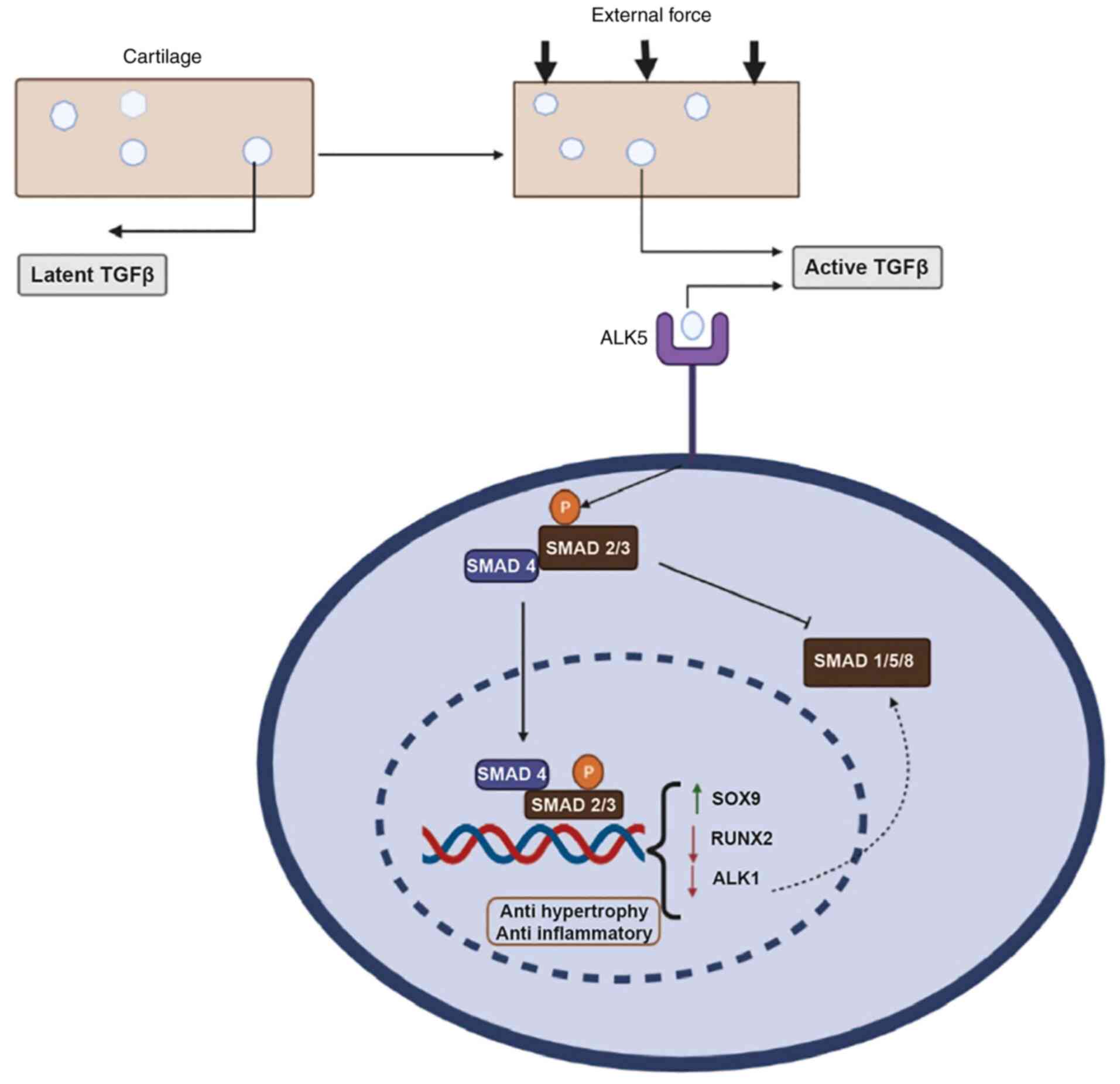
however, they have different tissue-specific expressions (13). They are generated in an inactive
form by chondrocytes, which are usually bound to the ECM of
cartilage. Shearing stress, a mechanical force caused by
compressive loading, activates these inactive chondrocytes
(13). Ligand binding stimulates
type I and type II receptors complex generation, while TGF binding
to the receptor complex is stabilized and facilitated by type III
receptors, which recruit receptor-regulated small mothers against
decapentaplegic (R-SMAD) protein (14). After R-SMAD is phosphorylated, a
complex is formed with SMAD4, which is transported to the nucleus
where it binds to transcription factors such as SRY-box
transcription factor 9 (SOX9) and runt-related transcription factor
2 (RUNX2) and initiates transcription. SMAD2/3 signaling is
associated with anti-hypertrophic and anti-inflammatory activity,
whereas SMAD1/5/8 signaling is associated with pro-hypertrophic
control of the ECM (15,16). The differential regulation of
SMAD2/3 and SMAD1/5/8 pathways depends on the presence of active
TGF-β concentration. SMAD1/5/8 signaling is stimulated with a
comparatively high TGF-β concentration (>5 ng/ml), while low
TGF-β concentration primarily stimulates SMAD2/3 signaling in human
fibroblasts (17). This process
is depicted in Fig. 2.
All three isoforms of the TGF-β superfamily can
induce chondrogenic differentiation of mesenchymal stem cells in
adult bone marrow. Compared to TGF-β1, TGF-β2 and TGF-β3 are more
efficient in stimulating chondrogenesis by aggregating
glycosaminoglycan (18).
Scientific evidence has confirmed embryonic lethality or various
bone defects of the hindlimbs and forelimbs in mice lacking TGF-β
isoforms, which indicates a key role of TGF-β in skeletogenesis
(19). It has been reported,
based on animal models, that the elevated expression of TGF-β1 is
involved in the development of OA. Injecting multiple
intra-articular doses of TGF-β into mouse joints shows similar
changes in the articular cartilage that occurs in experimental and
spontaneous mouse OA (20).
Similar results showing a higher concentration of active TGF-β1
leading to osteoarthritic changes in the bone and cartilage has
been demonstrated in mice subchondral bone (21). Synovial lining layer-induced
TGF-β1 expression in the murine knee joint also shows OA-like
features of chondro-osteophyte formation and hyperplasia of the
synovium (22).
Various in vitro and animal studies indicate
the involvement of TGF-β in OA but findings in human data are
limited (23,24). Suarez et al (23) report that 11 patients with hip OA
and 11 patients with femoral neck fracture had higher expression of
TGF-β isoforms. Wu et al (24) found that the protein expression
of TGF-β1 was 16-fold lower in OA cartilage than in healthy
cartilage, which indicate that TGF-β1 has a joint-specific effect
in OA. According to data collected from six hip OA patients and
four controls, TGF-β1 may also have a role in the hypertrophy stage
of the OA process (25).
Supplementary TGF-β can help in sustaining joint
homeostasis in a healthy joint when it is targeted to cartilage
with relatively low active levels of TGF-β. Low active levels could
be advantageous only when the chondrocyte TGF receptor expression
pattern supports hypertrophy inhibition to retain the
differentiated chondrocyte phenotype. Failing to fulfil such
conditions or exposing the whole joint to high TGF-β levels may
cause osteophyte formation and synovial fibrosis and may forcefully
cause articular chondrocyte hypertrophy (26). Systemic inhibition of TGF-β in
osteoarthritic joints may block its pathology but may influence
TGF-β′s crucial role in healthy cells, which may cause unwanted
adverse effects on healthy cartilage.
3. TGF-β family members in OA therapy
Multiple in vivo experiments have shown
cartilage defect treatment by adding extra TGF-β. Injecting
TGF-overexpressing fibroblasts, mesenchymal stem cells and
chondrocytes into rabbits enhances cartilage injury recovery
through cartilage regeneration (27,28). In the joints of rabbits with
experimental OA, intra-articular TGF-β1 transfection (used for its
overexpression) significantly reduces cartilage matrix degradation
(29). In the OA-affected
cartilage, TGF-β1 expression appears to be highly linked with SMAD3
expression, but this link is not observed in healthy cartilage.
Furthermore, TGF-β1 expression appears to be influenced by age, sex
and obesity. TGF-β1 switches its role from a protective agent to a
damage-causing agent in human OA cartilage, possibly via SMAD
independent pathway by augmenting MMP-13 expression, which is a
cartilage-degrading enzyme (30).
TGF-β2 also inhibits OA progression (31). It is reported to advance the
expression of specific tissue inhibitor of MMP-3 (TIMP), thereby
imparting cartilage protection (32). Furthermore, TGF-β2 inhibits
collagenase activity and proteoglycan degradation in OA by
downregulating IL-1β and tumor necrosis factor (TNF)-α (33,34). TGF-β2 regulates collagen
degradation of articular cartilage by downregulating collagenase
MMP-9 in OA (35). Despite
having a key protective role in chondrocyte homeostasis during OA
progression, a high concentration of TGF-β2 can lead to the
destruction of normal cartilage (23).
In a large animal investigation that included sheep,
Mrugala et al (36)
reported a favorable result in which bovine mesenchymal stem cells
(MSCs) with 50 ng of TGFβ-3 in a chitosan scaffold were utilized to
fill partial thickness defects generated in the inner region of the
patella. At two months, histological tests indicated the presence
of chondrocyte-like cells embedded in a hyaline cartilaginous
matrix that was entirely integrated into native cartilage tissue.
Another finding, by Tang et al (37), was the clinical enhancement
effect of TGF-β3 in vitro and in vivo on cartilage
formation with a suitable dose and scaffold carrier. In human MSCs,
TGF-β3 has shown influence on anabolic chondrogenic gene markers
such 1-collagen type II and cartilage oligomeric matrix protein.
For the in vivo study, TGF-β3 cultured with ovine MSCs in a
chitosan scaffold enhances the growth of hyaline cartilage that was
fully integrated into the sheep's host cartilage tissue (37).
A previous study also demonstrates dose- and
time-dependent expression of TGF-β3 on the chondrogenic gene
expression of cartilage oligomeric matrix protein, α1-collagen type
II and alkaline phosphatase at concentrations of 10, 20 or 60 ng/ml
(38). Furthermore, mode of
delivery is another crucial factor for effective chondrogenesis
effect of TGF-β3. In vivo studies have shown that hydrogels
implantation containing TGF-β3 (10 ng/ul) and rabbit chondrocytes
into 10 nude mice result in a substantial increase in
glycosaminoglycan (GAG), collagen and chondrocyte DNA content,
where continuous stimulation with them led to chondrogenesis for
two weeks (39,40). TGF-β3 release from
poly-lactic-co-glycolic acid (PLGA) microspheres embedded in
chitosan thermo-sensitive gels is shown to be linear ≤28 days, with
a concentration of roughly 3 ng/ml generating a 12-fold increase in
GAG synthesis from hMSCs. This data suggested that the mode of
delivery is equally important (41).
Another member of TGF-β family, BMP-6, has been
linked with chondrocyte differentiation. It is also reported to be
found in both normal and OA adult human articular cartilage
(37). Such endogenous
expression of BMP-6 in cartilage independent of the presence of OA
suggests that it serves a role in joint integrity maintenance and
might be used as a therapeutic molecule for cartilage regeneration
(42).
Applying all these growth factors with a suitable
platform for the purpose of cartilage lesion repair may represent
potential therapeutics which may aid hyaline cartilage regeneration
and thereby slow the progression towards OA. Most in vitro
investigations support chondrogenesis with TGF-β3; however, very
few in vivo studies exist and virtually no study has
investigated TGF-β3 application in human OA treatment for clinical
purpose (36,37,39,40). A comparative chart of different
TGF-β family members are shown in Table I.
 | Table IComparative role of different members
of TGF-β superfamily. |
Table I
Comparative role of different members
of TGF-β superfamily.
| Growth factors |
Characteristics | Effect on each
other | Function | Relation to OA |
|---|
| TGF-β1 | Most abundant and
widely expressed isoform. TGF-β1 and TGF-β2 share 71% sequence
identity. | TGF-β1 and BMP-2
have a synergistic effect in the production of hyaline-like
cartilage in serum-free chondrogenic differentiation of mesenchymal
stem cells. TGF-β1 and BMP-7 have a Synergistic Effect on
chondrogenesis and ECM synthesis. | Promotes cartilage
synthesis, articular chondrocyte growth, and cartilage repair.
Stimulates chondrocyte proliferation. Upregulates essential
glycolytic factors to promote maintenance of healthy articular
chondrocytes phenotype. | In mouse
experimental OA model, increased expression was found in developing
osteophytes and articular cartilage. Increased MMP-13 expression
causes cartilage destruction. |
| TGF-β2 | Expressed by
neurons in the embryonic and nervous system TGF-β1 and TGF-β2 share
71% sequence identity | Bone Morphogenetic
Protein-7 shows antagonistic behavior with TGF-β2 in human
trabecular meshwork cells. | Promotes
chondrogenesis in interstitial cells. Controls chondrocyte
differentiation, induce ECM formation and chondrocyte
proliferation. In the progress to TGF-β1 induced chondrogenic
differentiation, TGF-β2 alters from type I to type II
collagen. | Promotes the
expression of TIMP-3 imparting cartilage protection. |
| TGF-β3 | Found in lung
adenocarcinoma and kidney carcinoma cell lines. High expression
level in umbilical cord. TGF-β1 and TGF-β2 share 80% sequence
identity. | TGF-β3 shows
synergistic effects with FGF-18 in chondrogenic differentiation.
TGF-β3 and BMP-6 has shown improved chondrogenicity compared with
TGF-β3 alone. | Promotes in
vitro and in vivo cartilage formation; both stimulate
chondrogenic differentiation of cells, synthesize glycosaminoglycan
sulfate and increase extra chondral matrix components. | During mouse
experimental OA, enhanced expression was found in articular
cartilage and osteophytes development |
| BMP-2 | Found mainly in
lung, pancreas, kidney and spleen. | BMP-2 and TGF-β1
have shown synergistic behavior on rabbit bone marrow-derived
chondrogenesis. | Induces ECM
production and proliferation and bone formation. Skeletal repair
and regeneration. Supports expansion of the chondrogenic phenotype
of human articular chondrocytes. | Overexpressed in
osteoarthritic chondrocytes. Direct injection helps cartilage and
subchondral bone regeneration to treat large weight-bearing
osteochondral defects. Stimulates chondrocyte maturation and
hypertrophy in in vitro mesenchymal stromal cells. |
| BMP-7 | Expressed in liver,
brain, kidney, lung, heart, and pancreas. | TGF-β1 and BMP-7
show synergistic effect on extracellular matrix synthesis and
chondrogenesis. BMP-7 shows antagonistic behavior with TGF-β2 in
human trabecular meshwork cells. | Promotes ECM
synthesis and diminishes cartilage degradation through decreasing
expression of a number of ILs and MMPs
Function in postnatal maintenance of articular cartilage. | OA cartilage has
decreased levels of BMP-7. Intra-articular injection of rhBMP-7
inhibits articular cartilage degradation and blocks the synovial
membrane's production of inflammatory cytokines. |
| FGF-18 | Expressed mainly in
heart, skeletal muscle and pancreas. | Shows synergistic
behavior with TGF-β3 on the chondrogenic differentiation. | Stimulates
cartilage development, promotes regeneration of hyaline articular
cartilage potency and delays articular cartilage degeneration. Acts
chondroprotectively via regulating TIMP-1 expression. | In rats, promotes
repair of damaged cartilage in progressive OA. |
4. Stem cell and stem cell-derived
therapeutics in OA
Existing conventional treatments for OA include
physical therapy, chondroitin sulphate supplementation or surgical
therapy such as microfracture and abrasion arthroplasty which aim
to improve joint function and relieve pain. However, these
therapies have the limitation of being but poorly effective
(43).
Adult stem cells, particularly adipose-derived stem
cells (ASCs) and bone marrow-derived mesenchymal stem cells
(BMSCs), are extensively used for cartilage tissue engineering due
to their potential to differentiate into a chondrogenic lineage and
their ability to be matched to the patient (44,45). However, these cell lines have the
drawback of a limited number of cell passages. Adult stem cells
with a high passage number will have short telomeres. Telomere
shortening causes senescence and loss of function. This limitation
is circumvented by overexpressing human telomerase reverse
transcriptase (hTERT), a well-known approach for in vitro
chondrogenesis which prevents telomere shortening and makes ASCs
immortal (46).
MSCs are non-hematopoietic multipotent stem cells
which are commonly employed in laboratory research. These cells can
be obtained from tissues such as bone marrow, Wharton's jelly,
spleen, liver and adipose tissue (47,48). Due to the ability of MSCs to
develop into mesodermal tissues such as cartilage bone, muscle and
ligament under certain conditions (49), MSC therapy has been proven as
effective for treating OA (50).
Human adipose-derived mesenchymal stem cells (ADMSCs), are
multipotent stem cells and easier to harvest than are BMSCs. They
can be easily isolated from subcutaneous adipose tissue by using
lipoaspirates after enzymatic digestion (50). Moreover, their high abundance
makes cell culture expansion easy (51,52). Findings from a comparative study
on human adult MSCs derived from bone marrow, adipose tissue and
dermal tissue reveal that human adipose-derived stem cells (hASCs)
secrete the highest level of paracrine factors involved in tissue
regeneration; this feature makes the cell line more favorable for
regenerative therapies (53). In
addition, low immunogenicity, self-renewal potential, ability to
differentiate on multiple lineages and high rate of proliferation
give this cell type further advantage over other types of stem
cells (54-56). hASCs have also been linked to
tissue regeneration through immunomodulation and paracrine activity
(57). Spasovski et al
(58) first reported using
adipose tissue as a source of MSCs. Their findings regarding the
use of ADMSCs showed that nine patients diagnosed with OA were
treated with a single injection of ADMSCs. After six months of
follow up, their clinical examination using radiography showed
improvements, including the restoration of the hyaline articular
cartilage, with no significant adverse effects.
A number of recent findings reveal that the
therapeutic properties of stem cells have been attributed to the
paracrine secretion of anti-inflammatory and chondroprotective
mediators or trophic factors; in particular, small extracellular
vesicles (EVs) (59-61). An exosome, a major category of
EVs, is a nanosized vesicle that is surrounded by a phospholipid
membrane. It has a diameter of 30-200 nm and is present in various
biological fluids such as synovial fluid, saliva, blood, urine and
pleural fluid, or released by most cells, including joint cells
(60). Exosomes are
characterized by the presence of endosomal markers such as CD9,
TSG101, CD61 and CD83. They were previously considered to serve as
a means of removing undesired materials from the cell. However,
subsequent research has revealed that they have an important part
in intercellular signaling and infectious disease pathogenesis,
which indicates they have the paracrine nature of signaling
(61). Exosomes are discharged
via exocytosis from multivesicular bodies (i.e. late endosomes),
which fuse into the plasma membrane of target cells such as
chondrocytes and transfer their packaged cargo into the cytoplasm
(62). Exosomal cargo includes
lipids, transcription factors, ECM proteins and nucleic acids,
among other substances (e.g. mRNA) and noncoding RNA and trigger a
number of physiological processes, including epigenetic changes
(63).
As with healthy cells, apoptotic cells secrete
exosomes, called 'apoptotic exosomes'. They have an endosomal
origin, produced in a caspase 3- and 9-dependent manner. Apoptotic
exosomes share the same structural features in shape and size as
those produced by healthy cells; in addition, apoptotic exosomes
have the functional characteristic of being an intercellular
communicator. They also exhibit exosome-specific marker proteins
such as CD63, LAMP1, HSP70 (a stress-associated marker released
during apoptosis). However, what sets apoptotic exosomes apart is
the presence of sphingosine-1-phosphate receptors 1 and 3 (S1PR1
and S1PR3, respectively), a distinguished protein marker (64,65). Previous studies have confirmed
their role in inflammation, immunomodulation, cell signaling and
apoptotic cell clearance (64,66).
5. Adipose-derived stem cell (ASCs)-derived
exosome: A therapeutic and safe approach towards OA treatment
As stated previously, exosome-based therapy has
recently sparked interest in the scientific world due to its
well-known role in a number of pathobiological processes. A number
of studies have confirmed the stimulatory effect of BMSC-derived
exosomes on injured tissues, thereby causing cartilage and
subchondral bone regeneration and repair (67,68).
Cosenza et al (69) first demonstrated that EVs
produced by different cellular pathways have identical in
vivo functions in OA. They showed that microparticles and
exosomes isolated from BMSCs of adult mice have a similar
chondroprotective impact in a collagenase-induced OA model.
Exosomes from BMSCs which have been pre-treated with TGF-β3
markedly elevate anabolic marker gene expression, while decreasing
catabolic marker gene expression in osteoarthritic
chondrocytes.
Injecting intra-articular BMSCs exosomes results in
a decrement in articular cartilage impairment and subchondral bone
deterioration in a collagenase-induced mouse model (70). This study evaluates the effect of
exosomes and microparticles on OA-like murine chondrocytes and both
were able to restore the expression of anabolic chondrocyte markers
(e.g. aggrecan and type II collagen) in OA-like chondrocytes while
suppressing catabolic markers (e.g. ADAMTS5 and MMP-13) and
inflammatory markers (e.g. inducible nitric oxide synthase). The
two EVs also protect chondrocytes from apoptosis and suppress
macrophage activation.
Furthermore, exosomes generated from BMSCs may
influence the biological phenotype of other OA-related cells such
as synovial fibroblasts (SFBs) or macrophages. Findings by Jin
et al (71) demonstrate
that human BMSC-derived exosomes reduce the proliferation of
IL-1-treated SFBs and increased their apoptosis through an
miRNA-26a-5p-mediated reduction in PTGS2. Their data show that
hBMSC-derived exosomes overexpressing miR-26a-5p reduce
inflammation, proliferation and migration while promoting
apoptosis. Thus, these exosomes could attenuate OA damage by
repressing prostaglandin-endoperoxide synthase 2 (PTGS2). Mao et
al (72) demonstrate that,
among BMSC-derived exosomes overexpressing miR-92a3p, the
MSC-miR-92a-3p-exosome significantly upregulates the levels of
aggrecan, SOX9, COL9A1, COL2A1 and COMP and downregulates the
expression of COL10A1, RUNX2 and MMP13. This finding suggests that
BMSC-derived exosomes promote chondrogenesis and prevent cartilage
matrix degradation in a miR-92a-3p-dependent manner.
ASC-derived EVs have a potent function in OA
modulation. In Tofiño-Vian et al (73), MVs and exosomes were both
primarily responsible for the paracrine activity of AMSCs on
osteoarthritic osteoblasts. In IL-1-treated osteoblasts, EVs from
human AMSCs dramatically reduced IL-6 and PGE2 levels, increased
the release of IL-10 and downregulated mitochondrial membrane
potential. The findings of another study on the ADMSC-exosome by
Tofiño-Vian et al (74)
suggest that it had a chondroprotective function by using
anti-inflammatory effects. That study reports that ADMSC-exosomes
can reduce the secretion of proinflammatory cytokines such as IL-6,
TNF-α and IL-10 in OA chondrocytes. In addition, they also revealed
a decline in the cyclooxygenase-2 (COX-2) expression level, which
is an OA marker, and in the generation of prostaglandin E2 (PGE2),
which is a proinflammatory factor in the OA joint. The
intra-articular injection of ASC-EVs may efficiently protect the
cartilage from degradation and diminish OA progression in subacute
and chronic arthritic models, based on findings discussed in a
recent paper by Woo et al (75). In their findings, hASC-EVs
therapy effectively inhibits the IL-1β-mediated expression of
MMP-1, MMP-3, MMP-13 and ADAMTS-5, while increasing the expression
of type II collagen in chondrocytes. Their findings suggest that
hASC-EVs therapy increases chondrocyte proliferation and migration
while also mediating the balance between catabolic and anabolic
metabolism, thereby resulting in cartilage regeneration. In a study
by Zhao et al (76), in
which they extracted exosomes from donor adipose tissue by using
elective liposuction surgery, the investigators discovered that
adipose-derived stem cell (ADSC)-exosomes may reduce the expression
of proinflammatory genes while increasing the expression of
anti-inflammatory cytokines in activated SFBs and improving
periosteal cell proliferation and chondrogenic potential via
increasing miR145 and miR221. These findings add to the growing
body of evidence suggesting that AMSC-derived exosomes could offer
a novel perspective for the development of an efficient and
optimized OA therapy.
6. Safety perspective
The safety parameter of any product meant for human
therapeutic use has a vital role. It refers to the minimization of
the risk/benefit ratio associated with the product being employed
for patient treatment. ADSC-based therapy has a number of
applications in regenerative medicine involved in bone
regeneration, neurodegenerative diseases and autoimmune and
restoring wound defects, based on its efficiency and efficacy
(77). However, it also has the
adverse effect of blindness in SVF-treated patients, which puts the
credibility and accountability of these therapies at question
(78). Henceforth, for any
therapy, before entering the clinical setting, checking the safety
criteria of the product and the source is imperative.
A number of publications of ASC-derived cell
therapies to the construction of immortalized human adipose-derived
MSCs vouch for its safety to be used for public health. Vériter
et al (79) assesses the
safety and efficacy of ASC-derived cell therapies and demonstrates
the safety of autologous ASC transplantation in 17 patients without
any serious adverse events in grafted patients. Atat et al
(80) reveals that passaged ADSC
expansion has no effect on stem cell differentiation and does not
provide a malignant potential to the cells in vitro. Tátrai
et al (81) demonstrate
that transfecting BMI1 and TERT simultaneously into human
adipose-derived MSCs results in the production of successful
immortalized human adipose-derived MSCs without significantly
affecting their phenotype or biological behavior. The latest
research by Zhang et al (82), which used the same method,
indicates that immortalized MSCs are safe by using in vitro
and in vivo testing to confirm that immortalized MSCs are
not tumorigenic. They explore the efficacy and safety of
immortalized MSCs as a cellular drug carrier in brain tumor
treatment.
7. Regenerating cartilages by engineered
ASCs
As stated previously, BMSCs represent an appealing
cell source in cartilage tissue engineering. BMSCs can accomplish
chondrogenesis when stimulated with suitable growth factors such as
TGF-βs and BMPs, as indicated by the overexpression of SOX9, Col2A1
and ACAN (83). However,
chondro-induction of BMSCs is frequently accompanied by
osteogenesis and hypertrophy, which can lead to apoptosis and
calcification (84).
To date, ASCs have become the more desirable stem
cells used for cartilage regeneration due to the ease of production
and they can initiate chondrogenesis when stimulated in
chondrogenic media with TGF-β1, TGF-β3 and BMP-6 (85-87). BMSCs are superior to ASCs in the
chondrogenic potential; therefore, selecting the appropriate
combination of growth factors to induce ASCs chondrogenesis is
essential (88).
TGF-β3 is a robust chondrogenesis inducer, while
BMP-6 can synergistically induce the chondrogenesis potential of
TGF-β3. The combination of TGF-β3 and BMP-6 has an exceptional
potent chondrogenic effect on ASCs and will offer an ideal OA
therapy. Ude et al (89)
confirm the chondrogenic potential of ADSCs and BMSCs by using the
combination of TGF-β3 and BMP-6. They compared the effectiveness of
cartilage regeneration by chondrogenically induced ADSCs and BMSCs
by using a combination of TGF-β3 and BMP-6. On evaluating the
recovery of treated joints after 12 months, they discovered
cartilage regeneration in OA in the sheep knee after injecting them
with the combination of TGF-β3 and BMP-6.
Lu et al (90) designed genetically engineered
rabbit ASCs (rASCs) with sustained TGF-β3/BMP-6 expression using
baculovirus. They note that continued expression of TGF-β3/BMP-6
for two weeks enhances chondrogenesis, reduces
osteogenesis/hypertrophy and results in the development of
cartilaginous constructions with improved maturity and mechanical
qualities. Choi et al (91) show the significance of stem cells
as a cell source for chondrogenesis on induction with TGF-β3 and
BMP-6. The two growth factors exhibit a strong synergistic effect
of ≤281% when compared with control. In comparison with not only
controls, but also TGF-β3 or BMP-6 single treatments, the combined
therapy significantly boosts Sox9, aggrecan and collagen II
expression. All these findings validated the hypothesis that TGF-β3
and BMP-6 possess a strong chondrogenic potential for OA treatment,
compared to TGF-β3 alone.
As Cosenza et al (69) show, pre-activation of MSCs with
TGF-β can boost the anti-osteoarthritic potential of exosomes and
this approach of utilizing MSCs with growth factors would be an
efficacious platform which exploits the benefits of stem cells,
TGF-β3 and BMP-6, thus maintaining cartilage homeostasis and joint
health. To bring all these facts together, incorporating
MSC-derived exosomes along with the chondrogenic potential of
TGF-β3 and BMP-6 would be a superior approach to halt OA
progression and this would be a novel means to provide exceptional
treatment for OA patients.
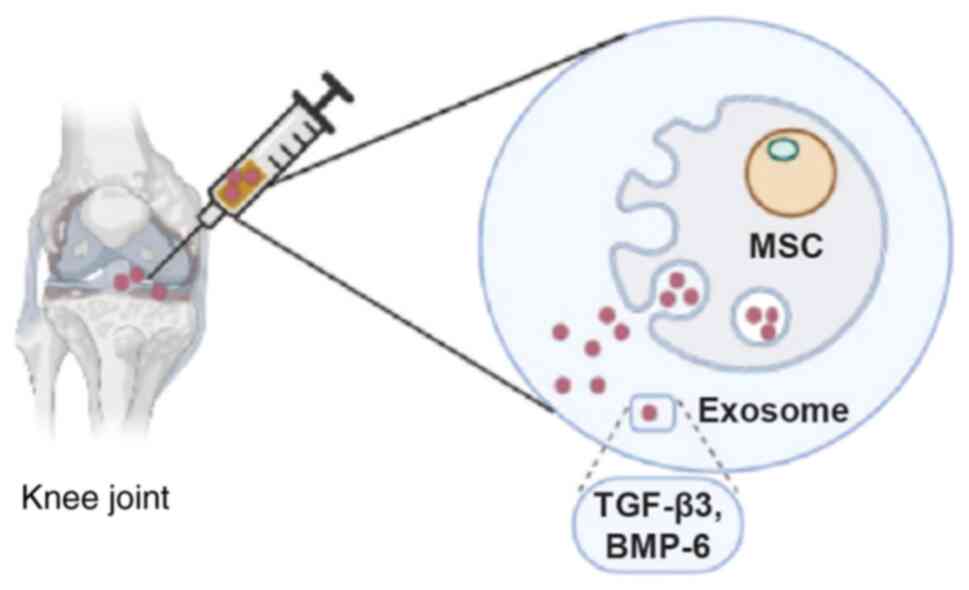
8. Bench to bedside
Exosomes, isolated from MSCs which themselves
possess chondroprotective function, besides the characteristic of
being a natural cargo carrier with the inherent property of low
immunogenicity, excellent specificity and high penetrability,
present an excellent candidate for targeted delivery of both TGF-β3
and BMP-6 into an injured joint for cure and treatment at the
cellular level without any side effects (Fig. 3).
As exosome-based therapy seems to be the improved
substitute for stem cell-based therapy, recently a number of
companies are developing stem cell derived exosomes-based
therapeutics for OA and joint injury. Exopharm is one such
Australian based company that has developed a stem cell derived
exosome-based drug for the treatment of osteoarthritis (Cevaris),
which is under pre-clinical development phase. (https://exopharm.com/). Similarly, another company
based in the Republic of Korea (Exostemtech) is conducting clinical
trials for exosomes produced from adipose derived stem cells.
(http://www.exostemtech.co.kr/).
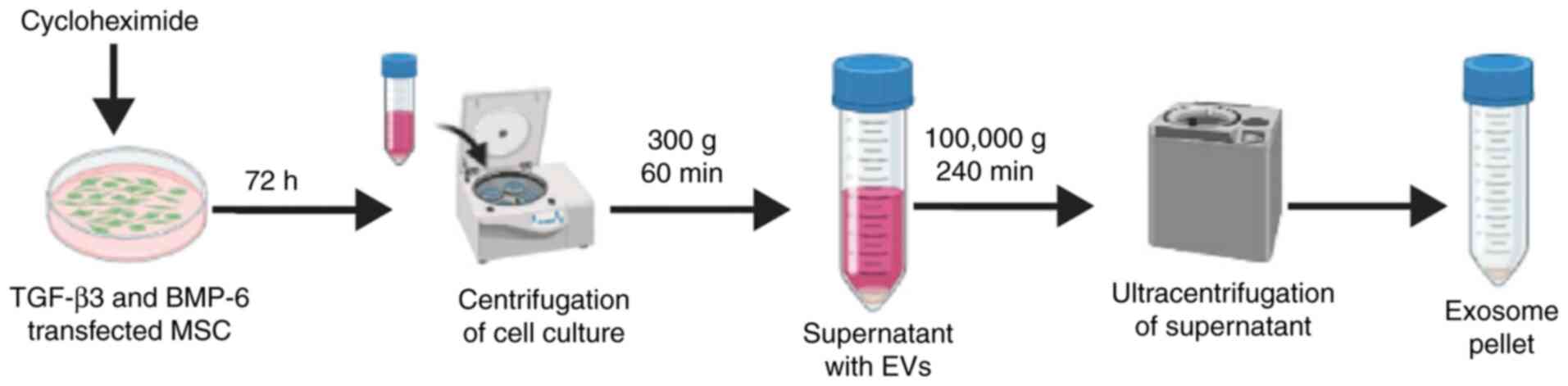
Similarly, CK-Exogene is a Republic of Korea based
company which recently launched its exosome based Covid-19 vaccine
against SARS Covid-2 infection (under the process of approval from
the Ministry of Food and Drug Safety) for its commercialization
(92). Following a similar
strategy, this company(CK-Exogene) is also planning to launch
exosome-based targeted therapy for osteoarthritis treatment. With
the company's patented technology of mass production of exosome
using apoptosis (Fig. 4), they
plan to develop targeted therapy for osteoarthritis using exosome
as a drug delivery vehicle by overexpressing both TGF-β3 with
BMP-6.
9. Conclusion
OA, although extensively researched, still lacks an
effective and safe treatment. The only current treatment option
available for advanced OA is joint replacement surgery. This
surgery may pose the risks of persistent pain, surgical
complications and limited implant lifespan. Existing therapy of
joint treatment has multiple adverse effects and ~20% of patients
receiving this surgery are dissatisfied with the outcome. In
addition, scientific efforts remain far behind with the development
of therapy that could slow the progression of OA or could reverse
the disease phenotype.
Of note, stem cell-based therapy is useful as an
alternative treatment before surgery. As stated previously, the
paracrine activity of MSCs and ADSCs has been attributed to
exosome. Multiple findings stating the role of exosomes in
chondroprotection have also shown exosomes may be a more favorable
choice than the source itself.
Additionally, considering the crucial role of. TGF-β
in the cartilage homeostasis, targeting it could be an alternative
therapeutic approach. Studies have confirmed TGF-β3 as a promising
candidate which has the chondrogenic potential to repair articular
cartilage degeneration. Combining TGF-β3 with BMP-6, which has
synergistic effect on chondrogenesis, with an efficient platform
such as exosomes, which themselves possess a chondroprotective
function, offers an innovative and more efficient approach to treat
injured cartilage. Investigating such strategies for use in
clinical practice for OA therapeutics would provide optimized
treatment without posing any adverse effects and would open a novel
avenue for targeted OA therapy in the future.
However, despite the findings of a number of studies
supporting the fact of MSC-/ASC-derived exosomes as a treatment
tool for OA, the lack of sufficient evidence makes their usage
challenging. More clinical studies using exosomes for OA treatment
are required to determine the repercussions and potential adverse
effects.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
KHY, NT, YJC and JK substantially contributed to the
conception and the design of the study. SHY, BJK, JOL and YNJ were
contributed to data acquisition and data analysis and
interpretation. And KHY, NT, YJC, SHY, BJK, JOL, YJ and JK were
involved in manuscript drafting and revision and critically revised
the manuscript for important intellectual content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The purification strategy for the mass production of
highly purified and concentrated exosomes is subject to Korean
patent application no. 10-2020-0062365 (Fig. 4), associated with CK-Exogene,
Inc. NT and JK are employees of CK-Exogene, Inc. However, the other
authors (KY, YC, SY, BK, JL and YJ) are not associated with
CK-Exogene, Inc. and declare that they have no competing
interests.
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
1
|
Felson DT: Clinical practice.
Osteoarthritis of the knee. N Engl J Med. 354:841–848. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring SR and Goldring MB: Changes in
the osteochondral unit during osteoarthritis: Structure, function
and cartilage-bone crosstalk. Nat Rev Rheumatology. 12:632–644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Urban H and Little CB: The role of fat and
inflammation in the pathogenesis and management of osteoarthritis.
Rheumatology (Oxford). 57(Suppl 4): pp. iv10–iv21. 2018, View Article : Google Scholar
|
|
4
|
Fox AJS, Bedi A and Rodeo SA: The basic
science of articular cartilage: Structure, composition and
function. Sports Health. 1:461–468. 2019.
|
|
5
|
Pearle AD, Warren RF and Rodeo SA: Basic
science of articular cartilage and osteoarthritis. Clin Sports Med.
24:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shoulders MD and Raines RT: Collagen
structure and stability. Annu Rev Biochem. 78:929–958. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aigner T, Zien A, Gehrsitz A, Gebhard PM
and McKenna L: Anabolic and catabolic gene expression pattern
analysis in normal versus osteoarthritic cartilage using
complementary DNA-array technology. Arthritis Rheum. 44:2777–2789.
2001. View Article : Google Scholar
|
|
8
|
Darling EM and Athanasiou KA:
Biomechanical strategies for articular cartilage regeneration. Ann
Biomed Eng. 31:1114–1124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kevorkian L, Young DA, Darrah C, Donell
ST, Shepstone L, Porter S, Brockbank SMV, Edwards DR, Parker AE and
Clark IM: Expression profiling of metalloproteinases and their
inhibitors in cartilage. Arthritis Rheum. 50:131–141. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Little CB, Barai A, Burkhardt D, Smith SM,
Fosang AJ, Werb Z, Shah M and Thompson EW: Matrix
metalloproteinase-13 deficient mice are resistant to osteoarthritic
cartilage erosion but not chondrocyte hypertrophy or osteophyte
development. Arthritis Rheum. 60:3723–3733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Latourte A, Cherifi C, Maillet J, Ea HK,
Bouaziz W, Brentano TF, Solal MC, Hay E and Richette P: Systemic
inhibition of IL-6/Stat3 signaling protects against experimental
osteoarthritis. Ann Rheum Dis. 76:748–755. 2017. View Article : Google Scholar
|
|
12
|
Jobling AI, Nguyen M, Gentle A and McBrien
NA: Isoform-specific changes in scleral transforming growth
factor-β expression and the regulation of collagen synthesis during
myopia progression. J Biol Chem. 279:18121–18126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Javelaud D and Mauviel A: Mammalian
transforming growth factor-betas: Smad signaling and
physio-pathological roles. Int J Biochem Cell Biol. 36:1161–1165.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Itoh S, Itoh F, Goumans MJ and Dijke PT:
Signaling of transforming growth factor-b family members through
Smad proteins. Eur J Biochem. 267:6954–6967. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finnson KW, Parker WL, Dijke PT, Thorikay
M and Philip A: ALK1 Opposes ALK5/Smad3 signaling and expression of
extracellular matrix components in human chondrocytes. J Bone Miner
Res. 23:896–906. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blaney Davidson EN, Remst DF, Vitters EL,
van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB and van der
Kraan PM: Increase in ALK1/ALK5 Ratio as a cause for elevated
MMP-13 expression in osteoarthritis in humans and mice. J Immunol.
182:7937–7945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Remst DF, Blaney Davidson EN, Vitters EL,
Bank RA, van den Berg WB and van der Kraan PM: TGF-β induces Lysyl
hydroxylase 2b in human synovial osteoarthritic fibroblasts through
ALK5 signaling. Cell Tissue Res. 355:163–171. 2014. View Article : Google Scholar
|
|
18
|
Barry F, Boynton RE, Liu B and Murphy M:
Chondrogenic differentiation of mesenchymal Stem cells from bone
marrow: Differentiation-dependent gene expression of matrix
components. Exp Cell Res. 268:189–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Enker ND and Krieglstein K: Targeted
mutations of transforming growth factor-beta genes reveal important
roles in mouse development and adult homeostasis. Eur J Biochem.
267:6982–6988. 2000. View Article : Google Scholar
|
|
20
|
van Beuningen HM, Glansbeek HL, van der
Kraan PM and van den Berg WB: Osteoarthritis-like changes in the
murine knee joint resulting from intra-articular transforming
growth factor-beta injections. Osteoarthritis Cartilage. 8:25–33.
2000. View Article : Google Scholar
|
|
21
|
Zhen G, Wen C, Jia XF, Li Y, Crane JL,
Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, et al: Inhibition
of TGF-β signaling in mesenchymal stem cells of subchondral bone
attenuates osteoarthritis. Nat Med. 19:704–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakker AC, van de Loo FA, van Beuningen
HM, Sime P, van Lent PL, van der Kraan PM, Richards CD and van den
Berg WB: Overexpression of active TGF-beta-1 in the murine knee
joint: Evidence for synovial-layer-dependent Chondro-osteophyte
formation. Osteoarthritis Cartilage. 9:128–136. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suarez MP, Oreja MTC, Calaza M, Reino JG
and Gonzalez A: Differential upregulation of the three transforming
growth factor beta isoforms in human osteoarthritic cartilage. Ann
Rheum Dis. 68:568–571. 2009. View Article : Google Scholar
|
|
24
|
Wu J, Liu W, Bemis A, Wang E, Qiu YC,
Morris EA, Flannery CR and Yang Z: Comparative proteomic
characterization of articular cartilage tissue from normal donors
and patients with osteoarthritis. Arthritis Rheum. 56:3675–3684.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verdier MP, Seite S, Guntzer K, Pujol JP
and Boumediene K: Immunohistochemical analysis of transforming
growth factor beta isoforms and their receptors in human cartilage
from normal and osteoarthritic femoral heads. Rheumatol Int.
25:118–124. 2005. View Article : Google Scholar
|
|
26
|
Lee WH, Song SU, Hwang TS, Yi Y, Oh IS,
Lee JY, Choi KB, Choi MS and Kim S: Regeneration of hyaline
cartilage by cell-mediated gene therapy using transforming growth
factor beta1-producing fibroblasts. Hum Gene Ther. 12:1805–1813.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song SU, Cha YD, Han JU, Oh IS, Choi KB,
Yi Y, Hyun JP, Lee HY, Chi GF, Lim CL, et al: Hyaline cartilage
regeneration using mixed human chondrocytes and transforming growth
factor-beta1-producing chondrocytes. Tissue Eng. 11:1516–1526.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo X, Zheng Q, Yang S, Shao Z, Yuan Q,
Pan Z, Tang S, Liu K and Quan D: Repair of full-thickness articular
cartilage defects by cultured mesenchymal stem cells transfected
with the transforming growth factor beta1 gene. Biomed Mater.
1:206–215. 2006. View Article : Google Scholar
|
|
29
|
Zhang P, Zhong ZH, Yu HT and Liu B:
Exogenous expression of IL-1Ra and TGF-β1 promotes in vivo repair
in experimental rabbit osteoarthritis. Scand J Rheumatol.
44:404–411. 2015. View Article : Google Scholar
|
|
30
|
Eshghi EA, Liu M, Harper PE, Doré J,
Martin G, Furey A, Green R, Rahman P and Zhai G: Overexpression of
MMP13 in human osteoarthritic cartilage is associated with the
SMAD-independent TGF-β signaling pathway. Arthritis Res Ther.
17:264–272. 2015. View Article : Google Scholar
|
|
31
|
Xie J, Zhang D and Lin Y: Anterior
Cruciate ligament transection-induced cellular and extracellular
events in menisci: Implications for osteoarthritis. Am J Sports
Med. 46:1185–1198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kudipudi PK, Galuska SP, Dietze R, Bobis
GS, Loveland KL and Konrad L: Betaglycan (TβRIII) is a key factor
in TGF-β2 signaling in prepubertal rat Sertoli cells. Int J Mol
Sci. 20:6214–6232. 2019. View Article : Google Scholar
|
|
33
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie J, Fu N, Cai LY, Gong TY, Li GY, Peng
Q and Ca XX: The effects of interleukin-1β in modulating
osteoclast-conditioned medium's influence on gelatinases in
chondrocytes through mitogen-activated protein kinases. Int J Oral
Sci. 7:220–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tchetina EV, Antoniou J, Tanzer M, Zukor
DJ and Poole AR: Transforming growth factor-beta2 suppresses
collagen cleavage in cultured human osteoarthritic cartilage,
reduces expression of genes associated with chondrocyte hypertrophy
and degradation, and increases prostaglandin E(2) production. Am J
Pathol. 168:132–1340. 2006. View Article : Google Scholar
|
|
36
|
Mrugala D, Bony C, Neves N, Caillot L,
Fabre S, Moukoko D, Jorgensen C and Noe D: Phenotypic and
functional characterization of ovine mesenchymal stem cells:
Application to a cartilage defect model. Ann Rheum Dis. 67:288–295.
2008. View Article : Google Scholar
|
|
37
|
Tang QO, Shakib K, Heliotis M and Tsiridis
E, Mantalaris A, Ripamonti A and Tsiridis E: TGF-beta3: A potential
biological therapy for enhancing chondrogenesis. Expert Opin Biol
Ther. 9:689–701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mehlhorn A, Schmal H, Kaiser S, Lepski G,
Finkenzeller G, Stark GB and Südkamp NP: Mesenchymal stem cells
maintain TGF-beta-mediated chondrogenic phenotype in alginate bead
culture. Tissue Eng. 12:1393–1403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bian L, Zhai DY, Tous E, Rai R, Mauck RL
and Burdick JA: Enhanced MSC chondrogenesis following delivery of
TGF-β3 from alginate microspheres within hyaluronic acid hydrogels
in vitro and in vivo. Biomaterials. 32:6425–6434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Choi SJ, Na K, Kim S, Woo DG, Sun BK,
Chung HM and Park KH: Combination of ascorbate and growth factor
(TGF beta-3) in thermo-reversible hydrogel constructs embedded with
rabbit chondrocytes for neocartilage formation. J Biomed Mater Res
A. 83:897–905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng ZH, Li YS, Gao X, Lei GH and Huard J:
Bone morphogenetic proteins for articular cartilage regeneration.
Osteoarthritis Cartilage. 26:1153–1161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hayashi M, Muneta T, Ju YJ, Mochizuki T
and Sekiya I: Weekly intra-articular injections of bone
morphogenetic protein-7 inhibits osteoarthritis progression.
Arthritis Res Ther. 10:R1182008. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hino K, Saito A, Kido M, Kanemoto S, Asada
R, Takai T, Cui M, Cui X and Imaizumi K: Master regulator for
chondrogenesis, Sox9, regulates transcriptional activation of the
endoplasmic reticulum stress transducer BBF2H7/CREB3L2 in
chondrocytes. J Biol Chem. 289:13810–13820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tan AR and Hung CT: Concise review:
Mesenchymal stem cells for functional cartilage tissue engineering:
Taking cues from chondrocyte-based constructs. Stem Cells Transl
Med. 6:1295–1303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gimble JM and Guilak F: Adipose-derived
adult stem cells: Isolation, characterization and differentiation
potential. Cytotherapy. 5:362–369. 2003. View Article : Google Scholar
|
|
46
|
Goldring MB: Immortalization of human
articular chondrocytes for generation of stable, differentiated
cell lines. Methods Mol Med. 100:23–36. 2004.PubMed/NCBI
|
|
47
|
L PK, Kandoi S, Misra R, Vijayalakshmi S,
Rajagopal K and Verma RS: The mesenchymal stem cell secretome: A
new paradigm towards cell-free therapeutic mode in regenerative
medicine. Cytokine Growth Factor Rev. 46:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheng L, Zhang K, Wu S, Cui M and Xu T:
Focus on mesenchymal stem cell-derived exosomes: Opportunities and
challenges in cell-free therapy. Stem Cells Int. 2017:63052952017.
View Article : Google Scholar
|
|
49
|
Lai RC, Arslan F, Lee MM, Sze NS, Choo A,
Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, et al:
Exosome secreted by MSC reduces myocardial ischemia/reperfusion
injury. Stem Cell Res. 4:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kanakaris NK and Giannoudis PV: Clinical
applications of bone morphogenetic proteins: Current evidence. J
Surg Orthop Adv. 17:133–146. 2008.PubMed/NCBI
|
|
51
|
Gentile P, Piccinno MS and Calabrese C:
Characteristics and potentiality of human adipose-derived stem
cells (hASCs) obtained from enzymatic digestion of fat graft.
Cells. 8:2822019. View Article : Google Scholar :
|
|
52
|
Galateanu B, Dinescu S, Cimpean A,
Dinischiotu A and Costache M: Modulation of adipogenic conditions
for prospective use of hADSCs in adipose tissue engineering. Int J
Mol Sci. 13:15881–15900. 2012. View Article : Google Scholar
|
|
53
|
Hsiao ST, Asgari A, Lokmic Z, Sinclair R,
Dusting GJ, Lim SY and Dilley RJ: Comparative analysis of paracrine
factor expression in human adult mesenchymal stem cells derived
from bone marrow, adipose and dermal tissue. Stem Cells Dev.
21:2189–2203. 2012. View Article : Google Scholar
|
|
54
|
Shukla L, Yuan Y, Shayan R, Greening DW
and Karnezis T: Fat therapeutics: The clinical capacity of
adipose-derived stem cells and exosomes for human disease and
tissue regeneration. Front Pharmacol. 11:1582020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hong P, Yang H, Wu Y, Li K and Tang Z: The
functions and clinical application potential of exosomes derived
from adipose mesenchymal stem cells: A comprehensive review. Stem
Cell Res Ther. 10:2422019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wong DE, Banyard DA, Santos PJF, Sayadic
LR, Evans GR and Widgerow AD: Adipose-derived stem cell
extracellular vesicles: A systematic review. J Plast Reconstr
Aesthet Surg. 72:1207–1218. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dinescu S, Hermenean A and Costache M:
Human adipose-derived stem cells for tissue engineering approaches:
Current challenges and perspectives. Stem Cells in Clinical
Practice and Tissue Engineering. InTech. Chapter-14. 2018,
View Article : Google Scholar
|
|
58
|
Spasovski D, Spasovski V, Baščarević Z and
Stojiljković M: Intra-articular injection of autologous
adipose-derived mesenchymal stem cells in the treatment of knee
osteoarthritis. J Gene Med. 20:e30022018. View Article : Google Scholar
|
|
59
|
Maumus M, Manferdini C, Toupet K,
Peyrafitte JA, Ferreira R, Facchini A, Gabusi E, Bourin P,
Jorgensen C, Lisignoli G and Noël D: Adipose mesenchymal stem cells
protect chondrocytes from degeneration associated with
osteoarthritis. Stem Cell Res. 11:834–844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qiu H, Liu S, Wu K, Zhao R, Cao L and Wang
H: Prospective application of exosomes derived from adipose-derived
stem cells in skin wound healing: A review. J Cosmet Dermatol.
19:574–581. 2020. View Article : Google Scholar
|
|
61
|
Kowal J, Tkach M and Thery C: Biogenesis
and secretion of exosomes. Curr Opin Cell Biol. 29:116–125. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Minciacchi RV, Freeman MR and Vizio DD:
Extracellular vesicles in cancer: Exosomes, microvesicles and the
emerging role of large oncosomes. Semin Cell Dev Biol. 40:41–51.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi DS, Kim DK, Kim YK and Gho YS:
Proteomics, transcriptomics and lipidomics of exosomes and
ectosomes. Proteomics. 13:1554–1571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park SJ, Kim JM, Kim J, Hur J, Park S, Kim
K, Shin HJ and Chwae YJ: Molecular mechanisms of biogenesis of
apoptotic exosome-like vesicles and their roles as
damage-associated molecular patterns. Proc Natl Acad Sci USA.
115:E11721–E11730. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Weichand B, Weis N, Weigert A, Grossmann
N, Levkau B and Brüne B: Apoptotic cells enhance
sphingosine-1-phosphate receptor 1 dependent macrophage migration.
Eur J Immunol. 43:3306–3313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kakarla R, Hur J, Kim YJ, Kim J and Chwae
YJ: Apoptotic cell-derived exosomes: Messages from dying cells.
Expe Mol Med. 52:1–6. 2020. View Article : Google Scholar
|
|
67
|
Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC
and Zhang CQ: Exosomes derived from miR-140-5p-overexpressing human
synovial mesenchymal stem cells enhance cartilage tissue
regeneration and prevent osteoarthritis of the knee in a rat model.
Theranostics. 7:180–1895. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vonk LA, van Dooremalen SFJ, Liv N,
Klumperman J, Coffe PJ, Saris DBF and Lorenowicz MJ: Mesenchymal
stromal/stem cell-derived extracellular vesicles promote human
cartilage regeneration in vitro. Theranostics. 8:906–920. 2018.
View Article : Google Scholar :
|
|
69
|
Cosenza S, Ruiz M, Toupet K, Jorgensen C
and Noël D: Mesenchymal stem cells derived exosomes and
microparticles protect cartilage and bone from degradation in
osteoarthritis. Sci Rep. 7:162142017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B,
Zhou J, Heng BC, Zou XH, Ouyang H and Liu H: Exosomes from
embryonic mesenchymal stem cells alleviate osteoarthritis through
balancing synthesis and degradation of cartilage extracellular
matrix. Stem Cell Res. 8:1892017.
|
|
71
|
Jin Z, Ren J and Qi S: Human bone
mesenchymal stem cells-derived exosomes overexpressing
microRNA-26a-5p alleviate osteoarthritis via down-regulation of
PTGS2. Int Immunopharmacol. 78:1059462020. View Article : Google Scholar
|
|
72
|
Mao G, Zhang Z, Hu S, Zhang Z, Chang Z,
Huang Z, Liao W and Kang Y: Exosomes derived from
miR-92a-3poverexpressing human mesenchymal stem cells enhance
chondrogenesis and suppress cartilage degradation via targeting
WNT5A. Stem Cell Res Ther. 9:2472018. View Article : Google Scholar
|
|
73
|
Tofiño-Vian M, Guillén MI, Pérez del Caz
MD, Silvestre A and Alcaraz MJ: Microvesicles from human adipose
tissue-derived mesenchymal stem cells as a new protective strategy
in osteoarthritic chondrocytes. Cell Physiol Biochem. 47:11–25.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tofiño-Vian M, Guillén MI, Pérez del Caz
MD, Castejón MA and Alcaraz MJ: Extracellular vesicles from
adipose-derived mesenchymal stem cells downregulate senescence
features in osteoarthritic osteoblasts. Oxid Med Cell Longev.
2017:71975982017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Woo CH, Kim HK, Jung GY, Jung YJ, Lee KS,
Yun YE, Han J, Lee J, Kim WS, Choi JS, et al: Small extracellular
vesicles from human adipose-derived stem cells attenuate cartilage
degeneration. J Extracell Vesicles. 9:17352492020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhao C, Chen JY, Peng WM, Yuan B, Bi Q and
Xu YJ: Exosomes from adipose-derived stem cells promote
chondrogenesis and suppress inflammation by upregulating miR-145
and miR-221. Mol Med Rep. 21:1881–1889. 2020.PubMed/NCBI
|
|
77
|
Stepien A, Dabrowska NL, Maciagowska M,
Macoch RP, Zolocinska A, Mazur S, Siennicka K, Frankowska E,
Kidzinski R, Chalimoniuk M and Pojda Z: Clinical application of
autologous adipose stem cells in patients with multiple sclerosis:
Preliminary results. Mediators Inflamm. Sep 28–2016.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kuriyan AE, Albini TA, Townsend JH,
Rodriguez M, Pandya HK, Leonard RE II, Parrott MB, Rosenfeld PJ,
Flynn HW Jr and Goldberg JL: Vision loss after intravitreal
injection of autologous 'stem cells' for AMD. N Engl J Med.
376:1047–1053. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Vériter S, André W, Aouassar N, Poirel HA,
Lafosse A, Docquier PL and Dufrane D: Human adipose-derived
mesenchymal stem cells in cell therapy: Safety and feasibility in
different 'Hospital Exemption' clinical applications. PLoS One.
10:e01395662015. View Article : Google Scholar
|
|
80
|
Atat OE, Antonios D, Hilal G, Hokayem N,
Abou-Ghoch J, Hashim H, Serhal R, Hebbo C, Moussa M and Alaaeddine
N: An evaluation of the stemness, paracrine and tumorigenic
characteristics of highly expanded, minimally passaged
adipose-derived stem cells. PLoS One. 11:e01623322016. View Article : Google Scholar
|
|
81
|
Tátrai P, Szepesi Á, Matula Z, Szigeti A,
Buchan G, Mádi A, Uher F and Német K: Combined introduction of
Bmi-1 and hTERT immortalizes human adipose tissue-derived stromal
cells with low risk of transformation. Biochem Biophys Res Commun.
422:28–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang Y, Liu J, Mo Y, Chen Z, Chen T, Li
Y, Zheng Y, Deng S, Xu X, Chen H, et al: Immortalized mesenchymal
stem cells: A safe cell source for cellular or cell membrane-based
treatment of glioma. Southern Medical University; 2021
|
|
83
|
Vater C, Kasten P and Stiehler M: Culture
media for the differentiation of mesenchymal stromal cells. Acta
Biomater. 7:463–477. 2011. View Article : Google Scholar
|
|
84
|
Pelttari K, Winter A, Steck E, Goetzke K,
Hennig T, Ochs BG, Aigner T and Richter W: Premature induction of
hypertrophy during in vitro chondrogenesis of human mesenchymal
stem cells correlates with calcification and vascular invasion
after ectopic transplantation in SCID mice. Arthritis Rheum.
54:3254–3266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Puetzer JL, Petitte JN and Loboa EG:
Comparative review of growth factors for induction of
three-dimensional in vitro chondrogenesis in human mesenchymal stem
cells isolated from bone marrow and adipose tissue. Tissue Eng Part
B Rev. 16:435–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Freyria AM and Mallein-Gerin F:
Chondrocytes or adult stem cells for cartilage repair: The
indisputable role of growth factors. Injury. 3:259–265. 2012.
View Article : Google Scholar
|
|
87
|
Santo VE, Gomes ME, Mano JF and Reis RL:
Controlled release strategies for bone, cartilage and osteochondral
engineering-part II: Challenges on the evolution from single to
multiple bioactive factor delivery. Tissue Eng Part B Rev.
19:327–352. 2013. View Article : Google Scholar :
|
|
88
|
Afizah H, Yang Z, Hui JH, Ouyang HW and
Lee EH: A comparison between the chondrogenic potential of human
bone marrow stem cells (BMSCs) and adipose-derived stem cells
(ADSCs) taken from the same donors. Tissue Eng. 13:659–666. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Ude CC, Shamsul BS, Ng MH, Chen HC, Ohnmar
H, Amaramalar SN, Rizal AR, Johan A, Norhamdan MY, Azizi M, et al:
Long-term evaluation of osteoarthritis sheep knee, treated with
TGF-β3 and BMP-6 induced multipotent stem cells. Exp Gerontol.
104:43–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Lu CH, Yeh TY, Yeh CL, Fang YH, Sung LY,
Lin SY, Yen TC, Chang YH and Hu YC: Regenerating cartilages by
engineered ASCs: Prolonged TGF-β3/BMP-6 expression improved
articular cartilage formation and restored zonal structure. Mol
Ther. 22:186–1895. 2014. View Article : Google Scholar
|
|
91
|
Choi S, Cho TJ, Kwon SK, Lee G and Cho J:
Chondrogenesis of periodontal ligament stem cells by transforming
growth factor-β3 and bone morphogenetic protein-6 in a normal
healthy impacted third molar. Int J Oral Sci. 5:7–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yoo KH, Thapa N, Kim BJ, Lee JO, Jang YN,
Chwae YJ and Kim J: Possibility of exosome-based coronavirus
disease 2019 vaccine (Review). Mol Med Rep. 25:262022. View Article : Google Scholar
|